Abstract
Galactose-deficient IgA1 (Gd-IgA1) plays a crucial role in the development of IgA nephropathy (IgAN). However, the pathogenic mechanisms driving Gd-IgA1 production have not been fully elucidated. Innate-immune activation via Toll-like receptor 9 (TLR9) is known to be involved in Gd-IgA1 production. A proliferation-inducing ligand (APRIL) and IL-6 are also known to enhance Gd-IgA1 synthesis in IgAN. Here, we investigated how TLR9 activation in IgA-secreting cells results in overproduction of nephritogenic IgA in the IgAN-prone ddY mouse model and in human IgA1-secreting cells. Injection of CpG-ODN (TLR9 ligand) increased production of aberrantly glycosylated IgA and IgG-IgA immune complexes (IC) in ddY mice that, in turn, exacerbated kidney injury. CpG-ODN-stimulated mice had elevated serum levels of APRIL that correlated with those of aberrantly glycosylated IgA and IgG-IgA IC. In vitro, TLR9 activation enhanced production of the nephritogenic IgA as well as APRIL and IL-6 in splenocytes of ddY mice and in human IgA1-secreting cells. However, siRNA knock-down of APRIL completely suppressed overproduction of Gd-IgA1 induced by IL-6. Neutralization of IL-6 reduced CpG-ODN-induced overproduction of Gd-IgA1. Furthermore, APRIL and IL-6 pathways each independently mediated TLR9-induced overproduction of Gd-IgA1. In summary, TLR9 activation enhanced synthesis of aberrantly glycosylated IgA that, in a mouse model of IgAN, further enhanced kidney injury. These findings indicate that APRIL and IL-6 synergistically, as well as independently, enhance synthesis of Gd-IgA1.
Keywords: IgA nephropathy, APRIL, IL-6, galactose deficient IgA1, immune complex
Introduction
IgA nephropathy (IgAN) is the most common primary glomerulonephritis2, and causes end-stage renal disease (ESRD) in 20–40% of patients within 20 years after onset of this disease.3 Although the pathogenesis of this disease remains to be fully elucidated, episodic macroscopic hematuria concurrent with an infection of upper-respiratory tract suggests that the mucosal immune system plays an important role in the clinical manifestations of IgAN.4
Aberrant glycosylation of IgA is central in the pathogenesis of IgAN. Glomerular IgA in IgAN patients is restricted to the IgA1 subclass and is enriched for molecules that have some hinge-region O-glycans deficient in galactose (galactose-deficient IgA1; Gd-IgA1).5,6 Furthermore, serum levels of Gd-IgA1 and IgA1-containing immune complexes (IC) with Gd-IgA1-specific autoantibodies are elevated in patients with IgAN.7–9 However, the mechanisms for production of Gd-IgA1 are still not fully understood.
We used the IgAN-prone ddY mouse model in this study. ddY mice develop glomerular injuries mimicking those of human IgAN.10 Although mice do not have IgA molecules with O-glycans typical for human IgA1, human IgAN and this IgAN-prone ddY mouse model share some disease-specific features, such as serum elevation of aberrantly glycosylated IgA and IgG-IgA IC.6,11 In fact, aberrant IgA glycosylation due to deficiency of β1,4-galactosylation of N-glycans induces murine IgAN due to elevation of serum levels of high-molecular-weight IgA and IgA-containing IC with their subsequent renal deposition and glomerular injury.12 ddY mice show proteinuria and a mesangial proliferative glomerulonephritis with co-deposition of IgA and C3 in the glomeruli. A genome-wide association study (GWAS) identified candidate loci linked with progression of IgAN in ddY mouse.13 The loci include genes functionally similar to those associated with human IgAN.14,15 These findings suggest that IgAN in ddY mice and in humans may be affected at least in part by the same susceptibility genes. Although the molecular features of murine IgA and human IgA1 differ, aberrantly glycosylated IgA tends to form polymeric IgA and IgG-IgA IC that subsequently drive progression of renal injury in the ddY murine model of IgAN as well as in human IgAN. Thus, the ddY mice model has some common features with human IgAN and can be a useful tool for analysis of various pathogenetic features of IgAN.
Toll like receptors (TLRs) are key molecules in the innate immune system and have been implicated in the pathogenesis of IgAN.16–20 Exogenous antigens derived from pathogens activate the TLR9-MyD88 signaling pathway.21 This process leads to significantly increased synthesis of inflammatory cytokines, such as type 1 IFN and IL-6.22 Recently, we demonstrated that TLR9 activation aggravated kidney injury in IgAN-prone ddY mice, with elevation of serum levels of IgA and IgG-IgA IC.16 Moreover, specific TLR9 polymorphisms associate with disease progression in patients with IgAN.16
Serum levels of TNF-α and IL-6 are elevated in patients with IgAN.23 Moreover, IL-6 and IL-4 increase production of IgA1 as well as accentuate the degree of galactose deficiency, increasing Gd-IgA1 synthesis by IgA1-secreting cell lines from IgAN patients.24 These findings suggest that IL-6 may be a key mediator of this process.24,25
The gene of tumor necrosis factor ligand superfamily member 13 (TNFSF13) encodes a proliferation inducing ligand (APRIL) that is a central cytokine for the maturation and survival of B cells.26 APRIL shares some signaling receptors important for B cell development with another TNF super family ligand, B cell activating factor (BAFF). BAFF is also involved in affinity maturation of B cells. A GWAS identified TNFSF13 as one of the candidate genes associated with IgAN.27 Indeed, in patients with IgAN, serum levels of APRIL are elevated28 and the levels are associated with renal prognosis.28
To gain a better understanding of the underlying mechanisms involved in the overproduction of aberrantly glycosylated IgA via TLR9 activation by the ligand, CpG oligonucleotides (ODN), we used IgAN-prone ddY mice and human IgA1-secreting cells.
Results
Activation of TLR9 aggravated renal injury in ddY mice via increased production of nephritogenic IgA
TLR9 can be activated by the corresponding ligands, such as CpG-ODN. To test the effect of TLR9 activation in a murine model of IgAN, we used injection of the ligand in IgAN-prone ddY mice. CpG-ODN-injected mice developed mesangial proliferation and extracellular matrix expansion (Figure 1A). Renal histological scores based on mesangial proliferation and mesangial matrix expansion in CpG-ODN-injected mice were significantly higher than those in control mice (P<0.01) (Figure 1A). Urinary albumin levels in the CpG-ODN-injected mice were significantly elevated compared with those of the control mice (P<0.01) (Figure 1A). Only the CpG-ODN-injected mice developed mesangial deposits of IgA, IgG, and complement C3 (Figure 1B). CpG-ODN-injected mice showed significantly higher serum levels of IgA (P<0.01) and IgG (P<0.01) than control mice (Figure 1C). Serum IgA from CpG-ODN-injected mice had lower reactivity with RCA-I and SNA lectins than that from control mice (Figure 1C), indicating a lower content of galactose and sialic acid on N-glycans of murine IgA. Furthermore, serum level of IgG-IgA IC in CpG-ODN-injected mice was significantly higher than that in control mice (P<0.05) (Figure 1C). These findings indicated that TLR9 activation by CpG ODN resulted in the overproduction of aberrantly glycosylated IgA and IgG-IgA IC, leading to immune-complex deposition and renal injury.
Figure 1. CpG-ODN stimulation induced production of nephritogenic IgA.
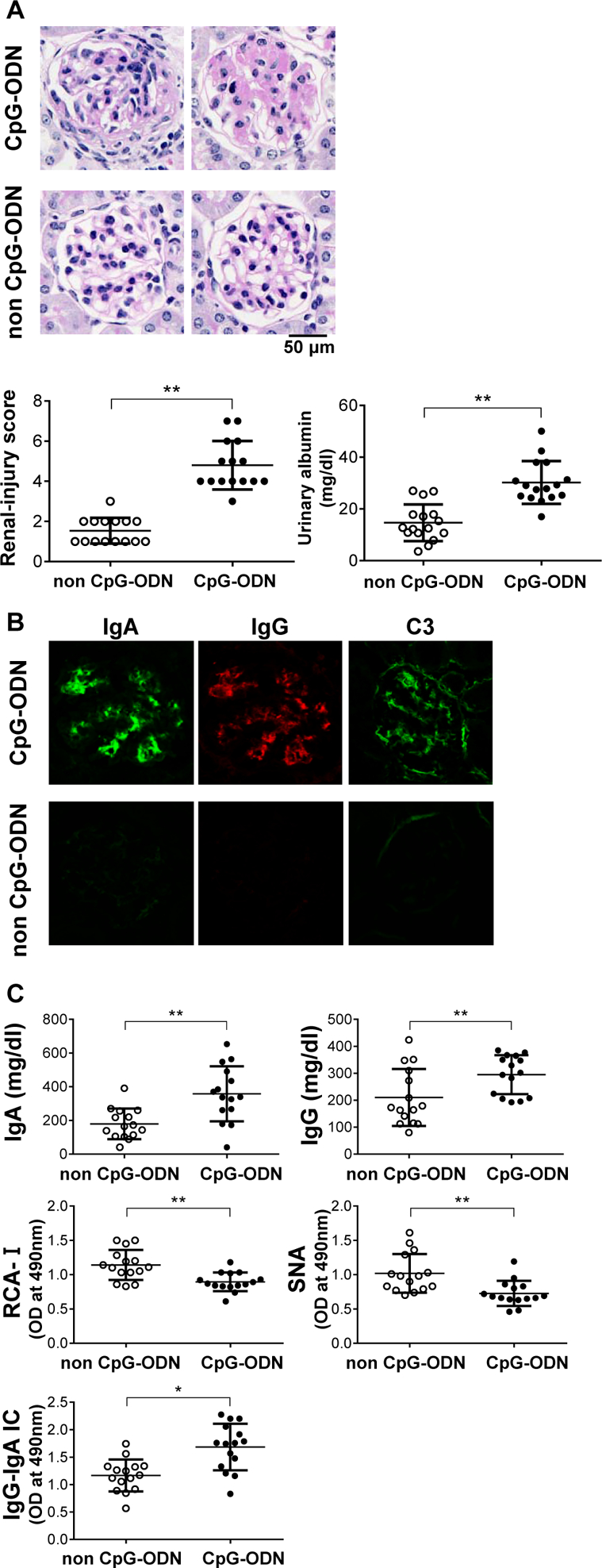
Injection with CpG-ODN increased blood levels of aberrantly glycosylated IgA and IgG-IgA IC and exacerbated kidney injury in ddY mice. (A) ddY mice before development of IgAN were intra-peritoneally injected with CpG-ODN thrice weekly for 12 weeks. In histological analysis of glomerular lesions, CpG-ODN-injected mice showed mesangial cellular proliferation and extracellular matrix expansion. Right panel shows evaluation of renal injuries by semi-quantitative scoring. Renal histological scores, evaluated by percentages of glomeruli with aforementioned lesions, showed that renal injury in the mice injected with CpG-ODN were exacerbated. Urinary albumin levels in the CpG-ODN-injected mice were significantly elevated compared with those of control mice. Bars represent the mean±SEM. **P<0.01. (B) Immunohistochemical analysis of glomerular IgA, IgG, and C3 deposits. Only CpG-ODN-injected mice developed mesangial deposits of IgA, IgG, and C3. (C) Serum levels of IgA, IgG, and IgG-IgA IC were significantly increased after CpG-ODN treatment for 12 weeks. *P<0.05, **P<0.01. Serum IgA in mice injected with CpG-ODN showed significantly lower reactivity with lectin RCA-I and SNA lectins than the IgA in control mice. **P<0.01. These results indicate that injection of CpG-ODN increased serum levels of aberrantly glycosylated IgA. Individual points are shown. *P<0.05, **P<0.01.
B cells and dendritic cells were involved in the CpG-ODN responses
We investigated which cell types are involved in the induction of overproduction of aberrantly glycosylated IgA in response to CpG-ODN. B cells and dendritic cells (DCs) were isolated from the spleens of ddY mice using anti-mouse CD19 and CD11c antibodies by FACS, respectively. CpG-ODN stimulation of isolated B cells induced overproduction of IgA. In addition, CpG-ODN significantly increased IgA production when B cells were co-cultured with DCs (Supplementary Figure). These results suggested that CpG-ODN directly activated B cells, and DCs further enhanced IgA production upon TLR9 activation.
Overproduction of APRIL enhanced synthesis of aberrantly glycosylated IgA and IgG-IgA IC
We assessed whether APRIL and/or BAFF was involved in the synthesis of nephritogenic IgA induced by TLR9 activation. Injection of CpG-ODN in ddY mice significantly elevated serum levels of APRIL and BAFF (Figure 2A). Expression levels of APRIL in splenocytes correlated with production of aberrantly glycosylated IgA and formation of IgG-IgA IC (Figure 2B). Expression levels of BAFF in splenocytes correlated with production of aberrantly glycosylated IgA, but not with formation of IgG-IgA IC (Figure 2B). Furthermore, serum levels of APRIL but not BAFF significantly correlated with elevated serum levels of aberrantly glycosylated IgA and IgG-IgA IC in the CpG-ODN-injected mice (Figure 2C). These findings suggest that both APRIL and BAFF may be involved in production of aberrantly glycosylated IgA, although APRIL may be more involved in the production of IgG-IgA IC.
Figure 2. Correlation between overproduction of APRIL and aberrantly glycosylated IgA in mice injected with CpG-ODN.
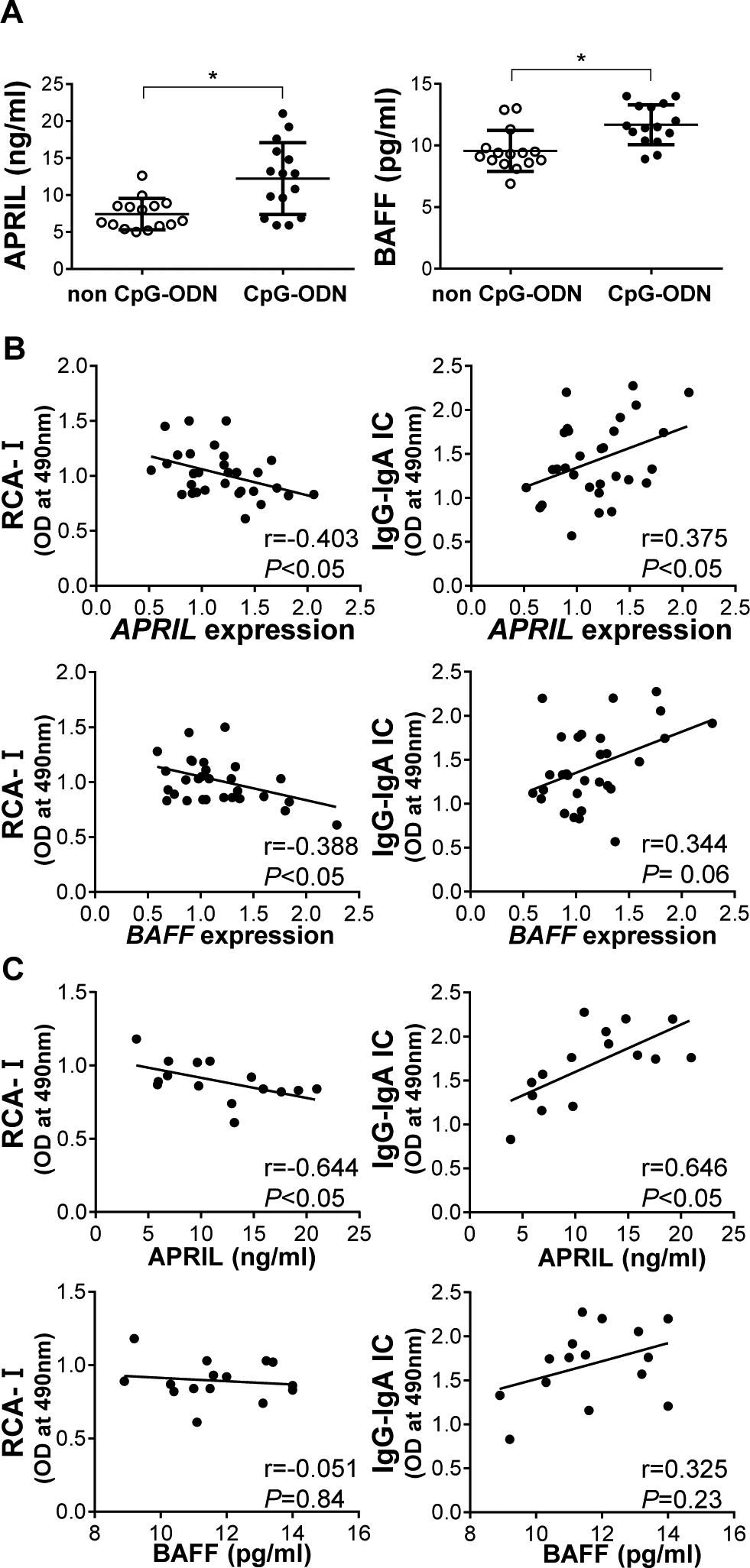
(A) Injection of ddY mice with CpG-ODN increased serum levels of BAFF and APRIL. Bars represent the mean±SEM. *P<0.05. (B) Expression levels of BAFF in whole splenocytes correlated with reduced reactivity with RCA-I lectin, but did not correlate with formation of IgG-IgA IC. Meanwhile, expression levels of APRIL were associated with production of aberrantly glycosylated IgA and formation of IgG-IgA IC. (C) In mice injected with CpG-ODN, serum levels of APRIL were associated with reduced reactivity of IgA with RCA-I lectin and elevated levels of IgG-IgA IC. Serum levels of BAFF did not correlate with production of glycosylated IgA and formation of IgG-IgA IC.
IL-6 induced production of aberrantly glycosylated IgA
Serum levels of IL-6 in CpG-ODN-injected mice were significantly higher compared with control mice (P<0.05) (Figure 3A). Using splenocytes of IgAN-prone ddY mice, we examined whether TLR9-induced cellular activation and overproduction of aberrantly glycosylated IgA was mediated by IL-6. CpG-ODN stimulation enhanced production of IL-6 by splenocytes in vitro. Addition of IL-6 to the culture medium of splenocytes increased IgA production. Moreover, IL-6 enhanced synthesis of aberrantly glycosylated IgA and formation of IgG-IgA IC, and increased APRIL production (Figure 3A). IL-6-neutralizing antibody reduced CpG-ODN-induced total IgA, aberrantly glycosylated IgA production, and IgG-IgA IC formation (Figure 3B). These findings indicate that TLR9 activation was mediated, at least in part, by IL-6. Furthermore, there may be another TLR9-mediated pathway(s) involved in production of aberrantly glycosylated IgA.
Figure 3. Effect of CpG-ODN and IL-6 on cultured splenocytes of ddY mice.
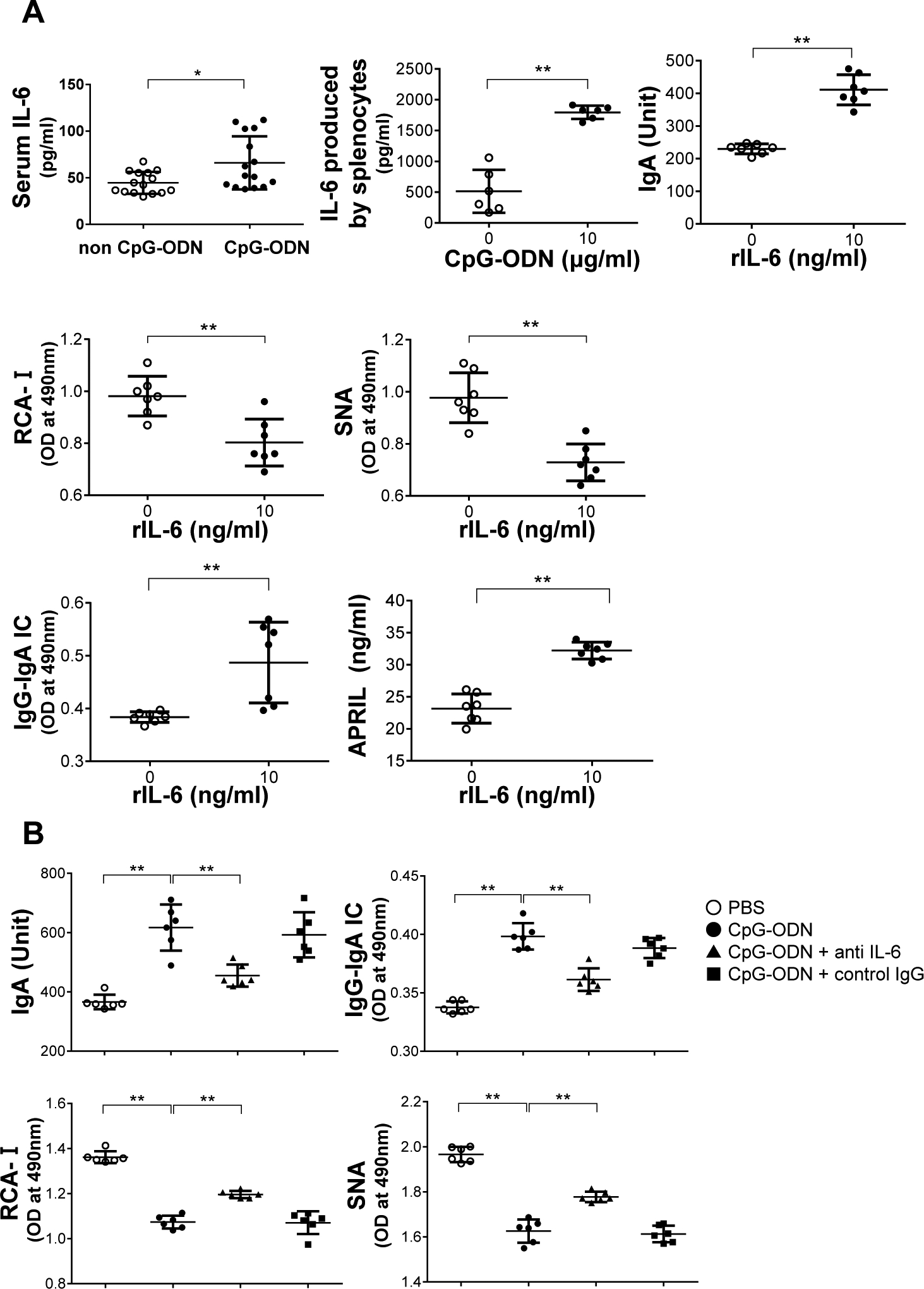
(A) Serum levels of IL-6 in CpG-ODN-injected mice were significantly higher compared with control mice. *P<0.05. CpG-ODN stimulation increased production of IL-6 of splenocytes of ddY mice in culture. Incubation of splenocytes of ddY mice with recombinant IL-6 increased production of total IgA and aberrantly glycosylated IgA, resulting in the formation of IgG-IgA IC. Recombinant IL-6 increased overproduction of APRIL. Bars represent the mean±SEM. **P<0.01. (B) Splenocytes in culture were stimulated with CpG-ODN in the presence or absence of IL-6-neutralizing antibody. Anti-IL-6 antibody reduced production of total IgA and aberrantly glycosylated IgA, and, consequently, reduced formation of IgG-IgA IC induced by CpG-ODN. **P<0.01. PBS, Phosphate-buffered saline.
APRIL and IL-6 enhanced synthesis of Gd-IgA1 in human IgA1-secreting cells
We further tested whether APRIL and IL-6 are common major mediators that enhance production of nephritogenic IgA in human IgA1-secreting cells. CpG-ODN supplementation enhanced increase of IL-6, expression of APRIL, production of APRIL protein, and production of IgA and Gd-IgA1 (Figure 4A). Stimulation with IL-6 as well as with APRIL enhanced production of IgA and Gd-IgA1 (Figure 4B, C). The effects of CpG-ODN stimulation on production of IgA and Gd-IgA1 were partly reduced by IL-6-neutralizing antibody (Figure 4D). Moreover, APRIL siRNA knock-down also reduced production of IgA and Gd-IgA1 induced by IL-6 stimulation (Figure 4B), indicating that APRIL and IL-6 synergistically promote the generation of Gd-IgA1.
Figure 4. Effect of CpG-ODN or APRIL supplementation on human IgA1-secreting cell lines on IgA1, Gd-IgA1, and IL-6 production.
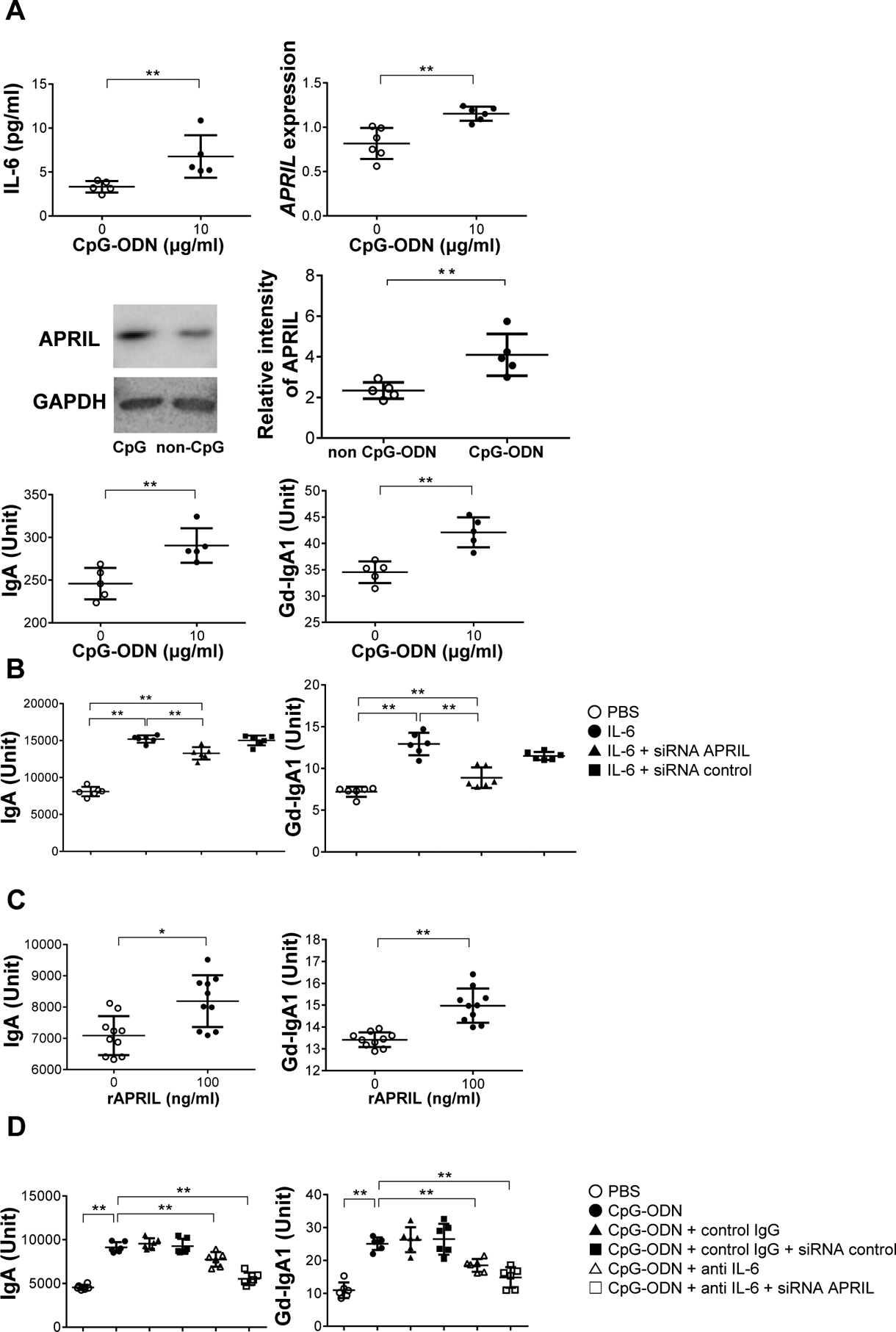
(A) CpG-ODN supplementation enhanced production of IL-6. CpG-ODN enhanced gene expression of APRIL. Western-blot analysis demonstrated that CpG-ODN increased protein levels of APRIL. Results were evaluated densitometrically. Stimulation with CpG-ODN induced production of IgA and Gd-IgA1. Bars represent the mean±SEM. *P<0.05, **P<0.01. (B) APRIL siRNA knock-down reduced IL-6-induced overproduction of IgA and Gd-IgA1. *P<0.05, **P<0.01. (C) IgA1-producing cells stimulated with recombinant APRIL produced more IgA and Gd-IgA1. (D) IgA1-secreting cells were incubated with anti-IL-6 antibody after CpG-ODN stimulation. The enhanced production of IgA and Gd-IgA1 induced by CpG-ODN was reduced by anti-IL-6 antibody and by siRNA knock-down of APRIL. PBS, Phosphate Buffered Saline.
Discussion
Mesangial IgA in human IgAN is exclusively of the IgA1 subclass that displays abnormal O-glycosylation.29,30 Human IgA1 has a hinge region with O-glycans that is absent from murine IgA.6,31,32 However, aberrant glycosylation of N-glycans is involved in the pathogenesis of a murine model of IgAN in ddY mice, with elevation of aberrantly glycosylated IgA and IgA-containing IC.11,12 Aberrant modifications of IgA carbohydrates in either O- or N-glycans are characteristic properties of nephritogenic IgA-containing IC.
Several studies have shown TLR9 activation in progression of IgAN.17–19 In the present study, we have shown that activation of TLR9 by intra-peritoneal injection of CpG-ODN in ddY mice aggravated renal injuries accompanied by glomerular IgA and IgG deposits, likely due to increased serum levels of aberrantly glycosylated IgA and IgG-IgA IC. Moreover, we showed that TLR9 activation was involved in synthesis of Gd-IgA1 in human IgA1-secreting cell lines.
We assessed the mechanism by which TLR9 activation mediated enhanced production of aberrantly glycosylated IgA. BAFF and APRIL are known for their roles in B-cell survival and differentiation. McCarthy et al. demonstrated that BAFF-overexpressing transgenic mice develop mesangial IgA deposits with renal injury and urinary abnormalities.33 Recently, we demonstrated that gene expression of APRIL is elevated in tonsillar germinal centers of patients with IgAN. Furthermore, overexpression of APRIL in tonsillar germinal centers correlated with serum levels of Gd-IgA1 and disease severity in patients with IgAN.34 These findings suggest that both BAFF and APRIL may be involved in the pathogenesis of IgAN. However, the association between BAFF/APRIL and TLR9 activation is unclear. In the present study, TLR9 activation increased gene expression and serum levels of APRIL. Serum levels of aberrantly glycosylated IgA correlated with expression levels of BAFF and APRIL in splenocytes. However, serum levels of IgG-IgA IC correlated with expression levels of APRIL but not BAFF. Furthermore, serum levels of APRIL were associated with overproduction of aberrantly glycosylated IgA and IgG-IgA IC in the TLR9-activated mice. In IgA1-secreting cells, TLR9 activation also induced overproduction of Gd-IgA1 via APRIL. These results suggested that APRIL plays an important role in TLR9-induced overproduction of nephritogenic IgA and formation of IgG-IgA IC.
The response by cell type to determine the role of the B and T cells and dendritic cells (DCs) should be clarified. In the present study, we assessed the role of B cells and plasmacytoid DCs. TLR9 is expressed by DCs, B cells, and macrophages, but not by T cells.35–37 The contribution of BAFF/APRIL to T cell-independent antibody response is known.38,39 Here, we tested whether TLR9 activation can enhance synthesis of aberrantly glycosylated IgA and formation of IgG-IgA IC via APRIL in a T cell-independent manner. We also used cloned human IgA1-secreting cell lines, and showed that TLR9 activation augmented production of Gd-IgA1 via APRIL and IL-6, suggesting the process is T cell-independent. Furthermore, we evaluated the roles of B cells and DCs. We showed that CpG-ODN specific for B cells and DCs increased production of aberrantly glycosylated IgA in whole splenocytes. Furthermore, we investigated whether CpG-ODN stimulation in vitro can enhance IgA production. CpG-ODN stimulation of isolated B cells induced overproduction of IgA. In addition, CpG-ODN significantly increased IgA production when B cells were co-cultured with DCs (Supplementary Figure S1). These results suggest that CpG-ODN activation resulting in greater IgA production and aberrant glycosylation is mediated by TLR9 in a T cell-independent manner.
IL-6 is known to accentuate proliferation of immunoglobulin-producing cells and the terminal differentiation of plasma cells.40–42 Furthermore, IL-6 may also be a primary-candidate cytokine for T follicular helper (Tfh) cell differentiation.43,44 Tfh cells are essential for B cell affinity maturation, class switch recombination, and memory B cell and plasma cell generation within the germinal center.43,44 In a lupus mouse model, IL-6 deficiency prevented Tfh cell differentiation and germinal-center B cell expansion, resulting in reduced autoantibody production.45 The present study clarified that TLR9 activation by CpG-ODN enhanced IL-6 production in splenocytes of murine IgAN. IL-6 induced overproduction of aberrantly glycosylated IgA and IgG-IgA IC in a murine model of IgAN. In human IgA1-secreting cells, we confirmed that IL-6 enhanced production of Gd-IgA1.24 Our findings suggest that IL-6 may be one of the major molecules inducing overproduction of nephritogenic IgA mediated by TLR9 activation.
APRIL plays an important role in IgA-class switching and plasmacytoid differentiation.46,47 IL-6 promotes the terminal differentiation of B cells to IgA-secreting plasma cells.41,42 The present study demonstrated that IL-6 stimulation induced production of APRIL. Then, we hypothesized there was an association between APRIL and IL-6 mediated by TLR9 activation inducing production of aberrantly glycosylated IgA. We found that IL-6-neutralizing antibody did not completely block CpG-ODN-induced overproduction of Gd-IgA1, and siRNA knock-down of APRIL reduced overproduction of Gd-IgA1 induced by IL-6. These data suggested that APRIL and IL-6 synergistically promoted the production of Gd-IgA1 mediated by TLR9 activation. Furthermore, both IL-6-neutralizing antibody and siRNA knock-down of APRIL reduced production of IgA and Gd-IgA1 more than did IL-6-neutralizing antibody alone. Thus, the TLR9 activation pathway involves IL-6 and APRIL synergistic effect on antibody production and glycosylation (Figure 5). Recent studies have also indicated that IL-6, in combination with APRIL, induced generation and survival of PCs.48,49 However, future studies are required to clarify intercellular signaling pathways between APRIL and IL-6 in the production of nephritogenic IgA.
Figure 5. Synergistic role between TLR9, APRIL, and IL-6 in IgA-producing cells in IgAN.
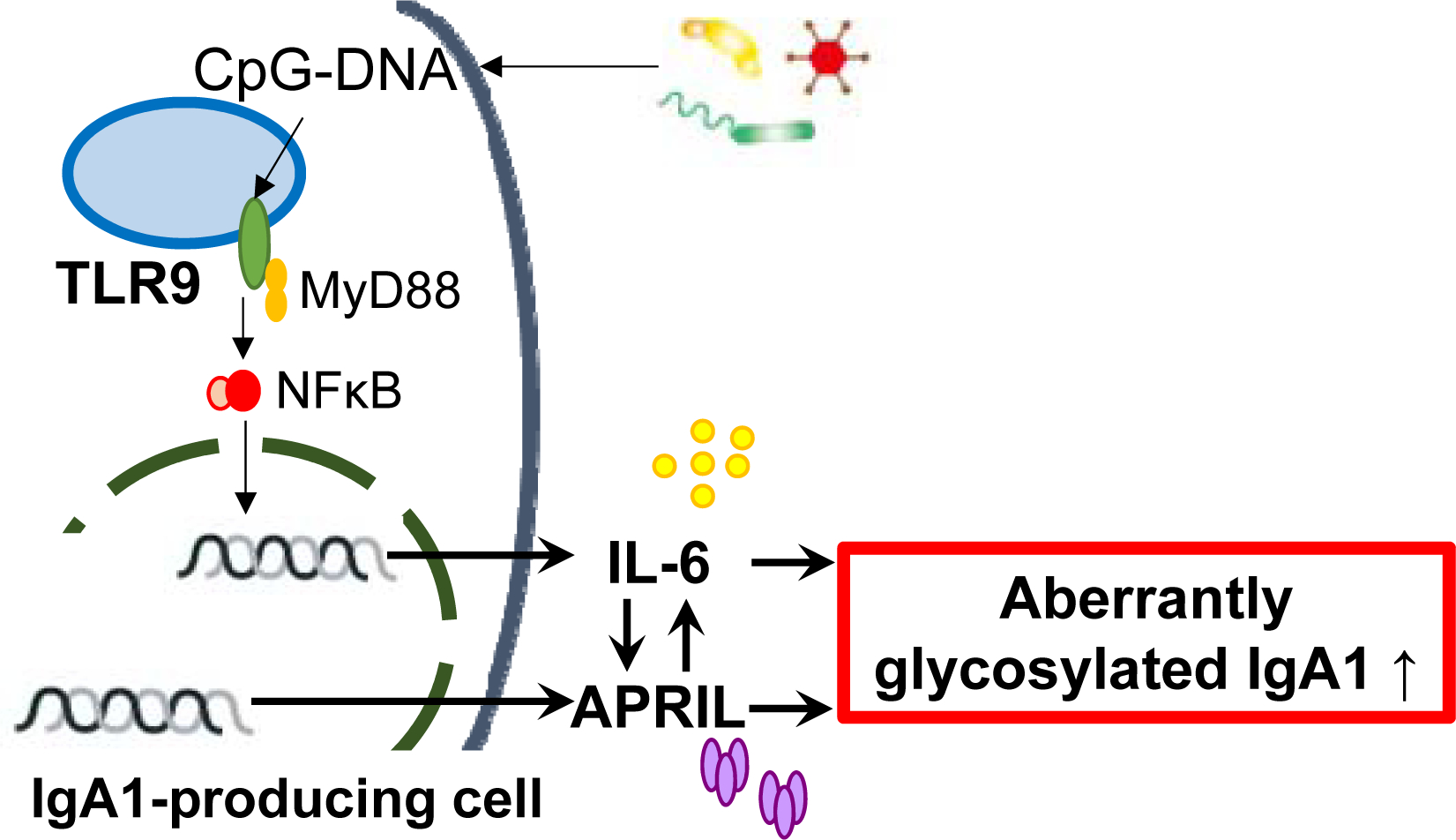
TLR9 activation induced production of APRIL and IL-6, resulting in overproduction of aberrantly glycosylated IgA. APRIL and IL-6 function synergistically to promote the generation of aberrantly glycosylated IgA. In addition to APRIL inducing production of IL-6, as previously reported, IL-6 can also enhance synthesis of APRIL. Furthermore, APRIL and IL-6 independently promote production of aberrantly glycosylated IgA.
In conclusion, TLR9 activation exacerbated renal injury in a murine model of IgAN by enhancing production of aberrantly glycosylated IgA and formation of IgG-IgA IC. In this process, the overproduction of APRIL and IL-6 induced by TLR9 activation enhanced production nephritogenic IgA. In addition, the present study clarified that APRIL and IL-6 function synergistically, as well as independently, to produce aberrantly glycosylated IgA. These findings may inform future development of new therapeutic approaches for IgAN.
Materials and Methods
Animals and experimental protocols
The IgAN-prone ddY mouse is a known model of spontaneous IgAN with variable incidence and extent of glomerular injury mimicking human IgAN.10 ddY mice are divided into three groups based on manifestation of kidney damage: mice with early or late onset and quiescent mice. Early-onset disease in ddY mice presents with proteinuria and IgA deposits in glomerular after 20 weeks of age.13 Therefore, we used ddY mice within 18 weeks of age to evaluate whether TLR9 activation aggravated IgAN in this model. A total of 30 female ddY mice (SLC Japan, Shizuoka, Japan) were maintained at the animal facility of Juntendo University, Tokyo, Japan. Mice were fed regular chow (Oriental Yeast Co. Ltd., Tokyo, Japan) and water ad libitum and were housed in a specific pathogen-free room. The experimental protocol was approved by the Ethics Review Committee for Animal Experimentation of Juntendo University Faculty of Medicine. At 6 weeks of age the mice were randomly divided into two groups. One group received CpG-ODN injected intra-peritoneal three times per week for 12 weeks; the second group (control) was injected with non-CpG-ODN. CpG-ODN and non-CpG-ODN were chemically synthesized (Invitrogen; Thermo Fisher Scientific, Kanagawa, Japan). Sequences of the ODN are TCGTCGTTTTCGGCGCGCGCCG or TGCTGCTTTTGGGGGGCCCCCC (5’→3’) for CpG or non-CpG ODNs, respectively.
Determination of IgA, IgG, IgG-IgA IC, and aberrantly glycosylated IgA in murine serum
Blood samples were collected from the buccal vein. Serum IgA, IgG, and IgG-IgA IC were measured by sandwich ELISA (Bethyl Laboratories, Montgomery, TX), using the modified method based on our previous report.50 Lectin-binding assays were used to access the glycoform of IgA. Biotinylated Ricinus communis agglutinin I (RCA-I; Vector Laboratories, Burlingame, CA) and Sambucus nigra bark lectin (SNA; Vector Laboratories) that recognized galactose and sialic acid attached to galactose (predominantly in α2,6 linkage), respectively, were used in this assay.51 Diluted sera were added at 100 ng of IgA per well. Microtiter plates coated with 1 mg/ml goat anti-mouse IgA (100 μl/well) for quantification of serum IgA were incubated with these samples, and biotinylated SNA or RCA-I was then added. Avidin–horseradish peroxidase conjugate (ExtrAvidin; Sigma-Aldrich, St. Louis, MO) was applied. Serum levels of aberrantly glycosylated bound IgA were expressed in Units (1 unit as OD 1.0 measured at 490 nm). Serum level of APRIL and BAFF were measured using mouse APRIL ELISA kit (MyBioSource, San Diego, CA) and mouse BAFF ELISA Kit (R&D Systems, Minneapolis, MN), according to the manufacturer’s protocols.
Assay for Gd-IgA1 produced by human IgA1-secreting cells
A sandwich ELISA for Gd-IgA1 was constructed using Gd-IgA1-specific antibody (IBL, Japan).52 Gd-IgA1-specific antibody (7.5 μg/mL) diluted in PBS was coated onto an ELISA plate for 18 h at room temperature. Samples were applied and incubated for 2 h at room temperature. Plates were washed and incubated for 2 h at room temperature with a 1:1,000-diluted HRP-conjugated mouse anti-human IgA1 α1-chain-specific monoclonal antibody (Southern Biotech, Birmingham, AL). After washing, plates were developed with SIGMAFAST o-phenylenediamine (OPD) solution (Sigma-Aldrich) and the reaction was stopped by addition of 1 M sulfuric acid (Wako, Osaka, Japan). The levels of Gd-IgA1 were extrapolated by referring to a standard curve (4-parameter logistic curve fitting) of OD at 490 nm and expressed in units. Enzymatically generated Gd-IgA1 from human plasma IgA152 was serially diluted (1.37–1,000 ng/mL) to generate a standard curve. In this study, 1 unit was defined as 1 ng/mL of the standard Gd-IgA1.52
Evaluation of renal injury in ddY mice
Kidneys were removed after perfusion with normal saline. Renal tissue specimens for microscopic examination were fixed in 20% formaldehyde, embedded in paraffin, cut into 3-μm-thick sections, and then stained with PAS. Specimens were quantitatively analyzed to determine the percentage of glomeruli with segmental and global sclerosis and/or mesangial cell proliferation and/or increase in mesangial matrix. Each section was scored semi-quantitatively for percentages of glomeruli with aforementioned lesions (0, 0%; 1, 1 to 24%; 2, 25 to 49%; and 3, >50% of 20 glomeruli).13,53,54 Total maximal score for each section was 9. Renal specimens for immunofluorescence were immersed in OCT compound (Sakura Finetek Japan Co. Ltd., Tokyo, Japan) and stored at −80°C. The specimens were cut into 3-μm thick sections, fixed with acetone at −20°C for 5 min, washed with PBS, blocked with a blocking agent (DS Pharma Biomedical Co. Ltd., Osaka, Japan) at room temperature for 30 min, and then incubated at room temperature for 2 h with the following primary antibodies: goat anti-murine IgA, Alexa conjugated goat anti-murine IgG and rat-anti-C3 (Bethyl Laboratories). After three more washes with PBS, the slides were mounted with mounting medium (Dako, Tokyo, Japan). Samples were analyzed and imaged using confocal laser microscopy (Olympus Corporation, Tokyo, Japan).
Real-Time Quantitative RT-PCR
RNA from spleen cells was extracted using Torizol solution (Invitrogen, Tokyo, Japan) and purified with RNeasy Mini Kit (74106; Qiagen, Valencia, CA). Real-time RT-PCR was performed using the Applied Biosystems 7500 Real-Time PCR System using SYBR Green PCR Master Mix (Applied Biosystems, Tokyo, Japan) and specific primers: mouse TLR9 forward primer TCTCCCAACATGGTTCTCCGTCG, reverse primer TGCAGTCCAGGCCATGA; mouse MyD88 forward primer GCACCTGTGTCTGGTCCATT, reverse primer CTGTTGGACACCTGGAGACA; and the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) forward primer CATTGTGGAAGGGCTCATGA, reverse primer TCTTCTGGGTGGCAGTGATG. The amplification conditions were as follows: preheating at 95°C for 20 s and then 40 cycles of denaturation at 95°C for 3 s, and annealing and extension at 60°C for 30 s. Homo sapiens-specific TaqMan gene expression assays (Life Technologies, Carlsbad, CA) were purchased for the primer of APRIL, Hs00601664_g1 and Mm03809849-s1, and BAFF, Mm01168134-m1. The TaqMan PCR cycling conditions were as follows: preheating at 95°C for 20 s and then 40 cycles of denaturation at 95°C for 3 s, and annealing and extension at 60°C for 30 s.
In vitro studies with splenocytes of ddY mice
Splenocytes were cultured in RPMI-1640 medium supplemented with 20% fetal calf serum, 100 μg/ml streptomycin, and 100 U/ml penicillin. Red blood cells were lysed in Red Blood Cell Lysing Buffer (R7757; Sigma-Aldrich) at room temperature for 1 min, followed by washing in RPMI-1640 medium.50 Cell cultures were kept in a humidified incubator at 37°C with 5% CO2. Class C CpG-ODN (TCGTCGTTTTCGGCGCGCGCCG) was used as the TLR9 ligand. Recombinant IL-6, anti-IL-6 antibody, and rat IgG1 isotype control were obtained from R&D Systems. Cells were cultured with either medium or recommended concentrations of CpG-ODN, 10 ng/ml recombinant IL-6, and 100 ng/ml anti-IL6 antibody. IgA, aberrantly glycosylated IgA, IgG-IgA IC and APRIL produced by splenocytes were measured by ELISA after 72 h incubation in vitro.
In vitro studies with human IgA1-secreting cell lines
B cells from the blood of a healthy person were immortalized with Epstein-Barr virus, subcloned to yield IgA1-secreting cell lines, and cultured as described.9 Furthermore, we confirmed expression of receptors for APRIL/BAFF and IL-6. Cells were cultured in RPMI-1640 medium supplemented with 20% fetal calf serum, streptomycin and penicillin in a humidified incubator at 37°C with 5% CO2. Class B CpG-ODN (TCGTCGTTTTGTCGTTTTGTCGTT) was used as the TLR9 ligand. Recombinant IL-6, recombinant APRIL, anti-IL-6 antibody and goat IgG control antibody were obtained from R&D Systems. Cells were cultured with either medium or recommended concentrations of CpG-ODN, 50 ng/ml recombinant IL-6, 40 ng/ml recombinant APRIL, and 100 ng/ml anti-IL-6 antibody for 72 h.
Western blotting
Supernatants from IgA1-secreting cells were separated by SDS-PAGE under reducing conditions using 5%−15% gradient slab gels. The gels were transferred to PVDF membranes and incubated with antibodies specific for APRIL (Abcam Inc., Cambridge, MA) and GAPDH (Abcam Inc). Blots were visualized by chemiluminescence using ECL (GE Healthcare, Tokyo, Japan) and luminescence detector (Konica Minolta Healthcare, Tokyo, Japan). Results were evaluated densitometrically.
APRIL siRNA knock-down
Human IgA1-secreting cells were cultured in 24-well culture plates and transfected with APRIL-specific siRNA (GS8741, Qiagen) or siRNA non-targeting control (GS10673, Qiagen) using the transfection protocol according to the manufacturer’s instructions. After 24 h incubation, the cells were incubated with 50 ng/ml recombinant IL-6 (R&D Systems) for 72 h. After incubation, cells and supernatants were harvested for measurement of IgA and Gd-IgA.
Statistical Analysis
The correlation between different parameters was analyzed using analysis of variance. Data were expressed as the mean ± standard deviation or median values. P values <0.05 were considered statistically significant. All statistical analyses were performed using GraphPad Prism version 6.0 for Windows (GraphPad Software, San Diego, CA).
Supplementary Material
CpG-oligodeoxynucleotide (ODN)-activated B cells and dendritic cells (DCs) further enhance IgA production. B cells and DCs were isolated from the spleens of ddY mice. CpG-ODN stimulation increased IgA production in the isolated B cells. This increase was further enhanced by co-culturing B cells with DCs. *P<0.05.
Cell sorting
Spleen cells of ddY mice were stained for 30 min at 4°C with the following mouse-specific monoclonal antibodies: PE Rat Anti-Mouse CD19 Clone 1D3 (BD Biosciences, Franklin Lakes, NJ) for B cells and APC Hamster Anti-Mouse CD11c Clone HL3 (BD Biosciences) for DCs. Gating was done using Summit software. Cells were sorted using Moflo Astrios EQs cell sorter (Beckman Coulter, CA) and collected directly into 50 ml tubes containing 10 ml of staining medium.
Translational Statement.
Disease-specific therapy targeting galactose-deficient IgA1 can constitute a future treatment of IgA nephropathy. Results from this study, that demonstrated the involvement of APRIL and IL-6 in overproduction of aberrantly glycosylated IgA, support the rationale for targeting APRIL and IL-6 in IgA nephropathy. In fact, a recent study demonstrated positive effects of anti-APRIL monoclonal antibody in murine IgAN,1 and a clinical study with neutralizing APRIL antibody is currently ongoing in patients with IgAN.
Acknowledgments
We thank Ms. Terumi Shibata and Aki Omori for their excellent technical assistance. We also thank Ms. Takako Ikegami and Ms. Tomomi Ikeda (Division of Molecular and Biochemical Research, Juntendo University Graduate School of Medicine), Ms. Tamami Sakanishi (Division of Cell Biology, Juntendo University Graduate School of Medicine) for their excellent technical assistance.
This study was supported, in part, by the JSPS KAKENHI Grant Number 18K08252 and Practical Research Project for Renal Diseases from the Japan Agency for Medical Research and development, AMED. JN and BAJ were supported in part by NIH grants DK078244 and DK082753.
Footnotes
In the interest of full disclosure, we report that B.A. Julian and J. Novak are co-founders of Reliant Glycosciences, LLC and co-inventors on the US patent application 14/318,082 (assigned to UAB Research Foundation that distributes royalties to the inventors).
All authors declared no competing interests.
Supplementary information is available at Kidney International’s website.
References
- 1.Myette JR, Kano T, Suzuki H, et al. A Proliferation Inducing Ligand (APRIL) targeted antibody is a safe and effective treatment of murine IgA nephropathy. Kidney Int 2019. [DOI] [PubMed] [Google Scholar]
- 2.Levy M, Berger J. Worldwide perspective of IgA nephropathy. Am J Kidney Dis 1988; 12: 340–347. [DOI] [PubMed] [Google Scholar]
- 3.D’Amico G The commonest glomerulonephritis in the world: IgA nephropathy. Q J Med 1987; 64: 709–727. [PubMed] [Google Scholar]
- 4.Feehally J, Beattie TJ, Brenchley PE, et al. Sequential study of the IgA system in relapsing IgA nephropathy. Kidney Int 1986; 30: 924–931. [DOI] [PubMed] [Google Scholar]
- 5.Wyatt RJ, Julian BA. IgA nephropathy. N Engl J Med 2013; 368: 2402–2414. [DOI] [PubMed] [Google Scholar]
- 6.Suzuki H, Yasutake J, Makita Y, et al. IgA nephropathy and IgA vasculitis with nephritis have a shared feature involving galactose-deficient IgA1-oriented pathogenesis. Kidney Int 2018; 93: 700–705. [DOI] [PubMed] [Google Scholar]
- 7.Glassock RJ. Analyzing antibody activity in IgA nephropathy. J Clin Invest 2009; 119: 1450–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moldoveanu Z, Wyatt RJ, Lee JY, et al. Patients with IgA nephropathy have increased serum galactose-deficient IgA1 levels. Kidney Int 2007; 71: 1148–1154. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki H, Moldoveanu Z, Hall S, et al. IgA1-secreting cell lines from patients with IgA nephropathy produce aberrantly glycosylated IgA1. J Clin Invest 2008; 118: 629–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Imai H, Nakamoto Y, Asakura K, et al. Spontaneous glomerular IgA deposition in ddY mice: an animal model of IgA nephritis. Kidney Int 1985; 27: 756–761. [DOI] [PubMed] [Google Scholar]
- 11.Okazaki K, Suzuki Y, Otsuji M, et al. Development of a model of early-onset IgA nephropathy. J Am Soc Nephrol 2012; 23: 1364–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nishie T, Miyaishi O, Azuma H, et al. Development of immunoglobulin A nephropathy- like disease in beta-1,4-galactosyltransferase-I-deficient mice. Am J Pathol 2007; 170: 447–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suzuki H, Suzuki Y, Yamanaka T, et al. Genome-wide scan in a novel IgA nephropathy model identifies a susceptibility locus on murine chromosome 10, in a region syntenic to human IGAN1 on chromosome 6q22–23. J Am Soc Nephrol 2005; 16: 1289–1299. [DOI] [PubMed] [Google Scholar]
- 14.Takei T, Iida A, Nitta K, et al. Association between single-nucleotide polymorphisms in selectin genes and immunoglobulin A nephropathy. Am J Hum Genet 2002; 70: 781–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gharavi AG, Yan Y, Scolari F, et al. IgA nephropathy, the most common cause of glomerulonephritis, is linked to 6q22–23. Nat Genet 2000; 26: 354–357. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki H, Suzuki Y, Narita I, et al. Toll-like receptor 9 affects severity of IgA nephropathy. J Am Soc Nephrol 2008; 19: 2384–2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakata J, Suzuki Y, Suzuki H, et al. Changes in nephritogenic serum galactose-deficient IgA1 in IgA nephropathy following tonsillectomy and steroid therapy. PLoS One 2014; 9: e89707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sato D, Suzuki Y, Kano T, et al. Tonsillar TLR9 expression and efficacy of tonsillectomy with steroid pulse therapy in IgA nephropathy patients. Nephrol Dial Transplant 2012; 27: 1090–1097. [DOI] [PubMed] [Google Scholar]
- 19.Coppo R, Camilla R, Amore A, et al. Toll-like receptor 4 expression is increased in circulating mononuclear cells of patients with immunoglobulin A nephropathy. Clin Exp Immunol 2010; 159: 73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maiguma M, Suzuki Y, Suzuki H, et al. Dietary zinc is a key environmental modifier in the progression of IgA nephropathy. PLoS One 2014; 9: e90558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawai T, Akira S. Signaling to NF-kappaB by Toll-like receptors. Trends Mol Med 2007; 13: 460–469. [DOI] [PubMed] [Google Scholar]
- 22.Kuwata H, Matsumoto M, Atarashi K, et al. IkappaBNS inhibits induction of a subset of Toll-like receptor-dependent genes and limits inflammation. Immunity 2006; 24: 41–51. [DOI] [PubMed] [Google Scholar]
- 23.Rostoker G, Rymer JC, Bagnard G, et al. Imbalances in serum proinflammatory cytokines and their soluble receptors: a putative role in the progression of idiopathic IgA nephropathy (IgAN) and Henoch-Schönlein purpura nephritis, and a potential target of immunoglobulin therapy? Clin Exp Immunol 1998; 114: 468–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suzuki H, Raska M, Yamada K, et al. Cytokines alter IgA1 O-glycosylation by dysregulating C1GalT1 and ST6GalNAc-II enzymes. J Biol Chem 2014; 289: 5330–5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamada K, Huang ZQ, Raska M, et al. Inhibition of STAT3 Signaling Reduces IgA1 Autoantigen Production in IgA Nephropathy. Kidney Int Rep 2017; 2: 1194–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schneider P, MacKay F, Steiner V, et al. BAFF, a novel ligand of the tumor necrosis factor family, stimulates B cell growth. J Exp Med 1999; 189: 1747–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kiryluk K, Li Y, Scolari F, et al. Discovery of new risk loci for IgA nephropathy implicates genes involved in immunity against intestinal pathogens. Nat Genet 2014; 46: 1187–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han SS, Yang SH, Choi M, et al. The Role of TNF Superfamily Member 13 in the Progression of IgA Nephropathy. J Am Soc Nephrol 2016; 27: 3430–3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hiki Y, Odani H, Takahashi M, et al. Mass spectrometry proves under-O-glycosylation of glomerular IgA1 in IgA nephropathy. Kidney Int 2001; 59: 1077–1085. [DOI] [PubMed] [Google Scholar]
- 30.Allen AC, Bailey EM, Brenchley PE, et al. Mesangial IgA1 in IgA nephropathy exhibits aberrant O-glycosylation: observations in three patients. Kidney Int 2001; 60: 969–973. [DOI] [PubMed] [Google Scholar]
- 31.Hiki Y O-linked oligosaccharides of the IgA1 hinge region: roles of its aberrant structure in the occurrence and/or progression of IgA nephropathy. Clin Exp Nephrol 2009; 13: 415–423. [DOI] [PubMed] [Google Scholar]
- 32.Mestecky J, Tomana M, Moldoveanu Z, et al. Role of aberrant glycosylation of IgA1 molecules in the pathogenesis of IgA nephropathy. Kidney Blood Press Res 2008; 31: 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCarthy DD, Kujawa J, Wilson C, et al. Mice overexpressing BAFF develop a commensal flora-dependent, IgA-associated nephropathy. J Clin Invest 2011; 121: 3991–4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muto M, Manfroi B, Suzuki H, et al. Toll-Like Receptor 9 Stimulation Induces Aberrant Expression of a Proliferation-Inducing Ligand by Tonsillar Germinal Center B Cells in IgA Nephropathy. J Am Soc Nephrol 2017; 28: 1227–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Latz E, Schoenemeyer A, Visintin A, et al. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat Immunol 2004; 5: 190–198. [DOI] [PubMed] [Google Scholar]
- 36.Hornung V, Rothenfusser S, Britsch S, et al. Quantitative expression of toll-like receptor 1–10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J Immunol 2002; 168: 4531–4537. [DOI] [PubMed] [Google Scholar]
- 37.Kadowaki N, Ho S, Antonenko S, et al. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J Exp Med 2001; 194: 863–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.He B, Xu W, Santini PA, et al. Intestinal bacteria trigger T cell-independent immunoglobulin A(2) class switching by inducing epithelial-cell secretion of the cytokine APRIL. Immunity 2007; 26: 812–826. [DOI] [PubMed] [Google Scholar]
- 39.Mackay F, Schneider P. Cracking the BAFF code. Nat Rev Immunol 2009; 9: 491–502. [DOI] [PubMed] [Google Scholar]
- 40.Kunimoto DY, Nordan RP, Strober W. IL-6 is a potent cofactor of IL-1 in IgM synthesis and of IL-5 in IgA synthesis. J Immunol 1989; 143: 2230–2235. [PubMed] [Google Scholar]
- 41.Beagley KW, Eldridge JH, Lee F, et al. Interleukins and IgA synthesis. Human and murine interleukin 6 induce high rate IgA secretion in IgA-committed B cells. J Exp Med 1989; 169: 2133–2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramsay AJ, Husband AJ, Ramshaw IA, et al. The role of interleukin-6 in mucosal IgA antibody responses in vivo. Science 1994; 264: 561–563. [DOI] [PubMed] [Google Scholar]
- 43.Nurieva RI, Chung Y, Martinez GJ, et al. Bcl6 mediates the development of T follicular helper cells. Science 2009; 325: 1001–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Poholek AC, Hansen K, Hernandez SG, et al. In vivo regulation of Bcl6 and T follicular helper cell development. J Immunol 2010; 185: 313–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jain S, Park G, Sproule TJ, et al. Interleukin 6 Accelerates Mortality by Promoting the Progression of the Systemic Lupus Erythematosus-Like Disease of BXSB.Yaa Mice. PLoS One 2016; 11: e0153059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Litinskiy MB, Nardelli B, Hilbert DM, et al. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat Immunol 2002; 3: 822–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Castigli E, Wilson SA, Scott S, et al. TACI and BAFF-R mediate isotype switching in B cells. J Exp Med 2005; 201: 35–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jourdan M, Cren M, Robert N, et al. IL-6 supports the generation of human long-lived plasma cells in combination with either APRIL or stromal cell-soluble factors. Leukemia 2014; 28: 1647–1656. [DOI] [PubMed] [Google Scholar]
- 49.Chu VT, Fröhlich A, Steinhauser G, et al. Eosinophils are required for the maintenance of plasma cells in the bone marrow. Nat Immunol 2011; 12: 151–159. [DOI] [PubMed] [Google Scholar]
- 50.Suzuki H, Suzuki Y, Aizawa M, et al. Th1 polarization in murine IgA nephropathy directed by bone marrow-derived cells. Kidney Int 2007; 72: 319–327. [DOI] [PubMed] [Google Scholar]
- 51.Chintalacharuvu SR, Emancipator SN. The glycosylation of IgA produced by murine B cells is altered by Th2 cytokines. J Immunol 1997; 159: 2327–2333. [PubMed] [Google Scholar]
- 52.Yasutake J, Suzuki Y, Suzuki H, et al. Novel lectin-independent approach to detect galactose-deficient IgA1 in IgA nephropathy. Nephrol Dial Transplant 2015; 30: 1315–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Katafuchi R, Kiyoshi Y, Oh Y, et al. Glomerular score as a prognosticator in IgA nephropathy: its usefulness and limitation. Clin Nephrol 1998; 49: 1–8. [PubMed] [Google Scholar]
- 54.Ballardie FW, Roberts IS. Controlled prospective trial of prednisolone and cytotoxics in progressive IgA nephropathy. J Am Soc Nephrol 2002; 13: 142–148. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
CpG-oligodeoxynucleotide (ODN)-activated B cells and dendritic cells (DCs) further enhance IgA production. B cells and DCs were isolated from the spleens of ddY mice. CpG-ODN stimulation increased IgA production in the isolated B cells. This increase was further enhanced by co-culturing B cells with DCs. *P<0.05.
Cell sorting
Spleen cells of ddY mice were stained for 30 min at 4°C with the following mouse-specific monoclonal antibodies: PE Rat Anti-Mouse CD19 Clone 1D3 (BD Biosciences, Franklin Lakes, NJ) for B cells and APC Hamster Anti-Mouse CD11c Clone HL3 (BD Biosciences) for DCs. Gating was done using Summit software. Cells were sorted using Moflo Astrios EQs cell sorter (Beckman Coulter, CA) and collected directly into 50 ml tubes containing 10 ml of staining medium.


