1. Introduction
During the outbreak of coronavirus disease 2019 (COVID-19) clinicians are increasingly involved in the observation of possible neurological complications due to the infection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1]. Among neurologic emergencies, new onset seizures and status epilepticus in non-epileptic patients are up to now, infrequently reported [2].
We hereby report a case of New Onset Refractory Status Epilepticus (NORSE) [3] in a patient with anti-NMDA receptor encephalitis and concomitant SARS-CoV-2 infection.
2. Case report
A 50-year-old man, with no significant pathology in his medical history except for mild hypertension, employed as a health care worker in the ambulance emergency service, presented on February 23rd an acute onset of psychiatric symptoms (confabulations and delirious ideas). He had fever (T 38 °C) without increased CRP or leucocytosis, respiratory symptoms (in particular no cough, cold or dyspnea) or diarrhea. After 4 days, focal motor seizures with impaired awareness and oro-facial dyskinesia/automatisms appeared. A first brain MRI was negative. He was treated with diazepam followed by adequate doses of valproic acid and lacosamide. Nevertheless, he suddenly developed a refractory status epilepticus (RSE) requiring admission to the intensive care unit (ICU) and anesthetics’ treatment (Fig. 1 ).
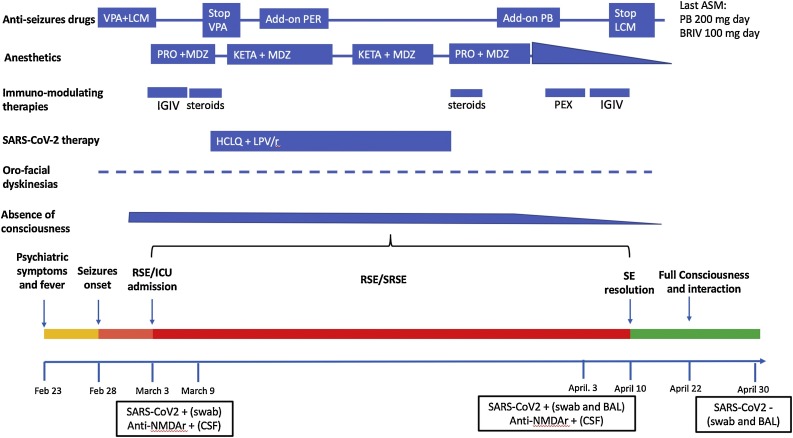
Fig. 1.
Temporal evolution of the condition in relation to diagnostic findings and treatments.
Pictorial evolution of the condition from February 23rd to April 30th 2020.
anti-NMDAr = antibodies against NMDA glutamate receptors; ASM = anti-seizures medications; BRV = brivaracetam; HCLQ = hydroxychloroquine; IGIV = intravenous immunoglobulins; LCM = lacosamide; LPV/r = lopinavir/ritonavir; MDZ = midazolam; KETA = Ketamine; PB = phenobarbital; PER = perampanel; PEX = plasma exchange; PRO = propofol; RSE = refractory status epilepticus; SRSE = super-RSE; VPA = valproate.
In the first week the patient underwent two spinal-taps with a CSF finding of 76 and 25 cells respectively plus oligoclonal bands. Search for the main neurotropic fungi, bacteria and viruses (and in particular for herpes viruses) was negative. On the other hand, anti-NMDA receptors antibodies positivity on CSF was found, while it was negative in serum (Table 1 ). A second brain MRI was unremarkable. Due to the rapid spread of COVID-19 cases in our district and in relation to the high occupational risk of the patient, on March the 3rd a throat swab for SARS-CoV-2 was acquired. Real-time reverse-transcription polymerase chain reaction analysis (PCR) confirmed SARS-CoV-2 infection. No sign on interstitial pneumonia were present on chest radiography and CT scan. The patient was then transferred to an ICU dedicated to COVID-19 patients.
Table 1.
Findings on cerebrospinal fluid and serum during the course of the disease.
| Cerebro Spinal Fluid | |||
|---|---|---|---|
| Investigations | |||
| Feb-25 | March-02 | April-03 | |
| Total cells count (n.v. < 4 cells /uL) | 76 | 25 | 16 |
| Proteins (n.v. 20−50 mg/dl) | slightly elevated | 48 | 105 |
| Glucose (n.v.40−80 mg/dl) | normal | 68 | 87 |
| Neurotropic viruses, bacteria and yeast PCR* | neg | neg | neg |
| SARS-Cov-2 PCR | – | – | neg |
| IgG Link index (n.v. < 0.70) | – | 0.67 | 1.45 |
| Isoelectrofocusing IgG | – | Mirror pattern + oligo-clonal bands | none |
| Autoimmune encephalitis and onconeurals Ab# | – | NMDA-R Ab + | NMDA-R Ab + |
| IL6 (pg/ml) ° | – | 4.58 | 5.75 |
| IL8 (pg/ml) ° | – | 40.1 | 744 |
| IL-1ß (pg/ml) ° | – | 0.314 | 0.287 |
| TNF-α (pg/ml) ° | – | 2.17 | 3.24 |
| Serum | |||
| Feb-25 | March-02 | April-03 | |
| Autoimmune encephalitis and onconeurals Ab# | – | neg | neg |
| IL6 (n.v. 0–10 pg/ml) | – | 52 | 206 |
CMV, EBV, HSV1, HSV2, HSV6, HSV7, HSV8, VZV, Adenovirus, Parvovirus, Influenza virus A and B, VRS, Enterovirus, Parechovirus,West Nile virus, Tuscany virus, K pneumoniae, E. coli, B. burgdoferi, T. pallidum, T gondii, L monocitogenes, S. agalactiae, S. pneumoniae, N meningitidis, H influenzae, Criptococcus neoformans and gattii.
NMDA-R, GABA-B R, AMPA-R 1–2, LGI1, CASPR2, DPPX, Amphiphysin, CV2, PNMA2, Ri, Yo, Hu, Recoverin, SOX1, Titin, Zic4, GAD65, Tr Ab.
IL-8, IL-6, Il-1ß and TNF-α were measured by commercially available multiplex bead immunoassays: ELLA Simple Plex ELISA, immunoassays, multianalyte platform (Bio-Techne - Abingdon UK). Bold indicates increased values respect with internal controls data. Cytokines control data were obtained from CSF samples from 12 subjects with functional neurologic disorders.
Therapeutic management was based on immunomodulating therapies typically used for anti-NMDA receptors antibodies encephalitis (metilprednisolone, IGIV, plasma-exchange) together with antiseizure drugs/anesthetics to control refractory/super refractory status (SRSE), as well as on empirical therapies used for SAR-CoV-2 infection (Fig. 1). The results of a third spinal tap confirmed positivity for anti-NMDA receptors antibodies (even at a lower title). Measurement of cytokines panel (IL-1 ß, Il6, IL8 and TNF-α) showed raised levels of IL6 and IL8 on CSF; IL6 was raised in serum too.
Forty-seven days from the beginning of SE and after having completed the first cycle of plasma-exchange and a second cycle of IGIV, seizures definitely ceased thus anesthetic therapy was stopped. The patient slowly regained consciousness, spontaneous breathing, active interaction with the examiner. At this stage PCR for SARs-CoV-2 on throat swab and on bronchial-alveolar lavage were negative. A progressive improvement of the EEG organization with the reappearance of a reactive alpha rhythm and reorganized sleep was observed (EEG evolution on e-figure). Finally, the patient underwent a total-body CT and PET in the search of tumours that were both negative. Four months after the onset the patient was discharged at home in good condition, autonomous, and without neurological deficits.
3. Discussion
This is, to our knowledge, the first NORSE reported in a patient with a concomitant SARS-CoV-2 infection. Regarding the association between SARS-CoV-2 infection and the development of autoimmune encephalitis, the case described does not allow to attribute a definite pathogenetic role to COVID-19. Indeed, the patient could have been an asymptomatic carrier of SARS-Cov-2 and had an unrelated anti NMDA receptor encephalitis. However, very recently another case report of anti-NMDA receptor encephalitis in COVID-19 with prominent psychotic symptoms has been published [4]. In our patients, the clinical onset characterized by fever, psychiatric disorders, and seizures, immediately oriented clinicians to investigate an autoimmune encephalitis, once a viral infection was excluded on the basis of CSF PCR negativity for the main neurotropic viruses. The patient had no interstitial pulmonary involvement, both at the beginning of the clinical history and during the evolution. Indeed, the search for SARS-CoV-2 infection was done mainly on the basis of the rapid increase in COVID-19 outbreak (the first COVID-19 patient was recorded on February 25 in Modena province), and on the high occupational risk of the patient. Anti-NMDA receptor encephalitis after infection with herpes simplex virus (HSV1) is a well know condition. A prospective study show that autoimmune encephalitis occurred in 27 % of patients with herpes simplex encephalitis [5]. In these cases, however, the development of autoimmune encephalitis is observed a few days, or more often weeks, after the end of the viral infection, and not concomitantly with it, as in our case. However, it is also not possible to exclude that SARS-CoV-2 infection played a role in the genesis of the autoimmune encephalitis in our patient, and only subsequent observations of cases with similar features can answer this question. Indeed, it is not possible to exclude a role of the so-called “cytokines storm”, often associated to COVID-19 infection, to maintain and promote refractory or super-refractory status epilepticus. IL6 is known to be elevated during the inflammatory phase of COVID infection. Moreover, increased CSF IL6 levels have been recognized to facilitate intrathecal autoantibody production in anti- NMDAR encephalitis [6]. As previously reported, IL6 and IL8 are both known pro-inflammatory cytokines/chemokines that have been found increased in CSF of patients with NORSE and febrile infection-related epilepsy syndrome (FIRES) where it could be speculated if their raise is the cause or the effect of SE [7].
Finally, an important aspect is the management and treatment of RSE/SRSE (and immunomodulating therapies generally used in anti-NMDA receptor encephalitis) in a patient with co-infection with SARS-CoV-2. Indeed, several enzyme-inducing antiepileptic drugs such as phenytoin, phenobarbital, which are often used for the treatment of SE can reduce plasma levels of antivirals, such as lopinavir/ritonavir, and remdesivir. Bidirectional pharmacokinetic interactions are also known for hydroxychloroquine/chloroquine and enzyme-inducers antiseizure medications. It is important to underline that the immunomodulating therapies used in our patient (metilprednisolone, IGIV, plasma-exchange) have not aggravated the SARS-CoV-2 infection, and besides having a positive effect on the management of the anti-NMDA encephalitis, they could also have been helpful in the treatment of COVID-19 due to immunosuppression/anti-inflammation properties, and among steroids especially dexamethasone [8].
A last but not less important aspect is that this case is paradigmatic to underline the importance of doing ‘whatever it takes’ and never give-up even if the clinical situation appears to be particularly difficult in young and healthy patients with NORSE.
Standard protocol approvals, registrations, and patient consents
The scientific advisory boards of our institution approved the research report. Written informed consent has been obtained from patient’s relative.
Data availability
Anonymized data will be shared by request of any qualified investigator.
Ethical publication statement
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure statement
Dr. Giovannini, Monti, Simone, Melegari, Bedin, Marudi, Pignatti, Bertellini, and Dr. Santangelo reports no disclosures. Prof. Meletti received research grant support from the Ministry of Health (MOH); has received personal compensation as scientific advisory board member for UCB and EISAI.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.seizure.2020.07.006.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Mao L., Jin H., Wang M., Hu Y., Chen S., He Q., et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020 doi: 10.1001/jamaneurol.2020.1127. Published online April 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu L., Xiong W., Liu D., Liu J., Yang D., Li N., et al. New onset acute symptomatic seizure and risk factors in Corona Virus Disease 2019: a retrospective multicenter study. Epilespia. 2020 doi: 10.1111/epi.16524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaspard N., Hirsch L.J., Sculier C., Loddenkemper T., van Baalen A., et al. New-onset refractory status epilepticus (NORSE) and febrile infection-related epilepsy syndrome (FIRES): state of the art and perspectives. Epilepsia. 2018;59:745–752. doi: 10.1111/epi.14022. [DOI] [PubMed] [Google Scholar]
- 4.Panariello A., Bassetti R., Radice A., Rossotti R., Puoti M., et al. Anti-NMDA receptor encephalitis in a psychiatric Covid-19 patient: a case report. Brain Behav Immnunity. 2020;87:179–181. doi: 10.1016/j.bbi.2020.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armangue T., Spatola M., Vlagea A., Mattozzi S., Carceles-Cordon M., et al. Frequency, symptoms, risk factors, and outcomes of autoimmune encephalitis after herpes simplex encephalitis: a prospective observational study and retrospective analysis. Lancet Neurol. 2018;17:760–772. doi: 10.1016/S1474-4422(18)30244-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Byun J.-I., Lee S.-T., Moon J., Jung J.H., Sunwoo J.S., Lim J.A., et al. Distinct intrathecal interleukin-17/interleukin-6 activation in anti-N-methyl-D-aspartate receptor encephalitis. J Neuroimmunol. 2016;297:141–147. doi: 10.1016/j.jneuroim.2016.05.023. Eur Cytokine Netw. 2019. 4:130-134. [DOI] [PubMed] [Google Scholar]
- 7.Sakuma H., Tanuma N., Kuki I., Takahashi Y., Shiomi M., et al. Intrathecal overproduction of proinflammatory cytokine and chemokines in febrile-infection related status epilepticus. J Neurol Neurosurg Psych. 2015;86:820–822. doi: 10.1136/jnnp-2014-309388. [DOI] [PubMed] [Google Scholar]
- 8.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J., et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymized data will be shared by request of any qualified investigator.