Introduction
More than three million cases of severe acute respiratory syndrome coronavirus-2 infections (SARS-CoV-2) have been documented in the United States.1 The prevalence of skin findings in coronavirus disease 2019 (COVID-19) infections has been reported as high as 20%.2 Two COVID-19 patients with associated transient unilateral livedo reticularis (LR) were previously described.3 We present a case of transient LR as the initial presenting sign in a case of COVID-19.
Patient
A previously healthy 34-year-old female health care worker with no medical history and known recent workplace exposures to SARS-CoV-2 experienced erythema of the left hand. Over 2 days, the rash progressed, and congestion, fever, and anosmia developed. Five days after the eruption, she had new-onset severe body aches and progression of rash to bilateral arms and thighs. The patient had no gastrointestinal complaints, shortness of breath, or cough. Nasopharyngeal polymerase chain reaction performed at this time confirmed SARS-CoV-2 infection.
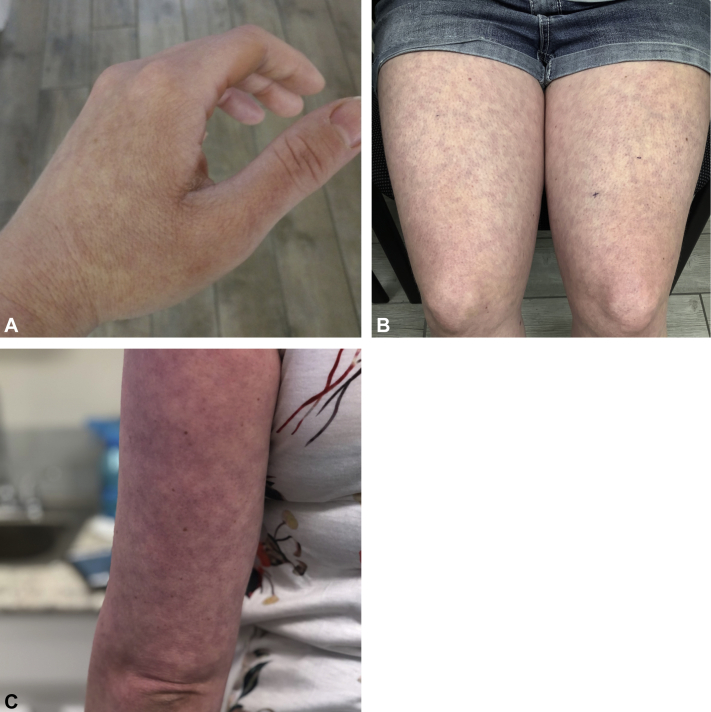
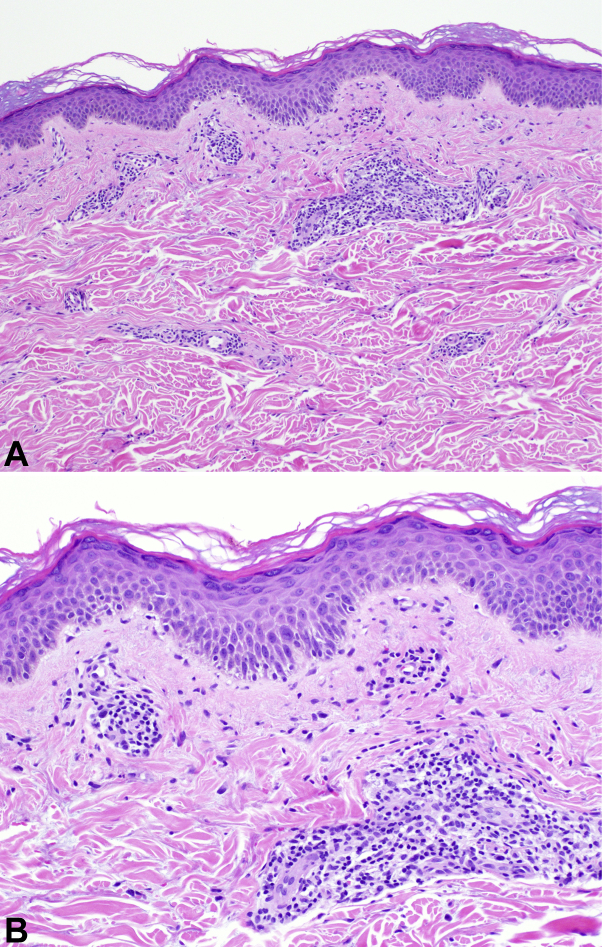
Despite resolution of her cold-like symptoms within 1 week from rash onset, her rash and body aches persisted. Dermatology examination found well-demarcated reticular lacy erythematous patches consistent with LR with overlying faint morbilliform exanthem of the left hand, bilateral thighs, and arms (Fig 1). Laboratory workup was unremarkable, with normal blood indices (white blood cells, 7.3 thousand/μL; lymphocytes, 2211/μL [30%]; and platelets, 356,000/μL), electrolytes, coagulation studies, D-dimer (0.38 μg/mL), liver-enzymes, ferritin, C-reactive protein, complement, fibrinogen, antinuclear antibodies (negative), and lupus anticoagulant (negative). Four-millimeter skin punch biopsies of the wrist and thighs found perivascular lymphocytic inflammation, increased superficial dermal mucin, and necrotic keratinocytes consistent with viral exanthem (Fig 2). Without treatment or hospitalization, the patient had full recovery with near-total resolution of her rash within 2 weeks.
Fig 1.
Morphologic features of SARS-CoV-2–associated LR. Photographs of the left hand (A), thighs (B), and arm (C).
Fig 2.
Histologic features of SARS-CoV-2–associated LR. (Hematoxylin-eosin stain. Original magnifications: A, ×100; B, ×200.)
Discussion
This is one of few reports of cutaneous changes preceding systemic signs/symptoms of COVID-19 infection.3, 4, 5, 6 This patient was healthy, with outpatient disease management, negative laboratory workup, and lack of newly initiated pharmacotherapy, arguing against other confounding LR triggers such as coagulopathy/vasculopathy and strengthening the association between LR and SARS-CoV-2. Of note, this patient was young, a demographic overrepresented in a high-rash-prevalence COVID-19 study.2 Similar to previous COVID-19-associated LR, the trunk was spared, as opposed to trunk predilection noted with other COVID-19 cutaneous findings.2
Histology identified viral exanthem, with other notable changes, including superficial deposition of mucin. Mucin is a pathologic feature of multiple skin lesions, including granuloma annulare, acute lupus erythematosus, dermatomyositis, pseudomyxoma cutis, and reactive skin conditions.7,8 It is unclear whether the observation of mucin in this biopsy was associated with subclinical disease that may have been unmasked with infection or whether it was directly associated with the exanthem.
Although histologic findings were consistent with viral exanthem, the gross morphologic feature of her rash was transient progressive LR, suggesting underlying vascular phenomena. Even in pathogenic LR, microscopic vascular changes are notoriously difficult to detect, as inflammation can be distributed in a segmental fashion, or, more simply, there can be low-grade inflammation, whereby patent vessels remain without discernible vasculitis or vasculopathy. An incisional wedge biopsy can certainly increase the potential diagnostic yield, permitting a more thorough assessment of cutaneous vessels, but this was not feasible in our patient's case.
The pathophysiology of LR involves hypercongestion of the cutaneous venous vasculature by restricted arterial inflow, exaggerated venous dilation, or impaired venous outflow.9,10 Endotheliitis, increasingly accepted as a disease feature of COVID-19,11, 12, 13 may have contributed to the etiopathogenesis of our patient's LR. SARS-CoV-2 is increasingly recognized to have wide-reaching tropism, with capacity to infect and replicate in various tissues and cell types, including lung, gut, kidney, and brain.14,15 There is increasing suspicion for direct endothelial cell infection by SARS-CoV-2,16 and one study has observed SARS-CoV-2 spike protein directly within the endothelial cell cytoplasm in COVID-19–associated chilblains lesions.17 Endotheliitis, possibly through such direct endothelial cell infection, may in turn result in vascular changes that drive altered coagulation and vascular homeostasis.11,18 This finding may ultimately result in dilated venous vasculature and the manifestation of LR, as was observed in this patient.
In this case, laboratory coagulation test results were normal and did not demonstrate classical COVID-19–associated coagulopathy (increased fibrinogen and d-dimer with minimal changes in the prothrombin and partial thromboplastin times).15 Importantly, COVID-19–associated coagulopathy is a laboratory finding associated with advanced and significant infection. In this patient with appropriate vitals and underwhelming clinical picture, the identification of vascular-associated skin findings argues in defense of direct viral influence on blood vessel behavior. Of note, it is not recommended to routinely evaluate for coagulation profiles in COVID-19 patients not admitted to the hospital.
Of interest, this patient was a health care worker, as were 3 previous COVID-19 patients who showed isolated cutaneous changes at onset of disease course.3,4,6 It remains to be determined whether health care providers are at greater risk for cutaneous changes, possibly because of differences in exposure, inoculation dose, or route of infection.
It is important for clinicians to be aware of dermatologic manifestations, including exanthems, with SARS-CoV-2 infections. As the pandemic continues and rapid identification of disease remains important, the observation of COVID-19–associated skin rashes such as COVID-19 LR may serve as an early sign of developing disease. The association between COVID-19 LR and disease severity remains to be determined. To better treat patients who have COVID-19 LR, we recommend documenting coagulopathic parameters,3 viral load versus time, and progression of clinical findings.
Footnotes
Funding sources: None.
Conflicts of interest: None disclosed.
References
- 1.Centers for Disease Control and Prevention COVID-19 Cases, Data, and Surveillance, Available at: https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html Accessed August 10, 2020.
- 2.Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34(5):e212–e213. doi: 10.1111/jdv.16387. [DOI] [PubMed] [Google Scholar]
- 3.Manalo I.F., Smith M.K., Cheeley J., Jacobs R. A dermatologic manifestation of COVID-19: transient livedo reticularis. J Am Acad Dermatol. 2020;83(2):700. doi: 10.1016/j.jaad.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henry D., Ackerman M., Sancelme E., Finon A., Esteve E. Urticarial eruption in COVID-19 infection. J Eur Acad Dermatol Venereol. 2020;34(6):e244–e245. doi: 10.1111/jdv.16472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alramthan A., Aldaraji W. A case of COVID-19 presenting in clinical picture resembling chilblains disease. First report from the Middle East. Clin Exp Dermatol. 2020;45(6):746–748. doi: 10.1111/ced.14243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Quintana-Castanedo L., Feito-Rodríguez M., Valero-López I., Chiloeches-Fernández C., Sendagorta-Cudós E., Herranz-Pinto P. Urticarial exanthem as early diagnostic clue for COVID-19 infection. JAAD Case Rep. 2020;6(6):498–499. doi: 10.1016/j.jdcr.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernandez-Flores A., Saeb-Lima M. Mucin as a diagnostic clue in dermatopathology. J Cutan Pathol. 2016;43(11):1005–1016. doi: 10.1111/cup.12782. [DOI] [PubMed] [Google Scholar]
- 8.Yokoyama E., Nakamura Y., Okita T., Nagai N., Muto M. CD34+ dermal dendritic cells and mucin deposition in dermatomyositis. World J Dermatol. 2016;5(2):65–71. [Google Scholar]
- 9.Gutierrez J., Katan M., Elkind M.S.V. Stroke: Pathophysiology, Diagnosis, and Management. 2016. Collagen Vascular and Infectious Diseases; pp. 619–631.e6. [Google Scholar]
- 10.Rose A.E., Saggar V., Boyd K.P., Patel R.R., McLellan B. Livedo reticularis. Dermatol Online J. 2013;19(12):20705. [PubMed] [Google Scholar]
- 11.Varga Z., Flammer A.J., Steiger P. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goshua G., Pine A.B., Meizlish M.L. Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre , cross-sectional study. Lancet Haematol. 2020;7(8):E575–E582. doi: 10.1016/S2352-3026(20)30216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ackermann M., Verleden S.E., Kuehnel M. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Puelles V.G., Lütgehetmann M., Lindenmeyer M.T. Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med. 2020;383(6):590–592. doi: 10.1056/NEJMc2011400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chu H., Chan J.F.-W., Yuen T.T.-T. Comparative tropism, replication kinetics, and cell damage profiling of SARS-CoV-2 and SARS-CoV with implications for clinical manifestations, transmissibility, and laboratory studies of COVID-19: an observational study. Lancet Microbe. 2020;1(1):E14–E23. doi: 10.1016/S2666-5247(20)30004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Becker R.C. COVID-19 update: Covid-19-associated coagulopathy. J Thromb Thrombolysis. 2020;50(1):54–67. doi: 10.1007/s11239-020-02134-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colmenero I., Santonja C., Alonso-Riaño M. SARS-CoV-2 endothelial infection causes COVID-19 chilblains: histopathological, immunohistochemical and ultraestructural study of 7 paediatric cases. Br J Dermatol. 2020 doi: 10.1111/bjd.19327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teuwen L.A., Geldhof V., Pasut A., Carmeliet P. COVID-19: the vasculature unleashed. Nat Rev Immunol. 2020;20:389–391. doi: 10.1038/s41577-020-0343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]