Abstract
Oral administration is a pillar of the pharmaceutical industry and yet it remains challenging to administer hydrophilic therapeutics by the oral route. Smart and controlled oral drug delivery could bypass the physiological barriers that limit the oral delivery of these therapeutics. Micro- and nanoscale technologies, with an unprecedented ability to create, control, and measure micro- or nanoenvironments, have found tremendous applications in biology and medicine. In particular, significant advances have been made in using these technologies for oral drug delivery. In this review, we briefly describe biological barriers to oral drug delivery and micro and nanoscale fabrication technologies. Micro and nanoscale drug carriers fabricated using these technologies, including bioadhesives, microparticles, micropatches, and nanoparticles, are described. Other applications of micro and nanoscale technologies are discussed, including fabrication of devices and tissue engineering models to precisely control or assess oral drug delivery in vivo and in vitro, respectively. Strategies to advance translation of micro and nanotechnologies into clinical trials for oral drug delivery are mentioned. Finally, challenges and future prospects on further integration of micro and nanoscale technologies with oral drug delivery systems are highlighted.
Keywords: Drug delivery devices, Micro and nanocarriers, Micro and nanoscale technologies, Oral drug delivery, Tissue models
Graphical abstract
1. Introduction
Oral delivery has been one of the most commonly used approaches for drug administration in the body due to its high patient compliance, low cost, non-invasiveness, and ease of use [1,2]. A multitude of therapeutic compounds, including synthetic small molecules and biologics, have been administered orally. However, oral drug delivery poses significant challenges to achieving efficient therapeutic outcomes. This is primarily due to the multitude of biological barriers that are present throughout the gastrointestinal (GI) tract that a drug carrier must navigate through. Some of these biological barriers to drug delivery include harsh acidic pH environments in the stomach, degrading enzymes that render drugs ineffective, the inefficient penetration of drugs across GI tissue barriers and into systemic circulation, and the eventual clearance of drugs through the GI tract, which may occur prior to drug release. To overcome these barriers, the delivered dosage of drugs is often higher than what is needed therapeutically, as the bioavailability of the compound is often reduced due to factors like enzymatic degradation and poor permeation through the intestinal wall. However, it is important that the drug concentration should not exceed the level that can cause toxicity in the body, as has been observed with some DNA and protein based drugs above critical concentrations [3,4]. On the other hand, if a drug at a non-toxic concentration level passes the physiological barriers of the GI tract, its delivered dosage to target site may not be effective [5].
An ideal oral drug delivery system has yet to be realized, by which drugs can be delivered to a biological target at appropriate concentrations with tunable dosage windows. Several limitations of delivery of drugs through the oral cavity are due to physiochemical properties of drugs. In particular, Lipinski rules or the Rule of Five (ROF) are used for discovering new drugs and to improve the efficacy of developed drugs [6]. The ROF details four properties of drug molecules including: that the molecular weight should not exceed 500 Da, logP values should be under 5, total number of hydrogen bond donors should be 5, and number of the hydrogen acceptors should not be more than 10. The total ROF score lies between 0 and 4. Molecules with an ROF score of more than 4 are considered to be marginal drug molecules and need to have further development. However, some drug molecules do not follow the Lipinski rules, such as proteins and RNA molecules. One of the most well-studied protein therapeutics for oral administration is insulin. Currently, insulin is administered via daily injections and 45-60% of diabetic patients intentionally skip insulin doses out of dread of injections [7]. It is therefore likely that oral administration of insulin would increase patient compliance and improve therapeutic outcomes in diabetic patients. However, the bioavailability of orally administered insulin is severely limited by the physiological barriers of the GI tract, such as acidic pH, presence of proteases, and the limited transport of insulin across GI epithelial barriers into the bloodstream [8].
By incorporating drugs such as insulin into materials-based carriers, it may be possible to overcome or circumvent the physiological barriers that limit oral administration efficacies. In designing materials for oral drug delivery, the drug carrier should preserve therapeutic efficacy of the drug cargo for effective use in humans. There are two major goals for designing materials for oral drug delivery: (1) the effective targeting of drugs to a GI section of interest, and (2) the release of drugs from the GI into the bloodstream for systemic circulation. For both of these goals, the design and development of drug delivering materials need to account for the mucosal microenvironment of the GI system, intestinal physiology, and target diseases. Moreover, chemistry, size, shape, metabolism, and bioavailability of drugs play a crucial role in the design of effective oral drug delivery systems.
Micro and nanotechnologies have seen widespread use in oral drug delivery systems with the goal of improving the efficiency of delivery systems. These technologies have been used for many applications, including drug discovery via development of high throughput screening assays [[9], [10], [11]], miniaturization of therapeutic and diagnostic tools, tissue engineering and, oral drug delivery [12,13]. Some major problems in oral drug delivery have been solved by fabrication of micro and nanocarriers with precise control over their architecture and size. These efforts are a part of controlled drug delivery systems dating back to the 1950s [14,15]. In recent years, dynamic oral delivery systems have been fabricated using micro and nanofabrication technologies by which sensing, recording, and stimulating biological systems can be achieved for optimized drug delivery [16,17]. In addition, microfabrication techniques have been used to make biomimetic GI tract in vitro models in which the body’s response to drugs can be recapitulated and used for better design of drugs.
In this work, a brief review of physiological barriers to oral drug delivery is given (Fig. 1 ). We then discuss micro- and nanofabrication techniques and the subsequently fabricated drug carriers that have been used as oral drug delivery systems, with a focus on the material components, fabrication technologies, and drug loading efficiencies. We describe in detail the general chemical and physical strategies to functionalize diverse types of drug carriers for oral drug delivery applications with a focus on bioadhesion and tissue barrier remodeling. Specific examples of how engineered drug carriers have been used successfully to navigate the GI tract and improve oral drug delivery are then provided. We then discuss applications of fabrication technologies for modeling of the GI tract. Following that, clinical trials in oral drug delivery systems using fabrication technologies are mentioned. Finally, we highlight challenges and future directions in using micro- and nanofabrication technologies for oral drug delivery.
Fig. 1.
Micro- and nanoscale technologies enable fabrication of oral drug carriers as well as human tissue-on-a-chip models for precision medicine applications.
2. Physiological barriers to oral drug delivery
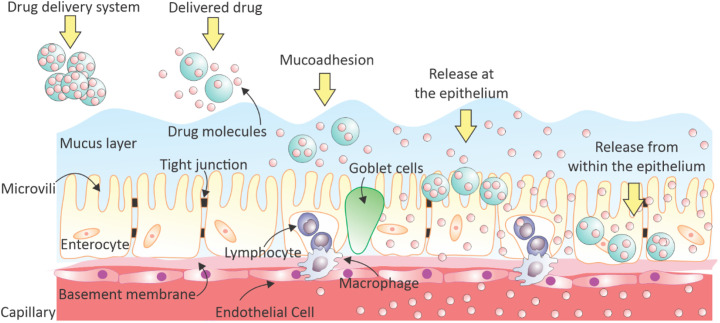
Some limitations of oral drug delivery systems are governed by GI anatomy, physiology, and biochemistry. The skin is the largest interface between the human body and the external environment [18,19]. In a healthy adult, the human skin has a surface area of approximately 2 m2. By comparison, the absorption mechanism of orally delivered drugs in the intestinal epithelium has more chemical and physical restrictions, as it has a much larger surface area (300 – 400 m2) [20]. In general, the drug is swallowed and enters the GI tract and it is released at the intestine proceeds by diffusing inside the mucus layer as shown in Fig. 2 . The mucus in the small intestine is discontinuous, whereas there are two layers in the stomach and large intestine (colon) [21]. The drug is delivered through the mucus layer and from there diffuses through pathways involving a long path through tight junctions (TJs) as well as epithelium cells. This process continues until the drug is carried all the way through the capillary layer covering the epithelium layer.
Fig. 2.
Schematic illustration of drug release and absorption mechanisms for orally delivered drugs in the large surface area of human intestinal epithelium.
The GI tract consists of the oral cavity, esophagus, stomach, small intestine and colon, each with different properties that need to be considered when designing delivery systems and studying drug release mechanisms (Table 1 ) [22]. In general, drug uptake in the GI tract is restricted by complex physiological barriers in the different GI tract regions. The GI tract has a naturally low permeability to the bloodstream and foreign molecules, such as orally delivered drugs [22]. The bottlebrush-like architecture of mucin in the lipid-rich matrix of mucus, embedded gastric glands in the stomach with the acidic environment, residence time, microbiome, and permeability across the intestinal epithelium should be considered for the design of carriers that facilitate oral delivery of small molecules, proteins, and peptides [23]. The main obstacles that exist for oral drug delivery are the biochemical, mucus diffusional, and cellular permeability barriers of the GI tract. The site of drug absorption is determined by the type of drug, as well as local environmental conditions such as pH, enzymes, mucus barriers, drug residence time, and GI surface area [24].
Table 1.
Characteristics of different segments of the human GI tract [22].
| pH | Length (cm) | Mean Diameter (cm) | Mucus Thickness (μm) | Mucus Turnover Rate (hours) | |
|---|---|---|---|---|---|
| Stomach | 0.8 – 5 | 20 | N/A | 245 ± 200 | 24 – 48 |
| Duodenum | ~ 7 | 17 – 56 | 4 | 15.5 | 24 – 48 |
| Jejunum | ≥7 | 280 – 1000 | 2 – 2.5 | 15.5 | |
| Ileum | ≥7 | 3 | 15.5 | ||
| Colon | 7 – 8 | 80 – 313 | 4 – 4.8 | 135 ± 25 | 24 – 48 |
2.1. Biochemical barriers
Enzymatic and pH degradation act together as the major biochemical barriers for the bioavailability of orally administered therapeutics (Fig. 3a). The presence of drug-degrading enzymes and acidic pH results in an approximately 94-98% loss of ingested biologic drugs due to deamidation, oxidation, or hydrolysis [25]. The stomach’s digestive fluid is composed of hydrochloric acid, protein-digesting enzyme pepsin, and mucus secreted by gastric glands, which cause an acidic environment (pH = 1.2–3). In addition to the harsh acidic environment of the stomach, digestive enzymes such as pepsin also pose challenges for oral drug delivery. Lipases in the stomach can also contribute to the hydrolysis of drugs with hydrophobic regions. The small intestine can also account for the digestion of drugs, as digestive enzymes, such as trypsins, chymotrypsins, carboxypeptidases, and elastases are present in high concentrations [26]. Finally, the colon provides a longer residence time of up to 20 h, low concentrations of digestive enzymes, and relatively neutral pH values of 6–6.7, as well as low fluid volumes to drug ratios [27].
Fig. 3.
A schematic of physiological barriers in oral drug delivery including: (a) biochemical barriers, (b) mucus barrier, and (c) cellular barriers to oral drug delivery. Reprinted by permission from Springer Nature [26] Copyright (2019).
2.2. Mucosal diffusion barrier
In addition to the above-mentioned physiological barriers (pH and enzymes), mucus with a viscoelastic and hydrogel- like structure creates a strong barrier for the penetration of therapeutics from the lumen to the underlying epithelium (Fig. 3b). The direct interaction of therapeutics with epithelial cells is restricted by two mucus layers: the outer loosely adherent layer and the inner firmly adherent layer [26]. Mucus is secreted by goblet cells, with turnover rates of every 24–48 h, to eliminate the attachment of potentially harmful compounds and bacteria. The majority of mucus is composed of mucin glycoproteins, which form a viscous gel to entrap foreign particles [26]. Mucus is also composed of proteins, carbohydrates, nucleic acids, lipids, salts, antibodies, and other active proteins [23]. Thus, it creates a safeguard and facilitates a nutrient-rich environment for bacterial colonization and antimicrobial molecules.
2.3. Cellular permeability barrier
The intestinal epithelium is the outermost layer of cells exposed to luminal contents. It is composed of TJs and three different kinds of cells: enterocytes, goblet cells, and Microfold cells (M-cells) (Fig. 3c) [28]. Enterocytes are the most abundant cells of the epithelium layer and enhance the transportation of nutrients and water from the gut lumen to the bloodstream. Mucus-secreting goblet cells comprise 10–20% of epithelial cells, while M-cells that cover Peyer's patches represent <1%. M-cells are responsible for antigen sampling and are important drug targets since they are less shielded by mucus [26]. TJs are paracellular barriers for the transportation of drugs between intestinal epithelial cells [29].
Methods for drug absorption into the bloodstream relies on interactions between the therapeutic and epithelial cells whether the drug is transported through the cell or between the cells through TJs. The absorption pathways are: a) transcellular pathways through epithelial cells; b) paracellular pathways through the TJs between adjacent epithelial cells; c) lymphatic absorption via M-cells of Peyer’s patches; d) receptor and transcytosis-mediated endocytosis, commonly conducted by the vitamin B12 uptake pathway or by hydrogen-coupled peptide transporters, transferring receptors, and IgG neonatal receptors [26]. In the next sections we will discuss how micro and nanoscale technologies allow for the bypass of biochemical and mucosal diffusion barriers to enable successful cellular uptake.
3. Micro and nanoscale fabrication techniques
Micro-/nanoscale fabrication technologies, including lithographic techniques and microfluidics, have opened up important opportunities to further develop the fields of tissue engineering and drug delivery [1,30]. Microfabrication has been implemented for drug delivery because of its capability to combine different characteristics, including the ability to make precise shapes and sizes (e.g. needles, non-symmetrical features) that increase the contact area of the drug delivery system with the GI tract and precise sizes or reservoirs (multiple or single) to control drug release. These microfabricated devices can be further engineered to be stimuli-responsive and bioadhesive. In addition to drug delivery applications, microfabrication technologies provide great advantages in the generation of biomimetic GI tract prototypes, integrating physiological cues, flow, and biomimetic structures [1,31]. There are a variety of methods to fabricate micro- and nanoscale systems for controlled drug delivery applications, including emulsion, assembly, photolithography, mold replication, micromachining, micromilling, deposition, etching, and laser ablation [32,33].
3.1. Emulsion and self-assembling systems
Emulsion and self-assembly based systems of fabrication are some of the most widely used techniques for the development of nano and microparticles. Emulsion fabrication, as well as nano-precipitation techniques, rely on the phase separation of hydrophobic polymers in aqueous solution [[34], [35], [36]]. Such oil-in-water emulsions are frequently used with biocompatible polymers, such as poly(lactic-co-glycolic acid) (PLGA) and polycaprolactone (PCL). These emulsion techniques are compatible with drug encapsulation, either with hydrophobic drugs and single emulsion techniques, or with hydrophilic drugs and a double emulsion technique, which creates water-in-oil-in-water particles. Emulsion techniques allow for the fabrication of particles of varying sizes, ranging from tens of nanometers to hundreds of microns [37]. The size of the particles is dictated by the shear imparted during the emulsification process which is affected by the properties of solutions, as well as the concentration of polymer and surfactant in the aqueous phase. The surfactant controls the phase separation behavior during emulsification [38]. PLGA-based reverse emulsions are not able to generate hydrophilic polymer particles in oil phase. Therefore, these emulsions are less common for clinical applications in oral drug delivery where the particle needs to stably traverse aqueous environments. However, in general, some PLGA-loaded particles have been used in clinic for other drug delivery systems.
Self-assembly fabrication approaches are directed by a variety of noncovalent forces such as hydrophobic-hydrophilic and electrostatic interactions. They can offer more precise control than standard emulsion or precipitation techniques and allow for the fabrication of diverse shapes including spherical, fibrillar, and ellipsoidal particles [[39], [40], [41]]. Self-assembling systems are typically size-limited to the nanoscale, with the particles commonly reported in the tens of nanometers to low hundreds of nanometers in diameter. Similar to emulsion techniques, self-assembling systems have been used to encapsulate both hydrophobic and hydrophilic cargo for oral drug delivery applications.
3.2. Electrospinning
In electrospinning, a high voltage source is used to make micro- or nanofibers from a polymer solution or melt. An electrostatic interaction between a grounded collector and charged polymer solution is formed when the polymer is expelled from a metal needle, forming a cone at the base of the needle called a Taylor cone. A fiber jet is ejected from the Taylor cone as the electric field strength exceeds the surface tension of the liquid. By travelling the fiber jet through the air, the solvent evaporates, consequently results in the deposition of solid polymer fibers on the collector. Fibers generated by this process usually have diameters on the order of hundreds of nanometers. The capability to simply generate materials at different sizes in a rapid and simple manner has made a great interest in electrospinning for tissue engineering and drug delivery applications [42,43]. Researchers have widely used this technology in drug delivery because it is easy to modulate the release profile of drugs based on properties of polymeric materials and it is compatible with a variety of drugs and biopolymers [44].
3.3. Lithography
Photolithography involves the transferring of a photomask’s pattern onto a photoresist by exposure to light [1,45,46]. The photoresist layer is employed to transfer the pattern to a material after development. The wavelength of light exposure is the dominant limitation of photolithography. High resolution nanostructures can be made using more advanced techniques, such as ion beam lithography and electron beam lithography [33].
Soft lithography is a complementary version of photolithography. While photolithography has worked well to deal with photoresists [47], soft lithography expands the capabilities of photolithography. Soft lithography can process a variety of elastomeric materials. Polydimethylsiloxane (PDMS) is commonly used for soft lithography applications due to its biocompatibility, low cost, chemical inertness, low toxicity, mechanical flexibility and durability, and versatile surface chemistry. Additionally, fabricating PDMS devices requires minimal equipment [48,49].
3.4. Microfluidics
Microfluidic systems are capable of handling and transporting small volumes of fluids through microchannels and they can be created with photo and soft lithography. Microfluidics have been used as a tool for designing drug delivery systems [45,50]. Drug delivery to target sites can be done in an efficient and well-controlled manner with desired rates using microfluidic platforms via implantation, localization, precise control, automation, and integration of the platform [51]. Microfluidics can fabricate materials with high precision and recapitulate in vivo conditions for drug screening [[52], [53], [54]] and drug discovery [54,55] because of its capability to provide physiologically relevant fluid flow [1]. This technology has become an essential part of cellular assays for the analysis of oral drug absorption.
Recently, different microfluidic-based platforms have been developed to produce and screen drug nanocarriers. Microfluidic drug development platforms provide high-throughput, reproducible, and low-cost methods for producing, screening, and optimizing nanocarriers. The properties of synthesized nanocarriers, such as morphology, drug loading capacity, and release kinetic parameters, can be easily and effectively be modified and optimized by adjusting the channel geometries and flow rate. Microfluidics facilitate the efficient and low cost production of various micro and nanoparticles, composed of different materials and therapeutic agents, with high loading capacity and controlled release at small scale, which minimizes the amount of required reagents, as compared to bulk mixing methods [56].
Microfluidic-based synthesizers are classified as diffusion and droplet-based methods (Fig. 4 ) [57]. Hasani-Sadrabadi et al. fabricated a microfluidic device for generating core-shell chitosan-based nanoparticles for oral delivery of hydrophobic anti-cancer drugs to treat colorectal cancer tumors [58]. The core of the nanoparticles was composed of a hydrophobic modified N-palmitoyl chitosan for efficient loading of hydrophobic drugs. The core also allowed for the formation of nanoparticles through the self-assembly of chitosan chains, without using a cross-linking agent. The self-assembly of chitosan chains occurred in the first microreactor with hydrodynamically focused flow controlling the mixing time of flow streams. A Tesla micromixer was also designed for efficient mixing and coating of nanoparticles by pH-responsive layer of Eudragit ((pH-sensitive poly(methyl methacrylate)) in a controlled manner. The Coanda effect generated in this Tesla-designed micromixer enhances mixing efficiency. It results in deflection of a part of the flow toward the narrow side of the channel and flow of other part through the curved side for an efficient mixing of two flow streams. In the latter study, the thickness of the shell in synthesized particles was controlled by the ratio of sheath flow rate to the main flow rate.
Fig. 4.
Microfluidic approaches to fabricate nanocarriers for oral drug delivery. Different diffusion- and droplet-based microfluidic platforms for preparation of nanoparticles including (a) microfluidic continuous flow, (b) microfluidic mixer, (c) microfluidic droplet generator, (d) microfluidic processor. Reprinted from [57] Copyright (2013), with permission from Elsevier.
Although the generation of particles can be controlled by tuning the flow rate in diffusion-based mixing methods, the continuous flow regime limits efficient diffusion and the reaction between materials boundaries between separate flow streams. In droplet-based techniques, each droplet serves as a microreactor for an independent reaction, resulting in higher production efficiency for drug-loaded nanocarriers. This method allows for precise control of nanocarrier size, drug loading efficiency, and total amount of nanocarrier produced. Nano-in-micro platforms can be introduced based on droplet-based methods to prepare nanoparticles encapsulated inside microparticles. Araujo et al. produced a multifunctional composite for the oral delivery of a mixture of glucagon-like peptide-1 and an enzymatic inhibitor (dipeptidyl peptidase 4) as antidiabetic drugs for synergistic therapy using droplet-based microfluidic techniques [59]. The glucagon-like peptide-1 was first loaded in different PLGA and mesoporous silicon biomaterials to limit its rapid degradation in the intestine. The resulting nanoparticles were further functionalized by mucoadhesive polymers, such as chitosan and cell penetrating peptides. Similar Nano-In-Micro platforms were utilized in several other investigations to encapsulate nanoparticles inside of microstructures, such as halloysite nanotubes-polymer, mesoporous silicon-solid lipid, and mesoporous silicon-polymer composites, for oral drug delivery applications [[60], [61], [62]].
3.5. Three-dimensional Printing
Three-dimensional (3D) printing technology is a promising fabrication technique that has received wide interest in biomedical engineering and drug delivery applications to provide complex drug release profiles, precise drug dosing, novel drug delivery devices, and 3D printed polypills [63]. 3D printing technology offers low-cost applications compared to conventional systems since it does not need several unit operations and requires minimal human intervention [[64], [65], [66], [67]]. Typically, 3D printing works through the digitally-controlled and layer-by-layer deposition of materials to make different 3D constructs and desired geometries without the need for molds or machining [[68], [69], [70]]. This technology can offer precise and personalized dosing for treatment of different patients [64,71]. However, 3D printing technology may need multiple steps and sophisticated equipment to synthesize oral delivery platforms in a commercial setting.
Spritam® [72] was approved as the first 3D printed drug tablet by the Food and Drug Administration (FDA) and this led to great interest into the implementation of 3D printed drug delivery systems. The capability to handle low volumes of fluids with spatial control facilitates the preparation of devices with interesting compositions and geometries. The flexibility of 3D printing enables the preparation of systems with multiple drugs and specific release profiles [73]. Various 3D printing technologies can be employed for the development of pharmaceutical formulations. Technologies such as digital light processing, selective laser sintering, continuous liquid interface production, stereolithography, fused deposition modelling (FDM), material jetting, inkjet deposition, and binder jetting are the most common 3D printing technologies that have been implemented in pharmaceutical research and customized drug formulation [68,74]. The most challenging part of using 3D printing for the fabrication of drug delivery systems is the development of functional inks that retain the features necessary for sustain release and bioadhesion. Some examples of such inks are commercially available (e.g., Resomer® filaments for 3D printing by Evonik). These could be potentially used for the development of oral delivery carriers.
Progress in 3D printing technology has led to innovative medical devices, as well as customized drug delivery systems. 3D printing technology offers multiple formulation options compared to conventional drug delivery systems. Moreover, it provides an opportunity to load multiple drugs into a single device and make multifunctional drug delivery systems and dimension-specific drug formulations to attain tunable drug release profiles [63]. More recently, two-photon lithography technologies were designed to offer 3D printing capabilities at nanoscale that could be interesting for oral delivery systems where a nanoparticle has to be of specific shape.
4. Micro and nanoscale carrier types
The unique physiochemical properties and high surface area to volume ratio of nanoparticles facilitate high loading of drugs through encapsulation or formation of chemical-physical bindings with their functional groups. The stability of some nanoparticles in aqueous physiological environments allows for successful loading and delivery of poorly water-soluble drugs [75]. Additionally, nanoparticles can be functionalized using different targeting or imaging agents in order to be utilized for imaging and targeted drug delivery applications [76]. Specifically, drug delivery using biocompatible nanocarriers has been introduced as an effective solution to overcome some challenges involved in oral drug administration, particularly for drugs with low stability, bioavailability, and solubility. Nanoparticles can protect drugs from the acidic environment of GI and the secretion of mucus to enhance membrane permeability, which promotes drug absorption and bioavailability. Bioadhesive properties of nanoparticles enhance the permeation of drugs by increasing residence time in the GI tract [77].
Microparticles are common oral delivery systems in addition to nanoparticles, and offer the means to improve the bioavailability of pharmaceuticals through the control over shape, size, geometry, and functional characteristics of the particle [[78], [79], [80], [81]]. During the past few decades, microparticle technology has been extensively applied for various applications in therapeutic and pharmaceutical fields, such as the delivery of anti-inflammatories [82,83], antibiotics [84,85], chemotherapeutics [86,87], proteins [88], and vitamins [89,90]. Microparticle sizes range from 1 to 1000 μm and they exist in various structures [91]. Microparticles may be characterized as either homogenous or heterogeneous structures depending on the formulation and processing.
Using the techniques described above, drug carriers can be fabricated in multiple size regimes from different materials, with tunable physical and chemical properties. We will briefly describe some of the different classes of carriers, before discussing functionalization strategies and methods of targeting the GI system or systemic bloodstream circulation via oral administration of these carriers.
4.1. Lipid-based nanoparticles
Lipid-based nanoparticles, such as solid lipid nanoparticles (SLNs) and liposomes, are a group of nanoparticles used extensively for oral drug delivery due to their excellent biocompatibility, similarity with biological membranes, and drug loading capacity. Liposomes were the first nanocarriers approved by FDA for clinical use [92]. They are composed of an aqueous core, which encapsulates hydrophilic drugs, and an amphiphilic lipid bilayer, which allows for the loading of hydrophobic drugs [93] (Fig. 5a). Additionally, they can be utilized as the carrier of biomolecules like peptides, antigens or antibodies which are covalently attached to the polyethylene glycol (PEG)-coated (PEGylated) surface of liposomes (Fig. 5c). Functionalization of liposomes with PEG limits their recognition by phagocytic cells, resulting in longer circulation time and enhanced biodistribution [94]. Furthermore, different targeting agents can be conjugated to the external surface of the liposomes for the enhanced targeted delivery of therapeutic agents to specific cells. The encapsulation of hydrophilic drugs and biologics inside of liposomes significantly improves their cellular absorption [95,96]. However, the efficiency of conventional phospholipid or cholesterol-based liposomes is seriously affected by instability of their lipid vesicles and phospholipid hydrolysis or oxidation in the GI tract. Therefore, chemical and physical functionalization is employed to increase their residence time in the intestine and enhance their stability, as we will discuss in later sections.
Fig. 5.
Fabrication and characterization of nanocarriers for oral drug delivery. (a) Schematic image of possibilities for drug loading and functionalization with different targeting and therapeutics ligands in liposomes. Reprinted from [92] with permission from Elsevier. (b) A strategy for loading hydrophilic drugs in the core of solid nanoparticles (blue color) by generation of a hydrophilic viscose phase in the core. Reprinted from [101], Copyright (2016), with permission from Elsevier. (c) A two-step preparation method for insulin-loaded core-shell nanoparticles composed of a modified chitosan core coated with thiolated hyaluronic acid through electrostatic [114]. Copyright (2018) Wiley-VCH Verlag GmbH & Co. KGaA. Reproduced with permission. (d) Self-assembly of cationic copolymers (yellow color) with anion biomacromolecules (green color) to form polymer micelles with targeting agents can improve mucoadhesion and can generate polymeric networks of micelles. Reprinted (adapted) with permission from [115] Copyright (2005) American Chemical Society.
SLNs contain a monolayer phospholipid shell and a solid lipid core [97]. These nanoparticles can be utilized for encapsulation of lipophilic ingredients and insoluble drugs. SLNs are prepared from synthetic or natural biodegradable and biocompatible lipids, such as fatty acids, triglycerides, steroids, and phospholipids. They have shown high stability in the harsh conditions of the GI, as compared to other lipid nanoparticles, such as liposomes. Functionalizing and non-covalent coating of SLNs with carboxymethyl chitosan can further enhance their stability and drug bioavailability [98]. Several investigations reported higher oral bioavailability of hydrophobic drugs, such as nitrendipine and nimodipine, loaded in SLNs [99,100]. However, the encapsulation of hydrophilic drugs in SLNs is limited due to the particle’s hydrophobic nature. Incorporation of a SLN core with hydrophilic viscosity-enhancing polymers, such as PEG, through a water-oil-water double emulsion method is proposed for higher loading efficiency of hydrophilic drugs in the core of SLNs. In this strategy, the core of orally administered SLNs consists of a solid lipid core and a hydrogen-bonded rich aqueous phase encapsulating insulin, which is either dispersed in the lipid phase or is formed like a central core in the lipid matrix (Fig. 5b) [101]. Although surface functionalization of SLNs with PEG enhances their hydrophilicity, a reduction in muco-adhesion of SLNs is also observed.
4.2. Polymeric nano and microparticles
Natural polymers, synthetic polymers or their combinations, cellulose derivatives, polysaccharides or proteins, and waxes of plant or animal origin can be used to prepare nano or microstructural oral drug delivery materials. Polymeric particles can interact with the mucus through electrostatic, van der Waals, hydrophobic, or hydrogen-bonding interactions, which may lead to long residence time of drugs in the absorption region [102]. However, more research is required to reduce their undesired adhesion to non-target regions [103]. Several studies have demonstrated more efficient absorbance of hydrophobic polymeric nanoparticles to the Peyer's patches when compared to less hydrophobic or hydrophilic particles [104], showing the important role of hydrophobicity in polymeric nanoparticles.
Polymeric nano and microparticles made of PLGA, poly(lactic acid) (PLA), PLA-PLGA copolymer, poly(acrylic acid) (Carbopol) and poly(N-isopropylacrylamide) have been extensively explored in the pharmaceutical field as carriers for oral drug delivery due to their biocompatibility, enzymatic degradation, bioadhesion, and rapid removal in the mucus. The biodegradability and biocompatibility of these polymers have been approved for various medical and pharmaceutical applications, including drug delivery by both the FDA and the European Medicine Agency [102].
Of the polymers available, PLGA is one of the most commonly researched polymers for oral drug delivery, owing to its FDA approval [105], biocompatibility, and biodegradability. In particular, PLGA is suitable for oral delivery of water insoluble anti-cancer drugs, such as paclitaxel and curcumin, due to its hydrophobic nature [106]. The encapsulation of hydrophobic anti-cancer drugs in PLGA nanoparticles resulted in enhanced bioavailability of drugs, which is due to improved aqueous stability of loaded drugs in PLGA nanoparticles, compared to free drug, and sustained drug release by degradation of the PLGA nanoparticles [106].
In addition to nanoparticles, different microparticle, polymer-based systems have been introduced for oral drug delivery for in vivo and in vitro studies which are summarized in Table 2 . A natural polymer-based microencapsulation system was proposed by Vasiliu et al. They prepared microparticles based on polyelectrolyte complexes between two polysaccharides (xanthan gum and gellan) and an acrylic ion exchange resin to obtain a novel antibiotic delivery system. The effects of contact time, temperature, and drug concentration on the patch efficacy were optimized using batch adsorption studies [107]. Wang et al. reported a monodisperse and temperature-induced self-bursting microcapsules for encapsulating hydrophobic compounds. The proposed microcapsules had a hydrophobic core and a thermo-responsive shell comprised of poly(N-isopropyl acrylamide) and embedded superparamagnetic Fe3O4 nanoparticles [108]. Koetting et al. designed hydrogel microparticles and used them for oral delivery of therapeutic proteins [109]. More efficient surface engineering technologies, advanced bioadhesive functionalization, and combination with smart materials will result in the development of highly functional microparticle-based oral drug delivery systems.
Table 2.
Microparticle systems for applications in oral drug delivery.
| Material | Model of Drug | Applications and Benefits | References |
|---|---|---|---|
| PLA | Insulin | A solvent extraction method was used to prepare different sized microcapsules and the highest insulin release profile was obtained in 7–12 h. | [116] |
| Lovastatin | PLA microspheres enhanced the bioavailability of drugs for gastroretentive drug delivery and prolonged the drug circulation time in vivo. | [117] | |
| PLGA | Amifostine | A solvent evaporation technique was used for Amifostine encapsulation and oral controlled release. It was observed that 50% of the drug was released within the first 6 h and 92% within 12 h. | [118] |
| Plasmid DNA (pDNA) | pDNA vaccine encapsulated PLGA microcapsules was synthesized via a solvent evaporation method. The pDNA was protected from degradation in the GI system. |
[119] |
|
| Insulin | Magnetic nanocrystals and insulin were encapsulated in PLGA microparticles to delay drug transition using a magnetic field. | [120] | |
| Curcumin | PLGA particles with different molecular weights were prepared by an emulsification-solvent evaporation method to encapsulate curcumin. The results showed that the bioavailability of high molecular weight PLGA particles was better than that of low molecular weight PLGA particles and curcumin. | [121] | |
| Physicochemical properties and in vivo therapeutic activities of porous and nonporous PLGA microparticles were studied. Ammonium bicarbonate was used to create the porosity and in vivo experiments showed that oral administration of porous microparticles exhibited therapeutic efficacy against Ulcerative colitis compared to nonporous microparticles. | [122] | ||
| PCL | Bovine serum albumin | PCL microparticles for use in oral vaccine applications were produced in sizes (5–10 microns) that can be taken by M cells in Peyer's patches. | [123] |
| Manidipine dihydrochloride | In order to treat high blood pressure, PCL microparticles containing Manidipine dihydrochloride with an antihypertensive effect for up to 24 h were developed. | [124] | |
| Polyvinyl alcohol (PVA) | Ornidazole | Controlled release of the drug molecule in the GI tissue was provided with PVA microparticles prepared using different ratios of PVA to starch. | [125] |
| Methylcellulose | Thymol | Methylcellulose and hydroxypropyl methylcellulose phthalate were used to produce Thymol encapsulated microspheres. In vivo pharmacokinetic studies showed that the microparticles could be used for local treatment of intestinal infections. | [126] |
| Ethylcellulose | Propranolol | Ethylcellulose microparticles containing Propranolol hydrochloride were prepared using a modified solvent evaporation method, and its use for the treatment of hypertension was studied. | [127] |
| Carboxymethyl cellulose sodium | Flurbiprofen | Chitosan-coated and uncoated sodium carboxymethyl cellulose and polyvinyl alcohol microspheres were synthesized and crosslinked with Fe3+ ions. The chitosan-coating provided a slower release and a lower burst effect. | [128] |
| Progesterone | Low methoxy amidated pectin-sodium carboxymethyl cellulose microspheres were prepared, and Zn2+ and Al3+ ions were used for crosslinking. The particles were tested in colon-targeted drug delivery. | [129] | |
| Chitosan | Ovalbumin | Porous chitosan microparticles, which can be taken up by the epithelium of the Peyer's patches, were synthesized and used as a vaccine delivery system. | [130] |
| Curcumin | A sustained release of curcumin in the intestinal tract was reported for N-trimethyl chitosan modified SLNs. | [131] | |
| Progesterone | Zn-pectinate/chitosan particles were made to increase the oral bioavailability of progesterone and to use the particles as the colon targeting system. | [132] | |
| Sodium hyaluronate | Vancomycin | Drug loading capacity of vancomycin in porous and degradable hyaluronic acid (HA) microparticles were increased by the HA porosity, and the drug release degree could be modified by the degradability of the particles. | [133] |
| Sodium alginate | Curcumin | Alginate microparticles crosslinked by ion gelation were used for controlled release curcumin solubilized in the lipid phase. | [134] |
| Insulin | The efficacy of microparticles prepared using different amounts of mucin and alginate on controlled insulin release was assessed. | [135] | |
| Gelatin | Vascular endothelial growth factor (VEGF) | Gelatin microparticles were designed for the controlled release of VEGF, and a regular controlled release was achieved by modifying the degree of microparticle crosslinking. | [136] |
| Bone morphogenetic protein-2 | Gelatin microparticles were evaluated for controlled release of bone morphogenetic protein-2, and the release profiles were compared with PLGA microparticles. | [137] | |
| Ciprofloxacin | Ciprofloxacin, a water-insoluble antimicrobial drug, was encapsulated in gelatin as a result of a one-step process by spray drying an aqueous solution. | [138] | |
| Polymethacrylic acid-polyethylene glycol-chitosan | Insulin | Surface thiolation was used to increase the drug release performance of hydrogel-based oral insulin delivery systems. | [139] |
| Chitosan-carboxymethyl starch | 5-aminosalicylic acid | Chitosan-carboxymethyl starch particles were synthesized via a casting technique with high encapsulation performance as a drug delivery system for the colon. | [140] |
| Chitosan-graft-polyacrylamide | Ibuprofen | Chitosan-graft-polyacrylamide copolymer was produced by cerium (IV) ammonium nitrate-induced free radical graft polymerization, and the release profile as a function of crosslinker amount and drug to polymer ratio was investigated. | [141] |
| Poly(butylmethacrylate-co-(2-dimethylaminoethyl) methacrylate-co-methylmethacrylate) | Micronutrients (iodine, zinc, iron, and vitamins (B2, B12, C, D, and A), biotin, folic acid, and niacin) | Poly(butylmethacrylate-co-(2-dimethylaminoethyl) methacrylate-co-methylmethacrylate) was used to encapsulate different micronutrients, and the encapsulation was shown to provide stability against a variety of factors. | [142] |
Another widely used nanocarrier for oral drug delivery is the polymer micelle, which is formed by self-assembly of amphiphilic polymers above the critical micelle concentration. This fabrication process provides a core-shell structure, which allows loading of poorly water soluble drugs in hydrophobic cores with enhanced bioavailability and stability [110,111]. For example, an amphiphilic block copolymer consisted of a micellar shell-forming PEG block and a core-forming poly(2-(4-vinylbenzyloxy)-N,N-diethylnicotinamide) block, while N,N-Diethylnicotinamide in the micellar inner core resulted in effective paclitaxel solubilization and stabilization [110]. Suitable copolymers for oral drug delivery should have self-assembling capabilities in water, biodegradability, biocompatibility, high stability, and residence time in the GI tract. Different polymeric micelles containing polyethers or polyesters have been proposed for oral drug delivery. Pluronics, composed of poly(ethylene oxide) (PEO)-poly(propylene oxide)-PEO copolymer, also known as of Poloxamer are commonly used polymers for micelle assembly. Some polymeric micelles exhibit pH-responsive disassembly with lower release rate in acidic environments, reducing initial burst release of drugs [112]. In addition to micelles, reverse micelles prepared in an oily solution with an interior hydrophilic core and exterior hydrophobic layer can be used for the encapsulation and sustained release of hydrophilic drugs [113].
4.3. Inorganic nano and microparticles
In addition to organic nano and microparticles, inorganic particles, including mesoporous silica, gold, silver, iron oxide, quantum dots, carbon nanotubes, and graphene oxide nanoparticles, have found wide applications for oral drug delivery due to their exceptional physiochemical properties [[143], [144], [145]]. High stability in aqueous conditions, along with acidic and enzymatic environments [1], feasibility of functionalization [2,3], large surface area with a high loading capacity [4,5], enhanced membrane permeability to the cells [6,7], and optical and magnetic properties [8,9] all suggest promising applications of inorganic nanoparticles not only in drug delivery, but also in bioimaging. However, their clinical applications are restricted by their poor biodegradability and biocompatibility, which requires functionalization with other biomaterials. Functionalization of inorganic nanoparticles with biocompatible ligands can be achieved during their synthesis procedure or after their preparation. Silica nanoparticles, with high porosity and surface area, possess silanol groups, which facilitate their functionalization. For example, silica nanoparticles coated with PEG slowed the release of insulin both in acidic and neutral pH [144]. PEG, chitosan, and alginate coated silicate nanoparticles have also been utilized for oral delivery of insulin to enhance their mucoadhesion and biocompatibility [144,146]. Poly(amidoamine)-functionalized multiwalled carbon nanotubes loaded with hydrophobic therapeutics were modified with a carboxylate group to increase loading capacity and drug dissolution [137]. Insulin-loaded silica nanoparticles ranging from 289 nm to 625 nm showed increased interaction with mucin when coated with chitosan [146].
4.4. Micropatches
Micropatches (also called wafers or films) can be designed as drug carriers with a typical size of 2-10 cm2 and a thickness of 20-500 μm [[147], [148], [149]]. They can be classified as melt away, rapid disintegrating, and sustained-release, referring to their different drug release rates and disintegration times as detailed by Kirsch et al. [150]. The drug release rate can be defined by the polymer used but can also be multilayered with varying disintegration times. Oral patches are usually fabricated with laminated structure, a drug-containing bioadhesive layer, and an impermeable backing layer for increased retention [151]. To improve the retention of micropatches within the intestinal lining, micropatches are designed to be thin and flat (Fig. 6a,b). This design also minimizes exposure to the constant flow of fluids from the intestine (Fig. 6c).
Fig. 6.
Micropatches in oral drug delivery. (a) Schematic representation showing a GI patch and (b) working mechanism of the hard capsule filled with mucoadhesive patches. Reprinted from [152], Copyright (2011), with permission from Elsevier. (c) In contrast to microspheres, asymmetric and planar microdevices facilitate proximal and unidirectional drug release, while increasing residence time in the GI tract. Reprinted from [29], Copyright (2015), with permission from Elsevier.
4.5. 3D fabricated microdevices
Encapsulation of drugs in polymeric matrices can be achieved using several methods, such as injection molding, pressing, and 3D printing [[153], [154], [155], [156]]. First, a drug polymer blend can be fabricated via mixing the polymer with the desired drug and then either molded or shaped in the form of filaments for 3D printing. The release rate can be tuned by manipulating the polymer or drug concentration.
Khaled et al. utilized 3D extrusion-based printing to fabricate a polypill for patients under complex medication regiments. The polypill contained five distinct drugs with two independently well-defined and controlled release profiles. The drug formulations aimed to improve the drug usage for patients taking a variety of different drugs and to allow for the tailoring of a drug regimen with distinct release kinetics for each individual. The polypill demonstrated rapid and sustained release profiles based on the excipient/active ratio [157]. Maroni et al. proposed printed 3D capsular and multi-compartment devices. The devices were separated by a 600 or 1200 μm thickness wall, for two-pulse oral drug delivery. The devices were manufactured by FDM 3D printing, which allows for larger scale production [158]. Melocchi et al. investigated FDM 3D printing for the manufacturing of capsular devices with a shell-thickness of 600 μm using a swellable and erodible polymer (hydroxypropyl cellulose) for oral pulsatile release. This study focused on the fabrication of hollow structures via FDM and the production of hydroxypropyl cellulose filaments by hot melt extrusion. After assembly of the capsular devices, the study reported an initial slow release followed by a quantitative and rapid liberation of the drug [159].
Li et al. showed that 3D printing can be utilized to fabricate oral drug delivery devices consisting of various materials with customized designs (Fig. 7a) [160]. FDM was employed for the 3D printing of glipizide-loaded filaments, which were fabricated by melt extrusion of PVA and glipizide [154]. Then, the drug-loaded filament was 3D printed into a tablet shape. The tablet was composed of a core and shell comprised of various contents of glipizide. By this new design, both controlled and delayed release were possible as the composition of the outer layer controlled the release behavior of the core. In another study, Maroni et al. employed FDM and IM technologies to fabricate a capsular device for two pulse oral drug administration (Fig. 7b) [158]. Commercial PVA and other formulations of polymers were used for FDM and injection molding. The capsules were composed of two hollow halves with desired thicknesses with a middle partition. Varied thicknesses and compositions in each half led to faster or slower drug release in the respective halves of the capsule. FDM enabled customization of the drug delivery system and injection molding was suitable for its high throughput production. Kirtane et al. designed a novel oral dosage form for weekly and sustained drug release (Fig. 7c) [161]. The dosage form released long-acting antiretroviral for the prevention and treatment of human immunodeficiency virus (HIV). The device was composed of an elastomeric core connected to six separate polymeric arms that were flexible enough to be folded and placed into a capsule. The arms were embraced by a polymeric shell and filled with a drug-polymer composite, which was fabricated by melt mixing. Poly(adipic anhydride), poly(sebacic anhydride), and PEG polymers and three antiretrovirals, dolutegravir, cabotegravir, and rilpivirine, were selected for the drug-polymer mixtures. Various polymers for drug-polymer composites enabled different drug release rates and a modular release system.
Fig. 7.
Examples of fabricated devices for oral drug delivery. (a) Caplets fabricated by 3D printing with various designs of multiple materials showed by different colors. Reprinted with permission from [160]. Copyright (2016) American Chemical Society. (b) 3D printed multi-compartment capsular devices with two phase release profiles. Reprinted from [158] Copyright (2017), with permission from Elsevier. (c) The device consisted of an elastomeric part (core) and six drug-loaded arms. Various polymers (blue, red and yellow) released the drug at different rates. Material from [161], published 2018, Nature Springer. (d) Scanning electron microscopic (SEM) image of the microcontainer filled with polymer and impregnated with ketoprofen. Scale bar is 100μm. Reprinted from [163] Copyright (2014), with permission from Elsevier. (e) Schematic of the drug- loaded micromotor and drug delivery in stomach. Reprinted by permission from [163]. Nature, Copyright (2017).
Reservoir-based microdevices are another type of microdevices designed to protect the drug against degradation and deactivation for an efficient drug release [[162], [163], [164], [165], [166]]. Precise control over the amount of drug loaded into a device can be obtained by drop-on demand inkjet printing of the drugs. Marizza et al. developed reservoir-based microdevices for oral drug delivery of active ingredients (Fig. 7d) [163]. They employed lithography techniques to fabricate microcontainers in desired dimensions. Then, the microcontainers were filled with a precise amount of polyvinylpyrrolidone (PVP) solution via inkjet printing and ketoprofen was soaked into the PVP when supercritical carbon dioxide was used as the loading medium. The amounts of the printed polymer and loaded drug were modulated by varying printing and soaking parameters. Thus, a controlled and reproducible drug release was achieved.
4.6. Dendrimers
Dendrimers have been extensively studied for oral drug delivery. Dendrimers have complex structure, divided into three parts: core, branch, and terminal groups. Dendrimers are monodispersed, usually symmetric and their molecular weight can be controlled. Additionally, physicochemical properties of dendrimers can be tuned based on their chemical structure, surface functionalization, and core structure. Most importantly, the terminal functional group in dendrimers has a significant role as it can be conjugated to various biological active molecules, such as enzymes and antibodies. Polyamidoamine is the most commonly used dendrimer with core structure consists of alkyl diamine and tertiary amine branches. Several drugs, such as clotrimazole, sulfamethoxazole, propranolol, ketoconazole, triclosan have been conjugated to polyamidoamine dendrimer to test their efficacy through oral drug delivery [[167], [168], [169]]. The latter studies proved that the activity of the drug molecules was significantly increased in conjugation with the dendrimer compared to their pure state.
5. Strategies to improve bioadhesion of drug carriers
Over the years, many types of micro- and nanocarriers have been designed and fabricated for oral drug delivery. A combination of different materials, versatile fabrication technologies, and different sizes and shapes of carriers have been explored to develop oral drug delivery systems. The aim of fabricated micro- and nanocarriers is to obtain dynamic and accurate control over the drug delivery process. These drug carriers can be engineered to improve their interactions with biological systems, such as mucus barriers. This can be achieved either through the increased adhesion and retention of the carrier within a biological environment (termed bioadhesive), which has been shown to improve oral drug delivery to various regions of the GI tract. To improve bioadhesion, two main strategies are used to functionalize the drug carriers, i.e., chemical modification to the surface of the carriers and engineering the physical and biological properties of the carriers.
The design of carriers with bioadhesive properties is considered an important aspect in oral, transmucosal, and transdermal delivery systems. The integration of bioadhesive properties improves the oral drug delivery of fabricated carriers. A key purpose of utilizing bioadhesive materials is to delay the transit of cargos for sustainable release of drugs at target sites, prolong the residence time in order to enhance the drug absorption process, and increase material-cellular contact, thus improving bioavailability. Bioadhesive materials have also demonstrated additional properties of inducing TJ rearrangement to enhance drug transport across epithelial barriers [29]. Achieving bioadhesive properties can be classified into chemical approaches and physical approaches. In this section, we describe strategies that are used across multiple applications in oral drug delivery to further functionalize drug carriers to improve their overall bioadhesion and the interactions with tissue barriers [1,29,170].
5.1. Chemical approaches to improve bioadhesion
Chemical bioadhesion is achieved through the combination of a material’s chemical composition and structure on its surface. A common chemical approach to enhance adhesion of a material is the immobilization of target lectin and carbohydrate binding proteins onto the surface of microparticles, microdevices, or micropatches, to increase the specificity of binding receptors of intestinal epithelial cell lines. As binding to cell surface receptor results in receptor-based endocytosis of nanocarriers, this approach facilitates target delivery of carriers into cells [171].
The surface charge of carriers is another effective parameter for particle uptake in oral administration. Considering the negative charge of sugar moieties on the mucins, positively charged nanoparticles can enhance mucoadhesion through the formation of electrostatic interactions [23]. Liu et al. showed the effect of surface chemical properties of a drug carrier in the GI tract. N3-O-toluyl-fluorouracil (TFu) was loaded into cationic SLNs (TFu-SLNs) and the particles were studied to improve the uptake of TFu. They observed that cation coated TFu-SLNs elevated the oral absorption of TFu about 2-fold in comparison with TFu suspension (Fig. 8c). The plausible mechanism to enhance the ability of controlled drug release is increased bioadhesion of the carrier by electrostatic interaction between the negatively charged absorption mucosal surface and positively charged colloidal particles [172].
Fig. 8.
Physical and chemical approaches to oral drug delivery. (a) Performance of traditional drug delivery platforms (left) compared to the developed tomato lectin-modified poly(methyl methacrylate) drug delivery microdevices. (b) SEM images of the microneedles fabricated via reactive ion etching technique (left) and insertion of needle tips into the epidermis (right). (c) Release curves of TFu-SLNs and TFu-Sol in artificial intestinal juice and artificial gastric juice. Reprinted with permission from [[172], [190], [193]].
Another important method for modifying drug carriers is through the incorporation of functional biomaterials into the design of oral drug delivery systems. Chemically modified polysaccharides, such as chitosan, alginate, pectin, gelatin, and dextran, are considered the most important natural polymers used in oral drug delivery due to their biocompatibility, bioadhesion, and enzymatic degradation. Chitosan with its positive charge enhances drug absorption and, consequently, coating nanoparticles with chitosan is an effective method to promote mucoadhesion [173]. Chitosan variants with higher molecular weight have shown better mucoadhesion [174]. Also, chemical modification of chitosan for enhanced mucoadhesion, physiological stability, permeability and bioavailability has been utilized for more efficient oral delivery of various drugs, including anti-cancer and peptide drugs [175] [176]. For instance, thiol functionalization of chitosan exhibited enhanced mucoadhesion, which is due to covalent bonds between the cysteine and thiol groups on mucus glycoproteins [177]. In a similar approach, Tian et al. introduced a two-step flash nanocomplexation process to fabricate core-shell nanoparticles coated with thiolated hyaluronic acid to be utilized for oral insulin delivery [114]. First, a positively charged insulin-loaded nanoparticle core was fabricated through electrostatic interaction between insulin and N-(2-hydroxypropyl)-3-trimethyl ammonium chloride-modified chitosan under turbulent mixing conditions. Subsequently, the prepared positively charged nanoparticle core was coated with polyanionic thiolated hyaluronic acid to synthesize the final product (Fig. 5c). A combination of chitosan with alginate and dextran have also been used for oral delivery of insulin [178].
Combining PLGA nanoparticles with chitosan has also been investigated to improve mucoadhesion of nanoparticles and facilitate their functionalization. In one example, Abd El Hady et al. studied a delivery system of diosmin in PLGA microparticles coated with chitosan and reported its effects on the gastric retention of diosmin [179]. In another study, folic acid (FA)-functionalized nanoparticles were prepared through a double emulsion method by surface coating insulin-loaded PLGA nanoparticles with chitosan-FA conjugates through electrostatic interactions to enhance their uptake and targeting abilities through FA receptors [180]. Multi-layered nanocapsules of PLGA and chitosan were prepared through layer-by-layer self-assembly of PLGA and chitosan via electrostatic interactions
Functionalization of copolymers with mucoadhesive functional groups is an efficient way to enhance the mucoadhesion of micelles as well. Dufresne et al. prepared a copolymer micelle based on PEG-poly-(2-(N,N-dimethylamino)ethyl methacrylate) and functionalized the PEG chains with thiol groups to form disulfide bonds with the mucin, leading to improved mucoadhesion of micelles [115]. In this method, thiolated copolymers with opposite charges were self-assembled in an aqueous solution to form thiolated polymer micelles with the potential for functionalization with targeting agents and improving mucoadhesion and the ability to form redox-sensitive polymer networks (Fig. 5d).
These strategies have also been applied to liposomal particles. Coating liposomes with mucoadhesive polymers, such as chitosan through non-covalent interactions increased the residence time and bioavailability of highly hydrophilic drugs with low permeability [181,182]. Modifying liposomes with thiol groups through functionalization with thiolated polymers (thiomers) imbued liposomes with desirable properties, such as mucoadhesion and enzyme inhibition [183,184]. In addition to mucoadhesive polymers, conjugation of liposomes with ligands, such as biotin, whose receptors are expressed in the intestine, can enhance the efficacy of conventional liposomes in oral drug delivery [185]. Glycans are binding sites for lectins in the membrane of cells in the GI tract; thus, functionalization of liposomes with lectin is another method to increase mucoadhesion [186].
In addition to mucoadhesive polymers, other hydrophilic polymers such as D-α-tocopheryl poly(ethylene glycol) 1000 succinate (TPGS) (a PEG-conjugated vitamin E) can be used to enhance the residence time and bioavailability of particles for oral drug delivery. TPGS can prolong the circulation time and cellular uptake of the coated nanoparticles. TPGS increases oral bioavailability by enhancing cell membrane permeability through the inhibition of P glycoproteins (P-gp) [187,188]. This is particularly important for increasing the oral bioavailability of anticancer drugs. For example, low concentrations of TPGS increased the intestinal permeability of paclitaxel, attributed to the inhibition of P-gp [189]. Therefore, TPGS stands as a potent adjuvant for orally administered chemotherapy.
5.2. Physical approaches to enhance bioadhesion
The physical properties of materials, such as mechanical properties, surface area, and surface morphology affect their adhesion properties to surfaces [29]. One of the physical methods to enhance bioadhesion is the utilization of different geometries/shapes of drug carriers. Tao and Desai made a direct comparison between conventional microspheres (multidirectional release) and their flat and thin device designed for unidirectional release, both coated with bioadhesive tomato lectin-poly(methyl methacrylate). They showed that despite the larger surface area of the microspheres compared to their device, the spheres remained less bounded to Caco-2 monolayers after consecutive washes. This was attributed to the smaller fraction of microsphere surface which is in direct contact to the cells [190]. Similarly, in nano-adhesive conditions, increasing bioadhesion of drug carriers is associated with the multivalence of adhesive elements per surface area. As the number of adhesive elements increases, Van der Waals adhesion also increases, resulting in greater absorption of the drug. The microvilli on mucosal epithelia have protrusions that increase surface area. Thus, fabricating nanostructured microdevices with multivalency could be a potential way to strengthen the bioadhesion of materials for targeting microvilli coated intestinal epithelium [191].
Microfabrication techniques are advanced technologies to design microdevice bodies with protruding microneedles and microposts. This permits the particle to interact with mucosa by strongly adhering to the mucosa surface and penetrating the mucus layer. As a result, an enhanced drug permeability occurs. Microneedle platforms were developed to increase the retention time in drug delivery systems (Fig. 8b). For instance, Guan et al. fabricated a crosslinked bilayered system made of PEG with chitosan microparticles. These fabricated systems were structured with self-folding arms. As expected, the arms anchored to the cell surface by penetrating into mucus layer. Thus, retention time of the device, as well as resistance to surface erosion of the mucus layer, increased [165][].
6. Targeting the gastrointestinal tract via oral administration with micro and nanoscale technologies
For both the gastric and intestinal tracts, innovative micro and nanoscale technologies have been developed for enhanced tissue targeting and oral drug delivery. These technologies range from polymeric nano and microparticles to 3D-printed wearables. Although many different oral drug delivery techniques have been developed so far, there are many considerations for any specific active ingredient and one delivery method does not fit all drugs. Thus, it is essential to customize the delivery technique based on the type of drugs and the patient needs. Selected examples of micro and nanotechnologies used for improving the efficacy of oral administration and drug delivery to the esophagus, stomach, intestines, and colon are described in this section.
6.1. Esophageal and stomach delivery
Delivery of drugs to the esophagus for treatment of esophageal diseases including infections, gastric reflux, and cancers is limited by the transient nature and low permeability of the esophagus. Therefore, designing an effective esophageal-targeted system with sufficient retention time during rapid transit through esophagus is of great importance. Drug-loaded nano and microparticles with prolonged contact and enhanced adhesion to the esophageal mucosa are introduced for targeting of therapeutic agents to the esophagus. Kockisch et al. developed an in vitro mucoadhesion tensile test and demonstrated efficient adhesion of polymeric microparticles, synthesized from carbopol, polycarbophil, and chitosan through water-in-oil emulsification to the porcine esophageal mucosa [194]. Additionally, magnetic systems are suggested for more efficient targeted therapy of esophageal cancer using an external magnetic force. Ito et al. prepared magnetic granules composed of ultrafine ferrite and a mixture of bioadhesive polymers containing hydroxypropyl cellulose and Carbopol 934. The granules were administered orally in rabbit models and were guided to the esophagus by applying an external magnet in less than 2 min, and results showed that almost all granules were retained in the target region for 2 h [195].
Rapid degradation, low stability, and poor absorption of drugs in the GI tract can be solved using stable drug delivery capsules to deliver drugs to the stomach, which is dominated by the gastric emptying time of 1-4 hours [196]. In one approach, Jiang et al. studied oral delivery of mussel-inspired and protein-functionalized electrospun nanofibers for treating gastric cancer [197]. Through the incorporation of a pH-responsive release mechanism, the authors demonstrated doxorubicin release from PCL nanofibers in acidic conditions of the stomach over neutral medium. In addition to the acidic stomach environment, there are a number of other challenges for the delivery of oral peptides, including low permeation through the intestinal epithelium, inactivation, and proteolytic degradation in the GI tract [198].
The incorporation of microneedles into pills that push into the GI lining (intra-enteral injection) to bypass these challenges can improve the bioavailability of a biologically active macromolecule [199]. An illustration of the working mechanism of such microneedle pills is represented in Fig. 9 . Recent studies suggest that macromolecule drug delivery may be possible via ingestible self-orienting millimeter-scale applicator (SOMA) capsules, which contain a tiny needle to autonomously position the capsule to engage the GI lining of the stomach in a safe manner to increase retention and enable the escape of insulin cargo from the GI into the bloodstream [196] (Fig. 10 ). The use of these methods is an alternative to injectable delivery of biologics medications, such as insulin and peptides, and enhances patient convenience, as well as increases safety and efficacy.
Fig. 9.
A microneedle approach for the delivery of biologics via oral administration. Delivery of biologics via the GI tract using a luminal unfolding microneedle injector (LUMI). Reprinted by permission from [199]. Nature, Copyright (2019).
Fig. 10.
(a) A schematic illustrating SOMA capsules for oral drug delivery. SOMA capsules reach a stable point of orientation and deliver biologics through the GI lining and into systemic circulation, (b) scale of fabricated SOMA, (c) the shape of SOMA capsules were inspired by the leopard tortoise shell, (d) mechanism of drug release after needle injection to the mucus through the spring ejection in caramelized sucrose. Reprinted from [196]. Reprinted with permission from AAAS.
Microneedle capsules can deliver not only small molecules but also proteins, peptides, vaccines, hormones, and other macromolecules. Traverso et al. successfully deployed a microneedle-containing device, modeled after current FDA-approved ingestible devices [200], and monitored the administration of insulin in the stomach [198].
Recently, some novel self-propelled microdevices, known as microrockets or micromotors, have been developed for delivery of drugs to target locations of organs. Microrockets should be biocompatible and be degraded in the gastric acid. Zhou et al. developed a self-propelled microdevice for target-based delivery of doxorubicin [202]. The microdevice consisted of a core of Zinc (Zn) surrounded by a thin layer of iron (Fe) and poly(aspartic acid) (PASP) microtube layer. Doxorubicin was adsorbed on the PASP surface by electrostatic interaction. The Zn particles electrodeposited onto the PASP/Fe layers could propel the microdevice by creating hydrogen bubbles in an acidic environment and prevent the oxidation of Fe. The microdevice was magnetically controlled due to presence of an Fe layer and navigated to the target site of the stomach by locating a strong magnet near it. The microdevice was trapped into gastric layer and started releasing the drug in an acidic environment. In another work, Ávila et al. proposed a magnesium (Mg)-based micromotor for active delivery of antibiotics for treatment of gastric bacterial infections [203]. The in vivo treatment of H. pylori infection using Mg-based micromotor loaded with clarithromycin was demonstrated in a mouse model. The micromotor consisted of a core of Mg microparticles coated with a thin layer of titanium oxide (TiO2) and then with a film of PLGA loaded with clarithromycin, while a small opening for the contact with acid was left. Then, the micromotor was coated with a thin layer of chitosan to enable electrostatic adhesion of the micromotor to the mucus of the stomach. In the acidic environment of stomach, Mg core reacted with acid and Mg gradually dissolved, while created hydrogen gas that led to self-propulsion of the micromotor in the gastric fluid and delivery of the drug. The in vivo studies illustrated high efficiency in delivery of antibiotics when using Mg-based micromotors compared to the passive drug delivery approaches.
6.2. Intestines and colon delivery
Enhancing intestinal drug delivery is primarily driven through the increased adhesion of materials to the intestinal lining and mucosal barriers, both to facilitate local release of drugs to intestinal drug targets, and to increase the release of drugs into the bloodstream for systemic circulation. Thus, the majority of oral drug delivery research is focused on delivery to this region of the GI tract. We will discuss significant examples of successful intestinal delivery of therapeutics, first discussing tissue-targeting strategies to achieve controlled drug release, and then discussing approaches focused at the cellular level of targeted intestinal drug delivery.
One of the most widely used drugs in oral drug delivery studies is insulin, with the goal of increasing insulin bioavailability via oral administration so that regular insulin injections are no longer required. One of the key goals with biologic delivery in the intestine is to protect the drug cargo from the harsh environment of the stomach prior to improving bioadhesion and controlled release of insulin within the intestines. Recently, Nemeth et al. reported on the loading of Eudragit® microwell devices via picoliter inkjet 3D printing for oral drug delivery applications [204]. This method provides a high throughput and reproducible means of loading biologics such as insulin into flat devices to increase tissue retention and improve oral drug delivery. In another study, Fox et al. employed a multi-step lithography process to develop a microdevice including a drug reservoir sealed by a nanostraw membrane [205]. The proposed device could facilitate drug loading and enable tunable release by manipulating the nanostraw inner diameter and density. The proposed microdevice could adhere to the GI tissue due to the presence of nanostructural topology (i.e., nanostraws) on the surface of the device. Thus, the drugs could be locally released over an adjustable time period, while the drugs were not exposed to drug-degrading biomolecules and digestive enzymes. The proposed microdevice can improve the oral absorption, which is of great importance for drugs with poor bioavailability such as insulin.
To increase the release of biomolecules from the intestine into the bloodstream, Abramson et al. developed a capsule named LUMI for the oral administration of various biomacromolecule drugs, using insulin as the potential target drug [206]. The device consisted of three degradable arms with drug-loaded microneedles packed into a capsule that was 9 mm in diameter and 30 mm in length. The microneedles were generated at the end of each arm and were composed of PVP with insulin powder at the tip. After taking the capsule, the poly(methacrylic acid-co-ethyl acrylate) coating was dissolved at pH levels above 5.5 and then the PEG coating embracing the compressed spring actuator was dissolved and the device was pushed out of the capsule when the arms were in a random direction to the intestinal wall. Then, the drug was delivered, and the rest of device was dissolved. The actuation time of the capsule could be tuned by varying the molecular weight of the PEG in an environment with the proper pH condition. After actuation, the capsule broke apart and was transported through GI tract. The LUMI device showed consistent release of drugs into the small intestinal mucosa during the in vivo studies. However, one limitation of this devices is that the LUMI delivery method may cause discomfort for patients due to scratching the intestine after activation of the device.
In another work, oral administration of insulin-loaded liposomes, containing three kinds of bile salts including sodium glycocholate, sodium taurocholate, and sodium deoxycholate, resulted in enhanced bioavailability and an increase in blood insulin for 20 h [207,208]. Liposomes containing ergosterol as the stabilizer, instead of cholesterol, have also shown a significant improvement in stability [209].
Recently, Lamson et al. reported on the development of anionic silica nanoparticles for the enhanced oral delivery of insulin [210]. The researchers found that both the size and charge of the silica nanoparticles influenced not only their interactions with the mucus barriers, but also the permeabilization of the epithelial barriers for enhanced insulin release into the bloodstream. Interestingly, 50 nm silica nanoparticles in juxtaposition to 20 nm or 100 nm particles, provided an optimal balance between increased mucus penetration and integrin-mediated TJ relaxation. Thus, 50 nm silica nanoparticles loaded with insulin displayed enhanced insulin bioavailability over what would normally be possible with oral drug delivery. This study lends support to other research that demonstrated nanostructural cues can influence TJ remodeling [29,205], and provides an example of the combined strengths of chemical and physical engineering of nanoscale drug carriers.
In addition to adjusting the physicochemical properties of drug carriers, the incorporation of stimuli-responsive materials in the reservoir-based drug delivery devices can advance the control over drug release. Using these materials, devices can be designed to be triggered and release the drug only under conditions simulating the GI tract. Nielsen et al. developed a platform of microwells for pH-triggered release of drugs [211]. The microwells were fabricated by hot embossing PLA. The microwells were then filled with amorphous sodium salt of furosemide (ASSF) powder via an improved screen-printing process, as a proof of principle for oral drug delivery. The release of ASSF occurred at pH 6.5 (intestinal pH). The proposed microwells can be used to protect the drug and prevent release before entering the intestine. Malachowski et al. developed a stimuli-responsive multi-fingered device that enabled site-specific delivery of various types of drugs by actively gripping the tissue at body temperature. It was proposed that gripping action increases the efficiency of drug delivery specially under flow conditions as this happens in the GI tract. The stimuli-responsive gripper was composed of rigid poly(propylene fumarate) and thermo-responsive poly(N-isopropylacrylamide-co-acrylic acid) hinges fabricated by photolithographic patterning [212]. The grippers had a porous structure that allowed loading of the device with commercially available drugs. When the device entered the body (above 32°C), it gripped the tissue and delivered the loaded drug over time (up to seven days for mesalamine and doxorubicin). Both in vitro and in vivo studies confirmed the improved delivery efficiency of doxorubicin.
In a different approach, scientists were inspired by transdermal patches to generate intestinal patches [152]. Intestinal patches are typically millimeters in size and have a mucoadhesive drug reservoir layer, a pH-sensitive layer, and a backing layer. Also, intestinal patches can be composed of water-insoluble polymers, pH-sensitive polymers, colors, fragrances, absorption enhancers, buffer substances, and preservatives [150]. While transdermal patches are designed for drug release of up to one week, intestinal patches are expected to release drugs over the course of hours [213]. Intestinal patches were invented to increase drug bioavailability, reduce the drug disruption by the GI tract, and prevent painful drug injection, such as anticancer drugs for cancer chemotherapy or insulin for diabetes [214].
Illustrations of various intestinal micropatch structures are shown in Fig. 11 . The two-layer patches comprised of a waterproof backing layer and a drug-laden mucoadhesive layer. The mucoadhesive layer provides strong adhesion to the intestinal mucosa. Cui et al. developed a novel two-layered micropatch drug delivery system for oral delivery of proteins. The micropatch consisted of bilaminated films, a hydrophobic ethylcellulose layer, a mucoadhesive chitosan-ethylenediaminetetraacetic acid hydrogel layer, and also carboxylated chitosan-grafted nanoparticles [215]. The adhesion of micropatches to the intestinal mucosa is driven by the attachment of cationic polymers to negative residues on mucin, or through polymer chain entanglement and hydrogen bonding. Banerjee et al. developed mucoadhesive intestinal patches that combine intestinal devices with dimethyl palmitoyl ammonia propane sulfonate as a permeation enhancer for oral delivery of insulin. The patches were delivered from a capsule coated with a pH-responsive coating. The patches adhered to intestinal mucosa, released cargo unidirectionally, and prevented enzymatic degradation in the gut [216]. As shown in Fig. 11, a typical 3-layer patch consists of a pH-responsive layer, a backing layer, and a drug-laden mucoadhesive layer. Four-layer patches typically have individual mucoadhesive and drug layers. Grabovac et al. made a three-layered oral delivery system for insulin delivery composed of thiolated polycarbophil as a polymeric matrix layer, a water-insoluble backing layer, and an enteric coating [217]. Eaimtrakarn et al. developed a four-layer patch consisting of a backing layer of ethyl cellulose to protect protein drug from enzymatic hydrolysis, a pH-sensitive surface layer, a drug-carrying middle layer, and a mucoadhesive layer. They tested three different pH-sensitive layers to achieve dissolution in different sections of the small intestine [218].
Fig. 11.
Examples of different intestinal patch structures including two-layered, three-layered, and four-layered patches. These patches deliver drugs with additional supportive layers. Reprinted from [216][], Copyright (2015), with permission from Elsevier.
Colon-targeting approaches have received increased attention in the field of oral drug delivery, as the longer retention time within the large intestine and colon, provide opportunities for sustained drug release and drug escape into the bloodstream. Varshosaz et al. used dextran to prepare a natural polymer-based microparticle system for targeted delivery to the colon of budesonide for ulcerative colitis treatment. Microcapsules were prepared with different drug-to-dextran ratios and three molecular weights of the polymer. Their results showed that budesonide-encapsulated microcapsules could target the colon, increasing the specificity of drug delivery, resulting in higher reduction of macroscopic damage and efficacy compared to mesalamine suspension [220].
In addition to microparticle systems, electrospun medicated shellac nanofibers have received interest for the fabrication of a colon-targeting delivery capsule to improve the bioavailability of poorly water soluble drugs and provide sustained release in the colon (Fig. 12 ) [221,222]. Wang et al. reported a simple method for the fabrication of capsule shells from a coaxial electrospinning method with a core fluid of shellac and ferulic acid (FA) and N,N-dimethylformamide as the shell [222]. Due to the insolubility of shellac at low pH, a small percentage of drug was released into solution at pH 2, mimicking the stomach pH, but sustained release was observed after 2 h incubation and transitioning to a neutral dissolution medium (Fig. 12b). Recently, there has been interest in the encapsulation of nanoparticles, such as micelles, liposomes, vesicles, and nanoparticles, in shellac nanofibers. In one study, Henning et al. developed liquid-filled shellac capsules to obtain colonic release of pectinate [223]. They showed that an external shellac layer significantly protected the pectinate from enzymes in the GI tract and extended the material retention time to several hours. Colon-targeted drug delivery has recently gained significant interest for bioactive proteins [224,197]. Ravi et al. developed a colon-targeted delivery system using inulin as an inner coating, followed by shellac as outer coating with diltiazem hydrochloride as a model drug. The release study showed that polymer-drug shellac tablets were insoluble in the stomach and intestinal environment and released the maximum amount of drug in the colonic environment to increase drug release into the bloodstream [221]. Wen et al. developed a core/shell structured nanofilm, using alginate as the outer shell layer and chitosan nanoparticles as the inner core layer for delivery of a model protein, bovine serum albumin [224]. In general, colon targeting fibrous materials could be useful for specific protein targeting the colon, with the aim of minimizing side effects and improving the local efficacy of proteins.
Fig. 12.
Preparation and characterization of shellac nanofibers and their applications in oral drug delivery. (a) A schematic illustrating the design strategy of medicated shellac nanofibers and the results of in vitro dissolution tests. (b) The FA release profiles and (c) SEM images (i, ii) just after dissolution, (iii, iv) 3h after dissolution, (v, vi) 7h after dissolution. Reprinted from [222], Copyright (2015), with permission from Elsevier.
In addition to controlling the drug carrier tissue-targeting properties, cellular interactions must also be controlled to improve bioadhesion and drug delivery efficacies. To target intestinal cells and improve cellular bioadhesion, chemical functionalization of materials is often performed. Antibodies and peptides specific for intestinal cells have recently been utilized for functionalization of drug carriers not only to increase adhesion, but also for target-based drug delivery to specific types of intestinal cells [23,103]. In one study, nanoparticles were grafted with trastuzumab (Herceptin, human epidermal growth factor receptor-2 antibody). The antibody-coated nanoparticles showed an increase in uptake by Caco-2 [225]. Another strategy is to use adhesion promoters, such as tethered or linear polymer chains to stimulate bioadhesion in drug delivery [226,227]. In another example, Ainslie et al. designed a microdevice with di-methacrylate hydrogel as a backbone to which avidin was covalently conjugated. Subsequently, biotinylated tomato lectin was added to increase the bioadhesion of the microdevice, as biotin has a strong binding affinity to avidin. In vitro testing results demonstrated that the Caco-2 epithelial colorectal cells were more attached to microdevices functionalized with lectin than non-functionalized devices [228]. Microdevices were conjugated with two lectins: (1) tomato, which is capable of attachment to Caco-2 cells, stable in low pH environments, and selective to small intestine epithelium; and (2) peanut, which has non-specific lectin sites. The lectin-conjugated microdevices showed a 2-4 fold higher degree of binding when compared to control microdevices. The tomato lectin conjugate showed significant difference of 2-folds over peanut lectin [229,230]. Similar strategies were applied for microspheres and micropatch systems to enhance the adhesion of the material.
To deliver drugs into the systemic circulation from the intestine, epithelial barriers and TJs must be overcome. Drug carriers have been shown to rearrange TJs and influence protein expression to enhance drug permeation across epithelial barriers. This is primarily achieved through the installation of nanotopography on microparticle carriers or on thin films. Uskokovic et al. demonstrated that nanowires protruding from the surface of silica microparticles facilitate the rearrangement of ZO-1 TJ proteins in Caco-2 monolayer models [231]. Subsequent work improved this design through the fabrication of planar nanowire-coated surfaces for enhanced tissue contact and TJ rearrangement (Fig. 13a) [232]. Nanostructured thin films have also been observed to reversibly loosen epithelial barriers, allowing for increased permeation of antibodies across TJs (Fig. 13b) [233]. In addition to influencing TJ behavior, recent work by Levy et al. demonstrated that PEG microdevices can inhibit P-gp efflux pump expression on Caco-2 monolayers, which may serve as a means of enhancing intestinal drug adsorption for oral drug delivery applications [234]. Although this study did not use nanostructured materials, it provides further evidence that physical parameters of nano and microtechnologies can influence cellular behavior to improve oral drug delivery applications. Taken together, these studies demonstrate that the physical engineering of nano and microscale materials can serve as important methods for enhancing drug penetration across epithelial barriers to improve systemic drug delivery via oral administration.
Fig. 13.
Physical approaches to modulate TJs for oral drug delivery. (a) Nanowire-coated silica microparticles and planar microdevices. Reprinted with permission from [232]. Copyright (2012) American Chemical Society (b) nanostructured thin films initiate ZO-1 TJ rearrangement to enhance drug penetration through epithelial barriers.Scale bars are 10 and 20 μm. Reprinted with permission from [233]. Copyright (2013) American Chemical Society.
7. Microfabricated in vitro models
In vitro modelling of the GI system is significantly helpful to investigate the performance of various oral drug delivery systems in GI tissues. In particular, in vitro GI tract models allow for the studying of drug permeation across biological barriers, as well as studying complex host-microbe interactions, while decreasing the costs and ethical issues involved with preclinical animal studies [235]. The physiological environment of the GI tract, including its functions and dynamic conditions, makes it a challenging organ to be modeled in vitro. Several in vitro models, such as organoids, trans-well co-culture, and macroscale bioreactors have been developed to study the GI tract and are reviewed in previous literature [236]. Here, we review systems that use microscale or nanoscale approaches to recapitulate the topography, motility, and flow present of the GI tract in microfabricated in vitro models and organ-on-a-chip platforms.
3D spheroid models aim to recapitulate in vivo biological conditions and have seen immense use as both basic science and drug discovery tools [237]. Micro and nanoscale technologies offer opportunities to improve on standard spheroid models and better mimic in vivo physiology. Several examples have been reported on the use of microparticles to direct cellular assembly and architecture in spheroid models [[238], [239], [240], [241]]. In a recent example, Samy et al. demonstrated that the growth of Caco-2 cells around a Matrigel microparticle initiates faster TJ formation when compared to the standard transwell model system [242]. This system also induces the proper polarization of apical and basal membranes, with actin and ZO-1 TJs displayed on the outer membrane of the spheroid, allowing easier drug access to the spheroid TJs in order to model intestinal drug transport in vitro.
Organ-on-a-chip platforms often provide a chamber to colonize cells in a specific arrangement that resembles the architecture of designed tissues or organs and mimics the physiological function of tissues or organs in a microfluidic system [243,244]. Kimura et al. developed a micro pumping system on-a-chip to model the gut epithelial using Caco-2 cells (Fig. 14 ). The device was used to investigate the perfusion and transportation of fluorescent compounds for applications in drug toxicity [245]. In further advancement of GI modeling on a chip, Imura et al. made a microchip-based system using Caco-2 cells to test drug absorption [246]. They also developed two organs-on-a-chip system using Caco-2 cells for intestine and HepG2 (liver hepatocellular) cells for liver. The intestinal absorption and hepatic metabolism were tested to demonstrate the feasibility of their device for in vitro assays [247]. In another work, Bricks et al. used a microfluidic platform to study the interaction between Caco-2 and HepG2. Their study showed that such coculture is important for proper metabolism and transformation of a tested compound [248]. A similar device was also used to study host-microbe molecular interactions in the gut. The intestinal epithelial barrier plays an important role in protecting our body from foreign organisms. To model this barrier, Tan et al. used a commercial chopstick-style electrode to record the trans epithelial electrical resistance and quantify the integrity of cellular lining in their system. The trans epithelial electrical resistance was also used to measure the permeability across the intestinal barrier [249]. In a more recent study, a modular GI tract-liver system was developed, using primary human intestinal epithelial cells and 3D liver micro-lobe like constructs [250]. The authors showed that the primary intestinal cells formed a monolayer and exhibited comparable cellular integrity to the native intestine.
Fig. 14.
The human gut-on-a-chip. (a) Schematic of the gut-on-a-chip device showing the porous ECM-coated membrane covered with gut epithelial cells and side vacuum chambers to apply mechanical strain on a membrane mimicking the role of peristaltic motion. Top channel (blue) represents the gut lumen and the bottom channel (red) represents the capillary bed underlaying the epithelial cells. (b) An actual image of the gut-on-a-chip device made of PDMS elastomer. Arrows show the flow direction and red and blue dyes in tubing correspond to the lower and upper microchannels, respectively, for channel visualization. (c) Schematics of intestinal monolayers cultured on the gut-on-a-chip porous membrane in the presence (right) or absence (left) of 30% mechanical strain applied by vacuum chambers and corresponding micrographs of epithelial cells on the porous membrane. Scale bar is 50 μm. Reproduced with permission. [255] Copyright 2012, Royal Society of Chemistry
The inclusion of dynamic conditions and fabrication of structures mimicking the intestinal architecture (e.g., crypts or villi) are important components in order to fully model the GI tract. To tackle this challenge, advanced models to recapitulate the microenvironment of the epithelium were recently developed. For instance, Costello et al. made in vitro small intestine tissue using polymeric scaffolds that mimic the 3D tissue architecture. The integrity data derived from trans epithelial electrical resistance showed that the inclusion of dynamic flow improved the integrity of the cell barrier as compared to static condition [251]. Shim et al. fabricated a collagen scaffold that mimics the intestinal villi to study the epithelium’s permeability [252]. This study showed that the inclusion of flow in the microfluidic tract, in addition to the incorporation of 3D structure, further increased the relevance of their model to the intestinal villi.
To further improve the biomimicry of in vitro models of GI, inclusion of gut motility is another mechanical cue that needs to be considered in the device design. To tackle this, a variety of cell stretchers were used to apply strain to a cell monolayer in different directions (uniaxial, biaxial, and radial stretching). Stretching is an important modulator of cell physiology in the GI tract. In particular, the epithelium in the intestine is stimulated by repetitive deformation caused by peristaltic movement and repetitive shortening of the villi. To investigate the effect of mechanical stimulation, Basson et al. cultured Caco-2 cells on an elastic membrane and subjected the cells to 10% strain. They showed that the cells proliferated faster and expressed brush border enzymes [253]. In a recent work, Caco-2 cell junctions were disrupted to analyze the impact of cyclic stretch on the TJ in cells. The results showed the increased paracellular permeability and re-organization of the junction's proteins after the disruption [254].
In one example of highly sophisticated human intestine models, known as “human gut-on-a-chip”, a microfluidic device was used to create both shear stress and cell stretching on a stretchable porous silicone membrane coated with extracellular matrix (ECM) proteins (Fig. 14a,b) [255]. The flow rate was adjusted to 30 μl/h to produce a shear stress of 0.02 dyne/cm2 to mimic the flow rate of the intestine. They also applied cyclic uniaxial strain (10%; 0.15 Hz) that mimicked the physiological peristaltic motions and facilitated the fast polarization of the epithelium (Fig. 14c). The cell monolayer developed with high integrity. This system was employed to co-culture intestinal epithelium cells with commensal microbes [256]. After one week, endotoxins and immune cells stimulated the epithelial cells to generate proinflammatory cytokines. The inflammatory cascade was induced by the villi injury and compromised the intestinal barrier function. The latter study showed that gut-on-a-chip is suitable to study the interaction between the microbiome and intestine in a pathophysiological environment. In a recent work, Kasendra et al. made a small intestine-on-a-chip system for culturing human intestinal vascular endothelium and primary epithelial cells [257]. The device also provided both uniaxial cyclic deformation and flow to the cells and showed the formation of villi-like structures (Fig. 15a) and functional gut epithelium and endothelium layers with TJs (Fig. 15b). This system also provided increased transcriptional similarities to the human duodenum.
Fig. 15.

Morphological and microscopical characterization of the primary human intestine on-a-chip. (a) Microscopic images of the intestinal epithelium grown on-a-chip after 12 days under cyclic strain and fluid flow showing the formation of epithelial villi-like protrusions. The images are stained for F-actin (magenta, brush border) and for nuclei (DAPI, blue). (b) Immunofluorescence images showing the intact TJs in the intestinal epithelium and underlying endothelium immunostained with ZO-1 (magenta), E-cadherin for epithelial cells (yellow), VE-cadherin for endothelial cells (green), and nuclei (DAPI, blue). Scale bars are 50 μm. Reproduced with permission [257]. Copyright 2018, Nature Publishing Group.
Taken together, the abovementioned studies are the most advanced micro-physiological models of the GI tract to date [258] Although the inclusion of a 3D and mature tissue composed of ECM, muscle cells, vasculature, and capillaries are missing from those models and would help to create a more biomimetic model of human GI tract. However, such simplified microphysiological models of GI tissues can be used to tackle challenges in oral drug delivery and improve our knowledge about the GI action in diverse microenvironments.
8. Bringing micro and nanoscale technologies into the clinic
It is crucial to push highly advanced drug delivery systems and devices towards clinical applications. Nanocarriers were first used in clinical trials in the 1960s to deliver small molecules that have poor pharmacokinetic profile, low solubility, and high off-target toxicity [259]. Among the developed particles for oral drug delivery, some polymer-based particles have been approved in clinical studies, and several liposomal formulations are under clinical investigations [260]. Additionally, other nanoparticle carriers, such as silica nanoparticles and calcium phosphate nanoparticles have been used in oral drug delivery and have begun to see translation into clinical trials [27]. These nanocarriers have been primarily used to deliver insulin, as well as small lipophilic peptides (~1–2 kDa molecular weight) with a cyclic structure, which are resistive to peptidase degradation.
Oral administration is a pillar of pharmaceutical industry. Currently 62% of FDA-approved drugs are orally administered drugs [262], thanks to ease of administration and high patient acceptance. While small hydrophobic molecules have been successful in oral administration, hydrophilic small molecules or peptides and proteins are yet to find their way to the clinical translation. Indeed, there is an unmet need for the delivery of proteins and peptides for which oral bioavailability is as low as <2% with current technologies [22]. Micro and nanotechnology have been showing to be promising to overcome these barriers. Although many microfabricated drug delivery systems are close to clinical trials, only a few formulations have seen translation into the clinic and numerous challenges remain for the future oral practice [263].
The oral delivery of biologicals could potentially improve the life of millions of patients and huge efforts are being put to push such solutions to the clinic [27][]. A self-emulsifying system for oral delivery is marketed for delivering immunosuppressing agent cyclosporine A (Sansimmune, CH). Similarly, self-emulsifying oral testosterone received FDA approval in 2019 (Jatenzo) [264]. Liposomal insulin formulation, which delivers drug to the liver, known as hepatocyte directed vesicular (HDV) insulin, showed promising results in phase 1 and 2 clinical trials [265]. The oral nanoformuation of this liposomal HDV insulin, composed of HDV conjugated insulin and a biotin-phosphatidylanolamine hepatic tag, is available now and is under phase 2, and 3 of clinical trials [266]. Liver-targeting liposomes for insulin delivery reached Phase III (NCT00814294). Silica nanoparticles also delivering insulin orally are currently in Phase II (NCT01973920, Oshadi Drug Administration, IL). Nanoparticles with a calcium phosphate core and pegylated salts of fatty acids, coated with carbomer and cellulose acetate phthalate for the delivery of insulin reached Phase I (ChiCTR-TRC-12 001 872, NOD/NodlinTM, CN). Nanomega Corp is encapsulating insulin in chitosan shelled gamma poly(g-glutamic acid)-based nanoparticles. These are key steppingstones that have to be leveraged for progressing micro and nanofabricated technologies for oral delivery of many therapeutic proteins. This knowledge can also be used for the delivery of hydrophilic small molecules. The challenges currently faced by micro and nanofabricated technologies are presented below.
Poor bioavailability has been the major bottleneck of translation of micro and nano technologies for oral delivery. Some practical and simple approaches have been proposed to tackle this problem. For example, nanoparticles can be coated with hydrophilic polymers, such as chitosan and PEG, and TPGS (a PEG-conjugated vitamin E) or can be synthesized with these polymers to further incorporate hydrophilic elements in the polymeric nanoparticles [267]. As a result, the permeability, solubility, stability, and thereby oral bioavailability of nanoparticles are enhanced.
Another important challenge for orally administered formulations is that nano- and microfabrication come at a cost that must be justified to make a product commercially cost-effective. This is achievable with high-throughput emulsion or self-assembly fabrication methods. However, more advanced microfabricated systems (3D printed or lithography enabled casting), have typically a complex engineering design and low-throughput manufacturing processes that result in high cost of fabrication [29,168,269,170]. For example, the current workflow for 3D printed drug delivery systems is multi-step (3D printing, drug loading, sealing, substrate release) and involves expensive equipment (3D printer, inkjet printer, spray coater) [270]. Recently, rapid and large-scale 3D printing was achieved using a mobile liquid interface that minimize heat buildup [271]. The evolution of such technology to print micro-scale elements in high-throughput could enable the progress of application of oral deliveries.
Expensive biologicals are frequently encapsulated in micro and nano-fabricated systems. Interestingly, a daily capsule of semaglutide requires a 100 times higher dose and more frequent administration, to achieve a similar efficacy compared to its weekly injection counterpart for the treatment of type 2 diabetes. A study was conducted to confirm that the additional costs incurred to produce extra semaglutide for the oral formulation would still generate a cost-effective oral formulation, given the greater quality of life experienced by the patients receiving the daily capsules [272]. This study testifies the need to carefully assess the dosing frequency against the efficacy when a molecule is selected for encapsulation in micro and nano-fabricated systems. This work also teaches the importance of direct comparison of oral delivery to other routes of administration.
Of particular interest is the applications of micro- and nanoscale technologies for delivery of therapies in preclinical and clinical trials for coronavirus disease 2019 (COVID-19). Antiviral drugs, such as remdesivir, chloroquine, and hydroxychloroquine have been proposed as promising drug candidates for treating coronavirus disease [[273], [274], [275]]. These antiviral drugs have been administered orally to inhibit virus infection with mild to moderate doses daily for a long time, which caused adverse events in patients. In order to overcome such issues, we suggest employing micro and nanotechnology-based drug delivery approaches to increase the efficacy of these drugs to prevent coronavirus infection.
Improving the standardization of preclinical parameters and procedures, including biodistribution, toxicity, protein adsorption, and device removal [27,269] will enable faster translation of technologies into the clinic. Despite these challenges, the opportunities for improving oral drug delivery using micro and nanoscale technologies is immense, and therefore the field should continue the push for translation into large animal models and eventual clinical trials.
9. Conclusion
During the past decade, researchers have had great leverage to create nano and microdevices that enable oral administration of different biomolecules. Indeed, the clinical translation of nanotechnology for drug delivery through the intravenous route, gave us a deep understanding of such systems. In parallel, the great progress of microfabrication methods was leveraged to serve the oral administration field. This progress was only recently reflected to marketed products. To unlock the full potential of such technologies, researchers should focus on inventing methods that would ensure robust manufacturing scale-up and solid proof of efficacy. It is crucial to push highly advanced drug delivery systems and devices towards clinical applications as they could be leading to an evolution of pharmaceutical industry towards more patient-friendly oral administration.
Acknowledgements
The authors have no competing interests. V.H. acknowledges the Swiss National Foundation Postdoctoral Fellowship (P2EZP2_187980). J.A.F. acknowledges the UCSF Health Innovation Via Engineering Postdoctoral Fellowship. S.S. acknowledges Fulbright-Nehru Postdoctoral Fellowship (Award No. 2395/FNPDR/2018). I.M. and M.M.S. acknowledge support from the British Heart Foundation Centre of Research Excellence (RE/18/4/34215). M.M.S. acknowledges support from the grant from the UK Regenerative Medicine Platform “Acellular / Smart Materials – 3D Architecture” (MR/R015651/1). F.N. acknowledges Schlumberger Foundation, Faculty for the Future Fellowship.
References
- 1.Sant S., Tao S.L., Fisher O.Z., Xu Q., Peppas N.A., Khademhosseini A. Microfabrication technologies for oral drug delivery. Adv. Drug Deliv. Rev. 2012;64(6):496–507. doi: 10.1016/j.addr.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sastry S.V., Nyshadham J.R., Fix J.A. Recent technological advances in oral drug delivery–a review. Pharmaceut Sci Tech Today. 2000;3(4):138–145. doi: 10.1016/s1461-5347(00)00247-9. [DOI] [PubMed] [Google Scholar]
- 3.Jiang D., England C.G., Cai W. DNA nanomaterials for preclinical imaging and drug delivery. J. Control. Release. 2016;239:27–38. doi: 10.1016/j.jconrel.2016.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maham A., Tang Z., Wu H., Wang J., Lin Y. Protein-based nanomedicine platforms for drug delivery. Small. 2009;5(15):1706–1721. doi: 10.1002/smll.200801602. [DOI] [PubMed] [Google Scholar]
- 5.Koziolek M., Grimm M., Schneider F., Jedamzik P., Sager M., Kuhn J.P., Siegmund W., Weitschies W. Navigating the human gastrointestinal tract for oral drug delivery: uncharted waters and new frontiers. Adv. Drug Deliv. Rev. 2016;101:75–88. doi: 10.1016/j.addr.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 6.Petit J., Meurice N., Kaiser C., Maggiora G. Softening the rule of five—where to draw the line? Bioorg. Med. Chem. 2012;20(18):5343–5351. doi: 10.1016/j.bmc.2011.11.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peyrot M., Rubin R.R., Kruger D.F., Travis L.B. Correlates of insulin injection omission. Diabetes Care. 2010;33(2):240–245. doi: 10.2337/dc09-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gedawy A., Martinez J., Al-Salami H., Dass C.R. Oral insulin delivery: existing barriers and current counter-strategies. J. Pharm. Pharmacol. 2018;70(2):197–213. doi: 10.1111/jphp.12852. [DOI] [PubMed] [Google Scholar]
- 9.Ahadian S., Civitarese R., Bannerman D., Mohammadi M.H., Lu R., Wang E., Davenport-Huyer L., Lai B., Zhang B., Zhao Y. Organ-on-a-chip platforms: a convergence of advanced materials, cells, and microscale technologies. Adv. Healthc. Mater. 2018;7(2) doi: 10.1002/adhm.201700506. [DOI] [PubMed] [Google Scholar]
- 10.Ahadian S., Ramón-Azcón J., Estili M., Obregón R., Shiku H., Matsue T. Facile and rapid generation of 3D chemical gradients within hydrogels for high-throughput drug screening applications. Biosens. Bioelectron. 2014;59:166–173. doi: 10.1016/j.bios.2014.03.031. [DOI] [PubMed] [Google Scholar]
- 11.Bae H., Chu H., Edalat F., Cha J.M., Sant S., Kashyap A., Ahari A.F., Kwon C.H., Nichol J.W., Manoucheri S., Zamanian B., Wang Y., Khademhosseini A. Development of functional biomaterials with micro- and nanoscale technologies for tissue engineering and drug delivery applications. J. Tissue Eng. Regen. Med. 2014;8(1):1–14. doi: 10.1002/term.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caldorera-Moore M., Peppas N.A. Micro- and nanotechnologies for intelligent and responsive biomaterial-based medical systems. Adv. Drug Deliv. Rev. 2009;61(15):1391–1401. doi: 10.1016/j.addr.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pan J., Chan S.Y., Lee W.G., Kang L. Microfabricated particulate drug-delivery systems. Biotechnol. J. 2011;6(12):1477–1487. doi: 10.1002/biot.201100237. [DOI] [PubMed] [Google Scholar]
- 14.Khademhosseini A., Langer R. Drug delivery and tissue engineering. Chem. Eng. Prog. 2006;102(2):38–42. [Google Scholar]
- 15.Park K. Controlled drug delivery systems: past forward and future back. J. Control. Release. 2014;190:3–8. doi: 10.1016/j.jconrel.2014.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dahan A., Hoffman A. Rationalizing the selection of oral lipid based drug delivery systems by an in vitro dynamic lipolysis model for improved oral bioavailability of poorly water soluble drugs. J. Control. Release. 2008;129(1):1–10. doi: 10.1016/j.jconrel.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 17.Son G.-H., Lee B.-J., Cho C.-W. Mechanisms of drug release from advanced drug formulations such as polymeric-based drug-delivery systems and lipid nanoparticles. Int. J. Pharm. Investig. 2017;47(4):287–296. [Google Scholar]
- 18.Prausnitz M.R., Elias P.M., Franz T.J., Schmuth M., Tsai J.-C., Menon G.K., Holleran W.M., Feingold K.R. Skin barrier and transdermal drug delivery. J Dermatology. 2012;3:2065–2073. [Google Scholar]
- 19.Groeber F., Holeiter M., Hampel M., Hinderer S., Schenke-Layland K. Skin tissue engineering—in vivo and in vitro applications. Adv. Drug Deliv. Rev. 2011;63(4–5):352–366. doi: 10.1016/j.addr.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 20.Prausnitz MR, Elias PM, Franz TJ, Schmuth M, Tsai J-C, Menon GK, Holleran WM, Feingold KR. Skin barrier and transdermal drug delivery. Dermatology. 2012;3:2065–2073. [Google Scholar]
- 21.Li H., Limenitakis J.P., Fuhrer T., Geuking M.B., Lawson M.A., Wyss M., Brugiroux S., Keller I., Macpherson J.A., Rupp S. The outer mucus layer hosts a distinct intestinal microbial niche. Nat. Commun. 2015;6(1):1–13. doi: 10.1038/ncomms9292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Homayun B., Lin X., Choi H.J. Challenges and Recent Progress in Oral Drug Delivery Systems for Biopharmaceuticals. Pharmaceutics. 2019;11(3):129. doi: 10.3390/pharmaceutics11030129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ensign L.M., Cone R., Hanes J. Oral drug delivery with polymeric nanoparticles: the gastrointestinal mucus barriers. Adv. Drug Deliv. Rev. 2012;64(6):557–570. doi: 10.1016/j.addr.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Renukuntla J., Vadlapudi A.D., Patel A., Boddu S.H., Mitra A.K. Approaches for enhancing oral bioavailability of peptides and proteins. Int. J. Pharm. 2013;447(1–2):75–93. doi: 10.1016/j.ijpharm.2013.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raja Moreno Miguel, Qi PeiLim, Yee Shan Wong, Gordon Xiong M., Zhang Yiming, Venkatraman Subbu. Nanocarriers for Drug Delivery. Elsevier; 2019. Polymeric Nanomaterials: Methods of Preparation and Characterization; pp. 557–653. [Google Scholar]
- 26.Brown T.D., Whitehead K.A., Mitragotri S. Materials for oral delivery of proteins and peptides. Nat. Rev. Mater. 2020;5:127–148. [Google Scholar]
- 27.Durán-Lobato M., Niu Z., Alonso M.J. Oral delivery of biologics for precision medicine. Adv. Mater. 2020;32 doi: 10.1002/adma.201901935. [DOI] [PubMed] [Google Scholar]
- 28.Han Y., Gao Z., Chen L., Kang L., Huang W., Jin M., Wang Q., Bae Y.H. Multifunctional oral delivery systems for enhanced bioavailability of therapeutic peptides/proteins. Acta Pharm. Sin. B. 2019;9(5) doi: 10.1016/j.apsb.2019.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fox C.B., Kim J., Le L.V., Nemeth C.L., Chirra H.D., Desai T.A. Micro/nanofabricated platforms for oral drug delivery. J. Control. Release. 2015;219:431–444. doi: 10.1016/j.jconrel.2015.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.LaVan D.A., Lynn D.M., Langer R. Moving smaller in drug discovery and delivery. Nat. Rev. Drug Discov. 2002;1(1):77–84. doi: 10.1038/nrd707. [DOI] [PubMed] [Google Scholar]
- 31.Belliveau N.M., Huft J., Lin P.J., Chen S., Leung A.K., Leaver T.J., Wild A.W., Lee J.B., Taylor R.J., Tam Y.K., Hansen C.L., Cullis P.R. Microfluidic Synthesis of Highly Potent Limit-size Lipid Nanoparticles for In Vivo Delivery of siRNA. Mol Ther Nucleic Acids. 2012;1:e37. doi: 10.1038/mtna.2012.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li X.J., Zhou Y. Elsevier; 2013. Microfluidic Devices for Biomedical Applications. [Google Scholar]
- 33.Nguyen N.T., Shaegh S.A., Kashaninejad N., Phan D.T. Design, fabrication and characterization of drug delivery systems based on lab-on-a-chip technology. Adv. Drug Deliv. Rev. 2013;65(11−12):1403–1419. doi: 10.1016/j.addr.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 34.Kawaguchi H. Functional polymer microspheres. Prog. Polym. Sci. 2000;25(8):1171–1210. [Google Scholar]
- 35.Makadia H.K., Siegel S.J. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers. 2011;3(3):1377–1397. doi: 10.3390/polym3031377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang Y., Fang Z., Chen X., Zhang W., Xie Y., Chen Y., Liu Z., Yuan W. An Overview of Pickering Emulsions: Solid-Particle Materials, Classification, Morphology, and Applications. Front. Pharmacol. 2017;8:287. doi: 10.3389/fphar.2017.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Varde N.K., Pack D.W. Microspheres for controlled release drug delivery. Expert. Opin. Biol. Ther. 2004;4(1):35–51. doi: 10.1517/14712598.4.1.35. [DOI] [PubMed] [Google Scholar]
- 38.McClements D.J. Advances in fabrication of emulsions with enhanced functionality using structural design principles. Curr. Opin. Colloid Interface Sci. 2012;17(5):235–245. [Google Scholar]
- 39.Branco M.C., Schneider J.P. Self-assembling materials for therapeutic delivery. Acta Biomater. 2009;5(3):817–831. doi: 10.1016/j.actbio.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rösler A., Vandermeulen G.W.M., Klok H.-A. Advanced drug delivery devices via self-assembly of amphiphilic block copolymers. Adv. Drug Deliv. Rev. 2012;64:270–279. doi: 10.1016/s0169-409x(01)00222-8. [DOI] [PubMed] [Google Scholar]
- 41.Torchilin V.P. Micellar nanocarriers: pharmaceutical perspectives. Pharm. Res. 2007;24:1–16. doi: 10.1007/s11095-006-9132-0. [DOI] [PubMed] [Google Scholar]
- 42.Sill T.J., Recum H.A. Electrospinning: applications in drug delivery and tissue engineering. Biomaterials. 2008;29(13):1989–2006. doi: 10.1016/j.biomaterials.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 43.Ignatious F., Sun L., Lee C.-P., Baldoni J. Electrospun nanofibers in Oral drug delivery. Pharm. Res. 2010;27(4):576–588. doi: 10.1007/s11095-010-0061-6. [DOI] [PubMed] [Google Scholar]
- 44.Thakkar S., Misra M. Electrospun polymeric nanofibers: new horizons in drug delivery. Eur. J. Pharm. Sci. 2017;107:148–167. doi: 10.1016/j.ejps.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 45.Riahi R., Tamayol A., Shaegh S.A.M., Ghaemmaghami A., Dokmeci M.R., Khademshosseini A. Microfluidics for Advanced Drug Delivery Systems. Curr Opin Chem Eng. 2015;7:101–112. doi: 10.1016/j.coche.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dou M., Sanjay S.T., Dominguez D.C., Liu P., Xu F., Li X. Multiplexed instrument-free meningitis diagnosis on a polymer/paper hybrid microfluidic biochip. Biosens. Bioelectron. 2017;87:865–873. doi: 10.1016/j.bios.2016.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weibel D.B., Diluzio W.R., Whitesides G.M. Microfabrication meets microbiology. Nat. Rev. Microbiol. 2007;5(3):209–218. doi: 10.1038/nrmicro1616. [DOI] [PubMed] [Google Scholar]
- 48.Whitesides G.M., Stroock A.D. Flexible methods for microfluidics. Phys. Today. 2001;54(6):42–48. [Google Scholar]
- 49.Catarino S.O., Rodrigues R.O., Pinho D., Miranda J.M., Minas G., Lima R. Blood Cells Separation and Sorting Techniques of Passive Microfluidic Devices: From Fabrication to Applications. Micromachines (Basel) 2019;10(9):593. doi: 10.3390/mi10090593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shamloo A., Abdorahimzadeh S., Nasiri R. Exploring contraction–expansion inertial microfluidic-based particle separation devices integrated with curved channels. AICHE J. 2019;65(11) [Google Scholar]
- 51.Sanjay S.T., Zhou W., Dou M., Tavakoli H., Ma L., Xu F., Li X. Recent advances of controlled drug delivery using microfluidic platforms. Adv. Drug Deliv. Rev. 2018;128:3–28. doi: 10.1016/j.addr.2017.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu M.H., Huang S.B., Lee G.B. Microfluidic cell culture systems for drug research. Lab Chip. 2010;10(8):939–956. doi: 10.1039/b921695b. [DOI] [PubMed] [Google Scholar]
- 53.Khademhosseini A., Langer R., Borenstein J., Vacanti J.P., et al. Proc. Natl. Acad. Sci. U. S. A. 2006;103(8):2480–2487. doi: 10.1073/pnas.0507681102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kang L., Chung B.G., Langer R., Khademhosseini A. Microfluidics for drug discovery and development: from target selection to product lifecycle management. Drug Discov. Today. 2008;13(1–2):1–13. doi: 10.1016/j.drudis.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dittrich P.S., Manz A. Lab-on-a-chip: microfluidics in drug discovery. Nat. Rev. Drug Discov. 2006;5(3):210–218. doi: 10.1038/nrd1985. [DOI] [PubMed] [Google Scholar]
- 56.Jun Y., Kang E., Chae S., Lee S.H. Microfluidic spinning of micro- and nano-scale fibers for tissue engineering. Lab Chip. 2014;14(13):2145–2160. doi: 10.1039/c3lc51414e. [DOI] [PubMed] [Google Scholar]
- 57.Tsui J.H., Lee W., Pun S.H., Kim J., Kim D.H. Microfluidics-assisted in vitro drug screening and carrier production. Adv. Drug Deliv. Rev. 2013;65(11–12):1575–1588. doi: 10.1016/j.addr.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hasani-Sadrabadi M.M., Taranejoo S., Dashtimoghadam E., Bahlakeh G., Majedi F.S., VanDersarl J.J., Janmaleki M., Sharifi F., Bertsch A., Hourigan K., Tayebi L., Renaud P., Jacob K.I. Microfluidic manipulation of Core/Shell nanoparticles for Oral delivery of chemotherapeutics: a new treatment approach for colorectal Cancer. Adv. Mater. 2016;28(21):4134–4141. doi: 10.1002/adma.201502697. [DOI] [PubMed] [Google Scholar]
- 59.Araujo F., Shrestha N., Shahbazi M.A., Liu D., Herranz-Blanco B., Makila E.M., Salonen J.J., Hirvonen J.T., Granja P.L., Sarmento B., Santos H.A. Microfluidic assembly of a multifunctional tailorable composite system designed for site specific combined Oral delivery of peptide drugs. ACS Nano. 2015;9(8):8291–8302. doi: 10.1021/acsnano.5b02762. [DOI] [PubMed] [Google Scholar]
- 60.Zhang H., Liu D., Shahbazi M.A., Makila E., Herranz-Blanco B., Salonen J., Hirvonen J., Santos H.A. Fabrication of a multifunctional nano-in-micro drug delivery platform by microfluidic templated encapsulation of porous silicon in polymer matrix. Adv. Mater. 2014;26(26):4497–4503. doi: 10.1002/adma.201400953. [DOI] [PubMed] [Google Scholar]
- 61.Liu D., Br Herranz-Blanco, Mäkilä E., Arriaga L.R., Mirza S., Weitz D.A., Sandler N., Salonen J., Hirvonen J., Santos HlA. Microfluidic templated mesoporous silicon–solid lipid microcomposites for sustained drug delivery. ACS Appl. Mater. Interfaces. 2013;5(22):12127–12134. doi: 10.1021/am403999q. [DOI] [PubMed] [Google Scholar]
- 62.Li W., Liu D., Zhang H., Correia A., Makila E., Salonen J., Hirvonen J., Santos H.A. Microfluidic assembly of a nano-in-micro dual drug delivery platform composed of halloysite nanotubes and a pH-responsive polymer for colon cancer therapy. Acta Biomater. 2017;48:238–246. doi: 10.1016/j.actbio.2016.10.042. [DOI] [PubMed] [Google Scholar]
- 63.Khan F.A., Narasimhan K., Swathi C.S.V., Mustak S., Mustafa G., Ahmad M.Z. Akhter S. 3D printing Technology in Customized Drug Delivery System: current state of the art, prospective and the challenges. Curr. Pharm. Des. 2018;24(42):5049–5061. doi: 10.2174/1381612825666190110153742. [DOI] [PubMed] [Google Scholar]
- 64.Khatri P., Shah M.K., Vora N. Formulation strategies for solid oral dosage form using 3D printing technology: a mini-review. J. Drug. Deliv. Sci. Technol. 2018;46:148–155. [Google Scholar]
- 65.Davoodi E., Montazerian H., Haghniaz R., Rashidi A., Ahadian S., Sheikhi A., Chen J., Khademhosseini A., Milani A.S., Hoorfar M. 3D-printed ultra-robust surface-doped porous silicone sensors for wearable biomonitoring. ACS Nano. 2020;14(2):1520–1532. doi: 10.1021/acsnano.9b06283. [DOI] [PubMed] [Google Scholar]
- 66.Montazerian H., Mohamed M., Montazeri M.M., Kheiri S., Milani A., Kim K., Hoorfar M. Permeability and mechanical properties of gradient porous PDMS scaffolds fabricated by 3D-printed sacrificial templates designed with minimal surfaces. Acta Biomater. 2019;96:149–160. doi: 10.1016/j.actbio.2019.06.040. [DOI] [PubMed] [Google Scholar]
- 67.Davoodi E., Fayazfar H., Liravi F., Jabari E., Toyserkani E. Drop-on-demand high-speed 3D printing of flexible milled carbon fiber/silicone composite sensors for wearable biomonitoring devices. Addit. Manuf. 2020;32 [Google Scholar]
- 68.Ashammakhi N., Ahadian S., Xu C., Montazerian H., Ko H., Nasiri R., Barros N., Khademhosseini A. Bioinks and bioprinting technologies to make heterogeneous and biomimetic tissue constructs. Materials Today Bio. 2019;1:1–23. doi: 10.1016/j.mtbio.2019.100008. 100008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fetah K., Tebon P., Goudie M.J., Eichenbaum J., Ren L., Barros N., Nasiri R., Ahadian S., Ashammakhi N., Dokmeci M.R. The emergence of 3D bioprinting in organ-on-chip systems. Progress in Biomedical Engineering. 2019;1(1) [Google Scholar]
- 70.Seyedmahmoud R., Çelebi-Saltik B., Barros N., Nasiri R., Banton E., Shamloo A., Ashammakhi N., Dokmeci M.R., Ahadian S. Three-dimensional bioprinting of functional skeletal muscle tissue using Gelatin Methacryloyl-alginate bioinks. Micromachines. 2019;10(10):679. doi: 10.3390/mi10100679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Alomari M., Mohamed F.H., Basit A.W., Gaisford S. Personalised dosing: printing a dose of one’s own medicine. Int. J. Pharm. 2015;494(2):568–577. doi: 10.1016/j.ijpharm.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 72.https://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/207958Orig1s000TOC.cfm
- 73.Prasad L.K., Smyth H. 3D printing technologies for drug delivery: a review. Drug Dev. Ind. Pharm. 2016;42(7):1019–1031. doi: 10.3109/03639045.2015.1120743. [DOI] [PubMed] [Google Scholar]
- 74.Lim S.H., Kathuria H., Tan J.J.Y., Kang L. 3D printed drug delivery and testing systems—a passing fad or the future? Adv. Drug Deliv. Rev. 2018;132:139–168. doi: 10.1016/j.addr.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 75.Nasrollahi F., Varshosaz J., Khodadadi A.A., Lim S., Jahanian-Najafabadi A. Targeted delivery of docetaxel by use of transferrin/poly (allylamine hydrochloride)-functionalized graphene oxide nanocarrier. ACS Appl. Mater. Interfaces. 2016;8(21):13282–13293. doi: 10.1021/acsami.6b02790. [DOI] [PubMed] [Google Scholar]
- 76.Nasrollahi F, Sana B, Paramelle D, Ahadian S, Khademhosseini A, Lim S. Incorporation of graphene quantum dots, Iron, and doxorubicin in/on ferritin Nanocages for bimodal imaging and drug delivery. Advanced Therapeutics. 2020;3:1900183. [Google Scholar]
- 77.Zhang L., Wang S., Zhang M., Sun J. Nanocarriers for oral drug delivery. J. Drug Target. 2013;21(6):515–527. doi: 10.3109/1061186X.2013.789033. [DOI] [PubMed] [Google Scholar]
- 78.Augustine R., Ashkenazi D.L., Arzi R.S., Zlobin V., Shofti R., Sosnik A. Nanoparticle-in-microparticle oral drug delivery system of a clinically relevant darunavir/ritonavir antiretroviral combination. Acta Biomater. 2018;74:344–359. doi: 10.1016/j.actbio.2018.04.045. [DOI] [PubMed] [Google Scholar]
- 79.Moreno J.A.S., Mendes A.C., Stephansen K., Engwer C., Goycoolea F.M., Boisen A., Nielsen L.H., Chronakis I.S. Development of electrosprayed mucoadhesive chitosan microparticles. Carbohydr. Polym. 2018;190:240–247. doi: 10.1016/j.carbpol.2018.02.062. [DOI] [PubMed] [Google Scholar]
- 80.Park C.G., Huh B.K., Kim S.N., Lee S.H., Hong H.R., Choy Y.B. Nanostructured mucoadhesive microparticles to enhance oral drug bioavailability. J. Ind. Eng. Chem. 2017;54:262–269. [Google Scholar]
- 81.Swider E., Koshkina O., Tel J., Cruz L.J., de Vries I.J.M., Srinivas M. Customizing poly(lactic-co-glycolic acid) particles for biomedical applications. Acta Biomater. 2018;73:38–51. doi: 10.1016/j.actbio.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 82.Lamprecht A., Rodero Torres H., Schäfer U., Lehr C.-M. Biodegradable microparticles as a two-drug controlled release formulation: a potential treatment of inflammatory bowel disease. J. Control. Release. 2000;69(3):445–454. doi: 10.1016/s0168-3659(00)00331-x. [DOI] [PubMed] [Google Scholar]
- 83.Valot P., Baba M., Nedelec J.M., Sintes-Zydowicz N. Effects of process parameters on the properties of biocompatible ibuprofen-loaded microcapsules. Int. J. Pharm. 2009;369(1–2):53–63. doi: 10.1016/j.ijpharm.2008.10.037. [DOI] [PubMed] [Google Scholar]
- 84.Petitti M., Barresi A.A., Vanni M. Controlled release of vancomycin from PCL microcapsules for an ophthalmic application. Chem. Eng. Res. Des. 2009;87(6):859–866. [Google Scholar]
- 85.Zhang Z., Zhu Y., Yang X., Li C. Preparation of azithromycin microcapsules by a layer-by-layer self-assembly approach and release behaviors of azithromycin. Colloids Surf. A Physicochem. Eng. Asp. 2010;362(1):135–139. [Google Scholar]
- 86.Moore C., Kosgodage U., Lange S., Inal J.M. The emerging role of exosome and microvesicle- (EMV-) based cancer therapeutics and immunotherapy. Int. J. Cancer. 2017;141(3):428–436. doi: 10.1002/ijc.30672. [DOI] [PubMed] [Google Scholar]
- 87.Tang K., Zhang Y., Zhang H., Xu P., Liu J., Ma J., Lv M., Li D., Katirai F., Shen G.X., Zhang G., Feng Z.H., Ye D., Huang B. Delivery of chemotherapeutic drugs in tumour cell-derived microparticles. Nat. Commun. 2012;3:1282. doi: 10.1038/ncomms2282. [DOI] [PubMed] [Google Scholar]
- 88.McQuilling J.P., Arenas-Herrera J., Childers C., Pareta R.A., Khanna O., Jiang B., Brey E.M., Farney A.C., Opara E.C. New alginate microcapsule system for angiogenic protein delivery and immunoisolation of islets for transplantation in the rat omentum pouch. Transplant. Proc. 2011;43(9):3262–3264. doi: 10.1016/j.transproceed.2011.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Glowka E., Stasiak J., Lulek J. Drug Delivery Systems for Vitamin D Supplementation and Therapy. Pharmaceutics. 2019;11(7):347. doi: 10.3390/pharmaceutics11070347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Han F.Y., Thurecht K.J., Whittaker A.K., Smith M.T. Bioerodable PLGA-Based Microparticles for Producing Sustained-Release Drug Formulations and Strategies for Improving Drug Loading. Front. Pharmacol. 2016;7(185) doi: 10.3389/fphar.2016.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lengyel M., Kállai-Szabó N., Antal V., Laki A.J., Antal I. Microparticles, microspheres, and microcapsules for advanced drug delivery. Sci. Pharm. 2019;87(3) [Google Scholar]
- 92.Noble G.T., Stefanick J.F., Ashley J.D., Kiziltepe T., Bilgicer B. Ligand-targeted liposome design: challenges and fundamental considerations. Trends Biotechnol. 2014;32(1):32–45. doi: 10.1016/j.tibtech.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 93.Çağdaş M., Sezer A.D., Bucak S. Liposomes as potential drug carrier systems for drug delivery. Application of nanotechnology in drug delivery. 2014:1–100. [Google Scholar]
- 94.Immordino M.L., Dosio F., Cattel L. Stealth liposomes: review of the basic science, rationale, and clinical applications, existing and potential. Int. J. Nanomedicine. 2006;1(3):297. [PMC free article] [PubMed] [Google Scholar]
- 95.Law S.L., Huang K.J., Chiang C.H. Acyclovir-containing liposomes for potential ocular delivery. Corneal penetration and absorption. J. Control. Release. 2000;63(1–2):135–140. doi: 10.1016/s0168-3659(99)00192-3. [DOI] [PubMed] [Google Scholar]
- 96.Li P., Nielsen H.M., Müllertz A. Oral delivery of peptides and proteins using lipid-based drug delivery systems. Expert. Opin. Drug. Del. 2012;9(10):1289–1304. doi: 10.1517/17425247.2012.717068. [DOI] [PubMed] [Google Scholar]
- 97.Mehnert W., Mäder K. Solid lipid nanoparticles: production, characterization and applications. Adv. Drug Deliv. Rev. 2012;64:83–101. doi: 10.1016/s0169-409x(01)00105-3. [DOI] [PubMed] [Google Scholar]
- 98.Sanjula B., Shah F.M., Javed A., Alka A. Effect of poloxamer 188 on lymphatic uptake of carvedilol-loaded solid lipid nanoparticles for bioavailability enhancement. J. Drug Target. 2009;17(3):249–256. doi: 10.1080/10611860902718672. [DOI] [PubMed] [Google Scholar]
- 99.Kumar V.V., Chandrasekar D., Ramakrishna S., Kishan V., Rao Y.M., Diwan P.V. Development and evaluation of nitrendipine loaded solid lipid nanoparticles: influence of wax and glyceride lipids on plasma pharmacokinetics. Int. J. Pharm. 2007;335(1–2):167–175. doi: 10.1016/j.ijpharm.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 100.Luo C.F., Yuan M., Chen M.S., Liu S.M., Zhu L., Huang B.Y., Liu X.W., Xiong W. Pharmacokinetics, tissue distribution and relative bioavailability of puerarin solid lipid nanoparticles following oral administration. Int. J. Pharm. 2011;410(1–2):138–144. doi: 10.1016/j.ijpharm.2011.02.064. [DOI] [PubMed] [Google Scholar]
- 101.Boushra M., Tous S., Fetih G., Korzekwa K., Lebo D.B., Xue H.Y., Wong H.L. Development and evaluation of viscosity-enhanced nanocarrier (VEN) for oral insulin delivery. Int. J. Pharm. 2016;511(1):462–472. doi: 10.1016/j.ijpharm.2016.07.016. [DOI] [PubMed] [Google Scholar]
- 102.Woodley J. Bioadhesion: new possibilities for drug administration? Clin. Pharmacokinet. 2001;40(2):77–84. doi: 10.2165/00003088-200140020-00001. [DOI] [PubMed] [Google Scholar]
- 103.des Rieux A., Fievez V., Garinot M., Schneider Y.-J., Préat V. Nanoparticles as potential oral delivery systems of proteins and vaccines: a mechanistic approach. J. Control. Release. 2006;116(1):1–27. doi: 10.1016/j.jconrel.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 104.Eldridge J.H., Hammond C.J., Meulbroek J.A., Staas J.K., Gilley R.M., Tice T.R. Controlled vaccine release in the gut-associated lymphoid tissues. I. Orally administered biodegradable microspheres target the Peyer’s patches. J. Control. Release. 1990;11(1–3):205–214. [Google Scholar]
- 105.https://www.accessdata.fda.gov/scripts/publications/search_result_record.cfm?id=63227
- 106.Khalil N.M., do Nascimento T.C.F., Casa D.M., Dalmolin L.F., de Mattos A.C., Hoss I., Romano M.A., Mainardes R.M. Pharmacokinetics of curcumin-loaded PLGA and PLGA–PEG blend nanoparticles after oral administration in rats. Colloids Surf. B: Biointerfaces. 2013;101:353–360. doi: 10.1016/j.colsurfb.2012.06.024. [DOI] [PubMed] [Google Scholar]
- 107.Vasiliu S., Bunia I., Racovita S., Neagu V. Adsorption of cefotaxime sodium salt on polymer coated ion exchange resin microparticles: kinetics, equilibrium and thermodynamic studies. Carbohydr. Polym. 2011;85(2):376–387. [Google Scholar]
- 108.Wang W., Liu L., Ju X.J., Zerrouki D., Xie R., Yang L., Chu L.Y. A novel thermo-induced self-bursting microcapsule with magnetic-targeting property. Chemphyschem. 2009;10(14):2405–2409. doi: 10.1002/cphc.200900450. [DOI] [PubMed] [Google Scholar]
- 109.Koetting M.C., Guido J.F., Gupta M., Zhang A., Peppas N.A. pH-responsive and enzymatically-responsive hydrogel microparticles for the oral delivery of therapeutic proteins: effects of protein size, crosslinking density, and hydrogel degradation on protein delivery. J. Control. Release. 2016;221:18–25. doi: 10.1016/j.jconrel.2015.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lee S.C., Huh K.M., Lee J., Cho Y.W., Galinsky R.E., Park K. Hydrotropic polymeric micelles for enhanced paclitaxel solubility: in vitro and in vivo characterization. Biomacromolecules. 2007;8(1):202–208. doi: 10.1021/bm060307b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Coimbra M., Rijcken C.J., Stigter M., Hennink W.E., Storm G., Schiffelers R.M. Antitumor efficacy of dexamethasone-loaded core-crosslinked polymeric micelles. J. Control. Release. 2012;163(3):361–367. doi: 10.1016/j.jconrel.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 112.Satturwar P., Eddine M.N., Ravenelle F., Leroux J.C. pH-responsive polymeric micelles of poly(ethylene glycol)-b-poly(alkyl(meth)acrylate-co-methacrylic acid): influence of the copolymer composition on self-assembling properties and release of candesartan cilexetil. Eur. J. Pharm. Biopharm. 2007;65(3):379–387. doi: 10.1016/j.ejpb.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 113.Vrignaud S., Anton N., Gayet P., Benoit J.P., Saulnier P. Reverse micelle-loaded lipid nanocarriers: a novel drug delivery system for the sustained release of doxorubicin hydrochloride. Eur. J. Pharm. Biopharm. 2011;79(1):197–204. doi: 10.1016/j.ejpb.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 114.Tian H., He Z., Sun C., Yang C., Zhao P., Liu L., Leong K.W., Mao H.Q., Liu Z., Chen Y. Uniform core–shell nanoparticles with thiolated hyaluronic acid coating to enhance oral delivery of insulin. Adv. Healthc. Mater. 2018;7(17) doi: 10.1002/adhm.201800285. [DOI] [PubMed] [Google Scholar]
- 115.Dufresne M.H., Gauthier M.A., Leroux J.C. Thiol-functionalized polymeric micelles: from molecular recognition to improved mucoadhesion. Bioconjug. Chem. 2005;16(4):1027–1033. doi: 10.1021/bc050007b. [DOI] [PubMed] [Google Scholar]
- 116.Ma X.Y., Pan G.M., Lu Z., Hu J.S., Bei J.Z., Jia J.H., Wang S.G. Preliminary study of oral polylactide microcapsulated insulin in vitro and in vivo. Diabetes Obes. Metab. 2000;2(4):243–250. doi: 10.1046/j.1463-1326.2000.00080.x. [DOI] [PubMed] [Google Scholar]
- 117.Guan Q., Chen W., Hu X. Development of lovastatin-loaded poly(lactic acid) microspheres for sustained oral delivery: in vitro and ex vivo evaluation. Drug Des Devel Ther. 2015;9:791–798. doi: 10.2147/DDDT.S76676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mandal T.K., Bostanian L.A., Graves R.A., Chapman S.R., Womack I. Development of biodegradable microcapsules as carrier for oral controlled delivery of amifostine. Drug Dev. Ind. Pharm. 2002;28(3):339–344. doi: 10.1081/ddc-120002849. [DOI] [PubMed] [Google Scholar]
- 119.Tian J., Sun X., Chen X., Yu J., Qu L., Wang L. The formulation and immunisation of oral poly(DL-lactide-co-glycolide) microcapsules containing a plasmid vaccine against lymphocystis disease virus in Japanese flounder (Paralichthys olivaceus) Int. Immunopharmacol. 2008;8(6):900–908. doi: 10.1016/j.intimp.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 120.Cheng J., Teply B.A., Jeong S.Y., Yim C.H., Ho D., Sherifi I., Jon S., Farokhzad O.C., Khademhosseini A., Langer R.S. Magnetically responsive polymeric microparticles for oral delivery of protein drugs. Pharm. Res. 2006;23(3):557–564. doi: 10.1007/s11095-005-9444-5. [DOI] [PubMed] [Google Scholar]
- 121.Prasad S., Tyagi A.K., Aggarwal B.B. Recent developments in delivery, bioavailability, absorption and metabolism of curcumin: the golden pigment from golden spice. Cancer Res. Treat. 2014;46(1):2–18. doi: 10.4143/crt.2014.46.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhou X., Chen Q., Ma Y., Huang Y., Gou S., Xiao B. Porous polymeric microparticles as an Oral drug platform for effective ulcerative colitis treatment. J. Pharm. Sci. 2019;108(7):2238–2242. doi: 10.1016/j.xphs.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 123.Benoit M.A., Baras B., Gillard J. Preparation and characterization of protein-loaded poly(epsilon-caprolactone) microparticles for oral vaccine delivery. Int. J. Pharm. 1999;184(1):73–84. doi: 10.1016/s0378-5173(99)00109-x. [DOI] [PubMed] [Google Scholar]
- 124.Barboza F.M., Machado W.M., Olchanheski Junior L.R., Padilha de Paula J., Zawadzki S.F., Fernandes D., Farago P.V. PCL/PHBV microparticles as innovative carriers for oral controlled release of manidipine dihydrochloride. ScientificWorldJournal. 2014;2014:268107. doi: 10.1155/2014/268107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chattopadhyay H., De A.K., Datta S. Novel Starch-PVA Polymer for Microparticle Preparation and Optimization Using Factorial Design Study. Int Sch Res Notices. 2015;2015:261476. doi: 10.1155/2015/261476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rassu G., Nieddu M., Bosi P., Trevisi P., Colombo M., Priori D., Manconi P., Giunchedi P., Gavini E., Boatto G. Encapsulation and modified-release of thymol from oral microparticles as adjuvant or substitute to current medications. Phytomedicine. 2014;21(12):1627–1632. doi: 10.1016/j.phymed.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 127.Pandav S., Preparation Naik J. In Vitro Evaluation of Ethylcellulose and Polymethacrylate Resins Loaded Microparticles Containing Hydrophilic Drug. J Pharm (Cairo) 2014;2014:904036. doi: 10.1155/2014/904036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bulut E. Chitosan coated- and uncoated-microspheres of sodium carboxymethyl cellulose/polyvinyl alcohol crosslinked with ferric ion: flurbiprofen loading and in vitro drug release study. J. Macromol. Sci. A. 2019;57(1):72–82. [Google Scholar]
- 129.Gadalla H.H., El-Gibaly I., Soliman G.M., Mohamed F.A., El-Sayed A.M. Amidated pectin/sodium carboxymethylcellulose microspheres as a new carrier for colonic drug targeting: development and optimization by factorial design. Carbohydr. Polym. 2016;153:526–534. doi: 10.1016/j.carbpol.2016.08.018. [DOI] [PubMed] [Google Scholar]
- 130.van der Lubben I.M., Verhoef J.C., van Aelst A.C., Borchard G., Junginger H.E. Chitosan microparticles for oral vaccination: preparation, characterization and preliminary in vivo uptake studies in murine Peyer’s patches. Biomaterials. 2001;22(7):687–694. doi: 10.1016/s0142-9612(00)00231-3. [DOI] [PubMed] [Google Scholar]
- 131.Ramalingam P., Ko Y.T. Enhanced oral delivery of curcumin from N-trimethyl chitosan surface-modified solid lipid nanoparticles: pharmacokinetic and brain distribution evaluations. Pharm. Res. 2015;32(2):389–402. doi: 10.1007/s11095-014-1469-1. [DOI] [PubMed] [Google Scholar]
- 132.Gadalla H.H., Soliman G.M., Mohammed F.A., El-Sayed A.M. Development and in vitro/in vivo evaluation of Zn-pectinate microparticles reinforced with chitosan for the colonic delivery of progesterone. Drug Deliv. 2016;23(7):2541–2554. doi: 10.3109/10717544.2015.1028602. [DOI] [PubMed] [Google Scholar]
- 133.Sahiner N., Suner S.S., Ayyala R.S. Mesoporous, degradable hyaluronic acid microparticles for sustainable drug delivery application. Colloids Surf. B: Biointerfaces. 2019;177:284–293. doi: 10.1016/j.colsurfb.2019.02.015. [DOI] [PubMed] [Google Scholar]
- 134.Zhang Z., Zhang R., Zou L., Chen L., Ahmed Y., Al Bishri W., Balamash K., McClements D.J. Encapsulation of curcumin in polysaccharide-based hydrogel beads: impact of bead type on lipid digestion and curcumin bioaccessibility. Food Hydrocoll. 2016;58:160–170. [Google Scholar]
- 135.Builders P.F., Kunle O.O., Okpaku L.C., Builders M.I. Attama AA, Adikwu MU. Preparation and evaluation of mucinated sodium alginate microparticles for oral delivery of insulin. Eur. J. Pharm. Biopharm. 2008;70(3):777–783. doi: 10.1016/j.ejpb.2008.06.021. [DOI] [PubMed] [Google Scholar]
- 136.Patel Z.S., Ueda H., Yamamoto M., Tabata Y., Mikos A.G. In vitro and in vivo release of vascular endothelial growth factor from gelatin microparticles and biodegradable composite scaffolds. Pharm. Res. 2008;25(10):2370–2378. doi: 10.1007/s11095-008-9685-1. [DOI] [PubMed] [Google Scholar]
- 137.Patel Z.S., Yamamoto M., Ueda H., Tabata Y., Mikos A.G. Biodegradable gelatin microparticles as delivery systems for the controlled release of bone morphogenetic protein-2. Acta Biomater. 2008;4(5):1126–1138. doi: 10.1016/j.actbio.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Silva D.M., Vyas H.K.N., Sanderson-Smith M.L., Sencadas V. Development and optimization of ciprofloxacin-loaded gelatin microparticles by single-step spray-drying technique. Powder Technol. 2018;330:201–209. [Google Scholar]
- 139.Sajeesh S., Vauthier C., Gueutin C., Ponchel G., Sharma C.P. Thiol functionalized polymethacrylic acid-based hydrogel microparticles for oral insulin delivery. Acta Biomater. 2010;6(8):3072–3080. doi: 10.1016/j.actbio.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 140.Saboktakin M.R., Tabatabaie R.M., Maharramov A., Ramazanov M.A. Synthesis and in vitro evaluation of carboxymethyl starch-chitosan nanoparticles as drug delivery system to the colon. Int. J. Biol. Macromol. 2011;48(3):381–385. doi: 10.1016/j.ijbiomac.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 141.Bulut E. Ibuprofen microencapsulation within acrylamide-grafted chitosan and methylcellulose interpenetrating polymer network microspheres: synthesis, characterization, and release studies. Artif Cells Nanomed Biotechnol. 2016;44(4):1098–1108. doi: 10.3109/21691401.2015.1011802. [DOI] [PubMed] [Google Scholar]
- 142.Anselmo A.C., Xu X., Buerkli S., Zeng Y., Tang W., McHugh K.J., Behrens A.M., Rosenberg E., Duan A.R., Sugarman J.L., Zhuang J., Collins J., Lu X., Graf T., Tzeng S.Y., Rose S., Acolatse S., Nguyen T.D., Le X., Guerra A.S., Freed L.E., Weinstock S.B., Sears C.B., Nikolic B., Wood L., Welkhoff P.A., Oxley J.D., Moretti D., Zimmermann M.B., Langer R., Jaklenec A. A heat-stable microparticle platform for oral micronutrient delivery. Sci. Transl. Med. 2019;11(518):eaaw3680. doi: 10.1126/scitranslmed.aaw3680. [DOI] [PubMed] [Google Scholar]
- 143.Mo R., Jiang T., Di J., Tai W., Gu Z. Emerging micro- and nanotechnology based synthetic approaches for insulin delivery. Chem. Soc. Rev. 2014;43(10):3595–3629. doi: 10.1039/c3cs60436e. [DOI] [PubMed] [Google Scholar]
- 144.Andreani T., de Souza A.L., Kiill C.P., Lorenzon E.N., Fangueiro J.F., Calpena A.C., Chaud M.V., Garcia M.L., Gremiao M.P., Silva A.M., Souto E.B. Preparation and characterization of PEG-coated silica nanoparticles for oral insulin delivery. Int. J. Pharm. 2014;473(1–2):627–635. doi: 10.1016/j.ijpharm.2014.07.049. [DOI] [PubMed] [Google Scholar]
- 145.Zheng X., Wang T., Jiang H., Li Y., Jiang T., Zhang J., Wang S. Incorporation of carvedilol into PAMAM-functionalized MWNTs as a sustained drug delivery system for enhanced dissolution and drug-loading capacity. Asian J. Pharm. Sci. 2013;8(5):278–286. [Google Scholar]
- 146.Andreani T., Miziara L., Lorenzon E.N., de Souza A.L., Kiill C.P., Fangueiro J.F., Garcia M.L., Gremiao P.D., Silva A.M., Souto E.B. Effect of mucoadhesive polymers on the in vitro performance of insulin-loaded silica nanoparticles: interactions with mucin and biomembrane models. Eur. J. Pharm. Biopharm. 2015;93:118–126. doi: 10.1016/j.ejpb.2015.03.027. [DOI] [PubMed] [Google Scholar]
- 147.Colley H.E., Said Z., Santocildes-Romero M.E., Baker S.R., D’Apice K., Hansen J., Madsen L.S., Thornhill M.H., Hatton P.V., Murdoch C. Pre-clinical evaluation of novel mucoadhesive bilayer patches for local delivery of clobetasol-17-propionate to the oral mucosa. Biomaterials. 2018;178:134–146. doi: 10.1016/j.biomaterials.2018.06.009. [DOI] [PubMed] [Google Scholar]
- 148.Banerjee A., Chen R., Arafin S., Mitragotri S. Intestinal iontophoresis from mucoadhesive patches: a strategy for oral delivery. J. Control. Release. 2019;297:71–78. doi: 10.1016/j.jconrel.2019.01.037. [DOI] [PubMed] [Google Scholar]
- 149.Whitehead K., Shen Z., Mitragotri S. Oral delivery of macromolecules using intestinal patches: applications for insulin delivery. J. Control. Release. 2004;98(1):37–45. doi: 10.1016/j.jconrel.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 150.Kirsch K., Hanke U., Weitschies W. An overview of intestinal wafers for oral drug delivery. Eur. J. Pharm. Biopharm. 2017;114:135–144. doi: 10.1016/j.ejpb.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 151.Shinkar D.M., Dhake A.S., Setty C.M. Drug delivery from the oral cavity: a focus on mucoadhesive buccal drug delivery systems. PDA J. Pharm. Sci. Technol. 2012;66(5):466–500. doi: 10.5731/pdajpst.2012.00877. [DOI] [PubMed] [Google Scholar]
- 152.Teutonico D., Ponchel G. Patches for improving gastrointestinal absorption: an overview. Drug Discov. Today. 2011;16(21−22):991–997. doi: 10.1016/j.drudis.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 153.Gioumouxouzis C.I., Katsamenis O.L., Bouropoulos N., Fatouros D.G. 3D printed oral solid dosage forms containing hydrochlorothiazide for controlled drug delivery. J. Drug. Deliv. Sci. Technol. 2017;40:164–171. [Google Scholar]
- 154.Li Q., Wen H., Jia D., Guan X., Pan H., Yang Y., Yu S., Zhu Z., Xiang R., Pan W. Preparation and investigation of controlled-release glipizide novel oral device with three-dimensional printing. Int. J. Pharm. 2017;525(1):5–11. doi: 10.1016/j.ijpharm.2017.03.066. [DOI] [PubMed] [Google Scholar]
- 155.Gioumouxouzis C.I., Baklavaridis A., Katsamenis O.L., Markopoulou C.K., Bouropoulos N., Tzetzis D., Fatouros D.G. A 3D printed bilayer oral solid dosage form combining metformin for prolonged and glimepiride for immediate drug delivery. Eur. J. Pharm. Sci. 2018;120:40–52. doi: 10.1016/j.ejps.2018.04.020. [DOI] [PubMed] [Google Scholar]
- 156.Buanz A.B.M., Belaunde C.C., Soutari N., Tuleu C., Gul M.O., Gaisford S. Ink-jet printing versus solvent casting to prepare oral films: effect on mechanical properties and physical stability. Int. J. Pharm. 2015;494(2):611–618. doi: 10.1016/j.ijpharm.2014.12.032. [DOI] [PubMed] [Google Scholar]
- 157.Khaled S.A., Burley J.C., Alexander M.R., Yang J., Roberts C.J. 3D printing of five-in-one dose combination polypill with defined immediate and sustained release profiles. J. Control. Release. 2015;217:308–314. doi: 10.1016/j.jconrel.2015.09.028. [DOI] [PubMed] [Google Scholar]
- 158.Maroni A., Melocchi A., Parietti F., Foppoli A., Zema L., Gazzaniga A. 3D printed multi-compartment capsular devices for two-pulse oral drug delivery. J. Control. Release. 2017;268:10–18. doi: 10.1016/j.jconrel.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 159.Melocchi A., Parietti F., Loreti G., Maroni A., Gazzaniga A., Zema L. 3D printing by fused deposition modeling (FDM) of a swellable/erodible capsular device for oral pulsatile release of drugs. J. Drug. Deliv. Sci. Technol. 2015;30:360–367. [Google Scholar]
- 160.Goyanes A., Wang J., Buanz A., Martinez-Pacheco R., Telford R., Gaisford S., Basit A.W. 3D printing of medicines: engineering novel Oral devices with unique design and drug release characteristics. Mol. Pharm. 2015;12(11):4077–4084. doi: 10.1021/acs.molpharmaceut.5b00510. [DOI] [PubMed] [Google Scholar]
- 161.Kirtane A.R., Abouzid O., Minahan D., Bensel T., Hill A.L., Selinger C., Bershteyn A., Craig M., Mo S.S., Mazdiyasni H., Cleveland C., Rogner J., Lee Y.L., Booth L., Javid F., Wu S.J., Grant T., Bellinger A.M., Nikolic B., Hayward A., Wood L., Eckhoff P.A., Nowak M.A., Langer R., Traverso G. Development of an oral once-weekly drug delivery system for HIV antiretroviral therapy. Nat. Commun. 2018;9(1):2. doi: 10.1038/s41467-017-02294-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lim S.H., Chia S.M., Kang L., Yap K.Y. Three-dimensional printing of carbamazepine sustained-release scaffold. J. Pharm. Sci. 2016;105(7):2155–2163. doi: 10.1016/j.xphs.2016.04.031. [DOI] [PubMed] [Google Scholar]
- 163.Marizza P., Keller S.S., Müllertz A., Boisen A. Polymer-filled microcontainers for oral delivery loaded using supercritical impregnation. J. Control. Release. 2014;173:1–9. doi: 10.1016/j.jconrel.2013.09.022. [DOI] [PubMed] [Google Scholar]
- 164.Marizza P., Pontoni L., Rindzevicius T., Alopaeus J., Su K., Zeitler J., Keller S.S., Kikic I., Moneghini M., De Zordi N. Supercritical impregnation of polymer matrices spatially confined in microcontainers for oral drug delivery: effect of temperature, pressure and time. J. Supercrit. Fluids. 2016;107:145–152. [Google Scholar]
- 165.Guan J., He H., Lee L.J., Hansford D.J. Fabrication of particulate reservoir-containing, capsulelike, and self-folding polymer microstructures for drug delivery. Small. 2007;3(3):412–418. doi: 10.1002/smll.200600240. [DOI] [PubMed] [Google Scholar]
- 166.Mazzoni C., Tentor F., Strindberg S.A., Nielsen L.H., Keller S.S., Alstrom T.S., Gundlach C., Mullertz A., Marizza P., Boisen A. From concept to in vivo testing: microcontainers for oral drug delivery. J. Control. Release. 2017;268:343–351. doi: 10.1016/j.jconrel.2017.10.013. [DOI] [PubMed] [Google Scholar]
- 167.D’Emanuele A., Jevprasesphant R., Penny J., Attwood D. The use of a dendrimer-propranolol prodrug to bypass efflux transporters and enhance oral bioavailability. J. Control. Release. 2004;95(3):447–453. doi: 10.1016/j.jconrel.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 168.Gardiner J., Freeman S., Leach M., Green A., Alcock J., D’Emanuele A. PAMAM dendrimers for the delivery of the antibacterial Triclosan. Journal of enzyme inhibition and medicinal chemistry. 2008;23(5):623–628. doi: 10.1080/14756360802205257. [DOI] [PubMed] [Google Scholar]
- 169.Winnicka K., Wroblewska M., Wieczorek P., Sacha P.T., Tryniszewska E. Hydrogel of ketoconazole and PAMAM dendrimers: formulation and antifungal activity. Molecules. 2012;17(4):4612–4624. doi: 10.3390/molecules17044612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Li H., Yu Y., Faraji Dana S., Li B., Lee C.Y., Kang L. Novel engineered systems for oral, mucosal and transdermal drug delivery. J. Drug Target. 2013;21(7):611–629. doi: 10.3109/1061186X.2013.805335. [DOI] [PubMed] [Google Scholar]
- 171.Tao S.L., Desai T.A. Microfabricated drug delivery systems: from particles to pores. Adv. Drug Deliv. Rev. 2003;55(3):315–328. doi: 10.1016/s0169-409x(02)00227-2. [DOI] [PubMed] [Google Scholar]
- 172.Liu D., Liu C., Zou W., Zhang N. Enhanced gastrointestinal absorption of N3-O-toluyl-fluorouracil by cationic solid lipid nanoparticles. J. Nanopart. Res. 2009;12(3):975–984. [Google Scholar]
- 173.Kawashima Y., Yamamoto H., Takeuchi H., Kuno Y. Mucoadhesive DL-lactide/glycolide copolymer nanospheres coated with chitosan to improve oral delivery of elcatonin. Pharm. Dev. Technol. 2000;5(1):77–85. doi: 10.1081/pdt-100100522. [DOI] [PubMed] [Google Scholar]
- 174.Takeuchi H., Thongborisute J., Matsui Y., Sugihara H., Yamamoto H., Kawashima Y. Novel mucoadhesion tests for polymers and polymer-coated particles to design optimal mucoadhesive drug delivery systems. Adv. Drug Deliv. Rev. 2005;57(11):1583–1594. doi: 10.1016/j.addr.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 175.Iqbal J., Shahnaz G., Perera G., Hintzen F., Sarti F., Bernkop-Schnurch A. Thiolated chitosan: development and in vivo evaluation of an oral delivery system for leuprolide. Eur. J. Pharm. Biopharm. 2012;80(1):95–102. doi: 10.1016/j.ejpb.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 176.Hosseinzadeh H., Atyabi F., Dinarvand R., Ostad S.N. Chitosan–Pluronic nanoparticles as oral delivery of anticancer gemcitabine: preparation and in vitro study. Int. J. Nanomedicine. 2012;7 doi: 10.2147/IJN.S26365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Bravo-Osuna I., Vauthier C., Farabollini A., Palmieri G.F., Ponchel G. Mucoadhesion mechanism of chitosan and thiolated chitosan-poly(isobutyl cyanoacrylate) core-shell nanoparticles. Biomaterials. 2007;28(13):2233–2243. doi: 10.1016/j.biomaterials.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 178.Sarmento B., Ferreira D., Jorgensen L., Van De Weert M. Probing insulin’s secondary structure after entrapment into alginate/chitosan nanoparticles. Eur. J. Pharm. Biopharm. 2007;65(1):10–17. doi: 10.1016/j.ejpb.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 179.Abd El Hady W.E., Mohamed E.A., Soliman O.A.E., El-Sabbagh H.M. In vitro-in vivo evaluation of chitosan-PLGA nanoparticles for potentiated gastric retention and anti-ulcer activity of diosmin. Int. J. Nanomedicine. 2019;14:7191–7213. doi: 10.2147/IJN.S213836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Xu B., Jiang G., Yu W., Liu D., Liu Y., Kong X., Yao J. Preparation of poly(lactic-co-glycolic acid) and chitosan composite nanocarriers via electrostatic self assembly for oral delivery of insulin. Mater. Sci. Eng. C Mater. Biol. Appl. 2017;78:420–428. doi: 10.1016/j.msec.2017.04.113. [DOI] [PubMed] [Google Scholar]
- 181.Hejazi R., Amiji M. Chitosan-based gastrointestinal delivery systems. J. Control. Release. 2003;89(2):151–165. doi: 10.1016/s0168-3659(03)00126-3. [DOI] [PubMed] [Google Scholar]
- 182.Kowapradit J., Apirakaramwong A., Ngawhirunpat T., Rojanarata T., Sajomsang W., Opanasopit P. Methylated N-(4-N,N-dimethylaminobenzyl) chitosan coated liposomes for oral protein drug delivery. Eur. J. Pharm. Sci. 2012;47(2):359–366. doi: 10.1016/j.ejps.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 183.Leitner V., Marschütz M., Bernkop-Schnürch A. Mucoadhesive and cohesive properties of poly (acrylic acid)-cysteine conjugates with regard to their molecular mass. Eur. J. Pharm. Sci. 2003;18(1):89–96. doi: 10.1016/s0928-0987(02)00245-2. [DOI] [PubMed] [Google Scholar]
- 184.Werle M., Hoffer M. Glutathione and thiolated chitosan inhibit multidrug resistance P-glycoprotein activity in excised small intestine. J. Control. Release. 2006;111(1–2):41–46. doi: 10.1016/j.jconrel.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 185.Zhang X., Qi J., Lu Y., He W., Li X., Wu W. Biotinylated liposomes as potential carriers for the oral delivery of insulin. Nanomedicine. 2014;10(1):167–176. doi: 10.1016/j.nano.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 186.Zhang N., Ping Q.N., Huang G.H., Xu W.F. Investigation of lectin-modified insulin liposomes as carriers for oral administration. Int. J. Pharm. 2005;294(1–2):247–259. doi: 10.1016/j.ijpharm.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 187.Collnot E.M., Baldes C., Schaefer U.F., Edgar K.J., Wempe M.F., Lehr C.M. Vitamin E TPGS P-glycoprotein inhibition mechanism: influence on conformational flexibility, intracellular ATP levels, and role of time and site of access. Mol. Pharm. 2010;7(3):642–651. doi: 10.1021/mp900191s. [DOI] [PubMed] [Google Scholar]
- 188.Zhang Z., Tan S., Feng S.-S. Vitamin E TPGS as a molecular biomaterial for drug delivery. Biomaterials. 2012;33(19):4889–4906. doi: 10.1016/j.biomaterials.2012.03.046. [DOI] [PubMed] [Google Scholar]
- 189.Varma M.V.S., Panchagnula R. Enhanced oral paclitaxel absorption with vitamin E-TPGS: effect on solubility and permeability in vitro, in situ and in vivo. Eur. J. Pharm. Sci. 2005;25(4–5):445–453. doi: 10.1016/j.ejps.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 190.Tao S.L., Desai T.A. Micromachined devices: the impact of controlled geometry from cell-targeting to bioavailability. J. Control. Release. 2005;109(1–3):127–138. doi: 10.1016/j.jconrel.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 191.Arzt E., Gorb S., Spolenak R. From micro to nano contacts in biological attachment devices. Proc. Natl. Acad. Sci. U. S. A. 2003;100(19):10603–10606. doi: 10.1073/pnas.1534701100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Henry S., McAllister D.V., Allen M.G., Prausnitz M.R. Microfabricated microneedles: a novel approach to transdermal drug delivery. J. Pharm. Sci. 1998;87(8):922–925. doi: 10.1021/js980042+. [DOI] [PubMed] [Google Scholar]
- 194.Kockisch S., Rees G.D., Young S.A., Tsibouklis J., Smart J.D. Polymeric microspheres for drug delivery to the oral cavity: an in vitro evaluation of mucoadhesive potential. J. Pharm. Sci. 2003;92(8):1614–1623. doi: 10.1002/jps.10423. [DOI] [PubMed] [Google Scholar]
- 195.Reddy P.C., Chaitanya K.S.C., Rao Y.M. A review on bioadhesive buccal drug delivery systems: current status of formulation and evaluation methods. DARU Journal of Pharmaceutical Sciences. 2011;19(6):385. [PMC free article] [PubMed] [Google Scholar]
- 196.Abramson A., Caffarel-Salvador E., Khang M., Dellal D., Silverstein D., Gao Y., Frederiksen M.R., Vegge A., Hubalek F., Water J.J., Friderichsen A.V., Fels J., Kirk R.K., Cleveland C., Collins J., Tamang S., Hayward A., Landh T., Buckley S.T., Roxhed N., Rahbek U., Langer R., Traverso G. An ingestible self-orienting system for oral delivery of macromolecules. Science. 2019;363(6427):611–615. doi: 10.1126/science.aau2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Jiang J., Xie J., Ma B., Bartlett D.E., Xu A., Wang C.H. Mussel-inspired protein-mediated surface functionalization of electrospun nanofibers for pH-responsive drug delivery. Acta Biomater. 2014;10(3):1324–1332. doi: 10.1016/j.actbio.2013.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Traverso G., Schoellhammer C.M., Schroeder A., Maa R., Lauwers G.Y., Polat B.E., Anderson D.G., Blankschtein D., Langer R. Microneedles for drug delivery via the gastrointestinal tract. J. Pharm. Sci. 2015;104(2):362–367. doi: 10.1002/jps.24182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Prausnitz M.R., Gomaa Y., Li W. Microneedle patch drug delivery in the gut. Nat. Med. 2019;25(10):1471–1472. doi: 10.1038/s41591-019-0606-0. [DOI] [PubMed] [Google Scholar]
- 200.https://www.fda.gov/media/107708/download
- 202.Zhou M., Hou T., Li J., Yu S., Xu Z., Yin M., Wang J., Wang X. Self-propelled and targeted drug delivery of poly (aspartic acid)/iron–zinc microrocket in the stomach. ACS Nano. 2019;13(2):1324–1332. doi: 10.1021/acsnano.8b06773. [DOI] [PubMed] [Google Scholar]
- 203.de Ávila B.E.-F., Angsantikul P., Li J., Lopez-Ramirez M.A., Ramírez-Herrera D.E., Thamphiwatana S., Chen C., Delezuk J., Samakapiruk R., Ramez V. Micromotor-enabled active drug delivery for in vivo treatment of stomach infection. Nat. Commun. 2017;8(1):1–9. doi: 10.1038/s41467-017-00309-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Nemeth C.L., Lykins W.R., Tran H., ElSayed M.E.H., Desai T.A. Bottom-up fabrication of multilayer enteric devices for the Oral delivery of peptides. Pharm. Res. 2019;36(6):89. doi: 10.1007/s11095-019-2618-3. [DOI] [PubMed] [Google Scholar]
- 205.Fox C.B., Cao Y., Nemeth C.L., Chirra H.D., Chevalier R.W., Xu A.M., Melosh N.A., Desai T.A. Fabrication of Sealed Nanostraw Microdevices for Oral Drug Delivery. ACS Nano. 2016;10(6):5873–5881. doi: 10.1021/acsnano.6b00809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Abramson A., Caffarel-Salvador E., Soares V., Minahan D., Tian R.Y., Lu X., Dellal D., Gao Y., Kim S., Wainer J., Collins J., Tamang S., Hayward A., Yoshitake T., Lee H.C., Fujimoto J., Fels J., Frederiksen M.R., Rahbek U., Roxhed N., Langer R., Traverso G. A luminal unfolding microneedle injector for oral delivery of macromolecules. Nat. Med. 2019;25(10):1512–1518. doi: 10.1038/s41591-019-0598-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Niu M., Lu Y., Hovgaard L., Guan P., Tan Y., Lian R., Qi J., Wu W. Hypoglycemic activity and oral bioavailability of insulin-loaded liposomes containing bile salts in rats: the effect of cholate type, particle size and administered dose. Eur. J. Pharm. Biopharm. 2012;81(2):265–272. doi: 10.1016/j.ejpb.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 208.Niu M., Lu Y., Hovgaard L., Wu W. Liposomes containing glycocholate as potential oral insulin delivery systems: preparation, in vitro characterization, and improved protection against enzymatic degradation. Int. J. Nanomedicine. 2011;6:1155. doi: 10.2147/IJN.S19917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Cui M., Wu W., Hovgaard L., Lu Y., Chen D., Qi J. Liposomes containing cholesterol analogues of botanical origin as drug delivery systems to enhance the oral absorption of insulin. Int. J. Pharm. 2015;489(1–2):277–284. doi: 10.1016/j.ijpharm.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 210.Lamson N.G., Berger A., Fein K.C., Whitehead K.A. Anionic nanoparticles enable the oral delivery of proteins by enhancing intestinal permeability. Nat Biomed Eng. 2020;4(1):84–96. doi: 10.1038/s41551-019-0465-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Nielsen L.H., Nagstrup J., Gordon S., Keller S.S., Ostergaard J., Rades T., Mullertz A., Boisen A. pH-triggered drug release from biodegradable microwells for oral drug delivery. Biomed. Microdevices. 2015;17(3):9958. doi: 10.1007/s10544-015-9958-5. [DOI] [PubMed] [Google Scholar]
- 212.Malachowski K., Breger J., Kwag H.R., Wang M.O., Fisher J.P., Selaru F.M., Gracias D.H. Stimuli-responsive theragrippers for chemomechanical controlled release. Angew. Chem. Int. Ed. Eng. 2014;53(31):8045–8049. doi: 10.1002/anie.201311047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Banerjee A., Mitragotri S. Intestinal patch systems for oral drug delivery. Curr. Opin. Pharmacol. 2017;36:58–65. doi: 10.1016/j.coph.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 214.Kaur G., Arora M., Ravi Kumar M.N.V. Oral Drug Delivery Technologies-A Decade of Developments. J. Pharmacol. Exp. Ther. 2019;370(3):529–543. doi: 10.1124/jpet.118.255828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Cui F., He C., Yin L., Qian F., He M., Tang C., Yin C. Nanoparticles incorporated in bilaminated films: a smart drug delivery system for oral formulations. Biomacromolecules. 2007;8(9):2845–2850. doi: 10.1021/bm070339e. [DOI] [PubMed] [Google Scholar]
- 216.Banerjee A., Lee J., Mitragotri S. Intestinal mucoadhesive devices for oral delivery of insulin. Bioeng Transl Med. 2016;1(3):338–346. doi: 10.1002/btm2.10015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Grabovac V., Foger F., Bernkop-Schnurch A. Design and in vivo evaluation of a patch delivery system for insulin based on thiolated polymers. Int. J. Pharm. 2008;348(1–2):169–174. doi: 10.1016/j.ijpharm.2007.06.052. [DOI] [PubMed] [Google Scholar]
- 218.Eaimtrakarn S., Itoh Y., Kishimoto J.I., Yoshikawa Y., Shibata N., Takada K. Retention and transit of intestinal mucoadhesive films in rat small intestine. Int. J. Pharm. 2001;224(1–2):61–67. doi: 10.1016/s0378-5173(01)00738-4. [DOI] [PubMed] [Google Scholar]
- 220.Varshosaz J., Ahmadi F., Emami J., Tavakoli N., Minaiyan M., Mahzouni P., Dorkoosh F. Microencapsulation of budesonide with dextran by spray drying technique for colon-targeted delivery: an in vitro/in vivo evaluation in induced colitis in rat. J. Microencapsul. 2011;28(1):62–73. doi: 10.3109/02652048.2010.529947. [DOI] [PubMed] [Google Scholar]
- 221.Ravi V., Siddaramaiah Pramod, Kumar T.M. Influence of natural polymer coating on novel colon targeting drug delivery system. J. Mater. Sci. Mater. Med. 2008;19(5):2131–2136. doi: 10.1007/s10856-007-3155-x. [DOI] [PubMed] [Google Scholar]
- 222.Wang X., Yu D.G., Li X.Y., Bligh S.W., Williams G.R. Electrospun medicated shellac nanofibers for colon-targeted drug delivery. Int. J. Pharm. 2015;490(1–2):384–390. doi: 10.1016/j.ijpharm.2015.05.077. [DOI] [PubMed] [Google Scholar]
- 223.Henning S., Leick S., Kott M., Rehage H., Suter D. Sealing liquid-filled pectinate capsules with a shellac coating. J. Microencapsul. 2012;29(2):147–155. doi: 10.3109/02652048.2011.635220. [DOI] [PubMed] [Google Scholar]
- 224.Wen P., Feng K., Yang H., Huang X., Zong M.-H., Lou W.-Y., Li N., Wu H. Electrospun core-shell structured nanofilm as a novel colon-specific delivery system for protein. Carbohydr. Polym. 2017;169:157–166. doi: 10.1016/j.carbpol.2017.03.082. [DOI] [PubMed] [Google Scholar]
- 225.Sun B., Ranganathan B., Feng S.S. Multifunctional poly(D,L-lactide-co-glycolide)/montmorillonite (PLGA/MMT) nanoparticles decorated by Trastuzumab for targeted chemotherapy of breast cancer. Biomaterials. 2008;29(4):475–486. doi: 10.1016/j.biomaterials.2007.09.038. [DOI] [PubMed] [Google Scholar]
- 226.Morishita M., Goto T., Nakamura K., Lowman A.M., Takayama K., Peppas N.A. Novel oral insulin delivery systems based on complexation polymer hydrogels: single and multiple administration studies in type 1 and 2 diabetic rats. J. Control. Release. 2006;110(3):587–594. doi: 10.1016/j.jconrel.2005.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Morishita M., Goto T., Peppas N.A., Joseph J.I., Torjman M.C., Munsick C., Nakamura K., Yamagata T., Takayama K., Lowman A.M. Mucosal insulin delivery systems based on complexation polymer hydrogels: effect of particle size on insulin enteral absorption. J. Control. Release. 2004;97(1):115–124. doi: 10.1016/j.jconrel.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 228.Ainslie K.M., Lowe R.D., Beaudette T.T., Petty L., Bachelder E.M., Desai T.A. Microfabricated devices for enhanced bioadhesive drug delivery: attachment to and small-molecule release through a cell monolayer under flow. Small. 2009;5(24):2857–2863. doi: 10.1002/smll.200901254. [DOI] [PubMed] [Google Scholar]
- 229.Tao S.L., Lubeley M.W., Desai T.A. Bioadhesive poly(methyl methacrylate) microdevices for controlled drug delivery. J. Control. Release. 2003;88(2):215–228. doi: 10.1016/s0168-3659(03)00005-1. [DOI] [PubMed] [Google Scholar]
- 230.Tao S.L., Lubeley M.W., Desai T.A. Synthesis of cytoadhesive poly(methylmethacrylate) for applications in targeted drug delivery. J. Biomed. Mater. Res. A. 2003;67(2):369–375. doi: 10.1002/jbm.a.10047. [DOI] [PubMed] [Google Scholar]
- 231.Uskoković V., Lee P.P., Walsh L.A., Fischer K.E., Desai T.A. PEGylated silicon nanowire coated silica microparticles for drug delivery across intestinal epithelium. Biomaterials. 2012;33(5):1663–1672. doi: 10.1016/j.biomaterials.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Uskokovic V., Lee K., Lee P.P., Fischer K.E., Desai T.A. Shape effect in the design of nanowire-coated microparticles as transepithelial drug delivery devices. ACS Nano. 2012;6(9):7832–7841. doi: 10.1021/nn3019865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Kam K.R., Walsh L.A., Bock S.M., Koval M., Fischer K.E., Ross R.F., Desai T.A. Nanostructure-mediated transport of biologics across epithelial tissue: enhancing permeability via nanotopography. Nano Lett. 2013;13(1):164–171. doi: 10.1021/nl3037799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Levy E.S., Samy K.E., Lamson N.G., Whitehead K.A., Kroetz D.L., Desai T.A. Reversible inhibition of efflux transporters by hydrogel microdevices. Eur. J. Pharm. Biopharm. 2019;145:76–84. doi: 10.1016/j.ejpb.2019.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Cencič A., Langerholc T. Functional cell models of the gut and their applications in food microbiology—a review. Int. J. Food Microbiol. 2010;141:S4–S14. doi: 10.1016/j.ijfoodmicro.2010.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Costa J., Advances Ahluwalia A. Current Challenges in Intestinal in vitro Model Engineering: A Digest. Front Bioeng Biotechnol. 2019;7(144):144. doi: 10.3389/fbioe.2019.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Edmondson R., Broglie J.J., Adcock A.F., Yang L. Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay and drug development technologies. 2014;12(4):207–218. doi: 10.1089/adt.2014.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Abbasi F., Ghanian M.H., Baharvand H., Vahidi B., Eslaminejad M.B. Engineering mesenchymal stem cell spheroids by incorporation of mechanoregulator microparticles. J. Mech. Behav. Biomed. Mater. 2018;84:74–87. doi: 10.1016/j.jmbbm.2018.04.026. [DOI] [PubMed] [Google Scholar]
- 239.Bratt-Leal A.M., Kepple K.L., Carpenedo R.L., Cooke M.T., McDevitt T.C. Magnetic manipulation and spatial patterning of multi-cellular stem cell aggregates. Integr Biol (Camb) 2011;3(12):1224–1232. doi: 10.1039/c1ib00064k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Cerchiari A.E., Samy K.E., Todhunter M.E., Schlesinger E., Henise J., Rieken C., Gartner Z.J., Desai T.A. Probing the luminal microenvironment of reconstituted epithelial microtissues. Sci. Rep. 2016;6(1):33148. doi: 10.1038/srep33148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Yajima Y., Yamada M., Utoh R., Seki M. Collagen microparticle-mediated 3D cell organization: a facile route to bottom-up engineering of thick and porous tissues. Acs Biomater-Sci Eng. 2017;3(9):2144–2154. doi: 10.1021/acsbiomaterials.7b00131. [DOI] [PubMed] [Google Scholar]
- 242.Samy K.E., Levy E.S., Phong K., Demaree B., Abate A.R., Desai T.A. Human intestinal spheroids cultured using sacrificial Micromolding as a model system for studying drug transport. Sci. Rep. 2019;9(1):1–12. doi: 10.1038/s41598-019-46408-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Bhatia S.N., Ingber D.E. Microfluidic organs-on-chips. Nat. Biotechnol. 2014;32(8):760–772. doi: 10.1038/nbt.2989. [DOI] [PubMed] [Google Scholar]
- 244.Christenson C., Baryeh K., Ahadian S., Nasiri R., Dokmeci M.R., Goudie M., Khademhosseini A., Ye J.Y. 112512J. International Society for Optics and Photonics; 2020. Enhancement of Label-Free Biosensing of Cardiac Troponin I. Label-Free Biomedical Imaging and Sensing (LBIS) 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Kimura H., Yamamoto T., Sakai H., Sakai Y., Fujii T. An integrated microfluidic system for long-term perfusion culture and on-line monitoring of intestinal tissue models. Lab Chip. 2008;8(5):741–746. doi: 10.1039/b717091b. [DOI] [PubMed] [Google Scholar]
- 246.Imura Y., Asano Y., Sato K., Yoshimura E. A microfluidic system to evaluate intestinal absorption. Anal. Sci. 2009;25(12):1403–1407. doi: 10.2116/analsci.25.1403. [DOI] [PubMed] [Google Scholar]
- 247.Imura Y., Sato K., Yoshimura E. Micro total bioassay system for ingested substances: assessment of intestinal absorption, hepatic metabolism, and bioactivity. Anal. Chem. 2010;82(24):9983–9988. doi: 10.1021/ac100806x. [DOI] [PubMed] [Google Scholar]
- 248.Bricks T., Paullier P., Legendre A., Fleury M.J., Zeller P., Merlier F., Anton P.M., Leclerc E. Development of a new microfluidic platform integrating co-cultures of intestinal and liver cell lines. Toxicol. in Vitro. 2014;28(5):885–895. doi: 10.1016/j.tiv.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 249.Tan H.-Y., Trier S., Rahbek U.L., Dufva M., Kutter J.P., Andresen T.L. A multi-chamber microfluidic intestinal barrier model using Caco-2 cells for drug transport studies. PLoS One. 2018;13(5) doi: 10.1371/journal.pone.0197101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Chen H.J., Miller P., Shuler M.L. A pumpless body-on-a-chip model using a primary culture of human intestinal cells and a 3D culture of liver cells. Lab Chip. 2018;18(14):2036–2046. doi: 10.1039/c8lc00111a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Costello C.M., Phillipsen M.B., Hartmanis L.M., Kwasnica M.A., Chen V., Hackam D., Chang M.W., Bentley W.E., March J.C. Microscale Bioreactors for in situ characterization of GI epithelial cell physiology. Sci. Rep. 2017;7(1):12515. doi: 10.1038/s41598-017-12984-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Shim K.Y., Lee D., Han J., Nguyen N.T., Park S., Sung J.H. Microfluidic gut-on-a-chip with three-dimensional villi structure. Biomed. Microdevices. 2017;19(2):37. doi: 10.1007/s10544-017-0179-y. [DOI] [PubMed] [Google Scholar]
- 253.Basson M.D., Li G.D., Hong F., Han O., Sumpio B.E. Amplitude-dependent modulation of brush border enzymes and proliferation by cyclic strain in human intestinal Caco-2 monolayers. J. Cell. Physiol. 1996;168(2):476–488. doi: 10.1002/(SICI)1097-4652(199608)168:2<476::AID-JCP26>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 254.Samak G., Gangwar R., Crosby L.M., Desai L.P., Wilhelm K., Waters C.M., Rao R. Cyclic stretch disrupts apical junctional complexes in Caco-2 cell monolayers by a JNK-2-, c-Src-, and MLCK-dependent mechanism. Am. J. Physiol. Gastrointest. Liver Physiol. 2014;306(11):G947–G958. doi: 10.1152/ajpgi.00396.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Kim H.J., Huh D., Hamilton G., Ingber D.E. Human gut-on-a-chip inhabited by microbial flora that experiences intestinal peristalsis-like motions and flow. Lab Chip. 2012;12(12):2165–2174. doi: 10.1039/c2lc40074j. [DOI] [PubMed] [Google Scholar]
- 256.Kim H.J., Li H., Collins J.J., Ingber D.E. Contributions of microbiome and mechanical deformation to intestinal bacterial overgrowth and inflammation in a human gut-on-a-chip. Proc. Natl. Acad. Sci. U. S. A. 2016;113(1) doi: 10.1073/pnas.1522193112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Kasendra M., Tovaglieri A., Sontheimer-Phelps A., Jalili-Firoozinezhad S., Bein A., Chalkiadaki A., Scholl W., Zhang C., Rickner H., Richmond C.A. Development of a primary human small intestine-on-a-Chip using biopsy-derived organoids. Sci. Rep. 2018;8(1):1–14. doi: 10.1038/s41598-018-21201-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Ronaldson-Bouchard K., Vunjak-Novakovic G. Organs-on-a-Chip: A Fast Track for Engineered Human Tissues in Drug Development. Cell Stem Cell. 2018;22(3):310–324. doi: 10.1016/j.stem.2018.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Ragelle H., Danhier F., Préat V., Langer R., Anderson D.G. Nanoparticle-based drug delivery systems: a commercial and regulatory outlook as the field matures. Expert opin. drug del. 2017;14(7):851–864. doi: 10.1080/17425247.2016.1244187. [DOI] [PubMed] [Google Scholar]
- 260.Ventola C.L. Progress in nanomedicine: approved and investigational nanodrugs. Pharmacy and Therapeutics. 2017;42(12):742. [PMC free article] [PubMed] [Google Scholar]
- 262.Zhong H., Chan G., Hu Y., Hu H., Ouyang D. A comprehensive map of FDA-approved pharmaceutical products. Pharmaceutics. 2018;10(4):263. doi: 10.3390/pharmaceutics10040263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Lu Y., Chen S.C. Micro and nano-fabrication of biodegradable polymers for drug delivery. Adv. Drug Deliv. Rev. 2004;56(11):1621–1633. doi: 10.1016/j.addr.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 264.https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/206089Orig1s000TOC.cfm
- 265.Geho W.B., Geho H.C., Lau J.R., Gana T.J. Hepatic-directed vesicle insulin: a review of formulation development and preclinical evaluation. J. Diabetes Sci. Technol. 2009;3(6):1451–1459. doi: 10.1177/193229680900300627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Caster J.M., Patel A.N., Zhang T., Wang A. Investigational nanomedicines in 2016: a review of nanotherapeutics currently undergoing clinical trials. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology. 2017;9(1) doi: 10.1002/wnan.1416. [DOI] [PubMed] [Google Scholar]
- 267.Mei L., Zhang Z., Zhao L., Huang L., Yang X.L., Tang J., Feng S.S. Pharmaceutical nanotechnology for oral delivery of anticancer drugs. Adv. Drug Deliv. Rev. 2013;65(6):880–890. doi: 10.1016/j.addr.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 269.Kam K.R., Desai T.A. Nano- and microfabrication for overcoming drug delivery challenges. J. Mater. Chem. B. 2013;1(14):1878–1884. doi: 10.1039/C3TB00048F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Vaut L., Juszczyk J.J., Kamguyan K., Jensen K.E., Tosello G., Boisen A. 3D printing of reservoir devices for Oral drug delivery: from concept to functionality through design improvement for enhanced Mucoadhesion. Acs Biomater-Sci Eng. 2020;6(4):2478–2486. doi: 10.1021/acsbiomaterials.9b01760. [DOI] [PubMed] [Google Scholar]
- 271.Walker D.A., Hedrick J.L., Mirkin C.A. Rapid, large-volume, thermally controlled 3D printing using a mobile liquid interface. Science. 2019;366(6463):360–364. doi: 10.1126/science.aax1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Abramson A., Halperin F., Kim J., Traverso G. Quantifying the value of orally delivered biologic therapies: a cost-effectiveness analysis of oral semaglutide. J. Pharm. Sci. 2019;108(9):3138–3145. doi: 10.1016/j.xphs.2019.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Gao J., Tian Z., Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Bioscience trends. 2020;14(1):72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 274.Gautret P., Lagier J.-C., Parola P., Meddeb L., Mailhe M., Doudier B., Courjon J., Giordanengo V., Vieira V.E., Dupont H.T. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020;56(1) doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 275.Grein J., Ohmagari N., Shin D., Diaz G., Asperges E., Castagna A., Feldt T., Green G., Green M.L., Lescure F.-X. Compassionate use of remdesivir for patients with severe Covid-19. N. Engl. J. Med. 2020;382(24):2327–2336. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]