Abstract
Rationale: Complement is crucial for host defense but may also drive dysregulated inflammation. There is limited understanding of alternative complement function, which can amplify all complement activity, during critical illness.
Objectives: We examined the function and key components of the alternative complement pathway in a series of critically ill patients and in a mouse pneumonia model.
Methods: Total classical (CH50) and alternative complement (AH50) function were quantified in serum from 321 prospectively enrolled critically ill patients and compared with clinical outcomes. Alternative pathway (AP) regulatory factors were quantified by ELISA (n = 181) and examined via transcriptomics data from external cohorts. Wild-type, Cfb−/−, and C3−/− mice were infected intratracheally with Klebsiella pneumoniae (KP) and assessed for extrapulmonary dissemination.
Measurements and Main Results: AH50 greater than or equal to median, but not CH50 greater than or equal to median, was associated with decreased 30-day mortality (adjusted odds ratio [OR], 0.53 [95% confidence interval (CI), 0.31–0.91]), independent of chronic liver disease. One-year survival was improved in patients with AH50 greater than or equal to median (adjusted hazard ratio = 0.59 [95% CI, 0.41–0.87]). Patients with elevated AH50 had increased levels of AP factors B, H, and properdin, and fewer showed a “hyperinflammatory” subphenotype (OR, 0.30 [95% CI, 0.18–0.49]). Increased expression of proximal AP genes was associated with improved survival in two external cohorts. AH50 greater than or equal to median was associated with fewer bloodstream infections (OR, 0.67 [95% CI, 0.45–0.98). Conversely, depletion of AP factors, or AH50 less than median, impaired in vitro serum control of KP that was restored by adding healthy serum. Cfb−/− mice demonstrated increased extrapulmonary dissemination and serum inflammatory markers after intratracheal KP infection compared with wild type.
Conclusions: Elevated AP function is associated with improved survival during critical illness, possibly because of enhanced immune capacity.
Keywords: complement, serum, host defense, pneumonia, critical illness
At a Glance Commentary
Scientific Knowledge on the Subject
The complement system is an evolutionarily ancient component of host defense that can drive dysregulated inflammation. The alternative complement pathway, in particular, is important for its ability to amplify complement activity and for its role in the hyperacute response to infection, including host defense in the lung. Little is known about the function of the alternative complement pathway during critical illness.
What This Study Adds to the Field
Critically ill patients exhibit a wide distribution of serum alternative complement pathway function, and those with elevated function demonstrate lower 30-day mortality and improved 1-year survival. Patients with elevated alternative pathway function displayed higher systemic levels of factor B, properdin, factor H, and a “hypoinflammatory” subphenotype, demonstrating fewer bloodstream infections and improved serum control of a multidrug-resistant gram-negative pathogen in vitro. Genetic deletion of factor B or complement component C3, which is crucial for alternative pathway amplification, increased extrapulmonary dissemination of bacteria, and enhanced inflammation in a mouse pneumonia model. These findings suggest that increased fluid-phase alternative complement pathway function is a protective mechanism during the biological stress of critical illness.
The complement system comprises an intricately regulated set of more than 40 proteins that are ancient components of the vertebrate immune system (1). Complement can act both in the “fluid-phase” of blood as well as at a cellular-molecular level in tissues throughout the body (1, 2). There are three well-characterized pathways of complement proteolysis and activation: the alternative, classical, and lectin pathways. The classical pathway is initiated primarily by antigen immunoglobulin complexes. In contrast, the alternative pathway is spontaneously activated but tightly controlled by regulatory proteins and protective host-cell surface inhibitory proteins. Therefore, the function of the complement system may be determined by a delicate balance between positive and negative regulation.
The alternative complement pathway is critically important for its ability to amplify complement activity (3, 4) and for its role in the hyperacute response to infection (5), including host defense in the lung (6, 7). Biological stressors, including infection (8–11), ischemia (12), trauma (13, 14), burns (15), and surgery (16, 17), have all been noted to activate the human complement system. These biological stressors can perturb the complement functional balance, leading to abnormal complement pathway function (8, 18–20) that may have clinical significance (21). Alternative complement pathway function, or AH50, can be quantified by measuring the hemolysis of rabbit erythrocytes after incubation with serum (22). Despite its role in immune function, overexuberant activation of the complement system is thought to contribute to the pathogenesis of sepsis and other critical illnesses (23, 24). We hypothesized that critical illness is associated with perturbations in alternative complement function and sought to characterize the clinical consequences of alternative pathway function. Some of the results of these studies have been previously reported in the form of an abstract (25, 26).
Methods
Subjects
We conducted a retrospective analysis of prospectively collected serum samples from 321 consecutively enrolled patients at the time of enrollment in the University of Pittsburgh Acute Lung Injury Registry and Biospecimen Repository (Institutional Review Board number PRO10110387) from October 2011 to December 2017 (see Methods and Figure E1 in the online supplement).
Microscaled Complement Functional Assays
We made minor modifications to manufacturer (Complement Tech) protocols to perform assays with a limited volume of patient serum and a standard, clear plastic, 96-well plate. Pooled reference serum from healthy volunteers was used to calibrate normal values of complement function for each assay (see Methods in the online supplement). Specific factor-depleted sera (Complement Tech) were used in validation and serum-mixing bacterial killing assays. The microscaled classical (CH50; see Figure E2A) and alternative (AH50; see Figure E2B) pathway function of serum from healthy volunteers were within the normal ranges provided by manufacturer.
Inflammatory Subphenotype Classification
Patients were classified into two distinct inflammatory subphenotypes, as previously described (27). Briefly, “hypoinflammatory” and “hyperinflammatory” phenotypes were identified by a previously validated three-variable model using IL-8, bicarbonate, and tumor necrosis factor receptor-1 values (28), which demonstrated excellent agreement with de novo latent class analysis models incorporating both clinical and biomarker variables in this cohort (27).
Alternative Complement Pathway Protein Analysis and Proximal Regulator Transcriptomics
Serum factor B, properdin, and factor H levels were determined by sandwich ELISA (see Methods in the online supplement). Publicly available gene expression data extracted from the EARLI (Early Assessment of Renal and Lung Injury) (29) and MARS (Molecular Diagnosis and Risk Stratification of Sepsis) (30) cohorts were examined in survivors and nonsurvivors (see Methods in the online supplement).
Serum Bacterial Growth Assay
Carbapenemase-producing Klebsiella pneumoniae (KPC5) clinical isolate from the bronchial washings of a critically ill patient is resistant to meropenem and colistin. The ability of various sera to control KPC5 growth was tested, as previously described (see Methods in the online supplement) (31).
Mouse Model
Wild-type (WT) C57BL/6J mice and mice genetically deficient for C3−/− (complement component C3; B6.129S4-C3tm1Crr/J, stock number 029661) were purchased from Jackson Laboratory and maintained using a protocol approved by the University of Pittsburgh Institutional Animal Care and Use Committee. In separate experiments, WT C57BL/6J and CFB (complement factor B) (Cfb−/−)-deficient mice (32, 33) were bred and maintained under pathogen‐free conditions at Washington University School of Medicine in St. Louis, MO, in accordance with institutional animal care guidelines. Eight-week to 12-week old, sex-matched mice were used in all experiments. Mice were intratracheally inoculated with a reference hypervirulent K. pneumoniae strain (43816; American Type Culture Collection) or KPC5. Necropsy with lung and spleen colony-forming unit counts; and, in select experiments, serum, BAL fluid, and lung tissue for cytokine analysis were collected at 24 hours postinfection, as previously described (31, 34, 35). Serum cytokine measurement was performed by multiplex assay, as previously described (see Methods in the online supplement) (36).
Statistical Analysis and Rigor
All assays were performed using deidentified serum specimens by researchers blinded to the clinical characteristics and outcomes of the associated registry patients. Distribution of continuous variables was assessed for normality and transformed if required. Student’s t tests and chi-squared tests were used to compare continuous and categorical variables between two groups of patients with high and low alternative pathway function defined by median AH50. Associations between complement activity and ICU and 30-day mortality were assessed using logistic regression. Probability of ICU death was calculated from postestimation after fitting a logistic model to confounders (see Methods in the online supplement), which was applied as a fractional polynomial function to measure association with ICU mortality. The associations with 1-year survival were assessed with Cox regression analysis (using time to death or last observation). Kruskal-Wallis test with Dunn’s test for multiple comparisons was applied to compare serum levels of factor B, properdin, and factor H in a subset of patients, as well as bacterial growth in healthy and factor-depleted sera. Cluster analysis of patients from external cohorts was conducted using hierarchical clustering with complete linkage (stop parameters by Duda-Hart indices) (37). The association between complement activity and bloodstream infection was tested using a multilevel mixed effect logistic regression model. Serum KPC5 killing was tested using a multilevel mixed effect linear regression model. We applied a mean-centering method to remove batch effect on complement activity (38). All analyses were performed after adjusting for confounders, which were selected among those variables considered plausible confounders.
Statistical models were checked for assumptions, including highly influential observations and proportionality of hazards. Statistical analysis was performed in Stata Version 15.1 (StataCorp).
Results
Clinical Characteristics for 321 Critically Ill Patients
Although others have shown perturbations of complement function in small series of patients with bacteremia (19, 39, 40), we sought to determine whether complement pathway function was associated with mortality in a large, heterogenous cohort of critically ill patients with acute respiratory failure. Table 1 displays clinical characteristics of the 321 consecutively enrolled research registry patients (median age, 57 yr; 44% female). Samples were collected within a mean of 2.1 days (SD, 2.2 d) after ICU admission. Consistent with the underlying demographics of the surrounding region, patients were primarily white (93%) and the majority were transferred from local outside hospitals (64%). The vast majority of patients (96%) required mechanical ventilation during their ICU course. Most patients were either at-risk (n = 125; 39%) or met diagnostic criteria for acute respiratory distress syndrome (ARDS) (n = 107; 33%). The remaining patients (n = 89; 28%) were mechanically ventilated for nonpulmonary critical illnesses such as heart failure or encephalopathy. Sepsis was confirmed or probable in 63% (n = 202) of patients and the median modified sequential organ failure assessment (SOFA) score was 7 (interquartile range, 5–9). We noted chronic liver disease (n = 33; 10%), immune suppression (n = 59; 18%), and active neoplasm (n = 19; 6%) in some patients. Ninety-three of 321 (29%) patients died within 30 days of their admission to the ICU.
Table 1.
Clinical Characteristics of 321 Critically Ill Patients Grouped by Median Alternative Complement Pathway Function
| Total (N = 321) | AH50 < Median (n = 160) | AH50 ≥ Median (n = 161) | P Value | |
|---|---|---|---|---|
| Age, yr, median (IQR) | 57 (45–67) | 56 (45–67) | 58 (45–67) | 0.56 |
| Sex, F, n (%) | 142 (44.2) | 69 (43.1) | 73 (45.3) | 0.69 |
| Race, n (%) |
0.41 | |||
| White | 297 (92.5) | 150 (93.8) | 147 (91.3) | |
| Black | 24 (7.5) | 10 (6.3) | 14 (8.7) | |
| Admission source OSH, n (%) | 205 (63.9) | 107 (66.9) | 98 (60.9) | 0.26 |
| ARDS, n (%) | 107 (33.3) | 53 (33.1) | 54 (33.5) | 0.94 |
| Suspected sepsis, n (%) | 202 (62.9) | 99 (61.9) | 103 (64.0) | 0.70 |
| Modified SOFA*, median (IQR) | 7 (5–9) | 8 (6–10) | 6 (4–8) | <0.001 |
| Chronic liver disease, n (%) | 33 (10.3) | 28 (17.5) | 5 (3.1) | <0.001 |
| Immune suppression, n (%) | 59 (18.4) | 29 (18.1) | 30 (18.6) | 0.91 |
| Active neoplasm, n (%) | 19 (5.9) | 5 (3.1) | 14 (8.7) | 0.034 |
| ICU mortality, n (%) | 84 (26.2) | 50 (31.3) | 34 (21.2) | 0.050 |
| 30-d mortality, n (%) | 93 (29.0) | 57 (35.6) | 36 (22.4) | 0.023 |
Definition of abbreviations: AH50 = alternative complement pathway function; ARDS = acute respiratory distress syndrome; IQR = interquartile range; OSH = outside hospital; SOFA = sequential organ failure assessment score.
Odds ratio for AH50 ≥ median is 0.58 (95% confidence interval, 0.34–1.00; P = 0.050) for ICU mortality and 0.53 (95% confidence interval, 0.31–0.91; P = 0.023) for 30-day mortality. Odds ratios are adjusted for AH50 batch effect, presence of active neoplasm, sex, SOFA, and patient age.
Modified SOFA score calculated with neurologic score equal to 0; therefore, maximum score is 20.
Values of CH50 and AH50 from serum collected at the time of enrollment were significantly correlated (Figure 1A; Spearman r = 0.50; P < 0.0001). Figure 1B displays the frequency distribution of AH50 values (range 6–1,008 U/ml serum). Given the wide distribution of complement function values, we examined whether there was an association between clinical outcomes and complement function in our registry patients.
Figure 1.

Increased alternative complement pathway activity is associated with decreased 30-day mortality and increased 1-year survival. (A) Scatter plot of classical pathway function (CH50) (U/ml serum; median, 130; interquartile range, 90–179) and alternative pathway function (AH50) (U/ml serum; median, 169; interquartile range, 136–204) values and (B) frequency distribution of AH50 values (grouped by increments of 50 U/ml serum; median value displayed) of serum prospectively collected from 321 consecutively enrolled critically ill patients at the time of enrollment. Normal value ranges are supplied by the manufacturer. (C) Probability of ICU mortality as a function of batch-adjusted AH50 (P = 0.015). (D) Patient survival from index ICU admission date by AH50 grouped by relationship to median (hazard ratio, 0.59 [95% confidence interval, 0.41–0.87]). Survival estimates are adjusted for AH50 batch effect, patient age, sex, sequential organ failure assessment score, chronic liver disease, and presence of active neoplasm. For AH50 less than median (n = 159), a single patient died within 24 hours of ICU admission and was therefore removed from this analysis rather than allocating an arbitrary duration of follow-up.
Complement Pathway Function Is Associated with Mortality
After adjusting for age, sex, SOFA, presence of active neoplasm, and batch effect, we found significantly decreased ICU (odds ratio [OR], 0.58 [95% confidence interval (CI), 0.34–1.00]; P = 0.050) and 30-day mortality (OR, 0.53 [95% CI, 0.31–0.91]; P = 0.023) in patients with AH50 greater than or equal to median (see Table 1). There were no significant differences in ARDS diagnosis, or confirmed or suspected sepsis, between groups, although modified SOFA scores were higher in patients with AH50 less than median. Increased numbers of patients diagnosed with chronic liver disease showed AH50 less than median (P < 0.001). However, the increased mortality observed in patients with lower alternative pathway function was not impacted by the presence of chronic liver disease in multivariate analysis (OR for 30-d mortality adjusted for chronic liver disease = 0.52 [95% CI, 0.30–0.90]; P = 0.020). Furthermore, when patients with history of chronic liver disease were removed from the analysis (n = 33 patients), the association between reduced 30-day mortality and higher AH50 was preserved in the remaining 288 patients (OR, 0.53 [95% CI, 0.30–0.93]; P = 0.027).
In contrast to AH50, we did not find a significant relationship between CH50 greater than or equal to median and ICU mortality (adjusted OR, 1.22 [95% CI, 0.72–2.09]; P = 0.46) nor 30-day mortality (adjusted OR, 1.02 [95% CI, 0.60–1.73]; P = 0.96) after adjustment for age, sex, presence of active neoplasm, SOFA, and batch effect. AH50 level as a continuous variable was associated with ICU mortality with decreasing probability of death in patients with higher complement pathway function (see Figure 1C; P = 0.015), whereas CH50 level was not predictive of ICU mortality (see Figure E3A; P = 0.31).
Others have shown associations between alternative pathway regulatory protein levels and long-term outcomes in patients with systolic heart failure (41). Therefore, we investigated differences in 1-year survival based on alternative pathway function. We found that AH50 greater than or equal to median was associated with increased 1-year survival (see Figure 1D; hazard ratio [HR], 0.59 [95% CI, 0.41–0.87]; adjusted P = 0.007) after multivariate adjustment, including the presence of chronic liver disease. Notably, CH50 values were not significantly associated with 1-year survival (see Figure E3B; HR, 1.00 [95% CI, 0.69–1.44]; adjusted P > 0.9). Limiting analysis to patients with sepsis (n = 202) provided a stronger survival association in patients with AH50 greater than or equal to median (HR, 0.51 [95% CI, 0.32–0.79]; adjusted P = 0.003).
Increased Alternative Complement Pathway Activity Is Associated with a Hypoinflammatory Subphenotype
Others have shown clinical subsets of ARDS patients characterized by hyperinflammatory versus hypoinflammatory subphenotypes, with implications for mortality during critical illness (28, 42). Notably, established ARDS subphenotypes may offer prognostic information, not only in patients with ARDS but also in mechanically ventilated patients at risk for ARDS (27).
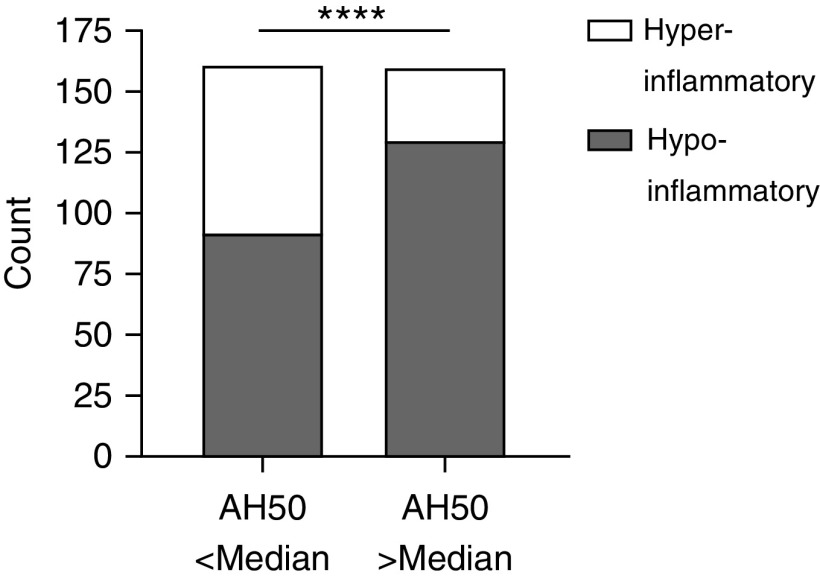
Therefore, we classified the inflammatory subphenotypes of 321 patients in our cohort using a previously validated model (28). Patients with elevated alternative complement activity were less likely to exhibit a hyperinflammatory subphenotype (Figure 2; OR, 0.30 [95% CI, 0.18–0.49]; P < 0.0001). We therefore sought to better understand the regulatory mechanisms that determine alternative pathway function in our cohort.
Figure 2.
Increased alternative complement pathway activity is associated with a hypoinflammatory phenotype. Alternative pathway function (AH50) greater than or equal to median is associated with a hypoinflammatory phenotype (odds ratio, 0.30 [95% confidence interval, 0.18–0.49]) as classified by a three-variable model consisting of bicarbonate, IL-8, and tumor necrosis factor receptor-1. Statistical analysis was by chi-square test. ****P < 0.0001.
Increased Alternative Complement Pathway Function Is Associated with Increased Serum Levels of Both Positive and Negative Regulatory Fluid-Phase Proteins
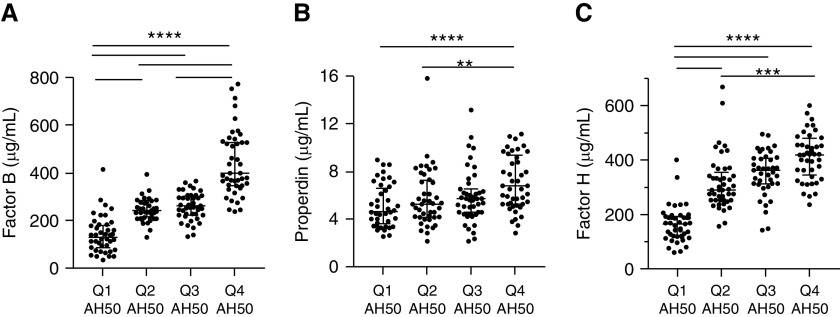
To investigate potential regulatory mechanisms that influence alternative pathway function, we examined serum levels of three major alternative pathway regulatory proteins within a stratified sample of patients from each quartile (N = 181). We found patients with the highest AH50 values had significantly higher levels of factor B (Figure 3A; Pearson correlation coefficient = 0.74; P < 0.001) and the positive regulator, properdin (see Figure 3B; Pearson correlation coefficient = 0.38; P < 0.001). Similarly, patients with the highest AH50 values had significantly higher levels of the negative regulator factor H (see Figure 3C; Pearson correlation coefficient = 0.58; P < 0.001). We note a trend toward improved survival in patients with factor B and factor H greater than the median that did not remain significant after multivariate adjustment (see Figure E4). Given the importance of alternative pathway regulatory factors in determining alternative pathway function, we next examined the transcriptional profile of alternative pathway regulatory factors in two external cohorts.
Figure 3.
Increased alternative complement pathway function (AH50) is associated with increased serum levels of both positive and negative regulatory fluid-phase proteins. Sera from 181 patients of 321 patients (n = 44, [quartile 1] Q1; n = 45, Q2; n = 45, Q3; and n = 47, Q4) were characterized for (A) factor B (μg/ml; Q1 median, interquartile range: 133, 92–176; Q4: 394, 347–526; P < 0.0001 for Q4 compared with all other quartiles), (B) properdin (μg/ml; Q1: 4.7, 3.7–6.5; Q4: 6.8, 5.4–9.3; P < 0.0001 compared with Q1), and (C) factor H (μg/ml; Q1: 166, 122–189; Q4: 419, 354–471; P < 0.0001 compared with Q1 and Q2). Statistical analysis was by Kruskal-Wallis test with Dunn’s post hoc test for multiple comparisons; horizontal lines represent post hoc statistical relationships. **P < 0.01, ***P < 0.001, and ****P < 0.0001.
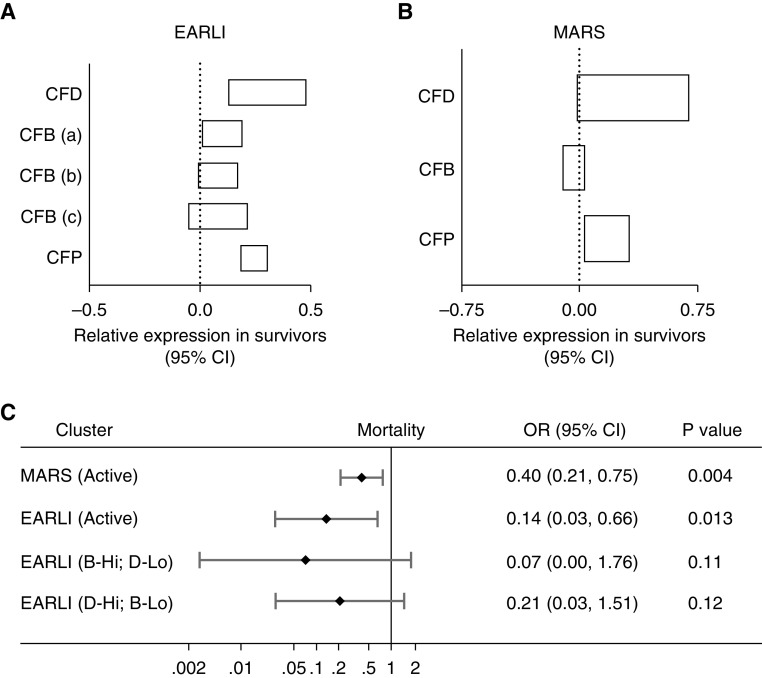
Increased Transcription of Fluid-Phase Alternative Pathway Components Is Associated with Improved Survival in Two External Critically Ill Cohorts
Both the EARLI and MARS cohorts report whole-blood transcriptional data of alternative pathway proximal CFD (complement factor D), CFB, and CFP (complement factor properdin) genes in critically ill patients with acute respiratory failure. We found increased relative expression of CFD, CFB (three separate transcripts), and CFP (Figure 4A) in EARLI patients who survived to 60 days. Similarly, we found increased relative expression of CFD and CFP in MARS patients who survived to 28 days. Because the AH50 is a total functional characterization of several proteins that contribute to alternative pathway activity, we sought to model alternative pathway activation by analysis of hierarchical clustering of gene expression data. Two clusters were identified in MARS: one cluster marked by elevated CFD and CFP expression (“Active”; n = 433) demonstrated decreased 28-day mortality (OR, 0.40 [95% CI, 0.21–0.75]; P = 0.004) compared with the cluster with lower expression. Four clusters were identified in EARLI: one cluster with elevated expression of CFD, CFB, and CFP (“Active”; n = 41) demonstrated decreased mortality (OR, 0.14 [95% CI, 0.03–0.66]; P = 0.013) compared with the reference cluster with decreased expression. Two other clusters, one with elevated CFB but decreased CFD (“B-Hi; D-Lo”, n = 4) and the other with elevated CFD but decreased CFB (“D-Hi; B-Lo”, n = 9) demonstrated a trend toward decreased mortality (OR, 0.07 [95% CI, 0.00–1.76] and OR, 0.21 [95% CI, 0.03–1.51], respectively) but did not reach statistical significance.
Figure 4.
Increased transcription of fluid-phase alternative pathway components is associated with improved survival in two external critically ill cohorts. Whole-blood transcription levels of CFD (complement factor D), CFB (complement factor B), and CFP (complement factor properdin) genes were increased in survivors of critical illness in the (A) EARLI (Early Assessment of Renal and Lung Injury) (n = 62; 48 survivors at 60 d) and (B) MARS (Molecular Diagnosis and Risk Stratification of Sepsis) (n = 479; 365 survivors at 28 d) cohorts as measured by Affymetrix gene array and published on the National Center for Biotechnology Information Gene Expression Omnibus. The 95% CI of relative gene expression as measured by t test is displayed. There are three separate CFB gene transcripts listed as CFB (a), CFB (b), and CFB (c) included in the EARLI data. (C) Cluster analysis using hierarchical clustering with complete linkage (stop parameters by Duda-Hart indices) revealed two MARS cohort clusters (active marked by increased CFD and CFP expression) and four EARLI cohort clusters (active marked by increased CFD, CFB, and CFP expression; B-Hi; D-Lo marked by elevated CFB and decreased CFD expression; and D-Hi; B-Lo marked by elevated CFD and decreased CFB expression) with decreased risk of mortality compared with the reference cluster in each cohort as measured by logistic regression. CI = confidence interval; OR = odds ratio.
Increased Alternative Complement Pathway Function Is Associated with Fewer Bloodstream Infections and Improved Serum Killing of a Nosocomial Lung Pathogen
To investigate whether complement activity influences the risk of infection during critical illness, we investigated the association between complement pathway function and the presence of documented bloodstream infection. The majority of patients (283 of 321; 88%) had one or more blood cultures drawn up to 24 hours before, or within 30 days after, ICU admission. Of the 48 (17%) patients with a blood culture positive for a pathogenic bacteria or yeast (Table 2), AH50 greater than or equal to median was associated with decreased risk of bloodstream infection (adjusted OR, 0.67 [95% CI, 0.45–0.98]; P = 0.040). We sought to further explore the role of complement function in host defense using a serum sensitivity assay. We used an extensively drug-resistant (XDR) nosocomial lung pathogen, K. pneumoniae strain KPC5, that is resistant to all antibiotics including meropenem and colistin but is sensitive to killing by serum from healthy volunteers (31). We found that serum from patients with elevated AH50 (quartile 4) demonstrated decreased growth of KPC5 in vitro compared with serum from patients with low AH50 (Figure 5A). Conversely, serum depleted of alternative complement pathway components factor D, factor B, and properdin demonstrated impaired ability to kill KPC5 that was restored by mixing equal parts serum from healthy volunteers (see Figure 5B).
Table 2.
Increased Alternative Complement Pathway Function Is Associated with Decreased Positive Blood Culture Burden
| Pathogen | Positive Blood Culture [n (% Patients with Blood Culture)] | AH50 ≥ Median [OR (95% CI)] | P Value | Adjusted* [OR (95% CI)] | P Value |
|---|---|---|---|---|---|
| Gram-negative bacteria† | 20 (7.1) | 0.67 (0.42–1.08) | 0.10 | 0.72 (0.44–1.19)‡§ | 0.20 |
| Gram-positive bacteria‖ | 31 (11.0) | 0.63 (0.34–1.15) | 0.13 | 0.73 (0.42–1.27) | 0.27 |
| Yeast¶ | 6 (2.1) | 0.53 (0.21–1.35) | 0.19 | 0.62 (0.23–1.70)‡§ | 0.35 |
| Any bacteria | 46 (16.3) | 0.68 (0.46–1.02) | 0.063 | 0.71 (0.48–1.05) | 0.082 |
| Any bacteria or yeast | 48** (17.0) | 0.65 (0.44–0.97) | 0.034 | 0.67 (0.45–0.98) | 0.040 |
Definition of abbreviations: AH50 = alternative complement pathway function; CI = confidence interval; OR = odds ratio.
Of 321 cohort patients, 283 had one or more blood cultures drawn from 24 hours before and 30 days after ICU admission. Multiple positive blood cultures collected on the same day were combined into a single result per organism.
Values were adjusted for age, sex, sequential organ failure assessment score, duration of ICU admission, total number of blood cultures drawn, the presence of chronic liver disease, and suspected sepsis.
Gram-negative bacteria include Acinetobacter baumannii, Escherichia coli, Klebsiella pneumoniae, K. variola, Pseudomonas aeruginosa, and Serratia marcescens.
Presence of chronic liver disease is not included in adjustment because the OR could not be calculated because no patients with chronic liver disease had gram-negative bacteria or yeast bloodstream infections.
Suspected sepsis is not included in adjustment because all patients with positive cultures were considered confirmed or probable cases of sepsis.
Gram-positive bacteria include Enterococcus faecalis, E. faecium, Staphylococcus aureus (both methicillin-susceptible and methicillin-resistant), Streptococcus gallolyticus, and Streptococcus pneumoniae.
Yeast include Candida albicans, C. glabrata, C. krusei, C. lusitaniae, C. parapsilosis, and C. tropicalis.
There were 48 patients with at least one positive culture, 9 of whom had more than one, for a total of 57 positive cultures.
Figure 5.
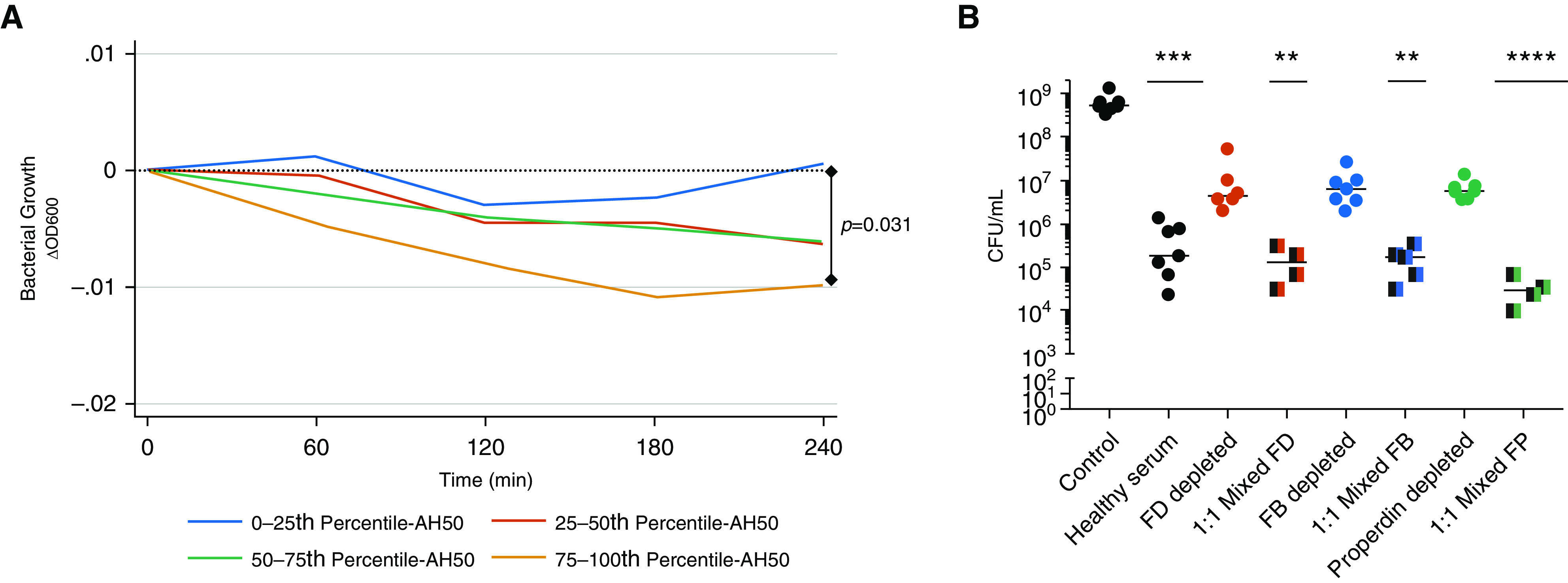
The alternative complement pathway contributes to in vitro serum control of an extensively drug-resistant Klebsiella pneumoniae lung pathogen. (A) Bacterial growth of the carbapenemase-producing K. pneumoniae clinical isolate KPC5 as measured by change in optical density 600 nm when grown in tryptic soy broth with 85% vol/vol serum from critically ill patients (n = 321). Median optical density 600 nm by quartile of AH50 is displayed. The association between complement activity and KPC5 growth was assessed by a multilevel mixed-effects linear regression model. (B) In separate experiments, KPC5 was grown for 4 hours in 5% vol/vol tryptic soy broth, serially diluted, plated, and counted (cfu/ml). Groups include KPC5 grown in 85% vol/vol solution of sterile phosphate buffered saline (control), healthy serum, and sera depleted of factor D (FD), factor B (FB), or properdin (FP) (n = 7 trials). In select experiments, healthy serum was also mixed 1:1 with sera depleted of FD (n = 4 trials), FB (n = 5 trials), and FP (n = 4 trials). Each point represents a single trial. Statistical analysis by Kruskal-Wallis test with Dunn’s post hoc significance compared with control is displayed. **P < 0.01, ***P < 0.001, and ****P < 0.0001. AH50 = alternative complement pathway function; ΔOD600 = change in optical density at 600 nm.
Cfb−/− and C3−/− Mice Demonstrate Increased Splenic Dissemination of K. pneumoniae after Acute Intrapulmonary Infection
To further explore the hypothesis that alternative complement pathway function supports host defense during infection, we used an acute intrapulmonary infection model with K. pneumoniae strains in mice genetically deficient for factor B (Cfb−/−), which effectively knocks out the alternative complement pathway, and C3 (C3−/−), which is crucial for alternative pathway amplification of all complement activity. We found similar lung bacterial burden (Figure 6A) but significantly increased splenic dissemination of a hypervirulent KP strain (ATCC 43816) 24 hours after intratracheal inoculation in Cfb−/− mice compared with WT (see Figure 6B), which was phenocopied in C3−/− mice. Serum cytokines revealed significantly elevated IL-1α, eotaxin, granulocyte colony-stimulating factor, IL-6, IL-12p40, and RANTES (regulated upon activation, normal T cell expressed and secreted) (see Figures 6C and E5) in Cfb−/− mice compared with WT. In separate experiments, we found minimal lung bacterial counts of the relatively avirulent XDR lung pathogen KPC5 in WT and C3−/− mice 24 hours postinfection (see Figure 6D). However, C3−/− mice demonstrated significantly increased splenic dissemination of KPC5 (see Figure 6E). Despite similar lung bacterial burden, C3−/− mice demonstrated higher BAL polymorphonuclear cell counts (see Figure 6F) and increased lung concentration of the cytokine IL-6 (see Figure 6G). There was no significant difference in BAL fluid protein or IgM levels between groups (see Figure E6).
Figure 6.
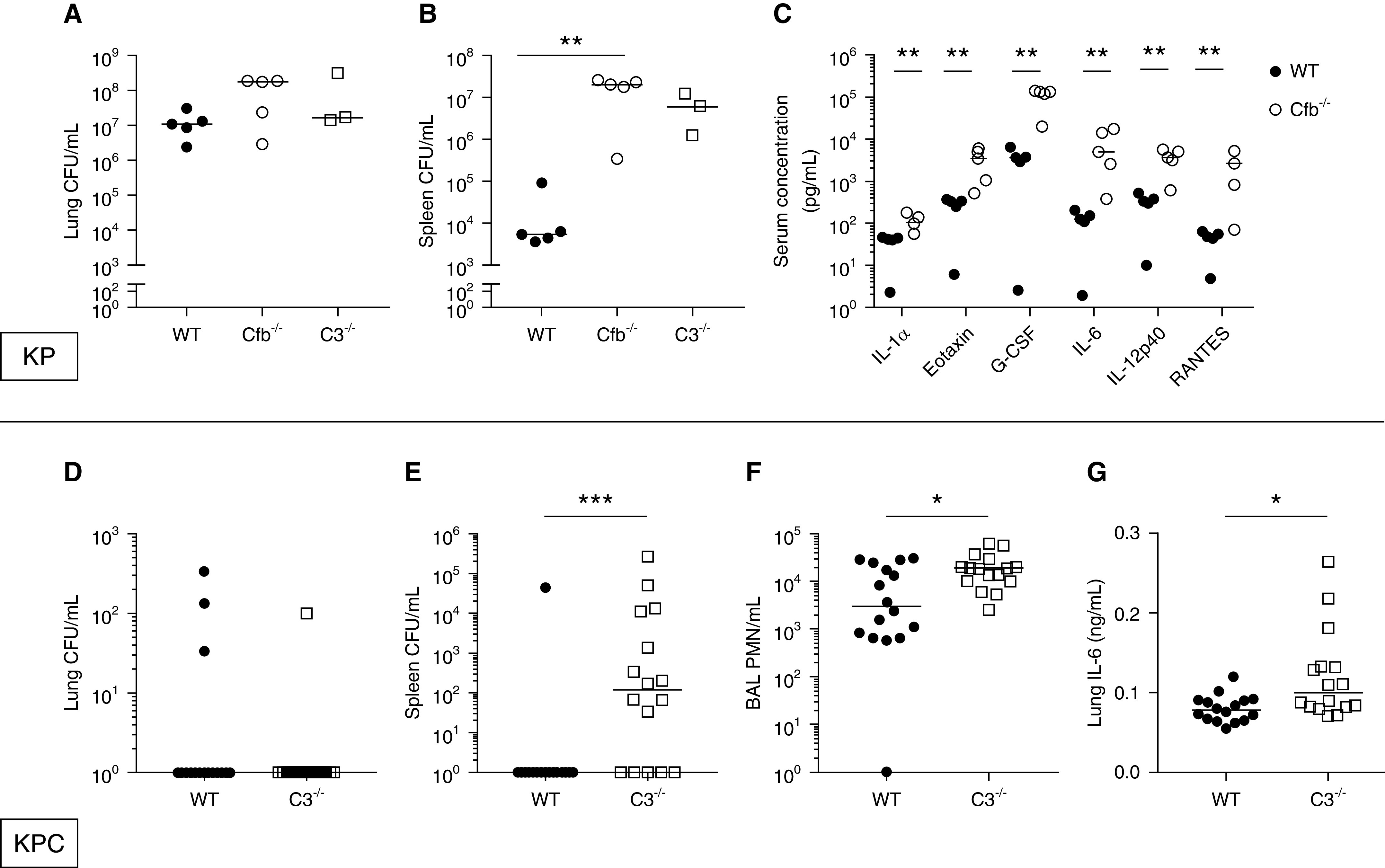
Cfb−/− and C3−/− mice demonstrate increased splenic dissemination of Klebsiella pneumoniae (KP) after acute intrapulmonary infection with both a hypervirulent and a nosocomial KPC strain. Wild-type (WT) (n = 5), Cfb−/− (n = 5), and C3−/− (n = 3) mice were intratracheally inoculated with 106 cfu of KP2 (American Type Culture Collection 43816), a hypervirulent reference KP strain. (A) Lung bacterial cfu/ml, (B) spleen cfu/ml, and (C) serum concentration (pg/ml) of select cytokines by multiplex obtained at necropsy 24 hours postinfection. In separate experiments, WT and C3−/− mice (n = 16 mice each group from two separate experiments) were intratracheally inoculated with 103 cfu of KPC5, a clinical isolate with muted virulence that is resistant to all tested antibiotics, and necropsy was performed at 24 hours. (D) Lung bacterial cfu/ml, (E) spleen bacterial cfu/ml, (F) BAL polymorphonucleated cells (PMN)/ml, and (F) lung IL-6 (ng/ml). Each point represents an individual mouse. (A and B) Statistical analysis by Kruskal-Wallis test with Dunn’s post hoc statistical significance compared with WT group displayed. (C–G) Statistical analysis by Mann-Whitney test. *P < 0.05, **P < 0.01, and ***P < 0.001. KPC = carbapenemase-producing Klebsiella pneumoniae; RANTES = regulated upon activation, normal T cell expressed and secreted.
Discussion
We examined the function of the classical and alternative complement pathways in 321 critically ill patients using a serum-sparing microscaled assay, which represents the largest data set characterizing complement function in a critically ill cohort to date. We found a wide distribution of complement function in our cohort and a novel association between increased alternative pathway function at ICU admission and reduced 30-day mortality. We further demonstrated that higher alternative pathway function was associated with increased survival one year after ICU admission. Patients with elevated alternative pathway function displayed a hypoinflammatory subphenotype, and higher levels of factor B and the positive regulatory protein, properdin, as well as the negative regulator, factor H. We found increased transcription of genes for proximal alternative pathway components factor D, factor B, and properdin in survivors of critical illness in publicly available data from two external cohorts. Within our cohort, there were fewer bloodstream infections in patients with AH50 greater than or equal to median, and serum from patients with AH50 greater than or equal to median showed enhanced control of XDR pathogen growth in vitro. Conversely, serum depleted of alternative pathway factors showed impaired control of the same XDR lung pathogen in vitro that corrected with the addition of healthy serum. Finally, we demonstrated that genetic deletion of factor B or C3, which is crucial to alternative pathway amplification, impairs host defense against extrapulmonary dissemination during acute intrapulmonary infection models in mice. Taken together, these findings suggest that increased fluid-phase alternative complement pathway function is a protective mechanism during the biological stress of critical illness.
Although the complement system has been previously examined in preclinical and clinical investigations of critical illness and respiratory failure (8–11, 18), few have addressed a specific role for alternative complement pathway function in clinical outcomes. Although others have studied complement activation in ARDS (43–46), we did not observe any difference in the frequency of ARDS in patients with AH50 greater than or equal to median and AH50 less than median in our cohort. Rather than investigating complement activation, we used the AH50 functional assay, which provides a global assessment of both positive and negative regulatory factors that determine total alternative complement pathway function. We propose two potential mechanisms for the observed benefit of increased alternative complement pathway function. First, elevated alternative complement pathway function could provide improved host defense, which may be crucial to protection during critical illness. Several preclinical models and clinical investigations have demonstrated that deficiencies in alternative complement function can impair host defense against pathogens (6, 7, 47–50). Furthermore, prior studies in humans during critical illness have identified upregulation of alternative complement and innate immune pathways in the glycoproteomic profile of sepsis survivors (21) and an association between genetic disposition toward alternative complement activation and decreased frequency of sepsis in critically ill children (51). Notably, the impaired ability of serum with low AH50 to control an XDR K. pneumoniae lung isolate invites the possibility that diminished alternative complement function during critical illness may increase the risk of some nosocomial infections. It is also possible that intact complement function may contribute to improved outcomes beyond the direct antimicrobial role of complement by supporting immune cell regulation such as cellular proliferation, metabolism, and survival (52, 53). For example, one intriguing concept is that, although immune cells can directly release complement into tissue microenvironments, circulating fluid-phase complement components may replenish complement activity in tissue microenvironments, contributing to critical local functions, including in the lung (2, 54). Therefore, the alternative complement pathway likely has important roles in host defense during critical illness.
The second mechanism of benefit we propose is that elevated alternative pathway function may imply a host with the ability to both rapidly initiate and deescalate an inflammatory response. In support of this concept, we found that patients with elevated alternative pathway function were more likely to demonstrate a hypoinflammatory subphenotype, which appears consistent with a recent report that elevated CFD transcription is associated with a beneficial subphenotype in patients with ARDS (30). Furthermore, we note the unexpected finding that patients with elevated alternative pathway function had elevated levels of a crucial negative regulator of the alternative pathway, factor H (55). Circulating levels of factor H have been reported to be stable between illness and recovery (11), and others have reported that factor H is not an acute phase reactant (56). Interestingly, we found factor H levels that are similar to prior reports in both critically ill patients (11) and stable outpatients (57–61). Therefore, it is possible that baseline factor H levels may be a major determinant of complement function during critical illness. Taken together, we propose that the ability to simultaneously initiate a robust host defense and deescalate an overly exuberant inflammatory response may have important implications for clinical outcomes (62–64).
Our study has several strengths, including characterization of total alternative complement pathway function in a heterogenous cohort of critically ill patients, evaluation of complement function and mortality, and translational models demonstrating potential mechanisms of protection provided by alternative complement function. However, one limitation is that findings of total alternative complement function are from a single academic medical center cohort. Notably, the data are supported by findings from two external cohorts that demonstrated increased transcription of proximal alternative pathway regulators in survivors of critical illness.
Conclusions
We have shown that increased alternative complement pathway function is associated with improved survival during critical illness, possibly due to improved host immune capacity. Further work remains to better define the biologic role of alternative complement pathway function during critical illness and to consider whether those pathways can be modulated in pursuit of improved patient outcomes (65).
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank the patients and patient families who have enrolled in the University of Pittsburgh Acute Lung Injury Registry. They also thank Dr. Faraaz Shah, Dr. John Evankovich, and Dr. Daniel Dunlap for their work to support the recruitment of patients and collection of biospecimens for the registry. They also thank Nancy Petro and Quanwei Yang for managing registry biospecimen processing, banking, and retrieval. They also thank Sean Ehinger for assistance with execution and quality control of complement factor assays. They also thank Dr. Nenoo Rawal of Complement Technology, Inc. for technical guidance in the development and validation of the microscaled complement pathway assays. They also thank Dr. John Atkinson of Washington University in St. Louis for kind provision of the Cfb−/− and C3−/− mouse colonies.
Footnotes
Supported by the NHLBI of the NIH under award numbers 4T32 HL007563, F32 HL142172, and L30 HL143734 (W.B.); K23 HL129987 (G.D.K); K08 HL148510 (H.S.K.); P01HL114453 (R.K.M., P.R., B.J.M., and J.S.L.); R01 HL097376 (B.J.M.); K24 HL123342 (A.M.); R01HL112937 (V.P.F.); R01 HL136143, R01 HL142084, and K24 HL143285 (J.S.L.); Career Development award number IK2 BX004886 from the U.S. Department of Veterans Affairs Biomedical Laboratory R&D (BLRD) Service (W.B.); American Heart Association Pre-Doctoral Fellowship 18PRE33960033 (T.F.O.); Lung Association and Children’s Discovery Institute of Washington University and St. Louis Children’s Hospital (PD-FR-2020–867) (H.S.K.); and the Vascular Medicine Institute, the Hemophilia Center of Western Pennsylvania, and the Institute for Transfusion Medicine (W.B). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, Department of Veterans Affairs, or any other sponsoring agency.
Author Contributions: W.B. performed the experiments; designed, analyzed, and interpreted the data; and wrote the manuscript. H.L., R.v.d.G., S.R.M., T.F.O., B.A., E.P., K.M., M.H., Z.X., and L.M. performed the experiments and analyzed the data. R.D., S.R., and M.S. performed critical data collection, database extraction, and management; and revised the work for important intellectual content. R.K.M., P.R., A.M., Y.D., and Y.Z. interpreted the data and revised the work for important intellectual content. G.D.K, H.S.K., B.J.M., and V.P.F. designed and interpreted the data and revised the work for important intellectual content. M.N. provided critical statistical expertise; designed, analyzed, and interpreted the data; and wrote the manuscript. J.S.L. conceived, designed, analyzed, and interpreted the data; and wrote the manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201910-2083OC on May 6, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Elvington M, Liszewski MK, Atkinson JP. Evolution of the complement system: from defense of the single cell to guardian of the intravascular space. Immunol Rev. 2016;274:9–15. doi: 10.1111/imr.12474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kulkarni HS, Elvington ML, Perng Y-C, Liszewski MK, Byers DE, Farkouh C, et al. Intracellular C3 protects human airway epithelial cells from stress-associated cell death. Am J Respir Cell Mol Biol. 2019;60:144–157. doi: 10.1165/rcmb.2017-0405OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harboe M, Ulvund G, Vien L, Fung M, Mollnes TE. The quantitative role of alternative pathway amplification in classical pathway induced terminal complement activation. Clin Exp Immunol. 2004;138:439–446. doi: 10.1111/j.1365-2249.2004.02627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blatt AZ, Pathan S, Ferreira VP. Properdin: a tightly regulated critical inflammatory modulator. Immunol Rev. 2016;274:172–190. doi: 10.1111/imr.12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zilow EP, Hauck W, Linderkamp O, Zilow G. Alternative pathway activation of the complement system in preterm infants with early onset infection. Pediatr Res. 1997;41:334–339. doi: 10.1203/00006450-199703000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Mueller-Ortiz SL, Drouin SM, Wetsel RA. The alternative activation pathway and complement component C3 are critical for a protective immune response against Pseudomonas aeruginosa in a murine model of pneumonia. Infect Immun. 2004;72:2899–2906. doi: 10.1128/IAI.72.5.2899-2906.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coonrod JD, Yoneda K. Comparative role of complement in pneumococcal and staphylococcal pneumonia. Infect Immun. 1982;37:1270–1277. doi: 10.1128/iai.37.3.1270-1277.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ecker EE, Seifter S, Dozois TF, Barr L. Complement in infectious disease in man. J Clin Invest. 1946;25:800–808. doi: 10.1172/JCI101766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCabe WR. Serum complement levels in bacteremia due to gram-negative organisms. N Engl J Med. 1973;288:21–23. doi: 10.1056/NEJM197301042880105. [DOI] [PubMed] [Google Scholar]
- 10.Fearon DT, Ruddy S, Schur PH, McCabe WR. Activation of the properdin pathway of complement in patients with gram-negative of bacteremia. N Engl J Med. 1975;292:937–940. doi: 10.1056/NEJM197505012921802. [DOI] [PubMed] [Google Scholar]
- 11.Whaley K, Schur PH, McCabe WR, Ruddy S. Modulation of the alternative complement pathway in patients with Gram-negative bacteraemia. Parasite Immunol. 1980;2:29–37. [Google Scholar]
- 12.Heideman M, Norder-Hansson B, Bengtson A, Mollnes TE. Terminal complement complexes and anaphylatoxins in septic and ischemic patients. Arch Surg. 1988;123:188–192. doi: 10.1001/archsurg.1988.01400260068008. [DOI] [PubMed] [Google Scholar]
- 13.Fosse E, Pillgram-Larsen J, Svennevig JL, Nordby C, Skulberg A, Mollnes TE, et al. Complement activation in injured patients occurs immediately and is dependent on the severity of the trauma. Injury. 1998;29:509–514. doi: 10.1016/s0020-1383(98)00113-2. [DOI] [PubMed] [Google Scholar]
- 14.Ganter MT, Brohi K, Cohen MJ, Shaffer LA, Walsh MC, Stahl GL, et al. Role of the alternative pathway in the early complement activation following major trauma. Shock. 2007;28:29–34. doi: 10.1097/shk.0b013e3180342439. [DOI] [PubMed] [Google Scholar]
- 15.Dobke M, Roberts C, Pearson G, Germany BA, Heck E, Baxter CR. A quantitative measurement of complement (C3) activation in severely burned patients. J Burn Care Rehabil. 1984;5:152–157. [Google Scholar]
- 16.Kvarnström A, Sokolov A, Swartling T, Kurlberg G, Mollnes TE, Bengtsson A. Alternative pathway activation of complement in laparoscopic and open rectal surgery. Scand J Immunol. 2012;76:49–53. doi: 10.1111/j.1365-3083.2012.02702.x. [DOI] [PubMed] [Google Scholar]
- 17.Rønholm E, Tomasdottir H, Runeborg J, Bengtsson A, Bengtson JP, Stenqvist O, et al. Complement system activation during orthotopic liver transplantation in man: indications of peroperative complement system activation in the gut. Transplantation. 1994;57:1594–1597. [PubMed] [Google Scholar]
- 18.Coonrod JD, Rylko-Bauer B. Complement levels in pneumococcal pneumonia. Infect Immun. 1977;18:14–22. doi: 10.1128/iai.18.1.14-22.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalter ES, Daha MR, ten Cate JW, Verhoef J, Bouma BN. Activation and inhibition of Hageman factor-dependent pathways and the complement system in uncomplicated bacteremia or bacterial shock. J Infect Dis. 1985;151:1019–1027. doi: 10.1093/infdis/151.6.1019. [DOI] [PubMed] [Google Scholar]
- 20.Burk A-M, Martin M, Flierl MA, Rittirsch D, Helm M, Lampl L, et al. Early complementopathy after multiple injuries in humans. Shock. 2012;37:348–354. doi: 10.1097/SHK.0b013e3182471795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeCoux A, Tian Y, DeLeon-Pennell KY, Nguyen NT, de Castro Brás LE, Flynn ER, et al. Plasma glycoproteomics reveals sepsis outcomes linked to distinct proteins in common pathways. Crit Care Med. 2015;43:2049–2058. doi: 10.1097/CCM.0000000000001134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morgan PB.Complement methods and protocolsTotowa, NJ: Humana Press; 2000 [Google Scholar]
- 23.Markiewski MM, DeAngelis RA, Lambris JD. Complexity of complement activation in sepsis. J Cell Mol Med. 2008;12:2245–2254. doi: 10.1111/j.1582-4934.2008.00504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karasu E, Nilsson B, Köhl J, Lambris JD, Huber-Lang M. Targeting complement pathways in polytrauma- and sepsis-induced multiple-organ dysfunction. Front Immunol. 2019;10:543. doi: 10.3389/fimmu.2019.00543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bain W, Olonisakin TF, Li H-H, Xiong Z, Nouraie SM, Kitsios G, et al. Reduced alternative complement activity is associated with impaired serum control of Carbapenemase-producing Klebsiella pneumoniae in a cohort of critically ill patients [abstract] Am J Respir Crit Care Med. 2018;197:A2788. [Google Scholar]
- 26.Bain W.Increased alternative complement pathway function is associated with reduced mortality in critically ill patients with acute respiratory failurePresented at the 15th Annual Respiratory Young Investigators’ Forum. 2019Chicago, IL [Google Scholar]
- 27.Kitsios GD, Yang L, Manatakis DV, Nouraie M, Evankovich J, Bain W, et al. Host-response subphenotypes offer prognostic enrichment in patients with or at risk for acute respiratory distress syndrome. Crit Care Med. 2019;47:1724–1734. doi: 10.1097/CCM.0000000000004018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Famous KR, Delucchi K, Ware LB, Kangelaris KN, Liu KD, Thompson BT, et al. ARDS Network. Acute respiratory distress syndrome subphenotypes respond differently to randomized fluid management strategy. Am J Respir Crit Care Med. 2017;195:331–338. doi: 10.1164/rccm.201603-0645OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kangelaris KN, Prakash A, Liu KD, Aouizerat B, Woodruff PG, Erle DJ, et al. Increased expression of neutrophil-related genes in patients with early sepsis-induced ARDS. Am J Physiol Lung Cell Mol Physiol. 2015;308:L1102–L1113. doi: 10.1152/ajplung.00380.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bos LDJ, Scicluna BP, Ong DSY, Cremer O, van der Poll T, Schultz MJ. Understanding heterogeneity in biologic phenotypes of acute respiratory distress syndrome by leukocyte expression profiles. Am J Respir Crit Care Med. 2019;200:42–50. doi: 10.1164/rccm.201809-1808OC. [DOI] [PubMed] [Google Scholar]
- 31.Olonisakin TF, Li H, Xiong Z, Kochman EJK, Yu M, Qu Y, et al. CD36 provides host protection against Klebsiella pneumoniae intrapulmonary infection by enhancing lipopolysaccharide responsiveness and macrophage phagocytosis. J Infect Dis. 2016;214:1865–1875. doi: 10.1093/infdis/jiw451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zou L, Feng Y, Li Y, Zhang M, Chen C, Cai J, et al. Complement factor B is the downstream effector of TLRs and plays an important role in a mouse model of severe sepsis. J Immunol. 2013;191:5625–5635. doi: 10.4049/jimmunol.1301903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsumoto M, Fukuda W, Circolo A, Goellner J, Strauss-Schoenberger J, Wang X, et al. Abrogation of the alternative complement pathway by targeted deletion of murine factor B. Proc Natl Acad Sci USA. 1997;94:8720–8725. doi: 10.1073/pnas.94.16.8720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bain W, Olonisakin T, Yu M, Qu Y, Hulver M, Xiong Z, et al. Platelets inhibit apoptotic lung epithelial cell death and protect mice against infection-induced lung injury. Blood Adv. 2019;3:432–445. doi: 10.1182/bloodadvances.2018026286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qu Y, Olonisakin T, Bain W, Zupetic J, Brown R, Hulver M, et al. Thrombospondin-1 protects against pathogen-induced lung injury by limiting extracellular matrix proteolysis. JCI Insight. 2018;3:e96914. doi: 10.1172/jci.insight.96914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee JS, Rosengart MR, Kondragunta V, Zhang Y, McMurray J, Branch RA, et al. Inverse association of plasma IL-13 and inflammatory chemokines with lung function impairment in stable COPD: a cross-sectional cohort study. Respir Res. 2007;8:64. doi: 10.1186/1465-9921-8-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duda RO, Hart PE, Stork DG.Pattern classification2nd ed.New York: John Wiley & Sons; 2001 [Google Scholar]
- 38.Goh WWB, Wang W, Wong L. Why batch effects matter in omics data, and how to avoid them. Trends Biotechnol. 2017;35:498–507. doi: 10.1016/j.tibtech.2017.02.012. [DOI] [PubMed] [Google Scholar]
- 39.Schreiber H, Rittirsch D, Flierl M, Brueckner U, Schneider M, Weiss M, et al. Complement activation during sepsis in humans. Adv Exp Med Biol. 2006;586:217–226. doi: 10.1007/0-387-34134-X_15. [DOI] [PubMed] [Google Scholar]
- 40.Unnewehr H, Rittirsch D, Sarma JV, Zetoune F, Flierl MA, Perl M, et al. Changes and regulation of the C5a receptor on neutrophils during septic shock in humans. J Immunol. 2013;190:4215–4225. doi: 10.4049/jimmunol.1200534. [DOI] [PubMed] [Google Scholar]
- 41.Shahini N, Michelsen AE, Nilsson PH, Ekholt K, Gullestad L, Broch K, et al. The alternative complement pathway is dysregulated in patients with chronic heart failure. Sci Rep. 2017;7:42532. doi: 10.1038/srep42532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Calfee CS, Delucchi K, Parsons PE, Thompson BT, Ware LB, Matthay MA NHLBI ARDS Network. Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials. Lancet Respir Med. 2014;2:611–620. doi: 10.1016/S2213-2600(14)70097-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Langlois PF, Gawryl MS, Zeller J, Lint T. Accentuated complement activation in patient plasma during the adult respiratory distress syndrome: a potential mechanism for pulmonary inflammation. Heart Lung. 1989;18:71–84. [PubMed] [Google Scholar]
- 44.Robbins RA, Russ WD, Rasmussen JK, Clayton MM. Activation of the complement system in the adult respiratory distress syndrome. Am Rev Respir Dis. 1987;135:651–658. doi: 10.1164/arrd.1987.135.3.651. [DOI] [PubMed] [Google Scholar]
- 45.Weigelt JA, Chenoweth DE, Borman KR, Norcross JF. Complement and the severity of pulmonary failure. J Trauma. 1988;28:1013–1019. doi: 10.1097/00005373-198807000-00017. [DOI] [PubMed] [Google Scholar]
- 46.Weinberg PF, Matthay MA, Webster RO, Roskos KV, Goldstein IM, Murray JF. Biologically active products of complement and acute lung injury in patients with the sepsis syndrome. Am Rev Respir Dis. 1984;130:791–796. doi: 10.1164/arrd.1984.130.5.791. [DOI] [PubMed] [Google Scholar]
- 47.Ram S, Lewis LA, Rice PA. Infections of people with complement deficiencies and patients who have undergone splenectomy. Clin Microbiol Rev. 2010;23:740–780. doi: 10.1128/CMR.00048-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ben-David I, Price SE, Bortz DM, Greineder CF, Cohen SE, Bauer AL, et al. Dynamics of intrapulmonary bacterial growth in a murine model of repeated microaspiration. Am J Respir Cell Mol Biol. 2005;33:476–482. doi: 10.1165/rcmb.2005-0053OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nypaver CM, Thornton MM, Yin SM, Bracho DO, Nelson PW, Jones AE, et al. Dynamics of human complement-mediated killing of Klebsiella pneumoniae. Am J Respir Cell Mol Biol. 2010;43:585–590. doi: 10.1165/rcmb.2009-0292OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stover CM, Luckett JC, Echtenacher B, Dupont A, Figgitt SE, Brown J, et al. Properdin plays a protective role in polymicrobial septic peritonitis. J Immunol. 2008;180:3313–3318. doi: 10.4049/jimmunol.180.5.3313. [DOI] [PubMed] [Google Scholar]
- 51.Agbeko RS, Fidler KJ, Allen ML, Wilson P, Klein NJ, Peters MJ. Genetic variability in complement activation modulates the systemic inflammatory response syndrome in children. Pediatr Crit Care Med. 2010;11:561–567. doi: 10.1097/PCC.0b013e3181d900ba. [DOI] [PubMed] [Google Scholar]
- 52.Arbore G, Kemper C, Kolev M. Intracellular complement: the complosome. In immune cell regulation. Mol Immunol. 2017;89:2–9. doi: 10.1016/j.molimm.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kolev M, Le Friec G, Kemper C. Complement: tapping into new sites and effector systems. Nat Rev Immunol. 2014;14:811–820. doi: 10.1038/nri3761. [DOI] [PubMed] [Google Scholar]
- 54.Elvington M, Liszewski MK, Bertram P, Kulkarni HS, Atkinson JPA. A C3(H20) recycling pathway is a component of the intracellular complement system. J Clin Invest. 2017;127:970–981. doi: 10.1172/JCI89412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ferreira VP, Pangburn MK, Cortés C. Complement control protein factor H: the good, the bad, and the inadequate. Mol Immunol. 2010;47:2187–2197. doi: 10.1016/j.molimm.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schwaeble W, Zwirner J, Schulz TF, Linke RP, Dierich MP, Weiss EH. Human complement factor H: expression of an additional truncated gene product of 43 kDa in human liver. Eur J Immunol. 1987;17:1485–1489. doi: 10.1002/eji.1830171015. [DOI] [PubMed] [Google Scholar]
- 57.Esparza-Gordillo J, Soria JM, Buil A, Almasy L, Blangero J, Fontcuberta J, et al. Genetic and environmental factors influencing the human factor H plasma levels. Immunogenetics. 2004;56:77–82. doi: 10.1007/s00251-004-0660-7. [DOI] [PubMed] [Google Scholar]
- 58.Sofat R, Mangione PP, Gallimore JR, Hakobyan S, Hughes TR, Shah T, et al. Distribution and determinants of circulating complement factor H concentration determined by a high-throughput immunonephelometric assay. J Immunol Methods. 2013;390:63–73. doi: 10.1016/j.jim.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 59.Nozal P, Garrido S, Alba-Domínguez M, Espinosa L, Peña A, Córdoba SR, et al. An ELISA assay with two monoclonal antibodies allows the estimation of free factor H and identifies patients with acquired deficiency of this complement regulator. Mol Immunol. 2014;58:194–200. doi: 10.1016/j.molimm.2013.11.021. [DOI] [PubMed] [Google Scholar]
- 60.Moreno-Navarrete JM, Martínez-Barricarte R, Catalán V, Sabater M, Gómez-Ambrosi J, Ortega FJ, et al. Complement factor H is expressed in adipose tissue in association with insulin resistance. Diabetes. 2010;59:200–209. doi: 10.2337/db09-0700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Silva AS, Teixeira AG, Bavia L, Lin F, Velletri R, Belfort R, Jr, et al. Plasma levels of complement proteins from the alternative pathway in patients with age-related macular degeneration are independent of Complement Factor H Tyr402His polymorphism. Mol Vis. 2012;18:2288–2299. [PMC free article] [PubMed] [Google Scholar]
- 62.Delano MJ, Ward PA. Sepsis-induced immune dysfunction: can immune therapies reduce mortality? J Clin Invest. 2016;126:23–31. doi: 10.1172/JCI82224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hotchkiss RS, Monneret G, Payen D. Immunosuppression in sepsis: a novel understanding of the disorder and a new therapeutic approach. Lancet Infect Dis. 2013;13:260–268. doi: 10.1016/S1473-3099(13)70001-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 65.Ali YM, Hayat A, Saeed BM, Haleem KS, Alshamrani S, Kenawy HI, et al. Low-dose recombinant properdin provides substantial protection against Streptococcus pneumoniae and Neisseria meningitidis infection. Proc Natl Acad Sci USA. 2014;111:5301–5306. doi: 10.1073/pnas.1401011111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.