Abstract
Opioid drugs are highly valued as potent analgesics; however, there are significant risks associated with long-term use because of their abuse liability. Opioids cause changes in ventral tegmental area (VTA) gene expression and cell activity that have been linked to addiction-related behaviors in rodent models. Here, we focus on VTA dopamine (DA) neurons and review the cellular, structural, and synaptic plasticity changes induced by acute and chronic opioid exposure. We also discuss many avenues for future research including determination of whether opioid neuroadaptations are specific for subpopulations of VTA DA neurons. A better understanding of the molecular adaptations within the cells and circuits that drive opioid abuse is crucial for the development of better treatments for substance use disorders and to create novel, safer pain-relieving therapeutics.
Opioid drugs have been used medically for centuries for their potent analgesic properties (Fields 2011). This class of drugs includes naturally occurring compounds derived from the opium poppy such as codeine and morphine as well as many synthetic derivatives such as heroin, oxycodone, and fentanyl. Although opioids remain among the most effective medications for acute pain relief, there are serious side effects that can occur with long-term opiate use, such as tolerance, physical dependence, and addiction (Ballantyne and LaForge 2007). In the United States, abuse of prescription drugs, and specifically pain-relieving opioids, has increased greatly since 1992 (Compton and Volkow 2006; Manchikanti et al. 2010; Han et al. 2017). Despite a recent strengthening of regulations that has decreased the number of opioid prescriptions, opioid-related deaths continue to rise, indicating that these measures alone are inadequate to combat the crisis (Manchikanti et al. 2018; Volkow and Koroshetz 2019). Although the ethics of chronic pain treatment and the potential for over- or underuse of opioid drugs can be debated (Fields 2011), there is no question that chronic opioid use causes neuroadaptations that lead to undesirable effects.
We will focus on the opioid-induced changes to midbrain ventral tegmental area (VTA) dopamine (DA) neurons, a key region and cell type in opioid addiction. Specifically, we discuss three types of opioid-induced VTA plasticity in response to acute and chronic exposure: synaptic plasticity—persistent changes in glutamatergic and γ-aminobutyric acid (GABA)ergic synaptic transmission (Lüscher and Malenka 2011; Langlois and Nugent 2017); cellular plasticity—homeostatic changes in intracellular signaling cascades (Williams et al. 2001; Nestler 2004); and structural plasticity—long-lasting changes in neuronal morphology (Russo et al. 2010). Because most preclinical opioid studies to date have utilized morphine and heroin, our discussion focuses on neuroadaptations induced by these opioid drugs. However, given the increased use and abuse of opioids such as fentanyl and oxycodone, future work will likely interrogate a wider range of opioid drugs. This is one of many ways that researchers hope to bridge the gap to translation, such that identification of the neuronal determinants of VTA plasticity helps to enable better therapeutics for opioid addiction and inform the design of safer drugs for pain relief.
VENTRAL TEGMENTAL AREA NEURON COMPLEXITY
The VTA has been widely studied in drug addiction because of its central role in reward-related behaviors. This heterogeneous region is composed of 60%–65% DA, 30%–35% GABA, and 2%–3% glutamate neurons (Swanson 1982; Nair-Roberts et al. 2008). While neuronal cell types do not strictly localize to one subnucleus of the VTA, they are organized in gradients, particularly across anteroposterior and mediolateral axes. Specifically, the ratio of GABA to DA neurons is higher in posterior sections of the VTA compared to anterior sections (Nair-Roberts et al. 2008) and glutamatergic neurons are concentrated in medial subnuclei (Yamaguchi et al. 2007). However, recent studies have shown that the VTA is even more heterogeneous than previously thought. VTA DA neurons have been historically defined using two main criteria: (1) expression of tyrosine hydroxylase (TH), the rate-limiting enzyme in DA synthesis, and (2) presence of a large hyperpolarization-activated (Ih) current. However, VTA DA neurons are now known to be capable of expressing and releasing multiple neurotransmitters (for review, see Barker et al. 2016; Morales and Margolis 2017). These combinatorial DA neurons have been classified as dual DA-glutamate when coexpressing TH and vesicular glutamate transporter 2 (VGLUT2) or dual DA-GABA through coexpression of TH and vesicular GABA transporter (VGAT) or glutamic acid decarboxylase (GAD) (Barker et al. 2016). Additionally, Ih current fails to fully distinguish between VTA DA and non-DA neurons, as there are subsets of DA neurons that express little to no Ih current (Margolis et al. 2006; Chieng et al. 2011). Thus, our understanding of opioid adaptations of VTA DA neurons may be incomplete, as many of the foundational studies preceded the identification of these subpopulations. The functional relevance of subpopulations of VTA DA neurons is currently an active area of research, and this is especially true for populations of VTA DA neurons defined by their projection target.
Individual dopaminergic projections from the VTA largely innervate only one region (Swanson 1982), with major targets including the nucleus accumbens (NAc), prefrontal cortex (PFC), and amygdala (Swanson 1982; Sesack and Grace 2010; Morales and Margolis 2017). These outputs are roughly organized topographically in the mediolateral axis. Because the NAc receives dense VTA DA projections, much work has focused on characterizing these neurons. For example, DA cell bodies located in more lateral portions of the VTA project to the NAc lateral shell (lShell), while more medial neurons project to the NAc core and NAc medial shell (mShell) (Lammel et al. 2008, 2014; Breton et al. 2019). There is a clear separation between DA projections terminating in NAc subregions, where lateral DA arborizations in the lShell minimally overlap with medial DA arborizations concentrated in the mShell (Beier et al. 2015). Moreover, these subpopulations are functionally distinct. For example, VTA DA projections targeting the ventral portion of the mShell are excited by unexpected aversive stimuli, in contrast to the inhibition seen in all other projection targets (de Jong et al. 2019). Because of VTA neuronal heterogeneity, it will be especially important to take factors such as cell type, cell body location, and projection into account when interpreting VTA DA adaptations in response to drugs of abuse.
The VTA also receives input from a variety of brain regions. Prominent afferents to VTA DA neurons include excitatory input originating from the medial PFC (mPFC), lateral habenula, bed nucleus of the stria terminalis, pedunculopontine tegmentum, and laterodorsal tegmentum nucleus (Sesack and Grace 2010; Morales and Margolis 2017) as well as inhibitory input arising from the NAc, rostromedial mesopontine tegmental nucleus, lateral hypothalamus, and ventral pallidum (Sesack and Grace 2010; Morales and Margolis 2017). There is also complexity at this level of regulation, as illustrated by examination of inhibitory inputs from the NAc to VTA DA neurons (Watabe-Uchida et al. 2012; Beier et al. 2015). NAc lShell neurons project indirectly to disinhibit lateral VTA DA neurons via synapses onto VTA GABAergic interneurons and result in reinforcement, as mice form a real-time place preference to lShell terminal stimulation and will self-stimulate these terminals (Yang et al. 2018). In contrast, mShell neurons predominately project directly to medial VTA DA neurons, inhibiting their activity (Yang et al. 2018). These studies demonstrate that inputs are capable of targeting discrete DA subpopulations and influencing a variety of behaviors, from aversion to reward (Lammel et al. 2014), and thus could also be differentially affected by opioid exposure.
ACUTE OPIOID EFFECTS ON VTA DA NEURONAL ACTIVITY
Like other classes of drugs of abuse, acute systemic or intra-VTA infusion of opioids such as morphine causes increased DA release into the NAc (DiChiara and Imperato 1988; Leone et al. 1991). This effect is primarily achieved through opioid binding to Gi/o-coupled µ-opioid receptors (MORs) located on VTA GABA neurons. Opioid binding hyperpolarizes presynaptic VTA GABA neurons and decreases their spontaneous firing rate, consequently disinhibiting DA neuron firing (Ostrowski et al. 1982; Gysling and Wang 1983; Matthews and German 1984; Johnson and North 1992). Moreover, opioid-induced increases in DA spontaneous firing rate can be blocked by systemic or local infusion of the MOR antagonist naloxone, consistent with a prominent inhibitory role of opioid action at MORs on GABA neurons (Gysling and Wang 1983; Matthews and German 1984). These MOR-mediated effects significantly contribute to opioid reward behaviors, as intra-VTA infusion of MOR agonists alone is capable of forming a conditioned place preference (CPP) (Bals-Kubik et al. 1993) and small interfering RNA knockdown of MORs in the VTA and substantia nigra prevents heroin CPP (Zhang et al. 2009). However, while the canonical model describes opioid action exclusively on VTA GABA neurons, postsynaptic MOR expression has been described on a subset of VTA DA neurons as well, where opioid peptides can elicit both excitation and inhibition of these neurons (Margolis et al. 2014). Although the contribution of the MOR-expressing DA subpopulation to behavior is not well understood, the possibility that these neurons could respond differentially to rewarding versus aversive stimuli (Brischoux et al. 2009; Lammel et al. 2012; Margolis et al. 2014) is an area worthy of future study.
SYNAPTIC PLASTICITY INDUCED BY ACUTE ADMINISTRATION OF OPIOIDS
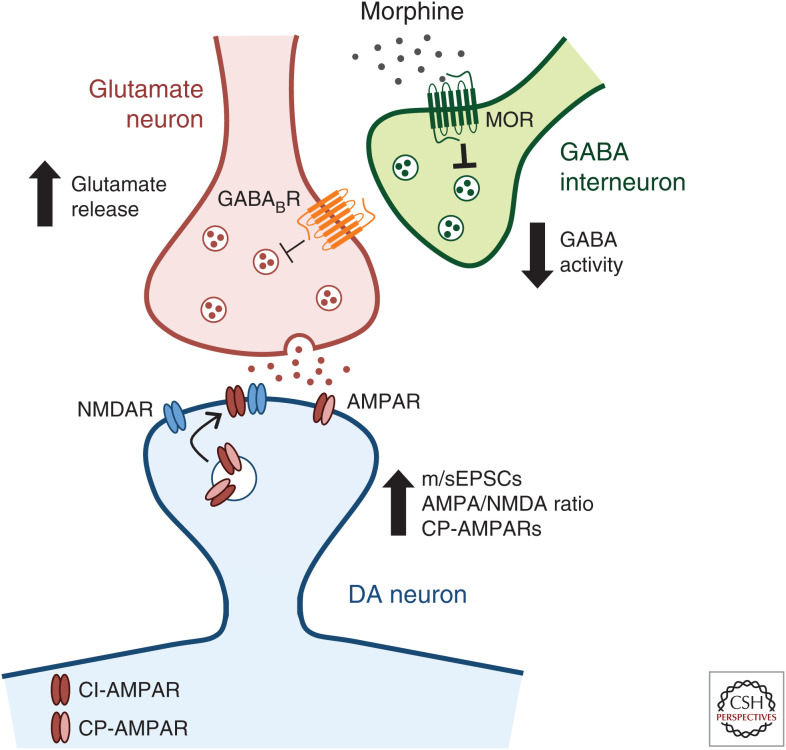
In addition to opioid actions via opioid receptors to alter VTA DA neuronal activity, acute opioid exposure also drives synaptic plasticity (glutamatergic and GABAergic) onto VTA DA neurons. Glutamatergic transmission in the VTA is critical for opioid responses, as pharmacological inhibition prevents expression of heroin self-administration (SA) (Xi and Stein 2002) and morphine CPP (Harris et al. 2004), and glutamatergic signaling is required for morphine-driven increases in VTA DA spontaneous firing rate and burst firing (Jalabert et al. 2011). Thus, much work has sought to define the regulation of the glutamate receptors (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor [AMPAR] and N-methyl-d-aspartic acid receptor [NMDAR]) that mediate excitatory postsynaptic currents (EPSCs) in the VTA following drug exposure. A single dose of morphine increases AMPA/NMDA ratio on VTA DA neurons, consistent with an increase in long-term potentiation (LTP) (Saal et al. 2003; Baimel and Borgland 2015; Authement et al. 2016), an effect similar to that observed with other drugs of abuse (Ungless et al. 2001; Saal et al. 2003). This is achieved in part through increased synaptic localization of AMPARs, measured through labeling of their constituent subunits (e.g., GluA1, GluA2, etc.). Specifically, acute morphine causes a shift in the distribution of GluA1 subunits from the cytoplasm to the plasmalemma in VTA DA neurons (Lane et al. 2008), suggesting an increase in postsynaptic AMPAR expression and enhanced LTP. At the same time, acute morphine treatment also drives insertion of calcium-permeable GluA2-lacking AMPARs in exchange for calcium-impermeable GluA2-containing AMPARs (Brown et al. 2010; Baimel and Borgland 2015; Authement et al. 2016). These results are linked to DA neuronal activity, as direct optogenetic activation of VTA DA neurons induces AMPAR redistribution and exchange of subunits similar to that following drug exposure (Brown et al. 2010). Together, these adaptations in receptor expression mediate enhanced glutamatergic transmission onto VTA DA neurons (Fig. 1), which is thought to contribute to the development of addiction (Wolf 2016).
Figure 1.
Ventral tegmental area (VTA) dopamine (DA)-glutamatergic synaptic plasticity is regulated by acute morphine. Morphine promotes the release of presynaptic glutamate in part through inhibition of VTA γ-aminobutyric acid (GABA) interneurons. Morphine binds to Gi/o-coupled µ-opioid receptors (MORs), causing a hyperpolarization of GABA neurons and decreasing their firing. This decreased GABAergic output onto glutamate neurons, via GABAB receptor signaling, results in increased frequency of miniature and spontaneous excitatory postsynaptic currents (m/sEPSCs) of DA neurons (Baimel and Borgland 2015; Chen et al. 2015). At a second level of DA-glutamatergic plasticity, morphine increases α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor/N-methyl-d-aspartic acid receptor (AMPA/NMDA) ratio, consistent with an increase in long-term potentiation (LTP), and causes the exchange of calcium-impermeable GluA2-containing AMPARs (CI-AMPARs) for calcium-permeable GluA2-lacking AMPARs (CP-AMPARs) (Saal et al. 2003; Baimel and Borgland 2015; Authement et al. 2016). Together, these mechanisms of synaptic plasticity increase glutamatergic signaling in the VTA.
Outside of adaptations within the VTA, opioids also induce changes in brain regions that project to the VTA. Following acute in vivo or ex vivo morphine exposure, there is an increased probability of presynaptic glutamate release onto VTA DA neurons, as measured by an increase in frequency of miniature and spontaneous EPSCs and paired-pulse depression (Baimel and Borgland 2015; Chen et al. 2015). Morphine promotes the release of presynaptic glutamate in part through inhibition of VTA GABA interneurons. Optogenetic stimulation of VTA GABA neurons decreases VTA DA excitatory responses, while inhibition of VTA GABA neurons has the opposite effect (Chen et al. 2015). Further, metabotropic GABAB receptors are located on glutamatergic terminals in the VTA, and inhibiting these receptors prevents the reduction in VTA DA excitatory responses caused by VTA GABA stimulation (Fig. 1; Chen et al. 2015). This increased glutamatergic input in combination with the depression of local GABA inhibitory input (discussed below [Nugent et al. 2007; Dacher and Nugent 2011; Authement et al. 2016]) shifts the balance of excitatory and inhibitory synaptic transmission onto VTA DA neurons (Baimel and Borgland 2015).
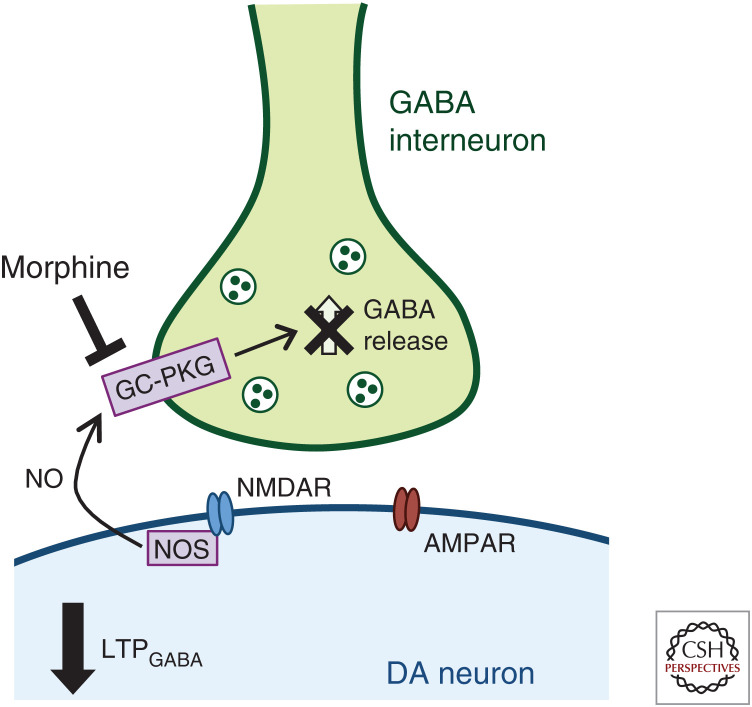
In addition to altering VTA DA neuron glutamatergic plasticity, drugs of abuse (including ethanol, morphine, nicotine, and cocaine) alter inhibitory synaptic plasticity in the VTA (Melis et al. 2002; Liu et al. 2005; Nugent et al. 2007; Niehaus et al. 2010). Unsurprisingly, opioids also regulate VTA DA neuron GABAergic responses (for review, see Langlois and Nugent 2017). VTA GABAergic LTP (LTPGABA) can be evoked by high-frequency electrical stimulation and results in facilitation of inhibitory postsynaptic currents (IPSCs) onto VTA DA neurons (Nugent et al. 2007). This form of inhibitory plasticity is driven by NMDAR-dependent release of nitric oxide (NO) (Nugent et al. 2007). NO acts as a retrograde messenger to activate guanylate cyclase (GC) in presynaptic GABA-ergic terminals, resulting in increased GABA release and initiating LTPGABA (Nugent et al. 2007). Acute in vivo morphine exposure blocks the induction of LTPGABA through disrupted NO−GC−protein kinase G signaling (Nugent et al. 2007, 2009; Niehaus et al. 2010). Amplitude and frequency of miniature IPSCs (mIPSCs) are also decreased following acute morphine exposure (Authement et al. 2016), consistent with evidence that LTPGABA is maintained presynaptically through a steady increase in GABA release (Nugent et al. 2007) and further supporting decreased inhibitory control of VTA DA neurons following opioid exposure. These data support disrupted inhibitory regulation of VTA DA neurons, contributing to increased VTA DA neuronal activity in response to acute morphine treatment (Fig. 2).
Figure 2.
Acute morphine disrupts ventral tegmental area (VTA) dopamine (DA)-γ-aminobutyric acid (GABA) synaptic plasticity. Acute morphine treatment prevents long-term potentiation of GABAergic (LTPGABA) synapses on VTA DA neurons. LTPGABA derives from NMDAR-dependent release of nitric oxide (NO) via activation of NO synthase (NOS) (Nugent et al. 2007). NO acts as a retrograde messenger to activate guanylate cyclase (GC) in presynaptic GABAergic terminals, resulting in increased GABA release and initiating LTPGABA (Nugent et al. 2007). Morphine prevents LTPGABA and disrupts normal inhibitory signaling in the VTA by blocking NO−GC−protein kinase G (PKG) signaling (Nugent et al. 2007, 2009; Niehaus et al. 2010).
Long-term depression (LTD) of GABAergic synapses onto VTA DA neurons (LTDGABA) has also been characterized (Dacher and Nugent 2011). LTDGABA is NMDA-independent and induced postsynaptically through D2 receptor (D2R) activation (Dacher and Nugent 2011; Dacher et al. 2013). Downstream of the D2R, inositol triphosphate receptor activation causes a local increase in intracellular calcium, resulting in internalization of GABAA receptors through protein kinase A signaling (Dacher et al. 2013). Acute morphine treatment blocks the induction of LTDGABA, thereby disrupting normal inhibitory control through a second mechanism, although the exact mechanism of action is not well understood (Dacher and Nugent 2011). Taken together, these studies indicate that opioids such as morphine both disrupt inhibitory input and strengthen excitatory input onto VTA DA neurons, altering the normal excitatory to inhibitory balance to increase VTA DA neuronal activity.
Although protein signaling mechanisms contribute to the expression and redistribution of glutamate and GABA receptors induced by opioids, recent evidence suggests that opioid-induced changes in VTA plasticity are also mediated via epigenetic regulation. Epigenetic mechanisms represent a class of changes capable of generating long-lasting repression or enhancement of gene regulation (Browne et al. 2019). Following a single morphine injection, expression of histone deacetylase (HDAC)2 is increased in VTA DA neurons, but not in substantia nigra DA neurons, demonstrating regional specificity (Authement et al. 2016). Bath application of an HDAC inhibitor (HDACi) was sufficient to eliminate glutamatergic plasticity onto VTA DA neurons, preventing the increase in AMPA/NMDA ratio seen with morphine treatments (Authement et al. 2016). HDACi treatment also rescued decreased mIPSC amplitude and frequency, restoring normal inhibitory control (Authement et al. 2016). Thus, epigenetic regulation could in part explain the long-lasting effects of opioids on synaptic plasticity. Collectively, this work clearly establishes that opioids alter plasticity onto VTA DA neurons that contributes to DA neuronal activity and output, and that these effects are mediated through multiple signaling and likely epigenetic mechanisms.
SYNAPTIC PLASTICITY INDUCED BY CHRONIC ADMINISTRATION OF OPIOIDS
Although research has focused on the effects of acute morphine on VTA DA physiology as described above, relatively little is known about the long-term consequences of opioid exposure. Because opioids are generally taken chronically, understanding whether similar mechanisms are induced by acute and chronic opioid exposure is critical to our understanding of opioid abuse. Similar to acute treatment, chronic morphine administration increases VTA DA neuronal firing, as morphine-dependent rats show a significantly increased in vivo firing rate and burst event frequency compared to drug-naive rats (Georges et al. 2006). Supporting this finding, similar effects are found in mice, both in vivo and in ex vivo slices (Mazei-Robison et al. 2011; Koo et al. 2012). This increased activity is in part mediated through potassium channel regulation, as the peak and sustained components of potassium currents are significantly decreased in VTA DA neurons of morphine pelleted mice (Koo et al. 2012). Chronic morphine also decreases VTA messenger RNA (mRNA) expression of potassium channel subunits (Mazei-Robison et al. 2011). Specifically, voltage-gated potassium channel subunit β2 (KCNAB2) and G-protein-gated inwardly rectifying potassium (GIRK) channel 3 show decreased permissive epigenetic markers and binding of RNA polymerase II (Pol II) at the gene promoters, consistent with decreased transcription (Mazei-Robison et al. 2011). These findings support enhanced excitability of VTA DA neurons following chronic morphine exposure; however, further research is needed to uncover the opioid-induced adaptations to ion channel expression and function that contribute to changes in cellular activity.
Altered VTA DA glutamatergic plasticity has also been demonstrated following chronic morphine; however, there is a gap in the literature compared to acute opioid mechanisms. Chronic escalating morphine injections increase GluA1 protein in the VTA (Fitzgerald et al. 1996) and VTA GluA1 overexpression potentiates morphine CPP (Carlezon et al. 1997), establishing this induction as behaviorally relevant. Ultrastructural studies using the same morphine administration paradigm have found increased GluA1 synaptic labeling in VTA DA neurons (Lane et al. 2008), consistent with increased LTP. These GluA1 effects are consistent with those observed following acute opioid treatment; however, VTA DA-glutamatergic and -GABAergic plasticity mechanisms remain largely unexplored following chronic exposure. To date, much of the research on long-term opioid-induced plasticity has focused on aspects of withdrawal. Although withdrawal is an important translational issue, mechanisms supporting withdrawal may be distinct from those elicited by chronic exposure, highlighting the need for synaptic plasticity studies that examine effects of long-term opioid exposure.
CELLULAR PLASTICITY INDUCED BY CHRONIC ADMINISTRATION OF OPIOIDS
Changes in VTA gene transcription and expression have been linked to changes in drug-related behaviors in mice (Lüscher and Malenka 2011; Wolf 2016). Discussion of brain-derived neurotrophic factor (BDNF) regulation is useful in this regard, as it shows the multiple levels at which opioids can influence transcription and expression. BDNF is a positive modulator of cellular and behavioral plasticity in the central nervous system, and chronic opioid exposure decreases bdnf expression in the VTA (Chu et al. 2007; Koo et al. 2012, 2015). This is a translationally relevant change, as human heroin addicts, as well as rodents in both passive and SA protocols, demonstrate decreased bdnf mRNA levels (Koo et al. 2015). Further, in rodent models of acute morphine exposure, a single injection was not sufficient to decrease bdnf expression (Koo et al. 2015), indicating that chronic drug use is required to induce these effects. This decrease in bdnf expression has been correlated with changes in epigenetic regulation of gene transcription and histone modifications. Chronic morphine causes Pol II stalling within bdnf promoter regions, indicative of gene suppression (Koo et al. 2015). Additionally, changes in histone modifications at bdnf-promotor 2 have been characterized, with decreased histone H3 acetylation (acH3) and increased trimethylation of histone H3 at Lys27 (H3K27me3), supporting evidence of decreased gene expression (Koo et al. 2015). Critically, these changes influence animal behavior. H3K27me3-mediated gene repression is necessary and sufficient for morphine CPP, as overexpression or deletion of enhancer of zeste homolog 2 (EZH2), a key protein for interacting with H3K27, enhances or blocks CPP, respectively (Koo et al. 2015). This is consistent with the idea that BDNF is a negative regulator of morphine reward. Infusion of BNDF into the VTA prevents morphine CPP while, in contrast, VTA bdnf gene knockout or decreased signaling via tyrosine receptor kinase B (TrkB) knockout increases morphine CPP (Koo et al. 2012). Further, decreased TrkB signaling specifically in VTA DA neurons is capable of yielding these effects (Koo et al. 2012), indicating DA neurons are an important cellular population. However, BDNF's role in the VTA is complex, as BDNF is also critical for the transition from inhibitory to excitatory GABAA receptor signaling on VTA GABA neurons that underlies the shift from DA-independent (opioid-naive) to DA-dependent (opioid-dependent) reward and motivation (Laviolette et al. 2004; Vargas-Perez et al. 2009). In these studies, exogenous BDNF shifted motivation to a DA-dependent state, but failed to diminish morphine CPP (Vargas-Perez et al. 2014). Importantly, BDNF is only one of hundreds of genes identified in large genome expression studies whose expression is regulated by chronic opioid administration (McClung et al. 2005; Heller et al. 2015), providing many candidate genes for future analyses.
Using RNA sequencing to identify novel gene targets, our group previously identified serum- and glucocorticoid-inducible kinase 1 (SGK1) as one of only five genes similarly up-regulated in the VTA of mice treated with chronic cocaine or morphine (McClung et al. 2005; Heller et al. 2015). In addition to regulation at the level of mRNA, SGK1 catalytic activity and phosphorylation at Ser78 are significantly increased by chronic, but not acute, morphine (Heller et al. 2015). To determine the behavioral relevance of these changes, we overexpressed mutant versions of SGK1 in the VTA of adult mice. A constitutively active version of SGK1 (S422D) promoted morphine sensitization (Heller et al. 2015), while decreased catalytic activity (K127Q) or phosphodeficiency (S78A) decreased morphine preference in a two-bottle choice task (Doyle et al. 2017). Current work explores whether these biochemical changes occur in VTA DA or GABA neurons as well as which population is responsible for driving the observed behavioral effects.
While the findings above are informative, they were derived from homogenization of the whole VTA, so it is unclear whether differences are driven specifically by expression in VTA DA neurons. Thus, it is critical to develop cell type–specific approaches to analyze changes in the morphine-induced transcriptome. For example, sgk1 gene induction is also seen in the NAc following chronic morphine exposure; however, single-cell RNA-sequencing has identified these changes predominantly in oligodendrocytes (Avey et al. 2018). To this end, we are using translating ribosome affinity purification (TRAP) to investigate VTA DA-specific transcriptional regulation following morphine treatment. Early work has demonstrated very little overlap between morphine-induced gene expression in whole VTA versus VTA DA neurons (Cooper et al. 2018), highlighting critical differences in cell type–specific gene regulation and the need for further research in this area.
STRUCTURAL PLASTICITY INDUCED BY CHRONIC ADMINISTRATION OF OPIOIDS
Whereas opioid-induced changes in VTA gene regulation and signaling and their link to behavior are being actively investigated, molecular changes also contribute to structural plasticity in the VTA. One of the best characterized adaptations following chronic opioid administration is decreased VTA DA neuron soma size, with no changes seen in neighboring substantia nigra DA neurons or non-DA VTA neurons (TH-negative, putative GABA neurons) (Sklair-Tavron et al. 1996). This effect has been seen with passive morphine administration across rodent species (Sklair-Tavron et al. 1996; Spiga et al. 2003; Chu et al. 2007; Russo et al. 2007; Mazei-Robison et al. 2011) as well as with heroin SA in rats (Russo et al. 2007). This morphological change is transient, as soma size is decreased at 1 and 14 days of withdrawal but returns to baseline by 30 days (Russo et al. 2007). Critically, postmortem samples from human heroin users also show a significant decrease in VTA DA soma size, suggesting this adaptation is translationally relevant (Mazei-Robison et al. 2011). The decrease in soma size is dependent on MOR activation and BDNF signaling, as effects could be blocked with concomitant systemic administration of the MOR antagonist naltrexone or local BDNF infusion (Sklair-Tavron et al. 1996). Moreover, neurotrophic factor signaling cascades appear critical for size changes, as decreased insulin receptor substrate 2 (IRS2)-Akt-mammalian target of rapamycin complex-2 (mTORC2) signaling promotes decreased soma size, whereas increased activity within the pathway prevents morphine-induced changes (Russo et al. 2007; Mazei-Robison et al. 2011).
Changes in VTA DA soma size have also been linked with neuronal activity (Coque et al. 2011). Like acute treatment, chronic morphine increases spontaneous and burst firing rates of VTA DA neurons, effects that are correlated with decreased soma size (Mazei-Robison et al. 2011; Koo et al. 2012). However, electrically evoked DA release into the NAc is decreased by chronic morphine, exhibiting decreased output (Mazei-Robison et al. 2011). These seemingly contradictory results are consistent with recent work by Liu et al. demonstrating that whole-VTA deletion of mTORC similarly decreased electrically evoked DA release in the NAc shell (Liu et al. 2018). In addition to these adaptations in electrophysiological properties and output, decreased soma size has been correlated with changes in reward behavior (Russo et al. 2007; Mazei-Robison et al. 2011). Specifically, both morphine CPP and soma size are decreased 1 and 14 days following morphine pellet exposure, but both measures are similar to sham surgical controls following 30 days of withdrawal (Russo et al. 2007). Similarly, these behavioral effects can be mimicked with biochemical manipulations of the IRS2-Akt-mTORC2 pathway that also influence VTA DA soma size. VTA overexpression of dominant-negative mutants of IRS2 or AKT are capable of decreasing morphine CPP and VTA DA soma size (Russo et al. 2007), supporting a link between soma size, neuronal activity, and reward behavior.
Early work from our laboratory showed that this decrease in size is specific to opioids, as other classes of drugs (ethanol, nicotine, or psychostimulants) did not affect VTA DA soma size (Mazei-Robison et al. 2014). However, Pitchers et al. have demonstrated that endogenous opiates (via natural reward) are also capable of significantly reducing soma size (Pitchers et al. 2014). Following chronic sexual experience, VTA DA neurons show a significant decrease in soma size, an effect that is blocked when mice are treated concomitantly with naloxone (Pitchers et al. 2014). No changes were observed in substantia nigra DA neurons or VTA TH-negative neurons (Pitchers et al. 2014), again paralleling previous findings elicited by morphine exposure. Moreover, these changes are similarly plastic, as decreases in soma size were seen 1 and 7 days after the final mating but returned to baseline by 31 days (Pitchers et al. 2014). Altogether, these data indicate that VTA DA soma size changes are elicited by both endogenous and exogenous opioids, although more work is needed to determine whether endogenous opioids similarly mediate soma size through the IRS2-Akt-mTORC2 pathway.
Structural plasticity also includes changes in dendritic spine density and dendritic morphology; however, these measures have been technically challenging to assess because of the complexity of the VTA. DA neurons in the VTA form a dense network of cell bodies and processes, making isolation of single neurons technically difficult to attain with transgenic-driven viral approaches that label the majority of VTA DA neurons. In contrast, Golgi staining achieves sparse labeling but requires a counterstain to identify the neurotransmitter profile of the selected cell. Stemming from these challenges, VTA spine adaptations in response to chronic opioid use remain wholly uncharacterized. However, the length of VTA DA neuron processes is significantly decreased following chronic morphine treatment (Sklair-Tavron et al. 1996), suggesting this may be a fruitful avenue to pursue if the technical challenges can be overcome. There is also support for potential opioid-induced changes in spine density based on data from the NAc. NAc shell, but not core, medium spiny neurons (MSNs) show an increase in filopodia-like spines with no change in total spine density (Graziane et al. 2016) following a single day of withdrawal from chronic morphine injections, while decreases in total spine density are observed after protracted withdrawal (Robinson and Kolb 1999; Robinson et al. 2002; Graziane et al. 2016). This may be a result of the weakening of synapses that are later pruned, based on the idea that spines are weakened by AMPAR internalization, as prevention of AMPAR internalization during morphine administration eliminates both short- and long-term withdrawal effects (Graziane et al. 2016). VTA biochemical studies also hint at potential opioid-induced changes in spine structure. Postsynaptic density-95 (PSD-95) is thought to play a critical role in synaptic assembly and maturation, as overexpression causes increased GluA1 synaptic clustering and spine density in primary hippocampal cultured neurons (El-Husseini et al. 2000). PSD-95 gene and protein expression are increased in the VTA of rats following morphine CPP (Wang et al. 2014), consistent with increased GluA1 synaptic labeling during morphine exposure (Lane et al. 2008). Although direct evidence for opioid-induced regulation remains lacking, other drugs of abuse regulate VTA spine density. Acute cocaine exposure increases spines on type I, but not type II, VTA neurons and is consistent with increased AMPA/NMDA ratio in only type I neurons (Sarti et al. 2007). However, cell type was originally based on morphology (Phillipson 1979), and while type 1 and type 2 cells were largely TH-positive, whether differences in afferent or efferent projections or neurotransmitter profile define cocaine-induced spine changes remains unclear. Given the connection between spine morphology and synaptic function, investigation of VTA DA spine density and dendritic morphology should be a promising future research direction. However, going forward, our knowledge of opioid-induced neuroadaptations within the VTA will likely be most improved by taking into account the complexity of VTA neurons, such as differences between populations defined by their anatomical position, neurotransmitter expression, and projection target.
HETEROGENEITY OF VTA DA NEURON RESPONSE TO OPIOIDS
There is considerable heterogeneity even within VTA DA neuronal populations, contributing to the diversity of effects seen in response to drugs of abuse (for review, see Juarez and Han 2016). Defining projection-specific effects of opioids is challenging, as broadly speaking, VTA neuronal cell types are widely mixed (Morales and Margolis 2017). Even within VTA DA neurons, DA projection populations fail to exclusively localize to a single subregion (Lammel et al. 2014; Beier et al. 2015) and biochemical markers for VTA DA populations are still under investigation (Poulin et al. 2018; Simmons et al. 2019). However, differences in baseline biochemical and electrophysiological properties have been characterized in VTA DA neurons projecting to the NAc versus PFC (Lammel et al. 2008, 2011). For example, PFC-projecting DA neurons lack functional somatodendritic D2Rs once thought of as a hallmark characteristic of VTA DA neurons (Lammel et al. 2008), implying functional distinctions based on projection target. There are also baseline differences in excitatory synaptic plasticity, where PFC- and NAc mShell-projecting neurons have increased AMPA/NMDA ratio compared to NAc lShell-projecting neurons (Lammel et al. 2011). These properties translate to divergent effects following acute drug administration, where acute cocaine treatment causes an increase in AMPA/NMDA ratio in NAc-projecting but not PFC-projecting neurons (Lammel et al. 2011). Although these projection differences remain understudied in the context of acute opioid treatment, there is evidence for circuit-level regulation. This is illustrated in findings that a single session of heroin SA predominantly activates VTA DA neurons projecting to the NAc mShell over those projecting to the NAc lShell (Corre et al. 2018). Thus, there is ample evidence for both basal differences in VTA subpopulations based on projection target as well as in response to opioid drugs.
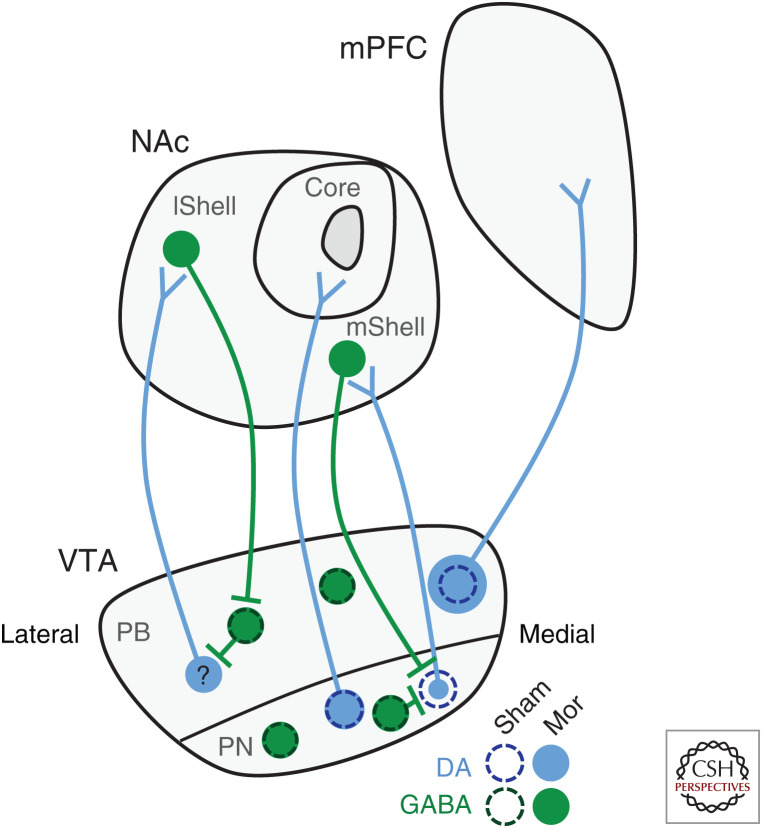
Although more work is needed to tease out differences induced by acute opioid treatment, a significant amount of effort has now focused on VTA DA subpopulations in the context of chronic opioid use. Building on early results showing decreased dendritic length by chronic morphine treatment (Sklair-Tavron et al. 1996), Lane et al. observed changes in VTA DA, but not nondopaminergic, dendrite diameter, with diameter decreased in the paranigral (PN) but increased in the parabrachial (PB) subregions of the VTA (Lane et al. 2008). As the PN predominantly contains cell bodies of NAc-projecting DA neurons, while the PB contains both NAc- and PFC-projecting DA neurons, these data support functional differences of DA subpopulations based on their projection target. Given this evolution in the field toward projection-specific information, our laboratory recently investigated changes in VTA DA soma size, focusing on the NAc and PFC as target regions. We confirmed a decrease in the surface area of VTA DA neurons projecting to the NAc following chronic morphine administration consistent with previous studies (Sklair-Tavron et al. 1996; Russo et al. 2007; Mazei-Robison et al. 2011). However, this change was only seen in neurons projecting to the NAc mShell, as neurons projecting to the NAc core did not differ from sham-treated mice (Simmons et al. 2019). Surprisingly, we observed an increase in soma size of VTA DA neurons projecting to the PFC following chronic morphine (Fig. 3; Simmons et al. 2019). While unexpected, these contrasting changes in soma size parallel increased dendrite diameter in PB but decreased dendrite diameter in PN regions mentioned earlier (Lane et al. 2008). As decreases in surface area have been linked to increased firing (Coque et al. 2011; Mazei-Robison et al. 2011), these opposing changes likely correlate with neuron activity and output, highlighting and further supporting circuit-specific changes within populations of VTA DA neurons.
Figure 3.
Morphine alters ventral tegmental area (VTA) dopamine (DA) soma size in a projection-specific manner. Chronic opioid exposure alters VTA DA, but not γ-aminobutyric acid (GABA), neuron soma size (Sklair-Tavron et al. 1996; Spiga et al. 2003; Chu et al. 2007; Russo et al. 2007; Mazei-Robison et al. 2011). However, the direction of change is projection specific. DA neurons projecting to the nucleus accumbens (NAc) medial shell (mShell) show the decrease in soma size while those projecting to the core are not different from sham controls (Simmons et al. 2019). It remains unknown whether neurons projecting to the NAc lateral shell (lShell) show a decrease in soma size as well. In contrast, VTA DA neurons projecting to the medial prefrontal cortex (mPFC) show a surprising increase in soma size (Simmons et al. 2019), (PB) Parabrachial, (PN) paranigral. (Circuit mapping summarized from Lammel et al. 2014 and Yang et al. 2018.)
Although VTA DA neurons are the major projection neurons from the VTA, there is evidence for drug-induced dysregulation of VTA GABAergic projections as well. Interestingly, VTA projections to the dorsal raphe (DR) are primarily GABAergic, with rostral VTA projections targeting DR GABA neurons (Li et al. 2019). Optogenetic inhibition of these terminals promotes real-time place preference, consistent with expression of MORs on these terminals, as DR infusion of a MOR agonist attenuates the aversive activation of these terminals (Li et al. 2019). Following chronic morphine exposure, this circuit shows decreased IPSCs of DR GABA neurons, indicating a deficit in inhibitory control from the VTA. In the vein of noncanonical VTA projections, the VTA also sends dopaminergic projections to the hippocampus (HPC). An acute morphine injection causes a potentiation of glutamatergic synapses in the HPC, dependent on morphine action in the VTA and HPC D1 receptor (D1R) signaling (Hu et al. 2014). Moreover, HPC D1R signaling is required for the formation of morphine CPP, indicating that VTA DA output plays a critical role in drug-associated learning and memories (Hu et al. 2014). These studies illustrate that opioids can induce changes within specific subpopulations of VTA neurons and circuit-specific changes may drive distinct aspects of opioid-related behavior.
CONCLUDING REMARKS
The VTA plays a critical role in drug reward, and opioid use causes dysregulation of VTA DA plasticity at the synaptic, cellular, and structural levels. Whereas the effect of acute opioid action on VTA DA neurons is relatively well understood, adaptations with respect to chronic use are understudied and remain an area of future investigation. Whether similar mechanisms are enacted with chronic opioid exposure seems particularly critical to address since opioids are often taken for long periods of time. VTA DA neuronal activity is clearly important for reward behavior, with synaptic plasticity onto VTA DA neurons a well-established mechanism for opioid adaptations. However, recent studies suggest that glial regulation of VTA DA neuronal activity may also be important for reward circuitry function. Notably, morphine exposure robustly activates VTA astrocytes (García-Pérez et al. 2014), and VTA astrocyte activation has been linked to decreased VTA DA neuronal activity and avoidance behavior (Gomez et al. 2019). There is also microglia activation in the VTA of morphine-dependent animals and this activation contributes to disrupted drug reward (Taylor et al. 2016). Interestingly, both astrocyte and microglia activation appear to affect VTA DA function via modulation of VTA GABA neurons (Taylor et al. 2015, 2016; Gomez et al. 2019), reinforcing the necessity of taking the cellular complexity of the VTA into account in future studies. Further, in addition to defining the role of subpopulations of VTA DA projection neurons in addiction-related behaviors, future work should also consider sex as a biological variable (Becker and Koob 2016). Although there are no baseline differences in VTA DA neuron electrophysiological properties (Chung et al. 2017), female rodents self-administer more oxycodone, morphine, and heroin (Cicero et al. 2003; Roth et al. 2004; Phillips et al. 2019), indicative of potential sex differences in the reward circuitry underlying these behaviors. Together, these additional layers of analysis will allow for greater understanding of the neural substrates and mechanisms in the VTA that contribute to the abuse of opioid drugs and may yield insight into novel therapeutic strategies.
Footnotes
Editors: R. Christopher Pierce, Ellen M. Unterwald, and Paul J. Kenny
Additional Perspectives on Addiction available at www.perspectivesinmedicine.org
REFERENCES
- Authement ME, Langlois LD, Kassis H, Gouty S, Dacher M, Shepard RD, Cox BM, Nugent FS. 2016. Morphine-induced synaptic plasticity in the VTA is reversed by HDAC inhibition. J Neurophysiol 116: 1093–1103. 10.1152/jn.00238.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avey D, Sankararaman S, Yim AKY, Barve R, Milbrandt J, Mitra RD. 2018. Single-cell RNA-Seq uncovers a robust transcriptional response to morphine by glia. Cell Rep 24: 3619–3629.e4. 10.1016/j.celrep.2018.08.080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baimel C, Borgland SL. 2015. Orexin signaling in the VTA gates morphine-induced synaptic plasticity. J Neurosci 35: 7295–7303. 10.1523/jneurosci.4385-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballantyne JC, LaForge KS. 2007. Opioid dependence and addiction during opioid treatment of chronic pain. Pain 129: 235–255. 10.1016/j.pain.2007.03.028 [DOI] [PubMed] [Google Scholar]
- Bals-Kubik R, Ableitner A, Herz A, Shippenberg TS. 1993. Neuroanatomical sites mediating the motivational effects of opioids as mapped by the conditioned place preference paradigm in rats. J Pharmacol Exp Ther 264: 489–495. [PubMed] [Google Scholar]
- Barker DJ, Root DH, Zhang S, Morales M. 2016. Multiplexed neurochemical signaling by neurons of the ventral tegmental area. J Chem Neuroanat 73: 33–42. 10.1016/j.jchemneu.2015.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Koob GF. 2016. Sex differences in animal models: focus on addiction. Pharmacol Rev 68: 242–263. 10.1124/pr.115.011163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier KT, Steinberg EE, DeLoach KE, Xie S, Miyamichi K, Schwarz L, Gao XJ, Kremer EJ, Malenka RC, Luo L. 2015. Circuit architecture of VTA dopamine neurons revealed by systematic input–output mapping. Cell 162: 622–634. 10.1016/j.cell.2015.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breton JM, Charbit AR, Snyder BJ, Fong PTK, Dias EV, Himmels P, Lock H, Margolis EB. 2019. Relative contributions and mapping of ventral tegmental area dopamine and GABA neurons by projection target in the rat. J Comp Neurol 527: 916–941. 10.1002/cne.24572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brischoux F, Chakraborty S, Brierley DI, Ungless MA. 2009. Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli. Proc Natl Acad Sci 106: 4894–4899. 10.1073/pnas.0811507106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MT, Bellone C, Mameli M, Labouèbe G, Bocklisch C, Balland B, Dahan L, Luján R, Deisseroth K, Lüscher C. 2010. Drug-driven AMPA receptor redistribution mimicked by selective dopamine neuron stimulation. PLoS ONE 5: e15870 10.1371/journal.pone.0015870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne CJ, Godino A, Salery M, Nestler EJ. 2019. Epigenetic mechanisms of opioid addiction. Biol Psychiatry S0006-3223(19)31510-0 10.1016/j.biopsych.2019.06.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA Jr, Boundy V, Haile C, Lane S, Kalb R, Neve R, Nestler E. 1997. Sensitization to morphine induced by viral-mediated gene transfer. Science 277: 812–815. 10.1126/science.277.5327.812 [DOI] [PubMed] [Google Scholar]
- Chen M, Zhao Y, Yang H, Luan W, Song J, Cui D, Dong Y, Lai B, Ma L, Zheng P. 2015. Morphine disinhibits glutamatergic input to VTA dopamine neurons and promotes dopamine neuron excitation eLife 4: e09275 10.7554/eLife.09275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chieng B, Azriel Y, Mohammadi S, Christie MJ. 2011. Distinct cellular properties of identified dopaminergic and GABAergic neurons in the mouse ventral tegmental area. J Physiol 589: 3775–3787. 10.1113/jphysiol.2011.210807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu NN, Zuo YF, Meng L, Lee DY, Han JS, Cui CL. 2007. Peripheral electrical stimulation reversed the cell size reduction and increased BDNF level in the ventral tegmental area in chronic morphine-treated rats. Brain Res 1182: 90–98. 10.1016/j.brainres.2007.08.086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung AS, Miller SM, Sun Y, Xu X, Zweifel LS. 2017. Sexual congruency in the connectome and translatome of VTA dopamine neurons. Sci Rep 7: 11120 10.1038/s41598-017-11478-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicero TJ, Aylward SC, Meyer ER. 2003. Gender differences in the intravenous self-administration of μ opiate agonists. Pharmacol Biochem Behav 74: 541–549. 10.1016/s0091-3057(02)01039-0 [DOI] [PubMed] [Google Scholar]
- Compton WM, Volkow ND. 2006. Abuse of prescription drugs and the risk of addiction. Drug Alcohol Depend 83 (Suppl. 1): S4–S7. 10.1016/j.drugalcdep.2005.10.020 [DOI] [PubMed] [Google Scholar]
- Cooper S, Garrison A, Hu Q, Heller E, Mazei-Robison M. 2018. Use of TRAP to identify morphine-induced changes in gene expression in ventral tegmental area dopamine neurons. In ACNP 57th Annual Meeting, Poster W244. Neuropsychopharmacology 43: 383–527. 10.1038/s41386-018-0268-5 [DOI] [Google Scholar]
- Coque L, Mukherjee S, Cao JL, Spencer S, Marvin M, Falcon E, Sidor MM, Birnbaum SG, Graham A, Neve RL, et al. 2011. Specific role of VTA dopamine neuronal firing rates and morphology in the reversal of anxiety-related, but not depression-related behavior in the ClockΔ19 mouse model of mania. Neuropsychopharmacology 36: 1478–1488. 10.1038/npp.2011.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corre J, van Zessen R, Loureiro M, Patriarchi T, Tian L, Pascoli V, Lüscher C. 2018. Dopamine neurons projecting to medial shell of the nucleus accumbens drive heroin reinforcement. eLife 7: e39945 10.7554/eLife.39945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacher M, Nugent FS. 2011. Morphine-induced modulation of LTD at GABAergic synapses in the ventral tegmental area. Neuropharmacology 61: 1166–1171. 10.1016/j.neuropharm.2010.11.012 [DOI] [PubMed] [Google Scholar]
- Dacher M, Gouty S, Dash S, Cox BM, Nugent FS. 2013. A-kinase anchoring protein-calcineurin signaling in long-term depression of GABAergic synapses. J Neurosci 33: 2650–2660. 10.1523/jneurosci.2037-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong JW, Afjei SA, Pollak Dorocic I, Peck JR, Liu C, Kim CK, Tian L, Deisseroth K, Lammel S. 2019. A neural circuit mechanism for encoding aversive stimuli in the mesolimbic dopamine system. Neuron 101: 133–151.e7. 10.1016/j.neuron.2018.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiChiara G, Imperato A. 1988. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci 85: 5274–5278. 10.1073/pnas.85.14.5274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle M, Bali V, Neve R, Werner C, Dietz D, Mazei-Robison M. 2017. W273. SGK1 phosphorylation and activity in the VTA regulates drug intake and reward. Neuropsychopharmacology 42: S648. [Google Scholar]
- El-Husseini AED, Schnell E, Chetkovich DM, Nicoll RA, Bredt DS. 2000. PSD-95 involvement in maturation of excitatory synapses. Science 290: 1364–1368. [PubMed] [Google Scholar]
- Fields HL. 2011. The doctor's dilemma: opiate analgesics and chronic pain. Neuron 69: 591–594. 10.1016/j.neuron.2011.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald LW, Ortiz J, Hamedani AG, Nestler EJ. 1996. Drugs of abuse and stress increase the expression of GluR1 and NMDAR1 glutamate receptor subunits in the rat ventral tegmental area: common adaptations among cross-sensitizing agents. J Neuorsci 16: 274–282. 10.1523/jneurosci.16-01-00274.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Pérez D, Luisa Laorden M, Núñez C, Victoria Milanes M. 2014. Glial activation and midkine and pleiotrophin transcription in the ventral tegmental area are modulated by morphine administration. J Neuroimmunol 274: 244–248. 10.1016/j.jneuroim.2014.07.017 [DOI] [PubMed] [Google Scholar]
- Georges F, Le Moine C, Aston-Jones G. 2006. No effect of morphine on ventral tegmental dopamine neurons during withdrawal. J Neurosci 26: 5720–5726. 10.1523/jneurosci.5032-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez JA, Perkins JM, Beaudoin GM, Cook NB, Quraishi SA, Szoeke EA, Thangamani K, Tschumi CW, Wanat MJ, Maroof AM, et al. 2019. Ventral tegmental area astrocytes orchestrate avoidance and approach behavior. Nat Commun 10: 1455 10.1038/s41467-019-09131-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziane NM, Sun S, Wright WJ, Jang D, Liu Z, Huang YH, Nestler EJ, Wang YT, Schlüter OM, Dong Y. 2016. Opposing mechanisms mediate morphine- and cocaine-induced generation of silent synapses. Nat Neurosci 19: 915–925. 10.1038/nn.4313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gysling K, Wang RY. 1983. Morphine-induced activation of A10 dopamine neurons in the rat. Brain Res 277: 119–127. 10.1016/0006-8993(83)90913-7 [DOI] [PubMed] [Google Scholar]
- Han B, Compton WM, Blanco C, Crane E, Lee J, Jones CM. 2017. Prescription opioid use, misuse, and use disorders in U.S. adults: 2015 National Survey on Drug Use and Health. Ann Intern Med 167: 293–301. 10.7326/M17-0865 [DOI] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Byrne R, Aston-Jones G. 2004. Glutamate-associated plasticity in the ventral tegmental area is necessary for conditioning environmental stimuli with morphine. Neuroscience 129: 841–847. 10.1016/j.neuroscience.2004.09.018 [DOI] [PubMed] [Google Scholar]
- Heller EA, Kaska S, Fallon B, Ferguson D, Kennedy PJ, Neve RL, Nestler EJ, Mazei-Robison MS. 2015. Morphine and cocaine increase serum- and glucocorticoid-inducible kinase 1 activity in the ventral tegmental area. J Neurochem 132: 243–253. 10.1111/jnc.12925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L, Jing XH, Cui CL, Xing GG, Zhu B. 2014. NMDA receptors in the midbrain play a critical role in dopamine-mediated hippocampal synaptic potentiation caused by morphine. Addict Biol 19: 380–391. 10.1111/adb.12010 [DOI] [PubMed] [Google Scholar]
- Jalabert M, Bourdy R, Courtin J, Veinante P, Manzoni OJ, Barrot M, Georges F. 2011. Neuronal circuits underlying acute morphine action on dopamine neurons. Proc Natl Acad Sci 108: 16446–16450. 10.1073/pnas.1105418108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SW, North RA. 1992. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neuorsci 12: 483–488. 10.1523/jneurosci.12-02-00483.1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juarez B, Han MH. 2016. Diversity of dopaminergic neural circuits in response to drug exposure. Neuropsychopharmacology 41: 2424–2446. 10.1038/npp.2016.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo J, Mazei-Robison MS, Chaudhury D, Juarez B, LaPlant Q, Ferguson D, Feng J, Sun H, Scobie KN, Damez-Werno D, et al. 2012. BDNF is a negative modulator of morphine action. Science 338: 124–128. 10.1126/science.1222265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo JW, Mazei-Robison MS, LaPlant Q, Egervari G, Braunscheidel KM, Adank DN, Ferguson D, Feng J, Sun H, Scobie KN, et al. 2015. Epigenetic basis of opiate suppression of Bdnf gene expression in the ventral tegmental area. Nat Neurosci 18: 415–422. 10.1038/nn.3932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Hetzel A, Häckel O, Jones I, Liss B, Roeper J. 2008. Unique properties of mesoprefrontal neurons within a dual mesocorticolimbic dopamine system. Neuron 57: 760–773. 10.1016/j.neuron.2008.01.022 [DOI] [PubMed] [Google Scholar]
- Lammel S, Ion DI, Roeper J, Malenka RC. 2011. Projection-specific modulation of dopamine neuron synapses by aversive and rewarding stimuli. Neuron 70: 855–862. 10.1016/j.neuron.2011.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Lim BK, Ran C, Huang KW, Betley MJ, Tye KM, Deisseroth K, Malenka RC. 2012. Input-specific control of reward and aversion in the ventral tegmental area. Nature 491: 212–217. 10.1038/nature11527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Lim BK, Malenka RC. 2014. Reward and aversion in a heterogeneous midbrain dopamine system. Neuropharmacology 76: 351–359. 10.1016/j.neuropharm.2013.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane DA, Lessard AA, Chan J, Colago EE, Zhou Y, Schlussman SD, Kreek MJ, Pickel VM. 2008. Region-specific changes in the subcellular distribution of AMPA receptor GluR1 subunit in the rat ventral tegmental area after acute or chronic morphine administration. J Neurosci 28: 9670–9681. 10.1523/jneurosci.2151-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois LD, Nugent FS. 2017. Opiates and plasticity in the ventral tegmental area. ACS Chem Neurosci 8: 1830–1838. 10.1021/acschemneuro.7b00281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviolette SR, Gallegos RA, Henriksen SJ, van der Kooy D. 2004. Opiate state controls bi-directional reward signaling via GABAA receptors in the ventral tegmental area. Nat Neurosci 7: 160–169. 10.1038/nn1182 [DOI] [PubMed] [Google Scholar]
- Leone P, Pocock D, Wise RA. 1991. Morphine-dopamine interaction: ventral tegmental morphine increases nucleus accumbens dopamine release. Pharmacol Biochem Behav 39: 469–472. 10.1016/0091-3057(91)90210-S [DOI] [PubMed] [Google Scholar]
- Li Y, Li CY, Xi W, Jin S, Wu ZH, Jiang P, Dong P, He XB, Xu FQ, Duan S, et al. 2019. Rostral and caudal ventral tegmental area GABAergic inputs to different dorsal raphe neurons participate in opioid dependence. Neuron 101: 748–761.e5. 10.1016/j.neuron.2018.12.012 [DOI] [PubMed] [Google Scholar]
- Liu QS, Pu L, Poo MM. 2005. Repeated cocaine exposure in vivo facilitates LTP induction in midbrain dopamine neurons. Nature 437: 1027–1031. 10.1038/nature04050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Li Y, Yu L, Vickstrom CR, Liu QS. 2018. VTA mTOR signaling regulates dopamine dynamics, cocaine-induced synaptic alterations, and reward. Neuropsychopharmacology 43: 1066–1077. 10.1038/npp.2017.247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüscher C, Malenka RC. 2011. Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron 69: 650–663. 10.1016/j.neuron.2011.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manchikanti L, Fellows B, Ailinani H, Pampati V. 2010. Therapeutic use, abuse, and nonmedical use of opioids: a ten-year perspective. Pain Physician 13: 401–435. [PubMed] [Google Scholar]
- Manchikanti L, Sanapati J, Benyamin RM, Atluri S, Kaye AD, Hirsch JA. 2018. Reframing the prevention strategies of the opioid crisis: focusing on prescription opioids, fentanyl, and heroin epidemic. Pain Physician 21: 309–326. 10.36076/ppj.2018.4.309 [DOI] [PubMed] [Google Scholar]
- Margolis EB, Lock H, Hjelmstad GO, Fields HL. 2006. The ventral tegmental area revisited: is there an electrophysiological marker for dopaminergic neurons? J Physiol 577: 907–924. 10.1113/jphysiol.2006.117069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Hjelmstad GO, Fujita W, Fields HL. 2014. Direct bidirectional μ-opioid control of midbrain dopamine neurons. J Neurosci 34: 14707–14716. 10.1523/jneurosci.2144-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews RT, German DC. 1984. Electrophysiological evidence for excitation of rat ventral tegmental area dopamine neurons by morphine. Neuroscience 11: 617–625. 10.1016/0306-4522(84)90048-4 [DOI] [PubMed] [Google Scholar]
- Mazei-Robison MS, Koo JW, Friedman AK, Lansink CS, Robison AJ, Vinish M, Krishnan V, Kim S, Siuta MA, Galli A, et al. 2011. Role for mTOR signaling and neuronal activity in morphine-induced adaptations in ventral tegmental area dopamine neurons. Neuron 72: 977–990. 10.1016/j.neuron.2011.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazei-Robison MS, Appasani R, Edwards S, Wee S, Taylor SR, Picciotto MR, Koob GF, Nestler EJ. 2014. Self-administration of ethanol, cocaine, or nicotine does not decrease the soma size of ventral tegmental area dopamine neurons. PLoS ONE 9: e95962 10.1371/journal.pone.0095962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung CA, Nestler EJ, Zachariou V. 2005. Regulation of gene expression by chronic morphine and morphine withdrawal in the locus ceruleus and ventral tegmental area. J Neurosci 25: 6005–6015. 10.1523/jneurosci.0062-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis M, Camarini R, Ungless MA, Bonci A. 2002. Long-lasting potentiation of GABAergic synapses in dopamine neurons after a single in vivo ethanol exposure. J Neuorsci 22: 2074–2082. 10.1523/jneurosci.22-06-02074.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales M, Margolis EB. 2017. Ventral tegmental area: cellular heterogeneity, connectivity and behaviour. Nat Rev Neurosci 18: 73–85. 10.1038/nrn.2016.165 [DOI] [PubMed] [Google Scholar]
- Nair-Roberts RG, Chatelain-Badie SD, Benson E, White-Cooper H, Bolam JP, Ungless MA. 2008. Stereological estimates of dopaminergic, GABAergic and glutamatergic neurons in the ventral tegmental area, substantia nigra and retrorubral field in the rat. Neuroscience 152: 1024–1031. 10.1016/j.neuroscience.2008.01.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ. 2004. Historical review: molecular and cellular mechanisms of opiate and cocaine addiction. Trends Pharmacol Sci 25: 210–218. 10.1016/j.tips.2004.02.005 [DOI] [PubMed] [Google Scholar]
- Niehaus JL, Murali M, Kauer JA. 2010. Drugs of abuse and stress impair LTP at inhibitory synapses in the ventral tegmental area. Eur J Neurosci 32: 108–117. 10.1111/j.1460-9568.2010.07256.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent FS, Penick EC, Kauer JA. 2007. Opioids block long-term potentiation of inhibitory synapses. Nature 446: 1086–1090. 10.1038/nature05726 [DOI] [PubMed] [Google Scholar]
- Nugent FS, Niehaus JL, Kauer JA. 2009. PKG and PKA signaling in LTP at GABAergic synapses. Neuropsychopharmacology 34: 1829–1842. 10.1038/npp.2009.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowski NL, Hatfield CB, Caggiula AR. 1982. The effects of low doses of morphine on the activity of dopamine-containing cells and on behavior. Life Sci 31: 2347–2350. 10.1016/0024-3205(82)90153-9 [DOI] [PubMed] [Google Scholar]
- Phillips AG, McGovern DJ, Lee S, Ro K, Huynh DT, Elvig SK, Fegan KN, Root DH. 2019. Oral prescription opioid-seeking behavior in male and female mice. Addict Biol e12828 10.1111/adb.12828 [DOI] [PubMed] [Google Scholar]
- Phillipson OT. 1979. A Golgi study of the ventral tegmental area of Tsai and interfascicular nucleus in the rat. J Comp Neurol 187: 99–115. 10.1002/cne.901870107 [DOI] [PubMed] [Google Scholar]
- Pitchers KK, Coppens CM, Beloate LN, Fuller J, Van S, Frohmader KS, Laviolette SR, Lehman MN, Coolen LM. 2014. Endogenous opioid-induced neuroplasticity of dopaminergic neurons in the ventral tegmental area influences natural and opiate reward. J Neurosci 34: 8825–8836. 10.1523/jneurosci.0133-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulin JF, Caronia G, Hofer C, Cui Q, Helm B, Ramakrishnan C, Chan CS, Dombeck DA, Deisseroth K, Awatramani R. 2018. Mapping projections of molecularly defined dopamine neuron subtypes using intersectional genetic approaches. Nat Neurosci 21: 1260–1271. 10.1038/s41593-018-0203-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. 1999. Morphine alters the structure of neurons in the nucleus accumbens and neocortex of rats. Synapse 33: 160–162. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Gorny G, Savage VR, Kolb B. 2002. Widespread but regionally specific effects of experimenter- versus self-administered morphine on dendritic spines in the nucleus accumbens, hippocampus, and neocortex of adult rats. Synapse 46: 271–279. 10.1002/syn.10146 [DOI] [PubMed] [Google Scholar]
- Roth ME, Cosgrove KP, Carroll ME. 2004. Sex differences in the vulnerability to drug abuse: a review of preclinical studies. Neurosci Biobehav Rev 28: 533–546. 10.1016/j.neubiorev.2004.08.001 [DOI] [PubMed] [Google Scholar]
- Russo SJ, Bolanos CA, Theobald DE, DeCarolis NA, Renthal W, Kumar A, Winstanley CA, Renthal NE, Wiley MD, Self DW, et al. 2007. IRS2-Akt pathway in midbrain dopamine neurons regulates behavioral and cellular responses to opiates. Nat Neurosci 10: 93–99. 10.1038/nn1812 [DOI] [PubMed] [Google Scholar]
- Russo SJ, Dietz DM, Dumitriu D, Morrison JH, Malenka RC, Nestler EJ. 2010. The addicted synapse: mechanisms of synaptic and structural plasticity in nucleus accumbens. Trends Neurosci 33: 267–276. 10.1016/j.tins.2010.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saal D, Dong Y, Bonci A, Malenka RC. 2003. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron 37: 577–582. 10.1016/S0896-6273(03)00021-7 [DOI] [PubMed] [Google Scholar]
- Sarti F, Borgland SL, Kharazia VN, Bonci A. 2007. Acute cocaine exposure alters spine density and long-term potentiation in the ventral tegmental area. Eur J Neurosci 26: 749–756. 10.1111/j.1460-9568.2007.05689.x [DOI] [PubMed] [Google Scholar]
- Sesack SR, Grace AA. 2010. Cortico-basal ganglia reward network: microcircuitry. Neuropsychopharmacology 35: 27–47. 10.1038/npp.2009.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons SC, Wheeler K, Mazei-Robison MS. 2019. Determination of circuit-specific morphological adaptations in ventral tegmental area dopamine neurons by chronic morphine. Mol Brain 12: 10 10.1186/s13041-019-0435-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sklair-Tavron L, Shi W, Lane S, Harris H, Bunney B, Nestler E. 1996. Chronic morphine induces visible changes in the morphology of mesolimbic dopamine neurons. Proc Natl Acad Sci 93: 11202–11207. 10.1073/pnas.93.20.11202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiga S, Serra GP, Puddu MC, Foddai M, Diana M. 2003. Morphine withdrawal-induced abnormalities in the VTA: confocal laser scanning microscopy. Eur J Neurosci 17: 605–612. 10.1046/j.1460-9568.2003.02435.x [DOI] [PubMed] [Google Scholar]
- Swanson LW. 1982. The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res Bull 9: 321–353. 10.1016/0361-9230(82)90145-9 [DOI] [PubMed] [Google Scholar]
- Taylor AM, Castonguay A, Taylor AJ, Murphy NP, Ghogha A, Cook C, Xue L, Olmstead MC, De Koninck Y, Evans CJ, et al. 2015. Microglia disrupt mesolimbic reward circuitry in chronic pain. J Neurosci 35: 8442–8450. 10.1523/jneurosci.4036-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AM, Castonguay A, Ghogha A, Vayssiere P, Pradhan AA, Xue L, Mehrabani S, Wu J, Levitt P, Olmstead MC, et al. 2016. Neuroimmune regulation of GABAergic neurons within the ventral tegmental area during withdrawal from chronic morphine. Neuropsychopharmacology 41: 949–959. 10.1038/npp.2015.221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungless MA, Whistler JL, Malenka RC, Bonci A. 2001. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature 411: 583–587. 10.1038/35079077 [DOI] [PubMed] [Google Scholar]
- Vargas-Perez H, Ting-A-Kee R, Walton CH, Hansen DM, Razavi R, Clarke L, Bufalino MR, Allison DW, Steffensen SC, van der Kooy D. 2009. Ventral tegmental area BDNF induces an opiate-dependent-like reward state in naive rats. Science 324: 1732–1734. 10.1126/science.1168501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas-Perez H, Bahi A, Bufalino MR, Ting-A-Kee R, Maal-Bared G, Lam J, Fahmy A, Clarke L, Blanchard JK, Larsen BR, et al. 2014. BDNF signaling in the VTA links the drug-dependent state to drug withdrawal aversions. J Neurosci 34: 7899–7909. 10.1523/jneurosci.3776-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Koroshetz WJ. 2019. The role of neurologists in tackling the opioid epidemic. Nat Rev Neurol 15: 301–305. 10.1038/s41582-019-0146-8 [DOI] [PubMed] [Google Scholar]
- Wang Z, Yan P, Hui T, Zhang J. 2014. Epigenetic upregulation of PSD-95 contributes to the rewarding behavior by morphine conditioning. Eur J Pharmacol 732: 123–129. 10.1016/j.ejphar.2014.03.040 [DOI] [PubMed] [Google Scholar]
- Watabe-Uchida M, Zhu L, Ogawa SK, Vamanrao A, Uchida N. 2012. Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron 74: 858–873. 10.1016/j.neuron.2012.03.017 [DOI] [PubMed] [Google Scholar]
- Williams JT, Christie MJ, Manzoni O. 2001. Cellular and synaptic adaptations mediating opioid dependence. Physiol Rev 81: 299–343. 10.1152/physrev.2001.81.1.299 [DOI] [PubMed] [Google Scholar]
- Wolf ME. 2016. Synaptic mechanisms underlying persistent cocaine craving. Nat Rev Neurosci 17: 351–365. 10.1038/nrn.2016.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Stein EA. 2002. Blockade of ionotropic glutamatergic transmission in the ventral tegmental area reduces heroin reinforcement in rat. Psychopharmacology (Berl) 164: 144–150. 10.1007/s00213-002-1190-3 [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Sheen W, Morales M. 2007. Glutamatergic neurons are present in the rat ventral tegmental area. Eur J Neurosci 25: 106–118. 10.1111/j.1460-9568.2006.05263.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, de Jong JW, Tak Y, Peck J, Bateup HS, Lammel S. 2018. Nucleus accumbens subnuclei regulate motivated behavior via direct inhibition and disinhibition of VTA dopamine subpopulations. Neuron 97: 434–449.e4. 10.1016/j.neuron.2017.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Landthaler M, Schlussman SD, Yuferov V, Ho A, Tuschl T, Kreek MJ. 2009. μ-Opioid receptor knockdown in the substantia nigra/ventral tegmental area by synthetic small interfering RNA blocks the rewarding and locomotor effects of heroin. Neuroscience 158: 474–483. 10.1016/j.neuroscience.2008.09.039 [DOI] [PMC free article] [PubMed] [Google Scholar]