Abstract
Purpose
Genomic tests can identify ER-positive HER2-negative localized breast cancer patients who may not benefit from adjuvant chemotherapy. Such tests seem especially interesting in “intermediate” clinico-pathological risk categories. The psychological impact of the decision uncertainty in these women remains largely unexplored. We assessed the clinical and psychological impact of EndoPredict® (EpClin), a clinico-genomic test, in these patients.
Methods
This multicenter, single arm prospective study (NCT02773004) enrolled patients for which adjuvant chemotherapy was uncertain, based on predefined criteria. The primary endpoint was the proportion of change between initial adjuvant decision and final administration of chemotherapy. Secondary endpoints included post-test (Day 17) and 1-year patient reported outcomes.
Results
One third of 200 evaluable patients had a high EpClin score (≥3.32867; 10 years cumulative risk of distance failure ≥10%). The overall change rate of chemotherapy decision was 72/200 (35.8%, 95% CI 29.2–42.4). Chemotherapy was withdrawn in 57 cases (28.4% [22.2–34.8]) and added in 15 (7.5% [3.8–11.2]. 6 changes (8%) were based on patients’ decisions. Anxiety and distress levels increased at Day 17 when adding chemotherapy after the test result (p < 10−7 and 0.00022 respectively), while stable in other situations. At 1-year, all patients had returned to the baseline anxiety and distress levels (mean anxiety 51.5, +/− SD = 2.5 [max. 80], mean distress 3±1 [max. 10]).
Conclusions
EndoPredict ® (EpClin) is clinically useful in deciding whether or not to administer adjuvant chemotherapy in patients with intermediate risk. A single-step decision is preferable since adding chemotherapy at a later stage increases anxiety and distress.
Keywords: Breast cancer, Genomic test, Chemotherapy, Patient reported outcomes, EndoPredict®
Highlights
-
•
EndoPredict ® (EpClin) allowed a chemotherapy decision modification in 35% of the patients included in the Adendom trial.
-
•
Patient-physician concertation is important: 8% of treatment changes are based on patients’ will.
-
•
A single-step decision including the test appears preferable to limit anxiety and distress.
1. Introduction
Over and above classical clinico-pathological parameters (such as age, tumor size, nodal status, tumor grade, and estrogen receptor expression), genomic signatures of early human epidermal growth factor receptor 2 (HER2)-negative breast cancers have emerged during the past 15 years allowing more precise evaluation of patients’ long-term prognosis. Currently, at least six gene signatures are commercially available (Oncotype DX™, MammaPrint®, Genomic Grade Index®, PAM50™, Breast Cancer Index™, and EndoPredict®) [1]. These signatures generally assess the patient’s risk in terms of distant relapse-free survival at 5 or 10 years. They were all developed to assist with chemotherapy decision making in the adjuvant setting. The majority, if not all of these tests, have essentially prognostic but not predictive value [2]. The utility of certain tests, for prognostic determination and chemotherapy decision making in patients with ER-positive HER2-negative localized breast cancer, has now widely been recognized [[1], [2], [3], [4], [5]]. These genomic tests do not always concord on the individual level, as demonstrated in the OPTIMA trial [4]. However, the tests provide prognostic information and help clinical decision making. First-generation prognostic tests (OncotypeDX™, Mammaprint®) are now used worldwide to guide decision making regarding adjuvant chemotherapy. These tests were validated initially based on substantial retrospective data and, more recent prospective data [[3], [4], [5]]. The most recently developed prognostic signatures add value by integrating clinical parameters to standard characteristics. They have demonstrated independent added value on top of tumor burden, tumor grade, estrogen receptor (ER) and progesterone receptor (PR) status, HER2 status, age and also tumor proliferation. EndoPredict® (EpClin) is one of such tests. It is based on the reverse transcription/polymerase chain reactions analysis of mRNA expression of eight tumor genes, in addition to 3 housekeeping genes, particularly relevant for disease progression [7]. The test then combines these genomic results with classical prognostic factors, nodal status and tumor size, for EPclin evaluation and 10-year distant relapse risk evaluation [7]. EpClin has been retrospectively validated in several independent clinical trials including long-term follow-up [[7], [8], [9], [10], [11]]. Several studies have assessed its impact under clinical conditions [[12], [13], [14]]. The evidence-based approach to clinical practice, for a genomic signature, as highlighted by Michiels et al. [2], includes evaluating the clinical impact: does the signature change risk prediction sufficiently to change recommended therapy? Which patients benefit from current genomic tests still remains unclear? Women with a clinically low-risk disease may not benefit [3]. Based on medico-economic data, tests are usually used empirically in patients with intermediate clinical risk, considered as a “grey zone” for whom the benefit of chemotherapy is debatable [15]. They reportedly increase oncologists’ and patients’ decision-making confidence, generally improving the balance of risk with the therapy decisions [16]. However, the patients’ point of view and their role in the decision making remain under-explored. Furthermore, patients’ reported outcomes and psychological consequences of genomic testing and subsequent decision changes need to be considered.
Our study aimed to assess the impact of EndoPredict® (EpClin) test on the final decision concerning adjuvant chemotherapy, in women with clinically “intermediate” risk ER-positive HER2-negative localized breast cancer, considered by their clinician to be in a “grey zone”. Our study is the first to evaluate the final decision, the decision made by the patient after receiving and discussing the genomic test results with her clinician. The study also aimed to assess the psychological consequences of the decision making process on patients.
2. Patients and methods
The present study (UC-0140/1505 – ADENDOM; 2015-A00528-41; UCBG 2–14; NCT02773004) was approved by the French Health authority (Agence Nationale de Sécurité du Medicament et des produits de santé) on October 16th, 2015 and by the National Ethics Committee (Comité de Protection des Personnes Sud Est 6) on November 19th, 2015. This multicenter, single arm prospective study enrolled patients from December 1st, 2015 until April 18th, 2016. During this time, no genomic test was reimbursed in routine practice for French breast cancer patients. Patients (performance status 0–1) with localized, fully resected, early breast cancer were eligible. In addition, the breast cancer needed to be classified as pN0 or pN1mi, ER-positive (>10% expression by immunohistochemistry [IHC]), HER2-negative (IHC 0/1 + or non-amplified). Furthermore, the local multidisciplinary team had to be unsure of the need for adjuvant chemotherapy, i.e. breast cancers of grade 2, or grade 3 and pT < 2 cm, or lobular histology, based on the current guidelines [17,18].
Our primary objective was to assess the clinical utility of the EndoPredict® (EpClin) genomic test. Whether the test influenced whether the patient was prescribed adjuvant CT or not. Secondary objectives were patient reported outcomes and evaluation of the feasibility of routine centralized EPclin testing. The primary outcome measure was the proportion of patients whose choice of treatment changed due to the EPclin results: from the initial choice made after surgery by the multidisciplinary team and the final choice made by the patients, with their clinician, after receiving the results. The main secondary outcome measures were the impact of the procedure and the genomic test results on patient’s anxiety levels, using the State Anxiety Inventory [19], and psychological distress, using the “Distress Thermometer” [20].
2.1. EndoPredict® (EpClin) testing methodology and results
The EpClin test was centralized at the Centre Jean Perrin, Clermont Ferrand and performed as recommended by the manufacturer. As recommended, pN1mi status was considered as pN1 for EpClin testing. Patients with an EpClin score ≥3.32867, equivalent to 10-year distant relapse risk ≥10%, were classified as “high-risk”, and those with a score <3.32867 were classified as “low-risk” [7]. In patients classified as “high-risk”, adjuvant chemotherapy followed by endocrine therapy was advised. While, for those classified as “low-risk”, endocrine therapy alone, without adjuvant chemotherapy, was advised.
Physicians and patients received the following results: the EpClin risk class (high/low risk), the EpClin risk score (expressed as a value on a curve), the molecular EndoPredict® score (displayed as a value on a bar), and the predicted 10-year distant relapse risk with endocrine therapy alone (as a percentage).
2.2. Trial process
The post-operative multidisciplinary meeting identified patients potentially eligible for the study. At the meeting, a first therapeutic decision (MTM1, adjuvant chemotherapy or not) was taken, using the standard clinical and histopathological markers and following the official guidelines [3]. The study was subsequently proposed to the patient by her oncologist. The uncertainty concerning the benefit of chemotherapy in her situation was explained. After obtaining written informed consent and study registration, the most representative block of the primary tumor on surgical specimen (or 10 paraffin slides) was centralized for EpClin testing. The EpClin results together with the recommended treatment (MTM2) were disclosed to the patient during a second visit, with her oncologist, around 15 days after the inclusion visit. The final decision, adjuvant chemotherapy or not, taken after this second visit was recorded.
2.3. Questionnaires
Patients completed the anxiety and distress evaluation questionnaires at inclusion, at day 15 (at the second visit when the test results are disclosure), at day 17, and at 1 year after inclusion.
2.4. Statistical analyses
The EpClin test was expected to classify 55–65% of ER-positive, HER2-negative, node-negative or pN1mi breast cancers as low risk [7]. The chemotherapy strategy change rate in this population was expected to be between 30 and 40%. A five steps Fleming design was planned, with a decision change rate of ≤15% considered as not acceptable (low clinical impact). The upper bound of the Fleming schedule was fixed at 30%, but was not to be used to stop the study. This design required 113 patients with a one-step design (unilateral α = 2.5% and β = 1%) (nPII software, De Rycke Y, Kramar A, Institut Curie 1999). To have an absolute accuracy of <7% for the final decision change rate, at least 170 inclusions were needed. To compensate for possible loss of information (non-informative tumor sample, etc.), we planned to include 200 patients. At each step of the Fleming schedule, 40 patients were to be included. The following low rejection bounds were fixed by calculations: none at the first step, ≤9 at the second (80 inclusions), 19 at the third, 29 at the fourth, and 40 at the fifth. During accrual in the stepwise Fleming design, the percentage of treatment decision changes was calculated on patients with informative tumor samples, and computed after completion of each group of 40 patients. If the study was not stopped prematurely for futility, the final decision change rate (primary endpoint), with its 95% confidence interval (CI), was to be calculated on all informative patients. Predictive clinical factors for decision-changes were also investigated, by univariate analysis, and then by logistic regression. These univariate analyses included Chi2 test with categorical parameters, Student t-test, ANOVA or Kruskal-Wallis H-test when one variable was quantitative and the other categorical (a non-parametric test was chosen if distributions were not Gaussian and/or in case of heteroscedasticity). Descriptive analyses were conducted for secondary endpoints using class sizes and proportions/percentages. The 95% CIs of means and percentages were calculated using the formula: means or percentage +/− (tN-1 x standard-deviation/N½). All tests were 2-sided and p-values less than 0.05 were considered significant. Clinsight software was used for data management (Ennov Inc. http://en.ennov.com/) and the SEM software [22] for all statistical analyses.
3. Results
3.1. Patient and tumor characteristics
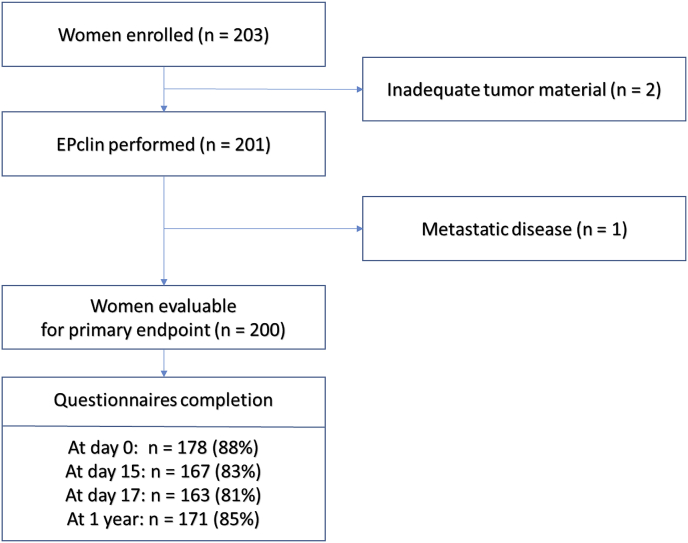
203 patients were included in 25 centers: 201 had an EPclin evaluation (inadequate material for 2 patients) and 200 were included in the primary endpoint assessment (1 patient was withdrawn due to diagnosis of metastatic disease after inclusion) (Fig. 1). The 25 centers comprised comprehensive cancer centers (n = 8), a university hospital (n = 1), general hospitals (n = 9), and private centers (n = 7). Patients’ characteristics are described in Table 1. Most patients had pT1 (75.5%) and grade 2 (74%) tumors, as well as node-negative disease (90.5%; the remaining being pN1mi). The majority of tumors (67%) were classified as EPclin low risk.
Fig. 1.
CONSORT flowchart.
Table 1.
Patients’ characteristics and disposition.
| N | Result | ||
|---|---|---|---|
| Median Age (range) | Years | 201 | 59 (23–81) |
| Tumor type | Ductal | 144 | 72% |
| Lobular | 30 | 15% | |
| Other | 27 | 13% | |
| Multiple foci | Yes | 24 | 12% |
| Tumor grade | 1 | 17 | 9% |
| 2 | 149 | 74% | |
| 3 | 35 | 17% | |
| ER-positive | 201 | 100% | |
| PR-positive | 166 | 82.5% | |
| Surgery type | Lumpectomy | 149 | 74% |
| Mastectomy | 52 | 26% | |
| Sentinel node dissection | 178 | 89% | |
| pT | pT1 | 151 | 75.5% |
| pT1a | 1 | ||
| pT1b | 34 | ||
| pT1c | 116 | ||
| pT2 | 44 | 22% | |
| pT3 | 5 | 2.5% | |
| pN | pN0 | 181 | 90.5% |
| pN1mi | 19 | 9.5% | |
| EPclin risk class | Low Risk | 135 | 67% |
| High risk | 66 | 33% | |
| Initial chemotherapy decision | Yes | 96 | 48% |
| No | 105 | 52% | |
| Final chemotherapy decisiona | Yes | 53 | 26% |
| No | 147 | 73% | |
One patient was withdrawn from the study due to metastatic disease discovered after accrual.
3.2. Analysis of primary endpoint
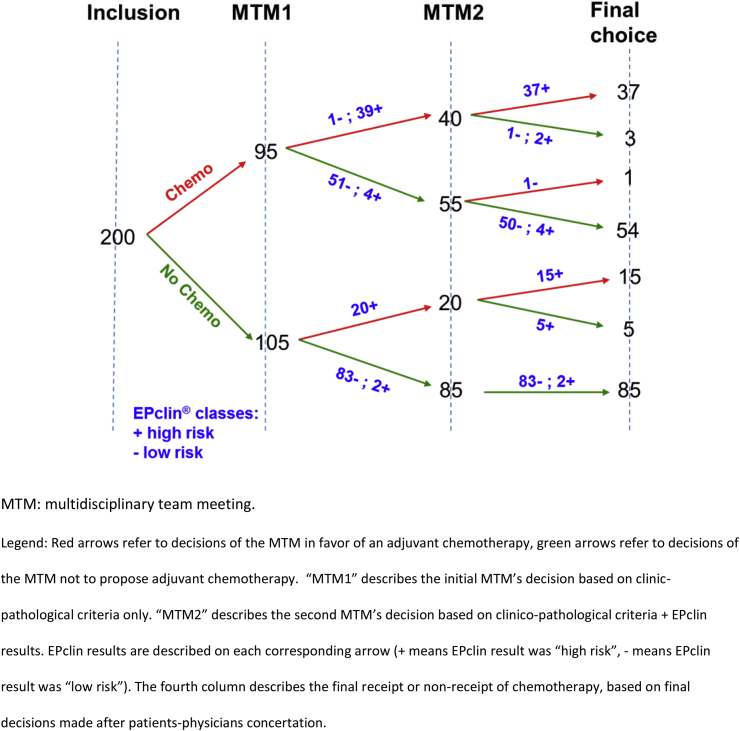
At each step of the Fleming schedule, the low rejection bound of 15% was largely exceeded. Thus, the study accrued patients until the target, of 200 patients, was reached. The final treatment decision change rate between primary decision (MTM1) and treatment administered, the primary endpoint, was 72/200 (36%, 95% CI 29.3–42.7) (Fig. 2). Chemotherapy was initially proposed to 48% of patients of which only 26% finally received chemotherapy (Table 1). In 57 cases, chemotherapy was withdrawn (28.4%, 95% CI 22.2–34.8) while in 15 cases (7.5%, 95% CI 3.8–11.2) chemotherapy was added. The decisions to change treatment were mostly related to the EPclin results (Fig. 2). However, in 7 cases (3.5%), the decision at MTM2 was discordant with the test results. After discussion with the patient, the final discordance rate between EPclin test results and final chemotherapy administration was 6.5% (13/200). Details concerning the decisions steps, including compliance/discrepancy between multidisciplinary teams’ decisions and final decisions, are provided in Fig. 2.
Fig. 2.
Patients’ flow, treatment decisions at three key steps of the trial.
3.3. Analyses of secondary outcomes
We then analyzed the determinants of discordant decisions between EPclin results and the final treatment administered (Table 2). These discordant decisions were not obviously linked to the 10-year metastatic risk evaluation level: 6 patients who eventually did not receive chemotherapy despite a high EPclin risk score, had a median 10-year distant relapse risk of 14% (range 11–19). In comparison, the median 10-year distant relapse risk in patients who received chemotherapy was 13% (range 10–18) in those where the initial multidisciplinary decision changed and 17% (range 10–45) in those where this decision was maintained.
Table 2.
Summary of treatment changes decisions according to test results (comparing initial and post-test decisions).
| CT added | CT maintained (No change) | CT Withdrawn | CT absent (No change) | |
|---|---|---|---|---|
|
Chemotherapy (CT) final administration N (%) according to EPclin class | ||||
| Low risk | 0 (0.0%) | 1 (0.7%) | 51 (37.8%) | 83 (61.5%) |
| High risk | 15 (23.1%) | 37 (56.9%) | 6 (9.2%) | 7 (10.8%) |
|
Total |
15 |
38 |
57 |
90 |
|
Median EPClin 10 year distant relapse risk per group, (%, [Range]) | ||||
| Low risk | – | 8% [8–8] | 6% [3–10] | 6% [2–10] |
| High risk | 13% [10–18] | 17% [10–45] | 14% [11–19] | 13% [11–20] |
3.3.1. MTM: multidisciplinary team meeting
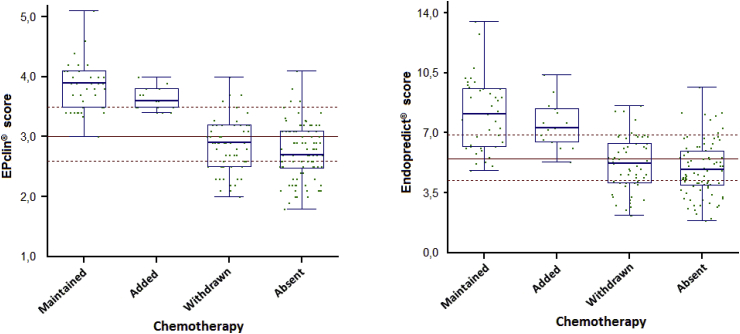
In univariate analyses, Endopredict® molecular score was slightly less discriminant than EPclin score for therapeutic decision changes (ANOVA: p = 0.00078 and 0.00013, respectively) (Fig. 3). In the multivariate analyses, the only independent predictor of the decision to not give/withdraw chemotherapy was EPclin risk class (logistic regression: p = 0.003; OR = 0.01, 95% CI 0.0–0.22), while Endopredict® score and EPclin risk level were not significant.
Fig. 3.
Box plots of the effect of EPclin score versus molecular score (Endopredict® score) on CT decision changes (CT maintained, CT added, CT withdrawn or CT absent).
Of note, initial treatment decisions and, subsequently, levels of treatment changes appeared heterogeneous across centers (Chi2 test: p = 0.001, Supplementary Table 1). The MTM2 decision to not administer chemotherapy varied between 12 and 70% across centers, while adding chemotherapy varied between 0 and 24%. A final decision discordant relatively to test result was taken after the final physician-patient concertation in 6 cases (8% of all changes).
3.3.2. Anxiety and distress
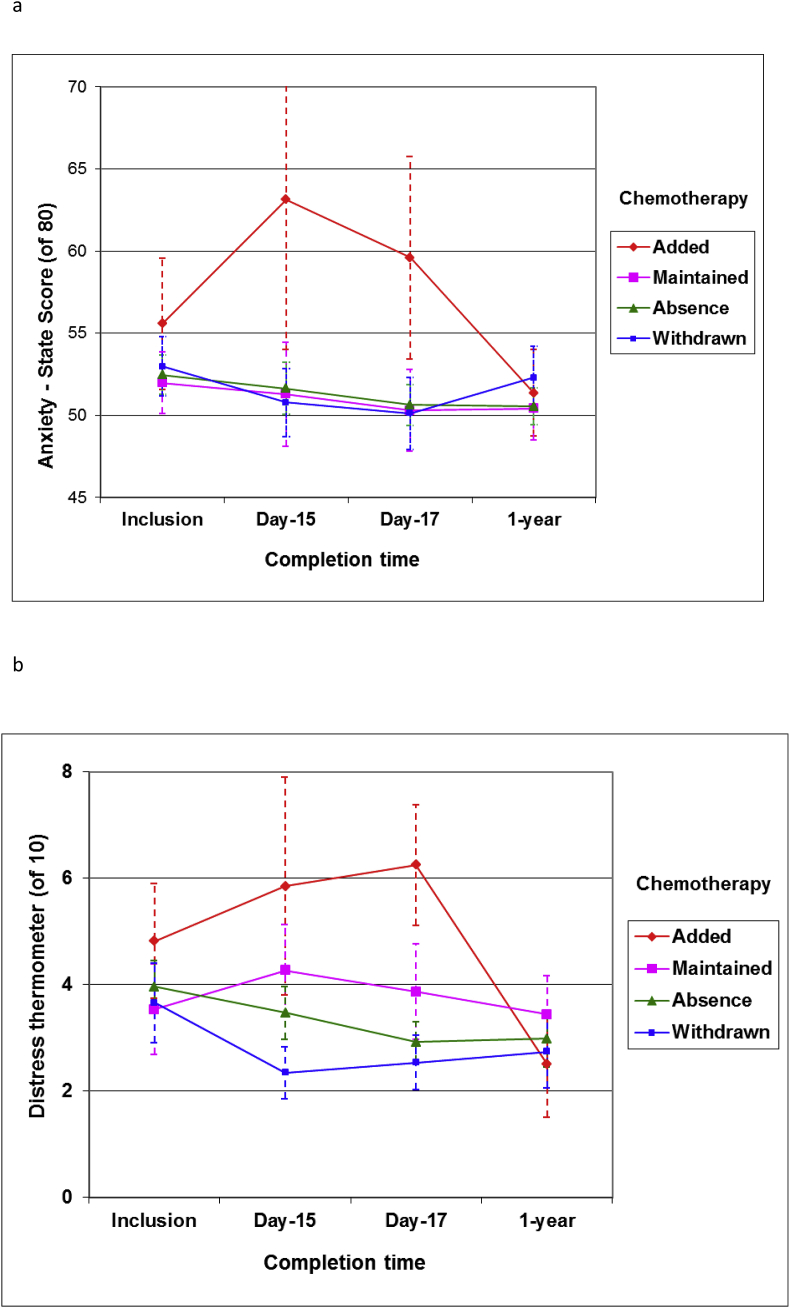
Patients had on average a moderate anxiety scores at baseline, around 52/82 points on the scale (Fig. 4a). Patients who had no change in chemotherapy decision and those for which chemotherapy was withdrawn upon test results, had a stable or non-significant decrease in anxiety levels at Day 17 (ANOVA: p = 0.10). In contrast, anxiety scores increased significantly at Day 17 in patients in whom chemotherapy was added upon test results (ANOVA: p < 10−7) (Fig. 4a). Similarly, distress levels were significantly higher in these patients (ANOVA: p < 10−7), even compared to patients who were initially proposed chemotherapy (ANOVA: p = 0.00022) (Fig. 4b). Distress levels at Day 17 were overall higher in patients where chemotherapy was planned compared to those where it was not planned (H-test: p = 0.0039) (Fig. 4b). There was a significant interaction between presence/absence of chemotherapy and when distress was assessed (ANOVA: p = 0.02): distress decreased over time in patients avoiding chemotherapy as compared to their counterparts.
Fig. 4.
Mean anxiety (a) and distress (b) levels and their evolution over time according to final chemotherapy decision (error bars correspond to 95% CI).
The relations between patient-related outcomes at Day 17 and decision changes are summarized in Table 3. Higher distress and anxiety levels are significantly correlated with a late decision and the adding of chemotherapy to the treatment plan.
Table 3.
Probabilities associated with relations between Patients Reported Outcomes over time and decision of chemotherapy (CT).
| Situation regarding chemo | Satisfaction (IN-PATSAT32) | Distress | State Anxiety |
|---|---|---|---|
| Any decision change | 0.59 | 0.94 | 0.018 |
| Addition versus no change or withdrawal | 0.61 | < 10−7 | < 10−7 |
| CT initially planned versus not planned | 0.47 | 0.19 | 0.54 |
At 1 year, however, all patients have at least returned to baseline anxiety and distress levels, independently of the final chemotherapy decision (Fig. 4a and b). The difference in the probabilities of distress level, by H-test, between women with “added chemotherapy” and other categories were 0.20 at inclusion, 0.0001 at Day 15, 0.000009 at Day 17, and 0.35 at 1 year.
4. Discussion
Our study met its primary endpoint and showed that in patients with early breast cancer at intermediate-risk, the use of EPclin to support the treatment decision resulted in a 35.8% change in whether adjuvant chemotherapy was administered or not. Finally, 26% and not 48% of patients received chemotherapy (a 54% relative and 22% absolute decrease). This is comparable with that observed in the Mindact study: a 14% absolute decrease in the study population and a 46% decrease in the clinical-high risk population [4]. The 35.8% decision change rate was as we expected. The confidence interval did not include 15%, the lower threshold below which the EndoPredict® test would be considered uninteresting. We thus conclude that the EndoPredict® test significantly impacted the clinical decision, whether or not to administer adjuvant chemotherapy, in our study population.
It is intuitive that an “intermediate” risk population (“grey zone”) would most benefit from genomic testing. However, this population’s definition remains empirical, as in our study. What is intermediate risk? Our study was conducted before the results of the Mindact trial were released [4]. Most of our patients would have been classified at low-clinical risk by AdjuvantOnline! (web-based tool) if accrued in Mindact (75% had pT1, 74% grade 2, and most had node-negative tumors). Based on the Mindact results, these patients would not have benefited from genomic testing. In contrast, in the Mindact trial, patients classified at “clinical-high” risk breast cancer could be efficiently reclassified by genomic testing to low or high genomic risk, which appeared be associated with a meaningful differences in distant disease-free survivals. In the Mindact study, using the genomic test decreased the chemotherapy prescription by 43% in patients with clinical-high risk disease and by only 14% in the study population. But Mindact included ER-negative and HER2-positive breast cancer patients.
In our study, patients were considered to be at intermediate risk by the clinicians and EPclin identified 33% of them as having a high genomic risk. One could question a possible over-sensitivity of EPclin as compared to Mammaprint®. As stated before, genomic tests seem to equally classify patients between low and high risk, although they lack concordance at the patient level [6]. Furthermore, clinico-genomic tests based on both clinico-pathological and genomic data, such as EPclin seem to be slightly more discriminant that purely genomic ones [23]. Indeed, on the ATAC cohort, Sestak et al. showed that the improvement of the likelihood ratio of early distant relapse risk prediction, over clinico-pathological classification, varied from 11 to more than 100% according to the different tests. However, not all of these tests include clinical data and had an intermediate risk categories [24]. In our study, the clinico-molecular EpClin score appears to be more discriminant than the purely molecular Endopredict® score (Fig. 3).
Our study highlights the importance of the shared decision process in the final decision whether or not to administer chemotherapy, as well as providing interesting data regarding patient reported outcomes. In all studies published so far, the patient’s point of view was not considered. Endpoints, such as the main one in Mindact, related purely to the test-derived decision (intent-to-treat) and not to the final decision taken after physician-patient discussions [4]. In our study, 6 out of 72 (8%) decision changes were exclusively the patients’ decisions and discordant with the test results. In Mindact, the compliance to chemotherapy randomized decisions varied from 80 to 89% in discordant groups, but it was not reported whether the high 10–11% non-compliance rate was due to the clinicians or to patients [4]. For tests assigning not only a dichotomous risk groups, but also numerical estimations of risk, and the individual risk on a risk curve, the discussion between physicians and patients may be crucial for the final chemotherapy decision. An estimation of an individual risk/benefit ratio of chemotherapy administration seems easier in these situations, although there is absolutely no proof for such use. Of note, the ongoing Rxponder trial (NCT01272037) assesses whether a specific cut-off of risk measured through OncotypeDX™, associated with a minimal chemotherapy benefit could be identified. However, risk alone, as observed by Michiels and colleagues, is unfortunately not a perfect decision criterion for chemotherapy administration. An ideal test would also include factors/properties to predict individual chemotherapy benefit [2].
To return to patients reported outcomes, our study provides important information regarding the decision process. We clearly showed that anxiety and distress significantly increase in patients, not initial considered for chemotherapy, but proposed chemotherapy after the genomic testing and who had delayed information regarding this decision. Higher distress and anxiety levels were significantly correlated with a late decision and the adding of chemotherapy to planned treatment. This pleads in favor of one-shot announcement of all the post-operative results, including genomic tests, whenever indicated. As well, one should consider how genomic tests and their results are presented to patients. Patients’ preferences have rarely been considered when genomic tests have been used for breast cancer adjuvant decisions [25,26].
Our study has limitations, mostly linked to the non-randomized methodology and open design. Biases in patient selection, in physicians’ decisions before testing, regarding indications of chemotherapy limit our study. Our “intermediate” target category remained quite vague and may have led to heterogeneity in patients accrued. Although, our study was designed in-line with the 2015 Saint Gallen prognostic classification and ESMO guidelines, we did not strictly adhere to them [17,18]. Centers were heterogeneous regarding their attitude to adjuvant chemotherapy, some “extreme” centers proposing chemotherapy in almost all cases, while others proposed almost no chemotherapy. Resulting in heterogeneous percentage changes across centers. However, this study was conducted in 25 centers of various statuses (public, private, comprehensive centers …), and the heterogeneity observed was mostly driven by a few “extreme” centers. If genomic tests cannot be offered to all patient, as in most countries including France, it is important that testing and patients’ information be controlled by cancers societies’ recommendations to avoid major decision biases. Genomic testing appears to result in more consistent final treatment decisions, across patients’ situations and centers. One only needs to define common criteria for genomic testing.
Another limitation concerns the patients’ decision role and patients’ preference. This study was not designed to thoroughly assess the role of the patients’ decision and data still lacks regarding this important point. Indeed, we did not collect data regarding the patients’ reasons for non-participation nor for chemotherapy refusal/demand.
5. Conclusion
EndoPredict® (EPclin) test has a confirmed impact on the de-escalation of adjuvant chemotherapy prescriptions under routine conditions. It allows a decrease in centers and physicians’ treatment decision heterogeneity. However, delayed decisions or decisions changes increase anxiety and distress in patients and should be avoided.
Funding
This academic study, sponsored by Unicancer, was funded by a grant from Myriad Genetics™. Myriad Genetics™ is the manufacturer of the EPclin test and provided the tests for free. All EPclin tests were conducted in an academic lab (Centre Jean Perrin). The manufacturer had no role in the design, conduct, analysis and interpretation of the study and results, nor in the writing of the present manuscript.
Compliance with ethical standards
All procedures performed in the present study were in accordance with the national and international ethical standards.
Disclosures
Frédérique Penault Llorca declared grants and honoraria from Myriad Genetics, Nanostring and Genomic Health. Suzette Delaloge declared institutional grants from Myriad Genetics and Genomic Health. Marianne Leheurteur declared honoraria from Genomic Health.
Contributor roles
Conceptualization FPL, SD; Formal Analysis FK; Funding Acquisition FPL, SD, JL; Investigation All authors; Methodology FK, FPL, SD; Project Administration JL, FPL, SD; Resources FPL; Supervision JL; Visualization FPL, SD, FK, JL; Writing – Original Draft Preparation FPL, SD, FK, JL; Writing – Review & Editing All authors.
Declaration of competing interest
All other authors have declared no conflicts of interest.
Acknowledgements
We thank all the patients for their participation into this trial, as well as Sibille Everhard and Saliha Ghanem for their logistic assistance; Anne Cayre and Amandine Bonhomme for their technical support (EPClin test). We thank Trevor Stanbury for editing the manuscript. We finally thank Myriad genetics for funding support of this independent academic study. We thank Myriad Genetics™ for funding this trial.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2019.10.013.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Krop I., Ismaila N., Andre F., Bast R.C., Barlow W., Collyar D.E. Use of biomarkers to guide decisions on adjuvant systemic therapy for women with early-stage invasive breast cancer: American Society of Clinical Oncology clinical practice guideline focused update. J Clin Oncol. 2017 Aug 20;35(24):2838–2847. doi: 10.1200/JCO.2017.74.0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Michiels S., Terne N., Rotolo F. Statistical controversies in clinical research: prognostic gene signatures are not (yet) useful in clinical practice. Ann Oncol. 2016;27:2160–2167. doi: 10.1093/annonc/mdw307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sparano J.A., Gray R.J., Makower D.F., Pritchard K.I., Albain K.S. Prospective validation of a 21-gene expression assay in breast cancer. N Engl J Med. 2015 Nov 19;373(21):2005–2014. doi: 10.1056/NEJMoa1510764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cardoso F., van’t Veer L.J., Bogaerts J. 70-Gene signature as an aid to treatment decisions in early-stage breast cancer. N Engl J Med. 2016;375:717–729. doi: 10.1056/NEJMoa1602253. [DOI] [PubMed] [Google Scholar]
- 5.Sparano J.A., Gray R.J., Makower D.F., Pritchard K.I., Albain K.S., Hayes D.F. Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Engl J Med. 2018 Jul 12;379(2):111–121. doi: 10.1056/NEJMoa1804710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartlett JM, Bayani J, Marshall A, Dunn JA, Campbell A. Comparing breast cancer multiparameter tests in the OPTIMA prelim trial: No test is more equal than the others. J Natl Cancer Inst. 2016 Apr 29 doi: 10.1093/jnci/djw050. 108(9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Filipits M., Rudas M., Jakesz R. A new molecular predictor of distant recurrence in ER-positive, HER2-negative breast cancer adds independent information to conventional clinical risk factors. Clin Cancer Res. 2011 Sep 15:17–18. doi: 10.1158/1078-0432.CCR-11-0926. [DOI] [PubMed] [Google Scholar]
- 8.Dubsky P., Filipits M., Jakesz R., Rudas M., Singer C.F., Greil R. Austrian Breast and colorectal Cancer Study Group (ABCSG). EndoPredict improves the prognostic classification derived from common clinical guidelines in ER-positive, HER2-negative early breast cancer. Ann Oncol. 2013 Mar;24(3):640–647. doi: 10.1093/annonc/mds334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin M., Brase J.C., Ruiz A., Prat A., Kronenwett R., Calvo L. Prognostic ability of EndoPredict compared to research-based versions of the PAM50 risk of recurrence (ROR) scores in node-positive, estrogen receptor-positive, and HER2-negative breast cancer. A GEICAM/9906 sub-study. Breast Canc Res Treat. 2016 Feb;156(1):81–89. doi: 10.1007/s10549-016-3725-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sestak I., Martín M., Dubsky P., Kronenwett R., Rojo F., Cuzick J. Prediction of chemotherapy benefit by EndoPredict in patients with breast cancer who received adjuvant endocrine therapy plus chemotherapy or endocrine therapy alone. Breast Canc Res Treat. 2019 Jul;176(2):377–386. doi: 10.1007/s10549-019-05226-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Filipits M., Dubsky P., Rudas M., Greil R., Balic M., Bago-Horvath Z. Prediction of distant recurrence using EndoPredict among women with ER(+), HER2(-) node-positive and node-negative breast cancer treated with endocrine therapy only. Clin Cancer Res. 2019 Jul 1;25(13):3865–3872. doi: 10.1158/1078-0432.CCR-19-0376. [DOI] [PubMed] [Google Scholar]
- 12.Thangarajah F., Eichler C., Fromme J., Malter W., Caroline Radosa J., Ludwig S. The impact of EndoPredict (®) on decision making with increasing oncological work experience: can overtreatment be avoided? Arch Gynecol Obstet. 2019 May;299(5):1437–1442. doi: 10.1007/s00404-019-05097-w. [DOI] [PubMed] [Google Scholar]
- 13.Mokbel K., Wazir U., Wazir A., Kasem A., Mokbel K. The impact of EndoPredict clinical score on chemotherapy recommendations in women with invasive ER(+)/HER2(-) breast cancer stratified as having moderate or poor prognosis by nottingham prognostic Index. Anticancer Res. 2018 Aug;38(8):4747–4752. doi: 10.21873/anticanres.12782. [DOI] [PubMed] [Google Scholar]
- 14.Ettl J., Klein E., Hapfelmeier A., Grosse Lackmann K., Paepke S., Petry C. Decision impact and feasibility of different ASCO-recommended biomarkers in early breast cancer: prospective comparison of molecular marker EndoPredict and protein marker uPA/PAI-1. PLoS One. 2017 Sep 6;12(9) doi: 10.1371/journal.pone.0183917. e0183917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hequet D., Callens C., Gentien D., Albaud B., Mouret-Reynier M.A., Dubot C. Prospective, multicenter French study evaluating the clinical impact of the Breast Cancer Intrinsic Subtype-Prosigna® Test in the management of early-stage breast cancers. PLoS One. 2017 Oct 18;12(10) doi: 10.1371/journal.pone.0185753. e0185753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fallowfield L., Matthews L., May S., Jenkins V., Bloomfield D. Enhancing decision-making about adjuvant chemotherapy in early breast cancer following EndoPredict testing. Psycho Oncol. 2018 Apr;27(4):1264–1269. doi: 10.1002/pon.4664. [DOI] [PubMed] [Google Scholar]
- 17.Coates A.S., Winer E.P., Goldhirsch A., Gelber R.D., Gnant M., Piccart-Gebhart M., Thürlimann B., Senn H.J. Panel members. Tailoring therapies–improving the management of early breast cancer: st gallen international expert consensus on the primary therapy of early breast cancer 2015. Ann Oncol. 2015 Aug;26(8):1533–1546. doi: 10.1093/annonc/mdv221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Senkus E., Kyriakides S., Ohno S., Penault-Llorca F., Poortmans P., Rutgers E., Zackrisson S., Cardoso F., ESMO Guidelines Committee Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015 Sep:26. Suppl 5:v8-30. [Google Scholar]
- 19.Spielberger C.D., Gorsuch R.L., Lushene R.D., Vagg P.R., Jacobs G.A. Consulting Psychologists Press; Palo Alto: 1983. Manual for the state-trait anxiety inventory (STAI) CA, USA.Zolnierek, K. B. H., & DiMatteo, M. R. (2009). Physician communication and patient adherence to treatment: a meta-analysis. Medical care, 47(8), 826. [Google Scholar]
- 20.Dolbeault S., Bredart A., Mignot V., Hardy P., Gauvain-Piquard A., Mandereau L. Screening for psychological distress in two French cancer centers: feasibility and performance of the adapted distress thermometer. Palliat Support Care. 2008;6(02):107–117. doi: 10.1017/S1478951508000187. [DOI] [PubMed] [Google Scholar]
- 22.Kwiatkowski F., Girard M., Hacene K., Berlie J. Sem: a suitable statistical software adapted for research in oncology. Bull Cancer. 2000;87(10):715–721. [PubMed] [Google Scholar]
- 23.Buus R., Sestak I., Kronenwett R., Denkert C., Dubsky P., Krappmann K. Comparison of EndoPredict and EPclin with oncotype DX recurrence score for prediction of risk of distant recurrence after endocrine therapy. J Natl Cancer Inst. 2016 Jul 10;(11):108. doi: 10.1093/jnci/djw149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sestak I., Buus R., Cuzick J., Dubsky P., Kronenwett R., Denkert C. Comparison of the performance of 6 prognostic signatures for estrogen receptor-positive breast cancer: a secondary analysis of a randomized clinical trial. JAMA Oncol. 2018 Apr 1;4(4):545–553. doi: 10.1001/jamaoncol.2017.5524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Albanell J., Svedman C., Gligorov J., Holt S.D., Bertelli G., Blohmer J.U., Rouzier R,Lluch A., Eiermann W. Pooled analysis of prospective European studies assessing the impact of using the 21-gene Recurrence Score assay on clinical decision making in women with oestrogen receptor-positive, human epidermal growth factor receptor 2-negative early-stage breast cancer. Eur J Cancer. 2016 Oct;66:104–113. doi: 10.1016/j.ejca.2016.06.027. [DOI] [PubMed] [Google Scholar]
- 26.Duric V.M., Stockler M.R., Heritier S. Patients’ preferences for adjuvant chemotherapy in early breast cancer: what makes AC and CMF worthwhile now? Ann Oncol. 2005;16:1786–1794. doi: 10.1093/annonc/mdi370. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.