Abstract
Milk production may involve a transient development of insulin resistance in nonmammary tissues to support redistribution of maternal macronutrients to match the requirements of the lactating mammary gland. In the current study, adipose and liver metabolic responses were measured in the fasting state and during a two-step (10 and 20 mU/m2/min) hyperinsulinemic-euglycemic clamp with stable isotopes, in 6-week postpartum women who were lactating (n = 12) or formula-feeding (n = 6) their infants and who were closely matched for baseline characteristics (e.g., parity, body composition, and intrahepatic lipid). When controlling for the low insulin concentrations of both groups, the lactating women exhibited a fasting rate of endogenous glucose production (EGP) that was 2.6-fold greater and a lipolysis rate that was 2.3-fold greater than the formula-feeding group. During the clamp, the groups exhibited similar suppression rates of EGP and lipolysis. In the lactating women only, higher prolactin concentrations were associated with greater suppression rates of lipolysis and lower intrahepatic lipid and plasma triacylglycerol concentrations. These data suggest that whole-body alterations in glucose transport may be organ specific and facilitate nutrient partitioning during lactation. Recapitulating a shift toward noninsulin-mediated glucose uptake could be an early postpartum strategy to enhance lactation success in women at risk for delayed onset of milk production.
Introduction
Data from detailed metabolic studies, such as the insulin clamp technique and i.v. glucose tolerance tests, suggest that from prepregnancy to late pregnancy, insulin sensitivity might become reduced by as much as 50% (1,2). Similar metabolic methods have been used in postpartum women following a pregnancy with normal glucose tolerance (NGT) or gestational diabetes mellitus (GDM), and data have suggested that lactation may restore whole-body insulin sensitivity to the prepregnancy state (3–5). The concept proposed by Neville et al. (6) suggested that after lactation has been established, the induction of hyperglycemia promotes glucose uptake in the mammary gland, which is a process that appears to be independent of insulin action. This theory is consistent with noninsulin-mediated glucose uptake (NIMGU) by the lactating mammary gland in humans, as has been documented in other mammalian species (7,8).
In observational studies, lactation has been found to be associated with a decreased incidence of maternal type 2 diabetes in a manner that is independent of ethnicity/race, gestational glucose tolerance, and postpartum weight change (9). Although the basis for this association is poorly understood, hypothesized mechanisms include the enhancement of known insulin-sensitivity systems during lactation (5,10). A contrasting view is that lactation in humans extends the period of physiological insulin resistance, established during pregnancy, into the postpartum period in one or more nonmammary tissues. Three observations support this latter hypothesis. First, lactating ruminants and rodents exhibit insulin resistance in nonmammary tissues, (7,11) and such findings of insulin resistance in nonhuman species set a precedent for consideration in human physiology. Second, indirect data from human studies have documented low circulating concentrations of adiponectin throughout lactation (12–18). High adiponectin is associated with insulin sensitivity (19), and thus, the observed reduction in this hormone in lactating women hints at decreased insulin sensitivity in one or more physiologic pathways. After weaning, adiponectin concentrations eventually recover to prepregnancy levels (18). Third, under conditions of hyperinsulinemia, lactating women have been shown to require the same amount of glucose infusion as nonpregnant, nonpostpartum women (3). If human mammary tissue exhibits NIMGU, these data suggest that at low insulin levels, more glucose may be taken-up in mammary tissue with less going to nonmammary tissues (peripheral insulin resistance). Such coordination between mammary and nonmammary tissues would increase the availability of substrates for milk production. Past studies in this field have investigated fasting and insulin-stimulated glucose metabolism in lactating women and included as the control group nonlactating, nonpostpartum women (3,5,20). The goal of the present project was to extend these studies by investigating the impact of lactation on liver and adipose tissue while controlling for the effects of the postpartum period by comparing lactating women to postpartum women choosing to formula-feed their infants. Given past observations of low fasting insulin levels in postpartum women, we used a hyperinsulinemic-euglycemic clamp method to test adipose and liver metabolism at insulin infusions that were at low (10 mU/m2/min) and medium (20 mU/m2/min) levels. An understanding of the hepatic and adipose physiology behind alterations in glucose uptake during lactation will facilitate development of strategies to ensure timely onset of ample milk secretion in women at risk for a late start, a predictor of short lactation duration (21).
Research Design and Methods
Subjects, Inclusion, and Exclusion Criteria
Informed consent was obtained from postpartum women. The protocol was first described at a scheduled prenatal visit during the third trimester of pregnancy and/or at the scheduled postpartum visit between 2 and 5 weeks past full-term delivery. Participants were lactating or nonlactating based on their choice of newborn feeding regimen with no more than 6 oz of formula per day (lactating group) or no breast milk (formula-feeding group). Visit 1 occurred during week 5 postpartum, while visits 2 and 3 occurred between 5 and 8 weeks postpartum. The research protocol was approved by the University of Texas Southwestern Institutional Review Board (number STU-092010–071), and the study was conducted according to the principles expressed in the Declaration of Helsinki. The inclusion criteria were BMI at 2 weeks postpartum 25–35 kg/m2, age 21–49 years, term delivery of one newborn, and intention to feed mostly breast milk (≤6 oz of formula per day) or to feed formula only. Women could have NGT or GDM (22) diagnosed during the third trimester of pregnancy and had been treated only with diet. Exclusion criteria included a history of preeclampsia, insulin-treated GDM, hormonal contraception or intrauterine device, pregnancy, type 2 diabetes, use of medications that interfere with prolactin release or nutrient metabolism, postpartum depression, contraindications for MRI, uncontrolled hypothyroidism, and liver or kidney disease. Lactation intensity was defined as the proportion of maternal milk to formula that was fed to the infant; exclusive lactation indicated feeding only maternal milk; and partial lactation reflected a combination of both.
Study Visits
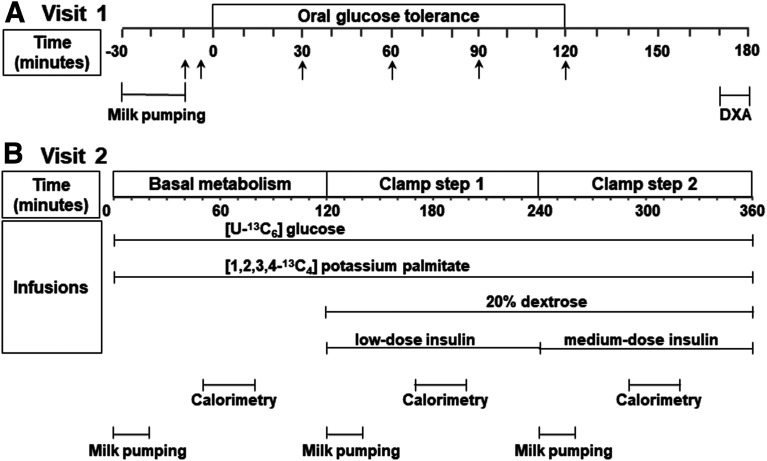
The overall research protocol is shown in Fig. 1, and subjects fasted before all three study visits. Visit 1 consisted of screening for inclusion and exclusion criteria, a complete medical history and physical exam, a urine pregnancy test, 2-h oral glucose tolerance test (OGTT), HbA1c, hemogram and platelet count, metabolic panel with liver and renal function, lipid panel, thyrotropin, and body composition by dual-energy X-ray absorptiometry. During visit 1, lactating women pumped breast milk using an electric, hospital-grade breast pump (Ameda) between 30 and 10 min before the 75 g of dextrose. Because prolactin levels fluctuate relative to time from nursing, peaking around 30 min from initiation of lactation (23), this approach allowed us to standardize the stimulation of prolactin relative to metabolic measurements. Visit 2 included the hyperinsulinemic-euglycemic clamp, and visit 3 included confirmation of a negative pregnancy test followed by proton MRS of the liver using a 3-Tesla magnet.
Figure 1.
Research protocols. A: Visit 1. Arrows indicate times of blood draws. B: Visit 2. DXA, dual-energy X-ray absorptiometry.
For the hyperinsulinemic-euglycemic clamp during visit 2, two anterograde lines were placed: one in the antecubital region for infusate administration and the other at a site between the wrist and the antecubital region of the contralateral arm for blood draws. Insulin was infused at 10 mU/m2/min (low-dose step) and at 20 mU/m2/min during step 2 (medium-dose step). Plasma glucose concentration was held constant at 80 mg/dL by a variable rate infusion of 20% dextrose adjusted every 5 min using the negative feedback principle described by DeFronzo et al. (24). The target plasma glucose of 80 mg/dL was selected because during lactation, fasting glucose is lower than in formula-feeding women (6,25). The low and medium insulin infusion rates were selected to mirror the low insulin concentrations seen in postpartum women and supported the study of metabolic changes in liver and adipose.
Later in this project, to expand the consideration of the impact of insulin infusion on adipose fatty acid flux, data were used from a second cohort of lactating (n = 6) and formula-feeding (n = 4) women who were infused with 10 mU/m2/min for step 1 and 40 mU/m2/min for step 2 (the same inclusion criteria, consenting procedures and infusion protocol of K+[1,2,3,4-13C4] palmitate were followed). Given the pattern of insulin suppression of adipose fatty acid release in the first cohort, these additional data were used to verify the insulin effect. The characteristics of the 28 postpartum participants (18 from the main cohort and 10 from the second cohort) are available in Supplementary Tables 1 and 2.
Also, during visit 2, infusions with stable isotopes were initiated in the fasting state (120 min before initiation of the two-step hyperinsulinemic-euglycemic clamp), and continued during the 4 h of the clamp (24). The i.v. line used for blood drawing was covered with a heating pad. Lactating women pumped breast milk simultaneously from both breasts for 20 min at the start of the basal fasting period (0–20 min) and during the first 20 min of each step of the clamp (120–140 min and 240–260 min). The plasma insulin concentration was acutely raised during the first 10 min of each step of the clamp and maintained steady after the prime by a continuous infusion during each 2-h period. Measurements of glucose every 5 min and insulin every 10 min during the last 30 min of each period were used to calculate plasma glucose and plasma insulin at steady state. Whole-body glucose disposal (Rd) was calculated during the last 30 min of each 2-h period. Tracer enrichments were measured at 10-min. intervals under basal and hyperinsulinemic conditions during the last 30 min of each phase. Glucose enrichments in plasma and infusates were measured by gas chromatography–mass spectrometry (Metabolic Solutions, Inc., Nashua, NH). Plasma palmitate enrichments were measured using a 6890N gas chromatography coupled to 5975 mass spectrophotometry detector (Agilent Technologies, Palo Alto, CA) as described previously (26). Fatty acid composition was measured by 6890N gas chromatography system (Agilent Technologies). Indirect calorimetry was performed at 50–80 min, 170–200 min, and 290–320 min during fasting and during each step of the clamp using a V Max Encore Calorimeter Model 29.NE (Palm Springs, CA). For fasting concentrations of prolactin, leptin, total and high-molecular-weight (HMW) adiponectin and estradiol, blood was drawn during the basal period at 25 and 30 min.
Stable Isotopes, Calculations, and Statistical Analysis
Stable isotopes were purchased from Cambridge Isotope Laboratory, Inc. (Tewksbury, MA). A primed-continuous infusion of [U-13C6]-glucose (20 µmol/kg over 1 min, followed by 0.4 µmol/kg/min) and a continuous infusion of K+[1,2,3,4-13C4] palmitate (8.05 µg/kg per minute) complexed to albumin (ratio of 2 mol fatty acid to 1 mol albumin), were administered to quantitate endogenous glucose production (EGP) and Ra of adipose free fatty acids (RaFFA), respectively. Rates of total Ra (sum of EGP and glucose infusion rate) and Rd were determined using Steele’s non-steady-state equations (27). To calculate NIMGU, a model similar to that published by Jumpertz et al. (28) was used. Accordingly, for each subject, the steady-state Rd glucose (y-axis) was plotted against the respective steady-state insulin concentrations (x-axis) during basal, low, and medium insulin infusion rates. Using these data, individual linear regression equations were calculated and Rd glucose at theoretical zero insulin concentration was extrapolated. The NIMGU reported is the mean for each group. The change in glucose disposal (∆Rd) was calculated as relative to the change in plasma insulin (∆Insulin) at steady state, according to the convention of Jensen and Neilsen (29) and Donner et al. (30). RaFFA was calculated as described previously (26), and FFA flux measurements were also normalized to insulin concentrations.
We calculated total area under the curve (AUC) values for glucose, insulin, and FFA using the trapezoidal rule. We used previously published equations to calculate HOMA for insulin resistance (HOMA-IR) and Adipo-IR (31). The Insulin Secretion Sensitivity Index 2, a measure analogous to the disposition index obtained from the intravenous glucose tolerance test, was calculated as the product of (AUCinsulin/AUCglucose) and Matsuda Index (32). The Stumvoll first-phase insulin secretion index was calculated as 1,194 + (4.724 × Ins0) − (117.0 × Gluc60) + (1.414 × Ins60). The Stumvoll second-phase index was calculated as 295 + (0.349 × Ins60) − (25.72 × Gluc60) + (1.107 × Ins0) (33). The Stumvoll indices use glucose in mmol/L and insulin in pmol/L. We converted the final insulin results from pmol/L to μU/mL.
Subject characteristics are presented as mean ± SD or median (25th–75th percentile). Two group comparisons were made using t tests or the Wilcoxon rank sum. Mixed-effects model repeated-measures analysis was conducted to simultaneously evaluate responses to infusion rates (0, 10, 20 mU/m2/min) and feeding method. Interactions between feeding method and infusion rates were assessed with repeated-measures analysis. As warranted, log or square-root transformations were made prior to analysis to meet assumptions of normality or variance homogeneity. Where assumptions were uncertain, nonparametric tests were made to assess robustness of parametric results. In the lactating group, Spearman correlation analyses were used to evaluate the associations between prolactin and other variables. A two-sided P value ≤0.05 was considered statistically significant. P values were not adjusted for multiple testing. Statistical analysis was performed with SAS version 9.4 (SAS Institute, Cary, NC).
Assays and Equipment
Glucose concentrations were analyzed by YSI (Model 2300-D Stat Plus; Yellow Springs, OH) and insulin by ELISA (ultrasensitive ALPCO kit 80-INSHUU-E01.1). The detection limit of the assay was 0.135 μU/mL. Each subject had 30 insulin duplicates during the clamp study. The intra-assay coefficient of variation with 30 samples ranged 3.0–6.0% (mean 4.2%). Leptin (kit HADK2MAG-61K; Millipore) and total and HMW adiponectin (kit 80-ADPHU-E01; ALPCO) were analyzed by ELISA and FFA by a colorimetric method (#991–34891; Wako). Prolactin and estradiol concentrations were analyzed by Quest clinical laboratories.
Data and Resource Availability
The data sets generated during the current study are available from the corresponding author upon reasonable request. No applicable resources were generated or analyzed during the current study.
Results
Anthropometrics and Demographics
Data collection took place during three visits at weeks 5.3 ± 0.7, 6.4 ± 0.8, and 7.1 ± 0.7 postpartum for visits 1, 2, and 3, respectively. As shown in Table 1, women who were lactating or formula-feeding were well matched for anthropometric characteristics including parity, prepregnancy weight, maternal term weight, maternal weight at 6 weeks postpartum, retention of weight at 6 weeks postpartum, BMI, waist-to-hip ratio, body composition, infant birth weight, and gestational age at delivery. Demographic data revealed that 75% of women who were lactating had a history of GDM, whereas 50% of women who were formula-feeding had a history of GDM during their most recent pregnancy (P = 0.36). Additionally, 67% of the lactating women and 67% of formula-feeding women had a family history of type 2 diabetes.
Table 1.
Characteristics of postpartum subjects
| Demographics and anthropometrics | Lactating (n = 12) | Formula (n = 6) | P value |
|---|---|---|---|
| Age (years) | 34 ± 3 | 33 ± 3 | 0.54 |
| Parity | 3.1 ± 0.8 | 2.5 ± 1.4 | 0.37 |
| Maternal weight (kg) | |||
| Before pregnancy | 74 ± 15 | 71 ± 10 | 0.68 |
| Term | 84 ± 15 | 82 ± 10 | 0.76 |
| At 6 weeks postpartum | 75 ± 16 | 72 ± 8 | 0.52 |
| Retention at 6 weeks postpartum | 1.5 ± 4.0 | 0.4 ± 2.2 | 0.45 |
| BMI at 6 weeks postpartum (kg/m2) | 30.0 ± 5.0 | 30.7 ± 2.0 | 0.70 |
| WHR at 6 weeks postpartum | 0.95 ± 0.04 | 0.97 ± 0.06 | 0.38 |
| Intrahepatic lipid (%) | 8.7 ± 4.6 | 10.4 ± 6.5 | 0.59 |
| DEXA at 6 weeks postpartum (kg) | |||
| Body fat | 31.3 ± 10.4 | 29.9 ± 4.9 | 0.70 |
| FFM | 41.9 ± 5.7 | 39.9 ± 3.9 | 0.39 |
| Infant birth weight (kg) | 3.54 ± 0.40 | 3.44 ± 0.55 | 0.73 |
| Exclusive lactation (%) | 83 | 0 | <0.0001 |
| Amount of formula fed (oz) | 1.4 ± 2.7 | 24.0 ± 0.0 | <0.0001 |
| Gestational age at delivery (days) | 276 ± 6 | 279 ± 9 | 0.60 |
| History of GDM | |||
| All pregnancies except for last (%) | 0 | 0 | |
| Most recent pregnancy (%) | 75 | 50 | 0.36 |
| Family history of type 2 diabetes (%) | 67 | 67 | 1.00 |
Data are mean ± SD unless otherwise indicated. DEXA, dual-energy X-ray absorptiometry; WHR, waist-to-hip ratio.
Biochemical Measurements
As shown in Table 2, except for their HbA1c levels (P = 0.05), both groups were matched for fasting concentrations of plasma glucose, insulin, FFA, lipid profiles, and for all measures of insulin sensitivity and secretion derived from fasting measurements and the 2-h OGTT. Plots of the OGTT with both glucose and insulin values are available in Supplementary Fig. 1. The respiratory quotient did not differ between the groups. As expected, prolactin levels were significantly higher (P < 0.001) and estradiol levels significantly lower (P = 0.01) in lactating women compared with formula-feeding women. No differences were observed between groups for concentrations of adiponectin and leptin. However, the adiponectin/leptin ratio was significantly higher (P = 0.04) in the lactating women compared with formula-feeding women.
Table 2.
Biochemistries, metabolic indicators, insulin sensitivity, and plasma hormones at 6 weeks postpartum
| Lactating (n = 12) | Formula (n = 6) | P value | |
|---|---|---|---|
| Biochemistries and metabolic indicators | |||
| HbA1c in percent (mmol/mol) | 5.8 ± 0.3 (40) | 5.5 ± 0.3 (37) | 0.05 |
| Fasting glucose (mg/dL) | 83 ± 9 | 87 ± 4 | 0.17 |
| Fasting insulin (μU/mL) | 2.6 ± 2.3 | 4.0 ± 2.7 | 0.30 |
| Fasting FFA (mmol/L) | 0.61 ± 0.12 | 0.67 ± 0.08 | 0.28 |
| Total cholesterol (mg/dL) | 197 ± 24 | 200 ± 50 | 0.92 |
| HDL cholesterol (mg/dL) | 51 ± 12 | 45 ± 5 | 0.12 |
| Triglycerides (mg/dL) | 125 ± 63 | 171 ± 95 | 0.31 |
| LDL cholesterol (mg/dL) | 121 ± 21 | 120 ± 34 | 0.96 |
| Matsuda Index | 10.5 ± 8.8 | 7.6 ± 5.3 | 0.40 |
| HOMA-IR | 1.1 ± 1.2 | 1.5 ± 1.2 | 0.54 |
| Adipo-IR | 3.2 ± 3.9 | 4.6 ± 3.0 | 0.42 |
| Insulin secretion sensitivity index 2 | 2.0 ± 0.6 | 2.5 ± 1.2 | 0.36 |
| First-phase Stumvoll (μU/mL) | 102 ± 72 | 168 ± 100 | 0.19 |
| Second-phase Stumvoll (μU/mL) | 29 ± 17 | 45 ± 25 | 0.21 |
| Fasting RQ | 0.82 ± 0.10 | 0.86 ± 0.03 | 0.17 |
| Clamp step 1 RQ | 0.84 ± 0.08 | 0.90 ± 0.06 | 0.08 |
| Clamp step 2 RQ | 0.88 ± 0.10 | 0.93 ± 0.09 | 0.33 |
| OGTT | |||
| Plasma glucose AUC (g/dL) · min | 18.3 ± 2.1 | 16.0 ± 5.4 | 0.20 |
| Plasma insulin AUC (mU/mL) · min | 4.9 ± 3.2 | 7.1 ± 5.9 | 0.49 |
| Plasma FFA AUC (mmol/L) · min | 36.4 ± 7.0 | 40.6 ± 11.7 | 0.35 |
| NGT:IFG:IGT:Combined IFG and IGT | 4:1:7:0 | 4:0:1:1 | 0.24 |
| Hormone concentrations | |||
| Prolactin (ng/mL) | 276 ± 142 | 11 ± 5 | <0.001 |
| Total adiponectin (μg/mL) | 4.05 ± 1.06 | 4.13 ± 1.14 | 0.89 |
| HMW adiponectin (μg/mL) | 1.98 ± 0.80 | 1.99 ± 0.63 | 0.99 |
| HMW/total adiponectin | 0.47 ± 0.09 | 0.48 ± 0.05 | 0.92 |
| Leptin (ng/mL) | 5.15 ± 4.06 | 6.76 ± 2.08 | 0.28 |
| Total adiponectin/leptin (μg/mL per ng/mL) | 1.12 ± 0.65 | 0.64 ± 0.22 | 0.04 |
| Estradiol (pg/mL) | 26.5 ± 14.6 | 74.2 ± 27.8 | 0.01 |
Data are mean ± SD unless otherwise indicated. Adipo-IR is calculated from fasting plasma FFA and insulin. IFG, impaired fasting glucose; IGT, impaired glucose tolerance; NGT, normal glucose tolerance; RQ, respiratory quotient.
Metabolic Adaptations in Lactating Women
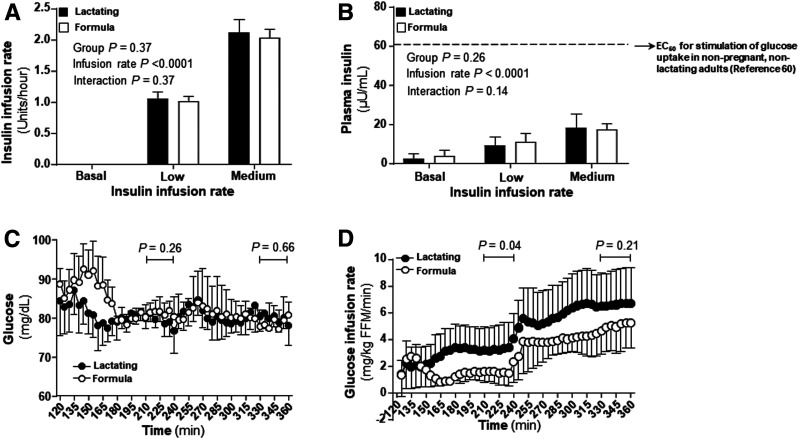
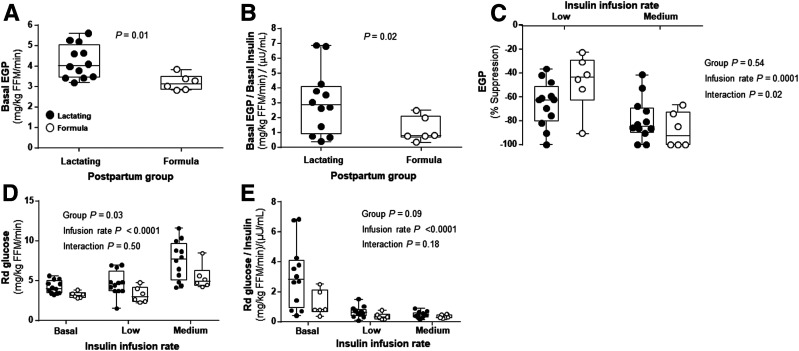
As shown in Fig. 2, during the hyperinsulinemic-euglycemic clamp, the insulin infusion rates (Fig. 2A), plasma insulin concentrations (Fig. 2B), and glucose levels (Fig. 2C) achieved steady state in both groups during the last 30 min of each clamp step. However, compared with formula-feeding women, lactating women required higher glucose infusion rates during step 1 (P = 0.04) (Fig. 2D). Interestingly, lactating women also exhibited a 1.3-fold-higher rate of basal EGP when measured in absolute units (4.22 ± 0.82 mg/kg fat-free mass [FFM]/min in lactating and 3.20 ± 0.39 mg/kg FFM/min in formula-feeding women) (P = 0.01) (Fig. 3A). When basal EGP was normalized for insulin, lactating women showed a 2.6-fold-higher rate (3.06 ± 2.17 and 1.20 ± 0.84 (mg/kg FFM/min)/(μU/mL) in lactating and formula-feeding women, respectively) (P = 0.02) (Fig. 3B). However, the suppression rates of EGP were not different between the groups during the clamp (P = 0.54) (Fig. 3C). Additionally, lactating women also increased their absolute Rd glucose compared with formula-feeding women (P = 0.03) (Fig. 3D). Both groups increased Rd glucose as insulin infusion rates increased during the clamp (P < 0.0001) (Fig. 3D). The group differences in Rd glucose were less apparent when normalized for the amount of insulin given during the clamp (P = 0.09) (Fig. 3E). Lactating women demonstrated higher glucose uptake at the lower basal insulin concentrations. Rd glucose analyzed across increasing levels of insulin, demonstrated that lactating women proportionally took up more glucose within the lower ranges of insulin concentrations.
Figure 2.
Insulin infusion rate, insulin and glucose concentrations, and glucose infusion rate. A: Insulin infusion rates, insulin and glucose concentrations, and glucose infusion rates. B: Insulin concentrations during the clamp compared with literature values (denoted by the top dashed line) for the mean insulin concentration that will half maximally stimulate glucose uptake in nonpregnant, nonlactating adults (60). C: Glucose concentrations during the clamp. D: Glucose infusion rates during the clamp. Data are mean ± SD. Filled bars and filled circles represent lactating women. Open bars and open circles represent formula-feeding women.
Figure 3.
EGP, EGP suppression, and whole-body glucose disposal. A: Basal EGP without adjustment for basal insulin concentration. B: Basal EGP with adjustment for basal insulin concentration. C: EGP suppression. D: Rd glucose without normalization for insulin concentrations at basal state and at low and medium insulin infusion rates. E: Rd glucose with normalization for insulin concentrations at basal state and at low and medium insulin infusion rates. Data are mean ± SD for lactating (n = 12, filled circles) and formula-feeding (n = 6, open circles) women.
The process of NIMGU does not include the effect of basal insulin on glucose uptake and represents 70–90% of the basal Rd in normoglycemic nonpregnant, nonpostpartum humans (34–37). In the current study, the mean NIMGU in lactating women was 1.95 ± 0.68 mg/kg total body weight/min or 81% of the mean basal EGP (data not shown). In the formula-feeding group, mean NIMGU was 1.41 ± 0.35 mg/kg total body weight/min or 79% of their mean basal EGP. Although the difference in absolute NIMGU between the groups was significant at P = 0.04, the proportion of basal EGP that was NIMGU was not different between the groups (P = 0.72).
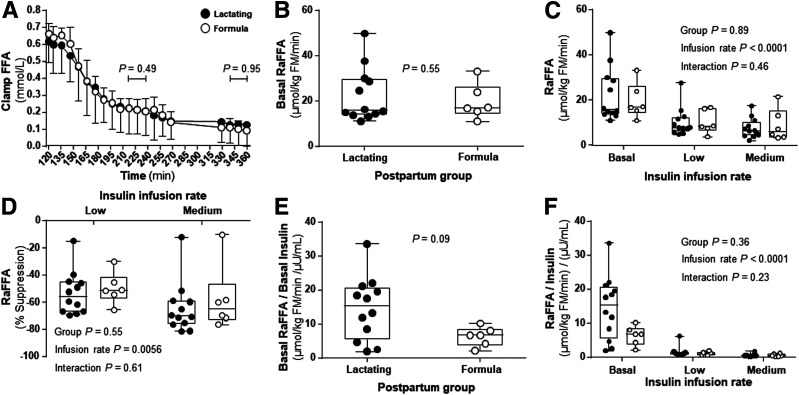
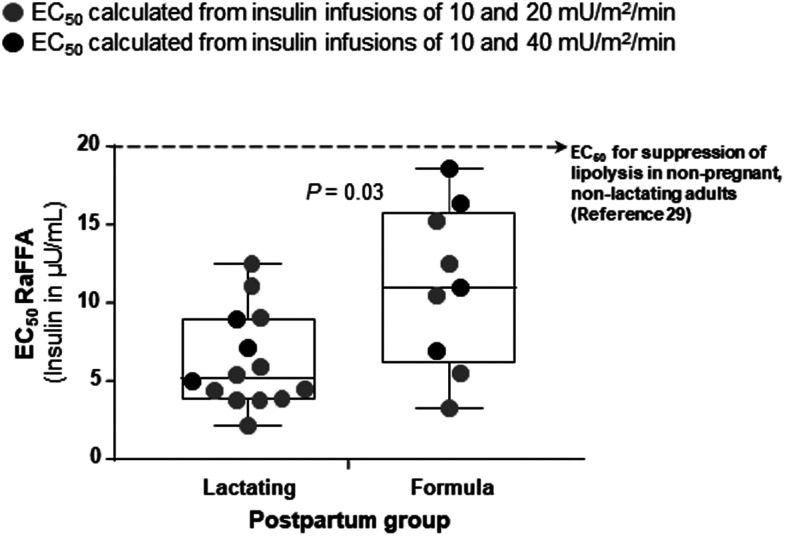
With regard to FFA metabolism, FFA concentrations, basal RaFFA, RaFFA during the clamp, and suppression of RaFFA from basal rates were similar between the two groups (Fig. 4A–D). Adjusting RaFFA for insulin concentrations showed a trend for a difference between the groups in the basal state (Fig. 4E) but not during the clamp (Fig. 4F). The shapes of the RaFFA curves from fasting to step 1 and step 2 were used to model the insulin concentration needed to reduce RaFFA by 50% (half-maximal effective concentration [EC50] RaFFA). The EC50 for the subjects studied at low and medium insulin infusion rates (depicted in Fig. 5 as gray symbols) appeared to support a higher insulin need to suppress adipose RaFFA in the formula-feeding subjects. To expand the sample size and more fully assess the adipose effect over a wider range of insulin concentrations, we added to the data set, results from 10 additional subjects who underwent higher insulin infusion rates during step 2 (40 mU/m2/min). The EC50 data from three lactating and four formula-feeding women are shown as black symbols in Fig. 5. Only 7 of the 10 subjects were added because 2 of them received no isotopes and 1 did not reach 50% suppression of RaFFA with the high-dose infusion. Combining data from both clamp groups, the calculated EC50 was significantly lower for lactating women (6.07 ± 3.34 µU/mL) compared with formula feeding women (9.42 ± 4.94 µU/mL, P = 0.03).
Figure 4.
Hyperinsulinemic-euglycemic clamp FFA concentrations and rates of lipolysis. A: FFA during the clamp. B: RaFFA in the basal state. C: RaFFA during clamp conditions. D: RaFFA suppression. E: RaFFA adjusted for insulin in the basal state. F: RaFFA adjusted for insulin during clamp conditions. Data are mean ± SD for lactating (n = 12, filled circles) and formula-feeding (n = 6, open circles) women.
Figure 5.
Insulin concentration EC50 for half maximal suppression of RaFFA. Data represent the EC50 RaFFA. Each gray-filled symbol represents datum from a lactating or formula-feeding woman who underwent the two-step hyperinsulinemic-euglycemic clamp at low and medium insulin levels (10 and 20 mU/m2/min, respectively). Only 16 of the 18 subjects are reported in the main analysis because 2 subjects did not reach 50% suppression of RaFFA with the medium-dose infusion. To increase the sample size and to determine whether the choice of clamp protocol insulin levels influenced the calculated EC50 RaFFA, an additional group of lactating and formula-feeding women (black-filled symbols) underwent an insulin infusion at 10 and 40 mU/m2/min insulin. Only 7 of the 10 subjects are reported from this additional group because 2 did not receive isotopes and 1 did not reach 50% suppression of RaFFA with the high-dose infusion. The box and whiskers graph represent minimum, maximum, median, and 25th and 75th percentiles. Lastly, the horizontal dotted line represents data from a published study (29) in nonpregnant, nonlactating women for comparison. In that study, the EC50 was calculated from a two-step clamp using insulin infusions of 0.25 and 2.5 mU/kg FFM/min (equivalent to 6 and 60 mU/m2/min when put in the units used in present study).
Impact of Elevated Prolactin During Lactation
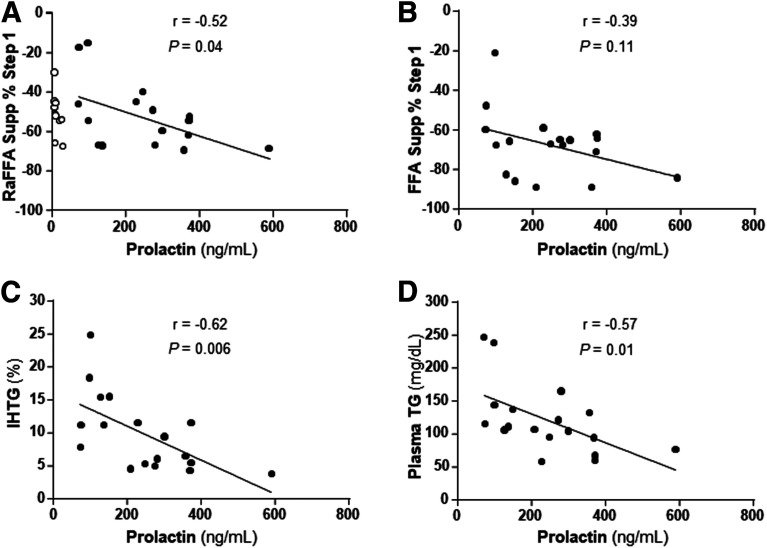
Higher prolactin levels are a distinguishing trait of lactating women and here, the range of prolactin concentrations in the lactating women was significantly associated with a greater suppression of RaFFA during the clamp step 1 (r = −0.52, P = 0.04) (Fig. 6A). A trend was observed for the suppression of FFA concentration during the clamp (r = −0.39, P = 0.11) (Fig. 6B). Higher prolactin concentrations in lactating women were also associated with lower intrahepatic triglyceride (TG) (r = −0.62, P = 0.006) (Fig. 6C) and lower plasma TG concentrations (r = −0.57, P = 0.01) (Fig. 6D).
Figure 6.
Relationships between prolactin and metabolic parameters. A: Data (filled circles) represent the relationship between prolactin concentration and suppression of RaFFA in lactating women during step 1 of the clamp (10 mU/m2/min insulin). Data from the nonlactating (formula-feeding, open circles) women were not included in these correlations (prolactin concentration = 11 ± 5 ng/mL). B–D: Relationships between the lactating women’s prolactin concentrations and the percent suppression of FFA concentrations during step 1 of the clamp (B), intrahepatic TG levels (C), and fasting plasma TG concentrations (D).
Discussion
Milk production is a dominant metabolic process during lactation, which means that nonmammary tissues have to adapt to meet the metabolic demands of the mammary gland during fasting and feeding (7). For example, glucose transport and lipoprotein lipase activity increase in mammary epithelium and decrease concurrently in adipose tissue (38,39). Although past human studies have focused on the potential influence of lactation to increase peripheral insulin sensitivity (primarily thought to occur in muscle), few studies have focused on the metabolic effects of breastfeeding on liver and adipose. Further, past studies have investigated fasting and insulin-stimulated glucose metabolism in lactating women and compared those data to results from nonlactating, nonpostpartum (and often leaner) women as the control group (3,5,20). To control for overall postpartum effects, the current study investigated women at 6 weeks postpartum, either lactating or formula-feeding their infants. Further, the subjects were closely matched for body composition, pregnancy and delivery history, and blood biochemistries. Low and medium levels of insulin were infused in a two-stage clamp to investigate liver and adipose metabolism and the findings considered in light of the large amount of literature existing on mammary glucose uptake in mammals. The results highlight the importance of peripheral organ metabolic coordination, particularly in the fasting state when it may be an advantage for peripheral tissues to exhibit insulin resistance, rather than increased insulin sensitivity.
Glucose and Fatty Acid Turnover
Focusing first on the fasting state, lactating and formula-feeding women exhibited low insulin concentrations that are characteristic of the postpartum period, relative to nonpregnant and nonpostpartum women, regardless of infant-feeding method (40). Even at these low insulin levels, compared with women who were formula-feeding, lactating women had 2.6-fold-greater basal EGP (P = 0.02). Then, under hyperinsulinemic-euglycemic low and medium insulin infusion rates, both groups of postpartum women achieved similar suppression rates of EGP. With regard to adipose metabolism, a novel contribution of this study was the 2.3-fold-greater rates of lipolysis (P = 0.09 for trend) in the fasting state. Tigas et al. (5) used a long (22 h) fasting protocol to measure RaGlycerol and found high levels of lipolysis that did not differ between lactating and nonpostpartum women. Those levels were twofold higher than levels observed in other studies of 12 h fasted healthy and obese subjects (41,42) and long fasting protocols may limit the ability to detect differences in metabolism due to lactation. We calculated the concentration of insulin needed to half-maximally suppress lipolysis (the EC50 RaFFA) and found it to be 36% lower in lactating women compared with the formula-feeding women (P = 0.03). Both groups’ EC50 insulin concentrations appeared lower than the values of 18.7 ± 3.8 µU/mL calculated in similarly obese, but nonpostpartum, nonlactating women (29). Thus, an improved adipose insulin sensitivity in the postpartum period may be further augmented by lactation. Lastly, a related but somewhat surprising observation was that within the lactating group, women with the highest prolactin concentrations exhibited better insulin-mediated suppression of lipolysis which resulted in the lowest levels of intrahepatic lipid and plasma TG, the latter being indicative of low liver fat since liver-derived VLDL carry the majority of plasma TG in the fasting state (26). These data provide a metabolic mechanism for the observation of Ajmera et al. (43), who found through a retrospective analysis that a longer duration of lactation was associated with a lower incidence of hepatic steatosis in the Coronary Artery Risk Development Study in Young Adults (CARDIA) study.
Glucose Uptake/Transport
The increases in fasting EGP in lactating women presented here were of similar magnitude to that in the studies of Haymond and colleagues (5,20). The net effect of glucose uptake regulation during lactation is to redistribute glucose to tissues that have an absolute requirement like brain and the mammary gland. Mammary gland glucose transport is mediated by the insulin-independent GLUT, GLUT1 (38), and low circulating insulin concentrations, such as during basal conditions and during the insulin infusion rates in this study, would favor systemic GLUT1-mediated glucose uptake (44). Lactation in animal models and transgenic overexpression of GLUT1 in skeletal muscle result in increased NIMGU and decreased insulin-mediated glucose uptake (45,46). These observations and the fact that the clamp conditions resulted in insulin concentrations below that needed to promote preferential glucose uptake by insulin-sensitive pathways, make it likely that glucose uptake was predominantly mediated by mechanisms independent of insulin.
NIMGU During the Postpartum Period
NIMGU represents 70–90% of the basal EGP in normoglycemic nonpregnant, nonpostpartum humans (34–37) and was calculated here using a model similar to that published by Jumpertz et al. (28). Mean NIMGU in the lactating subjects was 1.95 mg/kg total body weight/min or 81% of mean basal EGP. If NIMGU in brain and other tissues equals 1.40 mg/kg total body weight/min (35), then lactating mammary tissue contributed 0.55 mg/kg total body weight/min to NIMGU. In the lactating subjects with mean body weights of 75.3 kg, this equated to 60 g of glucose/day which is in good agreement with the published mammary glucose uptake of 50–75 g/day (5,47). By contrast, in the formula-feeding group, the mean NIMGU was 1.41 mg/kg total body weight/min (79% of basal EGP) which was nearly identical to the 1.40 mg/kg total body weight/min estimated to go to brain and other tissues (35). Thus, in the nonlactating subjects, the contribution of the involuting mammary tissue to NIMGU was negligible (0.01 mg/kg total body weight/min. or about 1 g of glucose/day). We assumed that total NIMGU, including the contribution of the lactating mammary gland to NIMGU, remains stable during basal and clamp conditions. We also considered that Rd glucose obtained during clamp studies included a NIMGU component. Because of that, we estimated insulin-mediated glucose uptake by subtracting the total estimated NIMGU (from mammary and nonmammary tissues) from Rd total during both steps of the clamp. Using this approach insulin-mediated glucose uptake was similar in both groups at low and medium insulin infusion rates (data not shown). Thus, it is likely that under the current study conditions, a large proportion of total glucose uptake was not mediated by insulin and that the mammary contribution to Rd via NIMGU suggests refinement of the term insulin sensitivity may be needed to take organ-to-organ coordination into consideration.
Postpartum Hormones
The intensity and duration of exposure to the lactation stimulus may influence tissue-specific responses to glucose as a result of changes in hormones, particularly prolactin, insulin, adiponectin, leptin, and estradiol. Prolactin is critical for the initiation of lactation, and its concentrations will decrease with time postpartum in all lactating women regardless of lactation intensity (48). Higher prolactin concentrations have been associated with lower adiponectin and insulin concentrations as well as lower insulin secretion rates during the postpartum period (5,13,49,50). The low adiponectin levels observed here further support the concept of peripheral insulin resistance, as has been shown in the general population (51). We speculate that reliance on NIMGU through GLUT1 during this period may promote a decrease in serum adiponectin. It has been shown that a proportion of adipocyte adiponectin is secreted at low insulin levels and increases in adipocyte glucose uptake via stimulation of the hexosamine pathway decrease both serum adiponectin and skeletal muscle glucose uptake (52). Thus, increased uptake of glucose by GLUT1 in adipose tissue could induce a secretory signal, such as low serum adiponectin intended to restrict glucose uptake in skeletal muscle and adaptively affect systemic glucose utilization to support lactogenesis. Lastly, lower estradiol levels are also a characteristic of lactating compared with formula-feeding women at 6–8 weeks postpartum (53). It has been proposed that lower physiologic levels of estradiol may contribute to lower fasting glucose and lower fasting insulin (53).
Glucose Tolerance, Insulin Secretion, and Insulin Sensitivity During Lactation in Women With and Without GDM
We have compared our results with those from five studies in which the postpartum participants were grouped by lactation status between 1 and 5 months postpartum. One study included women with NGT during pregnancy (53), another study included women with a spectrum of glucose tolerance during pregnancy (49), and three studies only included women with recent GDM (54–56). These studies reported lower fasting glucose and lower fasting insulin in the lactating groups regardless of prenatal glucose tolerance (53,55). Lactating women with and without a history of GDM appeared to have similar insulin secretion by Stumvoll indices after adjusting for BMI (49). The studies that only included postpartum women with recent GDM reported lower 2-h glucose, lower total AUC glucose or lower 2-h insulin in lactating compared with formula-feeding women (54,55). In addition, breastfeeding for 15 min during the 2-h OGTT decreased 2-h glucose levels by 5% on average at 6–9 weeks postpartum (56).
Current guidelines recommend reevaluation of glucose tolerance at 4–12 weeks postpartum among women with GDM (57). HbA1c testing is not routinely performed before 12 weeks postpartum to avoid interference with the interpretation of glycemic status by changes in RBC turnover, blood loss or prenatal glucose profile. The lactating women reported in the current study had a higher HbA1c than the formula-feeding group. Although one possible explanation for this is the higher number of mothers with GDM in the lactating group, we observed similar HbA1c levels in lactating women with and without a history of GDM (Supplementary Table 2). A group of investigators in Norway investigated the prevalence of HbA1c ≥5.7% (≥39 mmol/mol) at 14 weeks postpartum in a multiethnic group of 570 women with and without history of GDM. At 14 weeks postpartum they found a prevalence of HbA1c ≥5.7% for the total population of women without GDM of 17.6%. This study did not comment on lactation status (58). As for insulin sensitivity, a decrease of 50% between prepregnancy and late third trimester is a physiologic change independent of glucose tolerance during pregnancy (1,2,59). The clamps reported in the literature during lactation are from women with NGT (6).
Limitations
The primary limitation of this study relates to the nature of investigations utilizing hyperinsulinemic-euglycemic clamps and isotopic labeling which require intensive protocols that typically limit the sample size. These methods have an additional subject burden in new mothers who are challenged with the added activities of taking care of infants and alterations in their daily schedules and sleeping patterns. Even with these challenges, studies in this population provide critical information that is important to discovery of how the hormonal environment postpartum, and lactation status in particular, impact maternal health. Another limitation is our higher number of mothers with GDM and postpartum impaired glucose tolerance (IGT) in the lactating group, which may not be representative of the lactating population with prenatal and postnatal NGT. However, this is a group of strong clinical interest. Lastly, somatostatin was not infused during the clamp to calculate NIMGU, and except for insulin, all hormones were only measured in the basal state.
Conclusions
This study investigated the effect of the low insulin environment in postpartum women on hepatic glucose production, adipose FFA release, and intrahepatic lipid. High prolactin may stimulate nonmammary tissues to adapt to meet the metabolic demands of the mammary gland during fasting and feeding, which is optimized by the mammary gland’s higher NIMGU and fatty acid uptake. The present data suggest that lactation drives EGP and lipolysis in the fasting state to support lactogenesis. By contrast, in the fed state (mimicked here by the clamp) elevated prolactin balances these effects by suppressing lipolysis when dietary substrates are available for milk production. These forces would be predicted to protect against liver lipid accumulation and hyperlipidemia, even in obese subjects and highlight the need for future studies to investigate the impact of prolactin on fatty acid flux outside the mammary gland. Lastly, these data suggest that adaptations in glucose transport may confound the interpretation of whole-body and tissue-specific sensitivity to insulin in postpartum for women in general and particularly among lactating women. Future research, using methods that provide tissue-specific information on glucose and fatty acid uptake and handling will be required to more deeply probe the physiological impact of lactation on the cross-organ coordination of metabolic flux. Clamps limited to an acceptable number of hours for postpartum women would target suppression of insulin secretion and maximal insulin responses in glucose uptake. Optimally, investigations in this area will consider the contributions of time postpartum, intensity and duration of lactation to the outcome variables. These approaches would clarify the contribution of skeletal muscle to whole-body insulin sensitivity and responsiveness during lactation.
Article Information
Acknowledgments. The authors thank the postpartum participants, the research coordinating efforts of Linda Flores (Department of Internal Medicine, Division of Endocrinology, University of Texas Southwestern Medical Center), and the biostatistical assistance of Xilong Li (Department of Population and Data Sciences, University of Texas Southwestern Medical Center), senior database analyst. The authors used the services of the Clinical Research Unit and the Advanced Imaging Research Center at University of Texas Southwestern.
Funding. This work was supported by the American Diabetes Association (1-14-TS-33) and the National Institutes of Health (R03-DK-097463).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. M.A.R.-R. contributed to the execution of the study, analysis and interpretation of the results, and manuscript writing. M.M.S.-A. contributed to analysis and interpretation of the results and manuscript writing. B.A.-H. contributed to the statistical analysis and interpretation of the results. B.M.C. contributed to the execution of the study and the interpretation of the results. E.J.P. contributed to the analysis and interpretation of the results and manuscript writing. M.A.R.-R. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.12249938.
References
- 1.Catalano PM, Tyzbir ED, Wolfe RR, et al. Carbohydrate metabolism during pregnancy in control subjects and women with gestational diabetes. Am J Physiol 1993;264:E60–E67 [DOI] [PubMed] [Google Scholar]
- 2.Catalano PM, Huston L, Amini SB, Kalhan SC. Longitudinal changes in glucose metabolism during pregnancy in obese women with normal glucose tolerance and gestational diabetes mellitus. Am J Obstet Gynecol 1999;180:903–916 [DOI] [PubMed] [Google Scholar]
- 3.Neville MC, Casey C, Hay WW Jr. Endocrine regulation of nutrient flux in the lactating woman. Do the mechanisms differ from pregnancy? In Nutrient Regulation During Pregnancy, Lactation, and Infant Growth. Allen L, King J, Lonnerdal B, Eds. New York, Plenum Press, 1994, p. 85–98 [DOI] [PubMed] [Google Scholar]
- 4.McManus RM, Cunningham I, Watson A, Harker L, Finegood DT. Beta-cell function and visceral fat in lactating women with a history of gestational diabetes. Metabolism 2001;50:715–719 [DOI] [PubMed] [Google Scholar]
- 5.Tigas S, Sunehag A, Haymond MW. Metabolic adaptation to feeding and fasting during lactation in humans. J Clin Endocrinol Metab 2002;87:302–307 [DOI] [PubMed] [Google Scholar]
- 6.Neville MC, Hay WW Jr., Fennessey P. Physiological significance of the concentration of human milk glucose. Protoplasma 1990;159:118–128 [Google Scholar]
- 7.Baumgard LH, Collier RJ, Bauman DE. A 100-year review: regulation of nutrient partitioning to support lactation. J Dairy Sci 2017;100:10353–10366 [DOI] [PubMed] [Google Scholar]
- 8.Burnol AF, Leturque A, Ferré P, Girard J. Glucose metabolism during lactation in the rat: quantitative and regulatory aspects. Am J Physiol 1983;245:E351–E358 [DOI] [PubMed] [Google Scholar]
- 9.Gunderson EP, Lewis CE, Lin Y, et al. Lactation duration and progression to diabetes in women across the childbearing years: the 30-year CARDIA study. JAMA Intern Med 2018;178:328–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bajaj H, Ye C, Hanley AJ, et al. Prior lactation reduces future diabetic risk through sustained postweaning effects on insulin sensitivity. Am J Physiol Endocrinol Metab 2017;312:E215–E223 [DOI] [PubMed] [Google Scholar]
- 11.Vernon RG. Endocrine control of metabolic adaptation during lactation. Proc Nutr Soc 1989;48:23–32 [DOI] [PubMed] [Google Scholar]
- 12.Eriksson B, Löf M, Olausson H, Forsum E. Body fat, insulin resistance, energy expenditure and serum concentrations of leptin, adiponectin and resistin before, during and after pregnancy in healthy Swedish women. Br J Nutr 2010;103:50–57 [DOI] [PubMed] [Google Scholar]
- 13.Asai-Sato M, Okamoto M, Endo M, et al. Hypoadiponectinemia in lean lactating women: prolactin inhibits adiponectin secretion from human adipocytes. Endocr J 2006;53:555–562 [DOI] [PubMed] [Google Scholar]
- 14.Fuglsang J, Skjaerbaek C, Frystyk J, Flyvbjerg A, Ovesen P. A longitudinal study of serum adiponectin during normal pregnancy. BJOG 2006;113:110–113 [DOI] [PubMed] [Google Scholar]
- 15.Saucedo R, Zarate A, Basurto L, et al. Relationship between circulating adipokines and insulin resistance during pregnancy and postpartum in women with gestational diabetes. Arch Med Res 2011;42:318–323 [DOI] [PubMed] [Google Scholar]
- 16.McLachlan KA, O’Neal D, Jenkins A, Alford FP. Do adiponectin, TNFalpha, leptin and CRP relate to insulin resistance in pregnancy? Studies in women with and without gestational diabetes, during and after pregnancy. Diabetes Metab Res Rev 2006;22:131–138 [DOI] [PubMed] [Google Scholar]
- 17.Ozarda Y, Gunes Y, Tuncer GO. The concentration of adiponectin in breast milk is related to maternal hormonal and inflammatory status during 6 months of lactation. Clin Chem Lab Med 2012;50:911–917 [DOI] [PubMed] [Google Scholar]
- 18.Matuszek B, Burska A, Leszczyńska-Gorzelak B, Donica H, Nowakowski A. Comparative analysis of adiponectin isoform distribution in pregnant women with gestational diabetes mellitus and after delivery. Acta Obstet Gynecol Scand 2013;92:951–959 [DOI] [PubMed] [Google Scholar]
- 19.Combs TP, Berg AH, Rajala MW, et al. Sexual differentiation, pregnancy, calorie restriction, and aging affect the adipocyte-specific secretory protein adiponectin. Diabetes 2003;52:268–276 [DOI] [PubMed] [Google Scholar]
- 20.Mohammad MA, Sunehag AL, Chacko SK, Pontius AS, Maningat PD, Haymond MW. Mechanisms to conserve glucose in lactating women during a 42-h fast. Am J Physiol Endocrinol Metab 2009;297:E879–E888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nommsen-Rivers LA, Chantry CJ, Peerson JM, Cohen RJ, Dewey KG. Delayed onset of lactogenesis among first-time mothers is related to maternal obesity and factors associated with ineffective breastfeeding. Am J Clin Nutr 2010;92:574–584 [DOI] [PubMed] [Google Scholar]
- 22.Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. National Diabetes Data Group. Diabetes 1979;28:1039–1057 [DOI] [PubMed] [Google Scholar]
- 23.Howie PW, McNeilly AS, McArdle T, Smart L, Houston M. The relationship between suckling-induced prolactin response and lactogenesis. J Clin Endocrinol Metab 1980;50:670–673 [DOI] [PubMed] [Google Scholar]
- 24.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 1979;237:E214–E223 [DOI] [PubMed] [Google Scholar]
- 25.Butte NF, Hopkinson JM, Mehta N, Moon JK, Smith EO. Adjustments in energy expenditure and substrate utilization during late pregnancy and lactation. Am J Clin Nutr 1999;69:299–307 [DOI] [PubMed] [Google Scholar]
- 26.Barrows BR, Parks EJ. Contributions of different fatty acid sources to very low-density lipoprotein-triacylglycerol in the fasted and fed states. J Clin Endocrinol Metab 2006;91:1446–1452 [DOI] [PubMed] [Google Scholar]
- 27.Steele R, Wall JS, De Bodo RC, Altszuler N. Measurement of size and turnover rate of body glucose pool by the isotope dilution method. Am J Physiol 1956;187:15–24 [DOI] [PubMed] [Google Scholar]
- 28.Jumpertz R, Thearle MS, Bunt JC, Krakoff J. Assessment of non-insulin-mediated glucose uptake: association with body fat and glycemic status. Metabolism 2010;59:1396–1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jensen MD, Nielsen S. Insulin dose response analysis of free fatty acid kinetics. Metabolism 2007;56:68–76 [DOI] [PubMed] [Google Scholar]
- 30.Donner CC, Fraze E, Chen YD, Hollenbeck CB, Foley JE, Reaven GM. Presentation of a new method for specific measurement of in vivo insulin-stimulated glucose disposal in humans: comparison of this approach with the insulin clamp and minimal model techniques. J Clin Endocrinol Metab 1985;60:723–726 [DOI] [PubMed] [Google Scholar]
- 31.Lomonaco R, Ortiz-Lopez C, Orsak B, et al. Effect of adipose tissue insulin resistance on metabolic parameters and liver histology in obese patients with nonalcoholic fatty liver disease. Hepatology 2012;55:1389–1397 [DOI] [PubMed] [Google Scholar]
- 32.Retnakaran R, Ye C, Kramer CK, et al. Maternal serum prolactin and prediction of postpartum β-cell function and risk of prediabetes/diabetes. Diabetes Care 2016;39:1250–1258 [DOI] [PubMed] [Google Scholar]
- 33.Stumvoll M, Van Haeften T, Fritsche A, Gerich J. Oral glucose tolerance test indexes for insulin sensitivity and secretion based on various availabilities of sampling times. Diabetes Care 2001;24:796–797 [DOI] [PubMed] [Google Scholar]
- 34.Kolterman OG, Insel J, Saekow M, Olefsky JM. Mechanisms of insulin resistance in human obesity: evidence for receptor and postreceptor defects. J Clin Invest 1980;65:1272–1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gottesman I, Mandarino L, Gerich J. Estimation and kinetic analysis of insulin-independent glucose uptake in human subjects. Am J Physiol 1983;244:E632–E635 [DOI] [PubMed] [Google Scholar]
- 36.Baron AD, Brechtel G, Wallace P, Edelman SV. Rates and tissue sites of non-insulin- and insulin-mediated glucose uptake in humans. Am J Physiol 1988;255:E769–E774 [DOI] [PubMed] [Google Scholar]
- 37.Ferrannini E, Smith JD, Cobelli C, Toffolo G, Pilo A, DeFronzo RA. Effect of insulin on the distribution and disposition of glucose in man. J Clin Invest 1985;76:357–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Komatsu T, Itoh F, Kushibiki S, Hodate K. Changes in gene expression of glucose transporters in lactating and nonlactating cows. J Anim Sci 2005;83:557–564 [DOI] [PubMed] [Google Scholar]
- 39.Hamosh M, Clary TR, Chernick SS, Scow RO. Lipoprotein lipase activity of adipose and mammary tissue and plasma triglyceride in pregnant and lactating rats. Biochim Biophys Acta 1970;210:473–482 [DOI] [PubMed] [Google Scholar]
- 40.Syed-Abdul MM, Hu Q, Jacome-Sosa M, et al. Effect of carbohydrate restriction-induced weight loss on aortic pulse wave velocity in overweight men and women. Appl Physiol Nutr Metab 2018;43:1247–1256 [DOI] [PubMed] [Google Scholar]
- 41.Jensen MD. Regional glycerol and free fatty acid metabolism before and after meal ingestion. Am J Physiol 1999;276:E863–E869 [DOI] [PubMed] [Google Scholar]
- 42.Mittendorfer B, Horowitz JF, Klein S. Gender differences in lipid and glucose kinetics during short-term fasting. Am J Physiol Endocrinol Metab 2001;281:E1333–E1339 [DOI] [PubMed] [Google Scholar]
- 43.Ajmera VH, Terrault NA, VanWagner LB, et al. Longer lactation duration is associated with decreased prevalence of non-alcoholic fatty liver disease in women. J Hepatol 2019;70:126–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Doberne L, Greenfield MS, Rosenthal M, Widstrom A, Reaven G. Effect of variations in basal plasma glucose concentration on glucose utilization (M) and metabolic clearance (MCR) rates during insulin clamp studies in patients with non-insulin-dependent diabetes mellitus. Diabetes 1982;31:396–400 [DOI] [PubMed] [Google Scholar]
- 45.Buse MG, Robinson KA, Marshall BA, Mueckler M. Differential effects of GLUT1 or GLUT4 overexpression on hexosamine biosynthesis by muscles of transgenic mice. J Biol Chem 1996;271:23197–23202 [DOI] [PubMed] [Google Scholar]
- 46.Herman MA, Kahn BB. Glucose transport and sensing in the maintenance of glucose homeostasis and metabolic harmony. J Clin Invest 2006;116:1767–1775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Prentice AM, Prentice A. Energy costs of lactation. Annu Rev Nutr 1988;8:63–79 [DOI] [PubMed] [Google Scholar]
- 48.Díaz S, Serón-Ferré M, Cárdenas H, Schiappacasse V, Brandeis A, Croxatto HB. Circadian variation of basal plasma prolactin, prolactin response to suckling, and length of amenorrhea in nursing women. J Clin Endocrinol Metab 1989;68:946–955 [DOI] [PubMed] [Google Scholar]
- 49.Harreiter J, Vila G, Leitner K, et al. Decreased beta-cell function in breastfeeding obese and non-obese women: a prospective observational study. Clin Nutr 2019;38:2790–2798 [DOI] [PubMed] [Google Scholar]
- 50.Hennart P, Leclercq V, Delogne-Desnoeck J, Robyn C. Plasma glucose, insulin, glucagon and prolactin during long lasting lactation. Horm Metab Res 1984;16(Suppl. 1):183–185 [DOI] [PubMed] [Google Scholar]
- 51.Vega GL, Grundy SM. Metabolic risk susceptibility in men is partially related to adiponectin/leptin ratio. J Obes 2013;2013:409679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hazel M, Cooksey RC, Jones D, et al. Activation of the hexosamine signaling pathway in adipose tissue results in decreased serum adiponectin and skeletal muscle insulin resistance. Endocrinology 2004;145:2118–2128 [DOI] [PubMed] [Google Scholar]
- 53.Lenz S, Kühl C, Hornnes PJ, Hagen C. Influence of lactation on oral glucose tolerance in the puerperium. Acta Endocrinol (Copenh) 1981;98:428–431 [DOI] [PubMed] [Google Scholar]
- 54.Kjos SL, Henry O, Lee RM, Buchanan TA, Mishell DR Jr. The effect of lactation on glucose and lipid metabolism in women with recent gestational diabetes. Obstet Gynecol 1993;82:451–455 [PubMed] [Google Scholar]
- 55.Gunderson EP, Hedderson MM, Chiang V, et al. Lactation intensity and postpartum maternal glucose tolerance and insulin resistance in women with recent GDM: the SWIFT cohort. Diabetes Care 2012;35:50–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gunderson EP, Crites Y, Chiang V, et al. Influence of breastfeeding during the postpartum oral glucose tolerance test on plasma glucose and insulin. Obstet Gynecol 2012;120:136–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.American Diabetes Association 14. Management of diabetes in pregnancy: Standards of Medical Care in Diabetes—2020. Diabetes Care 2020;43(Suppl. 1):S183–S192 [DOI] [PubMed] [Google Scholar]
- 58.Waage C, Jenum AK, Mdala I, Berg JP, Richardsen K, Birkeland K. Associations between gestational diabetes mellitus and elevated HbA1c early postpartum in a multi-ethnic population. Prim Care Diabetes 2017;11:132–139 [DOI] [PubMed] [Google Scholar]
- 59.Catalano PM, Tyzbir ED, Roman NM, Amini SB, Sims EA. Longitudinal changes in insulin release and insulin resistance in nonobese pregnant women. Am J Obstet Gynecol 1991;165:1667–1672 [DOI] [PubMed] [Google Scholar]
- 60.Rizza RA, Mandarino LJ, Gerich JE. Dose-response characteristics for effects of insulin on production and utilization of glucose in man. Am J Physiol 1981;240:E630–E639 [DOI] [PubMed] [Google Scholar]