Oregano essential oil has multiple benefits in traditional medicine, cosmetics, and food industries. Carvacrol and its analog, thymol, are well-described components of oregano oil. Here, we show that these compounds inhibit HIV-target cell fusion independently of viral tropism. Our results suggest that carvacrol and thymol alter the cholesterol content of the viral membrane, blocking HIV-1 entry into the target cell. Resistance to carvacrol has selected for viruses with mutations in the viral envelope glycoprotein, gp41. This protein is known for its interaction with cholesterol present in membrane lipid rafts. Together, these results demonstrate the potential of therapies targeting the viral envelope membrane, and oregano oil is a safe supplement to antiretrovirals, potentially delaying disease progression and resistance development.
KEYWORDS: carvacrol, cholesterol depletion, oregano oil, thymol, entry inhibition, human immunodeficiency virus, viral membrane
ABSTRACT
Oregano essential oil has long been known for its health-promoting benefits. Here, we report its activity against viral replication. Oregano oil was found to specifically inhibit lentiviruses, such as human and simian immunodeficiency viruses (HIV and SIV), irrespective of virus tropism, but not hepatitis C virus, adenovirus 5 (ADV5), Zika virus, and influenza (H1N1) virus. Oregano oil’s most abundant components, carvacrol and its isomer, thymol, were shown to block virus-target cell fusion while not perturbing other stages of the virus life cycle. We detected changes in virus particle density, suggesting that cholesterol depletion from the HIV-1 envelope membrane reduces virus entry. Furthermore, infection was rescued by adding exogenous cholesterol. The evolution of viral resistance to carvacrol supported this mechanism of action with the identification of mutations in the viral gp41 fusion protein that counteracted cholesterol depletion. In addition, resistance to carvacrol emerged later than typically observed for other clinically used drugs, strengthening its antiviral potential. Structure-activity relationship studies revealed key motifs of carvacrol and thymol required for HIV neutralization and identified previously unknown active analogs. Carvacrol was also shown to additively cooperate with antiretroviral therapy. In sum, oregano oil and improved carvacrol and thymol analogs could be considered to supplement current HIV therapeutics.
IMPORTANCE Oregano essential oil has multiple benefits in traditional medicine, cosmetics, and food industries. Carvacrol and its analog, thymol, are well-described components of oregano oil. Here, we show that these compounds inhibit HIV-target cell fusion independently of viral tropism. Our results suggest that carvacrol and thymol alter the cholesterol content of the viral membrane, blocking HIV-1 entry into the target cell. Resistance to carvacrol has selected for viruses with mutations in the viral envelope glycoprotein, gp41. This protein is known for its interaction with cholesterol present in membrane lipid rafts. Together, these results demonstrate the potential of therapies targeting the viral envelope membrane, and oregano oil is a safe supplement to antiretrovirals, potentially delaying disease progression and resistance development.
INTRODUCTION
Human immunodeficiency virus type 1 (HIV-1) infects 36.9 million people worldwide (1). Antiretroviral therapy (ART) against HIV-1 has immensely improved the quality of life and life expectancy of infected individuals. However, it does not permanently eradicate the virus (1), since viral replication resumes upon treatment interruption. The emergence of drug resistance to ART is also a constant concern (2); thus, novel antiretrovirals (ARVs) are needed to address these issues.
The successful fusion of the HIV particle with the target cell membrane initiates the HIV life cycle. The HIV envelope protein spikes are anchored on the viral membrane; these consist of trimers of the surface glycoprotein subunit, gp120, and the associated transmembrane subunit, gp41. Upon viral attachment to the target cell, gp120 binds the cellular CD4 receptor and either the CXCR4 or the CCR5 coreceptor, depending on viral tropism. This interaction promotes fusion-inducing conformational changes in gp41, increasing the exposure of gp41 ectodomain 2 heptad repeat motifs, N-HR and C-HR, and insertion of the fusion peptide into the cellular membrane (3–5). Consequently, N-HR and C-HR fold in an antiparallel structure called a six-helix bundle, 6HB, triggering fusion between HIV and cell membranes (3–5). Only two HIV entry inhibitors are commercially available: maraviroc inhibits the envelope interaction with CCR5, while enfuvirtide, a fusion inhibitor peptide, targets gp41 (6, 7). Maraviroc is tropism dependent, while enfuvirtide is administered subcutaneously and is easily degraded; thus, novel antiviral entry inhibitors are needed in the anti-HIV portfolio.
Natural products have always been a valuable resource for the pharmaceutical industry, with many drugs being derived from or inspired by natural products greatly beneficial in virtually all clinical therapeutic areas (8, 9). The World Health Organization (WHO) has recommended the systematic testing of ethnomedicines and natural products against HIV due to their general safety and affordability (10). Oregano (Origanum vulgare) is a flowering plant from the Lamiaceae family, commonly known as the mint family of aromatic plants. Oregano grows in temperate areas of western-southwestern Eurasia and Mediterranean regions. Essential oils are a liquid concentrate of volatile hydrophobic chemical compounds derived from plants. Essential oils are considered safe for humans by the United States Food and Drug Administration (FDA) and have widespread applications in food preservation, flavoring, cosmetics, antiseptics, cleaners, and fresheners (11, 12).
Oregano oil has long been used in traditional medicine for its properties as an antimicrobial, anti-inflammatory, antifungal, and antioxidant (11), all while considered safe for eukaryotic cells and for the environment. Oregano oil is rich in the monoterpenic phenol carvacrol (also known as 5-isopropyl-2-methylphenol) and its isomeric analog, thymol (also known as 2-isopropyl-5-methylphenol). The precise composition of oregano oil depends on the plant’s growth conditions, origin of the extract (leaf, root, or stem), and the extraction method (11). This natural variation in composition can lead to variable biological properties for different oregano oil preparations.
Several ailments commonly associated with HIV infection, such as candidiasis and cryptosporidiosis, have been shown to benefit from oregano oil treatment (13, 14). Here, we investigated the effectiveness of oregano oil and individual, purified components in blocking HIV-1 replication in cell lines and primary CD4+ T cells. Our results show that oregano oil potently inhibits HIV-1 cell fusion, and this activity is mainly due to carvacrol and thymol. Our results suggest that these components alter the cholesterol content of the viral lipid membrane, blocking fusion with the target cell. Viral evolution to counteract oregano oil generated mutations in gp41 consistent with oregano oil’s proposed mode of action.
RESULTS
Oregano oil, carvacrol, and thymol inhibit HIV-1 replication.
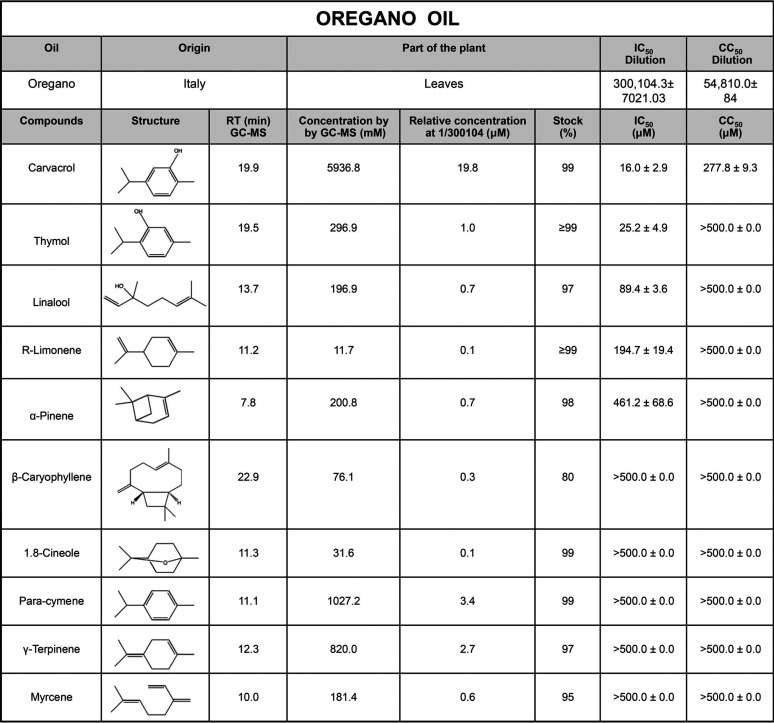
HIV-1 susceptibility to oregano oil was analyzed using a reporter cell line stably expressing the β-galactosidase (LacZ) gene under the control of the HIV promoter. This reporter line responds to the HIV-1 Tat protein expressed by the incoming virus. HeLa-CD4-LTR-LacZ cells were infected with the NL4-3 strain in the presence of decreasing dilutions of oregano oil, and LacZ activity was assessed 72 h later (Table 1). The inhibition of HIV replication was shown to be dose dependent, with a 50% inhibitory concentration (IC50) dilution of 1:300,104 ± 7,021 and 50% cytotoxic concentration (CC50) of 54,810 ± 84. Oregano oil procured from multiple companies displayed similar activity (data not shown).
TABLE 1.
Activity of oregano oil’s components against HIV-1 replicationa
HeLa-CD4-LTR-LacZ cells were infected with the HIV-1 NL4-3 strain in the presence of compounds for 72 h prior to CPRG assay. Viability assays were performed for 72 h on uninfected cells. Data are the means ± SEM (n = 2).
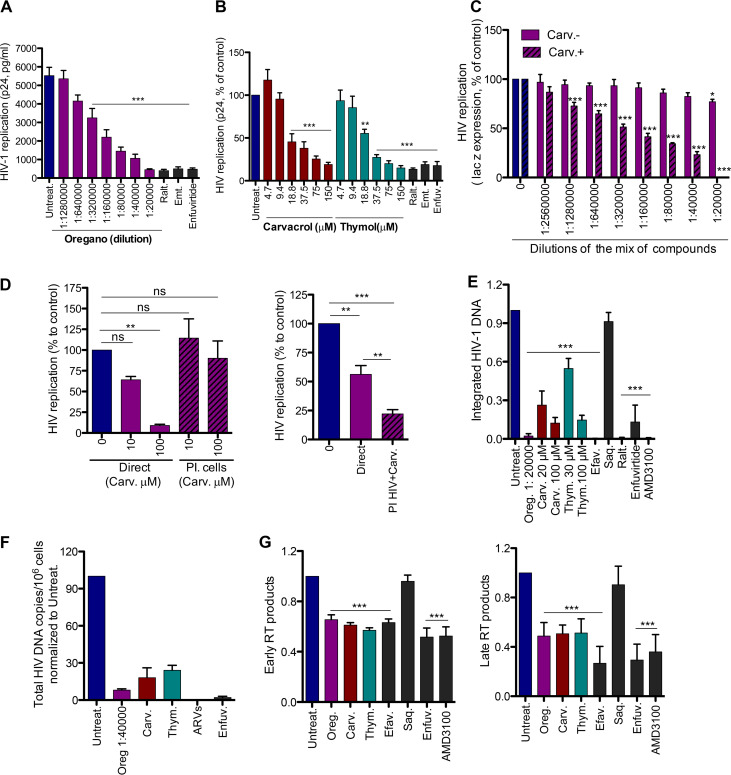
Gas chromatography-mass spectrometry (GC-MS) analysis of oregano oil at a 1:100 dilution identified 10 small-molecule components (Table 1). These were tested individually, revealing the antiviral activity of FDA-approved carvacrol and thymol at an IC50 of 16 ± 2.9 μM and 25.2 ± 4.9 μM, respectively. The inhibition of capsid p24 expression in the cell supernatant by p24 enzyme-linked immunosorbent assay (ELISA) confirmed these results (Fig. 1A and B). The integrase inhibitor raltegravir, the reverse transcriptase inhibitor emtricitabine, and the fusion inhibitor enfuvirtide were used as positive controls. To confirm carvacrol was the most effective antiviral agent in oregano oil preparations, we combined oregano oil’s individual components at the concentrations determined by GC-MS, with or without carvacrol, and tested the antiviral activity (Fig. 1C) of these preparations. The mixture without carvacrol was inactive, demonstrating its requirement for activity. Moreover, the concentration of carvacrol at the oregano oil IC50 dilution is 19.8 μM, which is similar to the IC50 of carvacrol alone (16 μM). That is not the case for thymol, which is present at a much lower level (1 μM) at oregano oil’s IC50 dilution. Moreover, the cytotoxicity of the mixture of compounds mimicked that of oregano oil itself (see Fig. S1A in the supplemental material). The activity of thymol was also found in red thyme oil, which is known for its higher abundance of thymol (Fig. S1B and C), supporting results shown in Table 1. Collectively, these data demonstrate that oregano oil, carvacrol, and thymol inhibit the HIV-1 NL4-3 strain, with carvacrol being the main antiviral ingredient in oregano oil.
FIG 1.
Oregano oil, carvacrol, and thymol inhibit HIV-1 infection. (A and B) HIV inhibition by oregano and related compounds. HeLa-CD4-LTR-LacZ cells were infected with HIV-1 NL4-3 in the presence of different dilutions of oregano (A) or carvacrol and thymol (B). Virus in cell supernatant was recovered 72 h later, and the level of p24 antigen was determined by sandwich ELISA. Results are means ± standard errors of the means (SEM) (n = 4). Raltegravir (Ralt., 200 nM), emtricitabine (Emt., 100 nM), and enfuvirtide (1 μg/ml) were used as controls. (C) Carvacrol (Carv.) is the active compound in oregano oil. The mixtures of compounds, with or without carvacrol, present in oregano oil as determined by GC-MS, were tested for the ability to block NL4-3 infection of HeLa-CD4-LTR-LacZ cells. Beta-galactosidase (β-gal) was measured by CPRG assay 72 h later. Results are means ± SEM (n = 3). (D) Carvacrol blocks the virus but not the target cells. Carvacrol was first incubated with TZM-bl cells for 3 h and then washed or incubated with NL4-3 and then diluted and added to cells. Luciferase was measured 72 h later. Results are means ± SEM (n = 3). ns, not significant; PI, preincubation. (E) Activity of the compounds on HIV-1 integration. HeLa-CD4-LTR-LacZ cells were infected with NL4-3 in the presence of compounds for 19 h. DNA was extracted and provirus integration quantified by Alu-PCR, followed by qPCR. Saquinavir (Saq., 200 nM), efavirenz (Efav., 200 nM), raltegravir (Ralt., 200 nM), and AMD3100/enfuvirtide (10 nM and 1 μg/ml, respectively) were used as controls. Oregano was used at 1:20,000 dilution and carvacrol and thymol (Thym.) at 100 μM. Results are means ± SEM (n = 6). (F) Activity on HIV-1 integration in human primary CD4+ T cells infected with NL4-3, in the presence of compounds, for 24 h. Oregano (1:40,000 dilution), carvacrol and thymol (100 μM), enfuvirtide (1 μg/ml), and ARVs (200 nM raltegravir, 200 nM lamivudine, and 100 nM efavirenz) were used. Data are means ± SEM (n = 2). (G) Activity of compounds on HIV-1 reverse transcription products. HeLa-CD4-LTR-LacZ cells were infected with NL4-3 in the presence of compounds for 10 h. DNA was extracted, and early and late reverse transcription products were measured by qPCR. Compound concentrations are as described for panel E. One-way ANOVA followed by Tukey’s posttest was used for statistical comparisons. **, P < 0.001; ***, P < 0.0001.
Next, we investigated whether carvacrol was active on the virus particle or on the target cells (Fig. 1D, left). For these experiments, we use TZM-bl cells that express luciferase under the control of the HIV long terminal repeat (LTR). As expected, the presence of carvacrol during NL4-3 infection of TZM-bl cells for 72 h, followed by Luciferase quantification, resulted in viral inhibition (solid bars on the left). However, pretreatment of TZM-bl cells with carvacrol for 3 h, prior to infection without carvacrol (hatched lines on the right), did not affect viral entry. To assess the specific activity of carvacrol on the virus particle, we incubated the virus with 100 μM carvacrol for 3 h (Fig. 1D, right). To eliminate most of the carvacrol after this period, the virus was diluted to 1/100 and 1/10 prior to TZM-bl cell infection in the absence of carvacrol. The 1/100 dilution of the virus was too low to detect viral infection even with the dimethyl sulfoxide (DMSO) control, and only the 1/10 dilution was detected. Since, in this situation, there is a 10 μM carryover of carvacrol, as a control, we used 10 μM directly on cells for an infection of a 1/10-diluted virus. The pretreatment of the virus with carvacrol had a significant negative impact on infectivity, which was further reduced by 60.6% compared to infection in the presence of 10 μM carvacrol without preincubation. Together, these results clearly suggest that carvacrol alters the infectivity of viral particles while having a negligible impact on the cellular membrane.
Oregano oil, carvacrol, and thymol inhibit HIV-1 entry.
Next, we investigated the mode of action of the oil and its functional compounds. We first assessed whether virus was blocked before or after integration into the host DNA. We measured the proviral DNA integration into HeLa-CD4-LTR-LacZ cells 19 h postinfection by Alu-PCR followed by quantitative real-time PCR (RT-qPCR) (Fig. 1E). Oregano oil, carvacrol, and thymol significantly decreased integrated HIV compared to the untreated control or treated with the protease inhibitor saquinavir. As expected, efavirenz (a reverse transcriptase inhibitor), raltegravir, AMD3100 (an entry inhibitor), and enfuvirtide (a fusion inhibitor) all inhibited the integration of HIV DNA. Results were confirmed by acute infection of primary human CD4+ T cells (Fig. 1F) without cell-associated toxicity (Fig. S2A). A cocktail of ARVs (raltegravir, lamivudine, and efavirenz) or enfuvirtide alone was used as a control.
Next, to determine whether reverse transcription (RT) activity was inhibited, we monitored the production of early and late RT products by RT-qPCR at 10 h postinfection of HeLa-CD4-LTR-LacZ cells (Fig. 1G). Oregano oil (1:20,000) and carvacrol and thymol (each at 100 μM) significantly inhibited the accumulation of early and late RT products, similarly to the positive controls (efavirenz, AMD3100, and enfuvirtide) but not the negative-control saquinavir. Efavirenz's potent activity on late RT products was previously reported (15). These results suggest that these compounds affect either RT activity or a step prior to that, such as HIV entry.
Comparing a compound’s effects on a time scale to that of reference drugs provides information on the possible target of each inhibitor (16). To discern between RT and viral entry as the specific mechanism of action, we added oregano oil, carvacrol, or thymol alongside entry and RT inhibitors, at different time points postinfection of HeLa-CD4-LTR-LacZ cells, and measured infectivity 72 h later (Fig. 2). Oregano oil, carvacrol, and thymol exhibited an antiviral pattern similar to that of AMD3100, in which antiviral activity was gradually lost when the compounds were added later after infection, when fusion with target cells had already occurred. These results suggest that oregano oil, carvacrol, and thymol are entry inhibitors of the CXCR4-tropic (X4) NL4-3 isolate.
FIG 2.
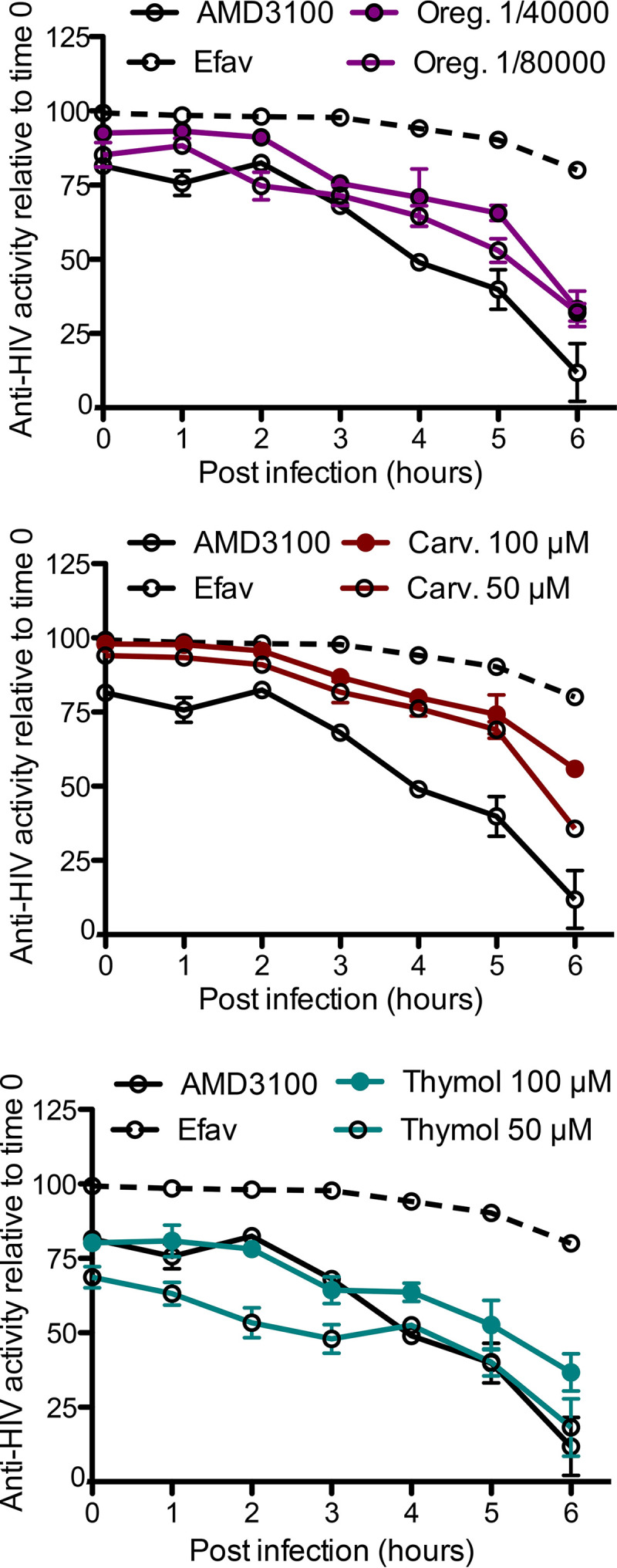
Oregano oil, carvacrol, and thymol inhibit HIV-1 entry in time-of-drug-addition assay. HeLa-CD4-LTR-LacZ cells were infected with NL4-3, compounds were added at the indicated times, and infection was revealed 72 h later. AMD3100 (4 nM) and efavirenz (10 nM) were used as controls. Data are means ± SEM (n = 2).
We next investigated their activity against viruses using the CCR5 coreceptor (R5), such as JRCSF and YU2 isolates. HeLa-CD4-CCR5 cells were infected with these viruses, and p24 capsid in the supernatant was monitored 72 h postinfection (Fig. 3A and B). Oregano oil, carvacrol, and thymol significantly inhibited viral replication at concentrations that did not impact cell viability (Fig. S3A and B). We also verified that the constitutive endocytosis of the receptor CD4 and coreceptors of HIV, a mechanism that could also explain entry inhibition, was not affected by these compounds, unlike phorbol 12-myristate 13-acetate (PMA), which was used as a control (Fig. S3C and D). These results suggest that oregano oil, carvacrol, and thymol inhibit HIV entry into the target cell irrespective of the entry coreceptor.
FIG 3.
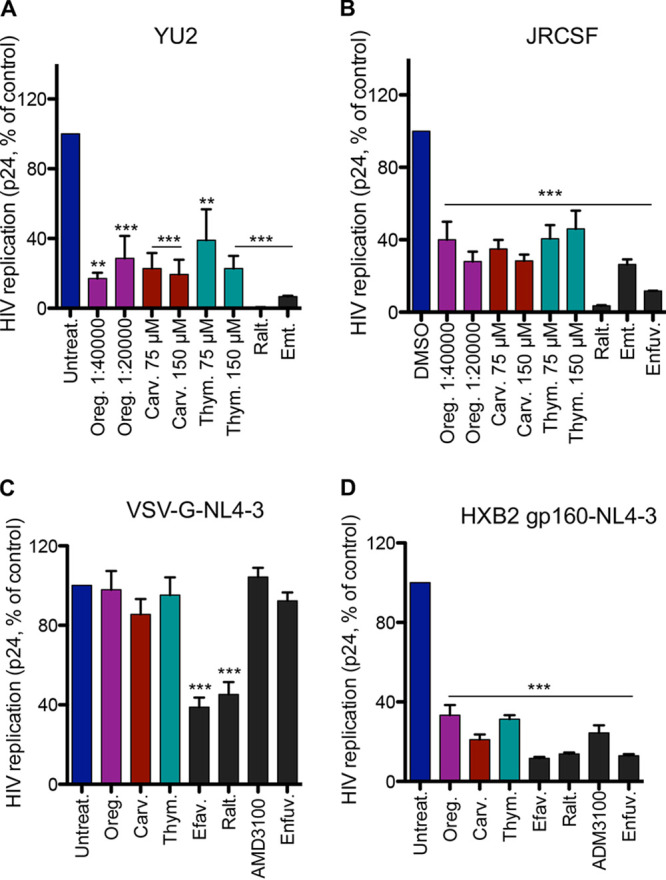
Oregano oil, carvacrol, and thymol inhibit HIV-1 entry, independent of the coreceptor and dependent on gp160. (A and B) Compound activity on R5-tropic HIV-1 replication. Cells were infected with YU2 (A) or JRCSF (B) strain. p24 in viral supernatants was determined 72 h postinfection. Raltegravir (100 nM), emtricitabine (100 nM), and enfuvirtide (1 μg/ml) were used as controls. Data are means ± SEM (A, n = 4; B, n = 3). (C) Oregano oil, carvacrol, and thymol do not inhibit infection of VSV-G pseudotyped NL4-3 infection of HEK293T cells. Results are means ± SEM (n = 4). (D) Activity of the compounds on HeLa-CD4-LTR-LacZ cells infected with HXB2-pseudotyped NL4-3. Results are means ± SEM (n = 4). Oregano oil (1:40,000 dilution), carvacrol, and thymol (100 μM) were used for panels C and D. Efavirenz (200 nM), raltegravir (200 nM), AMD3100 (10 to 20 nM), and enfuvirtide (1 μg/ml) were used as controls. One-way ANOVA followed by a Tukey’s posttest was used for statistical comparisons. **, P < 0.001; ***, P < 0.0001.
Next, to ensure that activity is dependent upon the HIV envelope, we assessed activity on viruses pseudotyped as being with or without the HIV-1 envelope. For this purpose, we infected HEK293T cells in the presence of oregano oil, carvacrol, and thymol with the NL4-3 virus pseudotyped with vesicular stomatitis virus G protein (VSV-G) envelop or HeLa-CD4-LTR-LacZ cells with NL4-3 pseudotyped with the HIV-1 HXB2 envelop (Fig. 3C and D). The supernatant p24 capsid was measured 72 h later. Oregano oil, carvacrol, or thymol inhibited HXB2 NL4-3 but not VSV-G pseudotyped viruses. As expected, AMD3100 and enfuvirtide inhibited the entry of the HXB2 but not the VSV-G pseudotyped viruses, while the postentry inhibitors, efavirenz and raltegravir, decreased levels of both pseudotyped viruses. These results suggest that a functional HIV-1 envelope is required for oregano oil, carvacrol, or thymol antiviral activity.
Oregano oil, carvacrol, and thymol block HIV fusion with the target cell.
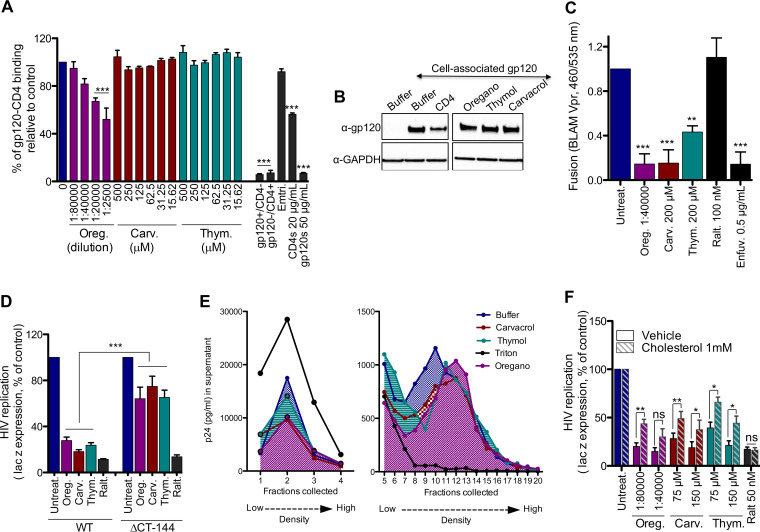
HIV gp120 interacts directly with the cell receptor CD4. As such, we investigated whether oregano oil, carvacrol, or thymol could block this interaction. An ELISA was performed with recombinant gp120 coated on the plate and soluble CD4-Ig titrated in the presence or absence of test compounds. The gp120-to-CD4-Ig interaction was revealed by the absorbance in solution (Fig. 4A). Carvacrol and thymol did not interfere with gp120–CD4-Ig binding; however, oregano oil at 1:20,000 and 1:2,500 dilutions inhibited the interaction by 40%. Thus, components of oregano oil other than carvacrol and thymol, or a specific combination of other ingredients, may be responsible for oregano oil’s inhibition of gp120–CD4-Ig binding (Table 1). As expected from controls, soluble gp120 and CD4, but not emtricitabine, inhibited the gp120–CD4-Ig interaction. In summary, the principal components of oregano oil, carvacrol, and thymol do not interfere with the gp120-CD4 interaction.
FIG 4.
Oregano oil, carvacrol, and thymol increased HIV density, preventing fusion. (A) Compounds do not block interaction of monomeric YU2 gp120 and CD4-Ig in ELISA. s, soluble. Emtricitabine (500 μM) and soluble gp120 or CD4 was used as a control. Results are means ± SEM (n = 3). (B) Compounds do not shed gp120 from gp41 from the surface of HEK293T cells transfected with JRCSF gp160. Oregano (1:20,000 dilution), carvacrol, and thymol (100 μM) were used. Data are representative of n = 3. CD4 was used as a control. (C) Oregano oil and related compounds inhibit viral fusion in BLAM-Vpr assays. Results are means ± SEM (n = 6). (D) Oregano oil and related compounds do not inhibit △CT-144 NL4-3 virus. Oregano (1:20,000 dilution), carvacrol and thymol (150 μM), and raltegravir (50 nM) were used. Results are means ± SEM (n = 5 to 7). (E) Oregano and related compounds increase density of HIV. Triton X-100 (0.1%) was used as a positive control. Oregano (1:10,000 dilution), carvacrol, and thymol (200 μM) were used. Data are representative of n = 4 experiments. (F) Compound activity on HIV is rescued by adding cholesterol. Compounds were incubated with NL4-3 and HeLa CD4-LTR-LacZ cells. β-Gal measured 72 h later. Results are means ± SEM (n = 4). t test was used for statistical comparison, except for panel F, where one-way ANOVA followed by a Tukey’s posttest were used. *, P < 0.01; **, P < 0.001; ***, P < 0.0001.
Shedding or detachment of gp120 from gp41 on the viral membrane may explain the block in viral entry. Thus, we investigated whether oregano oil, carvacrol, or thymol could promote shedding of gp120 at the cell membrane. We transfected HEK293T cells with JRCSF gp160, the precursor of gp120 and gp41, and allowed their membrane expression. Cells then were incubated with test compounds for 4 h, and the amount of remaining gp120 on the cell surface was detected by Western blotting with anti-gp120 serum (Fig. 4B). Except for the recombinant CD4 control, none of the compounds were found to induce the shedding of gp120 from the cell surface. Thus, oregano oil, carvacrol, and thymol do not promote shedding of gp120 from gp41.
We then investigated whether virus-cell fusion was inhibited by these compounds using the beta-lactamase (BLAM) assay. This assay relies on fluorescence emission upon the enzymatic cleavage of CCF2 dye by the β-lactamase Vpr fusion (BLAM-Vpr), which is transferred into the target cell after virus fusion (17) (Fig. 4C). HXB2 NL4-3 BLAM-Vpr pseudotyped virus was allowed to attach to the membrane of TZM-bl cells for 30 min. Cells were then incubated with test compounds for 90 min, before cell lysis, and wavelength measurement was performed at 460 nm for cleaved and 535 nm for uncleaved dye. Oregano oil, carvacrol, and thymol, as well as the fusion inhibitor enfuvirtide, significantly decreased the ratio of absorbance at 460 nm versus 535 nm, reflecting viral entry inhibition. As expected, the negative control, raltegravir, did not. Interestingly, an HIV isolate presenting a gp41 stop codon (ΔCytoplasmic Tail-144, ΔCT-144) (18) was not inhibited by oregano oil, carvacrol, and thymol (Fig. 4D). These data suggest that the activity of these compounds is dependent on virus-cell fusion and requires the CT of gp41. Although the function of the gp41 CT is not fully understood, it is well-established that it is required for HIV-1 envelope incorporation into virions (19, 20) and plays a role in regulating envelope fusogenicity (21).
The impairment of virus-cell fusion has also been linked to the modification of the buoyant density of the virus (22). As such, we incubated oregano oil, carvacrol, and thymol with NL4-3 virus for 6 h and then loaded the samples onto a 19.6% to 60% continuous sucrose-density gradient, followed by ultracentrifugation. A total of 22 fractions were collected, and the virus in fractions was determined by p24 ELISA (Fig. 4E). Virus was enriched in fraction 10 of the untreated control; however, exposure to oregano oil (1:10,000) and to carvacrol and thymol (200 μM) shifted virus to higher buoyant densities (fractions 12 and 11, respectively). As expected, Triton X-100, which disrupts the HIV-1 membrane, promoted virus accumulation at the top of the gradient. In summary, oregano oil, carvacrol, and thymol inhibit HIV fusion, seemingly by altering the viral membrane composition and increasing virion density.
It has been reported that cholesterol removal from virion membranes increases their buoyant density, leading to the loss of HIV-1 infectivity. Furthermore, adding exogenous cholesterol rescues viral infection (23). Thus, we investigated the effects of oregano oil, carvacrol, or thymol during the infection of HeLa-CD4-LTR-LacZ cells with NL4-3 in the presence of exogenous soluble cholesterol (Fig. 4F). HIV infection was rescued when cholesterol was added in the presence of oregano oil, carvacrol, and thymol but not with raltegravir, which was used as a control. Altogether, these results suggest that oregano oil, carvacrol, and thymol specifically inhibit HIV-cell fusion by stripping cholesterol from the HIV membrane or by binding to it, impairing viral fusion. The need for cholesterol during virus-cell fusion also seems to be dependent on the cytoplasmic tail of gp41.
Viral resistance studies identify mutations in gp41.
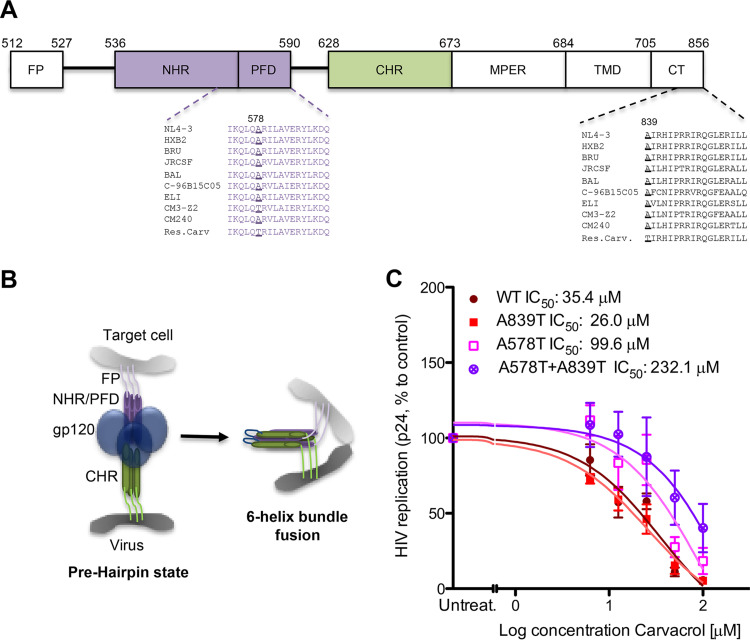
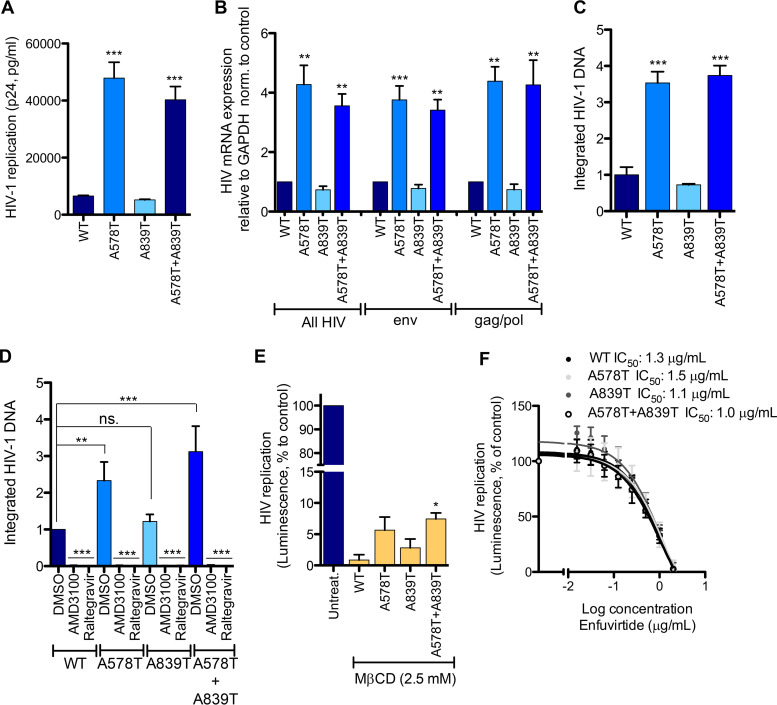
Viruses resistant to the most abundant compound in oregano oil, carvacrol, were obtained by passaging the viral isolate NL4-3 every week onto naive HeLa-CD4 cells in the presence of increasing concentrations of carvacrol. Resistance developed slowly over a 7-month period, and resistant viruses presented 3 novel variations in the envelope glycoproteins: (i) a single-point silent mutation at residue 294 in gp120 protein; (ii) a mutation, Ala578Thr, in the pocket-forming domain (PFD) in the N-terminal heptad repeat (NHR) domain of gp41; and (iii) a mutation, Ala839Thr, in the cytoplasmic tail domain of gp41 (Fig. 5A and B). The coding mutations were introduced individually or in combination in NL4-3 and tested in HIV replication assays in the presence of carvacrol (Fig. 5C). The single mutation Ala578Thr and the double mutant viruses showed resistance to carvacrol compared to the wild-type (WT) virus, with the double mutation conferring the highest resistance.
FIG 5.
Viruses resistant to carvacrol. (A) Schematic of gp41 protein, with annotated mutations found in carvacrol-resistant viruses. FP, fusion peptide; PFD, pocket-forming domain; NHR/CHR, N/C-terminal heptad repeat; MPER, membrane-proximal external region. (B) Schematic of the fusion of the viral and cell membranes. (C) Activity of carvacrol against carvacrol resistant viruses. TZM-bl cells were infected with the indicated viruses in the presence of increasing concentrations of carvacrol. Capsid p24 in the supernatant was measured 72 h later. Data are means ± SEM (n = 3).
To further characterize these resistant viruses, we measured HIV p24 capsid in the supernatant, HIV mRNA expression, and the level of integrated HIV DNA after infection of TZM-bl cells with identical amounts of virus without carvacrol. As shown in Fig. 6A, viruses A578T and Ala578Thr+Ala839Thr showed a significant 6- to 7-fold increase in p24 capsid release compared to that of the WT. Virus with single Ala839Thr mutation replicated similarly to the WT. HIV mRNA production (primers to nef region, env gene, and gag/pol genes) (Fig. 6B) as well as the integrated HIV DNA (Fig. 6C) confirmed this increase of viral replication. Upon infection of TZM-bl cells with WT NL4-3 or the different resistant viruses, we did not observe differences in gp120 and gp41 expression in the gp120/p24 capsid protein ratio (data not shown).
FIG 6.
Characterization of carvacrol-resistant viruses. (A to D) TZM-bl cells were infected with NL4-3- or carvacrol-resistant viruses. Cells were washed, and p24 capsid in supernatant (A), HIV mRNA expression (B), and integrated DNA (C) were measured 72 h or 19 h (D) later. Data are means ± SEM (n = 3). norm., normalized. (E) Carvacrol-resistant viruses are less sensitive to M-βCD. TZM-bl cells were infected with NL4-3- or carvacrol-resistant viruses in the presence of M-βCD. Luminescence was measured 72 h later. Data are means ± SEM (n = 3). (F) Carvacrol-resistant viruses are sensitive to the entry inhibitor enfuvirtide. Data are means ± SEM (n = 3). One-way ANOVA followed by Tukey’s posttest were used for statistical comparisons, except for panel D, where the Newman-Keuls posttest was used. *, P < 0.01; **, P < 0.001; ***, P < 0.0001.
To verify that this increase of virus fitness is due to a preintegration event, we measured viral integration by Alu-qPCR after 19 h of infection to avoid novel infection rounds. We observed a significant increase of integrated viral DNA with the Ala578Thr virus and the double mutated virus, suggesting an enhancement of preintegration events, namely, entry and fusion.
To assess if these viruses are less sensitive to cholesterol depletion, we treated TZM-bl cells with methyl-β-cyclodextrin (M-βCD), followed by infection with WT NL4-3 or resistant viruses, at a nontoxic M-βCD concentration (Fig. S4). Luminescence was measured 72 h later (Fig. 6E). A significant increase in viral replication compared to that of the WT was observed with Ala578Thr+Ala839Thr, while a nonsignificant but obvious increase was observed with the single mutant viruses. We also verified that these viruses were still inhibited by enfuvirtide (Fig. 6F).
Collectively, the evolution of viruses resistant to carvacrol supported inhibition of fusion as carvacrol’s mechanism of action against HIV, with the introduction of mutations in gp41 residues 578 and 839 that seem to regulate fusion events of clade B viruses.
Oregano oil, carvacrol, and thymol do not affect HIV postintegration stages.
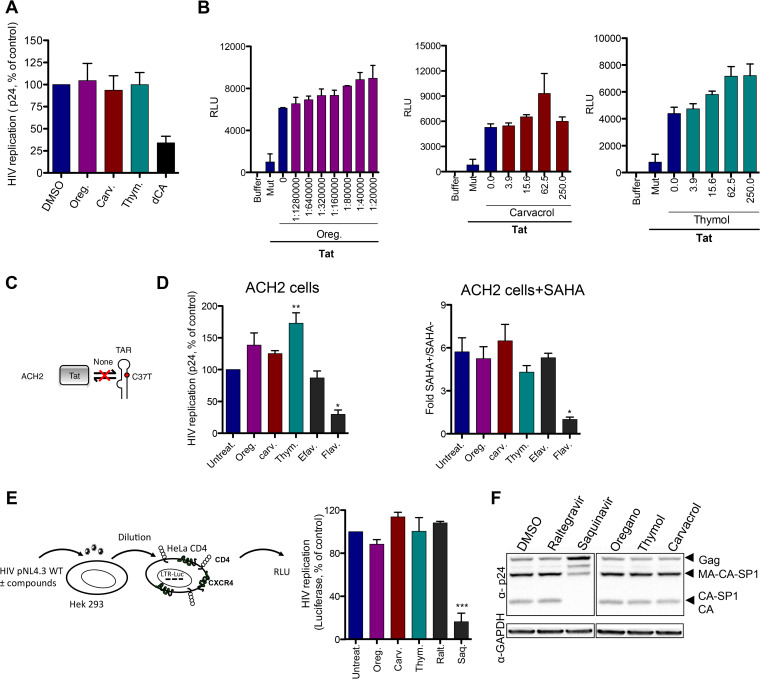
We also investigated the ability of these compounds to inhibit late-stage events in the HIV cycle. First, we studied the effects on HIV transcription. For these studies, oregano oil, carvacrol, and thymol were incubated with chronically infected HeLa CD4-LTR-LacZ cells (24) for 72 h (Fig. 7A). Apart from the Tat inhibitor didehydro-cortistatin A (dCA) (24), used as positive control, none of the compounds inhibited HIV replication. To confirm this result, we monitored HIV transcription triggered by the viral transactivator Tat in TZM-bl cells (Fig. 7B). While Tat mutated in its basic domain, used as a negative control, did not activate transcription, none of the tested compounds interfered with HIV Tat-mediated transcription. ACH2 cells contain a provirus with a mutation in TAR that blocks Tat binding. We also confirmed that these compounds, including the negative-control efavirenz, did not affect Tat-independent basal transcription (Fig. 7C and D), unlike the positive-control flavopiridol (CDK9 inhibitor). We also performed a stimulation of the cells with the histone deacetylase inhibitor SAHA, but no inhibition was observed. Collectively, these data suggest that oregano oil, carvacrol, and thymol do not inhibit HIV transcription.
FIG 7.
Oregano oil, carvacrol, and thymol do not affect late-stage HIV-1 infection. (A) Compounds and oil do not inhibit transcription of HeLa-CD4-LTR-LacZ cells chronically infected with NL4-3. Didehydro-cortistatin A (dCA; 100 nM) was used as a control. Results are means ± SEM (n = 2). (B) Compounds and oil do not inhibit Tat transactivation in TZM-bl cells. Tat mutated in the basic domain, Tat Mut, was used as a control. Data are the means ± SEM (n = 2). RLU, relative light units. (C) Schematic describing TAR mutation in ACH2 cells. (D) Compounds and oil do not inhibit SAHA (1 μM) reactivation of ACH2 cells. Flavopyridol (Flav., 100 nM) and efavirenz (100 nM) were used as controls. Results are means ± SEM (n = 4). (E) Compounds and oil do not affect maturation and assembly of the HIV-1 capsid. HEK293T cells were transfected with NL4-3 in the presence of compounds. After 72 h, the supernatant was collected and used to infect HeLa-CD4 cells expressing the luciferase gene under the control of the HIV promoter for another 72 h. Cells then were lysed and luciferase normalized to total protein. (F) Cell lysates from the first incubation in HEK293T cells from panel E were analyzed by Western blotting with anti-p24 antibody. Results are means ± SEM (n = 3) (E) and are representative of n = 3 experiments (F). Oregano (1:40,000 dilution), carvacrol, and thymol (100 μM) are shown in panels A, D, E, and F. Raltegravir (100 nM) and saquinavir (300 nM) were used. One-way ANOVA followed by Tukey’s posttest were used for statistical comparisons. **, P < 0.001; ***, P < 0.0001.
Next, we studied Gag maturation and viral assembly by incubating compounds with HEK293T cells transfected with NL4-3 plasmid for 72 h (Fig. 7E). The supernatant containing virus was recovered and used to infect the HeLa-CD4-LTR-luciferase promoter, and luminescence was measured 72 h later. In parallel, HEK293T cell lysates were analyzed by Western blotting with anti-p24 antibody. In the second-round infection assay as well as in the Gag protein maturation and assembly, oregano oil (1:40,000) and carvacrol and thymol (100 μM) performed similarly to raltegravir or the untreated control, while, as expected, the protease inhibitor saquinavir affected the maturation process (Fig. 7E). Taken together, these results demonstrate that oregano oil and its principal components impact the HIV entry without disturbing the late-stage events.
The activity of oregano oil, carvacrol, and thymol on viruses from multiple families.
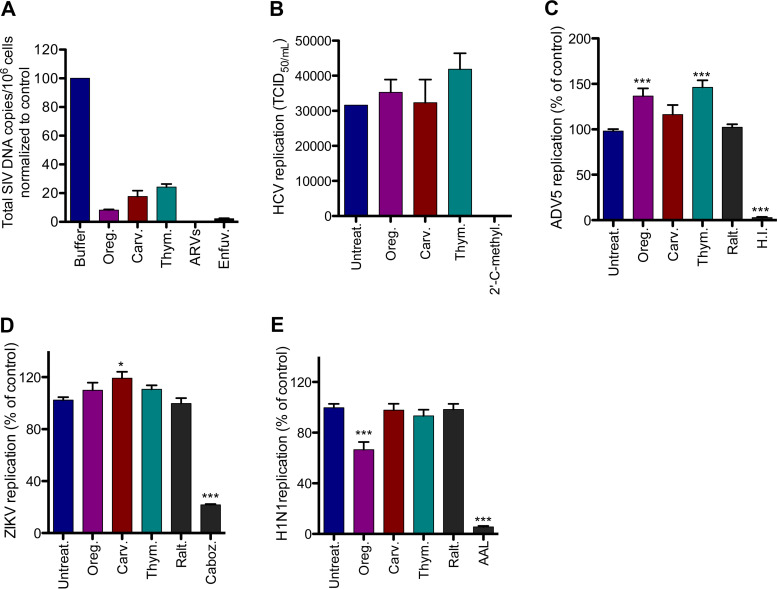
Carvacrol and thymol were previously reported to have inhibitory activity against several viruses, with various efficacies and therapeutic indices (25–27). We evaluated the activity of carvacrol, thymol, and oregano oil on viral replication of numerous viruses, such as simian immunodeficiency virus (SIV), hepatitis C virus (HCV), influenza A virus subtype H1N1, Zika virus (ZIKV) PB81 (Brazil strain), and adenovirus 5 (ADV5) (Fig. 8). Oregano oil, carvacrol, and thymol significantly inhibited SIVmac239 replication in primary rhesus macaque CD4+ T cells isolated from 3 independent macaques after a 6-day infection period (Fig. 8A). A cocktail of ARVs (raltegravir, emtricitabine, and tenofovir) was used as a control. We verified that these concentrations of compounds were not toxic for primary rhesus macaque CD4+ T cells (Fig. S5). We then evaluated their activity on Huh 7.5 cells infected with HCV and HeLa CD4-LTR-LacZ cells infected either with H1N1, ZIKV Brazil, or ADV5. Oregano oil, carvacrol, and thymol did not inhibit the replication of any of these viruses (Fig. 8B to E). 2′-C-methyladenosine (an inhibitor of RNA-dependent RNA polymerase), Aleuria aurantia lectin (binds to the influenza envelope hemagglutinin glycoprotein blocking H1N1 entry), carbozantinib (a tyrosine-kinase inhibitor known to block the activity of AXL, one of ZIKA’s main entry factors, expressed at the surface of HeLa-CD4 cells), and inactivated virus were used as positive controls for HCV, H1N1, ZIKV, and ADV5, respectively. Together, these results show the specificity of the activity of oregano oil, carvacrol, and thymol to HIV and SIV infections.
FIG 8.
Oregano oil, carvacrol, and thymol have no effect on HCV, ADV5, ZIKA, and H1N1. (A) Activity of the compounds and oil on SIV-infected primary rhesus macaque cells 6 days posttreatment. Virus replication was measured by integrated DNA content. Oregano (1:40,000 dilution), carvacrol (100 μM), and thymol (100 μM) were used. ARVs (raltegravir, emtracitabine, and tenofovir, 200 nM) were used. Results are means ± SEM (n = 2). (B) Compounds and oil do not inhibit HCV infection in Huh 7.5 cells. 2′-C-methyladenosine (2′-C-methyl., 10 μM) was used as a control. Oregano (1:40,000 dilution) and carvacrol and thymol (50 μM) were used. Data are means ± SEM (n = 2). (C) Compounds and oil did not affect H1N1 infection of HeLa-CD4-LTR-LacZ cells. Aleuria Aurantia lectin (AAL; 100 nM) and raltegravir (100 nM) were used as controls. Data are means ± SEM (n = 10). (D) Compounds and oil did not affect ZIKV Brazil infection of HeLa-CD4-LTR-LacZ cells. Cabozantinib (Caboz., 1 μM) and raltegravir (100 nM) were used as controls. Data are means ± SEM (n = 8). (E) No activity on ADV5 virus infection of HeLa-CD4-LTR-LacZ cells. Heat-inactivated virus (H.I.) and raltegravir (100 nM) were used as controls. Data are means ± SEM (n = 8). Oregano (1:20,000 dilution), carvacrol, and thymol (100 μM) were used in panels C, D, and E. One-way ANOVA followed by Tukey’s posttest were used for statistical comparisons. ***, P < 0.0001.
Structure-function relationships of carvacrol.
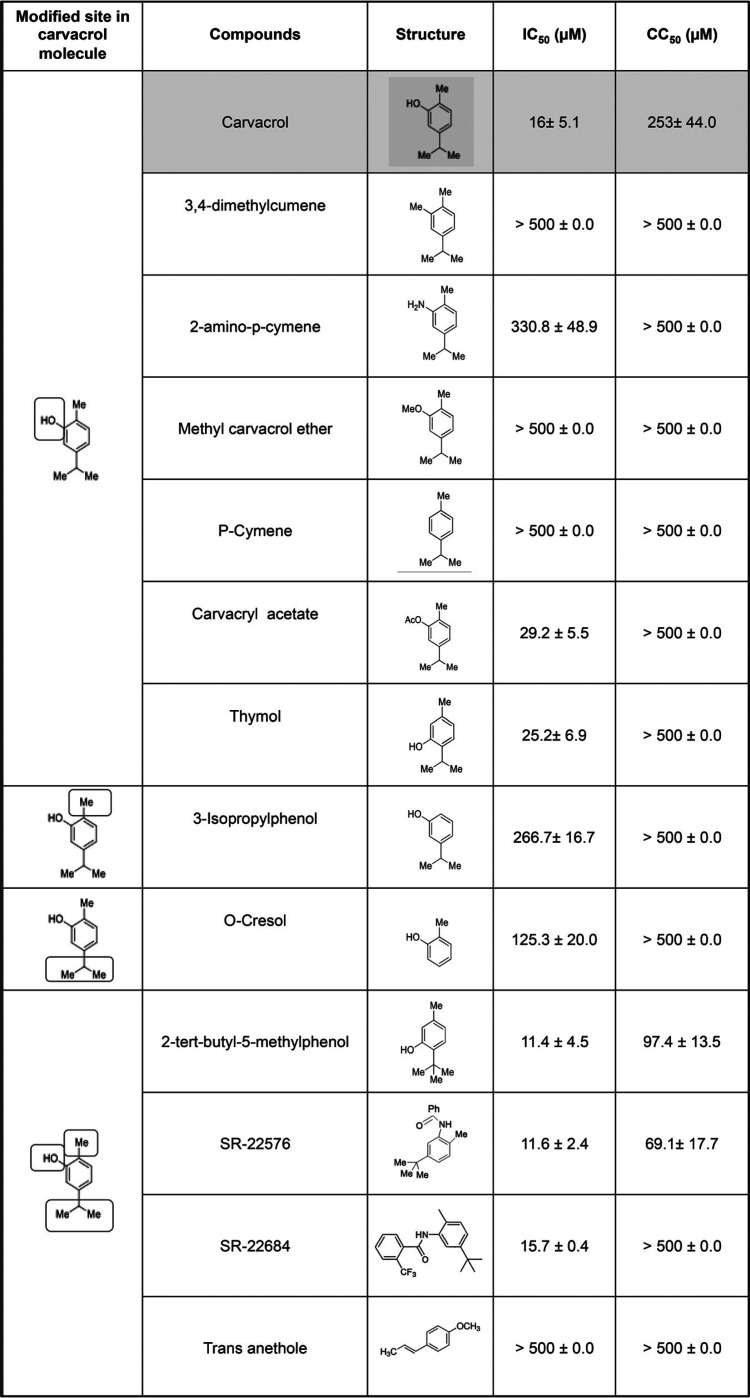
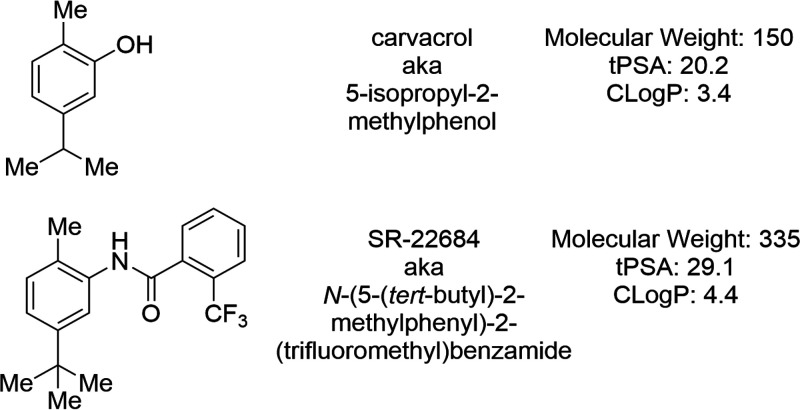
The metabolisms of carvacrol and thymol have been studied in rats, and both of these phenolic compounds, as expected, form sulfate or glucuronide conjugates, which are rapidly excreted in urine, along with unconjugated material (28). The oxidation of the methyl and isopropyl groups to hydroxyl- and carboxylate-containing metabolites was also noted. We wished to determine if analogs of carvacrol and, in particular, analogs that are expected to be more metabolically stable match or exceed its ability to prevent HIV entry.
We tested a series of analogs for their ability to inhibit NL4-3 replication in HeLa-CD4-LTR-LacZ cells (Table 2). Results show the importance of the presence and positioning of the OH, Me, and i-Pr groups (or possible replacements) with respect to HIV entry inhibition. While we did not identify analogs of carvacrol with significantly increased potency, the CC50 (and, thus, the therapeutic index) was improved for one analog (SR-22684) (Fig. 9). We have not evaluated the metabolism of this compound, although it lacks two of carvacrol’s sites of metabolism (the OH and i-Pr groups), while a third site (the methyl group) is more sterically encumbered. Molecular weight, hydrophobicity, and topological polar surface area all are increased for this compound, though not outside ranges normally desired for in vivo active compounds. High hydrophobicity is likely required for association with cholesterol, which is also hydrophobic.
TABLE 2.
Activity of carvacrol’s analogs against HIV-1 infectiona
HeLa-CD4-LTR-LacZ cells were infected with HIV-1 NL4-3 in the presence of compounds for 72 h prior to CPRG assay. Viability assays performed for 72 h on uninfected cells. Data are the means ± SEM (n = 2).
FIG 9.
Structure of carvacrol and SR-22684.
Carvacrol with anti-HIV drug cocktails.
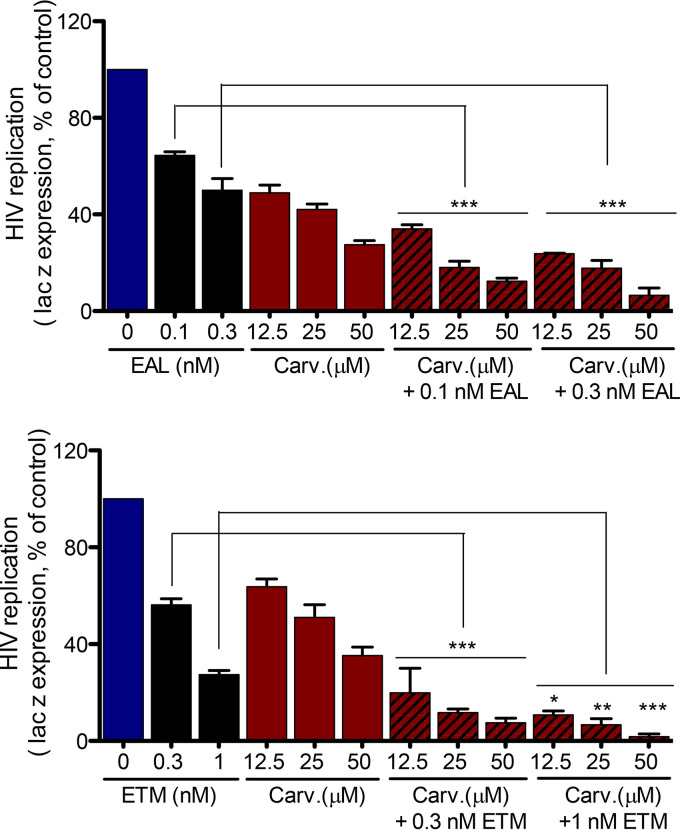
We assessed the activity of carvacrol in combination with two different cocktails of ART (Fig. 10): ETM (efavirenz, tenofovir, emtricitabine; ATRIPLA) and EAL (AZT, lamivudine and efavirenz; Combivir and Sustiva). In both cases, an additive activity is observed when carvacrol is mixed with these cocktails, rationalizing its use or the use of an improved carvacrol analog to complement current ART.
FIG 10.
Activity of carvacrol in combination with two cocktails of ARVs used in the clinic. HeLa-CD4-LTR-LacZ cells were infected with HIV-1 NL4-3 in the presence of different amounts of carvacrol or the indicated antiretrovirals (EAL, efavirenz, AZT, and lamivudine; ETM, efavirenz, tenofovir, and emtracitabine). β-Gal activity was measured 72 h later. Results represent the means ± SEM (n = 3). One-way ANOVA followed by Tukey’s posttest were used for statistical comparisons. *, P < 0.01; **, P < 0.001; ***, P < 0.0001.
DISCUSSION
Essential oils are safe and inexpensive and have been widely used within the pharmaceutical, cosmetic, and food industries. The FDA-approved formulation of oregano oil (from Origanum vulgare) has been of particular interest for its antibacterial and food-flavoring properties. Here, we describe a novel activity for oregano oil as an inhibitor of HIV replication and characterize its mechanism of action. Our results suggest that oregano oil acts by reducing cholesterol from the viral membrane (Fig. 4E and F). We observed a reduction in the buoyancy of virus treated with oregano oil, carvacrol, or thymol, suggesting cholesterol depletion (Fig. 4E), and a rescue of the infection when cholesterol was exogenously added back (Fig. 4F). Thus, oregano oil, carvacrol, or thymol blocks viral fusion with the target cell (Fig. 4C) through a mechanism that depends on the viral transmembrane gp41 protein (Fig. 4D, 5, and 6). This activity is independent of the virus tropism, as it blocks R5 or X4 viral isolates (Fig. 1 and 3). The antiviral properties of oregano oil have been attributed to two small molecules, carvacrol and thymol, with carvacrol generally being the more abundant component in oregano oil (Table 1 and Fig. 1A to C). Carvacrol and thymol have been shown to be active against a series of viruses (25–27), especially during pretreatment; however, to our knowledge, they had never been investigated against HIV or SIV.
Cholesterol is a major constituent of the HIV-1 envelope membrane bilayer (23, 29–31), important for virus stability and fusion efficacy (23, 29). In nonprogressor HIV-infected individuals, it was demonstrated that alterations in cholesterol metabolism in their antigen-presenting cells limited the transfer of HIV-1 to their CD4 helper T cells, reducing infection (32). The role of cholesterol during HIV-1 entry was highlighted using M-βCD-induced cholesterol depletion, which increased virion density and inhibited HIV replication (23). The cholesterol:phospholipid molar ratio of the viral membrane is about 2.5 times higher than that of the host cell membrane, and this composition is needed for proper fusion by gp120/gp41 envelope trimers (31). In agreement with this, the cholesterol depletion by oregano oil, carvacrol, or thymol blocked virus fusion to cells (Fig. 4C) while not affecting cell-to-cell fusion (data not shown). We also did not observe oregano oil, carvacrol, or thymol inhibition of VSV-G pseudotyped virus (Fig. 3C), consistent with reports that the infectivity of VSV-G entry is less sensitive to cholesterol depletion than HIV envelope-mediated fusion (33). Furthermore, exogenous cholesterol replenishment rescued viral infectivity after oregano, carvacrol, and thymol treatment (Fig. 4F). Carvacrol has been shown to alter cholesterol and sterol content in fungus and bacteria. Carvacrol interaction with ergosterol present in the fungal cell membranes leads to fungal membrane permeability and growth inhibition (12, 34). Similarly, carvacrol causes bacterial membrane permeability (35).
The dependence on cholesterol for viral entry has been shown for enveloped viruses, such as HCV and influenza (36–38). A specific role played by cholesterol in flavivirus Zika virus replication has not yet been reported. However, flaviviruses are known to be sensitive to the host membrane cholesterol content (39, 40). We did not observe any activity of oregano oil, carvacrol, or thymol on HCV, influenza, or Zika virus. We speculate that the compound activity is not only linked to cholesterol depletion but also dependent on specific immunodeficiency virus envelope membrane proteins, emphasized by the appearance of resistance mutations in gp41 protein (Fig. 5 and 6), the inhibition of SIV replication (Fig. 8A), and the loss of inhibition of VSV-G pseudotyped viruses (Fig. 3C). Future studies will investigate the direct binding of carvacrol to different envelope proteins from multiple viruses. The importance of the envelope cholesterol is not relevant for nonenveloped viruses, such as ADV5.
The gp41 CT domain is a complex region of about 150 residues, with several regions forming alpha helices that interact with lipid bilayers. It also contains palmitoylated cysteine residues required for envelope association with lipid rafts, which are discrete microdomains enriched in sphingolipids, and cholesterol required for efficient viral fusion (41). The truncation of this region and the interaction with cholesterol could explain the inability of oregano oil, carvacrol, and thymol to inhibit ΔCT-144 virus replication (Fig. 4D). The truncation of the gp41 CT also affects the conformation of the gp41 ectodomain (42) as well as gp120 (43), destabilizing the 6-helix bundle and affecting the fusion process (44, 45). We speculate that, similar to M-βCD (18), oregano oil depletion of virion membrane cholesterol blocks conformational changes or decreases the mobility of env, necessary for virus-cell fusion, a process that is independent of coreceptor usage. Future work will study the direct interaction of the compound with HIV cholesterol and conformational changes of the envelope protein.
Viruses growing in increasing concentrations of carvacrol developed a 2.8- to 6.6-fold resistance to carvacrol. These viruses appeared after a 7-month period, which is longer than the few weeks’ time observed for resistance development for commercially available ARVs (2). We identified mutations in residues Ala578Thr and Ala839Thr in the PFD and CT domains of gp41, respectively (Fig. 5A). Together, these two mutations increased viral replication by 4- to 5-fold (Fig. 6A and B), likely by facilitating viral entry, since we observed a 3-fold increase in integration (Fig. 6C and D) and an improved ability to infect during cholesterol depletion (Fig. 6E). The implication of these specific residues in HIV fusion has not been previously reported. However, the structure of the trimeric HIV envelope SOSIP.664 (5) shows Ala578, along with 3 other residues of the PFD in gp41 protein, associating with gp120. Nevertheless, when the cytoplasmic tail is missing (4), residue Ala578 is no longer needed for gp120 interaction. Similarly, carvacrol does not inhibit ΔCT-144 virus (Fig. 4D). Some HIV-1 strains, such as CRF07, frequently found in Asian countries, contain the Thr578Ala variation. The comparison of the crystal structure of the core region of CRF07 gp41 to clade B gp41 showed that three mutations, Thr578Ala, Lys633Arg, and Leu646Ile, abolished fusion, further supporting a critical role for residue 578 in the fusion process (46). On the other hand, these results suggest that carvacrol is less potent against certain HIV-1 Asian strains. It has been reported that a conserved sequence of gp41, 679LWYIK683, binds cholesterol and that mutations in this domain inhibit HIV infectivity. However, we did not identify variations that improve or reduce affinity for cholesterol in this region (47).
The activity of carvacrol was assessed in combination with two different cocktails of ART (Fig. 10). In both cases, an additive activity was observed with carvacrol. Of note, the concentration of carvacrol used in our experiments is lower than amounts considered toxic for animals (48). Carvacrol has, however, low bioavailability and is highly metabolized in vivo; thus, further work is needed to improve delivery and to achieve a lasting concentration of the active species. We have shown that carvacrol analogs, such as SR-22684 (Table 2 and Fig. 9), retain the activity of the natural product, although the bioavailability of such analogs has not yet been evaluated.
In summary, our results show that oregano oil, carvacrol, and thymol are inhibitors of HIV-1 entry. The HIV-1 glycoproteins associate with cholesterol at their envelope membranes to facilitate the interaction with the cellular membranes. Our results support a mechanism of action in which oregano oil, carvacrol, and thymol deplete cholesterol from the viral membrane, blocking viral fusion. The evolution of viral resistance to these molecules supports this mechanism of action, with the appearance of mutations in gp41 that we suspect improves viral fusion in the absence of cholesterol. We propose that gp41 residue 578 and/or 839 be considered in the design of future anti-gp41 drugs. In a general manner, these compounds have been found safe for human use by the FDA, and they are inexpensive, easy to synthesize, and can be formulated as tablets, liquids, or films. They also display additional properties that would benefit HIV-1-infected individuals on ART, such as antihyperlipidemia, candidiasis, and cryptosporidiosis (13, 14, 49–51). Interestingly, carvacrol and thymol have also been used to enhance the transdermal transport of AZT (52). Oregano oil, and perhaps carvacrol and thymol synthetic analogs, may offer promise as supplements to current HIV therapeutic strategies.
MATERIALS AND METHODS
Multiple-round infection.
HeLa-CD4-LTR-LacZ cells or TZM-bl cells were infected overnight (O/N) with wild-type NL4-3, NL4-3 pseudotyped with the HXB2 envelop, viruses resistant to carvacrol, or ΔCT-144 (gift from E. O. Freed) in the presence of increasing concentrations of testing compounds or control drugs. Cells were washed, and fresh medium containing compounds was added for 72 h. HIV-1 replication was assessed by measuring the viral capsid in the supernatant with p24 enzyme-linked immunosorbent assay (ELISA) from Advanced Bioscience Laboratories. The infection of HeLa-CD4-LTR-LacZ cells with NL4-3 was also monitored by measuring reporter β-galactosidase production using the chlorophenol red-β-d-galactopyranoside (CPRG) assay, as previously described (53). Water-soluble cholesterol (number C4951; Sigma) was used for the cholesterol rescue experiment. In TZM-bl cells, viral replication was assessed by measuring viral mRNA expression, as previously described (53), or by luminescence using Bright-Glo by following the manufacturer’s recommendations. The same protocol was used for Ghost/TZM-bl cell infection with JRCSF or YU2. In primary human and rhesus macaque CD4+ T cells, viral replication of HIV-1 and SIV was performed as previously described (54). To assess the activity of carvacrol on the virus, carvacrol was incubated with NL4-3 for 3 h. The mixture then was diluted 1/10 and incubated with TZM-bl cells in the absence of carvacrol. Similarly, to assess effects on cells, carvacrol was incubated with TZM-bl cells for 3 h and washed, and virus then was added to cells without carvacrol. Luciferase was measured 72 h later using Bright-Glo by following the manufacturer’s recommendations.
Single-round infection.
HeLa-CD4-LTR-LacZ cells were infected O/N with VSV-G–NL4-3 pseudotyped virus in the presence of oregano oil, carvacrol, thymol, or controls. Cells were washed, and fresh medium containing compounds was added for 72 h. p24 capsid in the supernatant was assessed by p24 ELISA.
Transactivation assay in HeLa-CD4-LTR-Luciferase cells.
TZM-bl cells were plated at 3 × 106 in a 10-cm2 culture dish and transfected with 5 μg of the constructs expressing Tat (PGK-Flag-Tat) and Tat Mut (PGK-Flag-Tat Mut), driven by the murine phosphoglycerate kinase-1 (PGK) promoter, using TransIT-LT1 transfection reagent (Mirus Bio LLC) according to the manufacturer’s instructions. Cells were split 24 h posttransfection and treated with compounds at different concentrations. Luciferase activity per total protein concentration of each sample was determined 48 h later.
Quantification of early/reverse transcription product and provirus integration.
HeLa-CD4-LTR-LacZ cells were plated at 1 × 105 cells per well in a six-well plate. Twenty-four hours later, cells were infected with NL4-3 virus in the presence of DMSO, oregano oil, carvacrol, thymol, or controls. At 10 and 19 h postinfection, genomic DNAs (gDNAs) were prepared using a DNeasy blood and tissue kit (Qiagen) according to the manufacturer’s protocol and subjected to quantitative PCR (qPCR) using Sensifast Sybr mix (Bioline). The early and late viral DNA products and the integrated proviruses were quantified as previously described (54).
Time-of-drug-addition assay.
HeLa-CD4-LTR-LacZ cells were plated at 5 × 103 in a 96-well plate and infected with NL4-3. Oregano oil and its related compounds, AMD3100 (4 nM) and efavirenz (10 nM), were added to the wells at different time points postinfection. The infection was allowed to proceed for 72 h. A CPRG assay was used to quantify viral replication.
gp120-CD4 interaction by ELISA.
The ELISA for gp120-CD4 interaction was performed as previously described (54).
Endocytosis of CD4 and CXCR4 in HeLa-CD4-LTR-LacZ cells revealed by flow cytometry.
Cells were plated at 5 × 105 in a 6-well plate. The next day, compounds were added and cells were collected at different time points for CD4 and CXCR4 expression analysis or 6 h later for CCR5. Cells were stained with phycoerythrin (PE)/Cy7 anti-human CD184 (for CXCR4; BioLegend), PE anti-human CD4 (BioLegend), PE anti-human CD195 (for CCR5; BioLegend), or PE Rat IgG2b (PE control; BioLegend) and fixed with 2% formaldehyde in phosphate-buffered saline (PBS). Cells were analyzed on an LSRII flow cytometer (BD Bioscience).
Shedding of gp120.
HEK293T cells were transfected with the constructs JRCSF gp160Δtail (1.5 μg) and pCMV Rev (0.5 μg) (gifts from M. Farzan) for 48 h with TransIT-2020 transfection reagent (Mirus Bio LLC) per the manufacturer’s protocol. JRCSF gp160Δtail was used, since it was shown to yield higher envelope trimer expression (55). Buffer, CD4-Ig (45 μg; gift from M. Farzan), oregano oil, carvacrol, or thymol was incubated with cells for 4 h. Cells then were washed several times with PBS–10 mM EDTA and lysed with radioimmunoprecipitation assay (RIPA) buffer supplemented with a cocktail of protease inhibitors (Roche). Protein concentration was determined by Bradford assay, and samples were analyzed by Western blotting with gp120 serum at 1:400 dilution (gift from M. Farzan).
Latently and chronically infected cells.
Chronically infected HeLa-CD4-LTR-LacZ cells (2.5 × 105 cells per well in a 6-well plate) and ACH2 cells (5 × 105 cells per well in a 6-well plate) were treated with compounds for 72 h, and p24 capsid in the supernatant was assessed by p24 ELISA. Cells were treated with oregano oil, carvacrol, thymol, dCA, efavirenz, or flavopiridol. ACH2 cells were also incubated with compounds in the presence of SAHA for 24 h.
Assessment of the maturation and assembly of the capsid by Western blotting.
HEK293T cells were transfected with the NL4-3 strain (12.5 μg) with TransIT-2020 transfection reagent (Mirus Bio LLC) per the manufacturer’s protocol. Six hours later, cells were washed, trypsinized, and split between the conditions to be tested. Cells were treated with control buffer, oregano oil, thymol, carvacrol, raltegravir, or saquinavir and incubated at 37°C for 72 h. Cells then were lysed with RIPA buffer supplemented with a cocktail of protease inhibitors (Roche) and analyzed by Western blotting with anti-p24 antibody (AIDS Reagent) at a 1:2,500 dilution. Anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody at a 1:1,500 dilution was used as a loading control.
HCV virus infectivity assessment by IHC and infection of HeLa-CD4-LTR-LacZ cells.
HCV virus infectivity (titer) assessment by immunohistochemistry (IHC) and infection of HeLa-CD4-LTR-LacZ cells with ZIKV Brazil, H1N1 2009, and AdV5 were performed as previously described (54).
BLAM assay.
HIV-1 pseudoviruses were added to cells and centrifuged at 2,095 × g at 4°C for 30 min to facilitate virus attachment to cells. Cells then were incubated with the compounds at 37°C for 90 min. The medium then was removed and CCF2 BLAM substrate was added. The plates were incubated at 12°C O/N to allow CCF2 cleavage by BLAM. The fluorescence intensity was measured by using a plate reader with excitation at 400 nm and emissions at 460 and 535 nm for the blue and green signals of the substrate, respectively. The fusion signal was calculated and expressed as a ratio of blue and green signals after subtracting the blank fluorescence signal from wells with substrate but without virus, using the following equation: fusion = (F460 − F460 blank)/(F535 − F535 blank).
Density of HIV using continuous sucrose-density equilibrium gradient.
Oregano oil, carvacrol, and thymol were incubated with NL4-3 virus for 6 h and then loaded on a 19.6% to 60% continuous sucrose-density equilibrium gradient, followed by ultracentrifugation. Several fractions were collected, and the virus present in these fractions was determined by p24 ELISA according to the manufacturer’s recommendations.
Measurement of compound and oil toxicity.
MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) assay (ATCC) or CellTiter-Glo luminescent cell viability (Promega) was performed in the presence of increasing concentrations of compounds according to the manufacturer’s protocol.
Statistical analysis.
P values were calculated using one-way analysis of variance (ANOVA) followed by a Tukey’s or Newman-Keuls post hoc test. The two-tailed paired t test was used when required. P values of <0.05 were considered statistically significant. Statistical analysis was performed using GraphPad Prism software (San Diego, CA, USA).
Acquisition or synthesis of analogs.
All compounds shown in Table 2 are commercially available, except for SR-22576 and SR-22684. These two compounds were made from commercial 2-methyl-5-(t-butyl)aniline by acylation with benzoyl chloride and o-trimethylbenzoyl chloride, respectively.
Supplementary Material
ACKNOWLEDGMENTS
We thank Ester H. Segal for kindly sharing reagents and Bill Webb for his help for the GC-MS analysis.
We have no conflicts of interest to declare.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Henderson LJ, Reoma LB, Kovacs JA, Nath A. 2019. Advances towards curing HIV-1 infection in tissue reservoirs. J Virol 94:e00375-19. doi: 10.1128/JVI.00375-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clutter DS, Jordan MR, Bertagnolio S, Shafer RW. 2016. HIV-1 drug resistance and resistance testing. Infect Genet Evol 46:292–307. doi: 10.1016/j.meegid.2016.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doms RW, Moore JP. 2000. HIV-1 membrane fusion: targets of opportunity. J Cell Biol 151:F9–F14. doi: 10.1083/jcb.151.2.f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee JH, Ozorowski G, Ward AB. 2016. Cryo-EM structure of a native, fully glycosylated, cleaved HIV-1 envelope trimer. Science 351:1043–1048. doi: 10.1126/science.aad2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pancera M, Zhou T, Druz A, Georgiev IS, Soto C, Gorman J, Huang J, Acharya P, Chuang GY, Ofek G, Stewart-Jones GB, Stuckey J, Bailer RT, Joyce MG, Louder MK, Tumba N, Yang Y, Zhang B, Cohen MS, Haynes BF, Mascola JR, Morris L, Munro JB, Blanchard SC, Mothes W, Connors M, Kwong PD. 2014. Structure and immune recognition of trimeric pre-fusion HIV-1 Env. Nature 514:455–461. doi: 10.1038/nature13808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dorr P, Westby M, Dobbs S, Griffin P, Irvine B, Macartney M, Mori J, Rickett G, Smith-Burchnell C, Napier C, Webster R, Armour D, Price D, Stammen B, Wood A, Perros M. 2005. Maraviroc (UK-427,857), a potent, orally bioavailable, and selective small-molecule inhibitor of chemokine receptor CCR5 with broad-spectrum anti-human immunodeficiency virus type 1 activity. Antimicrob Agents Chemother 49:4721–4732. doi: 10.1128/AAC.49.11.4721-4732.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duffalo ML, James CW. 2003. Enfuvirtide: a novel agent for the treatment of HIV-1 infection. Ann Pharmacother 37:1448–1456. doi: 10.1345/aph.1D143. [DOI] [PubMed] [Google Scholar]
- 8.Mousseau G, Clementz MA, Bakeman WN, Nagarsheth N, Cameron M, Shi J, Baran P, Fromentin R, Chomont N, Valente ST. 2012. An analog of the natural steroidal alkaloid cortistatin A potently suppresses Tat-dependent HIV transcription. Cell Host Microbe 12:97–108. doi: 10.1016/j.chom.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomford NE, Senthebane DA, Rowe A, Munro D, Seele P, Maroyi A, Dzobo K. 2018. Natural products for drug discovery in the 21st century: innovations for novel drug discovery. Int J Mol Sci 19:1578. doi: 10.3390/ijms19061578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anonymous. 1989. In vitro screening of traditional medicines for anti-HIV activity: memorandum from a WHO meeting. Bull World Health Organ 67:613–618. [PMC free article] [PubMed] [Google Scholar]
- 11.Leyva-Lopez N, Gutierrez-Grijalva EP, Vazquez-Olivo G, Heredia JB. 2017. Essential oils of oregano: biological activity beyond their antimicrobial properties. Molecules 22:989. doi: 10.3390/molecules22060989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bakkali F, Averbeck S, Averbeck D, Idaomar M. 2008. Biological effects of essential oils–a review. Food Chem Toxicol 46:446–475. doi: 10.1016/j.fct.2007.09.106. [DOI] [PubMed] [Google Scholar]
- 13.Gaur S, Kuhlenschmidt TB, Kuhlenschmidt MS, Andrade JE. 2018. Effect of oregano essential oil and carvacrol on Cryptosporidium parvum infectivity in HCT-8 cells. Parasitol Int 67:170–175. doi: 10.1016/j.parint.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Manohar V, Ingram C, Gray J, Talpur NA, Echard BW, Bagchi D, Preuss HG. 2001. Antifungal activities of origanum oil against Candida albicans. Mol Cell Biochem 228:111–117. doi: 10.1023/a:1013311632207. [DOI] [PubMed] [Google Scholar]
- 15.Abram ME, Ferris AL, Das K, Quinones O, Shao W, Tuske S, Alvord WG, Arnold E, Hughes SH. 2014. Mutations in HIV-1 reverse transcriptase affect the errors made in a single cycle of viral replication. J Virol 88:7589–7601. doi: 10.1128/JVI.00302-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lara HH, Ayala-Nunez NV, Ixtepan-Turrent L, Rodriguez-Padilla C. 2010. Mode of antiviral action of silver nanoparticles against HIV-1. J Nanobiotechnol 8:1. doi: 10.1186/1477-3155-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cavrois M, Neidleman J, Greene WC. 2014. HIV-1 fusion assay. Bio Protoc 4:e1212. doi: 10.21769/bioprotoc.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Waheed AA, Ablan SD, Roser JD, Sowder RC, Schaffner CP, Chertova E, Freed EO. 2007. HIV-1 escape from the entry-inhibiting effects of a cholesterol-binding compound via cleavage of gp41 by the viral protease. Proc Natl Acad Sci U S A 104:8467–8471. doi: 10.1073/pnas.0701443104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akari H, Fukumori T, Adachi A. 2000. Cell-dependent requirement of human immunodeficiency virus type 1 gp41 cytoplasmic tail for Env incorporation into virions. J Virol 74:4891–4893. doi: 10.1128/jvi.74.10.4891-4893.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murakami T, Freed EO. 2000. The long cytoplasmic tail of gp41 is required in a cell type-dependent manner for HIV-1 envelope glycoprotein incorporation into virions. Proc Natl Acad Sci U S A 97:343–348. doi: 10.1073/pnas.97.1.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wyss S, Dimitrov AS, Baribaud F, Edwards TG, Blumenthal R, Hoxie JA. 2005. Regulation of human immunodeficiency virus type 1 envelope glycoprotein fusion by a membrane-interactive domain in the gp41 cytoplasmic tail. J Virol 79:12231–12241. doi: 10.1128/JVI.79.19.12231-12241.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geuenich S, Goffinet C, Venzke S, Nolkemper S, Baumann I, Plinkert P, Reichling J, Keppler OT. 2008. Aqueous extracts from peppermint, sage and lemon balm leaves display potent anti-HIV-1 activity by increasing the virion density. Retrovirology 5:27. doi: 10.1186/1742-4690-5-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campbell SM, Crowe SM, Mak J. 2002. Virion-associated cholesterol is critical for the maintenance of HIV-1 structure and infectivity. AIDS 16:2253–2261. doi: 10.1097/00002030-200211220-00004. [DOI] [PubMed] [Google Scholar]
- 24.Mousseau G, Kessing CF, Fromentin R, Trautmann L, Chomont N, Valente ST. 2015. The Tat inhibitor didehydro-cortistatin A prevents HIV-1 reactivation from latency. mBio 6:e00465. doi: 10.1128/mBio.00465-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pilau MR, Alves SH, Weiblen R, Arenhart S, Cueto AP, Lovato LT. 2011. Antiviral activity of the Lippia graveolens (Mexican oregano) essential oil and its main compound carvacrol against human and animal viruses. Braz J Microbiol 42:1616–1624. doi: 10.1590/S1517-838220110004000049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanchez C, Aznar R, Sanchez G. 2015. The effect of carvacrol on enteric viruses. Int J Food Microbiol 192:72–76. doi: 10.1016/j.ijfoodmicro.2014.09.028. [DOI] [PubMed] [Google Scholar]
- 27.Sharifi-Rad J, Salehi B, Schnitzler P, Ayatollahi SA, Kobarfard F, Fathi M, Eisazadeh M, Sharifi-Rad M. 2017. Susceptibility of herpes simplex virus type 1 to monoterpenes thymol, carvacrol, p-cymene and essential oils of Sinapis arvensis L., Lallemantia royleana Benth. and Pulicaria vulgaris Gaertn. Cell Mol Biol 63:42–47. doi: 10.14715/cmb/2017.63.8.10. [DOI] [PubMed] [Google Scholar]
- 28.Austgulen LT, Solheim E, Scheline RR. 1987. Metabolism in rats of p-cymene derivatives: carvacrol and thymol. Pharmacol Toxicol 61:98–102. doi: 10.1111/j.1600-0773.1987.tb01783.x. [DOI] [PubMed] [Google Scholar]
- 29.Kalyana Sundaram RV, Li H, Bailey L, Rashad AA, Aneja R, Weiss K, Huynh J, Bastian AR, Papazoglou E, Abrams C, Wrenn S, Chaiken I. 2016. Impact of HIV-1 membrane cholesterol on cell-independent lytic inactivation and cellular infectivity. Biochemistry 55:447–458. doi: 10.1021/acs.biochem.5b00936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Richieri SP, Bartholomew R, Aloia RC, Savary J, Gore R, Holt J, Ferre F, Musil R, Tian HR, Trauger R, Lowry P, Jensen F, Carlo DJ, Maigetter RZ, Prior CP. 1998. Characterization of highly purified, inactivated HIV-1 particles isolated by anion exchange chromatography. Vaccine 16:119–129. doi: 10.1016/s0264-410x(97)00196-5. [DOI] [PubMed] [Google Scholar]
- 31.Aloia RC, Tian H, Jensen FC. 1993. Lipid composition and fluidity of the human immunodeficiency virus envelope and host cell plasma membranes. Proc Natl Acad Sci U S A 90:5181–5185. doi: 10.1073/pnas.90.11.5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rappocciolo G, Jais M, Piazza P, Reinhart TA, Berendam SJ, Garcia-Exposito L, Gupta P, Rinaldo CR. 2014. Alterations in cholesterol metabolism restrict HIV-1 trans-infection in nonprogressors. mBio 5:e01031-13. doi: 10.1128/mBio.01031-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guyader M, Kiyokawa E, Abrami L, Turelli P, Trono D. 2002. Role for human immunodeficiency virus type 1 membrane cholesterol in viral internalization. J Virol 76:10356–10364. doi: 10.1128/jvi.76.20.10356-10364.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nobrega RO, Teixeira AP, Oliveira WA, Lima EO, Lima IO. 2016. Investigation of the antifungal activity of carvacrol against strains of Cryptococcus neoformans. Pharm Biol 54:2591–2596. doi: 10.3109/13880209.2016.1172319. [DOI] [PubMed] [Google Scholar]
- 35.Gill AO, Holley RA. 2006. Inhibition of membrane bound ATPases of Escherichia coli and Listeria monocytogenes by plant oil aromatics. Int J Food Microbiol 111:170–174. doi: 10.1016/j.ijfoodmicro.2006.04.046. [DOI] [PubMed] [Google Scholar]
- 36.Aizaki H, Morikawa K, Fukasawa M, Hara H, Inoue Y, Tani H, Saito K, Nishijima M, Hanada K, Matsuura Y, Lai MM, Miyamura T, Wakita T, Suzuki T. 2008. Critical role of virion-associated cholesterol and sphingolipid in hepatitis C virus infection. J Virol 82:5715–5724. doi: 10.1128/JVI.02530-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun X, Whittaker GR. 2003. Role for influenza virus envelope cholesterol in virus entry and infection. J Virol 77:12543–12551. doi: 10.1128/jvi.77.23.12543-12551.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bajimaya S, Frankl T, Hayashi T, Takimoto T. 2017. Cholesterol is required for stability and infectivity of influenza A and respiratory syncytial viruses. Virology 510:234–241. doi: 10.1016/j.virol.2017.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Osuna-Ramos JF, Reyes-Ruiz JM, Del Ángel RM. 2018. The role of host cholesterol during flavivirus infection. Front Cell Infect Microbiol 8:388. doi: 10.3389/fcimb.2018.00388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carro AC, Damonte EB. 2013. Requirement of cholesterol in the viral envelope for dengue virus infection. Virus Res 174:78–87. doi: 10.1016/j.virusres.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 41.Bhattacharya J, Peters PJ, Clapham PR. 2004. Human immunodeficiency virus type 1 envelope glycoproteins that lack cytoplasmic domain cysteines: impact on association with membrane lipid rafts and incorporation onto budding virus particles. J Virol 78:5500–5506. doi: 10.1128/jvi.78.10.5500-5506.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Durham ND, Yewdall AW, Chen P, Lee R, Zony C, Robinson JE, Chen BK. 2012. Neutralization resistance of virological synapse-mediated HIV-1 infection is regulated by the gp41 cytoplasmic tail. J Virol 86:7484–7495. doi: 10.1128/JVI.00230-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Edwards TG, Wyss S, Reeves JD, Zolla-Pazner S, Hoxie JA, Doms RW, Baribaud F. 2002. Truncation of the cytoplasmic domain induces exposure of conserved regions in the ectodomain of human immunodeficiency virus type 1 envelope protein. J Virol 76:2683–2691. doi: 10.1128/jvi.76.6.2683-2691.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abrahamyan LG, Mkrtchyan SR, Binley J, Lu M, Melikyan GB, Cohen FS. 2005. The cytoplasmic tail slows the folding of human immunodeficiency virus type 1 Env from a late prebundle configuration into the six-helix bundle. J Virol 79:106–115. doi: 10.1128/JVI.79.1.106-115.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Klug YA, Rotem E, Schwarzer R, Shai Y. 2017. Mapping out the intricate relationship of the HIV envelope protein and the membrane environment. Biochim Biophys Acta Biomembr 1859:550–560. doi: 10.1016/j.bbamem.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 46.Du J, Xue H, Ma J, Liu F, Zhou J, Shao Y, Qiao W, Liu X. 2013. The crystal structure of HIV CRF07 B’/C gp41 reveals a hyper-mutant site in the middle of HR2 heptad repeat. Virology 446:86–94. doi: 10.1016/j.virol.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 47.Vincent N, Genin C, Malvoisin E. 2002. Identification of a conserved domain of the HIV-1 transmembrane protein gp41 which interacts with cholesteryl groups. Biochim Biophys Acta 1567:157–164. doi: 10.1016/s0005-2736(02)00611-9. [DOI] [PubMed] [Google Scholar]
- 48.Marchese A, Arciola CR, Coppo E, Barbieri R, Barreca D, Chebaibi S, Sobarzo-Sanchez E, Nabavi SF, Nabavi SM, Daglia M. 2018. The natural plant compound carvacrol as an antimicrobial and anti-biofilm agent: mechanisms, synergies and bio-inspired anti-infective materials. Biofouling 34:630–656. doi: 10.1080/08927014.2018.1480756. [DOI] [PubMed] [Google Scholar]
- 49.Aristatile B, Al-Numair KS, Veeramani C, Pugalendi KV. 2009. Antihyperlipidemic effect of carvacrol on D-galactosamine-induced hepatotoxic rats. J Basic Clin Physiol Pharmacol 20:15–27. doi: 10.1515/jbcpp.2009.20.1.15. [DOI] [PubMed] [Google Scholar]
- 50.Nagoor Meeran MF, Javed H, Al Taee H, Azimullah S, Ojha SK. 2017. Pharmacological properties and molecular mechanisms of thymol: prospects for its therapeutic potential and pharmaceutical development. Front Pharmacol 8:380. doi: 10.3389/fphar.2017.00380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nolan D. 2003. Metabolic complications associated with HIV protease inhibitor therapy. Drugs 63:2555–2574. doi: 10.2165/00003495-200363230-00001. [DOI] [PubMed] [Google Scholar]
- 52.Ham AS, Buckheit RW Jr. 2015. Current and emerging formulation strategies for the effective transdermal delivery of HIV inhibitors. Ther Deliv 6:217–229. doi: 10.4155/tde.14.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mediouni S, Chinthalapudi K, Ekka MK, Usui I, Jablonski JA, Clementz MA, Mousseau G, Nowak J, Macherla VR, Beverage JN, Esquenazi E, Baran P, de Vera IMS, Kojetin D, Loret EP, Nettles K, Maiti S, Izard T, Valente ST. 2019. Didehydro-cortistatin A inhibits HIV-1 by specifically binding to the unstructured basic region of Tat. mBio 10:e02662-18. doi: 10.1128/mBio.02662-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mediouni S, Jablonski JA, Tsuda S, Richard A, Kessing C, Andrade MV, Biswas A, Even Y, Tellinghuisen T, Choe H, Cameron M, Stevenson M, Valente ST. 2018. Potent suppression of HIV-1 cell attachment by Kudzu root extract. Retrovirology 15:64. doi: 10.1186/s12977-018-0446-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Binley JM, Cayanan CS, Wiley C, Schulke N, Olson WC, Burton DR. 2003. Redox-triggered infection by disulfide-shackled human immunodeficiency virus type 1 pseudovirions. J Virol 77:5678–5684. doi: 10.1128/jvi.77.10.5678-5684.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.