Introduction
Kidney dysfunction is frequently reported in patients with coronavirus disease 2019 (COVID-19). A recent large cohort study described an incidence of acute kidney injury (AKI) of 36% in patients who were hospitalized with COVID-19; AKI (often mild) was temporally linked with respiratory failure and associated with a poor prognosis.1 Many factors may be at play in determining kidney dysfunction in patients with COVID-19 who are critically ill; moreover, human kidneys have been reported as potential targets for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Most pathologic descriptions of kidney involvement in COVID-19 are derived from autopsy studies,S1–S5 with the main abnormality reportedly being acute tubular injury (ATI) claimed as being caused by viral infection. Few reports of kidney biopsy specimens obtained on clinical indications in patients with COVID-19 are currently available; interestingly, most of them describe a picture of collapsing glomerulopathy considered secondary to viral infection.2,3 Only Rossi et al.4 recently reported a case of ATI and focal acute tubular necrosis with no evidence of kidney viral localization in a critically ill patient with COVID-19. AKI in critically ill patients with COVID-19 can be multifactorial, and the toxicity of drugs used to treat septic status could contribute to kidney damage. We describe ATI and oxalate nephropathy likely caused by excessive vitamin C administration in 2 patients who underwent kidney biopsy procedures for slowly resolving AKI after acute respiratory distress syndrome (ARDS) due to COVID-19.
Case Presentations
Patient 1 was a 50-year-old white man hospitalized for the persistence of fever, cough, and dyspnea; an oropharyngeal/nasal swab was positive for SARS-CoV-2 5 days before admission. His medical history included arterial hypertension and Brugada syndrome; he had no history of kidney disease nor kidney stones. At presentation, a chest X-ray showed bilateral interstitial pneumonia, moderate respiratory insufficiency was present, and his kidney function was normal (serum creatinine, 1 mg/dl). Due to rapid deterioration of respiratory function, the patient was transferred to the intensive care unit on the same day where he was intubated and put on lung-protective mechanical ventilation. According to the internal protocol for COVID-19, he was started on hydroxychloroquine, lopinavir/ritonavir 200/50 mg/d (rapidly switched to darunavir-cobicistat 800/150 mg/d), and azithromycin 500 mg/d. To counteract cytokine storm, a single dose of subcutaneous tocilizumab was used. I.v. steroids and broad-spectrum antibiotic therapy were provided; considering his septic status, high-dose i.v. vitamin C (50 mg/kg 4 times/d) was administered. The patient was put on enteral nutrition. The clinical course was immediately complicated by septic shock with the need for inotropes, and the patient developed multiorgan failure with hepatic dysfunction and AKI with anuria; he was started on continuous venovenous hemodiafiltration on day 2. Meropenem was administered for Klebsiella pneumoniae bacteremia. The patient’s respiratory status improved, and he was extubated by day 15 from hospitalization; swabs for SARS-CoV-2 were negative from day 18. Despite an increase in urine output, kidney function did not show any improvement and the patient was transferred to the nephrology unit; at day 36 a biopsy specimen of the kidney was obtained. In the following days, partial improvement of kidney function was observed; the patient was discharged home on day 42.
Patient 2 was a 71-year-old white man who was hospitalized for fever, diarrhea, and worsening neurologic status. His medical history included type 2 diabetes mellitus treated with metformin plus long-acting insulin, arterial hypertension, hypercholesterolemia, and mild liver steatosis; he had no history of kidney disease or kidney stones. A chest X-ray showed bilateral interstitial pneumonia and an oropharyngeal/nasal swab was positive for SARS-CoV-2; at presentation, kidney function was normal (serum creatinine, 1.1 mg/dl). Because of worsening respiratory failure and rapid development of ARDS, he was immediately transferred to the intensive care unit where he was intubated and put on lung-protective mechanical ventilation. According to the internal protocol for COVID-19, he was started on hydroxychloroquine, darunavir-cobicistat 800/150 mg/d, and azithromycin 500 mg/d; i.v. steroids and broad-spectrum antibiotic therapy were provided. High-dose i.v. vitamin C (50 mg/kg 4 times/d) was administered according to internal management of septic patients. The patient was put on enteral nutrition. The clinical course was complicated by persistent hypotension with the need for inotropes. The patient developed progressive kidney dysfunction and needed kidney replacement treatment from day 8; he received 11 continuous venovenous hemodiafiltration sessions (2 of them with CytoSorb adsorber [CytoSorbents, Inc., Monmouth Junction, NJ]) with local citrate anticoagulation and was later switched to intermittent hemodialysis. The patient’s respiratory status improved, and he was extubated by day 15. During hospitalization, the patient received multiple antibiotic courses (piperacillin/tazobactam, ceftriaxone, linezolid, meropenem, daptomycin, and oxacillin), partly empirical to treat supposed bacterial pulmonary superinfection and partly guided on isolates (Staphylococcus epidermidis on blood cultures). The patient had a transient decrease in platelet count (attributed to prolonged linezolid treatment) and multifactorial anemia requiring blood transfusion. Since day 22, the patient had a negative SARS-CoV-2 swab. Despite regaining adequate diuresis, kidney function failed to improve and hemodialysis was continued; the patient was transferred to the nephrology unit, and on day 45 a biopsy specimen of the kidney was obtained. After the biopsy procedure, polyuria was induced; kidney function showed slow improvement and there was no further need for hemodialysis. The patient was discharged home on day 58 after admission. Clinical and laboratory parameters of the two patients are summarized in Table 1.
Table 1.
Relevant clinical and laboratory parameters of the 2 patients with severe COVID-19 and acute tubular injury and oxalate nephropathy after a kidney biopsy procedure
| Clinical and laboratory parameters | Patient 1 | Patient 2 |
|---|---|---|
| Treatments | ||
| Vitamin C total dose, g | 112 | 160 |
| Hydroxychloroquine dose, d | 400 mg bid (ND) | 400 mg bid (24) |
| Darunavir/cobicistat dose, d | 800/150 mg (unclear) | 800/150 mg (7) |
| Lopinavir/ritonavir, d | — | 200/50 mg (unclear) |
| Azithromycin dose, d | ND | 500 mg (5) |
| Tocilizumab | Yes | — |
| Steroids | Yes | Yes |
| Parenteral nutrition | — | — |
| Enteral nutrition | Yes | Yes |
| Interleukin-6 zenith, pg/ml | — | 123.5 |
| Polymerase chain reaction zenith, mg/dl | 44.4 | 28.3 |
| Days intubated | 15 | 15 |
| Need for inotropes | Yes | Yes |
| Anuria, d | 10 | 20 |
| Total time of kidney replacement treatment, d | 33 | 31 |
| S creatinine at admission, mg/dl | 1 | 1.1 |
| S creatinine at kidney biopsy procedure, mg/dl | 5.85 | 6.5 |
| Proteinuria, g/d | 0.2 | 1.2 |
| Microhematuria | No | Yes |
| Diuresis at kidney biopsy procedure, ml/d | 2000 | 3700 |
| S creatinine at hospital discharge, mg/dl | 4 | 4.44 |
| Diuresis at hospital discharge, ml/d | 1800 | 3900 |
| 24-h urine oxalate, d 50 | — | 42 mg |
b.i.d., twice a day; COVID-19, coronavirus disease 2019; ND, not determined, S, serum.
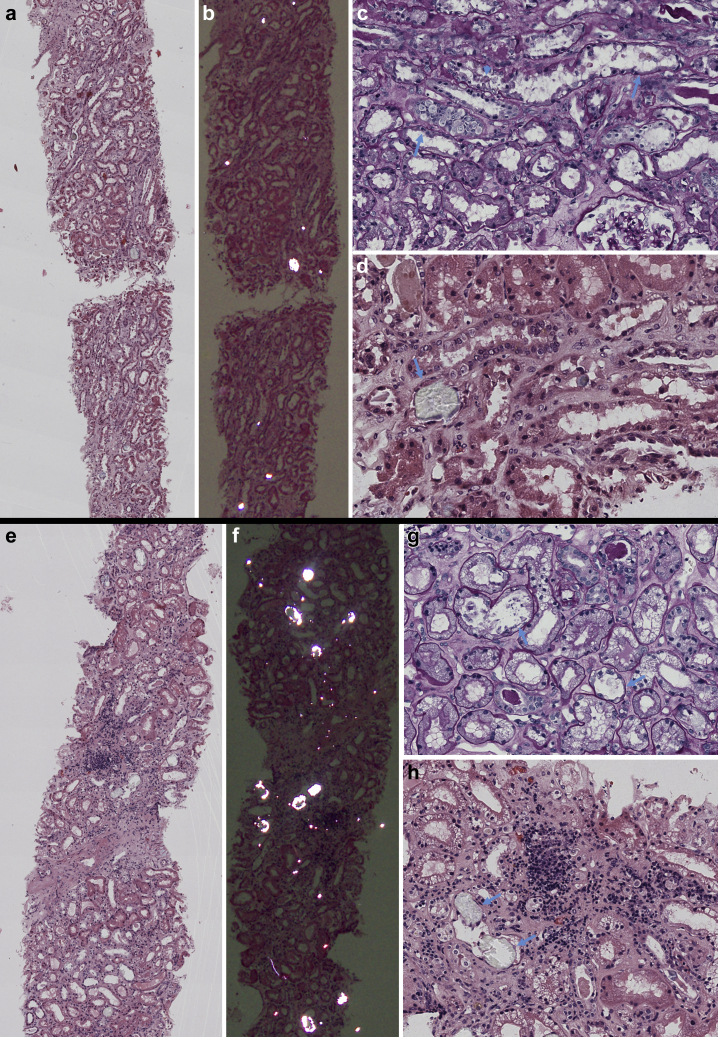
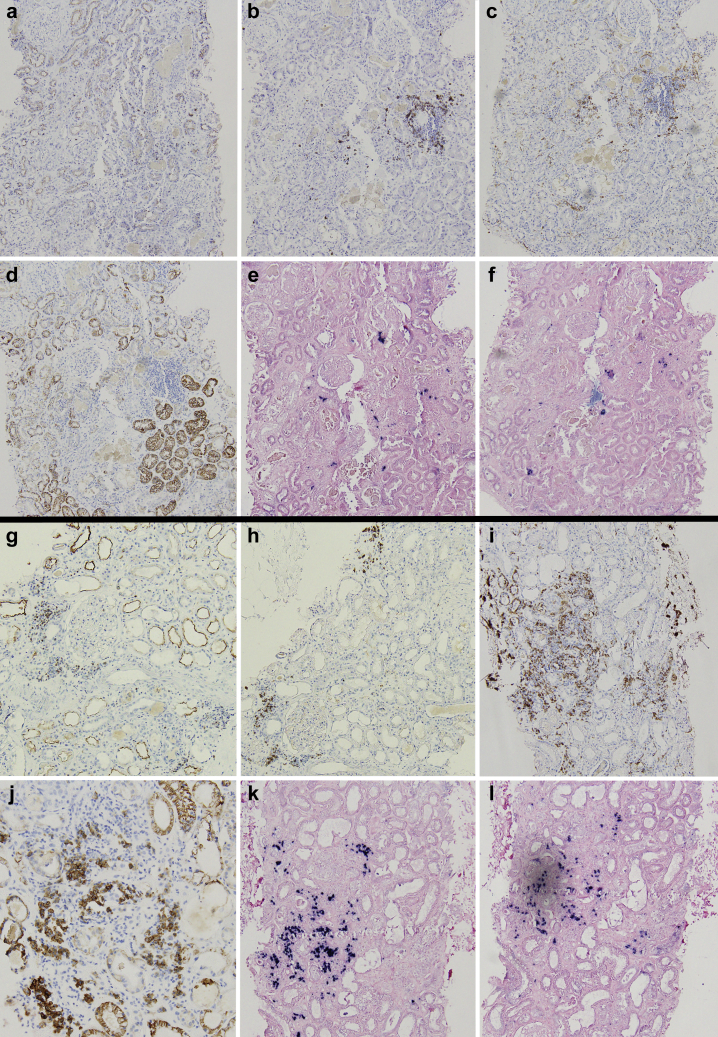
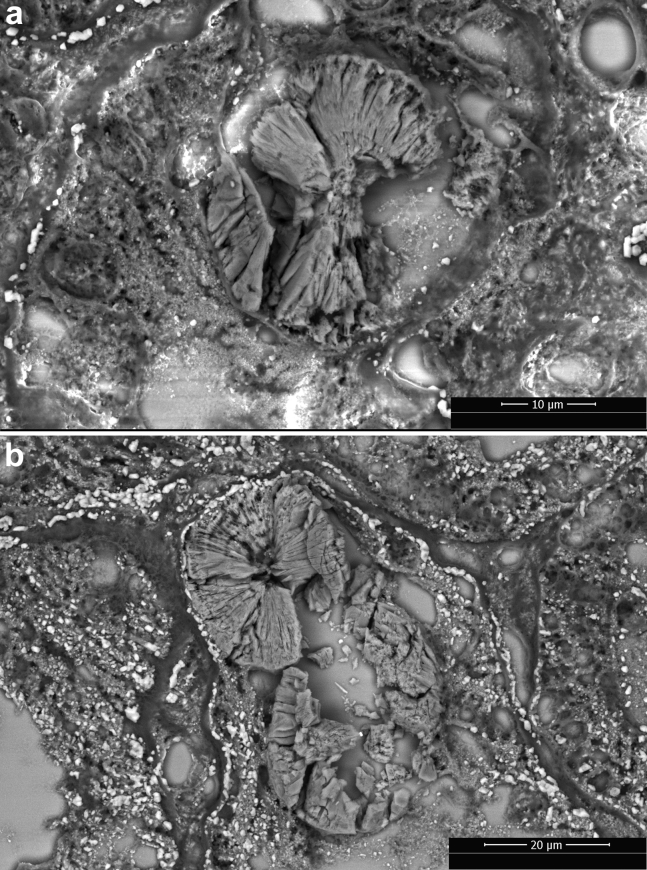
The kidney biopsy specimen of patient 1 (Figure 1) showed extensive ATI and focal acute tubular necrosis, with degenerative and reactive aspects of tubular cells (epithelial attenuation and focal detachment with presence of cellular debris in tubular lumen). Interestingly, calcium oxalate crystals (fan-shaped radially arranged translucent crystals on hematoxylin and eosin stain, birefringent when examined with polarized light) were identified in several tubular lumina. Few degenerated tubules contained periodic acid–Schiff–positive casts. There was mild focal interstitial inflammation; glomeruli showed no relevant alterations, apart from focal and segmental presence of inflammatory cells in capillary lumina; arteries and arterioles were normal. Immunohistochemical analysis characterized the inflammatory infiltrate as mild and mixed (Figure 2). Immunofluorescence on frozen tissue (immunostaining for IgG, IgA, IgM, complement component 3, complement component 1q, fibrinogen, kappa, and lambda) was negative or nonspecific. Scanning electron microscopy (EM; Nova NanoSEM 450, Fei Company, Hillsboro, OR) on paraffin-embedded slides confirmed the presence of diffuse calcium oxalate monohydrate crystals in the tubular lumina, identifying the typical rose-cut–shaped appearance. Energy-dispersive X-ray spectrometry (Bruker Quantax 200, Bruker Corporation, Billerica, MA) showed a common calcium matrix in both kinds of crystals and identified a stoichiometric atomic ratio compatible with calcium oxalate monohydrate (Supplementary Figure S1). Transmission EM analysis was not performed because of insufficient material.
Figure 1.
Light microscopy examination of kidney biopsy specimens obtained from patients 1 (a–d) and 2 (e–h). (a,e) Low-power view of kidney biopsy specimens with focus on tubule interstitium; several crystals are in the tubular lumens (hematoxylin and eosin [H&E] stain). (b,f) Same sections as a and c examined under polarized light: several birefringent crystals are visible in the tubular lumina (H&E stain). (c) Diffuse acute tubular injury with flattening of epithelial cell line (arrows) and focal cell detachment with intraluminal sloughing (arrowhead) (periodic acid–Schiff stain; original magnification ×400). (d) Detail of intratubular calcium oxalate crystal (arrow), with translucent appearance (H&E stain; original magnification ×400). (g) Diffuse acute tubular injury with extensive vacuolization of tubular epithelial cells and focal flattening of epithelial line (arrows) (periodic acid–Schiff stain; original magnification ×400). (h) Detail of intratubular calcium oxalate crystal (arrow), with translucent appearance (arrows); moderate mixed interstitial infiltrate in area of initial interstitial fibrosis (H&E stain; original magnification ×400).
Figure 2.
Immunohistochemistry characterization of interstitial inflammatory infiltrate of patients 1 (a–f) and 2 (g–l). Patient 1 showed minimal scattered interstitial infiltrate, composed mainly of B lymphocytes (b) and macrophages (c); patient 2 showed focal moderate interstitial inflammation with abundant presence of plasma cells (j) and macrophages (i); both T (g) and B (h) lymphocytes were present. (a,g) CD3 (T-lymphocytes; original magnification ×100); (b,h) CD20 (B-lymphocytes; original magnification ×100); (c,i) CD163 (macrophages; original magnification ×100); (d,j) CD138 (plasma cells; original magnifications: d, ×100; j, ×200); (e,k) kappa light chain (original magnification ×100); and (f,l) lambda light chain (original magnification ×100).
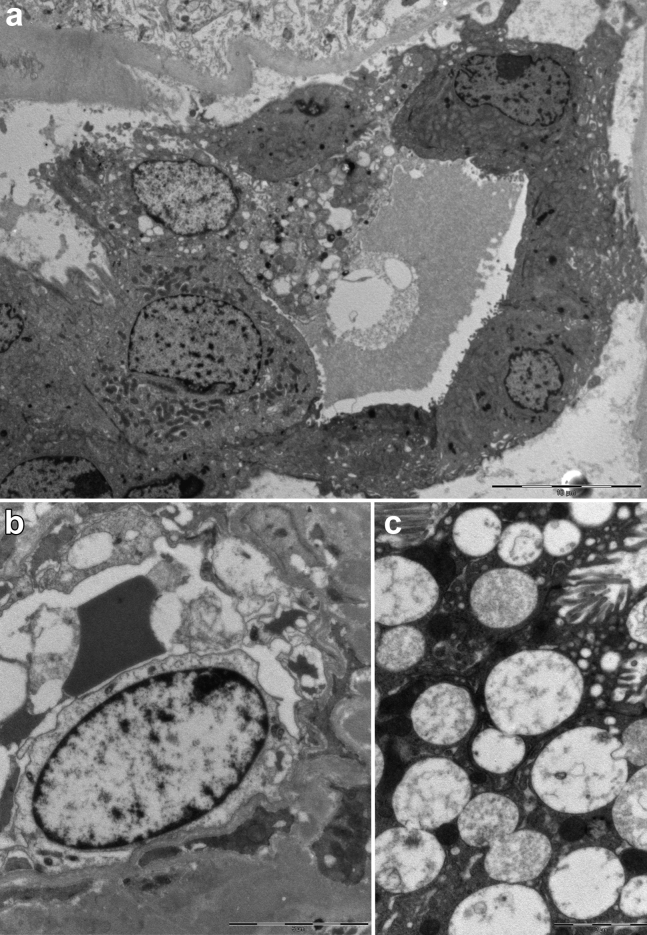
The kidney biopsy specimen of patient 2 (Figure 1) showed extensive ATI with marked coarse cytoplasmic vacuolization in the tubular epithelial cells (interpreted as being caused by ischemic injury), focal epithelial attenuation with thinning of the apical cytoplasm, focal tubulitis, and diffuse presence of calcium oxalate crystals in the tubular lumen. Moderate focal interstitial inflammation was present, more intense in areas of initial tubular atrophy; no glomerular abnormalities were identified; arteries showed mild intimal fibrosis, and there were no signs of thrombotic microangiopathy. Immunohistochemical analysis characterized the inflammatory infiltrate as mixed, with an abundance of plasma cells and macrophages (Figure 2). Immunofluorescence analysis (see above) was negative or nonspecific. On transmission EM examination (Figure 3), degenerative alterations involving proximal and distal tubules and peritubular capillaries were identified; few tubular lumina were filled up by the footprint of crystal aggregates likely extracted during preparation for the biopsy procedure. The extensive vacuolization of epithelial cells was confirmed; vacuoles contained amorphous material with low electron density, without evidence of structures suggestive for viral particles. No glomeruli were present in the examined material. Scanning EM (Figure 4) and energy-dispersive X-ray spectrometry analysis confirmed the presence of calcium oxalate monohydrate crystals.
Figure 3.
Ultrastructural analysis on transmission electron microscopy of kidney biopsy of patient 2. (a) Low magnification of a distal tubule with diffuse signs of acute injury (detachment of cells from tubular basement membrane; bar = 10 μm). (b) Peritubular capillary structure lined by a degenerated necrotic endothelial cell (bar = 5 μm). (c) Cytoplasmic vacuoles in a tubular epithelial cell; vacuoles are polymorphic in appearance, most often with low electron density content comprised of lamellar and corpuscular figures that are not suggestive for viral structures (diameter, >130 nm; bar = 2 μm).
Figure 4.
Scanning electron microscope photographs of calcium oxalate monohydrate crystals in the tubular lumen in the kidney biopsy specimen obtained from patients 1 (a) and 2 (b). Crystals show the typical rose-cut–shaped appearance.
Real-time polymerase chain reaction (performed according to Centers for Disease Control and Prevention guidelines, available at https://www.cdc.gov/coronavirus/2019-ncov/lab/) on frozen kidney tissue was negative for SARS-CoV-2 RNA in both patients.
Discussion
Critically ill patients are extremely prone to develop AKI. In the setting of COVID-19, where characteristics of sepsis and ARDS combine, several causes can contribute to kidney injury. Relative hypoperfusion of the kidney developing through alteration in microcirculation have been described in patients with sepsis.S6 Molecules released in the cytokine storm, which is considered central in the pathogenesis of organ damage in COVID-19, can damage tubular epithelial cells by activating them from the circulation via leukocyte stimulation or through the production of damage- and pathogen-associated molecular patterns from the tubular side.S6 Moreover, many potential mechanisms might be at play in the propagation of AKI in patients with ARDS (e.g., high intrathoracic pressures caused by mechanical ventilation, hypoxemia and systemic acidosis which influence kidney vascular resistance altering kidney perfusion pressures, and facilitation of release of proinflammatory cytokines).S7 Lastly, medications can have direct or indirect kidney toxicity.
Both of our patients had a clinical picture that was fully compatible with multifactorial ATI during sepsis and ARDSTable 1. Nevertheless, the failure to recover kidney function after several weeks from initial insult and after resolution of COVID-19 pneumonia, together with the uncertainty related to possible direct viral injury in the kidney, prompted histologic examination. In both cases, the kidney biopsy specimen showed ATI with mild reactive interstitial inflammation and no glomerular involvement; interestingly, tubular damage was often associated with the presence of calcium oxalate crystals in the tubular lumen, a picture that is suggestive of oxalate nephropathy.
Oxalate nephropathy (also named secondary oxalosis) is characterized by tubular crystalline deposits of calcium oxalate leading to acute and chronic tubular injury. Primary hyperoxaluria was unlikely and our patients had no history of kidney stones, and therefore we hypothesized that secondary hyperoxaluria somehow connected to the critical illness phase of COVID-19. Secondary hyperoxaluria is usually the result of increased dietary oxalate intake, increased intestinal oxalate availability, decreased intestinal oxalate degradation, or increased colonic permeability to oxalate.5 Given the clinical history of our patients, the most likely cause of high blood levels of oxalate causing hyperoxaluria and secondary oxalosis was vitamin C administration during the intensive care unit stay.
Preclinical studies showed that vitamin C was able to prevent sepsis-induced cytokine production that activated and sequestered neutrophils in the lungs.S8 The i.v. infusion of a high dose of vitamin C has been shown to significantly reduce the proinflammatory biomarkers C-reactive protein and procalcitonin in patients with sepsis,S9 and a recent trial indicated that the administration of 15 g/d of i.v. vitamin C for 4 days may decrease mortality in patients with sepsis-related ARDS.S10 There is mounting enthusiasm regarding the potential beneficial effects of vitamin C in limiting the cytokine storm in COVID-19,S11,S12 and a clinical trial is ongoing in China to test the effects of high-dose i.v. vitamin C administration in critically ill patients with COVID-19.S13 Indeed, vitamin C causes hyperoxaluria through the endogenous conversion of ascorbic acid to oxalate.6 In the setting of hyperoxaluria, calcium oxalate super saturation can occur and form crystal nuclei in urine; while crystals are quickly passed in healthy individuals, intratubular retention is believed to occur in areas of damaged and regenerating tubular epithelium, where molecules with potential crystal-binding capacity are expressed.6,7 There is no clear toxic dose for vitamin C, but a dose of 1000 mg/d can increase oxalate excretion by 6–13 mg/d and may induce calcium oxalate calculi,S14 and oxalate nephropathy has been reported after as few as 2 i.v. doses of vitamin C (dose unspecified) in a patient with preexisting kidney dysfunction.6 Our patients received a high dose load of vitamin C (>100 g in few days), and they were treated for a period of time that is longer than that tested in clinical trials (96 hours).S10 It is reasonable to hypothesize that high-dose vitamin C is not toxic per se, but consequent hyperoxaluria with secondary oxalosis can substantially aggravate kidney tubular injury of any type and jeopardize its recovery. We acknowledge that oxalate nephropathy caused by vitamin C administration is poorly described in the literature despite its widespread use in patients who are critically ill; it is also true that these patients offer several potentially clear clinical explanations for AKI and that they seldom undergo diagnostic kidney biopsy procedures. Indeed, oxalate nephropathy caused by vitamin C administration can be underreported. Considering the potentially beneficial effects of high-dose vitamin C in patients with sepsis, we believe that a cautious risk/benefit balance of its prolonged administration should be assessed on a case-by-case basis in patients with COVID-19 and kidney dysfunction.
In addition to vitamin C–induced hyperoxaluria, antibiotic administration and possible COVID-19–related enteritisS15 could have contributed to the picture of secondary oxalosis, favoring intestinal microbiota alterations and oxalate hyperabsorption. Moreover, some antibiotics (in particular ceftriaxone) can precipitate in the urine and cause the formation of crystalsS16; however, ceftriaxone crystals are generally needle-shaped,S16 which was not the morphology described in the ultrastructural analysis of our biopsy specimens, and their atomic content is different from what was described by energy-dispersive X-ray spectrometry in our cases. In addition, protease inhibitors, especially darunavir, have been reported to possibly form intratubular and cellular crystals.8 In our patients we were not able to recognize crystals with morphologic characteristics (i.e., needle-shaped or irregular, sometimes intracellular, multirefractive on polarized light,8 with a high atomic content of hydrogen and nitrogen on energy-dispersive X-ray spectrometry) compatible with protease inhibitor composition.
Direct infection of the kidney by SARS-CoV-2 is reasonably possible because most kidney cells, and especially proximal tubule epithelial cells, express angiotensin-converting enzyme 2, a specific viral receptor. Nevertheless, most of the available reports from autopsy studies that claimed to demonstrate direct viral entry in renal cells relied mainly on morphologic criteria (i.e., direct visualization of supposed viral structuresS1,S2) and have been extensively criticized.S4,S5 Moreover, SARS-CoV-2 has not been definitively proven to cause ATI. During previous coronaviruses outbreaks, renal tubular cells’ entry and direct cytopathic effect has been demonstrated in vitro for MERS-CoV but not for SARS-CoV9; interestingly, the latter, but not the former, shares the same cell receptor of SARS-CoV-2, angiotensin-converting enzyme 2. We did not identify SARS-CoV-2 in the kidney in our patients, neither through sensitive and specific reverse transcriptase polymerase chain reaction on frozen tissue nor through direct visualization of supposed viral particles in ultrastructure analysis with EM.
We believe that kidney injury in patients with severe COVID-19 is probably multifactorial and related to common causes of ATI in patients with sepsis: hypoperfusion caused by hypotension, cytokine storm, and drug toxicity. Nevertheless, we acknowledge that our patients underwent kidney biopsy procedures several days after the resolution of COVID-19 and with already no SARS-CoV-2 RNA detectable by reverse transcriptase polymerase chain reaction on oropharyngeal/nasal swab. Our report is then not able to rule out a potential role for SARS-CoV-2 in generating initial tubular injury, but at the same time it provides no elements to suggest it.
We describe 2 cases of a rare cause of iatrogenic AKI with ATI, secondary oxalosis caused by vitamin C supplementation, in the setting of COVID-19 and without a clear causative link with SARS-CoV-2 infection (Table 2).
Table 2.
Teaching points
| We describe 2 cases of multifactorial acute tubular injury with oxalate nephropathy in critically ill patients with COVID-19. |
| Oxalate nephropathy was caused by high-dose i.v. vitamin C supplementation, administered to counteract a COVID-19–related cytokine storm during sepsis. |
| Vitamin C causes hyperoxaluria through endogenous conversion of ascorbic acid to oxalate. |
| Oxalate nephropathy can substantially aggravate kidney tubular injury of any type and jeopardize its recovery. |
COVID-19, coronavirus disease 2019.
Our report has several limitations. First, we are describing only 2 cases; future studies on larger cohorts will be able to shed more light on the etiologies of kidney dysfunction in patients with COVID-19. Whether COVID-19 differs from other septic scenarios in terms of the pathogenesis of ATI and facilitation of crystallization of calcium oxalate in kidney tubules during high-dose vitamin C administration needs to be elucidated. Second, we were unfortunately not able to measure serum oxalate and vitamin C levels, and urine oxalate levels were measured only in 1 patient some weeks after vitamin C supplementation; nevertheless, the etiology of secondary oxalosis is based on the presence of potential causative factors, and we believe that the clinical history of our patients adequately fits our conclusive diagnosis. Third, we acknowledge that biopsy specimens were obtained after the resolution of SARS-CoV-2 infection; we were not able to demonstrate or suggest the presence of the virus in the kidney, but we cannot exclude that infection of kidney cells had happened during the acute phase of the disease.
Conclusion
Knowledge about COVID-19 infection pathophysiology and treatment is rapidly evolving; the causes of kidney dysfunction during SARS-CoV-2 infection are probably multiple and yet not fully elucidated. We believe it is of extreme importance to bring to the attention of the medical community cases of kidney biopsy procedures performed on clinical indication in patients with COVID-19; the study of pathologic lesions can contribute to a better understanding of the etiology of damage. Notorious side effects of drugs must be taken into consideration, and their risks and benefits balanced, before their widespread use in patients with COVID-19.
Disclosure
All the authors declared no competing interests.
Footnotes
Figure S1. Energy dispersive X-ray spectrometry (EDS) analysis of crystals: (A) preliminary EDS mapping showed a homogeneous pattern of the atomic with calcium (Ca)-based matrix. (B) The stoichiometric ratio was compatible with calcium oxalate monohydrate (CaC2O4), even if oxygen was relatively expressed lower than expected, possibly due to interference of the sodium (Na) and chloride (Cl) peaks due to inclusion in NaCl solution. C, carbon; O, oxygen; Mg, magnesium; Si, silicon.
Supplementary References.
Supplementary Material
References
- 1.Hirsch J.S., Ng J.H., Ross D.W. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98:209–218. doi: 10.1016/j.kint.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Larsen C.P., Bourne T.D., Wilson J.D. Collapsing glomerulopathy in a patient with coronavirus disease 2019 (COVID-19) Kidney Int Rep. 2020;5:935–939. doi: 10.1016/j.ekir.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peleg Y., Kudose S., D’Agati V. Acute kidney injury due to collapsing glomerulopathy following COVID-19 infection. Kidney Int Rep. 2020;5:940–945. doi: 10.1016/j.ekir.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rossi G.M., Delsante M., Pilato F.P. Kidney biopsy findings in a critically ill COVID-19 patient with dialysis-dependent acute kidney injury: a case against “SARS-CoV-2 nephropathy.”. Kidney Int Rep. 2020;5:1100–1105. doi: 10.1016/j.ekir.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lumlertgul N., Siribamrungwong M., Jaber B.L., Susantitaphong P. Secondary oxalate nephropathy: a systematic review. Kidney Int Rep. 2018;3:1363–1372. doi: 10.1016/j.ekir.2018.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cossey L.N., Rahim F., Larsen C.P. Oxalate nephropathy and intravenous vitamin C. Am J Kidney Dis. 2013;61:1032–1035. doi: 10.1053/j.ajkd.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 7.Verkoelen C.F., Verhulst A. Proposed mechanisms in renal tubular crystal retention. Kidney Int. 2007;72:13–18. doi: 10.1038/sj.ki.5002272. [DOI] [PubMed] [Google Scholar]
- 8.Soto K., Campos P., Manso R. Severe acute kidney injury and double tubulopathy due to dual toxicity caused by combination antiretroviral therapy. Kidney Int Rep. 2019;4:494–499. doi: 10.1016/j.ekir.2018.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eckerle I., Müller M.A., Kallies S. In-vitro renal epithelial cell infection reveals a viral kidney tropism as a potential mechanism for acute renal failure during Middle East Respiratory Syndrome (MERS) Coronavirus infection. Virol J. 2013;10:359. doi: 10.1186/1743-422X-10-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.