Abstract
Background
It is unknown what levels of pre-exposure prophylaxis (PrEP) use are needed to reduce racial disparities in HIV incidence among men who have sex with men (MSM). Using an agent-based model (ABM), we quantified the impact of achieving PrEP coverage targets grounded in equity on racial disparities in HIV incidence among MSM in an urban setting in the Southeastern United States.
Methods
An ABM was adapted to simulate HIV transmission in a network of Black/African American and White MSM aged 18 to 39 years in the Atlanta–Sandy Springs–Roswell metropolitan area over ten years (2015–2024). Scenarios simulated coverage levels consistent with targets based on the ratio of the number of individuals using PrEP to the number of individuals newly diagnosed in a calendar year (i.e., the “PrEP-to-need ratio”), ranging from 1 to 10. Incidence rate ratios and differences were calculated as measures of disparities.
Results
Without PrEP, the model predicted a rate ratio of 3.82 and a rate difference of 4.50 comparing HIV incidence in Black/African American and White MSM, respectively. Decreases in the rate ratio of at least 50% and in the rate difference of at least 75% were observed in all scenarios in which the PrEP-to-need ratio among Black/African American MSM was 10, regardless of the value among White MSM.
Conclusion
Significant increases in PrEP use are needed among Black/African American MSM to reduce racial disparities in HIV incidence. PrEP expansion must be coupled with structural interventions to address vulnerability to HIV infection among Black/African American MSM.
Keywords: HIV, pre-exposure prophylaxis, men who have sex with men, agent-based modeling, health disparities
INTRODUCTION
Between 2008 and 2015, HIV incidence among all adolescents and adults aged 13 years and older in the United States (U.S.) decreased by 15%.1 Despite these successes, there was essentially no change in incidence among who have sex with men (MSM) in this age range during this time period.1 Although incidence decreased among White MSM, these reductions were offset by increases among Hispanic/Latino MSM and stable rates among Black/African American MSM.1 Had incidence rates among Black/African American and Hispanic/Latino MSM been reduced to the levels observed among White MSM, overall incidence among MSM would have decreased by between at least 55%.2 Incidence rates among Black/African American MSM remain alarmingly high, and the lifetime risk of HIV infection among Black/African American MSM is the highest in the U.S.3
Daily use of tenofovir disoproxil fumarate co-formulated with emtricitabine (TDF/FTC) has demonstrated efficacy as pre-exposure prophylaxis (PrEP) to prevent HIV transmission among MSM.4 Studies have estimated that up to one-third of all new HIV infections over ten years could be averted if 40% of MSM at substantial risk for HIV infection used PrEP as prescribed.5 However, challenges have emerged in reaching this level of coverage, particularly among Black/African American and Hispanic/Latino MSM.6 Between 2014 and 2017, the proportion of MSM participating in the National HIV Behavioral Surveillance System who reported PrEP use increased from 5.7% to 35.1%,6 with White MSM most likely to use PrEP and Black/African American MSM least likely to use PrEP.6 Given these inequities, the beneficial impact of continued PrEP expansion may be greater for White MSM than for other MSM.7,8
The National HIV/AIDS Strategy for the U.S. included a goal of increasing the number of adults prescribed PrEP by at least 500%.9 However, no targets were set for ensuring that those most vulnerable to HIV infection were receiving PrEP. The PrEP-to-need ratio, defined as the ratio of the number of people using PrEP to the number of individuals newly diagnosed with HIV infection, has been proposed as one metric for measuring population-level coverage of PrEP.10 Given that this ratio is dependent on the underlying trends in new diagnoses,10 it may ensure that PrEP is deployed in an equitable fashion at levels commensurate with the current burden of HIV in specific communities. However, it is unclear what value of this ratio would maximize progress towards narrowing disparities. We used an agent-based network model to project the impact of achieving PrEP coverage levels consistent with targets based on the PrEP-to-need ratio on racial disparities in HIV incidence among Black/African American and White MSM.
METHODS
Study Setting
The Atlanta–Sandy Springs–Roswell metropolitan statistical area, a large region including nearly six million residents,11 represents the epicenter of the HIV epidemic in Georgia. Nearly two-thirds (65.8%) of all newly diagnosed cases of HIV infection in the state were reported in the region in 2017.12 MSM bear a disproportionate burden. Although it is estimated that 5.4% of men in the region have engaged in sexual behavior with other men,13 89.9% of all newly diagnosed cases of HIV infection among men in Atlanta were among MSM in 2017.12 Black/African American MSM were more than seven times more likely to be newly diagnosed with HIV infection compared to be White MSM as of 2017.12
Model Setting
In an agent-based model (ABM), an epidemic system is modeled as a collection of individual decision-making entities referred to as agents.14 Through stochastic processes, agents make decisions on the basis of a set of rules and execute various behaviors appropriate for the system they represent.14 As described previously, the TITAN model is an ABM created to represent infectious disease dynamics in networks of agents and evaluate the impact of prevention and treatment strategies on trajectories in HIV incidence.15
We adapted the TITAN model to simulate HIV transmission for ten years (2015 to 2024) in a population of 17,440 agents (61.1% White, 38.9% Black/African American), representing the estimated number of White and Black/African American MSM between 18 and 39 years old who reside in the Atlanta–Sandy Springs–Roswell region. This age range was selected based on the data underlying the model parameters. Most model parameters were derived from the published literature from two studies: InvolveMENt,16 a longitudinal cohort formed to understand sources of racial disparities in HIV incidence and prevalence among MSM conducted between 2010 and 2014; and the MAN Project,17 a cross-sectional assessment of racial differences in the sexual networks of MSM conducted between 2011 and 2013. Further information on input parameters and model processes are provided in the Supplemental File.
Model Processes
Sexual Network Formation and Sexual Behavior
A negative binomial distribution was used to represent the range of the total number of sexual partners per year for a given agent, with a median of 5 partners (IQR: 3–10) for Black/African American MSM and 7 partners (IQR: 4–15) for White MSM. For each agent, a value was drawn from this distribution at model initialization. This value became the mean value for a Poisson distribution specific to each agent that governed their number of sexual partners each year. At the beginning of each year, new numbers were drawn from these agent-specific distributions. This process allowed for each agent to exhibit tendencies with regard to the acquisition of new partners from year-to-year without holding this behavior constant.
During each time-step, agents had a defined probability of acquiring a new partner. When seeking a partner, a list of 100 partner-seeking agents that were able to pair with a given index agent was enumerated and one of these agents was selected at random. Partnerships were formed until each agent achieved the necessary number of partners for the current time-step. Partner selection was governed by the race of the index agent, where agents were more likely to select partners who were of the same race (with probabilities of same-race partnering of 76.5% for Black/African American MSM and 72.2% for White MSM).18 All partnerships were assigned a duration (45.6% between 1 and 3 months, 30.0% between 4 and 12 months, and 24.5% between 13 and 24 months),18 allowing partnerships to dissolve over time.
Following the creation of the initial network, interactions between agents were simulated. Each agent was assigned a target number of anal intercourse acts per partner per month. The actual number of anal intercourse acts per month was the average of the two agents’ target values. Each act was subject to a probability of condom use.19 Condom-protected acts were assumed to carry a negligible risk of transmission and were, therefore, not explicitly simulated.19
HIV Transmission and Treatment
Parameters governing the per-act probability of HIV transmission with anal intercourse were informed by a meta-analysis.20 An agent’s risk of HIV acquisition in a given time-step was dependent on the number of acts and whether the partner living with HIV infection was experiencing the acute stage of HIV infection, using antiretroviral treatment, or had achieved viral suppression.20 All parameters governing HIV testing and treatment were stratified by race: Black/African American MSM were less likely to be tested for HIV infection, diagnosed, use antiretroviral treatment, or achieve viral suppression than White MSM.21 The proportions of people living with HIV infection in each stage of the continuum of care for HIV treatment were held constant for the duration of the simulations.
Pre-Exposure Prophylaxis Implementation
In line with current guidelines,22 agents were eligible to initiate PrEP use if they engaged in condomless anal intercourse with two or more partners or they were in an ongoing partnership with a partner who was diagnosed with HIV infection. Agents who used PrEP could take four or more doses per week or two to three doses per week. An agent’s level of adherence impacted the level of protection conferred by PrEP.23 The probability of discontinuation was based on data from the PrEP program of the Fulton County Board of Health.24 All parameters were stratified by race, where Black/African American MSM were less likely to achieve optimal adherence and more likely to discontinue PrEP use.24
Model Calibration
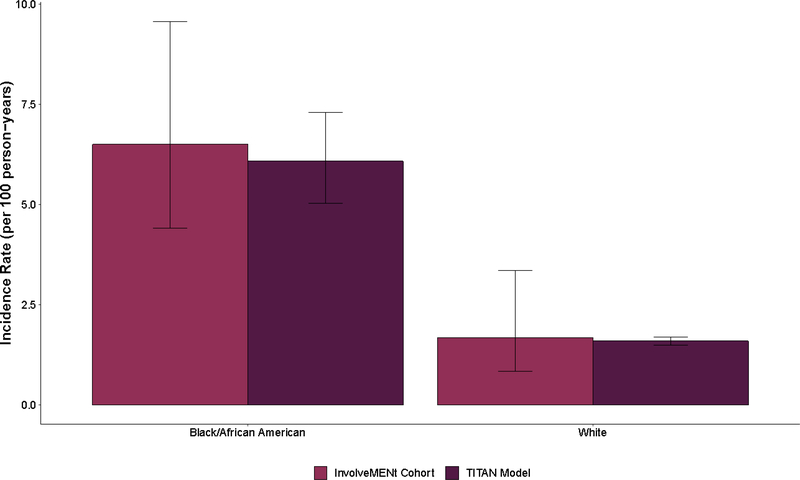
The primary calibration targets for the model were the observed incidence rates by race during the follow-up period of InvolveMENt (6.5 infections per 100 person-years for Black/African American MSM and 1.7 infections per 100 person-years for White MSM) (Figure 1).16 Model calibration was conducted using Latin hypercube sampling as described in the Supplemental File.25
Figure 1.
Comparison of observed HIV incidence among young Black/African American and White gay, bisexual, and other men who have sex with men (MSM) aged 18 to 39 years old residing in the Atlanta–Sandy Springs–Roswell metropolitan statistical area (MSA) who participated in InvolveMENt with simulations from the Treatment of Infectious Transmission in Agent-Based Networks (TITAN) model
Note: The error bars represent the 95% confidence interval for data from the InvolveMENt cohort and the 95% simulation interval from the TITAN model output.16
Model Scenarios
Model scenarios simulated race-specific PrEP-to-need ratios ranging from 1 (corresponding to ~7% of Black/African American MSM and ~2% of White MSM on PrEP) to 10 (corresponding to ~68% of Black/African American MSM and ~17% of White MSM). The resulting number of PrEP prescriptions made available in each scenario was based on the average number of agents newly diagnosed with HIV infection in a given calendar year predicted in the absence of PrEP.
Sensitivity Analyses
Sensitivity analyses were conducted to examine the robustness of the main analyses to uncertainty in key model parameters that were hypothesized to impact the observed effect of PrEP implementation. The parameters governing adherence to daily pill-taking and discontinuation following initiation of PrEP use were varied such that Black/African American MSM were as likely to adhere and discontinue as White MSM. To assess the impact of PrEP implementation in a potential future with large increases in viral suppression, we also varied parameters governing testing and treatment such that the Joint United Nations Programme on HIV/AIDS (UNAIDS) ‘90–90-90’ goals for the continuum of care were achieved.26
Outcome Measures
All outcome measures were considered relative to a base case scenario without PrEP implementation. The primary outcome measure was the incidence rate (expressed as the number of new infections per 100 person-years). This measure was calculated for the overall population and separately for each racial group. Incidence rate ratios were calculated as a measure of relative disparity while incidence rate differences were calculated as a measure of absolute disparity.27 All measures are summarized with the mean value across 100 simulations and presented with 95% simulation intervals (SIs), representing the middle 95% of the model output across these simulations.
RESULTS
In the absence of PrEP implementation, the model predicted 1,863 new infections among Black/African American MSM (95% SI: 1,769; 1,948) and 1,391 new infections among White MSM (95% SI: 1,310; 1,472) over the ten-year simulation period, corresponding to incidence rates of 6.10 new infections (95% SI: 5.75, 6.40) and 1.60 new infections (95% SI: 1.50, 1.70) per 100 person-years, respectively. The model predicted that the HIV incidence rate among Black/African American MSM was 3.82 times (95% SI: 3.53, 4.19) that among White MSM. The risk difference was 4.50 additional infections (95% SI: 4.17, 4.89) per 100 person-years among Black/African American MSM relative to White MSM.
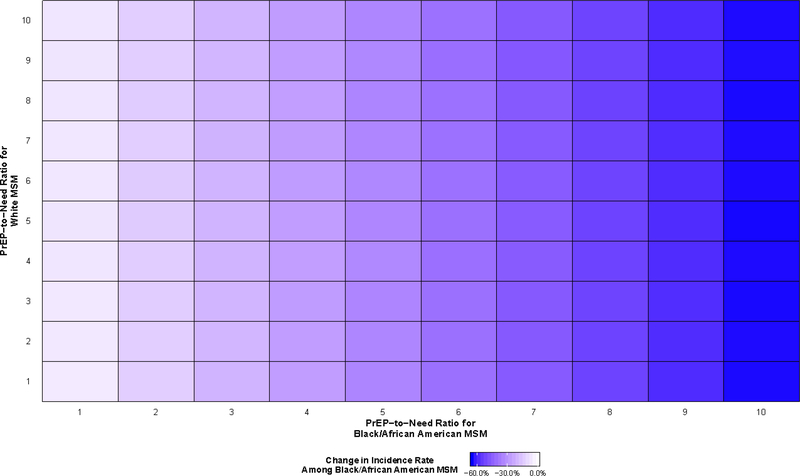
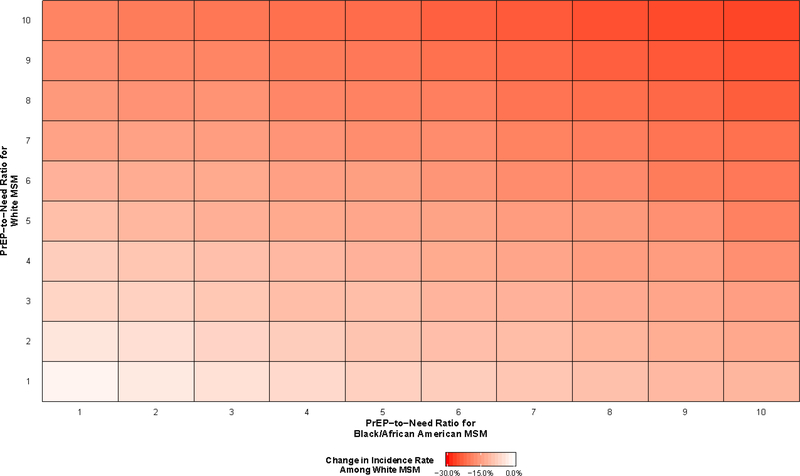
All PrEP scenarios resulted in decreases in HIV incidence among both Black/African American and White MSM (Figure 2 and Figure 3), although the differences varied greatly. The smallest decreases in race-specific incidence rates were observed when one agent used PrEP for every newly diagnosed agent in both racial groups (PrEP-to-need ratio equal to 1), with predicted decreases of 1.7% for the incidence among White MSM (95% SI: −8.2%, 4.5%) and 5.9% for the incidence among Black/African American MSM (95% SI: −11.5%, 0.0%). The largest decreases were observed when ten agents used PrEP for every newly diagnosed agent in both racial groups, with predicted decreases of 63.9% for Black/African American MSM (95% SI: −67.5%, −60.8%) and 26.3% for White MSM (95% SI: −30.8%, −21.4%).
Figure 2.
Predicted percent change in HIV incidence among Black/African American gay, bisexual, and other men who have sex with men (MSM) aged 18 to 39 years old residing in the Atlanta–Sandy Springs–Roswell metropolitan statistical area (MSA) between 2015 and 2024 relative to a scenario in which pre-exposure prophylaxis implementation (PrEP) does not occur
Figure 3.
Predicted percent change in HIV incidence among White gay, bisexual, and other men who have sex with men (MSM) aged 18 to 39 years old residing in the Atlanta–Sandy Springs–Roswell metropolitan statistical area (MSA) between 2015 and 2024 relative to a scenario in which pre-exposure prophylaxis (PrEP) implementation does not occur
There is evidence of an interaction effect between the PrEP-to-need ratios among Black/African American and White MSM with respect to reductions in HIV incidence among White MSM (Figure 3). However, this effect was not present with respect to reductions in HIV incidence among Black/African American MSM: decreases in HIV incidence among Black/African American MSM at any given PrEP-to-need ratio were fairly consistent regardless of the level of PrEP use among White MSM (Figure 2).
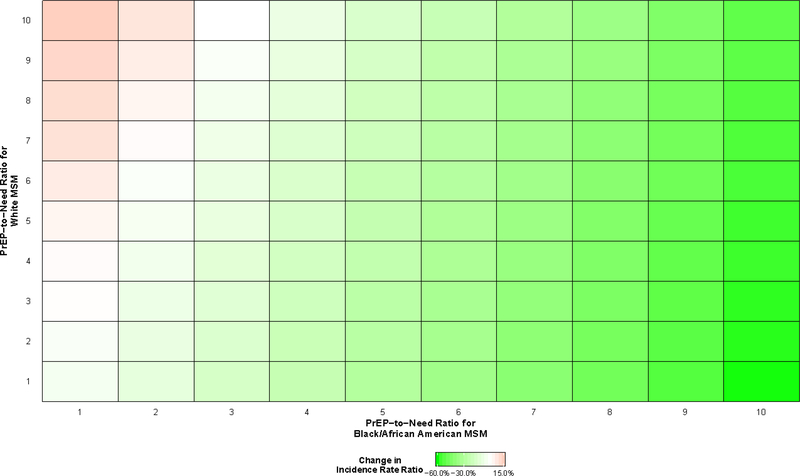
All scenarios resulted in changes in the rate ratio comparing Black/African American MSM to White MSM, with most scenarios resulting in decreases (Figure 4). The rate ratio increased when the PrEP-to-need ratio among White MSM greatly exceeded the PrEP-to-need ratio among Black/African American MSM. For example, when the PrEP-to-need ratio was 10 among White MSM and 1 among Black/African American MSM, the rate ratio increased by 14.7% (95% SI: 5.9%, 24.5%) to 4.38 (95% SI: 4.01, 4.75). The largest decrease in the rate ratio was predicted when these ratios were reversed, where the rate ratio decreased by 51.1% (95% SI: −55.6%, −45.4%) to 1.87 (95% SI: 1.70, 2.08). Decreases of this magnitude (>50%) were observed in all scenarios in which the PrEP-to-need ratio among Black/African American MSM was 10, regardless of the value among White MSM.
Figure 4.
Predicted changes in the incidence rate ratio between Black/African American and White gay, bisexual, and other men who have sex with men (MSM) aged 18 to 39 years old residing in the Atlanta–Sandy Springs–Roswell metropolitan statistical area (MSA) between 2015 and 2024, relative to a scenario in which pre-exposure prophylaxis (PrEP) implementation does not occur
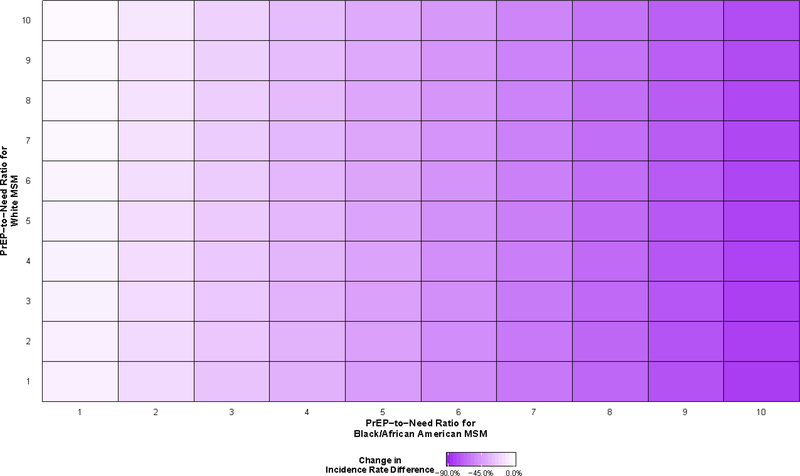
All scenarios resulted in decreases in the absolute measure of disparity comparing Black/African American MSM to White MSM (Figure 5). The smallest decrease was observed when ten White agents used PrEP for every newly diagnosed White agent and one Black/African American agent used PrEP for every newly diagnosed Black/African American agent (−2.5%; 95% SI: −8.8%, 4.5%). The largest decrease in the rate difference was predicted when these PrEP-to-need ratios were reversed, where the rate difference decreased by 82.8% (95% SI: −87.4%, −78.2%) to 0.78 (95% SI: 0.57, 0.98). Decreases of this magnitude (>75%) were observed among all scenarios where the PrEP-to-need ratio among Black/African American MSM was 10, regardless of the PrEP-to-need ratio among White MSM.
Figure 5.
Predicted changes in the incidence rate difference between Black/African American and White gay, bisexual, and other men who have sex with men (MSM) aged 18 to 39 years old residing in the Atlanta–Sandy Springs–Roswell metropolitan statistical area (MSA) between 2015 and 2024, relative to a scenario in which pre-exposure prophylaxis (PrEP) implementation does not occur
Sensitivity Analyses
The results of the main analyses were robust to improvements in the continuum of care (Supplemental Figure 1) and improvements in retention in PrEP care among Black/African American MSM (Supplemental Figure 3), where the relative changes in the rate ratio and rate difference with varying levels of PrEP use in both racial groups were similar in magnitude. The results were sensitive to improvements in adherence to PrEP among Black/African American MSM (Supplemental Figure 2), where the decreases in both measures of disparity were larger in all scenarios where Black/African American MSM had comparable adherence.
DISCUSSION
The current study demonstrates the utility of setting targets for PrEP expansion efforts guided by the PrEP-to-need ratio.10 Although previous studies have shown that relative disparities may widen even when equivalent proportions of Black/African American and White MSM use PrEP,7,8 deployment of effective prevention methods in a manner that is commensurate with epidemiological need have largely not been studied. Our results suggest that such a strategy can achieve the dual goals of reducing overall incidence and narrowing existing disparities. Further, unlike targets for increasing PrEP use that rely on percentage-based metrics that require estimates of the underlying population size (e.g., the proportion of MSM without HIV infection in a given jurisdiction who use PrEP), the PrEP-to-need ratio can be more easily measured by health departments, as its denominator only requires the number of individuals newly diagnosed with HIV infection as estimated through ongoing surveillance.28 As such, it represents a potential metric for monitoring the expansion of PrEP use and setting public health targets for PrEP use in the U.S.
HIV incidence among Black/African American MSM was largely insensitive to increasing levels of PrEP use among White MSM. These findings may be explained by differential assortative mixing patterns among White and Black/African American MSM and the large existing disparities in HIV prevalence. Previous studies have shown higher levels of race-based assortative mixing in the sexual networks of Black/African American MSM compared to White MSM.29,30 These differences in mixing may lead to beneficial spillover effects of PrEP use from the higher prevalence sexual networks of Black/African American MSM to the lower prevalence sexual networks of White MSM. By averting HIV infections among in the networks of Black/African American MSM, further forward transmission is prevented to White MSM. However, there is little or no beneficial spillover effect in the opposite direction – averting HIV infections among White MSM does little to prevent additional HIV infections among Black/African American MSM.
Nearly all modeled scenarios resulted in reductions in both absolute and relative measures of disparity in HIV incidence between Black/African American MSM and White MSM. In certain scenarios, the rate ratio increased while the rate difference decreased. Previous work has shown that increasing relative disparities but decreasing absolute disparities occur when the rate of improvement is smaller for the group with initially poorer outcomes.27 In the current study, these represent scenarios in which incidence was decreasing more slowly among Black/African American MSM relative to White MSM, suggesting that the PrEP coverage levels achieved by Black/African American MSM must result in a PrEP-to-need ratio that is at least equivalent to that among White MSM to narrow existing disparities. Notably, no scenario eliminated these disparities. These results suggest that, given the high diagnosis rate among Black/African American MSM in Atlanta and other urban centers in the Southeastern U.S.,31 achieving very high levels of PrEP use among Black/African American MSM is a crucial step in reducing incidence and narrowing disparities.32
The current study also suggests a continued need for high levels of PrEP use even in circumstances where there are dramatic improvements to the continuum of care. The relative reductions in HIV incidence and the associated measures of disparity were similar across scenarios when the observed continuum of care was maintained for the duration of the simulations and in sensitivity analyses when the ‘90–90-90’ goals for diagnosis, treatment, and viral suppression were achieved.26 Even under ideal circumstances where high levels of engagement in care are achieved and maintained among Black/African American MSM, incidence may still be three times higher relative to White MSM.33 Even if disparities in transmission were addressed by achieving high levels of viral suppression, the elevated prevalence of HIV infection among Black/African American MSM will continue to perpetuate disparities in incidence.33 Reversing these disparities will only be possible by achieving a sustained reduction in transmission through combinations of effective prevention and treatment.33
Sensitivity analyses further demonstrated the importance of the more distal stages of the continuum of PrEP care. Decreases in HIV incidence and the associated measures of disparity were larger for a given level of PrEP use in analyses where disparities in adherence to PrEP were eliminated. There were no substantial changes in the outcome measures in analyses where disparities in retention in clinical care among MSM who use PrEP were eliminated. However, we believe the lack of effect of improved retention in clinical care is artificial due to our modeling approach. In the current model, intervention coverage is implemented at model initialization and maintained for the duration of the simulation. Agents who discontinue PrEP use are immediately replaced with an additional agent eligible to initiate PrEP. Prior mathematical modeling studies have shown that improving retention in clinical care can result in further reductions in HIV incidence.34,35 In addition to supporting increased PrEP initiation, particularly among Black/African American MSM, further interventions, such as patient navigation and point-of-care adherence testing,36,37 are needed to assist individuals who use PrEP maintain optimal levels of adherence and use of the medication during periods where they are most at risk for HIV infection.38
These findings must be considered in light of their limitations. First, the model focused on HIV transmission alone and did not account for co-circulation of bacterial sexually transmitted infections (STIs). Given that a recent diagnosis with a bacterial STI is a behavioral indication for PrEP use among MSM, the number of agents considered eligible for PrEP use was likely underestimated.22,39 Second, a small proportion (~5%) of MSM in this population engaged in sexual behavior with female partners.40 These partnerships are not represented in the model, likely underestimating the total number of HIV infections averted, but we expect the magnitude of this underestimation to be negligible. Third, Murray and colleagues (2017) recently showed that ABMs that transport the risks and causal effects estimated in one population to another population will often result in bias.41 This lack of transportability is not unique to ABMs, but this potential bias is of greater concerns in ABMs over other approaches as their input parameters are taken from multiple different sources rather than a single source population.41 Given that most parameters in our model were taken from two studies drawn from a single source population, we believe that the magnitude of this bias, if present, is minimal. Fourth, the model represented HIV transmission dynamics specific to Black/African American and White MSM between 18 and 39 years old in Atlanta and, therefore, the findings are limited in their generalizability. However, given the commonalities in racial disparities in HIV incidence across urban areas in the Southeastern U.S.,31 we believe that these findings have implications for the epidemic in the region.
CONCLUSION
These findings suggest that the PrEP-to-need ratio represents a feasible and useful approach to monitoring PrEP engagement among MSM in a manner that is proportionate to the current burden of the epidemic. Doing so can result in significant reductions in overall incidence and in both absolute and relative measures of racial disparities in HIV incidence among MSM. Programs to increase access to and utilization of PrEP among Black/African American MSM should be a public health priority.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to acknowledge the Brown University Center for Computing and Visualization for providing access to the high-performance computing services utilized in this research. The authors would also like to thank Maximilian R.F. King, ScM and Jesse L. Yedinak, MPA for their research and administrative assistance. This research was supported with funding from the National Institute on Drug Abuse (DP2DA040236) and the National Institute of Mental Health (F31MH121112 and R25MH083620).
References
- 1.Singh S, Song R, Johnson AS, McCray E, Hall HI. HIV incidence, prevalence, and undiagnosed infections in U.S. men who have sex with men. Ann Intern Med. 2018;168(10):685–694. [DOI] [PubMed] [Google Scholar]
- 2.McCree DH, Williams AM, Chesson HW, et al. Changes in disparities in estimated HIV incidence rates among Black, Hispanic/Latino, and White men who have sex with men (MSM) in the United States, 2010–2015. J Acquir Immune Defic Syndr. 2019;81(1):57–62. [DOI] [PubMed] [Google Scholar]
- 3.Hess KL, Hu X, Lansky A, Mermin J, Hall HI. Lifetime risk of a diagnosis of HIV infection in the United States. Ann Epidemiol. 2017;27(4):238–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.United States Preventive Services Task Force. Preexposure prophylaxis for the prevention of HIV infection: US Preventive Services Task Force recommendation statement. JAMA. 2019;321(22):2203–2213. [DOI] [PubMed] [Google Scholar]
- 5.Jenness SM, Goodreau SM, Rosenberg ES, et al. Impact of the Centers for Disease Control’s HIV preexposure prophylaxis guidelines for men who have sex with men in the United States. J Infect Dis. 2016;214(12):1800–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finlayson T, Cha S, Xia M, et al. Changes in HIV preexposure prophylaxis awareness and use among men who have sex with men - 20 urban areas, 2014 and 2017. MMWR Morb Mortal Wkly Rep. 2019;68(27):597–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jenness SM, Maloney KM, Smith DK, et al. Addressing gaps in HIV preexposure prophylaxis care to reduce racial disparities in HIV incidence in the United States. Am J Epidemiol. 2019;188(4):743–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goedel WC, King MRF, Lurie MN, Nunn AS, Chan PA, Marshall BDL. Effect of racial inequities in pre-exposure use on racial disparities in HIV incidence among men who have sex with men: A modeling study. J Acquir Immune Defic Syndr. 2018;79(3):323–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Office of National AIDS Policy. National HIV/AIDS Strategy for the United States: Updated to 2020. Washington, District of Columbia: The White House; 2015. [Google Scholar]
- 10.Siegler AJ, Mouhanna F, Mera Giler R, et al. The prevalence of pre-exposure prophylaxis use and the pre-exposure prophylaxis-to-need ratio in the fourth quarter of 2017, United States. Ann Epidemiol. 2018;28(12):841–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Center for Health Statistics. Multiple Cause of Death File 2017 In. Wide-Ranging Online Database for Epidemiologic Research. Atlanta, Georgia: Centers for Disease Control and Prevention; 2018. [Google Scholar]
- 12.Georgia Department of Public Health. HIV Surveillance Summary - Georgia, 2017. Atlanta, Georgia: Georgia Department of Public Health;2019. [Google Scholar]
- 13.Grey JA, Bernstein KT, Sullivan PS, et al. Estimating the population sizes of men who have sex with men in US states and counties using data from the American Community Survey. JMIR Public Health Surveill. 2016;2(1):e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tracy M, Cerda M, Keyes KM. Agent-based modeling in public health: Current applications and future directions. Annu Rev Public Health. 2018;39:77–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marshall BDL, Friedman SR, Monteiro JFG, et al. Prevention and treatment produced large decreases in HIV incidence in a model of people who inject drugs. Health Aff (Millwood). 2014;33(3):401–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sullivan PS, Rosenberg ES, Sanchez TH, et al. Explaining racial disparities in HIV incidence in black and white men who have sex with men in Atlanta, GA: A prospective observational cohort study. Ann Epidemiol. 2015;25(6):445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hernández-Romieu AC, Sullivan PS, Rothenberg R, et al. Heterogeneity of HIV prevalence among the sexual networks of Black and White MSM in Atlanta: Illuminating a mechanism for increased HIV risk for young Black MSM. Sex Transm Dis. 2015;42(9):505–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.White D, Grey JA, Gorbach PM, Rothenberg RB, Sullivan PS, Rosenberg ES. Racial differences in partnership attributes, typologies, and risk behaviors among men who have sex with men in Atlanta, Georgia. Arch Sex Behav. 2017;46(4):961–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson WD, O’Leary A, Flores SA. Per-partner condom effectiveness against HIV for men who have sex with men. AIDS. 2018;32(11):1499–1505. [DOI] [PubMed] [Google Scholar]
- 20.Patel P, Borkowf CB, Brooks JT, Lasry A, Lansky A, Mermin J. Estimating per-act HIV transmission risk: A systematic review. AIDS. 2014;28(10):1509–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelley CF, Rosenberg ES, O’Hara BM, et al. Measuring population transmission risk for HIV: An alternative metric of exposure risk in men who have sex with men (MSM) in the US. PLoS One. 2012;7(12):e53284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.United States Public Health Service. Pre-exposure prophylaxis for the prevention of HIV infection in the United States - 2017 update. Atlanta, Georgia: Centers for Disease Control and Prevention; 2018. [Google Scholar]
- 23.Anderson PL, Glidden DV, Liu AY, et al. Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Sci Transl Med. 2012;4(151):151ra125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rolle C-PM, Onwubiko U, Jo J, Sheth AN, Kelley CF, Holland DP. PrEP implementation and persistence in a county health department in Atlanta, Georgia. Conference on Retroviruses and Opportunistic Infections; 2018; Boston, Massachusetts. [Google Scholar]
- 25.McKay MD, Beckman RJ, Conover WJ. A comparison of three methods for selecting values of input variables in the analysis of output from a computer code. Technometrics. 1979;21(2):239–245. [Google Scholar]
- 26.Joint United Nations Programme on HIV/AIDS. 90–90-90: An ambitious treatment target to help end the AIDS epidemic. Geneva, Switzerland: United Nations;2017. [Google Scholar]
- 27.Harper S, King NB, Meersman SC, Reichman RC, Breen N, Lynch J. Implicit value judgments in the measurement of health inequalities. Milbank Q. 2010;88(1):4–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenberg ES, Marcus JL. Progress and pitfalls in measuring HIV preexposure prophylaxis coverage in the United States. Ann Epidemiol. 2018;28(12):830–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goodreau SM, Rosenberg ES, Jenness SM, et al. Sources of racial disparities in HIV prevalence in men who have sex with men in Atlanta, GA, USA: A modelling study. Lancet HIV. 2017;4(7):e311–e320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Millett GA, Peterson JL, Flores SA, et al. Comparisons of disparities and risks of HIV infection in black and other men who have sex with men in Canada, UK, and USA: A meta-analysis. Lancet. 2012;380(9839):341–348. [DOI] [PubMed] [Google Scholar]
- 31.Rosenberg ES, Purcell DW, Grey JA, Hankin-Wei A, Hall E, Sullivan PS. Rates of prevalent and new HIV diagnoses by race and ethnicity among men who have sex with men, U.S. states, 2013–2014. Ann Epidemiol. 2018;28(12):865–873. [DOI] [PubMed] [Google Scholar]
- 32.Serota DP, Rosenberg ES, Sullivan PS, et al. Pre-exposure prophylaxis uptake and discontinuation among young Black men who have sex with men in Atlanta, Georgia: A prospective cohort study. Clin Infect Dis. 2019: Available online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosenberg ES, Millett GA, Sullivan PS, Del Rio C, Curran JW. Understanding the HIV disparities between black and white men who have sex with men in the USA using the HIV care continuum: A modeling study. Lancet HIV. 2014;1(3):e112–e118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chan PA, Goedel WC, Nunn AS, et al. Potential impact of interventions to enhance retention in care during real-world HIV pre-exposure prophylaxis implementation. AIDS Patient Care STDS. 2019;33(10):434–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khanna AS, Schneider JA, Collier N, et al. A modeling framework to inform preexposure prophylaxis initiation and retention scale-up in the context of ‘Getting to Zero’ initiatives. AIDS. 2019;33(12):1911–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Doblecki-Lewis S, Butts S, Botero V, Klose K, Cardenas G, Feaster DJ. A randomized study of passive versus active PrEP patient navigation for a heterogenous population at risk for HIV in South Florida. J Int Assoc Provid AIDS Care. 2019;18:2325958219848848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spinelli MA, Glidden DV, Anderson PL, et al. Short-term adherence marker to PrEP predicts future nonretention inn a large PrEP demo project: Implications for point-of-care adherence testing. J Acquir Immune Defic Syndr. 2019;81(2):158–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ezennia O, Geter A, Smith DK. The PrEP care continuum and Black men who have sex with men: A scoping review of published data on awareness, uptake, adherence, and retention in care. AIDS Behav. 2019;23(10):2654–2673. [DOI] [PubMed] [Google Scholar]
- 39.Jones J, Weiss K, Mermin J, et al. Proportion of incident human immunodeficiency virus cases among men who have sex with menn attributable to gonorrhea and chlamydia: A modelingn analysis. Sex Transm Dis. 2019;46(6):357–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sullivan PS, Peterson J, Rosenberg ES, et al. Understanding racial HIV/STI disparities in black and white men who have sex with men: a multilevel approach. PLoS One. 2014;9(3):e90514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murray EJ, Robins JM, Seage GR, Freedberg KA, Hernán MA. A comparison of agent-based models and the parametric g-formula for causal inference. Am J Epidemiol. 2017;186(2):131–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.