Although naturally present in HCV infection patient serum, the virological or immunological functions of the HCV F protein, which is a frameshift product of core coding sequences, remain unclear. Here, we report the effects of the HCV F protein between genotypes and discuss a potential explanation for the differential responses to type I IFN-based therapy among patients infected with different genotypes of HCV. Our study provides one step forward to understanding the host response during HCV infection and new insights for the prediction of the outcome of IFN-based therapy in HCV patients.
KEYWORDS: frameshift protein, hepatitis C virus, interferons, proteasome, type I interferon
ABSTRACT
Hepatitis C virus (HCV) has evolved mechanisms to evade innate immunity that are leading to chronic infections. The immunological function of the HCV frameshift (F) protein, which is a frameshift product of core coding sequences, has not been well characterized. The HCV F protein is produced during natural HCV infections and is found most commonly in genotype 1 HCV. In this study, we investigated whether the F protein plays a role in type I interferon (IFN) induction pathways. We engineered F expression constructs from core coding sequences of 4 genotypes (1a, 2a, 3a, and 4a) of HCV as well as the sequences which would only be able to produce core proteins. The peptide lengths and amino acids sequences of F proteins are highly variable. We hypothesized that F proteins from different genotypes might control the type I IFN production and response differently. We found that both IFN-beta (IFN-β) promoter activities are significantly higher in genotype 1a F protein (F1a)-expressing cells. Conversely, the IFN-β promoter activities are lower in genotype 2a F (F2a) protein-expressing cells. We also used real-time PCR to confirm IFN-β mRNA expression levels. By generating chimera F proteins, we discovered that the effects of F proteins were determined by the amino acid sequence 40 to 57 of genotype 1a. The regulation of type I IFN induction pathway is related but not limited to the activity of F1a to interact with proteasome subunits and to disturb the proteasome activity. Further molecular mechanisms of how F proteins from different genotypes of HCV control these pathways differently remain to be investigated.
IMPORTANCE Although naturally present in HCV infection patient serum, the virological or immunological functions of the HCV F protein, which is a frameshift product of core coding sequences, remain unclear. Here, we report the effects of the HCV F protein between genotypes and discuss a potential explanation for the differential responses to type I IFN-based therapy among patients infected with different genotypes of HCV. Our study provides one step forward to understanding the host response during HCV infection and new insights for the prediction of the outcome of IFN-based therapy in HCV patients.
INTRODUCTION
Hepatitis C virus (HCV) is a positive-stranded RNA virus that infects nearly 3% of the human population worldwide. It has been reported that HCV has evolved many mechanisms to evade innate immunity, leading to the establishment of persistent infection. For example, HCV core protein has been shown to induce suppressor of cytokine signaling 1 (SOCS1) and suppressor of cytokine signaling 3 (SOCS3), which would downregulate type I interferon (IFN) responses (1, 2). Both core and NS5A have been shown block STAT-1 phosphorylation, and NS5A was also able to block protein kinase R (PKR) phosphorylation and therefore inhibit type I interferon responses (3, 4). Recently, it has been shown that HCV NS3/4A protease cleaves critical adaptor proteins, MAVS- and TIR-domain-containing adapter-inducing interferon-β (TRIF), the adaptor protein of RIG-I, and Toll-like receptor 3 (TLR3) to attenuate interferon production and disrupt innate antiviral immunity (5, 6).
The HCV core protein is the first protein encoded by the HCV open reading frame, which is the nucleocapsid protein that packages the viral genome. It has been reported that alternative translation products of the core coding region could be produced as +1/−2 or −1/+2 frameshift protein, named F protein. Particularly, alternative reading frame protein (ARFP), or frameshift protein (F protein), also named core + 1 protein, was discovered in 1998 (7). The ribosome initiates the synthesis at the AUG codon of polyprotein and shifts to a +1 reading frame when it encounters the adenosine-rich sequence at codons 8 to 11 of the core protein sequence (8). The frameshifting is highly dependent on the 10-adenosine stretch (9). F proteins were found in all genotypes of HCV but most commonly in genotype 1a of HCV (10, 11). Amino acids 40 to 60 of genotype 1a F protein (F1a) are the binding sites of the α subunit of the 20S proteasome (12); thus, the half-life of F1a is only about 10 min. As F1a protein is a short-lived protein, its production is not only affected by proteasome but also has been shown to be modulated by core protein production (13, 14).
The F protein is produced during natural HCV infection, and the antibody of F protein has been detected (10, 15). Titers of the anti-F antibody are significantly higher in the group of chronically infected patients (16). Also, the presence of anti-F is associated with the outcome of interferon therapy (17). It has been reported that the F protein is not required for viral replication since the F-deficient subgenomic replicons of HCV can still replicate efficiently in Huh7 cells (18, 19). Previous studies showed that the F protein might regulate the expression of some genes, and those genes encode proteins involved in metabolism, signal transduction, DNA-dependent transcription regulation, cell processing and apoptosis, oncogenesis, and the immune response (20–22). However, the virological and immunological functions of the F protein are still unclear.
It has been reported that HCV core protein can interfere with the IFN signaling pathway (1, 2, 23, 24). However, these studies did not rule out the contributions of the F protein. It has been shown that HCV core protein can upregulate the 2′-5′-OAS gene promoter activity in noncancerous human hepatocyte PH5CH8 cells (25). The activation of the 2′-5′-OAS gene promoter is dependent on the first 20 amino acids at the N terminus; the deletion of amino acids 9 to 11 of the core protein completely lost the induction ability (23, 26). As previously mentioned, the frameshifting of F protein occurred at the adenosine-rich sequence at codons 8 to 11 of the core protein gene sequence, and the deletion of amino acids 9 to 11 of the core also disrupted the adenosine stretch to block the production of F proteins. A recent report showed that the F protein of the HCV JFH-1 strain suppressed RIG-I-dependent type I IFN induction (27). Based on these findings, we hypothesize that the F protein may contribute to the control of the type I interferon signaling pathway. However, the lengths of F proteins are different between genotypes, varying from 126 to 162 amino acids (8). Our goal is to determine whether HCV F proteins among different genotypes may regulate type I IFN induction and response pathways in a genotype-specific manner.
RESULTS
Genotypic regulation of IFN-β induction signaling pathway by F proteins.
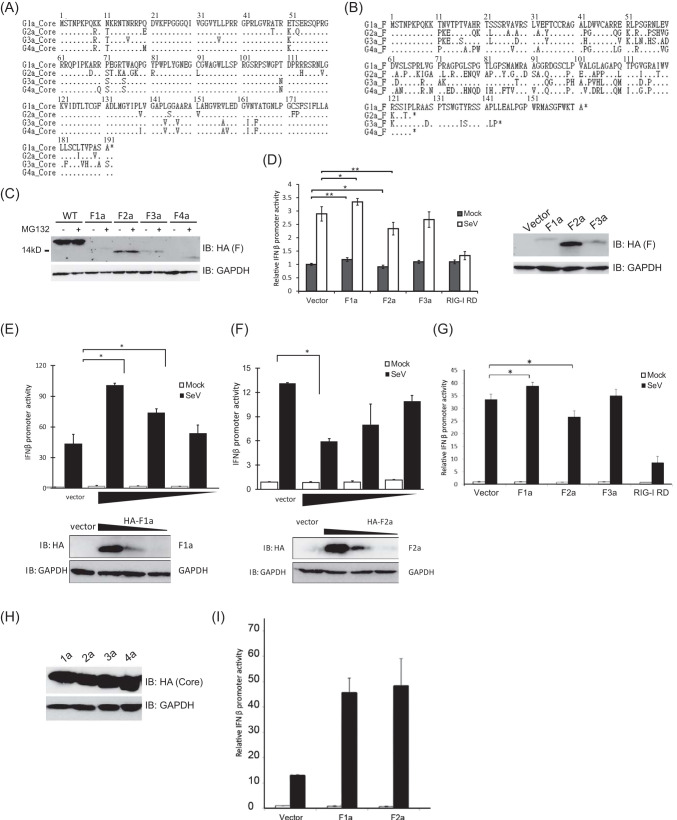
The goal of our research is to investigate whether the F protein plays a role in the interferon induction and signaling pathway. First, we engineered F protein expression constructs from core coding sequences of 4 genotypes (1a, 2a, 3a, and 4a) of HCV as well as the sequences which would only be able to produce core protein. We deleted a single adenosine from the 10-A stretch located at codons 8 to 11 and changed another nucleotide to generate a stop codon in the core protein reading frame in which there was a silent mutation for the F protein to ensure F protein production. To generate constructs that could only produce core protein, the adenosines at the 10-A stretch were replaced with guanosines to interrupt the sequence to avoid ribosomal frameshift without changing the amino acid sequences. The antigenic epitope of hemagglutinin (HA) was fused to the amino terminus of all of the constructs. We compared the nucleotide and amino acid sequences in the core coding regions of genotypes 1a, 2a, 3a, and 4a (Fig. 1A). We found that all four genotypes had the same lengths of core coding regions of 191 amino acids, and the sequences are highly conserved. However, the amino acid sequences of F protein from these four genotypes appeared to be different in their lengths, and the amino acid compositions of F proteins were highly variable (Fig. 1B). It has been reported that the genotype 1a F protein (F1a) is unstable and rapidly degraded by the proteasome and that proteasome inhibitor MG132 treatment could stabilize F1a (9, 12). However, very little is known about the expressions of F proteins of other genotypes. To see whether F proteins from different genotypes of HCV could be expressed and stabilized by proteasome inhibitors, F protein expression vectors were transfected into HEK293T cells treated with MG132 or mock (dimethyl sulfoxide [DMSO]) treated (Fig. 1C). F1a had the longest amino acid sequence and also the largest molecular weight among these four genotypes. Although the lengths of amino acid sequences of F proteins from genotype 2a and 4a were the same, the molecular weight of F4a was smaller. As expected, treatment of MG132 stabilized F1a (Fig. 1B). The expression levels of F proteins from genotypes 3a and 4a were also increased in cells treated with MG132 (Fig. 1C). However, F2a expression was not affected by MG132 treatment (Fig. 1C).
FIG 1.
The regulation of IFN-β induction signaling pathway by the F protein was genotype specific. (A) Alignment of core protein amino acid sequences of genotypes 1a, 2a, 3a, and 4a. The amino acid sequences of HCV core protein of four different genotypes contain 191 amino acids, and the compositions are highly conserved. (B) Alignment of F protein amino acid sequences of genotypes 1a, 2a, 3a, and 4a. The amino acid sequences of F proteins from different genotypes are different in length. Genotype 1a F protein contains 162 amino acids, genotypes 2a and 4a contain 126 amino acids, and genotype 3a contains 144 amino acids. Not only the length but also the compositions are highly variable. (C) Ectopic expression of F proteins in HEK293T cells. HEK293T cells were transfected with different constructs and harvested at 48 h posttransfection for immunoblotting. Cells were treated with MG132 for 6 h before harvesting. The expression levels of F proteins from genotypes 1a, 3a, and 4a were increased in cells treated with MG132. F2a expression was not affected by MG132 treatment. (D) The genotypic regulation of IFN-β induction signaling pathway by F protein in Huh7 cells. Huh7 cells were cotransfected with pIFN-β-Luc, pCMV-RLuc, and plasmids expressing F proteins. At 24 h posttransfection, these cells were infected with SeV (100 HAU/ml) for 18 h and then lysed to detect luciferase and renilla luciferase activities. The IFN-β promoter activity was enhanced in F1a-expressing cells. Conversely, the IFN-β promoter activity was suppressed in F2a protein-expressing cells. The promoter activity of genotype 3a-expressing cells had no difference from control. The protein expression levels were determined by immunoblotting. (E) F1a-enhanced IFN-β promoter activities in a dose-dependent manner. Increasing amounts of HA-F1a expression vector were transfected into Huh7 cells together with pIFN-β-Luc and pCMV-RLuc. At 24 h posttransfection, these cells were infected with SeV (100 HAU/ml) for 18 h and then lysed to detect luciferase and renilla luciferase activities. The protein levels of HA-F1a were determined by anti-HA immunoblotting. (F) F2a-suppressed IFN-β promoter activities in a dose-dependent manner. Increasing amounts of HA-F2a expression vector were transfected into Huh7 cells together with pIFN-β-Luc and pCMV-RLuc. At 24 h posttransfection, these cells were infected with SeV (100 HAU/ml) for 18 h and then lysed to detect luciferase and renilla luciferase activities. The protein levels of HA-F2a were determined by anti-HA immunoblotting. IB, immunoblot. (G) The genotypic regulation of IFN-β induction signaling pathway by F proteins in PH5CH8 cells. PH5CH8 cells were cotransfected with pIFN-β-Luc, pCMV-RLuc, and plasmids expressing F proteins. At 24 h posttransfection, cells were infected with SeV (100 HAU/ml) for 18 h and then lysed to detect luciferase and renilla luciferase activities. F1a expression enhanced IFN-β promoter activity during SeV infection, and F2a-expressing cells had reduced IFN-β promoter activity. IFN-β promoter activities in core proteins expressing Huh7 cells. Huh7 cells were cotransfected with pIFN-β-Luc, pCMV-RLuc, and plasmids expressing core proteins. At 24 h posttransfection, cells were infected with SeV (100 HAU/ml) for 18 h and then lysed to detect luciferase and renilla luciferase activities. (H) Expression of core proteins in HEK293T cells. HEK293T cells were transfected with different constructs and harvested at 48 h posttransfection for immunoblotting. (I) IFN-β promoter activities in core proteins expressing PH5CH8 cells. PH5CH8 cells were cotransfected with pIFN-β-Luc, pCMV-RLuc, and plasmids expressing core proteins. At 24 h posttransfection, cells were infected with SeV (100 HAU/ml) for 18 h and then lysed to detect luciferase and renilla luciferase activities. The IFN-β promoter activities were enhanced in both genotype 1a and genotype 2a core protein-expressing cells, but there was no difference among genotypes. **, P < 0.01; *, P < 0.05.
To investigate the function of the F protein in the type I interferon induction pathway, we used the Sendai virus (SeV) to stimulate the type I IFN signaling pathway. The reporter construct encoding the firefly luciferase under the control of the IFN-β promoter (pIFN-β-Luc) was used to monitor the activation of the type I IFN induction pathway in Huh7 cells cotransfected with F protein expression vectors. Twenty-four hours after transfection, cells were infected with SeV for 18 h, and the cell lysates were subjected to dual-luciferase activity assay, normalized by the cytomegalovirus (CMV) promoter-driven renilla luciferase activity. The IFN-β promoter activity was enhanced in F1a-expressing cells (Fig. 1D). Conversely, the IFN-β promoter activity was suppressed in genotype 2a F protein-expressing cells. The promoter activity of genotype 3a-expressing cells had no difference from control (Fig. 1D). This phenomenon could be found in uninfected cells and was magnified after SeV infection (Fig. 1D). To confirm our findings that F1a enhanced and F2a suppressed IFN-β promoter activities, we expressed increasing amounts of HA-F1a or HA-F2a in Huh7 cells transfected with IFN-β promoter (pIFN-β-Luc) and CMV promoter-driven renilla luciferase reporters, and we monitored the dual-luciferase activities in these cells upon SeV infection (Fig. 1E and F). The ectopic expression levels of HA-F1a and HA-F2a were determined by anti-HA immunoblotting (Fig. 1E and F). Indeed, the data showed that the IFN-β promoter activities were further enhanced with increased expression levels of HA-F1a (Fig. 1E) and were more suppressed with increased expression levels of HA-F2a (Fig. 1F). We adapted PH5CH8 cells to repeat the IFN-β reporter assay to confirm that the phenotype was not Huh7 specific (Fig. 1G). Indeed, the results were confirmed in PH5CH8 cells that F1a expression enhanced IFN-β promoter activities during SeV infection, and F2a expressing cells had reduced IFN-β promoter activities (Fig. 1G). Notably, the fold induction of IFN-β promoter activity was higher in PH5CH8 cells than that in Huh7 cells after SeV infection. It has been shown that HCV core protein can upregulate the 2′-5′-OAS gene promoter activity in noncancerous human hepatocyte PH5CH8 cells (25). We adapted the method and used Huh7 cells for the IFN-β reporter assay (Fig. 1H and I). We did not observe any difference between control and genotypes 1a and 2a core-expressing cells before SeV infection, and our data showed that core proteins enhanced the IFN-β promoter activities after virus infection both in Huh7 cells expressing HCV core protein from the 1a genotype and/or from the 2a genotype, but there was no difference among different genotypes. The regulation of IFN-β promoter activities by core protein was not genotype specific.
F1a interfered with the type I IFN induction pathway through IRF3.
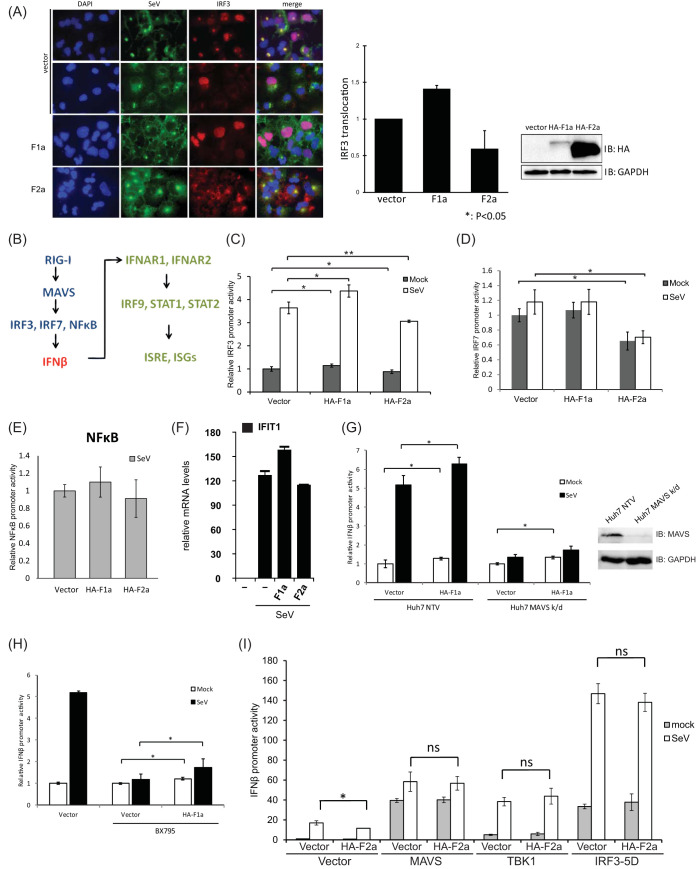
Based on these results, we next assessed whether the F protein controlled IFN-β induction. For activation, the IFN-β promoter should be bound with NF-κB, phospho-interferon regulatory factor 3 (IRF3), or phospho-IRF7 (28, 29). We first examined whether the nuclear translocation of IRF3 was regulated by the ectopic expression of either F1a or F2a (Fig. 2A). Huh7 cells were transfected with HA-F1a or HA-F2a expression vectors. The same batches of transfected cells were then split into two halves for immunoblotting and IRF3 immunofluorescence staining (Fig. 2A). Similar to our results in Fig. 1, the protein levels of HA-F1a were much lower than those of HA-F2a (Fig. 2A). However, when infected with SeV, the HA-F1a-expressing cells showed an increase in IRF3 nuclear localization, which was stained by Cy3-labeled antibodies, compared to the vector-transfected control cells (Fig. 2A). Notably, the intensities of anti-SeV staining were inversely correlated to the IRF3 nuclear localization (Fig. 2A). We then assessed at which molecular level F proteins were affecting the type I IFN induction pathway (Fig. 2B). Also, cells expressing HA-F2a showed a reduction in nuclear IRF3 upon SeV infection, which correlates to the previous IFN-β promoter reporter assay in Fig. 1 (Fig. 2A). We performed a luciferase reporter assay of the IRF3 promoter and found that IRF3 promoter activity was increased in F1a-expressing cells and decreased in F2a protein-expressing cells (Fig. 2C), and these differences were further amplified during SeV infection, of which results are consistent with the immunofluorescence staining of nuclear IRF3 during SeV infection (Fig. 2A and C). We also looked at IRF7 and NF-κB promoter activities (Fig. 2D and E). Both of the promoter reporters were not affected by F1a expression compared to the vector control; however, the IRF7 promoter activity was decreased in F2a-expressing cells (Fig. 2D), suggesting that F1a and F2a regulate type I IFN induction through different transcription factors. Nevertheless, the mRNA expression levels of IFIT1 and interferon-stimulated gene in F1a- and/or F2a-expressing cells during SeV infection showed the same trend as the phenotype observed by monitoring the promoter activities (Fig. 2F). These results indicated that genotype 1a F protein might interfere with IRF3 or upstream of IRF3, and the genotype 2a F protein might interfere upstream of both IRF3 and IRF7.
FIG 2.
F proteins affect downstream of MAVS and upstream of IRF3. (A) The genotypic effects of HCV F proteins in nuclear translocalization during SeV infection. Huh7 cells that were transfected with either HA-F1a or HA-F2a were split into 2 halves at 24 h posttransfection. One-half of the cells were lysed, and the expression levels of HA-F1a and HA-F2a were determined by anti-HA immunoblotting. Another half of transfected cells were then plated and infected with 100 HAU/ml of SeV for 18 h. The cells were fixed and stained with chicken anti-SeV and rabbit anti-IRF3 antibodies followed by fluorescein isothiocyanate (FITC)-labeled anti-chicken (green) and Cy3-labeled anti-rabbit (red) antibodies. The nucleus was stained by 4′,6-diamidino-2-phenylindole (DAPI) (blue). (B) An illustration of simplified RIG-I mediated IFN-β induction and response pathways. (C) The genotypic regulation of IRF3 promoter activity by F proteins in Huh7 cells. Huh7 cells were cotransfected with pIRF3-Luc, pCMV-RLuc, and plasmids expressing F proteins. At 24 h posttransfection, cells were infected with SeV (100 HAU/ml) for 18 h and then lysed to detect luciferase and renilla luciferase activities. IRF3 promoter activity was increased in F1a protein-expressing cells and decreased in F2a protein-expressing cells. (D) IRF7 promoter activities in F protein-expressing Huh7 cells. Huh7 cells were cotransfected with pIRF7-luc, pCMV-RLuc, and plasmids expressing F proteins. At 24 h posttransfection, cells were infected with SeV (100 HAU/ml) for 18 h and then lysed to detect luciferase and renilla luciferase activities. The IRF7 promoter activity was decreased in genotype 2a F protein-expressing cells. (E) NF-κB promoter activities in F proteins expressing Huh7 cells. Huh7 cells were cotransfected with pNFκB-Luc, pCMV-RLuc, and plasmids expressing F proteins. At 24 h posttransfection, cells were infected with SeV (100 HAU/ml) for 18 h and then lysed to detect luciferase and renilla luciferase activities. The NF-κB promoter reporter was not affected by either F1a or F2a expression. (F) mRNA levels of IFIT1 during SeV infection in F1a- and/or F2a-expressing cells. The mRNA levels of IFIT1 correlate with the IFN-β promoter activities observed during SeV infection in F1a- and/or F2a-expressing cells. (G) IFN-β promoter activities in F1a-expressing Huh7 MAVS k/d cells. Immunoblotting of Huh7 NTV cells and Huh7 MAVS k/d cells confirmed the expression levels of MAVS in these cells. Huh7 MAVS k/d cells were cotransfected with pIFN-β-Luc, pCMV-RLuc, and plasmids expressing F proteins. At 24 h posttransfection, cells were infected with SeV (100 HAU/ml) for 18 h and then lysed to detect luciferase and renilla luciferase activities. Although MAVS was knocked down, the IFN-β promoter activity was still greater in F1a protein-expressing cells and reduced in F2a protein-expressing cells compared to the control vector-transfected cella. (H) F1a protein controls downstream of TBK1. Huh7 were cotransfected with pIFN-β-Luc, pCMV-RLuc, and plasmids expressing F1a. At 24 h posttransfection, the cells were then treated with BX795 (10 μM) for 6 h, followed by SeV (100 HAU/ml) infection for 18 h. The cells were then lysed to detect luciferase and renilla luciferase activities. Enhanced IFN-β promoter activity in genotype 1a-expressing cells was still observed before and after SeV infection. (I) HA-F2a and a series of constructs, including MAVS, TBK1, and IRF3-5D, were cotransfected into Huh7 cells with pIFN-β-Luc and pCMV-RLuc. At 24 h posttransfection, cells were infected with SeV (100 HAU/ml) for 18 h and then lysed to detect the IFN-β promoter activities in these cells by luciferase reporter assays. **, P < 0.01; *, P < 0.05.
To further analyze at which step F1a enhanced the IFN-β promoter activity, we ectopically expressed F1a in Huh7 NTV (nontargeting vector) cells and Huh7 MAVS knockdown (k/d) cells to see whether F protein expression could still have an effect on controlling IFN-β expression. The immunoblotting results confirmed the expression levels of MAVS in Huh7 NTV and Huh7 MAVS k/d cells (Fig. 2G). We then repeated the luciferase assay to test the IFN-β promoter activity. After SeV infection, the IFN-β promoter activities were abolished in the Huh7 MAVs k/d cells as expected, suggesting that the differences in the promoter activities were mostly due to molecules downstream of MAVS (Fig. 2G). Also, when we focused on the promoter activity in the mock-infected MAVS k/d Huh7 cells, the IFN-β promoter activity was still greater in F1a-expressing cells than the vector control (Fig. 2G). These data indicated that F1a regulated the type I IFN induction pathway at the level downstream of MAVS. It has been well studied that after MAVS activation, TBK1 will be activated, and then phosphorylated IRF3 or IRF7, to induce the production of type I IFN (30–32) (Fig. 2B). To see if F1a affected TBK1, we pretreated Huh7 cells with a specific inhibitor of TBK1, BX795, prior to the SeV infection in the IFN-β promoter reporter assay. In the control vector-transfected cells, the IFN-β promoter activity was not significantly increased after SeV infection, suggesting that the pretreatment of 10 μM BX795 was efficient and sufficient to block TBK1-dependent IFN-β induction (Fig. 2H). Despite that BX795 pretreatment was sufficient, enhanced IFN-β promoter activities in genotype 1a-expressing cells were still observed before and after SeV infection (Fig. 2H). Based on the results, it was suggested that the F protein controlled downstream of TBK1 and was likely to be acting directly on IRF3.
To define the mechanism by which F2a downregulated the type I IFN induction pathway, we ectopically coexpressed HA-F2a and a series of constructs which could constitutively activate type I IFN induction, such as MAVS, TBK1, and IRF3-5D, and the IFN-β promoter activities in these cells were determined by luciferase reporter assays (Fig. 2I). Ectopic expression of MAVS or TBK1 can also activate IFN-β promoter activity (33). IRF3-5D, on the other hand, is the phosphomimetic mutant of IRF3, which is constitutively active to induce IFN-β expression (34). As expected, IFN-β promoter activities were increased in MAVS-, TBK1-, or IRF3-5D-overexpressing Huh7 cells (Fig. 2I). However, the coexpression of HA-F2a protein did not impair the signaling activities of MAVS, TBK1, and IRF3-5D through the IFN-β promoter, suggesting that F2a targeted the pathway upstream of MAVS. Taken together, our data showed that the HCV genotype 1a F protein affected downstream of TBK1 and possibly directly acted on IRF3, and the genotype 2a F protein targeted the pathway upstream of MAVS. In conclusion, the F protein of HCV was involved in the IFN-β induction signaling pathway; the genotype 1a F protein enhanced the signaling, but the genotype 2a F protein inhibited the IFN-β induction.
The differential regulation of the IFN-β induction signaling pathway by F1a and F2a proteins was dependent on amino acids 40 to 57.
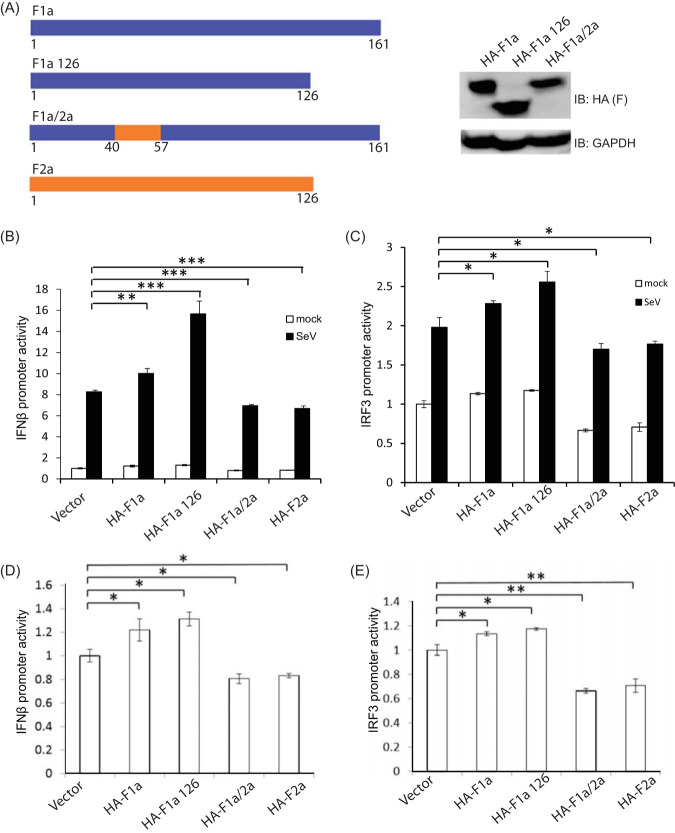
Our previous data showed that F1a and F2a contributed to the IFN-β induction pathway very differently. To investigate the molecular mechanism of these phenotypes, we compared F1a and F2a at the peptide level to design F protein mutants for further studies. Amino acids 40 to 60 of F1a and F2a are highly variable, and also, the lengths of these two proteins are distinct (Fig. 1B). To determine whether the different regulations of these two proteins were dependent on the internal region of amino acids 40 to 60 or on the C-terminal domain amino acids 127 to162 of F1a, we engineered two constructs, which were the short-form F1a (F1a126) and the chimeric F1a/2a with amino acids (aa) 40 to 57 substitution (Fig. 3A). F1a126 expressed a peptide with the same length as F2a, and the F1a/2a chimera contained the amino acids 40 to 57 of F2a in an F1a backbone (F1a/2a) (Fig. 3A). When transfected into Huh7 cells, these constructs appeared to be expressed at similar levels to F1a (Fig. 3A).
FIG 3.
The differential regulation of the IFN-β induction signaling pathway by F1a and F2a was dependent on amino acids 40 to 57. (A) Expression of F1a, F1a126, and F1a/2a proteins in Huh7 cells. Huh7 cells were transfected with different constructs and harvested at 48 h posttransfection for immunoblotting. Cells were treated with MG132 for 6 h before harvest. (B) IFN-β promoter activities in F protein-expressing Huh7 cells. Huh7 cells were cotransfected with pIFN-β-Luc, pCMV-RLuc, and plasmids expressing F proteins. At 24 h posttransfection, these cells were infected with SeV (100 HAU/ml) for 18 h and then lysed to detect luciferase and renilla luciferase activities. The IFN-β promoter activity was enhanced in F1a 1 to 126 protein-expressing cells. Conversely, the IFN-β promoter activity was suppressed in F1a/2a 40 to 57 protein-expressing cells. (C) IRF3 promoter activities in F proteins expressing Huh7 cells. Huh7 cells were cotransfected with pIRF3-luc, pCMV-RLuc, and plasmids expressing F proteins. At 24 h posttransfection, the cells were infected with SeV (100 HAU/ml) for 18 h and then lysed to detect luciferase and renilla luciferase activities. The IRF3 promoter activity was enhanced in F1a 1 to 126 protein-expressing cells. Conversely, the IRF3 promoter activity was suppressed in F1a/2a 40 to 57 protein-expressing cells. (D) The mock levels of panels B, C, and E are plotted for better resolution. ***, P < 0.001; **, P < 0.01; *, P < 0.05.
IFN-β promoter activities in mutant F protein-expressing cells were then examined during SeV infections (Fig. 3B). IFN-β promoter activity was still enhanced in F1a126-expressing cells, indicating that the regulation of the IFN-β signaling pathway was not dependent on the C-terminal domain of F1a (Fig. 3B). Conversely, the IFN-β promoter activity was decreased in F1a/2a-expressing cells, which indicated that the differential regulation of IFN-β promoter activity between F1a and F2a might be related to aa 40 to aa 57 (Fig. 3B). We also performed a dual-luciferase assay to test the IRF3 promoter activity, and the same phenotype was observed, showing that IRF3 promoter activity was increased in genotype F1a126-expressing cells and decreased in F1a/2a-expressing cells (Fig. 3C). These phenotypes could be found in uninfected cells and were magnified after SeV infection (Fig. 3B to E). This result was consistent with our previous findings (Fig. 1D to G), indicating that aa 40 to 57 of F1a contribute to the enhancement of type I IFN induction.
The enhancement of type I IFN induction by F1a is correlated by, but not limited to, the proteasome targeting activity.
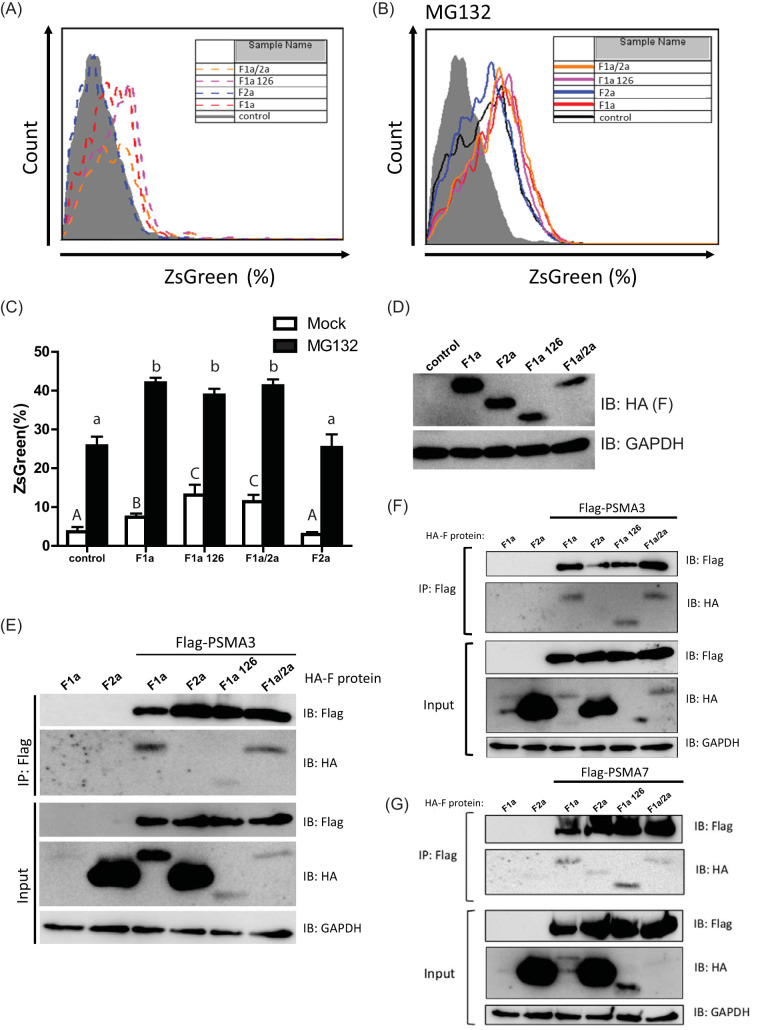
It has been reported that genotype 1a F protein is unstable and rapidly degraded by the proteasome, and the amino acids 40 to 60 of the genotype 1a F protein served as the binding domain for the proteasome α3 subunit (PSMA3) (12). Based on our results in Fig. 3, we hypothesized that the proteasome targeting activity of F1a may contribute to the regulation of the type I IFN induction pathway. We first generated stable Huh7 cells containing the pZsProSensor-1 plasmid. The mouse ornithine decarboxylase (MODC) degradation domain-fused ZsGreen expressed from pZsProSensor-1 is a protein with green fluorescence and would be degraded in a ubiquitin-independent, proteasome-dependent manner (35–37). The green fluorescence of MODC-ZsGreen would be accumulated intracellularly when the when normal proteasome function is retarded.
We then assessed whether the expression of F proteins and the mutants of different HCV genotypes may impair the degradation activity of proteasomes (Fig. 4A to C). As a control, we treated the cells with the proteasome inhibitor MG132 to ensure that the intracellular green fluorescence may reflect the reduced activity of proteasome (Fig. 4B). The green fluorescence of MODC-ZsGreen accumulated in the cells was determined by flow cytometry. As expected, ectopic expression of F1a resulted in the accumulation of green fluorescence in the cell when compared to the control (Fig. 4A), indicating that the proteasome activity was decreased by F1a, which is consistent with the previous report that F1a is rapidly degraded through interaction with PSMA3 (12). The ectopic expression of F2a, however, did not affect the proteasome activity, as the intracellular green fluorescence levels remained at a comparable level to the control (Fig. 4A). When the truncated F1a, F1a126, was expressed, the intracellular green fluorescence was accumulated at a higher level than that in the F1a-expressing cells (Fig. 4A), and this phenotype showed the same tendency as the enhancement of the type I IFN induction pathway (Fig. 3B and Fig. 4A). Nevertheless, although F1a/2a chimeric protein showed to decrease type I IFN induction (Fig. 3B and D), when we evaluated the effects of F1a/2a chimeric protein, it was still able to decrease the proteasome activity (Fig. 4A). Although the green fluorescence levels were increased in all cells treated with MG132, the phenotypes found in the mock-treated cells were retained (Fig. 4B). We graphed the data from mock- and MG132-treated cells together for a better presentation (Fig. 4C). Indeed, with similar ectopic expression levels of F1a, F2a, and their mutants (Fig. 4D), F1a-, F1a126-, and the F1a/2a-expressing cells showed accumulated MODC-ZsGreen in the cells, indicating the reduced state of proteasome activity. We then assessed the binding activity to PSMA3 of F1a, F1a126, F1a/2a, and F2a (Fig. 4E). Flag-tagged PSMA3 and HA-tagged F proteins were cotransfected into Huh7 cells. After 2 days, the cell lysates were subjected to immunoprecipitation. Consistent with previous reports, Flag-tagged PSMA3 was corecovered with HA-F1a (Fig. 4E and F). We found that the HA-tagged F2a, which showed high stability compared to F1a (Fig. 1B and 4E), could not interact with PSMA3 in both Huh7 and 293 cells (Fig. 4E and F). The truncated F1a, F1a126, was still able to interact with PSMA3 (Fig. 4E and F). Also, consistent with our proteasome activity analysis, Flag-tagged PSMA3 was able to be coimmunoprecipitated with HA-F1a/2a (Fig. 4E and F). This result, however, was much unexpected, as aa 40 to 60 of F1a were previously reported as the PSMA3-interacting domain (12). We further examined whether other proteasome subunits may interact with F proteins and their mutants (Fig. 4F). Similarly, Flag-tagged PSMA7 and HA-tagged F proteins were cotransfected into Huh7 cells, and the cell lysates were utilized for immunoprecipitation by anti-Flag antibodies. We found that among the F proteins and their mutants that we expressed, HA-F1a and HA-F1a126, two of the constructs that showed the most ability to enhance IFN-β promoter activation (Fig. 3), presented better binding activities to PSMA7 than those which could not enhance IFN-β promoter activation, i.e., F2a and F1a/2a (Fig. 4F). These results indicated that the effects of F proteins on the type I IFN induction pathway are correlated with their ability to interact with the proteasome subunits, of which interaction may regulate the proteasome activity and therefore affect the degradation of activated signaling molecules.
FIG 4.
The enhancement of type I IFN induction by F1a is correlated with the proteasome targeting activity. (A) Proteasome activity disturbance level by the ectopic F protein expressions was analyzed by flow cytometry for the ZsGreen-positive cells. F1a-, F1a126-, and F1a/2a-expressing cells had higher ZsGreen-positive cells than those in the control and F2a-expressing cells. (B) Similar to panel A, the experiment was repeated in the presence of MG132, and F1a-, F1a126-, and F1a/2a-expressing cells showed the same phenotype with higher ZsGreen-positive cells present than those in the control and F2a-expressing cells. (C) The bar graph shows the summarized flow cytometry results of panels A and B (n = 3). Means with the same letter are not significantly different from each other by t test (P < 0.05). (D) Ectopic expression of F proteins in Huh7 cells containing the pZsProSensor-1 plasmid. Huh7 cells containing pZsProSensor-1 plasmid cells were transfected with different constructs of F proteins and harvested at 48 h posttransfection for anti-HA immunoblotting. Cells were treated with MG132 for 6 h before harvesting. (E) Flag-tagged PSMA3 and HA-tagged F proteins were cotransfected into Huh7 cells. Proteins were recovered by anti-Flag co-IP and identified by anti-HA or anti-Flag immunoblot analysis. (F and G) Flag-tagged PSMA3 (F) or Flag-tagged PSMA7 (G) and HA-tagged F proteins were cotransfected into HEK293 cells. Proteins were recovered by anti-Flag co-IP and identified by anti-HA or anti-Flag immunoblot analysis. IP, immunoprecipitation.
DISCUSSION
The F protein has been shown to be a short-lived protein, but other studies only focused on HCV genotype 1a (9, 12). We cloned the F protein from different genotypes and found that F proteins of genotypes 1a, 3a, and 4a were unstable without treatment of MG132, but the genotype 2a F protein was not affected by MG132 (Fig. 1C), suggesting that not all genotypes of F proteins were sensitive to proteasomes, and F proteins from different genotypes might have very different biological and virological functions.
HCV core is a well-studied viral protein that regulates immune responses. For example, amino acids 70 and 91 of HCV core have been reported to be associated with patients’ responses to interferon monotherapy or interferon plus ribavirin combination therapy (38–40). HCV core protein can upregulate the 2′-5′-OAS gene promoter activity in noncancerous human hepatocyte PH5CH8 cells (25). 2′-5′-OAS is an interferon-stimulated gene (ISG); the production of 2′-5′-OAS is dependent on type I IFN. Our data showed that core proteins enhanced the IFN-β promoter activities after virus infection in both Huh7 and PH5CH8 cells, and there was no difference among different genotypes (Fig. 1H and I). However, the regulation of F proteins was genotype specific, e.g., F1a enhanced the signaling, and F2a reduced the signaling (Fig. 1E and F). The genotype-specific effects on the IFN-β promoter activities were not dependent on core proteins but F proteins. Of note is that while this current report was in preparation, Park et al. reported that the F protein of the HCV genotype 2a JFH-1 strain could suppress the RIG-I-dependent type I and type III IFN inductions (27). Our data validated these findings and extended to compare the effects of F proteins across different HCV genotypes.
It has been known that many HCV viral proteins are involved in interrupting innate immune signaling and NS3/4A protease plays a central role in innate immune evasion strategy by cleaving MAVS to attenuate the production of IFN-β (5, 41–43). HCV NS3/4A protease is required for processing HCV polyproteins to mature viral NS proteins to support HCV replication (44), and NS3/4A protease is distributed in intracellular membranes during HCV infection (45). The key adaptor protein of the RIG-I signaling pathway, MAVS, is also located on intracellular membranes such as mitochondria, peroxisome, and mitochondrial-associated membranes (MAMs) (33, 45, 46). It has been found that NS3/4A targets and cleaves the MAVS that are preferentially located on MAMs (45). Thus, the production of type I IFN would be attenuated for the virus to escape the innate immunity.
There are other hepatotropic viruses, such as hepatitis A virus (HAV) and GB virus B (GBVB), which may also encode a viral protease to cleave MAVS (47, 48). However, these hepatitis viruses do not generally cause chronic infection. Therefore, the cleavage of MAVS in early infection to interrupt the IFN-β induction signaling pathway may be necessary but not sufficient to support the progression to chronic infection. In our study, we determined that the F proteins were also involved in the regulation of IFN-β expression. Since F protein is translated from its own open reading frame (ORF) and does not require further cleavages by the NS3/4A protease, F protein might be the first protein to produce during HCV infection, suggesting that F protein may be the first viral protein to regulate the IFN-β induction signaling pathway prior to NS3/4A protease or other viral proteins.
There are other viruses that contain frameshift proteins which could affect host signaling pathways, such as Theiler’s murine encephalomyelitis virus (TMEV or Theiler’s virus) of Picornaviridae, which is a positive-sense single-stranded RNA virus encoding a polyprotein that is cleaved to produce 12 viral proteins. Persistent strains of TMEV contain an additional protein called L* protein, which is a frameshift protein encoded by the ORF that overlaps the main ORF, and it is a 156-amino-acid-long protein with a molecular mass of 18 kDa (49). The amino acid sequence of the L* protein is conserved in persistent strains of TMEV (50), and the L* ORF is required for persistence of the virus (51, 52). The L protein has been reported to be involved in inhibiting type I IFN production, and mutations in the L protein-coding sequence to disrupt L and L* proteins then would lose the phenotype (53). In our present study, we found that the frameshift proteins of HCV were also involved in regulating the type I IFN signaling pathway. Our data suggested that F1a enhanced the promoter activity of IRF3, and F2a suppressed the promoter activity of IRF3 and IRF7. Another paper announced that the F protein downregulates AP1-mediated transcription, reduces the production of hepcidin, and interferes with the iron metabolism (54). AP1 also binds to the IFN-β promoter and interferes with the production of IFN-β. Our data showed that F proteins interfered with the promoter activity of IFN-β (Fig. 1D to G) and also the promoter activity of the upstream transcription factor IRF3 (Fig. 2A and B). These results corresponded with previous studies that the F protein was involved in regulating the IFN-β induction signaling pathway.
In the molecular studies of mutant types F1a, we found that amino acids 127 to 162 at the C terminus were not responsible for the different regulations in IFN-β induction signaling pathway among F1a and F2a and that the interference depended on the amino acids 40 to 57 (Fig. 3B and C). Based on the previous studies, the amino acids 40 to 60 of genotype 1a F protein serve as the binding domain of the α3 subunit of the proteasome (12). That sequence was highly variable among genotypes (Fig. 1B). Also, the expression of F1a and F2a proteins was different (Fig. 1C); F1a expression was increased after MG132 treatment, but F2a was not affected by MG132. Therefore, we propose that the F protein of genotype 1a might control the signaling pathway by recruiting negative regulators to the proteasome for degradation and therefore enhancing the production of IFN-β and ISGs. However, our results from the proteasome activity assay suggested that the proteasome-targeting activity of F1a and its mutants may not be the only factor to regulate type I IFN induction, even though there might be some negative regulators involving in the IFN-β signaling pathway, which would interact with F1a. In 2005, HCV genotype 1a F protein has been reported to interact with different human proteins by using a yeast two-hybrid system (22). In the future, we will perform mass spectrometry to identify which negative regulator of the IFN-β induction pathway may be actively degraded in an F1a-dependent manner.
It has been reported that patients infected with genotype 1 HCV may have a high level of ISG expression before therapy, resulting in a poor response to the IFN-based treatment (55, 56) and that the sustained virological response (SVR) of patients infected with genotype 1 HCV only reaches approximately 50% when treated with ribavirin combined with pegylated IFN (peg-IFN). There is much research that announced that the ISGs expression is different among responders and nonresponders. Higher expression of ISG15, ISG16, OAS2, OAS3, IFIT1, MxA, USP18, and CEB1 is observed before the treatment of nonresponders (57, 58). In the present study, we observed that cells expressing genotype 1a F protein increased the IFN-β promoter activity (Fig. 1E). Current studies suggested that elevated ISG levels may result in nonresponders in IFN-α-based therapies of chronic HCV patients (56, 57). We are looking forward to identifying sequence algorithms of F proteins isolated from patients that may serve as indicators of whether these patients should receive IFN therapy in order to provide new insights for IFN therapy outcome prediction.
MATERIALS AND METHODS
Cell lines and DNA transfection.
Huh7 cells, PH5CH8 cells, and HEK293T cells were grown at 37°C in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS). Huh7 NTV cells and Huh7 MAVS k/d cells (59) were cultured in DMEM supplemented with 10% FBS and 1 μg/ml puromycin. FuGENE6 transfection reagent was used for transfections in Huh7 cells, PH5CH8 cells, HEK293T cells, Huh7 NTV cells, and Huh7 MAVS k/d cells according to the manufacturer’s instructions. Cells were harvested 48 to 72 h after transfection. F protein-expressing cells were treated with MG132 (1 μg/ml) for 6 h before harvest for immunoblotting.
Construction of expression plasmids.
Wild-type core protein-coding regions were cloned into pEFTak vector with an N-terminal HA tag. The sequences were further mutated to express F protein only and core protein only by site-directed mutagenesis (QuikChange Lighting site-directed mutagenesis kit; Agilent Technologies) following the manufacturer’s instructions. Selected colonies were amplified, and the plasmid DNA was extracted and purified by endotoxin-free NucleoBond Xtra Midi EF (Macherey-Nagel).
pEFtak/G1a HA-core, pEFtak/G2a HA-core, pEFtak/G3a HA-core, and pEFtak/G4a HA-core are plasmids that contain wild-type core coding sequences. F proteins from 4 different genotypes were designed and constructed based on published sequences (Uniprot accession number P0C044 for genotype 1a, GenPept accession number BAB32872 for genotype 2a, GenBank accession number YP_009272631.1 for genotype 3a, and GenBank accession number YP_009272634.1 for genotype 4a). pEFtak/G1a HA-F, pEFtak/G2a HA-F, pEFtak/G3a HA-F, and pEFtak/G4a HA-F are plasmids that express F protein only, of which adenosine was deleted from the 10-A stretches of the core coding sequences at codons 8 to 11. Early terminations in the core protein reading frame were introduced, of which mutations are silent for the F protein reading frame, to ensure the expression of F but not core proteins. pEFtak/G1a HA-core only, pEFtak/G2a HA-core only, pEFtak/G3a HA-core only, and pEFtak/G4a HA-core only are plasmids that could only be able to produce core protein by replacing adenosines with guanosines to avoid ribosomal frameshift at the 10-A stretch without changing the amino acid sequence.
Immunoblotting.
Cells were lysed in RIPA (50 mM Tris, 150 mM NaCl, 5 mM EDTA, 1% NP-40, 0.1% SDS, and 12 mM sodium deoxycholate) containing EDTA-free protease inhibitor. Protein concentrations were tested by Bradford protein assay. After separated by SDS-PAGE, the proteins were transferred from the gel to polyvinylidene difluoride (PVDF) membrane (Millipore) and then probed with primary antibodies, including anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibodies (GeneTex; catalog no. GTX100118) and anti-HA antibodies (Cell Signaling Technology; catalog no. 3724S) and secondary antibodies horseradish peroxidase (HRP)-linked donkey anti-rabbit IgG (GE Healthcare; catalog no. NA934V). HRP antibody signals were detected by chemiluminescence using SuperSignal West Pico chemiluminescent substrate (Thermo Fisher; catalog no. 34080) and/or mixed with SuperSignal West Femto maximum sensitivity substrates (Thermo Fisher; catalog no. 34096).
Virus infection, interferon, and BX795 treatment.
Sendai virus (100 hemagglutinating units [HAU]/ml) and IFN-β (100 IU/ml) were prepared in DMEM supplemented with 10% FBS. Cells seeded in 12-well plates or 48-well plates were inoculated with Sendai virus or treated with IFN-β for 6 or 18 h and then harvested. For the inhibition of TBK1, cells were treated with BX795 (10 μM for Huh7 cells) for 6 h before virus infection, and BX795 was dissolved in DMSO and diluted in 10% FBS-DMEM.
Luciferase assay.
For the dual-luciferase assay, firefly luciferase reporter plasmids, including pIFN-β-Luc containing the IFN-β gene promoter region, pIRF3-Luc containing the IRF3 gene promoter region, pIRF7-Luc containing the IRF7 gene promoter region, and p2X-NF-κB-Luc containing two NF-κB gene promoter regions, were transfected with a renilla luciferase reporter plasmid pCMV-RL as the internal control into Huh7 or PH5CH8 cells. Plasmids of interest, luciferase reporter plasmids, and renilla luciferase reporter plasmids were cotransfected into cells by FuGENE 6 transfection reagent. Cells were split from 1 well into 6 wells the next day for further experiments. At 24 h posttransfection, cells were infected with Sendai virus (100 HAU/ml) or treated with IFN-β (100 IU/ml) for 18 h. The reporter gene activity was measured by dual-luciferase reporter assay according to the manufacturer’s instructions (Promega).
Real-time PCR.
Total RNA was extracted by TRIzol reagent. Five hundred nanograms of RNA were used for reverse transcription by iScript cDNA synthesis kit (Bio-Rad) according to the manufacturer’s instructions. The complete mix was incubated with the following program in a PCR machine: 25°C for 5 min, 42°C for 30 min, 85°C for 5 min, and hold at 4°C. Relative amounts of an intracellular RNA of interest were quantified by real-time PCR on a StepOne real-time PCR system (ABI) and expressed as fold change using SYBR green (ABI) according to the manufacturer’s protocol. In brief, real-time PCR for each mRNA was carried out in triplicate, and each 10-μl reaction mixture included 5 μl of 2× SYBR green mix, 0.5 μl of primer stocks (forward and reverse, 10 nM each), 1 μl of cDNA, and 3.5 μl of diethyl pyrocarbonate (DEPC)-H2O. The reaction mixtures were incubated in a real-time PCR machine at 50°C for 2 min and 95°C for 10 min followed by 40 cycles each of 95°C for 15 s and 60°C for 1 min. All data presented are relative quantifications with efficiency corrections based on the relative expression of target genes versus GAPDH as the reference gene. The following primers were used: GAPDH_fwd, 5′-CCA CAT CGC TCA GAC ACC AT-3′; GAPDH_rev, 5′-AAA AGC CCT GGT GAC C-3′; IFNb_fwd, 5′-CTT TCC ATG AGC TAC AAC TTG C-3′; IFNb_rev, 5′-CAT TCA ATT GCC ACA GGA GC-3′; IFITM3_fwd 5′-ATG TCG TCT GGT CCC TGT TC-3′; IFITM3_rev, 5′-CCA ACC ATC TTC CTG TCC C-3′; OAS1_fwd, 5′-CCA AGC TCA AGA GCC TCA TC-3′; OAS1_rev, 5′-GAG CTC CAG GGC ATA CTG AG-3′; IFIT1_fwd, 5′-TCT CAG AGG AGC CTG GCT AA-3′; IFIT1_rev, 5′-TGA CAT CTC AAT TGC TCC AGA-3′; IFIT2_fwd, 5′-CGA ACA GCT GAG AAT TGC AC-3′; IFIT2_rev, 5′-CAA GTT CCA GGT GAA ATG GC-3′; SeV_fwd, 5′-GAC GCG AGT TAT GTG TTT GC-3′; and SeV_rev, 5′-TTC CAC GCT CTC TTG GAT CT-3′.
Proteasome activity analysis by flow cytometry.
HA-tagged F protein expression plasmids from different HCV genotypes were transfected into stable Huh7 cells containing the pZsProSensor-1 plasmid. Transfected cells were trypsinized at 48 h posttransfection and were fixed and permeabilized as previously described (59). For fluorescence analysis, cultured cells were stained with phycoerythrin (PE)-conjugated anti-HA.11 epitope tag (catalog no. 901517; BioLegend) at 4°C in the dark for 30 min. BD FACSCalibur was used for analyzing the fluorescence. PE-positive live cells were further analyzed for the intensity of their green fluorescence. The obtained data were analyzed using FlowJo software (Tree Star, Inc., Ashland, OR).
Statistical analysis.
All the experiments were repeated at least three times, and the results were presented as mean ± standard deviation (SD). Data were analyzed by two-tailed Student's t test. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
ACKNOWLEDGMENTS
We thank J.-H. James Ou for providing wild-type HCV core and F protein constructs. We thank our colleagues for reagents and members of the Liu laboratory for discussions.
This work was supported by Ministry of Science and Technology, Taiwan (MOST) grants MOST 104-2320-B-002-057 and MOST 105-2628-B-002-014-MY3 and by Network for Regional Healthcare Improvement (NRHI) grant NHRI-EX106-10417SC.
The authors whose names are listed immediately below certify that they have no affiliations with or involvement in any organization or entity with any financial interest or nonfinancial interest in the subject matter or materials discussed in the manuscript.
Y.-T.L. and H.M.L. conducted molecular studies of F protein interactions and signaling analyses. Y.-T.L., F.H., and Y.-M.L. performed experiments. H.M.L. directed the research. Y.-T.L. and H.M.L. wrote the manuscript.
REFERENCES
- 1.Bode JG, Ludwig S, Ehrhardt C, Albrecht U, Erhardt A, Schaper F, Heinrich PC, Haussinger D. 2003. IFN-alpha antagonistic activity of HCV core protein involves induction of suppressor of cytokine signaling-3. FASEB J 17:1–490. doi: 10.1096/fj.02-0664fje. [DOI] [PubMed] [Google Scholar]
- 2.Yoshida T, Hanada T, Tokuhisa T, Kosai K, Sata M, Kohara M, Yoshimura A. 2002. Activation of STAT3 by the hepatitis C virus core protein leads to cellular transformation. J Exp Med 196:641–653. doi: 10.1084/jem.20012127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gale M Jr, Blakely CM, Kwieciszewski B, Tan SL, Dossett M, Tang NM, Korth MJ, Polyak SJ, Gretch DR, Katze MG. 1998. Control of PKR protein kinase by hepatitis C virus nonstructural 5A protein: molecular mechanisms of kinase regulation. Mol Cell Biol 18:5208–5218. doi: 10.1128/mcb.18.9.5208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumthip K, Chusri P, Jilg N, Zhao L, Fusco DN, Zhao H, Goto K, Cheng D, Schaefer EA, Zhang L, Pantip C, Thongsawat S, O'Brien A, Peng LF, Maneekarn N, Chung RT, Lin W. 2012. Hepatitis C virus NS5A disrupts STAT1 phosphorylation and suppresses type I interferon signaling. J Virol 86:8581–8591. doi: 10.1128/JVI.00533-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li XD, Sun L, Seth RB, Pineda G, Chen ZJ. 2005. Hepatitis C virus protease NS3/4A cleaves mitochondrial antiviral signaling protein off the mitochondria to evade innate immunity. Proc Natl Acad Sci U S A 102:17717–17722. doi: 10.1073/pnas.0508531102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rehermann B. 2009. Hepatitis C virus versus innate and adaptive immune responses: a tale of coevolution and coexistence. J Clin Invest 119:1745–1754. doi: 10.1172/JCI39133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walewski JL, Keller TR, Stump DD, Branch AD. 2001. Evidence for a new hepatitis C virus antigen encoded in an overlapping reading frame. RNA 7:710–721. doi: 10.1017/s1355838201010111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu Z, Choi J, Yen TS, Lu W, Strohecker A, Govindarajan S, Chien D, Selby MJ, Ou J. 2001. Synthesis of a novel hepatitis C virus protein by ribosomal frameshift. EMBO J 20:3840–3848. doi: 10.1093/emboj/20.14.3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu ZM, Choi J, Lu W, Ou JH. 2003. Hepatitis C virus F protein is a short-lived protein associated with the endoplasmic reticulum. J Virol 77:1578–1583. doi: 10.1128/JVI.77.2.1578-1583.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walewski JL, Keller TR, Stump DD, Branch AD. 2001. Evidence for a new hepatitis C virus antigen encoded in an overlapping reading frame. RNA 7:710–721. doi: 10.1017/S1355838201010111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dalagiorgou G, Vassilaki N, Foka P, Boumlic A, Kakkanas A, Kochlios E, Khalili S, Aslanoglou E, Veletza S, Orfanoudakis G, Vassilopoulos D, Hadziyannis SJ, Koskinas J, Mavromara P. 2011. High levels of HCV core+1 antibodies in HCV patients with hepatocellular carcinoma. J Gen Virol 92:1343–1351. doi: 10.1099/vir.0.023010-0. [DOI] [PubMed] [Google Scholar]
- 12.Yuksek K, Chen WL, Chien D, Ou JH. 2009. Ubiquitin-independent degradation of hepatitis C virus F protein. J Virol 83:612–621. doi: 10.1128/JVI.00832-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vassilaki N, Boleti H, Mavromara P. 2007. Expression studies of the core+1 protein of the hepatitis C virus 1a in mammalian cells. The influence of the core protein and proteasomes on the intracellular levels of core+1. FEBS J 274:4057–4074. doi: 10.1111/j.1742-4658.2007.05929.x. [DOI] [PubMed] [Google Scholar]
- 14.Wolf M, Dimitrova M, Baumert TF, Schuster C. 2008. The major form of hepatitis C virus alternate reading frame protein is suppressed by core protein expression. Nucleic Acids Res 36:3054–3064. doi: 10.1093/nar/gkn111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Komurian-Pradel F, Rajoharison A, Berland J-L, Khouri V, Perret M, Van Roosmalen M, Pol S, Negro F, Paranhos-Baccalà G. 2004. Antigenic relevance of F protein in chronic hepatitis C virus infection. Hepatology 40:900–909. doi: 10.1002/hep.20406. [DOI] [PubMed] [Google Scholar]
- 16.Chuang WC, Allain JP. 2008. Differential reactivity of putative genotype 2 hepatitis C virus F protein between chronic and recovered infections. J Gen Virol 89:1890–1900. doi: 10.1099/vir.0.83677-0. [DOI] [PubMed] [Google Scholar]
- 17.Gao DY, Zhang XX, Hou G, Jin GD, Deng Q, Kong XF, Zhang DH, Ling Y, Yu DM, Gong QM, Zhan Q, Yao BL, Lu ZM. 2008. Assessment of specific antibodies to F protein in serum samples from Chinese hepatitis C patients treated with interferon plus ribavarin. J Clin Microbiol 46:3746–3751. doi: 10.1128/JCM.00612-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McMullan LK, Grakoui A, Evans MJ, Mihalik K, Puig M, Branch AD, Feinstone SM, Rice CM. 2007. Evidence for a functional RNA element in the hepatitis C virus core gene. Proc Natl Acad Sci U S A 104:2879–2884. doi: 10.1073/pnas.0611267104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vassilaki N, Friebe P, Meuleman P, Kallis S, Kaul A, Paranhos-Baccala G, Leroux-Roels G, Mavromara P, Bartenschlager R. 2008. Role of the hepatitis C virus core+1 open reading frame and core cis-acting RNA elements in viral RNA translation and replication. J Virol 82:11503–11515. doi: 10.1128/JVI.01640-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dou J, Liu P, Wang J, Zhang X. 2006. Effect of hepatitis C virus core shadow protein expressed in human hepatoma cell line on human gene expression profiles. J Gastroenterol Hepatol 21:1794–1800. doi: 10.1111/j.1440-1746.2006.04380.x. [DOI] [PubMed] [Google Scholar]
- 21.Tsao ML, Chao CH, Yeh CT. 2006. Interaction of hepatitis C virus F protein with prefoldin 2 perturbs tubulin cytoskeleton organization. Biochem Biophys Res Commun 348:271–277. doi: 10.1016/j.bbrc.2006.07.062. [DOI] [PubMed] [Google Scholar]
- 22.Huang YP, Cheng J, Zhang SL, Wang L, Guo J, Liu Y, Yang Y, Zhang LY, Bai GQ, Gao XS, Ji D, Lin SM, Shao Q. 2005. Screening of hepatocyte proteins binding to F protein of hepatitis C virus by yeast two-hybrid system. World J Gastroenterol 11:5659–5665. doi: 10.3748/wjg.v11.i36.5659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dansako H, Naka K, Ikeda M, Kato N. 2005. Hepatitis C virus proteins exhibit conflicting effects on the interferon system in human hepatocyte cells. Biochem Biophys Res Commun 336:458–468. doi: 10.1016/j.bbrc.2005.08.112. [DOI] [PubMed] [Google Scholar]
- 24.Lin W, Kim SS, Yeung E, Kamegaya Y, Blackard JT, Kim KA, Holtzman MJ, Chung RT. 2006. Hepatitis C virus core protein blocks interferon signaling by interaction with the STAT1 SH2 domain. J Virol 80:9226–9235. doi: 10.1128/JVI.00459-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Naganuma A, Nozaki A, Tanaka T, Sugiyama K, Takagi H, Mori M, Shimotohno K, Kato N. 2000. Activation of the interferon-inducible 2′-5′-oligoadenylate synthetase gene by hepatitis C virus core protein. J Virol 74:8744–8750. doi: 10.1128/jvi.74.18.8744-8750.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dansako H, Naganuma A, Nakamura T, Ikeda F, Nozaki A, Kato N. 2003. Differential activation of interferon-inducible genes by hepatitis C virus core protein mediated by the interferon stimulated response element. Virus Res 97:17–30. doi: 10.1016/s0168-1702(03)00218-1. [DOI] [PubMed] [Google Scholar]
- 27.Park SB, Seronello S, Mayer W, Ojcius DM. 2016. Hepatitis C virus frameshift/alternate reading frame protein suppresses interferon responses mediated by pattern recognition receptor retinoic-acid-inducible gene-I. PLoS One 11:e0158419. doi: 10.1371/journal.pone.0158419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Honda K, Takaoka A, Taniguchi T. 2006. Type I interferon gene induction by the interferon regulatory factor family of transcription factors. Immunity 25:349–849. doi: 10.1016/j.immuni.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 29.Thanos D, Maniatis T. 1995. Virus induction of human IFN beta gene expression requires the assembly of an enhanceosome. Cell 83:1091–1100. doi: 10.1016/0092-8674(95)90136-1. [DOI] [PubMed] [Google Scholar]
- 30.Barnes B, Lubyova B, Pitha PM. 2002. On the role of IRF in host defense. J Interferon Cytokine Res 22:59–71. doi: 10.1089/107999002753452665. [DOI] [PubMed] [Google Scholar]
- 31.Malmgaard L. 2004. Induction and regulation of IFNs during viral infections. J Interferon Cytokine Res 24:439–454. doi: 10.1089/1079990041689665. [DOI] [PubMed] [Google Scholar]
- 32.Fitzgerald KA, McWhirter SM, Faia KL, Rowe DC, Latz E, Golenbock DT, Coyle AJ, Liao SM, Maniatis T. 2003. IKK epsilon and TBK1 are essential components of the IRF3 signaling pathway. Nat Immunol 4:491–496. doi: 10.1038/ni921. [DOI] [PubMed] [Google Scholar]
- 33.Seth RB, Sun L, Ea CK, Chen ZJ. 2005. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell 122:669–682. doi: 10.1016/j.cell.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 34.Lin R, Mamane Y, Hiscott J. 1999. Structural and functional analysis of interferon regulatory factor 3: localization of the transactivation and autoinhibitory domains. Mol Cell Biol 19:2465–2474. doi: 10.1128/mcb.19.4.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li X, Zhao X, Fang Y, Jiang X, Duong T, Fan C, Huang CC, Kain SR. 1998. Generation of destabilized green fluorescent protein as a transcription reporter. J Biol Chem 273:34970–34975. doi: 10.1074/jbc.273.52.34970. [DOI] [PubMed] [Google Scholar]
- 36.Matz MV, Fradkov AF, Labas YA, Savitsky AP, Zaraisky AG, Markelov ML, Lukyanov SA. 1999. Fluorescent proteins from nonbioluminescent Anthozoa species. Nat Biotechnol 17:969–973. doi: 10.1038/13657. [DOI] [PubMed] [Google Scholar]
- 37.Vlashi E, Kim K, Lagadec C, Donna LD, McDonald JT, Eghbali M, Sayre JW, Stefani E, McBride W, Pajonk F. 2009. In vivo imaging, tracking, and targeting of cancer stem cells. J Natl Cancer Inst 101:350–359. doi: 10.1093/jnci/djn509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Akuta N, Suzuki F, Hirakawa M, Kawamura Y, Sezaki H, Suzuki Y, Hosaka T, Kobayashi M, Kobayashi M, Saitoh S, Arase Y, Ikeda K, Kumada H. 2011. Amino acid substitutions in hepatitis C virus core region predict hepatocarcinogenesis following eradication of HCV RNA by antiviral therapy. J Med Virol 83:1016–1022. doi: 10.1002/jmv.22094. [DOI] [PubMed] [Google Scholar]
- 39.Ogata F, Akuta N, Kobayashi M, Fujiyama S, Kawamura Y, Sezaki H, Hosaka T, Kobayashi M, Saitoh S, Suzuki Y, Suzuki F, Arase Y, Ikeda K, Kumada H. 2018. Amino acid substitutions in the hepatitis C virus core region predict hepatocarcinogenesis following eradication of HCV RNA by all-oral direct-acting antiviral regimens. J Med Virol 90:1087–1093. doi: 10.1002/jmv.25047. [DOI] [PubMed] [Google Scholar]
- 40.Toyoda H, Kumada T, Kaneoka Y, Maeda A. 2011. Amino acid substitutions in the hepatitis C virus core region are associated with postoperative recurrence and survival of patients with HCV genotype 1b-associated hepatocellular carcinoma. Ann Surg 254:326–332. doi: 10.1097/SLA.0b013e3182263b8e. [DOI] [PubMed] [Google Scholar]
- 41.Baril M, Racine ME, Penin F, Lamarre D. 2009. MAVS dimer is a crucial signaling component of innate immunity and the target of hepatitis C virus NS3/4A protease. J Virol 83:1299–1311. doi: 10.1128/JVI.01659-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Foy E, Li K, Sumpter R, Loo YM, Johnson CL, Wang CF, Fish PM, Yoneyama M, Fujita T, Lemon SM, Gale M. 2005. Control of antiviral defenses through hepatitis C virus disruption of retinoic acid-inducible gene-I signaling. Proc Natl Acad Sci U S A 102:2986–2991. doi: 10.1073/pnas.0408707102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meylan E, Curran J, Hofmann K, Moradpour D, Binder M, Bartenschlager R, Tschopp R. 2005. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature 437:1167–1172. doi: 10.1038/nature04193. [DOI] [PubMed] [Google Scholar]
- 44.Morikawa K, Lange CM, Gouttenoire J, Meylan E, Brass V, Penin F, Moradpour D. 2011. Nonstructural protein 3-4A: the Swiss army knife of hepatitis C virus. J Viral Hepat 18:305–315. doi: 10.1111/j.1365-2893.2011.01451.x. [DOI] [PubMed] [Google Scholar]
- 45.Horner SM, Liu HM, Park HS, Briley J, Gale M. 2011. Mitochondrial-associated endoplasmic reticulum membranes (MAM) form innate immune synapses and are targeted by hepatitis C virus. Proc Natl Acad Sci U S A 108:14590–14595. doi: 10.1073/pnas.1110133108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dixit E, Boulant S, Zhang YJ, Lee ASY, Odendall C, Shum B, Hacohen N, Chen ZJ, Whelan SP, Fransen M, Nibert ML, Superti-Furga G, Kagan JC. 2010. Peroxisomes are signaling platforms for antiviral innate immunity. Cell 141:668–681. doi: 10.1016/j.cell.2010.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen ZH, Benureau Y, Rijnbrand R, Yi JZ, Wang T, Warter L, Lanford RE, Weinman SA, Lemon SM, Martin A, Li K. 2007. GB virus B disrupts RIG-I signaling by NS3/4A-mediated cleavage of the adaptor protein MAVS. J Virol 81:964–976. doi: 10.1128/JVI.02076-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang Y, Liang YQ, Qu L, Chen ZM, Yi MK, Li K, Lemon SM. 2007. Disruption of innate immunity due to mitochondrial targeting of a picornaviral protease precursor. Proc Natl Acad Sci U S A 104:7253–7258. doi: 10.1073/pnas.0611506104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kong WP, Roos RP. 1991. Alternative translation initiation site in the DA strain of Theiler’s murine encephalomyelitis virus. J Virol 65:3395–3399. doi: 10.1128/JVI.65.6.3395-3399.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Michiels T, Jarousse N, Brahic M. 1995. Analysis of the leader and capsid coding regions of persistent and neurovirulent strains of Theiler’s virus. Virology 214:550–558. doi: 10.1006/viro.1995.0066. [DOI] [PubMed] [Google Scholar]
- 51.van Eyll O, Michiels T. 2002. Non-AUG-initiated internal translation of the L* protein of Theiler’s virus and importance of this protein for viral persistence. J Virol 76:10665–10673. doi: 10.1128/jvi.76.21.10665-10673.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ghadge GD, Ma L, Sato S, Kim J, Roos RP. 1998. A protein critical for a Theiler’s virus-induced immune system-mediated demyelinating disease has a cell type-specific antiapoptotic effect and a key role in virus persistence. J Virol 72:8605–8612. doi: 10.1128/JVI.72.11.8605-8612.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van Pesch V, van Eyll O, Michiels T. 2001. The leader protein of Theiler’s virus inhibits immediate-early alpha/beta interferon production. J Virol 75:7811–7817. doi: 10.1128/jvi.75.17.7811-7817.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kotta-Loizou I, Vassilaki N, Pissas G, Kakkanas A, Bakiri L, Bartenschlager R, Mavromara P. 2013. Hepatitis C virus core+1/ARF protein decreases hepcidin transcription through an AP1 binding site. J Gen Virol 94:1528–1534. doi: 10.1099/vir.0.050328-0. [DOI] [PubMed] [Google Scholar]
- 55.Chen L, Borozan I, Sun J, Guindi M, Fischer S, Feld J, Anand N, Heathcote J, Edwards AM, McGilvray ID. 2010. Cell-type specific gene expression signature in liver underlies response to interferon therapy in chronic hepatitis C infection. Gastroenterology 138:1123–1133.e3. doi: 10.1053/j.gastro.2009.10.046. [DOI] [PubMed] [Google Scholar]
- 56.Sarasin-Filipowicz M, Oakeley EJ, Duong FHT, Christen V, Terracciano L, Filipowicz W, Heim MH. 2008. Interferon signaling and treatment outcome in chronic hepatitis C. Proc Natl Acad Sci U S A 105:7034–7039. doi: 10.1073/pnas.0707882105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Asselah T, Bieche I, Narguet S, Sabbagh A, Laurendeau I, Ripault MP, Boyer N, Martinot-Peignoux M, Valla D, Vidaud M, Marcellin P. 2008. Liver gene expression signature to predict response to pegylated interferon plus ribavirin combination therapy in patients with chronic hepatitis C. Gut 57:516–524. doi: 10.1136/gut.2007.128611. [DOI] [PubMed] [Google Scholar]
- 58.Chen LM, Borozan I, Feld J, Sun J, Tannis LL, Coltescu C, Heathcote J, Edwards AM, McGilvray ID. 2005. Hepatic gene expression discriminates responders and nonresponders in treatment of chronic hepatitis C viral infection. Gastroenterology 128:1437–1444. doi: 10.1053/j.gastro.2005.01.059. [DOI] [PubMed] [Google Scholar]
- 59.Liu HM, Loo YM, Horner SM, Zornetzer GA, Katze MG, Gale M Jr. 2012. The mitochondrial targeting chaperone 14–3-3epsilon regulates a RIG-I translocon that mediates membrane association and innate antiviral immunity. Cell Host Microbe 11:528–537. doi: 10.1016/j.chom.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]