Abstract
Background
Dormant avascular micrometastases and single, or small groups of, non-proliferating cells are currently assumed to explain the multipeak dynamics of distant metastases (DM) following primary breast cancer surgical removal.
Methods
The hazard rate pattern for DM was analysed in 1518 premenopausal node-positive patients, enrolled in a series of randomized clinical trials on early breast cancer, which were carried out in Italy and Belgium. Patients underwent surgery alone (n = 397) or surgery plus adjuvant chemotherapy (n = 1121) and the minimal follow up was 15 years.
Results
The DM hazard rate for patients undergoing surgery alone displayed two early sharp peaks at 9 and 33 months, a wide intermediate one spanning from about 50 to 90 months and a late peak at 115–120 months. Adjuvant chemotherapy was associated with a prominent reduction of the two early peaks leaving a residual one at about 18 months and a reduction of the intermediate peak leaving two small peaks at about 50 and 80 months. The late peak remained unchanged.
Conclusions
Present results reveal the ability of adjuvant chemotherapy to reduce not only the rate of early relapses, but also the rate of intermediate relapses at about the sixth year of follow up. Adjuvant chemotherapy is not impacting on the development of metastases underlying the late peak detected at the tenth year. These findings suggest the existence of a previously unknown dormancy state that, at the primary tumour surgical removal, results in evolving chemo-sensitive metastatic processes, and, moreover, of a later chemo-refractory dormancy state.
Keywords: Breast cancer, Dormancy states, Surgery effects, Distant metastasis dynamics, Adjuvant chemotherapy effects
Statement of significance
-
•
Breast cancer removal wakes dormant micro foci causing peaks in recurrence dynamics.
-
•
Recurrence dynamics in premenopausal patients displays four peaks during 10 years.
-
•
Adjuvant chemo cuts peaks at years 1, 3, 6 while the peak at year 10 is unchanged.
-
•
Data support a multiplicity of chemo sensitive and chemo refractory dormant states.
Introduction
About 20 years ago, the concept that a continuous growth may explain breast cancer metastasis development during the sub-clinical phase following primary tumour surgical removal was established to be incompatible with clinical findings [1]. This discovery brought the “tumour dormancy” concept to the centre stage of clinical investigation. A further investigation of the recurrence dynamics in patients undergoing mastectomy without any added adjuvant treatment provided evidence that, following surgery, the recurrence risk displays peaks during the first three years and at the fifth year of follow-up [2]. These findings, in addition to computerized simulations, inspired a new paradigm of breast cancer metastatic development, involving the notions of tumour homeostasis, tumour quiescence in specific metastatic microscopic phases (single cells and foci lacking vascularization) and surgery-related acceleration of the metastatic process [3]. The involved biological model was later supported and refined by the analysis of recurrence dynamics for premenopausal and postmenopausal patients and for patients receiving adjuvant chemotherapy [3]. The proposed model, involving a statistical frailty concept [4], reasonably explained peak behaviour during the first four years after primary tumour removal for patients treated with surgery only or surgery plus adjuvant chemotherapy. Yet, the meaning of peaks occurring at later times received less attention. In particular, to explain the peak at about 5 years it was heuristically assumed that the metastatic pipeline from tumour cell seeding to metastasis clinical appearance is so long that it needs about 5 years, after the entrance spigot is turned off (primary removal), before it is depleted [5]. Conversely, the next less definite peaks were viewed as consequence of statistical fluctuations.
In the present analysis, which was carried out on a large case series from European historical clinical trials, we focused on the behaviour of such peaks in patients treated with or without adjuvant chemotherapy with the aim to explore their pattern and understand their possible biological meaning. To keep the heterogeneity of analysed data within suitable limits, we specifically focused on the premenopausal patients from these trials, who, when treated, received only similar adjuvant chemotherapy regimens.
Patients and methods
Patients
A series of randomized clinical trials on early breast cancer were carried out, after approval of an ethical committee, between 1973 and 1987 at the Istituto Nazionale dei Tumouri of Milan. In particular, investigations were focused on the effectiveness and optimal duration of adjuvant chemotherapy {Cyclophosphamide, Methotrexate, and Fluorouracil (CMF) ± Doxorubicin} for axillary node-positive patients who underwent primary tumour surgical removal by mastectomy or conservative surgery plus radiation therapy. Moreover, data from all patients undergoing mastectomy alone as primary treatment for operable breast cancer, who entered into different clinical trials since 1964, were extracted from the database of each individual trial, and compounded into a “historical untreated group” of patients receiving surgery alone without adjuvant chemo or endocrine therapy. Details of patients and treatments, following early reports on mentioned studies [2,[6], [7], [8]], have been repeatedly described. The long-term data of a three arm Belgian multicentre clinical trial [9] were investigated as well. The trial began in 1988 and compared two doses of Epirubicin plus Cyclophosphamide with the classical CMF regimen for node-positive patients who underwent primary tumour surgical removal by mastectomy or conservative surgery plus radiation therapy. In this trial, premenopausal patients received adjuvant chemotherapy only, while Tamoxifen was administered for 5 years only to postmenopausal patients with estrogen receptor (ER)-positive or ER-unknown tumours starting after the last cycle of chemotherapy. The study was approved by the ethics committee of the Institut Jules Bordet (Nr 2457, 23th of November 2017).
The pathological tumour size and axillary nodal status were available from clinical routine. Other prognostic factors such as grading and HER2 were not available for all series. ER status was systematically assessed (by biochemical method) for patients given adjuvant treatments while for patients undergoing surgery alone ER status was assessed “a posteriori” in a limited number of cases [10].
Data from premenopausal node-positive patients were extracted from these databases and grouped as follows:
-
•
Patients undergoing surgery only, who were obtained by merging the “historical untreated group” [2] and the untreated arm of the trial comparing adjuvant CMF to no further postoperative treatment [6].
-
•
Patients receiving six courses of adjuvant treatment (CMF ± Doxorubicin with different schedules) without any further systemic treatment, who were obtained from adjuvant chemotherapy studies [7,8].
-
•
Patients receiving six courses of adjuvant treatment (CMF, Cyclophosphamide + Epirubicin standard dose, Cyclophosphamide + Epirubicin higher dose), who were obtained from the Belgian trial [9].
Recurrence dynamics assessment
The objective of the current analysis was to compare the recurrence dynamics across these three groups, using distant metastasis (DM) occurrence as first event. Survival times were calculated as time elapsed since primary tumour removal to metastasis occurrence or to the last documented follow-up with no evidence of disease. Local-regional recurrences and second primary tumours, including contralateral breast cancers, and deaths without recurrence were considered as competing events and the corresponding survival times were censored at the time of their occurrence. The DM dynamics was studied by estimating with the life-table method the hazard rate for DM, i.e. the conditional probability of manifesting DM during a certain time span, given that the patient is clinically DM free at the beginning of the interval. A discretization of the time axis in six-month units was applied and a Kernel-like smoothing procedure [11] was adopted.
Results
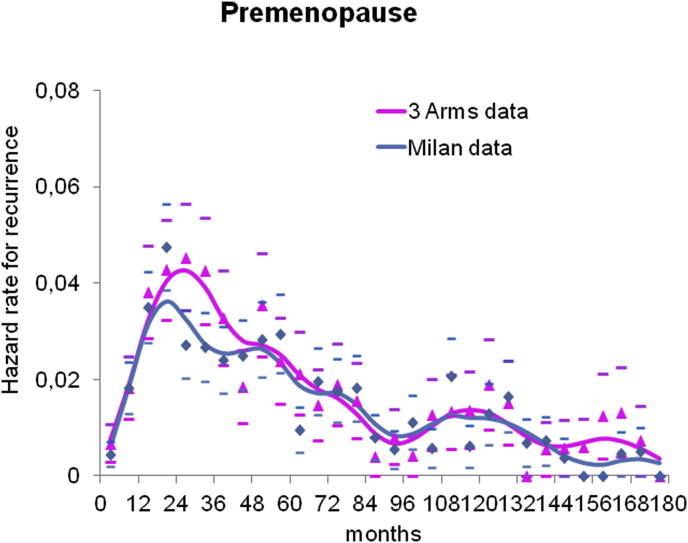
The main characteristics of the analysed premenopausal node-positive patients are reported in Table 1. When comparing the main clinical and pathological characteristics between the three groups of patients, we observed a higher rate of mastectomy in the untreated group, which may be explained by the historical dominance of mastectomy on conservative surgery in older trials. Despite the long time range during which the investigated clinical trials were carried out (about four decades), the distribution of pathologic tumour size and axillary nodal status are notably stable across the three groups. Moreover, in spite of the disparity of data source, the distribution of ER status does not display major changes among the three datasets. Given the similar distribution of the clinical and pathological characteristics and, mainly, the prominent coherence of DM dynamics regarding peak pattern behaviour between the treated groups from the Milan and Belgian trials (Fig. 1), we merged these two series for the remaining analyses.
Table 1.
Patient characteristics.
|
Characteristic |
Number of patients (%) |
||||
|---|---|---|---|---|---|
| Untreated n = 397 | CMF ± Dx 8 m n = 673 | CMF ± Epi 6 m n = 448 | Merged series n = 1121 | ||
| Type of surgery | |||||
| Mastectomy | 345 (87) | 289 (43) | 292 (65) | 581 (52) | |
| Conservative | 52 (13) | 293 (44) | 156 (35) | 449 (40) | |
| Other | – | 91 (13) | 91 (8) | ||
| pT, cm | |||||
| ≤ 2 | 168 (42) | 393 (58) | 186 (42) | 579 (51) | |
| 2-5 | 206 (52) | 280 (42)∗ | 165 (37) | 445 (40) | |
| > 5 | 23 (6) | – | 8 (1) | 8 (1) | |
| Unknown | – | – | 89 (20) | 89 (8) | |
| No. of positive nodes | |||||
| 1-3 | 272 (69) | 402 (60) | 271 (60) | 673 (60) | |
| ≥ 4 | 125 (31) | 271 (40) | 177 (40) | 448 (40) | |
| Patients with known ER status | 106 (27) | 620 (92) | 382 (85) | 1002 (89) | |
| Positive | 70 (66) | 470 (76) | 282 (74) | 752 (75) | |
| Negative | 36 (34) | 150 (24) | 100 (26) | 250 (25) | |
CMF = cyclophosphamide, methotrexate, and fluorouracil; Dx = Doxorubicin; Epi = Epirubicin; Tam = Tamoxifen; ∗ 2–5 cm and >5 cm cumulated; pT = pathologic tumour size; ER = Estrogen receptor.
Fig. 1.
Distant metastasis dynamics. The distant metastasis dynamics for premenopausal patients from Milan trials (673 patients) (blue) and the Belgian trial (448 patients) (pink) are reported. The curves reveal a prominent coherence of distant metastasis dynamics regarding peak pattern behaviour between patients from the Milan and Belgian trials. Cause-specific hazard rates were estimated within a six-month interval. Smoothed curves were obtained by a Kernel-like smoothing procedure. Standard deviation estimates for single points are also reported.
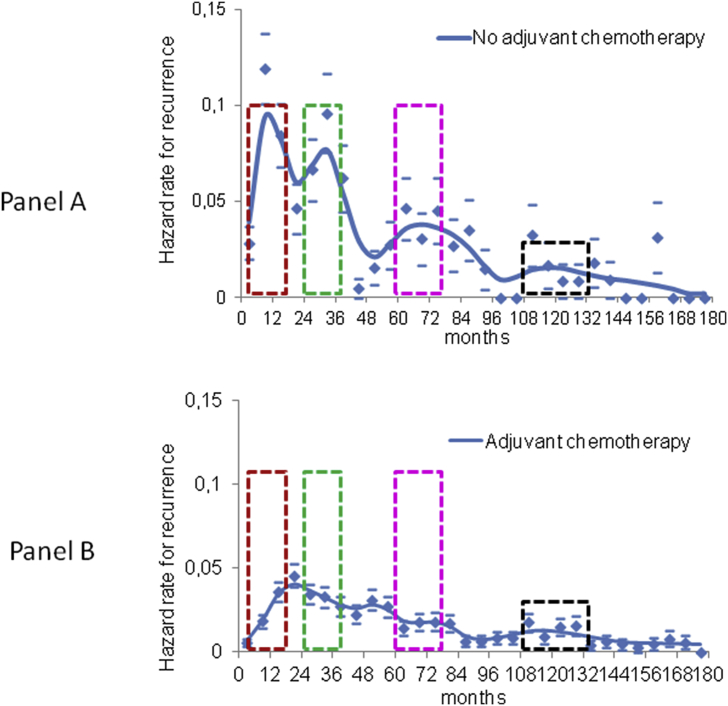
In Fig. 2, Panel A, we report the DM dynamics for patients undergoing primary tumour removal without any further systemic treatment. The hazard rate displays a multipeak pattern with two early sharp peaks at 9 and 33 months (early-1 and early-2 metastatic peaks), a wide intermediate peak spanning from about 50 to 90 months and reaching its top at about 70 months and, finally, a late peak at 115–120 months. Effects on DM dynamics associated with adjuvant chemotherapy administration are observable in Fig. 2, Panel B, where the early-1 and early-2 peaks are remarkably lower, leaving a residual peak in the middle region (about 18 months). The wide intermediate peak is lowered as well, leaving a residual couple of small peaks at about 50 and 80 months, while the late peak is unchanged. The comparison of DM dynamics in patients with or without adjuvant chemotherapy clearly indicates that the systemic treatment administration is associated with reductions of DM appearance at specified and temporally separate clusters occurring during the first, third and sixth year of follow-up, while the late one at about years ten-eleven is unchanged.
Fig. 2.
Distant metastasis dynamics changes related to adjuvant chemotherapy. Distant metastasis dynamics for 398 premenopausal patients undergoing primary tumour removal without any adjuvant systemic treatment (panel A) and for 1120 patients receiving adjuvant chemotherapy after primary tumour removal (panel B). The comparison between the two recurrence dynamics shows that the systemic treatment apparently acts on the clinical appearance of distant metastasis at specific and separate time clusters occurring during the first, third and sixth year of follow-up, while the late time cluster at about year ten does not change. The distant metastasis risk pattern is characterized by a sequence of metastasis appearance which are serially sensible (coloured dotted boxes) and refractory to administered adjuvant cytotoxic drugs. Cause-specific hazard rates were estimated within a six-month interval. Smoothed curves were obtained by a Kernel-like smoothing procedure. Standard deviation estimates for single points are also reported.
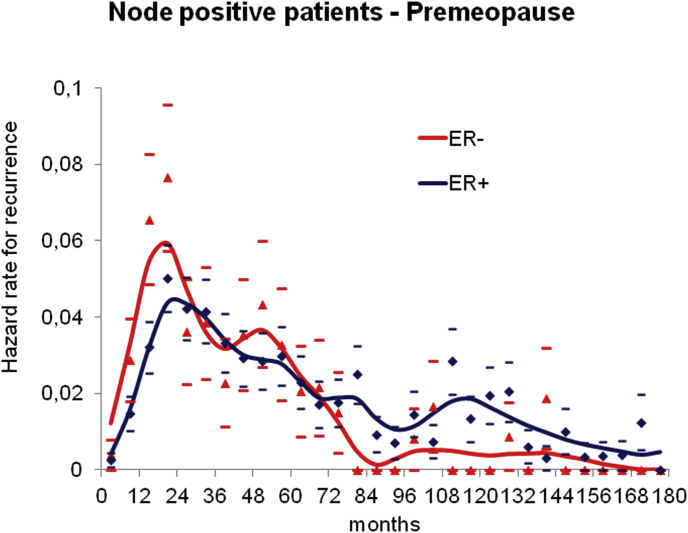
A separate analysis based on ER status was performed as well. Yet, as in ER-negative patients the DM events are mostly expected within the first five years, no major information on the late DM dynamics of ER-negative patients is obtainable in current case series (Fig. 3) and the late peak at about years ten-eleven is mostly related to ER-positive patients.
Fig. 3.
Distant metastasis dynamics by estrogen receptors. Distant metastasis dynamics for 752 ER-positive and 250 ER-negative premenopausal axillary node positive patients undergoing primary tumour removal and adjuvant systemic treatment. The DM dynamics is similar during the first 6 years with higher risk level for ER-negative patients, as expected. The comparison between the DM dynamics afterwards is not informative due to the insufficient number of patients at risk (133 patients) and events (8 events) for ER-negative patients in comparison with ER-positive patients (458 patients at risk and 77 events).
Discussion
The fact that recurrence risks remain high for more than 20 years after primary tumour surgical removal, even when patients were administered a few months of effective adjuvant chemotherapy and/or long-term hormone therapy, is an established finding [12,13], Yet, the statistical approach of present investigation adds the clinically relevant notion that this risk, in addition to being protracted, is also characterized by a structured pattern of quantitative levels, where risk peaks are spaced by lower, but not negligible, risk levels. Indeed, while meta-analysis allows more stable estimates across the heterogeneous case series involved, yet, it is also expected to introduce major bias on time patterns estimation. Therefore, in homogeneous case series, a continuous analysis on time according to a finer discretization is crucial for detecting hazard rate structures (according to the bias-variance trade-off principle of non-parametric estimation) [14].
About 15 years ago, we studied the recurrence risk reduction associated to adjuvant CMF administration during the first four years following mastectomy [3]. In the present investigation, the analysis is extended to later years, taking advantage from a larger series of patients, receiving similar adjuvant chemotherapy in the context of randomized clinical trials. The assessment of differential effects of adjuvant cytotoxic chemotherapy may be considered for revealing proliferative events occurring at the time of treatment administration associated to chemosensitivity, as a “pharmacological probe” of the underlying biological process of mestatatic development. Such reasoning was already applied in the above mentioned analysis on early recurrence dynamics for patients receiving adjuvant CMF, and revealed reduction of specific, temporally separate recurrence constellations at the first and third year [3]. These findings were considered coherent with a previously proposed model for breast cancer metastasis development, assuming tumour dormancy of microscopic metastases, due to homeostatic restraints from primary tumour. At least wo dormant states were considered: 1) single cells or nests containing a few cells, where most of them are non-dividing [15] and 2) micro-metastases of no more than the size of 1–2 mm without vascular support [16]. Following primary tumour surgical removal, a sudden acceleration of the metastatic process (tumour cell proliferation and/or recruitment of blood vessels by micrometastases [[17], [18], [19], [20]]) occurs. Recently, this effect has been directly evidenced in an experimental model system that definitively links surgery and the subsequent wound-healing response to the outgrowth of tumour cells at distant anatomical sites [21]. Therefore, effective cytotoxic chemotherapy targeting proliferating tumour cells, administered soon after the surgical treatment, is expected to reduce the recurrence rate at different, not consecutive times, as in fact occurs [3].
The results of the present investigation, while confirming previous ones, reveal the unexpected ability of adjuvant chemotherapy to also reduce the hazard rate level at the sixth year in node-positive premenopausal patients (Fig. 2). The observed reduction of the hazard rate level at the sixth year would correspond to a chemo-sensitive phase, while a chemo-refractory phase would underlie the late peak that does apparently not display major modifications. The former finding might be the result of direct chemotherapy cytotoxicity or even related to the induction of ovarian suppression in these premenopausal patients [22]. Anyway, whichever the biological process underlying this effect, it supports the concept that what was classified in the original version of the model as “single dormant cells” is likely to represent a few different metastatic biological conditions and dormancy states in a dynamic balance.
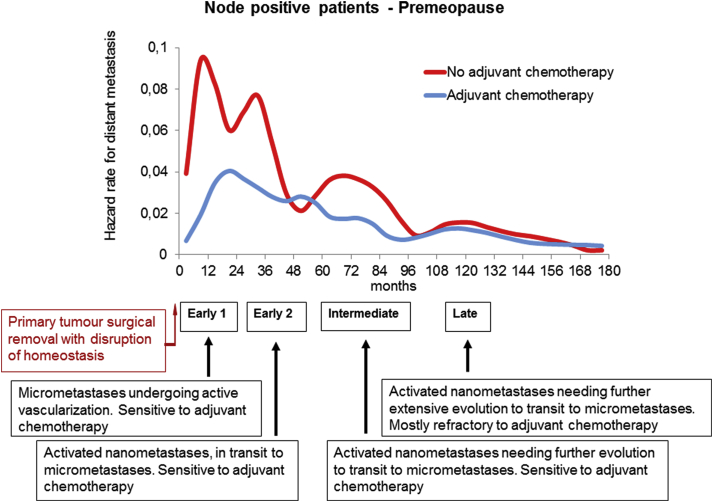
According to previous evidence [23], if the a-vascular foci (in the range of 105-106 cells) originating the early peak at the first year (early-1 peak) may be usefully called “micrometastases”, at a lower magnitude (putatively less than 103 cells), we could identify “nanometastases” related to biologic conditions originating the peaks at the third (early-2 peak), sixth (intermediate peak) and tenth (late peak) year. Since recurrence risk levels corresponding to metastases at third and sixth year are reduced when adjuvant cytotoxic chemotherapy is administered, the primary tumour removal should result in chemo-sensitive processes of the corresponding nanometastases. By contrast, the late peak at 10 years is unchanged, suggesting that the corresponding metastatic condition has a different biology. A possible outline of the extended model describing the metastatic process according to the new findings is reported in Fig. 4. This outline is a suggestion, as the knowledge of the biologic mechanisms underlying the multiplicity of nanometastatic dormancy are unknown. A range of possibilities may be assumed, such as a series of biologic steps to be followed in sequence by a neoplastic focus, or even the hypothesis of foci with different outcome evolving in parallel, or even the presence of reciprocal conditioning actions that determine the temporal evolution of the metastatic process. Only oriented investigations will be able to shed some light on this question, starting from the premise, however, that the investigated biology must explain the DM dynamics here observed.
Fig. 4.
Outline of the time extended model describing the metastatic process. Findings of present analysis are compatible with the presence of one micro-metastatic state (corresponding to the early-1 peak) and three different nano-metastatic states (corresponding to the early-2, intermediate and late peaks) that are under the effect of the homeostatic control from the primary tumour. Primary tumour removal disrupts the homeostasis and enhances transitions between dormancy states. If surgery induced transitions are chemo sensitive, the corresponding peaks will be dampened by adjuvant chemotherapy. On the base of such picture, the pattern of microscopic developing metastases may be described as follows: micrometastases undergoing surgery-driven active vascularization (sensitive to adjuvant chemotherapy); activated nanometastases, in transit to micrometastases (sensitive to adjuvant chemotherapy); activated nanometastases needing further evolution to transit to micrometastases (sensitive to adjuvant chemotherapy); activated nanometastases needing further extensive evolution to transit to micrometastases (mostly refractory to adjuvant chemotherapy).
The time extended model retains the core assumptions of the former one [3], exhibits internal coherence and, moreover, is in keeping with the evolving picture of the metastatic process. Indeed, the idea that the first step of the metastatic process is limited to a relocation within foreign tissues of single tumour cells from primary tumour, during the late phases of its development [24], is overly simplistic and is making way to a much more complex picture. A few traits are emerging, both at the cancer cell level (e.g. the policlonality of metastatic foci [25]) and at homing processes, which are well compatible with the here suggested multiplicity of nanometastases states. Indeed, the metastatic process implies the establishment of a favourable microenvironment in the seeded organ, the so-called metastatic niche [26,27]. Remarkably, niches may provide favourable conditions for tumour dormancy [28] within stable tissues, the so-called “sleepy niche” [29]. Therefore, early metastatic foci, the biology of which involves both cancer cells and niche cells [30], may display, even at nanometastatic level, a series of developmental processes correlated to stroma cells as well as to tumour cells, a few of which are apparently chemosensitive.
A few clinical considerations are suggested by present results. First of all, all past analyses on recurrence dynamics failed to identify specific tumour or host traits distinctive of patients recurring at different early risk peaks. Therefore, extending this concept to later peaks at least until opposing evidences, at present late recurring patients cannot be identified in advance. Present investigation on patients given relatively “old” adjuvant chemotherapies could have underestimated the possible long-lasting impact of current more effective treatment approaches [31]. However, it should be acknowledged that their influence on late recurrence risk is nearly unknown, mainly due to insufficient follow-up time [13]. Regarding hormone therapy, adjuvant Tamoxifen proved to reach similar therapeutic quantitative results, in comparison with untreated patients, as chemotherapy [32], and subsequent development of other endocrine therapies (luteinizing hormone-releasing hormone agonists, aromatase inhibitors and estrogen receptor antagonists) improved average 5-year DFS by about 3–5% [33,34], although a few subset of patients could benefit more [35]. Moreover, hormone treatment activity apparently relies on biological mechanisms mainly effective during early follow up times, while it is weaker (less than 4%), during subsequent administrations [[36], [37], [38]].
In our opinion, it is difficult to devise treatments focused on late recurrences due to the dramatic scarcity of knowledge about the tumour biology during a quite short time interval following primary removal. Events occurring in this time have a crucial influence on the disease course through at least a decade and interplay with other host factors (e.g. body adiposity [39]) mainly at late times. Therefore, no targeted treatment to be administered during late follow-up, can be supported by objective findings. However, future investigations on the dynamic biological conditions at nanometastatic level here evidenced, could result in new therapeutic approaches addressing late unfavourable events.
Conclusions
By means of data from premenopausal breast cancer patients treated in randomized clinical trials, we provided evidence that adjuvant chemotherapy is not only able to reduce the rate of early relapses, but also the rate of intermediate relapses at about the sixth year of follow up. Future studies are needed to evaluate whether similar observations can also be done in patients treated with adjuvant endocrine treatment.
These results suggest that the notion of solitary cancer cell dormancy turns out to be inadequate to explain late patterns of DM dynamics and may be usefully converted into the notion of dormant nanometastases. The multiplicity of dormancy states, revealed by the behaviour of the DM dynamics under adjuvant chemotherapy, is a clue of the complexity of such bio-system, with clinical consequences depending on (micro)environmental stimuli.
Despite the limit of analysing premenopausal patients who received adjuvant chemotherapy only and not adjuvant endocrine therapies, the time extended model suggests that the possibility of interfering with the metastatic process is wider than previously believed. Once again, tumour dormancy mechanisms knowledge emerges as a crucial condition to identify breast cancer treatments better than those acquired along present research lines.
Declarations
Ethics approval and consent to participate: All studies supplying the analysed databases were approved by the institutional ethics committees and review boards in accordance with the Declaration of Helsinki.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Romano Demicheli: Conceptualization, Formal analysis, Writing - original draft, Writing - review & editing. Christine Desmedt: Resources, Writing - review & editing. Mike Retsky: Writing - review & editing. Christos Sotiriou: Resources, Writing - review & editing. Martine Piccart: Resources, Writing - review & editing. Elia Biganzoli: Resources, Formal analysis, Writing - original draft, Writing - review & editing.
Declaration of competing interest
The authors declare that they have no competing interests.
Acknowledgements
We acknowledge the patients and investigators from the Milan clinical trials and from the Belgian 3-arms clinical trial, whose human experience and professional commitment underlay the neutral data we analysed.
Contributor Information
Romano Demicheli, Email: romano.demicheli@guest.unimi.it.
Christine Desmedt, Email: christine.desmedt@kuleuven.be.
Mike Retsky, Email: micheal.retsky@gmail.com.
Christos Sotiriou, Email: christos.sotiriou@bordet.be.
Martine Piccart, Email: martine.piccart@bordet.be.
Elia Biganzoli, Email: elia.biganzoli@unimi.it.
References
- 1.Demicheli R., Terenziani M., Valagussa P., Moliterni A., Zambetti, Bonadonna G. Local recurrences following mastectomy: support for the concept of tumor dormancy. J Natl Cancer Inst. 1994;86:45–48. doi: 10.1093/jnci/86.1.45. [DOI] [PubMed] [Google Scholar]
- 2.Demicheli R., Abbattista A., Miceli R., Valagussa P., Bonadonna G. Time distribution of the recurrence risk for breast cancer patients undergoing mastectomy: further support about the concept of tumor dormancy. Breast Canc Res Treat. 1996;41:177–185. doi: 10.1007/BF01807163. [DOI] [PubMed] [Google Scholar]
- 3.Demicheli R., Retsky M.W., Hrushesky W.J.M., Baum M. Tumor dormancy and surgery-driven dormancy interruption in breast cancer: learning from failures. Nat Clin Pract Oncol. 2007;4:699–710. doi: 10.1038/ncponc0999. [DOI] [PubMed] [Google Scholar]
- 4.Rancoita P.M., Valberg M., Demicheli R., Biganzoli E., Di Serio C. Tumor dormancy and frailty models: a novel approach. Biometrics. 2017;73:260–270. doi: 10.1111/biom.12559. Epub 2016 Jul 11. [DOI] [PubMed] [Google Scholar]
- 5.Retsky M., Demicheli R., Hrushesky W., Baum M., Gukas I. Surgery triggers outgrowth of latent distant disease in breast cancer: an inconvenient truth? Cancers. 2010;2:305–337. doi: 10.3390/cancers2020305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonadonna G., Brusamolino E., Valagussa P., Rossi A., Brugnatelli L., Brambilla C. Combination chemotherapy as an adjuvant treatment in operable breast cancer. N Engl J Med. 1976;294:405–410. doi: 10.1056/NEJM197602192940801. [DOI] [PubMed] [Google Scholar]
- 7.Moliterni A., Bonadonna G., Valagussa P., Ferrari L., Zambetti M. Cyclophosphamide, Methotrexate, and Fluorouracil with and without doxorubicin in the adjuvant treatment of resectable breast cancer with one to three positive axillary nodes. J Clin Oncol. 1991;9:1124–1130. doi: 10.1200/JCO.1991.9.7.1124. [DOI] [PubMed] [Google Scholar]
- 8.Buzzoni R., Bonadonna G., Valagussa P., Zambetti M. Adjuvant chemotherapy with doxorubicin plus Cyclophosphamide, Methotrexate, and Fluorouracil in the treatment of resectable breast cancer with more than three positive axillary nodes. J Clin Oncol. 1991;9:2134–2140. doi: 10.1200/JCO.1991.9.12.2134. [DOI] [PubMed] [Google Scholar]
- 9.Piccart M.J., Di Leo A., Beauduin M., Vindevoghel A., Michel J., Focan C. Phase III trial comparing two dose levels of Epirubicin combined with Cyclophosphamide with Cyclophosphamide, Methotrexate, and Fluorouracil in node-positive breast cancer. J Clin Oncol. 2001;19:3103–3110. doi: 10.1200/JCO.2001.19.12.3103. [DOI] [PubMed] [Google Scholar]
- 10.Bonadonna G., Moliterni A., Zambetti M., Daidone M.G., Pilotti S., Gianni L. 30 years’ follow up of randomized studies of adjuvant CMF in operable breast cancer: cohort study. BMJ. 2005;330(7485):217. doi: 10.1136/bmj.38314.622095.8F. Epub 2005 Jan 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramlau-Hansen Smoothing counting process intensities by means of Kernel functions. Ann Stat. 1983;11:453–466. [Google Scholar]
- 12.Pan H., Gray R., Braybrooke J., Davies C., Taylor C., McGale P. 20-Year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N Engl J Med. 2017;377:1836–1846. doi: 10.1056/NEJMoa1701830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colleoni M., Sun Z., Price K.N., Karlsson P., Forbes J.F., Thürlimann B. Annual hazard rates of recurrence for breast cancer during 24 Years of follow-up: results from the international breast cancer study group trials I to V. J Clin Oncol. 2016;34:927–935. doi: 10.1200/JCO.2015.62.3504. Epub 2016 Jan 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Demicheli R., Desmedt C., Piccart M., Biganzoli E. Tumor dormancy at bedside: a late awakening. Breast. 2019;45:61–63. doi: 10.1016/j.breast.2019.03.001. Epub 2019 Mar 2. [DOI] [PubMed] [Google Scholar]
- 15.Naumov G.N., MacDonald I.C., Weinmeister P.M., Kerkvliet N., Nadkarni K.V., Wilson S.M. Persistence of solitary mammary carcinoma cells in a secondary site: a possible contributor to dormancy. Canc Res. 2002;62:2162–2168. [PubMed] [Google Scholar]
- 16.Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst. 1990;82:4–6. doi: 10.1093/jnci/82.1.4. [DOI] [PubMed] [Google Scholar]
- 17.Demicheli R., Retsky M.W., Hrushesky W.J.M., Baum M., Gukas I.D. The effects of surgery on tumor growth: a century of investigations. Ann Oncol. 2008;19:1821–1828. doi: 10.1093/annonc/mdn386. Epub 2008 Jun 10. [DOI] [PubMed] [Google Scholar]
- 18.Gunduz N., Fisher B., Saffer E.A. Effect of surgical removal on the growth and kinetics of residual tumor. Canc Res. 1979;39:3861–3865. [PubMed] [Google Scholar]
- 19.Holmgren L., O’Reilly M.S., Folkman J. Dormancy of micrometastases: balanced proliferation and apoptosis in the presence of angiogenesis suppression. Nat Med. 1995;1:149–153. doi: 10.1038/nm0295-149. [DOI] [PubMed] [Google Scholar]
- 20.Hiller J.G., Perry N.J., Poulogiannis G., Riedel B., Sloan E.K. Perioperative events influence cancer recurrence risk after surgery. Nat Rev Clin Oncol. 2018;15:205–218. doi: 10.1038/nrclinonc.2017.194. Epub 2017 Dec 28. [DOI] [PubMed] [Google Scholar]
- 21.Krall J.A., Reinhardt F., Mercury O.A., Pattabiraman D.R., Brooks M.W., Dougan M. The systemic response to surgery triggers the outgrowth of distant immune-controlled tumors in mouse models of dormancy. Sci Transl Med. 2018;10(436) doi: 10.1126/scitranslmed.aan3464. pii: eaan3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Swain S.M., Jeong J.H., Geyer C.E., Jr., Costantino J.P., Pajon E.R., Fehrenbacher L. Longer therapy, iatrogenic amenorrhea, and survival in early breast cancer. N Engl J Med. 2010;362:2053–2065. doi: 10.1056/NEJMoa0909638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Demicheli R., Fornili M., Querzoli P., Pedriali M., Alberti S., Desmedt C. Microscopic tumor foci in axillary lymph nodes may reveal the recurrence dynamics of breast cancer. Canc Commun. 2019;39:35. doi: 10.1186/s40880-019-0381-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valastyan S., Weinberg R.A. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147:275–292. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hunter K.W., Amin R., Deasy S., Ha N.H., Wakefield L. Genetic insights into the morass of metastatic heterogeneity. Nat Rev Canc. 2018;18:211–223. doi: 10.1038/nrc.2017.126. Epub 2018 Feb 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Psaila B., Lyden D. The metastatic niche: adapting the foreign soil. Nat Rev Canc. 2009;9(4):285–293. doi: 10.1038/nrc2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaplan R.N., Riba R.D., Zacharoulis S., Bramley A.H., Vincent L., Costa C. VEGFR1-positive hematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sosa M.S., Bragado P., Aguirre-Ghiso J.A. Mechanisms of disseminated cancer cell dormancy: an awakening field. Nat Rev Canc. 2014;14:611–622. doi: 10.1038/nrc3793. Epub 2014 Aug 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peinado H., Zhang H., Matei I.R., Costa-Silva B., Hoshino A., Rodrigues G. Pre-metastatic niches: organ-specific homes for metastases. Nat Rev Canc. 2017;17:302–317. doi: 10.1038/nrc.2017.6. Epub 2017 Mar 17. [DOI] [PubMed] [Google Scholar]
- 30.Leyh B., Dittmer A., Lange T., Martens J.W., Dittmer J. Stromal cells promote anti-estrogen resistance of breast cancer cells through an insulin-like growth factor binding protein 5 (IGFBP5)/B-cell leukemia/lymphoma 3 (Bcl-3) axis. Oncotarget. 2015;6:39307–39328. doi: 10.18632/oncotarget.5624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cossetti R.J., Tyldesley S.K., Speers C.H., Zheng Y., Gelmon K.A. Comparison of breast cancer recurrence and outcome patterns between patients treated from 1986 to 1992 and from 2004 to 2008. J Clin Oncol. 2015;33:65–73. doi: 10.1200/JCO.2014.57.2461. Epub 2014 Nov 24. [DOI] [PubMed] [Google Scholar]
- 32.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomized trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 33.Pagani O., Regan M.M., Walley B.A., Fleming G.F., Colleoni M., Láng I. Adjuvant exemestane with ovarian suppression in premenopausal breast cancer. N Engl J Med. 2014;371:107–118. doi: 10.1056/NEJMoa1404037. Epub 2014 Jun 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Francis P.A., Pagani O., Fleming G.F., Walley B.A., Colleoni M., Láng I., Gómez H.L. Tailoring adjuvant endocrine therapy for premenopausal breast cancer. N Engl J Med. 2018;379:122–137. doi: 10.1056/NEJMoa1803164. Epub 2018 Jun 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pagani O., Francis P.A., Fleming G.F., Walley B.A., Viale G., Colleoni M. Absolute improvements in freedom from distant recurrence to tailor adjuvant endocrine therapies for premenopausal women: results from TEXT and SOFT. J Clin Oncol. 2019:JCO1801967. doi: 10.1200/JCO.18.01967. 10.1200/JCO.18.01967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Al-Mubarak M., Tibau A., Templeton A.J., Cescon D.W., Ocana A., Seruga B. Extended adjuvant Tamoxifen for early breast cancer: a meta-analysis. PloS One. 2014;9 doi: 10.1371/journal.pone.0088238. 10.1371/journal.pone.0088238.eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruhstaller T., Giobbie-Hurder A., Colleoni M., Jensen M.B., Ejlertsen B., de Azambuja E. Adjuvant letrozole and tamoxifen alone or sequentially for postmenopausal women with hormone receptor-positive breast cancer: long-term follow-up of the BIG 1-98 trial. J Clin Oncol. 2019;37:105–114. doi: 10.1200/JCO.18.00440. Epub 2018 Nov 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burstein H.J., Lacchetti C., Anderson H., Buchholz T.A., Davidson N.E., Gelmon K.A. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: ASCO clinical practice guideline focused update. J Clin Oncol. 2019;37:423–438. doi: 10.1200/JCO.18.01160. Epub 2018 Nov 19. [DOI] [PubMed] [Google Scholar]
- 39.Biganzoli E., Desmedt C., Fornili M., de Azambuja E., Cornez N., Ries F. Recurrence dynamics of breast cancer according to baseline body mass index. Eur J Canc. 2017;87:10–20. doi: 10.1016/j.ejca.2017.10.007. Epub 2017 Oct 31. [DOI] [PubMed] [Google Scholar]