Abstract
Respiratory viral infections remain a scourge, with seasonal influenza infecting millions and killing many thousands annually and viral pandemics, such as COVID-19, recurring every decade. Age, cardiovascular disease, and diabetes mellitus are risk factors for severe disease and death from viral infection. Immunometabolic therapies for these populations hold promise to reduce the risks of death and disability. Such interventions have pleiotropic effects that might not only target the virus itself but also enhance supportive care to reduce cardiopulmonary complications, improve cognitive resilience, and facilitate functional recovery. Ketone bodies are endogenous metabolites that maintain cellular energy but also feature drug-like signaling activities that affect immune activity, metabolism, and epigenetics. Here, we provide an overview of ketone body biology relevant to respiratory viral infection, focusing on influenza A and severe acute respiratory syndrome (SARS)-CoV-2, and discuss the opportunities, risks, and research gaps in the study of exogenous ketone bodies as novel immunometabolic interventions in these diseases.
Keywords: Ketones, Ketone bodies, β-hydroxybutyrate, COVID-19, SARS-CoV-2, Influenza, exogenous ketone, ketone ester, ketone salt, cytokine storm
Graphical Abstract
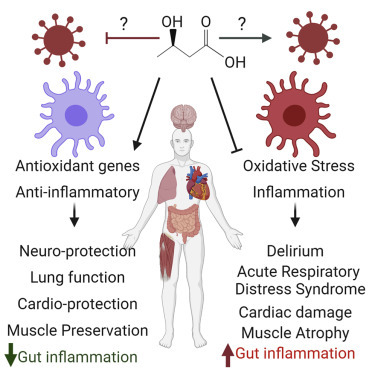
Stubbs et al. provide an overview of the known biological actions of ketone bodies that could be relevant to severe respiratory viral infection, using influenza A virus and SARS-CoV-2 as key examples, and describe how exogenous ketones could be a novel immunometabolic intervention in these diseases.
Background
Respiratory viral infection represents an ever-present public health threat, which can readily overwhelm our existing tools to prevent spread and widespread fatalities. Not only must we deal with seasonal outbreaks, such as respiratory influenza A virus (influenza), but throughout human history, we have faced the regular emergence of novel viruses with global pandemic potential, such as 2003 severe acute respiratory syndrome (SARS)-CoV, 2004 N5N1 influenza, 2009 H1N1/09 influenza, 2012 Middle East respiratory syndrome (MERS)-CoV, and most recently 2019 SARS-CoV-2. Influenza causes more than 20,000 deaths annually in the United States, incurring an economic burden in excess of $87 billion each year.1 , 2 Similarly, the 2019–2020 global pandemic of the novel coronavirus SARS-CoV-2, causative agent of COVID-19 respiratory disease, in only a few months infected millions of people, killed hundreds of thousands, and led to worldwide social and economic disruption. There is no efficacious universal vaccine for influenza or for SARS coronaviruses (SARS-CoV, SARS-CoV-2, and MERS); consequently, novel therapeutic approaches are vital for the treatment of these viral diseases.
Several population sub-groups appear to be at particularly high risk of complications with respiratory viral infections, such as influenza and SARS-CoV-2. First, older adults have increased risk of hospitalization and mortality.3, 4, 5, 6, 7, 8 Second, patients with diabetes (both type 1 and type 2) also appear to be particularly vulnerable5 , 9, 10, 11, 12; hallmarks of diabetes include hyperglycemia, glycemic variability, and/or insulin dysregulation, all shown to worsen outcomes from infection.13, 14, 15, 16, 17, 18, 19
Respiratory viruses, including influenza and SARS-CoV-2, can lead to acute respiratory distress syndrome (ARDS)20 , 21 and death from respiratory failure. ARDS is a complex syndrome that is characterized by lung vascular endothelial injury and alveolar epithelial injury and is associated histologically with alveolar filling with protein-rich fluid.22 The pathophysiology of ARDS includes complex host-pathogen immune interactions along with cellular damage and death resulting from a delayed, pathological hyperactive inflammatory response, hyperoxia, hypoxia, and oxidative stress.23 There is a critical need for mitigation strategies that attenuate these damage pathways, importantly without compromising the early, protective physiological immune response to viral infection. Even patients who recover from critical illness and ARDS can have long-term health consequences from prolonged critical illness, including cognitive impairment and physical disability, exacerbated with age,24 , 25 reinforcing that clinical strategies should focus on not only mitigating the acute disease process but also on supporting recovery.26
Metabolic therapies represent novel strategies that could be used to target viral disease progression. In the last decade, the field of immunometabolism research has uncovered multiple points where metabolism influences host-pathogen interactions, not only in altering infection risk and viral replication but also affecting the response of specific immune cell types, thus profoundly controlling disease outcomes.27, 28, 29, 30 One metabolic therapy with promise in this area is the induction of a state of ketosis, where blood ketone body concentrations are elevated. Ketone bodies are endogenous molecules synthesized from free fatty acids. The primary ketone body, beta-hydroxybutyrate (BHB), directly acts as both a highly efficient oxidative fuel and signaling metabolite.31 BHB has been shown to have diverse molecular effects, including metabolic regulation;32 increased cellular resistance to oxidative stress;33, 34, 35 inhibition of nuclear factor κB (NF-κB) signaling via HCAR2 receptor binding36, 37; decreased activity of components of the innate immune system, such as the nonobese diabetic (NOD)-, leucine-rich repeat (LRR)-, and pyrin domain-containing protein 3 (NLRP3) inflammasome;38, 39, 40 decreased systemic inflammatory burden;41 modifying gene expression;33 , 42 and acting as a fuel in the context of energetic stress.43 , 44 Ketogenic interventions have been used for decades in the treatment of intractable epilepsy45 and are under clinical investigation for their possible roles in targeting mechanisms of aging and utility in managing diabetes, heart failure, neurodegeneration, and other diseases.31 , 46 A recently registered clinical trial proposed use of ketogenic nutrition in intubated COVID-19 patients (NCT04358835). These multifaceted metabolites are not panaceas, but their pleotropic activities at the interface of aging, metabolism, and inflammation may be useful in mitigating aspects of respiratory viral infection, particularly among patients most susceptible to severe disease.
Blood ketone levels are normally only elevated during a period of fasting or when following a ketogenic diet (between 0.5 and 8 mM).47 It is important to distinguish between “physiological” levels of ketosis,48 which is an adaptive, regulated response to lowered carbohydrate availability and can be safely sustained over many months,45 , 49 and the acute pathological condition of ketoacidosis. In ketoacidosis, a fundamental metabolic derangement (such as insulin resistance or substance abuse) leads to uncontrolled ketone production and to ketone accumulation, which becomes a medical emergency.50 Barriers to the implementation of novel dietary strategies to trigger controlled, physiological endogenous ketosis, such as fasting or ketogenic diet, include difficulty with adherence51 , 52 and potential complications in disease states,53 especially when there may be a higher risk of promoting dysregulated ketoacidosis. For example, undernutrition and prolonged fasting can be harmful when applied to the intensive care unit (ICU),54 , 55 and infection (including COVID-19) may cause metabolic derangements that lead to development of ketoacidosis.56,57 Notably, acidosis itself is associated with mortality in COVID-19 patients,56 but it is unclear whether it plays a causative role versus being a marker of tissue hypoperfusion, renal failure, respiratory failure, or other pathologies.
Exogenous sources of ketones can directly raise circulating ketone levels equivalent to physiological levels (0.5–8 mM) within minutes, in a controlled manner without potentially burdensome dietary or pharmacologic interventions.57 As ketones are normal, endogenous metabolites, bio-identical exogenous ketones have a strong safety profile and low risk of toxicity, and some examples are classified as “generally recognized as safe” for use as food ingredients (GRAS) by the US Food and Drug Administration, although none have yet undergone formal testing as part of an investigational drug program.
One family of exogenous ketone compounds are ketone salts. These are inexpensive to synthesize and are widely available to the public in consumer health products; however, they only modestly increase blood BHB levels (∼1 mM BHB increase).57 Medium-chain triglycerides provide a longer lasting modest ketosis (∼0.5–1 mM BHB increase) without added salt load and have a body of clinical research supporting safety and efficacy for several metabolic outcomes in obese and diabetic patients and early-stage efficacy for improving metabolism in the aging brain and in Alzheimer’s disease.58, 59, 60, 61 Medium-chain triglycerides are also widely commercially available, though acute dosing can be limited by gastrointestinal side effects.62 Esters containing ketones and ketone precursors are also commercially available and result in higher blood BHB levels (∼1–5 mM BHB increase).57 The wide availability and extensive prior consumer use of most of these exogenous ketone compounds suggest that rapid clinical implementation would be feasible if and when clinical utility were to be demonstrated.
There may be immunological advantages of triggering a metabolic state that supports ketosis (i.e., lowered blood glucose and increased fatty acid oxidation), independent of increases in ketone bodies. For example, 4 h of fasting in mice, a time point that precedes detectable systemic elevations in ketone bodies, reduces circulating proinflammatory monocytes.63 Also, ketogenic diet, but not the exogenous ketone precursor, 1,3-butanediol, protected mice against lethal influenza infection via activation of lung-resident gamma-delta T cells.64 Given that different immune cell subsets express varying levels of ketogenic and ketolytic enzymes,65 the role of ketone bodies in immune responsiveness will likely vary depending on infection, background inflammation, and underlying metabolic context.
Exogenous and endogenous ketone biology could be relevant to precisely the patient groups at greater risk of respiratory viral infection. First, with respect to aging, nutritional strategies that increase circulating ketone body concentrations increase healthspan and lifespan in laboratory rodents by mitigating multiple age-related pathologies and improving overall health.66, 67, 68 Second, patients with diabetes, with accompanying obesity, hyperglycemia, glycemic variability, and insulin resistance, have elevated NLRP3 activity, increased systemic inflammation, and high oxidative stress.69 Endogenous and exogenous ketones have been demonstrated to reduce acute and/or chronic markers of glycemic load in healthy and diabetic populations.41 , 49 , 70, 71, 72, 73
We hypothesize that consumption of exogenous ketones may improve specific clinical outcomes of respiratory viral infection through already-understood molecular mechanisms. Older patients and patients with diabetes, in whom these mechanisms are most relevant, may be the groups most likely to benefit from exogenous ketone body administration. In the following sections, we outline the rationale and highlight the mechanisms through which BHB alters cellular biology and integrated physiology, which may have direct or indirect impact on critical illness caused by respiratory viral infection, using SARS coronaviruses and influenza as key examples. Finally, we discuss outstanding questions that may be a “cause to pause” as investigations into this hypothesis move from bench to bedside.
Molecular Mechanisms and Cellular Biology
Several known molecular mechanisms of the ketone body BHB have been implicated in respiratory virus life cycles and molecular pathogenesis (Figure 1 ). Most of these mechanisms are specifically implicated in viral replication. Here, we briefly summarize these BHB-regulated mechanisms and review the current state of knowledge of their roles in respiratory viruses.
Figure 1.
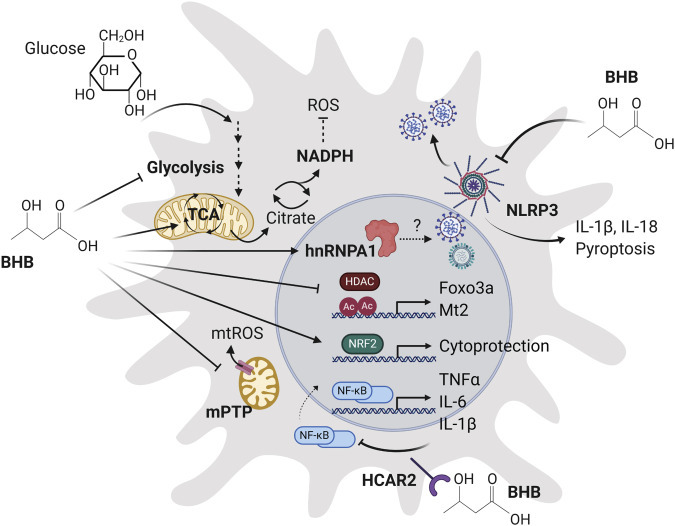
Molecular Mechanisms of Ketone Bodies Relevant to Viral Respiratory Infection
BHB Protects against Oxidative Stress
Oxidative stress from a variety of sources can contribute to lung epithelial damage during infection with influenza74 and subsequent ARDS.75 Cytoplasmic nicotinamide adenine dinucleotide phosphate (NADPH) plays a dual role in the body’s defense against oxidative stress. In immune cells, pentose-phosphate-pathway-derived NADPH serves as a cofactor for NADPH-oxidase-dependent reactive oxygen species (ROS) production, which plays a crucial role in the protective immune response but can also cause damage as part of pathological immune hyperactivation. In these cells, NADPH is also critical for reductive biosynthesis, activation-associated membrane expansion, and the production of lipid mediators, such as prostaglandins. However, in the cytoplasm of non-immune cells, NADPH reduces oxidized redox couples to sustain a protective antioxidant response (i.e., glutathione and thioredoxin systems). This is illustrated by data showing that maintenance of epithelial cell glutathione concentrations reduces viral replication following influenza infection,76 although influenza-mediated decrease in NADPH oxidase activity in immune cells leads to increased susceptibility to further bacterial infection.77
Pentose phosphate pathway substrates are typically derived from glucose metabolism. However, in cells with the capacity to oxidize BHB, ketone metabolism can also result in an increase of cytoplasmic NADPH (via citrate).78 Thus, in settings where glucose metabolism is unable to sufficiently maintain the cytoplasmic NADPH pool, BHB metabolism could provide an alternative route to reducing the cytosolic NADP/NADPH couple and protect against cell damage, although this effect still needs to be verified in the context of oxidative stress triggered by respiratory viral infection. Notably, the enzyme BHB dehydrogenase, which catalyzes the initial step in BHB oxidation, is not expressed in neutrophils or monocytes,79 , 80 and so elevated BHB would not be expected to affect cytosolic NADPH and have a stimulatory effect on either protective or pathological ROS production in these cells.
A second mechanism whereby exogenous ketones could mitigate oxidative stress is through upregulation of antioxidant genes. BHB activates the transcription factor Nrf2 to induce antioxidant response element (ARE) gene expression81, 82, 83 and induces local histone acetylation at the promoter of oxidative stress resistance genes (Foxo3a and Mt2) by inhibiting activity of histone deacetylases histone deacetylase 1 (HDAC1) and HDAC2.34 , 35 , 84 Both of these actions result in an increased expression of protective genes and cytoprotection, which was demonstrated in two independent studies using the kidney in a mouse model of chemically induced oxidative stress.33 , 81 Similar effects have been further demonstrated in other tissues in multiple preclinical models.34 , 35 , 82 , 83 Activation of Nrf2 is protective in multiple models of ARDS (reviewed in Liu et al.85). However, an inhibitory effect of BHB on HDAC activity may be counterproductive during viral infection. HDAC1 and 2 activity also play a protective role against influenza infection86 , 87; in fact, influenza actively dysregulates HDAC1 to allow effective replication.86 The recently published SARS-CoV-2 interactome88 indicates that this virus may also interact with HDAC2, although the importance of this interaction in infection is unknown. Finally, in addition to BHB-induced upregulation of antioxidant defenses, ketone bodies may have a direct, protective antioxidant effect. Both BHB and acetoacetate can act as scavengers for diverse free radicals in both in vitro and in vivo models.89 It should be noted that some evidence suggests that, under certain physiological conditions, such as hyperglycemia, acetoacetate (but not BHB) is associated with an increase in oxidative stress90 (further reviewed in Jain et al.91), emphasizing that controlling glucose and ketone levels together may be important for optimal effect. Altogether, the multiple possible protective effects of ketone bodies on oxidative stress provide a compelling case for further examination in the specific context of viral infection and systemic inflammation.
BHB Directly Inhibits Proinflammatory NLRP3 Activation
Inflammasomes are essential components of innate immunity that sense pathogen- or damage-associated molecular patterns. They are highly regulated multimeric protein complexes that facilitate caspase-1 activation for the secretion of interleukin-1β (IL-1β) and IL-18. Although some degree of inflammasome activation is required for the protective immune response and the clearance of certain infections, excessive pathological activation can lead to tissue damage and systemic inflammation. As described in more detail below, both SARS-CoV and influenza are directly sensed by the NLRP3 inflammasome. NLRP3, which is expressed primarily in innate immune cells, is the best characterized inflammasome, in part due to its strong linkage to metabolic inflammation (i.e., obesity and diabetes) and driving age-related inflammation.92 , 93 As obesity, diabetes, and age are also risk factors for respiratory viral infections, including influenza and SARS-CoV-2, collectively, these data strongly implicate inflammasome, and specifically NLRP3 activation, in their pathology.
Early clinical data from COVID-19 patients suggest a role for systemic inflammatory activation in the pathogenesis of severe disease, with higher levels of C-reactive protein (CRP), IL-6, IL-1β, and other proinflammatory cytokines and markers.94, 95, 96 Some of this may be driven by excessive NLRP3 activation. A study of 47 patients with confirmed COVID-19 in Wuhan found that increased lactate dehydrogenase (LDH) levels most significantly correlated with disease severity.97 LDH is released from cells undergoing an inflammatory form of cell death known as pyroptosis, which is induced by caspase-1 activation.98 Systemic inflammasome-mediated inflammation could also instigate emergency granulopoiesis, which would explain the neutrophilia commonly observed in severe COVID-19 patients.
Preclinical data support a key role for the NLRP3 inflammasome in mediating the pathogenesis of both influenza99 and SARS coronaviruses.100 NLRP3 has several mechanisms for detecting viral infections, such as influenza or CoV. Cytosolic influenza RNA is directly sensed by the NLRP3 inflammasome, and this is required for protection.101 , 102 Likewise, SARS-CoV proteins E, 3a, and 8b all activate NLRP3. E is a viroporin encoding a cation channel that increases intracellular sodium, calcium, and potassium flux, with the latter two being well-known inducers of NLRP3 activation and IL-1β production.103 The ion channel activity of E protein is crucial for both viral fitness and severity of disease in mouse-adapted SARS-CoV. Abrogation of the ion channel activity results in improved survival, reduced ARDS pathology, and reduced airway inflammatory markers, including IL-1β.104 Similarly, influenza virus encodes a proton channel, M2, that is required for virus fitness and stimulates NLRP3.105 SARS-CoV accessory protein 3a activates NLRP3 via TRAF3-mediated ubiquitination of apoptosis-associated speck-like protein containing a CARD (ASC), colocalizing with TRAF3 and ASC specks and increasing IL-1β production.106 3a is also a viroporin that encodes a potassium channel. Potassium efflux and mitochondrial ROS generation are required for activation of NLRP3 by 3a.107 Finally, SARS-CoV accessory protein 8b interacts with the LRR domain of NLRP3 and also stimulates NLRP3 activation and IL-1β release along with forming insoluble intracellular aggregates to activate autophagy and ER stress pathways.108 Altogether, studies of both influenza and SARS coronaviruses indicate a critical role for NLRP3 activation and IL-1β production in driving severe disease.
Although the possibility that additional inflammasome complexes contribute to COVID-19 morbidity has not been ruled out, it was recently reported that bats, the natural host of many zoonotic viruses and asymptomatically carry coronavirus, have reduced NLRP3 inflammasome activation in response to MERS and other stimuli.103 Bat species show reduced Nlrp3 transcriptional activation in response to inflammatory stimuli and reduced inflammasome activation and express an abundant splice variant with an exon 7 deletion that further reduces activation. These adaptations appear to all arise from positive selection within the LRR domain of NLRP3 and help bats avoid serious illness from RNA viruses, such as MERS and Ebola, without affecting viral load.103
Importantly, in multiple in vitro and animal studies that have utilized both dietary ketosis and exogenous ketone treatment, BHB inhibits NLRP3 activation in peripheral macrophages and neutrophils,38 in the retina,109 and in the CNS110 and reduced systemic inflammation in a rat model of gout, which is driven by NLRP3 activation.40 However, experiments attempting to repeat these findings in human subjects challenged with lipopolysaccharide (LPS) have produced inconsistent results. A recent study of healthy young men given intravenous (i.v.) BHB and LPS found BHB was associated with a small increase in circulating IL-1β111; similarly, a study by Neudorf et al.112 found a small increase in inflammatory markers in ex vivo LPS-stimulated peripheral blood mononuclear cells from healthy subjects given oral BHB compared with a calorie-free control. On the other hand, a follow-up study by the same group showed no additional effect of BHB on inflammatory markers in in ex vivo LPS-stimulated peripheral blood mononuclear from obese subjects following an oral glucose challenge,113 which suggests a context dependency of BHB-NLRP3 on circulating glucose or insulin concentrations. This is further underscored by a recent study demonstrating that changes in insulin and glucose concentrations modulate the effect of BHB on NLRP3 on human monocytes from diabetic patients in vitro,114 reinforcing the complexity of this interaction, which remains to be fully defined. In addition, it is not known whether BHB affects NLRP3 activation specifically in alveolar macrophages or lung epithelial cells as it does in blood macrophages. Given the strong preclinical evidence for BHB-mediated NLRP3 inhibition, and the key role of NLRP3 in both protective sensing of viral infection and the progression to pathological hyper-inflammation, it is vital to establish whether and how exogenous BHB may alter NLRP3 activity in the context of respiratory viral infection across a physiologically relevant range of BHB, glucose, and insulin concentrations. This would result in BHB dosing guidelines that account for timing of disease progression (i.e., possibly favoring late administration), background metabolic state (i.e., aging or diabetes), and a specific circulating level of BHB.
BHB Has Additional Anti-inflammatory Effects Mediated by HCAR2 and NF-κB
NF-κB is a potent proinflammatory transcription factor, induced by numerous signaling pathways, including the cell surface receptor HCAR2, and oxidative stress. Both NF-κB and HCAR2 are upregulated in diabetic patients,37 , 115 and NF-κB is strongly activated during viral infection. Upon activation, NF-κB translocates into the nucleus to induce expression of tumor necrosis factor alpha (TNF-α), IL-6, and IL-1β with multiple downstream effects, including inflammasome activation, apoptosis, and, importantly, further stimulation of NF-κB activation, which creates an inflammatory positive feedback loop. Despite clear evidence that NF-κB plays an important protective role in the immune response to influenza infection116 and that impaired production of downstream cytokines, such as IL-6, can increase influenza-related mortality,117 other studies have shown NF-κB can be “hijacked” to promote influenza replication,118 , 119 resulting in some debate over its overall role in disease progression.119 Inhibition of NF-κB has been suggested to be a possible treatment strategy for influenza infection.120 , 121 Although the role of NF-κB in SARS-CoV-2 has yet to be fully elucidated, the high levels of TNF-α and IL-6 in patients with severe COVID-19 infection are strongly suggestive of its activation.96 , 122
BHB strongly inhibits NF-κB-mediated inflammation by binding to HCAR2 on central-nervous-system-resident macrophages.123 , 124 Chronic administration of various exogenous ketone bodies fed ad libitum in rodents mobilizes fatty acid substrates and lowers NF-κB-related circulating cytokines IL-1β and IL-6, among others.125 The effect of BHB on HCAR2 and NF-κB in lung-resident macrophages has not yet been tested, although if an effect does occur, in vivo infection studies should consider timing of BHB administration to avoid interference with the protective effect of NF-κB, which could worsen overall disease outcomes.
BHB Reduces Apoptosis via Mitochondrial Permeability Transition Pore (mPTP) Closure
Prolonged opening of the mPTP is one of the mechanisms through which ROS can induce cellular injury and promote disease. Opening of the mPTP destroys the mitochondrial proton gradient that drives ATP production and allows entry of cations (Ca2+ and Mg2+) into the negatively charged inner mitochondrial membrane space, both of which severely impair mitochondrial function. Many viruses directly take advantage of this pathway to trigger apoptosis; for example, influenza PB1-F2 protein acts via mPTP opening to sensitize cells to apoptosis stimuli.126 Closure of the mPTP was suggested as a druggable target in a recent preprint describing a bioinformatics analysis of SARS-CoV-2 binding interactions.127
Oxidation of BHB closes the mPTP, providing cellular protection by maintaining the electrochemical potential gradient required for ATP generation through oxidative phosphorylation,128 thereby providing the energy required to restore normal Ca2+ and Mg2+ homeostasis and cellular volume regulation. BHB-mediated closure of the mPTP in conjunction with protection against ROS has been directly demonstrated in vitro35 and in vivo.129 A key question that must be addressed is whether physiological concentrations of BHB protect against virally triggered apoptosis via an mPTP-dependent mechanism.
BHB Interacts with RNA-Binding Ribonucleoprotein hnRNPA1
Several viruses are known to interact with host cells heterogeneous nuclear ribonucleoproteins (hnRNPs); a family of RNPs that regulate mRNA stability, splicing, and translation. The nucleocapsid proteins of the coronaviruses SARS-CoV,130 mouse hepatitis virus (MHV),131 and porcine epidemic diarrhea virus (PEDV)132 all interact in vitro and colocalize in vivo with human hnRNPA1, although influenza interacts with the related hnRNPA A2/B1.133 The preprint interactome of SARS-CoV-2 reveals only weak interactions of hnRNPA1 that do not meet stringency criteria.88 hnRNPs are thought to assist in viral replication.134 Overexpression of human hnRNPA1 accelerates MHV genome replication,132 and knockdown inhibits replication of both MHV and PEDV.132 A similar pattern has been found whereby knockdown of hnRNPA2/B1 reduces influenza replication136 and overexpression increases replication.135 Recently, BHB was reported to directly interact with hnRNPA1, in the first example of a BHB interaction target identified by a systematic binding screen.136 The BHB-hnRNPA1 interaction enhances stabilization of Oct4 mRNA, leading to reduced senescence in mouse vascular endothelial cells. The implications of this BHB-hnRNP interaction for replication of viruses, such as SARS-CoV-2 or influenza, are currently not known but could imply enhancement of viral replication.
BHB Inhibits Glycolysis
Viruses can induce changes to host cell metabolism, or use host-derived metabolites, to facilitate their replication. Metabolic plasticity is key to a successful antiviral immune response: upregulation of glycolytic programming is essential for activation and effector function of many immune cell types, but transition back to oxidative metabolism is required for establishment of long-lived memory CD8 T cells.137 , 138 Induction of non-immune cells by relatively common pathogens, such as influenza, rhinoviruses, and human respiratory syncytial virus, increases glycolytic rate and viral replication in infected cells.139, 140, 141 Complete inhibition of glycolysis by 2-deoxy-D-glucose (2-DG), an inhibitor of hexokinase (i.e., the committed step of glycolysis), effectively defends against infection by several viruses in cell culture, suppressing influenza replication in vitro 141 and rhinovirus infection in vivo.142 Although the primary mechanism for this is believed to be creation of energetic stress, secondary protective effects of 2-DG include activation of the unfolded protein response (UPR) to prevent the assembly of viral proteins143 and modulation of viral release through changes in glycosylation.144 Recent work demonstrated that non-toxic concentrations of 2-DG prevented SARS-CoV-2 replication in Caco-2 cells,145 and a 2-DG analog (WP1122) is under accelerated development for use in a COVID-19 clinical trial.
BHB directly inhibits glycolysis in multiple tissues, such as heart, brain, skeletal muscle, and in tumors.32,146, 147, 148 This occurs via an increase in cytoplasmic citrate, which decreases the activity of phosphofructokinase as well as a direct inhibitory effect on pyruvate dehydrogenase that is mediated by an increase in ketone-derived acetyl coenzyme A (CoA). Inhibition of glycolysis by ketones may not create the same energetic stress as 2-DG and analogs (given the availability of ketones) and may not have the same effects on the UPR or glycosylation. The current gap is to demonstrate that inhibition of glycolysis specifically reduces viral replication, and that this can be driven by exogenous application of BHB to infected epithelial cells. It would be important to expand this testing in an in vivo model to ensure that glycolysis required for immune cell function was not suppressed by BHB.
Integrated Physiology
Clinical outcomes in severe respiratory virus infection are driven not only by replication of the virus itself but by also the complex host-pathogen immune interactions that give rise to ARDS, by other organ-specific dysfunctions driven by the virus or by hypoxemia, and by complications of critical illness, such as loss of muscle function and the acute confusional state delirium. These disproportionately affect both mortality and long-term outcomes in survivors, such as cognitive and functional decline. Here, we review how the molecular mechanisms of ketone bodies described above may impact these crucial, complex manifestations of severe respiratory viral infection (Figure 2 ), including drawing analogies from existing literature of ketone bodies effects in, e.g., cardiac function and hypoxemia in other clinical contexts.
Figure 2.
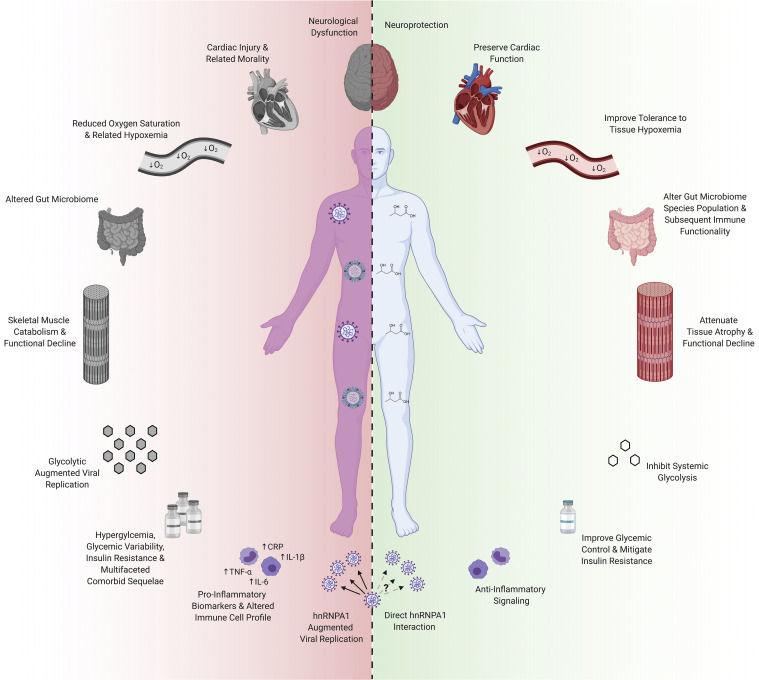
Physiological Mechanisms of Ketone Bodies Relevant to Severe Respiratory Viral Infection and the Syndromes of Critical Illness
Immunometabolic Modulation in ARDS
ARDS develops if the protective, early immune response is insufficient to contain viral infection. The pathophysiology of ARDS includes aberrant immune and inflammatory responses in the lung driven by innate immune activation that results in endothelial injury, interstitial and intra-alveolar edema, filling of alveoli with protein-rich cellular exudates, and interstitial fibrosis.23 Inflammatory markers, including IL-1β, IL-6, and TNF-α, are elevated in blood and alveolar fluid. Inflammation and mitochondrial dysfunction result in cell death and damage of epithelial cells.149 Iatrogenic injury from ventilation, including overpressure and hyperoxia, can cause further inflammatory and mechanical damage.150 Current therapy centers on supportive care that maintains adequate gas exchange while minimizing further injury.22
Modulation of innate immune cells activation by ketone bodies may be relevant to patients who have progressed to ARDS. As described above, BHB inhibits NLRP3 in peripheral macrophages to affect inflammation-mediated disease38 , 40 and inhibits NF-κB-mediated inflammation by binding to HCAR2 on central-nervous-system-resident macrophages.123 , 124 Oxidation of the ketone body acetoacetate by liver-resident macrophage-like Kupffer cells reduces fibrosis in mouse models of high-fat liver injury.79 Whether this modulation of innate immune responses extends to alveolar macrophages and the lung, and whether ketone bodies would thereby slow the inflammation and fibrosis of ARDS, is an area for investigation. Similarly, other effects of ketone bodies, including increasing mitochondrial energy production, reducing oxidative stress, and promoting resistance to ischemia and hypoxia (discussed further below), may be relevant to reducing alveolar injury in ARDS.
BHB Preserves Cardiac Function
Viral infection with influenza increases the risk of both acute and chronic cardiac injury and related mortality151, 152, 153, 154; early reports so far suggest that cardiac injury may be even more important in COVID-19.155 In early reports, cardiac complications and markers of cardiomyocyte injury are among the most important predictors of mortality in COVID-19.156 , 157 Mechanisms for cardiac injury in COVID-19 may include viral or inflammatory myocarditis, arrhythmias, cardiac stress from hypotension and hypoxemia, and acute coronary syndrome associated with the hypercoagulable state.155 Pre-existing heart failure is common in patients hospitalized with COVID-19 and associated with worse outcomes, but these manifestations also occur in patients without underlying cardiac disease. The link between heart failure and respiratory virus mortality is starkly illustrated by the 18% reduction in all-cause and cardiovascular death achieved with yearly influenza vaccination in patients with heart failure.158
Ketones are readily oxidized as a fuel for the heart, particularly under conditions of energetic stress, such as heart failure (reviewed in Puchalska and Crawford46). Data from both humans with heart failure and mouse models identify a metabolic switch to favor ketone body metabolism in cardiomyocytes,159 , 160 which is adaptive: genetic deletion of the enzymes required to metabolize BHB accelerates dysfunction and pathological remodeling.44 , 161 Ex vivo models have shown an ∼24% improvement in cardiac efficiency when ketones are metabolized compared with glucose alone.128 More recently, both in vivo and human clinical studies have shown significant improvements in cardiac function with intravenous ketone body infusion, including dose-dependent increases in ejection fraction and cardiac output.44 , 162 , 163 In preclinical models of acute cardiac ischemia, BHB protects against myocardial injury,164 likely through similar mechanisms as described below for hypoxemic injury. Ketone infusion is not part of any clinical care guideline for heart failure or cardiac ischemia but is under active clinical investigation.46 As mechanisms of cardiac injury in respiratory viral infection are clarified, investigation might be extended into this context as well.
BHB Improves Tolerance to Tissue Hypoxemia
Respiratory viral infections, such as influenza and COVID-19, commonly cause severe hypoxemia, which is also a defining feature of ARDS.22 Lung-protective ventilation strategies require tolerating moderate hypoxemia, and refractory hypoxemia eventually causes multiorgan failure and death. Hypoxia elevates systemic and tissue-specific (i.e., lung) inflammatory and oxidative stress biomarkers.165, 166, 167, 168 Activation of several key common pathways is conserved during hypoxia, infections, and lung injury, including hypoxia-inducible factor-a (HIF-1α) and inflammatory and oxidative pathways, such as NF-κB and NLRP3 (discussed above).169, 170, 171 HIF-1α appears to mediate its effect in these environments via adenosine receptor activation.169 , 170
Elevation of blood ketones has been shown to be broadly protective against hypoxia-related tissue damage, particularly in the brain. In a variety of models of hypoxic and ischemic cerebral injury, treatment with exogenous BHB maintained brain ATP levels, increased neuron survival, decreased cerebral infarct volume, decreased cerebral edema, and improved cognitive performance.172, 173, 174 Additionally, exogenous ketones mitigate inflammation and neuropathology in various model systems via adenosine 1a receptor activation,175, 176, 177 suggesting ketones may attenuate HIF-1α-induced inflammation and oxidative stress in hypoxia. Furthermore, exogenous sources of ketones protect cognitive function during hypoglycemia in mice (A.P.K., unpublished data) and humans,178 hypoxia in mice177 and humans (B.J.S. and A.P.K., unpublished data), and strenuous exercise.179 The effect of ketones on non-neuronal metabolism during hypoxia and in the specific context of respiratory viral infection is an open area for investigation.
BHB Improves Systemic Glycemic Control and Mitigates Insulin Resistance
Type 1 and 2 diabetes are distinct diseases that share the common hallmark of glycemic dysregulation with elevated circulating glucose levels, glycemic variability, elevated HbA1c, and sequelae of overlapping comorbidities. Both forms of diabetes impact several stages of viral respiratory disease.13, 14, 15, 16, 17 First, as discussed above, glucose availability and glycolytic metabolism has been directly implicated in increased viral replication.141 , 180 Second, glycemic dysregulation linked to diabetes increases systemic inflammation and oxidative stress,72,181 both processes that are also heavily implicated in disease progression of influenza77 and SARS-CoV-2.182 Avoiding both hypo- and severe hyperglycemia by insulin therapy is associated with improved outcomes in hospitalized and ICU patients183 and would be the standard of care applied to most patients with severe cases of influenza or COVID-19. A recent retrospective case study of COVID-19 patients with type 2 diabetes supports this, demonstrating that blood glucose between 3.9 and 10 mM (70 and 180 mg/dL) was associated with lower mortality.19 Taken together, this suggests that strategies that target inflammation and oxidative stress, improve insulin sensitivity, or improve glycemic control without adverse effects of hypoglycemia could potentially alter respiratory viral infection risk and/or progression to severe disease.
Acute exogenous ketone administration improves metrics of insulin sensitivity in both healthy and obese patients.41 , 70 , 71 Fasting-adapted ketosis prevents symptoms from even severe insulin-induced hypoglycemia.184 Furthermore, both acute and chronic administration of exogenous ketones lowers glucose levels, mitigates hypoglycemic injury, and improves disease outcomes in multiple animal models of multifactorial inflammatory disease.178 , 185 BHB-mediated lowering of blood glucose might have the further benefit of facilitating optimal ketone body action on inflammatory and oxidative stress pathways, which may be attenuated in the presence of high glucose91 , 114 (discussed above). Together, these data suggest that exogenous ketone bodies might attenuate glycemic dysregulation and its associated adverse outcomes in patients at high mortality risk from viral infection.
BHB Alters Gut Microbiome to Modulate Inflammation
Ketogenic diets alter the human and mouse gut microbiota.186 Separately, multiple groups have also observed a reduction in TH17 cells in human and mice exposed to ketogenic diet.187 , 188 Recent studies suggest a casual pathway wherein the ketone body BHB suppresses the growth of specific immunomodulatory members of the human gut microbiota (Bifidobacterium sp), leading to a decrease in small intestinal lamina propria TH17 cells.189 More work is needed to explore the mechanisms through which BHB impacts gut bacterial growth, host-microbiome interactions within the intestinal mucosa, and the broader consequences of these observations for systemic inflammation.
The impact of this specific pathway in COVID-19 is unknown, but there is evidence that the microbiome influences host inflammation and antiviral immunity in influenza.190 In antibiotic-treated mice, primary challenge with influenza results in higher viral titers within the lung and lower antigen-specific immune responses.190 In germ-free mice, the antibody-specific responses to influenza are diminished, and the innate signaling molecule TLR5 has been invoked as a specific mediator of the adjuvant effects of the gut microbiome.191 The effect of gut microbiota in human response to respiratory viral infections is still emerging. Although broad-spectrum antibiotics provided prior to influenza immunization did not affect initial responses to influenza vaccination, human subjects receiving broad spectrum antibiotics with low initial titer to vaccination had significantly lower immunoglobulin G1 (IgG1) and IgA1 on re-challenge.192 Though conceptual, the role of ketone bodies in augmenting human inflammatory responses and protective immunity to respiratory viruses through modulation of the microbiome could be relevant to individuals with COVID-19.
Ketone Bodies Attenuate Muscle Catabolism and Functional Decline
Muscle function and mass are strong predictors of morbidity and mortality in many clinical settings.193, 194, 195 Surviving critical illness and ARDS often does not mean a return to “normal life”—disability and loss of prior independent functioning are common, especially in older adults.196 More than half of survivors have significant post-recovery weakness197 and new disabilities in instrumental activities of daily living.198 Consequently, early mobilization aimed at preventing weakness and disability is a core element of new critical care practice guidelines.199 , 200 Even non-critical illness in hospitalized older adults commonly results in functional decline and loss of independence.201 , 202 Muscle atrophy, damage, and dysfunction, driven by immobility, inflammation, and catabolism, are major drivers of these phenomena.
Influenza can disrupt patient mobility and directly induce catabolism in a dose-dependent manner via augmented proinflammatory cytokines (TNF-α and IL-6) and myostatin and repressed anabolic signaling (insulin growth factor 1 [IGF-1]), which is exacerbated and/or prolonged with aging.203 , 204 In fact, proinflammatory cytokines (TNF-α, IL-6, and IL-1β),182 , 203, 204, 205, 206 increased markers of muscle catabolism, occurrence of excessive muscle breakdown, myalgia, myositis, and functional decrements have all been observed in patients with COVID-19207, 208, 209, 210 and influenza.211 Patients requiring ventilation also experience ubiquitin-associated diaphragmatic atrophy that predicts morbidity and mortality even 1 year following hospitalization.197 , 212, 213, 214
Exogenous ketone bodies can directly regulate muscle protein metabolism and prevent catabolism111 , 215, 216, 217, 218, 219, 220; a muscle-sparing effect is consistent with the evolutionary role of ketones to prolong survival during starvation. BHB infusion lowers blood urea nitrogen and urinary excretion215 , 219 similar to diet-induced ketosis. 221, 222, 223, 224 Anti-catabolic effects of exogenous ketones occur in multifaceted inflammatory settings and ubiquitin-driven atrophy.111 , 185 , 225 For example, a single dose of exogenous ketones attenuated infection-induced tissue catabolism by 46% while improving time to recovery from inflammation-induced catabolism.185 Furthermore, exogenous ketones mitigated muscle function decrements induced by depletive exercise training.226 A driver of this effect is believed to be a reduction in pro-catabolic inflammation mediated by BHB interaction with HCAR2 and NF-κB as discussed above.125 However, anti-catabolic effects of ketones can occur independently of NF-κB signaling.111 , 185 We demonstrated that ketone bodies can attenuate atrophy in multifactorial catabolic environments where alterations in proinflammatory cytokines, IGF-1/insulin, FOXO3a, and ubiquitin proteasome degradation pathway are observed,185 pathways mechanistically implicated in respiratory viral infection and aging.203 , 204 Altogether, mechanistic evidence suggests that ketones could mitigate muscle catabolism and functional impairment in patients with severe infection, although the specific catabolic pathways in the skeletal muscle of influenza or COVID-19 patients and the functional impact of ketones on ICU recovery have not yet been directly determined.
Immunometabolic Modulation in Delirium
Delirium is an acute confusional state that occurs in the majority of patients with critical illness and is most common in older adults.227 It is independently associated with mortality, prolonged ICU and hospital stays, and post-recovery cognitive decline.228 , 229 Delirium is not inevitable, as half of cases or more are preventable through systematic multicomponent clinical interventions,230 including in the ICU.231 The impact of delirium on both acute resource utilization and long-term outcomes makes it a crucial clinical target in viral pandemics.232 The pathophysiology of delirium is not well understood but features both systemic and central nervous system inflammatory activation as well as metabolic disturbances, leading to neuronal dysfunction.233 Unsurprisingly, delirium appears to be common in early descriptions of COVID-19.234 Whether delirium is specifically affected by SARS-CoV-2 is unknown, but it may be suggestive that inflammatory biomarkers similar to those elevated in COVID-19 are among the most common biomarkers associated with delirium (e.g., IL-1β, IL-6, IL-8, and CRP).235
Several of the ketone body mechanisms described above may be relevant to preventing or resolving delirium in severe respiratory illness. Inhibition of NLRP3 and NF-κB may reduce systemic or brain inflammatory activation. Protection from hypoxic stress responses or hypoglycemic energy deficits may protect neuronal function. Provision of ketone as energetic substrates, particularly in older adults with impaired cognitive function,234 may improve resilience to delirium or restore neuronal function. The utility of exogenous ketones in delirium is unknown, but elucidating their impact on molecular mechanisms and outcomes in preclinical models and clinical studies are important subjects for investigation.
Cause to Pause?
It is important to re-emphasize that the characteristics of ketone metabolism and biological effects in the specific context of viral infection are as yet largely unknown. Most work has been undertaken in model systems that do not always translate directly to humans, whether due to inter-species differences in ketone metabolism or the targeted cellular pathways. Furthermore, based on existing data, some effects of exogenous ketones could be considered either ineffective or detrimental in the context of severe illness. As we have highlighted throughout this review, some effects of BHB on cellular immune processes could be harmful if they interfered with the early immune response rather than dampened the late, pathological immune hyperactivation.
First, although in vitro and animal work consistently reinforce an inhibitory effect of BHB on NLRP3 activity, recent human studies have been equivocal and suggest that exogenous ketones might have no effect or even increase cytokine release under some conditions.111, 112, 113 It is also unclear whether inhibition of NLRP3 (should this occur) is helpful to control the excessive immune response in ARDS or in fact harmful by inhibiting the response that is vital to infection control. Therefore, the timing of exogenous ketone treatment may be an essential consideration. Second, some of the proposed mechanisms above might increase viral replication, for example, if the BHB-hnRNAPA1 interaction stabilizes or promotes the activity of that host RNP in RNA virus replication, or if BHB-mediated inhibition of HDAC1 and 2 increases viral replication. Third, several observed physiological effects of exogenous ketones may complicate severe illness and require careful monitoring. Acute cardiovascular changes, such as increasing resting heart rate162 , 163 and increasing blood flow to the brain and heart via vasodilation,162 , 163 , 236 are perceived as potentially therapeutic but would need to be monitored in severely ill patient groups. Studies have demonstrated a transient, mild metabolic acidosis following ketone ester administration, a mildly alkalizing effect of ketone salts,57 , 237 and a transient fall in plasma potassium with both esters and salts.57 , 162 Exogenous ketosis in healthy individuals decreases endogenous ketone production via inhibition of lipolysis and therefore should reduce the risk of uncontrolled ketoacidosis.72 , 238 However, any proposed exogenous ketone studies should proceed with caution and carefully monitor and control ketone and glucose levels, titrating administration as needed, which may be complicated given the increased incidence of ketoacidosis during infection.239 , 240
Mechanistic work using ketone bodies in relevant models is vital to support any move into clinical investigations, and any clinical testing should be appropriately staged and managed. As the relevant mechanisms of ketone action are defined, it would be important to monitor for mechanism-specific adverse effects; this could be informed by known pharmacological agents that target the same mechanism. Finally, it will be essential to investigate the ideal timing, dose, ketone compound (ester versus salt), and delivered ketone moiety (BHB versus acetoacetate) in order to effectively utilize exogenous ketones as an immunometabolic countermeasure.
Outstanding Research Questions
Despite a robust body of supporting basic science data underpinning these mechanisms of ketone bodies potentially relevant to severe respiratory viral illness, there is a clear need to directly demonstrate the role of these mechanisms in viral infection. A coordinated approach could simultaneously pursue clinical and preclinical studies in order to rapidly appraise the utility of exogenous ketones in viral infection, such as influenza and SARS-CoV-2 (Table 1 ). We provide to the community a detailed outline of proposed investigations at https://www.impactmetabolism.org/, including key mechanistic questions to be resolved in animal and in vitro models, and guidance on designing clinical studies of exogenous ketones targeting respiratory viruses or the complex syndromes of critical illness.
Table 1.
Key Outstanding Research Questions
| Key Questions |
|---|
| Preclinical |
| How do ketone bodies affect replication of influenza A or SARS-CoV-2? |
| What is the fate of ketone bodies in the lung? |
| How does treatment with ketones affect cellular metabolism, redox state, and mitochondrial energetics in lung immune and non-immune cells in health and during viral infection? |
| How do ketone bodies affect innate immunity in the lung following influenza or SARS-CoV-2 challenge? What is the role of NLRP3, HCAR2, and adenosine A1 receptor? |
| What are the mechanisms by which ketone bodies affect cardiomyocyte, skeletal muscle, and/or cognitive dysfunction caused by influenza or SARS-CoV-2? |
| Clinical |
| How might exogenous ketones affect viral replication and progression to disease in ambulatory influenza- or SARS-CoV-2-positive patients? |
| Do exogenous ketones affect systemic and local lung inflammation, innate immune activation, and progression to ARDS following viral infection? |
| In severely ill patients, can exogenous ketones support cardiac, skeletal muscle, and cognitive function through reduced oxidative stress and increased tolerance of hypoxemia? |
| How will exogenous ketones affect glycemia and hemodynamics in critically ill patients? |
| In older adults, can exogenous ketones help preserve muscle function in acute illness and/or affect the incidence or severity of delirium associated with inflammatory activation and metabolic dysfunction? |
Summary
There are multiple cellular and systemic mechanisms whereby ketone bodies might impact severe viral infections, such as influenza or SARS-CoV-2. Evidence across various model systems and/or clinical contexts support potentially relevant molecular mechanisms of ketone bodies, including provision of energetic support, attenuation of inflammation and oxidative stress, apoptosis resistance, maintenance of metabolic homeostasis, and others. These mechanisms have yet to be tested in the specific context of severe respiratory viral infection but could be hypothesized to prevent and mitigate disease as well as support recovery. Further investigation of ketone bodies in respiratory viral illness is warranted, informed by preclinical mechanistic work and with appropriate caution used in clinical studies. In considering the translatability of potential interventions, the natural history of ketone biology in humans provides strong evidence of safety, and the existence of multiple sources of exogenous ketones could facilitate translation. Consequently, exogenous ketone bodies represent a readily deployable tool that could impact multiple mechanisms directly linked to disease outcomes, making these compounds a priority for rapid investigation.
Acknowledgments
This work was supported by NIH K08 AG048354 (J.C.N.), Buck Institute institutional funds, and NIH T32 HL007185 (V.U.).
Author Contributions
Conceptualization, B.J.S., A.P.K., and J.C.N.; Writing – Original Draft, B.J.S., A.P.K., E.L.G., V.U., and J.C.N.; Figures, A.P.K. and E.L.G.; Writing – Review and Editing, all authors.
Declaration of Interests
J.C.N. and E.V. are co-founders of and shareholders in BHB Therapeutics, Ltd., which is developing products relating to ketone bodies. B.J.S. is a shareholder of HVMN, Inc., which markets products relating to ketone bodies and of BHB Therapeutics, Ltd. J.C.N., E.V., B.J.S., and A.P.K. are inventors on patents related to the use of ketone bodies. J.C.N. is on the scientific advisory board of Virta Health, Inc. E.V. is an advisor to Keto Fuel, Inc. P.J.T. is on the scientific advisory boards for Kaleido, Pendulum, Seres, and SNIPRbiome. All other authors declare no competing interests.
References
- 1.Molinari N.A., Ortega-Sanchez I.R., Messonnier M.L., Thompson W.W., Wortley P.M., Weintraub E., Bridges C.B. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine. 2007;25:5086–5096. doi: 10.1016/j.vaccine.2007.03.046. [DOI] [PubMed] [Google Scholar]
- 2.Thompson W.W., Weintraub E., Dhankhar P., Cheng P.Y., Brammer L., Meltzer M.I., Bresee J.S., Shay D.K. Estimates of US influenza-associated deaths made using four different methods. Influenza Other Respir. Viruses. 2009;3:37–49. doi: 10.1111/j.1750-2659.2009.00073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.CDC COVID-19 Response Team Severe outcomes among patients with coronavirus disease 2019 (COVID-19) - United States, February 12-March 16, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020;69:343–346. doi: 10.15585/mmwr.mm6912e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garg S., Kim L., Whitaker M., O’Halloran A., Cummings C., Holstein R., Prill M., Chai S.J., Kirley P.D., Alden N.B., et al. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019 - COVID-NET, 14 States, March 1-30, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020;69:458–464. doi: 10.15585/mmwr.mm6915e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., Wu Y., Zhang L., Yu Z., Fang M., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir. Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Quandelacy T.M., Viboud C., Charu V., Lipsitch M., Goldstein E. Age- and sex-related risk factors for influenza-associated mortality in the United States between 1997-2007. Am. J. Epidemiol. 2014;179:156–167. doi: 10.1093/aje/kwt235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thompson W.W., Shay D.K., Weintraub E., Brammer L., Bridges C.B., Cox N.J., Fukuda K. Influenza-associated hospitalizations in the United States. JAMA. 2004;292:1333–1340. doi: 10.1001/jama.292.11.1333. [DOI] [PubMed] [Google Scholar]
- 8.Iuliano A.D., Roguski K.M., Chang H.H., Muscatello D.J., Palekar R., Tempia S., Cohen C., Gran J.M., Schanzer D., Cowling B.J., et al. Global Seasonal Influenza-associated Mortality Collaborator Network Estimates of global seasonal influenza-associated respiratory mortality: a modelling study. Lancet. 2018;391:1285–1300. doi: 10.1016/S0140-6736(17)33293-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.CDC COVID-19 Response Team Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019 - United States, February 12-March 28, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020;69:382–386. doi: 10.15585/mmwr.mm6913e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwong J.C., Campitelli M.A., Rosella L.C. Obesity and respiratory hospitalizations during influenza seasons in Ontario, Canada: a cohort study. Clin. Infect. Dis. 2011;53:413–421. doi: 10.1093/cid/cir442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zarychanski R., Stuart T.L., Kumar A., Doucette S., Elliott L., Kettner J., Plummer F. Correlates of severe disease in patients with 2009 pandemic influenza (H1N1) virus infection. CMAJ. 2010;182:257–264. doi: 10.1503/cmaj.091884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanslik T., Boelle P.Y., Flahault A. Preliminary estimation of risk factors for admission to intensive care units and for death in patients infected with A(H1N1)2009 influenza virus, France, 2009-2010. PLoS Curr. 2010;2:RRN1150. doi: 10.1371/currents.RRN1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simonsen J.R., Harjutsalo V., Järvinen A., Kirveskari J., Forsblom C., Groop P.H., Lehto M., FinnDiane Study Group Bacterial infections in patients with type 1 diabetes: a 14-year follow-up study. BMJ Open Diabetes Res. Care. 2015;3:e000067. doi: 10.1136/bmjdrc-2014-000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Livingstone S.J., Levin D., Looker H.C., Lindsay R.S., Wild S.H., Joss N., Leese G., Leslie P., McCrimmon R.J., Metcalfe W., et al. Scottish Diabetes Research Network epidemiology group. Scottish Renal Registry Estimated life expectancy in a Scottish cohort with type 1 diabetes, 2008-2010. JAMA. 2015;313:37–44. doi: 10.1001/jama.2014.16425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conway B.N., Lopes-Virella M.F., Blot W.J. Late adulthood mortality among African-American and white American people with type 1 diabetes according to age at diabetes diagnosis. Diabet. Med. 2018;35:729–736. doi: 10.1111/dme.13617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McAlister F.A., Majumdar S.R., Blitz S., Rowe B.H., Romney J., Marrie T.J. The relation between hyperglycemia and outcomes in 2,471 patients admitted to the hospital with community-acquired pneumonia. Diabetes Care. 2005;28:810–815. doi: 10.2337/diacare.28.4.810. [DOI] [PubMed] [Google Scholar]
- 17.Casqueiro J., Casqueiro J., Alves C. Infections in patients with diabetes mellitus: a review of pathogenesis. Indian J. Endocrinol. Metab. 2012;16:S27–S36. doi: 10.4103/2230-8210.94253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marshall R.J., Armart P., Hulme K.D., Chew K.Y., Brown A.C., Hansbro P.M., Bloxham C.J., Flint M., Ronacher K., Bielefeldt-Ohmann H., et al. Glycemic variability in diabetes increases the severity of influenza. MBio. 2020;11:e02841-19. doi: 10.1128/mBio.02841-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu L., She Z.G., Cheng X., Qin J.J., Zhang X.J., Cai J., Lei F., Wang H., Xie J., Wang W., et al. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metab. 2020;31:1068–1077.e3. doi: 10.1016/j.cmet.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S., Huang H., Zhang L., Zhou X., Du C., et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern. Med. 2020 doi: 10.1001/jamainternmed.2020.0994. Published online March 13, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalil A.C., Thomas P.G. Influenza virus-related critical illness: pathophysiology and epidemiology. Crit. Care. 2019;23:258. doi: 10.1186/s13054-019-2539-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fan E., Brodie D., Slutsky A.S. Acute respiratory distress syndrome: advances in diagnosis and treatment. JAMA. 2018;319:698–710. doi: 10.1001/jama.2017.21907. [DOI] [PubMed] [Google Scholar]
- 23.Matthay M.A., Zemans R.L., Zimmerman G.A., Arabi Y.M., Beitler J.R., Mercat A., Herridge M., Randolph A.G., Calfee C.S. Acute respiratory distress syndrome. Nat. Rev. Dis. Primers. 2019;5:18. doi: 10.1038/s41572-019-0069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Azoulay E., Vincent J.L., Angus D.C., Arabi Y.M., Brochard L., Brett S.J., Citerio G., Cook D.J., Curtis J.R., Dos Santos C.C., et al. Recovery after critical illness: putting the puzzle together-a consensus of 29. Crit. Care. 2017;21:296. doi: 10.1186/s13054-017-1887-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sasannejad C., Ely E.W., Lahiri S. Long-term cognitive impairment after acute respiratory distress syndrome: a review of clinical impact and pathophysiological mechanisms. Crit. Care. 2019;23:352. doi: 10.1186/s13054-019-2626-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brummel N.E., Ferrante L.E. Integrating geriatric principles into critical care medicine: the time is now. Ann. Am. Thorac. Soc. 2018;15:518–522. doi: 10.1513/AnnalsATS.201710-793IP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pearce E.J., Pearce E.L. Immunometabolism in 2017: driving immunity: all roads lead to metabolism. Nat. Rev. Immunol. 2018;18:81–82. doi: 10.1038/nri.2017.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pålsson-McDermott E.M., O’Neill L.A.J. Targeting immunometabolism as an anti-inflammatory strategy. Cell Res. 2020;30:300–314. doi: 10.1038/s41422-020-0291-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kedia-Mehta N., Finlay D.K. Competition for nutrients and its role in controlling immune responses. Nat. Commun. 2019;10:2123. doi: 10.1038/s41467-019-10015-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smallwood H.S., Duan S., Morfouace M., Rezinciuc S., Shulkin B.L., Shelat A., Zink E.E., Milasta S., Bajracharya R., Oluwaseum A.J., et al. Targeting metabolic reprogramming by influenza infection for therapeutic intervention. Cell Rep. 2017;19:1640–1653. doi: 10.1016/j.celrep.2017.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Newman J.C., Verdin E. β-hydroxybutyrate: a signaling metabolite. Annu. Rev. Nutr. 2017;37:51–76. doi: 10.1146/annurev-nutr-071816-064916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robinson A.M., Williamson D.H. Physiological roles of ketone bodies as substrates and signals in mammalian tissues. Physiol. Rev. 1980;60:143–187. doi: 10.1152/physrev.1980.60.1.143. [DOI] [PubMed] [Google Scholar]
- 33.Shimazu T., Hirschey M.D., Newman J., He W., Shirakawa K., Le Moan N., Grueter C.A., Lim H., Saunders L.R., Stevens R.D., et al. Suppression of oxidative stress by β-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science. 2013;339:211–214. doi: 10.1126/science.1227166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kong G., Huang Z., Ji W., Wang X., Liu J., Wu X., Huang Z., Li R., Zhu Q. The ketone metabolite β-hydroxybutyrate attenuates oxidative stress in spinal cord injury by suppression of class I histone deacetylases. J. Neurotrauma. 2017;34:2645–2655. doi: 10.1089/neu.2017.5192. [DOI] [PubMed] [Google Scholar]
- 35.Kim D.Y., Davis L.M., Sullivan P.G., Maalouf M., Simeone T.A., van Brederode J., Rho J.M. Ketone bodies are protective against oxidative stress in neocortical neurons. J. Neurochem. 2007;101:1316–1326. doi: 10.1111/j.1471-4159.2007.04483.x. [DOI] [PubMed] [Google Scholar]
- 36.Taggart A.K.P., Kero J., Gan X., Cai T.Q., Cheng K., Ippolito M., Ren N., Kaplan R., Wu K., Wu T.J., et al. (D)-beta-hydroxybutyrate inhibits adipocyte lipolysis via the nicotinic acid receptor PUMA-G. J. Biol. Chem. 2005;280:26649–26652. doi: 10.1074/jbc.C500213200. [DOI] [PubMed] [Google Scholar]
- 37.Liu T., Zhang L., Joo D., Sun S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017;2:17023. doi: 10.1038/sigtrans.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Youm Y.-H., Nguyen K.Y., Grant R.W., Goldberg E.L., Bodogai M., Kim D., D’Agostino D., Planavsky N., Lupfer C., Kanneganti T.D., et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat. Med. 2015;21:263–269. doi: 10.1038/nm.3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamanashi T., Iwata M., Kamiya N., Tsunetomi K., Kajitani N., Wada N., Iitsuka T., Yamauchi T., Miura A., Pu S., et al. Beta-hydroxybutyrate, an endogenic NLRP3 inflammasome inhibitor, attenuates stress-induced behavioral and inflammatory responses. Sci. Rep. 2017;7:7677. doi: 10.1038/s41598-017-08055-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goldberg E.L., Asher J.L., Molony R.D., Shaw A.C., Zeiss C.J., Wang C., Morozova-Roche L.A., Herzog R.I., Iwasaki A., Dixit V.D. β-hydroxybutyrate deactivates neutrophil NLRP3 inflammasome to relieve gout flares. Cell Rep. 2017;18:2077–2087. doi: 10.1016/j.celrep.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Soto-Mota A., Vansant H., Evans R.D., Clarke K. Safety and tolerability of sustained exogenous ketosis using ketone monoester drinks for 28 days in healthy adults. Regul. Toxicol. Pharmacol. 2019;109:104506. doi: 10.1016/j.yrtph.2019.104506. [DOI] [PubMed] [Google Scholar]
- 42.Xie Z., Zhang D., Chung D., Tang Z., Huang H., Dai L., Qi S., Li J., Colak G., Chen Y., et al. Metabolic regulation of gene expression by histone lysine β-hydroxybutyrylation. Mol. Cell. 2016;62:194–206. doi: 10.1016/j.molcel.2016.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kirsch J.R., D’Alecy L.G. Hypoxia induced preferential ketone utilization by rat brain slices. Stroke. 1984;15:319–323. doi: 10.1161/01.str.15.2.319. [DOI] [PubMed] [Google Scholar]
- 44.Horton J.L., Davidson M.T., Kurishima C., Vega R.B., Powers J.C., Matsuura T.R., Petucci C., Lewandowski E.D., Crawford P.A., Muoio D.M., et al. The failing heart utilizes 3-hydroxybutyrate as a metabolic stress defense. JCI Insight. 2019;4:e124079. doi: 10.1172/jci.insight.124079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kossoff E.H., Krauss G.L., McGrogan J.R., Freeman J.M. Efficacy of the Atkins diet as therapy for intractable epilepsy. Neurology. 2003;61:1789–1791. doi: 10.1212/01.wnl.0000098889.35155.72. [DOI] [PubMed] [Google Scholar]
- 46.Puchalska P., Crawford P.A. Multi-dimensional roles of ketone bodies in fuel metabolism, signaling, and therapeutics. Cell Metab. 2017;25:262–284. doi: 10.1016/j.cmet.2016.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krebs H.A. The regulation of the release of ketone bodies by the liver. Adv. Enzyme Regul. 1966;4:339–354. doi: 10.1016/0065-2571(66)90027-6. [DOI] [PubMed] [Google Scholar]
- 48.Krebs H.A., Williamson D.H., Bates M.W., Page M.A., Hawkins R.A. The role of ketone bodies in caloric homeostasis. Adv. Enzyme Regul. 1971;9:387–409. [Google Scholar]
- 49.Hallberg S.J., McKenzie A.L., Williams P.T., Bhanpuri N.H., Peters A.L., Campbell W.W., Hazbun T.L., Volk B.M., McCarter J.P., Phinney S.D., et al. Effectiveness and safety of a novel care model for the management of type 2 diabetes at 1 year: an open-label, non-randomized, controlled study. Diabetes Ther. 2018;9:583–612. doi: 10.1007/s13300-018-0373-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Laffel L. Ketone bodies: a review of physiology, pathophysiology and application of monitoring to diabetes. Diabetes Metab. Res. Rev. 1999;15:412–426. doi: 10.1002/(sici)1520-7560(199911/12)15:6<412::aid-dmrr72>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 51.Gardner C.D., Trepanowski J.F., Del Gobbo L.C., Hauser M.E., Rigdon J., Ioannidis J.P.A., Desai M., King A.C. Effect of low-fat vs low-carbohydrate diet on 12-month weight loss in overweight adults and the association with genotype pattern or insulin secretion: the DIETFITS randomized clinical trial. JAMA. 2018;319:667–679. doi: 10.1001/jama.2018.0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dansinger M.L., Gleason J.A., Griffith J.L., Selker H.P., Schaefer E.J. Comparison of the Atkins, Ornish, Weight Watchers, and Zone diets for weight loss and heart disease risk reduction: a randomized trial. JAMA. 2005;293:43–53. doi: 10.1001/jama.293.1.43. [DOI] [PubMed] [Google Scholar]
- 53.Gullett N.P., Mazurak V.C., Hebbar G., Ziegler T.R. Nutritional interventions for cancer-induced cachexia. Curr. Probl. Cancer. 2011;35:58–90. doi: 10.1016/j.currproblcancer.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Doig G.S., Heighes P.T., Simpson F., Sweetman E.A., Davies A.R. Early enteral nutrition, provided within 24 h of injury or intensive care unit admission, significantly reduces mortality in critically ill patients: a meta-analysis of randomised controlled trials. Intensive Care Med. 2009;35:2018–2027. doi: 10.1007/s00134-009-1664-4. [DOI] [PubMed] [Google Scholar]
- 55.Shpata V., Kreka M., Mjekaj E., Naço M., Gjyzari A., Soxhuku A. Malnutrition affects negatively the outcome of intensive care unit (ICU) patients: 12AP3-6. Eur. J. Anaesthesiol. 2013;30:189. [Google Scholar]
- 56.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stubbs B.J., Cox P.J., Evans R.D., Santer P., Miller J.J., Faull O.K., Magor-Elliott S., Hiyama S., Stirling M., Clarke K. On the metabolism of exogenous ketones in humans. Front. Physiol. 2017;8:848. doi: 10.3389/fphys.2017.00848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fortier M., Castellano C.A., Croteau E., Langlois F., Bocti C., St-Pierre V., Vandenberghe C., Bernier M., Roy M., Descoteaux M., et al. A ketogenic drink improves brain energy and some measures of cognition in mild cognitive impairment. Alzheimers Dement. 2019;15:625–634. doi: 10.1016/j.jalz.2018.12.017. [DOI] [PubMed] [Google Scholar]
- 59.Croteau E., Castellano C.A., Richard M.A., Fortier M., Nugent S., Lepage M., Duchesne S., Whittingstall K., Turcotte É.E., Bocti C., et al. Ketogenic medium chain triglycerides increase brain energy metabolism in Alzheimer’s disease. J. Alzheimers Dis. 2018;64:551–561. doi: 10.3233/JAD-180202. [DOI] [PubMed] [Google Scholar]
- 60.Eckel R.H., Hanson A.S., Chen A.Y., Berman J.N., Yost T.J., Brass E.P. Dietary substitution of medium-chain triglycerides improves insulin-mediated glucose metabolism in NIDDM subjects. Diabetes. 1992;41:641–647. [PubMed] [Google Scholar]
- 61.Clegg M.E. Medium-chain triglycerides are advantageous in promoting weight loss although not beneficial to exercise performance. Int. J. Food Sci. Nutr. 2010;61:653–679. doi: 10.3109/09637481003702114. [DOI] [PubMed] [Google Scholar]
- 62.Ööpik V., Timpmann S., Medijainen L., Lemberg H. Effects of daily medium-chain triglyceride ingestion on energy metabolism and endurance performance capacity in well-trained runners. Nutr. Res. 2001;21:1125–1135. [Google Scholar]
- 63.Jordan S., Tung N., Casanova-Acebes M., Chang C., Cantoni C., Zhang D., Wirtz T.H., Naik S., Rose S.A., Brocker C.N., et al. Dietary intake regulates the circulating inflammatory monocyte pool. Cell. 2019;178:1102–1114.e17. doi: 10.1016/j.cell.2019.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Goldberg E.L., Molony R.D., Kudo E., Sidorov S., Kong Y., Dixit V.D., Iwasaki A. Ketogenic diet activates protective γδ T cell responses against influenza virus infection. Sci. Immunol. 2019;4:eaav2026. doi: 10.1126/sciimmunol.aav2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Heng T.S., Painter M.W., Immunological Genome Project Consortium The Immunological Genome Project: networks of gene expression in immune cells. Nat. Immunol. 2008;9:1091–1094. doi: 10.1038/ni1008-1091. [DOI] [PubMed] [Google Scholar]
- 66.Edwards C., Canfield J., Copes N., Rehan M., Lipps D., Bradshaw P.C. D-beta-hydroxybutyrate extends lifespan in C. elegans. Aging (Albany N.Y.) 2014;6:621–644. doi: 10.18632/aging.100683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Newman J.C., Covarrubias A.J., Zhao M., Yu X., Gut P., Ng C.P., Huang Y., Haldar S., Verdin E. Ketogenic diet reduces midlife mortality and improves memory in aging mice. Cell Metab. 2017;26:547–557.e8. doi: 10.1016/j.cmet.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Roberts M.N., Wallace M.A., Tomilov A.A., Zhou Z., Marcotte G.R., Tran D., Perez G., Gutierrez-Casado E., Koike S., Knotts T.A., et al. A ketogenic diet extends longevity and healthspan in adult mice. Cell Metab. 2017;26:539–546.e5. doi: 10.1016/j.cmet.2017.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Oguntibeju O.O. Type 2 diabetes mellitus, oxidative stress and inflammation: examining the links. Int. J. Physiol. Pathophysiol. Pharmacol. 2019;11:45–63. [PMC free article] [PubMed] [Google Scholar]
- 70.Myette-Côté É., Neudorf H., Rafiei H., Clarke K., Little J.P. Prior ingestion of exogenous ketone monoester attenuates the glycaemic response to an oral glucose tolerance test in healthy young individuals. J. Physiol. 2018;596:1385–1395. doi: 10.1113/JP275709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Myette-Côté É., Caldwell H.G., Ainslie P.N., Clarke K., Little J.P. A ketone monoester drink reduces the glycemic response to an oral glucose challenge in individuals with obesity: a randomized trial. Am. J. Clin. Nutr. 2019;110:1491–1501. doi: 10.1093/ajcn/nqz232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mikkelsen K.H., Seifert T., Secher N.H., Grøndal T., van Hall G. Systemic, cerebral and skeletal muscle ketone body and energy metabolism during acute hyper-D-β-hydroxybutyratemia in post-absorptive healthy males. J. Clin. Endocrinol. Metab. 2015;100:636–643. doi: 10.1210/jc.2014-2608. [DOI] [PubMed] [Google Scholar]
- 73.LaFountain R.A., Miller V.J., Barnhart E.C., Hyde P.N., Crabtree C.D., McSwiney F.T., Beeler M.K., Buga A., Sapper T.N., Short J.A., et al. Extended ketogenic diet and physical training intervention in military personnel. Mil. Med. 2019;184:e538–e547. doi: 10.1093/milmed/usz046. [DOI] [PubMed] [Google Scholar]
- 74.Liu M., Chen F., Liu T., Chen F., Liu S., Yang J. The role of oxidative stress in influenza virus infection. Microbes Infect. 2017;19:580–586. doi: 10.1016/j.micinf.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 75.Matthay M.A., Zemans R.L. The acute respiratory distress syndrome: pathogenesis and treatment. Annu. Rev. Pathol. 2011;6:147–163. doi: 10.1146/annurev-pathol-011110-130158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cai J., Chen Y., Seth S., Furukawa S., Compans R.W., Jones D.P. Inhibition of influenza infection by glutathione. Free Radic. Biol. Med. 2003;34:928–936. doi: 10.1016/s0891-5849(03)00023-6. [DOI] [PubMed] [Google Scholar]
- 77.Sun K., Metzger D.W. Influenza infection suppresses NADPH oxidase-dependent phagocytic bacterial clearance and enhances susceptibility to secondary methicillin-resistant Staphylococcus aureus infection. J. Immunol. 2014;192:3301–3307. doi: 10.4049/jimmunol.1303049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kashiwaya Y., King M.T., Veech R.L. Substrate signaling by insulin: a ketone bodies ratio mimics insulin action in heart. Am. J. Cardiol. 1997;80(3A):50A–64A. doi: 10.1016/s0002-9149(97)00458-x. [DOI] [PubMed] [Google Scholar]
- 79.Puchalska P., Martin S.E., Huang X., Lengfeld J.E., Daniel B., Graham M.J., Han X., Nagy L., Patti G.J., Crawford P.A. Hepatocyte-macrophage acetoacetate shuttle protects against tissue fibrosis. Cell Metab. 2019;29:383–398.e7. doi: 10.1016/j.cmet.2018.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Uhlén M., Fagerberg L., Hallström B.M., Lindskog C., Oksvold P., Mardinoglu A., Sivertsson Å., Kampf C., Sjöstedt E., Asplund A., et al. Proteomics. Tissue-based map of the human proteome. Science. 2015;347:1260419. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 81.Wei T., Tian W., Liu F., Xie G. Protective effects of exogenous β-hydroxybutyrate on paraquat toxicity in rat kidney. Biochem. Biophys. Res. Commun. 2014;447:666–671. doi: 10.1016/j.bbrc.2014.04.074. [DOI] [PubMed] [Google Scholar]
- 82.Izuta Y., Imada T., Hisamura R., Oonishi E., Nakamura S., Inagaki E., Ito M., Soga T., Tsubota K. Ketone body 3-hydroxybutyrate mimics calorie restriction via the Nrf2 activator, fumarate, in the retina. Aging Cell. 2018;17:e12699. doi: 10.1111/acel.12699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Meroni E., Papini N., Criscuoli F., Casiraghi M.C., Massaccesi L., Basilico N., Erba D. Metabolic responses in endothelial cells following exposure to ketone bodies. Nutrients. 2018;10:E250. doi: 10.3390/nu10020250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shimazu T., Hirschey M.D., Newman J., He W., Shirakawa K., Le Moan N., Grueter C.A., Lim H., Saunders L.R., Stevens R.D., et al. Suppression of oxidative stress by β-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science. 2013;339:211–214. doi: 10.1126/science.1227166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu Q., Gao Y., Ci X. Role of Nrf2 and its activators in respiratory diseases. Oxid. Med. Cell. Longev. 2019;2019:7090534. doi: 10.1155/2019/7090534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nagesh P.T., Husain M. Influenza A virus dysregulates host histone deacetylase 1 that inhibits viral infection in lung epithelial cells. J. Virol. 2016;90:4614–4625. doi: 10.1128/JVI.00126-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nagesh P.T., Hussain M., Galvin H.D., Husain M. Histone deacetylase 2 is a component of influenza A virus-induced host antiviral response. Front. Microbiol. 2017;8:1315. doi: 10.3389/fmicb.2017.01315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gordon D.E., Jang G.M., Bouhaddou M., Xu J., Obernier K., White K.M., O’Meara M.J., Rezelj V.V., Guo J.Z., Swaney D.L., et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020 doi: 10.1038/s41586-020-2286-9. Published online April 30, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Haces M.L., Hernández-Fonseca K., Medina-Campos O.N., Montiel T., Pedraza-Chaverri J., Massieu L. Antioxidant capacity contributes to protection of ketone bodies against oxidative damage induced during hypoglycemic conditions. Exp. Neurol. 2008;211:85–96. doi: 10.1016/j.expneurol.2007.12.029. [DOI] [PubMed] [Google Scholar]
- 90.Jain S.K., Rains J.L., Croad J.L. High glucose and ketosis (acetoacetate) increases, and chromium niacinate decreases, IL-6, IL-8, and MCP-1 secretion and oxidative stress in U937 monocytes. Antioxid. Redox Signal. 2007;9:1581–1590. doi: 10.1089/ars.2007.1577. [DOI] [PubMed] [Google Scholar]
- 91.Jain S.K., McVie R., Bocchini J.A., Jr. Hyperketonemia (ketosis), oxidative stress and type 1 diabetes. Pathophysiology. 2006;13:163–170. doi: 10.1016/j.pathophys.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 92.Vandanmagsar B., Youm Y.H., Ravussin A., Galgani J.E., Stadler K., Mynatt R.L., Ravussin E., Stephens J.M., Dixit V.D. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat. Med. 2011;17:179–188. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Youm Y.H., Grant R.W., McCabe L.R., Albarado D.C., Nguyen K.Y., Ravussin A., Pistell P., Newman S., Carter R., Laque A., et al. Canonical Nlrp3 inflammasome links systemic low-grade inflammation to functional decline in aging. Cell Metab. 2013;18:519–532. doi: 10.1016/j.cmet.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y., Xie C., Ma K., Shang K., Wang W., et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa248. Published online March 12, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mo P., Xing Y., Xiao Y., Deng L., Zhao Q., Wang H., Xiong Y., Cheng Z., Gao S., Liang K., et al. Clinical characteristics of refractory COVID-19 pneumonia in Wuhan, China. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa270. Published online March 16, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Han Y., Zhang H., Mu S., Wei W., Jin C., Xue Y., Tong C., Zha Y., Song Z., Gu G. Lactate dehydrogenase, a risk factor of severe COVID-19 patients. medRxiv. 2020 doi: 10.1101/2020.03.24.20040162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Boise L.H., Collins C.M. Salmonella-induced cell death: apoptosis, necrosis or programmed cell death? Trends Microbiol. 2001;9:64–67. doi: 10.1016/s0966-842x(00)01937-5. [DOI] [PubMed] [Google Scholar]
- 99.Tate M.D., Mansell A. An update on the NLRP3 inflammasome and influenza: the road to redemption or perdition? Curr. Opin. Immunol. 2018;54:80–85. doi: 10.1016/j.coi.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 100.Fung T.S., Liu D.X. Human coronavirus: host-pathogen interaction. Annu. Rev. Microbiol. 2019;73:529–557. doi: 10.1146/annurev-micro-020518-115759. [DOI] [PubMed] [Google Scholar]
- 101.Thomas P.G., Dash P., Aldridge J.R., Jr., Ellebedy A.H., Reynolds C., Funk A.J., Martin W.J., Lamkanfi M., Webby R.J., Boyd K.L., et al. The intracellular sensor NLRP3 mediates key innate and healing responses to influenza A virus via the regulation of caspase-1. Immunity. 2009;30:566–575. doi: 10.1016/j.immuni.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Allen I.C., Scull M.A., Moore C.B., Holl E.K., McElvania-TeKippe E., Taxman D.J., Guthrie E.H., Pickles R.J., Ting J.P. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity. 2009;30:556–565. doi: 10.1016/j.immuni.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ahn M., Anderson D.E., Zhang Q., Tan C.W., Lim B.L., Luko K., Wen M., Chia W.N., Mani S., Wang L.C., et al. Dampened NLRP3-mediated inflammation in bats and implications for a special viral reservoir host. Nat. Microbiol. 2019;4:789–799. doi: 10.1038/s41564-019-0371-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nieto-Torres J.L., DeDiego M.L., Verdiá-Báguena C., Jimenez-Guardeño J.M., Regla-Nava J.A., Fernandez-Delgado R., Castaño-Rodriguez C., Alcaraz A., Torres J., Aguilella V.M., Enjuanes L. Severe acute respiratory syndrome coronavirus envelope protein ion channel activity promotes virus fitness and pathogenesis. PLoS Pathog. 2014;10:e1004077. doi: 10.1371/journal.ppat.1004077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ichinohe T., Pang I.K., Iwasaki A. Influenza virus activates inflammasomes via its intracellular M2 ion channel. Nat. Immunol. 2010;11:404–410. doi: 10.1038/ni.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Siu K.L., Yuen K.S., Castaño-Rodriguez C., Ye Z.W., Yeung M.L., Fung S.Y., Yuan S., Chan C.P., Yuen K.Y., Enjuanes L., Jin D.Y. Severe acute respiratory syndrome coronavirus ORF3a protein activates the NLRP3 inflammasome by promoting TRAF3-dependent ubiquitination of ASC. FASEB J. 2019;33:8865–8877. doi: 10.1096/fj.201802418R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chen I.Y., Moriyama M., Chang M.F., Ichinohe T. Severe acute respiratory syndrome coronavirus viroporin 3a activates the NLRP3 inflammasome. Front. Microbiol. 2019;10:50. doi: 10.3389/fmicb.2019.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shi C.S., Nabar N.R., Huang N.N., Kehrl J.H. SARS-Coronavirus Open Reading Frame-8b triggers intracellular stress pathways and activates NLRP3 inflammasomes. Cell Death Discov. 2019;5:101. doi: 10.1038/s41420-019-0181-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Trotta M.C., Maisto R., Guida F., Boccella S., Luongo L., Balta C., D’Amico G., Herman H., Hermenean A., Bucolo C., D’Amico M. The activation of retinal HCA2 receptors by systemic beta-hydroxybutyrate inhibits diabetic retinal damage through reduction of endoplasmic reticulum stress and the NLRP3 inflammasome. PLoS ONE. 2019;14:e0211005. doi: 10.1371/journal.pone.0211005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Qian J., Zhu W., Lu M., Ni B., Yang J. D-β-hydroxybutyrate promotes functional recovery and relieves pain hypersensitivity in mice with spinal cord injury. Br. J. Pharmacol. 2017;174:1961–1971. doi: 10.1111/bph.13788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Thomsen H.H., Rittig N., Johannsen M., Møller A.B., Jørgensen J.O., Jessen N., Møller N. Effects of 3-hydroxybutyrate and free fatty acids on muscle protein kinetics and signaling during LPS-induced inflammation in humans: anticatabolic impact of ketone bodies. Am. J. Clin. Nutr. 2018;108:857–867. doi: 10.1093/ajcn/nqy170. [DOI] [PubMed] [Google Scholar]
- 112.Neudorf H., Durrer C., Myette-Cote E., Makins C., O’Malley T., Little J.P. Oral ketone supplementation acutely increases markers of NLRP3 inflammasome activation in human monocytes. Mol. Nutr. Food Res. 2019;63:e1801171. doi: 10.1002/mnfr.201801171. [DOI] [PubMed] [Google Scholar]
- 113.Neudorf H., Myette-Côté É., P Little J. The impact of acute ingestion of a ketone monoester drink on LPS-stimulated NLRP3 activation in humans with obesity. Nutrients. 2020;12:E854. doi: 10.3390/nu12030854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kim S.R., Lee S.G., Kim S.H., Kim J.H., Choi E., Cho W., Rim J.H., Hwang I., Lee C.J., Lee M., et al. SGLT2 inhibition modulates NLRP3 inflammasome activity via ketones and insulin in diabetes with cardiovascular disease. Nat. Commun. 2020;11:2127. doi: 10.1038/s41467-020-15983-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Liu F., Fu Y., Wei C., Chen Y., Ma S., Xu W. The expression of GPR109A, NF-kB and IL-1β in peripheral blood leukocytes from patients with type 2 diabetes. Ann. Clin. Lab. Sci. 2014;44:443–448. [PubMed] [Google Scholar]
- 116.Maelfait J., Roose K., Bogaert P., Sze M., Saelens X., Pasparakis M., Carpentier I., van Loo G., Beyaert R. A20 (Tnfaip3) deficiency in myeloid cells protects against influenza A virus infection. PLoS Pathog. 2012;8:e1002570. doi: 10.1371/journal.ppat.1002570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dienz O., Rud J.G., Eaton S.M., Lanthier P.A., Burg E., Drew A., Bunn J., Suratt B.T., Haynes L., Rincon M. Essential role of IL-6 in protection against H1N1 influenza virus by promoting neutrophil survival in the lung. Mucosal Immunol. 2012;5:258–266. doi: 10.1038/mi.2012.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wurzer W.J., Ehrhardt C., Pleschka S., Berberich-Siebelt F., Wolff T., Walczak H., Planz O., Ludwig S. NF-kappaB-dependent induction of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) and Fas/FasL is crucial for efficient influenza virus propagation. J. Biol. Chem. 2004;279:30931–30937. doi: 10.1074/jbc.M403258200. [DOI] [PubMed] [Google Scholar]
- 119.Dam S., Kracht M., Pleschka S., Schmitz M.L. The influenza A virus genotype determines the antiviral function of NF-κB. J. Virol. 2016;90:7980–7990. doi: 10.1128/JVI.00946-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yan H., Wang H., Ma L., Ma X., Yin J., Wu S., Huang H., Li Y. Cirsimaritin inhibits influenza A virus replication by downregulating the NF-κB signal transduction pathway. Virol. J. 2018;15:88. doi: 10.1186/s12985-018-0995-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.McCarty M.F., Barroso-Aranda J., Contreras F. Practical strategies for targeting NF-kappaB and NADPH oxidase may improve survival during lethal influenza epidemics. Med. Hypotheses. 2010;74:18–20. doi: 10.1016/j.mehy.2009.04.052. [DOI] [PubMed] [Google Scholar]
- 122.Herold T., Jurinovic V., Arnreich C., Lipworth B.J., Hellmuth J.C., von Bergwelt-Baildon M., Klein M., Weinberger T. Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. J. Allergy Clin. Immunol. 2020 doi: 10.1016/j.jaci.2020.05.008. Published online May 18, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rahman M., Muhammad S., Khan M.A., Chen H., Ridder D.A., Müller-Fielitz H., Pokorná B., Vollbrandt T., Stölting I., Nadrowitz R., et al. The β-hydroxybutyrate receptor HCA2 activates a neuroprotective subset of macrophages. Nat. Commun. 2014;5:3944. doi: 10.1038/ncomms4944. [DOI] [PubMed] [Google Scholar]
- 124.Zandi-Nejad K., Takakura A., Jurewicz M., Chandraker A.K., Offermanns S., Mount D., Abdi R. The role of HCA2 (GPR109A) in regulating macrophage function. FASEB J. 2013;27:4366–4374. doi: 10.1096/fj.12-223933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Poff A., Koutnik A., Deblasi J., Rogers C., Kesl S., Ward N., D'Agostino D. Characterizing the physiologic effects of exogenous ketone supplements – an alternative or adjuvant to the ketogenic diet. FASEB J. 2018;32:812.38. [Google Scholar]
- 126.Zamarin D., García-Sastre A., Xiao X., Wang R., Palese P. Influenza virus PB1-F2 protein induces cell death through mitochondrial ANT3 and VDAC1. PLoS Pathog. 2005;1:e4. doi: 10.1371/journal.ppat.0010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nabirotchkin S., Peluffo A.E., Bouaziz J., Cohen D. Focusing on the unfolded protein response and autophagy related pathways to reposition common approved drugs against COVID-19. Preprints. 2020;2020:2020030302. [Google Scholar]
- 128.Sato K., Kashiwaya Y., Keon C.A., Tsuchiya N., King M.T., Radda G.K., Chance B., Clarke K., Veech R.L. Insulin, ketone bodies, and mitochondrial energy transduction. FASEB J. 1995;9:651–658. doi: 10.1096/fasebj.9.8.7768357. [DOI] [PubMed] [Google Scholar]
- 129.Kim D.Y., Simeone K.A., Simeone T.A., Pandya J.D., Wilke J.C., Ahn Y., Geddes J.W., Sullivan P.G., Rho J.M. Ketone bodies mediate antiseizure effects through mitochondrial permeability transition. Ann. Neurol. 2015;78:77–87. doi: 10.1002/ana.24424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang Y., Zhang X. The nucleocapsid protein of coronavirus mouse hepatitis virus interacts with the cellular heterogeneous nuclear ribonucleoprotein A1 in vitro and in vivo. Virology. 1999;265:96–109. doi: 10.1006/viro.1999.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Luo H., Chen Q., Chen J., Chen K., Shen X., Jiang H. The nucleocapsid protein of SARS coronavirus has a high binding affinity to the human cellular heterogeneous nuclear ribonucleoprotein A1. FEBS Lett. 2005;579:2623–2628. doi: 10.1016/j.febslet.2005.03.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Li Z., Zeng W., Ye S., Lv J., Nie A., Zhang B., Sun Y., Han H., He Q. Cellular hnRNP A1 interacts with nucleocapsid protein of porcine epidemic diarrhea virus and impairs viral replication. Viruses. 2018;10:127. doi: 10.3390/v10030127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chang C.-K., Chen C.J., Wu C.C., Chen S.W., Shih S.R., Kuo R.L. Cellular hnRNP A2/B1 interacts with the NP of influenza A virus and impacts viral replication. PLoS ONE. 2017;12:e0188214. doi: 10.1371/journal.pone.0188214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Shi S.T., Yu G.Y., Lai M.M. Multiple type A/B heterogeneous nuclear ribonucleoproteins (hnRNPs) can replace hnRNP A1 in mouse hepatitis virus RNA synthesis. J. Virol. 2003;77:10584–10593. doi: 10.1128/JVI.77.19.10584-10593.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wang Y., Zhou J., Du Y. hnRNP A2/B1 interacts with influenza A viral protein NS1 and inhibits virus replication potentially through suppressing NS1 RNA/protein levels and NS1 mRNA nuclear export. Virology. 2014;449:53–61. doi: 10.1016/j.virol.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Han Y.M., Bedarida T., Ding Y., Somba B.K., Lu Q., Wang Q., Song P., Zou M.H. β-hydroxybutyrate prevents vascular senescence through hnRNP A1-mediated upregulation of Oct4. Mol. Cell. 2018;71:1064–1078.e5. doi: 10.1016/j.molcel.2018.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Stienstra R., Netea-Maier R.T., Riksen N.P., Joosten L.A.B., Netea M.G. Specific and complex reprogramming of cellular metabolism in myeloid cells during innate immune responses. Cell Metab. 2017;26:142–156. doi: 10.1016/j.cmet.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 138.Pearce E.L., Poffenberger M.C., Chang C.H., Jones R.G. Fueling immunity: insights into metabolism and lymphocyte function. Science. 2013;342:1242454. doi: 10.1126/science.1242454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Thai M., Graham N.A., Braas D., Nehil M., Komisopoulou E., Kurdistani S.K., McCormick F., Graeber T.G., Christofk H.R. Adenovirus E4ORF1-induced MYC activation promotes host cell anabolic glucose metabolism and virus replication. Cell Metab. 2014;19:694–701. doi: 10.1016/j.cmet.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Thaker S.K., Ch’ng J., Christofk H.R. Viral hijacking of cellular metabolism. BMC Biol. 2019;17:59. doi: 10.1186/s12915-019-0678-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kohio H.P., Adamson A.L. Glycolytic control of vacuolar-type ATPase activity: a mechanism to regulate influenza viral infection. Virology. 2013;444:301–309. doi: 10.1016/j.virol.2013.06.026. [DOI] [PubMed] [Google Scholar]
- 142.Gualdoni G.A., Mayer K.A., Kapsch A.M., Kreuzberg K., Puck A., Kienzl P., Oberndorfer F., Frühwirth K., Winkler S., Blaas D., et al. Rhinovirus induces an anabolic reprogramming in host cell metabolism essential for viral replication. Proc. Natl. Acad. Sci. USA. 2018;115:E7158–E7165. doi: 10.1073/pnas.1800525115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Leung H.J., Duran E.M., Kurtoglu M., Andreansky S., Lampidis T.J., Mesri E.A. Activation of the unfolded protein response by 2-deoxy-D-glucose inhibits Kaposi’s sarcoma-associated herpesvirus replication and gene expression. Antimicrob. Agents Chemother. 2012;56:5794–5803. doi: 10.1128/AAC.01126-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Payne L.G., Kristensson K. Effect of glycosylation inhibitors on the release of enveloped vaccinia virus. J. Virol. 1982;41:367–375. doi: 10.1128/jvi.41.2.367-375.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bojkova D., Klann K., Koch B., Widera M., Krause D., Ciesek S., Cinatl J., Münch C. Proteomics of SARS-CoV-2-infected host cells reveals therapy targets. Nature. 2020 doi: 10.1038/s41586-020-2332-7. Published online May 14, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Cox P.J., Kirk T., Ashmore T., Willerton K., Evans R., Smith A., Murray A.J., Stubbs B., West J., McLure S.W., et al. Nutritional ketosis alters fuel preference and thereby endurance performance in athletes. Cell Metab. 2016;24:256–268. doi: 10.1016/j.cmet.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 147.Shukla S.K., Gebregiworgis T., Purohit V., Chaika N.V., Gunda V., Radhakrishnan P., Mehla K., Pipinos I.I., Powers R., Yu F., Singh P.K. Metabolic reprogramming induced by ketone bodies diminishes pancreatic cancer cachexia. Cancer Metab. 2014;2:18. doi: 10.1186/2049-3002-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lund T.M., Ploug K.B., Iversen A., Jensen A.A., Jansen-Olesen I. The metabolic impact of β-hydroxybutyrate on neurotransmission: reduced glycolysis mediates changes in calcium responses and KATP channel receptor sensitivity. J. Neurochem. 2015;132:520–531. doi: 10.1111/jnc.12975. [DOI] [PubMed] [Google Scholar]
- 149.Capelozzi V.L., Allen T.C., Beasley M.B., Cagle P.T., Guinee D., Hariri L.P., Husain A.N., Jain D., Lantuejoul S., Larsen B.T., et al. Molecular and immune biomarkers in acute respiratory distress syndrome: a perspective from members of the pulmonary pathology society. Arch. Pathol. Lab. Med. 2017;141:1719–1727. doi: 10.5858/arpa.2017-0115-SA. [DOI] [PubMed] [Google Scholar]
- 150.Thiel M., Chouker A., Ohta A., Jackson E., Caldwell C., Smith P., Lukashev D., Bittmann I., Sitkovsky M.V. Oxygenation inhibits the physiological tissue-protecting mechanism and thereby exacerbates acute inflammatory lung injury. PLoS Biol. 2005;3:e174. doi: 10.1371/journal.pbio.0030174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ni Z., Guo Z., Chen X., Wang Q., Qiu Y., Wu T., Yang Y., Zhao L. Cardiac injury in patients with pandemic 2009 influenza A (H1N1) infection. Acta Cardiol. 2011;66:427–432. doi: 10.1080/ac.66.4.2126589. [DOI] [PubMed] [Google Scholar]
- 152.Greaves K., Oxford J.S., Price C.P., Clarke G.H., Crake T. The prevalence of myocarditis and skeletal muscle injury during acute viral infection in adults: measurement of cardiac troponins I and T in 152 patients with acute influenza infection. Arch. Intern. Med. 2003;163:165–168. doi: 10.1001/archinte.163.2.165. [DOI] [PubMed] [Google Scholar]
- 153.Gao C., Wang Y., Gu X., Shen X., Zhou D., Zhou S., Huang J.A., Cao B., Guo Q., Community-Acquired Pneumonia–China Network Association between cardiac injury and mortality in hospitalized patients infected with avian influenza A (H7N9) virus. Crit. Care Med. 2020;48:451–458. doi: 10.1097/CCM.0000000000004207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Madjid M., Aboshady I., Awan I., Litovsky S., Casscells S.W. Influenza and cardiovascular disease: is there a causal relationship? Tex. Heart Inst. J. 2004;31:4–13. [PMC free article] [PubMed] [Google Scholar]
- 155.Akhmerov A., Marbán E. COVID-19 and the heart. Circ. Res. 2020;126:1443–1455. doi: 10.1161/CIRCRESAHA.120.317055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Shi S., Qin M., Shen B., Cai Y., Liu T., Yang F., Gong W., Liu X., Liang J., Zhao Q., et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.0950. Published online March 25, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Guo T., Fan Y., Chen M., Wu X., Zhang L., He T., Wang H., Wan J., Wang X., Lu Z. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1017. Published online March 27, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Modin D., Jørgensen M.E., Gislason G., Jensen J.S., Køber L., Claggett B., Hegde S.M., Solomon S.D., Torp-Pedersen C., Biering-Sørensen T. Influenza vaccine in heart failure. Circulation. 2019;139:575–586. doi: 10.1161/CIRCULATIONAHA.118.036788. [DOI] [PubMed] [Google Scholar]
- 159.Aubert G., Martin O.J., Horton J.L., Lai L., Vega R.B., Leone T.C., Koves T., Gardell S.J., Krüger M., Hoppel C.L., et al. The failing heart relies on ketone bodies as a fuel. Circulation. 2016;133:698–705. doi: 10.1161/CIRCULATIONAHA.115.017355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Bedi K.C., Jr., Snyder N.W., Brandimarto J., Aziz M., Mesaros C., Worth A.J., Wang L.L., Javaheri A., Blair I.A., Margulies K.B., Rame J.E. Evidence for intramyocardial disruption of lipid metabolism and increased myocardial ketone utilization in advanced human heart failure. Circulation. 2016;133:706–716. doi: 10.1161/CIRCULATIONAHA.115.017545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Schugar R.C., Moll A.R., André d’Avignon D., Weinheimer C.J., Kovacs A., Crawford P.A. Cardiomyocyte-specific deficiency of ketone body metabolism promotes accelerated pathological remodeling. Mol. Metab. 2014;3:754–769. doi: 10.1016/j.molmet.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Nielsen R., Møller N., Gormsen L.C., Tolbod L.P., Hansson N.H., Sorensen J., Harms H.J., Frøkiær J., Eiskjaer H., Jespersen N.R., et al. Cardiovascular effects of treatment with the ketone body 3-hydroxybutyrate in chronic heart failure patients. Circulation. 2019;139:2129–2141. doi: 10.1161/CIRCULATIONAHA.118.036459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Gormsen L.C., Svart M., Thomsen H.H., Søndergaard E., Vendelbo M.H., Christensen N., Tolbod L.P., Harms H.J., Nielsen R., Wiggers H., et al. Ketone body infusion with 3-hydroxybutyrate reduces myocardial glucose uptake and increases blood flow in humans: a positron emission tomography study. J. Am. Heart Assoc. 2017;6:e005066. doi: 10.1161/JAHA.116.005066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zou Z., Sasaguri S., Rajesh K.G., Suzuki R. dl-3-hydroxybutyrate administration prevents myocardial damage after coronary occlusion in rat hearts. Am. J. Physiol. Heart Circ. Physiol. 2002;283:H1968–H1974. doi: 10.1152/ajpheart.00250.2002. [DOI] [PubMed] [Google Scholar]
- 165.Møller P., Loft S., Lundby C., Olsen N.V. Acute hypoxia and hypoxic exercise induce DNA strand breaks and oxidative DNA damage in humans. FASEB J. 2001;15:1181–1186. doi: 10.1096/fj.00-0703com. [DOI] [PubMed] [Google Scholar]
- 166.Araneda O.F., Tuesta M. Lung oxidative damage by hypoxia. Oxid. Med. Cell. Longev. 2012;2012:856918. doi: 10.1155/2012/856918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Fröhlich S., Boylan J., McLoughlin P. Hypoxia-induced inflammation in the lung: a potential therapeutic target in acute lung injury? Am. J. Respir. Cell Mol. Biol. 2013;48:271–279. doi: 10.1165/rcmb.2012-0137TR. [DOI] [PubMed] [Google Scholar]
- 168.Eltzschig H.K., Carmeliet P. Hypoxia and inflammation. N. Engl. J. Med. 2011;364:656–665. doi: 10.1056/NEJMra0910283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Rabbani Z.N., Mi J., Zhang Y., Delong M., Jackson I.L., Fleckenstein K., Salahuddin F.K., Zhang X., Clary B., Anscher M.S., Vujaskovic Z. Hypoxia inducible factor 1alpha signaling in fractionated radiation-induced lung injury: role of oxidative stress and tissue hypoxia. Radiat. Res. 2010;173:165–174. doi: 10.1667/RR1816.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Gessi S., Merighi S., Stefanelli A., Fazzi D., Varani K., Borea P.A. A(1) and A(3) adenosine receptors inhibit LPS-induced hypoxia-inducible factor-1 accumulation in murine astrocytes. Pharmacol. Res. 2013;76:157–170. doi: 10.1016/j.phrs.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 171.Huang J.J., Xia J., Huang L.L., Li Y.C. HIF-1α promotes NLRP3 inflammasome activation in bleomycin-induced acute lung injury. Mol. Med. Rep. 2019;20:3424–3432. doi: 10.3892/mmr.2019.10575. [DOI] [PubMed] [Google Scholar]
- 172.Wood T.R., Stubbs B.J., Juul S.E. Exogenous ketone bodies as promising neuroprotective agents for developmental brain injury. Dev. Neurosci. 2018;40:451–462. doi: 10.1159/000499563. [DOI] [PubMed] [Google Scholar]
- 173.Prins M.L. Cerebral metabolic adaptation and ketone metabolism after brain injury. J. Cereb. Blood Flow Metab. 2008;28:1–16. doi: 10.1038/sj.jcbfm.9600543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Zhao M., Huang X., Cheng X., Lin X., Zhao T., Wu L., Yu X., Wu K., Fan M., Zhu L. Ketogenic diet improves the spatial memory impairment caused by exposure to hypobaric hypoxia through increased acetylation of histones in rats. PLoS ONE. 2017;12:e0174477. doi: 10.1371/journal.pone.0174477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kovács Z., D’Agostino D.P., Dobolyi A., Ari C. Adenosine A1 receptor antagonism abolished the anti-seizure effects of exogenous ketone supplementation in Wistar Albino Glaxo Rijswijk rats. Front. Mol. Neurosci. 2017;10:235. doi: 10.3389/fnmol.2017.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kovács Z., D’Agostino D.P., Ari C. Anxiolytic effect of exogenous ketone supplementation is abolished by adenosine A1 receptor inhibition in Wistar Albino Glaxo/Rijswijk rats. Front. Behav. Neurosci. 2018;12:29. doi: 10.3389/fnbeh.2018.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kovács Z., D’Agostino D.P., Diamond D.M., Ari C. Exogenous ketone supplementation decreased the lipopolysaccharide-induced increase in absence epileptic activity in Wistar Albino Glaxo Rijswijk rats. Front. Mol. Neurosci. 2019;12:45. doi: 10.3389/fnmol.2019.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Page K.A., Williamson A., Yu N., McNay E.C., Dzuira J., McCrimmon R.J., Sherwin R.S. Medium-chain fatty acids improve cognitive function in intensively treated type 1 diabetic patients and support in vitro synaptic transmission during acute hypoglycemia. Diabetes. 2009;58:1237–1244. doi: 10.2337/db08-1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Evans M., Egan B. Intermittent running and cognitive performance after ketone ester ingestion. Med. Sci. Sports Exerc. 2018;50:2330–2338. doi: 10.1249/MSS.0000000000001700. [DOI] [PubMed] [Google Scholar]
- 180.Reading P.C., Allison J., Crouch E.C., Anders E.M. Increased susceptibility of diabetic mice to influenza virus infection: compromise of collectin-mediated host defense of the lung by glucose? J. Virol. 1998;72:6884–6887. doi: 10.1128/jvi.72.8.6884-6887.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.de Rekeneire N., Peila R., Ding J., Colbert L.H., Visser M., Shorr R.I., Kritchevsky S.B., Kuller L.H., Strotmeyer E.S., Schwartz A.V., et al. Diabetes, hyperglycemia, and inflammation in older individuals: the health, aging and body composition study. Diabetes Care. 2006;29:1902–1908. doi: 10.2337/dc05-2327. [DOI] [PubMed] [Google Scholar]
- 182.Gong J., Dong H., Xia S.Q., Huang Y.Z., Wang D., Zhao Y., Liu W., Tu S., Zhang M., Wang Q., Lu F. Correlation analysis between disease severity and inflammation-related parameters in patients with COVID-19 pneumonia. medRxiv. 2020 doi: 10.1101/2020.02.25.20025643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Qaseem A., Humphrey L.L., Chou R., Snow V., Shekelle P., Clinical Guidelines Committee of the American College of Physicians Use of intensive insulin therapy for the management of glycemic control in hospitalized patients: a clinical practice guideline from the American College of Physicians. Ann. Intern. Med. 2011;154:260–267. doi: 10.7326/0003-4819-154-4-201102150-00007. [DOI] [PubMed] [Google Scholar]
- 184.Drenick E.J., Alvarez L.C., Tamasi G.C., Brickman A.S. Resistance to symptomatic insulin reactions after fasting. J. Clin. Invest. 1972;51:2757–2762. doi: 10.1172/JCI107095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Koutnik A.P., Poff A.M., Ward N.P., DeBlasi J.M., Soliven M.A., Romero M.A., Roberson P.A., Fox C.D., Roberts M.D., D'Agostino D.P. Ketone bodies attenuate wasting in models of atrophy. J. Cachexia Sarcopenia Muscle. 2020 doi: 10.1002/jcsm.12554. Published April 2, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Olson C.A., Vuong H.E., Yano J.M., Liang Q.Y., Nusbaum D.J., Hsiao E.Y. The gut microbiota mediates the anti-seizure effects of the ketogenic diet. Cell. 2018;174:497. doi: 10.1016/j.cell.2018.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ni F.-F., Li C.R., Liao J.X., Wang G.B., Lin S.F., Xia Y., Wen J.L. The effects of ketogenic diet on the Th17/Treg cells imbalance in patients with intractable childhood epilepsy. Seizure. 2016;38:17–22. doi: 10.1016/j.seizure.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 188.Kim D.Y., Hao J., Liu R., Turner G., Shi F.D., Rho J.M. Inflammation-mediated memory dysfunction and effects of a ketogenic diet in a murine model of multiple sclerosis. PLoS ONE. 2012;7:e35476. doi: 10.1371/journal.pone.0035476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Ang Q.Y., Alexander M., Newman J.C., Tian Y., Cai J., Upadhyay V., Turnbaugh J.A., Verdin E., Hall K.D., Leibel R.L., et al. Ketogenic diets alter the gut microbiome resulting in decreased intestinal Th17 cells. Cell. 2020;181:1263–1275.e16. doi: 10.1016/j.cell.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Ichinohe T., Pang I.K., Kumamoto Y., Peaper D.R., Ho J.H., Murray T.S., Iwasaki A. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc. Natl. Acad. Sci. USA. 2011;108:5354–5359. doi: 10.1073/pnas.1019378108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Oh J.Z., Ravindran R., Chassaing B., Carvalho F.A., Maddur M.S., Bower M., Hakimpour P., Gill K.P., Nakaya H.I., Yarovinsky F., et al. TLR5-mediated sensing of gut microbiota is necessary for antibody responses to seasonal influenza vaccination. Immunity. 2014;41:478–492. doi: 10.1016/j.immuni.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Hagan T., Cortese M., Rouphael N., Boudreau C., Linde C., Maddur M.S., Das J., Wang H., Guthmiller J., Zheng N.Y., et al. Antibiotics-driven gut microbiome perturbation alters immunity to vaccines in humans. Cell. 2019;178:1313–1328.e13. doi: 10.1016/j.cell.2019.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Prado C.M., Purcell S.A., Alish C., Pereira S.L., Deutz N.E., Heyland D.K., Goodpaster B.H., Tappenden K.A., Heymsfield S.B. Implications of low muscle mass across the continuum of care: a narrative review. Ann. Med. 2018;50:675–693. doi: 10.1080/07853890.2018.1511918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Laukkanen P., Heikkinen E., Kauppinen M. Muscle strength and mobility as predictors of survival in 75-84-year-old people. Age Ageing. 1995;24:468–473. doi: 10.1093/ageing/24.6.468. [DOI] [PubMed] [Google Scholar]
- 195.Srikanthan P., Karlamangla A.S. Muscle mass index as a predictor of longevity in older adults. Am. J. Med. 2014;127:547–553. doi: 10.1016/j.amjmed.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Desai S.V., Law T.J., Needham D.M. Long-term complications of critical care. Crit. Care Med. 2011;39:371–379. doi: 10.1097/CCM.0b013e3181fd66e5. [DOI] [PubMed] [Google Scholar]
- 197.Dinglas V.D., Aronson Friedman L., Colantuoni E., Mendez-Tellez P.A., Shanholtz C.B., Ciesla N.D., Pronovost P.J., Needham D.M. Muscle weakness and 5-year survival in acute respiratory distress syndrome survivors. Crit. Care Med. 2017;45:446–453. doi: 10.1097/CCM.0000000000002208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Hopkins R.O., Suchyta M.R., Kamdar B.B., Darowski E., Jackson J.C., Needham D.M. Instrumental activities of daily living after critical illness: a systematic review. Ann. Am. Thorac. Soc. 2017;14:1332–1343. doi: 10.1513/AnnalsATS.201701-059SR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Fan E., Zakhary B., Amaral A., McCannon J., Girard T.D., Morris P.E., Truwit J.D., Wilson K.C., Thomson C.C. Liberation from mechanical ventilation in critically ill adults. An official ATS/ACCP clinical practice guideline. Ann. Am. Thorac. Soc. 2017;14:441–443. doi: 10.1513/AnnalsATS.201612-993CME. [DOI] [PubMed] [Google Scholar]
- 200.Ely E.W. The ABCDEF bundle: science and philosophy of how ICU liberation serves patients and families. Crit. Care Med. 2017;45:321–330. doi: 10.1097/CCM.0000000000002175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Covinsky K.E., Pierluissi E., Johnston C.B. Hospitalization-associated disability: “She was probably able to ambulate, but I’m not sure”. JAMA. 2011;306:1782–1793. doi: 10.1001/jama.2011.1556. [DOI] [PubMed] [Google Scholar]
- 202.Gill T.M., Allore H.G., Gahbauer E.A., Murphy T.E. Change in disability after hospitalization or restricted activity in older persons. JAMA. 2010;304:1919–1928. doi: 10.1001/jama.2010.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Bartley J.M., Pan S.J., Keilich S.R., Hopkins J.W., Al-Naggar I.M., Kuchel G.A., Haynes L. Aging augments the impact of influenza respiratory tract infection on mobility impairments, muscle-localized inflammation, and muscle atrophy. Aging (Albany N.Y.) 2016;8:620–635. doi: 10.18632/aging.100882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Radigan K.A., Nicholson T.T., Welch L.C., Chi M., Amarelle L., Angulo M., Shigemura M., Shigemura A., Runyan C.E., Morales-Nebreda L., et al. Influenza A virus infection induces muscle wasting via IL-6 regulation of the E3 ubiquitin ligase atrogin-1. J. Immunol. 2019;202:484–493. doi: 10.4049/jimmunol.1701433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J., HLH Across Speciality Collaboration, UK COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Conti P., Ronconi G., Caraffa A., Gallenga C.E., Ross R., Frydas I., Kritas S.K. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J. Biol. Regul. Homeost. Agents. 2020;34:1. doi: 10.23812/CONTI-E. [DOI] [PubMed] [Google Scholar]
- 207.Mao L., Jin H., Wang M., Hu Y., Chen S., He Q., Chang J., Hong C., Zhou Y., Wang D., et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:1–9. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Jin M., Tong Q. Rhabdomyolysis as potential late complication associated with COVID-19. Emerg. Infect. Dis. 2020;26:1618–1620. doi: 10.3201/eid2607.200445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Cheng Y., Luo R., Wang K., Zhang M., Wang Z., Dong L., Li J., Yao Y., Ge S., Xu G. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97:829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Li Z., Wu M., Yao J., Guo J., Liao X., Song S., Li J., Duan G., Zhou Y., Wu X., et al. Anti-2019-nCoV Volunteers Caution on kidney dysfunctions of COVID-19 patients. medRxiv. 2020 doi: 10.1101/2020.02.08.20021212. [DOI] [Google Scholar]
- 211.Ayala E., Kagawa F.T., Wehner J.H., Tam J., Upadhyay D. Rhabdomyolysis associated with 2009 influenza A(H1N1) JAMA. 2009;302:1863–1864. doi: 10.1001/jama.2009.1582. [DOI] [PubMed] [Google Scholar]
- 212.Medrinal C., Prieur G., Frenoy É., Robledo Quesada A., Poncet A., Bonnevie T., Gravier F.E., Lamia B., Contal O. Respiratory weakness after mechanical ventilation is associated with one-year mortality - a prospective study. Crit. Care. 2016;20:231. doi: 10.1186/s13054-016-1418-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Goligher E.C., Dres M., Fan E., Rubenfeld G.D., Scales D.C., Herridge M.S., Vorona S., Sklar M.C., Rittayamai N., Lanys A., et al. Mechanical ventilation-induced diaphragm atrophy strongly impacts clinical outcomes. Am. J. Respir. Crit. Care Med. 2018;197:204–213. doi: 10.1164/rccm.201703-0536OC. [DOI] [PubMed] [Google Scholar]
- 214.Tobin M.J., Laghi F., Jubran A. Narrative review: ventilator-induced respiratory muscle weakness. Ann. Intern. Med. 2010;153:240–245. doi: 10.1059/0003-4819-153-4-201008170-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Sherwin R.S., Hendler R.G., Felig P. Effect of ketone infusions on amino acid and nitrogen metabolism in man. J. Clin. Invest. 1975;55:1382–1390. doi: 10.1172/JCI108057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Miles J.M., Haymond M.W., Gerich J.E. Suppression of glucose production and stimulation of insulin secretion by physiological concentrations of ketone bodies in man. J. Clin. Endocrinol. Metab. 1981;52:34–37. doi: 10.1210/jcem-52-1-34. [DOI] [PubMed] [Google Scholar]
- 217.Beylot M., Khalfallah Y., Riou J.P., Cohen R., Normand S., Mornex R. Effects of ketone bodies on basal and insulin-stimulated glucose utilization in man. J. Clin. Endocrinol. Metab. 1986;63:9–15. doi: 10.1210/jcem-63-1-9. [DOI] [PubMed] [Google Scholar]
- 218.Nair K.S., Welle S.L., Halliday D., Campbell R.G. Effect of beta-hydroxybutyrate on whole-body leucine kinetics and fractional mixed skeletal muscle protein synthesis in humans. J. Clin. Invest. 1988;82:198–205. doi: 10.1172/JCI113570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Maiz A., Moldawer L.L., Bistrian B.R., Birkhahn R.H., Long C.L., Blackburn G.L. Monoacetoacetin and protein metabolism during parenteral nutrition in burned rats. Biochem. J. 1985;226:43–50. doi: 10.1042/bj2260043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Fearon K.C., Borland W., Preston T., Tisdale M.J., Shenkin A., Calman K.C. Cancer cachexia: influence of systemic ketosis on substrate levels and nitrogen metabolism. Am. J. Clin. Nutr. 1988;47:42–48. doi: 10.1093/ajcn/47.1.42. [DOI] [PubMed] [Google Scholar]
- 221.Cahill G.F., Jr. Fuel metabolism in starvation. Annu. Rev. Nutr. 2006;26:1–22. doi: 10.1146/annurev.nutr.26.061505.111258. [DOI] [PubMed] [Google Scholar]
- 222.Felig P., Owen O.E., Wahren J., Cahill G.F., Jr. Amino acid metabolism during prolonged starvation. J. Clin. Invest. 1969;48:584–594. doi: 10.1172/JCI106017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Owen O.E., Felig P., Morgan A.P., Wahren J., Cahill G.F., Jr. Liver and kidney metabolism during prolonged starvation. J. Clin. Invest. 1969;48:574–583. doi: 10.1172/JCI106016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Phinney S.D., Bistrian B.R., Evans W.J., Gervino E., Blackburn G.L. The human metabolic response to chronic ketosis without caloric restriction: preservation of submaximal exercise capability with reduced carbohydrate oxidation. Metabolism. 1983;32:769–776. doi: 10.1016/0026-0495(83)90106-3. [DOI] [PubMed] [Google Scholar]
- 225.Koutnik A.P., D’Agostino D.P., Egan B. Anticatabolic effects of ketone bodies in skeletal muscle. Trends Endocrinol. Metab. 2019;30:227–229. doi: 10.1016/j.tem.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 226.Poffé C., Ramaekers M., Van Thienen R., Hespel P. Ketone ester supplementation blunts overreaching symptoms during endurance training overload. J. Physiol. 2019;597:3009–3027. doi: 10.1113/JP277831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Inouye S.K., Westendorp R.G., Saczynski J.S. Delirium in elderly people. Lancet. 2014;383:911–922. doi: 10.1016/S0140-6736(13)60688-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Ely E.W., Shintani A., Truman B., Speroff T., Gordon S.M., Harrell F.E., Jr., Inouye S.K., Bernard G.R., Dittus R.S. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291:1753–1762. doi: 10.1001/jama.291.14.1753. [DOI] [PubMed] [Google Scholar]
- 229.Pandharipande P.P., Girard T.D., Jackson J.C., Morandi A., Thompson J.L., Pun B.T., Brummel N.E., Hughes C.G., Vasilevskis E.E., Shintani A.K., et al. BRAIN-ICU Study Investigators Long-term cognitive impairment after critical illness. N. Engl. J. Med. 2013;369:1306–1316. doi: 10.1056/NEJMoa1301372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Hshieh T.T., Yue J., Oh E., Puelle M., Dowal S., Travison T., Inouye S.K. Effectiveness of multicomponent nonpharmacological delirium interventions: a meta-analysis. JAMA Intern. Med. 2015;175:512–520. doi: 10.1001/jamainternmed.2014.7779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Pun B.T., Balas M.C., Barnes-Daly M.A., Thompson J.L., Aldrich J.M., Barr J., Byrum D., Carson S.S., Devlin J.W., Engel H.J., et al. Caring for critically ill patients with the ABCDEF bundle: results of the ICU Liberation Collaborative in over 15,000 adults. Crit. Care Med. 2019;47:3–14. doi: 10.1097/CCM.0000000000003482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.LaHue S.C., James T.C., Newman J.C., Esmaili A.M., Ormseth C.H., Ely E.W. Collaborative delirium prevention in the age of COVID-19. J. Am. Geriatr. Soc. 2020;68:947–949. doi: 10.1111/jgs.16480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Maldonado J.R. Delirium pathophysiology: an updated hypothesis of the etiology of acute brain failure. Int. J. Geriatr. Psychiatry. 2018;33:1428–1457. doi: 10.1002/gps.4823. [DOI] [PubMed] [Google Scholar]
- 234.Helms J., Kremer S., Merdji H., Clere-Jehl R., Schenck M., Kummerlen C., Collange O., Boulay C., Fafi-Kremer S., Ohana M., et al. Neurologic features in severe SARS-CoV-2 infection. N. Engl. J. Med. 2020;382:2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Toft K., Tontsch J., Abdelhamid S., Steiner L., Siegemund M., Hollinger A. Serum biomarkers of delirium in the elderly: a narrative review. Ann. Intensive Care. 2019;9:76. doi: 10.1186/s13613-019-0548-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Svart M., Gormsen L.C., Hansen J., Zeidler D., Gejl M., Vang K., Aanerud J., Moeller N. Regional cerebral effects of ketone body infusion with 3-hydroxybutyrate in humans: Reduced glucose uptake, unchanged oxygen consumption and increased blood flow by positron emission tomography. A randomized, controlled trial. PLoS ONE. 2018;13:e0190556. doi: 10.1371/journal.pone.0190556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Dearlove D.J., Faull O.K., Rolls E., Clarke K., Cox P.J. Nutritional ketoacidosis during incremental exercise in healthy athletes. Front. Physiol. 2019;10:290. doi: 10.3389/fphys.2019.00290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Shivva V., Cox P.J., Clarke K., Veech R.L., Tucker I.G., Duffull S.B. The population pharmacokinetics of D-β-hydroxybutyrate following administration of (R)-3-hydroxybutyl (R)-3-hydroxybutyrate. AAPS J. 2016;18:678–688. doi: 10.1208/s12248-016-9879-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Li J., Wang X., Chen J., Zuo X., Zhang H., Deng A. COVID-19 infection may cause ketosis and ketoacidosis. Diabetes Obes. Metab. 2020 doi: 10.1111/dom.14057. Published online April 20, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Seth P., Kaur H., Kaur M. Clinical profile of diabetic ketoacidosis: a prospective study in a tertiary care hospital. J. Clin. Diagn. Res. 2015;9:OC01–OC04. doi: 10.7860/JCDR/2015/8586.5995. [DOI] [PMC free article] [PubMed] [Google Scholar]


