Abstract
Nitroxyl (HNO), the one-electron reduced and protonated congener of nitric oxide (NO), is a chemically unique species with potentially important biological activity. Although HNO-based pharmaceuticals are currently being considered for the treatment of chronic heart failure or stroke/transplant-derived ischemia, the chemical events leading to therapeutic responses are not established. The interaction of HNO with oxidants results in the well-documented conversion to NO, but HNO is expected to be readily reduced as well. Recent thermodynamic calculations predict that reduction of HNO is biologically accessible. Herein, kinetic analysis suggests that the reactions of HNO with several mechanistically distinct reductants are also biologically feasible. Product analysis verified that the reductants had in fact been oxidized and that in several instances HNO had been converted to hydroxylamine. Moreover, a theoretical analysis suggests that in the reaction of HNO with thiol reductants, the pathway producing sulfinamide is significantly more favorable than that leading to disulfide. Additionally, simultaneous production of HNO and NO yielded a biphasic oxidative capacity.
Keywords: Nitroxyl, Kinetics, Nitric oxide, Reduction, Nitrogen oxides, Thiols, Free radicals
In recent years, nitroxyl (HNO) has been demonstrated to be a chemically unique species with potentially important pharmacological activity [1,2]. Unlike many other biologically relevant nitrogen oxides (e.g., nitric oxide (NO), nitrogen dioxide (NO2), nitrite (NO2−)), for which an abundance of biochemical data has been amassed, the physiologically relevant chemistry of HNO has been largely obfuscated by its metastability. The propensity of HNO to dimerize and then dehydrate to produce nitrous oxide (N2O) [3,4] precludes storage and necessitates the use of donor compounds. Angeli’s salt (sodium trioxodinitrate, Na2N2O3) [5] has been the primary HNO donor for chemical and pharmacological studies. Temposil (citrated calcium carbimide), which is metabolically activated to release HNO, has been used for over half a century as a behavioral therapeutic for chronic alcoholism [6] and works via an HNO-mediated modification of the catalytic cysteine residue of aldehyde dehydrogenase [7].
Other potential therapeutic uses for HNO have been reported more recently. For example, HNO from Angeli’s salt enhances cardiac contractility and output in a chronic heart failure model [8]. Additionally, HNO promotes smooth muscle relaxation and vasodilation [9,10] and decreases lobar arterial pressure without changing left atrial pressure [11]. Diverse cellular processes such as ryanodine receptor activation [12], transcription factor modification [13], cyclooxygenase activation [14], heme oxygenase induction [15], voltage gated potassium channel activation [16], protein thiol modification [17], increased arginine uptake [18], cAMP fluctuation [1], and calcitonin gene-related peptide release [19] can be moderated by HNO. Interestingly, HNO seems to specifically affect calcium signaling inmyocardial tissue by interacting with a variety of functionally distinct thiol proteins ([2] and references therein). However, the biochemistry responsible for many of these effects of HNO is not well understood.
Toxicological outcomes have also been associated with exogenous application, and potentially endogenous production, of HNO. In this regard, HNO may mediate the inflammatory effects of ischemia–reperfusion by enhancing dexamethasone-inhibitable [20] neutrophil invasion [21]. Additionally, the etiology of amyotrophic lateral sclerosis has been suggested to involve the pro-oxidative chemistry associated with aberrant HNO production [22].
The biological targets of HNO have been reported to include protein thiols [17], transition metals [23], iron–sulfur clusters [24], and DNA [25,26]. Reaction with oxidants results in conversion of HNO to NO [27]. HNO is also recognized as an electrophile [28], which suggests that reduction may also occur. Recent thermodynamic calculations predict that reduction of HNO is accessible under biological conditions [29,30], but the kinetic proclivity toward reduction has not been elucidated.
Notably, compounds that can be considered structurally similar to HNOare readily reduced in diverse chemical and physiological settings. For example, oxoammonium cations (R2NO+) could serve to mechanistically model two-electron transfer to HNO (albeit oxoammonium cations will be much more oxidizing than HNO). Two-electron transfer to R2NO+from NADH proceeds with a 1:1 stoichiometry [31]. Oxoammonium electrophiles are also able to oxidize alcohols under relatively mild conditions [32], which may correspond to the reaction of HNO with ascorbate. There is also precedent in the reduction of S-nitrosothiols (RSNOs) by NADH and ascorbate. The stoichiometry for reduction of S-nitrosoglutathione by Hantzsch dihydropyridines is 1:1, with concurrent production of one equivalent of oxidized pyridine [33]. Enzymatic reduction of RSNOs by dihydropyridine-dependent dehydrogenase activity is also well known [34]. Additionally, ascorbate reduces RSNOs in a metal-independent manner [35].
Considering that the cytosolic environment of cells is reducing in nature, reductive metabolism of HNO may be facile and thus important to its pharmacology and ultimate fate. As a first step in addressing this hypothesis, the reduction of HNO by several mechanistically distinct reductants has been examined. The findings of this study indicate that HNO reduction is a likely metabolic fate and confirm the idea that thiols and thiol proteins are likely targets for HNO activity. Moreover, generation of both the disulfide and the sulfinamide products from this interaction is energetically accessible, although sulfinamide formation is more favored than disulfide formation, with the latter being driven by the availability of other reactive thiols. As disulfide is readily reversible in vivo, whereas sulfinamide is thought not to be, this may have potential implications for the pharmacology of HNO and the reversibility of its effects.
Experimental procedures
Chemicals and solutions
Reduced nicotinamide adenine dinucleotide (NADH), oxidized nicotinamide adenine dinucleotide (NAD+), glutathione, and glutathione disulfide were purchased from Sigma (St. Louis, MO, USA). L-Ascorbic acid was purchased from Fischer Scientific, and L-dehydroascorbic acid was from Sigma. Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid), hydroxylamine hydrochloride, and isobutyl nitrate were purchased from Aldrich (Milwaukee, WI, USA). All other chemicals were obtained from commercial suppliers and were of the highest purity available. Rigorous exclusion of metals was performed through the use of deionized water, treatment with Chelex resin, and inclusion of diethylenetriamine pentaacetate (DTPA) chelator. Use of glass containers was avoided or, when required, glassware was demetallated by holding full volumes of stirred 1% nitric acid over night.
Angeli’s salt synthesis
Angeli’s salt (AS) was used as a source of HNO and synthesized according to a method modified from that of Smith and Hein [3] but utilizing a process whereby sodium hydroxide was replaced by sodium methoxide equivalents dissolved in methanol. The reagents employedwere hydroxylamine hydrochloride and isobutyl nitrate, the ratio of NH2OH·HCl:RONO2:MeONa was 3:1:5, and the primary contaminants were nitrite and hydroxylamine, totaling b3% of the solid AS preparation. Stock solutions of Angeli’s salt were made up in at least fourfold excess sodium hydroxide immediately before use and kept on ice. All stocks were from the same batch of Angeli’s salt and purity was determined by the absorbance at 248 nm (extinction coefficient 8100 M−1 cm−1 [5]) of an Angeli’s salt preparation in 0.1 N NaOH before assays, periodically throughout the experiments, and to ensure that complete decomposition of prodrug had occurred in the product analysis studies.
Kinetics of reduction
Direct reaction
Solutions of reductants were made up in distilled water. Reaction was initiated by the addition of Angeli’s salt. Typically, 100–1000× AS in 0.1 M NaOH was rapidly mixed into water containing 0.1 M potassium phosphate buffer (pH 7.4) and the reductant at final concentration. Reactions were carried out in triplicate or greater unless otherwise noted, under either aerobic or anaerobic (argon-saturated) conditions at room temperature for 1–2 min (at 23°C and pH 7.45 the t1/2 of AS decomposition was 17 min (6.33×10−4 s−1 as determined here)). Loss of chromophore was linear over this period, and initial rates were derived from these data.
Competition reaction
Competition kinetics were determined as in the direct reaction studies, but the competitor (10–100× stocks in water) was added to the reaction mixture immediately before Angeli’s salt addition.
Mathematical modeling
The loss of reductant chromophore in these kinetic runs was quantified, and the data were analyzed with the freely available kinetic software DYNAFIT [36]. The kinetic constants used were from the literature and are shown in Scheme 1. Shown below is an example of a reaction mechanism and the differential equations utilized to determine the second-order rate constant for the reaction of HNO with NH2OH.
Scheme 1.
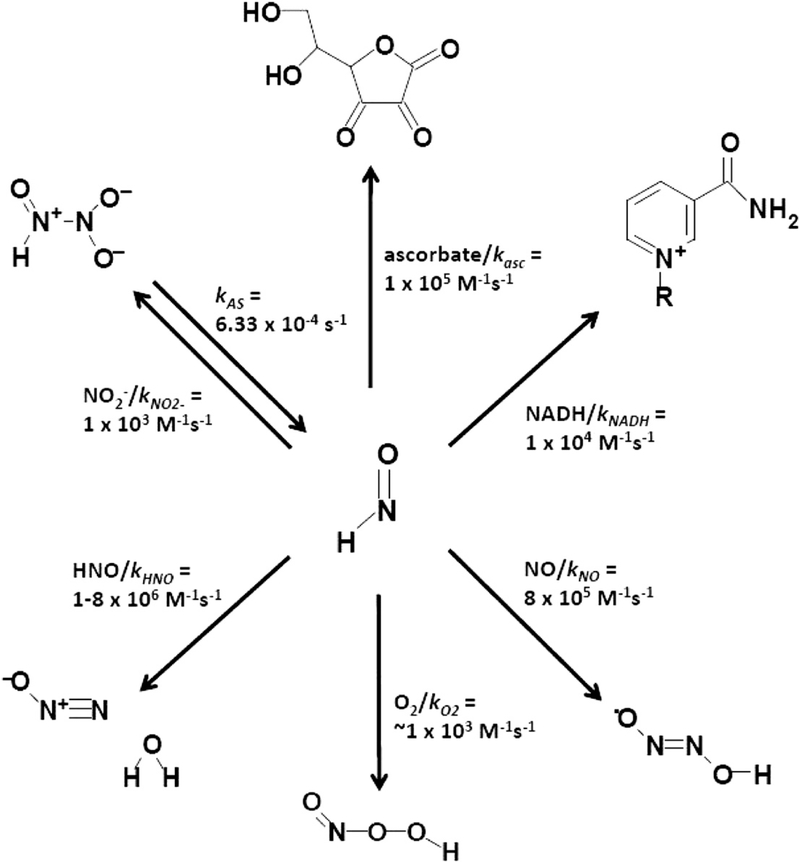
Kinetic scheme describing the combined processes under consideration. This was used to process initial rate data through DYNAFIT for direct and competition reactions. The coreactant leading to product is shown adjacent the reaction arrow, followed by the published rate constant. References: kAS, determined here; kNO2−[48]; kHNO [29,85]; kNO [65]; kO2 [29,48].
The reaction mechanism is as follows:
The differential equations used are:
In addition to DYNAFIT, the kinetic reactions were also modeled using MATLAB version 7.01 SP1. In general, the reactions under consideration, the known rate constants, and a crude estimate of the unknown rate constants were determined. The programs then set up a series of simultaneous differential equation solvers to derive predicted rate constants based on actual initial rates of experimental data. A subsequent comparison of the fit between model-predicted data and the experimental data using the newly developed rate constant for NAD (P)H determines whether the user-defined reactions were necessary and sufficient to describe the system under study. All variables were nondimensionalized with respect to the initial concentration of each species or with the parent compound of each species if the initial concentration was zero. The set of differential equations was solved using the stiff ordinary differential equation solver “ode15s” with the absolute error tolerance set to 10−12 and the relative error tolerance set to 10−6. Optimization of unknown reaction coefficients was achieved using the “fminsearch” function, which performs a multidimensional unconstrained nonlinear minimization based on the Nelder–Mead method. The objective function was defined as the sum of the square error of the theoretical prediction from the experimentally derived values for the formation of a selected species normalized to the experimental values. Various initial conditions were tested to ensure the results were not due to trapping within a local minima.
NADH and NAD+ analysis
NADH was determined by monitoring the absorbance at 340 nm (λ= 6.2×103 M−1 cm−1). The formation of NAD+ was monitored using a fluorometric assay published previously [37]. Fluorescence was measured using a Perkin–Elmer LS50B spectrofluorometer (excitation at 365 nm; emission at 460 nm), against an NAD+ standard curve. Reactions were typically performed in 400 μl of 50mMChelex-treated sodium phosphate buffer, pH 7.45, containing 10 μM DTPA. After complete decomposition of AS (greater than five half-lives), the NADH remaining and NAD+ produced were measured from the same incubation.
Ascorbate and dehydroascorbate analysis
Ascorbate was determined by monitoring the absorbance at 265 or 285 nm (ɛ265= 1.4×104 M−1 cm−1, ɛ285= 5.44×103 M−1 cm−1, determined in this study). Dehydro-1-ascorbate produced from the reaction of ascorbate with Angeli’s salt was assayed as the hemiketal methanol adduct, absorbing at 346 nm, according to Badrakhan et al. [38]. After complete decomposition of AS (greater than five half-lives), the ascorbate remaining and dehydroascorbate produced were measured from parallel incubations with methanol. The reactions were carried out in 400 μl of 50 mM Chelextreated sodium phosphate buffer, pH 7.45, containing 10 μM DTPA.
Hydroxylamine (NH2OH) analysis
The formation of NH2OH from the reactions of HNO with the various reductants was determined using the indoxine test [39]. In summary, a 0.4-ml sample was mixed with an equal volume of 8-hydroxyquinoline (1%w/v in absolute ethanol) and Na2CO3 (10% w/v in absolute ethanol). The solution was reacted at 95°C for 5 min. Upon cooling to room temperature the absorbance at 710 nm was determined using a Shimadzu UV-2501PC spectrophotometer. Occasionally the assay was carried out in microplate format with heating via thermocycler (Perkin–Elmer) and spectrophotometry performed on a microplate reader (Emax; Molecular Devices). Quantitation was accomplished using a standard curve generated from known concentrations of authentic NH2OH●HCl (Sigma).
Theoretical calculations
All structures were initially optimized using the B3LYP density functional theory method with a 6–311+G(d) basis set implemented in the Gaussian 03 program [40–42]. Gas phase energies were computed for all structures using the high-accuracy CBS-QB3 multicomponent method, which generally gives average errors of ±1 kcal/mol compared to experimentally measured data for the G3 data set [43]. Aqueous solvation energies were calculated as single points on the B3LYP/6–311+G(d) gas phase-optimized geometries using B3LYP/6–31+G(d) in the SM6 continuum solvation model. The SM6 model has been demonstrated to give aqueous solvation free energies with an average error of ∼0.5 kcal/mol for a limited test set of neutral solutes [44]. Aqueous solvation energies were applied to the CBS-QB3 gas phase energies to obtain free energies in aqueous solution.
Results
Reaction rate constants
Prior reports indicate that HNO may be subject to reduction under biological conditions [45]. In an attempt to assess the feasibility of this process, HNO derived from the donor Angeli’s salt was exposed to a variety of common biologically relevant reductants. To obtain the order of the reaction with respect to reductant, it is desirable to maintain the reactions under excess HNO. Given the necessity to produce HNO in situ and the relatively slow decay of the principal HNO donor Angeli’s salt, such conditions are difficult to achieve. The consumption of HNO by dimerization (Scheme 1) further complicates achieving a high steady-state concentration of HNO. Therefore, the reactions seem to be nearly zero order in reductant for much of the examined concentration range (Fig. 1A; NADH or ascorbate as reductant). To examine the dependence of reaction rate on Angeli’s salt and HNO concentration, constant NADH or ascorbate (100–500 μM) was reacted with varying Angeli’s salt (0.1–2000 μM) for 1–2 min, whereas the rate of loss of reductant was monitored spectrophotometrically (Fig. 1B).
Fig. 1.
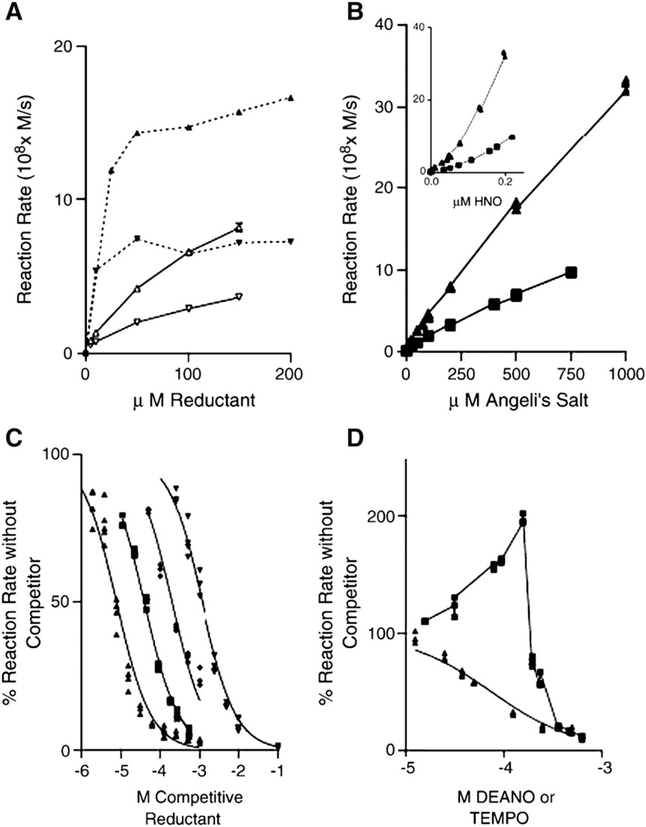
Direct and competition reactions of HNO with reductants. A. Plot of [reductant] versus rate of reductant oxidation. (▼) 200 μM Angeli’s salt and (▲) 500 μM Angeli’s salt: filled symbols, NADH (average of duplicates; open symbols, ascorbate (n= 3 B. Plot of [donor] (Angeli’s salt, 1–1000 μM) versus rate of reductant oxidation. NADH (▲, 85 μM), ascorbate (■, 100 μM). B inset. Plot of [HNO] versus rate of reductant oxidation. NADH (▲, 85 μM), ascorbate (■, 100 μM). [HNO] was derived from the method of Liochev and Fridovich [49]. C. Competition assay. Angeli’s salt (500 μM) and NADH (85 μM) were reacted together in the presence of increasing concentrations of competing reductant, and the loss of NADH was monitored as in A. Glutathione (■), ascorbate (▼), trolox (●), and hydroxylamine (♦) data are shown, with the values predicted by DYNAFIT shown as a solid line superimposed on the data. D. Anomolous behaviour of NO in the competition assay. Conditions were as in C, but with nitric oxide donor, DEANO (■), as the competitor. A typical response, exhibited by TEMPO (▼) is shown for comparison.
Correlating the effect of HNO treatment with Angeli’s salt concentration is often not informative; ideally the effect would be plotted versus the concentration of HNO ([HNO]). This is especially crucial in the determination of reaction rate constants. To address this, others have predicted HNO concentrations based upon steady-state assumptions of nitroxyl, governed by donor decomposition (vf), dimerization to N2O (vd), and the reaction under study (vr) (Eqs. (1) and (2), where RH2 is any reductant) [12,46–48]. Cheong et al. [12] used the steady-state equation, vf= vd, to plot ryanodine receptor activation versus [HNO]. By including another component in the steady state, vr, Liochev and Fridovich [49] predict [HNO] more accurately (Eq. (3)). They observed rates of product formation (or loss of reactant) and determined rate constants for reaction with, for example, superoxide dismutase. Utilizing a fitting parameter from plots of Angeli’s salt against binding velocity Bari et al. [46] and Marti et al. [50] have published rate constants for the reaction of HNO with metalloporphyrins. Using end-product analysis instead, Miranda et al. [1] determined kr after complete decomposition of nitroxyl donor (Eq. (4)):
| (1) |
| (2) |
| (3) |
| (4) |
These general strategies provide insight into the potency and stoichiometry of HNO reactions, but the equations are not readily solvable in the presence of multiple species capable of reaction with HNO. A further limitation of these equations is their lack of a time-dependent component.
Because it is clear that neither Angeli’s salt concentrations nor simple calculations of HNO concentration will enable determination of second-order rate constants for the reaction of nitroxyl with a scavenger, we quantified the loss of reductant via kinetic analysis using the software programs DYNAFIT and MATLAB according to Scheme 1, in which the donor (AS) produces HNO and multiple paths lead to its consumption (for example O2, NO, reductant, nitrite). We determined the value of kNADH for the HNO/NADH reaction to be 1.1 (±0.2) × 104 M−1 s−1. When we used our reactant concentrations and observed the velocities of reaction to calculate kNADH with the equations of Liochev and Fridovich [48] or Bari et al. [46], the resultant values ranged from 0.9×104 to 2.2×104 M−1 s−1. The reaction of HNO with ascorbate was difficult to monitor directly as the UV absorbance of Angeli’s salt overlaps with that of ascorbate, necessitating the use of off-maximum ascorbate absorbances for quantitation. Additionally, we noted that authentic dehydroascorbate, a product of the HNO/ascorbate reaction, produces an absorbance at 273 nm over time in solution, blunting our observed loss of ascorbate. Because many biomolecules absorb minimally in the visible region, NADH was used as an indicator in the assessment of the propensity of other reductants to reduce HNO via a competition assay. The degree to which common reductants competed with the oxidation of NADH by HNO is shown in Fig. 1C. The values predicted by DYNAFIT, based on Scheme 1, are superimposed as solid lines on the observed data points. The fit is reasonable, providing validation of the analysis model utilized in the competition assay. Control experiments verified that addition of competing reductants did not affect the NADH concentration or reverse of the HNO-induced bleaching of NADH. Table 1 shows the rate constants proposed for the reaction of nitroxyl with glutathione, thiosulfate, tris-carboxyethylphosphine, the water-soluble vitamin E analog trolox, ascorbic acid, and hydroxylamine. Trolox was nearly insoluble above low-millimolar concentrations, leading to a deviation from predicted values at higher concentrations. Interestingly, selenomethionine reacted with a rate constant of around 1×104 M−1 s−1 but methionine was unreactive up to its limit of solubility (not shown). The selenide is therefore much more reactive than its sulfide analog. From Table 1, it is clear that nitroxyl is capable of reaction with a diverse array of mechanistically distinct reductants, with second-order reaction rate constants that span several orders of magnitude.
Table 1.
Rate constants for the reaction of HNO with reductants
| Reductant | Kred × 10−5 M−1 s−1 | % Error | kred/k1NADH |
|---|---|---|---|
| Hydroxylamine | 0.04 | 7.4 | 0.31 |
| Selenomethionine | 0.09 | 12 | 0.67 |
| NAD(P)H | 0.11 | 9.1 | 1 |
| Thiosulfate | 0.21 | 8.4 | 1.58 |
| Trolox | 0.2 | 14 | 1.72 |
| TEMPO | 0.63 | 13 | 4.38 |
| Ascorbate | 1.1 | 9.8 | 6.1 |
| Glutathione | 76 | 9.5 | 42.80 |
| Tris-carboxyethylphosphine | 84 | 21 | 53.42 |
Enhanced oxidative capacity of HNO/NO mixtures
As HNO can be readily oxidized to NO, it is possible that HNO and NO can be present simultaneously in systems examining HNO. Because HNO and NO react with each other to form a putative oxidant (vide infra), there is the need to determine whether these reaction products can be oxidizing. To assess the oxidative potential of the product of the reaction of HNO with NO in our system, the NO donor DEA/NO [51] was titrated with constant Angeli’s salt and NADH. DEA/NO decays with a half-life similar to that of Angeli’s salt but produces two equivalents of NO. At concentrations up to 600 μM, DEA/NO alone resulted in no oxidation of NADH. In the presence of Angeli’s salt, the rate of NADH oxidation was dependent on the concentration of DEA/NO in a biphasic manner (Fig. 1D).
The association of HNO and NO produces the hyponitrous radical (HN2O2) [52], which at pH 7.4 is predominantly deprotonated as the hyponitrite radical anion (N2O2−). Poskrebyshev et al. [53] reported an experimental value for the potential of the N2O2−/N2O22− couple of +0.96 V, indicating that N2O2− is a reasonably strong oxidant. In contrast, a markedly different value for this couple of −0.4 V has been reported by us based on theoretical analysis with CBS-QB3 level theory and (C)PCM solvation mode [54]. This negative potential suggested that HNO/NO mixtures should actually be relatively reducing, an idea that has been challenged recently [55]. Because the reaction of HNO with NO clearly produces an oxidant and thermodynamic calculations involving solvation of a dianion are known to be prone to significant error, we have reinvestigated the potential of the N2O2−/N2O22− couple herein. The SM6 continuum solvation model was utilized as it is better able predict thermodynamic parameters than the (C)PCM model when a dianion is present in the reaction [56]. The recalculated potential of the N2O2−/N2O22− couple based on the SM6 model is 0.34 ± 0.1 V. Additionally, as the pKa1 and pKa2 of hyponitrous acid are 7.05 and 11.4, respectively, a predominate product of the HNO/NO product reduction reaction under physiological conditions will be the monoprotonated hyponitrous anion. Thus, we have calculated the potential of the more relevant proton-coupled reduction of N2O2− to HN2O2 as a significantly more favorable 1.0±0.1 V, consistent with recent arguments [55].
Reduction products
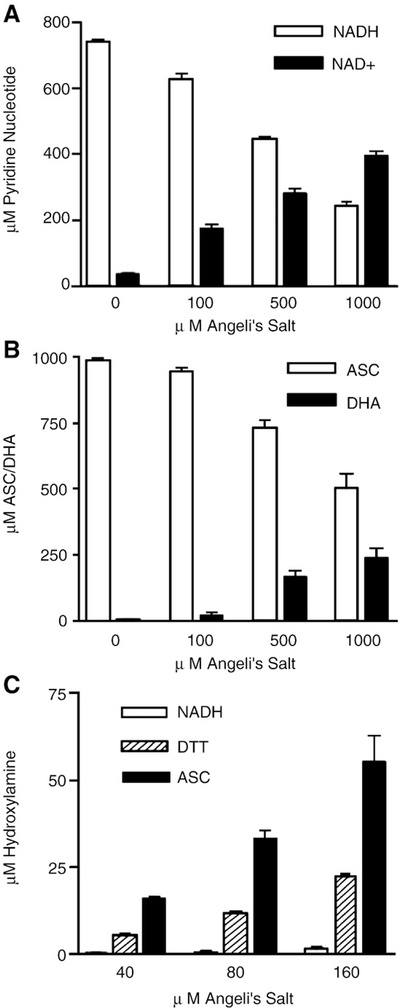
To qualitatively validate the proposed reaction scheme, the products of oxidation of NADH and ascorbate (Figs. 2A and B, respectively) and of HNO reduction (Fig. 2C) were analyzed. The HNO-dependent loss of NADH or ascorbate monitored spectroscopically was accompanied by an increase in NAD+or dehydroascorbate, albeit nonquantitatively. Reduction of HNO to NH2OH is inherently not quantitative owing to the reaction of HNO with NH2OH [57] and the interference of excess reductants in the 8-hydroxyyquinoline/indooxine assay [58] (shown for comparison is the effect elicited by a strong reductant, dithiothreitol). Plots of NH2OH formed versus increasing reductant or Angeli’s salt were therefore invariably bell shaped (higher concentrations not shown). We currently do not have an explanation for the apparent lack of NH2OH production in the HNO/NAD(P)H reaction (Fig. 2C). NAD(P)H interfered to a lesser extent with the indooxine assay, yet the reaction with HNO produced disproportionately less NH2OH than might be expected from the relative magnitude of the rate constant. This lack of NH2OH production in the reaction of HNO with NADH has been previously reported by Reif and coworkers [59]. To address the possibility that NH2OH could be an intermediate that is further reduced (to NH3, for example), the reaction between NH2OH and all reductants was examined. In all cases, the direct reaction of NH2OH with reductants did not lead to reductant oxidation (data not shown), indicating that NH2OH is not further reduced under the conditions of our experiments.
Fig. 2.
Product formation from the reaction of HNO with reductant. (A) 800 μMNADH or (B) 1000 μM ascorbate was reacted for 90 min in 50 mM potassium phosphate with the indicated concentrations of Angeli’s salt before assay for remaining reductant and oxidized products. (C) Hydroxylamine was assayed using the indooxine test after reaction under conditions as in (A) and (B). ASC, ascorbate; DHA, dehydroascorbate; DTT, dithiothreitol.
Reaction of HNO with NAD(P)H
NADH/NADPH
The ratio of reduced to oxidized dihydropyridine nucleotides in mammalian systems differs for the NADH and NADPH pools [60]. Therefore we sought to ascertain whether the 2′ phosphate plays a role in the relative reactivity of NADPH versus NADH, as has been reported for reaction with ferric iron [61]. To this end, the rate of reaction of NADPH with HNO was examined. As this rate constant (1.3 (±0.4)×104 M−1 s−1) did not differ significantly from that of NADH, the reaction of nitroxyl with dihydropyridine nucleotides can be generally signified as HNO+ NAD(P)H → products.
Reaction with oxygen
Monoprotonated nitroxyl reacts with dioxygen with a published bimolecular rate constant of kO2<<105 M−1 s−1 (29) or kO2 ∼103 M−1 s−1 [1]. Although the reaction is thermodynamically favorable [62] the kinetic relevance of this reaction under biological conditions is questionable, as it has a significant energy of activation. The identity of the product of HNO autoxidation has been a matter of debate. The resulting oxidant has been argued to be either peroxynitrite (ONOO−) [63] or an as yet unidentified and distinct species [64]. The reaction of HNO with O2 would provide an additional pathway for HNO loss (Scheme 1), and its inclusion in the kinetic analysis scheme slightly improves the fits of modeling software predictions to experimental data; however, the rates of reaction of HNO with NADH in the presence and absence of dioxygen were not significantly different over the short time periods employed (<2 min). The rate constant under anaerobic conditions was calculated as 0.9 (±0.3) × 104 M−1 s−1, versus 1.1 (±0.2) × 104 M−1 s−1 in the presence of oxygen. Inclusion of ONOO− in the model as a downstream oxidant of NADH (kr = 4×103 M−1 s−1 [65]) worsened the fits of experimental data, suggesting that ONOO− is not consuming NADH in this system. Furthermore, the rate constant (0.9 ± 0.3×104 M−1 s−1) for NADH consumption was not significantly altered in the presence of bicarbonate (20 or 50 mM, pH 7.4–7.6), which rapidly scavenges ONOO− [66]. Therefore, although autoxidation of HNO is a viable consumption pathway, we concur with Reif and coworkers [59] that the resulting oxidant leading to the loss of NAD(P)H is not ONOO−. Thus, it is not likely that any of the biological activity of HNO is due to formation of ONOO−. This is further supported by recent work indicating that the cardiovascular actions of HNO and ONOO− are distinct [67].
Hydroxyl radical formation
HNO has been suggested to produce hydroxyl radical activity either via a secondary decomposition pathway of the hyponitrous acid formed upon HNO dimerization [68] or by elimination from the hyponitrous radical formed from the reaction of HNO with trace NO [63]. To examine whether NAD(P)H oxidation was mediated through ●OH-type chemistry, the impact of mechanistically distinct ●OH scavengers was examined. Because neither methionine nor salicylate, at concentrations up to 10 mM, inhibited NADH oxidation (data not shown), it is concluded that significant ●OH is not produced in this system. This is consistent with the findings of Buchholz and Powell [68], who reported that a radical mechanism is promoted only in the presence of metal ion or nitrous acid.
Thermodynamic favorability of the reaction of HNO with thiols
After the initial association of HNO with a thiol, the resulting N-hydroxysulfenamide (RSNHOH) is short-lived and can either react with an additional thiol equivalent to form the disulfide and NH2OH [45,69] or isomerize to a sulfinamide [45]. Together, these pathways account for the modifications of thiol-dependent proteins by HNO, which can be either reversible or irreversible by dithiothreitol [17].
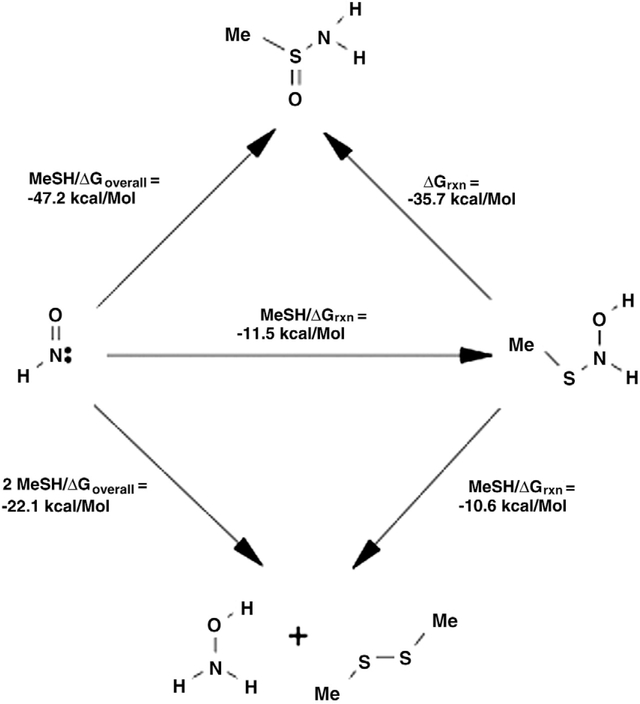
The relative changes in free energy for these reactions of HNO with a simple thiol have been calculated (Fig. 3). The formation of N-hydroxysulfenamide was found to be thermodynamically favorable (ΔGaq of −11.5 ± 1 kcal/mol). Additionally, both the subsequent reaction with thiol (to give the disulfide and hydroxylamine) and the rearrangement to sulfinamide were calculated to be exergonic (ΔGaq of −10.6 and −35.7 ± 1 kcal/mol, respectively).
Fig. 3.
Thermodynamic scheme describing reactions of HNO with thiol (B3LYP/6–31G(d) method with solvation via SM6 model).
Discussion
In this study we demonstrate that HNO can react with several biological reductants with rate constants that would predict potential biological relevance. The reductant we utilized as a primary tool of investigation was NADH. Wink and coworkers first examined the reactivity of Angeli’s salt with NADPH [26] and indicated the presence of both aerobic and anaerobic pathways. Reif et al. [59] further concluded that nitroxyl was the actual oxidant of NADPH and that hydroxylamine was not likely to be one of the reduced products of HNO. Liochev and Fridovich have examined the NADPH/HNO reaction in some detail [49], including reaction rates and inhibition by nitroxyl scavengers. These studies provide conflicting estimates for the stoichiometry of the HNO/NAD(P)H reaction and, although reaction rates have been examined, no second-order rate constant was proposed. We have determined this rate constant to be on the order of 104 M−1 s−1 and also demonstrate as a corollary that the stoichiometry for the reaction is 1:1. In addition, our experimental data support the findings of Reif et al. [59] that HNO is the proximal oxidant, not peroxynitrite, and that hydroxylamine is apparently not formed as a product in this reaction.
The rate constants in Table 1 enable considerations of the relative contribution that these reductive pathways might make to HNO metabolism as a whole. A similar assessment, based on final product analysis after complete decomposition of HNO donor rather than initial rates of reaction, was performed by Miranda et al. for GSH, cysteine, and a short series of oxidants [1]. The rate constant for the reaction of HNO with GSH determined here is within fivefold of the previously published value (6×105M−1 s−1).We are therefore in agreement that among the common biological reductants, GSH is likely to be one of the predominant targets of HNO, based on the calculated rate constant and relatively high intracellular GSH concentrations.
The case of vitamin E (tocopherol) is more ambivalent. Studies here with the water-soluble analog trolox do not accurately account for the localization of tocopherol in lipid membranes [70]. Similar to NO and O2 [71], HNO is expected to partition into the membrane. The rate of the reaction of HNO with tocopherol in cells may thus be significantly higher than that reported here for trolox, which may define a lower limit for tocopherol reactivity.
It is possible that HNO and NO may be found in proximity to one another in chemical and biological settings; however, the relative amounts will differ greatly depending on formation conditions and the cellular environment [27]. As these two nitrogen oxide congeners are observed to have orthogonal biological properties [72], differing ratios of HNO to NO may be expected to stimulate markedly varying biochemical responses. Indeed, it has been previously noted that NO has a biphasic effect on product yields in reactions of HNO with benzoic acid or NADH [63]. Similarly, varying NO:HNO ratios produced opposing effects on the activity of a copper-thiolate-dependent transcription factor, Ace-1 [13]. Additionally, Miranda and coworkers recently invoked an HNO/NO-dependent reaction sequence to explain the scavenging effects of superoxide dismutase [1].
The reaction of HNO with NO initially produces HN2O2, which at pH 7.4 largely exists as the deprotonated species, N2O2−. This anion can either react with an additional NO to produce N3O3−, which is a relatively unreactive species, or carry out chemistry of its own. To account for the observed increase in oxidative capacity exhibited by combinations of HNO with low amounts of NO, Kirsch et al. [63] have proposed elimination of ●OH from HN2O2. However, at physiological pH the ratio of HN2O2 to N2O2− will not exceed 1/100 [53] and will perhaps be as low as 1/10,000 [73], thus limiting the pathway for ●OH release from HN2O2 (N2O2− is not likely to eliminate the oxyl radical anion, O●−) [55]. Additionally, although elimination of ●OH from HN2O2 is thermodynamically favorable (ΔGaq of −28.1 kcal/mol [63]), Poskrebyshev has shown that both HN2O2 and N2O2− are kinetically stable to self-decomposition and are more likely to participate in redox reactions [53]. We thus propose that at lower NO concentrations, HNO reacts with NO to form N2O2−, which instead of subsequently producing OH, directly oxidizes NADH with a rate constant greater than that of HNO. We are in agreement with Kirsch and de Groot [63] regarding the inhibition of oxidation at higher ratios of NO to HNO, namely that the hyponitrite radical is itself trapped by additional NO to form N3O3−, which is not reactive in this system. DYNAFIT predictions based upon data like that shown in Fig. 1B (inset) led to a calculated rate constant of ∼1×106M−1 s−1 for the reaction of N2O2− with NADH. Although there is significant error (±30%) associated with this prediction, the proposed reaction sequence generally describes the changes in the rate of NADH oxidation after concomitant exposure to HNO and NO.
The biological relevance of N2O2−-mediated oxidation relies on the likelihood that it can be generated from simultaneous and localized NO and HNO production. Although it is not established that HNO is generated endogenously, its possible pharmacological utility (vide supra) makes its interaction with endogenously generated NO a distinct possibility. Clearly, the presence of HNO and NO together will divert both from their respective biological targets (i.e., soluble guanylate cyclase for NO and thiol proteins for HNO) to form an oxidant that can react with cellular reductants. Clearly, this will be an important factor in evaluating the pharmacology/toxicology of HNO donors as drugs.
The kinetic and thermodynamic data presented here support the growing consensus that thiols are a primary target of HNO in vivo. Of the two known reactions of HNO with thiols (Fig. 3), sulfinamide formation was calculated to be thermodynamically more favorable than disulfide production. This may have significant implications for the biological effects of HNO. However, it is likely the reaction pathway chosen will be highly dependent on the proximity of other reactive thiols, allowing disulfide formation to be competitive with the thermodynamically favorable sulfinamide pathway. Regardless, sulfinamide formation from glutathione and proteins after treatment with an HNO donor has been recently shown [74,75]. It is difficult to speculate on the biological relevance of the initial adduct of the HNO and RSH reaction, as few published data address RSNHOH directly. However, both the N-hydroxysulfenamide and the sulfinamide products are intriguing as protein thiol modifications. For example, a putative N-hydroxysulfenamide form of glyceraldehyde-3-phosphate dehydrogenase (GAPDH)was shown to have inhibited dehydrogenase activity but increased acylphosphatase activity [76]. The acylphosphatase activity of GAPDH has been linked to a futile cycle that increases glycolytic flux but decreases energy yield by hydrolyzing the product of its own dehydrogenase reaction [77]. We, and others, have seen that HNO is indeed a potent inhibitor of GAPDH dehydrogenase activity in vitro [78] and in yeast cells [79], but it remains to be seen whether HNO can induce acylphosphatase activity and futile cycling.
Of note, yeast cells obligatorily dependent upon glycolysis were more susceptible to HNO toxicity than respiring cells [79]. Additionally, HNO has been shown to be more toxic to cancer cell lines, which are strongly dependent upon glycolysis, than to non-cancer cell types [80]. This may have important implications for treatments in which the mechanistic basis for therapy rests upon the modification of glycolysis.
Rearrangement of the N-hydroxysulfenamide to the corresponding sulfinamide may be relevant in the peroxiredoxin H2O2 scavenging system. Sulfinamides are relatively stable at pH 7.4 [81], but could potentially be subject to hydrolysis in acidic subcellular compartments or by cellular amidases and esterases to form the corresponding sulfinic acids. The discovery of a sulfinate reductase [82], which acts upon peroxiredoxin sulfinates, led to the hypothesis that this “overoxidation” acts as a molecular floodgate allowing peroxide-dependent signal transduction to occur [83]. Whereas the reaction of thiol with H2O2 to form a sulfinate is second order in H2O2, the reaction of HNO with RSH is first order in oxidant. To the best of our knowledge, HNO is the only species that can form the sulfinic oxidation state (RS(O)NH2, RSO2H) via reaction of a thiol with only one equivalent of oxidant. Thus, HNO is a potentially valuable tool in the investigation of signal transduction. It is also interesting to note that HNO may potentially alter the redox state of the NADPH/NADP+ couple either by direct oxidation of NADPH or by oxidizing cellular thiols, leading to indirect NADPH oxidation as cellular glutathione and thioredoxin reductases reduce the disulfides so formed.
In summary, with the intracellular environment being generally reducing in nature, reduction of HNO might be a facile, if not the primary, metabolic event. The significance of these findings lies in the possibility that the conversion of HNO to a reduced species may account in part for the observed pharmacological effects of HNO donors. Clearly, it will be important for future work in this area to include the possibility of reduced species in the overall biology of HNO. Significantly, NH2OH, one of the most likely reduction products, has recently been shown to be capable of being oxidized by peroxidases to HNO [84], allowing for a possible recycling mechanism involving reducing species (e.g., thiols), H2O2, and HNO/NH2OH.
Acknowledgments
This work was funded by grants from the National Science Foundation (0096380; J.M.F.), the USPHS National Research Service Award (GM08496; A.D.), and the National Institute of General Medical Sciences, National Institutes of Health (GM 59446; K.N.H.).
References
- [1].Miranda KM; Paolocci N; Katori T; Thomas DD; Ford E; Bartberger MD; Espey MG; Kass DA; Feelisch M; Fukuto JM; Wink DA A biochemical rationale for the discrete behavior of nitroxyl and nitric oxide in the cardiovascular system. Proc. Natl. Acad. Sci. USA 100:9196–9200; 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Paolocci N; Jackson MI; Lopez BE; Miranda K; Tocchetti GC; Wink DA; Hobbs AJ; Fukuto JM The pharmacology of nitroxyl and its therapeutic potential: not just the Janus face of NO. Pharmacol. Ther. 113:442–458; 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Smith PAS; Hein GE The alleged role of nitroxyl in certain reactions of aldehydes and alkyl halides. J. Am. Chem. Soc. 82:5731–5740; 1960. [Google Scholar]
- [4].Kohout FC; Lampe FW On the role of the nitroxyl molecule in the reaction of hydrogen atoms with nitric oxide. J. Am. Chem. Soc. 87:5795–5796; 1965. [Google Scholar]
- [5].Bonner FT; Ravid B Thermal-decomposition of oxyhyponitrite (sodium trioxodinitrate) in aqueous-solution. Inorg. Chem. 14:558–563; 1975. [Google Scholar]
- [6].Smith JA;Wolford JA;Weber M; McLean D Use of citrated calcium carbimide (temposil) in treatment of chronic alcoholism. J. Am. Med. Assoc. 165:2181–2183; 1957. [DOI] [PubMed] [Google Scholar]
- [7].DeMaster EC; Shirota FN; Nagasawa HT The metabolic activation of cyanamide to an inhibitor of aldehyde dehydrogenase is catalyzed by catalase. Biochem. Biophys. Res. Commun. 122:358–365; 1984. [DOI] [PubMed] [Google Scholar]
- [8].Paolocci N; Katori T; Champion HC; St. John ME; Miranda KM; Fukuto JM; Wink DA; Kass DA Positive inotropic and lusitropic effects of HNO/NO− in failing hearts: independence from beta-adrenergic signaling. Proc. Natl. Acad. Sci. USA 100:5537–5542; 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Irvine JC; Favaloro JL; Kemp-Harper BK NO− activates soluble guanylate cyclase and Kv channels to vasodilate resistance arteries. Hypertension 41: 1301–1307; 2003. [DOI] [PubMed] [Google Scholar]
- [10].Fukuto JM; Chiang K; Hszieh R; Wong P; Chaudhuri G The pharmacological activity of nitroxyl: a potent vasodilator with activity similar to nitric oxide and/or endothelium-derived relaxing factor. J. Pharmacol. Exp. Ther. 263: 546–551; 1992. [PubMed] [Google Scholar]
- [11].De Witt BJ; Marrone JR; Kaye AD; Keefer LK; Kadowitz PJ Comparison of responses to novel nitric oxide donors in the feline pulmonary vascular bed. Eur. J. Pharmacol. 430:311–315; 2001. [DOI] [PubMed] [Google Scholar]
- [12].Cheong E; Tumbev V; Abramson J; Salama G; Stoyanovsky DA Nitroxyl triggers Ca2+ release from skeletal and cardiac sarcoplasmic reticulum by oxidizing ryanodine receptors. Cell Calcium 37:87–96; 2005. [DOI] [PubMed] [Google Scholar]
- [13].Cook NM; Shinyashiki M; Jackson MI; Lal FA; Fukuto JM Nitroxyl-mediated disruption of thiol proteins: inhibition of the yeast transcription factor Ace1. Arch. Biochem. Biophys. 410:89–95; 2003. [DOI] [PubMed] [Google Scholar]
- [14].Aniruddha S; Vidwans TFU; Hewett JA; Hewett SJ Differential modulation of prostaglandin H synthase-2 by nitric oxide-related species in intact cells. Biochemistry 40:11533–11542; 2001. [DOI] [PubMed] [Google Scholar]
- [15].Naughton P; Foresti R; Bains SK; Hoque M; Green CJ; Motterlini R Induction of heme oxygenase 1 by nitrosative stress: a role for nitroxyl anion. J. Biol. Chem. 277:40666–40674; 2002. [DOI] [PubMed] [Google Scholar]
- [16].Costa G; Labadia A; Triguero D; Jimenez E; Garcia-Pascual A Nitrergic relaxation in urethral smooth muscle: involvement of potassium channels and alternative redox forms of NO. Naunyn Schmiedebergs Arch. Pharmacol. 364: 516–523; 2001. [DOI] [PubMed] [Google Scholar]
- [17].DeMaster EG; Redfern B; Nagasawa HT Mechanisms of inhibition of aldehyde dehydrogenase by nitroxyl, the active metabolite of the alcohol deterrent agent cyanamide. Biochem. Pharmacol. 200 (7–2015):55; 1998. [DOI] [PubMed] [Google Scholar]
- [18].Bae SY; Xu Q; Hutchinson D Colton, Y+ and Y+L arginine transporters in neuronal cells expressing tyrosine hydroxylase. Biochim. Biophys. Acta 1745: 65–73; 2005. [DOI] [PubMed] [Google Scholar]
- [19].Paolocci N; Saavedra WF; Miranda KM; Martignani C; Isoda T; Hare JM; Espey MG; Fukuto JM; Feelisch M; Wink DA; Kass DA Nitroxyl anion exerts redox-sensitive positive cardiac inotropy in vivo by calcitonin gene-related peptide signaling. Proc. Natl. Acad. Sci. USA 98:10463–10468; 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Takahira R; Yonemura K; Fujise Y; Hishida A Dexamethasone attenuates neutrophil infiltration in the rat kidney in ischemia/reperfusion injury: the possible role of nitroxyl. Free Radic. Biol. Med. 31:809–815; 2001. [DOI] [PubMed] [Google Scholar]
- [21].Vanuffelen BE; Van Der Zee J; De Koster BM; Vansteveninck J; Elferink JG Intracellular but not extracellular conversion of nitroxyl anion into nitric oxide leads to stimulation of human neutrophil migration. Biochem. J. 330 (Pt. 2): 719–722; 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Vaananen AJ; Liebkind R; Kankuri E; Liesi P; Rauhala P Angeli’s salt and spinal motor neuron injury. Free Radic. Res. 38:271–282; 2004. [DOI] [PubMed] [Google Scholar]
- [23].Doyle MP; Mahapatro SN Nitric oxide dissociation from trioxodinitrate(II) in aqueous solution. J. Am. Chem. Soc. 106:3678–3679; 1984. [Google Scholar]
- [24].Maraj SR; Khan S; Cui XY; Cammack R; Joannou CL; Hughes MN Interactions of nitric oxide and redox-related species with biological targets. Analyst 3:699–703; 1995. [Google Scholar]
- [25].Ohshima H; Gilibert I; Bianchini F Induction of DNA strand breakage and base oxidation by nitroxyl anion through hydroxyl radical production. Free Radic. Biol. Med. 26:1305–1313; 1999. [DOI] [PubMed] [Google Scholar]
- [26].Wink DA; Feelisch M; Fukuto J; Christodoulou D; Jourd’heuil D; Grisham M; Vodovotz V; Cook JA; Krishna M; DeGraff W; Kim S; Gamson J; Mitchell JB The cytotoxicity of nitroxyl: possible implications for the pathophysiological role of NO. Arch. Biochem. Biophys. 351:66–74; 1998. [DOI] [PubMed] [Google Scholar]
- [27].Fukuto JM; Hobbs AJ; Ignarro LJ Conversion of nitroxyl (HNO) to nitric oxide (NO) in biological systems: the role of physiological oxidants and relevance to the biological activity of HNO. Biochem. Biophys. Res. Commun. 196: 707–713; 1993. [DOI] [PubMed] [Google Scholar]
- [28].Bartberger MD; Fukuto JM; Houk KN On the acidity and reactivity of HNO in aqueous solution and biological systems. Proc. Natl. Acad. Sci. USA 98:2194–2198; 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Shafirovich V; Lymar SV Nitroxyl and its anion in aqueous solutions: spin states, protic equilibria, and reactivities toward oxygen and nitric oxide. Proc. Natl. Acad. Sci. USA 99:7340–7345; 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Dutton AS; Fukuto JM; Houk KN Theoretical reduction potentials for nitrogen oxides from CBS-QB3 energetics and (C)PCM solvation calculations. Inorg. Chem. 44:4024–4028; 2005. [DOI] [PubMed] [Google Scholar]
- [31].Goldstein S; Merenyi G; Russo A; Samuni A The role of the oxoammonium cation in the SOD-mimic activity of cyclic nitroxides. J. Am. Chem. Soc. 125: 789–795; 2002. [DOI] [PubMed] [Google Scholar]
- [32].Kernag CA; Bobbitt JM; McGrath DV Mild and convenient oxidation of aromatic heterocyclic primary alcohols by 4-acetylamino-2,2,6,6-tetramethylpiperidine-1-oxoammonium perchlorate. Tetrahedron Lett. 40:1635–1636; 1999. [Google Scholar]
- [33].Mao YZ; Jin MZ; Liu ZL; Wu LM Oxidative reactivity of S-nitrosoglutathione with Hantzsch 1,4-dihydropyridine. Org. Lett. 2:741–742; 2000. [DOI] [PubMed] [Google Scholar]
- [34].Haqqani AS; Do SK; Birnboim HC The role of a formaldehyde dehydrogenase–glutathione pathway in protein S-nitrosation in mammalian cells. Nitric Oxide 9:172–181; 2003. [DOI] [PubMed] [Google Scholar]
- [35].Holmes AJ; Williams DLH Reaction of ascorbic acid with S-nitrosothiols: clear evidence for two distinct reaction pathways. Perkin Trans. 2 (8):1639–1644; 2000. [Google Scholar]
- [36].Kuzmic P Program DYNAFIT for the analysis of enzyme kinetic data: application to HIV proteinase. Anal. Biochem. 237:260–273; 1996. [DOI] [PubMed] [Google Scholar]
- [37].Kaplan NO; Colowick SP; Barnes CC The effect of alkali on diphospho pyridine nucleotide. J. Biol. Chem. 191:461–472; 1951. [PubMed] [Google Scholar]
- [38].Badrakhan CD; Petrat F; Holzhauser M; Fuchs A; Lomonosova EE; de Groot H; Kirsch M The methanol method for the quantification of ascorbic acid and dehydroascorbic acid in biological samples. J. Biochem. Biophys. Methods 58: 207–218; 2004. [DOI] [PubMed] [Google Scholar]
- [39].Magee WE; Burris RH Fixation of N2 and utilization of combined nitrogen by Nostoc muscorum. Am. J. Bot. 41:777–782; 1954. [Google Scholar]
- [40].Becke AD Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 98:5648–5652; 1993. [Google Scholar]
- [41].Frisch MJ; Trucks GW; Schlegel HB; Scuseria GE; Robb MA; Cheeseman JR; Montgomery JA Jr.; Vreven T; Kudin KN; Burant JC; Millam JM; Iyengar SS; Tomasi J; Barone V; Mennucci B; Cossi M; Scalmani G; Rega N; Petersson GA; Nakatsuji H; Hada M; Ehara M; Toyota K; Fukuda R; Hasegawa J; Ishida M; Nakajima T; Honda Y; Kitao O; Nakai H; Klene M; Li X; Knox JE; Hratchian HP; Cross JB; Bakken V; Adamo C; Jaramillo J; Gomperts R; Stratmann RE; Yazyev O; Austin AJ; Cammi R; Pomelli C; Ochterski JW; Ayala PY; Morokuma K; Voth GA; Salvador P; Dannenberg JJ; Zakrzewski VG; Dapprich S; Daniels AD; Strain MC; Farkas O; Malick DK; Rabuck AD; Raghavachari K; Foresman JB; Ortiz JV; Cui Q; Baboul AG; Clifford S; Cioslowski J; Stefanov BB; Liu G; Liashenko A; Piskorz P; Komaromi I; Martin RL; Fox DJ; Keith T; Al-Laham MA; Peng CY; Nanayakkara A; Challacombe M; Gill PMW; Johnson B; Chen W; Wong MW; Gonzalez C; Pople JA Gaussian 03, revision C.02. Gaussian, Inc., Wallingford, CT; 2004. [Google Scholar]
- [42].Lee C; Yang W; Parr RG Development of the Colle–Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 37:785–789; 1998. [DOI] [PubMed] [Google Scholar]
- [43].Montgomery JA; Ochterski JW; Peterson GA; complete basis set model chemistry, A.; An improved atomic pair natural orbital method, I. V. J. Chem. Phys. 101:5900–5909; 1994. [Google Scholar]
- [44].Kelly CP; Cramer CJ; Truhlar DG SM6: a density functional theory continuum solvation model for calculating aqueous solvation free energies of neutrals, ions, and solute-water clusters. J. Chem. Theory Comput. 1:1133–1152; 2005. [DOI] [PubMed] [Google Scholar]
- [45].Wong PSY; Hyun J; Fukuto JM; Shirota FN; DeMaster EG; Shoeman DW; Nagasawa HT Reaction between S-nitrosothiols and thiols: generation of nitroxyl (HNO) and subsequent chemistry. Biochemistry 37:5362–5371; 1998. [DOI] [PubMed] [Google Scholar]
- [46].Bari SE; Marti MA; Amorebieta VT; Estrin DA; Doctorovich F Fast nitroxyl trapping by ferric porphyrins. J. Am. Chem. Soc. 125:15272–15273; 2003. [DOI] [PubMed] [Google Scholar]
- [47].Liochev SI; Fridovich I Copper–zinc superoxide dismutase as a univalent NO− oxidoreductase and as a dichlorofluorescin peroxidase. J. Biol. Chem. 276: 35253–35257; 2001. [DOI] [PubMed] [Google Scholar]
- [48].Liochev SI; Fridovich I The mode of decomposition of Angeli’s salt (Na2N2O3) and the effects thereon of oxygen, nitrite, superoxide dismutase, and glutathione. Free Radic. Biol. Med. 34:1399–1404; 2003. [DOI] [PubMed] [Google Scholar]
- [49].Liochev SI; Fridovich I Nitroxyl (NO−): A substrate for superoxide dismutase. Arch. Biochem. Biophys. 402:166–171; 2002. [DOI] [PubMed] [Google Scholar]
- [50].Marti MA; Bari SE; Estrin DA; Doctorovich F Discrimination of nitroxyl and nitric oxide bywater soluble Mn(III) porphyrins. J. Am. Chem. Soc. 127:4680–4684; 2005. [DOI] [PubMed] [Google Scholar]
- [51].Dutton AS; Fukuto JM; Houk KN The mechanism of NO formation from the decomposition of dialkylamino diazeniumdiolates: density functional theory and CBS-QB3 predictions. Inorg. Chem. 43:1039–1045; 2004. [DOI] [PubMed] [Google Scholar]
- [52].Lymar SV; Shafirovich V; Poskrebyshev GA One electron reduction of aqueous nitric oxide: a mechanistic revision. Inorg. Chem. 44:5212–5221; 2005. [DOI] [PubMed] [Google Scholar]
- [53].Poskrebyshev GA; Shafirovich V; Lymar SV Hyponitrite radical, a stable adduct of nitric oxide and nitroxyl. J. Am. Chem. Soc. 126:891–899; 2004. [DOI] [PubMed] [Google Scholar]
- [54].Dutton AS; Fukuto JM; Houk KN Theoretical reduction potentials for nitrogen oxides from CBS-QB3 energetics and (C)PCM solvation calculations. Inorg. Chem. 44:7687–7688; 2005. [DOI] [PubMed] [Google Scholar]
- [55].Poskrebyshev GA; Shafirovich V; Lymar SV Disproportionation pathways of aqueous hyponitrite radicals (HN2O2/N2O2−). J. Phys. Chem. 112:8295–8302; 2008. [DOI] [PubMed] [Google Scholar]
- [56].Kelly CP; Cramer CJ; Truhlar DG SM6: a density functional theory continuum solvation model for calculating aqueous solvation free energies of neutrals, ions and solute-water clusters. J. Chem. Theor. Comput. 1:1133–1152; 2005. [DOI] [PubMed] [Google Scholar]
- [57].Bonner FT; Dzelzkalns LS; Bonucci JA Properties of nitroxyl as intermediate in the nitric oxide-hydroxylamine reaction and in trioxodinitrate decomposition. Inorg. Chem. 17:2487–2494; 1978. [Google Scholar]
- [58].Stojanovic S; Stanic D; Nikolic M; Spasic M; Niketic V Iron catalyzed conversion of NO into nitrosonium (NO+) and nitroxyl (HNO/NO−) species. Nitric Oxide 11:256–262; 2004. [DOI] [PubMed] [Google Scholar]
- [59].Reif A; Zecca L; Riederer P; Feelisch M; Schmidt HH Nitroxyl oxidizes NADPH in a superoxide dismutase inhibitable manner. Free Radic. Biol. Med. 30:803–808; 2001. [DOI] [PubMed] [Google Scholar]
- [60].Schafer FQ; Buettner GR Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic. Biol. Med. 30:1191–1212; 2001. [DOI] [PubMed] [Google Scholar]
- [61].Brumaghim JL; Li Y; Henle E; Linn S Effects of hydrogen peroxide upon nicotinamide nucleotide metabolism in Escherichia coli: changes in enzyme levels and nicotinamide nucleotide pools and studies of the oxidation of NAD(P)H by Fe (III). J. Biol. Chem. 278:42495–42504; 2003. [DOI] [PubMed] [Google Scholar]
- [62].Miranda KM; Dutton AS; Ridnour LA; Foreman CA; Ford E; Paolocci N; Katori T; Tocchetti CG; Mancardi D; Thomas DD; Espey MG; Houk KN; Fukuto JM; Wink DA Mechanism of aerobic decomposition of Angeli’s salt (sodium trioxodinitrate) at physiological pH. J. Am. Chem. Soc. 127:722–731; 2005. [DOI] [PubMed] [Google Scholar]
- [63].Kirsch M; de Groot H Formation of peroxynitrite from reaction of nitroxyl anion with molecular oxygen. J. Biol. Chem. 277:13379–13388; 2002. [DOI] [PubMed] [Google Scholar]
- [64].Miranda KM; Yamada K; Espey MG; Thomsa DD; DeGraff W; Mitchell JB; Krishna MC; Colton CA; Wink DA Further evidence for distinct reactive intermediates from nitroxyl and peroxynitrite: effects of buffer composition on the chemistry of Angeli’s salt and synthetic peroxynitrite. Arch. Biochem. Biophys. 401:134–144; 2002. [DOI] [PubMed] [Google Scholar]
- [65].Kobayashi K; Miki M; Tagawa S Kinetic behavior of nitric oxide studied by pulse radiolysis. Furi Rajikaru no Rinshu 10:78–84; 1996. [Google Scholar]
- [66].Uppu RM; Pryor WA Carbon dioxide catalysis of the reaction of peroxynitrite with ethyl acetoacetate: an example of aliphatic nitration by peroxynitrite. Biochem. Biophys. Res. Commun. 229:764–769; 1996. [DOI] [PubMed] [Google Scholar]
- [67].Katori T; Donzelli S; Tocchetti CG; Miranda KM; Cormaci G; Thomas DD; Ketner EA; Lee MJ; Mancardi D; Wink DA; Kass DA; Paolocci N Peroxynitrite and myocardial contractility: in vivo versus in vitro effects. Free Radic. Biol. Med. 41:1606–1618; 2006. [DOI] [PubMed] [Google Scholar]
- [68].Bucholz J; Powell R The decomposition of hyponitrous acid. II. The chain reaction. J. Am. Chem. Soc. 87:2350–2353; 1965. [Google Scholar]
- [69].Doyle MP; Mahapatro SN; Broene RD; Guy JK Oxidation and reduction of heme proteins by trioxodinitrate(II): the role of nitrosyl hydride and nitrite. J. Am. Chem. Soc. 110:593–599; 1988. [Google Scholar]
- [70].Fragata M; Bellemare F Model of singlet oxygen scavenging by [alpha]-tocopherol in biomembranes. Chem. Phys. Lipids 27:93–99; 1980. [Google Scholar]
- [71].Liu X; Miller MJ; Joshi MS; Thomas DD; Lancaster JR Jr. Accelerated reaction of nitric oxide with O2 within the hydrophobic interior of biological membranes. Proc. Natl. Acad. Sci. USA 95:2175–2179; 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Wink DA; Miranda KM; Katori T; Mancardi D; Thomas DD; Ridnour L; Espey MG; Feelisch M; Colton CA; Fukuto JM; Pagliaro P; Kass DA; Paolocci N Orthogonal properties of the redox siblings nitroxyl and nitric oxide in the cardiovascular system: a novel redox paradigm. Am. J. Phys. Heart Circ. Phys. 285:H2264–H2276; 2003. [DOI] [PubMed] [Google Scholar]
- [73].Seddon WA; Fletcher JW; Sopchyshyn FC Pulse radiolysis of nitric oxide in aqueous solution. Can. J. Chem. 51:1123–1130; 1973. [Google Scholar]
- [74].Shen B; English AM Mass spectrometric analysis of nitroxyl-mediated protein modification: comparison of products formed with free and protein-based cysteines. Biochemistry 44:14030–14044; 2005. [DOI] [PubMed] [Google Scholar]
- [75].Donzelli S; Espey MG; Thomas DD; Mancardi D; Tocchetti CG; Ridnour LA; Paolocci N; King SB; Miranda KM; Lazzarino G; Fukuto JM; Wink DA Discriminating formation of HNO from other reactive nitrogen oxide species. Free Radic. Biol. Med. 40:1056–1066; 2005. [DOI] [PubMed] [Google Scholar]
- [76].Allison WS; Connors MJ The activation and inactivation of the acyl phosphatase activity of glyceraldehyde-3-phosphate dehydrogenase. Arch. Biochem. Biophys. 136:383–391; 1970. [DOI] [PubMed] [Google Scholar]
- [77].Arutyunov DY; Muronetz VI The activation of glycolysis performed by the non-phosphorylating glyceraldehyde-3-phosphate dehydrogenase in the model system. Biochem. Biophys. Res. Commun. 300:149–154; 2003. [DOI] [PubMed] [Google Scholar]
- [78].DeMaster EG; Kaplan E; Shirota FN; Nagasawa HT Metabolic activation of cyanamide by liver mitochondria, a requirement for the inhibition of aldehyde dehydrogenase enzymes. Biochem. Biophys. Res. Commun. 107:1333–1339; 1982. [DOI] [PubMed] [Google Scholar]
- [79].Lopez BE; Rodriguez CE; Pribadi M; Cook NM; Shinyashiki M; Fukuto JM Inhibition of yeast glycolysis by nitroxyl (HNO): a mechanism of HNO toxicity and implications to HNO biology. Arch. Biochem. Biophys. 442:140–148; 2005. [DOI] [PubMed] [Google Scholar]
- [80].Stoyanovsky DA; Schor NF; Nylander KD; Salama G Effects of pH on the cytotoxicity of sodium trioxodinitrate (Angeli’s salt). J. Med. Chem. 47:210–217; 2004. [DOI] [PubMed] [Google Scholar]
- [81].Zenser TV; Lakshmi VM; Hsu FF; Davis BB Methemoglobin oxidation of Nacetylbenzidine to form a sulfinamide. Drug Metab. Dispos. 29:401–406; 2001. [PubMed] [Google Scholar]
- [82].Biteau B; Labarre J; Toledano MB ATP-dependent reduction of cysteine–sulphinic acid by S. cerevisiae sulphiredoxin. Nature 425:980–984; 2003. [DOI] [PubMed] [Google Scholar]
- [83].Rhee SG; Kang SW; Jeong W; Chang TS; Yang KS;Woo HA Intracellular messenger function of hydrogen peroxide and its regulation by peroxiredoxins. Curr. Opin. Cell Biol. 17:183–189; 2005. [DOI] [PubMed] [Google Scholar]
- [84].Donzelli S; Espey MG; Flore-Santana W; Switzer CH; Yeh GC; Huang J; Stuehr DJ; King SB; Miranda KM; Wink DA Generation of nitroxyl by heme protein-mediated peroxidation of hydroxylamine but not N-hydroxy-L-arginine. Free Radic. Biol. Med. 45:578–584; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Rabai G; Epstein IR Systematic design of chemical oscillators. 63. Large amplitude pH oscillation in the oxidation of hydroxylamine by iodate in a continuous-flow stirred tank reactor. J. Phys. Chem. 94:6361–6365; 1990. [Google Scholar]