Abstract
Background
Evidence on how weight loss correlates to health-related quality-of-life (HRQOL) among obese breast cancer (BC) patients is limited. We aimed to evaluate associations between weight changes and HRQOL.
Methods
We included 993 obese women with stage I-II-III BC from CANTO, a multicenter, prospective cohort collecting longitudinal, objectively-assessed anthropometric measures and HRQOL data (NCT01993498). Associations between weight changes (±5% between diagnosis and post-treatment [shortly after completion of surgery, adjuvant chemo- or radiation-therapy]) and patient-reported HRQOL (EORTC QLQ-C30/B23) were comprehensively evaluated. Changes in HRQOL and odds of severely impaired HRQOL were assessed using multivariable generalized estimating equations and logistic regression, respectively.
Results
14.1% women gained weight, 67.3% remained stable and 18.6% lost weight. Significant decreases in functional status and exacerbation of symptoms were observed overall post-treatment. Compared to gaining weight or remaining stable, obese women who lost weight experienced less of a decline in HRQOL, reporting better physical function (mean change [95%CI] for gain, stability and loss: −12.9 [-16.5,-9.3], −6.9 [-8.2,-5.5] and −6.2 [-8.7,-3.7]; pinteraction[weight-change-by-time] = 0.006), less dyspnea (+18.9 [+12.3,+25.6], +9.2 [+6.5,+11.9] and +3.2 [-1.0,+7.3]; pinteraction = 0.0003), and fewer breast symptoms (+22.1 [+16.8,+27.3], +18.0 [+15.7,+20.3] and +13.4 [+9.0,+17.2]; pinteraction = 0.044). Weight loss was also significantly associated with reduced odds of severe pain compared with weight gain (OR [95%CI] = 0.51 [0.31–0.86], p = 0.011) or stability (OR [95%CI] = 0.62 [0.41–0.95], p = 0.029). No associations between weight loss and worsening of other physical or psychosocial parameters were found.
Conclusions
This large contemporary study suggests that weight loss among obese BC patients during early survivorship was associated with better patient-reported outcomes, without evidence of worsened functionality or symptomatology in any domain of HRQOL.
Keywords: Breast cancer, Survivorship, Obesity, Weight change, Weight loss, Health-related quality-of-life, Patient-reported outcomes
Highlights
-
•
One-in-five women was obese at diagnosis of early breast cancer.
-
•
Patient-reported quality of life significantly worsened after breast cancer treatment.
-
•
Weight changes were associated to changes in quality of life post-treatment.
-
•
Weight loss was not associated with worse functionality or symptomatology.
1. Introduction
Obesity is a risk factor for a number of chronic diseases, including diabetes and cardiovascular disease, as well as for early mortality [1]. Several physical, psychological, and social dimensions of health that contribute to the self-perception of quality of life (QOL), referred to as health-related (HR)-QOL, are also negatively impacted by obesity [2].
Obesity has increasingly become recognized as a risk factor also for cancer and as a prognostic factor for individuals diagnosed with early-stage malignancies. Compelling evidence points at the strong link between obesity and breast cancer: excess weight represents a risk factor for postmenopausal breast cancer [3], increases the risk of recurrent and second primary breast cancer [4] and that of overall and breast cancer-specific mortality [5]. Obesity may also interfere with adequate breast cancer treatment delivery [6], exacerbating toxicities and burdening healthcare costs [7]. As the prevalence of obese has risen around the world, the number of obese patients with breast cancer has dramatically increased [8].
Weight changes occurring after diagnosis of early breast cancer are common and post-treatment weight gain is associated with poor HRQOL, body image issues and psychological distress, further aggravating the deterioration of HRQOL that is frequently caused by cancer treatment [9]. Randomized clinical trials reported on some benefits of weight loss on physical function and fitness level of obese individuals [10,11]. Nevertheless, such trials did not address the impact of weight loss on overall HRQOL during or shortly after cancer treatment, and often focused on selected patient groups (e.g. postmenopausal women). In addition, recent large systematic reviews among the general population remarked that studies on obesity, weight loss, and HRQOL have been very heterogeneous in terms of design, study population, and HRQOL assessment [12,13], recommending that research should focus on prospective studies with high retention rates and carefully-chosen HRQOL measures [14].
Because only limited evidence suggests that weight loss in obese breast cancer patients consistently improves HRQOL [14], we aimed to evaluate if weight changes occurring between breast cancer diagnosis and shortly after primary treatment completion are associated with HRQOL. To do so, we used CANTO, a large, contemporary, prospective clinical study of breast cancer survivors that accesses extensive and longitudinal information, including comprehensive serial assessments of patient-reported outcomes (PROs) [15].
2. Methods
CANTO (CANcer TOxicities, ClinicalTrials.gov/NCT01993498) enrolled patients with stage I-II-III breast cancer [16] across 26 French centers. For this sub study, we used data collected at diagnosis (baseline) and during the first visit after primary treatment completion, defined as completion of definitive breast surgery, adjuvant chemotherapy or radiotherapy, as appropriate (adjuvant endocrine therapy and anti-human epidermal growth-factor receptor (HER)-2 therapy were allowed to be ongoing). Study protocol was approved by a central ethical committee for human subjects. Informed consent was obtained at patient inclusion. Details about the CANTO study procedures were previously published [15].
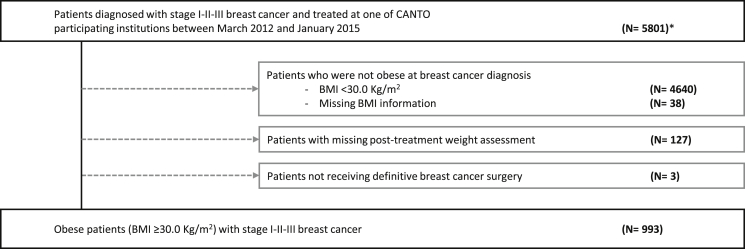
We accessed information from 5801 women diagnosed from 2012 to 2015. We excluded patients who were not obese at diagnosis (Body Mass Index [BMI]<30.0 kg/m2 [N = 4640] or missing [N = 38]), 127 patients with missing post-treatment weight reassessment, and three patients not treated with curative intent (Fig. 1).
Fig. 1.
CONSORT diagram of patient population. ∗Note: total accrual in CANTO 12012 patients. We accessed information from 5801 women who were enrolled from March 2012 to January 2015.
Outcome variables. We assessed PROs using the European Organisation for Research and Treatment of Cancer (EORTC) quality-of-life-questionnaires QLQ-C30 and QLQ-BR23. The EORTC QLQ-C30 is a 30-item questionnaire that includes a) a global health status subscale, b) five multi-item functional subscales for physical, emotional, social, cognitive, and role functioning, c) three multi-item symptom scales for fatigue, pain, and nausea/vomiting, and d) six single-item symptom scales assessing other cancer-related symptoms including sleep disturbance, dyspnea, appetite loss, constipation, diarrhea, and financial difficulties. The EORTC QLQ-BR23 is the breast cancer-specific companion module to the EORTC QLQ-C30 and consists of 23 items that include a) four functional scales for body image, sexual functioning, sexual enjoyment, and future perspective, and b) four symptom scales for systemic side-effects, breast symptoms, arm symptoms, and upset by hair loss. Questionnaires include two 7-point Likert scale items with response ranging from ‘very poor’ to ‘excellent’ for global health, and 4-point Likert scale items with possible responses of ‘not at all’, ‘a little’, ‘quite a bit, and ‘very much’ for functioning and symptoms. A standard scoring algorithm was used to convert responses to all items to a 0–100 scale. For global health and functional scales, higher scores reflect a better level of QOL and function, whereas for symptom scales higher scores reflect greater symptom severity compared with lower scores. A validated French version of EORTC QLQ-C30 and QLQ-B23 was used in CANTO [15,[17], [18], [19], [20]] PROs were comprehensively modeled: (1) as continuous; and dichotomizing HRQOL scores by clinical severity, namely (2) HRQOL deterioration from baseline to post-treatment (change ≥ 10 points on global, functional or symptoms scales); (3) prevalence of patients scoring <60 on global/functional or ≥40 on symptoms scales, which defined ‘poor functions’ and ‘severe symptoms’, respectively; and (4) prevalence of patients transitioning from non-poor function or non-severe symptom at baseline to reporting so post-treatment. All cut-offs were based on previously validated thresholds defining a change as at least “moderate” [21] or considered to define problems of substantial clinical relevance [22] from the patient’s perspective.
Independent variables. Weight change between baseline and post-treatment was defined as weight gain ≥5%, stable weight within ±5%, and weight loss ≥5%. These cut-offs were based on evidence that a weight change as low as 5% of baseline can be clinically meaningful, including being associated with cardiovascular and metabolic disease risk factors and outcomes [[23], [24], [25], [26]].
Covariates. These included clinical variables, socioeconomic status, psychological variables (as per the Hospital Anxiety and Depression Scale [27]), health behaviors (including physical activity as per Global Physical Activity Questionnaire-16 [28]), and type of breast cancer treatment received. Variables were categorized as per Table 1.
Table 1.
Baseline cohort characteristics.
| Characteristic, N (%) | Overall | By weight change | |||
| Gain ≥5% | Stable ±5% | Loss ≥5% | p-value^ | ||
| 993 (100) | 140 (14.1) | 668 (67.3) | 185 (18.6) | ||
| Baseline BMI, kg/m2 | |||||
| Mean (SD) | 34.5 (4.1) | 34.4 (3.8) | 34.4 (3.9) | 35.2 (4.7) | 0.185 |
| Missing | – | – | – | – | |
| Baseline BMI, WHO categories53 | |||||
| Obese class I | 640 (64.4) | 92 (65.7) | 439 (65.7) | 109 (58.9) | 0.2140 |
| Obese class II | 248 (25.0) | 34 (24.3) | 169 (25.3) | 45 (24.3) | |
| Obese class III | 105 (10.6) | 14 (10.0) | 60 (9.0) | 31 (16.8) | |
| Missing | – | – | – | – | |
| Baseline weight, continuous, kg | |||||
| Mean (SD) | 89.4 (12.8) | 89.0 (12.1) | 88.9 (12.7) | 91.5 (13.2) | 0.099 |
| Missing | – | – | – | – | |
| Age at diagnosis, years | |||||
| Mean (SD) | 59.1 (10.5) | 54.2 (10.8) | 60.1 (10.5) | 59.3 (9.2) | <.0001 |
| Missing | – | – | – | – | |
| Age at diagnosis, years | |||||
| < 50 | 194 (19.5) | 44 (31.4) | 118 (17.7) | 32 (17.3) | <.0001 |
| 50-64 | 471 (47.4) | 72 (51.4) | 302 (45.2) | 97 (52.4) | |
| ≥65 | 328 (33.1) | 24 (17.2) | 248 (37.1) | 56 (30.3) | |
| Missing | – | – | – | – | |
| Marital status | |||||
| In a relationship | 687 (74.9) | 107 (83.6) | 463 (74.4) | 117 (70.1) | 0.325 |
| Not in a relationship∗ | 230 (25.1) | 21 (16.4) | 159 (25.6) | 50 (29.9) | |
| Missing | 76 | 12 | 46 | 18 | |
| Highest education level achieved | |||||
| Primary or lower | 256 (27.9) | 34 (25.6) | 180 (29.0) | 42 (24.8) | 0.176 |
| High school | 451 (49.1) | 67 (52.3) | 308 (49.6) | 76 (45.0) | |
| College graduate or higher | 211 (23.0) | 27 (21.1) | 133 (21.4) | 51 (30.2) | |
| Missing | 75 | 12 | 47 | 16 | |
| Employment status | |||||
| Professionally active | 379 (40.3) | 72 (55.4) | 245 (38.4) | 62 (36.1) | 0.019 |
| Professionally inactive | 561 (59.7) | 58 (44.6) | 393 (61.6) | 110 (63.9) | |
| Missing | 53 | 10 | 30 | 13 | |
| Monthly total householdincome | |||||
| <1500 Euro | 185 (21.0) | 27 (22.5) | 124 (20.8) | 34 (20.7) | 0.579 |
| 1500–3000 Euro | 421 (47.8) | 55 (45.8) | 294 (49.3) | 72 (43.9) | |
| ≥3000 Euro | 274 (31.1) | 38 (31.7) | 178 (29.9) | 58 (35.4) | |
| Missing | 113 | 20 | 72 | 21 | |
| Menopausal status | |||||
| Premenopausal | 249 (25.6) | 55 (40.4) | 151 (23.1) | 43 (23.6) | 0.001 |
| Postmenopausal | 724 (74.4) | 81 (59.6) | 504 (76.9) | 139 (76.4) | |
| Missing | 20 | 4 | 13 | 3 | |
| Charlson comorbidity index | |||||
| 0 | 614 (68.9) | 91 (71.6) | 410 (68.4) | 113 (68.5) | 0.594 |
| 1+ | 277 (31.1) | 36 (28.4) | 189 (31.5) | 52 (31.5) | |
| Missing | 102 | 13 | 69 | 20 | |
| Anxiety, score | |||||
| Mean (SD) | 8.8 (4.2) | 8.6 (4.1) | 8.9 (4.4) | 8.6 (3.9) | 0.644 |
| Missing | 62 | 11 | 40 | 11 | |
| Anxiety, categorical | |||||
| Non-case | 387 (41.6) | 50 (38.8) | 269 (42.8) | 68 (39.1) | 0.769 |
| Doubtful case | 246 (26.4) | 41 (31.8) | 153 (24.4) | 52 (29.9) | |
| Case | 298 (32.0) | 38 (29.4) | 206 (32.8) | 54 (31.0) | |
| Missing | 62 | 11 | 40 | 11 | |
| Depression, score | |||||
| Mean (SD) | 4.5 (3.7) | 4.4 (3.6) | 4.4 (3.6) | 4.7 (3.9) | 0.775 |
| Missing | 62 | 11 | 40 | 11 | |
| Depression, categorical | |||||
| Non-case | 736 (79.0) | 97 (75.2) | 504 (80.2) | 135 (77.6) | 0.202 |
| Doubtful case | 131 (14.1) | 25 (19.4) | 82 (13.1) | 24 (13.8) | |
| Case | 64 (6.9) | 7 (5.4) | 42 (6.7) | 15 (8.6) | |
| Missing | 62 | 11 | 40 | 11 | |
| Smoking behavior | |||||
| Current smoker | 106 (10.8) | 28 (20.4) | 63 (9.6) | 15 (8.2) | 0.017 |
| Former smoker | 200 (20.5) | 32 (23.4) | 132 (20.1) | 36 (19.7) | |
| Never smoker | 671 (68.7) | 77 (56.2) | 462 (70.3) | 132 (72.1) | |
| Missing | 16 | 3 | 11 | 2 | |
| Baseline alcohol consumption | |||||
| Daily consumption | 859 (89.9) | 17 (12.7) | 60 (9.3) | 19 (10.6) | 0.256 |
| Less than daily consumption | 96 (10.1) | 117 (87.3) | 582 (90.6) | 160 (89.4) | |
| Missing | 38 | 6 | 26 | 6 | |
| Physical Activity, MET-hours/week | |||||
| Median (IQR) | 8.0 (0.0–26.7) | 12.0 (0.0–48.0) | 8.0 (0.0–26.0) | 8.0 (0.0–19.0) | 0.215 |
| Missing | 70 | 13 | 45 | 12 | |
| Tumor stage | |||||
| I | 438 (44.1) | 56 (40.0) | 317 (47.5) | 65 (35.1) | 0.007 |
| II | 431 (43.4) | 64 (45.7) | 272 (40.8) | 95 (51.4) | |
| III | 123 (12.4) | 20 (14.3) | 78 (11.7) | 25 (13.5) | |
| Missing | 1 | – | 1 | – | |
| Tumor subtype | |||||
| HR+/HER- | 786 (79.6) | 97 (69.3) | 557 (83.8) | 132 (72.1) | <.0001 |
| HR±/HER2+ | 127 (12.8) | 22 (15.7) | 71 (10.7) | 34 (18.6) | |
| HR-/HER2- | 75 (7.6) | 21 (15.0) | 37 (5.6) | 17 (9.3) | |
| Missing | 5 | – | 3 | 2 | |
| Breast surgery | |||||
| Partial surgery | 746 (75.1) | 107 (76.4) | 508 (76.0) | 131 (70.8) | 0.471 |
| Mastectomy | 247 (24.9) | 33 (23.6) | 160 (24.0) | 54 (29.2) | |
| Missing | – | – | – | – | |
| Axillary surgery | |||||
| Axillary dissection | 436 (43.9) | 70 (50.0) | 289 (42.3) | 77 (41.6) | 0.386 |
| Sentinel lymph node∗∗ | 557 (56.1) | 70 (50.0) | 379 (56.7) | 108 (58.4) | |
| Missing | – | – | – | – | |
| Adjuvant radiation therapy | |||||
| Yes | 908 (91.6) | 129 (92.1) | 615 (92.3) | 164 (88.6) | 0.354 |
| No | 83 (8.4) | 11 (7.9) | 51 (7.6) | 21 (11.3) | |
| Missing | 2 | – | 2 | – | |
| (Neo) adjuvant chemotherapy | |||||
| Yes∗∗∗ | 532 (53.6) | 84 (60.0) | 314 (47.0) | 134 (72.4) | <.0001 |
| No | 461 (46.4) | 56 (40.0) | 354 (53.0) | 51 (27.5) | |
| Missing | – | – | – | – | |
| Adjuvant endocrine therapy | |||||
| Yes∗∗∗∗ | 829 (83.5) | 105 (75.0) | 575 (86.1) | 149 (80.5) | 0.0008 |
| No | 164 (16.5) | 35 (25.0) | 93 (13.9) | 36 (19.5) | |
| Missing | – | – | – | – | |
| Adjuvant anti-HER2 therapy | |||||
| Yes | 97 (9.8) | 18 (12.9) | 49 (7.3) | 30 (16.2) | 0.0006 |
| No | 896 (90.2) | 122 (87.1) | 619 (92.7) | 155 (83.8) | |
| Missing | – | – | – | – | |
BMI= Body Mass Index; WHO= World Health Organization; SD= Standard Deviation; IQR = interquartile range; HR= Hormone-receptor; HER2 = Human Epidermal Growth Factor receptor 2; MET = Metabolic Equivalent of Task.
^Jonckheere-Terpstra test statistics ∗Includes separated, divorced, widowed, unmarried ∗∗Includes 7 patients who did not receive any axillary lymph node surgical procedure; ∗∗∗Includes approximately 90% women receiving an anthracycline + taxane chemotherapy combination; ∗∗∗∗Patients receiving endocrine therapy were at a median of 3.9 months (IQR 3.0–5.5 months) since treatment initiation at the time of this analysis.
2.1. Statistical analysis
Baseline characteristics were tabulated and compared by category of weight change using descriptive statistics.
Associations between outcomes and the independent variable were then examined using different sets of multivariable-adjusted models, which also accounted for baseline HRQOL score (details in respective table footnotes and figure legends).
First, we explored the association between weight change category and HRQOL scores as continuous outcomes. Repeated measurements of HRQOL scores collected from diagnosis to the post-treatment visit were analyzed using the GENMOD procedure and fitting multivariate generalized estimating equations (GEE) with independent correlation structure. We obtained: a) Model-derived least square mean values for HRQOL scores at baseline and post-treatment and b) respective mean least square differences between baseline and post-treatment, according to weight change category (as independent variable). Standard errors and respective 95% Confidence Intervals (CIs) were also calculated. All models accounted for weight change, time, interaction weight-change-by-time, and covariates (ie, age, menopausal status, baseline Body Mass Index, comorbidities, marital status, education, anxiety and depression, smoking status, physical activity, breast and axillary surgery, receipt of chemotherapy, radiation therapy, endocrine therapy, anti-HER2 therapy). To test the hypothesis that the population-averaged HRQOL domain scores differ by weight change category, p-values for the effect of weight change (pgroup), time (ptime) and weight-change-by-time interaction (pinteraction) were obtained.
Then, we fit distinct multivariable logistic regression models for each dichotomous outcome to assess odds of HRQOL deterioration and of reporting poor functions or severe symptoms, which returned adjusted odds ratios (aORs) and respective 95% CIs. Logistic regression models were also adjusted for all covariates detailed above.
In sensitivity analyses, we repeated the models by categorizing weight change as ±10% of baseline and as continuous percent-unit change of baseline.
P values < 0.05 were considered significant. All tests were two-sided. Analyses were conducted with SAS v9.4.
3. Results
Our analytic cohort included 993 obese patients. Excluded patients did not differ from those included in the analytic cohort (data not shown). Median time from diagnosis to post-treatment was 10.5 months (interquartile range [IQR] 7.8–12.5). Mean age at diagnosis was 59.1 years (Standard Deviation = 10.5). All patients in this cohort received breast cancer surgery, 91.6% received radiation therapy, and 53.6% received adjuvant chemotherapy. Complete cohort characteristics are displayed in Table 1.
Mean baseline BMI was 34.5 kg/m2 (range, 30.0–59.0), mean baseline weight was 89.4 Kg (range, 61.0–153.0). The majority of women, 67.3%, remained stable, 14.1% gained ≥5%, and 18.6% lost ≥5% of baseline weight. Women who lost weight were more likely to be older, postmenopausal, and never smokers (Table 1). Physical activity was associated with weight changes: 59.1%, 63.7% and 73.6% of patients among those who gained, remained stable, or lost weight reported at least same or higher amounts of physical activity post-treatment compared to baseline, respectively (Cochran-Armitage trend test p = 0.009; Table 2).
Table 2.
Metrics of change in weight and physical activity from baseline to post-treatment.
| Characteristic Total | Overall N (%) = 993 (100) | By weight change category |
||
|---|---|---|---|---|
| Gain ≥5% N (%) =140 (14.1) | Stable ±5% N (%) = 668 (67.3) | Loss ≥5% N (%) =185 (18.6) | ||
| Post-treatment BMI, kg/m2 | ||||
| Mean (SD) | 34.3 (4.4) | 37.3 (4.3) | 34.4 (3.9) | 31.8 (4.7) |
| Missing | – | – | – | – |
| Post-treatment weight, kg | ||||
| Mean (SD) | 88.9 (13.4) | 96.5 (13.3) | 89.1 (12.7) | 82.4 (12.7) |
| Missing | – | – | – | – |
| Change in weight, kg | ||||
| Absolute change, mean (95% CI) | −0.5 (−0.9,-0.2) | +7.5 (+6.9,+8.0) | +0.1 (−0.1,+0.3) | −9.0 (−9.7,-8.3) |
| Percent change, mean (95% CI) | −0.5 (−0.9,-0.1) | +8.4 (+7.8,+9.1) | +0.2 (−0.01,+0.4) | −9.9 (−10.6,-9.2) |
| Missing | – | – | – | – |
| Total Physical Activity behavior, N (%) | ||||
| Maintained/Increased | 559 (65.0) | 68 (59.1) | 371 (63.7) | 120 (73.6) |
| Reduced | 301 (35.0) | 47 (40.9) | 211 (36.2) | 43 (26.4) |
| Missing | 133 | 25 | 86 | 22 |
BMI= Body Mass Index; SD= Standard Deviation; CI= Confidence Interval; IQR = interquartile range; MET = Metabolic Equivalent of Task.
Median completion rate of EORTC-QLQs was 93.6% (IQR 91.0%–94.5%) at baseline and 91.7% (91.1%–92.1%) post-treatment (Supplementary Table 1).
We observed a significant reduction in functional scores and increased symptom scores across the majority of HRQOL domains overall (ptime<.0001; Supplementary Table 1) and by weight change (Table 3). Compared to those who gained weight or remained stable, women who lost weight reported less of a decline in HRQOL, including scoring better in physical function (mean change for weight gain, stability and loss [95% Confidence Interval]: −12.9 [-16.5,-9.3], −6.9 [-8.2,-5.5] and −6.2 [-8.7,-3.7], respectively; pinteraction = 0.006), dyspnea (+18.9 [+12.3,+25.6], +9.2 [+6.5,+11.9] and +3.2 [-1.0,+7.3], respectively; pinteraction = 0.0003), and breast symptoms (+22.1 [+16.8,+27.3], +18.0 [+15.7,+20.3] and +13.4 [+9.0,+17.2], respectively; pinteraction = 0.044). Similar patterns suggesting a smaller decrement in HRQOL among those who lost weight were found for other domains (Table 3). Finally, in order to evaluate whether the relationship between weight change and changes in HRQOL differed by receipt of chemotherapy, we introduced weight-change-by-chemotherapy interaction terms in the models, which were not significant (pinteraction = 0.256 for physical function, pinteraction = 0.690 for dyspnea, and pinteraction = 0.544 for breast symptoms).
Table 3.
Post-treatment health-related quality of life and changes from baseline by category of weight change.
| HRQOL domain | Gain ≥5% N (%) = 140 (14.1) | Stable ±5% N (%) = 668 (67.3) | Loss ≥5% N (%) = 185 (18.6) | ||||||||||||||
| Mean° | SE | Mean change§ | 95% CI for the change | Mean° | SE | Mean change§ | 95% CI for the change | Mean | SE | Mean change§ | 95% CI for the change | pgroup | pinteraction | ||||
| Lower | Upper | Lower | Upper | Lower | Upper | ||||||||||||
| EORTC QLQ-C30 Functional Scales | |||||||||||||||||
| Global Health | 64.8 | 2.2 | −3.3 | −7.3 | +0.6 | 62.2 | 1.6 | −2.5 | −4.3 | −0.6 | 66.1 | 2.1 | −0.1 | −3.3 | +3.2 | 0.048 | 0.346 |
| Physical Function | 67.9 | 2.3 | −12.9 | −16.5 | −9.3 | 72.6 | 1.6 | −6.9 | −8.2 | −5.5 | 75.6 | 2.0 | −6.2 | −8.7 | −3.7 | 0.043 | 0.006 |
| Emotional Function | 73.3 | 2.5 | +1.9 | −2.0 | +5.8 | 73.9 | 2.0 | +4.0 | +1.5 | +6.3 | 75.6 | 2.4 | +5.6 | +1.7 | +9.5 | 0.876 | 0.425 |
| Social Function | 78.4 | 3.1 | −12.1 | −17.5 | −6.7 | 80.7 | 2.0 | −10.8 | −13.1 | −8.6 | 84.6 | 2.4 | −9.7 | −13.6 | −5.8 | 0.035 | 0.770 |
| Cognitive Function | 71.8 | 3.5 | −6.4 | −11.2 | −1.5 | 74.3 | 2.2 | −4.6 | −6.8 | −2.5 | 74.7 | 2.7 | −5.4 | −9.2 | −1.5 | 0.674 | 0.799 |
| Role Function | 68.3 | 3.2 | −13.4 | −19.0 | −7.9 | 71.4 | 2.1 | −9.7 | −12.1 | −7.2 | 76.2 | 2.8 | −8.0 | −12.9 | −3.1 | 0.058 | 0.335 |
| EORTC QLQ-C30 Symptom Scales | |||||||||||||||||
| Fatigue | 46.8 | 3.4 | +16.7 | +11.2 | +22.3 | 43.5 | 2.2 | +10.9 | +8.6 | +13.1 | 42.6 | 3.0 | +10.3 | +5.6 | +15.0 | 0.930 | 0.138 |
| Pain | 36.1 | 3.4 | +17.0 | +11.7 | +22.3 | 36.0 | 2.4 | +14.4 | +11.9 | +16.9 | 31.2 | 3.1 | +12.4 | +7.5 | +17.4 | 0.162 | 0.466 |
| Insomnia | 45.6 | 4.6 | +8.2 | +0.7 | +15.7 | 44.7 | 3.3 | +2.3 | −0.9 | +5.5 | 41.7 | 4.2 | +1.6 | −4.0 | +7.3 | 0.535 | 0.321 |
| Nausea/Vomit | 11.3 | 2.2 | +4.3 | +0.7 | +7.9 | 8.4 | 1.3 | +3.2 | +1.7 | +4.7 | 9.4 | 1.8 | +1.1 | −2.1 | +4.4 | 0.103 | 0.398 |
| Dyspnea | 44.6 | 3.9 | +18.9 | +12.3 | +25.6 | 31.9 | 2.6 | +9.2 | +6.5 | +11.9 | 24.5 | 3.1 | +3.2 | −1.0 | +7.3 | 0.0003 | 0.0003 |
| Appetite Loss | 12.3 | 3.0 | −1.7 | −6.8 | +3.4 | 12.7 | 1.9 | −0.6 | −2.8 | +1.6 | 16.8 | 2.5 | +2.3 | −2.6 | +7.2 | 0.329 | 0.492 |
| Constipation | 17.3 | 3.4 | +8.4 | +2.7 | +14.1 | 15.9 | 2.2 | +5.7 | +3.4 | +8.0 | 16.6 | 3.2 | +10.3 | +5.6 | +15.1 | 0.693 | 0.199 |
| Diarrhea | 15.8 | 3.4 | +5.1 | −0.3 | +10.6 | 17.0 | 2.1 | +3.4 | +0.9 | +5.8 | 18.6 | 3.1 | +3.9 | −0.7 | +8.6 | 0.472 | 0.854 |
| Financial Difficulties | 15.7 | 3.3 | +0.9 | −5.4 | +7.1 | 15.3 | 2.2 | +4.2 | +2.1 | +6.3 | 16.8 | 2.8 | +8.7 | +4.2 | +13.2 | 0.555 | 0.101 |
| EORTC QLQ-B23 Functional Scales | |||||||||||||||||
| Body Image | 68.0 | 3.3 | −17.6 | −23.4 | −11.9 | 71.0 | 2.3 | −12.9 | −15.6 | −10.3 | 74.8 | 2.8 | −11.4 | −15.9 | −6.8 | 0.236 | 0.228 |
| Sexual Function | 18.6 | 3.1 | +1.0 | −3.6 | +5.7 | 17.4 | 2.2 | −2.0 | −3.9 | −0.2 | 18.9 | 2.8 | +0.6 | −3.9 | +5.1 | 0.980 | 0.329 |
| Sexual Enjoyment^ | 52.5 | 4.8 | −6.3 | −14.2 | +1.6 | 51.5 | 4.1 | −8.2 | −12.1 | −4.4 | 51.7 | 4.1 | −5.3 | −15.0 | +4.4 | 0.915 | 0.805 |
| Future Perspective | 56.2 | 3.8 | +3.4 | −3.23 | +10.1 | 53.4 | 2.7 | +2.5 | −0.5 | +5.6 | 53.5 | 3.2 | +7.1 | +1.7 | +12.4 | 0.308 | 0.345 |
| EORTC QLQ-B23 Symptom Scales | |||||||||||||||||
| Side Effects | 24.1 | 2.3 | +10.9 | +6.9 | +14.8 | 22.3 | 1.4 | +10.3 | +8.8 | +11.8 | 21.4 | 1.9 | +10.8 | +8.0 | +13.6 | 0.353 | 0.922 |
| Breast Symptoms | 33.1 | 3.2 | +22.1 | +16.8 | +27.3 | 28.2 | 1.7 | +18.0 | +15.7 | +20.3 | 28.1 | 2.4 | +13.4 | +9.0 | +17.2 | 0.215 | 0.044 |
| Arm Symptoms | 34.8 | 3.8 | +20.4 | +13.6 | +27.2 | 31.0 | 2.3 | +14.2 | +11.6 | +16.8 | 29.5 | 3.0 | +14.4 | +9.5 | +19.3 | 0.650 | 0.243 |
| Upset by Hair Loss | 39.2 | 8.3 | −8.4 | −31.6 | +14.8 | 42.8 | 6.3 | +16.0 | +4.7 | +27.3 | 9.3 | 3.0 | +0.2 | −22.6 | +23.0 | 0.313 | 0.123 |
HRQOL= Health-related Quality of life; EORTC QLQ = European Organisation for Research and Treatment of Cancer Quality-of-Life questionnaire; SE= Standard Error; CI= Confidence Interval; °Model-based least square means for HRQOL scores post-treatment by category of weight change; §Model-based differences in least squares means from baseline to post-treatment by category of weight change. Estimates were obtained using generalized estimating equations, adjusted for weight change category (group), time, interaction weight change category by time, age, menopausal status, Body Mass Index, comorbidities, marital status, education, smoking status, physical activity, breast and axillary surgery, receipt of chemotherapy, radiation therapy, endocrine therapy, anti-HER2 therapy, anxiety, and depression. P-values for the effect of weight change category (pgroup) and of interaction weight change category by time (pinteraction) on differences in least squares means are reported.
A substantial proportion of patients (range 15.6–56.5%) experienced at least moderate HRQOL-deterioration (Supplementary Fig. 1A). In several domains, including dyspnea and body image, weight loss was associated with a smaller prevalence of patients experiencing such deterioration. Women who lost weight seemed also more likely to report reduced appetite over time (Supplementary Fig. 1B).
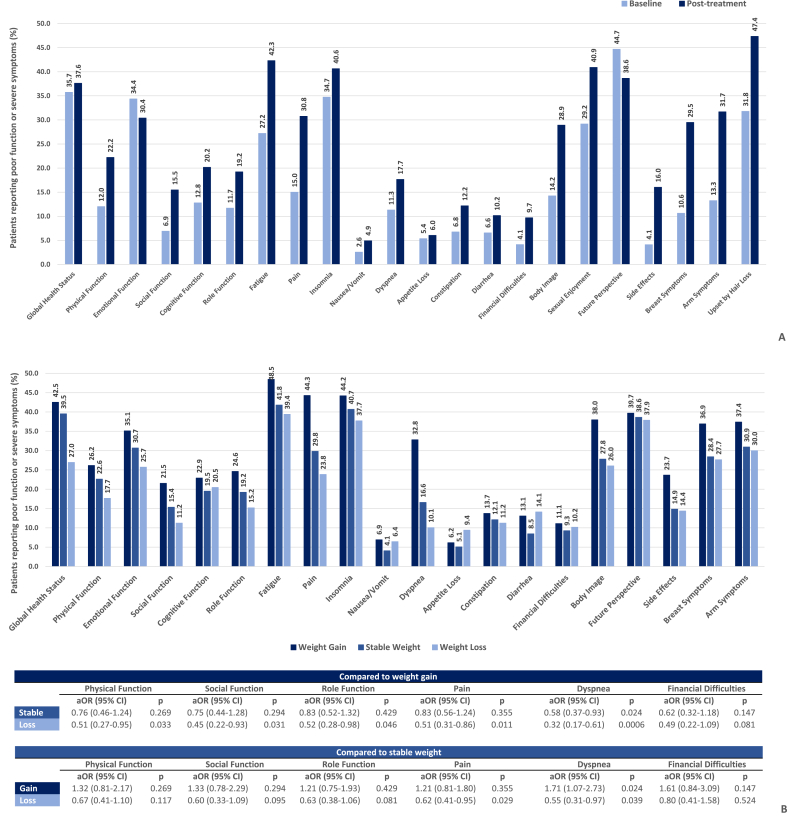
Women tended to report poor function and severe symptoms more often post-treatment (Fig. 2A), although this happened less frequently among women who lost weight (Fig. 2B). Particularly, weight loss was associated with a significant reduction in the odds of reporting poor physical function (aOR [95% CI] = 0.51 [0.27–0.95], p = 0.033), poor social function (aOR [95% CI] = 0.45 [0.22–0.93], p = 0.031), poor role function (aOR [95% CI] = 0.52 [0.28–0.98], p = 0.046), severe pain (aOR [95% CI] = 0.51 [0.31–0.86], p = 0.011), and severe dyspnea (aOR [95% CI] = 0.32 [0.17–0.61], p = 0.0006) compared to weight gain, and with those of reporting severe pain (aOR [95% CI] = 0.62 [0.41–0.95], p = 0.029) and severe dyspnea (aOR [95% CI] = 0.55 (0.31–0.97), p = 0.039) compared to stable weight (Fig. 2B). Consistent results were found in sensitivity analyses (data not shown).
Fig. 2.
Prevalence (%) of patients reporting poor function or severe symptoms at baseline and post-treatment in the whole cohort (2A). Prevalence (%) of patients reporting poor function or severe symptoms post-treatment by category of weight change (2B). Poor function is defined by a score <60, severe symptoms by a score ≥40 on the respective EORTC QLQ-C30 and QLQ-B23 scales. Prevalence of poor sexual function is not displayed because of scaling of the y-axis (prevalence > 80% in all subgroups). Adjusted odds ratios (aOR) and respective 95% Confidence Intervals (CI) were obtained from multivariable logistic regression models and are reported for selected domains. All models were adjusted for time, age, menopausal status, Body Mass Index, comorbidities, marital status, education, smoking status, physical activity, breast and axillary surgery, receipt of chemotherapy, radiation therapy, endocrine therapy, anti-HER2 therapy, anxiety, and depression.
Among the patients who had not reported severe dyspnea at baseline, 13.1% started to report so post-treatment (Supplementary Fig. 2A). Odds of onset of severe post-treatment dyspnea were elevated among women who gained weight (aOR [95% CI] = 1.84 [1.02–3.33], p = 0.044) and reduced among women who lost weight (aOR ([95% CI] = 0.39 [0.17–0.86], p = 0.019) compared to those whose weight remained stable (Supplementary Fig. 2B).
4. Discussion
In this large study, we report on the relationship between weight changes and HRQOL in a cohort of obese women with early breast cancer. Overall, patients tended to report reduced HRQOL after breast cancer treatment compared to assessments obtained at diagnosis. Nevertheless, women who gained weight seemed to score the worst in several HRQOL domains, whereas weight loss was associated with more favorable variations in HRQOL. Importantly, there was no association between weight loss and worsened HRQOL after treatment.
Consistently with recent World Health Organization-Europe data, and reflecting the prevalence of obesity in the general French population, obese patients account for almost 20% of the CANTO cohort [29]. Avoiding functional decline and preventing symptom exacerbation among obese women with breast cancer are therefore priorities that concern many providers and patients. In line with past reports, we describe worsening of numerous physical symptoms including fatigue, pain, breast discomfort, and body image issues following breast cancer treatment [30]. Weight gain was previously shown to contribute to deterioration of objective physical and cardio-metabolic parameters [31], and here we report that it is also linked with more bothersome, subjective, patient-reported HRQOL-changes. Beyond impaired well-being, post-treatment functional decline leads to loss of independence [32], worse social and cost-related outcomes [33], and possibly impairs cancer-specific outcomes [34], while increased symptom burden is associated with higher rates of non-adherence to adjuvant treatment and worse social rehabilitation in breast cancer survivors [35,36]. Our results inform further studies aimed at testing whether weight control can modulate some of these negative consequences.
In our primary analysis, we reported negative changes in HRQOL after early breast cancer treatment overall, although among women who lost weight we found smaller or negligible changes. According to guidelines provided by Cocks et al. [37], differences in mean HRQOL-changes among women that lost weight were at most trivial-to-small for domains such as physical function and dyspnea (−6.2 and + 3.2 points, respectively), whereas differences in those that gained weight were significantly greater, reaching the range of medium-to-large size (−12.9 and + 18.9 points, respectively). In additional analyses, we adopted particularly conservative cut-offs to categorize PROs (lower than median reference value for functions and higher than median for symptoms) [38], and found that weight loss was also associated with smaller proportions of patients reporting severely impaired HRQOL.
Obesity is now defined as a disease state, and the American Society of Clinical Oncology endorses weight gain prevention and weight loss facilitation for all obese cancer patients [39]. Expert consensus suggests that, for obese women, moderate weight loss of up to one Kg/week can be pursued at any time post breast cancer diagnosis, also during active adjuvant treatment if under appropriate monitoring [40]. However, robust evidence supporting this statement is still lacking. A novel aspect of the present study is that our results did not show that weight loss during or shortly after completion of adjuvant treatment was linked to worsening in HRQOL. Women in our study had a diagnosis of early-stage breast cancer, and this specific setting is partly responsible for our findings. Prior studies have reported that weight loss during treatment correlates with higher levels of toxicity and poorer outcomes, including cancer-specific survival [41,42], but these studies mostly evaluated disease groups other than breast cancer or patients with more advanced disease. In addition, our study included exclusively obese women at diagnosis, for whom a beneficial effect of weight loss on health outcomes could be expected, particularly in physical parameters [43].
Lifestyle interventions conducted in overweight or obese breast cancer survivors and that looked at HRQOL include the ENERGY and the LISA studies. ENERGY recruited patients with early-stage breast cancer diagnosed within the previous five years [10], and LISA reported data in women who completed chemotherapy at least four weeks before inclusion [11]. Findings of these studies are consistent with ours, in that a mean weight loss of 6% of baseline in the interventional arm of ENERGY was linked to a more likely preservation of physical function, while participants in the LISA intervention experienced a mean weight loss of 4–5 Kg and reported a greater increase in physical condition, compared to respective control arms [10,11]. Our results expand on this prior work for obese breast cancer patients, highlighting how weight changes occurring during and shortly after adjuvant therapy are related to longer-term HRQOL. There is indeed scarce data from studies that follow up patients with breast cancer from initial diagnosis to post-primary treatment. CANTO offered an unparalleled opportunity to explore the relationship between obesity, weight changes, and HRQOL in early breast cancer survivors in one of the largest contemporary, prospective, longitudinal studies with comprehensive HRQOL assessments. By longitudinally collecting data at several time points after diagnosis, CANTO will also be informative about future questions on the relationship between weight changes and HRQOL occurring later on during the survivorship trajectory [15].
Nevertheless, we acknowledge some limitations. First, it was not possible to assess whether weight loss was purposeful and to establish intentionality of weight changes. However, despite the possibility that some percentages of the observed weight loss were actually unintentional, our comprehensive PRO analyses showed that weight loss was not associated with worsened patient-reported condition in any of the explored outcomes. To improve our understanding of patients’ behavioral characteristics, we also assessed other metrics, and found increased physical activity levels among patients who lost weight, an important mediator of successful attempts of weight management [43]. Although it is possible that we could not account for some unmeasured confounders, all our models were also adjusted for change in physical activity behavior, as well as for several important clinical and treatment-related factors. Second, we used a cut-off of 5% to define weight change, based on previously observed clinically meaningful benefits of a weight loss of such magnitude [23]. There has also been substantial variability in values previously used to establish thresholds for HRQOL [44], and universal definitions for EORTC QLQ-C30 and QLQ-BR23 domains are still lacking. However, we provided rationale to support the notion that the chosen cut-offs were clinically relevant. Third, we used self-reported instruments, subject to some recall and reporting biases, but CANTO adopted questionnaires that had already been consistently proven to be reliable and valid instruments for observational studies [45]. Finally, French law does not allow collection of race/ethnicity data, which could have provided relevant information in this context.
5. Conclusions
In conclusion, we have reported several significant associations between weight changes and differential variations in HRQOL of obese women undergoing breast cancer treatment. Our findings were consistent across several domains of general and breast cancer-specific HRQOL, particularly suggesting that weight loss was not associated with worse HRQOL. Weight loss among obese individuals is a complex process, which includes substantial behavioral changes based primarily on modification of dietary habits and increased energy expenditure. Weight loss interventions are now deemed feasible and also safe in obese breast cancer survivors [11] and randomized trials of weight loss are underway, holding the promise to improve breast cancer outcomes and PROs over the first years following diagnosis [46]. Our study suggests that prevention of weight gain and purposeful weight loss during the early survivorship period should be tested in dedicated studies as strategies to mitigate many downstream sequelae of primary breast cancer treatment. Answering this question has important implications, as it would help reduce the burden that secondary effects of breast cancer treatment pose on the care of obese survivors. Finally, from a patient’s perspective, the cancer journey contains many “teachable moments” to improve health behaviors, including engaging in weight loss programs. If approached not only as a way to improve one’s general well-being and reduce excess weight-related morbidity, but also to pursue functional preservation and symptom management during and after cancer treatment, the goal of weight loss may be more favorably embraced, making attempts more likely successful [31].
Ethical approval
The CANTO study was approved by the national regulatory authorities of France (ID-RCB: 2011-A01095-36) and by the ethics committee CPP IDF VII (11–039). Informed consent for study participation was obtained at patient enrollment.
Data availability
CANTO data is available upon request to a dedicated study Executive Committee (http://www.unicancer.fr/rd-unicancer/letude-canto).
Authorship
ADM and IVL: conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, resources, software, supervision, validation, visualization, writing - original draft, and writing - review and editing. MEM and ADM: data curation. ADM: formal analysis. SM, AP, FA, and JL: conceptualization, supervision, validation, draft writing. All authors gave substantial contribution to interpretation of data, revised this manuscript for important intellectual content, reviewed and approved its final version.
Funding
This research was supported by a Clinical Research Fellowship from the European Society for Medical Oncology (ESMO) to Antonio Di Meglio, a Career Catalyst Research grant from Susan G. Komen (CCR17483507) to Ines Vaz-Luis, funding from Odyssea to Ines Vaz-Luis and from Foundation Gustave Roussy to Ines Vaz-Luis. The CANTO study is supported by the French Government under the “Investment for the Future” program managed by the National Research Agency (ANR), grant n° ANR-10-COHO-0004. Lee W Jones is supported by grants from AKTIV Against Cancer, Kavli Trust, and the Memorial Sloan Kettering Cancer Center Support Grant/Core Grant (P30 CA008748).
Previous presentations
Results of this study were partly presented as a poster display during the European Society for Medical Oncology (ESMO) Congress 2018 (Munich, Germany - October 22, 2018). Annals of Oncology (2018) 29 (suppl_8): viii603-viii640.10.1093/annonc/mdy300.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgments
We would like to thank Yuki Takahashi for her help in manuscript proofreading.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2020.04.002.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Prevalence of deteriorated HRQOL in the overall cohort (1A). Distribution of patients reporting selected deteriorated HRQOL domains by weight change (1B). Deterioration was defined as a decrease or an increase of at least 10 points from baseline on functional and symptom scales, respectively. Adjusted Odds ratios (aORs) and respective 95% Confidence Intervals (CI) were derived from multivariable logistic regression, modeling the odds of HRQOL deterioration. Models are adjusted by age, menopausal status, Body Mass Index, comorbidities, marital status, education, smoking status, physical activity, breast and axillary surgery, receipt of chemotherapy, radiation therapy, endocrine therapy, anti-HER2 therapy, anxiety, and depression.
Distribution of changes in HRQOL status from baseline to post-treatment in the overall cohort (2A). % patients reporting non-poor/non-severe HRQOL at baseline that started reporting so post-treatment is highlighted in dark blue. Distribution of patients reporting non-poor/non-severe HRQOL at baseline that started reporting so post-treatment, according to weight change (2B). Adjusted Odds Ratios (aORs) and respective 95% Confidence Intervals (CI) derived from multivariable logistic regression models are reported, adjusted for age, menopausal status, Body Mass Index, comorbidities, marital status, education, smoking status, physical activity, breast and axillary surgery, receipt of chemotherapy, radiation therapy, endocrine therapy, radiation therapy, anti-HER2 therapy, anxiety, and depression.
References
- 1.Adams K.F., Schatzkin A., Harris T.B., Kipnis V., Mouw T., Ballard-Barbash R. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 Years old. N Engl J Med. 2006;355:763–778. doi: 10.1056/NEJMoa055643. [DOI] [PubMed] [Google Scholar]
- 2.Kolotkin R.L., Meter K., Williams G.R. Quality of life and obesity. Obes Rev An Off J Int Assoc Study Obes. 2001;2:219–229. doi: 10.1046/j.1467-789x.2001.00040.x. [DOI] [PubMed] [Google Scholar]
- 3.Schoemaker M.J., Nichols H.B., Wright L.B., Brook M.N., Jones M.E., O’Brien K.M. Association of body mass index and age with subsequent breast cancer risk in premenopausal women. JAMA Oncol. 2018;4 doi: 10.1001/jamaoncol.2018.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li C.I., Daling J.R., Porter P.L., Tang M.-T.C., Malone K.E. Relationship between potentially modifiable lifestyle factors and risk of second primary contralateral breast cancer among women diagnosed with estrogen receptor-positive invasive breast cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2009;27:5312–5318. doi: 10.1200/JCO.2009.23.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan D.S.M., Vieira A.R., Aune D., Bandera E.V., Greenwood D.C., McTiernan A. Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Ann Oncol Off J Eur Soc Med Oncol. 2014;25:1901–1914. doi: 10.1093/annonc/mdu042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi M., Fuller C.D., Wang S.J., Siddiqi A., Wong A., Thomas C.R. Effect of body mass index on shifts in ultrasound-based image-guided intensity-modulated radiation therapy for abdominal malignancies. Radiother Oncol J Eur Soc Ther Radiol Oncol. 2009;91:114–119. doi: 10.1016/j.radonc.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kent S., Green J., Reeves G., Beral V., Gray A., Jebb S.A. Hospital costs in relation to body-mass index in 1·1 million women in England: a prospective cohort study. Lancet Publ Heal. 2017;2:e214–e222. doi: 10.1016/S2468-2667(17)30062-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goodwin P.J., Stambolic V. Impact of the obesity epidemic on cancer. Annu Rev Med. 2015;66:281–296. doi: 10.1146/annurev-med-051613-012328. [DOI] [PubMed] [Google Scholar]
- 9.Ligibel J.A., Partridge A., Giobbie-Hurder A., Campbell N., Shockro L., Salinardi T. Physical and psychological outcomes among women in a telephone-based exercise intervention during adjuvant therapy for early stage breast cancer. J Wom Health (Larchmt) 2010;19:1553–1559. doi: 10.1089/jwh.2009.1760. [DOI] [PubMed] [Google Scholar]
- 10.Demark-Wahnefried W., Colditz G.A., Rock C.L., Sedjo R.L., Liu J., Wolin K.Y. Quality of life outcomes from the Exercise and Nutrition Enhance Recovery and Good Health for You (ENERGY)-randomized weight loss trial among breast cancer survivors. Breast Canc Res Treat. 2015;154:329–337. doi: 10.1007/s10549-015-3627-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goodwin P.J., Segal R.J., Vallis M., Ligibel J.A., Pond G.R., Robidoux A. Randomized trial of a telephone-based weight loss intervention in postmenopausal women with breast cancer receiving letrozole: the LISA trial. J. Clin Oncol. Off. J. Am. Soc. Clin. Oncol. 2014;32:2231–2239. doi: 10.1200/JCO.2013.53.1517. [DOI] [PubMed] [Google Scholar]
- 12.Warkentin L.M., Das D., Majumdar S.R., Johnson J.A., Padwal R.S. The effect of weight loss on health-related quality of life: systematic review and meta-analysis of randomized trials. Obes Rev An Off J Int Assoc Study Obes. 2014;15:169–182. doi: 10.1111/obr.12113. [DOI] [PubMed] [Google Scholar]
- 13.Driscoll S., Gregory D.M., Fardy J.M., Twells L.K. Long-term health-related quality of life in bariatric surgery patients: a systematic review and meta-analysis. Obesity. 2016;24:60–70. doi: 10.1002/oby.21322. [DOI] [PubMed] [Google Scholar]
- 14.Kolotkin R.L., Andersen J.R. A systematic review of reviews: exploring the relationship between obesity, weight loss and health-related quality of life. Clin Obes. 2017;7:273–289. doi: 10.1111/cob.12203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vaz-Luis I., Cottu P., Mesleard C., Martin A.L., Dumas A., Dauchy S. UNICANCER: French prospective cohort study of treatment-related chronic toxicity in women with localised breast cancer (CANTO) ESMO Open. 2019;4 doi: 10.1136/esmoopen-2019-000562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.AJCC - American joint committee on cancer n.d. https://cancerstaging.org/Pages/default.aspx
- 17.Aaronson N.K., Ahmedzai S., Bergman B., Bullinger M., Cull A., Duez N.J. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 18.Sprangers M.A., Groenvold M., Arraras J.I., Franklin J., te Velde A., Muller M. The European Organization for Research and Treatment of Cancer breast cancer-specific quality-of-life questionnaire module: first results from a three-country field study. J Clin Oncol. 1996;14:2756–2768. doi: 10.1200/JCO.1996.14.10.2756. [DOI] [PubMed] [Google Scholar]
- 19.Fayers P., Aaronson N.K., Bjordal K., Groenvold M., Curran D., Bottomley A. European Organisation for Research and Treatment of Cancer; 2001. EORTC QLQ-C30 scoring manual. [Google Scholar]
- 20.Questionnaires | EORTC – quality of Life : EORTC – quality of life n.d. https://qol.eortc.org/questionnaires/ (accessed March 3, 2020)
- 21.Osoba D., Rodrigues G., Myles J., Zee B., Pater J. Interpreting the significance of changes in health-related quality-of- life scores. J Clin Oncol. 1998;16:139–144. doi: 10.1200/JCO.1998.16.1.139. [DOI] [PubMed] [Google Scholar]
- 22.Oberguggenberger A., Hubalek M., Sztankay M., Meraner V., Beer B., Oberacher H. Is the toxicity of adjuvant aromatase inhibitor therapy underestimated? Complementary information from patient-reported outcomes (PROs) Breast Canc Res Treat. 2011;128:553–561. doi: 10.1007/s10549-011-1378-5. [DOI] [PubMed] [Google Scholar]
- 23.Saquib N., Flatt S.W., Natarajan L., Thomson C.A., Bardwell W.A., Caan B. Weight gain and recovery of pre-cancer weight after breast cancer treatments: evidence from the women’s healthy eating and living (WHEL) study. Breast Canc Res Treat. 2007;105:177–186. doi: 10.1007/s10549-006-9442-2. [DOI] [PubMed] [Google Scholar]
- 24.Ball K., Brown W., Crawford D. Who does not gain weight? Prevalence and predictors of weight maintenance in young women. Int J Obes Relat Metab Disord J Int Assoc Study Obes. 2002;26:1570–1578. doi: 10.1038/sj.ijo.0802150. [DOI] [PubMed] [Google Scholar]
- 25.Harris T.B., Savage P.J., Tell G.S., Haan M., Kumanyika S., Lynch J.C. Carrying the burden of cardiovascular risk in old age: associations of weight and weight change with prevalent cardiovascular disease, risk factors, and health status in the Cardiovascular Health Study. Am J Clin Nutr. 1997;66:837–844. doi: 10.1093/ajcn/66.4.837. [DOI] [PubMed] [Google Scholar]
- 26.Meltzer A.A., Everhart J.E. Self-reported substantial 1-year weight change among men and women in the United States. Obes Res. 1995;3(Suppl 2):123s–134s. doi: 10.1002/j.1550-8528.1995.tb00455.x. [DOI] [PubMed] [Google Scholar]
- 27.Zigmond A.S., Snaith R.P. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 28.Global physical activity questionnaire analysis guide GPAQ analysis guide global physical activity questionnaire (GPAQ) analysis guide. [n.d].
- 29.Data and statistics. 2019. [Google Scholar]
- 30.Bower J.E., Ganz P.A., Desmond K.A., Bernaards C., Rowland J.H., Meyerowitz B.E. Fatigue in long-term breast carcinoma survivors. Cancer. 2006;106:751–758. doi: 10.1002/cncr.21671. [DOI] [PubMed] [Google Scholar]
- 31.McInnes J.A., Knobf M.T. Weight gain and quality of life in women treated with adjuvant chemotherapy for early-stage breast cancer. Oncol Nurs Forum. 2001;28:675–684. [PubMed] [Google Scholar]
- 32.Yabroff K.R., Lawrence W.F., Clauser S., Davis W.W., Brown M.L. Burden of illness in cancer survivors: findings from a population-based national sample. J Natl Cancer Inst. 2004;96:1322–1330. doi: 10.1093/jnci/djh255. [DOI] [PubMed] [Google Scholar]
- 33.Morey M.C., Snyder D.C., Sloane R., Cohen H.J., Peterson B., Hartman T.J. Effects of home-based diet and exercise on functional outcomes among older, overweight long-term cancer survivors: renew: a randomized controlled trial. J Am Med Assoc. 2009;301:1883–1891. doi: 10.1001/jama.2009.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holmes M.D., Chen W.Y., Feskanich D., Kroenke C.H., Colditz G.A. Physical activity and survival after breast cancer diagnosis. J Am Med Assoc. 2005;293:2479–2486. doi: 10.1001/jama.293.20.2479. [DOI] [PubMed] [Google Scholar]
- 35.Serum assessment of non-adherence to adjuvant endocrine therapy (ET) among premenopausal patients in the prospective multicenter CANTO cohort | OncologyPRO n.d. https://oncologypro.esmo.org/Meeting-Resources/ESMO-2018-Congress/Serum-assessment-of-non-adherence-to-adjuvant-endocrine-therapy-ET-among-premenopausal-patients-in-the-prospective-multicenter-CANTO-cohort (accessed February 18, 2019).
- 36.Luis I.M.V.D., O’Neill A.M., Sepucha K., Miller K.D., Dang C.T., Northfelt D.W. Symptom burden and employment status in breast cancer (BC) survivors. J Clin Oncol. 2018;36 doi: 10.1200/JCO.2018.36.15_suppl.10073. 10073–10073. [DOI] [Google Scholar]
- 37.Cocks K., King M.T., Velikova G., Martyn St-James M., Fayers P.M., Brown J.M. Evidence-based guidelines for determination of sample size and interpretation of the European organisation for the research and treatment of cancer quality of life questionnaire core 30. J Clin Oncol. 2011;29:89–96. doi: 10.1200/JCO.2010.28.0107. [DOI] [PubMed] [Google Scholar]
- 38.Scott N.W., Fayers P.M., Aaronson N.K., Bottomley A., De Graeff A., Groenvold M. 2008. EORTC QLQ-C30 reference values this manual presents reference data for the QLQ-C30 based upon data provided by EORTC quality of life group members and other users of the QLQ-C30 sprangers on behalf of the EORTC quality of life group EORTC quality of life group. [Google Scholar]
- 39.Ligibel J.A., Alfano C.M., Courneya K.S., Demark-Wahnefried W., Burger R.A., Chlebowski R.T. American society of clinical oncology position statement on obesity and cancer. J Clin Oncol. 2014;32:3568–3574. doi: 10.1200/JCO.2014.58.4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Demark-Wahnefried W., Campbell K.L., Hayes S.C. Weight management and its role in breast cancer rehabilitation. Cancer. 2012;118:2277–2287. doi: 10.1002/cncr.27466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meyerhardt J.A., Kroenke C.H., Prado C.M., Kwan M.L., Castillo A., Weltzien E. Association of weight change after colorectal cancer diagnosis and outcomes in the kaiser permanente northern California population. 2017. [DOI] [PMC free article] [PubMed]
- 42.Mutschler N.S., Scholz C., Friedl T.W.P., Zwingers T., Fasching P.A., Beckmann M.W. Prognostic impact of weight change during adjuvant chemotherapy in patients with high-risk early breast cancer: results from the ADEBAR study. Clin Breast Canc. 2018;18:175–183. doi: 10.1016/j.clbc.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 43.Ligibel J.A., Basen-Engquist K., Bea J.W. Weight management and physical activity for breast cancer prevention and control. Am Soc Clin Oncol Educ B. 2019 Jan;39:e22–e33. doi: 10.1200/EDBK_237423. Epub 2019 May 17. Review. PubMed PMID: 31099634. [DOI] [PubMed] [Google Scholar]
- 44.Giesinger J.M., Kuijpers W., Young T., Tomaszewski K.A., Friend E., Zabernigg A. Thresholds for clinical importance for four key domains of the EORTC QLQ-C30: physical functioning, emotional functioning, fatigue and pain. Health Qual Life Outcome. 2016;14:87. doi: 10.1186/s12955-016-0489-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Troiano R.P., Berrigan D., Dodd K.W., Mâsse L.C., Tilert T., McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40:181–188. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 46.Ligibel J.A., Barry W.T., Alfano C., Hershman D.L., Irwin M., Neuhouser M. Randomized phase III trial evaluating the role of weight loss in adjuvant treatment of overweight and obese women with early breast cancer (Alliance A011401): study design. NPJ Breast Canc. 2017;3:37. doi: 10.1038/s41523-017-0040-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Prevalence of deteriorated HRQOL in the overall cohort (1A). Distribution of patients reporting selected deteriorated HRQOL domains by weight change (1B). Deterioration was defined as a decrease or an increase of at least 10 points from baseline on functional and symptom scales, respectively. Adjusted Odds ratios (aORs) and respective 95% Confidence Intervals (CI) were derived from multivariable logistic regression, modeling the odds of HRQOL deterioration. Models are adjusted by age, menopausal status, Body Mass Index, comorbidities, marital status, education, smoking status, physical activity, breast and axillary surgery, receipt of chemotherapy, radiation therapy, endocrine therapy, anti-HER2 therapy, anxiety, and depression.
Distribution of changes in HRQOL status from baseline to post-treatment in the overall cohort (2A). % patients reporting non-poor/non-severe HRQOL at baseline that started reporting so post-treatment is highlighted in dark blue. Distribution of patients reporting non-poor/non-severe HRQOL at baseline that started reporting so post-treatment, according to weight change (2B). Adjusted Odds Ratios (aORs) and respective 95% Confidence Intervals (CI) derived from multivariable logistic regression models are reported, adjusted for age, menopausal status, Body Mass Index, comorbidities, marital status, education, smoking status, physical activity, breast and axillary surgery, receipt of chemotherapy, radiation therapy, endocrine therapy, radiation therapy, anti-HER2 therapy, anxiety, and depression.
Data Availability Statement
CANTO data is available upon request to a dedicated study Executive Committee (http://www.unicancer.fr/rd-unicancer/letude-canto).