Abstract
Aims
This study aimed to use an umbrella review methodology to capture the range of outcomes that were associated with low‐dose aspirin and to systematically assess the credibility of this evidence.
Methods
Aspirin is associated with several health outcomes, but the overall benefit/risk balance related to aspirin use is unclear. We searched three major databases up to 15 August 2019 for meta‐analyses of observational studies and randomized controlled trials (RCTs) including low‐dose aspirin compared to placebo or other treatments. Based on random‐effects summary effect sizes, 95% prediction intervals, heterogeneity, small‐study effects and excess significance, significant meta‐analyses of observational studies were classified from convincing (class I) to weak (class IV). For meta‐analyses of RCTs, outcomes with random effects P‐value < .005 and a moderate/high GRADE assessment, were classified as strong evidence. From 6802 hits, 67 meta‐analyses (156 outcomes) were eligible.
Results
Observational data showed highly suggestive evidence for aspirin use and increased risk of upper gastrointestinal bleeding (RR = 2.28, 95% CI: 1.97–2.64). In RCTs of low‐dose aspirin, we observed strong evidence for lower risk of CVD in people without CVD (RR = 0.83; 95% CI: 0.79–0.87) and in general population (RR = 0.83; 95% CI: 0.79–0.89), higher risk of major gastrointestinal (RR = 1.47; 95% CI: 1.26–1.72) and intracranial bleeding (RR = 1.34; 95% CI: 1.18–1.53), and of major bleedings in people without CVD (RR = 1.62; 95% CI: 1.26–2.08).
Conclusion
Compared to other active medications, low‐dose aspirin had strong evidence for lower risk of bleeding, but also lower comparative efficacy. Low‐dose aspirin significantly lowers CVD risk and increases risk of bleeding. Evidence for multiple other health outcomes is limited.
Keywords: aspirin, cancer, cardiovascular disease, meta‐analysis, umbrella review
1. INTRODUCTION
Low‐dose aspirin, defined as less than 325 mg daily, is widely used worldwide, particularly for the prevention of cardiovascular disease (CVD). 1 The United States Preventive Services Task Force (USPSTF) recommends aspirin for primary CVD prevention in adults with a 10‐year risk of heart attack or stroke exceeding 10% in individuals who are not at increased risk of bleeding and after individualized informed decisions, 2 , 3 whilst other societies recommend low‐dose aspirin use for secondary CVD prevention only. 4 In the US, over 30% of adults take aspirin for CVD prevention, but use in recent years is probably decreasing. 4
Aspirin irreversibly inhibits cyclo‐oxygenase 1 (COX‐1) which leads to inhibition of platelet thromboxane A2 and thrombus formation in arteries. 5 Beyond CVD, low‐dose aspirin use has been linked to lower risk of cancers, overall mortality and other chronic conditions. 4 The veracity of these claimed non‐cardiovascular effects is unclear. European and American guidelines currently do not support aspirin for cancer prevention, 4 , 6 but the issue is unsettled. 7 , 8 However, despite possible benefits, low‐dose aspirin is also associated with an increased risk of bleeding 9 and any clinical benefits need to be balanced against adverse effects. At the same time, prescription of low‐dose aspirin in many primary care settings is suboptimal 10 , 11 and many patients who would probably benefit remain untreated. 12 Improving appropriate use of aspirin is therefore essential. 11 The body of research on low dose aspirin is constantly increasing with new studies and meta‐analyses thereof being published during the last few years. At the same time, the breadth of outcomes examined has expanded to cover a wide range of outcomes not limited to CVD. We used the umbrella review methodology in order to capture the breadth of outcomes reported and assess the totality of evidence of low dose aspirin on a number of outcomes. 13 In this sense, umbrella reviews (i.e. reviews of previously published systematic reviews/meta‐analyses consisting in the replication of the meta‐analyses following a uniform statistical approach for all factors to allow their comparison) have been created for overcoming the inherent limitations of meta‐analyses. 13
Here we aimed to capture the breadth of outcomes that have been associated with low‐dose aspirin intake and systematically assess the quality, strength and credibility of the associations. We used the umbrella review methodology to combine evidence from a wide range of outcomes and populations and we present results separately for observational studies and randomized controlled trials (RCTs).
2. METHODS
2.1. Data sources and searches
We conducted an umbrella review, 14 searching the MEDLINE, Scopus, Embase databases from inception until 15 August 2019 with: “(Meta‐Analysis[ptyp] OR metaanaly*[tiab] OR meta‐analy*[tiab] OR Systematic review [ptyp] OR “systematic review” [tiab]) AND (aspirin [tiab])”. In addition, we hand‐searched the reference lists of eligible articles.
2.2. Study selection
Eligible articles were systematic reviews with meta‐analyses of observational/intervention studies, which investigated low‐dose aspirin in relation to any clinical outcome. Four authors (J.D., G.P., T.B., S.C.) independently performed title and abstract screening in couples. Disagreements were resolved through consensus with another independent author (N.V.). Full texts of all potentially eligible articles were then retrieved by the same four authors and any disagreement was resolved with another independent author (M.S.).
We included meta‐analyses that investigated effects of low‐dose aspirin, defined as at least 75 mg and less than 325 mg daily 15 , 16 or use of the regular 325 mg aspirin dose three or more times a week (but not daily) for at least six months. 17 Both meta‐analyses of observational studies that investigated the association of low‐dose aspirin with any clinical outcome and meta‐analyses of RCTs were considered. Meta‐analyses were included only if they reported study‐specific information (i.e. effect size, 95% confidence intervals [CIs], sample size) or if those metrics could be inferred from the data presented. The RCT meta‐analyses were divided into meta‐analysis of placebo/no active control and active control groups (e.g. heparins, vitamin K antagonists). Studies were excluded if aspirin was accompanied by additional co‐administered medications (e.g. clopidogrel, heparins).
2.3. Data extraction
Four independent investigators (J.D., G.P., T.B., S.C.) extracted the following information for each meta‐analysis, independently, in pairs: first author name; publication year; number of studies; study population; type of effect size; study design; number of participants with (cases) and without (controls) events for each study. We also extracted the study‐specific estimated relative risk for health outcome (risk ratio, RR; odds ratio OR; hazard ratio, HR; mean difference, MD; standardized mean difference, SMD) and 95% CIs. We finally extracted the data for the Assessment of Multiple Systematic Reviews (AMSTAR)‐2 tool. 18
When more than one meta‐analysis on the same research question using the same study design (observational or RCTs) was identified, the one with the largest number of participants was selected.
2.4. Data synthesis and analysis
For each meta‐analysis, we estimated the summary effect size and its 95% CI by using the random‐effects Hartung‐Knapp‐Sidik‐Jonkman (HK) estimator. 19 This estimator consistently results in more adequate error rates than the DerSirmonian‐Leird method, especially when the number of studies is small. 19 We also estimated the prediction interval (PIs) and its 95% CI, which further accounts for between‐study effects and estimates the certainty of the association if a new study addresses that same association. 20 , 21 , 22 In order to estimate whether any large studies were available, for the largest study of each meta‐analysis, we calculated the standard error (SE) of the effect size. If the SE was less than 0.10, then the 95% CI would be lower than 0.20. Between‐study inconsistency was estimated with the I 2 metric, with values ≥50% indicative of high heterogeneity. 23
We calculated the evidence of small‐study effects (i.e. whether small studies inflated effect sizes) using the regression asymmetry test 24 with a P‐value < .10. 25
Finally, we applied the excess of significance test. 26 Because of the limited statistical power of this test, a lenient significance threshold (P < .10) was adopted. 27 We considered the effect size of the largest dataset and based on this we estimated the power of each constituent study with an algorithm using a non‐central t distribution. Excess significance for each meta‐analysis was considered whenever P < .10.
All statistical analyses were conducted in Stata, version 14.0 (StataCorp), and R, version 3.3.0 (R Foundation for Statistical Computing).
2.5. Grading the evidence
For observational studies, using the criteria mentioned above, significant associations (i.e. P < .05) were categorized into strong, highly suggestive, suggestive, or weak evidence, following a grading scheme that has already been applied in various fields, 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 as reported in Table 1. We assessed the methodological quality of the included meta‐analyses of observational studies using AMSTAR‐2, 18 , 36 which ranks the quality of a meta‐analysis from critically low to high according to 16 predefined items. For each association in the convincing or highly suggestive categories, we reassessed the evidence keeping only prospective observational studies in an attempt to address reverse causality and applying the credibility ceiling at 10%. However, application of the 10% credibility ceiling did not affect any class I associations. Finally, for each association in the convincing category, we reassessed the evidence taking in account the AMSTAR‐2 evaluation.
TABLE 1.
Credibility assessment criteria for meta‐analyses of observational studies
| Evidence classification | Criteria |
|---|---|
| Convincing (class I) |
Associations with P < .000001; >1,000 cases (or >20 000 participants for continuous outcomes) having the event of interest; the largest component study reporting a nominal statistically significant result (P < .05); a 95% PI that excluded the null; no large heterogeneity (I2 < 50%); no evidence of small‐study effect (P > .10); no excess significance bias (P > .10); Studies survived the 10% credibility ceiling test. |
| Highly suggestive (class II) |
Associations with P < .000001; >1000 cases (or >20 000 participants for continuous outcomes) having the event of interest; the largest component study reporting a statistically significant result (P < .05). |
| Suggestive (class III) |
Associations with P < .001; >1000 cases (or >20 000 participants for continuous outcomes) having the event of interest |
| Weak (class IV) | Remaining statistically significant associations with P < .05. |
Abbreviations: PI = prediction interval; RCT = randomized controlled trial.
Evidence from meta‐analyses of RCTs was assessed in terms of the significance of the summary effect, using a P‐value of <.005 as the threshold for statistical significance, as recently proposed. 37 , 38 We used stringent P‐values when evaluating the findings of RCTs in order to decrease the possibility of “false‐positives” (i.e. to claim that an effect is present when there is none in reality). 39 When the P‐value for the random effect was <.005, we evaluated the evidence using the GRADE (Grading of Recommendations, Assessment, Development and Evaluation) assessment. 40 Outcomes having a P‐value of <.005 and a moderate/high GRADE assessment were classified as strong evidence. We also considered 95% PIs (excluding the null or not), the presence of large heterogeneity (I 2 > 50%), small study effects (P > .10), and excess significance (P > .10) as possible indicators of heterogeneity and bias in the available evidence.
3. RESULTS
3.1. Literature review
Overall, we identified 6802 papers (Figure 1); 578 publications were selected as potentially eligible and 67 meta‐analyses (corresponding to 156 different outcomes) were finally included in this study (references in Supplementary Material ).
FIGURE 1.
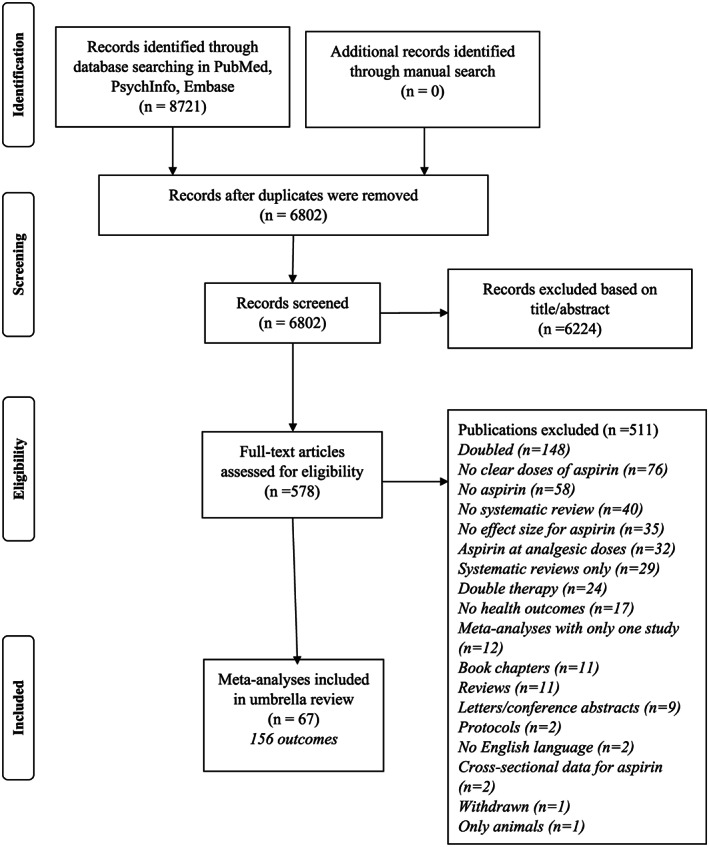
Flow chart
3.2. Meta‐analyses of observational studies
The median number of studies of meta‐analyses including observational studies for each outcome was three (range 2–32), the median number of participants was 11 894 (range 520 to 1059 682), and the median number of cases was 1114 (range 10 to 144 373) (Table S1).
The majority of the meta‐analyses included studies on general populations, followed by patients with cancer or diabetes. Overall, 11 out of the 41 outcomes reported nominally significant summary results (P < .05), but only two associations survived the application of the more stringent P‐value (P < 10−6), i.e. higher risk of upper gastrointestinal bleeding in general population and in people undergoing coronary artery bypass graft.
The study with the largest number of participants had an SE of less than 0.10 in 12 outcomes and a more conservative effect compared to the random‐effects model in 11 of these 12 outcomes. Heterogeneity among studies was modest and 24 outcomes presented low heterogeneity (I 2 < 50%). Three associations presented 95% PIs excluding the null value. Evidence for excess statistical significance was present in five out of 41 outcomes and small‐study effects were also seen in five out of 41 of the outcomes. Publication bias was present in six of the 41 outcomes.
Based on the above criteria, no outcome presented convincing evidence, only one outcome presented highly suggestive evidence (class II: higher incidence of upper gastrointestinal bleeding in the general population; RR = 2.28, 95% CI: 1.97–2.64), two outcomes presented suggestive evidence (class III: lower incidence of prostate cancer and cancer specific death in the general population) and eight outcomes weak evidence. Using the AMSTAR‐2, all the meta‐analyses included were evaluated as having a critically low rating mainly because the risk of bias was not accurately assessed and the sources of funding for the included studies were not reported (Table S2).
In a sensitivity analysis, we included only prospective cohort studies in each meta‐analysis (Table S3). Two outcomes presented suggestive evidence (lower cancer‐specific death in people affected by colorectal cancer and higher risk of upper gastrointestinal bleeding in the general population) and four were classified as weak evidence. Both outcomes with suggestive evidence had a low AMSTAR‐2 score.
3.3. Meta‐analyses of RCTs (vs placebo/no treatment)
The median number of RCTs meta‐analyses using placebo/no treatment for each outcome was five (range 2–23), the number of participants was, in median, 12 184 (71 to 1126 384), and the median number of cases was 377 (4 to 7087) (Table S4).
Overall, 76 outcomes were included. Of these, 25 reported significant results (P < .05), but only five survived the application of a more stringent P‐value (P < .005): lower risk of serious CVD in people without CVD (RR = 0.83; 95% CI: 0.79–0.87) and of CVD in the general population (RR = 0.83; 95% CI: 0.78–0.89), higher risk of major gastrointestinal (RR = 1.47; 95% CI: 1.26–1.72) and intracranial bleeding (RR = 1.34; 95% CI: 1.18–1.53) in the general population, and major bleeding in people without CVD at baseline (RR = 1.62; 95% CI: 1.26–2.08). Using the GRADE assessment, as reported in Table 2, we observed strong evidence for all the outcomes in primary prevention, having a P‐value of <.005, except for major bleeding in primary prevention (presence of publication bias).
TABLE 2.
GRADE evidence for randomized controlled trials comparing low dose aspirin vs placebo/no intervention
| Certainty assessment | Summary of findings | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of participants (studies) | Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | Overall certainty of evidence | Study event rates (%) | Relative effect (95% CI) | Anticipated absolute effects | |||
| With placebo/no intervention | With aspirin | Risk with placebo/no intervention | Risk difference with aspirin | Median follow‐up in years | ||||||||
| CVD events a in general population | ||||||||||||
| 113 204 (23 RCTs) | Not serious | Not serious | Not serious | Not serious | None | ⊕⊕⊕⊕high | 3836/56646 (6.8%) | 3243/56558 (5.7%) | RR 0.831 (0.779 to 0.885) | 68 per 1000 | 11 fewer per 1,000 (15 fewer to 8 fewer) | 5z |
| Major bleedings in people without CVD | ||||||||||||
| 98 311 (10 RCTs) | Not serious | Not serious | Not serious | Not serious | None | ⊕⊕⊕⊕high | 285/49157 (0.6%) | 456/49154 (0.9%) | RR 1.618 (1.258 to 2.080) | 6 per 1000 | 4 more per 1,000 (2 more to 6 more) | 4.5 |
| Intracranial bleeding (primary prevention) | ||||||||||||
| 160 404 (11 RCTs) | Not serious | Not serious | Not serious | Not serious | None | ⊕⊕⊕⊕high | 256/79419 (0.3%) | 349/80985 (0.4%) | RR 1.341 (1.178 to 1.528) | 3 per 1000 | 1 more per 1,000 (1 more to 2 more) | 6 |
| Major gastrointestinal bleeding (primary prevention) | ||||||||||||
| 140 792 (10 RCTs) | Not serious | Not serious | Not serious | Not serious | None | ⊕⊕⊕⊕high | 308/70456 (0.4%) | 593/70336 (0.8%) | RR 1.557 (1.355 to 1.790) | 4 per 1000 | 2 more per 1,000 (2 more to 3 more) | 6 |
| Major bleeding (primary prevention) | ||||||||||||
| 157 248 (10 RCTs) | Not serious | Not serious | Not serious | Not serious | Publication bias strongly suspected a | ⊕⊕⊕⊕◯ moderate | 901/77835 (1.2%) | 1301/79413 (1.6%) | RR 1.474 (1.263 to 1.721) | 12 per 1000 | 5 more per 1,000 (3 more to 8 more) | 6 |
| Serious CVD in people without CVD at the baseline | ||||||||||||
| 107 074 (11 RCTs) | Not serious | Not serious | Not serious | Not serious | None | ⊕⊕⊕⊕high | 2505/52587 (4.8%) | 2392/54487 (4.4%) | RR 0.831 (0.791 to 0.872) | 48 per 1000 | 8 fewer per 1,000 (10 fewer to 6 fewer) | 5 |
Abbreviations: CABG: coronary artery disease bypass graft; CI: confidence interval; CVD: cardiovascular disease; MD: mean difference; OR: odds ratio; RCT: randomized controlled trial; RR: risk ratio.
CVD events included coronary artery disease events, coronary artery disease mortality, stroke, serious vascular events, serious vascular events mortality.
The largest study, in terms of sample size, had an SE of less than 0.10 in only 15 outcomes. Heterogeneity among studies was low in 60 out of 76 studies, with 40 reporting an I 2 of 0%. Nine outcomes presented 95% PIs excluding the null value. Finally, evidence for excess statistical significance was present in five out of 74 outcomes and small‐study effects were present in six out of 76 outcomes.
As reported in Table S5, only two out of 76 rated “high”, six rated “low” according to the AMSTAR‐2 criteria, whilst the other meta‐analyses were rated as “critically low”.
3.4. Meta‐analyses of RCTs (vs active controls)
As reported in Table S6, the median number of studies of meta‐analyses including intervention studies using active controls for each outcome was three (range 2–15), the median number of participants was 3607 (193 to 33 435), and the median number of cases was 121 (4 to 1364).
In these meta‐analyses, 16 (41%) out of the 39 outcomes reported nominally significant summary results (P < .05), and, of them, two treatment effects had a summary effect with a P‐value of <.005 (Table 3). Using the GRADE assessment, we observed strong evidence for associations between aspirin use and higher risk of subarachnoid bleeding in cerebrovascular conditions (compared to cilostazol) and higher incidence of pulmonary embolism in cancer under chemotherapy (compared to heparins). Three meta‐analyses were rated as low quality according to the criteria suggested by the AMSTAR‐2, and the others were rated as critically low (Table S7).
TABLE 3.
GRADE evidence for randomized controlled trials comparing low dose aspirin vs. active controls
| Certainty assessment | Summary of findings | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of participants (studies) follow‐up | Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | Overall certainty of evidence | Study event rates (%) | Relative effect (95% CI) | Anticipated absolute effects | |||
| With active control | With aspirin | Risk with active control | Risk difference with aspirin | Median follow‐up in years | ||||||||
| Subarachnoid bleeding in stroke or TIA: aspirin vs cilostazol | ||||||||||||
| 2740 (2 RCTs) | Not serious | Not serious | Not serious | Not serious | Publication bias strongly suspected strong association a | ⊕⊕⊕⊕ high | 10/1371 (0.7%) | 32/1369 (2.3%) | RR 3.121 (2.885–3.376) | 7 per 1000 | 15 more per 1000 (4 more to 38 more) | 1.7 |
| Pulmonary embolism in cancer under chemotherapy: aspirin vs LMWH | ||||||||||||
| 781 (2 RCTs) | Not serious | Not serious | Not serious | Not serious | Publication bias strongly suspected very strong association a | ⊕⊕⊕⊕ high | 1/385 (0.3%) | 7/396 (1.8%) | RR 8.488 (1.653 to 43.59) | 3 per 1000 | 19 more per 1000 (3 more to 81 more) | 1.2 |
Abbreviations: CI: confidence interval; DAPT: dual antiplatelet therapy; LMWH: low molecular weight heparins; OR: odds ratio; RCT: randomized controlled trial; RR: risk ratio; TIA: transient ischaemic attack; VKA: vitamin K antagonists.
Few studies
Heterogeneity among studies was low, with the majority of outcomes (32 out of 39) (82%) having an I 2 < 50%. However, nine out of 39 outcomes (23%) presented summary effects with 95% PIs excluding the null value. Only one study showed evidence of excess significance, whilst no outcomes showed evidence for statistically significant small‐study effects. No meta‐analysis showed evidence of publication bias.
3.5. Comparison of findings from observational studies and clinical trials
As reported in Table S8, ten outcomes were examined by both meta‐analyses of observational studies and meta‐analyses of RCTs using placebo/no intervention as controls. The direction of the association/effect was concordant for seven of the 10 outcomes. In one case (stroke in patients with type 2 diabetes), the 95% CIs of RCTs excluded the null from the estimated effect size, but they were in the opposite direction, while in the other nine topics, the 95% CIs overlapped.
4. DISCUSSION
With this work, we provide a comprehensive overview of the associations between low‐dose aspirin and a wide range of health outcomes.
In a large epidemiological study, it is reported that about one third of American people take low‐dose aspirin for primary and secondary prevention, even if the prevalence of people taking aspirin has been declining in the last years. 4 In a more recent study, a consistent proportion of American people were taking aspirin without a physician's recommendation, corresponding to about 6.6 million adults. Nearly half of people at least 70 years of age in the survey, 44.6%, were on aspirin for primary CVD prevention. 41 Therefore, systematic knowledge of the efficacy and risk of low‐dose aspirin use is of great clinical importance.
The topic of the use of low‐dose aspirin in primary prevention is of great interest. Our umbrella review found that for primary prevention, use of low‐dose aspirin was associated with 17% lower CVD incidence (including serious events, i.e. non‐fatal myocardial infarction, non‐fatal stroke or vascular death). Moreover, low‐dose aspirin was associated with 34% higher risk of bleeding in primary prevention (major and intracranial). These risks and benefits need to be weighted in formal decision analysis to guide aspirin use in primary prevention. Taken together, these findings suggest that in the balance between prevention and risk one should consider the risk of bleedings. Three recent RCTs were published last year. 42 , 43 , 44 The ARRIVE (Aspirin to Reduce the Risk of Initial Vascular Events) trial enrolled participants at high risk for CVD events without diabetes. 42 The results of this trial suggested no significant effect of low‐dose aspirin on the reduction of CVD incidence, but a significantly increased risk of gastrointestinal bleedings. 42 Another recent randomized controlled trial, the ASCEND study (A Study of Cardiovascular Events in Diabetes), documented a significant benefit of aspirin among people with diabetes in preventing CVD events (of about 12%), but at the cost of a significant increase in the rate of major bleeding events, 43 in a manner similar to our findings. In a third randomized controlled trial, data from ASPREE (Aspirin in Reducing Events in the Elderly) showed no benefit of aspirin on CVD events in older people and also demonstrated a significantly increased risk of major bleeding in those taking aspirin in this older age group. 44 Taken together these findings and those from the present umbrella review suggest that the benefits and risks of low‐dose aspirin for the primary prevention of CVD events in the modern era of preventive management in middle‐aged people (i.e., involving statins, anti‐hypertension medications, smoking cessation, obesity management and other similar interventions) are closely balanced, calling into question the use of aspirin in those without a prior cardiovascular disease event.
A topic of great clinical relevance in clinical settings is low‐dose aspirin in primary prevention specifically for those at high CVD risk, such as people with diabetes. Observational and intervention studies in this review show little evidence that low‐dose aspirin prevents overall and specific CVD events in diabetes (Table S1), indicating that the widespread use of this medication may not be justified in this population. 45 As shown in Table S9, these findings can be applied in other conditions at higher risk of CVD such as women with antiphospholipid antibodies. Unfortunately, we were unable to take the same conditions into consideration for secondary prevention due to the limited data available.
The prevention of cancer is another topic of interest. 46 In our umbrella review, we identified several meta‐analyses, including observational studies, which investigated the effect of aspirin on risk of cancer/cancer progression/cancer‐specific death. Low‐dose aspirin was associated with a reduced risk of prostate cancer with suggestive evidence in observational studies, whilst the evidence regarding mortality in colorectal cancer patients was weak. The USPSTF guidelines suggested that low‐dose aspirin is efficacious in reducing the incidence of colorectal cancer, even if this benefit is not apparent until 10 years after aspirin therapy is started. 46 However, this work was not eligible for our umbrella review, since, among four eligible studies, two used doses of aspirin ≥325 mg and another one used a vitamin supplementation together with aspirin. 46 Other guidelines specifically from cancer‐related societies suggest that low‐dose aspirin should not be used in the general population, but only in some specific conditions, such as Lynch's syndrome. 47 In meta‐analyses of the RCTs (vs placebo/no intervention), low‐dose aspirin was associated with a nominally statistically significant reduction in cancer‐specific death in people affected by cancer at baseline or in the general population, but this evidence remains poor.
As reported in Table S9, we found, in observational studies, highly suggestive evidence or at least suggestive evidence that low‐dose aspirin is associated with a higher risk of upper and overall gastrointestinal bleeding in the general population, i.e. in the primary prevention setting. The risk of these events is more than doubled compared to non‐users, 48 an observation confirmed in the RCTs vs placebo/no intervention. However, the risk of bleeding (overall, major, gastrointestinal) was lower in people taking low‐dose aspirin compared to several other medications, including clopidogrel.
Our study has some shortcomings that we should acknowledge. First, we used evidence assessment criteria, which can be biased, as they are based on already established tools for observation and interventional studies. 33 , 49 Moreover, since the meta‐analyses included studies with significant differences in design, population and other basic characteristics, large heterogeneity may be worrisome. We consequently used an I 2 of 50% as one of the criteria for having convincing outcomes. However, I 2 estimates can also carry substantial uncertainty 50 and often clinical heterogeneity might be of importance, even in the absence of statistical heterogeneity. It is known that meta‐analyses have important limitations 51 and their results may also depend on choices made about what estimates to select from each study and how to report them in the meta‐analysis (e.g. in our umbrella review several meta‐analyses did not report information regarding aspirin dosage). 52 Applying the criteria suggested by the AMSTAR‐2 for evaluating the quality of meta‐analyses, we observed the presence of low/critically low rating. This evidence is mainly due to missing information in items 2 (protocol published before the meta‐analysis), 7 (list of excluded studies), or 11 (no appropriate meta‐analytic approach, particularly the absence of investigation in case of high heterogeneity). Furthermore, low‐dose aspirin covers a substantial range of dosing regimens and these may not have exactly the same efficacy and harms, but this was beyond the discerning ability of our study design. 53 The umbrella review was limited to outcomes studied in the respective meta‐analyses and does not provide in‐depth data on disease severity, dose–response effects, or specific subgroups such as by sex or age. At the same time, it is also possible that some studies were included in two or more outcomes (e.g. in intracranial and major bleedings) in major bleeding that is a cumulative outcome: however, we believe that this includes a limited portion of the studies included, over 156 outcomes. We decided to include the data from observational studies that are, per se, biased in their nature. As a number of outcomes (34/41) were only examined in observational settings, we included in this review data from observational studies acknowledging their limitations. However, a large majority of the outcomes included in the observational studies (34/41) were not included in those of RCTs, highlighting the importance of their inclusion. Finally, this umbrella review could not explore fully the possibility of risk stratification for clinical use, especially taking into account potential risk factors for adverse outcomes.
In conclusion, in this umbrella review including 67 independent meta‐analyses and 156 outcomes, we found that low‐dose aspirin decreased the risk of CVD events in the general population (when compared to placebo/no intervention) with strong evidence according to GRADE criteria, whilst the data for individual CVD outcomes are limited. Moreover, when limiting to only observational studies, moderate evidence for associations between aspirin intake and lower risk of specific cancers in the general population was observed. However, this finding should be interpreted with caution given the inherent bias of observational study designs. The risk of bleeding (particularly gastrointestinal and intracranial) is, however, also strong and substantial, suggesting that physicians should accurately consider the risks and benefits of prescribing aspirin. Despite many dozens of other clinical outcomes having been assessed, evidence for them remains weak and therefore should not be a major determinant in decision‐making regarding use of low‐dose aspirin.
COMPETING INTERESTS
Dr Demurtas received an honorary consultancy from Bayer; Dr Stubbs is supported by Health Education England and the National Institute for Health Research HEE/NIHR ICA Programme Clinical Lectureship (ICA‐CL‐2017‐03‐001), and is part supported by the National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care South London at King's College Hospital NHS Foundation Trust. The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health and Social Care; Dr Firth is supported by a Blackmores Institute Fellowship; Dr Koyanagi's work was supported by the Miguel Servet contract financed by the CP13/00150 and PI15/00862 projects, integrated into the National R+D+I and funded by the ISCIII – General Branch Evaluation and Promotion of Health Research – and the European Regional Development Fund (ERDF‐FEDER); Dr Theodoratou is supported by a Cancer Research UK (CRUK) Career Development Fellowship (C31250/A22804). The other authors don't have any financial arrangements, organizational affiliations or other relationships that might give rise to any conflict of interest regarding the subject matter of the manuscript submitted.
CONTRIBUTORS
N.V. and I.T. conceived the study. N.V., M.S. and A.K. did the statistical analyses. N.V., M.S., A.K., A.P. and B.S. wrote the first draft of the paper; B.S., N.V., J.D., G.P., S.C. and T.B. conducted the searches, carried out screening and data extraction, and contributed in writing the paper; B.S. and S.M. contributed to data interpretation; T.T., B.S., E.T., G.O., A.V., J.F., L.S. and J.P.A.I. contributed towards the intellectual conception of the review, and revised the manuscript; I.T. supervised the study and contributed in writing the paper and interpreting the findings.
Supporting information
Table S1. Health outcomes and evidence class reported in included meta‐analyses of observational studies
Table S2: AMSTAR‐2 quality assessment of meta‐analyses of observational studies
Table S3. Health outcomes and evidence class reported in included meta‐analyses of only cohort studies
Table S4. Health outcomes and evidence class reported in included meta‐analyses of randomized controlled trials, with placebo/no treatment as controls
Table S5: AMSTAR‐2 quality assessment of meta‐analyses of RCT with placebo/no treatment as controls
Table S6. Health outcomes and evidence class reported in included meta‐analyses of randomized controlled trials versus active controls
Table S7: AMSTAR‐2 quality assessment of meta‐analyses of RCT with active controls
Table S8. Overlap between meta‐analyses of observational studies and low dose aspirin randomized controlled trials
Table S9. Summary of evidence grading for meta‐analyses including observational studies and randomized controlled trials
Table S10. References of the included meta‐analyses
Veronese N, Demurtas J, Thompson T, et al. Effect of low‐dose aspirin on health outcomes: An umbrella review of systematic reviews and meta‐analyses. Br J Clin Pharmacol. 2020;86:1465–1475. 10.1111/bcp.14310
Nicola Veronese, Jacopo Demurtas and Trevor Thompson equally contributed to the work.
REFERENCES
- 1. Kim C, Beckles GL. Cardiovascular disease risk reduction in the behavioral risk factor surveillance system. Am J Prev Med. 2004;27(1):1‐7. [DOI] [PubMed] [Google Scholar]
- 2. Ittaman SV, VanWormer JJ, Rezkalla SH. The role of aspirin in the prevention of cardiovascular disease. Clin Med Res. 2014;12(3‐4):147‐154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arnett DK, Blumenthal RS, Albert MA, et al. ACC/AHA Guideline on the primary prevention of cardiovascular disease. J Am Coll Cardiol. 2019;2019:26029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stuntz M, Bernstein B. Recent trends in the prevalence of low‐dose aspirin use for primary and secondary prevention of cardiovascular disease in the United States, 2012–2015. Prev Med Rep. 2017;5:183‐186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bartolucci AA, Tendera M, Howard G. Meta‐analysis of multiple primary prevention trials of cardiovascular events using aspirin. Am J Cardiol. 2011;107(12):1796‐1801. [DOI] [PubMed] [Google Scholar]
- 6. Piepoli MF, Hoes AW, Agewall S, et al. 2016 European guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of 10 societies and by invited experts) developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J. 2016;37(29):2315‐2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Patrignani P, Patrono C. Aspirin and cancer. J Am Coll Cardiol. 2016;68(9):967‐976. [DOI] [PubMed] [Google Scholar]
- 8. Sutcliffe P, Connock M, Gurung T, et al. Aspirin for prophylactic use in the primary prevention of cardiovascular disease and cancer: a systematic review and overview of reviews. Health Technol Assess (Winch Eng). 2013;17(43):1‐253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cryer B. Gastrointestinal safety of low‐dose aspirin. Am J Manag Care. 2002;8:S701‐S708. [PubMed] [Google Scholar]
- 10. McCallum AK, Whincup PH, Morris RW, Thomson A, Walker M, Ebrahim S. Aspirin use in middle‐aged men with cardiovascular disease: are opportunities being missed? Br J Gen Pract. 1997;47(420):417‐421. [PMC free article] [PubMed] [Google Scholar]
- 11. Short D, Frischer M, Bashford J, Ashcroft D. Why are eligible patients not prescribed aspirin in primary care? A qualitative study indicating measures for improvement. BMC Fam Pract. 2003;4:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sweeney K. How can evidence‐based medicine help patients in general practice? Fam Pract. 1996;13(6):489‐490. [DOI] [PubMed] [Google Scholar]
- 13. Fusar‐Poli P, Radua J. Ten simple rules for conducting umbrella reviews. Evid Based Ment Health. 2018;21:95‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ioannidis JP. Integration of evidence from multiple meta‐analyses: a primer on umbrella reviews, treatment networks and multiple treatments meta‐analyses. CMAJ. 2009;181(8):488‐493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lloyd J, Bochner F. Aspirin: how low is low dose? Australian Prescriber. 1996;19:79‐81. [Google Scholar]
- 16. Goodman LS. Goodman and Gilman's the pharmacological basis of therapeutics. New York: McGraw‐Hill; 1996. [Google Scholar]
- 17. Sostres C, Lanas A. Epidemiology of low dose aspirin damage in the lower gastrointestinal tract. Curr Pharm Des. 2015;21:5094‐5100. [DOI] [PubMed] [Google Scholar]
- 18. Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non‐randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. IntHout J, Ioannidis JP, Borm GF. The Hartung‐Knapp‐Sidik‐Jonkman method for random effects meta‐analysis is straightforward and considerably outperforms the standard DerSimonian‐Laird method. BMC Med Res Methodol. 2014;14:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. IntHout J, Ioannidis JPA, Rovers MM, Goeman JJ. Plea for routinely presenting prediction intervals in meta‐analysis. BMJ Open. 2016;6(7):e010247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Higgins JP, Thompson SG, Spiegelhalter DJ. A re‐evaluation of random‐effects meta‐analysis. J R Stat Soc Ser A Stat Soc. 2009;172:137‐159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Serghiou S, Goodman SN. Random‐effects meta‐analysis: summarizing evidence with caveats. JAMA. 2018;321(3):301‐302. [DOI] [PubMed] [Google Scholar]
- 23. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med. 2002;21(11):1539‐1558. [DOI] [PubMed] [Google Scholar]
- 24. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629‐634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Carvalho AF, Kohler CA, Brunoni AR, et al. Bias in peripheral depression biomarkers. Psychother Psychosom. 2016;85(2):81‐90. [DOI] [PubMed] [Google Scholar]
- 26. Ioannidis JP, Trikalinos TA. An exploratory test for an excess of significant findings. Clin Trials. 2007;4(3):245‐253. [DOI] [PubMed] [Google Scholar]
- 27. Ioannidis JP. Clarifications on the application and interpretation of the test for excess significance and its extensions. J Math Psychol. 2013;57:184‐187. [Google Scholar]
- 28. Aromataris E, Fernandez R, Godfrey CM, Holly C, Khalil H, Tungpunkom P. Summarizing systematic reviews: methodological development, conduct and reporting of an umbrella review approach. Int J Evid Based Healthc. 2015;13(3):132‐140. [DOI] [PubMed] [Google Scholar]
- 29. Belbasis L, Savvidou MD, Kanu C, Evangelou E, Tzoulaki I. Birth weight in relation to health and disease in later life: an umbrella review of systematic reviews and meta‐analyses. BMC Med. 2016;14:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bellou V, Belbasis L, Tzoulaki I, Evangelou E, Ioannidis JP. Environmental risk factors and Parkinson's disease: an umbrella review of meta‐analyses. Parkinsonism Relat Disord. 2016;23:1‐9. [DOI] [PubMed] [Google Scholar]
- 31. Dinu M, Pagliai G, Casini A, Sofi F. Mediterranean diet and multiple health outcomes: an umbrella review of meta‐analyses of observational studies and randomized trials. Nutr Metab Cardiovasc Dis. 2017;27(1):e21. [DOI] [PubMed] [Google Scholar]
- 32. Kyrgiou M, Kalliala I, Markozannes G, et al. Adiposity and cancer at major anatomical sites: umbrella review of the literature. BMJ. 2017;356:j477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li X, Meng X, Timofeeva M, et al. Serum uric acid levels and multiple health outcomes: umbrella review of evidence from observational studies, randomised controlled trials, and Mendelian randomisation studies. BMJ. 2017;357:j2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Theodoratou E, Tzoulaki I, Zgaga L, Ioannidis JPA. Vitamin D and multiple health outcomes: umbrella review of systematic reviews and meta‐analyses of observational studies and randomised trials. BMJ. 2014;348:g2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Veronese N, Solmi M, Caruso MG, et al. Dietary fiber and health outcomes: an umbrella review of systematic reviews and meta‐analyses. Am J Clin Nutr. 2018;107(3):436‐444. [DOI] [PubMed] [Google Scholar]
- 36. Towheed TE, Hochberg MC, Shea BJ, Wells G. WITHDRAWN: analgesia and non‐aspirin, non‐steroidal anti‐inflammatory drugs for osteoarthritis of the hip. Cochrane Database Syst Rev. 2007;(1):CD000517. [DOI] [PubMed] [Google Scholar]
- 37. Ioannidis JA. The proposal to lower p value thresholds to .005. JAMA. 2018;319(14):1429‐1430. [DOI] [PubMed] [Google Scholar]
- 38. Benjamin DJ, Berger JO, Johannesson M, et al. Redefine statistical significance. Nat Hum Behav. 2018;2(1):6‐10. [DOI] [PubMed] [Google Scholar]
- 39. Ioannidis JP. Publishing research with P‐values: prescribe more stringent statistical significance or proscribe statistical significance? Eur Heart J. 2019;40:2553. [DOI] [PubMed] [Google Scholar]
- 40. Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. O'Brien CW, Juraschek SP, Wee CC. Prevalence of aspirin use for primary prevention of cardiovascular disease in the United States: results from the 2017 National Health Interview Survey. Ann Intern Med. 2019;171(8):596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gaziano JM, Brotons C, Coppolecchia R, et al. Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): a randomised, double‐blind, placebo‐controlled trial. The Lancet. 2018;392:1036‐1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Group ASC . Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med. 2018;379:1529‐1539. [DOI] [PubMed] [Google Scholar]
- 44. McNeil JJ, Nelson MR, Woods RL, et al. Effect of aspirin on all‐cause mortality in the healthy elderly. N Engl J Med. 2018;379:1519‐1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Simpson SH, Gamble JM, Mereu L, Chambers T. Effect of aspirin dose on mortality and cardiovascular events in people with diabetes: a meta‐analysis. J Gen Intern Med. 2011;26:1336‐1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Karmali KN, Huffman MD. I do not have heart disease—should I be taking aspirin? JAMA Cardiol. 2017;2:824. [DOI] [PubMed] [Google Scholar]
- 47. Lok P, Dijk S. Offer daily aspirin to cut risk of colorectal cancer in people with Lynch syndrome, says NICE. BMJ (Online). 2019;366:l5010. [DOI] [PubMed] [Google Scholar]
- 48. Garcia Rodriguez LA, Martin‐Perez M, Hennekens CH, Rothwell PM, Lanas A. Bleeding risk with long‐term low‐dose aspirin: a systematic review of observational studies. PLoS ONE. 2016;11:e0160046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ioannidis JP, Trikalinos TA. The appropriateness of asymmetry tests for publication bias in meta‐analyses: a large survey. CMAJ. 2007;176(8):1091‐1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ioannidis JP, Patsopoulos NA, Rothstein HR. Reasons or excuses for avoiding meta‐analysis in forest plots. BMJ. 2008;336(7658):1413‐1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ioannidis JP. The mass production of redundant, misleading, and conflicted systematic reviews and meta‐analyses. Milbank Q. 2016;94(3):485‐514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kavvoura FK, Liberopoulos G, Ioannidis JP. Selection in reported epidemiological risks: an empirical assessment. PLoS Med. 2007;4:e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Fisher M, Knappertz V. The dose of aspirin for the prevention of cardiovascular and cerebrovascular events. Curr Med Res Opin. 2006;22(7):1239‐1248. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Health outcomes and evidence class reported in included meta‐analyses of observational studies
Table S2: AMSTAR‐2 quality assessment of meta‐analyses of observational studies
Table S3. Health outcomes and evidence class reported in included meta‐analyses of only cohort studies
Table S4. Health outcomes and evidence class reported in included meta‐analyses of randomized controlled trials, with placebo/no treatment as controls
Table S5: AMSTAR‐2 quality assessment of meta‐analyses of RCT with placebo/no treatment as controls
Table S6. Health outcomes and evidence class reported in included meta‐analyses of randomized controlled trials versus active controls
Table S7: AMSTAR‐2 quality assessment of meta‐analyses of RCT with active controls
Table S8. Overlap between meta‐analyses of observational studies and low dose aspirin randomized controlled trials
Table S9. Summary of evidence grading for meta‐analyses including observational studies and randomized controlled trials
Table S10. References of the included meta‐analyses


