Abstract
Background:
Voxel-based morphometry (VBM) is an objective structural magnetic resonance imaging (MRI) technique which allows researchers to investigate group-level differences in regional gray matter (GM) volume or density over the whole brain. In the last decade, VBM studies in restless leg syndrome (RLS) have exhibited inconsistent and conflicting findings.
Methods:
Studies will be identified through a computerized literature search of the following databases: PubMed, Web of Science, and Embase until October 1, 2018 and updated on March 1, 2020. This protocol will be performed in accordance with the Preferred Reporting Items for Systematic review and Meta-Analysis Protocols (PRISMA-P). In addition, we will follow the recent guidelines and recommendations for coordinate-based meta-analysis (CBMA). This CBMA will be performed with the seed-based d mapping with permutation of subject images (SDM-PSI) software.
Results:
This CBMA will offer the latest evidence of GM alterations in RLS.
Conclusions:
To our knowledge, this will be the first CBMA that pooled VBM findings in RLS. This quantitative evidence of GM alterations will characterize brain morphometry of RLS.
PROSPERO registration number:
CRD42018117014.
Keywords: coordinate-based meta-analysis, gray matter, restless leg syndrome, seed-based d mapping, voxel-based morphometry
1. Introduction
Restless leg syndrome (RLS) is a common sensorimotor disorder characterized by a distressing urge to move the legs due to unpleasant sensations, usually occurring or worsening during rest or at bedtime.[1,2] RLS is of major clinical and public health significance due to its high prevalence, adverse effect on sleep and health-related quality of life, and a significant personal and social burden due to its increased risk of significant morbidity.[2–5] Although the exact pathogenesis of RLS remains to be elucidated, brain dopaminergic dysfunction and iron deficiency play critical roles.[6]
Voxel-based morphometry (VBM) is an objective structural magnetic resonance imaging (MRI) technique which allows researchers to investigate group-level differences in regional gray matter (GM) volume or density over the whole brain. In the last decade, VBM studies in RLS have exhibited inconsistent and conflicting findings. The discrepancies in the VBM studies might be attributed to the small sample sizes in each single study, differences in methodological protocols (from magnetic MRI acquisition to statistics), and/or heterogeneity of RLS populations. Prior qualitative reviews therefore proposed that there might be no GM alterations in RLS.[7] However, these VBM findings have not been quantitatively analyzed yet.
Coordinate-based meta-analysis (CBMA) is a powerful and invaluable approach to quantify voxel-based neuroimaging findings. In this study, we will use seed-based d mapping with permutation of subject images (SDM-PSI), to identify consistent and robust GM alterations in RLS.
2. Methods
2.1. Protocol and registration
This protocol will be performed in accordance with the Preferred Reporting Items for Systematic review and Meta-Analysis Protocols (PRISMA-P).[8] The protocol of this meta-analysis was registered at PROSPERO (http://www.crd.york.ac.uk/PROSPERO) (registration number: CRD42018117014).
2.2. Data sources and study selection
Studies will be identified through a computerized literature search of the following databases: PubMed, Web of Science, and Embase until October 1, 2018. The search keywords used were (“voxel-based morphometry” OR “vbm” OR “gray matter” OR “grey matter” OR “voxel∗”) AND (“Willis Ekbom Disease” OR “restless legs syndrome”). No restriction to the publication language was used for the search. Additional qualified articles were obtained from the reference lists of relevant studies and reviews. The final search was updated on March 1, 2020.
2.3. Eligibility criteria
Studies will be included if they: enrolled patients with idiopathic RLS patients according to the accepted criteria and matched healthy controls (HCs); employed VBM analysis to investigate GM volume or density differences between RLS patients and HCs; reported significant imaging results with 3-dimensional coordinates either in Montreal Neurological Institute (MNI) or Talairach stereotactic space or null findings; were published as peer-reviewed and original articles in English.
Studies will be excluded if: the sample size in either the RLS group or the HC group was fewer than 7 individuals[9]; peak coordinates of significant results could not be obtained from the published articles even the authors had been contacted; region of interest (ROI) analysis or small volume correction (SVC) analysis was applied; when the patient group was overlapped in multiple studies, only the study with the largest sample size was selected; publications were not original articles, such as conference abstracts, letters, case reports, research protocols, reviews, and editorials. Figure 1 presents the study selection process in accordance with the PRISMA flowchart.
Figure 1.
Study selection process in accordance with the PRISMA flowchart. PRISMA = Preferred Reporting Items of Systematic Review and Meta-Analysis.
2.4. Data collection and extraction
For each included study, we will extract the following variables: name of the first author, publication year, sample size, age, sex distribution, International Restless Legs Syndrome Study Group (IRLSSG) severity scale (IRLS) score, illness duration, MRI field strength, MRI sequence, voxel size, imaging processing software package, template, modulation, processing methods, modulation, smooth kernel, covariate, statistical threshold, peak coordinates (x, y, and z), corresponding t statistics (z value or P value), and their stereotactic reference space, were extracted according to a predefined and standardized data extraction form.
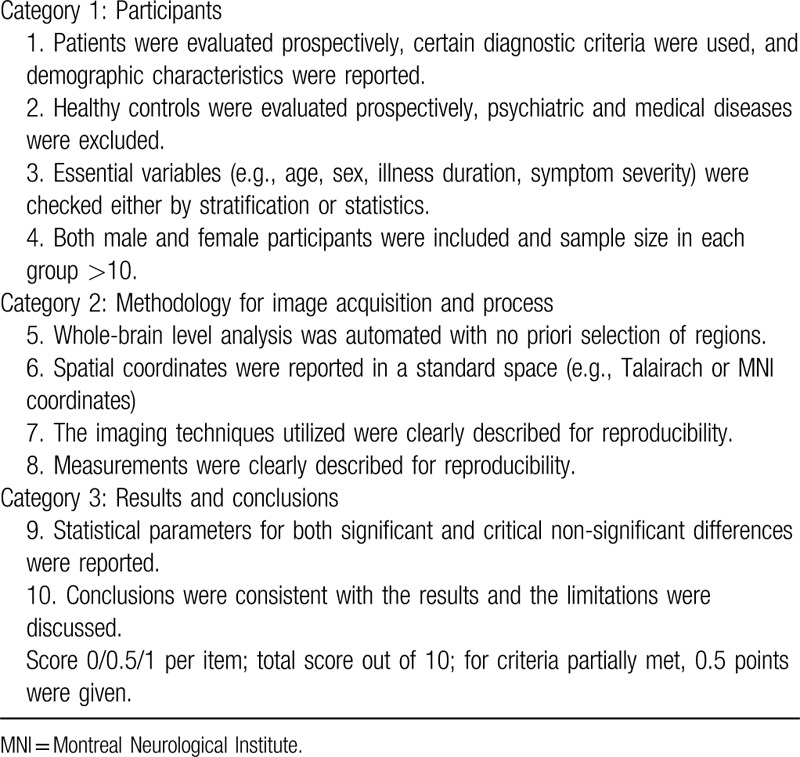
2.5. Study quality assessment
Study quality of each study included will be assessed with a 10-point checklist based on previous neuroimaging CBMA.[10] This checklist assesses aspects of clinical and demographic characteristics and imaging-specific methodology used in the studies (details in Table 1).
Table 1.
Quality evaluation checklists.
2.6. Data analysis
2.6.1. Voxel-wise CBMA
This CBMA will be performed with the SDM-PSI software (www.sdmproject.com). SDM-PSI has been described in detail elsewhere.[11,12] We briefly summarized the standard processes here. First, we collected and organized the information regarding the peak coordinates of significant GM differences between RLS and HCs. Second, the lower and upper bounds of possible effect size images were estimated within a GM mask. Third, effect sizes were analyzed using MetaNSUE based on multiple imputations algorithms.[13] Fourth, Rubin rules are used to voxel wisely combine the meta-analysis images from the different imputed datasets.[13] Finally, subject images were recreated in order to run a standard permutation test and the maximum statistic of the combined meta-analysis image is saved that the distribution of the maximum statistic is used to family-wise error-correct for multiple comparisons. The statistical threshold for this analysis was set to a corrected P < .05 (threshold-free cluster enhancement [TFCE]-based familywise error rate [FWER]) and voxels extent ≥10.
2.6.2. Sensitivity analysis
To test the reliability of the results, sensitivity analysis will be performed by iteratively repeating the analysis leaving out 1 dataset each time.[14,15]
2.6.3. Assessment of heterogeneity and potential publication bias
If there were significant results regarding consistent GM differences between RLS and HCs in the CBMA, we extracted the values from relevant peaks using PSI-SDM. Heterogeneity between studies was assessed with the I2 statistic using a random effects model. An I2 > 50% were regarded as indicators of heterogeneity. In addition, we applied funnel plots and Egger tests to assess the publication bias. An asymmetric plot and P-values <.05 were considered significant.
2.6.4. Meta-regression analysis
Meta-regression analysis will be carried out to examine the effects of potential confounds, such age, female percentage in the sample, IRLS score, and illness duration on GM alterations across studies if these variables were reported in >10 datasets. The statistical threshold for this analysis was set to a P < .05 (TFCE-based FWER corrected) and voxels extent ≥10.
2.6.5. Ethical principles and publication
No ethical approval is required because this coordinate-based meta-analysis will be performed based on published studies. The results of this review will be published in peer-reviewed journals.
3. Discussion
There is a debate regarding GM alterations in RLS. To our knowledge, this is the first CBMA that pooled VBM findings in RLS. Our CBMA will offer the quantitative evidence of GM alterations in RLS. The strength of this study is that this CMBA uses the latest technique, SDM-PSI, for the CBMA.[11,12] Compared with previous CBMA methods, such as the old versions of SDM, Activation Likelihood Estimation (ALE), and Multilevel Kernel Density Analysis (MKDA), SDM-PSI makes major improvements, such as applying a standard subject-based permutation test to control the FWER and use of unbiased estimation of effect sizes, random-effects models, Freedman-Lane-based permutations, and TFCE statistics.[11,12] One of the limitations of this study is that the CBMA is based on peak coordinates information, rather on statistical parametric maps, which may bias the results.
VBM is a popular technique to investigate differences in regional GM volume or density over the whole brain at the group-level. However, it has been suggested that many imaging and methodological factors may affect the results, such as imaging acquisition, preprocessing (realignment and segmentation), modulation, model definition, and statistical analysis.[10,16] We then will review all studies included to address these points.
Author contributions
Conceptualization: HaiCun Shi, ZhenYu Dai, PingLei Pan.
Data curation: LiQin Sheng, PanWen Zhao, HaiRong Ma.
Formal analysis: HaiRong Ma, LiQin Sheng.
Funding acquisition: PingLei Pan.
Investigation: LiQin Sheng, PanWen Zhao, Liang Qi.
Methodology: LiQin Sheng, PingLei Pan.
Project administration: LiQin Sheng, PingLei Pan.
Resources: PanWen Zhao, YuanYuan Shi.
Software: LiQin Sheng.
Supervision: HaiCun Shi, ZhenYu Dai, PingLei Pan.
Validation: PingLei Pan.
Visualization: YuanYuan Shi, LiQin Sheng.
Writing – original draft: LiQin Sheng, PanWen Zhao, HaiRong Ma.
Writing – review & editing: HaiCun Shi, JianGuo Zhong, ZhenYu Dai, PingLei Pan.
Footnotes
Abbreviations: CBMA = coordinate-based meta-analysis, FWHM = full width half maximum, GM = gray matter, HC = healthy control, IRLS = International Restless Legs Syndrome Study Group (IRLSSG) severity scale, IRLSSG = International Restless Legs Syndrome Study Group, MRI = magnetic resonance imaging, PRISMA = Preferred Reporting Items of Systematic Review and Meta-Analysis, RLS = restless leg syndrome, ROI = regions of interest, SDM-PSI = seed-based d mapping with permutation of subject images, SVC = small volume correction, TFCE = threshold-free cluster enhancement, VBM = voxel-based morphometry.
How to cite this article: Sheng L, Zhao P, Ma H, Qi L, Yi Z, Shi Y, Zhong J, Shi H, Dai Z, Pan P. Gray matter alterations in restless legs syndrome: a coordinate-based meta-analysis. Medicine. 2020;99:29(e21374).
HCS, ZYD, and PLP have contributed equally to this work.
This work was supported by the National Natural Science Foundation of China (81601161) and Jiangsu Commission of Health (LGY2018039, QNRC 2016466).
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Allen RP, Picchietti DL, Garcia-Borreguero D, et al. Restless legs syndrome/Willis-Ekbom disease diagnostic criteria: updated International Restless Legs Syndrome Study Group (IRLSSG) consensus criteria--history, rationale, description, and significance. Sleep Med 2014;15:860–73. [DOI] [PubMed] [Google Scholar]
- [2].Picchietti DL, Hensley JG, Bainbridge JL, et al. Consensus clinical practice guidelines for the diagnosis and treatment of restless legs syndrome/Willis-Ekbom disease during pregnancy and lactation. Sleep Med Rev 2015;22:64–77. [DOI] [PubMed] [Google Scholar]
- [3].Innes KE, Selfe TK, Agarwal P. Restless legs syndrome and conditions associated with metabolic dysregulation, sympathoadrenal dysfunction, and cardiovascular disease risk: a systematic review. Sleep Med Rev 2012;16:309–39. [DOI] [PubMed] [Google Scholar]
- [4].Ohayon MM, O’Hara R, Vitiello MV. Epidemiology of restless legs syndrome: a synthesis of the literature. Sleep Med Rev 2012;16:283–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Trenkwalder C, Allen R, Hogl B, et al. Restless legs syndrome associated with major diseases: a systematic review and new concept. Neurology 2016;86:1336–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Khan FH, Ahlberg CD, Chow CA, et al. Iron, dopamine, genetics, and hormones in the pathophysiology of restless legs syndrome. J Neurol 2017;264:1634–41. [DOI] [PubMed] [Google Scholar]
- [7].Rizzo G, Li X, Galantucci S, et al. Brain imaging and networks in restless legs syndrome. Sleep Med 2017;31:39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 2016;350:g7647. [DOI] [PubMed] [Google Scholar]
- [9].Tahmasian M, Sepehry AA, Samea F, et al. Practical recommendations to conduct a neuroimaging meta-analysis for neuropsychiatric disorders. Hum Brain Mapp 2019;40:5142–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Luo R, Pan P, Xu Y. No reliable gray matter changes in essential tremor. Neurol Sci 2019;40:2051–63. [DOI] [PubMed] [Google Scholar]
- [11].Albajes-Eizagirre A, Solanes A, Vieta E, et al. Voxel-based meta-analysis via permutation of subject images (PSI): theory and implementation for SDM. Neuroimage 2019;186:174–84. [DOI] [PubMed] [Google Scholar]
- [12].Albajes-Eizagirre A, Solanes A, Fullana MA, et al. Meta-analysis of voxel-based neuroimaging studies using seed-based d mapping with permutation of subject images (SDM-PSI). J Vis Exp 2019;1–7. [DOI] [PubMed] [Google Scholar]
- [13].Albajes-Eizagirre A, Solanes A, Radua J. Meta-analysis of non-statistically significant unreported effects. Stat Methods Med Res 2019;28:3741–54. [DOI] [PubMed] [Google Scholar]
- [14].Radua J, Mataix-Cols D. Voxel-wise meta-analysis of grey matter changes in obsessive-compulsive disorder. Br J Psychiatry 2009;195:393–402. [DOI] [PubMed] [Google Scholar]
- [15].Radua J, Rubia K, Canales-Rodriguez EJ, et al. Anisotropic kernels for coordinate-based meta-analyses of neuroimaging studies. Front Psychiatry 2014;5:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Celle S, Delon-Martin C, Roche F, et al. Desperately seeking grey matter volume changes in sleep apnea: a methodological review of magnetic resonance brain voxel-based morphometry studies. Sleep Med Rev 2016;25:112–20. [DOI] [PubMed] [Google Scholar]


