Abstract
Virus-specific T cell-mediated immunity is severely impaired in chronic hepatitis B virus (HBV) patients. HBV-specific T cells in chronic HBV patients show a low ability to produce cytokines and to exert their cytotoxic activity. A prominent characteristic of these exhausted T cells is overexpression of inhibitory receptor molecules which negatively regulate T cell function. In this study, we examined in vitro regulation of two inhibitory receptor expressions, programmed death 1 (PD-1) and T cell immunoglobulin mucin domain-containing molecule 3 (TIM-3). Peripheral blood mononuclear cells (PBMCs) obtained from healthy individuals were in vitro stimulated with a panel of cytokines. PD-1 and TIM-3 expression levels on CD4+ and CD8+ T cells were examined at days 2 and 7 post stimulation. We demonstrated that PD-1 and TIM-3 were induced via polyclonal (anti-CD3) and cytokine (interleukin 15 [IL-15]) stimulations. Noteworthy, there was a significantly increased induction of TIM-3 on CD8+ T cells as compared to CD4+ T cells. Our study thus contributes to further understanding the regulation of T cell exhaustion markers PD-1 and TIM-3.
Keywords: Inhibitory receptors, interleukin 15, PD-1, T cells, TIM-3
Introduction
An estimated 350 million individuals are chronically infected with the hepatitis B virus (HBV), and these infections cause significant morbidity and mortality worldwide. HBV-infected patients are at risk for chronic progressive liver disease such as liver cirrhosis and hepatocellular carcinoma [1]. Current treatment of chronic HBV using pegylated interferon-α (PEG-IFN-α)-based therapy or nucleos(t)ide analogs (NUC) have improved clinical outcome [2]. However, still a substantial number of patients fails to respond to the treatment. Moreover, several limitations of PEG-IFN-based treatment remain such as frequent side-effects on multiple organ systems that lead to discontinuation of the therapy in a group of patients. For NUC-based therapy, the side-effects are lower, yet the patients require long duration of treatments [2]. Immune-modulation therapy, either as mono or combination therapy, has been considered to achieve improved control of the viruses [3].
The persistence of HBV in hepatocytes of infected hosts has been facilitated by weak or absent HBV-specific immune responses [4,5]. Due to continuously high viral antigen pressure, the HBV-specific T cells in chronic HBV patients have a decreased ability to perform anti-viral actions either through direct cytolytic (production of perforin and granzymes) and non-cytolytic (production of IFN-γ) pathways [6]. The impaired T cell activity, termed as T cell exhaustion, was first demonstrated in chronic lymphocytic choriomeningitis virus (LCMV)-infected mice [7] and subsequently identified in chronic human viral infections, including human immunodeficiency virus (HIV), HBV and hepatitis C virus (HCV) as well as in cancer [8]. Noteworthy, due to the availability of tetramer staining, many studies focused on T cell exhaustion in CD8+ T cells, although it can also occur in CD4+ T cells [9-12].
T cell function is regulated by several extrinsic regulatory pathways, such as via the inhibitory cytokines interleukin 10 (IL-10) and transforming growth factor β (TGF-β) as well as regulatory T cells (Treg) [8]. In addition, T cell function is intrinsically modulated by expression of inhibitory receptor molecules such as PD-1, cytotoxic T-lymphocyte associated protein 4 (CTLA-4) and TIM-3 on the surface of T cells [8]. In chronic HBV and HCV patients, it has been shown that overexpression of these inhibitory receptors may have a negative impact on the functionality of T cells. Total and HBV- and HCV-specific T cells in chronic HBV and HCV patients overexpress many inhibitory receptor molecules, such as PD-1, 2B4 and TIM-3 [13-17]. Importantly, single or combination blockade of these inhibitory receptor molecules in vitro can enhance the functionality of exhausted T cells, as indicated by increased proliferation, cytokine production and cytotoxic activity [12,18,19].
This approach to reactivate exhausted virus-specific T cells was rapidly translated into clinical setting, especially for HCV [20,21]. Out of 56 chronic HCV patients who received a single dose of anti-PD-1 antibody, 6 patients showed a decline in their serum HCV RNA level of more than 0.5 log [21]. Tremelimumab, a fully human monoclonal antibody against CTLA-4, has been tested in advanced hepatocellular carcinoma (HCC) in combination with ablative therapies. A significant portion of patients with quantifiable HCV demonstrated a notable reduction in viral load [22]. Nivolumab, an anti-PD-1 human monoclonal antibody is currently being tested in Phase 1/2 trials for HBV- and HCV-associated HCC (NCT01658878). The role of inhibitory receptor molecules during chronic HBV and HCV infections and also their potential to be manipulated as a novel target for immunotherapy have previously been extensively reviewed by us [23,24] and others [25,26].
Despite significant achievements in understanding the immunoregulatory role of inhibitory receptors on HBV-specific T cells and their application in the clinic, their regulation of expression is still poorly understood. In fact, this is a foundation for understanding T cell responses during acute and chronic HBV and HCV, as well as other viral infections. Therefore, we investigated whether cytokines could modulate the expression of PD-1 and TIM-3 to improve our understanding on T cell regulation during inflammation or acute infections. During those conditions, T cells are highly exposed to various cytokines produced by immune or non-immune cells. Thus, our study may contribute to further understanding the regulation of inhibitory receptors PD-1 and TIM-3. In translational settings, findings of this study may provide guidance to more optimize immune-based therapy targeting the inhibitory receptors in chronic HBV and HCV infections.
Methods
Isolation and cullture of peripheral blood mononuclear cells (PBMCs)
PBMCs of healthy individuals were isolated from venous blood by ficoll separation according to the manufacturer instructions (Ficoll-PaqueTM plus, Amersham). PBMCs were cultured at 2×105 cells per well in RPMI 1640 medium supplemented with 5% human serum, penicillin, streptomycin, HEPES and L-glutamine.
In vitro stimulation of PBMCs
Cells were stimulated with complete medium alone or with 400 ng/ml soluble anti-CD3 clone OKT3 (eBioscience), 1 µg/ml anti-CD28 clone CD28.6 (eBioscience), 10 ng/ml IFN-α (Schering-Plough), 10 ng/ml IFN-γ (Miltenyi Biotec), 10 ng/ml IL-15 (PeproTech), 25 ng/ml IL-10 (R&D Systems), 1 ng/ml TGF-β (PeproTech), and a pro-inflammatory cytokine cocktail consisting of 10 ng/ml IL-1β (Miltenyi Biotec), 10 ng/ml IL-6 (Miltenyi Biotec) and 2.5 ng/ml tumor necrosis factor (TNF) (R&D Systems). Experiments were performed in 4 wells per condition and repeated at least three times. All cells were cultured at 37°C with 5% CO2.
On day 2 and 7, cells were pooled and stained with anti-CD3 FITC clone UCHT1, anti-CD8 PE clone B9.11 (both from Beckman Coulter), anti-CD4 APC-H7 clone SK3 (BD Biosciences), anti-PD-1 PerCPeFluor 710 clone eBioJ105, and anti-TIM-3 APC clone F38-2E2 (both from eBiosciences). Cells were measured using FACSCanto II and analyzed by FACSDiva software (both from BD Biosciences).
Study approval
A written informed consent was signed by the healthy volunteers who agreed to participate, and data was anonymized. Ethical approval was obtained from the ethical review board of the Erasmus MC.
Statistical analysis
Statistical analysis was performed using the nonpaired, nonparametric test (Mann-Whitney test; GraphPad Prism software, GraphPad Software Inc., La Jolla, CA). P values < 0.05 were considered statistically significant.
Results
PD-1 expression on CD4+ and CD8+ T cells following in vitro cell stimulation
In order to investigate the regulation of inhibitory receptors PD-1 and TIM-3, PBMCs from healthy donors were stimulated with various stimulation conditions. The frequency of PD-1+ and TIM-3+ within CD4+ and CD8+ T cells were analyzed following 2 and 7 days of total PBMC stimulation and compared with the frequency of positive cells at baseline (day 0).
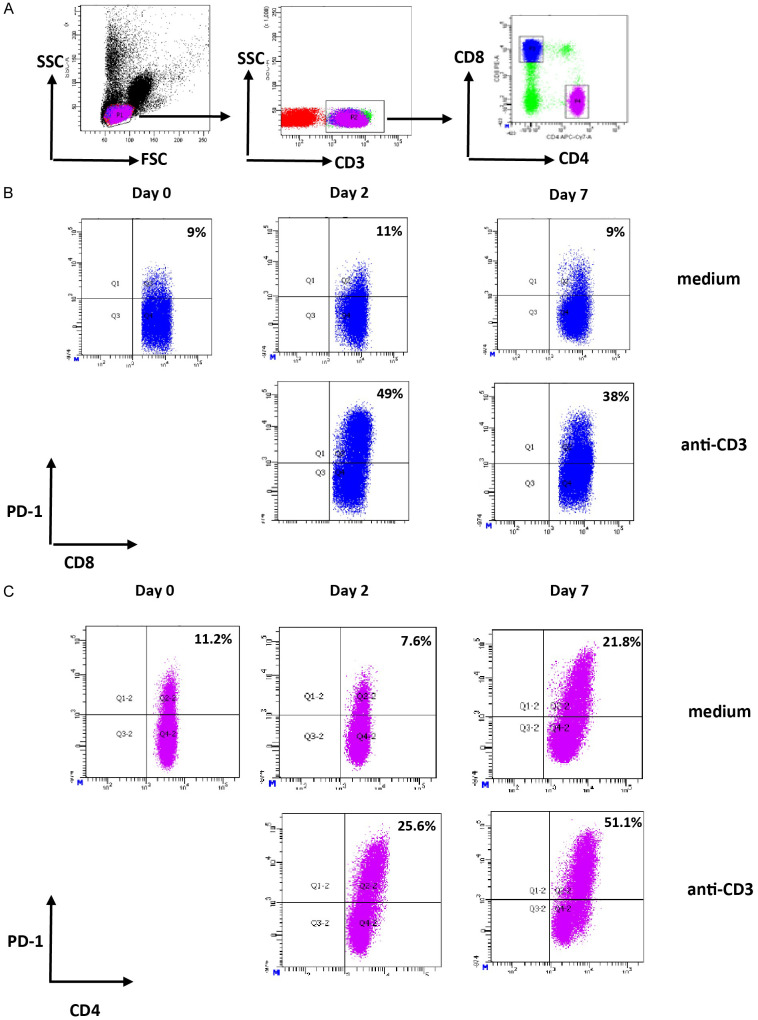
Gating strategy to identify CD4+ and CD8+ T cells after culture with medium or anti-CD3 was shown in Figure 1A. Representative figures to identify PD-1+ expressing CD8+ and CD4+ T cells were shown in Figure 1B and 1C, respectively. The mean frequency of PD-1+ expressing CD8+ T cells at baseline (day 0) was 14.2% (ranging from 8.5% until 20.1%). PBMCs stimulated with IL-15 demonstrated increased frequency of PD-1+ CD8+ T cells (the mean frequencies of PD-1+ CD8+ T cells were 10.1%; 13.4%; and 21.7% at day 0, 2, and 7 respectively; P < 0.05 for comparison between day 0 and day 7) (Figure 2A). For other stimuli, including IFN-α, IFN-γ, IL-10, TGF-β, and combination of IL-1β, IL-6 and TNF, no significant differences were observed (Figure 2A). Analysis on CD4+ T cell populations demonstrated similar findings, in which only IL-15 significantly induced the expression of PD-1 (Figure 2A).
Figure 1.
Identification of PD-1-expressing CD4+ and CD8+ T cells. (A) Gating strategy to identify CD4+ and CD8+ T cells. Lymphocytes were gated based on FSC/SSC profile. CD3+ T cells were identified within the lymphocyte gate to separate CD4+ T cells and CD8+ T cells. (B and C) Representative dot plots of PD-1 expression on CD8+ T cells (B) or CD4+ T cells (C) in medium condition and after stimulation of PBMCs with anti-CD3. CD3+ CD8+ T cells or CD3+ CD4+ T cells were analysed and frequency of PD-1+ CD8+ T cells (B) or PD-1+ CD4+ T cells (C) at day 2 and day 7 were compared with the frequency at baseline (day 0). Values in the upper right quadrant indicate the percentage of PD-1 positive cells within CD8+ or CD4+ T cells.
Figure 2.
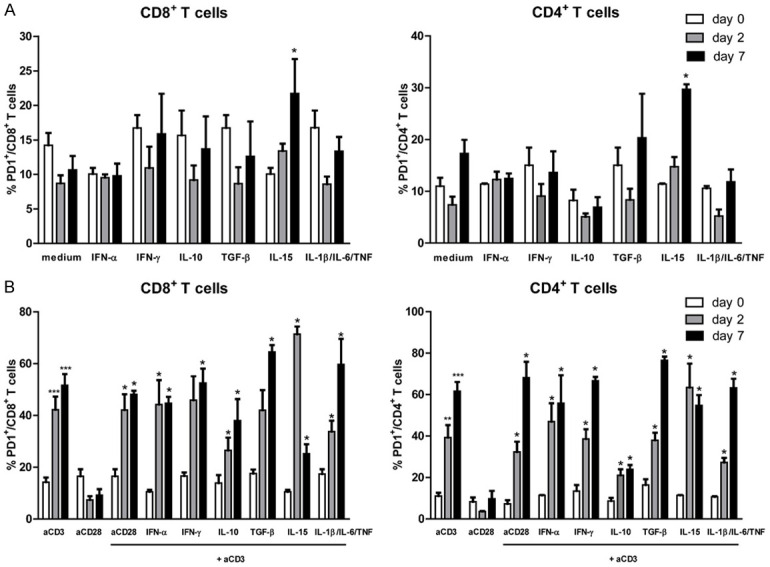
Regulation of PD-1 expression on CD4+ and CD8+ T cells following various cytokine stimulations (A) and combination of cytokines and anti-CD3 stimulation (B). Data sets represent the mean and standard error of the mean (SEM) from eight (for medium and anti-CD3 stimulation) or three (for other stimuli) independent experiments. Comparison of the frequency of PD-1+ CD8+ T cells (left panel) and PD-1+ CD4+ T cells (right panel) between 0, 2 and 7 days after stimulations are shown. The P value is calculated in comparison with the frequency of positive cells at day 0 (* = P < 0.05; *** = P < 0.001).
Next, we combined those cytokines with anti-CD3 stimulation to examine the influence of those cytokines during anti-CD3-mediated T cell activation [27]. Combination of anti-CD3 and IL-15 stimulation resulted in a trend towards increased frequency of PD-1+ CD8+ T cells compared to anti-CD3 stimulation alone at day 2, but not at day 7 (Figure 2B). The differences between mean frequency of PD-1+ CD8+ T cells at day 2 and day 0 were 28.0% for anti-CD3 versus 60.8% for combined anti-CD3 and IL-15 stimulation (P < 0.01). There was a marked decrease of PD-1+ CD8+ T cells (14.7%) at day 7 with anti-CD3 and IL-15 stimulation (Figure 2B and Supplementary Figure 1). For CD4+ T cells, we found a similar trend only at day 2, although the difference was not significant. The differences between mean frequency of PD-1+ CD4+ T cells at day 2 and day 0 were 28.3% for anti-CD3 versus 52.0% for combined anti-CD3 and IL-15 stimulation (Figure 2B and Supplementary Figure 1).
Interestingly, there was a trend towards lower induction of PD-1 expression with anti-CD3 and IL-10 stimulation as compared to anti-CD3 stimulation alone. The differences between mean frequency of PD-1+ CD8+ T cells at day 2 and day 0 were 28.0% for anti-CD3 versus 12.6% for combined anti-CD3 and IL-10 stimulation (P = n.s.). Between day 7 and day 0, the differences in mean frequency were 37.4% for anti-CD3 and 24.1% for combined anti-CD3 and IL-10 stimulation (P = n.s). We also found a trend towards lower induction of PD-1 expression with anti-CD3 and IL-10 stimulation compared to anti-CD3 stimulation alone in CD4+ T cells. The differences between mean frequency of PD-1+ CD4+ T cells at day 2 and day 0 were 28.3% for anti-CD3 versus 12.5% for combined anti-CD3 and IL-10 stimulation (P = n.s). Between day 7 and day 0, the differences in mean frequency were 50.6% for anti-CD3 and 15.3% for combined anti-CD3 and IL-10 stimulation (P < 0.01). Combination of anti-CD3 and other stimuli were not able to modulate PD-1 expression on both CD4+ and CD8+ T cells (Figure 2B).
TIM-3 expression on CD4+ and CD8+ T cells following in vitro cell stimulation
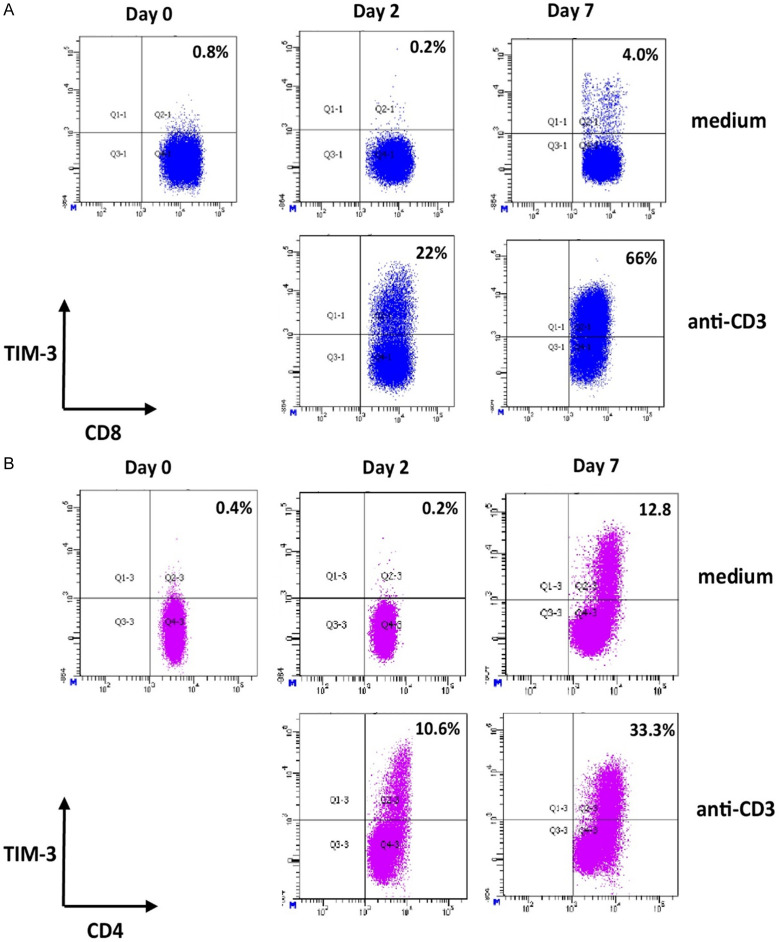
Representative figures to identify TIM-3+ expressing CD8+ and CD4+ T cells were shown in Figure 3A and 3B, respectively. In contrast to PD-1, the mean frequency of TIM-3+ expressing CD8+ T cells at baseline was very low (0.6%, ranging from 0.1% until 1.1%) (Figures 3A and 4A). Similar with PD-1 expression, only IL-15 stimulation was able to induce the expression of TIM-3 on CD8+ T cells (the mean frequencies of TIM-3+ CD8+ T cells were 0.4%; 14.9%; and 42.8% at day 0, 2, and 7 respectively; P < 0.05) (Figure 4A). For other stimuli, including IFN-α, IFN-γ, IL-10, TGF-β, and combination of IL-1β, IL-6 and TNF, no significant differences of TIM-3 inductions were observed (Figure 4A). Similarly, analysis on CD4+ T cell populations demonstrated that only IL-15 induced the expression of TIM-3 (Figure 4A).
Figure 3.
Identification of TIM-3-expressing CD4+ and CD8+ T cells. Representative dot plots of TIM-3 expression on CD8+ T cells (A) or CD4+ T cells (B) in medium condition and after stimulation of PBMCs with anti-CD3. CD3+ CD8+ T cells within the lymphocyte gate were analysed and frequency of TIM-3+ CD8+ T cells (A) or TIM-3+ CD4+ T cells (B) at day 2 and day 7 were compared with the frequency at baseline (day 0). The gating strategy to identify CD4+ and CD8+ T cells was similar as shown in Figure 1A. Values in the upper right quadrant indicate the percentage of TIM-3 positive cells within CD4+ or CD8+ T cells.
Figure 4.
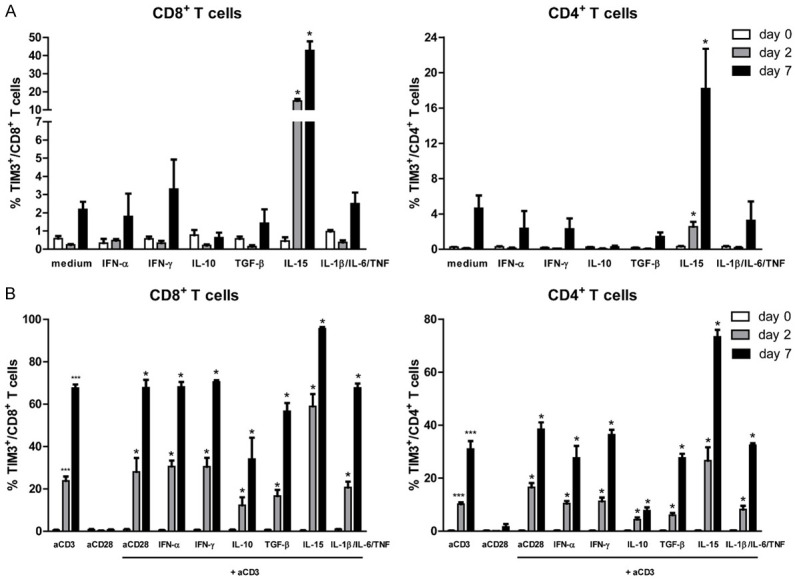
Regulation of TIM-3 expression on CD4+ and CD8+ T cells following various cytokine stimulations (A) and combination of cytokines and anti-CD3 stimulation (B). Data sets represent the mean and standard error of the mean (SEM) from eight (for medium and anti-CD3 stimulation) or three (for other stimuli) independent experiments. Comparison of the frequency of TIM-3+ CD8+ T cells (left panel) and TIM-3+ CD4+ T cells (right panel) between 0, 2 and 7 days after stimulations are shown. The p value is calculated in comparison with the frequency of positive cells at day 0 (* = P < 0.05; *** = P < 0.001).
We then combined those cytokines with anti-CD3 stimulation to examine the influence of those various cytokines during anti-CD3-mediated T cell activation [27]. Combination of anti-CD3 and IL-15 stimulation showed a trend towards increased up-regulation of TIM-3+ CD8+ T cells compared to anti-CD3 stimulation alone (Figure 4B). The differences between mean frequency of TIM-3+ CD8+ T cells at day 2 and day 0 were 23.2% for anti-CD3 stimulation versus 58.7% for combined anti-CD3 and IL-15 stimulation (P < 0.01). Between day 7 and day 0, the differences in mean frequency of TIM-3+ CD8+ T cells were 67.0% for anti-CD3 and 95.4% for the combination of anti-CD3 and IL-15 stimulation (P < 0.01) (Figure 4B and Supplementary Figure 1). Similar with PD-1 expression, there was a trend towards lower induction of TIM-3 expression with combination of anti-CD3 and IL-10 stimulation as compared to anti-CD3 stimulation alone. For combination of anti-CD3 and IL-10 stimulation, the difference between mean frequency of TIM-3+ CD8+ T cells at day 2 and day 0 was 11.7% (P < 0.05) and at day 7 and day 0 was 33.5% (P < 0.01) (Figure 4B and Supplementary Figure 1).
We obtained similar trends on TIM-3 induction CD4+ T cells following combined stimulation with anti-CD3. We found that combination of anti-CD3 and IL-15 stimulation resulted in a trend towards increased frequency of TIM-3+ CD4+ T cells compared to anti-CD3 stimulation alone. The differences between mean frequency of TIM-3+ CD4+ T cells at day 2 and day 0 were 9.9% for anti-CD3 stimulation versus 26.3% for combined anti-CD3 and IL-15 stimulation (P < 0.01). Between day 7 and day 0, the differences in mean frequency of TIM-3+ CD4+ T cells were 30.8% for anti-CD3 and 73.2% for the combination of anti-CD3 and IL-15 stimulation (P < 0.01) (Figure 4B and Supplementary Figure 1). We also found a trend towards lower induction of TIM-3 expression with anti-CD3 and IL-10 stimulation compared to anti-CD3 stimulation alone. For combination of anti-CD3 and IL-10 stimulation, the difference between mean frequency of TIM-3+ CD4+ T cells at day 2 and day 0 was 4.2% (P < 0.01) and at day 7 and day 0 was 7.5% (P < 0.01) (Figure 4B and Supplementary Figure 1). Combination of anti-CD3 and other stimuli were not able to modulate TIM-3 expression on CD4+ T cells compared to anti-CD3 stimulation alone (Figure 4B).
Differential inductions of PD-1 and TIM-3 expression on CD4+ and CD8+ T cells
Since anti-CD3 and IL-15 stimulation induced the expression of PD-1 and TIM-3 expression on both CD4+ and CD8+ T cells, we determined whether any difference exists in their inductions levels. PD-1+ expression showed a comparable induction between CD4+ and CD8+ T cells at day 2 and at day 7 following anti-CD3 stimulation [the mean induction of PD-1+ after correction for the background in the medium condition were 31.9% and 33.5% at day 2 (P = n.s) and 44.4% and 41.0% at day 7 (P = n.s) for CD4+ and CD8+ T cells, respectively] (Figure 5A). A similar trend was observed with IL-15 stimulation at day 7, but the difference was not significant (Figure 5B). For combined anti-CD3 and IL-15 stimulation, there was a similar induction of PD-1 on CD4+ and CD8+ T cells at day 2. However, at day 7 post stimulation, the induction level was notably higher in CD4+ compared to CD8+ T cells. This was due to considerable downregulation of PD-1 on CD8+ T cells at day 7 [the mean induction of PD-1+ 43.3% and 14.7% at day 7 (P < 0.05) for CD4+ and CD8+ T cells, respectively] (Figure 5C).
Figure 5.
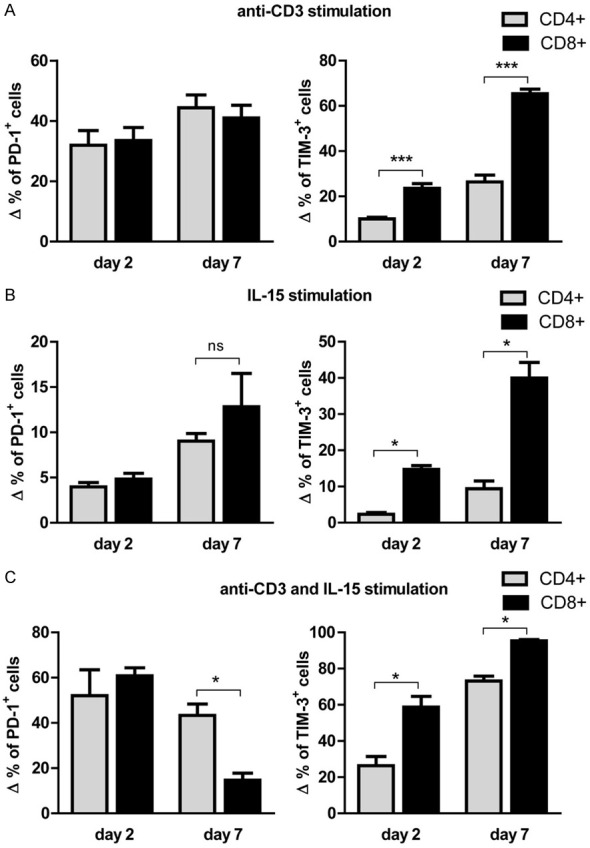
Differential induction of PD-1 and TIM-3 expression on CD4+ and CD8+ T cells following anti-CD3 and IL-15 stimulation. A. The Δ frequencies of PD-1+ (left panel) or TIM-3+ (right panel) of CD4+ and CD8+ T cells were calculated by subtracting medium condition from anti-CD3 stimulation at day 2 or day 7. B. The Δ frequencies of PD-1+ (left panel) or TIM-3+ (right panel) of CD4+ and CD8+ T cells were calculated by subtracting medium condition from IL-15 stimulation at day 2 or day 7. C. The Δ frequencies of PD-1+ (left panel) or TIM-3+ (right panel) of CD4+ and CD8+ T cells were calculated by subtracting day 0 condition from combined anti-CD3 and IL-15 stimulation at day 2 or day 7 (* = P < 0.05; *** = P < 0.001).
For TIM-3 induction, there was a significantly increased frequency of TIM-3+ on CD8+ T cells compared to CD4+ T cells both at day 2 and day 7 following stimulation with anti-CD3 [the mean frequencies of TIM-3+ after correction for the background in the medium condition were 10.0% and 23.5% at day 2 (P < 0.001) and 26.4% and 65.4% at day 7 (P < 0.001) for CD4+ and CD8+ T cells, respectively] (Figure 5A). Similar findings were observed with IL-15 stimulation and also combined anti-CD3 and IL-15 stimulation (Figure 5B and 5C).
Discussion
Understanding regulation of T cell function is highly important to delineate T cell response during viral infections as well as in cancer. These responses can be manipulated to develop immune-based therapy that has currently been used in cancer treatment. Our results demonstrated that of the separate cytokines studied, only IL-15 was found to induce expression of PD-1 and TIM-3 on T cells. For TIM-3 expression, this induction was more significant on CD8+ T cells than on CD4+ T cells. Our study, therefore, provide a basis for understanding T cell function by examining the regulation of inhibitory receptor expressions PD-1 and TIM-3.
In order to investigate the regulation of inhibitory receptors PD-1 and TIM-3, we selected several stimulation conditions for different reasons. IFN-α and IFN-γ have been reported to induce up-regulation of PD-1 or TIM-3 ligands (PD-L1 and galectin-9 [gal-9], respectively) [28-31]. In addition, IFN-α has been used in the clinic for chronic HBV and HCV therapy [2,32]. However, it is unknown whether IFN-α and IFN-γ can modulate PD-1 and TIM-3 expression. IL-10 and TGF-β are two inhibitory cytokines that negatively regulate T cell function in chronic HBV and HCV infections [33]. Whether these cytokines exert their suppressive effects in part by inducing inhibitory receptor expression on T cells have not been investigated yet. PD-1 and TIM-3 are highly expressed in acute hepatitis B and C patients. It is unknown whether pro-inflammatory cytokines induced during acute infection, such as IL-1β, IL-6 and TNF, can influence the expression of PD-1 and TIM-3. Since it is known that IL-15 induces T cell expansion [34], we were also interested to see whether IL-15 could up-regulate inhibitory receptor molecule expression as part of a negative feedback mechanism.
Understanding T cell immunity in chonic viral infections is highly important to comprehend pathogenesis of these high burden diseases [4]. Our study contributes to further understanding the regulation of T cell inhibitory receptors PD-1 and TIM-3. We demonstrate that PD-1 and TIM-3 can be up-regulated following polyclonal (anti-CD3) and cytokine (IL-15) stimulation, suggesting their role during T cell activation. Interestingly, co-stimulation through anti-CD28 was not able to induce expression of PD-1 and TIM-3. In addition, pro-inflammatory cytokines, such as IL-1β, IL-6 and TNF, which are normally enriched in acute inflammatory states, did not modulate PD-1 and TIM-3 expression.
It is hypothesized that induction of PD-1 and TIM-3 expression during T cell activation may be necessary to inhibit excessive activation of naïve T cells. Indeed, it has been shown that blockade of TIM-3 during in vitro CD4+ T cell stimulation with anti-CD3 and anti-CD28 increased the production of several cytokines, such as IL-2, IL-6, IL-17 and IFN-γ [35]. Two inhibitory cytokines investigated in our study, IL-10 and TGF-β, were unable to induce PD-1 and TIM-3 expression. However, combination of anti-CD3 and IL-10 stimulation reduced the mean frequency of PD-1+ and TIM-3+ T cells compared to anti-CD3 stimulation alone. This finding is consistent with the direct or indirect effects of IL-10 to inhibit T cell activation process [36]. Anti-viral cytokines IFN-α and IFN-γ had no effect on PD-1 and TIM-3 expression. Therefore, it seems that up-regulation of PD-L1 and gal-9 by IFN-α and IFN-γ is one of the mechanisms to prevent excessive host damage in inflamed tissues [28-31]. The effects of IFN-α and IFN-γ on the expression of other inhibitory receptors should be further investigated to determine whether negative feedback mechanisms may exist through upregulation of inhibitory receptors on T cells.
The only cytokine investigated in this study which was able to induce expression of PD-1 and TIM-3 is IL-15. IL-15 is a T cell growth factor required for proliferation and maintenance of naïve and memory T cell pools [37,38]. Mechanistically, IL-15 triggers multiple downstream signaling pathways resulting in decreased apoptosis and enhanced cell growth, activation, and migration of T cells and innate immune cells, including natural killer (NK) cells [39]. Here, IL-15 stimulation alone was sufficient to induce expression of PD-1 and TIM-3 on T cells. This observation could also be mediated by the effects of IL-15 on other innate immune cells which then influence T cells. This possibility was because of the use of PBMC in our study [39]. In addition, T cells stimulated with anti-CD3 and IL-15 together had higher PD-1 and TIM-3 expression compared to anti-CD3 stimulation alone, suggesting that these two pathways can function in a synergistic manner. It suggests that IL-15 may negatively modulate the survival of T cells by inducing PD-1 and TIM-3 expression since PD-1+ and TIM-3+ T cells are less functional in producing cytokines and are more susceptible to apoptosis [40,41]. Indeed, there was a significant decrease of PD-1+ CD8+ T cells at day 7 with anti-CD3 and IL-15 stimulation (Figure 2B and Supplementary Figure 1), which might be the result of excessive stimulation of T cells or apoptosis of PD-1 expressing cells at day 7.
The finding that antigen-independent stimulation through IL-2, IL-7 and IL-15 can induce PD-1 and TIM-3 expression [41,42] may partially explain why the frequency of PD-1+ and TIM-3+ is increased not only on virus-specific T cells but also on total T cell population in chronic HBV and HCV patients as compared to normal individuals [19,43-45]; since these cytokines may increase in chronic inflammatory state. IL-15 can be used as a vaccine adjuvant to improve immunity againts many pathogens [38]. IL-15 has also been tested to reactivate exhausted T cells during chronic simian immunodefiency virus (SIV) infection in macaques [46] and is considered as cytokine therapy in chronic HIV patients to boost HIV-specific T cells [37]. Considering the effect of IL-15 on PD-1 and TIM-3 induction and also the fact that exhausted HIV-specific T cells have increased expression of PD-1 and TIM-3 [47,48], it should be considered that this approach is preceded with blockade of inhibitory receptor molecules to more optimally restore T cell function [49].
Conclusions
One limitation of our study that we used PBMC collected from healthy individuals. Thus, findings of this study might be different in PBMC collected from chronically infected HBV and HCV patients. In conclusion, our study demonstrated that PD-1 and TIM-3 were induced via polyclonal (anti-CD3) and cytokine (IL-15) stimulation. We observed a significantly increased up-regulation of TIM-3 on CD8+ T cells compared to CD4+ T cells. Thus, our study contributes to further understanding the expression regulation of T cell exhaustion markers PD-1 and TIM-3.
Acknowledgements
M. S. H. is financially supported by the Directorate General of Higher Education, Ministry of Education and Culture, Indonesia (2011-2013).
Disclosure of conflict of interest
None.
Abbreviations
- CTLA-4
T-lymphocyte associated protein 4
- gal-9
galectin-9
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- HIV
human immunodeficiency virus
- IL
interleukin
- LCMV
lymphocytic choriomeningitis virus
- NK
natural killer
- NUC
nucleos(t)ide analogs
- PBMCs
peripheral blood mononuclear cells
- PD-1
programmed death 1
- PEG-IFN-α
pegylated interferon-α
- SIV
simian immunodefiency virus
- TGF-β
transforming growth factor β
- TIM-3
T cell immunoglobulin mucin domain-containing molecule 3
- Treg
regulatory T cells
Supporting Information
References
- 1.Sarpel D, Baichoo E, Dieterich DT. Chronic hepatitis B and C infection in the United States: a review of current guidelines, disease burden and cost effectiveness of screening. Expert Rev Anti Infect Ther. 2016;14:511–521. doi: 10.1586/14787210.2016.1174066. [DOI] [PubMed] [Google Scholar]
- 2.Tillmann HL, Samuel G. Current state-of-the-art pharmacotherapy for the management of hepatitis B infection. Expert Opin Pharmacother. 2019;20:873–885. doi: 10.1080/14656566.2019.1583744. [DOI] [PubMed] [Google Scholar]
- 3.Bertoletti A, Le Bert N. Immunotherapy for chronic hepatitis B virus infection. Gut Liver. 2018;12:497–507. doi: 10.5009/gnl17233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shin EC, Sung PS, Park SH. Immune responses and immunopathology in acute and chronic viral hepatitis. Nat Rev Immunol. 2016;16:509–523. doi: 10.1038/nri.2016.69. [DOI] [PubMed] [Google Scholar]
- 5.Peeridogaheh H, Meshkat Z, Habibzadeh S, Arzanlou M, Shahi JM, Rostami S, Gerayli S, Teimourpour R. Current concepts on immunopathogenesis of hepatitis B virus infection. Virus Res. 2018;245:29–43. doi: 10.1016/j.virusres.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 6.Boni C, Fisicaro P, Valdatta C, Amadei B, Di Vincenzo P, Giuberti T, Laccabue D, Zerbini A, Cavalli A, Missale G, Bertoletti A, Ferrari C. Characterization of hepatitis B virus (HBV)-specific T-cell dysfunction in chronic HBV infection. J Virol. 2007;81:4215–4225. doi: 10.1128/JVI.02844-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zajac AJ, Blattman JN, Murali-Krishna K, Sourdive DJ, Suresh M, Altman JD, Ahmed R. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med. 1998;188:2205–2213. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol. 2015;15:486–499. doi: 10.1038/nri3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raziorrouh B, Ulsenheimer A, Schraut W, Heeg M, Kurktschiev P, Zachoval R, Jung MC, Thimme R, Neumann-Haefelin C, Horster S, Wachtler M, Spannagl M, Haas J, Diepolder HM, Gruner NH. Inhibitory molecules that regulate expansion and restoration of HCV-specific CD4+ T cells in patients with chronic infection. Gastroenterology. 2011;141:1422–31. 1431, e1–6. doi: 10.1053/j.gastro.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 10.Kasprowicz V, Schulze Zur Wiesch J, Kuntzen T, Nolan BE, Longworth S, Berical A, Blum J, McMahon C, Reyor LL, Elias N, Kwok WW, McGovern BG, Freeman G, Chung RT, Klenerman P, Lewis-Ximenez L, Walker BD, Allen TM, Kim AY, Lauer GM. High level of PD-1 expression on hepatitis C virus (HCV)-specific CD8+ and CD4+ T cells during acute HCV infection, irrespective of clinical outcome. J Virol. 2008;82:3154–3160. doi: 10.1128/JVI.02474-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dong Y, Li X, Zhang L, Zhu Q, Chen C, Bao J, Chen Y. CD4(+) T cell exhaustion revealed by high PD-1 and LAG-3 expression and the loss of helper T cell function in chronic hepatitis B. BMC Immunol. 2019;20:27. doi: 10.1186/s12865-019-0309-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacobi FJ, Wild K, Smits M, Zoldan K, Csernalabics B, Flecken T, Lang J, Ehrenmann P, Emmerich F, Hofmann M, Thimme R, Neumann-Haefelin C, Boettler T. OX40 stimulation and PD-L1 blockade synergistically augment HBV-specific CD4 T cells in patients with HBeAg-negative infection. J Hepatol. 2019;70:1103–1113. doi: 10.1016/j.jhep.2019.02.016. [DOI] [PubMed] [Google Scholar]
- 13.Bengsch B, Seigel B, Ruhl M, Timm J, Kuntz M, Blum HE, Pircher H, Thimme R. Coexpression of PD-1, 2B4, CD160 and KLRG1 on exhausted HCV-specific CD8+ T cells is linked to antigen recognition and T cell differentiation. PLoS Pathog. 2010;6:e1000947. doi: 10.1371/journal.ppat.1000947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vali B, Jones RB, Sakhdari A, Sheth PM, Clayton K, Yue FY, Gyenes G, Wong D, Klein MB, Saeed S, Benko E, Kovacs C, Kaul R, Ostrowski MA. HCV-specific T cells in HCV/HIV co-infection show elevated frequencies of dual Tim-3/PD-1 expression that correlate with liver disease progression. Eur J Immunol. 2010;40:2493–2505. doi: 10.1002/eji.201040340. [DOI] [PubMed] [Google Scholar]
- 15.Fisicaro P, Valdatta C, Massari M, Loggi E, Ravanetti L, Urbani S, Giuberti T, Cavalli A, Vandelli C, Andreone P, Missale G, Ferrari C. Combined blockade of programmed death-1 and activation of CD137 increase responses of human liver T cells against HBV, but not HCV. Gastroenterology. 2012;143:1576–1585. e4. doi: 10.1053/j.gastro.2012.08.041. [DOI] [PubMed] [Google Scholar]
- 16.Kroy DC, Ciuffreda D, Cooperrider JH, Tomlinson M, Hauck GD, Aneja J, Berger C, Wolski D, Carrington M, Wherry EJ, Chung RT, Tanabe KK, Elias N, Freeman GJ, de Kruyff RH, Misdraji J, Kim AY, Lauer GM. Liver environment and HCV replication affect human T-cell phenotype and expression of inhibitory receptors. Gastroenterology. 2014;146:550–561. doi: 10.1053/j.gastro.2013.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Owusu Sekyere S, Suneetha PV, Kraft AR, Zhang S, Dietz J, Sarrazin C, Manns MP, Schlaphoff V, Cornberg M, Wedemeyer H. A heterogeneous hierarchy of co-regulatory receptors regulates exhaustion of HCV-specific CD8 T cells in patients with chronic hepatitis C. J Hepatol. 2015;62:31–40. doi: 10.1016/j.jhep.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 18.Nakamoto N, Cho H, Shaked A, Olthoff K, Valiga ME, Kaminski M, Gostick E, Price DA, Freeman GJ, Wherry EJ, Chang KM. Synergistic reversal of intrahepatic HCV-specific CD8 T cell exhaustion by combined PD-1/CTLA-4 blockade. PLoS Pathog. 2009;5:e1000313. doi: 10.1371/journal.ppat.1000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McMahan RH, Golden-Mason L, Nishimura MI, McMahon BJ, Kemper M, Allen TM, Gretch DR, Rosen HR. Tim-3 expression on PD-1+ HCV-specific human CTLs is associated with viral persistence, and its blockade restores hepatocyte-directed in vitro cytotoxicity. J Clin Invest. 2010;120:4546–4557. doi: 10.1172/JCI43127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sangro B, Gomez-Martin C, de la Mata M, Inarrairaegui M, Garralda E, Barrera P, Riezu-Boj JI, Larrea E, Alfaro C, Sarobe P, Lasarte JJ, Perez-Gracia JL, Melero I, Prieto J. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol. 2013;59:81–88. doi: 10.1016/j.jhep.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 21.Gardiner D, Lalezari J, Lawitz E, DiMicco M, Ghalib R, Reddy KR, Chang KM, Sulkowski M, Marro SO, Anderson J, He B, Kansra V, McPhee F, Wind-Rotolo M, Grasela D, Selby M, Korman AJ, Lowy I. A randomized, double-blind, placebo-controlled assessment of BMS-936558, a fully human monoclonal antibody to programmed death-1 (PD-1), in patients with chronic hepatitis C virus infection. PLoS One. 2013;8:e63818. doi: 10.1371/journal.pone.0063818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duffy AG, Ulahannan SV, Makorova-Rusher O, Rahma O, Wedemeyer H, Pratt D, Davis JL, Hughes MS, Heller T, ElGindi M, Uppala A, Korangy F, Kleiner DE, Figg WD, Venzon D, Steinberg SM, Venkatesan AM, Krishnasamy V, Abi-Jaoudeh N, Levy E, Wood BJ, Greten TF. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J Hepatol. 2017;66:545–551. doi: 10.1016/j.jhep.2016.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hakim MS, Spaan M, Janssen HL, Boonstra A. Inhibitory receptor molecules in chronic hepatitis B and C infections: novel targets for immunotherapy? Rev Med Virol. 2014;24:125–138. doi: 10.1002/rmv.1779. [DOI] [PubMed] [Google Scholar]
- 24.Boeijen LL, Hoogeveen RC, Boonstra A, Lauer GM. Hepatitis B virus infection and the immune response: the big questions. Best Pract Res Clin Gastroenterol. 2017;31:265–272. doi: 10.1016/j.bpg.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 25.Cho H, Kang H, Lee HH, Kim CW. Programmed cell death 1 (PD-1) and cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) in viral hepatitis. Int J Mol Sci. 2017;18:1517. doi: 10.3390/ijms18071517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ye B, Liu X, Li X, Kong H, Tian L, Chen Y. T-cell exhaustion in chronic hepatitis B infection: current knowledge and clinical significance. Cell Death Dis. 2015;6:e1694. doi: 10.1038/cddis.2015.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith-Garvin JE, Koretzky GA, Jordan MS. T cell activation. Annu Rev Immunol. 2009;27:591–619. doi: 10.1146/annurev.immunol.021908.132706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maier H, Isogawa M, Freeman GJ, Chisari FV. PD-1:PD-L1 interactions contribute to the functional suppression of virus-specific CD8+ T lymphocytes in the liver. J Immunol. 2007;178:2714–2720. doi: 10.4049/jimmunol.178.5.2714. [DOI] [PubMed] [Google Scholar]
- 29.Chen L, Zhang Z, Chen W, Zhang Z, Li Y, Shi M, Zhang J, Chen L, Wang S, Wang FS. B7-H1 up-regulation on myeloid dendritic cells significantly suppresses T cell immune function in patients with chronic hepatitis B. J Immunol. 2007;178:6634–6641. doi: 10.4049/jimmunol.178.10.6634. [DOI] [PubMed] [Google Scholar]
- 30.Mengshol JA, Golden-Mason L, Arikawa T, Smith M, Niki T, McWilliams R, Randall JA, McMahan R, Zimmerman MA, Rangachari M, Dobrinskikh E, Busson P, Polyak SJ, Hirashima M, Rosen HR. A crucial role for Kupffer cell-derived galectin-9 in regulation of T cell immunity in hepatitis C infection. PLoS One. 2010;5:e9504. doi: 10.1371/journal.pone.0009504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muhlbauer M, Fleck M, Schutz C, Weiss T, Froh M, Blank C, Scholmerich J, Hellerbrand C. PD-L1 is induced in hepatocytes by viral infection and by interferon-alpha and -gamma and mediates T cell apoptosis. J Hepatol. 2006;45:520–528. doi: 10.1016/j.jhep.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 32.European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C 2018. J Hepatol. 2018;69:461–511. doi: 10.1016/j.jhep.2018.03.026. [DOI] [PubMed] [Google Scholar]
- 33.Claassen MA, de Knegt RJ, Turgut D, Groothuismink ZM, Janssen HL, Boonstra A. Negative regulation of hepatitis C virus specific immunity is highly heterogeneous and modulated by pegylated interferon-alpha/ribavirin therapy. PLoS One. 2012;7:e49389. doi: 10.1371/journal.pone.0049389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zeng R, Spolski R, Finkelstein SE, Oh S, Kovanen PE, Hinrichs CS, Pise-Masison CA, Radonovich MF, Brady JN, Restifo NP, Berzofsky JA, Leonard WJ. Synergy of IL-21 and IL-15 in regulating CD8+ T cell expansion and function. J Exp Med. 2005;201:139–148. doi: 10.1084/jem.20041057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hastings WD, Anderson DE, Kassam N, Koguchi K, Greenfield EA, Kent SC, Zheng XX, Strom TB, Hafler DA, Kuchroo VK. TIM-3 is expressed on activated human CD4+ T cells and regulates Th1 and Th17 cytokines. Eur J Immunol. 2009;39:2492–2501. doi: 10.1002/eji.200939274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sabat R, Grutz G, Warszawska K, Kirsch S, Witte E, Wolk K, Geginat J. Biology of interleukin-10. Cytokine Growth Factor Rev. 2010;21:331–344. doi: 10.1016/j.cytogfr.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 37.Fehniger TA, Caligiuri MA. Interleukin 15: biology and relevance to human disease. Blood. 2001;97:14–32. doi: 10.1182/blood.v97.1.14. [DOI] [PubMed] [Google Scholar]
- 38.Patidar M, Yadav N, Dalai SK. Interleukin 15: a key cytokine for immunotherapy. Cytokine Growth Factor Rev. 2016;31:49–59. doi: 10.1016/j.cytogfr.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 39.Robinson TO, Schluns KS. The potential and promise of IL-15 in immuno-oncogenic therapies. Immunol Lett. 2017;190:159–168. doi: 10.1016/j.imlet.2017.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Petrovas C, Casazza JP, Brenchley JM, Price DA, Gostick E, Adams WC, Precopio ML, Schacker T, Roederer M, Douek DC, Koup RA. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J Exp Med. 2006;203:2281–2292. doi: 10.1084/jem.20061496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mujib S, Jones RB, Lo C, Aidarus N, Clayton K, Sakhdari A, Benko E, Kovacs C, Ostrowski MA. Antigen-independent induction of Tim-3 expression on human T cells by the common gamma-chain cytokines IL-2, IL-7, IL-15, and IL-21 is associated with proliferation and is dependent on the phosphoinositide 3-kinase pathway. J Immunol. 2012;188:3745–3756. doi: 10.4049/jimmunol.1102609. [DOI] [PubMed] [Google Scholar]
- 42.Kinter AL, Godbout EJ, McNally JP, Sereti I, Roby GA, O’Shea MA, Fauci AS. The common gamma-chain cytokines IL-2, IL-7, IL-15, and IL-21 induce the expression of programmed death-1 and its ligands. J Immunol. 2008;181:6738–6746. doi: 10.4049/jimmunol.181.10.6738. [DOI] [PubMed] [Google Scholar]
- 43.Nebbia G, Peppa D, Schurich A, Khanna P, Singh HD, Cheng Y, Rosenberg W, Dusheiko G, Gilson R, ChinAleong J, Kennedy P, Maini MK. Upregulation of the Tim-3/galectin-9 pathway of T cell exhaustion in chronic hepatitis B virus infection. PLoS One. 2012;7:e47648. doi: 10.1371/journal.pone.0047648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Golden-Mason L, Palmer B, Klarquist J, Mengshol JA, Castelblanco N, Rosen HR. Upregulation of PD-1 expression on circulating and intrahepatic hepatitis C virus-specific CD8+ T cells associated with reversible immune dysfunction. J Virol. 2007;81:9249–9258. doi: 10.1128/JVI.00409-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peng G, Li S, Wu W, Tan X, Chen Y, Chen Z. PD-1 upregulation is associated with HBV-specific T cell dysfunction in chronic hepatitis B patients. Mol Immunol. 2008;45:963–970. doi: 10.1016/j.molimm.2007.07.038. [DOI] [PubMed] [Google Scholar]
- 46.Picker LJ, Reed-Inderbitzin EF, Hagen SI, Edgar JB, Hansen SG, Legasse A, Planer S, Piatak M, Lifson JD, Maino VC, Axthelm MK, Villinger F. IL-15 induces CD4 effector memory T cell production and tissue emigration in nonhuman primates. J Clin Invest. 2006;116:1514–1524. doi: 10.1172/JCI27564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, Mncube Z, Duraiswamy J, Zhu B, Eichbaum Q, Altfeld M, Wherry EJ, Coovadia HM, Goulder PJ, Klenerman P, Ahmed R, Freeman GJ, Walker BD. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 48.Jones RB, Ndhlovu LC, Barbour JD, Sheth PM, Jha AR, Long BR, Wong JC, Satkunarajah M, Schweneker M, Chapman JM, Gyenes G, Vali B, Hyrcza MD, Yue FY, Kovacs C, Sassi A, Loutfy M, Halpenny R, Persad D, Spotts G, Hecht FM, Chun TW, McCune JM, Kaul R, Rini JM, Nixon DF, Ostrowski MA. Tim-3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV-1 infection. J Exp Med. 2008;205:2763–2779. doi: 10.1084/jem.20081398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ha SJ, West EE, Araki K, Smith KA, Ahmed R. Manipulating both the inhibitory and stimulatory immune system towards the success of therapeutic vaccination against chronic viral infections. Immunol Rev. 2008;223:317–333. doi: 10.1111/j.1600-065X.2008.00638.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.