Abstract
Taurine has been shown to have positive effects on bone mass, which are thought to be due in part to its cytoprotective effects on osteoblasts and here we show that taurine also protects osteocytes against cell death due to reactive oxygen species. Using the IDG-SW3 cell line, the expression of the taurine uptake transporter Taut/Slc6a6 is increased during osteoblast to osteocyte differentiation. Taurine had no effect on genes associated with osteoblast to osteocyte differentiation such as Dmp1, Phex or osteocalcin, even at high doses, but a slight yet significant inhibition of alkaline phosphatase was observed at the highest dose (50 mM). No effect was seen on the osteoclast regulatory genes Rankl and Opg, however the wnt antagonist Sost/sclerostin was potently and dose-dependently downregulated in response to taurine supplementation. Taurine also significantly inhibited Dkk1 mRNA expression, but only at 50mM. Interestingly, osteocytes were found to also be able to synthesize taurine intracellularly, potentially as a self-protective mechanism, but do not secrete the metabolite. A highly significant increase in the expression of cysteine dioxygenase (Cdo), a key enzyme necessary for the production of taurine, was observed with osteoblast to osteocyte differentiation along with a decrease in methionine, the precursor of taurine. For the first time, we describe the synthesis of taurine by osteocytes, potentially to preserve viability and to regulate bone formation through inhibition of sclerostin.
Keywords: Osteocyte, Metabolism, Taurine, Sclerostin, Wnt signaling
Introduction
Osteocytes are the longest-lived and most numerous of bone cells and play essential roles during bone growth and homeostasis [1]. During terminal differentiation, an osteoblast on the bone surface becomes embedded in a collagenous extracellular matrix, which is mineralized by hydroxyapatite deposits as the cell matures into an osteocyte. The mature osteocyte expresses several genes that have important functions in regulating bone remodeling. These include Sost/sclerostin and Dikkopf-1 (Dkk1), which inhibit the Wnt signaling pathway and subsequently bone formation [2–4], in addition to Receptor activator of nuclear factor kappa-B ligand (Rankl) and Osteoprotegerin (Opg), which promote and inhibit bone resorption respectively [5]. Therefore, the osteocyte is an important target for therapeutics to maintain bone mass and health.
Taurine is a non-essential amino acid in humans, which can be synthesized from the sulfur-containing amino acids methionine and cysteine [6], but is mainly obtained from dietary sources such as meat and fish. The main sites for the biosynthesis of taurine are the liver and the pancreas, but it has also been found to be produced in neuronal cells such as astrocytes and neurons [7] as well as adipocytes [8]. In tissues that do not produce endogenous taurine, it is instead taken up from the plasma by the taurine uptake transporter TauT (encoded by the gene Slc6a6) [9].
There is an increased interest in the biological activities of taurine due to its effects as a cytoprotective agent. Taurine supplementation has been shown to protect against cardiomyopathy by regulating intracellular calcium levels, reducing blood pressure and acting as an antioxidant [10]. It is also known to reduce inflammation in alcoholic liver disease [11] and to protect against inflammation and disease progression in a mouse model of Parkinson’s disease [12]. Furthermore, mice lacking the taurine transporter TauT have a reduced exercise capacity and increased muscle fatigability [13].
There is some evidence that taurine may play a beneficial role in regulating bone mass. Male mice given a diet supplemented with 2% taurine for 6 weeks had lower urinary calcium excretion and higher femur bone mineral content, compared to mice fed a control diet [14]. In addition, a twice-weekly dose of 50mg/kg taurine was found to protect against trabecular and cortical bone loss in male mice fed a low protein diet [15]. In female rats, administration of a 2% taurine diet for 6 weeks showed protective effects against the loss of bone mineral content (BMC) following ovariectomy. However, the beneficial effects of taurine may be dependent on calcium, as taurine supplementation was unable to prevent the decrease in BMC in ovariectomized rats fed a low calcium diet [16, 17]. In humans, urinary taurine levels were decreased in post-menopausal Chinese women, with taurine found to be consistently and significantly reduced in osteoporosis [18].
The mechanisms by which taurine regulates bone mass have not been fully elucidated. The taurine transporter, TauT, is expressed in both osteoblasts [19] and osteoclasts [20]. In RAW264.7 cells and primary bone marrow macrophages, osteoclast differentiation in response to RANKL was decreased by taurine treatment and this was attenuated by inhibiting TauT expression by siRNA targeting Slc6a6 [20]. In human osteoblasts in vitro, taurine supplementation increased proliferation through activating the ERK1/2 signaling pathway [21]. Taurine was also shown to promote the differentiation of human mesenchymal stem cells into osteoblasts and upregulate the expression of osteoblast markers osterix, Runx2, osteopontin and alkaline phosphatase via ERK1/2 signaling [22]. In addition to promoting differentiation, taurine was also able to protect against serum deprivation-induced apoptosis in pre-osteoblastic MC3T3-E1 cells by activating ERK1/2 [23]. Interestingly though, culturing these cells under calcium-free conditions prevented taurine uptake [24]. This suggests that similar to the protective effects on the ovariectomized rats, interplay between taurine and calcium is required.
In contrast to osteoblasts and osteoclasts, the effect of taurine on osteocytes has not yet been explored. Given the beneficial effects of taurine on bone in vivo and the essential role of osteocytes in maintaining bone mass, we hypothesized that taurine may exert some of its beneficial effects by acting directly on osteocytes, for example by maintaining osteocyte viability or affecting the expression of osteocyte-secreted regulators of bone remodeling As osteocytes are long-lived cells isolated within a mineralized environment, we used the osteocyte cell lines IDG-SW3 and MLO-Y4 to investigate the effects of taurine supplementation on osteocyte viability and the expression of bone remodeling genes. Furthermore, as cells outside of the liver and pancreas such as neural cells have been shown to make taurine, we investigated whether osteocytes are capable of synthesizing their own, a more readily available source of taurine.
Methods
MLO-Y4 cell death assay
To determine whether taurine could protect against oxidative stress-induced cell death in osteocyte-like cells, MLO-Y4 cells were seeded at a density of 1×104 cells/cm2 on a collagen-coated 96 well plate in αMEM supplemented with 2.5% fetal bovine serum (FBS), 2.5% calf serum, 100 U/ml penicillin and 50 μg/ml streptomycin (Themo-Fisher, Waltham, MA). Cells were pre-treated with varying concentrations of taurine (1–50 mM) for 24 hours, followed by treatment with 0.3 mM hydrogen peroxide (EMD Millipore, Burlington, MA) for 4 hours to induce approximately 20 % cell death. 100 μM 5-Aminoimidazole-4-carboxamide ribonucleotide (AICAR, Sigma, St Louis, MO), which activates AMPK and protects against cell death, was used as a positive control. Cells were stained with 2 μM ethidium homodimer 1 (Themo-Fisher) for 20 min to detect dead cells. Percentage of cell death was calculated as EthD-1 positive cells divided by the total number of cells stained with 5 μg/mL Hoechst 33342 (Thermo-Fisher) as a nuclear counterstain.
IDG-SW3 cell culture
IDG-SW3 cells were cultured as previously described [25, 26].
Lactate Dehydrogenase (LDH) Assay
As the cell death assay as performed on MLO-Y4 cells cannot be performed on the mineralized IDG-SW3 cells, the CyQuant LDH Cytotoxicity Assay (Thermo-Fisher) was used to determine whether taurine could protect against oxidative stress-induced cytotoxicity in mature osteocyte-like cells. The assay was performed according to the manufacturer’s instructions. Briefly, day 28 IDG-SW3 cells were pre-treated with increasing concentrations of taurine (1–100 mM) for 24 hours. The media was then replaced with fresh differentiation media containing taurine and 0.7 mM hydrogen peroxide. 10X lysis buffer was used as a positive control to induce cell membrane damage (maximum LDH activity). After 6 hours, 50 μl culture media was harvested and transferred to a 96-well culture plate. 50 μl of reaction mixture was added to each sample and the positive controls and incubated at room temperature for 30 minutes. After stopping the reaction with 50 μl stop solution, the plate was read in a spectrophotometer (Synergy HTX, BioTek, Winooski, VT) at 490 and 680 nm. To determine LDH activity, the 680 nm values were subtracted from the 490 nm values. This data was used to calculate the % cytotoxicity using the following equation: % cytotoxicity= (compound-treated LDH activity - spontaneous LDH activity/maximum LDH activity – spontaneous LDH activity) x 100.
Metabolic Profiling of IDG-SW3 cells
For metabolic profiling, the cells were seeded at a density of 4×104 cells/cm2 in a collagen-coated T75 culture flask (Corning Inc., Corning, NY) and cultured under proliferation conditions (33°C and 5% CO2) for 48 hours in growth media (α-MEM with 10% FBS, 100 U/ml penicillin, 50 μg/ml streptomycin and 50U/ml IFN-γ), until confluent. The media was then changed for differentiation media (α-MEM with 10% FBS, 100 U/ml penicillin, 50 μg/ml streptomycin, 50 μg/ml ascorbic acid and 4 mM β-glycerophosphate) and the cells were cultured at 37°C and 8% CO2 to induce differentiation and matrix mineralization. The media was replenished every 3 days during differentiation and 48 hours prior to harvesting at each time point.
In order to investigate the levels of taurine and related metabolites during differentiation, culture media was removed from the flask at day 4, 9, 18 and 28 and frozen at −80°C. The cell layer was washed three times with ice-cold PBS (Hyclone, GE Healthcare, Chicago, IL) and then the cells were scraped in 2 ml icecold methanol and transferred to a glass vial (SUN-SRi, Rockwood, TN) on ice. 2 ml chloroform was added and the vials vortexed and incubated on ice for 10 minutes. 2 ml water was added, vortexed and then incubated overnight at 4°C. The mixture was centrifuged at 4000 rpm at 4°C for 30 minutes to separate the fractions. The top layer was carefully removed and frozen at −80°C for subsequent NMR analysis.
Gene Expression Profiling
To investigate the effects of exogenous taurine on IDG-SW3 cell gene expression, IDG-SW3 cells were seeded at a density of 4×104 cells/cm2 in 12 well plates (Corning) and cultured until confluent under proliferation conditions. The cells were then cultured for 28 days under differentiation conditions to acquire a mature osteocyte-like phenotype [25, 26]. The cells were then cultured in differentiation media supplemented with 1–50 mM taurine (Sigma) for 24 hours and the cell lysate was harvested for RNA or protein isolation as described below.
Primary bone cell isolation and culture
To examine whether primary bone cells at different stages of differentiation also synthesize taurine, bone cells were isolated from 7 day old mouse pups. This age of mice was chosen due to the higher yield of bone cells required for metabolomics analysis by NMR. Tibiae and femora were dissected from eight C57Bl/6 mice, the soft tissue removed and the periosteum scraped with a scalpel. The epiphyses were removed and the marrow flushed with a 27 gauge needle and sterile PBS. The bone was cut into 1–2 mm pieces and washed three times in sterile HBSS (Hyclone). The bone pieces were then digested in 2 mg/ml collagenase (Sigma) for 25 minutes at 37°C on a rotating platform. The bone pieces were then washed three times in HBSS and digested a second time in 2 mg/ml collagenase for 25 minutes. After a further three washes in HBSS the bone pieces were incubated in a solution of 5 mM EDTA/0.1% BSA (Sigma) for 25 mins. The bone pieces were then washed in HBSS and divided between three T75 flasks (Corning) in growth media (αMEM, 10% FBS, 100 U/ml penicillin, 50 μg/ml streptomycin) and incubated at 37°C and 5% CO2 for 14 days to allow cells to grow out of the bone chips.
The outgrown cells were plated into T25 flasks (Corning) at a density of 2×104 cells/cm2 and cultured in growth media at 37°C and 5% CO2 for 72 hours, until confluent. The media was then changed for differentiation media (α-MEM with 10% FBS, 100 U/ml penicillin, 50 μg/ml streptomycin, 50 μg/ml ascorbic acid and 4 mM β-glycerophosphate) and the cells were cultured for 28 days, with media changes every three days. Culture media and cell lysates were harvested at day 7 (early differentiation stage) and day 28 (late differentiation) as for the IDG-SW3 cells. Media was changed 24 hours prior to harvesting.
To extract metabolites from the primary cells, methanol/chloroform/water extraction was used as for the IDG-SW3 cells, but using 1 ml volume of solvents.
RT-PCR
Total RNA was isolated from the cell cultures using Trizol according to the manufacturer’s instructions (Thermo-Fisher). 1 μg RNA was reverse transcribed into cDNA using the High Capacity cDNA synthesis kit (Thermo-Fisher). Real-time PCR was performed using 25 ng of template cDNA and either Taqman Gene Arrays (Thermo-Fisher) or PrimeTime qPCR Primer Assays (Integrated DNA Technologies, Coralville, Iowa). The data was normalized to the housekeeping gene Actb. Relative expression was determined using the 2-ΔΔCt method [27].
Western blotting
Western blotting was performed to examine the effects of taurine supplementation on intracellular sclerostin levels. IDG-SW3 cells differentiated for 28 days were treated with 50 mM taurine for 24 hours. Culture media was then removed and the cell layers were washed three times with PBS and lysed in ice-cold RIPA buffer (100 mM Tris, 300 mM NaCl, 1% sodium deoxycholate, 2% NP-40, 0.2% SDS) (Boston Bioproducts, Ashland, MA) containing proteinase inhibitors (Sigma). All steps were performed on ice to prevent protein degradation. 10 μg total protein from each sample was loaded onto a 4–20% Tris-glycine mini Proteon gel (Biorad, Hercules, CA) and separated by SDS PAGE electrophoresis. The protein was electroblotted to a PVDF membrane (GE Heathcare, Pittsburg, PA) for 45 minutes at 360 mA on ice. Background staining was blocked by incubating the membrane in 5% BSA/1% milk prior to overnight incubation with polyclonal anti-sclerostin antibody AF1589 (R&D Systems, Minneapolis, MN) at 1:500 dilution. HRP-conjugated rabbit anti-goat (1:1000, Thermo-Fisher) was used as the secondary antibody. Membranes were stripped using Restore stripping buffer (Thermo-Fisher) and re-probed using a monoclonal, HRP-conjugated β-actin antibody (1:25,000 dilution, Sigma) as a loading control. Immunoreactive bands were detected using the SuperSignal West Dura Chemiluminescence kit (Thermo-Fisher) and the FujiFilm LAS 4000 gel documentation system (FujiFilm, Tokyo, Japan). Densitometry was performed using ImageJ software (NIH, Bethesda, MD).
Sclerostin ELISA
To examine the effects of taurine on sclerostin secretion, an enzyme-linked immunosorbent assay (ELISA) was performed. IDG-SW3 cells were cultured as described previously and differentiated for 28 days into a mature osteocyte-like phenotype. The cells were then treated with increasing concentrations of taurine (1–50 mM) for 48 hours. Cell culture media was then harvested, centrifuged at 2000 x g for 5 mins to remove cellular debris and frozen at −80. The media was used to run the sclerostin ELISA (R&D Systems) according to the manufacturer’s instructions. Briefly, 50 μl of media or standards were added to a 96-well plate conjugated to the primary antibody and incubated with 50 μl diluent solution for 3 hours at room temperature. 100 μl mouse SOST conjugate was then added to each well and incubated at room temperature for 1 hour. 100 μl of substrate was then added and incubated for 30 minutes. The reaction was stopped with 100 μl stop solution and the plate was read at 450 nm with a wavelength correction of 540 nm. The sclerostin concentration was determined by interpolating from a four parameter logistic curve (Prism 8, GraphPad).
Nuclear Magnetic Resonance (NMR) spectroscopy data collection and processing
The intracellular and extracellular levels of taurine and related metabolites were determined by nuclear magnetic resonance spectroscopy. Culture media was filtered through a 10 kD MWCO Amicon filter (EMD Millipore, Burlington, MA) to remove large molecular weight proteins. The methanol fraction from the cell lysate was dried in a vacuum centrifuge and resuspended in deuterated sodium phosphate buffer (Sigma). Chenomx reference standard solution (Chenomx Inc., Canada) containing 4,4-dimethyl-4silapentane-1-sulfonic acid-d6 (DSS-d6) was added for NMR analysis.
NMR data were acquired on a Bruker Avance III 700MHz NMR spectrometer with a TXI triple resonance probe operating at 25C. Spectra were collected with a 1D NOESY pulse sequence covering 12 ppm. The spectra were digitized with 32768 points during a 3.9 second acquisition time. The mixing time was set to 100 ms and the relaxation delay between scans was set to 2.0 seconds.
The data were processed using Advanced Chemistry Development Spectrus Processor (version 2016.1, Toronto, Canada). The spectra were zero filled to 65536 points, apodized using a 0.3Hz decaying exponential function and fast Fourier transformed. Automated phase correction and linear baseline correction was applied to all samples. Metabolite concentrations were quantified using the Chenomx NMR Suite (version 8.2, Edmonton, Canada). The DSS-d6 was used as a chemical shift and quantification reference for all spectra and was set to a chemical shift of 0.00 and a concentration of 0.5 mM. Quantitative fitting of each spectrum was carried out in batch mode, followed by manual adjustment for some spectra to correct for errors arising from spectral overlap.
Statistical analysis
Graphpad Prism 8 (GraphPad, La Jolla, CA) was used to perform a one-way ANOVA with Tukey’s post-hoc test or Student’s t-test. All data is shown as mean±SD, with *P<0.05, **P<0.01 and ***P<0.001.
Results
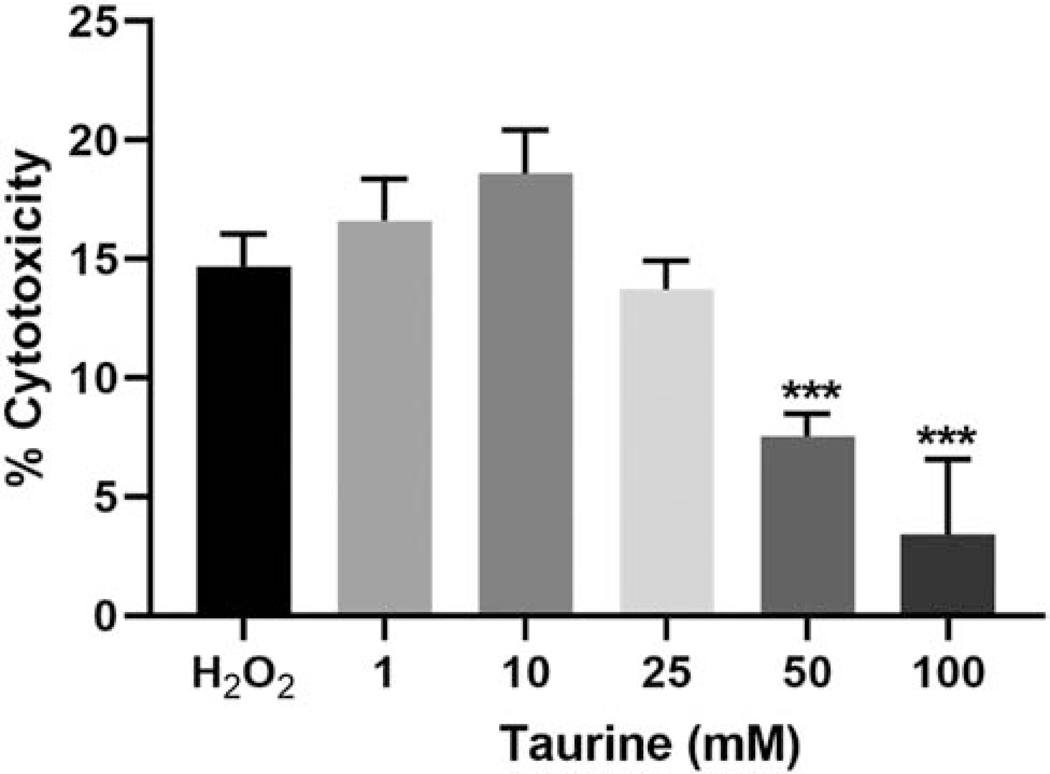
Taurine protects against hydrogen peroxide-induced cytotoxicity of osteocyte-like cells.
Taurine has previously been shown to protect against cell death in cells such as cardiomyocytes, neurons and pancreatic β-cells [6]. To examine whether taurine may play a similar role in maintaining osteocyte viability, we induced oxidative stress in differentiated IDG-SW3 cells using 0.7 mM hydrogen peroxide. H2O2 treatment resulted in approximately 15% cytotoxicity, as measured by lactate dehydrogenase release (Figure 1). Pretreatment with taurine was able to ameliorate the cytotoxicity, particularly at the higher concentrations. The potential protective effect of taurine against oxidative stress-induced cell death was also examined in the early osteocyte cell line MLO-Y4. Similar to IDG-SW3 cells, taurine was able to significantly reduce cell death in H2O2-treated MLO-Y4 cells at 25 and 50 mM concentrations, although a small but significant increase in cell death was observed at 1 mM (Supplemental Figure 1).
Figure 1. Exogenous taurine protects against hydrogen peroxide-induced cytotoxicity in IDG-SW3 osteocyte cells.
Pretreatment with 50 and 100 mM taurine significantly reduced cytotoxicity induced in IDG-SW3 cells by treatment with 0.7 mM hydrogen peroxide (n=6 ± SD, ***=p<0.001. One-way ANOVA with Tukey’s post hoc test).
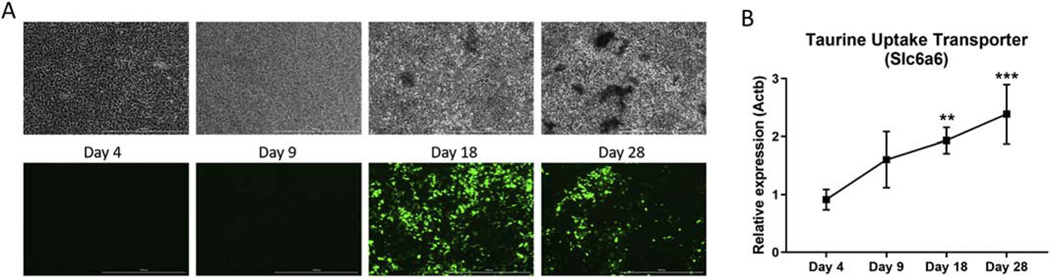
Taurine uptake transporter expression is increased during osteoblast to osteocyte differentiation
Given the ability of taurine supplementation to regulate bone mineral content and protect against bone loss in rodents [14, 16], we hypothesized that some of these effects may be due to taurine regulation of osteocyte function. To examine this we used the IDG-SW3 cell line, which has been shown to recapitulate the osteoblast to osteocyte differentiation process in vitro, characterized by robust extracellular matrix mineralization and mature osteocyte marker gene expression. The cells also express GFP under control of the dentin matrix 1 (Dmp1) promoter, which is expressed during osteocyte differentiation [25]. At early stages of differentiation the IDG-SW3 cells showed no evidence of matrix mineralization and minimal Dmp1-GFP expression, but by day 18 robust GFP expression was observed co-localized with areas of matrix mineralization (Figure 2A). The increase in mineral deposition and Dmp1-GFP expression was correlated with elevated expression of the taurine uptake transporter Taut/Slc6a6 mRNA, which reached maximal levels at day 28 (Figure 2B).
Figure 2. Taurine uptake transporter expression is increased during osteoblast to osteocyte differentiation.
(A) Phase contrast (top) and Dmp1-GFP (bottom) images of IDG-SW3 cells during osteoblast to osteocyte differentiation. The scale bar represents 1000 μm. (B) mRNA expression of the taurine uptake transporter Slc6a6 increased during IDG-SW3 cell differentiation and peaked at day 28 (Ct values = 17–18.5). A significant difference was observed at days 18 and 28 compared to day 4 (n=3 ± SD, ** = p<0.01, ***=p<0.00. One-way ANOVA with Tukey’s post hoc test1).
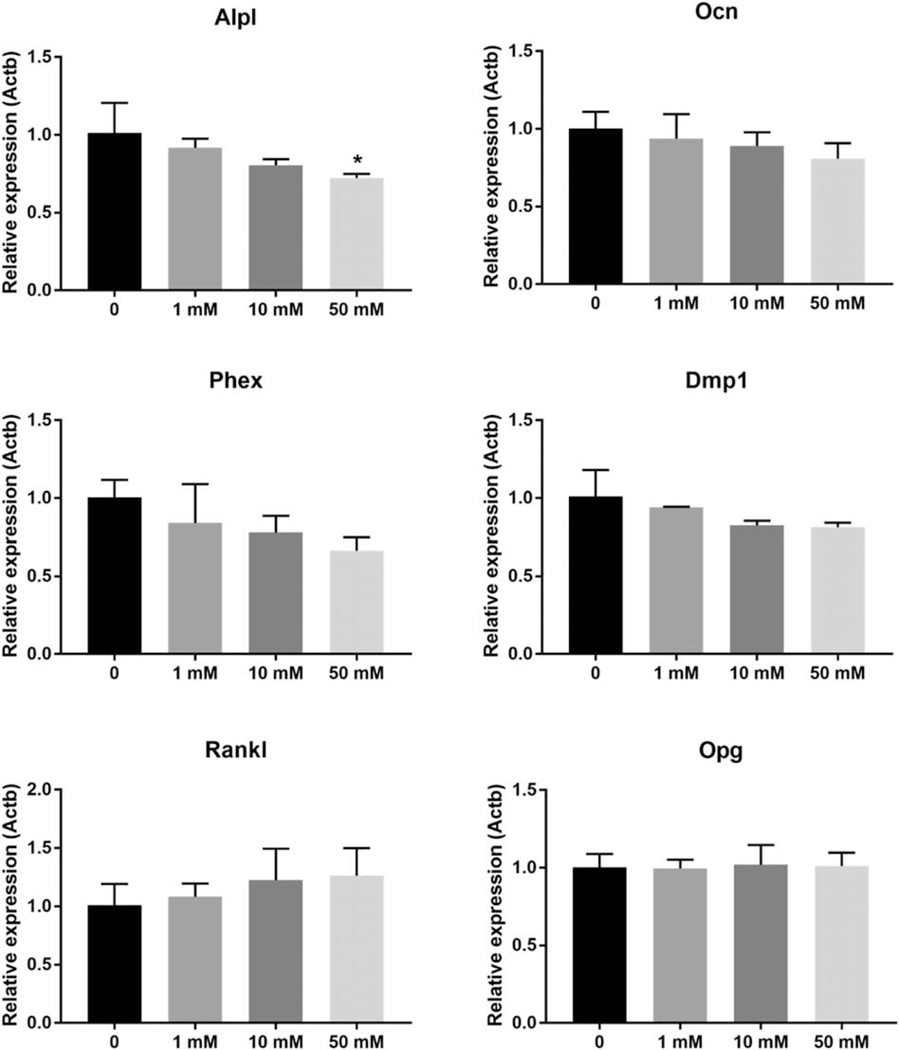
Taurine regulates the expression of Wnt signaling inhibitors in IDG-SW3 cells
To determine the potential effects of taurine on osteocyte function, IDG-SW3 cells were differentiated for 28 days into a mature osteocyte phenotype and treated with increasing concentrations of taurine for 24 hours. The expression of genes associated with matrix mineralization and positive regulation of bone formation, Osteocalcin (Ocn), Phosphate-regulating endopeptidase, X-linked (Phex) and Dmp1 were all unaffected by taurine supplementation, even at the higher concentrations (Figure 3). Alkaline phosphatase (Alpl) expression was slightly decreased at 50 mM taurine. Expression of Rankl, which promotes osteoclast formation and Opg, which inhibits osteoclast formation were not affected by taurine treatment (Figure 3).
Figure 3. Exogenous taurine supplementation has minimal effects on genes associated with matrix mineralization and osteoclast differentiation.
50 mM taurine slightly decreased the expression of Alpl in differentiated day 28 IDG-SW3 cells, but other genes associated with mineralization and bone resorption were unaffected (n=3 ± SD, * = p<0.05. One-way ANOVA with Tukey’s post hoc test).
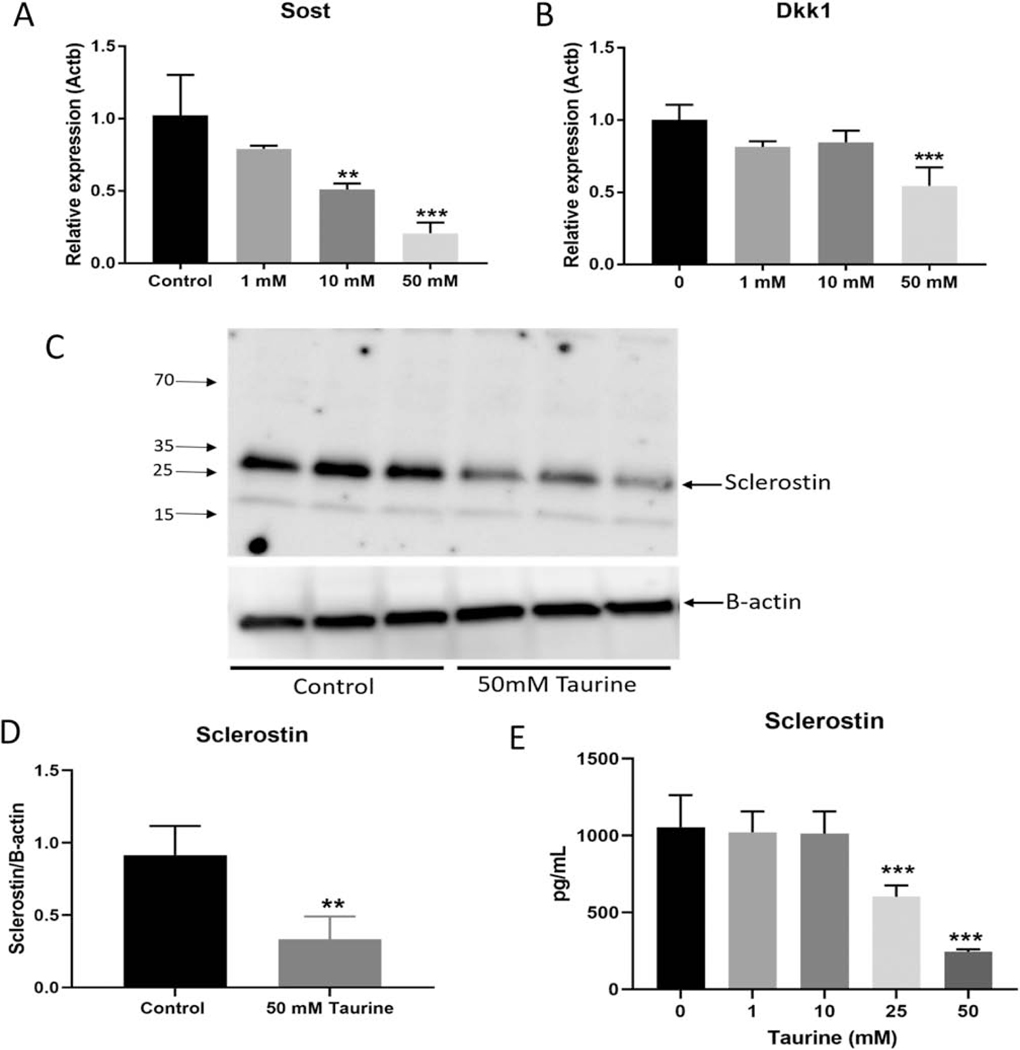
Interestingly, Sost mRNA expression showed a robust and dose-dependent decrease in response to taurine (Figure 4A). As the protein product of Sost, sclerostin, is a well-known inhibitor of the Wnt signaling pathway [28], we also investigated the expression of another Wnt inhibitor, Dkk1. Similar to Sost, Dkk1 expression was also decreased in response to taurine, although the effects were less dramatic than Sost and only reached significance at the highest dose (50 mM) (Figure 4B).
Figure 4. Taurine dose-dependently decreased the expression of Wnt signaling inhibitors.
(A) Sost and (B) Dkk1 mRNA expression were downregulated in day 28 IDG-SW3 cells in response to exogenous taurine supplementation (n=3 ± SD). (C) and (D) 50 mM taurine significantly inhibited intracellular sclerostin protein expression in the mature IDG-SW3 cells and (E) significantly inhibited secretion of sclerostin in media (n=3 ± SD ** = p<0.01, *** = p<0.001. One-way ANOVA with Tukey’s post hoc test).
To confirm that sclerostin is indeed a target of taurine, we examined sclerostin protein expression in IDG-SW3 cells treated with 50 mM taurine by Western blotting and by ELISA. Intracellular sclerostin levels were reproducibly downregulated in response to 24 hours of taurine supplementation (Figure 3C). Densitometric analysis of sclerostin staining revealed a 70% reduction in sclerostin protein levels when normalized to beta-actin (Figure 3D). Additionally, 25 and 50 mM taurine was able to inhibit secretion of sclerostin into the culture medium by 40 and 75% respectively compared to untreated control cells (Figure 3E).
IDG-SW3 and primary bone cells synthesize taurine
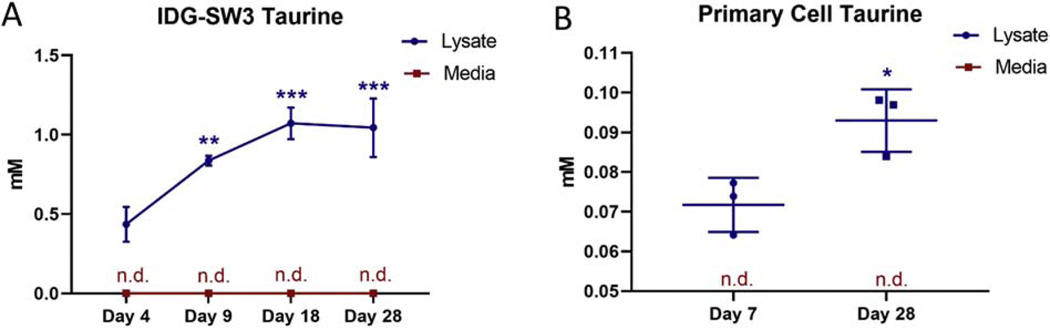
To examine whether osteocytes may be a source of taurine, we examined culture media and cell lysate from IDG-SW3 cells at different stages of osteoblast to osteocyte differentiation by NMR. NMR analysis of the cell lysate detected the presence of taurine in the early differentiated cultures and this was increased from 0.4±0.1 mM at day 4 to 1.1±0.09 mM at day 18 and remained elevated at day 28 (Figure 5A). Interestingly, taurine was not detectable by NMR analysis of the cell culture media at any time point (Figure 5A). This suggests that the taurine synthesized by these cells is retained within the cell and not secreted into the surrounding media at least at the levels of detection by NMR. The absence of taurine peaks in the NMR spectra of cell media is demonstrated in Supplemental Figure 3.
Figure 5. Taurine synthesis increases with differentiation in IDG-SW3 and primary bone cells.
(A) Taurine levels peak at day 18 in the cell lysate but are undetectable in the media at all time points (n=4 ± SD, **=p<0.01, ***=p<0.001. One-way ANOVA with Tukey’s post hoc test). (B) Taurine is also present in the lysate of cells isolated from mouse long bone and is increased with differentiation. Taurine was not detected in the media (n.d. = not detected) (n=3 ± SD, * = p<0.05. Two-tailed t-test).
To confirm that the biosynthesis of taurine is not confined to cell lines, we examined the production of taurine in primary bone cells isolated from 7 day old mouse long bones. Isolated cells were differentiated for 7 or 28 days into an osteoblastic or osteocytic differentiation stage. Matrix mineralization was not seen at day 7 of differentiation, but strong mineral deposition was observed by day 28 (data not shown). NMR analysis showed the presence of taurine in the cell lysate, but not in the culture media, similar the IDG-SW3 cells. Similar to the IDG-SW3 cells, taurine levels in the primary cell lysate was increased with differentiation (Figure 5B). However, the intracellular taurine levels were lower than those observed for the IDG-SW3 cells. Therefore, unlike cells in the liver and pancreas, osteoblasts/osteocytes produce taurine but do not appear to secrete it.
IDG-SW3 cells express genes essential for taurine biosynthesis and uptake
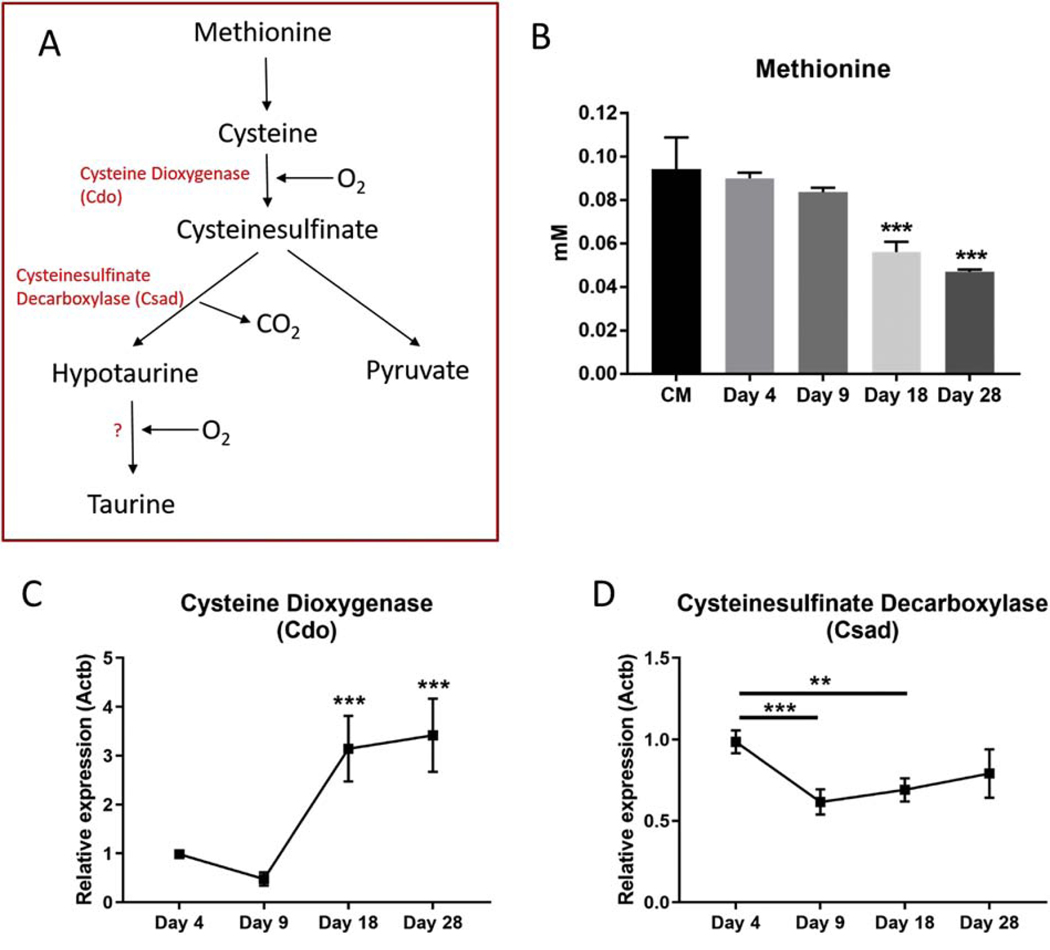
The process of taurine biosynthesis involves the conversion of methionine and cysteine into taurine via the production of several intermediates. Cysteine is converted to cysteinesulfinate by the enzyme cysteine dioxygenase, which is decarboxylated into hypotaurine by cysteinesulfinate decarboxylase. Hypotaurine is oxidized into taurine by a yet undefined mechanism [29](outlined in Figure 6A). We found that methionine levels in the culture media of the IDG-SW3 cells decreased with differentiation of the cells, consistent with it being used in the synthesis of taurine (Figure 6B). Cysteine was undetectable in the culture media or lysate (data not shown). The intermediates cysteinsulfinate and hypotaurine were also undetectable in either the culture media or the cell lysate by NMR. The levels of pyruvate, an alternative product of methionine metabolism, decreased in the culture media similar to methionine. Pyruvate levels in the cell lysate were very low and showed a temporal increase at day 9, but not at day 18 and 28 (Supplemental Figure 2).
Figure 6. Regulators of taurine biosynthesis and transport are highly expressed in IDG-SW3 cells.
(A) Taurine biosynthesis pathway. (B) Levels of the cysteine/taurine precursor, methionine were decreased in IDG-SW3 culture media during differentiation. (C) Cdo1 and (D) Csad mRNA expression levels during IDG-SW3 differentiation (Ct values = 17–19 for Cdo1, 19–21 for Csad). (n=4 ± SD, ** = p<0.01, *** = p<0.001. One-way ANOVA with Tukey’s post hoc test).
Cdo, the gene encoding for cysteine dioxygenase, was very highly expressed in IDG-SW3 cells (Ct values 17–19) and increased 3-fold in expression by day 18 of differentiation (Figure 6C). Csad, the gene encoding cysteinesulfinate decarboxylase, was also highly expressed (Ct values, 19–20), although its expression was decreased during differentiation (Figure 6D). Therefore, in addition to being responsive to exogenous taurine supplementation, osteocytes also possess the cellular machinery for taurine biosynthesis.
Discussion
Taurine has been shown to have beneficial effects on bone in vivo, however the mechanisms by which taurine exerts its effects have not been fully described. One of the primary functions of taurine in many different cell types is to protect against cell stress, due to conditions such as aging, disease and inflammation [6]. We found that taurine supplementation was able to protect IDG-SW3 and MLO-Y4 osteocyte-like cells from cell death triggered by H2O2 treatment. This suggests that taurine may have similar protective properties on osteocytes. In the liver, taurine was shown to protect against reactive oxygen species-induced apoptosis and mitochondrial damage by acting as a free radical scavenger [30]. Similar effects have been observed in cardiomyocytes [31], skeletal muscle [32], chondrocytes [33, 34] and neuronal cells [35]. In MC3T3 pre-osteoblastic cells, taurine was shown to protect against apoptosis by activating ERK1/2 signaling pathway [23].
In addition to protecting against cell death, our data also suggests that taurine may directly modulate genes that are important for osteocyte function and bone homeostasis. We found that the gene encoding for the taurine uptake transporter, Slc6a6, was highly expressed in IDG-SW3 cells, particularly during late differentiation. This suggests that osteocytes may be responsive to exogenous taurine supplementation, therefore we examined the effects of taurine on osteocyte gene expression. We found that treating differentiated IDG-SW3 cells with taurine did not affect the expression of Rankl or Opg, genes that regulate osteoclast differentiation and activity. We also found no change in the expression of Ocn, Phex and Dmp1, genes associated with extracellular matrix mineralization, although a small decrease in Alpl expression was observed in cells treated with high dose (50 mM) taurine. This result is in contrast to that found by Zhou et al. [22], where taurine supplementation increased alkaline phosphatase expression in mesenchymal stem cells suggesting that effects may be due to stage of differentiation (osteoblast precursor cells as opposed to mature osteocyte-like cells).
Interestingly, the expression of Sost mRNA was dose dependently decreased by taurine supplementation. Similar effects were also observed in sclerostin protein levels, which were strongly downregulated by taurine. Sclerostin is a well known negative regulator of bone formation [4], which acts via inhibiting the Wnt signaling pathway. Another Wnt inhibitor, Dkk1, was also decreased by taurine supplementation, although to a lesser degree than Sost. Positive effects of taurine supplementation have been observed on the bone of mice and rats [14, 15], however the mechanism behind these beneficial effects have not yet been elucidated. Our results raise the intriguing possibility that taurine may be regulating sclerostin levels in bone to enhance Wnt signaling and increase bone mass.
Surprisingly, we found that taurine was synthesized by IDG-SW3 cells and primary bone cells ex-vivo. The lower taurine levels detected in the primary cells is likely due in part to the fewer number of cells cultured, as the primary cells were cultured at a density of 2×104 cells/cm2 in 25 cm2 flasks versus 4×104 cells/cm2 in 75 cm2 flasks for the IDG-SW3 cells. Additionally, the primary cells may be a more heterogeneous population containing some cells that are not osteoblasts or osteocytes and do not synthesize taurine. Although taurine has previously been detected in the bone in vivo [36], the taurine was thought to originate from other sources such as the liver or pancreas, which are considered to be the main sites of taurine production [37, 38]. Interestingly, taurine was only detected in the cell lysate in both the IDG-SW3 and primary cells, but not in the culture media. This suggests that the taurine was retained intracellularly.
The importance of intracellular taurine levels in osteoblasts and osteocytes is unknown, but taurine is known to play a key role in regulating essential intracellular pathways in other cell types. For example, taurine has been shown to regulate intracellular calcium levels in cardiomyocytes [39]. In microglia, taurine reduces inflammatory cytokine release and inhibits activation of the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-ĸB) pathway [40]. Furthermore, depletion of taurine in heart muscle resulted in inhibition of the key energy metabolism pathways oxidative phosphorylation and fatty acid oxidation [41]. The inhibition of sclerostin secretion of osteocytes by taurine may also represent a mechanism by which taurine indirectly affects the activity of osteoblasts or osteoclasts.
The biosynthesis of taurine requires the conversion of the sulfur-containing amino acids methionine and cysteine into taurine via several intermediate steps. We found that methionine levels in IDG-SW3 cell culture media were decreased with differentiation, concomitant with increased taurine levels, therefore, suggesting that some of the extracellular methionine is being used for the biosynthesis of taurine. Interestingly, we did not detect cysteine in the media of these cells, but found that IDG-SW3 cells express very high levels of Cdo and Csad mRNA, which encode for enzymes that convert cysteine into cysteinesulfinate and hypotaurine, respectively. Thus, any cysteine initially present may have been converted into these intermediate metabolites. Furthermore, the decreased methionine levels at day 18 and 28 of differentiation did not result in increased pyruvate in the culture media or lysate at these time points, suggesting that the decreased methionine levels are not due to pyruvate synthesis.
In summary, we show for the first time that osteocytes are capable of synthesizing taurine but do not secrete detectable taurine in vitro. Furthermore, taurine supplementation reduced the expression of sclerostin and protected cells against oxidative stress-induced cell death. These findings may help explain the beneficial effects of taurine supplementation on bone in vivo. Further studies are needed to examine whether taurine may also regulate essential osteocyte functions such as mechanosensation and mechanotransduction.
Supplementary Material
Highlights.
We describe a potential mechanism for the benefits of taurine on bone
Taurine protects from oxidative-stress induced cytotoxicity in osteocyte-like cells
Taurine downregulates Sost mRNA and sclerostin protein expression in vitro
Osteocytes can synthesize taurine in vitro
Acknowledgements
This work was supported by NIH grant PO1AG039355 to LFB and Project STEM funding to MK.
Footnotes
Credit Author Statement
Matt Prideaux: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Data curation, Writing – Original draft, Writing – Review and editing, Visualization, Supervision
Yukiko Kitase: Methodology, Validation, Formal analysis, Investigation, Data curation, Writing – Review and editing, Visualization, Supervision
Morris Kimble: Formal analysis, Investigation
Thomas O’Connell: Conceptualization, Methodology, Validation, Resources, Formal analysis, Investigation, Data curation, Writing – Original draft, Writing – Review and editing, Visualization
Lynda F. Bonewald: Conceptualization, Methodology, Validation, Resources, Writing – Original draft, Writing – Review and editing, Visualization, Supervision, Project administration, Funding acquisition
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dallas SL, Prideaux M, and Bonewald LF, The osteocyte: an endocrine cell ... and more. Endocr Rev, 2013. 34(5): p. 658–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li J, et al. , Dkk1-mediated inhibition of Wnt signaling in bone results in osteopenia. Bone, 2006. 39(4): p. 754–66. [DOI] [PubMed] [Google Scholar]
- 3.Robling AG, et al. , Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J Biol Chem, 2008. 283(9): p. 5866–75. [DOI] [PubMed] [Google Scholar]
- 4.Li X, et al. , Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J Bone Miner Res, 2008. 23(6): p. 860–9. [DOI] [PubMed] [Google Scholar]
- 5.Xiong J, et al. , Osteocytes, not Osteoblasts or Lining Cells, are the Main Source of the RANKL Required for Osteoclast Formation in Remodeling Bone. PLoS One, 2015. 10(9): p. e0138189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schaffer S and Kim HW, Effects and Mechanisms of Taurine as a Therapeutic Agent. Biomol Ther (Seoul), 2018. 26(3): p. 225–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vitvitsky V, Garg SK, and Banerjee R, Taurine biosynthesis by neurons and astrocytes. J Biol Chem, 2011. 286(37): p. 32002–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ueki I and Stipanuk MH, 3T3-L1 adipocytes and rat adipose tissue have a high capacity for taurine synthesis by the cysteine dioxygenase/cysteinesulfinate decarboxylase and cysteamine dioxygenase pathways. J Nutr, 2009. 139(2): p. 207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson CM, et al. , Taurine uptake across the human intestinal brush-border membrane is via two transporters: H+-coupled PAT1 (SLC36A1) and Na+- and Cl(−)-dependent TauT (SLC6A6). J Physiol, 2009. 587(Pt 4): p. 731–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu YJ, et al. , The potential health benefits of taurine in cardiovascular disease. Exp Clin Cardiol, 2008. 13(2): p. 57–65. [PMC free article] [PubMed] [Google Scholar]
- 11.Wu G, et al. , Effect of taurine on alcoholic liver disease in rats. Adv Exp Med Biol, 2009. 643: p. 313–22. [DOI] [PubMed] [Google Scholar]
- 12.Hou L, et al. , Taurine protects noradrenergic locus coeruleus neurons in a mouse Parkinson’s disease model by inhibiting microglial M1 polarization. Amino Acids, 2018. 50(5): p. 547–556. [DOI] [PubMed] [Google Scholar]
- 13.Warskulat U, et al. , Taurine transporter knockout depletes muscle taurine levels and results in severe skeletal muscle impairment but leaves cardiac function uncompromised. FASEB J, 2004. 18(3): p. 577–9. [DOI] [PubMed] [Google Scholar]
- 14.Choi MJ and Seo JN, Effect of taurine feeding on bone mineral density and bone markers in rats. Adv Exp Med Biol, 2013. 776: p. 51–8. [DOI] [PubMed] [Google Scholar]
- 15.Moon PD, et al. , Taurine, a major amino acid of oyster, enhances linear bone growth in a mouse model of protein malnutrition. Biofactors, 2015. 41(3): p. 190–7. [DOI] [PubMed] [Google Scholar]
- 16.Choi MJ and DiMarco NM, The effects of dietary taurine supplementation on bone mineral density in ovariectomized rats. Adv Exp Med Biol, 2009. 643: p. 341–9. [DOI] [PubMed] [Google Scholar]
- 17.Choi MJ, Effects of taurine supplementation on bone mineral density in ovariectomized rats fed calcium deficient diet. Nutr Res Pract, 2009. 3(2): p. 108–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu L, et al. , Association between metabolic profiles in urine and bone mineral density of pre- and postmenopausal Chinese women. Menopause, 2019. 26(1): p. 94–102. [DOI] [PubMed] [Google Scholar]
- 19.Yuan LQ, et al. , Taurine transporter is expressed in osteoblasts. Amino Acids, 2006. 31(2): p. 157–63. [DOI] [PubMed] [Google Scholar]
- 20.Yuan LQ, et al. , Taurine inhibits osteoclastogenesis through the taurine transporter. Amino Acids, 2010. 39(1): p. 89–99. [DOI] [PubMed] [Google Scholar]
- 21.Jeon SH, et al. , Taurine increases cell proliferation and generates an increase in [Mg2+]i accompanied by ERK 1/2 activation in human osteoblast cells. FEBS Lett, 2007. 581(30): p. 592934. [DOI] [PubMed] [Google Scholar]
- 22.Zhou C, et al. , Taurine promotes human mesenchymal stem cells to differentiate into osteoblast through the ERK pathway. Amino Acids, 2014. 46(7): p. 1673–80. [DOI] [PubMed] [Google Scholar]
- 23.Zhang LY, et al. , Taurine inhibits serum deprivation-induced osteoblast apoptosis via the taurine transporter/ERK signaling pathway. Braz J Med Biol Res, 2011. 44(7): p. 618–23. [DOI] [PubMed] [Google Scholar]
- 24.Kang YS and Kim SJ, The Change of Taurine Transport in Osteocytes by Oxidative Stress, Hypertonicity and Calcium Channel Blockers. Biomolecules & Therapeutics, 2008. 16(3): p. 219225. [Google Scholar]
- 25.Woo SM, et al. , Cell line IDG-SW3 replicates osteoblast-to-late-osteocyte differentiation in vitro and accelerates bone formation in vivo. J Bone Miner Res, 2011. 26(11): p. 2634–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prideaux M, et al. , Parathyroid Hormone Induces Bone Cell Motility and Loss of Mature Osteocyte Phenotype through L-Calcium Channel Dependent and Independent Mechanisms. PLoS One, 2015. 10(5): p. e0125731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Livak KJ and Schmittgen TD, Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods, 2001. 25(4): p. 402–8. [DOI] [PubMed] [Google Scholar]
- 28.Bonewald LF and Johnson ML, Osteocytes, mechanosensing and Wnt signaling. Bone, 2008. 42(4): p. 606–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ripps H and Shen W, Review: taurine: a “very essential” amino acid. Mol Vis, 2012. 18: p. 267386. [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Z, et al. , Taurine supplementation reduces oxidative stress and protects the liver in an iron-overload murine model. Mol Med Rep, 2014. 10(5): p. 2255–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang Y, et al. , Exogenous taurine attenuates mitochondrial oxidative stress and endoplasmic reticulum stress in rat cardiomyocytes. Acta Biochim Biophys Sin (Shanghai), 2013. 45(5): p. 35967. [DOI] [PubMed] [Google Scholar]
- 32.Thirupathi A, et al. , Modulatory effects of taurine on metabolic and oxidative stress parameters in a mice model of muscle overuse. Nutrition, 2018. 54: p. 158–164. [DOI] [PubMed] [Google Scholar]
- 33.Bian Y, Wang H, and Sun S, Taurine alleviates endoplasmic reticulum stress in the chondrocytes from patients with osteoarthritis. Redox Rep, 2018. 23(1): p. 118–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bian Y, Zhang M, and Wang K, Taurine protects against knee osteoarthritis development in experimental rat models. Knee, 2018. 25(3): p. 374–380. [DOI] [PubMed] [Google Scholar]
- 35.Liu Q, et al. , Role of Taurine in BDE 209-Induced Oxidative Stress in PC12 Cells. Adv Exp Med Biol, 2017. 975 Pt 2: p. 897–906. [DOI] [PubMed] [Google Scholar]
- 36.Lubec B, et al. , Distribution and disappearance of the radiolabeled carbon derived from Larginine and taurine in the mouse. Life Sci, 1997. 60(26): p. 2373–81. [DOI] [PubMed] [Google Scholar]
- 37.Huxtable RJ, Physiological actions of taurine. Physiol Rev, 1992. 72(1): p. 101–63. [DOI] [PubMed] [Google Scholar]
- 38.De Luca A, Pierno S, and Camerino DC, Taurine: the appeal of a safe amino acid for skeletal muscle disorders. J Transl Med, 2015. 13: p. 243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takahashi K, et al. , Effect of taurine on angiotensin II-induced hypertrophy of neonatal rat cardiac cells. J Cardiovasc Pharmacol, 1997. 30(6): p. 725–30. [DOI] [PubMed] [Google Scholar]
- 40.Che Y, et al. , Taurine protects dopaminergic neurons in a mouse Parkinson’s disease model through inhibition of microglial M1 polarization. Cell Death Dis, 2018. 9(4): p. 435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schaffer SW, et al. , Impaired energy metabolism of the taurinedeficient heart. Amino Acids, 2016. 48(2): p. 549–58. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.