Version Changes
Revised. Amendments from Version 1
The following updates have been made in response to reviewers’ recommendations. We introduced figure 1, which describes the seroprevalence of anti- T. gondii IgG, IgM and both IgG and IgM among children and pregnant women. The results section has been updated accordingly Table 2b a new addition provides information on seropositivity of specific immunoglobulins in a particular settlement type. Table 3b. provided on the seroprevalence of the T. gondii specific immunoglobulins G and M in the trimesters of pregnancy. Table 4: Introduces additional information on risk factors An update was made on the haematological information in Tables 6 a-c
Abstract
Background: Toxoplasma gondii is an obligate, intracellular, apicomplexan parasite that causes toxoplasmosis. Although the global prevalence of toxoplasmosis has been estimated to be approximately 30%, there is limited seroprevalence data in Ghana, with a dearth of information on the impact of T. gondii on haematological parameters in exposed persons.
Methods: Questionnaires were administered to 300 consenting individuals to obtain demographic information and assessment of their risk of exposure to T. gondii. Using anti- T. gondii IgG/IgM combo test kits, seropositivity to parasite-specific IgG and/or IgM was determined. A haematological analyser was used to measure haematological parameters.
Results: There was an overall seroprevalence of 50.3% (n=151), with 49.7% (n=149) of the study participants seropositive for IgG and 1% (n=3) testing positive for IgM. Furthermore, the observed seroprevalence among pregnant women was 56.4% (n=62). With regard to settlement type, a seroprevalence of 55.6% was observed in the rural community, 50.6% in the peri-urban community and 47.1% in the urban community. The study identified cat ownership, contact with cat litter, contact with raw meat [RR (95% CI: 1.76 (1.23-2.53), 1.66 (1.03-2.67), 1.25(1.00-1.57)] and age (p<0.001) as risk factors for infection. Analyses of haematological data revealed significant reduction in the white blood cell, lymphocytes and mean corpuscular volume levels in seropositive males (p=0.0223, 0.0275, and 0.0271) respectively. Only the mean corpuscular volume of seropositive females reduced significantly as compared to the seronegative counterparts (p=0.0035).
Conclusions: About half of the study population, including women of reproductive age carried antibodies against T. gondii, raising concerns about the risk of congenital toxoplasmosis and anaemia. We, therefore, recommend that screening for Toxoplasma gondii be included in the routine screening of pregnant women seeking antenatal care and further investigation should be conducted on the haematological implications of infection in humans.
Keywords: Toxoplasma gondii, Haematology, Seroprevalence, IgG, Exposure, Risk Factors
Introduction
Toxoplasma gondii — the causative organism for toxoplasmosis — is an obligate, intracellular, apicomplexan parasite with a wide geographic distribution and the ability to infect virtually any cell type across a broad host range, including humans, companion animals, livestock and wildlife 1. About a third of the world’s population is infected with T. gondii but the parasite does not usually cause clinically significant disease 2. Until recently, latent infections in humans were assumed to be asymptomatic; however, results from animal studies, personality and behavioural profiles, as well as psychomotor performance tests have led to a reconsideration of this assumption 3, 4. Certain individuals, including foetuses, neonates, and the immune-compromised are at high risk for life-threatening complications from toxoplasmosis 4. Congenital transmission of T. gondii carries the risk of miscarriage or stillbirth, and children born with toxoplasmosis are likely to suffer from severe symptoms, such as hydrocephalus, calcifications of the brain or retinochoroiditis later in life if not treated 5.
People are typically infected by either accidentally ingesting infectious oocysts or eating undercooked meat containing tissue cysts (bradyzoites). Toxoplasma oocysts can be found in soil or water contaminated with cat faeces, making the consumption of raw vegetables and water from unsafe drinking sources important risk factors for infection. The consumption of raw or undercooked meat is also a risk factor as livestock and game often harbour bradyzoites. The parasite can also be transmitted in utero to a developing foetus if a woman is infected for the first time while pregnant. Solid-organ transplantation such as the heart, liver and kidney transplants are another means by which T. gondii infection can occur, although this is very rare 6, 7.
During acute primary infection with T. gondii, anti- T. gondii immunoglobulin M (IgM) is initially produced. However, IgM titres decline over the next few months, becoming undetectable within a year. The immune system also produces anti- T. gondii IgG a few weeks after the initial infection. IgG antibody levels usually peak within one or two months after the infection but are still detectable throughout the lifetime of the infected individual 4. The seroprevalence of antibodies against T. gondii has been reported across the globe, and ranges from 51% to 72% in several countries in Latin America and the Caribbean, including Argentina, Brazil, Cuba, Jamaica, and Venezuela. In Scandinavia, the seroprevalence of antibodies specific to T. gondii is reported to vary between 11% and 28%, while in Southeast Asia, China and Korea, seropositivity to T. gondii exposure has been estimated to range from 4% to 39% in women of reproductive age 8. Furthermore, both active and latent infections of T. gondii have been reported in many African countries, particularly in individuals suffering from HIV/AIDS. For example, 88.2% of HIV-positive individuals seeking healthcare at Arba Minch Hospital in Ethiopia were also seropositive for T. gondii infection 9, and a study conducted in Burkina Faso among pregnant women revealed an overall seroprevalence of 31.1% 10.
In Ghana, Sefa-Boakye et al., 11 and Ayi et al., 12 reported seroprevalence rates of 83.6% and 92.5% among pregnant women in Kumasi and Accra, respectively. In the Central region of Ghana, Abu et al., 13 reported an overall seroprevalence of 85% in a population-based study that investigated risk factors for T. gondii infection. A follow-up study later revealed that 2.6% of the study participants showed signs of ocular toxoplasmosis 14. In the Greater Accra region, Kwoffie et al., 15 analysed placental tissue samples from IgG-seropositive women post-delivery and estimated the risk of congenital transmission of the infection to be 39.8% based on the presence of T. gondii DNA in the placental samples.
In this study, we screened 300 individuals from three hospitals in the Ashanti region of Ghana and estimated the seroprevalence of T. gondii infection. Our primary objective was to investigate the seroprevalence, associated risk factors and haematological consequences of T. gondii infection in the Ashanti region of Ghana. Specifically, we determined 1) the seroprevalence of T. gondii-specific IgG and IgM; 2) the risk factors associated with T. gondii infection; and 3) the haematological consequences of T. gondii exposure in the Ashanti region of Ghana.
Methods
Ethical consideration
Prior to the commencement of the study, approval was obtained from all three hospitals. Ethical approval for the study was also given by the Committee on Human Research Publications and Ethics at the Kwame Nkrumah University of Science and Technology and Komfo Anokye Teaching Hospital in Kumasi, Ghana (reference number: CHRPE/AP/018/18). Written informed consent was obtained from all the study participants.
Study design
A cross-sectional study design was employed. Study participants were recruited during hospital visits that occurred between 30 th January and 28 th February 2018. Questionnaires were administered to obtain data on demographics and risk factors.
Study site
The study was conducted at three hospitals; namely, Kumasi South Hospital (KSH), Agona Government Hospital (AGH) and Kuntanase Government Hospital (KGH), all in the Ashanti region of Ghana. KSH is located in Atonsu, a community within the Asokwa Sub-metropolitan Assembly of the Kumasi Metropolis which has a population size of 1,730,249. Recruitment and sample collection were conducted from 31st January to 8th of February 2018 at KSH. At KGH, recruitment and data collection were carried out from 15 th–16 th and 27 th–28 th February 2018. KGH is situated in Kuntanase, the capital of the Bosumtwi district of the Ashanti Region with a population of 93,910 and located at about 28km from the capital city, Kumasi. AGH is located at about 37 km from Kumasi and is situated in the Sekyere South District, which has a population of 94,009. Recruitment and selection of participants took place from 19 th–21 st February 2018 at the Agona Government Hospital.
Participant selection
To be included in the study, individuals had to be at least six months of age and had to have been referred to the laboratories of one of the study hospitals (KSH, KGH and AGH). Pregnant women were preferentially recruited into the study due to our interest in congenital toxoplasmosis. The selection of study subjects was based on their willingness to participate after being briefed about the study, and confirmed by signing or thumb-printing an informed consent form. In the case of minors, consent was obtained from parents or legal guardians. Critically ill patients (individuals in need of urgent medical attention) and children below 6 months were excluded from the study. We provided a written questionnaire to each of the participants as they waited for their turn at the laboratory of the various hospitals. The questionnaires were administered in the form of an interview to all participants by the investigators.
Variables
Data obtained from the questionnaires on factors that predispose respondents to infection (cat ownership, contact with cat litter, eating raw meat, consumption of raw or undercooked vegetables and sources of drinking water) were analysed. As part of the demographic data, age, sex, location (rural, urban or peri-urban), education and employment were also analysed. These variables were treated as predictor variables and compared with the anti- T. gondii serological results (endpoint variables).
Bias
The female to male ratio was identified to be biased. This was due to priority being given to the enrolment of pregnant women.
Sample size determination
A minimum sample size of 207 was determined using the binomial model. This was calculated with a 95% CI and precision level of 5%: N o= [Z 2 (P) (1-P)] /(d) 2 using overall seroprevalence of 83.4% observed by Sefah-Boakye et al. 11 in the Ashanti region. In this equation, N o is the sample size, Z is the critical value of the binomial distribution at the 5% level (1.96), p is the overall seroprevalence (0.84), q = 1 – p, and d (error) is the precision level (5%).
Quantitative variables
Participants who tested positive for parasite-specific IgG, IgM or both were classified as seropositive, while those with negative tests were described as seronegative. We compared the median haematological parameter values of the seropositive and seronegative categories to investigate the effects of infection on the different haematological indices.
Haematological analyses
Using sterile needles and syringes, qualified phlebotomists obtained 3 mL of venous blood from each participant. The blood samples were dispensed into labelled EDTA tubes to prevent clotting and were later used for haematological analyses. Complete blood counts were run on the blood using the Sysmex XP-300 Automated Haematology Analyzer. The analyser provided cell count data on red blood cells (RBC), white blood cells (WBC), lymphocytes, neutrophils and platelets. Other parameters measured included haemoglobin (Hb), mean corpuscular volume (MCV), mean corpuscular haemoglobin (MCH), mean platelet volume (MPV), plateletcrit and red blood cell distribution width (RDW).
Serological analyses
The blood samples were tested for the presence of anti- T. gondii IgG/IgM using the commercially available OnSite Toxo IgG/IgM rapid combo test kit manufactured by CTK Biotech, USA. This test kit works with either whole blood or serum. Following the manufacturer’s protocol, a 10 µL sample of blood was dropped into the sample/buffer well on the kit. Two drops of buffer were then added and allowed to stand for 10–15 min. Results were recorded as positive if both the test control line and M and/or G line(s) developed. Test results were seropositive for IgG if the G line developed in addition to the control (C) while blood samples were seropositive for IgM if the M line appeared in addition to the control. Seropositive results to IgG and IgM were obtained when both the M and G lines developed in addition to the control (C) band.
Data analysis
Data obtained from serological examination and questionnaires were keyed into Microsoft Excel (2016). Seroprevalence was calculated by expressing the number of seropositive individuals as a percentage of the total number tested. The chi-square and Mann-Whitney U tests were carried out in GraphPad Prism 6 (GraphPad Software, Inc., San Diego, CA, USA) to investigate the association between demographics and seropositivity, as well as to compare the differences in the median haematological values of seropositive and seronegative individuals respectively. STATA 14 was also used to determine the relative risk of seropositivity for each risk factor. Analysed results were considered statistically significant if p ≤ 0.05.
Results
In total, 300 participants were recruited for the study. Out of this number, 80.6% (n=242) were female, of which 45.5% (n=110) were pregnant. The median age for all participants was 27 years.
Seroprevalence of T. gondii
50.3% (n=151) of the 300 participants were seropositive for anti- T. gondii antibodies. Among the seropositive population, 98% (n=148) and 2% (n=3) of the participants were seropositive for IgG and IgM respectively, with 0.3% (n=1) of the participants seropositive for IgM but seronegative for IgG (IgM only), 49.3% (n=148) seropositive for IgG but seronegative for IgM (IgG only) and 1.3% (n=2) of the 151 seropositive participants seropositive for both IgG and IgM ( Table 1). Furthermore, 56.4% (n=62) of the pregnant women were seropositive for IgG only, with 0.9 % (n=1) testing positive for IgM only and both IgG and IgM. Out of the 41 children tested, 17.1% (n=7) were seropositive for IgG only whereas 2.4% (n=1) were seropositive for IgM only. None of the children were seropositive for both IgG and IgM ( Figure 1).
Figure 1. Seroprevalence of anti- T. gondii antibodies in children and pregnant women.
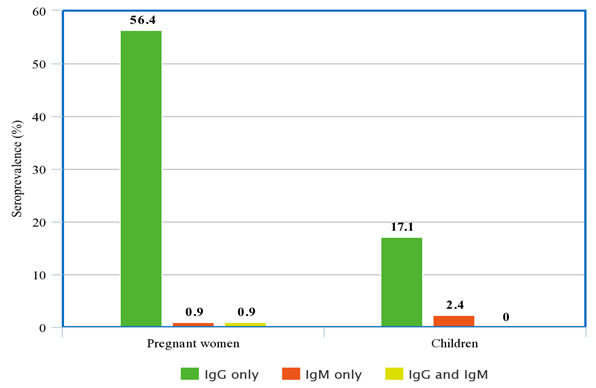
Table 1. Seroprevalence of anti- T gondii immunoglobulins.
| Tests | Positive (n %) | Negative (n%) |
|---|---|---|
| IgG and/or IgM | 151 (50.3) | 149 (49.7) |
| IgM only | 1 (0.3) | 299 (99.7) |
| IgG only | 148 (49.3) | 152 (50.7) |
| IgG and IgM | 2 (0.7) | 298 (99.3) |
The communities within which the hospitals are located were classified as urban (Kumasi South Hospital), rural (Agona Government Hospital) and peri-urban (Kuntanase Government Hospital) based on their distance from Kumasi, the capital city. (Our classification is based on the population of the communities, where urban is highly populated city area, followed by peri-urban then, the rural area with the lowest population). Seroprevalence rates of 47.1%, 50.6% and 55.6% were therefore observed from the urban, rural and peri-urban areas, respectively ( Table 2a). Seroprevalence of anti- T. gondii IgG per study sites were 49.4% (n=39), 55.6% (n=45) and 47.1% (n=66) in the rural, peri-urban and urban areas respectively. Seropositivity for IgG and both IgG and IgM were 1.42% (n=2) in the urban area whereas seropositivity of 1.26% (n=1) for IgM was recorded in the rural area ( Table 2b).
Table 2a. Seroprevalence of Toxoplasma gondii based on the type of community.
| Setting | Total tested | Seropositive (n %) | χ 2 | P-value |
|---|---|---|---|---|
| 1.457 | 0.482 | |||
| Urban | 140 | 66 (47.1) | ||
| Peri-Urban | 81 | 45 (55.6) | ||
| Rural | 79 | 40 (50.6) |
Table 2b. Sero-prevalence of anti T. gondii IgG and IgM per study site location.
| Location | IgG (n%) | IgM (n%) | IgG & IgM (n%) |
|---|---|---|---|
| Urban | 66 (47.1) | 2(1.42) | 2 (1.42) |
| Peri-Urban | 45 (55.6) | 0 | 0 |
| Rural | 39 (49.4) | 1(1.26) | 0 |
A total of 110 pregnant women were recruited into the study, including 37 in their first trimester, 41 in the second trimester and 32 in the third trimester. The women ranged in age from 16 to 45 years, with a mean age of 28.6 years. Out of the 110 pregnant women, sixty-two were seropositive, indicating a general seroprevalence of 54.6%. Seropositivity for T. gondii was observed in 18 (48.6%) of the pregnant women in their first trimester, 22 (53.7%) in their second trimester and another 22 (68.8%) in their third trimester ( Table 3a). Almost all seropositive pregnant women within the first 16.4%(n=18), second 20% (n=22) and third 20% (n=22) trimesters were seropositive for only anti- T. gondii IgG. ( Table 3b).
Table 3a. Seroprevalence of anti- Toxoplasma gondii immunoglobulins in pregnant women.
| Total tested | Positive (n %) | Negative (n%) | χ2 | RR (95% CI) | P-value | |
|---|---|---|---|---|---|---|
| Gestation period | 2.53 | 1.27(0.94-1.72) | 0.1119 | |||
| First trimester | 37 | 18 (48.6) | 19 (51.4) | |||
| Second trimester | 41 | 22 (53.7) | 19 (46.3) | |||
| Third trimester | 32 | 22 (68.8) | 10 (31.3) |
Table 3b. Sero-prevalence of anti T. gondii IgG and IgM among pregnant women.
| Gestational Period | IgG (n%) | IgM (n%) | IgG & IgM (n%) |
|---|---|---|---|
| First trimester | 18 (16.4) | 0 | 0 |
| Second trimester | 22 (20) | 0 | 0 |
| Third trimester | 22 (20) | 1 (0.9) | 1 (0.9) |
To identify risk factors for T. gondii infection, the risk-ratio and chi-square tests were conducted to investigate the association between a few predisposing factors and seropositivity. Cat ownership (p=0.002) and contact with cat litter (p=0.003) were strongly associated with seropositivity for T. gondii. Handling of raw meat was also identified as a risk factor for exposure (p=0.05) ( Table 4).
Table 4. Association between Toxoplasma gondii seropositivity and possible risk factors.
| Possible risk factors | Total tested | Positive n (%) | Negative n (%) | χ 2 | RR (95%CI) | P-value |
|---|---|---|---|---|---|---|
| Cat ownership | 10.10 | 1.76(1.23-2.53) | 0.002** | |||
| Yes | 92 | 59 (64.1) | 33 (35.9) | |||
| No | 208 | 91 (43.8) | 117 (56.3) | |||
| Contact with cat litter | 4.50 | 1.66(1.03-2.67) | 0.003** | |||
| No | 241 | 113 (46.9) | 128 (53.1) | |||
| Yes | 59 | 37 (62.7) | 22 (37.3) | |||
| Source of drinking water | 1.66 | 0.99(0.81-1.04) | 0.198 | |||
| Wells | 71 | 40 (56.3) | 31 (43.7) | |||
| Pipe | 223 | 107 (48.0) | 116 (52.0) | |||
| River | 6 | 3 (50.0) | 3 (50.0) | |||
| Handling of raw meat | 3.85 | 1.25(1.00-1.57) | 0.050* | |||
| Yes | 151 | 84 (55.6) | 67 (44.4) | |||
| No | 149 | 66 (44.3) | 83 (55.7) | |||
|
Eating raw/undercooked
vegetables |
1.98 | 1.05(0.98-1.14) | 0.16 | |||
| Yes | 269 | 139(52.2) | 130 (47.8) | |||
| No | 29 | 11 (33.3) | 18 (66.7) |
Certain demographic factors were also investigated to ascertain their association with seropositivity. The results revealed that the location, sex, education and employment status of participants were not predisposing factors to seropositivity. Age was, however, significantly associated with seropositivity (p-value = 1.3*10 -6) ( Table 5).
Table 5. Association between demographic factors and seropositivity of Toxoplasma gondii.
| Demographics | Total tested | Positive (n %) | Negative (n%) | χ 2 | RR (95% CI) | P-value |
|---|---|---|---|---|---|---|
| Location | 1.07 | 1.12 (0.90-1.38) | 0.3 | |||
| Rural | 81 | 45 (55.6) | 36 (44.4) | |||
| Urban | 140 | 66 (47.1) | 74 (52.9) | |||
| Peri-Urban | 79 | 40 (50.6) | 39 (49.4) | |||
| Sex | 3.28 | 0.65 (0.40-1.04) | 0.07 | |||
| Male | 58 | 23 (39.7) | 35 (60.3) | |||
| Female | 242 | 127 (52.5) | 115 (47.5) | |||
| Education | 0.95 | 0.96 (0.88-1.05) | 0.33 | |||
| None | 40 | 23 (57.5) | 17 (42.5) | |||
| Basic | 56 | 27 (48.2) | 29 (51.8) | |||
| JHS | 84 | 51 (60.7) | 33 (39.3) | |||
| SHS | 86 | 38 (44.2) | 48 (55.8) | |||
| Tertiary | 30 | 10 (33.3) | 20 (66.7) | |||
| Adult education | 4 | 2 (50.0) | 2 (50.0) | |||
| Employment | 2.69 | 1.24 (0.96-1.6) | 0.1 | |||
| Unemployed | 145 | 75 (51.7) | 70 (48.3) | |||
| Employed | 152 | 75 (49.3) | 77 (50.7) | |||
| Retired | 3 | 2 (66.7) | 1 (33.3) | |||
| Age | 16.66 | 1.29 (1.14-1.46) | <0.001 | |||
| 0–18 | 67 | 19 (25.4) | 56 (83.6) | |||
| 19–44 | 175 | 92 (52.7) | 73 (41.7) | |||
| 45-> | 58 | 40 (67.0) | 20 (34.5) | |||
Effect of T. gondii infection on haematological parameters
T. gondii is capable of infecting virtually any nucleated cell, including erythroblasts. The effect of T. gondii on certain blood parameters (WBC, LYM, NEUT, RBC, Hb and MCV) was therefore investigated. Comparisons were made between seropositive and seronegative individuals for males and females. A further comparison was made between being pregnant or not for seropositive and seronegative females. The median and interquartile ranges were calculated, where p-values ≤ 0.05 were considered significant. We obtained significant differences in the WBC, LYM and MCV for males (p-values=0.0223, 0.0275, and 0.0271) respectively ( Table 6a) whereas for the females, significant association was obtained only in the MCV (p-value=0.0035) ( Table 6b). For the pregnant women who were seropositive, significant reduction was obtained in the LYM count (p=0.0369) ( Table 6c).
Table 6a. Effect of Toxoplasma gondii infection on haematological parameters in males.
| Haematological parameters | Seropositive | Seronegative | P-value |
|---|---|---|---|
| Median (IQR) | |||
| White blood cells (103/µL) | 2.80 (2.60-3.30) | 4.10 (2.60-4.80) | 0.0223 * |
| Lymphocytes (103/µL) | 1.90 (1.30-2.30) | 2.30 (1.80-3.30) | 0.0275 * |
| Neutrophils (103/µL) | 1.00 (0.60-1.30) | 1.10 (0.80-1.80) | 0.2587 |
| Red blood cell (106/µL) | 4.64 (3.92-5.59) | 4.99 (4.37-5.30) | 0.6731 |
| Haemoglobin (g/dl) | 12.40 (11.70-14.60) | 12.20 (11.00-13.90) | 0.47 |
| Mean corpuscular volume (/fL) | 97.80 (94.20-102.90) | 94.40 (87.90-100.00) | 0.0271 * |
P-values ≤ 0.05
Table 6b. Effect of Toxoplasma gondii infection on haematological parameters in females.
| Haematological parameters | Seropositive | Seronegative | P-value |
|---|---|---|---|
| Median (IQR) | |||
| White blood cells (10 3/µL) | 3.20 (2.00-5.13) | 3.45 (2.20-5.20) | 0.3727 |
| Lymphocytes (10 3/µL) | 1.50 (0.70-2.60) | 1.55 (0.38-2.70) | 0.955 |
| Neutrophils (10 3/µL) | 0.70 (0.40-1.10) | 0.80 (0.38-1.20) | 0.7711 |
| Red blood cell (10 6/µL) | 4.24 (3.75-4.56) | 4.33 (3.94-4.64) | 0.0825 |
| Haemoglobin (g/dl) | 11.35 (10.20-12.30) | 11.40 (10.30-12.50) | 0.531 |
| Mean corpuscular volume (/fL) | 100.10 (92.03-104.50) | 96.40 (87.35-101.40) | 0.0035 ** |
P-values ≤ 0.01
Table 6c. Effect of Toxoplasma gondii infection on haematological parameters in pregnant women.
| Haematological parameters | Seropositive | Seronegative | P-value |
|---|---|---|---|
| Median (IQR) | |||
| White blood cells (10 3/µL) | 2.95 (1.80-5.95) | 3.30 (1.65-5.88) | 0.87 |
| Lymphocytes (10 3/µL) | 1.00 (0.00-1.63) | 0.00 (0.00-1.33) | 0.0369 * |
| Neutrophils (10 3/µL) | 0.50 (0.00-0.80) | 0.30 (0.00-0.80) | 0.4237 |
| Red blood cell (10 6/µL) | 4.21 (3.58-4.37) | 4.06 (3.70-4.51) | 0.6363 |
| Haemoglobin (g/dl) | 11.15 (10.20-11.90) | 11.20 (10.25-12.73) | 0.4645 |
| Mean corpuscular volume (/fL) | 98.00 (00.00-103.50) | 92.40 (00.00-101.70) | 0.139 |
* P-values ≤ 0.05
Discussion
Toxoplasma gondii is an intracellular parasite with a wide geographical distribution and an extremely broad host range. Despite the high prevalence of T. gondii infections in many parts of the world, there is limited data on the epidemiology of the infection in Ghana, and a majority of the few studies available are restricted to Accra, the capital city. Studies investigating the epidemiology of the infection typically do so by determining seroprevalence rates based on the detection of T. gondii-specific antibodies. T. gondii-specific IgG is associated with previous exposure to the parasite and is used as a marker for latent infection while IgM is used as a marker for exposure or acute infection 16.
In this study, an overall seroprevalence of 50.3% was observed, which is similar to a study from Accra that reported a seroprevalence rate of 49.7% 17, in contrast to another Accra-based study that reported 92.5% seroprevalence 12. The study also found a seroprevalence rate of 1.0% for parasite-specific IgM, suggesting active infections in these study participants. Other studies have previously reported fairly low seroprevalence rates of IgM, including 6.0% in the Central region of Ghana and 2.5% in Southwestern Ethiopia 13, 18. However, anti- T. gondii IgM seroprevalence rates as high as 29.7%, 39.1% and, even, 76.1% have been reported in Accra and Kumasi 11, 12, 17. Variations in the seroprevalence of anti- T. gondii antibodies from different studies may be due to methodological differences, including the choice of commercial ELISA kits.
As the three hospitals were located in different settlement types (urban, peri-urban and rural), we assumed that the patient population at each hospital primarily comprised individuals from the surrounding communities. We, therefore, compared seroprevalence rates among participants from the urban, peri-urban and rural communities. In many instances, rural inhabitants regularly come into contact with potentially oocyst-contaminated soil as part of their normal farming activities. However, we found no significant association between seropositivity and settlement type, which agrees with findings from Munoz-Zanzi et al., 19, who also found no obvious differences in seroprevalence between rural villages and urban slums. In contrast, Kawashima et al., 20 showed that seropositivity was significantly higher in rural communities compared to urban communities.
Acute infection by T. gondii in pregnancy can be detrimental to the foetus, resulting in stillbirth, chorioretinitis, hydrocephalus or even death 21. The ability of almost all subclasses of IgG — with the notable exception of IgG2 — to cross the placenta provides some form of protection to the foetus 22. The period of pregnancy in which the expectant mother becomes infected greatly influences the incidence and severity of the disease. Infection within the first trimester of pregnancy carries an approximately 6% risk of transmission to the foetus, while in the second and third trimesters, the risk of congenital transmission is about 33–47% and 60–81%, respectively 21. Contrary to the gestational risk of transmission, the risk of developing clinical symptoms of the disease (congenital toxoplasmosis) is highest when maternal infection occurs within the first trimester and gradually reduces through the second and third trimesters 21. Our study estimated an overall anti- T. gondii seroprevalence of 56.4% among pregnant women. This observation is lower than the 83.6% reported by Sefah-Boakye et al., in the same region (Ashanti) 11. Furthermore, we observed a trend of increasing seroprevalence with gestational period, with similar observations made in other studies from Kumasi, Accra and Southwestern Ethiopia 11, 18, 23.
In disease control, prevention and elimination, identification of the risk factors for infection are key. Horizontal transmission of T. gondii may occur via contact with cat litter, consumption of raw meat, eating of raw or undercooked vegetables, organ transplant and blood transfusion. We identified cat ownership, contact with cat litter and handling of raw meat as important risk factors for infection among our study population. Since T. gondii undergoes sexual reproduction in the gastrointestinal tract of domestic felines, individuals who owned cats or had regular contact with cat litter had 76% and 66% greater risk of T. gondii infection respectively compared to individuals who neither owned cats nor handled cat litter. Furthermore, since T. gondii is capable of encysting in the tissues of infected animals, people who regularly handle raw meat are also at greater risk of infection 24. We also investigated the association between certain demographic parameters and seropositivity. Consistent with findings from other studies in Ghana and Iran, seropositivity increased with age 13, 15, 25. This is due to the fact that infection has strongly been associated with contact with the soil where the oocyst prevails for years. The longer an individual survives, the greater the likelihood of coming into contact with contaminated soil hence the increase in seroprevalence with age.
T. gondii is capable of invading any nucleated cell, including immature red blood cells 26. As a mechanism of survival, intracellular parasites need to exit and re-infect other cells. The mechanism by which T. gondii exits infected cells is still not clearly understood. Some in vitro studies have demonstrated that T. gondii exits the cell by exerting tension on the host cell membrane, often causing the cell to rupture 27. Other studies have suggested that the parasite exits the host cell by disrupting the cytoskeleton in a manner similar to Plasmodium, resulting in cell lysis 28. In the previous report by Agordzo et al., (2019), results obtained for seropositive and seronegative individuals were generally similar 29. This is not different from the present study where the haematological data had been expanded and comparisons made between similar groups. These observations agree with other findings where haematological parameters sometimes remain stable even in times of infection 30. With the exception of pregnant women, we found significant differences in the mean corpuscular volumes of T. gondii seropositive and seronegative males and females. The mean corpuscular volumes increased significantly in seropositive individuals for both males and females as compared to the seronegative groups. Furthermore, the mean corpuscular volume was reported to have reduced significantly in seropositive pregnant women in Libya which is not the case in our study 30. We observed an increase in the MCV of seropositive pregnant women, even though this was not significant. Consistent with other findings in Libya and Iraq, infection by T. gondii did not have any significant effect on the red blood cells count and haemoglobin level in pregnant women 30, 31. The WBCs are known to play a major role in protection against infection. Whiles the immune system is considered to be fighting infections in cases of elevated WBC count, a significant decrease can be considered to be detrimental to one’s health. Even though a general decrease in WBC counts was observed in all categories under consideration, a significant reduction was obtained for only seropositive males. Furthermore, our findings for the WBC count for pregnant women does not agree with the reports of Hassen et al., (2019) which reported a significant decrease in the white blood cell for seropositive pregnant women 30. An investigation of the lymphocytes and neutrophils, which are components of the WBC and promote host defence by inducing anti-inflammatory response also reveal decreased counts in seropositive individuals for males and females whereas an increase in their counts were observed for pregnant women. The lymphocyte levels in seropositive males reduced significantly as opposed to the seronegative groups. In the case of the pregnant women, the lymphocyte count increased significantly in the seropositive group and this conforms with the findings of Hassen et al., (2019), even though they did not report a significant observation 30. Per the experimental observations of Biswas et al., (2017) 32, depletion of neutrophils results in a reduction in the IFN-γ production which resulted in an increase parasite burden in the central nervous system. While the exact molecular processes driving these differences are still unclear, there is increasing evidence that latent toxoplasmosis may cause chronic low-grade inflammation 33, which may, in turn, lead to anaemia 34.
Conclusion
The seroprevalence of T. gondii infection was high among our study population, including among pregnant participants. In addition, cat ownership, contact with cat litter and age were identified as major risk factors for infection. Furthermore, additional research is needed to fully clarify the links between latent toxoplasmosis and anaemia. In conclusion, we recommend that testing for infection by the parasite be included in routine screening of pregnant women seeking antenatal care and further studies should investigate the impact of infection on blood parameters of humans.
Data availability
Underlying data
Harvard Dataverse: Replication Data for: Seroprevalence, Risk Factors and Impact of Toxoplasma gondii Infection on Haematological Parameters in the Ashanti Region of Ghana: A Cross-Sectional Study, https://doi.org/10.7910/DVN/HXJONT 35.
This project contains the following underlying data:
-
-
Data includes, demography of study participants, risk factors, IgG response and measurement of haematological parameters
-
-
Data dictionary
Extended data
Harvard Dataverse: Replication Data for: Seroprevalence, Risk Factors and Impact of Toxoplasma gondii Infection on Haematological Parameters in the Ashanti Region of Ghana: A Cross-Sectional Study, https://doi.org/10.7910/DVN/HXJONT 35.
This project contains the following extended data:
-
-
Questionnaire used in the present study.
Data are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication).
Acknowledgements
We acknowledge the Kuntanase, Agona and Kumasi South Hospitals for allowing us the space in their laboratories. We are also grateful to all participants for their voluntary participation in this study. The KNUST Graduate Assistantship Program has been of immense support to Samuel Kekeli Agordzo and Abdul-Hakim Mutala.
Funding Statement
AHM and SKA received graduate assistantship from the KNUST College of Science. KB received funds for laboratory supplies from the Africa Research Excellence Fund (AREF) Research Development Fellowship [MRF-157-0007-F-BADU]. Kingsley Badu is an Affiliate of the African Academy of Sciences (2017-2022).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 2; peer review: 2 approved]
References
- 1. Dubey JP, Jones JL: Toxoplasma gondii infection in humans and animals in the United States. Int J Parasitol. 2008;38(11):1257–78. 10.1016/j.ijpara.2008.03.007 [DOI] [PubMed] [Google Scholar]
- 2. Montoya JG, Liesenfeld O: Toxoplasmosis. Lancet. 2004;363(9425):1965–76. 10.1016/S0140-6736(04)16412-X [DOI] [PubMed] [Google Scholar]
- 3. Flegr J: Effects of Toxoplasma on human behavior. Schizophr Bull. 2007;33(3):757–60. 10.1093/schbul/sbl074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Montoya JG: Laboratory diagnosis of Toxoplasma gondii infection and toxoplasmosis. J Infect Dis. 2002;185(Suppl 1):S73–82. 10.1086/338827 [DOI] [PubMed] [Google Scholar]
- 5. Robbins JR, Zeldovich VB, Poukchanski A, et al. : Tissue barriers of the human placenta to infection with Toxoplasma gondii. Infect Immun. 2012;80(1):418–28. 10.1128/IAI.05899-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Galvan-Ramirez Mde L, Troyo R, Roman S, et al. : A systematic review and meta-analysis of Toxoplasma gondii infection among the Mexican population. Parasit Vectors. 2012;5(1):271. 10.1186/1756-3305-5-271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Galván-Ramírez ML, Covarrubias X, Rodríguez R, et al. : Toxoplasma gondii antibodies in Mexican blood donors. Transfusion. 2005;45(2):281–2. 10.1111/j.1537-2995.2004.00442.x [DOI] [PubMed] [Google Scholar]
- 8. Muralikrishna P, Sunil B, Savita RK, et al. : Toxoplasmosis-The Public Health significance. International Journal of Science, Environment and Technology. 2017;6(5):2752–2758. Reference Source [Google Scholar]
- 9. Yohanes T, Debalke S, Zemene E: Latent Toxoplasma gondii Infection and Associated Risk Factors among HIV-Infected Individuals at Arba Minch Hospital, South Ethiopia. AIDS Res Treat. 2014;2014:652941. 10.1155/2014/652941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bamba S, Cissé M, Sangaré I, et al. : Seroprevalence and risk factors of Toxoplasma gondii infection in pregnant women from Bobo Dioulasso, Burkina Faso. BMC Infect Dis. 2017;17(1):482. 10.1186/s12879-017-2583-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sefah-Boakye J, Frimpong EH, Dompreh A, et al. : Seroprevalence of Toxoplasma gondii Infection among Pregnant Women in the Ashanti Region of Ghana. Int J Microbiol Adv Immunol. 2016;4(1):70–74. 10.19070/2329-9967-1600013 [DOI] [Google Scholar]
- 12. Ayi I, Edu SA, Apea-Kubi KA, et al. : Sero-epidemiology of toxoplasmosis amongst pregnant women in the greater accra region of ghana. Ghana Med J. 2009;43(3):107–14. 10.4314/gmj.v43i3.55325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Abu EK, Boampong JN, Ayi I, et al. : Infection risk factors associated with seropositivity for Toxoplasma gondii in a population-based study in the Central Region, Ghana. Epidemiol Infect. 2015;143(9):1904–12. 10.1017/S0950268814002957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Abu EK, Boampong JN, Amoabeng JK, et al. : Epidemiology of Ocular Toxoplasmosis in Three Community Surveys in the Central Region of Ghana, West Africa. Ophthalmic Epidemiol. 2016;23(1):14–9. 10.3109/09286586.2015.1089579 [DOI] [PubMed] [Google Scholar]
- 15. Kwofie KD, Ghansah A, Osei JH, et al. : Indication of Risk of Mother-to-Child Toxoplasma gondii Transmission in the Greater Accra Region of Ghana. Matern Child Health J. 2016;20(12):2581–2588. 10.1007/s10995-016-2084-z [DOI] [PubMed] [Google Scholar]
- 16. Dhakal R, Gajurel K, Pomares C, et al. : Significance of a Positive Toxoplasma Immunoglobulin M Test Results in the United States. J Clin Microbiol. 2015;53(11):3601–5. 10.1128/JCM.01663-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ayeh-Kumi PF, Opoku AG, Kwakye-Nuako G, et al. : Sero-prevalence of toxoplasmosis among patients visiting the Korle-Bu Teaching Hospital, Accra, Ghana. RIF. 2010;1(3):147–150. Reference Source [Google Scholar]
- 18. Zemene E, Yewhalaw D, Abera S, et al. : Seroprevalence of Toxoplasma gondii and associated risk factors among pregnant women in Jimma town, Southwestern Ethiopia. BMC Infect Dis. 2012;12(1):337. 10.1186/1471-2334-12-337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Munoz-Zanzi C, Campbell C, Berg S: Seroepidemiology of toxoplasmosis in rural and urban communities from Los Rios Region, Chile. Infect Ecol Epidemiol. 2016;6(1):30597. 10.3402/iee.v6.30597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kawashima T, Khin-Sane-Win, Kawabata M, et al. : Prevalence of antibodies to Toxoplasma gondii among urban and rural residents in the Philippines. Southeast Asian J Trop Med Public Health. 2000;31(4):742–6. [PubMed] [Google Scholar]
- 21. Dunn D, Wallon M, Peyron F, et al. : Mother-to-child transmission of toxoplasmosis: risk estimates for clinical counselling. Lancet. 1999;353(9167):1829–33. 10.1016/S0140-6736(98)08220-8 [DOI] [PubMed] [Google Scholar]
- 22. Palmeira P, Quinello, C, Silveira-Lessa AL, et al. : IgG placental transfer in healthy and pathological pregnancies. Clin Dev Immunol. 2012;2012:985646. 10.1155/2012/985646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ayi I, Sowah AO, Blay EA, et al. : Toxoplasma gondii infections among pregnant women, children and HIV-seropositive persons in Accra, Ghana. Trop Med Health. 2016;44(1):17. 10.1186/s41182-016-0018-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Swai ES, Schoonman L: Seroprevalence of Toxoplasma gondii infection amongst residents of Tanga district in north-east Tanzania. Tanzan J Health Res. 2009;11(4):205–9. 10.4314/thrb.v11i4.50178 [DOI] [PubMed] [Google Scholar]
- 25. Jafari R, Sadaghian M, Safari M: Seroprevalence of Toxoplasma gondii infection and related risk factors in Tabriz City, Iran, 2008. J Res Health Sci. 2012;12(2):119–121. [PubMed] [Google Scholar]
- 26. Joiner KA, Dubremetz JF: Toxoplasma gondii: a protozoan for the nineties. Infect Immun. 1993;61(4):1169–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lund E, Lycke E, Sourander P: A cinematographic study of Toxoplasma gondii in cell cultures. Br J Exp Pathol. 1961;42(4):357–62. [PMC free article] [PubMed] [Google Scholar]
- 28. Lavine MD, Arrizabalaga G: Exit from host cells by the pathogenic parasite Toxoplasma gondii does not require motility. Eukaryot Cell. 2008;7(1):131–140. 10.1128/EC.00301-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Agordzo SK, Badu K, Addo MG, et al. : Seroprevalence, risk factors and impact of Toxoplasma gondii infection on haematological parameters in the Ashanti region of Ghana: a cross-sectional study. [version 1; peer review: 2 approved with reservations]. AAS Open Res. 2019;2:166 10.12688/aasopenres.13022.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hassen AH, Ali MS, Ekhnafer AM, et al. : Effect of Toxoplasma Gondii Infection on Haematological and Liver Function Parameters among Abortive Women in El-Beida City. 10.21276/sjbr.2019.4.8.4 [DOI] [Google Scholar]
- 31. Mahmood OI: Effect of Toxoplasmosis on hematological, biochemical and immunological parameters in pregnant women in Tikrit city, Iraq. Tikrit Journal of Pure Science. 2018;21(3):24–27. Reference Source [Google Scholar]
- 32. Biswas A, French T, Düsedau HP, et al. : Behavior of Neutrophil Granulocytes during Toxoplasma gondii Infection in the Central Nervous System. Front Cell Infect Microbiol. 2017;7:259. 10.3389/fcimb.2017.00259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bay-Richter C, Petersen E, Liebenberg N, et al. : Latent toxoplasmosis aggravates anxiety- and depressive-like behaviour and suggest a role of gene-environment interactions in the behavioural response to the parasite. Behav Brain Res. 2019;364:133–139. 10.1016/j.bbr.2019.02.018 [DOI] [PubMed] [Google Scholar]
- 34. Wang Z, Zhang DX, Zhao Q: Infection-stimulated anemia results primarily from interferon gamma-dependent, signal transducer and activator of transcription 1-independent red cell loss. Chin Med J (Engl). 2015;128(7):948–55. 10.4103/0366-6999.154303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Badu K: "Replication Data for: Seroprevalence, Risk Factors and Impact of Toxoplasma gondii Infection on Haematological Parameters in the Ashanti Region of Ghana: A Cross-Sectional Study". Harvard Dataverse, V2, UNF: 6:m7qejmGOTFGSAD5ay0wQkw== [fileUNF].2019. 10.7910/DVN/HXJONT [DOI] [PMC free article] [PubMed]


