Abstract
Introduction
Breast cancer patients undergo extended treatments that affect their psychological state and quality of life. There is a lack of studies examining the effects of holistic stress management interventions (that combine stress perception, cognitive and lifestyle interventions) on mental health and biological indices (e.g. cortisol concentrations) of breast cancer patients.
Materials and methods
This pilot randomized controlled trial provided the first assessment of the effects of a novel, cognitive-based intervention, the Pythagorean Self-Awareness Intervention (PSAI), on psychological symptoms, quality of life, sleep quality and lifestyle as well as on stress-related biological measures of breast cancer patients undergoing adjuvant therapy. Standardized questionnaires were administered at baseline and 8-weeksafter the intervention to evaluate quality of life, stress, depression, and anxiety (primary outcomes). Sleep quality, lifestyle and hair cortisol concentrations were also assessed (secondary outcomes).
Results
Forty-five breast cancer patients undergoing adjuvant therapy were randomly assigned to the PSAI group (n = 25) or the control group (n = 20).Women in the PSAI group reported significant improvements post-intervention in total Quality of Life, specific aspects of Quality of Life [Physical well-being, Social well-being, Emotional well-being, Functional well-being, Breast cancer concerns] as well as Perceived stress, depression, anxiety and stress. Improvements in secondary outcomes included increase in sleep quality, empowerment for healthy lifestyle and reduction of hair cortisol concentrations.
Conclusions
The PSAI was beneficial as complementary therapy in the women studied. Larger randomized controlled trials with longer follow-up are needed to ascertain these findings.
Keywords: Breast cancer, Cognitive interventions, Hair cortisol, Quality of life, Stress, Management, Adjuvant therapy
Abbreviations: BCC, Breast Cancer Concerns; BMI, Body Mass Index; CBT, Cognitive Behavioral Therapy; DMN, Default Mode Network; EWB, Emotional Well-Being; FACT-B, Functional Assessment in Cancer Treatment-Breast; FWB, Functional Well-Being; HCC, Hair Cortisol Concentration; HLPCQ, Healthy Lifestyle and Personal Control Questionnaire; PSAI, Pythagorean Self-Awareness Intervention; PSS, Perceived Stress Scale; PSQI, Pittsburgh Sleep Quality Index; PWB, Physical Well-Being; SD, Standard Deviation; SWB, Social Well-Being
Highlights
-
•
Breast cancer patients face physical and psychological cancer-related stressors.
-
•
Pythagorean self-awareness is a novel stress-management intervention (PSAI).
-
•
Breast cancer patients were allocated to PSAI group and control group.
-
•
Mental health, quality of life and sleep were ameliorated in PSAI group.
-
•
Hair cortisol concentrations were reduced significantly in the PSAI group.
1. Introduction
According to global statistics, breast cancer is enlisted as the most common diagnosis of female malignancy, with 2,088,849 new cases reported in 2018 [1]. Women with breast cancer face several physical and psychological cancer-related stressors that may impact on patients for many years following treatment [2].
Such stressors, include treatment-related side effects (mastectomy, lymphedema, menopausal symptoms, alopecia, weight changes, surgical wounds and scars etc.) that may lead to body image distortion, sexual dysfunction/intimacy issues as well as low self-esteem [3,4]. Also, breast cancer per se and breast cancer treatments seem to burden the multiple role performance of women in today’s society by disrupting family functioning, professional and social life [5].
As psychological distress and anxiety are highly prevalent all through disease trajectory, breast cancer patients seem to experience twice the degree of such issues, compared to the general female population, thus increasing their vulnerability to depression [6].
Furthermore, breast cancer survivors often report concerns about cancer recurrence which may lead to long-term anxiety and depressive symptoms [7]. Additionally, distress symptoms of breast cancer patients are underreported as medical screening may condone the psychological status of this population [8].
Psychological symptoms of these patients should not be overlooked [9] as they are linked to exacerbation of physical symptoms and poorer quality of life [10,11].
Lifestyle of breast cancer patients as a stressor is also overlooked. Women with breast cancer tend to abstain from physical activity and gain weight [12,13] that lead to increased body cortisol, increased visceral fat and disturbance of metabolic parameters, such as glucose, cholesterol, C-reactive protein etc., that have been linked to poor breast cancer prognosis [[12], [13], [14], [15]].
Researchers of stress support that stress is caused by the individuals’ cognitive appraisal of situational relevance to well-being, rather than by the stressor itself [16].
The cognitive processing and appraisal of an illness diagnosis, plays a key role in adjustment [17]. Research in patients with breast cancer or other chronic diseases has shown that maladaptive cognitive responses (i.e. irrational, automatic and intrusive thoughts) decrease adjustment to the disease and increase vulnerability to stress, anxiety and depression [18].
Τhe first significant step towards cognitive reappraisal of these cognitive distortions is self-awareness. Self-awareness is the capacity to introspection, identification of stressors, evaluation and comparison of one’s current behavior to personal internal standards and values with an aim to become self-conscious through objective self-evaluation [19]. Self-awareness as a tool of cognitive reappraisal of the breast cancer diagnosis, may empower patients to make conscious rather than impulsive and instinctive choices based on negative thoughts about life-threatening consequences. Self-awareness shapes the individual’s perception of stressful events and situations and reduces the response to them [20], increases self-control (i.e. the ability to handle personal feelings and emotions under difficult conditions) and self-efficacy (i.e. individual’s internal beliefs about the ability to have an impact on the events that affect his/her live) [21], thus improving the overall QoL of these patients.
Stress management interventions in oncology care seem imperative while all aforementioned are crucial when designing such interventions for breast cancer patients.
Therefore, several cancer organizations such as the American Cancer Society [22], the Canadian Cancer Society [23] or Lifestyle Medicine health providers [24] have published specific recommendations not only for cancer prevention but also for better cancer prognosis. These recommendations include health awareness, adoption of a healthy lifestyle (healthy weight, healthy eating, active living, improvement of sleep quality and lymphedema awareness) as well as management of emotional issues, that should be taken into account shortly after diagnosis [25].
Among the most common techniques in dealing with breast cancer’s psychological issues, cognitive-behavioral therapy (CBT) showed to be effective in dealing with distress symptoms and improving QoL in breast cancer patients and survivors, as it affects emotions and behaviors [26]. Also, mindfulness interventions have shown to successfully improve fatigue, cognitive functions and psychological issues including emotional wellbeing, anxiety, distress and depression, in women with breast cancer [27]. However, there is a lack of studies examining the effects of holistic stress management interventions (that combine stress perception, cognitive and lifestyle interventions) on mental health and biological indices (e.g. cortisol concentrations) of breast cancer patients.
Α recently introduced cognitive-based stress management technique, the “Pythagorean Self-Awareness Intervention (PSAI)”, seems to have promising effects on patients suffering from obesity, insomnia, multiple sclerosis, mild cognitive impairment or acne vulgaris. In a recent study of Simos et al. [28], implementation of the PSAI for 8 weeks in adults with obesity, resulted in statistically significant decreases in perceived stress, serum and saliva cortisol concentrations, body mass index (BMI) and obesity-related biomarkers. Also, in the study of Darviri et al. [29], patients with mild cognitive impairment who took part in PSAI, showed significant improvements in cognition, stress perception, anxiety, depression and self-efficacy. Furthermore, the application of PSAI in patients with multiple sclerosis improved significantly their physical and psychosocial well-being and QoL [30]. In another study [31], subjects suffering from chronic insomnia showed improvement after participating in the PSAI for their stress management. Finally, in a pilot study of individuals with acne vulgaris, implementation of the PSAI improved the stage of acne [32].
Based on the necessity for improving mental and physical health we hypothesized that PSAI along with lifestyle modifications, used as complementary therapy in breast cancer, would benefit patients through cognitive reappraisal of distortions, mobilization of personal resources to managing cancer related-stressors and cope with the disease, empowerment in increasing control over health and by improving QoL in general. The aim of this study was to explore, for the first time, the effects of the PSAI on mental health (stress, anxiety, depression) and QoL during cancer treatment (primary outcomes), as well as on sleep quality, lifestyle and hair cortisol concentrations (secondary outcomes) of women with breast cancer undergoing adjuvant therapy.
2. Materials and Methods
2.1. Study design - participants
This pilot, non-blinded, randomized, two-armed study was conducted at the outpatient Breast Department of the General Anti-Cancer Hospital Agios Savvas in Athens, Greece, over a period of 10 months, from February 2018 to December 2018. The study protocol was approved by the Hospital’s Scientific and Ethics Committee (Protocol n.12350/16-11-2017) and was consistent with the Declaration of Helsinki. Participants were informed by the researcher (MC), about the purposes and processes of the research and were enrolled in the study only after submitting written informed consent. Inclusion criteria were age older than 18 years, diagnosis of primary malignancy of the breast confirmed with biopsy and active anti-cancer adjuvant treatment (chemotherapy, radiation therapy or hormonal therapy). Exclusion criteria included any psychiatric co-morbidity (i.e. major depression, psychosis or drug abuse), any metastasis or autoimmune disease, systematic corticosteroid intake, previous participation in any study related to stress management and inability to read or write in the Greek language.
2.2. Randomization
Study participants were randomized into two groups, the Pythagorean Self-Awareness Intervention (PSAI) group and the control group, based on random numbers generated by an online random number generator (www.random.org).
3. Measurements
3.1. Socio-demographic, health and disease-related data
Participants were asked about their age, personal status, educational level, professional status, parity and smoking habits. Disease-related information, such as stage of cancer based on American Joint Committee on Cancer TNM system (0-III), type of surgery (mastectomy or lumpectomy), active adjuvant therapy (chemotherapy, radiation therapy, hormonal therapy), hormonal status as well as weight and height, were retrieved from patients’ medical records.
3.2. Questionnaires
A battery of self-report questionnaires was administered to participants before initiation and after the end of the intervention.
3.2.1. Perceived Stress Scale (PSS)
Perceived stress was evaluated using the Greek version of the PSS questionnaire, which consists of 14 items, each scored on a 5-point Likert scale. There are seven positive and seven negative items and the total score ranges from 0 to 56. Higher PSS scores indicate higher levels of perceived stress over the past month [33,34]. Reliability and internal consistency of PSS was very good in both baseline and final measurements (Cronbach’s alpha: 0.91 and 0.96, respectively).
3.2.2. Depression, anxiety, stress scale (DASS-21)
Symptoms of depression, anxiety and distress were evaluated using the Greek version of the DASS-21questionnaire which consists of 21 items that generate 3 subscales (depression, anxiety, stress). Each item is scored on a 5-point Likert scale. Specific cut-off scores for each symptom describe the degree of severity (normal, mild, moderate, severe, extremely severe) [35,36]. The reliability and internal consistency in each axis was satisfactory (Cronbach’s alpha initially DassDepression 0.93, DassAnxiety 0.92, DassStress 0.90, finally DassDepression 0.95, DassAnxiety 0.93, DassStress 0.92).
3.2.3. Functional Assessment of cancer therapy-breast module (FACT-B)
Quality of life was assessed with the FACT-B questionnaire (version 4), which consists of five subscales: physical well-being (PWB), social well-being (SWB), emotional well-being (EWB), functional well-being (FWB), and breast cancer concerns (BCC). Subscales’ scores ranges are 0–28 for PWB, 0–28 for SWB, 0–24 for EWB, 0–28 for FWB, 0–40 for BCC and the FACT-B total score range is 0–148 [37].Reliability and internal consistency in each category was satisfactory (Cronbach’s alpha initially PWB 0.91, SWB 0.81, EWB 0.72, FWB 0.92 BCS 0.83, FACT-B 0.90and finally PWB 0.92, SWB 0.85, EWB 0.75, FWB 0.95, BCC 0.86, FACT- B 0.97).
3.2.4. Pittsburgh Sleep Quality Index (PSQI)
The quality of sleep was assessed with the use of the Greek version of the PSQI questionnaire [38]. The PSQI consists of 19 self-reference questions, grouped in 7 components (subjective sense of quality of sleep, awaking time, latency, duration, usual productivity of sleep, use of medication for sleep and dysfunction during the day). Scoring ranges from 0 to 3, which results in a global score that ranges from 0 = high quality of sleep to 21 = low quality of sleep. A total score ≥5 indicates poor sleep [39]. The reliability and internal consistency in each category was satisfactory (Cronbach’s alpha PSQIpre 0.85 and PSQIpost 0.88, respectively).
3.2.5. Healthy Lifestyle and Personal Control Questionnaire (HLPCQ)
Participants were assessed about their health-related daily activities with the use of the HLPCQ. The HLPCQ is designed to assess the degree of someone’s control over ones’ daily activities i.e. diet, everyday program, physical activity, socialization, and pessimistic thoughts. The questionnaire consists of 26 items scored on a 4-pointLikert scale ranging from 1 = never/rarely to 4 = always. A higher score indicates increased health empowerment [40]. The reliability and internal consistency in each category was satisfactory (Cronbach’s alpha for HLPCQpre 0.88 and HLPCQpost0.91, respectively).
3.2.6. Hair cortisol concentrations
To objectively measure the long-term stress and aggregation of endogenous cortisol levels, hair tufts were collected from each study participant, at baseline and 1 month after completion of the intervention. Hair was collected from the posterior vertex of the scalp and was cut off as close to the scalp as possible. The hair tufts were taped to a piece of paper and remained in storage at room temperature until analysis. The methodology for the analysis has been described previously [41]. Hair has a fairly predictable growth rate of approximately 1 cm/month. Therefore the most proximal 1 cm segment to the scalp approximates the past month’s cortisol production; the second most proximal 1 cm segment approximates the production during the month before that and so on [42]. Analyses were performed at the Unit of Clinical and Translational Research in Endocrinology of the First Department of Pediatrics, School of Medicine, National and Kapodistrian University of Athens, at the Choremeio Research Laboratory in Athens, Greece.
3.3. Pythagorean Self-Awareness Intervention
Pythagorean Self-Awareness is based on the 71 Golden Verses of Pythagoras [43] which are comprised of 71 lines of moral exhortations, attributed to the ancient Ionian Greek philosopher Pythagoras of Samos (c. 570 – c. 495 BCE) [44]. The PSAI is a mental technique that incorporates the golden verses of Pythagoras to build the notion of self-awareness in the individuals by adjusting the way they view themselves and the others. The technique is comprised of 3 successive stages; 1st stage -sequential recall of daily events; 2nd stage -primary contemplation on thoughts and emotions related to each event; and 3rd stage -critical appraisal of the individual’s attitude as an observer.
Unlike other techniques, this method does not use any mental “trick” i.e. focusing on objects, senses, breathing or words, to achieve its purpose. Mindfulness and meditation, practice non-judgmental consciousness without the use of critical self-evaluation [[45], [46], [47]] which is a key difference from PSAI that encourages the individual to evaluate and modify lifestyle, relationships, behavior, by using its critical self as an “internal observer” and according to the Golden Verses moral framework.
In the Pythagorean Self-Awareness technique, thinking processes and feelings cooperate and interact. To “activate and train” the “inside observer” the individual must focus on inner consciousness; use mental concentration and memory, while the regularity of technique application is extremely important for achieving its benefits.
The key element of the neurophysiologic basis of the technique is the activation of the Default Mode Network (DMN) that is a neural brain connection system regarding self-relevant processes of cognition [48]. Neuroimaging research has shown that the DΜΝ is involved in discrete aspects of memory retrieval [49]. The DMN is important in metacognitive processes of introspection (internal cognition) and it is the basic element for alternating a person’s behavior via inhibition of impulses and sentimental behavior [50].
Through recall of the actions of the day, self-observation and self-assessment (verses 41, 42), individuals detect wrong choices and unhealthy behaviors and are encouraged to initiate the process of correction (verse 44). This internal dialogue promotes self-referential awareness and mindfulness, resulting in correct choices and the installation of a healthier lifestyle. Through self-observation the individual has the opportunity to observe also other aspects of one’s behavior, achieve a moral cognitive reconstruction as dictated by the golden verses, and head towards self-improvement. This may reduce stress and anxiety and improve daily life [51].
3.4. PSAI implementation
A total of 8 sessions were delivered by MC (MSc in stress management, specialized in lymphedema of breast cancer patients) and CD (Professor of health promotion and stress management) to the intervention (PSAI) group. In the 1st individualized session, participants completed the questionnaires and both groups received the same information about distress and its effects on health. They were also encouraged to adopt a healthy lifestyle i.e. adhere to the Mediterranean diet, retain or lower their body mass index (BMI), become physically active and adopt asleep routine. Information about lymphedema and specific exercises as well as training in diaphragmatic breathing were also delivered to each study participant. Patients of the PSAI group were given pedometers as an incentive for physical activity. Afterwards, participants of both groups were monitored on a weekly basis. Patients in the control group were contacted once per week via telephone. In each telephone call, participants were briefly asked about their physical and mental status with no further discussion or in-depth counseling. The PSAI group was monitored through face-to-face group sessions. In the 2nd session, the intervention group was introduced to the Pythagorean Self-Awareness Intervention. Participants were instructed to practice the PSAI technique twice a day (at bedtime and in the morning) in a quiet place at home. The technique was presented to the participants in 5 simple steps, as shown in Table 1.
Table 1.
Steps of PSAI implementation.
| Step 1: | Sit at bedside, breath diaphragmatically for 5–10 min and then read the golden verses, with emphasis on verses 9–45. |
| Step 2: | Recall every event of the day in the exact sequence that it happened |
| Step 3: | Visualize yourself as an observer of another person. To enhance recall of events, categorize them as follows: diet, physical activity, sleep, interpersonal contacts (verses 32–35) and assess accomplishment of goals set during morning practice. |
| Step 4: | Critically appraise each selected experience using three questions (verse 42): “In what have I done wrong ? What have I done right ? What have I omitted that I ought to have done?”. Remember to stay emotionally detached, examine the performed actions and disapprove or endorse accordingly (verses 9–45). |
| Step 5: | In the morning, immediately after waking up, quickly summarize the conclusions of the previous night practice and set goals for the upcoming day. |
PSAI: Pythagorean Self-Awareness Intervention.
The next 6 sessions of the program were based on the golden verses (9–45)exhortations about health awareness and lifestyle modifications, and included lectures about health-awareness [52], lifestyle modifications [53], circadian rhythms [54,55], memory [56], cognitive reconstruction and appraisal of interpersonal relationships [57]. With reference to the golden verses, for example, to verses 32–35 (verse 32 “In no way neglect the health of your body”; verse 33 “But give it drink and food in due measure, and also the exercise of which it needs”) participants were given scientific advice according to current lifestyle medicine recommendations in order to optimize overall health [52], about diet (Mediterranean diet) [52], physical activity (10.000steps/day self-monitored with pedometers) [58] and healthy routine of sleep [59], always in respect of their doctor’s approval.
The PSAI sessions took place once a week and lasted for 180 min with 10 min interval. Each session was divided into two sections; the first section (50 min) included feedback from the participants about their experiences with the technique (i.e. questions or difficulties) and clarifications to queries about the technique; the second section (2 h with a 10 min interval in between) included several thematic lectures and presentations on the session’s topic.
During the final session, final assessments were made. Compliance to the technique was assessed by weekly diaries that participants had to keep and submit to the researchers in each session.
3.5. Statistical methods
Between-group comparisons for baseline data were performed with the use of Pearson’s exact chi-square and Mann-Whitney U tests for categorical and interval characteristics, respectively. Absolute differences (Δ = final measurement minus baseline measurement) were used as dependent variables in the Mann-Whitney U tests for between-group comparisons. The effect sizes of the intervention were calculated by the following formula: rho = Z/N0.5, where rho is the effect size (<0.3 small, 0.3–0.5 moderate and >0.5 large effect size), Z is the score of each Mann-Whitney U test, and N is the study sample. The level of significance was set p < 0.05 for all analyses. BMI was calculated as the ratio of body weight to the square of height (kg/m2). Statistical calculations were performed using the SPSS for Windows (version 25.0) statistical software (SPSS Inc., Chicago, IL, USA).
4. Results
4.1. Study participants’ characteristics
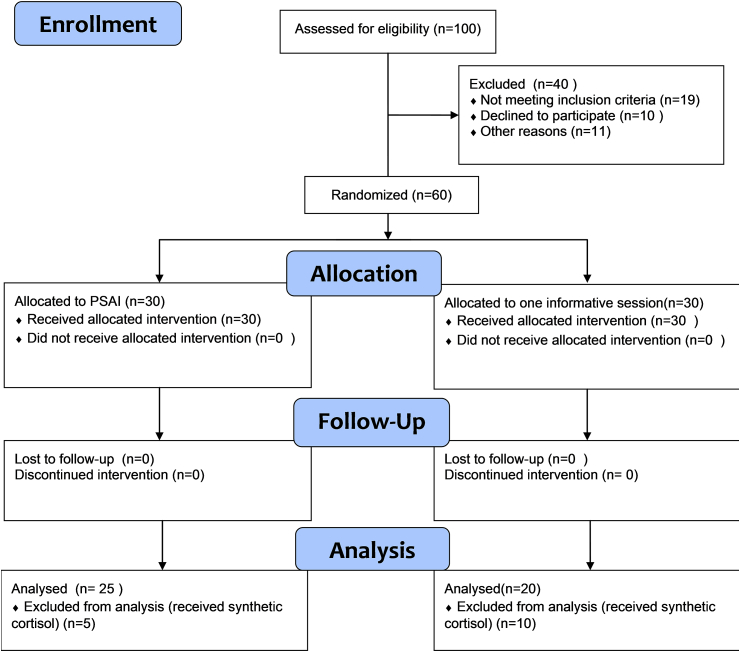
The study sample initially consisted of 60 female patients, of which 30 were assigned to the intervention group and 30 to the control group. The flow diagram of the study is illustrated in Fig. 1. No drop-outs were noted during the follow up in either group. However, 15 patients from both groups were not included in the analysis, even though they had completed the program, because they had to receive orally synthetic cortisol as part of their treatment regimen, that was decided after study recruitment. Finally, 45 patients were analyzed (PSAI group N = 25/Control group N = 20). Patients in the intervention group showed full compliance with the PSAI, as assessed by the weekly diaries and full participation in all sessions. No harm or side-effects were reported by any participant. Participants’ baseline characteristics are presented in Table 2.
Fig. 1.
Flow Diagram of the study.
Table 2.
Baseline socio-demographic and disease-related characteristics of study participants.
| Referential measurements | PSAI group (N = 25) | Control group (N = 20) | p value |
|---|---|---|---|
| Age (mean ± SD) | 52.8 ± 7.88 | 51.8 ± 8.63 | 0.73 |
| Personal status | |||
| Single | 3 (12%) | 7 (35%) | 0.35 |
| Married | 10 (40%) | 5 (25%) | |
| Divorced | 11 (44%) | 6 (30%) | |
| Widowed | 1 (4%) | 2(10%) | |
| Parity | |||
| Yes | 16 (64%) | 9 (45%) | 0.21 |
| No | 9 (36%) | 11 (55%) | |
| Educational level | |||
| Primary school (6 years) | 0% | 1 (5%) | 0.07 |
| Secondary (9 years) | 0% | 3(15%) | |
| High school (12 years) | 9 (36%) | 6 (30%) | |
| Higher education (≥14 years) | 16 (64%) | 10 (50%) | |
| Working status | |||
| Retired | 4 (16%) | 4 (20%) | 0.40 |
| Unemployed | 2 (8%) | 4 (20%) | |
| Employed | 19 (76%) | 12 (60%) | |
| BMI | 28.3 ± 5.12 | 26.5 ± 6.18 | 0.34 |
| Smoking | |||
| Yes | 5 (20%) | 4 (20%) | 0.78 |
| No | 11 (44%) | 10 (50%) | |
| Ex-smoking | 9 (36%) | 6 (30%) | |
| Type of surgery | |||
| Lumpectomy | 12 (48%) | 7 (35%) | 0.21 |
| Single mastectomy | 13 (52%) | 10 (45%) | |
| Double mastectomy | 3 (15%) | ||
| Cancer Stage | |||
| In situ | 5 (20%) | 4 (20%) | 0.88 |
| I | 10 (40%) | 6(30%) | |
| IIA | 3 (12%) | 4 (20%) | |
| IIB | 3 (12%) | 4 (20%) | |
| IIIA | 4 (16%) | 2 (10%) | |
| Adjuvant Therapy | |||
| Hormonal therapy | 6 (24%) | 4 (20%) | 0.52 |
| Chemotherapy | 3 (12%) | 4 (20%) | |
| Radiation therapy | 16 (66%) | 12 (60%) | |
| Hormonal Status | |||
| Pre-menopausal | 12 (48%) | 11 (55%) | 0.65 |
| Menopause | 13 (52%) | 9 (45%) | |
PSAI: Pythagorean Self-Awareness Intervention, BMI: Body Mass Index
There were no significant differences between study groups at baseline. The majority of participants was middle-aged (mean age ± SD 52.7 ± 8.5 years), non-smokers(46.7%), divorced (37.8%), had children (56.6%), had completed higher education(57.8%) and were employed (68.9%). As for their health and disease-related characteristics, participants were generally overweight (mean BMI ± SD 27.8 ± 5.6kg/m2), almost half had undergone single mastectomy (51.1%) and were in premenopausal status (51.1%), more than a third were diagnosed with breast cancer stage I (35.6%), and the majority were treated with radiation therapy (62.2%) at the time of assessment. Regarding psychological symptoms, participants showed mild to moderate depression and distress and a moderate degree of anxiety (DASS 21).Patients had a moderate quality of life (FACT-B) during breast cancer treatment and very poor quality of sleep (PSQI). Finally, lifestyle and personal control measurements were poor (HLPCQ) for both groups (Table 3).
Table 3.
Psychometric measurements and hair cortisol concentrations of study groups.
| Referential measurements | PSAI group (N = 25) | Control group (N = 20) | p value |
|---|---|---|---|
| PSS (mean ± SD) | 29.55 ± 8.82 | 31.06 ± 9.09 | 0.62 |
| DassDepression (mean ± SD) | 6.05 ± 6.34 | 8.8 ± 6.41 | 0.21 |
| DassAnxiety (mean ± SD) | 6 ± 5.8 | 6.4 ± 6.66 | 0.85 |
| DassStress (mean ± SD) | 8.5 ± 5.91 | 10.2 ± 5.77 | 0.40 |
| FACT-B (mean ± SD) | 75.6 ± 18.45 | 80.3 ± 17.58 | 0.45 |
| PWB (mean ± SD) | 18.35 ± 5.61 | 21 ± 2.64 | 0.10 |
| SWB (mean ± SD) | 13.85 ± 4.92 | 12.6 ± 6.33 | 0.51 |
| EWB (mean ± SD) | 13.25 ± 4.76 | 12.46 ± 5.5 | 0.65 |
| FWB (mean ± SD) | 9.75 ± 4.58 | 10.73 ± 5.36 | 0.56 |
| BCC (mean ± SD) | 20.40 ± 4.88 | 23.53 ± 6.22 | 0.10 |
| PSQI (mean ± SD) | 9 ± 5.09 | 9.4 ± 3.79 | 0.80 |
| HLPCQ (mean ± SD) | 35.65 ± 13.37 | 35.26 ± 12.33 | 0.93 |
| HCCmean (min-max) | 20.72 (7.01–37) | 17.62 (6.21–32.53) | 0.25 |
BCC: Breast Cancer Concerns, BMI: Body Mass Index, EWB: Emotional Well-Being, FACT-B: Functional Assessment in Cancer Treatment-Breast, FWB: Functional Well-Being, HCC: Hair Cortisol Concentration, HLPCQ: Healthy Lifestyle and Personal Control Questionnaire, PSAI: Pythagorean Self-Awareness Intervention, PSS: Perceived Stress Scale, PSQI: Pittsburgh Sleep Quality Index, PWB: Physical Well-Being, SD: Standard Deviation, SWB: Social Well-Being.
Frequencies were analyzed by Pearson’s chi-square (categorical by categorical comparisons) and non-parametric Mann-Whitney U test (categorical by quantitative comparisons).
*Level of significance p < 0.05.
4.1.1. Primary endpoint analyses
Adjusted mean differences, standard deviations, p values, and effect sizes for the PSAI group versus the Control group for each primary outcome are presented in Table 4.
Table 4.
Comparisons of outcomes’ differences across study groupsa.
| Measurements (mean ± SD) | PSAI group (N = 25) | Control group (N = 20) | 95% CI | p value | Effect size |
|---|---|---|---|---|---|
| ΔBMI score | - 0.77 ± 0.86 | 0.35 ± 1.73 | −1.93,-0.16 | 0.037* | 0.3 |
| ΔPSS score | −15.6 ± 10.09 | 2.6 ± 8.44 | −23.86, −10.83 | 0.000* | 0.8 |
| ΔDepression score | −3.7 ± 5.22 | −0.2 ± 2.27 | −6.35, −0.67 | 0.017* | 0.4 |
| Δanxiety score | −3.95 ± 5.99 | 0.53 ± 1.12 | −7,54, −1.35 | 0.007* | 0.5 |
| Δstress score | −4.5 ± 5.52 | −0.26 ± 0.72 | −7.20, −1.04 | 0.003* | 0.5 |
| ΔFACT-B scoreb | 44.05 ± 17.04 | −11.2 ± 17.99 | 40.16, 65.31 | 0.004* | 0.6 |
| ΔPWB scoreb | 7.1 ± 4.49 | −3.86 ± 6.99 | 6.17, 14.27 | 0.025* | 0.4 |
| ΔSWB scoreb | 5.25 ± 4.43 | −0.13 ± 2.41 | 2.94, 7.93 | 0.001* | 0.6 |
| ΔEWB scoreb | 8.7 ± 4.54 | −2.06 ± 5.4 | 6.83, 13.89 | 0.002* | 0.6 |
| ΔFWB scoreb | 10 ± 4 | −2.06 ± 3.71 | 8.80, 14.32 | 0.001* | 0.7 |
| ΔBCC scoreb | 13 ± 4.24 | −3.06 ± 3.91 | 12.11, 18.38 | 0.001* | 0.6 |
| ΔPSQI score | −4.21 ± 6.45 | 2.35 ± 3.75 | −10.66, −2.47 | 0.002* | 0.6 |
| ΔHLPCQ scoreb | 17.4 ± 12.65 | −3.86 ± 6.23 | 12.86, 27.43 | 0.004* | 0.6 |
| ΔHCC | −8.14 ± 5.83 | 1.17 ± 3.46 | −12.67, −5.95 | 0.000* | 0.7 |
BCC: Breast Cancer Concerns, BMI: Body Mass Index, EWB: Emotional Well-Being, FACT-B: Functional Assessment in Cancer Treatment-Breast, FWB: Functional Well-Being, HCC: Hair Cortisol Concentration, HLPCQ: Healthy Lifestyle and Personal Control Questionnaire, PSAI: Pythagorean Self-Awareness Intervention, PSS: Perceived Stress Scale, PSQI: Pittsburgh Sleep Quality Index, PWB: Physical Well-Being, SD: Standard Deviation, SWB: Social Well-Being.
*Level of significance p < 0.05.
Effect size is calculated as rho = Z/N0.5.
Non-parametric Mann-Whitney U tests for categorical by numerical comparisons.
Positive difference on these scales indicate improvement.
Statistically significant improvements in favor of the PSAI group were noted for BMI(p = 0.037), perceived stress (p = 0.000), depression (p = 0.017), anxiety (p = 0.007) and distress (p = 0.003). Moreover, statistical improvements were noted in all subscales[PWB (p = 0.025), SWB (p = 0.001), EWB (p = 0.002), FWB (p = 0.001), BCS (p = 0.001)and FACT-B total score treatment (p = 0.004)] that comprise quality of life during cancer therapy. Furthermore, statistically significant improvements were noticed in sleep quality (p = 0.002), healthy lifestyle and personal control (p = 0.004) and total hair cortisol concentrations (p = 0.000). According to the effect size, PSAI had a large impact on perceived stress, FACT-B total score, SWB, EWB, FWB, sleep quality, healthy lifestyle empowerment, and total hair cortisol concentration. Moderate effect size was found in depression, anxiety, stress (DASS) and physical well-being. Finally, a small effect size was found in BMI.
5. Discussion
The present study shows, for the first time, the advantages of the novel Pythagorean Self-Awareness Intervention for breast cancer patients undergoing adjuvant therapy. Our hypothesis that a holistic program of cognitive-based stress management combined with lifestyle counseling, would improve psychological issues (distress, anxiety, depression), QoL during cancer treatment, sleep quality, lifestyle and hair cortisol concentrations of breast cancer patients during active therapy was supported by the study findings. The strongest effect size was observed in Perceived stress, QoL, Sleep quality, Healthy lifestyle empowerment and total hair cortisol concentrations. Many studies have investigated the effects of different stress management methods in breast cancer patients. Specifically, a recent meta-analysis of breast cancer patients showed that CBT can improve perceived stress and QoL [60]. Indeed, the decrease of perceived stress and also the improvement of the QoL in breast cancer patients have been associated with better adjustment to the disease and could even serve as a prognostic factor along with medical parameters [61]. Our results in sleep quality are similar to the study of Pelekasis et al. [62] who tested CBT in combination with lifestyle modifications in 53 women who were receiving chemotherapy. Sleep improvement as an outcome in oncologic populations interventions, is an important finding as there is evidence that poor quality of sleep may be associated with increased breast cancer mortality [63]. Sleep quality improvement can be attributed to every night’s practice of self-evaluation and self-judgment just before sleep. Guided by the “compass” of the golden verses and not by consolidated beliefs, the night’s practice settles the events of the day, thus decreasing non-beneficial internal dialogues and enhancing perceived self-efficacy. Research in breast cancer patients shows that perceived self-efficacy affects actions and coping behaviors and assists in the adjustment process after a cancer diagnosis [64,65].
In terms of healthy lifestyle empowerment research has shown that unhealthy lifestyle choices increase the risk for cancer and non-cancer mortality in women with breast cancer [66,67]. Our finding of lifestyle empowerment following the PSAI, suggests patients’ motivation and mobilization to take action and increase of their “fighting spirit”, that has been shown to enhance the effectiveness of the immune system that is imperative for survival [68].
Following the PSAI, hair cortisol concentrations decreased significantly. To our knowledge this is the first study investigating the effect of a cognitive-based stress management intervention on hair cortisol. Other studies focus on serum or saliva cortisol measurements that represent cortisol concentrations at a single point in time and are subject to major physiological daily fluctuations, making the assessment of long-term systemic exposure to cortisol difficult. Hence, a single measurement cannot reflect the aggregation of systemic exposure [69] which, on the other hand, is reflected in hair cortisol measurement.
Study limitations include the small sample size, the lack of long-term follow-up, the lack of measurement of cancer related biomarkers, the lack of a patient group of different educational level or comparison with another cognitive-based stress management technique. As such, generalization and validity of the results cannot be safely verified.
6. Conclusions
The PSAI improved psychological symptoms, QoL, sleep quality and lifestyle as well as the stress-related biological index of hair cortisol in breast cancer patients under adjuvant anti-cancer treatment. Future large-scale randomized controlled trials, not only of cancer patients but also of cancer survivors, of longer follow-up, measuring more sophisticated indices (such as immune biomarkers) are warranted to fully explore the potential of PSAI to be used as an effective complementary tool to alleviate stress and improve the QoL in this patient population.
Data availability
We state that data of the study is available on request from the corresponding author.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical approval
The study protocol was approved by the Scientific and Ethics Committee of the General Anti-Cancer Hospital Agios Savvas in Athens, Greece (Protocol n.12350/16-11-2017) and was consistent with the Declaration of Helsinki.
Declaration of competing interest
None.
Acknowledgments
We thank all women for participating in the study, as well as the nurses and surgeons of the 1st and 2nd Departments of Breast of the General Anti-Cancer Hospital Agios Savvas in Athens, Greece. Also, special thanks to Clinical Chemist Aimilia Mantzou for her assistance with hair cortisol analysis.
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Pinquart M., Fröhlich C., Silbereisen R.K. Cancer patients’ perceptions of positive and negative illness-related changes. J Health Psychol. 2007;12(6):907–921. doi: 10.1177/1359105307082454. [DOI] [PubMed] [Google Scholar]
- 3.Hewitt M., Herdman R., Holland J. The National Academies Press; Washington, DC: 2004. Meeting psychosocial needs of women with breast cancer. [PubMed] [Google Scholar]
- 4.Prates A.C.L., Freitas-Junior R., Prates M.F.O. Influence of body image in women undergoing treatment for breast cancer. Rev Bras Ginecol Obstet. 2017;39:175–183. doi: 10.1055/s-0037-1601453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keesing S., Rosenwax L., McNamara B. The implications of women’s activity limitations and role disruptions during breast cancer survivorship. Women’s Health. 2018 doi: 10.1177/1745505718756381. 1745505718756381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burgess C., Cornelius V., Love S., Graham J., Richards M., Ramirez A. Depression and anxiety in women with early breast cancer: five year observational cohort study. Br Med J. 2005;330:702–705. doi: 10.1136/bmj.38343.670868.D3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dunn L.B., Langford D.J., Paul S.M., Berman M.B., Shumay D.M., Kober K. Trajectories of fear of recurrence in women with breast cancer. Support Care Cancer. 2015;23(7):2033–2043. doi: 10.1007/s00520-014-2513-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pasquini M., Biondi M. Depression in cancer patients: a critical review. Clin Pract Epidemiol Ment Health. 2007;3:2. doi: 10.1186/1745-0179-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kang D.H., Park N.J., McArdle T. Cancer-specific stress and mood disturbance: implications for symptom perception, quality of life, and immune response in women shortly after diagnosis of breast cancer. ISRN Nurs. 2012 doi: 10.5402/2012/608039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fann J.R., Thomas-Rich A.M., Katon W.J. Major depression after breast cancer: a review of epidemiology and treatment. Gen Hosp Psychiatry. 2008;30:112–126. doi: 10.1016/j.genhosppsych.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 11.Perry S., Kowalski T.L., Chang C.H. Quality of life assessment in women with breast cancer: benefits, acceptability and utilization. Health Qual Life Outcomes. 2007;5(1):24. doi: 10.1186/1477-7525-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phillips S.M., Dodd K.W., Steeves J., McClain J., Alfano C.M., McAuley E. Physical activity and sedentary behavior in breast cancer survivors: new insight into activity patterns and potential intervention targets. Gynecol Oncol. 2015;138(2):398–404. doi: 10.1016/j.ygyno.2015.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goodwin P.J., Pritchard K.I. Obesity and hormone therapy in breast cancer: an unfinished puzzle. J Clin Oncol. 2010:3405–3407. doi: 10.1200/JCO.2010.29.5113. [DOI] [PubMed] [Google Scholar]
- 14.Sephton S.E., Sapolsky R.M., Kraemer H.C., Spiegel D. Diurnal cortisol rhythm as a predictor of breast cancer survival. J Natl Cancer Inst. 2000;21(12):994–1000. doi: 10.1093/jnci/92.12.994. 92. [DOI] [PubMed] [Google Scholar]
- 15.Obradović M.M., Hamelin B., Manevski N., Couto J.P., Sethi A., Coissieux M.M. Glucocorticoids promote breast cancer metastasis. Nature. 2019;567(7749):540. doi: 10.1038/s41586-019-1019-4. [DOI] [PubMed] [Google Scholar]
- 16.Hamilton N.A., Kitzman H., Guyotte S. Enhancing health and emotion: mindfulness as a missing link between cognitive therapy and positive psychology. J Cogn Psychother. 2006;20(2):123–134. doi: 10.1891/jcop.20.2.123. [DOI] [Google Scholar]
- 17.Greenberg M.A. Cognitive processing of traumas: the role of intrusive thoughts and reappraisals. J Appl Soc Psychol. 1995;25:1262–1296. doi: 10.1111/j.1559-1816.1995.tb02618.x. [DOI] [Google Scholar]
- 18.Dupont A., Bower J.E., Stanton A.L., Ganz P.A. Cancer-related intrusive thoughts predict behavioral symptoms following breast cancer treatment. Health Psychol. 2014;33(2):155. doi: 10.1037/a0031131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wicklund R.A. vol 8. Academic Press; 1975. pp. 233–275. (Objective self-awareness. Advances in experimental social psychology). [DOI] [Google Scholar]
- 20.Goleman D. Bloomsbury Publishing; London: 1995. Emotional intelligence. [Google Scholar]
- 21.Hartman D., Zimberoff D. Higher stages of human development. J Heart-Centred Ther. 2008;11(2):3–95. [Google Scholar]
- 22.American Cancer Society Breast cancer. https://www.cancer.org/cancer/breast-cancer.html
- 23.Canadian cancer society, http://www.cancer.ca/en/prevention-andscreening/reduce-cancer-risk/make-healthy-choices/?region=bc [accessed 10 December 2019].
- 24.Bodai B.I., Tuso P. Breast cancer survivorship: a comprehensive review of long-term medical issues and lifestyle recommendations. Perm J. 2015;19(2):48. doi: 10.7812/TPP/14-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamer J., Warner E. Lifestyle modifications for patients with breast cancer to improve prognosis and optimize overall health. CMAJ (Can Med Assoc J) 2017;189(7):E268–E274. doi: 10.1503/cmaj.160464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang M., Huang L., Feng Z., Shao L., Chen L. Effects of cognitive behavioral therapy on quality of life and stress for breast cancer survivors: a meta-analysis. Minerva Med. 2017:84–93. doi: 10.23736/S0026-4806.16.04528-6. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Q., Zhao H., Zheng Y. Effectiveness of mindfulness-based stress reduction (MBSR) on symptom variables and health-related quality of life in breast cancer patients—a systematic review and meta-analysis Support Care. Cancer. 2019;27:771. doi: 10.1007/s00520-018-4570-x. [DOI] [PubMed] [Google Scholar]
- 28.Simos D.S., Kokkinos A., Tentolouris N., Dimosthenopoulos C., Mantzou E., Artemiadis A. Pythagorean self awareness intervention (PSAI): a novel cognitive stress management technique for body weight control. Eur J Clin Investig. 2019:e13164. doi: 10.1111/eci.13164. [DOI] [PubMed] [Google Scholar]
- 29.Darviri C., Zavitsanou C., Delikou A., Giotaki A., Artemiadis A., Terentiou A. A novel non-pharmaceutical treatment for patients with mild cognitive impairment. Psychology. 2016;7:678–686. doi: 10.4236/psych.2016.75070. [DOI] [Google Scholar]
- 30.Darviri C., Zavitsanou C., Delikou A., Giotaki A., Artemiadis A., Anagnostouli M. Pythagorean self-awareness serves successfully as a new cognitive behavioral based technique in multiple sclerosis physical and psychosocial well-being and quality of life. Psychology. 2016;7:572. doi: 10.4236/psych.2016.74059. 04. [DOI] [Google Scholar]
- 31.Chatzikonstantinou F., Miskedaki A., Antoniou C., Chatzikonstantinou M., Chrousos G., Darviri C. A novel cognitive stress management technique for acne vulgaris: a short report of a pilot experimental study. Int J Dermatol. 2019;58(2):218–220. doi: 10.1111/ijd.14227. [DOI] [PubMed] [Google Scholar]
- 32.Tsoli S., Vasdekis S., Tigani X., Artemiadis A., Chrousos G., Darviri C. A novel cognitive behavioral treatment for patients with chronic insomnia: a pilot experimental study. Complement Ther Med. 2018;37:61–63. doi: 10.1016/j.ctim.2018.01.015. [DOI] [PubMed] [Google Scholar]
- 33.Cohen S., Kamarck T., Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–396. doi: 10.2307/2136404. [DOI] [PubMed] [Google Scholar]
- 34.Andreou E., Alexopoulos E.C., Lionis C., Varvogli L., Gnardellis C., Chrousos G.P. Perceived stress scale: reliability and validity study in Greece. Int J Environ Res Public Health. 2011;8:3287–3298. doi: 10.3390/ijerph8083287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lovibond S.H., Lovibond P.F. 2nd.Ed. Psychology Foundation; Sydney: 1995. Manual for the depression anxiety stress scales. [Google Scholar]
- 36.Lyrakos G.N., Arvaniti C., Smyrnioti M., Kostopanagiotou G. Translation and validation study of the depression anxiety stress scale in the Greek general population and in a psychiatric patient’s sample. Eur Psychiatry. 2011;26:1731. doi: 10.1016/S0924-9338(11)73435-6. [DOI] [Google Scholar]
- 37.Brady M.J., Cella D.F., Mo F., Bonomi A.E., Tulsky D.S., Lloyd S.R. Reliability and validity of the functional assessment of cancer therapy-breast quality –of-life instrument. J Clin Oncol. 1997;15(3):974–986. doi: 10.1200/JCO.1997.15.3.974. [DOI] [PubMed] [Google Scholar]
- 38.Kotronoulas G.C., Papadopoulou C.N., Papapetrou A., Patiraki E. Psychometric evaluation and feasibility of the Greek Pittsburgh Sleep Quality Index (GR-PSQI) in patients with cancer receiving chemotherapy. Support Care Cancer. 2011;19(11):1831–1840. doi: 10.1007/s00520-010-1025-4. [DOI] [PubMed] [Google Scholar]
- 39.Buysse D.J., Reynolds C.F., Monk T.H., Berman S.R., Kupfer D.J. The Pittsburghsleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 40.Darviri C., Alexopoulos E.C., Artemiadi A.K., Tigani X., Kraniotou C., Darvyri P. The Healthy Lifestyle and Personal Control Questionnaire (HLPCQ): a novel tool for assessing self-empowerment through a constellation of daily activities. BMC Public Health. 2014;14:995. doi: 10.1186/1471-2458-14-995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wester V.L., Van Rossum E.F. Clinical applications of cortisol measurements in hair. Eur J Endocrinol. 2015;173(4) doi: 10.1530/EJE-15-0313. M1-10. [DOI] [PubMed] [Google Scholar]
- 42.Wennig R. Potential problems with the interpretation of hair analysis results Forensic. Sci Int. 2000;107:5–12. doi: 10.1016/S0379-0738(99)00146-2. [DOI] [PubMed] [Google Scholar]
- 43.The golden verses of Pythagoras. https://en.wikipedia.org/wiki/The_golden_verses_of_Pythagoras. [accessed 10 December 2019].
- 44.William Keith Chambers Guthrie . Cambridge University Press; Cambridge: 1978. A history of Greek philosophy, the earlier Presocratics and the Pythagoreans. [Google Scholar]
- 45.Kabat-Zinn, Full J. Bantam Dell; New York: 2013. Catastrophe living: using the wisdom of your body and mind to face stress, pain, and illness. [Google Scholar]
- 46.Creswell J.D. Mindfulness interventions. Annu Rev Psychol. 2017;68:491–516. doi: 10.1146/annurev-psych-042716-051139. [DOI] [PubMed] [Google Scholar]
- 47.Walsh Roger, Shapiro Shauna L. The meeting of meditative disciplines and western psychology: a mutually enriching dialogue. Am Psychol. 2006;61(3):227–239. doi: 10.1037/0003-066X.61.3.227. [DOI] [PubMed] [Google Scholar]
- 48.Buckner R.L., Andrews-Hanna J.R., Schacter D.L. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124(1):1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 49.Marchand W.R. Neural mechanisms of mindfulness and meditation: evidence from neuroimaging studies. World J Radiol. 2014;6(7):471. doi: 10.4329/wjr.v6.i7.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shapira-Lichter I., Oren N., Jacob Y., Gruberger M., Hendler T. Portraying the unique contribution of the default mode network to internally driven mnemonic processes. Proc Natl Acad Sci. 2013;110(13):4950–4955. doi: 10.1073/pnas.1209888110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Basten U., Christine S., Christian J.F. Trait anxiety and the neural efficiency of manipulation in working memory. Cognit Affect Behav Neurosci. 2012;12(3):571–588. doi: 10.3758/s13415-012-0100-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ford E.S., Bergmann M.M., Kröger J., Schienkiewitz A., Weikert C., Boeing H. Healthy living is the best revenge: findings from the European Prospective Investigation into Cancer and Nutrition-Potsdam study. Arch Intern Med. 2009;169(15):1355–1362. doi: 10.1001/archinternmed.2009.237. [DOI] [PubMed] [Google Scholar]
- 53.Lee I.M., Shiroma E.J., Kamada M., Bassett D.R., Matthews C.E., Buring J.E. Association of step volume and intensity with all-cause mortality in older women. J Am Intern Med. 2019 doi: 10.1001/jamainternmed.2019.0899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Klarsfeld A., Serge B., François R. L’horlogecircadienne à l’heure Nobel-PrixNobel de Médecine 2017: jeffrey C. Hall, Michael Rosbashet Michael W.Young. M-S (Med Sci) 2018;34(5):480–484. doi: 10.1051/medsci/20183405023. [DOI] [PubMed] [Google Scholar]
- 55.Circadian rhythms. https://en.wikipedia.org/wiki/Circadian_rhythm.[accessed 10 December 2019].
- 56.Baird B., Smallwood J., Gorgolewski K.J., Margulies D.S. Medial and lateral networks in anterior prefrontal cortex support metacognitive ability for memory and perception. J Neurosci. 2013;33(42):16657–16665. doi: 10.1523/JNEUROSCI.0786-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tagini A., Antonino R. The ‘I’ and the ‘Me’ in self-referential awareness: aneurocognitive hypothesis. Cogn Process. 2010;11(1):9–20. doi: 10.1007/s10339-009-0336-1. [DOI] [PubMed] [Google Scholar]
- 58.World Health Organization WHO Library Cataloguing in Publication Data Pacific physical activity guidelines for adults: framework for accelerating the communication of physical activity guidelines. 2008. https://www.who.int/dietphysicalactivity/publications/pacific_pa_guidelines.pdf SBN 978 92 9061 394 7. [PubMed]
- 59.NationalSleepFoundation.https://www.sleepfoundation.org/articles/healthysleep-tips [accessed 10 December 2019].
- 60.Ye M., Du K., Zhou J., Zhou Q., Shou M., Hu B. A meta analysis of the efficacy of cognitive behavior therapy on quality of life and psychological health of breast cancer survivors and patients. Psycho Oncol. 2018;27:1695–1703. doi: 10.1002/pon.4687. [DOI] [PubMed] [Google Scholar]
- 61.Antoni M.H., Lechner S.C., Kazi A., Wimberly S.R., Sifre T., Urcuyo K.R. How stress management improves quality of life after treatment for breast cancer. J Consult Clin Psychol. 2006;74(6):1143. doi: 10.1037/0022-006X.74.6.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pelekasis P., Zisi G., Koumarianou A., Marioli A., Chrousos G., Syrigos K. Forming a stress management and health promotion program for women undergoing chemotherapy for breast cancer: a pilot randomized controlled trial. Integr Cancer Ther. 2016;15(2):165–174. doi: 10.1177/1534735415598225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lehrer S., Green S., Ramanathan L., Rosenzweig K.E. Insufficient sleep associated with increased breast cancer mortality. Sleep Med. 2013;14:469. doi: 10.1016/j.sleep.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 64.Loh S.Y., Quek K.F. Cancer-behavior-coping in women with breast cancer: effect of a cancer self-management program. Intern J Appl Basic Med Res. 2011;1(2):84. doi: 10.4103/2229-516X.91150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rottmann N., Dalton S.O., Christensen J., Frederiksen K., Johansen C. Self-efficacy, adjustment style and well-being in breast cancer patients: a longitudinal study. Qual Life Res. 2010;19:827. doi: 10.1007/s11136-010-9653-1. [DOI] [PubMed] [Google Scholar]
- 66.Ibrahim E.M., A-Homaidh A. Physical activity and survival after breast cancer diagnosis: meta-analysis of published studies. Med Oncol. 2011;28:753–765. doi: 10.1007/s12032-010-9536-x. [DOI] [PubMed] [Google Scholar]
- 67.Dandamudi A., Tommie J., Nommsen-Rivers L., Couch S. Dietary patterns and breast cancer risk: a systematic review. Anticancer Res. 2018;38(6):3209–3222. doi: 10.21873/anticanres.12586. [DOI] [PubMed] [Google Scholar]
- 68.Antoni M., Lutgendorf S., Cole S.W., Dhabhar F.S., Sephton S.E., McDonald P.G. The influence of bio-behavioural factors on tumour biology: pathways and mechanisms. Nat Rev Cancer. 2006;6:240–248. doi: 10.1038/nrc1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Russell E., Koren G., Rieder M., Van Uum S. Hair cortisol as a biological marker of chronic stress: current status, future directions and unanswered questions. Psychoneuroendocrinology. 2012;37(5):58901. doi: 10.1016/j.psyneuen.2011.09.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
We state that data of the study is available on request from the corresponding author.