Abstract
We have recently reported that the sublingual (s.l.) mucosa is an efficient site for inducing systemic and mucosal immune responses. In this study, the potential of s.l. immunization to induce remote Ab responses and CD8+ cytotoxic responses in the female genital tract was examined in mice by using a nonreplicating Ag, OVA, and cholera toxin (CT) as an adjuvant. Sublingual administration of OVA and CT induced Ag-specific IgA and IgG Abs in blood and in cervicovaginal secretions. These responses were associated with large numbers of IgA Ab-secreting cells (ASCs) in the genital mucosa. Genital ASC responses were similar in magnitude and isotype distribution after s.l., intranasal, or vaginal immunization and were superior to those seen after intragastric immunization. Genital, but not blood or spleen, IgA ASC responses were inhibited by treatment with anti-CCL28 Abs, suggesting that the chemokine CCL28 plays a major role in the migration of IgA ASC progenitors to the reproductive tract mucosa. Furthermore, s.l. immunization with OVA induced OVA-specific effector CD8+ cytolytic T cells in the genital mucosa, and these responses required coadministration of the CT adjuvant. Furthermore, s.l. administration of human papillomavirus virus-like particles with or without the CT adjuvant conferred protection against genital challenge with human papillomavirus pseudovirions. Taken together, these findings underscore the potential of s.l. immunization as an efficient vaccination strategy for inducing genital immune responses and should impact on the development of vaccines against sexually transmitted diseases.
The genital mucosa constitutes a major portal of transmission of infections such as syphilis, genital herpes, human papillomavirus (HPV),3 AIDS, Chlamydia, chancroid, and gonorrhea. To date, only two injectable vaccines against a sexually transmissible disease, genital human papillomavirus infection, have been registered recently and appear to confer protection by genital IgG Abs derived from the circulation (1, 2). An ideal vaccine against genital infections should also induce mucosal immune responses and local immunological memory, attributes that are generally better achieved by mucosal immunization (3–6).
IgG Abs predominate over secretory IgA Abs in cervicovaginal secretions (7), but the respective roles of these Ab isotypes in protection against sexually transmitted pathogens is still debated. These genital Abs can be produced locally, but a significant proportion is derived by transudation from blood. Mucosal T cells and, notably, CTLs may also play a critical role in the clearance of sexually transmitted intracellular pathogens (8–12).
Given the high degree of compartmentalization of the secretory immune system, selection of appropriate routes or combinations of immunization routes must be given special attention when designing vaccines that provide the right type of immunity at the desired body site. All licensed mucosal vaccines, except for only one intranasal (i.n.) live-attenuated influenza vaccine, are given orally and induce protective immune responses in the intestinal tract (3) but in general fail to induce secretory Ab responses in genital secretions. In contrast to oral vaccination (13–15), i.n. vaccination induces strong serum Ab responses requiring lower vaccine doses and stimulates immune responses in a variety of mucosal tissues, including the reproductive tract mucosa (9, 13, 16). How such responses disseminate to the genital tract is by and large still unknown. Local intravaginal (i.vag.) immunization has been shown to induce genital Abs in humans and mice, but the effectiveness of this route depends on the host’s hormonal status (13, 17–19), and this mode of immunization faces obvious programmatic limitations for community-based vaccine campaigns. Interestingly, i.n. immunization has been shown to be effective in inducing strong IgA and IgG Abs in the genital tract (13, 16, 20). Compared with i.vag. immunization, i.n. immunization generally requires lower doses of Ag to induce genital Ab responses of the same magnitude and is theoretically less sensitive to the host hormonal status (18). However, the use of the i.n. route has raised certain concerns associated to the potential redirection of live-attenuated organisms or toxin-based adjuvants to the CNS (21–24).
We have recently reported that the sublingual (s.l.) mucosa is an efficient site for inducing broad spectrum immune responses, including secretory and systemic Ab responses and mucosal as well as systemic CTLs (25, 26). In this study, we present evidence that s.l. immunization of female mice with a nonreplicating Ag, OVA, and cholera toxin (CT) adjuvant induces remote mucosal immune responses associated with the migration of Ag-specific IgA Ab-secreting cells (ASCs) and CTLs in the genital tract mucosa. Furthermore, we show that HPV virus-like particles (VLPs) administered sublingually (s.l.) induced HPV-neutralizing Abs in serum, virus-specific Abs in genital tissues, and protection against genital challenge with HPV even in the absence of CT.
Materials and Methods
Mice
Female BALB/c and C57BL/6 mice were used at 6–8 wk of age. All mice were bred and maintained on an OVA-free diet and under specific pathogen-free conditions in the animal care facilities of Nice Medical School (Nice, France), the International Vaccine Institute (Seoul, South Korea), and the U.S. National Cancer Institute (Bethesda, MD) in accordance with national and international guidelines, and all experiments described in this article were approved by the respective ethical committees for animal experimentation.
Immunizations
Progesterone (Depo-Provera)-treated mice were used in all experiments. Grade V chicken egg OVA (Sigma-Aldrich) and CT (List Biological Laboratories) were diluted in isotonic, pyrogen-free saline. Unless otherwise indicated, OVA (200 μg) alone or admixed with CT (2 μg) was administered on days 0, 7 and 21, and mice were sacrificed 7 days after the last immunization. These doses were selected for inducing the most consistent serum Ab responses to OVA (25). For s.l. and i.n. immunization, mice were heavily anesthetized with ketamine/xylazine and a total volume of 5 μl of OVA solution with/without CT in saline was topically administered onto the s.l. mucosa or split into each nostril, respectively. Animals were maintained with the head placed in an anteflexion position for 30 min to keep the Ag solutions on the s.l. mucosa. Sham-treated control animals received 5 μl of saline solution. For i.vag. immunization, mice were given 20 μl of OVA admixed with CT in saline. For intragastric (i.g.) immunization, mice were given 0.2 ml of OVA (1 mg) admixed with CT (2 μg) in antacid buffer consisting of 3% (w/v) sodium bicarbonate and administered with a feeding needle.
For HPV vaccination, mice were immunized with HPV16 L1 VLPs on days 0, 7, and 21. HPV16 L1 VLPs were produced as described previously (27, 28). Anesthetized mice were s.l. administered 10 μl of saline containing either 5 μg of HPV16 L1 VLPs alone or admixed with 2 μg of CT. A separate group of mice received an i.m. injection of 50 μl of HPV16 L1 VLPs adsorbed with alum (Pierce).
Preparation of cell suspensions
Mice were anesthetized with pentobarbital and 125 IU of heparin (Sigma-Aldrich) in 0.2 ml saline was injected i.p. Blood was drawn directly from the heart and the mice were sacrificed by cervical dislocation. The vagina and uterus were dissected out and cut finely in RPMI 1640 medium containing heparin (1 IU/ml) before digestion for 1 h at 37°C with 2 mg/ml collagenase-dispase (Boehringer Mannheim) in RPMI 1640 medium (Invitrogen) supplemented with 0.1 mg/ml DNase 1 (Boehringer Mannheim). Single cell suspensions were collected by filtration through a cell strainer. Cell suspensions from spleen, submandibular lymph nodes (SMLNs), and ileosacral lymph nodes (ILNs) were obtained by pressing the organs through nylon sieves. All suspensions were freed from erythrocytes by treatment with ammonium chloride.
Measurement of specific Abs
Vaginal washes were collected from anesthetized mice by flushing 50 μl of PBS using a micropipette. Saliva was collected after i.p. injection of pilocarpine (100 μg per animal). All mucosal samples were clarified by centrifugation and immediately stored at −20°C. Blood was collected from the tail vein and plasma was separated by centrifugation and stored at −20°C. Plasma and mucosal secretions were assayed for Ab levels to CT, OVA, and HPV16 L1 VLPs by means of ELISA using HRP-conjugated, affinity-purified goat Abs to mouse IgG or IgA (Southern Biotechnology Associates) as detection reagents (29). The titer of a sample was defined as the reciprocal of the highest sample dilution yielding an absorbance value at least equal to 3-fold that of background. For each experimental group of mice, results were expressed as geometric mean Ab titer (GMT) + SD.
In vitro neutralization of HPV pseudovirions
The in vitro neutralization of HPV pseudovirions has been described previously (30). Sera from individual mice were collected and serial 4-fold dilutions were incubated at 4°C for 1 h with pseudovirions carrying pYSEAP and expressing secreted alkaline phosphatase (SEAP). The pseudovirus/sera mixtures were then used to infect 293TT cells. Supernatants were analyzed for SEAP activity after 72 h by using Great EscAPe SEAP chemiluminescence kit (Clontech) with minor modifications. Briefly, 15 μl of culture supernatant were mixed with 45 μl of 1× dilution buffer and incubated at 65°C for 30 min. SEAP substrate (45 μl) was added and incubated for 1 h at room temperature. The luminescent activity was measured using a microplate reader (POLARstar OPTIMA; BMG LABTECH) with the glow end point set at 0.20 s/well for an average reading from the handling of raw data. The neutralization titer was defined as the reciprocal of the highest dilution of serum yielding a 50% reduction in SEAP activity.
ELISPOT assays
Spleen, lymph node, and lung cell suspensions were assayed for numbers of specific ASCs by the ELISPOT assay (31, 32). Briefly, graded numbers of cells were incubated for 3 h at 37°C in nitrocellulose-bottomed 96-well plates previously coated with OVA (30 μg/ml) or the B subunit of CT (5 μg/ml). Plates were then washed extensively and wells were developed by stepwise incubations with HRP-conjugated goat Abs to mouse IgG and IgA (Southern Biotechnology Associates) and washing with wash buffer (PBS) followed by the addition of AEC-H2O2 (:Where AEC is 3-amino-9-ethylcarbazol) as a chromogenic substrate (Sigma-Aldrich) as previously described (13). Spots were enumerated using an automated reader.
Chemotaxis assay
Lymph node cells were isolated from the SMLNs and ILNs of mice immunized s.l. or i.vag., respectively. Cells were suspended in complete medium and allowed to rest for 1 h at 37°C with 5% CO2. Rested cells were placed in the upper chamber of an 8-μm Transwell plate (Corning Costar) at 106 cells per well. In some wells, 106 cells were placed directly in the lower chamber as a positive control for maximum migration. The lower chamber contained medium alone or medium containing chemokines at concentrations determined to be optimal (250 mM for CCL28, 300 nM for CCL25, and 100 nM for CXCL12). After incubation at 37 °C with 5% CO2 for 4 h, cells that had migrated were harvested from the lower chamber. The number of migrated Ag-specific IgA and IgG ASCs was determined by ELISPOT.
In vivo Ab-mediated inhibition of ASC migration
Groups of five female BALB/c mice were primed s.l. with 5 μg of CT. One month later, mice were boosted s.l. with 5 μg of CT. To neutralize CCL28 activity, mice were injected i.p. on day s1, 3, and 5 after a boost with 100 μg of rat monoclonal anti-CCL28 Ab or rat Ig2b isotype control (R&D Systems). On day 7 after the boost, mice were sacrificed and blood, genital tract, SMLNs, and spleen were harvested. The frequency of Ag-specific ASCs in these organs was determined by ELISPOT.
Cytokine assays
Triplicate cultures of SMLN or spleen cells were seeded at 4 × 105 cells per flat-bottom well of 96-well culture plates (Falcon; BD Biosciences) together with 1 × 105 accessory cells. Accessory cells were prepared by depleting naive syngeneic spleen cell suspensions with magnetic beads coated with anti-CD3 (clone KT3–1.1) and anti-rat Ig following the manufacturer’s (Dynal Biotech) protocol. After incubation at 37°C with 5% CO2 for 72 h in the presence or absence of 2 mg/ml OVA, culture supernatants were assayed for IFN-γ, IL-4, and IL-10 contents by means of calibrated ELISAs as described previously (33).
Flow cytometry detection of tissue CTLs
An in vivo CTL assay was performed essentially as described (34) with minor modifications. Spleen cell suspensions were prepared from C57BL/6 mice and split into two fractions. One fraction was labeled with 4 μM CFSE (nominal CFSEhigh cells) for 5 min at room temperature and pulsed for 45 min with 1 μM OVA257–264 SIINFEKL peptide (Proimmune) at 37°C with 5% CO2. The other cell fraction consisted of cells labeled with 0.4 M CFSE for 5 min at room temperature (nominal CFSElow cells) and without peptide pulse. A total of 15 × 106 cells comprising equal numbers of CFSEhigh and CFSElow cells in 200 μl of saline were injected i.v. into sham (control) mice and into mice that had been previously immunized with CT (2 μg) and/or OVA (200 μg). For detection of genital CTLs, 2 × 106 target cells were injected directly into the vaginal mucosa of anesthetized mice using an insulin syringe (35). Single cell suspensions from ILN, spleen, and genital tract mucosa were prepared 24 h after cell transfer and analyzed for differential CFSE staining by flow cytometry. The uterus and vagina were excised and digested for 45 min at 37°C with dispase II (1.2 U/ml in RPMI 1640) (Roche Diagnostics) before analysis. Specific killing activity was calculated according to the formula 1 × (ratio of CFSElow/CFSEhigh cells in control mice/ratio of CFSElow/CFSEhigh cells in immunized mice) × 100 and expressed as percentage of specific lysis.
Production of HPV16 pseudovirus
HPV16 pseudovirions containing the reporter plasmid pCLucf or pYSEAP, which encodes luciferase and GFP or a secreted form of alkaline phosphatase, respectively, was produced as described previously (27, 28). Detailed protocols and plasmid maps are accessible on the website of the Laboratory of Cellular Oncology, National Cancer Institute, National Institutes of Health (Bethesda, MD; home.ccr.cancer.gov/Lco/). Plasmid purity and capsid content were determined on 10% SDS-Tris-glycine gels (Bio-Rad). For HPV16 pseudovirions carrying the pCLucf plasmid, the infectious titer was determined by flow cytometry on 293TT cells 48 h postinfection. For HPV16 pseudovirions carrying the pYSEAP plasmid, infectivity was determined on 293TT cells by SEAP detection 72 h postinfection.
Mouse model of vaginal HPV16 infection
All mice were challenged for HPV genital infection 2 wk after the third immunization. The protocol for in vivo genital infection has been previously described in detail (36). Two weeks after the third immunization, mice were injected s.c. with 3 mg of medroxyprogesterone acetate (Depo-Provera) for 4 days before challenge with HPV pseudovirions. Mice were pretreated i.vag. with 50 μl of nonoxynol-9 (Conceptrol) to permeabilize the epithelium and challenged 5 h later with an i.vag. instillation of 2 × 107 IU of HPV pseudovirions diluted in carboxymethyl cellulose 2% (w/v). HPV infection was monitored by measuring luciferase expression in the genital tract on day 2 postchallenge. Anesthetized mice were instilled i.vag. with 20 μl of luciferine (Sigma-Aldrich) and imaged 3 min later during a 1-min exposure using a Xenogen IVIS in vivo imager (Caliper Science).
Statistical analyses
The corrected (Bonferroni) nonparametric Mann-Whitney U test was used for pair wise multiple comparisons between experimental groups. A value of p < 0.05 was considered significant.
Results
Sublingual administration of CT and OVA induces Ag-specific Abs in the cervicovaginal secretions
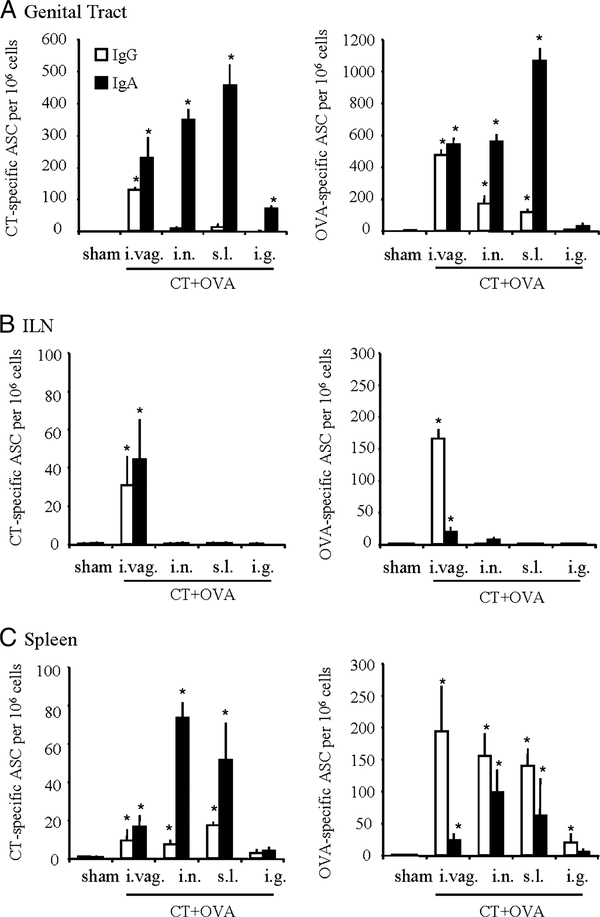
Groups of 8–15 mice were immunized s.l. with different doses of OVA (10, 50, and 200 μg) given together with a CT (2 μg) adjuvant, and the presence of specific Abs was examined in genital secretions 1 wk after the last of three consecutive immunizations (Fig. 1A). Genital IgA Ab responses to OVA were readily detected in mice immunized with 10 μg of OVA plus CT adjuvant, and these responses were comparable between animals immunized by the s.l. and the i.n. routes. Increased doses (50 and 200 μg) of OVA yielded more pronounced and more consistent responses (Fig. 1A). Genital Ab titers induced by s.l. immunization were comparable to those observed after i.vag. immunization of mice treated with progesterone but higher than those of untreated mice (Fig. 1B and data not shown). Furthermore, s.l. administration of OVA alone induced significant IgA and IgG Ab titers that were enlarged when OVA was coadministered with CT (Fig. 1B). Overall, s.l. immunization induced IgA Abs in genital secretions at levels comparable to those seen after i.n. or i.vag. immunization, but markedly higher than after i.g. immunization (Fig. 1B). Whereas OVA-specific Ab responses in blood were predominantly of the IgG class (Fig. 1C), genital secretions from animals immunized by the s.l. route with OVA and CT comprised comparable proportions of IgA and IgG Abs to OVA (Fig. 1B). Similar findings were obtained in animals immunized by either the i.n. or the i.vag. routes (Fig. 1, B and C). In contrast, i.g. immunization with OVA plus CT induced weak or negligible Ab responses in genital secretions (Fig. 1B).
FIGURE 1.
Sublingual immunization induces Ab responses in genital secretions. Mice were immunized by the s.l. or the i.n. route with various doses of OVA and CT (2 μg) adjuvant (A). For the results presented in B and C the mice were immunized by the s.l., i.n., i.vag., or i.g. routes with OVA (200 μg) alone or coadministered with CT (2 μg) in saline. Blood samples and vaginal washes were collected 1 wk after the third of three consecutive immunizations (days 0, 7, and 21) and samples were assayed for IgG and IgA Abs. Data are expressed as geometric mean titer (histogram bars) + SD (lines) and were determined from groups of 8–15 mice (three experiments). Asterisks (*) denote statistical (p < 0.05; Mann-Whitney U test) differences between immunized and sham-treated groups (A) and between mice immunized by the s.l., i.n., or i.vag. route and mice immunized by the i.g. route (B and C).
Consistent with our previous study (23), s.l. immunization with CT and OVA gave rise to specific Ab responses in blood, and these responses were comparable to those observed after i.n. or i.vag. immunization, but significantly stronger than after oral immunization (Fig. 1C). Sublingual immunization with CT also elicited high anti-CT IgA and IgG Ab titers in serum and genital secretions (data not shown).
Sublingual administration of CT and OVA induces Ag-specific ASCs in the genital tract mucosa but not in the ILN
To further determine to which extent genital Ab responses observed after s.l. immunization result from local Ab formation, ELISPOT analyses of enzymatically dispersed genital tissue specimens were conducted 7 days after the last of three immunizations with OVA plus CT. As shown in Fig. 2A, s.l. immunization induced large numbers of OVA-specific and CT-specific ASCs in the genital tract. These responses were markedly dominated by IgA ASCs and were comparable to or even higher than those seen after i.n. and i.vag. immunization (Fig. 2A). ILNs were devoid of ASCs after either s.l. or i.n. immunization with CT plus OVA but contained large numbers of IgG ASCs in animals immunized by the vaginal route (Fig. 2B). Splenic ASC responses to CT induced by s.l. immunization were comparable in magnitude and isotype distribution to the corresponding responses induced by i.n. or i.vag. immunization, being comprised of larger numbers of IgG ASCs, especially after i.vag. immunization (Fig. 2C). Surprisingly, spleen ASC responses to OVA were predominantly of the IgA isotype and were larger after i.n. and s.l. immunization than after i.vag. immunization. The i.g. administration of OVA plus CT failed to induce appreciable genital ASC responses and very weak splenic ASC responses.
FIGURE 2.
Sublingual immunization with CT and OVA induces systemic and genital Ab-secreting cells. Mice were immunized on days 0, 7, and 21 with saline by the s.l. route (sham) or with 200 μg of OVA together with 2 μg of CT given in saline by the s.l., i.n. i.vag., or i.g. routes and assayed 1 wk after the last immunization for OVA-specific IgA (filled bars) and IgG (open bars) ASCs in the genital tract (A), ILN (B), and spleen (C). Data are expressed as mean ASC numbers per million cells + S.D. (vertical bars) determined from groups of five to eight mice and are representative of three separate experiments. Asterisks denote significant differences between immunized mice and sham-immunized control mice (*, p < 0.05; Mann-Whitney U test).
These findings suggest that genital IgA Ab responses induced by s.l. immunization are to a large extent contributed by local ASCs, whereas IgG responses appear to be derived from an extragenital source, probably from blood and peripheral lymphoid tissues such as the spleen. Furthermore, these results indicate that genital IgA ASCs induced by s.l. and i.n. immunization do not originate from draining ILNs, contrary to IgA ASCs induced by local genital immunization.
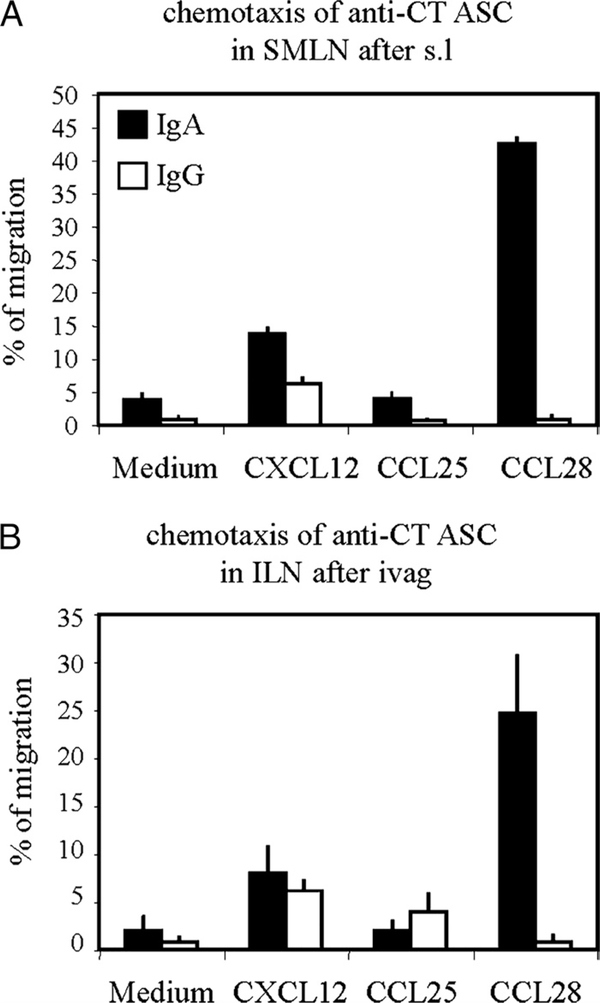
Sublingual immunization induces CCL28-dependent selective migration of IgA ASCs to the genital mucosa
The observation that IgA and IgG ASCs induced by s.l. immunization markedly differ with respect to their anatomic distribution suggested that IgA and IgG ASCs induced in lymph nodes draining the site of immunization, i.e., SMLNs, comprise ASC populations with different migratory properties. To address this issue, in vitro migration experiments were performed with SMLN cells collected after s.l. immunization with CT and subjected to various chemokine gradients. As seen in Fig. 3A, CT-specific IgA ASCs isolated from SMLNs migrated to CCL28, a common mucosa-associated epithelial chemokine known to selectively attract IgA plasmablasts (37). These ASCs failed to respond to CCL25, a chemokine mediating extravasation of IgA ASCs into the small intestine (38). In striking contrast, SMLN IgG ASCs failed to respond to CCL28 and CCL25 (Fig. 3A). In addition, IgG and IgA ASCs did not differ with regard to their potential to migrate against CXCL12. Because both IgA and IgG ASCs to CT could be induced in ILN by local i.vag. immunization (Fig. 2B), similar experiments were performed with ILN cells from i.vag. immunized mice. Fig. 3B shows that indeed CT-specific IgA ASCs but not IgG ASCs from ILNs obtained after i.vag. immunization with CT responded to CCL28, and both populations failed to respond to CCL25. These findings suggest that CCL28 attracts preferentially IgA ASCs formed in draining as well as in remote lymph nodes.
FIGURE 3.
Differential chemotactic responses of IgG and IgA ASCs after s.l. (A) or i.vag. (B) immunization. Mice were immunized on days 0, 7, and 21 with 2 μg of CT in saline by the s.l. or i.vag. route. Mononuclear cells isolated from draining SMLNs (A) and ILNs (B) were migrated to optimal concentrations of CCL28, CCL25, or CXCL12 chemokines. The numbers of migrated and input CT-specific IgG and IgA ASCs were determined by ELISPOT, and data are expressed as arithmetic mean percentages of migrated IgG (clear bar) and IgA (black bar) ASCs + SD, determined in triplicate Transwells and pooled from two separate experiments.
To further ascertain the role of CCL28 in the selective migration of IgA ASCs induced by s.l. immunization, separate groups of mice were immunized twice s.l. with CT and treated with 100 μg of CCL28 or isotype control Abs given on days 1, 3, and 5 after the second immunization. ASCs were then enumerated in vaginal, blood, and spleen cell suspensions on day 7 after s.l. booster immunization. As shown in Fig. 4, treatment with anti-CCL 28 inhibited the migration of IgA ASCs into the genital mucosa but had no effect on the migration of IgA ASCs (and IgG ASCs) into the blood or spleens of s.l. immunized mice (Fig. 4). Collectively, these results indicate that CCL28 plays a critical role in the selective homing of IgA ASCs into genital tissues.
FIGURE 4.
In vivo blockade of CCL28 inhibits expansion of IgA ASC in the genital tract. Separate groups of mice were s.l. immunized twice, 1 mo apart, with CT (5 μg) and treated with an anti-CCL28 mAb or an isotype control mAb given on days 1, 3, and 5 after the second immunization. On day 7 after a s.l. booster, CT-specific ASCs were enumerated by ELISPOT in cell suspensions from genital tissue (top left), SMLN (top right), spleen (bottom left), and blood (bottom right). Data represent mean CT-specific IgA ASC numbers + SD determined from groups of five mice in two separate experiments.
Sublingual immunization with CT and OVA induces mixed Th1 and Th2 T cell response
To examine the consequences of s.l. immunization on the subsequent polarization of T cells, mononuclear cells of the SMLN and spleen were prepared 1 wk after the last of three s.l. immunizations with OVA given alone or together with CT and stimulated in vitro with OVA. The s.l. immunization with OVA alone failed to induce detectable amounts of IL-10 and IFN-γ in the culture supernatants of mononuclear cells isolated from the SMLN or spleen in contrast to s.l. immunization with CT and OVA, which induced production of IL-10 and IFN-γ (Table I). These data indicate that s.l. immunization with CT as an adjuvant promotes mixed Th1 and Th2 responses to coadministered OVA Ag.
Table I.
Sublingual immunization with CT and OVA promotes Th1/Th2 cytokinea
| SMLN Cellsb |
Spleen Cellsb |
|||
|---|---|---|---|---|
| Immunization |
IFN-γ |
IL-10 |
IFN-γ |
IL-10 |
| Sham | <10c | <10c | <10c | <10c |
| OVA (s.l.) | <10c | 24 ± 15 | <10c | <10c |
| CT + OVA (s.l.) | 6919 ± 1603 | 813 ± 255 | 9413 ± 1792 | 467 ± 111 |
Mice were immunized on days 0, 7, and 21. One week after the last immunization, mononuclear cells from the SMLN or spleen were stimulated with OVA in vitro and 72-h supernatants were assayed for cytokine contents.
Values are expressed as mean cytokine concentration determined in triplicate cultures for each immunization group with four mice per group.
Not detectable.
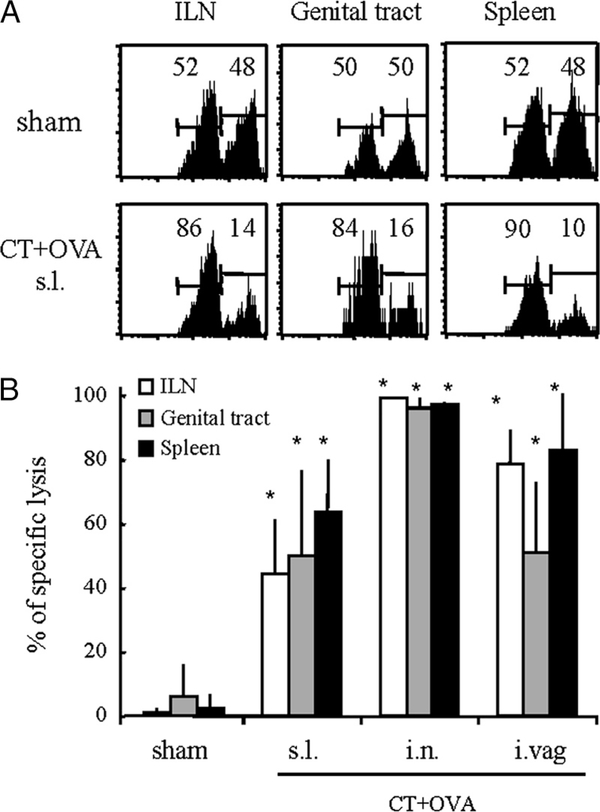
Sublingual immunization with CT and OVA induces remote mucosal CTLs
We next examined whether s.l. immunization could induce remote CD8+ T cells and cytolytic activity in the genital tract mucosa. A modified in vivo cytolysis assay in which labeled target spleen cells pulsed with a peptide entailing a prominent CTL epitope on OVA (OVA257–264) are directly injected into the vaginal wall was established for measuring putative genital CTL responses. In parallel, CTL responses were measured in spleen and ILN suspensions after i.v. injection of target cells. As shown in Fig. 5A, in vivo specific cytotoxic activity was readily detected in the genital tracts and ILNs of mice immunized s.l. with CT and OVA, and these responses were comparable to those seen after local i.vag. immunization and somewhat lower than after i.n. immunization (Fig. 5B). The s.l. immunization with CT and OVA also induced specific lysis in the SMLN (data not shown) and in the spleen (Fig. 5B). These data indicate that s.l. immunization can induce effector CTL responses in genital tissues.
FIGURE 5.
Sublingual immunization with CT and OVA induces effector CTLs in the genital tract mucosa. A, C57BL/6 mice were immunized on days 0, 7, and 21 with OVA given with CT. On day 28, mice were infused a mixture of CFSE-labeled target and control cells as described in Materials and Methods. Specific target cell killing was determined by flow cytometry analyses of cell suspensions from spleen, ILN, and genital mucosa. B, Comparative analyses of systemic and genital CTL responses induced by s.l., i.n., and i.vag. immunization. Data are expressed as mean percentage of specific killing + SEM determined from groups of 6–8 individual mice per experimental group and pooled from two separate experiments. Asterisks denote significant differences (*, p < 0.05; MannWhitney U test) between experimental and control (sham-treated) groups.
Sublingual vaccination with HPV VLPs induces serum neutralizing Abs and genital Ab responses
Prior studies have shown that HPV VLPs are not only potent immunogens for systemic immunization but are also strongly immunogenic when administered i.n. or in the lungs (39, 40).
Mice were immunized on days 0 and 7 s.l. with 5 μg of HPV16 VLP with or without CT or i.m. with 5 μg of HPV16 L1 VLP adsorbed with alum. On day 21, serum samples were collected and assayed for HPV16-neutralizing activity. Sera from mice immunized s.l. with HPV16 L1 VLP alone or in combination with CT had respectively moderate (geometric mean titer, 1114; range, 640–2560) to strong neutralization titers (geometric mean titer, 13511; range, 10240–40960) against HPV16 (Fig. 6A). Parenteral (i.m.) immunization with VLP plus alum induced significantly higher neutralizing titer (geometric mean titer, 31041; range, 10240–40960) than s.l. immunization with either VLP alone or VLP plus CT groups (Fig. 6A). IgG Ab responses to HPV16 L1 VLP were readily detected in mice immunized s.l. with VLP alone (geometric mean titer, 15136; range, 12150–36450), and these responses were enlarged in mice immunized with HPV16 L1 VLP adjuvanted with CT (geometric mean titer, 70,464; range, 36450–109350) (Fig. 6B). Vaginal IgA Abs were detected in mice immunized s.l. with HPV16 L1 VLP only (geometric mean titer, 173; range, 100–250) or together with CT (geometric mean titer, 302; range, 125–800). Of note, i.m. immunization with HPV16 L1 VLP and alum induced lower vaginal IgA responses (geometric mean titer, 17; range, 10–25) than those of s.l. immunized mice. Conversely, i.m. immunized mice exhibited stronger vaginal IgG Ab responses to HPV16 L1 VLP (geometric mean titer, 252; range, 30–1,200) than s.l. immunized mice. Furthermore, s.l. immunization with HPV VLP given alone or with CT adjuvant induced virus-neutralizing responses in genital secretions, and these responses were comparable to those evoked by i.m. immunization (Table II). Taken together, these results indicate that s.l. immunization induced both serum IgG and genital IgA Ab responses.
FIGURE 6.
Sublingual immunization with HPV VLP elicits serum-neutralizing Abs and anti-VLP IgA in cervicovaginal secretions. Mice were immunized on days 0 and 7 by the s.l. route with saline (sham), 2 μg of CT only, 5 μg of HPV16 L1 VLP only, or together with 2 μg of CT. A group of mice was immunized i.m. with 5 μg of HPV16 L1 VLP in 2 mg of alum (Pierce). Blood samples were collected 2 wk after the second immunization and sera were assayed for HPV-neutralizing Abs (A) and HPV16 L1 VLP-specific IgG Abs (B). Genital secretions collected 2 wk after the second immunization were assayed for HPV16 L1 VLP-specific IgA (filled histograms), and IgG (open histograms) Abs (C). Serum-neutralizing Ab levels are expressed as geometric mean titers giving 50% inhibition of infection as determined from five to seven mice per group (A). Serum and genital Ab levels are expressed as geometric mean end point titers as determined from groups of five to seven mice. Asterisks denote significant differences (*, p < 0.05; Mann-Whitney U test) between s.l. vs i.m. immunized groups.
Table II.
Sublingual immunization with HPV VLP elicits neutralizing Abs in cervicovaginal secretiona
| Neutralizing Ab Titer in Genital Secretionsb |
||
|---|---|---|
| Immunogen |
Geometric mean |
Range |
| Sham | <20 | NAc |
| CT | <20 | NAc |
| HPV16 L1 VLP (s.l.) | 46 | 20–80 |
| HPV16 L1 VLP + CT (s.l.) | 61 | 20–320 |
| HPV16 L1 VLP + alum (i.m.) | 32 | 20–80 |
Mice were immunized on days 0 and 7 by the s.l. route with saline (sham), 2μg of CT only, 5μg of HPV16 L1 VLP alone or together with 2 μg of CT. A group of mice was immunized i.m. with 5 μg of HPV16 L1 VLP in alum. Vaginal washes were collected 2 wk after the second immunization and assayed for neutralization activity.
Determined on groups of five mice.
Not applicable
Sublingual vaccination with HPV VLPs protects mice against genital challenge with HPV pseudovirions
A model mouse model of genital HPV infection based on the use of HPV pseudovirions carrying a luciferase reporter gene has been developed recently and recapitulates the early steps of HPV natural infection (36, 41). Mice were immunized on days 0, 7, and 21 and challenged i.vag. 2 wk after the last immunization with HPV16 pseudovirions carrying a luciferase gene. On day 2 after challenge the intensity of infection was monitored by measuring luciferase activity (expressed in photon/s (p/s)) in the genital area using a Xenogen IVIS imager. As illustrated in Fig. 7B, s.l. immunization with HPV16 VLPs given alone conferred complete protection against HPV16 genital infection compared with sham treated mice (Fig. 7A). Similar results were obtained in mice given s.l. VLP with CT adjuvant or i.m. VLP with alum. Thus, despite marked differences in the levels of serum and genital IgA and IgG Ab responses, s.l. immunization with VLPs even in the absence of CT adjuvant conferred protection as good as that of i.m. administration of the same dose of alum-adjuvanted VLPs (Fig. 7B).
FIGURE 7.
Sublingual immunization with HPV16 L1 VLP protects mice against genital challenge with HPV16 pseudovirions. Groups of 7–10 mice were immunized on days 0, 7, and 21 by the s.l. route with saline (sham), 2 μg of CT alone, 5 μg of HPV16 L1 VLP (16VLP) alone, or admixed with 2 μg of CT adjuvant. A group of 10 mice was immunized i.m. with 5 μg of HPV16 L1 VLP adsorbed with 2 mg of alum (Pierce) adjuvant. Two weeks after the last immunization, mice were challenge i.vag. with 2 ×107 IU of HPV16 pseudovirions carrying a luciferase gene. Luciferase expression was measured 24 h after genital challenge using an in vivo imager (IVIS). A, Visualization of luciferase expression is shown for sham-treated mice (top) and for mice immunized s.l. with VLP alone (bottom). B, Arithmetic mean numbers of infectious units (extrapolated from levels of luciferase activity) + SD are depicted and have been pooled from two experiments. Asterisks denote significant differences between groups of immunized vs sham-treated control animals (*, p < 0.01; Mann-Whitney U test).
Discussion
In this study, we show for the first time that s.l. immunization can induce secretory Ab responses and CTLs in the reproductive tract mucosa. We have previously shown that secretory Ab responses and bona fide mucosal CTLs can be induced in the respiratory mucosa after s.l. immunization (25). The present study further expands on these initial findings and demonstrates that, much in the same way as i.n. immunization, this route of vaccine administration is exceptionally potent for inducing disseminated mucosal effector B and T cell responses.
The magnitude and isotype distribution of genital Ab responses in animals immunized s.l., i.n., or i.vag. were comparable. The fact that IgA Ab responses dominated in genital secretions whereas IgG Abs were predominant in blood indicates that a proportion of these Abs are formed locally and not only result from the mere transudation of serum Abs through the genital epithelium. This interpretation is supported by the finding of large numbers of IgA ASCs, and to a lesser extent IgG ASCs, in genital tissues from s.l. (and for that matter also i.n. and i.vag.) immunized animals. It is also noteworthy that IgA ASCs predominated the genital ASC responses after s.l. immunization whereas IgG responses were predominant in serum. This suggests that genital IgA responses were contributed mainly by local ASCs, whereas the IgG Abs detected in genital secretions were to a large extent contributed by blood-derived Abs.
The fact that large numbers of specific ASCs could be detected in genital lymph nodes after local i.vag. immunization, confirming previous reports (13, 20), but not after s.l. (and i.n.) immunization suggests that genital ASCs induced by s.l. immunization originate from remote inductive site(s), presumably SMLN draining the sublingual mucosa. The finding that IgA ASCs predominated the genital response whereas the responses in SMLN comprised comparable numbers of IgA and IgG ASCs (23) suggests that IgA ASC precursors induced by s.l. immunization have unique migratory properties. The latter hypothesis is supported by the finding that SMLN IgA ASCs, but not IgG ASCs from s.l. immunized mice, migrated in vitro in response to CCL28, a major chemokine involved in the selective migration of IgA plasmablasts to mucosal tissues (37, 42, 43). Furthermore, in vivo treatment of mice with anti-CCL28 Ab markedly inhibited genital IgA ASC responses after s.l. immunization but had negligible if any effect on IgG and IgA ASC responses in the blood or spleen. Taken together, these results indicate that s.l. vaccination induces the migration of Ag-specific IgA ASCs into the vaginal mucosa in a CCL28-dependent manner. The finding that the female genital mucosa is an enriched source of CCL28 (H.-R. Cha and M. Kweon, manuscript in preparation) is compatible with this interpretation and further supports the notion that CCL28 plays an important role in the selective migration of IgA immunoblasts into genital tissues (44). The observation that local vaginal immunization with CT induced comparable IgA and IgG ASC responses in the genital tract mucosa and draining lymph nodes tends to indicate that CT modifies the genital microenvironment by inducing chemoattractive signals for both IgA and IgG ASCs. Experiments are ongoing to address this issue.
Given the marked degree of compartmentalization of the mucosal immune system (3, 5), the choice of the most appropriate route for immunization is of critical importance to develop effective vaccination strategies against genital infections. To date, the vaginal and nasal routes have proven to be effective for inducing genital immune responses, albeit the effectiveness of i.vag. immunization appears to depend on the host hormonal status (18, 19). Several studies have documented that i.n. immunization can induce genital immune responses characterized by the presence of ASCs and memory CD8+ T cells in the genital mucosa (9, 13, 18, 44, 45). We previously reported that, similarly as i.n. immunization, s.l. immunization with a nonreplicating Ag combined with CT as an adjuvant induced mucosal Abs and CTLs in the systemic compartment and in the upper aerodigestive tract mucosa (25). A major finding of this study was that s.l. administration of a nonreplicating protein Ag was also able to induce systemic (splenic) and local genital expansion of effector CTLs. These responses, detected by means of an in vivo cytolysis assay, were also induced by i.n. and i.vag. immunizations and also required the coadministration of a CT adjuvant. This finding builds on our previous study and is consistent with early reports showing that CT can act as adjuvant for enhancing CTL responses to coadministered protein Ags (46–48). In addition, the proportion and avidity of Ag-specific CD8+ T cells in the gut have been shown to be compartmentalized to tissues proximal to the sites of immunization (46, 47). The question of whether CTLs induced by remote mucosal immunization may display functional properties different from those induced by local (i.vag.) immunization remains to be addressed.
The vaginal mucosa is under the control of sex hormones that can modify the ability of this tissue to serve as a site of induction and/or expression of immune responses (19, 49–51). In our hands, progesterone pretreatment of female mice enhanced the genital immune responses induced by i.vag. immunization but had little if any effect on immune responses induced by s.l. or i.n. immunization (H.-R. Cha and M. Kweon, manuscript in preparation), which is in line with data reported after i.n. and i.vag. immunization in humans (50, 51). Furthermore, the s.l. route of administration does not carry the risk of retrograde transport of Ags, including replicating and nonreplicating Ags or adjuvants, including CT or lymphotoxin, into the brain as has been reported for certain Ags administered i.n. (24). Together, these features should confer a safety advantage for the s.l. route to deliver vaccine against genital pathogens.
Two injectable VLP-based HPV vaccines (Gardasil and Cervarix) have recently been shown to confer protection against HPV-associated cervical intraepithelial neoplasia and adenocarcinoma (1, 2). IgG Abs transuding from blood into genital tissues and secretions are considered the main effector mechanism conferring protection. In the present study, s.l. vaccination with HPV VLPs, given either alone or together with CT adjuvant, induced HPV-specific IgG Abs in serum and IgA Abs in vaginal secretions. In addition, HPV-neutralizing activity was demonstrated in both serum and genital secretions after s.l. immunization. These data are reminiscent of earlier studies showing that the delivery of HPV VLPs into the lower respiratory tract induced HPV-neutralizing Abs in serum and in genital secretions (39, 40).
The observation that serum IgG Ab and neutralizing responses to HPV were lower after s.l. vaccination compared with i.m. vaccination, whereas genital IgA Ab responses were higher after s.l. immunization is intriguing, given the fact that both types of immunization induced complete protection against genital HPV challenge. A plausible explanation is that the threshold level of neutralizing Abs required to achieve complete protection was induced by either route. Another explanation is that genital IgA Abs, which were induced mainly after s.l. immunization, could provide an additional and perhaps distinct protective mechanism against genital HPV infection by interfering with the attachment and subsequent entry of HPV peudovirions into the cervicovaginal epithelium. In this regard, secretory IgA Abs have been shown to prevent the entry of viruses into mucosal epithelial cells, interfere with transcytosis across epithelial cells, and even neutralize virus replication within epithelial cells (52–56).
The finding that s.l. administration of HPV VLPs evoked unusually strong mucosal immune responses in the absence of any added adjuvant is also particularly surprising and underscores the exceptional immunogenicity of such VLPs. Such immunogenic properties could be related to their remarkable ability to bind several types of professional APCs, including B cells, dendritic cells, and macrophages (57, 58), induce the production of proinflammatory cytokines (59), and activate innate immune responses (58, 60). These properties may explain why the HPV vaccine has been so efficient and why s.l. vaccination with VLPs in the absence of any added adjuvant is sufficient to induce systemic and genital Ab responses.
This first demonstration that s.l. vaccination with nonreplicating Ags can induce simultaneous systemic and genital Ab and CTL responses provides a foundation for further evaluation of this alternative form of vaccination against genital pathogens. Clinical studies are ongoing to assess the applicability of this concept in humans.
Acknowledgments
This work was supported by the Bill and Melinda Gates Foundation, INSERM (France), the Agence Nationale de Recherches sur le SIDA (France), the Association Ensemble Contre le SIDA (SIDACTION) (France), the Association Faire face au SIDA (France), the Swedish Science Council (Medicine) Grant K2000–06X-03382, and the Knut and Alice Wallenberg Foundation (Sweden). The International Vaccine Institute is supported in part by grants from the governments of the Republic of Korea, Kuwait, and Sweden (SIDA).
Footnotes
Disclosures
The authors have no financial conflict of interest.
Abbreviations used in this paper: ASC, Ab-secreting cell; CT, cholera toxin; HPV, human papillomavirus; i.g., intragastric; i.n., intranasal; ILN, ileosacral lymph node; i.vag., intravaginal; SEAP, secreted alkaline phosphatase; s.l., sublingual; SMLN, submandibular lymph node; VLP, virus-like particle.
References
- 1.Stanley M 2008. Human papillomavirus vaccines versus cervical cancer screening. Clin. Oncol. (R. Coll. Radiol.) 20: 388–394. [DOI] [PubMed] [Google Scholar]
- 2.Frazer IH, Lowy DR, and Schiller JT 2007. Prevention of cancer through immunization: prospects and challenges for the 21st century. Eur. J. Immunol 37(Suppl. 1): S148–S155. [DOI] [PubMed] [Google Scholar]
- 3.Holmgren J, and Czerkinsky C 2005. Mucosal immunity and vaccines. Nat. Med 11: S45–S53. [DOI] [PubMed] [Google Scholar]
- 4.Morrison RP, and Caldwell HD 2002. Immunity to murine chlamydial genital infection. Infect. Immun 70: 2741–2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neutra MR, and Kozlowski PA 2006. Mucosal vaccines: the promise and the challenge. Nat. Rev. Immunol 6: 148–158. [DOI] [PubMed] [Google Scholar]
- 6.Parr MB, and Parr EL 2003. Vaginal immunity in the HSV-2 mouse model. Int. Rev. Immunol 22: 43–63. [DOI] [PubMed] [Google Scholar]
- 7.Mestecky J 1987. The common mucosal immune system and current strategies for induction of immune responses in external secretions. J. Clin. Immunol 7: 265–276. [DOI] [PubMed] [Google Scholar]
- 8.Belyakov IM, Derby MA, Ahlers JD, Kelsall BL, Earl P, Moss B, Strober W, and Berzofsky JA 1998. Mucosal immunization with HIV-1 peptide vaccine induces mucosal and systemic cytotoxic T lymphocytes and protective immunity in mice against intrarectal recombinant HIV-vaccinia challenge. Proc. Natl. Acad. Sci. USA 95: 1709–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gallichan WS, and Rosenthal KL 1996. Long-lived cytotoxic T lymphocyte memory in mucosal tissues after mucosal but not systemic immunization. J. Exp. Med 184: 1879–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gallichan WS, Woolstencroft RN, Guarasci T, McCluskie MJ, Davis HL, and Rosenthal KL 2001. Intranasal immunization with CpG oligodeoxynucleotides as an adjuvant dramatically increases IgA and protection against herpes simplex virus-2 in the genital tract. J. Immunol 166: 3451–3457. [DOI] [PubMed] [Google Scholar]
- 11.Roan NR, Gierahn TM, Higgins DE, and Starnbach MN 2006. Monitoring the T cell response to genital tract infection. Proc. Natl. Acad. Sci. USA 103: 12069–12074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao X, Deak E, Soderberg K, Linehan M, Spezzano D, Zhu J, Knipe DM, and Iwasaki A 2003. Vaginal submucosal dendritic cells, but not Langerhans cells, induce protective Th1 responses to herpes simplex virus-2. J. Exp. Med 197: 153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johansson EL, Rask C, Fredriksson M, Eriksson K, Czerkinsky C, and Holmgren J 1998. Antibodies and antibody-secreting cells in the female genital tract after vaginal or intranasal immunization with cholera toxin B subunit or conjugates. Infect. Immun 66: 514–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kozlowski PA, Cu-Uvin S, Neutra MR, and Flanigan TP 1997. Comparison of the oral, rectal, and vaginal immunization routes for induction of antibodies in rectal and genital tract secretions of women. Infect. Immun 65: 1387–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parr EL, Parr MB, and Thapar M 1988. A comparison of specific antibody responses in mouse vaginal fluid after immunization by several routes. J. Reprod. Immunol 14: 165–176. [DOI] [PubMed] [Google Scholar]
- 16.Rudin A, Johansson EL, Bergquist C, and Holmgren J 1998. Differential kinetics and distribution of antibodies in serum and nasal and vaginal secretions after nasal and oral vaccination of humans. Infect. Immun 66: 3390–3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gallichan WS, and Rosenthal KL 1996. Effects of the estrous cycle on local humoral immune responses and protection of intranasally immunized female mice against herpes simplex virus type 2 infection in the genital tract. Virology 224: 487–497. [DOI] [PubMed] [Google Scholar]
- 18.Kozlowski PA, Williams SB, Lynch RM, Flanigan TP, Patterson RR, Cu-Uvin S, and Neutra MR 2002. Differential induction of mucosal and systemic antibody responses in women after nasal, rectal, or vaginal immunization: influence of the menstrual cycle. J. Immunol 169: 566–574. [DOI] [PubMed] [Google Scholar]
- 19.Wira C, Fahey J, Wallace P, and Yeaman G 2005. Effect of the menstrual cycle on immunological parameters in the human female reproductive tract. J. Acquir. Immune Defic. Syndr 38(Suppl. 1): S34–S36. [DOI] [PubMed] [Google Scholar]
- 20.Gallichan WS, and Rosenthal KL 1998. Long-term immunity and protection against herpes simplex virus type 2 in the murine female genital tract after mucosal but not systemic immunization. J. Infect. Dis 177: 1155–1161. [DOI] [PubMed] [Google Scholar]
- 21.Armstrong ME, Lavelle EC, Loscher CE, Lynch MA, and Mills KH 2005. Proinflammatory responses in the murine brain after intranasal delivery of cholera toxin: implications for the use of AB toxins as adjuvants in intranasal vaccines. J. Infect. Dis 192: 1628–1633. [DOI] [PubMed] [Google Scholar]
- 22.Fujihashi K, Koga T, van Ginkel FW, Hagiwara Y, and McGhee JR 2002. A dilemma for mucosal vaccination: efficacy versus toxicity using enterotoxin-based adjuvants. Vaccine 20: 2431–2438. [DOI] [PubMed] [Google Scholar]
- 23.Lemiale F, Kong WP, Akyurek LM, Ling X, Huang Y, Chakrabarti BK, Eckhaus M, and Nabel GJ 2003. Enhanced mucosal immunoglobulin A response of intranasal adenoviral vector human immunodeficiency virus vaccine and localization in the central nervous system. J. Virol 77: 10078–10087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Ginkel FW, Jackson RJ, Yuki Y, and McGhee JR 2000. Cutting edge: the mucosal adjuvant cholera toxin redirects vaccine proteins into olfactory tissues. J. Immunol 165: 4778–4782. [DOI] [PubMed] [Google Scholar]
- 25.Cuburu N, Kweon MN, Song JH, Hervouet C, Luci C, Sun JB, Hofman P, Holmgren J, Anjuere F, and Czerkinsky C 2007. Sublingual immunization induces broad-based systemic and mucosal immune responses in mice. Vaccine 25: 8598–8610. [DOI] [PubMed] [Google Scholar]
- 26.Song JH, Nguyen HH, Cuburu N, Horimoto T, Ko SY, Park SH, Czerkinsky C, and Kweon MN 2008. Sublingual vaccination with influenza virus protects mice against lethal viral infection. Proc. Natl. Acad. Sci. USA 105: 1644–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pastrana DV, Vass WC, Lowy DR, and Schiller JT 2001. NHPV16 VLP vaccine induces human antibodies that neutralize divergent variants of HPV16. Virology 279: 361–369. [DOI] [PubMed] [Google Scholar]
- 28.Buck CB, Pastrana DV, Lowy DR, and Schiller JT 2004. Efficient intracellular assembly of papillomaviral vectors. J. Virol 78: 751–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anjuere F, George-Chandy A, Audant F, Rousseau D, Holmgren J, and Czerkinsky C 2003. Transcutaneous immunization with cholera toxin B subunit adjuvant suppresses IgE antibody responses via selective induction of Th1 immune responses. J. Immunol 170: 1586–1592. [DOI] [PubMed] [Google Scholar]
- 30.Pastrana DV, Buck CB, Pang YY, Thompson CD, Castle PE, FitzGerald PC, Kruger Kjaer S, Lowy DR, and Schiller JT 2004. Reactivity of human sera in a sensitive, high-throughput pseudovirus-based papillomavirus neutralization assay for HPV16 and HPV18. Virology 321: 205–216. [DOI] [PubMed] [Google Scholar]
- 31.Czerkinsky CC, Nilsson LA, Nygren H, Ouchterlony O, and Tarkowski A 1983. A solid-phase enzyme-linked immunospot (ELISPOT) assay for enumeration of specific antibody-secreting cells. J. Immunol. Methods 65: 109–121. [DOI] [PubMed] [Google Scholar]
- 32.Sedgwick JD, and Holt PG 1983. A solid-phase immunoenzymatic technique for the enumeration of specific antibody-secreting cells. J. Immunol. Methods 57: 301–309. [DOI] [PubMed] [Google Scholar]
- 33.Anjuere F, Luci C, Lebens M, Rousseau D, Hervouet C, Milon G, Holmgren J, Ardavin C, and Czerkinsky C 2004. In vivo adjuvant-induced mobilization and maturation of gut dendritic cells after oral administration of cholera toxin. J. Immunol 173: 5103–5111. [DOI] [PubMed] [Google Scholar]
- 34.Coles RM, Mueller SN, Heath WR, Carbone FR, and Brooks AG 2002. Progression of armed CTL from draining lymph node to spleen shortly after localized infection with herpes simplex virus 1. J. Immunol 168: 834–838. [DOI] [PubMed] [Google Scholar]
- 35.Luci C, Hervouet C, Rousseau D, Holmgren J, Czerkinsky C, and Anjuere F 2006. Dendritic cell-mediated induction of mucosal cytotoxic responses following intravaginal immunization with the nontoxic B subunit of cholera toxin. J. Immunol 176: 2749–2757. [DOI] [PubMed] [Google Scholar]
- 36.Roberts JN, Buck CB, Thompson CD, Kines R, Bernardo M, Choyke PL, Lowy DR, and Schiller JT 2007. Genital transmission of HPV in a mouse model is potentiated by nonoxynol-9 and inhibited by carrageenan. Nat. Med 13: 857–861. [DOI] [PubMed] [Google Scholar]
- 37.Lazarus NH, Kunkel EJ, Johnston B, Wilson E, Youngman KR, and Butcher EC 2003. A common mucosal chemokine (mucosae-associated epithelial chemokine/CCL28) selectively attracts IgA plasmablasts. J. Immunol 170: 3799–3805. [DOI] [PubMed] [Google Scholar]
- 38.Bowman EP, Kuklin NA, Youngman KR, Lazarus NH, Kunkel EJ, Pan J, Greenberg HB, and Butcher EC 2002. The intestinal chemokine thymus-expressed chemokine (CCL25) attracts IgA antibody-secreting cells. J. Exp. Med 195: 269–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Balmelli C, Roden R, Potts A, Schiller J, De Grandi P, and Nardelli-Haefliger D 1998. Nasal immunization of mice with human papillomavirus type 16 virus-like particles elicits neutralizing antibodies in mucosal secretions. J. Virol 72: 8220–8229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nardelli-Haefliger D, Roden R, Balmelli C, Potts A, Schiller J, and De Grandi P 1999. Mucosal but not parenteral immunization with purified human papillomavirus type 16 virus-like particles induces neutralizing titers of antibodies throughout the estrous cycle of mice. J. Virol 73: 9609–9613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alphs HH, Gambhira R, Karanam B, Roberts JN, Jagu S, Schiller JT, Zeng W, Jackson DC, and Roden RB 2008. Protection against heterologous human papillomavirus challenge by a synthetic lipopeptide vaccine containing a broadly cross-neutralizing epitope of L2. Proc. Natl. Acad. Sci. USA 105: 5850–5855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hieshima K, Kawasaki Y, Hanamoto H, Nakayama T, Nagakubo D, Kanamaru A, and Yoshie O 2004. CC chemokine ligands 25 and 28 play essential roles in intestinal extravasation of IgA antibody-secreting cells. J. Immunol 173: 3668–3675. [DOI] [PubMed] [Google Scholar]
- 43.Kunkel EJ, and Butcher EC 2003. Plasma-cell homing. Nat. Rev. Immunol 3: 822–829. [DOI] [PubMed] [Google Scholar]
- 44.Iwasaki A 2007. Mucosal dendritic cells. Annu. Rev. Immunol 25: 381–418. [DOI] [PubMed] [Google Scholar]
- 45.Eriksson K, Quiding-Jarbrink M, Osek J, Moller A, Bjork S, Holmgren J, and Czerkinsky C 1998. Specific-antibody-secreting cells in the rectums and genital tracts of nonhuman primates following vaccination. Infect. Immun 66: 5889–5896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Belyakov IM, Kuznetsov VA, Kelsall B, Klinman D, Moniuszko M, Lemon M, Markham PD, Pal R, Clements JD, Lewis MG, et al. 2006. Impact of vaccine-induced mucosal high-avidity CD8+ CTLs in delay of AIDS viral dissemination from mucosa. Blood 107: 3258–3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Belyakov IM, Isakov D, Zhu Q, Dzutsev A, and Berzofsky JA 2007. A novel functional CTL avidity/activity compartmentalization to the site of mucosal immunization contributes to protection of macaques against simian/human immunodeficiency viral depletion of mucosal CD4+ T cells. J. Immunol 178: 7211–7221. [DOI] [PubMed] [Google Scholar]
- 48.Bowen JC, Nair SK, Reddy R, and Rouse BT 1994. Cholera toxin acts as a potent adjuvant for the induction of cytotoxic T-lymphocyte responses with non-replicating antigens. Immunology 81: 338–342. [PMC free article] [PubMed] [Google Scholar]
- 49.Black CA, Rohan LC, Cost M, Watkins SC, Draviam R, Alber S, and Edwards RP 2000. Vaginal mucosa serves as an inductive site for tolerance. J. Immunol 165: 5077–5083. [DOI] [PubMed] [Google Scholar]
- 50.Kaushic C, Ashkar AA, Reid LA, and Rosenthal KL 2003. Progesterone increases susceptibility and decreases immune responses to genital herpes infection. J. Virol 77: 4558–4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Johansson EL, Wassen L, Holmgren J, Jertborn M, and Rudin A 2001. Nasal and vaginal vaccinations have differential effects on antibody responses in vaginal and cervical secretions in humans. Infect. Immun 69: 7481–7486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mazanec MB, Kaetzel CS, Lamm ME, Fletcher D, and Nedrud JG 1992. Intracellular neutralization of virus by immunoglobulin A antibodies. Proc. Natl. Acad. Sci. USA 89: 6901–6905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Corthésy B, Benureau Y, Perrier C, Fourgeux C, Parez N, Greenberg H, and Schwartz-Cornil I 2006. Rotavirus anti-VP6 secretory immunoglobulin A contributes to protection via intracellular neutralization but not via immune exclusion. J. Virol 80: 10692–10699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wright A, Yan H, Lamm ME, and Huang YT 2006. Immunoglobulin A antibodies against internal HIV-1 proteins neutralize HIV-1 replication inside epithelial cells. Virology 356: 165–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huang YT, Wright A, Gao X, Kulick L, Yan H, and Lamm ME 2005. Intraepithelial cell neutralization of HIV-1 replication by IgA. J. Immunol 174: 4828–4835. [DOI] [PubMed] [Google Scholar]
- 56.Bélec L, Ghys PD, Hocini H, Nkengasong JN, Tranchot-Diallo J, Diallo MO, Ettiègne-Traore V, Maurice C, Becquart P, Matta M, et al. 2001. Cervicovaginal secretory antibodies to human immunodeficiency virus type 1 (HIV-1) that block viral transcytosis through tight epithelial barriers in highly exposed HIV-1-seronegative African women. J. Infect. Dis 184: 1412–1422. [DOI] [PubMed] [Google Scholar]
- 57.Lenz P, Thompson CD, Day PM, Bacot SM, Lowy DR, and Schiller JT 2003. Interaction of papillomavirus virus-like particles with human myeloid antigen-presenting cells. Clin. Immunol 106: 231–237. [DOI] [PubMed] [Google Scholar]
- 58.Lenz P, Day PM, Pang YY, Frye SA, Jensen PN, Lowy DR, and Schiller JT 2001. Papillomavirus-like particles induce acute activation of dendritic cells. J. Immunol 166: 5346–5355. [DOI] [PubMed] [Google Scholar]
- 59.Lenz P, Lowy DR, and Schiller JT 2005. Papillomavirus virus-like particles induce cytokines characteristic of innate immune responses in plasmacytoid dendritic cells. Eur. J. Immunol 35: 1548–1556. [DOI] [PubMed] [Google Scholar]
- 60.Yang R, Murillo FM, Delannoy MJ, Blosser RL, Yutzy W. H. t., Uematsu S, Takeda K, Akira S, Viscidi RP, and Roden RB 2005. B lymphocyte activation by human papillomavirus-like particles directly induces Ig class switch recombination via TLR4-MyD88. J. Immunol 174: 7912–7919. [DOI] [PubMed] [Google Scholar]