Abstract
Objectives
To describe the clinical characteristics and predictors of major outcomes in patients treated with tocilizumab (TCZ) for severe COVID-19 pneumonia.
Patients and methods
Case series of all sequential patients with severe COVID-19 pneumonia treated with TCZ at an Academic Spanish hospital (March 12 - May 2, 2020). Clinical outcomes: death, length of hospital stay. An early clinical response to TCZ (48-72 h after the administration) was assessed by variations in respiratory function markers, Brescia COVID Respiratory Severity Scale (BCRSS), inflammatory parameters, and patients' and physicians' opinion. Associations were tested by multiple logistic regression.
Results
From a cohort of 236 patients, 77 patients treated with TCZ were included (median age 62 years (IQR 53.0–72.0), 64.9% were males), 42.9% had Charlson index ≥3; hypertension (41.6%), obesity (34.7%), and diabetes (20.8%).
Median follow-up was 83.0 days (78.0–86.5), no patient was readmitted. ICU admission was required for 42 (54.5%), invasive mechanical ventilation in 38 (49.4%) and 10 patients died (12.9% global, 23.8% at ICU admitted). After multivariate adjustment, TCZ response by BCRSS (OR 0.03 (0.01–0.68), p = 0.028), and Charlson index (OR 3.54 (1.20–10.44), p = 0.022) has been identified as independent factors associated with mortality. Median of hospital stay was 16.0 days (11.0–23.0); BCRSS, physician subjective and D-dimer response were associated with shorter hospitalization stay.
Conclusions
In a Mediterranean cohort, use of tocilizumab for severe COVID-19 show 12.9% of mortality. Early TCZ-response by BCRSS and low comorbidity were associated with increased survival. Early TCZ-response was related to shorter median hospital stay.
Keywords: COVID19 pneumonia, Tocilizumab, Mortality, Mechanical invasive ventilation, Case series
Highlights
-
•
In severe COVID-19 pneumonia, use of tocilizumab (TCZ) was associated with low mortality, and no major safety concerns.
-
•
Early TCZ-response by BCRSS and low comorbidity were associated with increased survival.
-
•
An early tocilizumab response was related to shorter median hospital stay.
1. Introduction
Given the paucity of evidence to guide Coronavirus disease 2019 (COVID-19) treatment, clinical trials are urgently needed and in the meanwhile, to exploit available observational data can be crucial [1].
In COVID-19, significant lung damage is thought to be caused by an exaggerated immune response (a cytokine storm) that can progress to cardiovascular collapse, multiple organ dysfunction and death [2]. Here, interleukin-6 (IL-6) likely plays a relevant role, so IL-6 blocking agents, such as tocilizumab (TCZ), could theoretically dampen the inflammatory cascade and improve clinical outcomes [3]. Although data in patients with COVID-19 remains very limited and conflicting, the Spanish Health Ministry (SHM) authorized and funded the emergency use of TCZ owing to the severe epidemic involving our country.
This study aims to describe the initial clinical experience with TCZ in patients with severe COVID-19 pneumonia and to determine the predictors of major outcomes in the follow-up.
2. Material and methods
2.1. Patients and study design
Retrospective cohort of the 306 patients admitted for COVID-19 pneumonia in a tertiary center from March 12 to May 2, 2020. Eighty-two (26.7%) patients were treated with TCZ according to the local protocol. The study population was categorized into two groups: maximum care (intensive care unit (ICU) and intubation as needed) and patients with limited therapeutic effort (LTE). The attending team agreed with the families the suitable approach for each individual, considering patients and disease characteristics (age, comorbidities, frailty, short pre-admission life expectancy, and extremely severe or advanced irreversible disease) and registered it in records. Antivirals, anti-inflammatories, and non-invasive ventilation were administered according to individual assessment. For this study, only maximum care population, 236 patients (77 with TCZ), was included in the analysis.
Antiviral treatment included hydroxychloroquine, lopinavir/ritonavir, and azithromycin. The COVID-19 clinical-team advised TCZ in case of rapid progression of respiratory failure, severe systemic inflammatory response (IL-6 >40 pg/mL, ferritin >1000 mg/L, C-reactive protein >5 mg/dL, lymphocytes <900 cells/mm3, lactate dehydrogenase >300U/L and/or D-Dimers >500 μg/mL) or significant radiological progression. TCZ dose: initial 600 mg, with a second or third dose (400 mg) in case of persistent or progressive disease; from March 30 onward, the SHM restricted TCZ to a single dose. If unsuccessful, individualized subsequently treatment with corticosteroids.
2.2. Variables and data collection
Data were extracted from electronic medical records of admissions and follow-up after discharge by phone calls.
2.2.1. Explanatory variables
The main explanatory variable of the present analysis was the early response to TCZ. However, no tools to assess response to TCZ in COVID-19 are available, as well as a proven timing to consider it. However, in COVID-19, reported median time from dyspnea onset to ARDS development is around 2.5 days [4]. Accordingly, the period up to 72 h after infusion was established the timing of interest by the authors. Similarly, clinical response to TCZ was defined as the absence of progression or improvement in respiratory function (response was defined as lower oxygen support requirements, and/or breathing rate), inflammatory parameters (response was reductions in two or more of C-reactive protein, ferritin, lactate dehydrogenase and D-dimers), as well as referred by the patient and the responsible physician, according to clinical records. Combinations of respiratory and inflammatory response (either or both of them) were also explored. Moreover, Brescia COVID Respiratory Severity Scale (BCRSS) [5] was also applied; a reduction or no changes in the score 72 h after TCZ infusion defined the positive response.
2.2.2. Outcomes
1) All-cause mortality (either in-hospital or after discharge) and associated factors. 2) The impact of an early clinical response to TCZ in hospital and ICU stay. 3) Evaluate safety of TCZ therapy.
Cause of death were registered
2.3. Statistical analysis
Categorical and continuous variables are given as frequencies (percentages) and as median (interquartile range), respectively. Patients treated and not treated with TCZ were compared by Mann-Whitney's U, chi-squared and Fisher's exact tests.
For logistic regression, for the following variables, standard categorizations were followed: estimated glomerular filtration rate <60 ml/min/1.73 m2 (by CKD-EPI formula), oximetry <94% [6], CURB65 Severity Score for Community-Acquired Pneumonia ≥3 [7], systolic and diastolic blood pressures <100 and 60 mmHg, respectively, heart rate >100bpm and respiratory rate >24 rpm.
Cumulative incidences of outcomes for each explanatory variable were registered. The final date of follow up was June 9, 2020, unless censored. Associations were evaluated by chi-squared test. Multiple logistic regression models were built to explore risk factors at presentation associated with subsequent mortality; odds ratios (OR) with 95% confidence intervals (95%CI) were estimated. Variables were included as covariates if shown associations in simple models with p-values below 0.100. The model was considered valid if the patients not included, due to losses in the collection of variables, were less than 20% of the study population. Some covariates could be excluded in case of been highly correlated, >20% of missing values or number of events was too small to calculate odds ratios. IBM SPSS Statistics v25 (Armonk, NY) was used for analyses. P < 0.050 defined statistical significance.
HGUA-ISABIAL ethics committee approved the study (expedient no. 200145); being a retrospective study, informed consent from patients was not required.
3. Results
3.1. General characteristics of the study population
Seventy-seven patients (32.6% of the maximum care cohort with severe pneumonia) were treated with TCZ with a median age of 62 years (53.0–72.0), 64.9% were males and 42.9% had a Charlson index≥ 3. Table 1 shows the general comparative characteristics of TCZ and non-TCZ subpopulations. These subpopulations were similar in age, gender, comorbidities, and usual care (hydroxychloroquine 98.7% vs 92.5%, plus azithromycin 76.6% vs 70.3%, respectively). However, TCZ-subpopulation clearly differed at admission in fever and dyspnea as clinical presentation features, worse respiratory function, higher inflammatory parameters, more frequent extensive lung opacities, and lower lymphocytes count, all indicators of a more severe disease. It is not surprising therefore, the lower mortality rate (3/159, 1.9%) and shorter length of hospitalization stay (5 days (7–9)) (both p = 0.0001), in non-TCZ against TCZ subpopulation (see below).
Table 1.
Comparison of general characteristics of the study population according to having been treated with TCZ.
| Population without TCZ [n = 159] | TCZ subpopulation [n = 77] | p | |
|---|---|---|---|
| Demographics | |||
| Age (median), years | 57.0 (44.0–70.0) | 62.0 (53.0–72.0) | .061 |
| Males, % | 57.2 (91/159) | 64.9 (50/77) | .258 |
| Nosocomial, % | 3.8 (6/159) | 2.6 (2/76) | .652 |
| Long-term care resident, % | 0.6 (1/159) | 0.0 (0/77) | .486 |
| Health professional, % | 11.9 (19/159) | 11.7 (9/77) | .954 |
| Comorbidities | |||
| Hypertension, % | 40.3 (64/159) | 41.6 (32/77) | .848 |
| Diabetes, % | 16.4 (26/159) | 20.8 (16/77) | .552 |
| Body mass index, kg/m2 | 27.6 (24.2–31.6) | 28.1 (25.0–32.2) | .348 |
| Obesity, % | 35.5 (54/152) | 34.7 (26/75) | .899 |
| Cardiovascular disease, % | 8.2 (13/159) | 7.8 (6/77) | .779 |
| Chronic respiratory disease, % | 16.5 (26/159) | 14.3 (11/77) | .736 |
| Immunosuppression, % | 10.1 (16/159) | 5.2 (4/77) | .203 |
| Charlson comorbidity index | 2 (0–4) | 2.0 (1.0–3.0) | .662 |
| Charlson index ≥3, % | 38.4 (61/159) | 42.9 (33/77) | .509 |
| 10-years expected survivala | 90.2 (53.4–98.3) | 90.0 (74.0–95.9) | .264 |
| Clinical Presentation | |||
| Clinical duration, daysb | 7.0 (3.0–9.0) | 7.0 (4.0–10.0) | .442 |
| Fever, % | 73.6 (117/159) | 90.9 (70/77) | .002 |
| Dry cough, % | 64.8 (103/159) | 68.4 (52/76) | .582 |
| Wet cough, % | 15.7 (25/159) | 20.8 (16/77) | .336 |
| Dyspnea, % | 44.3 (70/158) | 64.5 (49/76) | .004 |
| Diarrhoea, % | 25.6 (40/156) | 35.1 (27/77) | .135 |
| Confusion, % | 6.4 (10/156) | 6.6 (5/76) | 1.000 |
| Fatigue, % | 41.7 (63/151) | 54.7 (41/75) | .066 |
| Myalgias-arthralgias, % | 33.1 (51/154) | 32.0 (24/75) | .866 |
| Anosmia-dysgeusia, % | 15.1 (23/152) | 13.5 (10/74) | .746 |
| Initial Assessment | |||
| Oximetry at room air (%) | 96.0 (94.0–98.0) | 93.0 (89.0–95.3) | .000 |
| PaO2:FiO2, mm Hg | 361.9 (310.0–442.0) | 307.0 (269.8–341.3) | .000 |
| Respiratory rate, breaths/min | 16.0 (16.0–18.0) | 24.0 (17.0–32.0) | .000 |
| Systolic BP, mmHg | 132.0 (116.0–145.0) | 127.0 (111.0–144.0) | .509 |
| Diastolic BP, mmHg | 82.0 (73.0–91.0) | 74.0 (67.0–84.5) | .002 |
| Heart rate, beats/min | 96.0 (83.0–106.0) | 95.0 (87.0–107.0) | .959 |
| CURB65 | 0.0 (0.0–1.0) | 1.0 (0.0–2.0) | .004 |
| eGFR, ml/min/m2 | 89.4 (70.0–90.0) | 83.4 (68.9–90.0) | .326 |
| eGFR <60 ml/min/m2, % | 18.2 (29/159) | 14.7 (11/75) | .498 |
| Leukocytes, per mm3 | 6410.0 (5065.0–8402.5) | 6000.0 (4920.0–8290.0) | .445 |
| Lymphocytes, per mm3 | 1200.0 (860.0–1510.0) | 860.0 (625.0–1055.0) | .000 |
| C-reactive protein, mg/dL | 4.0 (1.7–8.5) | 11.0 (5.8–17.2) | .000 |
| Procalcitonin, ng/mL | 0.1 (0.1–0.2) | 0.1 (0.1–0.3) | .000 |
| Ferritin, mg/L | 551.0 (281.5–978.0) | 1220.5 (532.3–2263.0) | .000 |
| Lactate dehydrogenase, U/L | 241.0 (202.0–317.0) | 349.0 (275.8–441.0) | .000 |
| D-dimers, mg/mL | 0.6 (0.4–0.9) | 0.8 (0.5–1.2) | .008 |
| Interleukin 6, pg/mL | 18.0 (8.0–34.0) | 66.5 (31.3–158.0) | .000 |
| Troponin T, ng/L | 7.0 (5.0–14.3) | 10.0 (6.0–17.0) | .022 |
| Brain natriuretic peptide, pg/mL | 64.0 (23.0–191.0) | 104.0 (33.0–320.5) | .161 |
| Creatine phosphokinase, U/L | 79.0 (51.5–137.5) | 97.0 (64.0–152.5) | .064 |
| Aspartate aminotransferase, U/L | 30.0 (22.0–44.5) | 43.5 (29.5–76.3) | .000 |
| Alanine aminotransferase, U/L | 26.0 (16.0–43.0) | 34.5 (19.5–55.0) | .009 |
| Opacities >50% of lung surface on X-rays, % | 8.2 (13/159) | 34.2 (26/76) | <0.001 |
Data shown as % unless specified otherwise. In bold, statistically significant differences.
10-years expected survival derived from Charlson comorbidity index score.
Days of symptoms before admission. OR: odds ratio, 95%CI: 95% confidence interval.
In TCZ-subpopulation, at admission, 90.9% of patients were febrile, 68.4% had dyspnea, with a median (IQR) oximetry of 93.0% (89.0–95.3) at room air. In the chest X-rays, 34.2% showed opacities with involvement greater than 50% of the lung surface.
TCZ was administered a median of 10.0 days (7.5–12.0) after disease onset, and 2.0 days (1.0–4.0) after admission. Median of hospital stay was 16.0 days (11.0–23.0). At the time of analysis, two patients remained hospitalized (one at ICU), and cumulative follow-up was 83.0 days (78–86.5). No readmissions occurred in those treated with TCZ, and no patient was lost to follow-up.
Forty-two patients (54.5% of the TCZ-cohort) were admitted at ICU, median stay of 12.0 days (7.0-23.0); 38 (49.4%) required invasive mechanical ventilation (IMV) for a median of 8.0 days (6.0–15.0), 44.7% of them (17) along with the administration of TCZ. Two patients were transferred from other centers under IMV, so TCZ was administered afterwards.
One patient required extracorporeal membrane oxygenation support. In non-TCZ subpopulation six were admitted at ICU (3.8%), none required IMV.
Serious bacterial infections occurred in 14.2% of the cohort, 26.1% of ICU patients, 7 ventilation-associated pneumonia (VAP) and 7 catheter-related bacteremia (three patients suffered both). The incidence of VAP was 14.6 per 1.000 days of intubation. No fungal infection was diagnosed.
3.2. Mortality and associated factors
Overall mortality was 12.9% (10/77), occurring a median of 26 days (11.5–33.7) after symptoms onset, all at ICU and under invasive mechanical ventilation requirements. Kaplan-Meyer survival function is represented in Fig. S1 (supplementary material). Three were COVID-19 related, six VAP, and one due to a difficult intubation-related cardiac arrest. Timing of IMV showed no difference in regards of mortality (23.5% in early IMV versus 26.3% in late IMV, p = 1.000).
Table 2 shows the associations between mortality and clinical features at admission in the TCZ-subpopulation. Baseline predictors of mortality were PaO2:FiO2, CURB65 score ≥3 and LDH; whereas Charlson index, confusion, and D-dimer, were close to statistical significance, and were also included in the multivariate model.
Table 2.
Mortality outcome and predictors at admission.
| TCZ subpopulation (n = 77) | OR (95%CI) | p | |
|---|---|---|---|
| Demographics | |||
| Age | 1.04 (0.97–1.09) | .235 | |
| Gender | |||
| - Females | 2/27 (7.4) | 1.00 (ref) | – |
| - Males | 8/50 (16.0) | 2.38 (0.47–12.1) | .296 |
| Nosocomial case | |||
| - No | 9/75 (12.0) | 1.00 (ref) | - |
| - Yes | 1/2 (50.0) | 7.22 (0.41–125.87) | .175 |
| Long-term care resident | |||
| - No | 10/77(13.0) | NC | – |
| - Yes | 0/0 (0.0) | ||
| Health professional | |||
| - No | 10/68 (14.7) | NC | – |
| - Yes | 0/9 (0.0) | ||
| Comorbidities | |||
| Hypertension | |||
| - No | 5/45 (11.1) | 1.00 (ref) | - |
| - Yes | 5/32 (15.6) | 1.48 (0.39–5.61) | .563 |
| Diabetes | |||
| - No | 6/61 (9.8) | 1.00 (ref) | - |
| - Yes | 4/16 (25.0) | 3.06 (0.75–12.53) | .121 |
| Obesity | |||
| - No | 6/49 (12.2) | 1.00 (ref) | - |
| - Yes | 3/26 (11.5) | 0.94 (0.21–4.09) | .929 |
| Cardiovascular disease | |||
| - No | 6/71 (8.4) | 1.00 (ref) | - |
| - Yes | 2/6 (33.3) | 4.17 (0.62–27.77) | .140 |
| Chronic respiratory disease | |||
| - No | 9/66 (13.6) | 1.00 (ref) | - |
| - Yes | 1/11 (9.1%) | 0.61 (0.07–5.37) | .657 |
| Immunosuppression | |||
| - No | 10/73 (13.7) | NC | - |
| - Yes | 0/4 (0.0) | – | |
| Charlson index | 1.42 (0.99–2.04) | .052 | |
| - <3 | 4/44 (9.1) | 1.00 (ref) | - |
| - ≥3 | 6/33 (18.2) | 2.22 (0.57–8.62) | .248 |
| 10-years expected survivala | |||
| - ≥90% | 3/38 (7.9) | 1.00 (ref) | - |
| - <90% | 6/36 (16.7) | 2.33 (0.54–10.14) | .258 |
| Clinical Presentation | |||
| Clinical durationb | |||
| - ≥7 | 4/40 (10.0) | 1.00 (ref) | - |
| - <7 | 6/37 (16.2) | 0.57 (0.15–2.22) | .421 |
| Fever | |||
| - No | 0/7 (0) | NC | - |
| - Yes | 10/70 (14.3) | – | |
| Dry cough | |||
| - No | 1/25 (4.0) | 1.00 (ref) | - |
| - Yes | 9/52 (17.3) | 4.81 (0.57–40.39) | .148 |
| Wet cough | |||
| - No | 8/61 (13.1) | 1.00 (ref) | - |
| - Yes | 2/16 (12.5) | 0.95 (0.18–4.96) | .948 |
| Dyspnea | |||
| - No | 3/28 (10.7) | 1.00 (ref) | - |
| - Yes | 7/49 (14.3) | 1.33 (0.32–5.64) | .696 |
| Diarrhoea | |||
| - No | 7/50 (14.0) | 1.00 (ref) | - |
| - Yes | 3/27 (11.1) | 0.77 (0.18–3.25) | .720 |
| Confusion | |||
| - No | 8/72 (11.1) | 1.00 (ref) | - |
| - Yes | 2/5 (40.0) | 5.25 (0.76–36.33) | .093 |
| Fatigue | |||
| - No | 3/34 (8.8) | 1.00 (ref) | - |
| - Yes | 7/41 (17.1) | 2.13 (0.51–8.96) | .303 |
| Myalgias-arthralgias | |||
| - No | 6/51 (11.8) | 1.00 (ref) | - |
| - Yes | 4/24 (16.7) | 1.50 (0.38–5.91) | .562 |
| Anosmia-dysgeusia | |||
| - No | 9/64 (14.1) | 1.00 (ref) | - |
| - Yes | 1/10 (10.0) | 0.68 (0.08–6.02) | .728 |
| Initial Assessment | |||
| Oximetry at room air | |||
| - ≥94% | 2/25 (8.0) | 1.00 (ref) | - |
| - <94% | 8/49 (16.3) | 2.24 (0.44–11.47) | .332 |
| PaO2:FiO2 | 0.98 (0.98–0.99) | .007 | |
| - ≥300 | 2/47 (4.3) | 1.00 (ref) | - |
| - <300 | 8/29(27.6) | 8.57 (1.67–43.91) | .010 |
| Respiratory rate | |||
| - ≤24 | 2/16 (12.5) | 1.00 (ref) | - |
| - >24 | 4/25 (16.0) | 1.33 (0.21–8.28) | .758 |
| Systolic BP | |||
| - ≥100 | 8/68 (11.8) | 1.00 (ref) | - |
| - <100 | 2/5 (40.0) | 5.00 (0.72–34.63) | .103 |
| Diastolic BP | |||
| - ≥60 | 9/65 (13.8) | 1.00 (ref) | - |
| - <60 | 1/8 (12.5) | 0.89 (0.09–8.11) | .917 |
| Heart rate | |||
| - ≤100 | 6/48 (12.5) | 1.00 (ref) | - |
| - >100 | 4/26 (15.4) | 1.27 (0.33–4.99) | .729 |
| CURB65 | |||
| - <3 | 2/31 (6.5) | 1.00 (ref) | - |
| - ≥3 | 4/6 (66.7) | 29.00 (3.15–267.37) | .003 |
| eGFR | |||
| - ≥60 | 7/64 (10.9) | 1.00 (ref) | - |
| - <60 | 3/11 (27.3) | 3.05 (0.65–14.27) | .156 |
| Leukocytes | 1.00 (1.00–1.00) | .127 | |
| Lymphocytes | .99 (.99–1.01) | .201 | |
| C-reactive protein | 1.03 (0.96–1.11) | .393 | |
| Procalcitonin | .88 (0.36–2.14) | .777 | |
| Ferritin | 1.00 (0.99–1.00) | .415 | |
| Lactate dehydrogenase | 1.01 (1.00–1.01) | .034 | |
| D-dimers | 1.06 (0.99–1.13) | .090 | |
| Interleukin 6 | 0.99 (0.98–1.01) | .294 | |
| Troponin T | 1.01 (0.97–1.05) | .598 | |
| Brain natriuretic peptide | 1.00 (0.99–1.01) | .884 | |
| Creatine phosphokinase | 0.99 (0.99–1.01) | .562 | |
| Aspartate aminotransferase | .99 (.973–1.01) | .489 | |
| Alanine aminotransferase | 0.99 (0.97–1.01) | .477 | |
| Opacities of lung surface on X-rays | |||
| - ≤50% | 4/52 (7.6) | 1.00 (ref) | - |
| - >50% | 5/25 (20.0) | 2.35 (0.61–9.02) | .213 |
Data shown as n (%) unless specified otherwise. For units of the variable, please refer to Table 1. In bold, statistically significant differences.
10-years expected survival derived from Charlson comorbidity index score.
Days of symptoms before admission. OR: odds ratio, 95%CI: 95% confidence interval, NC: not calculable.
Table S1 (supplementary material) shows the associations between mortality and TCZ early response. BCRSS scale (51/77, 65.8%) was the only response assessment found associated with a lower mortality risk (OR (95% CI) 0.14 (0.03–0.74), p = 0.021). Neither combined response (respiratory function, inflammatory, or both), patient and physician subjective response, or their individual parameters, were predictors of survival.
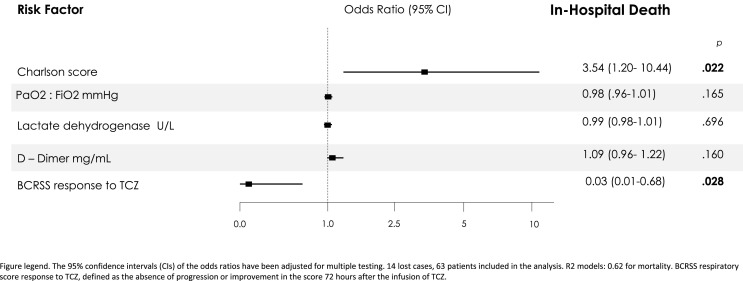
After multivariate adjustment (Fig. 1 ), TCZ response by BCRSS (OR 0.03 (0.01–0.68), p = 0.028), and Charlson index (OR 3.54 (1.20–10.44), p = 0.022) has been identified as independent factors associated with mortality. The inclusion of age and confusion (not included in the initial model due to the number of cases lost), do not modify the independent association between mortality and comorbidity – Charlson index; BCRSS response remained protective with a trend towards significance (adjusted OR 0.039, p = 0.06). Regarding response to TCZ according to the BCRSS score, there were no differences in terms of clinical and imaging features at presentation, length of days from disease onset to admission or to TCZ administration, and in number of TCZ doses used.
Fig. 1.
Predictors of In-Hospital Death from Multivariable Logistic-Regression Analysis. Figure legend. The 95% confidence intervals (CIs) of the odds ratios have been adjusted for multiple testing. 14 lost cases, 63 patients included in the analysis. R2 models: 0.62 for mortality. BCRSS respiratory score response to TCZ, defined as the absence of progression or improvement in the score 72 h after the infusion of TCZ.
The logistic regression analysis was not able to define predictive features of BCRSS - TCZ response. Some variables show trends towards significance, such as the presence of a PaO2:FiO2 <300 mmHg (OR 0.43 (0.16–1.17) p = 0.09) or oximetry at room air <94% (OR 0.37(0.12–1.17) p = 0.093, as poor response factors, and a longer clinical course, defined as a longer time between the onset of COVID-19 symptoms and the need to establish treatment with TCZ (OR 1.14 (0.97–1.33) p = 0.091), as a factor that favors the response.
3.3. Early clinical response to TCZ and length of hospital and ICU stay
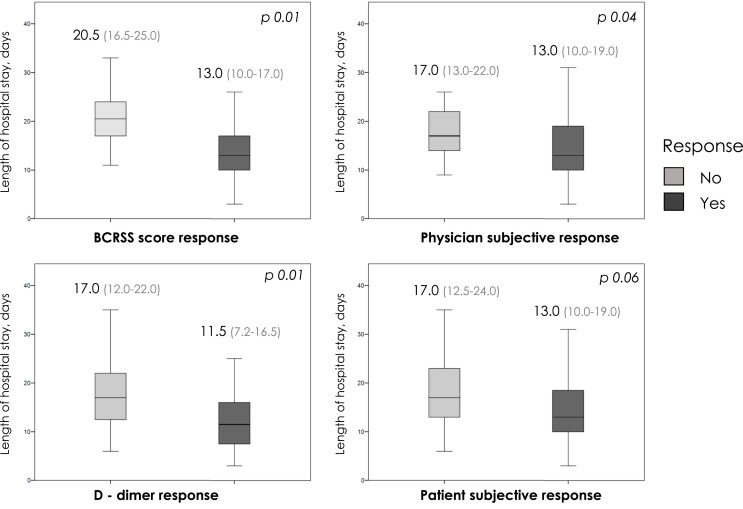
BCRSS score response, physician subjective response, and D-dimer response were associated with shorter hospitalization stay in surviving patients (Fig. 2 ); with patient subjective response near to the significance. Also, a decrease in the BCRSS score or D-dimer 72 h after TCZ infusion (Spearman's Rho +0.417 (p < 0.0001) and +0.303 (p = 0.016), respectively) were correlated with a shorter mean stay.
Fig. 2.
Early clinical response to tocilizumab and length of hospital stay. Figure legend. Boxplot represents the degree of dispersion of the length of hospital stay, horizontal line denote the median. Length of hospital stay between responders a non-responders were compared by Mann-Whitney's U. BCRSS, Brescia COVID Respiratory Severity Scale.
The rest of evaluated responses (combined or individual) showed no differences in hospital stay. The inclusion in the analysis of patients with fatal outcome entailed no changes in the length of stay in relation to BCRSS score (p = 0.014) or patient subjective response (p = 0.054), whereas D-dimer response remained close to the significance (p = 0.080) and physician subjective response loss the significance (p = 0.649). There was no differences in ICU stay depending on all assessed TCZ-responses. Timing of IMV showed no impact in ICU stay (p = 0.639), length of IMV (p = 0.458) or hospital stay (p = 0.779).
4. Discussion
This was a middle-aged and high-comorbidity population, with severe COVID-19 pneumonia and rapid respiratory aggravation and raised inflammatory markers. Patients were treated with TCZ an average of 10 days since disease onset, and followed a median of 83 days. Overall mortality was 12.9% (23.8% in ICU), and 49.4% patients required IMV. A TCZ response at 72 h defined by BCRSS as predictor of favorable evolution and Charlson index as poor outcome, were independent associated with mortality, and probably are able to predict survival. Response to TCZ at 72 h, as defined by BCRSS score, physician's opinion and D-dimers, were associated with a shorter hospital stay in surviving patients.
Our mortality rate results contrast with previous reports. In the largest series published outside of China, 21.0% of 2653 patients analyzed died [8]. In China, Zhou et al. [9] reported 28.2% mortality in 191 patients, while in other series, in younger and milder populations, rates were 11.0–11.7% [10,11]. ICU data are even more remarkable, with mortality of 45.0–61.5% [12,13]; 81–97% in patients on IMV [9,13], though in USA 24.5% is reported [8]. Despite potential confounders and different populations, 12.9% mortality (26.3% in IMV) reported here in TCZ-treated severe pneumonia seems lower than expected in patients with severe pneumonia and clinical progression. Despite questioned use [14], most patients received hydroxychloroquine and azithromycin without significant complications.
In order to contextualize our findings, it is important to consider three basic aspects of the COVID-19 approach, dealing with indication, elective candidates, and predicts of response and outcomes.
First, TCZ indication in severe pneumonia due to COVID-19. There is no solid evidence at the expense of randomized clinical trials for TCZ use in severe COVID-19, and the benefit-risk ratio of using an immunomodulatory agent in an acute infectious disease remains a matter of debate [15]. While trials are ongoing, extrapolating from clinical experience may benefit the multidisciplinary teams caring for patients with severe COVID-19.
Five case series have reported use of TCZ in COVID-19. Xu et al. [16] reported 21 patients – four critical and 17 severe (two required intubation and one non-invasive ventilation), 90.5% were discharged after 13.5 days without serious adverse events; no deaths were registered. Luo et al. [17] published the clinical outcomes of 15 patients a week after TCZ treatment (seven critical, six severe, two moderate), with three deaths (15.7%; 46% in critical), two disease aggravation, and 10 (52%) with a rapid response (9 clinical stabilization and one clinical improvement). Alattar et al. [18] reported 25 patients, 36% achieved primary outcome (discharge alive from ICU by day 14), with a mortality rate of 12% and 52% were still in ICU. Toniati et al. [19] published the clinical outcomes of 100 patients (43 in ICU) at 10 days, 69 with a rapid improvement, 8 stabilized and a mortality rate of 20% (24% in ICU). According to our results, this series also described a rapid clinical improvement in the first 24–72 in 58% of the cases that received TCZ. Unfortunately, long-term outcome is not reported. And recently, Capra et al. [20] have provided a comparative between standard care (26 patients) and TCZ plus standard care (62 patients) in non-ICU severe COVID-19 pneumonia. Patients receiving TCZ showed significantly greater survival rate as compared to control patients (hazard ratio for death, 0.035 (0.004–0.347), adjusting for age, comorbidities and PCR; however, selection bias and unknown outcome (62.9% TCZ-patients were still hospitalized), limit the study.
Regarding safety, no TCZ-related severe events occurred in the present study. Secondary or hospital-acquired infections is a major concern, suffered by 5.1%–38.9% Chinese patients, and by 4.8%–27.4% of patients in Western countries. However, data are significantly biased by limited follow-up in these series, especially for those patients admitted at ICU [21]. In our long-term follow-up cohort, and taking into account the aforementioned limitations, infection incidence was intermediate with respect to the western series [[22], [23], [24]]. Detected causal microorganisms were the usual at our center, and no fungal or opportunistic infections occurred, unlike other series [10,13,24,25]. Finally, VAP incidence (14.6 per 1.000 days of intubation) was similar to the local ICU registry from 2018 (16.7; 95%CI 13.1–20.0) and was responsible for late mortality. Prophylaxis of hepatitis B reactivation was used as needed. Despite several shortcomings, our low mortality rate and TCZ safety, combined with reported data by others authors, reinforces the rationale for TCZ use in COVID-19 pneumonia.
Second, which patients are candidates for TCZ therapy ? . Given the high frequency of non-severe presentations, intensive interventions such as TCZ should be reserved for selected patients (i.e. those with poor prognostic factors or worsening diseases). Nonetheless, except for advanced age, elevated body mass index, and comorbid conditions, robust prognostic factors in COVID-19 are lacking [26], especially for the TCZ-treated population.
In the present study, patients treated with TCZ were indeed different at time of admission, with fever and dyspnea as prominent presentation features, worse respiratory function, higher inflammatory parameters, more severe lymphopenia and more extensive lung opacities. Thus, all indicators of a more severe disease, translating into higher mortality and longer average stay. Remarkably, without differences in age, gender, and comorbidities compared to global cohort.
The severe disease occurring in these patients may suggest a cytokine release syndrome (CRS), though how to diagnose it in COVID-19 is debatable. Experience with immunotherapy-triggered CRS suggests that TCZ should be restricted to severe cases [15]. This principle is probably not shared by viral infections, such as COVID-19, in which a timely intervention in at milder stages may prevent progression. Here, TCZ was administered at detection of worsening, a median of two days after admission. This early use could explain the reduced total and ICU mortality rates; Capra et al. reported data [20] using TCZ within 4 days from admission, thus reinforcing this hypothesis. Also, our data suggest that in patients with a worse respiratory status, the administration of TCZ along with the establishment of IMV seems an effective approach, with similar results in length of IMV, average stay in hospital and final outcome.
And third, predictive baseline factors of fatal outcome and assessment of TCZ response. In the present case series of severe COVID-19 pneumonia treated with TCZ, baseline clinical status, opacities extension or inflammatory markers could not discriminate subgroups of worse evolution.
In the absence of validated scales for TCZ response in COVID-19, a reasonable approach is using an objective early response from combining different clinical and analytical parameters, and testing their capacity of predicting lower mortality. However, neither combined response (respiratory function, inflammatory, or both), patient and physician subjective response, or their individual parameters, were useful predictors. The absence of statistical significance translates that they are not adequate response parameters, at least in the short term, to assess the patient's evolution. It is a complex disease, with a pathophysiology not completely clarified, such us genetic components and virus-host relationship, that probably hides unknown prognostic markers. Only TCZ response at 72 h defined by BCRSS, applied retrospectively, was associated with a lower mortality risk in univariate analysis, and this association persist after adjustment for other confounders.
BCRSS is a COVID Respiratory Severity Scale (BCRSS 0 to 8, with higher scores indicating higher severity), recently published [5], and employed as an improvement outcome measure in acute respiratory distress syndrome related to COVID-19 pneumonia [19]. BCRSS is an objective respiratory scale, which takes into account not only respiratory function, but also the type and ventilatory support maneuvers required. The scale simplifies the clinical summary of a patient's status, and allows clinicians to compare among patients and to track the evolution of a patient's respiratory severity over time. To note, the grade of inflammation is not taken into account in BCRSS, that, together with the integrated approach of the scale (respiratory function - ventilatory support), could explain the absence of association with survival of other responses evaluated.
Our findings place comorbidity as an independent mortality factor, downplaying the role of age in the TCZ subpopulation. These results replicate the findings in community-acquired pneumonia (CAP), whereas age itself was reported to not contribute to CAP mortality until 80 years of age if patients have at least one comorbidity [27].
Regarding inflammatory issues, having routine (ferritin, LDH, CPR, D-dimer) and non-routine markers (IL-6, other citokines) evolution during disease duration and confronting to daily clinical evaluation, would be of great value in predicting CRS, risk stratification and prognosis [15]. However, at present, no cut-off value has yet been proposed for any of these markers. A previous cohort study suggested that IL-6 levels were significantly elevated in COVID-19 patients but varied considerably among both ICU and non-ICU patients [28]. However, according to our series and other reports [15,29,30], in patients treated with TCZ, IL-6 levels at presentation are not associated with differences in subsequent mortality. Thus, IL-6 measurement may be an indispensable part of the grading system or to favors using anti-inflammatory agents, but apparently is not able to predict further outcomes once the treatment is begun.
In TCZ treated patients, the absence of impact of the basal inflammatory parameters, including IL-6 and CRP, and of the inflammatory response 72 h after the administration of TCZ, calls into question the exclusive indication of TCZ based on these parameters, as postulated by some authors [15]. However, reduction in serum levels might not translate in improvement of inflammation in targeted organs such as the lungs, or the response there may occur later. The present results likely support this interpretation.
The effect of an early TCZ-response on the length of hospital admission stay is a relevant issue that has not been reported in the literature. The evaluation of TCZ-response impact in admission stay from different perspectives, showed a median reduction in 7.5 days for BCRSS response, 5 days for D-dimer response and 4 days for physician subjective response.
The optimal timing of TCZ during the disease course remains undefined. Considering current knowledge about COVID-19 course, antivirals and immune boosters should be initiated promptly after symptom onset, whereas immunosuppressants should be administered at the very start of the cytokine storm. A disease severity grading system, based on prognostic factors with impact on mortality rate, may provide an objective tool to assess the most appropriate timing to initiate TCZ treatment. Unfortunately, our data is unable to identify baseline prognostic factors, capable of predicting a fatal outcome in these patients.
Some limitations, as lacking a control group, the clinical, observational, the retrospective nature of the study and the absence of validated TCZ response scores for COVID-19, must be acknowledged. The evaluation of TCZ response by objective data, exhaustive revision of records and almost three months of censored time, is likely to ameliorate these shortcomings. It is possible that 48–72 h is not the correct timeframe to evaluate response to TCZ, nonetheless, in COVID-19, reported median time from dyspnea onset to ARDS development is around 2.5 days [4], and also 72 h was chosen as is a crucial time to predict outcomes and modulate the use of antibiotics in other infection diseases, such as CAP [31,32], therefore it seems a more than reasonable approach. Also, the long follow-up time, that comprises mortality and hospital stay, allows having, from our perspective, a complete assessment of the impact of TCZ in the clinical course of COVID-19. Although BCRSS score was applied retrospectively, the tool was designed to guide treatment decisions in clinical practice [5]; despite this, was successfully identified as an independent predictor of survival. To gauge the impact in disease course in early response, we performed a separate analysis considering TCZ initiation along with intubation, with similar findings.
In summary, this experience provides the second largest series of patients with severe COVID-19 pneumonia treated with TCZ, but the one with the longest follow-up, six-fold the current published series. In severe COVID-19 pneumonia, use of TCZ was associated with low mortality, even in those under IMV, and no major safety concerns. Low comorbidity and TCZ early response defined by BCRSS are associated with increased survival, confirming that this tool is helpful to follow COVID-19 patients treated with TCZ. Finally, an early TCZ response was related to shorter median hospital stay. These findings must be replicated in other long-term observational studies and ongoing clinical trials, but combined data reinforces the rationale for TCZ use in COVID-19 pneumonia.
Declaration of competing interest
MA declares speaking fees from Roche Pharma (<10,000$).
Acknowledgments
COVID multidisciplinary team: Santos Asensio, Cleofé Fernandez, Alfredo Candela, Ma del Mar García, Ignacio Gayá, José M. Ramos, Sergio Reus, Paloma Ruiz, Diego Torrús, Pilar Gonzalez-De-La-Aleja, Violeta Esteban, Ma del Mar García-Mullor, Mar Blanes, Jaime Guijarro, José Carlos Pascual, Iris Gonzalez, Pedro Sanso, Jaime Javaloy, Clara Llopis, Olga Coronado, Esther García, Gonzalo Rodríguez, Paola Melgar, Mariano Franco, Félix Lluís, Carmen Zaragoza, Cándido Alcaraz, Ana Carrión, Celia Villodre, Emilio Ruiz de la Cuesta, Cristina Alenda, Francisca Peiró, María Planelles, Laura Greco, Sandra Silvia, Antonio Francia, Iván Verdú, Juan Sales, Ana Palacios, Hortensia Ballester, Antonio García-Valentín, Marta Márquez, Eva Canelo, Andrea Juan, Elena Vives, Andrea Revert, Gonzalo Fuente, Ester Nofuentes, Carolina Mangas, Eva Vera, Alicia Ferradas, Isabel Rimen, Helena López, Cristian Herrera, Beatriz López, Marina Morillas, Vanesa Rodríguez, Mercedes Khartabi, Mario Giménez, Ernesto Tovar, Estela Martínez, Lucia Medina, Sandra Baile, Carlos Salazar, Norma Guerra, Sarai Moliner, M° Carmen López, Blanca Figueres.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.jaut.2020.102523.
Funding section
There are no sources of financing.
Transparency declarations section
MA declares speaking fees from Roche Pharma (<10,000$).
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- 1.Bhimraj A., Morgan R.L., Shumaker A.H., Lavergne V., Baden L., Cheng V.C.-C., Edwards K.M., Gandhi R., Muller W.J., O'Horo J.C., Shoham S., Murad M.H., Mustafa R.A., Sultan S., Falck-Ytter Y. Infectious diseases society of America guidelines on the treatment and management of patients with COVID-19. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang C., Wu Z., Li J.-W., Zhao H., Wang G.-Q. Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int. J. Antimicrob. Agents. 2020;55:105954. doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sanders J.M., Monogue M.L., Jodlowski T.Z., Cutrell J.B. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. J. Am. Med. Assoc. 2020 doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- 4.Razonable R.R., Pennington K.M., Meehan A.M., Wilson J.W., Froemming A.T., Bennett C.E., Marshall A.L., Virk A., Carmona E.M. A collaborative multidisciplinary approach to the management of coronavirus disease 2019 in the hospital setting. Mayo Clin. Proc. 2020;95:1467–1481. doi: 10.1016/j.mayocp.2020.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duca A., Piva S., Focà E., Latronico N., Rizzi M. Calculated decisions: brescia-COVID respiratory severity scale (BCRSS)/Algorithm. Emerg. Med. Pract. 2020;22:CD1–CD2. [PubMed] [Google Scholar]
- 6.Ranieri V.M., Rubenfeld G.D., Thompson B.T., Ferguson N.D., Caldwell E., Fan E., Camporota L., Slutsky A.S. Acute respiratory distress syndrome: the Berlin Definition. J. Am. Med. Assoc. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. ARDS Definition Task Force. [DOI] [PubMed] [Google Scholar]
- 7.Chalmers J.D., Singanayagam A., Akram A.R., Mandal P., Short P.M., Choudhury G., Wood V., Hill A.T. Severity assessment tools for predicting mortality in hospitalised patients with community-acquired pneumonia. Systematic review and meta-analysis. Thorax. 2010;65:878–883. doi: 10.1136/thx.2009.133280. [DOI] [PubMed] [Google Scholar]
- 8.Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W., the Northwell COVID-19 Research Consortium. Barnaby D.P., Becker L.B., Chelico J.D., Cohen S.L., Cookingham J., Coppa K., Diefenbach M.A., Dominello A.J., Duer-Hefele J., Falzon L., Gitlin J., Hajizadeh N., Harvin T.G., Hirschwerk D.A., Kim E.J., Kozel Z.M., Marrast L.M., Mogavero J.N., Osorio G.A., Qiu M., Zanos T.P. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. J. Am. Med. Assoc. 2020 doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y., Chen H., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., Xia J., Yu T., Zhang X., Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu K., Fang Y.-Y., Deng Y., Liu W., Wang M.-F., Ma J.-P., Xiao W., Wang Y.-N., Zhong M.-H., Li C.-H., Li G.-C., Liu H.-G. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province: Chinese Med J. 2020;133:1025–1031. doi: 10.1097/CM9.0000000000000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. J. Am. Med. Assoc. 2020;323:1239. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 13.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., Wu Y., Zhang L., Yu Z., Fang M., Yu T., Wang Y., Pan S., Zou X., Yuan S., Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.COVID-19 Treatment Guidelines Panel Coronavirus disease 2019 (COVID-19) treatment guidelines. National institutes of Health. https://www.covid19treatmentguidelines.nih.gov/ [PubMed]
- 15.Liu B., Li M., Zhou Z., Guan X., Xiang Y. Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)? J. Autoimmun. 2020;111 doi: 10.1016/j.jaut.2020.102452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu X., Han M., Li T., Sun W., Wang D., Fu B., Zhou Y., Zheng X., Yang Y., Li X., Zhang X., Pan A., Wei H. Effective treatment of severe COVID-19 patients with tocilizumab. Proc. Natl. Acad. Sci. U.S.A. 2020;117:10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luo P., Liu Y., Qiu L., Liu X., Liu D., Li J. Tocilizumab treatment in COVID-19: a single center experience. J. Med. Virol. 2020;92:814–818. doi: 10.1002/jmv.25801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alattar R., Ibrahim T.B.H., Shaar S.H., Abdalla S., Shukri K., Daghfal J.N., Khatib M.Y., Aboukamar M., Abukhattab M., Alsoub H.A., Almaslamani M.A., Omrani A.S. Tocilizumab for the treatment of severe coronavirus disease 2019. J. Med. Virol. 2020;jmv:25964. doi: 10.1002/jmv.25964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toniati P., Piva S., Cattalini M., Garrafa E., Regola F., Castelli F., Franceschini F., Airò P., Bazzani C., Beindorf E.-A., Berlendis M., Bezzi M., Bossini N., Castellano M., Cattaneo S., Cavazzana I., Contessi G.-B., Crippa M., Delbarba A., De Peri E., Faletti A., Filippini M., Filippini M., Frassi M., Gaggiotti M., Gorla R., Lanspa M., Lorenzotti S., Marino R., Maroldi R., Metra M., Matteelli A., Modina D., Moioli G., Montani G., Muiesan M.-L., Odolini S., Peli E., Pesenti S., Pezzoli M.-C., Pirola I., Pozzi A., Proto A., Rasulo F.-A., Renisi G., Ricci C., Rizzoni D., Romanelli G., Rossi M., Salvetti M., Scolari F., Signorini L., Taglietti M., Tomasoni G., Tomasoni L.-R., Turla F., Valsecchi A., Zani D., Zuccalà F., Zunica F., Focà E., Andreoli L., Latronico N. Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: a single center study of 100 patients in Brescia, Italy. Autoimmun. Rev. 2020;19 doi: 10.1016/j.autrev.2020.102568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Capra R., De Rossi N., Mattioli F., Romanelli G., Scarpazza C., Sormani M.P., Cossi S. Impact of low dose tocilizumab on mortality rate in patients with COVID-19 related pneumonia. Eur. J. Intern. Med. 2020;76:31–35. doi: 10.1016/j.ejim.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Antinori S., Galimberti L., Milazzo L., Ridolfo A.L. Bacterial and fungal infections among patients with SARS-CoV-2 pneumonia. Inf. Med. 2020;28:29–36. [PubMed] [Google Scholar]
- 22.Arentz M., Yim E., Klaff L., Lokhandwala S., Riedo F.X., Chong M., Lee M. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington state. J. Am. Med. Assoc. 2020 doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barrasa H., Rello J., Tejada S., Martín A., Balziskueta G., Vinuesa C., Fernández-Miret B., Villagra A., Vallejo A., San Sebastián A., Cabañes S., Iribarren S., Fonseca F., Maynar J. Alava COVID-19 study investigators, SARS-CoV-2 in Spanish intensive care units: early experience with 15-day survival in vitoria. Anaesth Crit Care Pain Med. 2020 doi: 10.1016/j.accpm.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morena V., Milazzo L., Oreni L., Bestetti G., Fossali T., Bassoli C., Torre A., Cossu M.V., Minari C., Ballone E., Perotti A., Mileto D., Niero F., Merli S., Foschi A., Vimercati S., Rizzardini G., Sollima S., Bradanini L., Galimberti L., Colombo R., Micheli V., Negri C., Ridolfo A.L., Meroni L., Galli M., Antinori S., Corbellino M. Off-label use of tocilizumab for the treatment of SARS-CoV-2 pneumonia in Milan, Italy. Eur. J. Intern. Med. 2020;76:36–42. doi: 10.1016/j.ejim.2020.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prattes J., Valentin T., Hoenigl M., Talakic E., Reisinger A.C., Eller P. Invasive pulmonary aspergillosis complicating COVID-19 in the ICU - a case report. Med Mycol Case Rep. 2020 doi: 10.1016/j.mmcr.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jamilloux Y., Henry T., Belot A., Viel S., Fauter M., El Jammal T., Walzer T., François B., Sève P. Should we stimulate or suppress immune responses in COVID-19? Cytokine and anti-cytokine interventions. Autoimmun. Rev. 2020;19:102567. doi: 10.1016/j.autrev.2020.102567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luna C.M., Palma I., Niederman M.S., Membriani E., Giovini V., Wiemken T.L., Peyrani P., Ramirez J. The impact of age and comorbidities on the mortality of patients of different age groups admitted with community-acquired pneumonia. Annals ATS. 2016;13:1519–1526. doi: 10.1513/AnnalsATS.201512-848OC. [DOI] [PubMed] [Google Scholar]
- 28.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sinha P., Matthay M.A., Calfee C.S. Is a “cytokine storm” relevant to COVID-19? JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.3313. [DOI] [PubMed] [Google Scholar]
- 30.Sciascia S., Aprà F., Baffa A., Baldovino S., Boaro D., Boero R., Bonora S., Calcagno A., Cecchi I., Cinnirella G., Converso M., Cozzi M., Crosasso P., De Iaco F., Di Perri G., Eandi M., Fenoglio R., Giusti M., Imperiale D., Imperiale G., Livigni S., Manno E., Massara C., Milone V., Natale G., Navarra M., Oddone V., Osella S., Piccioni P., Radin M., Roccatello D., Rossi D. Pilot prospective open, single-arm multicentre study on off-label use of tocilizumab in patients with severe COVID-19. Clin. Exp. Rheumatol. 2020;38:529–532. [PubMed] [Google Scholar]
- 31.Uranga A., España P.P., Bilbao A., Quintana J.M., Arriaga I., Intxausti M., Lobo J.L., Tomás L., Camino J., Nuñez J., Capelastegui A. Duration of antibiotic treatment in community-acquired pneumonia: a multicenter randomized clinical trial. JAMA Intern Med. 2016;176:1257–1265. doi: 10.1001/jamainternmed.2016.3633. [DOI] [PubMed] [Google Scholar]
- 32.Halm E.A., Fine M.J., Marrie T.J., Coley C.M., Kapoor W.N., Obrosky D.S., Singer D.E. Time to clinical stability in patients hospitalized with community-acquired pneumonia: implications for practice guidelines. J. Am. Med. Assoc. 1998;279:1452–1457. doi: 10.1001/jama.279.18.1452. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.