 Four weeks of exposure to cigarette smoke aggravated the progression of diabetic kidney injury including inflammation, fibrosis, and oxidative stress.
Four weeks of exposure to cigarette smoke aggravated the progression of diabetic kidney injury including inflammation, fibrosis, and oxidative stress.
Abstract
Diabetic kidney disease (DKD) is the leading cause of end-stage kidney disease. Epidemiological studies have demonstrated that cigarette smoke or nicotine is a risk factor for the progression of chronic kidney injury. The present study analyzed the kidney toxicity of cigarette smoke in experimental rats with DKD. Experimental diabetes was induced in 7-week-old Sprague-Dawley rats by a single intraperitoneal injection of streptozotocin (60 mg kg–1). Four weeks after the induction of diabetes, rats were exposed to cigarette smoke (200 μg L–1), 4 h daily, and 5 days per week for 4 weeks. Cigarette smoke did not affect the levels of plasma glucose, hemoglobin A1c, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol or non-esterified fatty acids in both control and diabetic rats under the experimental conditions. Cigarette smoke, however, significantly increased diabetes-induced glomerular hypertrophy and urinary kidney injury molecule-1 (KIM-1) and neutrophil gelatinase-associated lipocalin (NGAL) excretion, suggesting exacerbation of diabetic kidney injury. Cigarette smoke promoted macrophage infiltration and fibrosis in the diabetic kidney. As expected, cigarette smoke increased oxidative stress in both control and diabetic rats. These data demonstrated that four weeks of exposure to cigarette smoke aggravated the progression of DKD in rats.
1. Introduction
Cigarette smoke is a major risk factor for cardiovascular diseases, pulmonary disorders, and cancers.1–3 A large number of epidemiological studies also demonstrated that cigarette smoke exacerbates the development and progression of chronic kidney disease (CKD).4–7 Smoking induces oxidative stress, inflammation, and lipid accumulation, which lead to endothelial dysfunction, upregulation of inflammatory mediators, and accumulation of advanced glycation end products.8 All these events result in thickening of glomerular basement membrane and mesangial expansion, which ultimately progress to glomerulosclerosis and interstitial fibrosis of the kidney.8,9
Diabetic kidney disease (DKD) is the leading cause of end-stage kidney disease.10 Chronic hyperglycemia exacerbates the progression of kidney disease.11,12 Cigarette smoke impairs glucose regulation in patients with type 1 and 2 diabetes.13,14 Smokers with type 2 diabetes exhibited increased blood glucose concentrations compared with nonsmokers.13 Cigarette smoke also increased postprandial blood glucose levels in diabetic patients.15 Heavy smoking (≥20 cigarettes per day, at least 1 year) significantly increased not only plasma hemoglobin A1c (HbA1c) and triglyceride (TG) levels but also proteinuria leading to progression of kidney injury in patients with type 1 diabetes.14
Mechanistically, cigarette smoke accelerates oxidative stress, which is an important risk factor for the initiation and progression of DKD.16 Cigarette smoke contains more than 4000 different chemicals17 and produces harmful gases, which enhance reactive oxygen species (ROS) generation. In addition, cigarette smoke stimulates NADPH oxidases (Noxs) to generate ROS,18 which leads to inflammation and endothelial dysfunction.19,20 Noxs are major sources of ROS in the kidney as well as other organs.21 Nox4 expression and activity were increased in diabetic kidneys.22–24 Nicotine exposure further increased Nox4 expression in the kidney of db/db mice, an experimental animal model of type 2 diabetes.25 Nox inhibitors effectively blocked nicotine-induced proliferation and fibronectin synthesis in human mesangial cells.26
While TGF-β is the major profibrotic cytokine produced in CKD including DKD,27 cigarette smoke or nicotine stimulates TGF-β expression and extracellular matrix (ECM) synthesis in atrial tissue, periodontal tissue, lung epithelial cells, and hepatic stellate cells.28–31 Tobacco consumption has been identified as a risk factor for atrial fibrosis.32
Studies investigating the role of nicotine may advance our understanding of reno-toxic mechanisms induced by exposure to electronic cigarettes as well as cigarette smoke. However, an experimental model elucidating the reno-toxic mechanism of cigarette smoke inhalation in DKD is needed. In this study, we developed an experimental model to investigate the kidney toxicity of cigarette smoke and demonstrated its role in the progression of DKD.
2. Materials & methods
2.1. Kentucky reference cigarettes
We purchased 3R4F reference cigarettes from the Center for Tobacco Reference Products of the University of Kentucky (Lexington, KY, USA). Cigarette smoke was generated by a JB2080 automatic 30-port cigarette smoking machine (CH Technologies, Westwood, NJ, USA) in conformity with ISO standard 3308 regimen: 35 mL puff volume, 2 s puff duration, 60 s puff interval, without blocking the vent. The cigarette smoke generated was diluted with filtered clean air to adjust for the total particulate matter (TPM) concentration and sent to the NITC-36 nose-only inhalation chamber (HCT, Icheon, Korea) housing the rats. The non-smoking group was exposed to filtered clean air instead of cigarette smoke.
2.2. Animals and experimental protocol
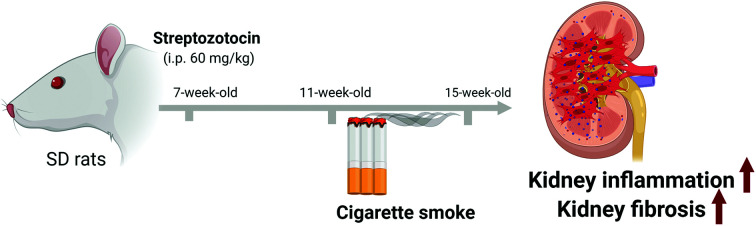
Sprague-Dawley rats (male, 6-week-old) were purchased from Orient Bio (Seongnam, Korea) and all the experimental exposures were conducted at Korea Conformity Laboratories (KCL, Incheon, Korea) following the approval of the Institutional Animal Care and Use Committee of KCL (IA 17-00342). The rats were housed in the laboratory animal facility and acclimated for a week before starting the experiments. The laboratory animal facility was maintained at a constant temperature and humidity with a 12 h light/dark cycle. Food and water were provided ad libitum. Animals were randomly divided into 4 groups: control group (n = 8), cigarette smoke-exposed group (CS control, n = 6), diabetes mellitus group (DM, n = 8), and cigarette smoke-exposed diabetes mellitus group (CS DM, n = 8). Experimental diabetes was induced in 7-week-old rats via a single intraperitoneal injection of streptozotocin (STZ, 60 mg kg–1) according to our previous study.33 STZ was dissolved in 0.1 M citrate buffer solution (pH 4.5) and kept on ice until ready for injection. Four weeks after the induction of diabetes, CS control rats and CS DM rats were exposed to cigarette smoke at a TPM concentration of 200 μg L–1, which was based on our preliminary study, for 4 hours daily, and 5 days per week for 4 weeks. All rats were euthanized at 8 weeks post-STZ or vehicle injection.
2.3. Measurement of blood parameters
Blood was collected from the abdominal aorta using 0.16% K3-EDTA as an anticoagulant. Blood samples were collected and centrifuged at 10 000g for 2 min to obtain the plasma. Parameters including plasma HbA1c, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), TG, non-esterified fatty acids (NEFA), and blood urea nitrogen (BUN) were analyzed using a 7180 clinical analyzer (Hitachi, Tokyo, Japan).
2.4. Measurement of urine parameters
Urine samples were collected in metabolic cages and centrifuged at 3000 rpm for 20 min. The levels of urinary kidney injury molecule-1 (KIM-1, R&D Systems, Minneapolis, MN, USA), neutrophil gelatinase-associated lipocalin (NGAL, Immunology Consultants Laboratory, Inc., Portland, OR, USA), and albumin excretion (ALPCO, Westlake, OH, USA) were determined using ELISA kits.
2.5. Histology and immunohistochemical (IHC) staining
Rat kidneys were fixed with 4% paraformaldehyde-lysine-periodate before dehydrating and embedding in paraffin. The 5 μm tissue sections were stained with periodic acid-Schiff (PAS, Abcam, Cambridge, MA, USA). The glomerular volume, tuft area, and fractional mesangial area (FMA) were determined based on an analysis of randomly selected 15–20 glomeruli obtained from each stained kidney section. To evaluate the collagen network in the kidney, specimens were stained with Masson's trichome (MT) staining kit (Sigma-Aldrich, St Louis, MO, USA). The IHC analysis was performed using anti-NGAL (1 : 400, Abcam, Cambridge, MA, USA), anti-collagen 1 (COL-1, 1 : 400, Southern Biotech, Birmingham, AL, USA), anti-cluster of differentiation 68 (CD68, 1 : 400; Santa Cruz Biotechnology Inc., CA, USA), anti-nitrotyrosine (NT, 1 : 200, Southern Biotech, Birmingham, AL, USA), and anti-8-hydroxy-2′-deoxyguanosine (8-oxo-dG,1 : 200, Trevigen, Gaithersburg, MD, USA) antibodies. Images were captured with a Zeiss microscope equipped with Axio Cam HRC digital camera and Axio Cam software (Carl Zeiss, Thornwood, NY, USA). Staining intensities were quantified using Image-Pro Plus 4.5 software (Cybernetics, Silver Spring, MD, USA).
2.6. Statistical analysis
All results were expressed as mean ± standard error (SE). ANOVA was used to assess the differences between multiple groups, followed by Fisher's LSD test. The level of statistical significance was set at a p-value less than 0.05.
3. Results
3.1. Physical and biochemical parameters
STZ-induced diabetic rats showed significantly reduced body weight, increased blood glucose, HbA1c, HDL-c, LDL-c, TG, and NEFA compared with age-matched control rats (Table 1). In rats with DM, kidney weight-to-body weight ratio, urine volume, and BUN were also significantly increased. Interestingly, exposure to cigarette smoke significantly decreased body weight of controls, but not diabetic rats. Cigarette smoke also reduced plasma TG in both control and diabetic rats. Other physical and biochemical parameters in control and diabetic rats were not affected by cigarette smoke.
Table 1. Physical and biochemical parameters of experimental animals.
| Control (n = 8) | CS control (n = 6) | DM (n = 8) | CS DM (n = 8) | |
| Body weight (g) | 524 ± 7 | 425 ± 13* | 246 ± 13* | 246 ± 16* |
| Blood glucose (mg dL–1) | 177 ± 5 | 147 ± 3 | 487 ± 24* | 483 ± 19* |
| HbA1c (%) | 5.4 ± 0.4 | 5.2 ± 0.4 | 11.5 ± 0.6* | 10.6 ± 0.5* |
| HDL-c (mg dL–1) | 22 ± 2 | 22 ± 1 | 34 ± 2* | 37 ± 3* |
| LDL-c (mg dL–1) | 6.6 ± 0.5 | 7.8 ± 0.6 | 10.7 ± 1.5* | 9.1 ± 1.2 |
| LDL-c/HDL-c | 0.3 ± 0.3 | 0.4 ± 0.0 | 0.3 ± 0.1 | 0.3 ± 0.1 |
| TG (mg dL–1) | 154 ± 14 | 69 ± 16* | 667 ± 97* | 420 ± 62*# |
| NEFA (μEq per L) | 195 ± 29 | 225 ± 38 | 391 ± 24* | 408 ± 33* |
| Kidney/body weight (10–3) | 3.0 ± 0.0 | 3.0 ± 0.1 | 6.0 ± 0.2* | 6.0 ± 0.2* |
| Urine volume (mL) | 3.7 ± 0.4 | 2.2 ± 0.5 | 7.6 ± 1.4* | 10.2 ± 2.0* |
| BUN (mg dL–1) | 14 ± 1 | 11 ± 0 | 25 ± 1* | 21 ± 2* |
3.2. Effect of cigarette smoke on kidney injury
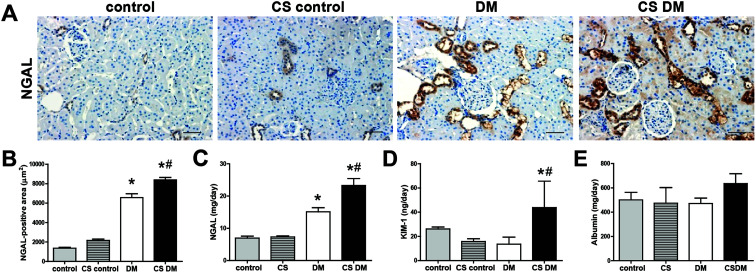
NGAL and KIM-1 are established markers of kidney tubular injury.34,35 The levels of kidney and urinary NGAL were significantly increased in DM compared with control rats (Fig. 1A–C). Urinary KIM-1 and albumin excretion, however, were not significantly increased in DM under our experimental conditions (Fig. 1D and E). Cigarette smoke significantly increased kidney NGAL expression and urinary NGAL and KIM-1 excretion in diabetic rats, but not in controls. Additionally, urinary albumin excretion tended to increase in CS DM compared with DM (p = 0.101), although it did not reach statistical significance.
Fig. 1. Effect of cigarette smoke on kidney injury. Paraffin-embedded kidney sections (5 μm-thick) were stained with NGAL antibody (A) and quantified as shown (B). Original magnification, 200×; scale bar, 50 μm. The levels of urinary NGAL (C), KIM-1 (D), and albumin (E) were measured using an ELISA kit. Data are expressed as mean ± SE of 6–8 rats per group. *p < 0.05 vs. control rats; #p < 0.05 vs. DM rats.
3.3. Effect of cigarette smoke on kidney morphology
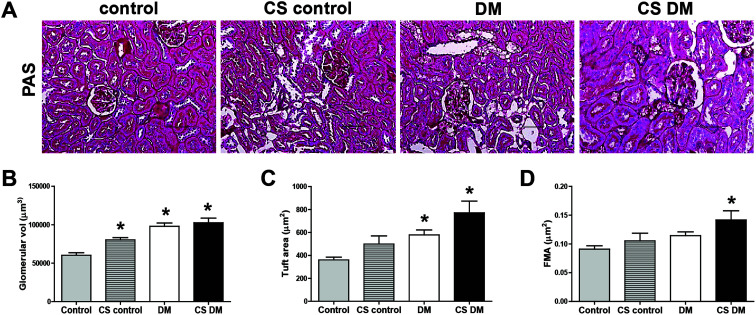
To determine the effect of cigarette smoke on kidney morphology, PAS staining was performed (Fig. 2A). Diabetes increased glomerular volume and tuft area compared with control (Fig. 2B and C). Cigarette smoke significantly increased glomerular volume in control rats. Cigarette smoke also tended to increase tuft area (p = 0.076) and FMA (p = 0.140) in diabetic rats. FMA was only significantly increased in CS DM among the 4 groups (Fig. 2D).
Fig. 2. Effect of cigarette smoke on kidney morphology. Kidney sections were stained with a PAS staining kit (A). Original magnification, 200×; scale bar, 50 μm. Glomerular volume (B), tuft area (C), and FMA (D) were analyzed using Image-Pro Plus 4.5.1. Data are expressed as mean ± SE of 6–8 rats per group. *p < 0.05 vs. control rats.
3.4. Effect of cigarette smoke on kidney inflammation and fibrosis
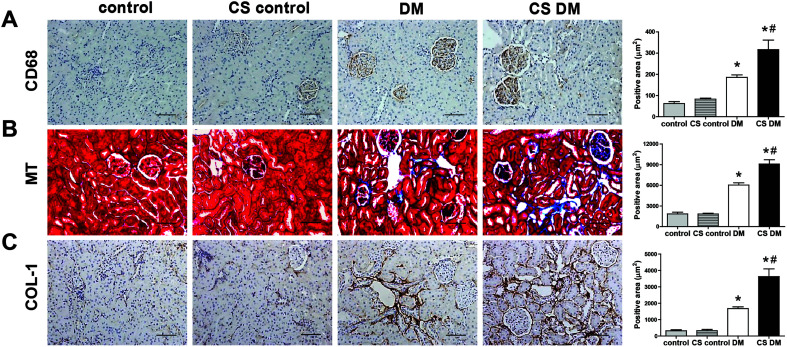
Next, we measured kidney inflammation and fibrosis by staining the respective marker proteins. Positive staining for CD68 and MT/COL-1 suggested increased macrophage infiltration and collagen accumulation, respectively, in diabetic kidney (Fig. 3A–C). Furthermore, cigarette smoke significantly aggravated macrophage infiltration and collagen accumulation in diabetic rats, but not in controls.
Fig. 3. Effect of cigarette smoke on kidney inflammation and fibrosis. Kidney sections were stained with CD68 antibody (A), MT (B), and COL-1 (C). Original magnification, 200×; scale bar, 50 μm. *p < 0.05 vs. control rats; #p < 0.05 vs. DM rats.
3.5. Effect of cigarette smoke on oxidative stress
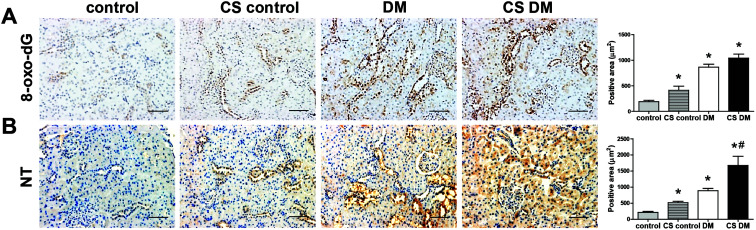
Oxidative stress plays a crucial role in the progression of DKD.16,21 As expected, diabetic kidney showed a significantly increased staining of 8-oxo-dG and NT (Fig. 4A and B). Cigarette smoke significantly increased 8-oxo-dG and NT staining in control kidney, and NT staining in diabetic kidney.
Fig. 4. Effect of cigarette smoke on oxidative stress. Kidney sections were stained with 8-oxo-dG antibody (A) and NT (B). Original magnification, 200×; scale bar, 50 μm. *p < 0.05 vs. control rats; #p < 0.05 vs. DM rats.
4. Discussion
The present data showed that inhalation of cigarette smoke exacerbated kidney injury in STZ-induced diabetic rats. Cigarette smoke significantly increased diabetic kidney tubular injury, inflammation, fibrosis, and oxidative stress in rats. Our present data are consistent with previous clinical studies, which showed that cigarette smoke is a risk factor for the progression of kidney disease.4–7 In addition, cigarette smoke increased the kidney mesangial/glomerular area, and the expression of fibronectin, and TGF-β in db/db mice, regardless of its effect on control.36 Nicotine, a major component of cigarette smoke, also increased kidney glomerular volume, FMA, expression of fibronectin and Nox4 protein, NT accumulation, and albuminuria in db/db mice, but not in control db/m mice.25 Similarly, chronic nicotine exposure also increased the kidney expression of KIM-1 protein and ROS production in mice with ischemia-reperfusion (IR)-induced kidney injury but not in control.37
Short-term exposure to cigarette smoke did not significantly affect plasma glucose or lipid parameters, such as HbA1c, HDL-c, LDL-c and NEFA in both diabetic as well as control rats under the experimental conditions. Our study was consistent with other studies showing that blood glucose was not affected by cigarette smoke in high-fat diet (HFD)-fed mice.38 However, smokers with type 1 diabetes showed worse glycemic control and higher plasma TG levels in a clinical study.14 Species and the amount/duration of cigarette smoke may determine the effect of cigarette smoke on systemic metabolism.
Oxidative stress plays an important role in triggering diabetic microvascular complications including DKD.16,21 Cigarette smoke leads to redox imbalance. Nicotine increased oxidative stress via upregulation of pro-oxidative p66shc protein expression and downregulation of anti-oxidative MnSOD expression in HFD-fed mice kidneys.38 Nicotine significantly increased the protein level of Nox4,25 which is the major source of ROS in the kidney. Interestingly, non-selective Nox inhibitor, diphenyleneiodonium and apocynin, significantly decreased nicotine-induced human mesangial cell proliferation and fibronectin protein expression.26 VAS2870, a selective Nox inhibitor, also significantly decreased nicotine-induced ROS generation, and other antioxidants such as N-acetyl-l-cysteine and 2,2,6,6-tetramethylpiperidinyl-1-oxy, prevented nicotine-induced apoptosis in podocytes.39 In addition to nicotine, cigarette smoke contains tar, carbon monoxide, heavy metals, N-nitrosamines, polycyclic aromatic hydrocarbons, nitro compounds, and aldehydes.40 Exposure to heavy metals such as mercury, lead, and arsenic generates ROS and induces oxidative stress in the kidney.41 Tar and TPM of cigarette smoke contain high concentrations of free radicals, such as semiquinones and carbon-centered radicals, which also lead to oxidative stress.42 Additionally, nicotine-derived nitrosamine ketone, a tobacco-specific N-nitrosamine, increases oxidative DNA damage in lung and liver tissues of mice and rats.43,44
Cigarette smoke may accelerate the progression of DKD in part via non-neuronal nicotinic acetylcholine receptors (nAChR) α3, α4, α7, and β1, which are the major subtypes in the kidney.24,37,38 Treatment with nAChR α7-selective antagonist or non-selective nAChR antagonist inhibited nicotine-stimulated oxidative stress and apoptosis in podocytes39 or human proximal tubular cells.45 Nicotine-induced human mesangial cell proliferation was also blocked by α-bungarotoxin (nAChR α7 antagonist), α-lobeline (nAChR α4/β2 antagonist), and dihydro-β-erythroidine (nAChR α3/β2 and α4/β2 antagonist).26 Nicotine-activated nAChRα7 inhibited miR133 and miR590 expression in cultured atrial fibroblasts.28 The expression of miR133 and miR590 inhibits translation of TGF-β and TGFRII, leading to collagen accumulation. However, nicotine had an anti-inflammatory effect and prevented IR-induced kidney injury.46 Treatment with nAChR α3β4 agonist prevented diet-induced obesity and induced energy expenditure.47 Interestingly, cigarette smoke significantly decreased body weight in control, but not in diabetic, rats in the present study. Gene Expression Omnibus database (GSE30529 and GSE30528) analysis revealed lower levels of nAChR expression in the kidney tubules of DKD patients and higher nAChR expression in the glomeruli of DKD patients compared with healthy controls (Fig. S1†). A comprehensive study investigating the role of nAchR in cigarette smoke-induced DKD is needed.
The clinical relevance of cigarette smoke exposure cannot be quantified based on animal studies alone. Human smoking behavior differs from animal behavior analyzed in experimental studies due to the diversity of tobacco products available, the differences in individual smoking habits, as well as the amount and duration of smoking in human. In our preliminary study, we tested various concentrations of TPM (100, 200, 400, and 600 μg L–1) in rats under normal conditions for 4 weeks (unpublished data). Lethality was observed in rats exposed to 400 and 600 μg L–1 TPM, presumably due to CO intoxication. Therefore, 200 μg L–1 TPM represented the highest possible concentration that diabetic rats were exposed to for 4 weeks. The dose of 200 μg L–1 TPM was within the ranges generally used in a number of animal toxicology studies.48–52 Smoking a cigarette per day elevated plasma cotinine level to 10.9–19.3 ng mL–1 in human clinical studies.53–55 Based on the data, exposure to 200 μg L–1 TPM, which may result in 140 50 or 330 ng mL–1 (ref. 48) of plasma cotinine, corresponds to the consumption of approximately 7–30 cigarettes per day. Accordingly, the exposure level in this study did not exceed that of heavy smokers.
5. Conclusion
Cigarette smoke inhalation exacerbates diabetes-induced kidney injury including inflammation, fibrosis, and oxidative stress, although significant kidney toxicity of cigarette smoke is not detected in control rats. Our understanding of mechanisms underlying kidney toxicity following exposure to cigarette smoke may be further enhanced using the study protocol reported.
Conflicts of interest
There are no conflicts of interest to declare.
Supplementary Material
Acknowledgments
This study was supported by the Ministry of Food and Drug Safety [Grand No. 19183MFDS163]; and Korea Health Technology R&D project through Korea Health Industry Development Institute [Grand No. HI18C0695], Republic of Korea.
Footnotes
†Electronic supplementary information (ESI) available. See DOI: 10.1039/c9tx00201d
References
- Salahuddin S., Prabhakaran D., Roy A. Glob. Heart. 2012;7:113–120. doi: 10.1016/j.gheart.2012.05.003. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Minagawa S., Araya J., Sakamoto T., Hara H., Tsubouchi K., Hosaka Y., Ichikawa A., Saito N., Kadota T., Sato N., Kurita Y., Kobayashi K., Ito S., Utsumi H., Wakui H., Numata T., Kaneko Y., Mori S., Asano H., Yamashita M., Odaka M., Morikawa T., Nakayama K., Iwamoto T., Imai H., Kuwano K. Nat. Commun. 2019;10:3145–3158. doi: 10.1038/s41467-019-10991-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S., Jee S. H., Shin H. R., Park E. H., Shin A., Jung K. W., Hwang S. S., Cha E. S., Yun Y. H., Park S. K., Boniol M., Boffetta P. BMC Cancer. 2014;14:406. doi: 10.1186/1471-2407-14-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gombos P., Langer R. M., Korbely R., Varga M., Kaposi A., Dinya E., Müller V. Transplant. Proc. 2010;42:2357–2359. doi: 10.1016/j.transproceed.2010.05.143. [DOI] [PubMed] [Google Scholar]
- Orth S. R., Ritz E., Schrier R. W. Kidney Int. 1997;51:1669–1677. doi: 10.1038/ki.1997.232. [DOI] [PubMed] [Google Scholar]
- Orth S. R., Stöckmann A., Conradt C., Ritz E., Ferro M., Kreusser W., Piccoli G., Rambausek M., Roccatello D., Schäfer K., Sieberth H. G., Wanner C., Watschinger B., Zucchelli P. Kidney Int. 1998;54:926–931. doi: 10.1046/j.1523-1755.1998.00067.x. [DOI] [PubMed] [Google Scholar]
- Orth S. R. Tob. Induced Dis. 2002;1:137–155. doi: 10.1186/1617-9625-1-2-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakkarwar V. A. World J. Diabetes. 2012;3:186–195. doi: 10.4239/wjd.v3.i12.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sliwińska-Mossoń M., Milnerowicz H. Diabetes Vasc. Dis. Res. 2017;14:265–276. doi: 10.1177/1479164117701876. [DOI] [PubMed] [Google Scholar]
- Alicic R. Z., Rooney M. T., Tuttle K. R. Clin. J. Am. Soc. Nephrol. 2017;12:2032–2045. doi: 10.2215/CJN.11491116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UK Prospective Diabetes Study (UKPDS) Group Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- Wong M. G., Perkovic V., Chalmers J., Woodward M., Li Q., Cooper M. E., Hamet P., Harrap S., Heller S., MacMahon S., Mancia G., Marre M., Matthews D., Neal B., Poulter N., Rodgers A., Williams B., Zoungas S. Diabetes Care. 2016;39:694–700. doi: 10.2337/dc15-2322. [DOI] [PubMed] [Google Scholar]
- Chidozie N. J., Okorie E. A., Chima O. E., Sally N. O. I., Amadi A. N., Dozie I. N. S., Iwuji S. C., Nwaokoro A. A. Asian J. Med. Sci. 2014;5:63–71. [Google Scholar]
- Feodoroff M., Harjutsalo V., Forsblom C., Thorn L., Wadén J., Tolonen N., Lithovius R., Groop P. H. Acta Diabetol. 2016;53:525–533. doi: 10.1007/s00592-015-0822-0. [DOI] [PubMed] [Google Scholar]
- Sari M. I., Sari N., Darlan D. M., Prasetya R. J. Open Access Maced. J. Med. Sci. 2018;6:634–637. doi: 10.3889/oamjms.2018.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha H., Hwang I. A., Park J. H., Lee H. B. Diabetes Res. Clin. Pract. 2008;82:42–45. doi: 10.1016/j.diabres.2008.09.017. [DOI] [PubMed] [Google Scholar]
- Roemer E., Stabbert R., Rustemeier K., Veltel D. J., Meisgen T. J., Reininghaus W., Carchman R. A., Gaworski C. L., Podraza K. F. Toxicology. 2004;195:31–52. doi: 10.1016/j.tox.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Yao H., Yang S.-R., Kode A., Rajendrasozhan S., Caito S., Adenuga D., Henry R., Edirisinghe I., Rahman I. Biochem. Soc. Trans. 2007;35:1151–1155. doi: 10.1042/BST0351151. [DOI] [PubMed] [Google Scholar]
- Chang K. H., Park J. M., Lee C. H., Kim B., Choi K. C., Choi S. J., Lee K., Lee M. Y. Toxicol. In Vitro. 2017;38:49–58. doi: 10.1016/j.tiv.2016.10.013. [DOI] [PubMed] [Google Scholar]
- Kim M., Han C. H., Lee M. Y. Toxicol. Res. 2014;30:149–157. doi: 10.5487/TR.2014.30.3.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha J. C., Banal C., Chow B. S. M., Cooper M. E., Jandeleit-Dahm K. Antioxid. Redox Signaling. 2016;25:657–684. doi: 10.1089/ars.2016.6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorin Y., Block K., Hernandez J., Bhandari B., Wagner B., Barnes J. L., Abboud H. E. J. Biol. Chem. 2005;280:39616–39626. doi: 10.1074/jbc.M502412200. [DOI] [PubMed] [Google Scholar]
- Sedeek M., Callera G., Montezano A., Gutsol A., Heitz F., Szyndralewiez C., Page P., Kennedy C. R. J., Burns K. D., Touyz R. M., Hébert R. L. Am. J. Physiol.: Renal Physiol. 2010;299:1348–1358. doi: 10.1152/ajprenal.00028.2010. [DOI] [PubMed] [Google Scholar]
- Li X., Cai W., Lee K., Liu B., Deng Y., Chen Y., Zhang X., He J. C., Zhong Y. Sci. Rep. 2018;8:4294–4304. doi: 10.1038/s41598-018-22371-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua P., Feng W., Ji S., Raij L., Jaimes E. A. Am. J. Physiol.: Renal Physiol. 2010;299:732–739. doi: 10.1152/ajprenal.00293.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaimes E. A., Tian R. X., Raij L. Am. J. Physiol.: Heart Circ. Physiol. 2007;292:76–82. doi: 10.1152/ajpheart.00693.2006. [DOI] [PubMed] [Google Scholar]
- Böttinger E. P., Bitzer M. J. Am. Soc. Nephrol. 2002;13:2600–2610. doi: 10.1097/01.asn.0000033611.79556.ae. [DOI] [PubMed] [Google Scholar]
- Shan H., Zhang Y., Lu Y., Zhang Y., Pan Z., Cai B., Wang N., Li X., Feng T., Hong Y., Yang B. Cardiovasc. Res. 2009;83:465–472. doi: 10.1093/cvr/cvp130. [DOI] [PubMed] [Google Scholar]
- Ebrahimpour A., Shrestha S., Bonnen M. D., Eissa N. T., Raghu G., Ghebre Y. T. J. Pharmacol. Exp. Ther. 2019;368:169–178. doi: 10.1124/jpet.118.252650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeda J., Morgan M., McKee C., Mouralidarane A., Lin C. I., Roskams T., Oben J. A. Biochem. Biophys. Res. Commun. 2012;417:17–22. doi: 10.1016/j.bbrc.2011.10.151. [DOI] [PubMed] [Google Scholar]
- Takeuchi-Igarashi H., Kubota S., Tachibana T., Murakashi E., Takigawa M., Okabe M., Numabe Y. Odontology. 2016;104:35–43. doi: 10.1007/s10266-014-0177-y. [DOI] [PubMed] [Google Scholar]
- Goette A., Lendeckel U., Kuchenbecker A., Bukowska A., Peters B., Klein H. U., Huth C., Röcken C. Heart. 2007;93:1056–1063. doi: 10.1136/hrt.2005.087171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E. A., Seo J. Y., Jiang Z., Yu M. R., Kwon M. K., Ha H., Lee H. B. Kidney Int. 2005;67:1762–1771. doi: 10.1111/j.1523-1755.2005.00274.x. [DOI] [PubMed] [Google Scholar]
- Han W. K., Bailly V., Abichandani R., Thadhani R., Bonventre J. V. Kidney Int. 2002;62:237–244. doi: 10.1046/j.1523-1755.2002.00433.x. [DOI] [PubMed] [Google Scholar]
- Devarajan P. Scand. J. Clin. Lab. Invest., Suppl. 2008;241:89–94. doi: 10.1080/00365510802150158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obert D. M., Hua P., Pilkerton M. E., Feng W., Jaimes E. A. Am. J. Med. Sci. 2011;341:126–130. doi: 10.1097/MAJ.0b013e3181f6e3bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arany I., Grifoni S., Clark J. S., Csongradi E., Maric C., Juncos L. A. Am. J. Physiol.: Renal Physiol. 2011;301:125–133. doi: 10.1152/ajprenal.00041.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arany I., Hall S., Reed D. K., Reed C. T., Dixit M. Nicotine Tob. Res. 2016;18:1628–1634. doi: 10.1093/ntr/ntw029. [DOI] [PubMed] [Google Scholar]
- Lan X., Lederman R., Eng J. M., Shoshtari S. S. M., Saleem M. A., Malhotra A., Singhal P. C. PLoS One. 2016;11:e0167071. doi: 10.1371/journal.pone.0167071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roemer E., Schramke H., Weiler H., Buettner A., Kausche S., Weber S., Berges A., Stueber M., Muench M., Trelles-Sticken E., Pype J., Kohlgrueber K., Voelkel H., Wittke S. Beitrage zur Tab. Int. Contrib. to Tob. Res. 2012;25:316–335. [Google Scholar]
- Salazar-Flores J., Torres-Jasso J. H., Rojas-Bravo D., Reyna-Villela Z. M., Torres-Sánchez E. D. J. Heavy Met. Toxic. Dis. 2019;4:1–16. [Google Scholar]
- Valavanidis A., Vlachogianni T., Fiotakis K. Int. J. Environ. Res. Public Health. 2009;6:445–462. doi: 10.3390/ijerph6020445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa J., Prokopczyk B., Desai D., Amin S., EI-Bayoumy K. Carcinogenesis. 1998;19:1783–1788. doi: 10.1093/carcin/19.10.1783. [DOI] [PubMed] [Google Scholar]
- Sipowicz M. A., Amin S., Desai D., Kasprzak K. S., Anderson L. M. Cancer Lett. 1997;117:87–91. doi: 10.1016/s0304-3835(97)00208-5. [DOI] [PubMed] [Google Scholar]
- Kim C. S., Choi J. S., Joo S. Y., Bae E. H., Ma S. K., Lee J. U., Kim S. W. PLoS One. 2016;11:e0152591. doi: 10.1371/journal.pone.0152591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeboah M. M., Xue X., Javdan M., Susin M., Metz C. N. Am. J. Physiol.: Renal Physiol. 2008;295:654–661. doi: 10.1152/ajprenal.90255.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemmensen C., Jall S., Kleinert M., Quarta C., Gruber T., Reber J., Sachs S., Fischer K., Feuchtinger A., Karlas A., Simonds S. E., Grandl G., Loher D., Sanchez-Quant E., Keipert S., Jastroch M., Hofmann S. M., Nascimento E. B. M., Schrauwen P., Ntziachristos V., Cowley M. A., Finan B., Müller T. D., Tschöp M. H. Nat. Commun. 2018;9:4304. doi: 10.1038/s41467-018-06769-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter C. A., Misra M. Toxicol. Pathol. 2010;38:402–415. doi: 10.1177/0192623310364028. [DOI] [PubMed] [Google Scholar]
- Carter C. A., Misra M., Maronpot R. R. J. Toxicol. Pathol. 2012;25:201–207. doi: 10.1293/tox.25.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaworski C. L., Dozier M. M., Gerhart J. M., Rajendran N., Brennecke L. H., Aranyi C., Heck J. D. Food Chem. Toxicol. 1997;35:683–692. doi: 10.1016/s0278-6915(97)00033-1. [DOI] [PubMed] [Google Scholar]
- Rithidech K., Chen B. T., Mauderly J. L., Whorton Jr. E. B., Brooks A. L. Environ. Mol. Mutagen. 1989;14:27–33. doi: 10.1002/em.2850140106. [DOI] [PubMed] [Google Scholar]
- Choi S. J., Lee S. H., Lee S. J., Yang M. J., Lee K. Mol. Cell. Toxicol. 2016;12:313–325. [Google Scholar]
- Pérez-Stable E. J., Herrera B., Jacob 3rd P., Benowitz N. L. JAMA, J. Am. Med. Assoc. 1998;280:152–156. doi: 10.1001/jama.280.2.152. [DOI] [PubMed] [Google Scholar]
- Ahijevych K., Szalacha L. A., Tan A. Nicotine Tob. Res. 2019;21:1189–1197. doi: 10.1093/ntr/nty170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz N. L., Dains K. M., Dempsey D., Wilson M., Jacob P. Nicotine Tob. Res. 2011;13:772–783. doi: 10.1093/ntr/ntr072. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.