Abstract
Study Objectives
For most women, the menopause is accompanied by hot flashes and sleep problems. Although hot flashes reportedly wake women from sleep, in the few studies that have used objective measures of both sleep and hot flashes, links between hot flashes and nocturnal awakening have been inconsistent. In a well-characterized cohort of midlife women, we examined the association between objectively assessed hot flashes and actigraphically defined wake from sleep. We hypothesized that wake episodes would be more likely during an objective hot flash relative to minutes without a hot flash.
Methods
Peri- and postmenopausal midlife women underwent simultaneous objective measurement of hot flashes (sternal skin conductance) and sleep (actigraphy) over 24 hours in the home. The likelihood of waking in the minutes during the hot flash relative to the minutes preceding the hot flash was compared using generalized estimating equations.
Results
We studied 168 women with at least one objective nocturnal hot flash and actigraphy data. Actigraphy-assessed wake episodes were concurrent with 78% of the objective hot flashes. We found an increased likelihood of wake in the minutes during the objective hot flash (0 to +5 min: OR [95% CI] = 5.31 (4.46 to 6.33); p < .0001) relative to the minutes preceding it (–10 to –1 min). The increased likelihood of wake occurred irrespective of whether the women reported the objective hot flash.
Conclusion
Among these women who underwent objective measurement of sleep and hot flashes, nocturnal wakefulness was observed with the majority of hot flashes.
Keywords: hot flashes, vasomotor symptoms, night sweats, menopause, sleep, actigraphy
Statement of Significance.
The menopause is typically accompanied by symptoms such as hot flashes and sleep problems. Although women often report hot flashes waking them from sleep, research on the covariation of hot flashes and sleep has produced mixed findings. This study used objective measures of sleep (actigraphy) and hot flashes (sternal skin conductance) among 168 midlife women studied in their home. Findings showed actigraphic wake episodes were observed with 78% of objective hot flashes. Further, an increased likelihood of wake during the hot flash relative to the minutes preceding the objective hot flash was observed. These data indicate that hot flashes are associated with awakening from sleep.
Introduction
The menopause transition is universal for women as they age. For most women, the menopause transition is accompanied by characteristic symptoms such as hot flashes (also known as night sweats or vasomotor symptoms) and sleep disturbance (e.g. trouble falling asleep, nighttime waking, and early-morning wakening). Hot flashes are experienced by more than 70% of women [1] and sleep problems are reported by 40%–60% of women during the menopause transition [2–5]. Both symptoms often persist for years [6, 7] and are linked to significant distress and impairment [8, 9].
Despite the longstanding recognition of and high prevalence of these symptoms, the precise relationship between hot flashes and disturbed sleep is not well understood. Many women report that hot flashes wake them up [10], with reported hot flashes among the strongest and most consistent correlates of reported sleep disturbance during the menopause transition [5, 11, 12]. Typically, reports of overnight hot flashes are provided the following morning, yet these reports can be affected by memory, mood, and the quality of sleep the night before [13]. Relationships are less clear in the few studies that have used “objective” measures of sleep (e.g. actigraphy and polysomnography) and hot flashes (e.g. sternal skin conductance). Using objective measures to address these questions is useful, as these symptoms occur during and around sleep, when precise reporting may be difficult. One early study of 14 women found evidence of a concurrent association between objective hot flashes and objective awakenings [14]. However, subsequent studies found no such associations [15–17]. A larger 2007 study of 102 women did not support an association between objective hot flashes and sleep efficiency but did find objective hot flashes in the first half of the night and subjective sleep quality [18]. Two recent studies are notable. In one such study, 34 women underwent objective assessments of sleep and hot flashes, and 69% of the objective hot flashes were associated with awakenings [19]. Another recent study of 28 premenopausal women who underwent pharmacologic suppression of the reproductive axis did not find objective hot flashes associated with transitions to wake, yet did find that 66% of hot flashes occurred within 5 min of awakening [20]. Thus, given the mixed findings in the extant literature, paired with relatively small samples sizes, methodological limitations, and highly specific samples in some cases (e.g. premenopausal women undergoing pharmacological suppression of the reproductive axis) [20], further investigation of relationships between hot flashes and sleep is warranted.
We examined the association between physiologically assessed hot flashes and actigraphically defined wake episodes from sleep. We tested this question in a well-characterized cohort of 168 peri- and postmenopausal women who underwent simultaneous sternal skin conductance measurement of hot flashes and actigraphy-assessed sleep over 24 h in the home environment. We hypothesized that wake episodes would be more likely during an objectively detected hot flash than in the preceding minutes before the hot flash. We further investigated whether any association between hot flash occurrence and wake varied as a function of whether the hot flash was concurrently reported by the participant. In exploratory analyses, we tested whether the association between hot flash occurrence and wake varied as a function of whether the woman identified herself at screening as typically having hot flashes. Further, because both sleep and hot flashes show marked differences between racial/ethnic groups [1, 21], we also tested whether associations between hot flashes and waking from sleep varied as a function of race/ethnicity.
Study Participants
Participants included 304 nonsmoking women aged 40–60 years recruited for a study investigating relationships between menopausal symptoms and cardiovascular disease risk as described in detail elsewhere [22]. Briefly, women were recruited from the community via local advertisements, mailings, and online message board postings. By design, half of the women reported daily hot flashes, and half reported no hot flashes in the past 3 months [23]. Women were either late perimenopausal (2–12 months amenorrhea) or postmenopausal (≥12 months amenorrhea), and exclusion criteria included current smoking, hysterectomy, and/or bilateral oophorectomy; history of heart disease, stroke, arrhythmia, ovarian/gynecological cancer; current pregnancy; current chemotherapy; or having used the following medications in the past 3 months: oral/transdermal estrogen or progesterone, selective estrogen receptor modulators, selective serotonin reuptake inhibitors, serotonin–norepinephrine reuptake inhibitors, gabapentin, insulin, β-blockers, calcium channel blockers, α-2 adrenergic agonists, or other antiarrhythmic agents. Of the 184 women who showed nocturnal hot flashes, 16 were excluded from this analysis due to a lack of valid actigraphy data, yielding 168 participants in this analysis. These 16 women did not differ from the women included in this analysis on study variables (ps > .05).
Design and Procedures
Women underwent telephone and in-person screening procedures, physical measurements, and 24 h of ambulatory hot flash and actigraphy sleep monitoring, and questionnaire completion. Procedures were approved by the University of Pittsburgh Institutional Review Board. Participants provided written informed consent.
Measures
Hot flashes
Hot flashes were measured over 24 h via the VU-AMS (VU University Amsterdam, the Netherlands) [24, 25], a portable ambulatory monitor that quantifies hot flashes via sternal skin conductance, a validated measure of hot flashes [26, 27]. After monitoring, hot flash data were downloaded, reviewed, and scored via UFI software (DPSv3.7; Morro Bay, CA) according to standard, validated methods [26–28] that have demonstrated reliability including in the present laboratory (ĸ = .86) [29]. A 20-min lockout period was implemented after the start of the hot flash, during which no subsequent hot flashes were coded. Women were also asked to report hot flashes they subjectively experienced by pressing event mark buttons on the hot flash monitor and the wrist actigraph, which provided date and time-stamped event marks. Hot flashes were classified as occurring during the nighttime sleep period or during the daytime wake period. The nocturnal sleep period was defined by the daily sleep diary and further delimited by actigraphic sleep onset.
Actigraphic sleep
Women wore an actigraph unit (Actiwatch 2, Respironics, Inc., Murrysville, PA) [30] on the wrist of the nondominant hand and completed a daily sleep diary [31] during monitoring. The sleep diary was used to determine bedtime (time tried to go to sleep) and rise time (final wake time), which comprised the sleep interval for actigraphy data. Actigraphy data were collected in 1-min epochs and analyzed with Philips Actiware, v6.0.0 software, with a wake threshold of 40, and number of epochs for sleep onset/offset of 10 (i.e. 10 min). For these analyses, data points falling within the nocturnal sleep interval (defined by sleep diary and further delimited by the first actigraphic sleep onset of at least 10 min in length and final actigraphic sleep offset) were considered for analysis. Wake during the sleep interval was defined as minutes with an activity threshold of 40 or above.
Additional variables
Demographics, medical history, and medication use were assessed by standard instruments. Menopause status was obtained from self-reported menstrual bleeding patterns [23]. Height and weight were measured by fixed stadiometer and balance beam scale, respectively, and body mass index (BMI) calculated.
Data Analysis
Variables were examined for outliers, cell sizes, and deviations from normality. Consistent with prior work [32, 33], we evaluated the odds of waking episodes in the minutes during (0–5 min following the onset of the hot flash) and after the hot flash (6–15 min following the onset of the hot flash) relative to before the hot flash (10–1 min before the onset of the hot flash) in generalized estimating equations (GEE) with a logit link and an unstructured correlation structure. GEE have the advantage of accounting for the lack of independence of hot flash or waking episodes within a woman. We tested interactions by whether the hot flash was reported (based on diary and/or button press). As the study included both women who did and did not self-identify as having hot flashes (classified based on screening interview), we further tested interactions by a woman’s reported hot flash status at screening; models were stratified where significant interactions were observed. Similar interactions were tested for race/ethnicity. Further, in sensitivity analyses, we examined relationships using different time intervals around the hot flash (e.g. hot flash interval being 1 min before to 4 min following the onset of the hot flash). Although in this primarily within-woman analysis in which between-woman covariates are less relevant, we considered covariates age, race/ethnicity, education, BMI, shift work, and sleep medication use in additional models. Finally, we additionally conducted primary models excluding women who were shift workers (n = 7), women who were taking sleep medications (hypnotics, melatonin, over the counter sleep aids, n = 6). Analyses were performed with SAS, v9.4 (SAS Institute, Cary, NC). Models were two-sided, α = 0.05.
Results
Participants were on average 54 years old and postmenopausal (Table 1). We observed 591 objective hot flashes across the participants, 128 (22%) of which were concurrently reported by participants at the time of their occurrence.
Table 1.
Participant characteristics
| N | 168 |
|---|---|
| Age, M (SD) | 53.89 (3.82) |
| Race/ethnicity, N (%) | |
| Non-Hispanic white | 114 (67.86) |
| Black | 47 (27.98) |
| Other | 7 (4.17) |
| Education, N (%) | |
| High school/some college/vocational | 83 (49.40) |
| College or higher | 85 (50.60) |
| Menopause stage, N (%) | |
| Perimenopausal | 25 (14.88) |
| Postmenopausal | 143 (85.12) |
| Women reporting having hot flashes* N (%) | 119 (70.83) |
| Number of physiologic overnight hot flashes, Median (IQR) | 3 (2, 5) |
| Total sleep time (actigraphy), M (SD) min | 379.8 (79.2) |
| WASO (actigraphy), Median (IQR) | 48.95 (26.83) |
IQR, interquartile range; M, mean; SD, standard deviation; WASO, wake after sleep onset.
*Reported at screening, past 3 months.
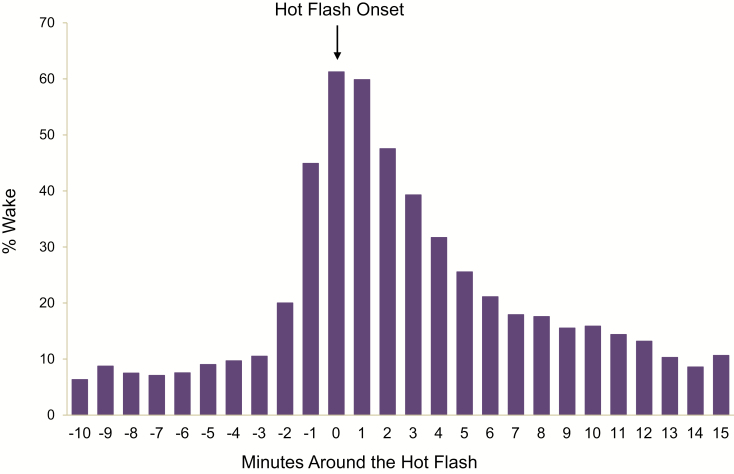
We first considered whether hot flashes were associated with actigraphically defined wake episodes. We found an increased likelihood of wake during the objectively detected hot flash relative to the minutes before the onset of the hot flash (Table 2; Figure 1). In fact, across the participants, wake episodes were observed with 362 (78%) of the objective hot flashes. Thirty-six percent of the total wake episodes occurred with an objective hot flash.
Table 2.
Likelihood of actigraphy-assessed wakefulness in minutes around physiologically assessed hot flashes
| OR (95% CI) | P-value | |
|---|---|---|
| Timing relative to hot flash onset | ||
| Before (–1 to –10 min) | Ref | — |
| During (0 min to +5 min) | 5.31 (4.46 to 6.33) | <.0001 |
| After (+6 to +15 min) | 1.11 (0.94 to 1.32) | .22 |
N = 168 women, 591 physiologic hot flashes. OR, odds ratio.
Figure 1.
Wake/sleep status around the onset of hot flashes, N = 168 women.
We also considered whether associations between hot flash and wake episodes varied as a function of whether the hot flash was concurrently reported. The association between hot flashes and wake was strongest for objective hot flashes that were concurrently reported, yet was also apparent for hot flashes participants did not report (interaction by whether the hot flash was reported, p < .0001; Table 3).
Table 3.
Likelihood of actigraphy-assessed wakefulness in minutes around physiologically assessed hot flashes by whether the hot flash was concurrently reported
| Physiologic hot flash that was reported | Physiologic hot flash that was not reported | |
|---|---|---|
| OR (95% CI) | OR (95% CI) | |
| Timing relative to hot flash onset | ||
| Before (–1 to –10 min) | Ref | Ref |
| During (0 min to +5 min) | 11.63 (8.46 to 15.98)*** | 4.41 (3.69 to 5.27)*** |
| After (+6 to +15 min) | 1.37 (1.04 to 1.81)* | 1.02 (0.83to 1.26) |
CI, confidence interval; OR, odds ratio.
*p < .05; ***p < .0001; N = 168 women, 128 physiological hot flashes reported, 463 physiological hot flashes not reported.
We conducted several secondary analyses. At screening, 71% (n = 119) of the women described themselves as having daily hot flashes, and 29% (n = 49) of the women denied having hot flashes in the past 3 months. The interaction between a woman’s reported hot flash status at study screening and objective hot flashes in relation to wake was statistically significant (p = .046). Stratified models indicated that relationships between hot flashes and wake were slightly more pronounced among women who self-identified as having daily hot flashes (during the hot flash [0 to +5 min]: OR = 5.96 [4.89 to 7.27]; p < .00001; after the hot flash [+6 to +15 min]: OR = 1.21 [1.00 to 1.47]; p = .05, relative to before the hot flash [–1 to –10 min]), yet hot flash–wake relationships were also observed among women who did not report having hot flashes in the past 3 months (during the hot flash: OR = 3.71 [2.69 to 5.11]; p < .00001; after the hot flash: OR = .80 [0.61–1.06]; p = .13, relative to before the hot flash).
We also considered race/ethnicity, finding that associations between wake and hot flashes varied by race/ethnicity (interaction p = .04). Both non-Hispanic white and nonwhite (principally African American) women showed an increased likelihood of wake with hot flashes, yet for the nonwhite women, the wake episodes persisted after the hot flash (Supplementary Table 1). Further, whereas covariates were not included in these largely within-subject models, findings were unchanged when we additionally included age, race, education, BMI, shift work, and use of sleep medications as covariates (Supplementary Table 2). Further, we repeated primary models excluding women who were either shift workers or who used sleep medications; findings were comparable with primary models (Supplementary Table 3). Finally, because it appeared that the wake episode might have occurred slightly before the onset of the hot flash, we defined the 5-min period surrounding the hot flash as starting from a minute before the hot flash and extending to 4 min after the hot flash. In these models, the relationship between hot flashes and wake was particularly strong and persisted for up to 15 min following the hot flash (Supplementary Table 4).
Discussion
In the largest study to date to investigate the relationship between hot flashes and wakefulness using objective measures of hot flashes and sleep, we found that nocturnal wakefulness was associated with hot flashes. These associations were observed irrespective of whether the specific hot flash was concurrently reported by the participant. Associations among hot flashes and wakefulness were also observed irrespective of whether the participant described herself as someone who typically had hot flashes. These data support the hypothesis that wakefulness typically occurs in conjunction with hot flashes and underscore the importance of hot flashes to the quality of women’s sleep during the menopause transition.
Women typically report that their hot flashes wake them up at night. In fact, these observations based on self-reported data fueled the long-held “domino hypothesis” that hot flashes caused sleep problems, which, in turn, affect mood among midlife women [34, 35]. However, in the few studies that used objective measures of both hot flashes and sleep, associations are less clear. Two recent polysomnography studies have addressed this question. In a study of 34 women who underwent objective assessments of sleep and hot flashes, 69% of the objective hot flashes were associated with awakenings [19]. In another study of 28 premenopausal women who underwent pharmacological suppression of the reproductive axis, objective hot flashes were not associated with increased transitions to wake, yet 66% of hot flashes occurred within 5 min before or after an awakening [20]. Although yielding important findings, these studies were limited by their small sample sizes of women, and in one of these studies, investigation of premenopausal women, the generalizability of whom to menopausal women is not fully established. Our study, investigating a large number of women in their home environments, showed a fivefold increased odds of wakefulness during an objective hot flash relative to the minutes before the hot flash. Notably, wakefulness from sleep was observed in conjunction with 78% of the objective hot flashes studied. Of bouts of wakefulness across the night, a third were accompanied by a hot flash.
We further examined whether the associations between hot flashes and wakefulness varied as a function of whether the hot flash was reported by the participant. The relationship between hot flashes and awakenings was most pronounced when a woman reported the hot flash, with an over 11-fold increased odds of wake with a reported hot flash. This finding would be expected as the woman would need to be awake to report the hot flash, and the act of reporting itself (button press) would likely register as movement on the actigraph. However, it is notable that the relationship between hot flashes and wake was also apparent when the hot flash was not reported at the time of its occurrence, with an over fourfold odds of wake with these non-reported hot flashes. These data further add to the body of literature showing the importance of objectively detected hot flashes for women’s sleep irrespective of whether the hot flash is reported.
Consistent with our prior work [33], a substantial number of women showing hot flashes on objective monitoring did not report having hot flashes at the study entry. Even among these women, we detected objective hot flashes and observed an increased likelihood of wake with these hot flashes. The phenomenon of underreporting of hot flashes relative to objective monitoring is well documented [27, 36], and we previously demonstrated that these hot flashes show characteristic autonomic nervous system changes, supporting their validity as hot flashes [33]. Notably, many of the women who did not report having hot flashes at entry (which queried about the past three months) did report having had them in the past, and newer findings indicate that hot flashes often persist for a decade or more [7]. Thus, it is possible these women are still having hot flashes and that these hot flashes—even though not perceived—may be relevant to sleep.
Several additional findings are of note. We found that whereas both non-Hispanic white and nonwhite (principally African American) women tended to show wake episodes during the hot flash, the non-Hispanic white women tended to fall asleep within the 10 min after the hot flash onset. Conversely, the nonwhite women continued to show a greater likelihood of wake well after the objective hot flash, suggesting that nonwhite women fell back asleep more slowly than did white women. These data are consistent with findings that African American midlife women showing more difficulty initiating and maintaining sleep than comparably aged white women [21]. More than a third of all of the wakeful episodes occurred with hot flashes. These data indicate the potential importance of hot flashes to awakenings during the night but also underscore that a range of factors are likely determinants of awakenings and should be comprehensively investigated in future work. Further, we a priori defined the “during hot flash” interval as beginning at the time of onset of the hot flash (i.e. minute 0), yet when visualizing the data, we observed the potential of wake preceding the hot flash. When we defined the “during the hot flash” interval starting at 1 minute before the hot flash, associations were particularly pronounced. Assertions about a temporal ordering of hot flashes and wake should be regarded with caution given several methodological issues that may limit the degree of precision required to draw firm conclusions regarding the time course of hot flashes and wakefulness from sleep, including requirements for very precise device time synchronization, 1-min actigraphy epochs, and relatively slow changes characteristic of sternal skin conductance. However, these findings do point to a potential phenomenon of wake preceding the hot flash that should be further investigated future work using additional methodologies.
Several limitations warrant mention. First, this was an observational study. We cannot assert causal relationships between hot flashes and awakening; further experimental studies may yield greater insight into the causality nature of these relationships. Second, wrist actigraphy is an assessment of movement, not electrophysiological awakening as measured with electroencephalography. Therefore, it is possible that there is imprecision or inaccuracy in the definition of wake. However, actigraphy has been demonstrated to have strong validity for assessment of wakefulness after sleep onset relative to polysomnography [37]. Third, although a third of the participants were nonwhite, most of the nonwhite women were African American, and we cannot make conclusions about other racial/ethnic groups. Further work should continue to investigate the possibility of racial/ethnic differences in relationships between hot flashes and sleep in diverse groups of women. Fourth, women with less frequent flashing, who were smokers, who were taking certain medications, or who had had certain gynecological surgeries (e.g. hysterectomy and bilateral oophorectomy) were not included here; results may not be generalized to these women. Finally, the aim of this work was to determine relationships between hot flashes and actigraphic wake episodes; future work should consider the implications of these hot flash-related wake episodes for women’s health and functioning.
This work also had notable strengths. We studied a large number of midlife women monitored in their home environment. Objective measures of both hot flashes and actigraphy were used to examine study questions, rather than relying solely on self-report which, although of critical clinical importance, may incorporate influences such as mood and memory that make them less ideal for studies of the precise relationship between sleep and hot flashes [13, 38]. We examined relationships in a time series fashion, which allowed for more rigorous questions about the temporality of hot flash–wake relationships, in contrast to work that examined summative hot flash or sleep indices over the night.
In summary, in this study of 168 midlife women studied in their home environments who underwent both objective assessments of hot flashes and sleep, we found that waking from sleep occurred in conjunction with 78% of objectively detected hot flashes. We further observed a fivefold increased odds of wake in the minutes during the hot flash compared with the minutes preceding the hot flash. These associations were observed irrespective of whether the woman reported the hot flash or even whether the women identified themselves as typically having hot flashes. Nonwhite women may have particularly prolonged wake following the hot flash. These data underscore the importance of hot flashes to women’s sleep during midlife and the menopause transition.
Funding
This work was supported by the National Institutes of Health (NIH), National Heart Lung and Blood Institute (R01HL105647, K24HL123565 to Thurston) and the University of Pittsburgh Clinical and Translational Science Institute (NIH Grant UL1TR000005).
Conflict of interest statement. Dr. Thurston consults for Pfizer and Procter & Gamble.
Supplementary Material
References
- 1. Gold EB, et al. Longitudinal analysis of the association between vasomotor symptoms and race/ethnicity across the menopausal transition: study of women’s health across the nation. Am J Public Health. 2006;96(7):1226–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. NIH. State-of-the Science Conference statement. Management of menopause-related symptoms. Ann Intern Med. 2005;142:1003–1013. [PubMed] [Google Scholar]
- 3. Kravitz HM, et al. Sleep difficulty in women at midlife: a community survey of sleep and the menopausal transition. Menopause. 2003;10(1):19–28. [DOI] [PubMed] [Google Scholar]
- 4. Polo-Kantola P. Sleep problems in midlife and beyond. Maturitas. 2011;68(3):224–232. [DOI] [PubMed] [Google Scholar]
- 5. Kravitz HM, et al. Sleep disturbance during the menopausal transition in a multi-ethnic community sample of women. Sleep. 2008;31(7):979–990. [PMC free article] [PubMed] [Google Scholar]
- 6. Krystal AD, et al. Sleep in peri-menopausal and post-menopausal women. Sleep Med Rev. 1998;2(4):243–253. [DOI] [PubMed] [Google Scholar]
- 7. Avis NE, et al. ; Study of Women’s Health Across the Nation Duration of menopausal vasomotor symptoms over the menopause transition. JAMA Intern Med. 2015;175(4):531–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bolge SC, et al. Burden associated with chronic sleep maintenance insomnia characterized by nighttime awakenings among women with menopausal symptoms. Menopause. 2010;17(1):80–86. [DOI] [PubMed] [Google Scholar]
- 9. Avis NE, et al. Change in health-related quality of life over the menopausal transition in a multiethnic cohort of middle-aged women: Study of Women’s Health Across the Nation. Menopause. 2009;16(5):860–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kronenberg F. Hot flashes: epidemiology and physiology. Ann N Y Acad Sci. 1990;592:52–86; discussion 123. [DOI] [PubMed] [Google Scholar]
- 11. Kravitz HM, et al. Relationship of day-to-day reproductive hormone levels to sleep in midlife women. Arch Intern Med. 2005;165(20):2370–2376. [DOI] [PubMed] [Google Scholar]
- 12. Burleson MH, et al. Daily vasomotor symptoms, sleep problems, and mood: using daily data to evaluate the domino hypothesis in middle-aged women. Menopause. 2010;17(1):87–95. [DOI] [PubMed] [Google Scholar]
- 13. Fu P, Matthews K, Thurston R. How well do different measurement modalities estimate the number of vasomotor symptoms? Findings from the Study of Women’s Health Across the Nation FLASHES Study. Menopause 2014;21(2):124–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Erlik Y, et al. Association of waking episodes with menopausal hot flushes. JAMA. 1981;245(17):1741–1744. [PubMed] [Google Scholar]
- 15. Woodward S, et al. The thermoregulatory effects of menopausal hot flashes on sleep. Sleep. 1994;17(6):497–501. [DOI] [PubMed] [Google Scholar]
- 16. Freedman RR, et al. Lack of sleep disturbance from menopausal hot flashes. Fertil Steril. 2004;82(1):138–144. [DOI] [PubMed] [Google Scholar]
- 17. Freedman RR, et al. Sleep disturbance in menopause. Menopause. 2007;14(5):826–829. [DOI] [PubMed] [Google Scholar]
- 18. Freedman RR, et al. Effects of REM sleep and ambient temperature on hot flash-induced sleep disturbance. Menopause. 2006;13(4):576–583. [DOI] [PubMed] [Google Scholar]
- 19. de Zambotti M, et al. Magnitude of the impact of hot flashes on sleep in perimenopausal women. Fertil Steril. 2014;102(6):1708–1715.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bianchi MT, et al. Nocturnal hot flashes: relationship to objective awakenings and sleep stage transitions. J Clin Sleep Med. 2016;12(7):1003–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hall MH, et al. Race and financial strain are independent correlates of sleep in midlife women: the SWAN sleep study. Sleep. 2009;32(1):73–82. [PMC free article] [PubMed] [Google Scholar]
- 22. Thurston RC, et al. Menopausal hot flashes and carotid intima media thickness among midlife women. Stroke. 2016;47(12):2910–2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Harlow SD, et al. ; STRAW + 10 Collaborative Group Executive summary of the stages of reproductive aging workshop + 10: addressing the unfinished agenda of staging reproductive aging. J Clin Endocrinol Metab. 2012;97(4):1159–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. de Geus EJ, et al. Ambulatory measurement of respiratory sinus arrhythmia and respiration rate. Biol Psychol. 1995;41(3):205–227. [DOI] [PubMed] [Google Scholar]
- 25. Willemsen GH, et al. Ambulatory monitoring of the impedance cardiogram. Psychophysiology. 1996;33(2):184–193. [DOI] [PubMed] [Google Scholar]
- 26. Freedman RR. Laboratory and ambulatory monitoring of menopausal hot flashes. Psychophysiology. 1989;26(5):573–579. [DOI] [PubMed] [Google Scholar]
- 27. Carpenter JS, et al. Feasibility and psychometrics of an ambulatory hot flash monitoring device. Menopause. 1999;6(3):209–215. [DOI] [PubMed] [Google Scholar]
- 28. Thurston RC, et al. Improving the performance of physiologic hot flash measures with support vector machines. Psychophysiology. 2009;46(2):285–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Thurston RC, et al. Support Vector Machines to improve physiologic hot flash measures: application to the ambulatory setting. Psychophysiology. 2011;48(7):1015–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ancoli-Israel S, et al. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26(3):342–392. [DOI] [PubMed] [Google Scholar]
- 31. Monk TH, et al. The pittsburgh sleep diary. J Sleep Res. 1994;3(2):111–120. [PubMed] [Google Scholar]
- 32. Thurston RC, et al. Hot flashes and cardiac vagal control during women’s daily lives. Menopause. 2012;19(4):406–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Thurston RC, et al. Changes in heart rate variability during vasomotor symptoms among midlife women. Menopause. 2016;23(5):499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Avis NE, et al. Longitudinal study of hormone levels and depression among women transitioning through menopause. Climacteric. 2001;4(3):243–249. [PubMed] [Google Scholar]
- 35. Gibson CJ, et al. Negative affect and vasomotor symptoms in the Study of Women’s Health Across the Nation Daily Hormone Study. Menopause. 2011;18(12):1270–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Thurston RC, et al. Adiposity and hot flashes in midlife women: a modifying role of age. J Clin Endocrinol Metab. 2011;96(10):E1588–E1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Marino M, et al. Measuring sleep: accuracy, sensitivity, and specificity of wrist actigraphy compared to polysomnography. Sleep. 2013;36(11):1747–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Thurston RC, et al. Are vasomotor symptoms associated with sleep characteristics among symptomatic midlife women? Comparisons of self-report and objective measures. Menopause. 2012;19(7):742–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.