Abstract
BACKGROUND
Liddle syndrome (LS), an autosomal dominant disorder, is a common monogenic hypertension in pediatrics. In this study, we reported a novel SCNN1G variant in a Chinese family with pediatric LS, and conduct a systematic review of epithelial sodium channel (ENaC)-gene-positive LS cases to conclude the clinical genetic features of LS in childhood.
METHODS
Next-generation sequencing and in silico analysis were performed in the proband to discover candidate variants. Sanger sequencing was used to identify the predicted likely pathogenic variant. LS patients in this family were treated with amiloride. The Medline database was searched to summarize clinical features of pediatric LS cases whose age at genetic diagnosis was not more than 18 years.
RESULTS
Genetic analysis identified a novel SCNN1G missense variant (c.1874C>T, p.Pro625Leu) in the proband with LS in childhood. In silico analysis revealed this heterozygous variant was highly conserved and deleterious. A total of 38 publications described pediatric LS associated with 25 pathogenic variants in SCNN1B and SCNN1G in 54 children. Despite the phenotypic heterogeneity, early-onset hypertension is the most common feature. All LS patients in this family or the reviewed cases showed significantly improvements in hypertension and hypokalemia after treatment with ENaC inhibitors.
CONCLUSIONS
This study identified a novel SCNN1G missense variant in a patient with pediatric LS, expanding the genetic spectrum of SCNN1G and demonstrating the PY motif of γ-ENaC as a potential mutant region. Early identification and specific management of LS in children and adolescents are important to prevent the development of hypertensive end-organ disease.
Keywords: blood pressure, hypertension, Pediatric Liddle syndrome, SCNN1G gene
Hypertension has become as an important health issue in the pediatric population in recent years1; the prevalence of hypertension in children and adolescents in China is 3.1%.2 Inherited pathogenic factors make a substantial contribution to pediatric hypertension. In particular, monogenic hypertension is an increasingly recognized type of hypertension in children.3,4 Keeping genetic conditions in mind is helpful when diagnosing hypertension in children, because the younger the child, the more likely it is that their hypertension is due to a secondary cause.5
Liddle syndrome (LS, OMIM #177200) is a common monogenic form of hypertension in children, with an autosomal dominant pattern of inheritance.4 In 1963, Liddle et al. first reported a 16-year-old index case with low renin resistant hypertension, severe hypokalemia, and metabolic alkalosis in a Caucasian family.6 Further exploration showed that LS is caused by gain-of-function pathogenic variants in the epithelial sodium channel (ENaC) genes.7 The prevalence of LS was shown to be 1.52% (5/330)8 and 0.91% (7/766)9 in patients with early-onset hypertension; that is, those diagnosed at less than 40 years of age. The actual prevalence of LS in the pediatric hypertensive population remains unknown, due to genetic testing is not widespread. Thus, LS might be largely underdiagnosed with a much higher prevalence than currently estimated.10,11
In this study, we reported a male adolescent proband with LS and a novel missense variant in exon 13 of the SCNN1G gene. The identified variant was detected in 3 additional relatives. We conducted a systematic review to identify LS cases that were genetically confirmed during childhood. Our review indicated that genetic testing is essential for early identification of LS, especially in children and adolescents with early-onset hypertension.
METHODS
Participants
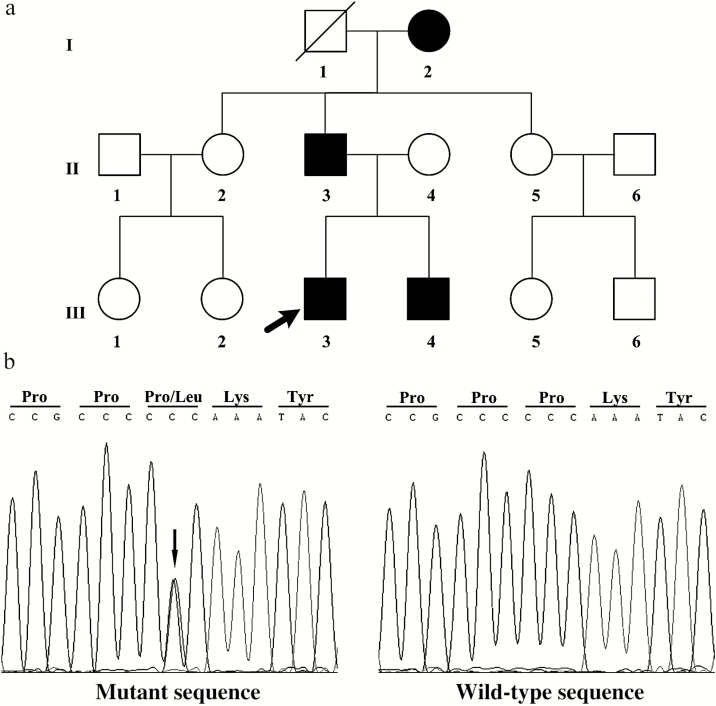
The index patient (subject III-3), at the age of 14 years, was found to have an average of 3 measurements of blood pressure (BP) during 3 different clinic visits of 150/100 mm Hg, which was above the sex- and age-specific 99th percentile (138/90 mm Hg).12 To identify the etiology of early-onset hypertension, the patient was admitted to the hypertension ward of Fuwai Hospital. As well as the proband, 10 family members (Figure 1a) were enrolled in this study for genetic analysis. To exclude common genetic variants, candidate variants were identified in 100 unrelated hypertension patients and 100 normotensive subjects.
Figure 1.
Pedigree and Sanger sequencing results of the 3-generation family with Liddle syndrome. (a) Solid symbols represent the identified variant carriers; open symbols represent normal individuals without the identified variant; the black arrow indicates the proband. (b) Sanger sequencing showing mutant and wild-type sequences in patients with Liddle syndrome and normal individuals, respectively, in this study. The black arrow indicates the identified variant site (c.1874C>T, p.Pro625Leu) in the mutant sequence.
This study was approved by the Ethics Committee of Fuwai Hospital and was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all participants.
Biochemical examination
Plasma renin concentration (PRC) and plasma aldosterone concentration were measured by chemiluminescence immunoassay using the LIAISON Direct Renin kit (DiaSorin S.p.A, Vercelli, Italy) and the LIAISON Aldosterone kit (DiaSorin Inc., Stillwater, MN, USA). Plasma was collected from patients after 2 hours of upright position for measuring PRC and plasma aldosterone concentration.
Genetic testing
Genomic DNA was extracted from peripheral blood leukocytes using the QIAamp DNA Blood Mini kit (QIAGEN, Hilden, Germany). We performed targeted sequencing in the proband, with a panel of 41 genes associated with monogenic hypertension (Supplementary Table S1 online). Sanger sequencing in all subjects was used to identify candidate variants, which were selected by means of in silico analysis. Exon 13 of SCNN1G was amplified by polymerase chain reaction using a pair of primers according to Yang et al.13 The polymerase chain reaction products were sequenced using an ABI Prism 377 DNA sequencer (Applied Biosystems, Foster City, CA, USA).
Systematic review
To clarify reported genetic and clinical features of ENaC-gene-positive LS patients in childhood, we retrieved papers on LS published in English on Medline from 1963 to November 2019, using the terms “Liddle syndrome,” “Liddle’s syndrome,” and “pseudoaldosteronism.” In addition, data and references on ENaC gene variants were enriched after searching the Human Gene Mutation Database (www.hgmd.org). Cases were included based on the following criteria: LS patients must have been identified by genetic testing and the patients were aged ≤18 years. Two authors completed the study selection independently, with consultation from a third author in case of discrepancies. Statistical analysis was performed using SPSS (SPSS 16.0; SPSS Inc., Chicago, IL, USA).
RESULTS
Clinical features
At the time of hospitalization, the proband had high BP (163/96 mm Hg), low-normal potassium level (3.58 mmol/l, normal range 3.5–5.3 mmol/l), and hypoaldosteronism in the supine position (2.9 ng/dl). He denied a history of vomiting, diarrhea, and use of licorice or other medications. Other hormonal determinations, including PRC, serum cortisol, adrenocorticotropin, catecholamine, metanephrine, normetanephrine, thyroid, and gonadal hormones were within normal limits. Echocardiography and computed tomography showed no abnormality of the heart, aorta, kidney, and adrenal or renal arteries. Polysomnography monitoring excluded sleep apnea syndrome.
Pedigree investigation revealed that the proband’s grandmother, father, and younger brother had early-onset hypertension. His grandmother and father failed to control BP within normal range, even using 3 kinds of antihypertensive medications. The proband’s grandmother (subject I-2) suffered a stroke at the age of 60 years. His grandmother and father had hypokalemia, hyporeninemia, and hypoaldosteronism (Table 1).
Table 1.
Clinical and biochemical characteristics of patients with Liddle syndrome in this family
| Subjects | Gender | BMI, kg/m2 | Onset age of hypertension, y | BPa, mm Hg | Serum K+, mmol/l | PAC, ng/dl | PRC, μIU/ml | ADRR | Treated with amiloride | |
|---|---|---|---|---|---|---|---|---|---|---|
| BP, mm Hg | Serum K+, mmol/l | |||||||||
| I-2 | F | 17.6 | 28 | 180/110 | 3.04 | 2.9 | 3.7 | 0.784 | 140/90 | 3.89 |
| II-3 | M | 24.8 | 30 | 180/120 | 2.94 | 2.5 | 4.0 | 0.625 | 135/85 | 3.96 |
| III-3 | M | 27.8 | 14 | 160/100 | 3.58 | 3.6 | 8.8 | 0.409 | 120/80 | 4.13 |
| III-4 | M | 15.7 | 3 | 120/80 | 3.20 | 2.8 | 4.2 | 0.667 | 95/65 | 4.05 |
Abbreviations: ADRR, plasma aldosterone to direct renin ratio; BMI, body mass index; BP, blood pressure; F, female; K+, potassium level (normal range: 3.5–5.3 mmol/l); M, male; PAC, plasma aldosterone concentration (normal range: 3.0–35.3 ng/dl); PRC, plasma renin concentration (normal range: 4.4–46.1 μIU/ml).
aThe maximum blood pressure was measured more than 3 times.
Genetic analysis
A novel heterozygous variant in exon 13 of SCNN1G, c.1874C>T, was found in the proband (Figure 1b). This missense variant altered proline to leucine at codon 625 (p.Pro625Leu), resulting in a change in the PY motif. To date, the p.Pro625Leu variant in SCNN1G had not been reported in the Exome Aggregation Consortium database (http://exac.broadinstitute.org/), the 1000 Genomes Project database (http://browser.1000genomes.org), or the Human Gene Mutation Database (http://www.hgmd.org). In silico analysis predicted this variant to be deleterious, using the SIFT (http://sift.jcvi.org), Provean (http://provean.jcvi.org/index.php), PolyPhen-2 (http://genetics.bwh.harvard.edu/pph2), and MutationTaster (http://www.mutationtaster.org). In addition, Pro625 in γ-ENaC protein is highly conserved in vertebrates. No pathogenic variant in SCNN1B or other genes was identified (Supplementary Table S1 online). The predicted likely pathogenic variant was found in the 3 affected relatives (I-2, II-3, and III-4), but not in other family members. Moreover, none of 100 unrelated patients with hypertension or 100 normotensive individuals had this SCNN1G variant (c.1874C>T), indicating that it is not a common genetic variant.
Tailored therapy for LS patients
ENaC inhibitors are effective in the treatment of LS. There are only compound amiloride (amiloride 2.5 mg and hydrochlorothiazide 25 mg each tablet) in the market. The proband and 3 family members were treated with compound amiloride (1–4 tablets/day), and BP and plasma potassium level were reexamined after 1 month of tailored therapy. At reexamination, all patients showed significant improvements in BP and potassium levels (Table 2).
Table 2.
Systematic review data of clinical characteristics of 54 pediatric patients with Liddle syndrome with identified mutations in epithelial sodium channel genes
| Parameters | Summary data | Number of cases available |
|---|---|---|
| Male, n (%) | 33 (61.11) | 54 |
| Median age at genetic diagnosis, y | 14 (0.1, 18) | 51 |
| Hypertension, n (%) | 48 (88.89) | 54 |
| Median age at hypertension onset, y | 12.75 (0.1, 17) | 48 |
| Median Max. systolic/diastolic BP, mm Hg | ||
| Before treatment | 153/100 (240/180, 100/51) | 50 |
| After treatmenta | 120/78 (150/110, 90/40) | 35 |
| Hypokalemia, n (%) | 39 (78) | 50 |
| Median Min. serum or plasma K+, mmol/l | ||
| Before treatment | 3.2 (1.8, 4.6) | 48 |
| After treatmenta | 4.1 (3.01, 4.9) | 25 |
| Suppressed PAC, n (%) | 37 (72.55) | 51 |
| Suppressed PRA or PRC, n (%) | 44 (86.27) | 51 |
| Family history, n (%) | 36 (90) | 40 |
Abbreviations: BP, blood pressure; PAC, plasma aldosterone concentration; PRA, plasma renin activity; PRC, plasma renin concentration.
aPediatric Liddle syndrome patients were treated with amiloride or triamterene.
Systemic review on genetic and clinical characteristics of pediatric LS
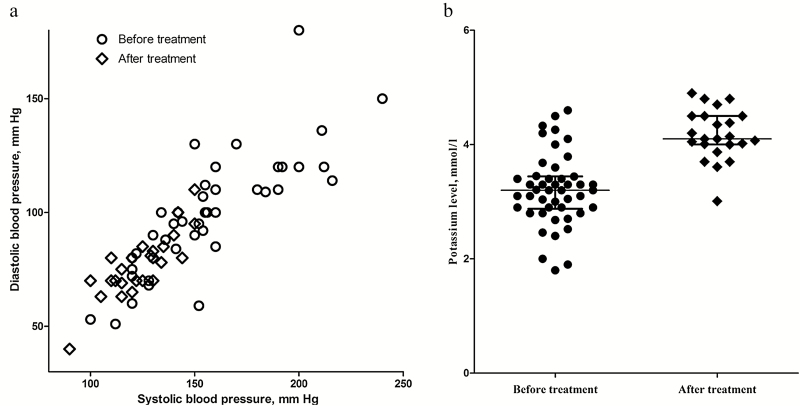
Our review of the literatures found 54 cases (male, 61.11%) of genetically confirmed pediatric LS, including the 2 young patients in the present study. A total of 25 pathogenic variants (including missense, nonsense, and frameshift variants) in SCNN1B and SCNN1G were identified in pediatric LS (Supplementary Table S2 online). The median age at hypertension onset was 12.75 years, ranging from 0.1 to 17 years, and the median age at genetic diagnosis was 14 years. Phenotypic heterogeneity was reflected in the incidence of hypertension (88.89%), hypokalemia (78%), hypoaldosteronism (72.55%), and hyporeninemia (86.27%). After treatment with ENaC inhibitors, BP (median maximum value: 153/100 vs. 120/78 mm Hg) and potassium level (median minimum value: 3.2 vs. 4.1 mmol/l) were significantly improved (Figure 2). Only 10% cases were sporadic, 90% of patients had a family history of LS (Table 2).
Figure 2.
Systematic review data on blood pressure and potassium level before and after treatment with epithelial sodium channel (ENaC) inhibitors in pediatric patients with Liddle syndrome. (a) Reduction in systolic and diastolic blood pressure; (b) significant increase (improvement) in potassium level.
DISCUSSION
In this paper, we reported a novel heterozygous missense variant (c.1874C>T, p.Pro625Leu) in SCNN1G in a Chinese family with LS and conducted a systemic review on genetic and clinical features of pediatric LS. A diagnosis of LS should be strongly considered in pediatric cases of monogenic hypertension.
The ENaC, together with renal outer medullar K+ channel and Na+/K+ ATPase, plays essential roles in hydroelectrolytic homeostasis and blood volume.14 In the kidney, ENaC is expressed in the luminal membrane of the principal cells of the distal nephron, and consists of 3 partly homologous subunits (α-ENaC, β-ENaC, and γ-ENaC).15 Mutations in ENaC genes impair expression of proline-rich regions (PY motif), resulting in loss of regulatory binding sites for Nedd4-2, an ubiquitin ligase associated with breakdown of ENaC.16,17 Therefore, uncontrolled ENaC is constitutively activated with increased Na+ reabsorption and subsequent intravascular volume expansion, resulting in high BP.18,19 Hypokalemia is secondary to excess Na+ reabsorption promoting extrusion of K+ via the Na+/K+ ATPase pumps.20 Renin and aldosterone are suppressed by both the elevated sodium level and the volume expansion.5 This molecular mechanism explains the typical clinical manifestations observed in LS patients.
Pediatric LS is mainly affected by pathogenic variants in SCNN1B and SCNN1G (located on chromosome 16p), which encode the β and γ subunits of ENaC, respectively. In the pediatric LS literatures, 25 ENaC gene pathogenic variant sites have been reported, consisting of 19 SCNN1B variants and 6 SCNN1G variants (including this study). These pathogenic variants either cause a missense variant of a key residue in the PY motif or generate a nonsense or frameshift (deletions and insertions) variant that truncates the cytoplasmic carboxyl terminus with loss of the PY motif.21
SCNN1G pathogenic variants are relatively infrequent in the spectrum of ENaC variants. To date, only 9 different pathogenic variants have been identified in SCNN1G (5 in pediatric LS). In particular, SCNN1G missense variants are quite rare. Hiltunen et al. described the first missense variant located in the extracellular domain of γ-ENaC associated with increased ENaC activity and LS.22 Then, in 2018, Lata et al. reported a missense variant (c.1874C>G, p. Pro625Arg) of a key residue in the PY motif in a white male, who presented with hypertension and hypokalemia at 13 years of age.23 The present study identified the second missense variant (c.1874C>T, p.Pro625Leu) in the same nucleotide position, changing cytosine to thymine. A predicted likely pathogenic SCNN1G variant may locate in the key residue in the PY motif, resulting in LS.
Among LS patients, phenotypic heterogeneity is very common, especially in children. In this family, the proband’s grandmother, father, and younger brother all presented with typical LS symptoms, such as severe hypertension, hypokalemia, hyporeninemia, and hypoaldosteronism. However, the proband manifested only early-onset hypertension with low-normal levels of potassium, plasma aldosterone concentration, and PRC. According to the literature review (Table 2), phenotypic heterogeneity in pediatric LS is mainly reflected in the following aspects. Early-onset hypertension is the most common (88.89%) clinical feature of pediatric LS, with a median maximum BP of 153/100 mm Hg before management. Overall, 78% of pediatric cases had hypokalemia (median minimum potassium level: 3.2 mmol/l). Hyporeninemia were observed in 72.55% and 86.27% of pediatric pathogenic variants carriers, respectively. Tetti et al. reported that, in 200 LS patients diagnosed by genetic testing, 92.4% had hypertension, 71.8% had hypokalemia, and 58.2% had hypoaldosteronemia.24 In 54 genetically confirmed LS probands, 100% had hypertension, 94% had hypokalemia, 84% had hypoaldosteronism, and 94% had hyporeninemia.25 Thus, pediatric LS patients are more likely to be normotensive. LS patients lacking a family history have also been noted. Cui et al. reported that de novo pathogenic variants accounted for 50% of cases,26 and Yang et al. found that 86% index cases with LS had a family history.25 Consistent with our review in this study, only 4 (10%) pediatric cases were isolated with de novo pathogenic variants.13,26,27 Although a family history of childhood hypertension is a clue for pediatric LS, physicians should consider the isolated LS individual with a de novo pathogenic variant.
It is helpful for early diagnosis to define the molecular basis of LS in childhood (Supplementary Figure S1 online). Usually, genetic diagnosis occurs at a later age than the onset of hypertension.25 In pediatric cases of LS, the median ages at genetic diagnosis and hypertension onset are 14 and 12.75 years, respectively. Actual age at genetic diagnosis should be much later, because not all LS children included in this study were probands. Misdiagnosis or underdiagnosis without genetic confirmation misses the best opportunity for early control of disorders in pediatric patients with LS.
Comprehensive management (Supplementary Figure S1 online) for LS patients consists of a healthy lifestyle (low-sodium diet, with <2 g of NaCl/day, not smoking, and getting more exercise) and treatment with ENaC antagonists.28,29 Patients with LS do respond to treatment with ENaC inhibitors, such as amiloride and triamterene. ENaC inhibitors can improve arterial hypertension and hypokalemia, because they reverse the pathological volume expansion and excessive sodium reabsorption.30 In contrast, mineralocorticoid receptor antagonists are not helpful in the management of LS, because Na+ reabsorption is independent of mineralocorticoid receptor activation.31 Hence, the lack of response to mineralocorticoid receptor antagonists should increase suspicions about LS.32
We acknowledge several limitations of this study. We did not conduct electrophysiological measurements of the amiloride-sensitive sodium current. The systematic review data lacked information on long-term prognosis, thus we could not evaluate management efficacy.
In conclusion, we identified a novel and predicted likely pathogenic SCNN1G missense variant in a pediatric LS proband in a Chinese family. This discovery expands the genetic spectrum of SCNN1G pathogenic variants and demonstrates the PY motif of γ-ENaC to be a potential mutant region. We recommend that LS should be considered in children with early-onset hypertension, regardless of potassium level, plasma aldosterone concentration, or PRC, and even in the absence of a family history. Furthermore, once a genetic diagnosis of LS is confirmed, at-risk family members should be screened for the same variant to allow early identification. For pediatric patients with LS, it is crucial to make a precise diagnosis as early as possible, because tailored therapy can prevent the development of hypertensive end-organ damage and decrease the morbidity and mortality associated with long-term and uncontrolled hypertension.33
SUPPLEMENTARY MATERIAL
Supplementary data are available at American Journal of Hypertension online.
Supplemental Figure S1. Flow chart for diagnosis and treatment of Liddle syndrome. ACTH, adrenocorticotropic hormone; AME, apparent mineralocorticoid excess; CAH, congenital adrenal hyperplasia; COX2, cyclooxygenase-2; DOC, deoxycorticosterone; ENaC, epithelial sodium channel; GRS, glucocorticoid-resistance syndrome; GS, Geller syndrome; K, potassium level; N, normal; NASIDs, nonsteroidal anti-inflammatory drugs.
FUNDING
This work was supported by CAMS Innovation Fund for Medical Sciences (2016-I2M-1-002), the National Key Research and Development Program of China (2016YFC1300100), National Natural Science Foundation of China (81600305), Beijing Nova Program (Z171100001117026), PUMC Youth Fund and the Fundamental Research Funds for the Central Universities (3332015205 and 3332019134), and PUMC Graduate Innovation Fund (2018-1002-01-14).
DISCLOSURE
The authors declared no conflict of interest.
REFERENCES
- 1. Bruyne PD, Walle JV. Management of hypertension in children and adolescents. Acta Clin Belg 2015; 70:87–94. [DOI] [PubMed] [Google Scholar]
- 2. Meng L, Liang Y, Liu J, Hu Y, Yan Y, Mi J. Prevalence and risk factors of hypertension based on repeated measurements in Chinese children and adolescents. Blood Press 2013; 22:59–64. [DOI] [PubMed] [Google Scholar]
- 3. Aggarwal A, Rodriguez-Buritica D. Monogenic hypertension in children: a review with emphasis on genetics. Adv Chronic Kidney Dis 2017; 24:372–379. [DOI] [PubMed] [Google Scholar]
- 4. Simonetti GD, Mohaupt MG, Bianchetti MG. Monogenic forms of hypertension. Eur J Pediatr 2012; 171:1433–1439. [DOI] [PubMed] [Google Scholar]
- 5. Raina R, Krishnappa V, Das A, Amin H, Radhakrishnan Y, Nair NR, Kusumi K. Overview of monogenic or Mendelian forms of hypertension. Front Pediatr 2019; 7:263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liddle GW, Bledsoe T, Coppage WS Jr. A familial renal disorder simulating primary aldosteronism but with negligible aldosterone secretion. Trans Assoc Am Phys 1963; 76:199–213. [Google Scholar]
- 7. Yang KQ, Xiao Y, Tian T, Gao LG, Zhou XL. Molecular genetics of Liddle’s syndrome. Clin Chim Acta 2014; 436:202–206. [DOI] [PubMed] [Google Scholar]
- 8. Wang LP, Yang KQ, Jiang XJ, Wu HY, Zhang HM, Zou YB, Song L, Bian J, Hui RT, Liu YX, Zhou XL. Prevalence of Liddle syndrome among young hypertension patients of undetermined cause in a Chinese population. J Clin Hypertens (Greenwich) 2015; 17:902–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liu K, Qin F, Sun X, Zhang Y, Wang J, Wu Y, Ma W, Wang W, Wu X, Qin Y, Zhang H, Zhou X, Wu H, Hui R, Zou Y, Jiang X, Song L. Analysis of the genes involved in Mendelian forms of low-renin hypertension in Chinese early-onset hypertensive patients. J Hypertens 2018; 36:502–509. [DOI] [PubMed] [Google Scholar]
- 10. Ceccato F, Mantero F. Monogenic forms of hypertension. Endocrinol Metab Clin North Am 2019; 48:795–810. [DOI] [PubMed] [Google Scholar]
- 11. Pagani L, Diekmann Y, Sazzini M, De Fanti S, Rondinelli M, Farnetti E, Casali B, Caretto A, Novara F, Zuffardi O, Garagnani P, Mantero F, Thomas MG, Luiselli D, Rossi E. Three reportedly unrelated families with Liddle syndrome inherited from a common ancestor. Hypertension 2018; 71:273–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu LS; Writing Group of 2010 Chinese Guidelines for the Management of Hypertension 2010 Chinese guidelines for the management of hypertension. Zhonghua Xin Xue Guan Bing Za Zhi 2011; 39:579–615. [PubMed] [Google Scholar]
- 13. Yang KQ, Lu CX, Xiao Y, Liu YX, Jiang XJ, Zhang X, Zhou XL. A novel frameshift mutation of epithelial sodium channel β-subunit leads to Liddle syndrome in an isolated case. Clin Endocrinol (Oxf) 2015; 82:611–614. [DOI] [PubMed] [Google Scholar]
- 14. Hanukoglu I, Hanukoglu A. Epithelial sodium channel (ENaC) family: phylogeny, structure-function, tissue distribution, and associated inherited diseases. Gene 2016; 579:95–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Canessa CM, Schild L, Buell G, Thorens B, Gautschi I, Horisberger JD, Rossier BC. Amiloride-sensitive epithelial Na+ channel is made of three homologous subunits. Nature 1994; 367:463–467. [DOI] [PubMed] [Google Scholar]
- 16. Rotin D, Staub O. Role of the ubiquitin system in regulating ion transport. Pflugers Arch 2011; 461:1–21. [DOI] [PubMed] [Google Scholar]
- 17. Benos DJ, Stanton BA. Functional domains within the degenerin/epithelial sodium channel (Deg/ENaC) superfamily of ion channels. J Physiol 1999; 520 Pt 3:631–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Botero-Velez M, Curtis JJ, Warnock DG. Brief report: Liddle’s syndrome revisited—a disorder of sodium reabsorption in the distal tubule. N Engl J Med 1994; 330:178–181. [DOI] [PubMed] [Google Scholar]
- 19. Martinez-Aguayo A, Fardella C. Genetics of hypertensive syndrome. Horm Res 2009; 71:253–259. [DOI] [PubMed] [Google Scholar]
- 20. Vehaskari VM. Heritable forms of hypertension. Pediatr Nephrol 2009; 24:1929–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Warnock DG, Kusche-Vihrog K, Tarjus A, Sheng S, Oberleithner H, Kleyman TR, Jaisser F. Blood pressure and amiloride-sensitive sodium channels in vascular and renal cells. Nat Rev Nephrol 2014; 10:146–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hiltunen TP, Hannila-Handelberg T, Petäjäniemi N, Kantola I, Tikkanen I, Virtamo J, Gautschi I, Schild L, Kontula K. Liddle’s syndrome associated with a point mutation in the extracellular domain of the epithelial sodium channel gamma subunit. J Hypertens 2002; 20:2383–2390. [DOI] [PubMed] [Google Scholar]
- 23. Lata S, Marasa M, Li Y, Fasel DA, Groopman E, Jobanputra V, Rasouly H, Mitrotti A, Westland R, Verbitsky M, Nestor J, Slater LM, D’Agati V, Zaniew M, Materna-Kiryluk A, Lugani F, Caridi G, Rampoldi L, Mattoo A, Newton CA, Rao MK, Radhakrishnan J, Ahn W, Canetta PA, Bomback AS, Appel GB, Antignac C, Markowitz GS, Garcia CK, Kiryluk K, Sanna-Cherchi S, Gharavi AG. Whole-exome sequencing in adults with chronic kidney disease: a pilot study. Ann Intern Med 2018; 168:100–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tetti M, Monticone S, Burrello J, Matarazzo P, Veglio F, Pasini B, Jeunemaitre X, Mulatero P. Liddle syndrome: review of the literature and description of a new case. Int J Mol Sci 2018; 19:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yang KQ, Lu CX, Fan P, Zhang Y, Meng X, Dong XQ, Luo F, Liu YX, Zhang HM, Wu HY, Cai J, Zhang X, Zhou XL. Genetic screening of SCNN1B and SCNN1G genes in early-onset hypertensive patients helps to identify Liddle syndrome. Clin Exp Hypertens 2018; 40:107–111. [DOI] [PubMed] [Google Scholar]
- 26. Cui Y, Tong A, Jiang J, Wang F, Li C. Liddle syndrome: clinical and genetic profiles. J Clin Hypertens (Greenwich) 2017; 19:524–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang Y, Zheng Y, Chen J, Wu H, Zheng D, Hui R. A novel epithelial sodium channel gamma-subunit de novo frameshift mutation leads to Liddle syndrome. Clin Endocrinol (Oxf) 2007; 67:801–804. [DOI] [PubMed] [Google Scholar]
- 28. Gao L, Wang L, Liu Y, Zhou X, Hui R, Hu A. A family with Liddle syndrome caused by a novel missense mutation in the PY motif of the beta-subunit of the epithelial sodium channel. J Pediatr 2013; 162:166–170. [DOI] [PubMed] [Google Scholar]
- 29. Melcescu E, Phillips J, Moll G, Subauste JS, Koch CA. 11Beta-hydroxylase deficiency and other syndromes of mineralocorticoid excess as a rare cause of endocrine hypertension. Horm Metab Res 2012; 44:867–878. [DOI] [PubMed] [Google Scholar]
- 30. Awadalla M, Patwardhan M, Alsamsam A, Imran N. Management of Liddle syndrome in pregnancy: a case report and literature review. Case Rep Obstet Gynecol 2017; 2017:6279460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Athimulam S, Lazik N, Bancos I. Low-renin hypertension. Endocrinol Metab Clin North Am 2019; 48:701–715. [DOI] [PubMed] [Google Scholar]
- 32. Mulatero P, Verhovez A, Morello F, Veglio F. Diagnosis and treatment of low-renin hypertension. Clin Endocrinol (Oxf) 2007; 67:324–334. [DOI] [PubMed] [Google Scholar]
- 33. Guzman-Limon M, Samuels J. Pediatric hypertension: diagnosis, evaluation, and treatment. Pediatr Clin North Am 2019; 66:45–57. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.