Abstract
Purpose:
An inverse association between physical activity (PA) and risk of coronary heart disease has been seen in many studies, but evidence for benefits of PA after myocardial infarction (MI) in reducing mortality is limited.
Methods:
Using data from the Health Professionals Follow-up Study cohort, we followed male survivors of MI. Short term and long term changes in PA from before to after MI were calculated, and participants without ambulation impairment were classified into maintained low, decreased, increased, or maintained high PA categories. Cox models were used to estimate hazard ratios (HR) and 95% confidence intervals (CI) for mortality across PA and PA change categories.
Results:
During a mean of 14 years of follow-up of 1651 incident non-fatal MI cases, we documented 678 deaths, 307 were due to CVD. Adjusted HRs for all-cause mortality comparing ≥ 21 with ≤ 1.5 METs/week of PA before MI was 0.73; (95% CI, 0.59–0.89; Ptrend=0.03). Compared with men who maintained low PA before and after MI, men who maintained high PA had a 39% (95% CI, 25–50) lower risk of all-cause mortality, and those who had a long-term increase in PA from before to after MI had a 27% (95% CI, 6–43) lower risk. Walking for ≥ 30 minutes/day after MI was associated with a 29% lower mortality (HR=0.71; 95% CI, 0.58–0.84), independent of walking pace, and walking pace after MI was inversely associated with mortality (HR=0.67; 95% CI, 0.49–0.92).
Conclusions:
Maintaining a high PA or having a long-term increase in PA from before to after MI was associated with lower mortality among male MI survivors. Walking time and walking pace after MI were each inversely associated with mortality.
Keywords: exercise, myocardial infarction, survival, walking, risk factors
Introduction
Physical activity has been associated with lower risk of coronary heart disease (CHD) in many epidemiologic studies(1–3). Several mechanisms are likely to explain this association including improved blood lipid profile and blood pressure, decreased blood coagulability, increased insulin sensitivity, and better weight control(4–6). Since the 1950s, exercise has been an essential component of cardiac rehabilitation (CR) for patients with CHD(7–9). Exercise and other components of the CR programs, namely healthy diet, weight loss, smoking cessation, and blood pressure/glucose/lipid control, were found to reduce the rate of hospital readmission and cardiovascular disease (CVD) mortality(7, 8). Despite the substantial evidence for the benefits of CR, only a small percentage of patients with MI are referred to, participate, or complete CR programs(10). Moreover, in recent analyses, the financial sustainability of CR’s has been questioned(10, 11). While efforts are being made to enhance the outreach of CR, some have alternatively advocated for transforming CR to a broader healthy lifestyle prevention program (11, 12) to reach a larger patient population and extend beyond the traditional CR’s 12-week window(12). Given this interest in longer term interventions, there is a growing need for additional information on habitual physical activity (PA) and long-term outcomes among patients with MI.
We therefore evaluated discretionary physical activity, its intensity and changes, among male survivors of MI in the Health Professionals Follow-Up Study cohort, utilizing repeated measurements of PA, diet, and smoking during three decades of follow-up.
Methods
Study Population
The Health Professionals Follow Up Study (HPFS) was initiated in 1986 after enrolling 51,529 male health professionals, 40 to 75 years of age. Mailed questionnaires were administered biennially to obtain updated medical, lifestyle and other health related information.
From the 45,396 men who were free of CVD or cancer at the time of enrollment, we included participants who survived a documented incident MI during follow up between 1988 and January 31, 2010 until the end of the 2-year follow up period during which the MI occurred (n=2476). We excluded those who reported cancer (n=156) or stroke (n=40) at or before the time of MI diagnosis or difficulty in walking eight blocks or climbing a flight of stairs before MI diagnosis (n=226). Participants were also excluded if they reported impaired physical activity on the first questionnaire cycle post-MI diagnosis (n=301), had body mass index (BMI) < 18.5 kg/m2 (n=21), or had missing data on PA in the last questionnaire before MI (n=3) or first questionnaire after reporting MI (n=428). After exclusion for one or more reasons, a total of 1651 eligible participants were followed up until 2016.
The study protocol was approved by the institutional review boards of Brigham and Women’s Hospital and the Harvard T.H. Chan School of Public Health.
Ascertainment of MI
MI was initially self-reported and confirmed by medical records documenting symptoms and either diagnostic electrocardiographic changes or elevated cardiac specific enzyme levels(13). Medical records were reviewed by physicians blinded to the participants’ exposure status. For those whose medical records were unavailable, the diagnosis was considered probable if supported by telephone interview or additional corroborating information.
Ascertainment of Mortality
Deaths were identified from searches of vital records, the National Death Index, and reports by the participant’s next of kin or the postal system(14). Cardiovascular mortality included fatal MI, fatal stroke, and coronary heart disease, confirmed by a review of death certificates, medical records, and/or autopsy reports after obtaining the permission of the next of kin.
Assessment of Physical Activity (PA)
We assessed total and moderate to vigorous PA (MVPA), as the latter has been strongly associated with incidence of CVD(1, 15). Participants reported the average time spent per week in the previous year on specific activities by choosing one of the 10 different duration options, ranging from 0 to ≥ 11 hours/ week. MVPA included brisk walking, moderate and heavy outdoor work, weight training, jogging (<10 minutes/mile), running (≥ 10 minutes/mile), bicycling, lap swimming, tennis/squash/racquetball, and rowing. Data on walking pace, categorized as easy (< 2 miles/hour), normal (2 – 2.9 miles/hour), brisk (3 – 3.9 miles/hour), or striding (≥ 4 miles/hour), were also collected(16). A weekly energy expenditure score (MET-hours/week) was computed for each participant after summing up the metabolic equivalent task (MET) score associated with each activity(17). The validity and reproducibility of these measurements have been previously reported(18, 19). Briefly, correlations between PA reported in diaries and that in the questionnaire was 0.62 in women and 0.58 in men. A new validation study conducted on a subgroup of our HPFS cohort and other non-health professional participants in 2011–2013 showed moderate correlation between our physical activity questionnaire and the doubly labelled water (DLW) determined physical activity energy expenditure (r=0.40 for total PA and 0.43 for MVPA), as well as with two accelerometer measures (r=0.44 for total PA and 0.39 for MVPA) (unpublished data). In addition, independent associations were observed between PA and several biomarkers of obesity and CVD risk such as HDL cholesterol, leptin, and C-peptide(20).
PA before MI was derived from the last questionnaire before MI diagnosis. PA after MI was analyzed from the first questionnaire after reporting MI diagnosis. The difference between these PA measurements represented short term PA change in this study. To represent long-term PA change, we calculated the difference between the cumulative average of repeated measurements of PA in the periods before and after MI.
Assessment of Covariates
Covariates including age, race, marital status, family history of diabetes, MI and cancer, in addition to a dietary quality index (in quintiles), alcohol consumption (0, 0.1–4.9, 5.0–14.9, 15–29.9, or ≥30.0 g per day), cigarette smoking (never smoked, previously smoked, currently smokes 1–14, 15–24, or ≥ 25 cigarettes per day, or not reported), year of MI diagnosis, and aspirin use were used in the multivariate adjustment. Dietary information was collected from validated food frequency questionnaires every 4 years (21, 22). Diet quality was assessed using the 2010 Alternate Healthy Eating Index(23) (AHEI); scores range between 0 and 110 with higher scores indicating a healthier diet. Heart failure during hospitalization, incidence of cancer, and stroke were further included in the models assessing the association of PA after MI with mortality to minimize confounding. BMI, diabetes, hypertension, hypercholesterolemia, and their respective medications were included in a sensitivity analysis since these are likely to be in the causal pathway relating PA to mortality, and therefore could potentially attenuate the true association of PA with mortality. To account for potential bias attributed to MI severity, we further adjusted in a sensitivity analysis for other available clinical data collected during hospital admission: peak levels of cardiac enzymes (> median levels of peak hospital troponin or CKMB) and left ventricular ejection fraction dysfunction (ejection fraction <50%), or the occurrence of ST elevation myocardial infarction. To account for changes in medical treatment of MI participants across the study period, we included indicator variables to reflect year of MI diagnosis.
Statistical Analysis
Hazard ratios and 95% confidence intervals were estimated using Cox proportional hazards models, with number of months since the return of the first questionnaire after MI diagnosis as the time scale. Person-time was calculated from the time of the return of the first post-MI questionnaire until death or end of follow up, whichever came first. Pre-MI data were obtained on average 13 months before MI diagnosis and post-MI data on average 34 months after MI diagnosis.
MVPA before and after MI were categorized as ≤1.5, 1.6–7.4, 7.5–20.9, and ≥21 MET-hours/week. 21 MET-hours/week is equivalent to 7 hours/week of moderate intensity exercise. Changes in PA were classified based on cutoffs of 7.5 MET-hours/week which is equivalent to 2.5 hours/week of moderate intensity exercise: maintained low, decreased, increased, and maintained high.
Effect modifications were assessed by including interaction terms between continuous PA and each of BMI, age at MI diagnosis, alcohol drinking, and smoking.
For analyses of linear trends, medians of PA categories were modeled as a continuous variable. Nonlinear trends were tested with likelihood ratio tests of restricted cubic splines.
To reduce potential bias from excluding participants who did not survive until the time of the first questionnaire cycle after MI diagnosis or had missing post-MI PA data, a sensitivity analysis was conducted using pre-MI PA. We included those who died at the time of or after MI (thus including fatal CHD and sudden cardiac deaths) but did not answer a subsequent questionnaire, those who had missing post-MI PA data, and those who reported PA impairment in the first questionnaire cycle post-MI. Because changes in PA might be confounded by the underlying health status, we generated a new variable and combined data on long-term changes in PA, weight, and diet quality from before to after MI in a sensitivity analysis. For example, poor health status would be likely if a participant reduced his PA from high to low, while losing weight and not improving diet quality.
Data were analyzed in SAS software, (version 9.4-SAS institute) at a two-tailed alpha level of 0.05.
Results
Study Participants
A total of 1651 participants with incident non-fatal MI, were included in the analysis with a mean age of 65 years (range 40–89) at MI diagnosis.
Characteristics of participants categorized by MVPA levels after and before MI, are presented in Table 1 and Supplemental Table1 (see Appendix, Supplemental Digital Content 1, supplemental data). Physically more active men tended to have a higher AHEI score, consumed more alcohol, and were less likely to be current smokers or diabetic as compared to those with lower PA levels after MI.
Table 1.
Age-adjusted characteristics of participants in the Health Professionals Follow up Study by categories of moderate to vigorous physical activity (MET-hours/week) after MI (N=1651).
| ≤ 1.5 (N=280) | 1.6–7.4 (N=243) | 7.5–20.9 (N=415) | ≥ 21 (N=713) | p-value‡ | |
|---|---|---|---|---|---|
| Continuous Variables | Mean (Standard Deviation) or Median (Interquartile Range) | ||||
| Mod to vig PA (MET-hrs/week) | 0 (0−0) | 4.3 (2.5−5.8) | 12.7 (10−16) | 40.0 (28.0−59.0) | <0.001 |
| Mod to vig PA (hrs/week) | 0 (0−0) | 1.0 (0.5−1.2) | 2.5 (2.1−3.5) | 8.2 (6.0−11.5) | <0.001 |
| Total PA (hrs/week) | 1.0 (0−2.7) | 2.0 (1.0−3.5) | 4.0 (2.7−5.7) | 9.2 (6.5−13.5) | <0.001 |
| Total PA (MET-hours/week) | 3.0 (0−8.0) | 7.2 (4.5−12.5) | 16.9 (13.0−22.5) | 43.4 (31.0−63.5) | <0.001 |
| Age at diagnosis* | 66.9 (9.9) | 66.1 (9.2) | 64.8 (8.5) | 64.5 (8.4) | <0.001 |
| BMI (Kg/m2) | 26.4 (3.8) | 26.4 (3.9) | 26.3 (3.5) | 25.7 (3.5) | 0.01 |
| Alternate Healthy Eating Index score | 56 (11) | 57 (13) | 59 (12) | 62 (12) | <0.001 |
| Alcohol intake (g/day) | 8.1 (13.2) | 10.6 (15.3) | 10.8 (14.0) | 10.5 (14.0) | 0.03 |
| Categorical variables | % | % | % | % | |
| White race | 90 | 93 | 93 | 92 | 0.84 |
| Married | 72 | 76 | 80 | 82 | <0.001 |
| Family history of MI | 40 | 44 | 44 | 42 | 0.99 |
| Family history of Diabetes | 26 | 22 | 24 | 24 | 0.68 |
| Family history of cancer | 34 | 33 | 32 | 36 | 0.59 |
| Types of physical activity | |||||
| Walking ≥ 1 hr/week | 54 | 67 | 75 | 78 | <0.001 |
| Running ≥ 1 hr/week | 0 | 0 | 0.2 | 5 | 0.002 |
| Jogging and rowing ≥ 1 hr/week | 0 | 4 | 21 | 36 | <0.001 |
| Biking and swimming ≥ 1 hr/week | 0 | 14 | 34 | 35 | <0.001 |
| Racquet sports ≥ 1 hr/week | 0 | 1 | 4 | 11 | <0.001 |
| Mod-heavy outdoor work ≥ 1 hr/week | 0 | 15 | 40 | 61 | <0.001 |
| Weight training ≥ 1 hr/week | 0.4 | 2 | 12 | 27 | <0.001 |
| Smoking | |||||
| Never smoker | 36 | 40 | 37 | 40 | 0.41 |
| Former smoker | 58 | 53 | 60 | 58 | 0.62 |
| Current smoker | 7 | 6 | 4 | 3 | 0.001 |
| Comorbidities | |||||
| Diabetes | 22 | 13 | 14 | 11 | <0.001 |
| Hypertension | 59 | 53 | 55 | 54 | 0.43 |
| Hypercholesterolemia | 63 | 65 | 67 | 70 | 0.02 |
| Medication use | |||||
| Aspirin | 75 | 80 | 82 | 86 | <0.001 |
| Anti-hypertensives | 80 | 80 | 83 | 78 | 0.67 |
| Anti-hypercholesterolemia | 50 | 54 | 63 | 68 | <0.001 |
| Heart Failure during hospital admission | 11 | 9 | 7 | 6 | 0.01 |
| ST Elevation Myocardial Infarction | 65 | 60 | 58 | 62 | 0.74† |
| Abnormal Ejection Fraction | 45 | 36 | 33 | 33 | 0.01† |
| Peak cardiac enzymes > median | 79 | 82 | 82 | 81 | 0.53† |
| Cancer after MI | 7 | 11 | 8 | 9 | 0.35 |
| Stroke after MI | 3 | 4 | 2 | 2 | 0.11 |
Not age adjusted; PA: Physical Activity; Mod to vig: Moderate to vigorous intensity physical activity; Racquet sports included tennis, squash, and racquetball. Peak cardiac enzymes include peak troponin or CKMB levels. Variables were assessed on average 34 months post-MI diagnosis.
based on valid data for 1191 (ST Elevation Myocardial Infarction), 996 (abnormal ejection fraction), and 634 (peak cardiac enzymes) participants.
p-value for linear trends.
All-cause and CVD mortality
We documented 678 deaths from all-causes and 307 deaths from CVD among survivors of MI during a mean of 14 years of follow-up after the first post-MI questionnaire. MVPA before and after MI diagnosis were both inversely associated with all-cause mortality (HR comparing ≥ 21 to ≤ 1.5 MET-hours/week=0.73 (95% CI 0.59–0.89, Ptrend=0.03) before MI and 0.74 (95% CI, 0.60–0.92, Ptrend=0.02) after MI diagnosis. A significant non-linear inverse association was noted between PA before MI and CVD mortality (P for non-linearity=0.03, Table 2). Further adjustment for BMI, hypertension, diabetes, and hypercholesterolemia and their respective medications modestly attenuated these associations (Table 2) and were therefore not included in further models.
Table 2:
Age- and multivariate-adjusted Hazard Ratios (95% CI) of all-cause and CVD mortality according to categories of moderate to vigorous intensity physical activity in MET-hours/week, shortly before and after myocardial infarction diagnosis (N=1651).
| Moderate-vigorous Physical Activity (MET-hours/week) | Nevents (Ntotal) | Age adjusted | Multivariate Adjusted * | + comorbidities† | + BMI | +comorbidities + BMI |
|---|---|---|---|---|---|---|
| Before MI diagnosis | ||||||
| All-cause mortality | ||||||
| ≤ 1.5 | 168 (338) | Reference | Reference | Reference | Reference | Reference |
| 1.6–7.4 | 131 (287) | 0.79 (0.62, 0.99) | 0.81 (0.64, 1.02) | 0.87 (0.69, 1.10) | 0.84 (0.66, 1.06) | 0.89 (0.70, 1.13) |
| 7.5–20.9 | 158 (404) | 0.68 (0.55, 0.85) | 0.71 (0.57, 0.89) | 0.76 (0.61, 0.96) | 0.74 (0.59, 0.93) | 0.79 (0.63 0.99) |
| ≥ 21 | 221 (622) | 0.68 (0.56, 0.83) | 0.73 (0.59, 0.89) | 0.76 (0.62, 0.94) | 0.76 (0.61, 0.93) | 0.79 (0.64, 0.98) |
| pnonlinearity | 0.13 | 0.13 | 0.24 | 0.19 | 0.24 | |
| plinearity | 0.005 | 0.03 | 0.04 | 0.06 | 0.07 | |
| CVD mortality | ||||||
| ≤ 1.5 | 87 (338) | Reference | Reference | Reference | Reference | Reference |
| 1.6–7.4 | 56 (287) | 0.65 (0.46, 0.91) | 0.69 (0.47, 0.94) | 0.70 (0.50, 0.99) | 0.69 (0.49, 0.98) | 0.72 (0.51, 1.03) |
| 7.5–20.9 | 69 (404) | 0.57 (0.42, 0.79) | 0.60 (0.43, 0.83) | 0.63 (0.45, 0.87) | 0.62 (0.45, 0.86) | 0.65 (0.46, 0.90) |
| ≥ 21 | 95 (622) | 0.56 (0.42, 0.76) | 0.62 (0.46, 0.84) | 0.65 (0.48, 0.88) | 0.64 (0.47, 0.87) | 0.66(0.49, 0.91) |
| pnonlinearity | 0.007 | 0.03 | 0.04 | 0.04 | 0.047 | |
| plinearity | 0.005 | 0.04 | 0.05 | 0.06 | 0.07 | |
| After MI diagnosis | ||||||
| All-cause mortality | ||||||
| ≤ 1.5 | 147 (280) | Reference | Reference | Reference | Reference | Reference |
| 1.6–7.4 | 113 (243) | 0.77 (0.60, 0.98) | 0.79 (0.62, 1.01) | 0.82 (0.64, 1.06) | 0.79 (0.61, 1.01) | 0.82 (0.64, 1.06) |
| 7.5–20.9 | 176 (405) | 0.83 (0.67, 1.04) | 0.91 (0.73, 1.14) | 0.95 (0.75, 1.19) | 0.91 (0.73, 1.14) | 0.95 (0.76, 1.19) |
| ≥ 21 | 242 (713) | 0.66 (0.54, 0.81) | 0.74 (0.60, 0.92) | 0.78 (0.63, 0.97) | 0.76 (0.61, 0.94) | 0.80 (0.64, 0.99) |
| pnonlinearity | 0.57 | 0.89 | 0.80 | 0.96 | 0.73 | |
| plinearity | <.001 | 0.02 | 0.05 | 0.04 | 0.08 | |
| CVD mortality | ||||||
| ≤ 1.5 | 72 (280) | Reference | Reference | Reference | Reference | Reference |
| 1.6–7.4 | 52 (243) | 0.73 (0.51, 1.05) | 0.77 (0.53, 1.10) | 0.81 (0.56, 1.17) | 0.76 (0.53, 1.09) | 0.81 (0.56, 1.17) |
| 7.5–20.9 | 75 (405) | 0.71 (0.52, 0.99) | 0.81 (0.58, 1.13) | 0.85 (0.61, 1.20) | 0.80 (0.57, 1.12) | 0.85 (0.61, 1.19) |
| ≥ 21 | 108 (713) | 0.60 (0.49, 0.81) | 0.71 (0.52, 0.97) | 0.77 (0.56, 1.06) | 0.72 (0.53, 0.99) | 0.79 (0.57, 1.08) |
| pnonlinearity | 0.14 | 0.43 | 0.67 | 0.42 | 0.66 | |
| plinearity | 0.005 | 0.09 | 0.21 | 0.13 | 0.29 | |
Adjusted for age, race, family history of myocardial infarction, cancer, and diabetes, smoking, marital status, alcohol consumption, alternate healthy eating index score, year of MI diagnosis, and aspirin use. Models using PA after MI diagnosis were further adjusted for heart failure during hospital admission and incidence of stroke and cancer after MI diagnosis.
included diabetes, in addition to hypertension, hypercholesterolemia, and their respective medications.
In a model with simultaneous adjustment for PA before and after MI diagnosis, MVPA before MI remained an important predictor for all-cause and CVD mortality. The association of post-MI PA with mortality became non-significant (HR comparing ≥ 21 to ≤ 1.5 MET-hours/week=0.82 (95% CI 0.65, 1.04, Ptrend =0.20) [Supplemental Table 2, (see Appendix, Supplemental Digital Content 1, supplemental data)].
Change in PA from before to after MI diagnosis
After MI diagnosis, participants tended to increase their PA and the majority of those in the highest pre-MI PA categories (≥ 7.5 MET-hours/week) remained active [Supplemental Table 3, (see Appendix, Supplemental Digital Content 1, supplemental data)]. Compared to men who remained in the low PA category, those who maintained high PA both before and after MI had 24% lower all-cause mortality (HR= 0.76; 95% CI, 0.62–0.92) and 30% lower CVD mortality risk (HR=0.70; 95% CI, 0.52–0.95) (Table 3). Short term increase in PA was not associated with lower risk of mortality. In analyses of long-term PA change, compared to men who maintained low long-term PA, those who increased from low to high PA or maintained their high PA from before to after MI were at a lower risk of mortality [(HR= 0.73; 95% CI, 0.57–0.94) for those who increased PA and HR=0.61; 95% CI, 0.50–0.75 for those who maintained high PA] (Table 3). To address the possibility that men may have reduced their PA due to disease symptoms during the follow up period, i.e., reverse causation, we stopped updating the data on PA once a participant reported ambulation impairment anytime during the follow-up. Similar associations were observed, and also a lower mortality risk was observed among those who changed from high to low cumulative average PA as compared to those who remained physically inactive (Table 3).
Table 3:
Multivariate adjusted Hazard Ratios (95% CI) of all-cause and CVD mortality according to Moderate to vigorous physical activity (PA) change from before to after MI. Medians (Interquartile Range) before and after MI are presented for each category of PA change.
| Short term Change (MET-hrs) | Median (IQR) pre-MI | Median (IQR) post-MI | Ndeaths | All-Cause Mortality HR† (95% CI) | NCVDdeaths | CVD Mortality HR† (95% CI) |
| Low- Low (N=322) | 0.8 (0–3) | 0.8 (0–3) | 170 | 1 | 78 | 1 |
| Low-High (N=303) | 2.1 (0–5) | 18.9 (12–37) | 129 | 0.93 (0.73, 1.17) | 65 | 1.04 (0.74, 1.46) |
| High-Low (N=201) | 17.5 (12–34) | 2.0 (0–5) | 90 | 0.74 (0.57, 0.96) | 46 | 0.86 (0.59, 1.26) |
| High-High (N=825) | 28.5 (16–49) | 30 (18–47) | 289 | 0.76 (0.62, 0.92) | 118 | 0.70 (0.52, 0.95) |
| Cumulative Average Change (MET-hrs) | Median (IQR) pre-MI | Median (IQR) post-MI | HR† (95% CI) | HR† (95% CI) | ||
| Low- Low (N=252) | 1.7 (0–4) | 2.6 (1–5) | 144 | 1 | 71 | 1 |
| Low-High (N=281) | 3.7 (1–5) | 16.4 (12–26) | 123 | 0.73 (0.57, 0.94) | 63 | 0.73 (0.51, 1.04) |
| High-Low (N=152) | 14.6 (11–23) | 3.6 (2–6) | 75 | 0.83 (0.62, 1.11) | 30 | 0.69 (0.44, 1.07) |
| High-High (N=966) | 25.1 (15–39) | 30.4 (18–45) | 336 | 0.61 (0.50, 0.75) | 143 | 0.54 (0.40, 0.73) |
| Cumulative Average Change * (MET-hrs) | Median (IQR) pre-MI | Median (IQR) post-MI | HR† (95% CI) | HR† (95% CI) | ||
| Low- Low (N=234) | 1.8 (0–4) | 2.2 (0.5–5) | 140 | 1 | 71 | 1 |
| Low-High (N=286) | 3.7 (1–5) | 16.4 (11–26) | 127 | 0.69 (0.54, 0.89) | 63 | 0.67 (0.47, 0.96) |
| High-Low (N=137) | 15 (11–23) | 3.8 (1–6) | 71 | 0.73 (0.54, 0.98) | 30 | 0.66 (0.42, 1.02) |
| High-High (N=971) | 25 (15–39) | 31 (18–46) | 340 | 0.61 (0.50, 0.75) | 143 | 0.53 (0.39, 0.71) |
Using cumulative average before and stopping to update the data after reporting physical activity impairment during the follow-up after MI
Adjusted for age, race, family history of myocardial infarction, cancer, and diabetes, smoking, marital status, alcohol consumption, alternate healthy eating index score, year of MI diagnosis, and aspirin use, heart failure during hospital admission, and incidence of cancer and stroke after MI. PA were classified based on cutoffs of 7.5 MET-hours/week which is equivalent to 2.5 hours/week of moderate intensity exercise. Low-Low represents those whose moderate to vigorous physical activity remained <7.5 MET-hours/week from before to after MI.
Because long-term changes in PA could be confounded by the underlying health status of a participant, a more detailed analysis was carried out among participants with available data on their cumulative average weight and diet in the periods before and after MI (N=1541). Compared to those who maintained low PA and did not experience weight loss from before to after MI, participants who maintained low PA while experiencing weight loss without diet improvement were at a 54% higher risk of all-cause mortality (HR=1.54; 95% CI, 1.04, 2.27). In contrast, those who maintained their high PA levels, reduced their weight, and improved their diet were at a lower risk of mortality (HR= 0.61; 95% CI, 0.40, 0.94) [Supplemental Table 4, (see Appendix, Supplemental Digital Content 1, supplemental data)].
After further adjustment for MI severity, the associations between PA post-MI and change in PA in relation with all-cause and CVD mortality were similar [Supplemental Table 5, (see Appendix, Supplemental Digital Content 1, supplemental data)].
The inverse associations between PA (pre and post-MI) and mortality were similar across categories of BMI, age, and alcohol drinking groups (p>0.05, for interaction), but were stronger among never smokers as compared to ever smokers (p = 0.03 for interaction) [Supplemental Table 6, (see Appendix, Supplemental Digital Content 1, supplemental data)].
Type of Physical Activity
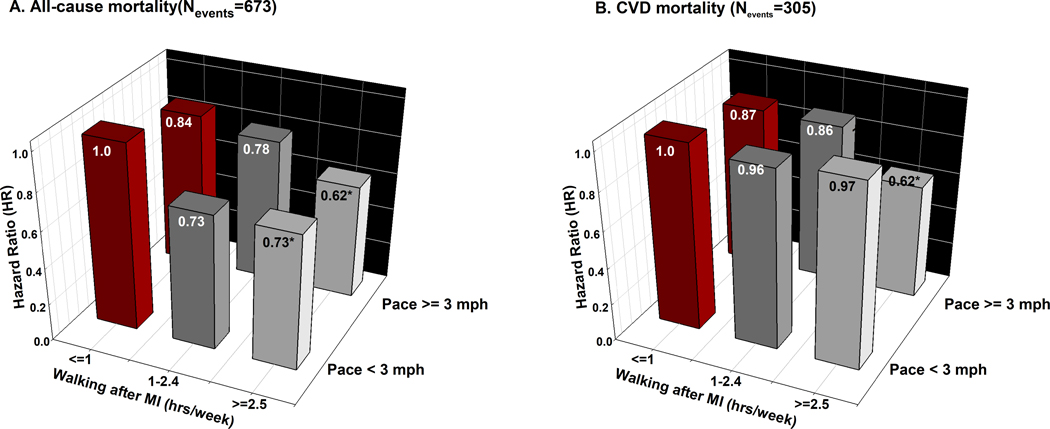
Walking for 2.5 hours or more per week after MI was associated with lower all-cause mortality; (HR=0.71; 95% CI, 0.58– 0.87) for 2.5–5.0 hours/week, and (HR=0.65; 95% CI, 0.51–0.85) for >5 hours/week, P<0.001 for non-linearity. Brisk walking (pace ≥ 3 mph) was independently associated with lower mortality as compared to walking at an easy pace, HR=0.71; 95% CI, 0.51–0.97) [Supplemental Tables 7 and 8, (see Appendix, Supplemental Digital Content 1, supplemental data)]. The joint associations between walking and pace of walking in relation to risks of all-cause and CVD mortality are shown in Figure 1. In comparison with men who walked one hour or less per week at a low pace after MI, men who walked 2.5 hours or more had a 27% lower risk of all-cause mortality if their pace was < 3 mph and 38% lower risk if their pace was ≥ 3 mph [Figure 1, Supplemental Table 9, (see Appendix, Supplemental Digital Content 1, supplemental data)]. Similar associations were observed with simultaneous adjustment for walking duration and pace before their MI. When including all other types of activities in the same model, walking remained inversely associated with all-cause mortality.
Figure 1: Multivariate adjusted Hazard Ratios of all-cause and CVD mortality for the joint association between walking and pace of walking after MI diagnosis (N=1651).
Models were adjusted for age, race, family history of myocardial infarction, cancer, and diabetes, smoking, marital status, alcohol consumption, alternate healthy eating index score, year of MI diagnosis, aspirin use, heart failure during hospital admission, and incidence of cancer and stroke after MI. Walking ≤ 1 hour/week at a pace < 3mph was considered as the reference group. P-value for interaction between number of walking hours and pace of walking after MI was 0.06 with all-cause mortality and 0.19 with CVD mortality. * represents statistical significance
To minimize any confounding of the association between walking and MI by high intensity PA, we restricted the analysis to men who reported less than 1 hour of weekly vigorous exercise (<6 MET-hour/week, n=961). Compared to men who walked one hour or less at a low pace after MI, those who walked 2.5 hours or more were at a lower risk of all-cause mortality (HR=0.66; 95% CI, 0.50–0.86) for walking pace < 3 mph and HR= 0.63; 95% CI, 0.46–0.87 for walking pace ≥ 3 mph [Supplemental Figure 1, (see Appendix, Supplemental Digital Content 1, supplemental data)].
In an exploratory analysis of other types of physical activity, practicing racquet sports after MI for more than 1 hour/ week was associated with 36% (95% CI, 4–57) lower risk of all-cause mortality as compared to participants who did not practice this activity, after accounting for all other types of physical activity. Spending > 1 hour/week on biking or rowing after MI was independently associated with higher risk of mortality [Supplemental Tables 10–13, (see Appendix, Supplemental Digital Content 1, supplemental data)].
Inclusion of fatal and all non-fatal MIs
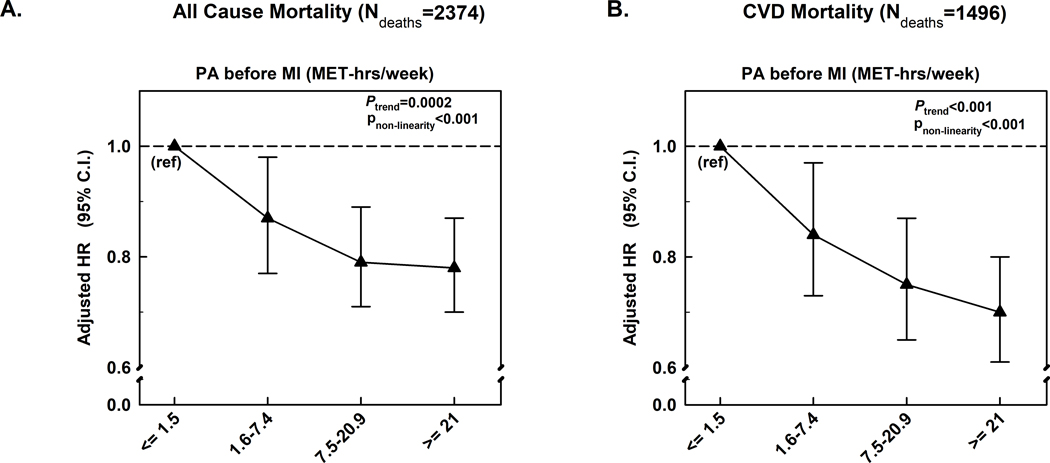
We also evaluated pre-MI PA in an analysis that included men who died of CHD before returning a post MI questionnaire, including those who died before hospitalization. This included 3419 participants among whom 2347 died, including 1496 CVD deaths. Higher pre-MI levels of MVPA remained inversely associated with all-cause and CVD mortality [Figure 2, Supplemental Table 14, (see Appendix, Supplemental Digital Content 1, supplemental data)].
Figure 2: Moderate to vigorous physical activity (MET-hours/week) before MI in relation to all-cause and CVD mortality among all 3419 men with MI, including those who did not survive up to the post-MI questionnaire cycle or had missing post-MI PA data.
Models were adjusted for age, race, family history of myocardial infarction, cancer, and diabetes, smoking, marital status, alcohol consumption, alternate healthy eating index score, year of MI diagnosis, and aspirin use.
Discussion
In this prospective study of male survivors of MI, higher levels of PA before an acute MI were associated with improved survival after MI, and maintaining high PA or having a long-term increase in PA from before to after MI was associated with a lower post MI risk of all-cause and CVD mortality. Walking at least 2.5 hours per week after MI diagnosis was associated with substantially lower all-cause mortality, independent of pace or other types of physical activity.
To our knowledge, this study is the first analysis of PA to include a large number of male survivors of myocardial infarction whose physical activity data have been repeatedly measured before and after MI using a validated PA questionnaire, and had a long period of follow up after their MI diagnosis. Utilizing the prospective design of the cohort and the updated assessment of PA and other important lifestyle factors every two years for over a mean of 15 years of follow up after MI, we were able to analyze short-term and long-term change in PA from before to after MI in relation to mortality. Several sensitivity analyses were conducted to reduce biases from reverse causation and account for MI severity. Selection bias was also reduced with the inclusion of all fatal CHD and non-fatal MI cases in an analysis examining the association of PA before MI and risk of mortality.
Our results are consistent with previous studies examining change in PA and mortality among survivors of myocardial infarction. Only two previous studies have examined this association in a cohort design(24, 25). The first study(24) included a small number of MI patients (n=407) followed over 7 years, using an unvalidated instrument to measure PA. The second study focused on 856 postmenopausal women(25), with a median of 7.2 years of follow up after MI. Our results are also consistent with meta-analyses of randomized controlled trials of exercise-based cardiac rehabilitation programs which reported lower risks of cardiac mortality in the cardiac rehabilitation group as compared to the comparison group(7, 8), although these programs often include other life-style modifications in addition to physical activity. The associations observed between PA post-MI and PA changes and mortality were not however as strong as those reported using population-based cohort data of Swedeheart registry (26, 27). This could be attributed to the differences in study design in terms of inclusion/exclusion criteria, the use of a validated questionnaire for measuring PA, frequency and time for measuring PA post-MI, definition of PA change as the Swedeheart study defined PA change as the difference in PA between two post-MI visits (at 6–10 weeks and 10–12 months post MI), duration of follow up post-MI, and most importantly the approaches taken to minimize potential reverse causality biases. However, the direction of associations was similar to our findings, and together these findings lend support to the benefits of physical activity post MI. In our study, we also extended the research and examined the association between short term and long-term change in PA with mortality taking into account important lifestyle factors, in addition to adjusting for disease severity in a sensitivity analysis. We also examined the different types of PA including walking in relation to mortality.
Exercise can favorably modulate several physiological and biochemical processes after the cardiac event. In animal models exercise increases contractility and myofilament Ca2+ sensitivity(28, 29). It also favorably modulates the renin angiotensin aldosterone system post MI(30) and myocardial fibrosis and remodeling(31). In addition, increasing physical activity can improve cardiovascular risk factors including lipid profile, insulin sensitivity, blood pressure, and weight control (4, 5).
On the other hand, further research is needed to confirm the findings observed for the different types of physical activity, other than walking, in relation to mortality. In our study, these activities were practiced by a very few participants, and therefore analyzing participants who practice these activities more frequently in other cohorts would give a better understanding of the association between these activities post-MI and mortality.
The present study has several limitations. Participants had to survive to the post-MI questionnaire cycle so that short-term PA change could be measured. Therefore, those with severe MI who died at the time of the event or shortly thereafter were not included in the primary analysis.
We conducted a sensitivity analysis using pre-diagnosis PA after including these patients, who are normally missed in other clinical studies, and the inverse association between pre-MI PA and all-cause and CVD mortality was similar. Also, the use of self-reported PA measurements inevitably includes measurement errors, but in previous validation studies these assessments were reasonably correlated with detailed assessments of PA(18). The use of repeated assessments of physical activity in our study will reduce measurement error, but the apparent benefit of physical activity in our study is almost certainly underestimated. A new validation study conducted on a subgroup of our HPFS cohort and other non-health professional participants in 2011–2013 showed moderate correlation between our physical activity questionnaire and the doubly labelled water (DLW) determined physical activity energy expenditure (r=0.40 for total PA and 0.43 for MVPA), as well as with two accelerometer measures (r=0.44 for total PA and 0.39 for MVPA) (unpublished data). Independent associations between reported physical activity and biomarkers of obesity and CVD risk were previously reported(20).
Although we adjusted for major confounders, residual and unmeasured confounding is possible. Changes in clinical management of MI over time could have also modified the results. However, we controlled for the year of MI diagnosis, which indirectly accounts for changes in coronary disease management. Lack of a national registry for MI is a limitation, but medical records were reviewed by physicians blinded to the participants’ exposure status to ascertain health outcomes. Finally, our patients are mostly non-Hispanic white men, drawn from a cohort of health professionals whose socioeconomic status may not represent the overall population, thus affecting the generalizability of our study findings. However, this homogeneity would have helped to minimize unmeasured confounding related to socioeconomic status.
In summary, our results support the most recent 2018 Physical Activity Guidelines for Americans(32) and the current ACC/AHA guidelines for the management of myocardial infarction patients which endorse a post-hospitalization plan of care that includes physical activity and other appropriate lifestyle factor modifications(33). Our findings emphasize the value of maintaining regular PA throughout adult life because greater PA was associated with both lower risk of MI and also better survival among those who experienced an MI. Walking, a common activity among older population, appears to confer a substantial reduction in the risk of mortality. In all cases, cardiac patients are advised to consult their healthcare providers about the type and amount of physical activity appropriate for their conditions.
Supplementary Material
Acknowledgement
The cohorts were supported by grants of UM1 CA167552, and R01 HL35464 from the National Institutes of Health. We would like to thank the participants and staff of the Health Professionals Study for their valuable contributions. The results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation. The authors assume full responsibility for analyses and interpretation of these data. The results of the present study do not constitute endorsement by ACSM.
Laila Al-Shaar received research support from the American Heart Association (17PRE33660133).
Footnotes
Conflict of Interest
The authors have no conflict of interest.
References
- 1.Tanasescu M, Leitzmann MF, Rimm EB, Willett WC, Stampfer MJ, Hu FB. Exercise type and intensity in relation to coronary heart disease in men. Jama. 2002;288(16):1994–2000. Epub 2002/10/22. PubMed PMID: 12387651. [DOI] [PubMed] [Google Scholar]
- 2.Chomistek AK, Henschel B, Eliassen AH, Mukamal KJ, Rimm EB. Frequency, Type, and Volume of Leisure-Time Physical Activity and Risk of Coronary Heart Disease in Young Women. Circulation. 2016;134(4):290–9. Epub 2016/07/28. doi: 10.1161/circulationaha.116.021516. PubMed PMID: 27462052; PubMed Central PMCID: PMCPMC4966899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manson JE, Greenland P, LaCroix AZ, Stefanick ML, Mouton CP, Oberman A, et al. Walking compared with vigorous exercise for the prevention of cardiovascular events in women. The New England journal of medicine. 2002;347(10):716–25. Epub 2002/09/06. doi: 10.1056/NEJMoa021067. PubMed PMID: 12213942. [DOI] [PubMed] [Google Scholar]
- 4.Nystoriak MA, Bhatnagar A. Cardiovascular Effects and Benefits of Exercise. Frontiers in cardiovascular medicine. 2018;5:135. Epub 2018/10/17. doi: 10.3389/fcvm.2018.00135. PubMed PMID: 30324108; PubMed Central PMCID: PMCPMC6172294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thompson Paul D, Buchner D, Piña Ileana L, Balady Gary J, Williams Mark A, Marcus Bess H, et al. Exercise and Physical Activity in the Prevention and Treatment of Atherosclerotic Cardiovascular Disease. Circulation. 2003;107(24):3109–16. doi: 10.1161/01.CIR.0000075572.40158.77. [DOI] [PubMed] [Google Scholar]
- 6.Ahmed HM, Blaha MJ, Nasir K, Rivera JJ, Blumenthal RS. Effects of physical activity on cardiovascular disease. The American journal of cardiology. 2012;109(2):288–95. Epub 2011/10/21. doi: 10.1016/j.amjcard.2011.08.042. PubMed PMID: 22011559. [DOI] [PubMed] [Google Scholar]
- 7.Lawler PR, Filion KB, Eisenberg MJ. Efficacy of exercise-based cardiac rehabilitation post-myocardial infarction: a systematic review and meta-analysis of randomized controlled trials. American heart journal. 2011;162(4):571–84.e2. Epub 2011/10/11. doi: 10.1016/j.ahj.2011.07.017. PubMed PMID: 21982647. [DOI] [PubMed] [Google Scholar]
- 8.Anderson L, Oldridge N, Thompson DR, Zwisler AD, Rees K, Martin N, et al. Exercise-Based Cardiac Rehabilitation for Coronary Heart Disease: Cochrane Systematic Review and Meta-Analysis. Journal of the American College of Cardiology. 2016;67(1):1–12. Epub 2016/01/15. doi: 10.1016/j.jacc.2015.10.044. PubMed PMID: 26764059. [DOI] [PubMed] [Google Scholar]
- 9.O’Connor GT, Buring JE, Yusuf S, Goldhaber SZ, Olmstead EM, Paffenbarger RS Jr., et al. An overview of randomized trials of rehabilitation with exercise after myocardial infarction. Circulation. 1989;80(2):234–44. Epub 1989/08/01. PubMed PMID: 2665973. [DOI] [PubMed] [Google Scholar]
- 10.Arena R, Williams M, Forman DE, Cahalin LP, Coke L, Myers J, et al. Increasing referral and participation rates to outpatient cardiac rehabilitation: the valuable role of healthcare professionals in the inpatient and home health settings: a science advisory from the American Heart Association. Circulation. 2012;125(10):1321–9. Epub 2012/02/01. doi: 10.1161/CIR.0b013e318246b1e5. PubMed PMID: 22291128. [DOI] [PubMed] [Google Scholar]
- 11.Sandesara PB, Lambert CT, Gordon NF, Fletcher GF, Franklin BA, Wenger NK, et al. Cardiac rehabilitation and risk reduction: time to “rebrand and reinvigorate”. Journal of the American College of Cardiology. 2015;65(4):389–95. Epub 2015/01/31. doi: 10.1016/j.jacc.2014.10.059. PubMed PMID: 25634839. [DOI] [PubMed] [Google Scholar]
- 12.Lavie CJ, Arena R, Franklin BA. Cardiac Rehabilitation and Healthy Life-Style Interventions: Rectifying Program Deficiencies to Improve Patient Outcomes. Journal of the American College of Cardiology. 2016;67(1):13–5. Epub 2016/01/15. doi: 10.1016/j.jacc.2015.09.103. PubMed PMID: 26764060. [DOI] [PubMed] [Google Scholar]
- 13.Niedziela J, Hudzik B, Niedziela N, Gąsior M, Gierlotka M, Wasilewski J, et al. The obesity paradox in acute coronary syndrome: a meta-analysis. European Journal of Epidemiology. 2014;29(11):801–12. doi: 10.1007/s10654-014-9961-9. PubMed PMID: PMC4220102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rich-Edwards JW, Corsano KA, Stampfer MJ. Test of the National Death Index and Equifax Nationwide Death Search. Am J Epidemiol. 1994;140(11):1016–9. PubMed PMID: 7985649. [DOI] [PubMed] [Google Scholar]
- 15.Lee IM, Sesso HD, Oguma Y, Paffenbarger RS Jr. Relative intensity of physical activity and risk of coronary heart disease. Circulation. 2003;107(8):1110–6. PubMed PMID: 12615787. [DOI] [PubMed] [Google Scholar]
- 16.Health Professionals Follow-Up Study Questionnaires; https://sites.sph.harvard.edu/hpfs/hpfs-questionnaires/. Accessed on 27/09/2018.
- 17.Chomistek AK, Chiuve SE, Jensen MK, Cook NR, Rimm EB. Vigorous physical activity, mediating biomarkers, and risk of myocardial infarction. Med Sci Sports Exerc. 2011;43(10):1884–90. doi: 10.1249/MSS.0b013e31821b4d0a. PubMed PMID: 21448079; PubMed Central PMCID: PMCPMC3249756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chasan-Taber S, Rimm EB, Stampfer MJ, Spiegelman D, Colditz GA, Giovannucci E, et al. Reproducibility and validity of a self-administered physical activity questionnaire for male health professionals. Epidemiology (Cambridge, Mass). 1996;7(1):81–6. Epub 1996/01/01. PubMed PMID: 8664406. [DOI] [PubMed] [Google Scholar]
- 19.Wolf AM, Hunter DJ, Colditz GA, Manson JE, Stampfer MJ, Corsano KA, et al. Reproducibility and validity of a self-administered physical activity questionnaire. International journal of epidemiology. 1994;23(5):991–9. Epub 1994/10/01. PubMed PMID: 7860180. [DOI] [PubMed] [Google Scholar]
- 20.Fung TT, Hu FB, Yu J, Chu NF, Spiegelman D, Tofler GH, et al. Leisure-time physical activity, television watching, and plasma biomarkers of obesity and cardiovascular disease risk. American journal of epidemiology. 2000;152(12):1171–8. Epub 2000/12/29. PubMed PMID: 11130623. [DOI] [PubMed] [Google Scholar]
- 21.Willett WC, Sampson L, Browne ML, Stampfer MJ, Rosner B, Hennekens CH, et al. The use of a self-administered questionnaire to assess diet four years in the past. American journal of epidemiology. 1988;127(1):188–99. Epub 1988/01/01. PubMed PMID: 3337073. [DOI] [PubMed] [Google Scholar]
- 22.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and Validity of an Expanded Self-Administered Semiquantitative Food Frequency Questionnaire among Male Health Professionals. American Journal of Epidemiology. 1992;135(10):1114–26. doi: 10.1093/oxfordjournals.aje.a116211. [DOI] [PubMed] [Google Scholar]
- 23.Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, et al. Alternative dietary indices both strongly predict risk of chronic disease. The Journal of nutrition. 2012;142(6):1009–18. Epub 2012/04/20. doi: 10.3945/jn.111.157222. PubMed PMID: 22513989; PubMed Central PMCID: PMCPMC3738221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steffen-Batey L, Nichaman Milton Z, Goff David C, Frankowski Ralph F, Hanis Craig L, Ramsey David J, et al. Change in Level of Physical Activity and Risk of All-Cause Mortality or Reinfarction. Circulation. 2000;102(18):2204–9. doi: 10.1161/01.CIR.102.18.2204. [DOI] [PubMed] [Google Scholar]
- 25.Gorczyca AM, Eaton CB, LaMonte MJ, Manson JE, Johnston JD, Bidulescu A, et al. Change in Physical Activity and Sitting Time After Myocardial Infarction and Mortality Among Postmenopausal Women in the Women’s Health Initiative-Observational Study. Journal of the American Heart Association. 2017;6(5). Epub 2017/05/17. doi: 10.1161/jaha.116.005354. PubMed PMID: 28507059; PubMed Central PMCID: PMCPMC5524089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ek A, Ekblom O, Hambraeus K, Cider A, Kallings LV, Borjesson M. Physical inactivity and smoking after myocardial infarction as predictors for readmission and survival: results from the SWEDEHEART-registry. Clinical research in cardiology : official journal of the German Cardiac Society. 2019;108(3):324–32. Epub 2018/09/01. doi: 10.1007/s00392-018-1360-x. PubMed PMID: 30167806; PubMed Central PMCID: PMCPMC6394466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ekblom O, Ek A, Cider A, Hambraeus K, Borjesson M. Increased Physical Activity Post-Myocardial Infarction Is Related to Reduced Mortality: Results From the SWEDEHEART Registry. Journal of the American Heart Association. 2018;7(24):e010108. Epub 2018/12/19. doi: 10.1161/jaha.118.010108. PubMed PMID: 30561263; PubMed Central PMCID: PMCPMC6405601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Waard MC, van der Velden J, Bito V, Ozdemir S, Biesmans L, Boontje NM, et al. Early exercise training normalizes myofilament function and attenuates left ventricular pump dysfunction in mice with a large myocardial infarction. Circulation research. 2007;100(7):1079–88. Epub 2007/03/10. doi: 10.1161/01.res.0000262655.16373.37. PubMed PMID: 17347478. [DOI] [PubMed] [Google Scholar]
- 29.Schober T, Knollmann BC. Exercise after myocardial infarction improves contractility and decreases myofilament Ca2+ sensitivity. Circulation research. 2007;100(7):937–9. Epub 2007/04/14. doi: 10.1161/01.res.0000265138.06052.08. PubMed PMID: 17431194. [DOI] [PubMed] [Google Scholar]
- 30.Wan W, Powers AS, Li J, Ji L, Erikson JM, Zhang JQ. Effect of post-myocardial infarction exercise training on the renin-angiotensin-aldosterone system and cardiac function. The American journal of the medical sciences. 2007;334(4):265–73. Epub 2007/11/22. doi: 10.1097/MAJ.0b013e318068b5ed. PubMed PMID: 18030183. [DOI] [PubMed] [Google Scholar]
- 31.Xu X, Wan W, Powers AS, Li J, Ji LL, Lao S, et al. Effects of exercise training on cardiac function and myocardial remodeling in post myocardial infarction rats. Journal of molecular and cellular cardiology. 2008;44(1):114–22. Epub 2007/11/06. doi: 10.1016/j.yjmcc.2007.10.004. PubMed PMID: 17980387; PubMed Central PMCID: PMCPMC2244592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Piercy KL, Troiano RP, Ballard RM, et al. The physical activity guidelines for Americans. JAMA. 2018;320(19):2020–8. doi: 10.1001/jama.2018.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gara PT, Kushner FG, Ascheim DD, Casey DE, Chung MK, de Lemos JA, et al. 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction. Journal of the American College of Cardiology. 2013;61(4):e78. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.