Abstract
The co-existence of humans and gut microbiota started millions of years ago. Until now, a balance gradually developed between gut bacteria and their hosts. It is now recognized that gut microbiota are key to form adequate immune and metabolic functions and, more in general, for the maintenance of good health. Gut microbiota are established before birth under the influence of maternal nutrition and metabolic status, which can impact the future metabolic risk of the offspring in terms of obesity, diabetes, and cardiometabolic disorders during the lifespan. Obesity and diabetes are prone to disrupt the gut microbiota and alter the gut barrier permeability, leading to metabolic endotoxaemia with its detrimental consequences on health. Specific bacterial sequences are now viewed as peculiar signatures of the metabolic syndrome across life stages in each individual, and are linked to pathogenesis of cardiovascular diseases (CVDs) via metabolic products (metabolites) and immune modulation. These mechanisms have been linked, in association with abnormalities in microbial richness and diversity, to an increased risk of developing arterial hypertension, systemic inflammation, nonalcoholic fatty liver disease, coronary artery disease, chronic kidney disease, and heart failure. Emerging strategies for the manipulation of intestinal microbiota represent a promising therapeutic option for the prevention and treatment of CVD especially in individuals prone to CV events.
Introduction
The World Health Organizations affirmed that cardiovascular diseases (CVDs) are responsible for the death of 17.9 million people per year, which corresponds to 31% of all deaths [1]. Of these, 85% are attributed to heart attack and stroke. The spectrum of noncommunicable disorders generally classified as CVDs includes arterial hypertension, coronary artery disease (CAD), and cardiomyopathies that promote heart failure (HF) and cerebrovascular diseases in later stages. Vasculature instabilities at the level of endothelial function and arterial stiffness can instigate the development of CVDs. Significant progress has accompanied our recent understanding of the relationship linking gut microbiota to metabolic disorders, particularly diabetes, and obesity, foretelling the onset and progression of CVDs. This review will explore the main findings supporting a functional role for gut microbiota in the pathophysiology and progression of CVDs and their metabolic correlates. Insights on therapeutic approaches aiding microbiota restoration in connection with CVD will also be discussed.
Background, molecules and mechanisms of microbiota actions
The microbiota are a complex ecosystem composed of about 1014 microorganisms. They include bacteria, eukaryotes, viruses, and archaea, located in body regions that interact with the external world such as the skin and organs belonging to the respiratory, gastrointestinal, and urogenital systems. Up to 80% of microbiota is represented by Firmicutes and Bacteroidetes [2], with other phyla including Proteobacteria, Verrucomicrobia, Actinobacteria, and Fusobacteria. The whole genetic heritage of the microbiota, the microbiome, approximately encloses 3.3 millions of genes. High-throughput sequencing technologies have dramatically improved our understanding of the complex relationship linking the microbiome to the host through the encoding of multiple active metabolites that are capable of bidirectional interactions, both at the molecular and cellular level. The human microbiota work as a dynamic entity competent for self-modulation (resilience and resistance phenomenon) in response to external stressors during conventional life events [3]. Exogenous factors occurring early in life like the type of birthing, the type of milk feeding, antibiotics, and dietary habits can influence intra- and interindividual qualitative and quantitative variability of microbiota. Intrinsic factors are also known to modulate microbial composition and abundance, such as pH, gut motility, mucus, and antimicrobial peptides. Arumugam et al. [4] proposed the division of the human microbiota into three main subgroups, termed enterotypes. These include Bacteroides, Prevotella, and Ruminococcus, which shape the individual core microbiota and tend to remain stable after temporary perturbations.
Locally, an adequate gut microbiota is key to maintain unaltered the integrity and permeability of the intestinal epithelial barrier. At the systemic level, gut microbiota contribute to promote the maturation of the enteric nervous system, develop innate and adaptive immunity, and maintain body homeostasis [5–8]. Metabolic phenotyping of microbiota enterotypes displays intrinsic functions that, depending on bacterial strain prevalence, are instrumental to (a) help the host to absorb fats and fat-soluble vitamins contained in the diet, (b) digest complex carbohydrates and plant polysaccharides via enzymes that are not expressed by the human genome, and (c) partake in bile acid-related metabolism. Intestinal processing through fermentation of different nondigestible food components present in the human diet by colonic microbes leads to the production of short-chain fatty acids (SCFAs; acetate, propionate, and butyrate), trimethylamine-N-oxide (TMAO), bile acids, incretin hormones, polyamines, polyphenols, and vitamins. In turn, SCFAs act as signaling molecules and source of energy for colonic epithelial cells [9, 10]. Other molecules can trigger genetic and epigenetic pathways so as to promote the reprogramming of the cell genome in response to environmental stimuli, or to shape the immune system during the weaning phase [11]. In physiological conditions, the activity of the gut microbiota and the stability of the gut barrier are preserved through the function of tight junction proteins, a normal endocannabinoid system tone and lipopolysaccharide (LPS) detoxification by intestinal alkaline phosphatase [12]. LPS is a microorganism-derived proinflammatory component that is continuously released into the colon upon the death of gram-negative bacteria. High-fat diet (HFD) is particularly effective in altering microbiota composition [13] and favor LPS absorption across the intestinal barrier through chylomicrons [14], thereby prompting a condition of systemic inflammation. A healthy lifestyle and an appropriate dietary intake are thus essential components for the balance between microbiota and host.
Quantitative and qualitative alterations of microbiota communities, a condition often referred to as dysbiosis, has been shown to be associated with several human disorders such as obesity, diabetes mellitus, CVDs, asthma, inflammatory bowel disease, as well as neurodegenerative and psychiatric disorders. A metagenome-wide association study (MGWAS) on people with type 2 diabetes showed a moderate degree of gut microbial dysbiosis, decreased butyrate-producing bacteria and increased opportunistic pathogens conventionally capable of inducing bacteraemic infections (Bacteroides caccae, Cl. hathewayi, Cl. ramosum, Cl. symbiosum, Eggerthella lenta, and E. coli), as well as an enrichment of other microbial functions conferring sulfate reduction and oxidative stress resistance [15].
The role of metabolic phenotypes
Obesity and diabetes are accepted emblems of metabolic impairment. However, obesity is per se a heterogeneous condition, as exemplified by different phenotypes that can be identified in association with metabolic and CVD risk. Recognizing the significance of this spectrum is key to understand the varying degree of metabolic impairment associated with nonobese and obese phenotypes. These include metabolically unhealthy obese (MUO), metabolically healthy obese (MHO), and normal weight obese subjects (NWO) [16]. MUO are generally characterized by a body mass index (BMI) > 30 kg/m2, high visceral fat mass, increases in proinflammatory adipocytokines exacerbating adipose tissue (AT) dysfunction, proatherogenic state, and dyslipidemia. Hence, MUO individuals are particularly prone to develop type 2 diabetes, nonalcoholic fatty liver disease (NAFLD), and atherosclerotic CVD [17–20]. Conversely, MHO individuals show a BMI > 30 kg/m2 with a percentage of body fat (BF) mass > 30% and waist circumference > 90 cm, but they harbor normal lipid and BP profiles, and show a rather healthy insulin sensitivity [21]. Patients with the MHO profile are thus less exposed to the risk of metabolic complications normally associated with obesity, possibly as the result of early-onset obesity (<20 years), which would allow implementation of adaptive mechanisms and preserve insulin sensitivity. Also relevant is the proportion of visceral fat and its related detrimental effects on insulin sensitivity, being relatively lower in MHO than in MUO. However, when endothelial parameters of MHO are plotted against those of lean subjects, an increase in intima media thickness and a reduced endothelial function can be documented in MHO subjects, in spite of their apparently normal metabolic profile [22]. Moreover, lifestyle and diet change have no effect on BMI modifications in these subjects [23].
As far as the NWO phenotype is regarded, this describes the profile of subjects with normal body weight and BMI (<25 kg/m2) who harbor an increased total body fat mass percentage (>30% in female; >25% in male) [24, 25]. The NWO phenotype thus presents with normal weight and metabolically healthy AT excess, but is characterized by chronic low-grade inflammation and is thus at increased risk for CVDs. In addition to NWO, researchers have also conceptualized another metabolic phenotype represented by persons with metabolically obese normal weight (MONW), in whom premature cardiometabolic impairment hyperinsulinemia, dyslipidemia, and propensity to increased CVD risk may occur in spite of a normal BMI [26–28]. In particular, NWOs do not carry the overtly adipose body composition of MHOs, and are distinguished from MONWs owing to a relatively healthier metabolic profile that does not predispose them to the metabolic syndrome. However, NWO are exposed to the consequences of a chronic low-grade inflammatory state. Individuals with NWO show, in fact, increased circulating levels of interleukin (IL)-1α, IL-1β, IL-6, IL-8, and tumor necrosis factor-α, which are correlated with the increased fat mass percentage [29]. In addition, CV risk markers are borderline normal in NWO whereas some CVD risk indices, i.e., those linked to an atherogenic lipid profile and chronic proinflammatory state, are reminiscent of those documented in populations with overt obesity [30]. Moreover, glutathione, nitric oxide (NO) metabolites (NO2−/NO3−), and antioxidant nonprotein capacity levels are generally lower in NWO women than controls from the normal population. In contrast, lipid hydroperoxide levels are higher in NWO compared with normal weight subjects, highlighting that NWO women are also unprotected against oxidative stress related to metabolic abnormalities [31]. Further, the NWO syndrome can be associated with the presence of polymorphisms responsible for inflammation, CVD, and sarcopenia [32, 33]. Corroborating these findings, other studies reported that (a) NWO is independently associated with cardiometabolic deregulation and risk of CV mortality, (b) blood pressure (BP) values and prevalence of dyslipidaemia/hyperglycemia are higher in NWO than lean women and, (c) odd ratios for the presence of at least two nonadipose components of the metabolic syndrome increase with the severity of NWO and associate with an increased CVD risk independent of BMI [34, 35]. Lastly, NWO women manifest increased anxiety scores and attention to weight control, with an excessive body dissatisfaction and drive for thinness [36].
In NWO and obese women, modulation of the microbiota with selected probiotics (Streptococcus thermophilus, Bifidobacterium animalis subsp lactis, Streptococcus thermophiles, Lactobacillus bulgaricus, Lactococcus lactis subsp lactis, Lactobacillus acidophilus, Lactobacillus Plantarum, Lactobacillus Reuteri) [37] has been found to be associated with a change in body composition parameters, such as weight, waist girth, body water, and BF while improving the depression status, anxiety, body dissatisfaction, and eating behavior [38].
Hence, it is crucial to identify phenotypes at CV risk not only by assessing anthropometric parameters (e.g., BMI), but also by measuring the total amount of BF, regional fat distribution, visceral, and/or ectopic fat accumulation that is responsible for the secretion of adipokines and bioactive molecules both related to metabolic disorders and gut microbiota [39, 40].
Impact of nutriepigenetic and personalized nutrition on microbiota
Epigenetic factors suggested to mediate interactions between the environment and the genome [41] involve DNA methylation, histone modifications, and regulation of genes by small noncoding regulatory RNAs (miRNA), which are capable of posttranscriptionally repression of gene expression through interaction with the complementary sites in the 3ʹ untranslated regions of target genes. In this context, DNA methylation has received particular attention [42]. Among the nutrients that play a role in modulating DNA methylation are methionine, folic acid, choline, betaine and vitamins B2, B6, and B12 [43]. In addition, also polyphenols, including those contained in green tea, soy, and turmeric, such as epigallocatechin-3-gallate, genistein, and curcumin, show epigenetic effects, modulating gene expression, chromatin remodeling, and DNA methylation [44, 45]. In the last decade, the role of methylation has been thoroughly investigated in the attempt to correlate microbiota changes with the development of metabolic diseases [46]. Gut microbiota may alter host histone acetylation and methylation in human tissues. SCFAs production seems particularly relevant for epigenetic regulation of inflammatory reactions. In fact, a reduction in the consumption of fiber-rich foods resulted in lower production of SCFAs by gut bacteria, with consequent alteration of chromatin [47, 48].
Despite the need for more accurate information on how diet can change epigenetics via microbiota, evidence exists that gut bacteria can communicate with the host through their metabolites, influencing gene transcription and potentially driving the development of noncommunicable diseases.
Microbiota and cardiovascular diseases
Obesity and the metabolic syndrome
Epidemiologic studies highlighted direct and indirect effects of obesity on several CV risk factors such as dyslipidaemia, hypertension, glucose intolerance and diabetes, CAD as well as several types of cancer [20, 49]. AT expansion and dysfunction modulate, in coordinated fashion with infiltrating macrophages and leukocytes, the secretory profile of adipocytokines that are capable of ensuing a proinflammatory state linked with the metabolic complications of obesity. Gut microbiota is postulated not to be an innocent bystander but, rather, one of the factors influencing the genesis of obesity and the different components of the metabolic syndrome (Fig. 1). Mechanisms proposed to explain this role include the regulation of energy extraction from nutrients and the ability of microorganism to ferment undigested dietary polysaccharides generating SCFAs [50]. Generally, an “obese microbiota” has been characterized to be able of extracting more energy from the diet [51]. SCFAs have been shown to induce lipogenesis, increase triglyceride levels, and favor transcription factor-1 binding, the sterol regulatory elements involved in lipogenesis [50]. Another mechanism resides in the ability of microbiota to decrease liver fatty acid oxidation by suppressing the adenosine monophosphate kinase (AMPK), a mediator of cellular energy expressed in the liver and muscle fibers [52]. It has also been demonstrated that fasting-induced adipose factor, a circulating lipoprotein lipase inhibitor, increased cellular uptake of fatty acids, and adipocyte triglyceride accumulation [53]. Interestingly, overgrowth of Lactobacillus, E. coli, and Faecalibacterium show increased ability for the extraction of calories from food and may contribute to the positive energy state associated with obesity [54]. Evidence has also accumulated linking the metabolic syndrome to dysbiosis and metabolic endotoxemia, with toll-like receptor-2 (TLR-2)-mediated inflammatory response being stimulated by LPS, which subsequently increases the secretion of proinflammatory cytokines [55].
Fig. 1. Interplay between metabolic dysfunction, microbiota and cardiometabolic problems.
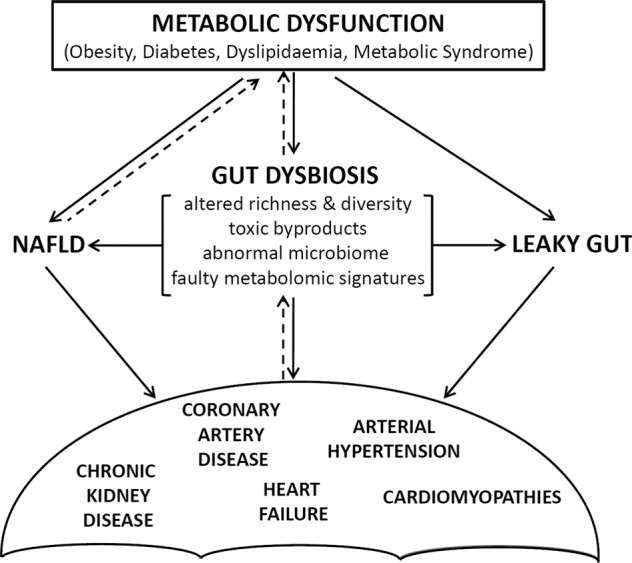
Summary of the relationships between cardiovascular diseases and gut microbiota through chemical and mechanical microbial activities. NAFLD nonalcoholic fatty liver disease.
Bacterial populations of obese subjects show low levels of microbial diversity with respect to lean subjects [56, 57]. Under specific conditions, this form of dysbiosis can contribute to the development of the diseases [58]. The crosstalk between gut microbiota, diet, and immune system activates mediators and signaling pathways, which influence metabolism and disease related to BF accumulation. A reduction of Bifidobacterium and Atopobium was observed in obese compared with the nonobese rats in combination with higher abundance of Clostridium cluster XIVa and Lactobacillus. The specific role of diet as a factor contributing to the obese phenotype is corroborated by the finding that HFD induces a reduction in the Clostridium cluster XIVa (Clostridium coccoides) group, Bifidobacterium, and Bacteroides while increasing Firmicutes [59]. Angelakis et al. [54] reported a higher abundance of Bacteroidetes phylum in the gut microbiota of obese and overweight adults compared with lean individuals. However, the type of diet does not seem to change the balance between Firmicutes and Bacteroidetes in the obese state, where an increase in Firmicutes is generally observed [60, 61]. A similar profile of increased Firmicutes/Bacteroidetes ratio is observed in the fecal microbiota of obese humans [62, 63].
The open question, therefore, remains whether dysbiosis observed in obese subjects is a consequence of host genotype or another result of overnutrition. The simultaneous presence within the gastrointestinal tract of obese subjects of H2-producing Prevotellaceae and H2-utilizing methanogenic Archaea, like Firmicutes, is claimed to explain the accelerated fermentation, the increase in the production of SCFAs, and high energy uptake [64].
Intriguingly, the severity of obesity elicits an additional effect on microbiota diversity and expression. A reduced microbial gene richness has been documented in 75% of patients with severe obesity versus 40% of those with moderate obesity [65, 66]. Significant differences exist in α diversity, β diversity, and species between patients with severe obesity and controls [67]. In extremely obese classes, however, low bacterial richness is only modestly influenced by bariatric surgery despite underlying metabolic improvements [65]. This suggests that weight loss and metabolic amelioration determined by bariatric surgery may not be directly related to changes in bacterial species and bacterial richness.
Diabetes mellitus and dyslipidemia
Evidence on the role of dysbiosis in glycaemia and lipid abnormalities provides a tool for understanding the pathophysiology and the treatment of diseases such as diabetes mellitus and dyslipidemias. Significant differences in microbiota composition exist between healthy and diabetic subjects, and the mechanisms proposed to explain the influence of microbiota on insulin resistance include metabolic endotoxemia, low-grade chronic inflammation, modifications in the secretion of the incretins and butyrate production [68, 69]. Metagenome studies in Chinese and Scandinavian diabetic cohorts identified a reduced abundance of butyrate-producing bacteria (Roseburia intestinalis and Faecalibacterium prausnitzii) and an increased expression of microbial genes involved in oxidative stress, LPS generation, and intestinal permeability, all representing proinflammatory signatures [15, 70]. People with obesity and diabetes have been repeatedly shown to host excessive proliferation of gram-negative bacteria, abnormal LPS release with consequent increased intestinal permeability and endotoxemia, which act on TLR and innate immunity to determine the release of proinflammatory cytokines and define a condition of systemic inflammation [15, 68, 71]. By contrast, human and animal studies provided evidence that some bacterial species, such as the mucin-degrading gram-negative Akkermansia muciniphila, can benefit metabolic homeostasis, glucose tolerance, and systemic inflammation. In fact, its abundance is inversely correlated with the presence of diabetes and obesity [72–74] and increases during metformin treatment [75].
With regards to lipid metabolism, intestinal microbes are involved in hepatic lipid and bile metabolism, reverse cholesterol transport, energy expenditure, and insulin sensitivity [76–79]. Of particular interest is bile acid metabolism, since intestinal microbiota are indispensable for the transformation of primary bile acids into secondary acids [79]. In turn, primary and secondary bile acids can act as ligands for specific liver receptors, such as the farnesoid X receptor, which regulates triglyceride turnover as well as the export of VLDL (very-low-density lipoprotein) and de novo lipogenesis, or the Takeda G protein-coupled receptor 5, which acts on glucose metabolism and insulin sensitivity [80–82]. Through still unclear mechanisms, the microbiota can act on lipid metabolism also through SCFAs and TMAO [82–85].
Studies in rodents investigating the relationship between intestinal microbiota and plasma lipids showed that cholesterol and triglyceride levels were reduced in plasma of conventionally raised mice compared with germ-free mice, whereas they were increased in the AT and liver [77]. A clinical study conducted on 893 subjects found 34 bacterial taxa associated with BMI and blood lipids, and revealed that microbiota explain 6% of the variance in triglycerides and 4% of that of high-density lipoproteins (HDL) independent of age, gender, and genetic risk factors [78]. These authors added evidence that the gut microbiome provides a small but significant contribution to the variation in TG (triglycerides) and HDL in part independently of BMI variations.
Arterial hypertension
Arterial hypertension is a multifactorial disorder relating to genetic and environmental factors. In addition to the recognized role of obesity, dietary factors, physical activity, and chronic stress, there is accumulating evidence of a role for microbiota in inducing and maintaining high levels of BP. The linking mechanisms include effects mediated by SCFAs, systemic inflammation, and a number of vasoactive metabolites, such as serotonin, dopamine, norepinephrine, p-cresol sulfate, indoxyl sulfate, and TMAO [86]. The effect of SCFAs acetate, propionate, and butyrate on BP seemingly depends on their binding to the G protein-coupled receptor GPR41 (e.g., free fatty acid receptor 3), GPR43, GPR109A, and GPCR olfactory receptor 51E2 (e.g., Olfr78 in mice and OR51E2 in humans) [87]. The consequence of receptor binding varies depending on the ligand. In the renal afferent arteriole, for example, Olfr78 stimulation causes renin release and activation of the renin–angiotensin system, while GPR41 stimulation promotes local vasodilation, as it happens for propionate [88, 89]. It has also been demonstrated that the absence of gut microbiota can protect mice from Angiotensin II-induced arterial hypertension, vascular dysfunction, and hypertension-induced end-organ damage [90]. Noticeably, these receptors are expressed in smooth muscle cells of small resistance vessels, and GPR41 stimulation determines an increase in energy expenditure through activation of the sympathetic nervous system, leading to an increase in BP [88, 91]. Studies in mice also suggested that dysfunctional gut-sympathetic communications can associate with increased gut permeability, dysbiosis, and systemic inflammation, all of which play a role in hypertension [92, 93]. In mice and in small human cohorts, it has also been shown that a salt-rich diet encourages a depletion in Lactobacillus species, which associates with increased TH17 cells and increased BP [94].
With regards to TMAO, its association with arterial hypertension is not currently clear, although animal data suggest a propensity of elevated TMAO levels to influence the susceptibility to elevated BP [95].
Interestingly, fecal transplant from hypertensive to normotensive mice promoted an increase in systolic BP of the recipient mice, while fecal transplant from normotensive to hypertensive rats was insufficient to reduce systolic BP or normalize dysbiosis [96, 97]. Transplant of pathological microbiota from obstructive sleep apnea-induced hypertensive rats to normotensive rats has been shown to induce hypertension and dysbiosis after only 1 week [98]. As suggested by Marques et al., the hypotensive effect of a diet high in fiber could depend on the change of the microbiota with an increase in some acetate-producing bacteria such as Bacteroides acidifaciens [99].
Although evidence of a link between microbiota and hypertension in humans is limited, there are studies showing a lower richness and diversity of intestinal microbiota in hypertensive patients compared with normotensive counterparts [95, 100, 101]. An MGWAS revealed 53,953 microbial genes differently distributed between hypertensive patients and controls: opportunistic pathogenic spp. were more frequently distributed in hypertensive gut microbiota, whereas SCFAs-producing spp. were higher in controls, showing a correlation between hypertension-associated spp. and the severity of disease [100]. Of note, microbiota alterations typical of hypertension can be already identified in prehypertensive subjects, suggesting that these changes could precede the clinical manifestations of the disorder and/or contribute to the development of hypertension [101].
Nonalcoholic fatty liver disease
NAFLD is a proxy of the metabolic syndrome and is growingly recognized as a marker of CVD, both directly and indirectly through its association with other cardiometabolic disorders. The mesenteric blood supply conveying to the liver drains microbial products originating from intestinal saccharolytic and proteolytic fermentation that contribute to NAFLD pathogenesis [102]. Patients with NAFLD present changes in the expression and distribution of tight junction protein critical for gut barrier permeability. This event promotes liver exposure to gut-derived endotoxins ensuing per se the development of NAFLD [103, 104]. Also, microbial activity increases TLR activation and initiates nuclear transcription factors resulting in the release of proinflammatory cytokines, which ultimately lead to hepatic injury and fibrosis. Such a proinflammatory outcome has also been observed for ethanol-producing bacteria via nuclear factor-κB (NF-κB) signaling pathways [105]. Moreover, gut microbiota modulate liver fatty acid oxidation by acting on AMPK, the enzyme involved in liver and muscle cells as a gauge of cell energy. AMPK inhibition decreases fatty acid oxidation and enhances fat accumulation. Also important is the activity of TMAO not only as a predictor of NAFLD but also for a putative role in liver triglycerides accumulation and cholesterol input into the bloodstream [106].
Cross-sectional studies in nonobese humans reported a relationship between an increased Bacteroidetes to Firmicutes ratio and NAFLD progression. A decrease in butyrate-producing Ruminococcaceae has been observed in patients with NAFLD independent of obesity. Complementing these findings, intervention studies found that hepatic mitochondria are the main target of the beneficial effect of butyrate-based compounds in reverting insulin resistance and fat accumulation in diet-induced obese mice [107], and supplementation of SCFAs in animals reduced liver fat accumulation, inflammation, and cholesterol synthesis via mechanisms relating to the AMPK–acetyl-CoA carboxylase pathway and hepatic fatty acid synthase activity [108–110].
Atherosclerosis and vasculopathies
Atherosclerosis is a chronic inflammatory disease and a leading cause of vascular disease worldwide [111, 112]. It mainly affects large and medium-sized arteries and is characterized by the presence of plaques (atheromas) leading to vasculopaties. Atherosclerosis often remains asymptomatic until arterial narrowing reaches thrombotic occlusion. CAD includes all conditions associated with insufficient blood flow and consequent oxygen depletion of the heart muscle. Koren et al. [113] suggested that the gut microbiota of CVD patients produce more proinflammatory molecules. These authors found that individuals with atherosclerotic plaques display similarities in bacterial representation between the atherosclerotic plaque and oral cavity/intestine, suggesting that some microbial communities may contribute to plaque instability. Equally, Calandrini et al. [114] documented higher abundance of oral microbacterial spp. P. gingivalis and Aggregatibacter actinomycetemcomitans in atheromas. Also important is the role of TMAO. It acts on monocytes/macrophages by promoting the uptake of cholesterol and thus increasing the potential thrombogenic effect via formation of foam cells [83]. Moreover, TMAO has been claimed to yield proatherogenic actions via production of inflammatory molecules [115] that play a central role in atherosclerosis development, such as IL-6, cyclooxygenase (COX)-2, E-selectin, and intercellular adhesion molecule-1. Recently, Zhu et al. showed that circulating levels of TMAO directly modulate platelet hyperreactivity (e.g., activation, adhesion, and aggregation) and the rate of clot formation in vivo [116]. Qualitative and quantitative alterations in microbiota can, in turn, also be induced by acute myocardial infarction (AMI). Wu et al. [117] found microbial and gut barrier alterations in a rat model with increased levels of Synergistetes pylum and Lachnospiraceae family until 7 days post AMI. The comparison of gut microbiota in CAD patients revealed higher abundance of Lactobacillales and lower levels of Bacteroides and Prevotella than controls [118]. These findings indirectly suggest that an appropriate richness and diversity of human microbiota could be crucial for primary prevention in patients prone to CAD.
Chronic kidney disease
Although the prevalence of chronic kidney disease (CKD) is globally varied, its incidence is increasing worldwide and represents a major public health burden [119]. CKD patients exhibit increased all-cause and CV mortality in comparison with the general population when standardized by age and gender [120]. In the last decade, a growing number of studies have focused on the alterations of gut microbiota in CKD patients. Accumulation of uremic toxins alters the gut microenvironment and impairs the host immune response, resulting in a condition of gut dysbiosis [121]. Dysbiosis in CKD induces bacterial proteolysis with further production of uremic toxins such as indoxyl sulfate, p-cresyl sulfate, and TMAO. In particular, indoxyl sulfate acts as a predictor of CV and all-cause mortality in CKD patients. An inverse correlation between indoxyl sulfate and glomerular filtration rate (GFR), and a direct correlation with endothelial function tests, such as pulse wave velocity and the aortic calcifications, is observed in CKD patients [122]. Likewise, plasma levels of p-cresyl sulfate are associated with an increased risk of death and CV events in hemodialysis patients [123].
Evidence of a correlation between TMAO levels and the decrease of GFR exists in patients affected by moderate to severe CKD [124]. The increased levels of these uremic toxins originating from the gut microbiota are not only associated with an enhanced risk of CVD but also with a faster progression of CKD [125]. Understanding the specific mechanism/s by which the CKD toxic compounds can impair gut microbiota composition should be a goal of future investigations [126].
Cardiomyopathies and heart failure
An association has been described between gut microbiota and proteomic/metabolomic determinants of cardiomyopathy phenotypes [127], and host–microbe interactions have been found to impact proteins involved in epithelial function, lipid metabolism, and central nervous system function relating to CVD [128]. Interestingly, succinate, a tricarboxylic acid intermediate metabolite, can cause murine cardiac hypertrophy in a GPR91-dependent manner and its levels increase in patients with hypertrophic cardiomyopathy, the condition of left ventricle wall thickening leading to defective pump function [129]. Likewise, indoxyl sulfate can stimulate hypertrophy and fibrosis of cardiomyocytes in vitro and in vivo [130]. With regards to ischemic cardiomyopathy, it has been observed that patients with ischemic cardiomyopathy harbor higher levels of TMAO and betaine that those with dilated cardiomyopathy, potentially reflecting a more negative prognosis in the former [131].
Several studies focused on the relationship between gut microbiota and HF, a multisystem disorder resulting from cardiomyopathies of different origin. In HF, hemodynamic alterations can impair gut epithelial function and lead to the translocation of bacteria-derived endotoxins across the gut epithelial barrier, which can in turn induce systemic inflammatory responses [132, 133] via increased LPS concentrations [134, 135]. The severity of HF has been associated with the disadvantageous overgrowth of Campylobacter, Shigella, Salmonella, as well as Candida spp [136]. Using bacterial 16S rRNA gene sequences, lower diversity indices and core intestinal microbiota (Coriobacteriaceae, Erysipelotrichaceae, and Ruminococcaceae) were found in HF patients compared with controls [137]. Changes in core intestinal microbiota, depletion in butyrate-producer Lachnospiraceae family, as well as correlations between microbial changes and increased levels of soluble CD25, a marker of T cell and macrophage activation, partly explain the immune abnormalities seen in HF patients [138]. When further analyzed according to age, lower proportions of Bacteroidetes and larger quantities of Proteobacteria have been described in older compared with younger patients with HF [139].
HF patients show an imbalanced production of butyrate and TMAO, which associates with distinct metabolomic signatures relating to enhanced expression of microbial genes associated with LPS biosynthesis [140]. In experimental HF, TMAO directly acts on inflammatory pathways known to contribute to CVD [115, 141, 142], and its pharmacologic manipulation has been shown to blunt diet-induced inflammation, cardiac dysfunction, fibrosis, and atherosclerosis [143].
Cardiovascular mortality
Studies on mortality predictors have focused on circulating products activity rather than microbiota strains. TMAO is by far the most investigated product, with particular reference to its prognostic impact in patients with established atherosclerosis. A study in medically treated patients with stable CAD showed that high TMAO predicted a fourfold increased mortality risk in 5 years; after multiple adjustments, the mortality risk remained nearly doubled [144]. Parallel findings were obtained in medically treated patients with peripheral artery disease [145]. Also, TMAO levels predicted a poor prognosis in patients with HF and a threefold increase in 5-year mortality risk (HR, 3.4) even after multiple adjustments [146]. In contrast, a study on patients with venous thromboembolism (VTE) found no change in the risk of recurrent VTE across TMAO categories, although a marginally significant U-shaped association was reported between TMAO and mortality risk, with the lowest mortality risk observed for TMAO levels of 4 μmol/L [147]. In a systematic review and meta‐analysis of prospective studies (19,256 participants, 3315 incident cases), elevated TMAO was associated with increased relative risk (RR) for major adverse CV events (MACE; RR, 1.62) and all-cause mortality (RR, 1.63), independent of traditional risk factors [148]. The dose–response study showed that increasing TMAO levels by 1 μmol/L conferred a significant 2–5% higher risk of MACE. In another meta-analysis of 14 studies on 16 cohorts enrolling 15,662 subjects, TMAO levels were associated with all-cause mortality (HR, 1.91) and major adverse cardio- and cerebrovascular events (MACCE; HR, 1.67). The dose–response subanalysis documented a 7.6% increased RR for all-cause mortality for each 10 μmol/L increment in TMAO levels [149]. Lastly, TMAO predicted mortality and multiple CVR more strongly in individuals with diabetes than in those without. In patients with diabetes and acute coronary syndrome, high TMAO levels were associated with increased mortality (HR = 2.7), myocardial infarction (HR = 4.0), HF (HR = 4.6), unstable angina (HR = 9.1), and all CV events (HR = 2.0), while in patients without diabetes, associations were “only” significant for death (HR, 2.7) and HF (HR, 1.9) [150].
Modulation of gut microbiota as a therapeutic strategy
Interventions during neonatal life
Early postnatal life is critical for adult health. Microbiota colonization of the host occurs early, i.e., during prenatal life, birthing process and upon postnatal exposure to external factors [151–153]. Birth mode and infant gut microbiota mediate the association between maternal prepregnancy overweight and early childhood overweight [154]. They are also correlated with children’s BMI at age 12 years [155]. Altered α- and β-diversity of the offspring fecal microbial community and abnormal Firmicutes/Bacteroides ratio have also been observed in association with maternal HFD, maternal obesity, and overnutrition possibly via SCFAs production and energy extraction from the diet [156–160].
The use of probiotics during pregnancy is a promising tool to circumvent the problem. In a double-blind study, administration to mothers of Lactobacillus rhamnosus from 4 weeks before delivery to 6 months after delivery alleviated the initial, but not late, weight gain among children who then became overweight compared with placebo [161]. Also, prebiotics have shown encouraging results in modulating the offspring gut microbiota, the development of the intestinal tract, and the expression of satiety hormones and genes associated with healthy glucose metabolism. In 2–12-week-old infants, supplementation with short-chain oligosaccharides with or without long-chain oligosaccharides showed a strong bifidogenic effect compared with traditional infant formulas [162].
Dietary approaches: prebiotics, probiotics, postbiotics, and polyphenols
Preventive strategies aimed at reducing the burden of obesity and noncommunicable diseases should consider genetic makeup and its interaction with dietary intake as a continuum. The association between unhealthy nutrition and inflammation or oxidative stress applies to chronic diseases consequent to abnormal microbiota colonization of the gut. The HFD is paradigmatic for its ability to trigger systemic inflammation via inflammatory mediators, such as activated NF-κB, as well as products of leukocyte activation, such as CD11A, CD11B, and CD62L [163]. In postprandial conditions, the inflammatory response related to endotoxemia and bacterial LPS synthesis exerts forceful inflammatory and proatherogenic effects [164]. Likewise, a meat-based diet can increase bile-tolerant microorganisms (e.g., Alistipes, Bilophila, and Bacteroides) and decrease the abundance of Firmicutes, which metabolize dietary plant polysaccharides (Roseburia, Eubacterium rectale, and Ruminococcus bromii) [165]. Inversely, diets rich in polyunsaturated (PUFA) and monounsaturated (MUFA) fatty acids, and poor in animal fats and glucose-dense food have been largely shown to advantage microbiota and benefit CV health.
Healthy dietary approaches expressing this peculiar micronutrient profile include Mediterranean, Japanese, and vegetarian diets [166, 167]. The usefulness of the Mediterranean Diet (MD) in preventing CVD risk and other noncommunicable chronic diseases [168, 169] has been extensively shown owing to high contents of vegetables, fresh fruit, cereals, olive oil, resveratrol-containing foods and other antioxidant-rich compounds. A simple change in the meal energy load from lipids to carbohydrates equivalent to ≈25% improves postprandial proatherogenic factors [170], while adherence to the MD can reduce LDL oxidized proteins and promote significant variation in gene expression of antioxidant and prooxidant genes [171].
It is also recognized that dietary interventions can modify gut microbiota in a fast, diet-specific fashion [165]. A plethora of compounds have been found to modulate the gut microbiota and activate specific patterns of gene expression that, in turn, act to restore the hormetic mechanisms capable of decreasing cellular stress and inflammation [172–175]. As such, four classes of compounds constitute effective nutritional interventions: prebiotics, probiotics, postbiotics, and phytochemicals [176].
Prebiotics belong to fiber, vitamins A, B1, B2, B6, B3, B12, D, α, β, γ, δ-tocopherol family, α, β, γ, δ-tocotrienol, MUFA, and PUFA (ω-9, ω-6), iron, zinc, phytosterols, and inulin, which act to control and re-establish innate and adaptive immune efficiency, improving immune functions, and stress response [177].
Probiotics, such as Bacteroides, Clostridium, Faecalibacterium, Eubacterium, Peptococcus, Peptostreptococcus, and Bifidobacterium can correct the altered intestinal microbiota and restore innate and adaptive immunity [178]. Clinical trials have been conducted to evaluate the effects of probiotics on BP and NAFLD. There is indirect evidence that probiotic treatment could be a new target in the treatment of hypertension, since reversing dysbiosis could solve one of the mechanisms underlying hypertension. A meta-analysis of nine randomized trials showed a significant decrease in both systolic and diastolic BP in patients who consumed a daily dose of ≥109 CFU of probiotics [179]. Li et al. demonstrated that probiotic supplementation improves insulin resistance, hepatic histology, and total fatty acid content in mice with steatohepatitis [180]. In humans, supplementation with fructo-oligosaccharide (or fructo-oligosaccharide and Bifidobacterium longum) was associated with reductions in NAFLD and TNFα levels [181, 182]. Furthermore, the use of a mix of probiotic strains decreased levels of TNFα and reduced hepatic inflammatory signaling in liver steatosis suggesting that probiotic administration could ameliorate oxidative and inflammatory liver damage associated with NAFLD.
Postbiotics can improve epithelial barrier function, increase the production of mucins by the goblet cells, and are able to decrease inflammatory processes through downregulation of proinflammatory cytokine production by intestinal epithelial cells [183–185]. Such phytochemicals include curcumin, polyphenols like caffeic acid, flavonoids like catechin, epicatechin, quercetin, procyanidins, and phenolic acids, hydroxytyrosol and resveratrol, which are highly effective in blocking NF-κB activation, hence peroxidation of low density lipoprotein cholesterol [173, 186, 187]. Many of these compounds yield anti-inflammatory activity by modulating the gut-associated lymphoid tissues, responsible for upregulation of caspases, p53, NF-E2-related factor, Bcl-2-associated X, and IL-10 and downregulation of protein kinase-C, protein kinase-D, lipoxygenase, COX-2, sirtuins, TNF-α, uncoupling protein-2, dual-oxidase 2 gene, IL-1 receptor-associated kinase-2 gene, catalase, and C-X-C motif chemokine ligand 12 genes [34, 188, 189].
Fecal transplant
One emerging strategy to modulate the intestinal microbiota in patients with dysbiosis is to use a fecal microbiota transplant (FMT) [190]. This is a key treatment for Clostridium difficile infections [191–193]. Studies have shown the possibility of transmission of obesity, inflammatory bowel disease, depression [194], mood disorders [195], and other conditions in animal through FMT, giving strength to the argument that microbiota may be actively implicated in the pathogenesis of metabolic diseases. Gregory et al. [196] showed that atherosclerosis susceptibility can be transmitted with a gut microbial transplant through TMAO-induced effects, while others observed that Akkermansia muciniphila can reverse western diet-induced atherosclerosis and endotoxemia in ApoE-knockout mice [197]. This approach can prevent inflammation and suppress the formation of atheroma via reduced macrophage infiltration [198]. In humans, the use of FMT from metabolically healthy donors to subjects with metabolic syndrome prompted improvements in peripheral and hepatic insulin sensitivity measured by the hyperglycemic clamp, suggesting the possibility of manipulating microbiota to treat diabetes [199].
Conclusions
CVD is intimately associated with alterations in microbiota. Lifestyle changes and elimination of associated risk factors remain the main strategy to reduce CV morbidity and mortality. The complex interaction between gut microbiota and the CV system warrants further research to clarify the intra- and interindividual variations in this relationship and plan intervention strategies. There is, however, emerging evidence that chronic inflammation, CVD and CV mortality can be reduced by improvements in long-term composition of the gut microbiota. In this perspective, manipulation of intestinal microbiota could represent a novel therapeutic option for the treatment of CVD, even if further studies are necessary to clarify the long-term effects of gut microbiota in prevention and treatment of CV disease.
Acknowledgements
Obesity Programs of nutrition, Education, Research and Assessment (OPERA) group members served as collaborators and approved the final version of the manuscript: Colao Annamaria, Savastano Silvia, Barrea Luigi, Muscogiuri Giovanna, Alviggi Carlo, Angrisani Luigi, Annunziata Giuseppe, Beguinot Francesco, Belfiore Annamaria, Belfiore Antonino, Bellastella Giuseppe, Biondi Bernadette, Bonaduce Domenico, Bordoni Laura, Brasacchio Caterina, Capaldo Brunella, Caprio Massimiliano, Cataldi Mauro, Cignarelli Angelo, Cittadini Antonello, Conforti Alessandro, Cuomo Rosario, De Placido Giuseppe, De Siena Marina, Di Carlo Costantino, Di Luigi Luigi, Di Nisio Andrea, Di Renzo Laura, Di Somma Carolina, Docimo Ludovico, Donini Lorenzo Maria, Federici Massimo, Foresta Carlo, Gabbianelli Rosita, Gambineri Alessandra, Gastaldelli Amalia, Giallauria Francesco, Giardiello Cristiano, Gnessi Lucio, Guida Brunella, Laudisio Daniela, Lenzi Andrea, Macchia Paolo Emidio, Manno Emilio, Marzullo Paolo, Migliaccio Silvia, Muratori Fabrizio, Musella Mario, Nardone Gerardo, Nicasto Vincenzo, Piazza Luigi, Pilone Vincenzo, Pivari Francesca, Pivonello Rosario, Pugliese Gabriella, Riccardi Gabriele, Ritieni Alberto, Salzano Ciro, Sanduzzi Alessandro, Sbraccia Paolo, Sesti Giorgio, Soldati Laura, Taglialatela Maurizio, Trimarco Bruno, Tuccinardi Dario.
Funding
The 2019 OPERA meeting was organized by Panta Rei Srl and sponsored by Novo Nordisk, Therascience, Bruno Pharma, Merck, Savio Pharma Italia Srl, IBSA Institut Biochimique SA, Bioitalia Srl, Cohesion Pharmaceutical, and Specchiasol Srl. Publication of this article as part of a supplement was sponsored by Panta Rei Srl, Naples, Italy. The meeting sponsors and organizer did not have access to the manuscripts and the authors maintained control of the content.
Author contributions
PM and LDR equally contributed to the work. The authors’ responsibilities were as follows: PM, LDR, GP, MDS, LB, GM, and SS were responsible for the concept of this paper and drafted the manuscript; GM, AC, and SS provided a critical review of the paper. OPERA Group members participated to the revision of the manuscript. All authors and OPERA Group Members contributed to and agreed on the final version of the manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization (WHO). Fact-sheets on cardiovascular disease. World Health Organization; Geneva, Switzerland, 2017. https://www.who.int/en/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds).
- 2.Bäckhed F, Fraser CM, Ringel Y, Sanders ME, Sartor RB, Sherman PM, et al. Defining a healthy human gut microbiome: current concepts, future directions, and clinical applications. Cell Host Microbe. 2012;12:611–22. doi: 10.1016/j.chom.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 3.Sommer F, Anderson JM, Bharti R, Raes J, Rosenstiel P. The resilience of the intestinal microbiota influences health and disease. Nat Rev Microbiol. 2017;15:630–8. doi: 10.1038/nrmicro.2017.58. [DOI] [PubMed] [Google Scholar]
- 4.Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, et al. Enterotypes of the human gut microbiome. Nature. 2011;473:174–80. doi: 10.1038/nature09944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Natividad JMM, Verdu EF. Modulation of intestinal barrier by intestinal microbiota: pathological and therapeutic implications. Pharmacol Res. 2013;69:42–51. doi: 10.1016/j.phrs.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 6.Gensollen T, Iyer SS, Kasper DL, Blumberg RS. How colonization by microbiota in early life shapes the immune system. Science. 2016;352:539–44. doi: 10.1126/science.aad9378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud D-J, Bakker BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. 2013;54:2325–40. doi: 10.1194/jlr.R036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang M, Chekan JR, Dodd D, Hong P-Y, Radlinski L, Revindran V, et al. Xylan utilization in human gut commensal bacteria is orchestrated by unique modular organization of polysaccharide-degrading enzymes. Proc Natl Acad Sci. 2014;111:E3708–17. doi: 10.1073/pnas.1406156111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W, et al. Host-gut microbiota metabolic interactions. Science. 2012;336:1262–7. doi: 10.1126/science.1223813. [DOI] [PubMed] [Google Scholar]
- 10.Macfarlane S, Macfarlane GT. Regulation of short-chain fatty acid production. Proc Nutr Soc. 2003;62:67–72. doi: 10.1079/PNS2002207. [DOI] [PubMed] [Google Scholar]
- 11.Bhat MI, Kapila R. Dietary metabolites derived from gut microbiota: critical modulators of epigenetic changes in mammals. Nutr Rev. 2017;75:374–89. doi: 10.1093/nutrit/nux001. [DOI] [PubMed] [Google Scholar]
- 12.Everard A, Geurts L, Van Roye M, Delzenne NM, Cani PD. Tetrahydro iso-alpha acids from hops improve glucose homeostasis and reduce body weight gain and metabolic endotoxemia in high-fat diet-fed mice. PLoS ONE. 2012;7:e33858. doi: 10.1371/journal.pone.0033858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cani PD, Neyrinck AM, Fava F, Knauf C, Burcelin RG, Tuohy KM, et al. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia. 2007;50:2374–83. doi: 10.1007/s00125-007-0791-0. [DOI] [PubMed] [Google Scholar]
- 14.Ghoshal S, Witta J, Zhong J, de Villiers W, Eckhardt E. Chylomicrons promote intestinal absorption of lipopolysaccharides. J Lipid Res. 2009;50:90–7. doi: 10.1194/jlr.M800156-JLR200. [DOI] [PubMed] [Google Scholar]
- 15.Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;26:55–60. doi: 10.1038/nature11450. [DOI] [PubMed] [Google Scholar]
- 16.De Lorenzo A. New obesity classification criteria as a tool for bariatric surgery indication. World J Gastroenterol. 2016;22:681. doi: 10.3748/wjg.v22.i2.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abenavoli L, Luigiano C, Guzzi PH, Milic N, Morace C, Stelitano L, et al. Serum adipokine levels in overweight patients and their relationship with non-alcoholic fatty liver disease. Panminerva Med. 2014;56:189–93. [PubMed] [Google Scholar]
- 18.Després J-P. Body fat distribution and risk of cardiovascular disease: an update. Circulation. 2012;126:1301–13. doi: 10.1161/CIRCULATIONAHA.111.067264. [DOI] [PubMed] [Google Scholar]
- 19.Alwardat N, Di Renzo L, de Miranda RC, Alwardat S, Sinibaldi Salimei P, De Lorenzo A. Association between hypertension and metabolic disorders among elderly patients in North Jordan. Diabetes Metab Syndr. 2018;12:661–6. doi: 10.1016/j.dsx.2018.04.011. [DOI] [PubMed] [Google Scholar]
- 20.Shah A, Mehta N, Reilly MP. Adipose inflammation, insulin resistance, and cardiovascular disease. J Parenter Enter Nutr. 2008;32:638–44. doi: 10.1177/0148607108325251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karelis AD, Faraj M, Bastard J-P, St-Pierre DH, Brochu M, Prud’homme D, et al. The metabolically healthy but obese individual presents a favorable inflammation profile. J Clin Endocrinol Metab. 2005;90:4145–50. [DOI] [PubMed]
- 22.Chang Y, Kim B-K, Yun KE, Cho J, Zhang Y, Rampal S, et al. Metabolically-healthy obesity and coronary artery calcification. J Am Coll Cardiol. 2014;63:2679–86. doi: 10.1016/j.jacc.2014.03.042. [DOI] [PubMed] [Google Scholar]
- 23.Camhi SM, Crouter SE, Hayman LL, Must A, Lichtenstein AH. Lifestyle behaviors in metabolically healthy and unhealthy overweight and obese women: a preliminary study. PLoS ONE. 2015;10:e0138548. [DOI] [PMC free article] [PubMed]
- 24.Oliverosa E, Somersa VK, Sochora O, Goela K, Lopez-Jimeneza F. The concept of normal weight obesity. Prog Cardiovas Dis. 2014;56:426–33. doi: 10.1016/j.pcad.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 25.Marques-Vidal P, Pecoud A, Hayoz D, et al. Prevalence of normal weight obesity in Switzerland: effect of various definitions. Eur J Nutr. 2008;47:251–7. doi: 10.1007/s00394-008-0719-6. [DOI] [PubMed] [Google Scholar]
- 26.Lee S-H, Han K, Yang HK, Kim H-S, Cho J-H, Kwon H-S, et al. A novel criterion for identifying metabolically obese but normal weight individuals using the product of triglycerides and glucose. Nutr Diabetes. 2015;5:e149. doi: 10.1038/nutd.2014.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruderman N, Chisholm D, Pi-Sunyer X, Schneider S. The metabolically obese, normal-weight individual revisited. Diabetes. 1998;47:699–713. doi: 10.2337/diabetes.47.5.699. [DOI] [PubMed] [Google Scholar]
- 28.De Lorenzo A, Martinoli R, Vaia F, Di Renzo L. Normal weight obese (NWO) women: an evaluation of a candidate new syndrome. Nutr Metab Cardiovas Dis. 2006;16:513–23. doi: 10.1016/j.numecd.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 29.Di Renzo L, Del Gobbo V, Bigioni M, Premrov MG, Cianci R, De Lorenzo A. Body composition analyses in normal weight obese women. Eur Rev Med Pharmacol Sci. 2006;10:191–6. [PubMed] [Google Scholar]
- 30.De Lorenzo A, Del Gobbo V, Premrov MG, Bigioni M, Galvano F, Di Renzo L. Normal-weight obese syndrome: early inflammation? Am J Clin Nutr. 2007;85:40–5. doi: 10.1093/ajcn/85.1.40. [DOI] [PubMed] [Google Scholar]
- 31.Di Renzo L, Galvano F, Orlandi C, Bianchi A, Di Giacomo C, La Fauci L, et al. Oxidative stress in normal-weight obese syndrome. Obesity. 2010;18:2125–30. doi: 10.1038/oby.2010.50. [DOI] [PubMed] [Google Scholar]
- 32.Di Renzo L, Sarlo F, Petramala L, Iacopino L, Monteleone G, Colica C, et al. Association between -308 G/A TNF- α polymorphism and appendicular skeletal muscle mass index as a marker of sarcopenia in normal weight obese syndrome. Dis Markers. 2013;35:615–23. doi: 10.1155/2013/983424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Di Renzo L, Gratteri S, Sarlo F, Cabibbo A, Colica C, De Lorenzo A. Individually tailored screening of susceptibility to sarcopenia using p53 codon 72 polymorphism, phenotypes, and conventional risk factors. Dis Markers. 2014;2014:1–10. doi: 10.1155/2014/743634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Romero-Corral A, Somers VK, Sierra-Johnson J, Korenfeld Y, Boarin S, Korinek J, et al. Normal weight obesity: a risk factor for cardiometabolic dysregulation and cardiovascular mortality. Eur Heart J. 2010;31:737–46. doi: 10.1093/eurheartj/ehp487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marques-Vidal P, Pécoud A, Hayoz D, Paccaud F, Mooser V, Waeber G, et al. Normal weight obesity: relationship with lipids, glycaemic status, liver enzymes and inflammation. Nutr Metab Cardiovasc Dis. 2010;20:669–75. doi: 10.1016/j.numecd.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 36.Di Renzo L, Tyndall E, Gualtieri P, Carboni C, Valente R, Ciani AS, et al. Association of body composition and eating behavior in the normal weight obese syndrome. Eat Weight Disord. 2016;21:99–106. doi: 10.1007/s40519-015-0215-y. [DOI] [PubMed] [Google Scholar]
- 37.De Lorenzo A, Costacurta M, Merra G, Gualtieri P, Cioccoloni G, Marchetti M, et al. Can psychobiotics intake modulate psychological profile and body composition of women affected by normal weight obese syndrome and obesity? A double blind randomized clinical trial. J Transl Med. 2017;15:135. doi: 10.1186/s12967-017-1236-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huffman DM, Barzilai N. Role of visceral adipose tissue in aging. Biochim Biophys Acta. 2009;1790:1117–23. doi: 10.1016/j.bbagen.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bays H. Central obesity as a clinical marker of adiposopathy; increased visceral adiposity as a surrogate marker for global fat dysfunction. Curr Opin Endocrinol Diabetes Obes. 2014;21:345–51. doi: 10.1097/MED.0000000000000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jokela M, Hintsanen M, Hakulinen C, Batty GD, Nabi H, Singh-Manoux A, et al. Association of personality with the development and persistence of obesity: a meta-analysis based on individual-participant data. Obes Rev. 2013;14:315–23. doi: 10.1111/obr.12007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Däbritz J, Menheniott TR. Linking immunity, epigenetics, and cancer in inflammatory bowel disease. Inflamm Bowel Dis. 2014;20:1638–54. doi: 10.1097/MIB.0000000000000063. [DOI] [PubMed] [Google Scholar]
- 42.Harris RA, Nagy-Szakal D, Pedersen N, Opekun A, Bronsky J, Munkholm P, et al. Genome-wide peripheral blood leukocyte DNA methylation microarrays identified a single association with inflammatory bowel diseases. Inflamm Bowel Dis. 2012;18:2334–41. doi: 10.1002/ibd.22956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anderson OS, Sant KE, Dolinoy DC. Nutrition and epigenetics: an interplay of dietary methyl donors, one-carbon metabolism and DNA methylation. J Nutr Biochem. 2012;23:853–9. doi: 10.1016/j.jnutbio.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Remely M, Lovrecic L, de la Garza AL, Migliore L, Peterlin B, Milagro FI, et al. Therapeutic perspectives of epigenetically active nutrients: therapeutic epigenetically active nutrients. Br J Pharmacol. 2015;172:2756–68. doi: 10.1111/bph.12854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reuter S, Gupta SC, Park B, Goel A, Aggarwal BB. Epigenetic changes induced by curcumin and other natural compounds. Genes Nutr. 2011;6:93–108. doi: 10.1007/s12263-011-0222-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scarpa M, Stylianou E. Epigenetics: concepts and relevance to IBD pathogenesis. Inflamm Bowel Dis. 2012;18:1982–96. doi: 10.1002/ibd.22934. [DOI] [PubMed] [Google Scholar]
- 47.Krautkramer KA, Kreznar JH, Romano KA, Vivas EI, Barrett-Wilt GA, Rabaglia ME, et al. Diet-microbiota interactions mediate global epigenetic programming in multiple host tissues. Mol Cell. 2016;64:982–92. doi: 10.1016/j.molcel.2016.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eeckhaut V, Ducatelle R, Sas B, Vermeire S, Van Immerseel F. Progress towards butyrate-producing pharmabiotics: Butyricicoccus pullicaecorum capsule and efficacy in TNBS models in comparison with therapeutics. Gut. 2014;63:367. doi: 10.1136/gutjnl-2013-305293. [DOI] [PubMed] [Google Scholar]
- 49.Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease. J Am Coll Cardiol. 2009;53:1925–32. doi: 10.1016/j.jacc.2008.12.068. [DOI] [PubMed] [Google Scholar]
- 50.Khan MJ, Gerasimidis K, Edwards CA, Shaikh MG. Role of gut microbiota in the aetiology of obesity: proposed mechanisms and review of the literature. J Obes. 2016;2016:1–27. doi: 10.1155/2016/7353642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tilg H, Moschen AR, Kaser A. Obesity and the microbiota. Gastroenterology. 2009;136:1476–83. doi: 10.1053/j.gastro.2009.03.030. [DOI] [PubMed] [Google Scholar]
- 52.Hardie DG. AMPK: a key regulator of energy balance in the single cell and the whole organism. Int J Obes. 2008;32:S7–12. doi: 10.1038/ijo.2008.116. [DOI] [PubMed] [Google Scholar]
- 53.Backhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA. 2004;101:15718–23. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Angelakis E, Armougom F, Million M, Raoult D. The relationship between gut microbiota and weight gain in humans. Future Microbiol. 2012;7:91–109. doi: 10.2217/fmb.11.142. [DOI] [PubMed] [Google Scholar]
- 55.Beynon AL, Brown MR, Wright R, Rees MI, Sheldon IM, Davies JS. Ghrelin inhibits LPS-induced release of IL-6 from mouse dopaminergic neurones. J Neuroinflammation. 2013;10:40. doi: 10.1186/1742-2094-10-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Avolio E, Gualtieri P, Romano L, Pecorella C, Ferraro S, Di Renzo L, et al. Obesity and body composition in man and woman: associated diseases and new role of gut microbiota. Curr Med Chem. 2020;27:216-29. [DOI] [PubMed]
- 57.Graessler J, Bornstein TD, Goel D, Bhalla VP, Lohmann T, Wolf T, et al. Lipidomic profiling before and after Roux-en-Y gastric bypass in obese patients with diabetes. Pharmacogenomics J. 2014;14:201–7. doi: 10.1038/tpj.2013.42. [DOI] [PubMed] [Google Scholar]
- 58.Nyangale EP, Mottram DS, Gibson GR. Gut microbial activity, implications for health and disease: the potential role of metabolite analysis. J Proteome Res. 2012;11:5573–85. doi: 10.1021/pr300637d. [DOI] [PubMed] [Google Scholar]
- 59.Respondek F, Gerard P, Bossis M, Boschat L, Bruneau A, Rabot S, et al. Short-chain fructo-oligosaccharides modulate intestinal microbiota and metabolic parameters of humanized gnotobiotic diet induced obesity mice. PLoS ONE. 2013;8:e71026. [DOI] [PMC free article] [PubMed]
- 60.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–3. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 61.Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457:480–4. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Furet J-P, Kong L-C, Tap J, Poitou C, Basdevant A, Bouillot J-L, et al. Differential adaptation of human gut microbiota to bariatric surgery–induced weight loss. Diabetes. 2010;59:3049–57. doi: 10.2337/db10-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kong L-C, Tap J, Aron-Wisnewsky J, Pelloux V, Basdevant A, Bouillot J-L, et al. Gut microbiota after gastric bypass in human obesity: increased richness and associations of bacterial genera with adipose tissue genes. Am J Clin Nutr. 2013;98:16–24. doi: 10.3945/ajcn.113.058743. [DOI] [PubMed] [Google Scholar]
- 64.Zhang H, DiBaise JK, Zuccolo A, Kudrna D, Braidotti M, Yu Y, et al. Human gut microbiota in obesity and after gastric bypass. Proc Natl Acad Sci USA. 2009;106:2365–70. doi: 10.1073/pnas.0812600106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Aron-Wisnewsky J, Prifti E, Belda E, Ichou F, Kayser BD, Dao MC, et al. Major microbiota dysbiosis in severe obesity: fate after bariatric surgery. Gut. 2019;68:70–82. doi: 10.1136/gutjnl-2018-316103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cotillard A, Kennedy SP, Kong LC, Prifti E, Pons N, Le Chatelier E, et al. Dietary intervention impact on gut microbial gene richness. Nature. 2013;500:585–8. doi: 10.1038/nature12480. [DOI] [PubMed] [Google Scholar]
- 67.Wang F-G, Bai R-X, Yan W-M, Yan M, Dong L-Y, Song M-M. Differential composition of gut microbiota among healthy volunteers, morbidly obese patients and post-bariatric surgery patients. Exp Ther Med. 2019;17:2268–78. doi: 10.3892/etm.2019.7200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Larsen N, Vogensen FK, van den Berg FWJ, Nielsen DS, Andreasen AS, Pedersen BK, et al. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS ONE. 2010;5:e9085. doi: 10.1371/journal.pone.0009085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gao Z, Yin J, Zhang J, Ward RE, Martin RJ, Lefevre M, et al. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes. 2009;58:1509–17. doi: 10.2337/db08-1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Karlsson FH, Tremaroli V, Nookaew I, Bergström G, Behre CJ, Fagerberg B, et al. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature. 2013;498:99–103. doi: 10.1038/nature12198. [DOI] [PubMed] [Google Scholar]
- 71.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–72. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 72.Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci USA. 2013;110:9066–71. doi: 10.1073/pnas.1219451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Everard A, Lazarevic V, Derrien M, Girard M, Muccioli GG, Muccioli GM, et al. Responses of gut microbiota and glucose and lipid metabolism to prebiotics in genetic obese and diet-induced leptin-resistant mice. Diabetes. 2011;60:2775–86. doi: 10.2337/db11-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Karlsson CLJ, Onnerfält J, Xu J, Molin G, Ahrné S, Thorngren-Jerneck K. The microbiota of the gut in preschool children with normal and excessive body weight. Obesity. 2012;20:2257–61. doi: 10.1038/oby.2012.110. [DOI] [PubMed] [Google Scholar]
- 75.Shin N-R, Lee J-C, Lee H-Y, Kim M-S, Whon TW, Lee M-S, et al. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut. 2014;63:727–35. doi: 10.1136/gutjnl-2012-303839. [DOI] [PubMed] [Google Scholar]
- 76.Ghazalpour A, Cespedes I, Bennett BJ, Allayee H. Expanding role of gut microbiota in lipid metabolism. Curr Opin Lipidol. 2016;27:141–7. doi: 10.1097/MOL.0000000000000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Velagapudi VR, Hezaveh R, Reigstad CS, Gopalacharyulu P, Yetukuri L, Islam S, et al. The gut microbiota modulates host energy and lipid metabolism in mice. J Lipid Res. 2010;51:1101–12. doi: 10.1194/jlr.M002774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fu J, Bonder MJ, Cenit MC, Tigchelaar EF, Maatman A, Dekens JAM, et al. The gut microbiome contributes to a substantial proportion of the variation in blood lipids. Circ Res. 2015;117:817–24. doi: 10.1161/CIRCRESAHA.115.306807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sayin SI, Wahlström A, Felin J, Jäntti S, Marschall H-U, Bamberg K, et al. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013;17:225–35. doi: 10.1016/j.cmet.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 80.Zhang Y, Ge X, Heemstra LA, Chen W-D, Xu J, Smith JL, et al. Loss of FXR protects against diet-induced obesity and accelerates liver carcinogenesis in ob/ob mice. Mol Endocrinol. 2012;26:272–80. doi: 10.1210/me.2011-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Thomas C, Gioiello A, Noriega L, Strehle A, Oury J, Rizzo G, et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009;10:167–77. doi: 10.1016/j.cmet.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Khan MJ, Gerasimidis K, Edwards CA, Shaikh MG. Role of gut microbiota in the aetiology of obesity: proposed mechanisms and review of the literature. J Obes. 2016;2016:7353642. doi: 10.1155/2016/7353642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shih DM, Wang Z, Lee R, Meng Y, Che N, Charugundla S, et al. Flavin containing monooxygenase 3 exerts broad effects on glucose and lipid metabolism and atherosclerosis. J Lipid Res. 2015;56:22–37. doi: 10.1194/jlr.M051680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Warrier M, Shih DM, Burrows AC, Ferguson D, Gromovsky AD, Brown AL, et al. The TMAO-generating enzyme flavin monooxygenase 3 is a central regulator of cholesterol balance. Cell Rep. 2015;10:326–38. doi: 10.1016/j.celrep.2014.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kang Y, Cai Y. Gut microbiota and hypertension: from pathogenesis to new therapeutic strategies. Clin Res Hepatol Gastroenterol. 2018;42:110–7. doi: 10.1016/j.clinre.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 87.Jose PA, Raj D. Gut microbiota in hypertension. Curr Opin Nephrol Hypertens. 2015;24:403–9. doi: 10.1097/MNH.0000000000000149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pluznick JL, Protzko RJ, Gevorgyan H, Peterlin Z, Sipos A, Han J, et al. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc Natl Acad Sci USA. 2013;110:4410–5. doi: 10.1073/pnas.1215927110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pluznick J. A novel SCFA receptor, the microbiota, and blood pressure regulation. Gut Microbes. 2014;5:202–7. doi: 10.4161/gmic.27492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Karbach SH, Schönfelder T, Brandão I, Wilms E, Hörmann N, Jäckel S, et al. Gut microbiota promote angiotensin II-induced arterial hypertension and vascular dysfunction. J Am Heart Assoc. 2016;5:e003698. [DOI] [PMC free article] [PubMed]
- 91.Kimura I, Inoue D, Maeda T, Hara T, Ichimura A, Miyauchi S, et al. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41) Proc Natl Acad Sci USA. 2011;108:8030–5. doi: 10.1073/pnas.1016088108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Santisteban MM, Qi Y, Zubcevic J, Kim S, Yang T, Shenoy V, et al. Hypertension-linked pathophysiological alterations in the gut. Circ Res. 2017;120:312–23. doi: 10.1161/CIRCRESAHA.116.309006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Manco M, Putignani L, Bottazzo GF. Gut microbiota, lipopolysaccharides, and innate immunity in the pathogenesis of obesity and cardiovascular risk. Endocr Rev. 2010;31:817–44. doi: 10.1210/er.2009-0030. [DOI] [PubMed] [Google Scholar]
- 94.Wilck N, Matus MG, Kearney SM, Olesen SW, Forslund K, Bartolomaeus H, et al. Salt-responsive gut commensal modulates TH17 axis and disease. Nature. 2017;551:585–9. doi: 10.1038/nature24628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tang WHW, Hazen SL. Microbiome, trimethylamine N-oxide, and cardiometabolic disease. Transl Res. 2017;179:108–15. doi: 10.1016/j.trsl.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yang T, Santisteban MM, Rodriguez V, Li E, Ahmari N, Carvajal JM, et al. Gut dysbiosis is linked to hypertension. Hypertension. 2015;65:1331–40. doi: 10.1161/HYPERTENSIONAHA.115.05315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Adnan S, Nelson JW, Ajami NJ, Venna VR, Petrosino JF, Bryan RM, et al. Alterations in the gut microbiota can elicit hypertension in rats. Physiol Genom. 2017;49:96–104.. doi: 10.1152/physiolgenomics.00081.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Durgan DJ, Ganesh BP, Cope JL, Ajami NJ, Phillips SC, Petrosino JF, et al. Role of the gut microbiome in obstructive sleep apnea-induced hypertension. Hypertension. 2016;67:469–74. doi: 10.1161/HYPERTENSIONAHA.115.06672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Marques FZ, Nelson E, Chu PY, Horlock D, Fiedler A, Ziemann M, et al. High fibre diet and acetate supplementation change the gut microbiota and prevent the development of hypertension and heart failure in DOCA-salt hypertensive mice. Circulation. 2017;135:964–77. doi: 10.1161/CIRCULATIONAHA.116.024545. [DOI] [PubMed] [Google Scholar]
- 100.Yan Q, Gu Y, Li X, Yang W, Jia L, Chen C, et al. Alterations of the gut microbiome in hypertension. Front Cell Infect Microbiol. 2017;7:381. doi: 10.3389/fcimb.2017.00381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li J, Zhao F, Wang Y, Chen J, Tao J, Tian G, et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome. 2017;5:14. doi: 10.1186/s40168-016-0222-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Duseja A, Chawla YK. Obesity and NAFLD: the role of bacteria and microbiota. Clin Liver Dis. 2014;18:59–71. doi: 10.1016/j.cld.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 103.Miele L, Valenza V, La Torre G, Montalto M, Cammarota G, Ricci R, et al. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology. 2009;49:1877–87. doi: 10.1002/hep.22848. [DOI] [PubMed] [Google Scholar]
- 104.Le Roy T, Llopis M, Lepage P, Bruneau A, Rabot S, Bevilacqua C, et al. Intestinal microbiota determines development of non-alcoholic fatty liver disease in mice. Gut. 2013;62:1787–94. [DOI] [PubMed]
- 105.Yao C, Muir J, Gibson P. Insights into colonic protein fermentation, its modulation and potential health implications. Aliment Pharmacol Ther. 2016;43:181–96. doi: 10.1111/apt.13456. [DOI] [PubMed] [Google Scholar]
- 106.Chen YM, Liu Y, Zhou RF, et al. Associations of gut-flora-dependent metabolite trimethylamine-N-oxide, betaine and choline with nonalcoholic fatty liver disease in adults. Sci Rep. 2016;6:19076. doi: 10.1038/srep19076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mollica MP, Mattace Raso G, Cavaliere G, Trinchese G, De Filippo C, Aceto S, et al. Butyrate regulates liver mitochondrial function, efficiency, and dynamics in insulin-resistant obese mice. Diabetes. 2017;66:1405–18. doi: 10.2337/db16-0924. [DOI] [PubMed] [Google Scholar]
- 108.den Besten G, Bleeker A, Gerding A, van Eunen K, Havinga R, van Dijk TH, et al. Short-chain fatty acids protect against high-fat diet-induced obesity via a PPARγ-dependent switch from lipogenesis to fat oxidation. Diabetes. 2015;64:2398–408. doi: 10.2337/db14-1213. [DOI] [PubMed] [Google Scholar]
- 109.Kondo T, Kishi M, Fushimi T, Kaga T. Acetic acid upregulates the expression of genes for fatty acid oxidation enzymes in liver to suppress body fat accumulation. J Agric Food Chem. 2009;57:5982–6. doi: 10.1021/jf900470c. [DOI] [PubMed] [Google Scholar]
- 110.Sakakibara S, Yamauchi T, Oshima Y, Tsukamoto Y, Kadowaki T. Acetic acid activates hepatic AMPK and reduces hyperglycemia in diabetic KK-A (y) mice. Biochem Biophys Res Commun. 2006;344:597–604. doi: 10.1016/j.bbrc.2006.03.176. [DOI] [PubMed] [Google Scholar]
- 111.Herrington W, Lacey B, Sherliker P, Armitage J, Lewington S. Epidemiology of atherosclerosis and the potential to reduce the global burden of atherothrombotic disease. Circ Res. 2016;118:535–46. doi: 10.1161/CIRCRESAHA.115.307611. [DOI] [PubMed] [Google Scholar]
- 112.Dalen JE, Alpert JS, Goldberg RJ, Weinstein RS. The epidemic of the 20th century: coronary heart disease. Am J Med. 2014;127:807–12. doi: 10.1016/j.amjmed.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 113.Koren O, Spor A, Felin J, Fak F, Stombaugh J, Tremaroli V, et al. Human oral, gut, and plaque microbiota in patients with atherosclerosis. Proc Natl Acad Sci USA. 2011;108:4592–8. doi: 10.1073/pnas.1011383107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Calandrini C, Ribeiro A, Gonnelli A, Ota-Tsuzuki C, Rangel L, Saba-Chujfi E, et al. Microbial composition of atherosclerotic plaques. Oral Dis. 2014;20:e128–34. doi: 10.1111/odi.12205. [DOI] [PubMed] [Google Scholar]
- 115.Ma G, Pan B, Chen Y, Guo C, Zhao M, Zheng L, et al. Trimethylamine N-oxide in atherogenesis: impairing endothelial self-repair capacity and enhancing monocyte adhesion. Biosci Rep. 2017;37:BSR20160244. doi: 10.1042/BSR20160244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhu W, Gregory JC, Org E, Buffa JA, Gupta N, Wang Z, et al. Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell. 2016;165:111–24. doi: 10.1016/j.cell.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wu ZX, Su-Fang L, Hong C, Jun-Xian S, Yuan-Feng G, Feng Z, et al. The changes of gut microbiota after acute myocardial infarction in rats. PLoS ONE. 2017;12:e0180717. [DOI] [PMC free article] [PubMed]
- 118.Yamashita T, Emoto T, Sasaki N, Hirata K. Gut microbiota and coronary artery disease. Int Heart J. 2016;57:9. doi: 10.1536/ihj.16-414. [DOI] [PubMed] [Google Scholar]
- 119.De Nicola L, Minutolo R. Worldwide growing epidemic of CKD: fact or fiction? Kidney Int. 2016;90:482–4. doi: 10.1016/j.kint.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 120.Bruck K, Stel VS, Gambaro G, Hallan S, Volzke H, Arnlov J, et al. CKD prevalence varies across the european general population. J Am Soc Nephrol. 2016;27:2135–47. doi: 10.1681/ASN.2015050542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Castillo-Rodriguez E, Fernandez-Prado R, Esteras R, Perez-Gomez MV, Gracia-Iguacel C, Fernandez-Fernandez B, et al. Impact of altered intestinal microbiota on chronic kidney disease progression. Toxins. 2018;10:E300. doi: 10.3390/toxins10070300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Barreto FC, Barreto DV, Liabeuf S, Meert N, Glorieux G, Temmar M, European Uremic Toxin Work, G et al. Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin J Am Soc Nephrol. 2009;4:1551–8. doi: 10.2215/CJN.03980609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bammens B, Evenepoel P, Keuleers H, Verbeke K, Vanrenterghem Y. Free serum concentrations of the protein-bound retention solute p-cresol predict mortality in hemodialysis patients. Kidney Int. 2006;69:1081–7. doi: 10.1038/sj.ki.5000115. [DOI] [PubMed] [Google Scholar]
- 124.Missailidis C, Hallqvist J, Qureshi AR, Barany P, Heimburger O, Lindholm B, et al. Serum trimethylamine-N-oxide is strongly related to renal function and predicts outcome in chronic kidney disease. PLoS ONE. 2016;11:e0141738. doi: 10.1371/journal.pone.0141738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Anders HJ, Andersen K, Stecher B. The intestinal microbiota, a leaky gut, and abnormal immunity in kidney disease. Kidney Int. 2013;83:1010–6. doi: 10.1038/ki.2012.440. [DOI] [PubMed] [Google Scholar]
- 126.Ramezani A, Massy ZA, Meijers B, Evenepoel P, Vanholder R, Raj DS. Role of the gut microbiome in uremia: a potential therapeutic target. Am J Kidney Dis. 2016;67:483–98. doi: 10.1053/j.ajkd.2015.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nomura S, et al. Genetic and non-genetic determinants of clinical phenotypes in cardiomyopathy. J Cardiol. 2019;73:187–90. doi: 10.1016/j.jjcc.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 128.Zhernakova DV, Le TH, Kurilshikov A, Atanasovska B, Bonder MJ, Sanna S, et al. LifeLines cohort study; BIOS consortium. Individual variations in cardiovascular-disease-related protein levels are driven by genetics and gut microbiome. Nat Genet. 2018;50:1524–32. doi: 10.1038/s41588-018-0224-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Aguiar CJ, Rocha-Franco JA, Sousa PA, Santos AK, Ladeira M, Rocha-Resende C, et al. Succinate causes pathological cardiomyocyte hypertrophy through GPR91 activation. Cell Commun Signal. 2014;12:78. doi: 10.1186/s12964-014-0078-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lekawanvijit S, Adrahtas A, Kelly DJ, Kompa AR, Wang BH, Krum H. Does indoxyl sulfate, a uraemic toxin, have direct effects on cardiac fibroblasts and myocytes? Eur Heart J. 2010;31:1771–9. doi: 10.1093/eurheartj/ehp574. [DOI] [PubMed] [Google Scholar]
- 131.Troseid M, Ueland T, Hov JR, Svardal A, Gregersen I, Dahl CP, et al. Microbiota-dependent metabolite trimethylamine-N-oxide is associated with disease severity and survival of patients with chronic heart failure. J Intern Med. 2015;277:717–26. doi: 10.1111/joim.12328. [DOI] [PubMed] [Google Scholar]
- 132.Sandek A, Bauditz J, Swidsinski A, Buhner S, Weber-Eibel J, von Haehling S, et al. Altered intestinal function in patients with chronic heart failure. J Am Coll Cardiol. 2007;50:1561–9. doi: 10.1016/j.jacc.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 133.Kamo T, Akazawa H, Suzuki JI, Komuro I. Novel concept of a heart-gut axis in the pathophysiology of heart failure. Korean Circ J. 2017;47:663–9. doi: 10.4070/kcj.2017.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Peschel T, Schonauer M, Thiele H, Anker SD, Schuler G, Niebauer J. Invasive assessment of bacterial endotoxin and inflammatory cytokines in patients with acute heart failure. Eur J Heart Fail. 2003;5:609–14. doi: 10.1016/s1388-9842(03)00104-1. [DOI] [PubMed] [Google Scholar]
- 135.Sandek A, Bjarnason I, Volk HD, Crane R, Meddings JB, Niebauer J, et al. Studies on bacterial endotoxin and intestinal absorption function in patients with chronic heart failure. Int J Cardiol. 2012;157:80–85. doi: 10.1016/j.ijcard.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 136.Pasini E, Aquilani R, Testa C, Baiardi P, Angioletti S, Boschi F, et al. Pathogenic gut flora in patients with chronic heart failure. JACC Heart Fail. 2016;4:220–7. doi: 10.1016/j.jchf.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 137.Luedde M, Winkler T, Heinsen FA, Rühlemann MC, Spehlmann ME, Bajrovic A, et al. Heart failure is associated with depletion of core intestinal microbiota. ESC Heart Fail. 2017;4:282–90. doi: 10.1002/ehf2.12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kummen M, Mayerhofer CCK, Vestad B, Broch K, Awoyemi A, Storm-Larsen C, et al. Gut microbiota signature in heart failure defined from profiling of 2 independent cohorts. J Am Coll Cardiol. 2018;71:1184–6. doi: 10.1016/j.jacc.2017.12.057. [DOI] [PubMed] [Google Scholar]
- 139.Kamo T, Akazawa H, Suda W, Saga-Kamo A, Shimizu Y, Yagi H, et al. Dysbiosis and compositional alterations with aging in the gut microbiota of patients with heart failure. PLoS ONE. 2017;12:e0174099. doi: 10.1371/journal.pone.0174099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Cui X, Ye L, Li J, Jin L, Wang W, Li S, et al. Metagenomic and metabolomic analyses unveil dysbiosis of gut microbiota in chronic heart failure patients. Sci Rep. 2018;8:635. doi: 10.1038/s41598-017-18756-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Organ CL, Otsuka H, Bhushan S, Wang Z, Bradley J, Trivedi R, et al. Choline diet and its gut microbe-derived metabolite, trimethylamine N-oxide. exacerbate pressure overload-induced heart failure. Circ Heart Fail. 2016;9:e002314. doi: 10.1161/CIRCHEARTFAILURE.115.002314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sun X, Jiao X, Ma Y, Liu Y, Zhang L, He Y, et al. Trimethylamine N-oxide induces inflammation and endothelial dysfunction in human umbilical vein endothelial cells via activating ROS-TXNIP-NLRP3 inflammasome. Biochem Biophys Res Commun. 2016;481:63–70. doi: 10.1016/j.bbrc.2016.11.017. [DOI] [PubMed] [Google Scholar]
- 143.Wang Z, Roberts AB, Buffa JA, Levison BS, Zhu W, Org E, et al. Non-lethal Inhibition of gut microbial trimethylamine production for the treatment of atherosclerosis. Cell. 2015;163:1585–95. doi: 10.1016/j.cell.2015.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Senthong V, Wang Z, Li XS, Fan Y, Wu Y, Tang WH, et al. Intestinal microbiota-generated metabolite trimethylamine-N-oxide and 5-year mortality risk in stable coronary artery disease: the contributory role of intestinal microbiota in a COURAGE-like patient cohort. J Am Heart Assoc. 2016;5:e002816. doi: 10.1161/JAHA.115.002816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Senthong V, Wang Z, Fan Y, Wu Y, Hazen SL, Tang WH. Trimethylamine N-oxide and mortality risk in patients with peripheral artery disease. J Am Heart Assoc. 2016;5:e004237. doi: 10.1161/JAHA.116.004237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tang WH, Wang Z, Fan Y, Levison B, Hazen JE, Donahue LM, et al. Prognostic value of elevated levels of intestinal microbe-generated metabolite trimethylamine-N-oxide in patients with heart failure: refining the gut hypothesis. J Am Coll Cardiol. 2014;64:1908–14. doi: 10.1016/j.jacc.2014.02.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Reiner MF, Müller D, Gobbato S, Stalder O, Limacher A, Bonetti NR, et al. Gut microbiota-dependent trimethylamine-N-oxide (TMAO) shows a U-shaped association with mortality but not with recurrent venous thromboembolism. Thromb Res. 2019;174:40–7. [DOI] [PubMed]
- 148.Heianza Y, Ma W, Manson JE, Rexrode KM, Qi L. Gut microbiota metabolites and risk of major adverse cardiovascular disease events and death: a systematic review and meta-analysis of prospective studies. J Am Heart Assoc. 2017;6:e004947. doi: 10.1161/JAHA.116.004947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Schiattarella GG, Sannino A, Toscano E, Giugliano G, Gargiulo G, Franzone A, et al. Gut microbe-generated metabolite trimethylamine-N-oxide as cardiovascular risk biomarker: a systematic review and dose-response meta-analysis. Eur Heart J. 2017;38:2948–56. doi: 10.1093/eurheartj/ehx342. [DOI] [PubMed] [Google Scholar]
- 150.Lever M, George PM, Slow S, Bellamy D, Young JM, Ho M, et al. Trimethylamine-N-oxide as predictors of cardiovascular outcomes show different patterns in diabetes mellitus: an observational study. PLoS ONE. 2014;9:e114969. doi: 10.1371/journal.pone.0114969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Perez PF, Donnet-Hughes A, Schrffrin Schiffrin EJ, Marteau P, Brassart D. Presentation of microbial signals via maternal cells. an evolutionary advantage of mammals. In: Intestinal microbiota in health and disease. Modern concepts. Boca Raton, FL (USA). CRC Press; 2014. EJ
- 152.Collado MC, Rautava S, Aakko J, Isolauri E, Salminen S. Human gut colonisation may be initiated in utero by distinct microbial communities in the placenta and amniotic fluid. Sci Rep. 2016;6:23129. [DOI] [PMC free article] [PubMed]
- 153.Aagaard K, Ma J, Antony KM, Ganu R, Petrosino J, Versalovic J. The placenta harbors a unique microbiome. Sci Transl Med. 2014;6:237ra265 37. [DOI] [PMC free article] [PubMed]
- 154.Tun HM, Bridgman SL, Chari R, Field CJ, Guttman DS, Becker AB, Canadian Healthy Infant Longitudinal Development (CHILD) Study Investigators et al. Roles of birth mode and infant gut microbiota in intergenerational transmission of overweight and obesity from mother to offspring. JAMA Pediatr. 2018;172:368–77. doi: 10.1001/jamapediatrics.2017.5535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Stanislawski MA, Dabelea D, Wagner BD, Iszatt N, Dahl C, Sontag MK, et al. Gut microbiota in the first 2 years of life and the association with body mass index at age 12 in a norwegian birth cohort. MBio. 2018;9:e01751-18. doi: 10.1128/mBio.01751-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Jumpertz R, Le DS, Turnbaugh PJ, Trinidad C, Bogardus C, Gordon JI, et al. Energy-balance studies reveal associations between gut microbes, caloric load, and nutrient absorption in humans. Am J Clin Nutr. 2011;94:58–65. [DOI] [PMC free article] [PubMed]
- 157.Chang PV, Hao L, Offermanns S, Medzhitov R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc Natl Acad Sci USA. 2014;111:2247–52. [DOI] [PMC free article] [PubMed]
- 158.Park J, Kim M, Kang SG, Jannasch AH, Cooper B, Patterson J, et al. Short-chain fatty acids induce both effector and regulatory t cells by suppression of histone deacetylases and regulation of the mtor-s6k pathway. Mucosal Immunol. 2015;8:80–93. doi: 10.1038/mi.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wankhade UD, Zhong Y, Kang P, Alfaro M, Chintapalli SV, Thakali KM, et al. Enhanced offspring predisposition to steatohepatitis with maternal high-fat diet is associated with epigenetic and microbiome alterations. PLoS ONE. 2017;12:e0175675. doi: 10.1371/journal.pone.0175675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Bruce-Keller AJ, Fernandez-Kim SO, Townsend RL, Kruger C, Carmouche R, Newman S, et al. Maternal obese-type gut microbiota differentially impact cognition, anxiety and compulsive behavior in male and female offspring in mice. PLoS ONE. 2017;12:e0175577. doi: 10.1371/journal.pone.0175577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Luoto R, Laitinen K, Nermes M, Isolauri E Impact of maternal probiotic-supplemented dietary counselling on pregnancy outcome and prenatal and postnatal growth: a double-blind, placebo-controlled study. Br J Nutr. 2010;103:1792–9. [DOI] [PubMed]
- 162.Borewicz K, Suarez-Diez M, Hechler C, Beijers R, de Weerth C, Arts I, et al. The effect of prebiotic fortified infant formulas on microbiota composition and dynamics in early life. Sci Rep. 2019;9:2434. doi: 10.1038/s41598-018-38268-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.van Oostrom AJ, Rabelink TJ, Verseyden C, et al. Activation of leuko- cytes by postprandial lipemia in healthy volunteers. Atherosclerosis. 2004;177:175–82. doi: 10.1016/j.atherosclerosis.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 164.Erridge C, Attina T, Spickett CM, Webb DJ. A high-fat meal induces low-grade endotoxemia: evidence of a novel mechanism of postprandial inflammation. Am J Clin Nutr. 2007;86:1286–92. doi: 10.1093/ajcn/86.5.1286. [DOI] [PubMed] [Google Scholar]
- 165.David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–63. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.De Lorenzo A, Bernardini S, Gualtieri P, Cabibbo A, Perrone MA, Giambini I, et al. Mediterranean meal versus Western meal effects on postprandial ox-LDL, oxidative and inflammatory gene expression in healthy subjects: a randomized controlled trial for nutrigenomic approach in cardiometabolic risk. Acta Diabetol. 2016;54:141–9. doi: 10.1007/s00592-016-0917-2. [DOI] [PubMed] [Google Scholar]
- 167.Calabrese V, Cornelius C, Trovato A, Cavallaro M, Mancuso C, Di Renzo L, et al. The hormetic role of dietary antioxidants in free radical-related diseases. Curr Pharm Des. 2010;16:877–83. doi: 10.2174/138161210790883615. [DOI] [PubMed] [Google Scholar]
- 168.Di Daniele N, Petramala L, Di Renzo L, Sarlo F, Della Rocca DG, Rizzo M, et al. Body composition changes and cardiometabolic benefits of a balanced Italian Mediterranean Diet in obese patients with metabolic syndrome. Acta Diabetol. 2012;50:409–16. doi: 10.1007/s00592-012-0445-7. [DOI] [PubMed] [Google Scholar]
- 169.De Lorenzo A, Noce A, Bigioni M, Calabrese V, Della Rocca DG, Di Daniele N, et al. The effects of Italian Mediterranean organic diet (IMOD) on health status. Curr Pharm Des. 2010;16:814–24. doi: 10.2174/138161210790883561. [DOI] [PubMed] [Google Scholar]
- 170.Maffeis C, Pinelli L, Surano MG, Fornari E, Cordioli S, Gasperotti S. Pro-atherogenic postprandial profile: meal-induced changes of lipoprotein sub-fractions and in-flammation markers in obese boys. Nutr Metab Cardiovasc Dis. 2012;22:959–65. doi: 10.1016/j.numecd.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 171.Di Renzo L, Marsella LT, Carraro A, Valente R, Gualtieri P, Gratteri S, et al. Changes in LDL oxidative status and oxidative and inflammatory gene expression after red wine intake in healthy people: a randomized trial. Mediators Inflamm. 2015;2015:317348. doi: 10.1155/2015/317348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Di Renzo L, Merra G, Botta R, Gualtieri P, Manzo A, Perrone MA, et al. Post-prandial effects of hazelnut-enriched high fat meal on LDL oxidative status, oxidative and inflammatory gene expression of healthy subjects: a randomized trial. Eur Rev Med Pharmacol Sci. 2017;21:1610–26. [PubMed] [Google Scholar]
- 173.Di Renzo L, Carraro A, Valente R, Iacopino L, Colica C, De Lorenzo A. Intake of red wine in different meals modulates oxidized LDL level, oxidative and inflammatory gene expression in healthy people: a randomized crossover trial. Oxid Med Cell Longev. 2014;2014:681318. doi: 10.1155/2014/681318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Di Renzo L, Cioccoloni G, Sinibaldi Salimei P, Ceravolo I, De Lorenzo A, Gratteri S. Alcoholic beverage and meal choices for the prevention of noncommunicable diseases: a randomized nutrigenomic trial. Oxid Med Cell Longev. 2018:5461436. [DOI] [PMC free article] [PubMed]
- 175.Di Renzo L, Di Pierro D, Bigioni M, Sodi V, Galvano F, Cianci R, et al. Is antioxidant plasma status in humans a consequence of the antioxidant food content influence? Eur Rev Med Pharmacol Sci. 2007;11:185–92. [PubMed] [Google Scholar]
- 176.Soldati L, Di Renzo L, Jirillo E, Ascierto PA, Marincola FM, De Lorenzo A. The influence of diet on anti-cancer immune responsiveness. J Transl Med. 2018;16:75. doi: 10.1186/s12967-018-1448-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Mocchegiani E, Costarelli L, Giacconi R, Piacenza F, Basso A. Malavolta R. Zinc, metallothioneins and immunosenescence: effect of zinc supply as nutrigenomic approach. Biogerontology. 2011;12:455–65. doi: 10.1007/s10522-011-9337-4. [DOI] [PubMed] [Google Scholar]
- 178.Macpherson AJ, Harris NL. Interactions between commensal intestinal bacteria and the immune system. Nat Rev Immunol. 2004;4:478–85. doi: 10.1038/nri1373. [DOI] [PubMed] [Google Scholar]
- 179.Khalesi S, Sun J, Buys N, Jayasinghe R. Effect of probiotics on blood pressure: a systematic review and meta-analysis of randomized, controlled trials. 2014;64:897–903. [DOI] [PubMed]
- 180.Li Z, Yang S, Lin H, Huang J, Watkins PA, Moser AB, et al. Probiotics and antibodies to TNF inhibit inflammatory activity and improve nonalcoholic fatty liver disease. Hepatology. 2003;37:343–50. doi: 10.1053/jhep.2003.50048. [DOI] [PubMed] [Google Scholar]
- 181.Daubioul C, Horsmans Y, Lambert P, Danse E, Delzenne NM. Effects of oligofructose on glucose and lipid metabolism in patients with nonalcoholic steatohepatitis: results of a pilot study. Eur J Clin Nutr. 2005;59:723–6. doi: 10.1038/sj.ejcn.1602127. [DOI] [PubMed] [Google Scholar]
- 182.Malaguarnera M, Vacante M, Antic T, Giordano M, Chisari G, Acquaviva R, et al. Bifidobacterium longum with fructo-oligosaccharides in patients with non alcoholic steatohepatitis. Dig Dis Sci. 2012;57:545–53. doi: 10.1007/s10620-011-1887-4. [DOI] [PubMed] [Google Scholar]
- 183.Ong HS, Yim HCH. Microbial factors in inflammatory diseases and cancers. Adv Exp Med Biol. 2017;1024:153–74. doi: 10.1007/978-981-10-5987-2_7. [DOI] [PubMed] [Google Scholar]
- 184.Wang Y, Kasper LH. The role of microbiome in central nervous system disorders. Brain Behav Immun. 2014;38:1–12. doi: 10.1016/j.bbi.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kelly D, King T, Aminov R. Importance of microbial colonization of the gut in early life to the development of immunity. Mutat Res. 2007;622:58–69. doi: 10.1016/j.mrfmmm.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 186.Colica C, Di Renzo L, Trombetta D, Smeriglio A, Bernardini S, Cioccoloni G, et al. Antioxidant effects of a hydroxytyrosol-based pharmaceutical formulation on body composition, metabolic state, and gene expression: a randomized double-blinded, placebo-controlled crossover trial. Oxid Med Cell Longev. 2017;2017:2473495. doi: 10.1155/2017/2473495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Bachmeier BE, Mohrenz IV, Mirisola V, Schleicher E, Romeo F, Hohneke C, et al. Curcumin downregulates the inflammatory cytokines CXCL1 and-2 in breast cancer cells via NFkB. Carcinogenesis. 2008;29:779–89. doi: 10.1093/carcin/bgm248. [DOI] [PubMed] [Google Scholar]
- 188.Kawaguchi K, Matsumoto T, Kumazawa Y. Effects of antioxidant polyphenols on TNF-alpha-related diseases. Curr Topo Med Chem. 2011;11:1767–679. doi: 10.2174/156802611796235152. [DOI] [PubMed] [Google Scholar]
- 189.Tsao R, Li H. Antioxidant properties in vitro and in vivo: realistic assessments of efficacy of plant extracts. CAB Rev. 2012;7:9. [Google Scholar]
- 190.Scott KP, Antoine J-M, Midtvedt T, van Hemert S. Manipulating the gut microbiota to maintain health and treat disease. Microb Ecol Health Dis. 2015;26:25877. doi: 10.3402/mehd.v26.25877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Cammarota G, Ianiro G, Tilg H, Rajilić-Stojanović M, Kump P, Satokari R, et al. European consensus conference on faecal microbiota transplantation in clinical practice. Gut. 2017;66:569–80. doi: 10.1136/gutjnl-2016-313017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.McDonald LC, Gerding DN, Johnson S, Bakken JS, Carroll KC, Coffin SE, et al. Clinical practice guidelines for clostridium difficile infection in adults and children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA) Clin Infect Dis. 2018;66:e1–48. doi: 10.1093/cid/cix1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Liubakka A, Vaughn BP. Clostridium difficile Infection and fecal microbiota transplant. AACN Adv Crit Care. 2016;27:324–37. doi: 10.4037/aacnacc2016703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Kelly JR, Borre Y, O’ Brien C, Patterson E, El Aidy S, Deane J, et al. Transferring the blues: depression-associated gut microbiota induces neurobehavioural changes in the rat. J Psychiatr Res. 2016;82:109–18. doi: 10.1016/j.jpsychires.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 195.Mangiola F. Gut microbiota in autism and mood disorders. World J Gastroenterol. 2016;22:361. doi: 10.3748/wjg.v22.i1.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Gregory JC, Buffa JA, Org E, Wang Z, Levison BS, Zhu W, et al. Transmission of atherosclerosis susceptibility with gut microbial transplantation. J Biol Chem. 2015;290:5647–60. doi: 10.1074/jbc.M114.618249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.de Groot PF, Frissen MN, de Clercq NC, Nieuwdorp M. Fecal microbiota transplantation in metabolic syndrome: history, present and future. Gut Microbes. 2017;8:253–67. doi: 10.1080/19490976.2017.1293224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Li J, Lin S, Vanhoutte PM, Woo CW, Xu A. Akkermansiamuciniphila protects against atherosclerosis by preventing metabolic endotoxemia-induced inflammation in Apoe−/− mice. Circulation. 2016;133:2434–46. doi: 10.1161/CIRCULATIONAHA.115.019645. [DOI] [PubMed] [Google Scholar]
- 199.Vrieze A, Van Nood E, Holleman F, Salojärvi J, Kootte RS, Bartelsman J, et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143:913–6.e7. doi: 10.1053/j.gastro.2012.06.031. [DOI] [PubMed] [Google Scholar]


