Abstract
Stem cell aging contributes to aging-associated tissue degeneration and dysfunction. Recent studies reveal a mitochondrial metabolic checkpoint that regulates stem cell quiescence and maintenance, and dysregulation of the checkpoint leads to functional deterioration of aged stem cells. Here, we present the evidence supporting the mitochondrial metabolic checkpoint regulating stem cell aging and demonstrating the feasibility to target this checkpoint to reverse stem cell aging. We discuss the mechanisms by which mitochondrial stress leads to stem cell deterioration. We speculate the therapeutic potential of targeting the mitochondrial metabolic checkpoint for rejuvenating aged stem cells and improving aging tissue functions.
Keywords: stem cell aging, SIRT2, SIRT3, SIRT7, NLRP3
The stem cell theory of aging postulates that aging is the result of the inability of tissue-specific stem cells to repair and maintain tissues. A large body of evidence supports the notion that stem cell functions decline with aging, consistent with the degeneration and dysfunction of aging regenerative tissues (de Haan and Lazare, 2018; Lopez-Otin et al., 2013; Rossi et al., 2008). These observations suggest the importance of stem cells in understanding the biology of aging and in developing effective therapies for treating diseases of aging. Genetic regulators of aging are critical for stem cell maintenance (Chen et al., 2008; Juntilla et al., 2010; Miyamoto et al., 2007; Tothova et al., 2007). Prominently, some genetic regulators of aging become dysregulated during aging and reversion of such dysregulations has been shown to improve the maintenance and function of aged stem cells (Brown et al., 2013; Luo et al., 2017; Mohrin et al., 2015). These findings strongly support the stem cell theory of aging and provide an entry point for understanding the molecular mechanism of stem cell aging and the feasibility of rejuvenating aged stem cells and reversing aging-associated tissue degeneration.
Mitochondrial Metabolic Checkpoint in Stem Cell Maintenance and Aging
Stem cells in many tissues convert between two metabolically distinctive states: metabolically inactive quiescent state and metabolically active proliferative state. In adult animals, stem cells are mostly quiescent due to a lack of physiological demand to proliferate. Compelling evidence indicates that stem cell quiescence is also a protective mechanism to prevent stem cell death and the depletion of the stem cell pool (Cheung and Rando, 2013; Ren et al., 2017). Quiescent stem cells have fewer mitochondria and primarily rely on glycolysis to support the energy demand (Ito and Suda, 2014). Upon the transition from quiescence to proliferation, mitochondrial biogenesis occurs and OXPHOS is upregulated to meet the increased energy demand of proliferating cells (Mohrin et al., 2018). This metabolic switch from glycolysis to OXPHOS is also necessary to support stem cell differentiation (Anso et al., 2017; Inoue et al., 2010; Khacho et al., 2016; Tang et al., 2016; Tormos et al., 2011; Vannini et al., 2016; Zhang et al., 2013; Zheng et al., 2016).
Increased mitochondrial biogenesis during the transition from stem cell quiescence to proliferation is inevitably concomitant with increased mitochondrial stress, such as mitochondrial oxidative stress and mitochondrial protein folding stress, which makes the cells prone to death (Mohrin et al., 2018). Thus, a cellular condition that needs to be monitored during the restriction point, a cellular checkpoint at the transition of G0 to G1 state of the cell cycle, is the mitochondrial health. Because cells are committed to proliferation once they pass the restriction point, monitoring the mitochondrial health at the restriction point ensures that only cells with healthy mitochondria and metabolic competence can enter the cell cycle.
Recent studies revealed sirtuins, a family of protein deacetylases, as critical regulators of the mitochondrial metabolic checkpoint in stem cells. SIRT3 is a mitochondrial deacetylase that modifies mitochondrial antioxidant SOD2 to reduce oxidative stress (Qiu et al., 2010). It is highly expressed in hematopoietic stem cells (HSCs) and its expression is suppressed in differentiated hematopoietic cells (Brown et al., 2013). SIRT3 deletion results in loss of HSC quiescence and compromised HSC maintenance and function at an old but not young age. SIRT7 is a histone deacetylase that represses the expression of mitochondrial ribosomal proteins to regulate the mitochondrial unfolded protein response and to reduce mitochondrial protein folding stress (Mohrin et al., 2015; Shin et al., 2013). SIRT7 ablation leads to loss of HSC quiescence and aspects of HSC aging phenotypes, such as reduced regenerative capacity per cell and myeloid-biased differentiation (Mohrin et al., 2015). Interestingly, the expression of SIRT3 and SIRT7 is reduced in HSCs during aging, and overexpression of SIRT3 or SIRT7 improves the function of aged HSCs (Brown et al., 2013; Chambers et al., 2007; Mohrin et al., 2015). These studies demonstrate that dysregulation of the mitochondrial metabolic checkpoint is an underlying cause of stem cell aging and may be targeted for rejuvenation.
Mitochondrial Quality Control in Stem Cells
Mitochondrial damage can also be mitigated by mitochondria fusion/fission, which occurs to dilute or segregate the damage (Chen and Chan, 2009; Twig et al., 2008). Damaged mitochondria can also be cleared by the process of mitophagy (Ding and Yin, 2012; Twig et al., 2008). Consistent with the view that mitochondrial stress critically regulates stem cell fate and aging, these mitochondrial quality control mechanisms have been shown to be essential for stem cell maintenance.
The asymmetric division of stem cells allows them to generate two daughter cells with distinct cell fates (Katajisto et al., 2015; Morrison and Kimble, 2006). The daughter cell that inherited fewer aged and possibly damaged mitochondria preserves the stemness (Katajisto et al., 2015). Silencing dynamin-related protein 1 (DRP1), a component required for mitochondrial fission, prevents the asymmetric segregation of mitochondria during cell division and leads to the subsequent loss of stemness in the daughter cells (Katajisto et al., 2015). Inactivation of DRP1 upon transplantation stress results in the accumulation of damaged mitochondria and loss of HSC regenerative potential (Hinge et al., 2020).
Loss of mitofusin1/2 (MFN1/2) or OPA1, mitochondrial fusion proteins, leads to aberrant mitochondrial fragmentation, a shift toward oxidative respiration, and excessive ROS production in neural stem cells (NSCs) (Khacho et al., 2016). This structural disruption of mitochondria impairs the self-renewal of NSCs, further depletes the NSCs pool, blocks neurogenesis and causes cognitive dysfunction (Khacho et al., 2016). Similarly, HSCs have higher expression of MFN2 and longer mitochondrial length compared to the progeny (Luchsinger et al., 2016). Interestingly, MFN2 is preferentially required for the maintenance of HSCs primed for lymphopoiesis (Luchsinger et al., 2016). This observation is in keeping with the notion that mitochondrial stress is a cause of HSC aging, as characteristics of HSC aging include reduced lymphopoiesis and increased myelopoiesis. It will be worth examining whether modulating mitochondrial fusion/fission could affect the lineage potential of HSCs during aging and reverse HSC aging.
Adult stem cells have high levels of autophagy under the physiological state (Salemi et al., 2012). This autophagic activity is necessary for the self-renewal and differentiation capacities of stem cells from various tissue origins, including HSCs, muscle stem cells (MuSCs), and NSCs (Ito et al., 2016; Mortensen et al., 2011; Salemi et al., 2012; Sun et al., 2015). Loss of autophagy increases mitochondrial oxidative stress and leads to senescence in young MuSCs (Garcia-Prat et al., 2016), while increases autophagy via chemical uncoupling enhances engraftment ability of HSCs (Vannini et al., 2016). Lysosomal sequestration of mitochondrial enhances the regenerative capacity of HSCs (Liang et al., 2020). Autophagy is overall diminished in aged stem cells, contributing to the loss of quiescence, senescence, and ultimately degeneration (Garcia-Prat et al., 2016; Sun et al., 2015).
Mechanisms underlying mitochondrial stress regulation of stem cells
The mitochondrial genome encodes genes that are essential for mitochondrial respiratory chain including OXPHOS subunits, ribosomal RNAs and transfer RNAs. Mitochondrial oxidative stress could result in mitochondrial DNA (mtDNA) mutations due to proximity. Indeed, mtDNA mutations accumulate with age in stem cells, and impair mitochondrial oxidative respiration in the progeny (Kang et al., 2016; McDonald et al., 2008; Taylor et al., 2003). In NSCs, mitochondrial 8-oxoguanine DNA glycosylase (OGG1), an enzyme for oxidized DNA lesion repair, is required to maintain mtDNA integrity and support mitochondrial biogenesis during proper differentiation (Wang et al., 2011; Wang et al., 2010). Moreover, mtDNA defects have been shown causative to stem cell dysfunction in mice carrying a proofreading-defective mitochondrial DNA polymerase (POLG-mutator mice) (Ahlqvist et al., 2012; Chen et al., 2009; Kujoth et al., 2005; Norddahl et al., 2011; Trifunovic et al., 2004). POLG-mutator mice develop severe somatic mtDNA mutations with accelerated aging and reduced lifespan (Kujoth et al., 2005; Trifunovic et al., 2004). These mice have defective NSCs and HSCs, and exhibit some premature HSC aging phenotypes including anemia, lymphopenia, and myeloid differentiation (Ahlqvist et al., 2012; Chen et al., 2009; Norddahl et al., 2011). These findings indicate that mtDNA integrity is needed for proper mitochondrial function and stem cell fate decisions.
Recent studies uncovered a signaling event that mediates the effect of mitochondrial stress on the degeneration of HSCs during aging. The NACHT, LRR and PYD domains-containing protein 3 (NLRP3) inflammasome is an innate immune sensor that can be activated by metabolic danger signals such as ROS, leading to proinflammatory cytokine secretion and/or caspase 1-dependent cell death (Grant and Dixit, 2013; Guo et al., 2015; Nakahira et al., 2011). While extensively studied in myeloid cells, NLRP3 has been found to be expressed and function in HSCs (Luo et al., 2019). The NLRP3 inflammasome is activated in aged HSCs due to increased mitochondrial stress. Ameliorating mitochondrial stress by overexpressing SIRT3 or SIRT7 reduces the caspase 1 activity and improves the function of aged HSCs (Brown et al., 2013; Luo et al., 2019; Mohrin et al., 2015). NLRP3 is a bona fide substrate of SIRT2, which deacetylates NLRP3 and inhibits the activation of the NLRP3 inflammasome (He et al., 2020). The expression of SIRT2 is reduced in aged HSCs, resulting in aberrant activation of the NLRP3 inflammasome and increased susceptibility to mitochondrial stress-induced stem cell deterioration (Luo et al., 2019).
Therapeutic Implications
The findings that mitochondrial stress is a main driver of stem cell deterioration during aging and the mitochondrial protective programs may be targeted to reverse stem cell aging have important therapeutic implications (Brown et al., 2013; Luo et al., 2019; Mohrin et al., 2015). Sirtuin activation is likely to improve mitochondrial integrity and stem cell maintenance. NAD+ is the co-factor for the sirtuin family proteins that catalyze protein deacetylation (Houtkooper et al., 2012). Indeed, replenishing NAD+ levels by dietary nicotinamide riboside (NR), a key NAD+ precursor, increased sirtuin activity, reduced mitochondrial stress within HSCs, and improved the regenerative function of HSCs (Vannini et al., 2019). It significantly expanded the pool of progenitors, without concurrent HSC exhaustion, boosted survival by 80%, and accelerated blood reconstitution after lethal irradiation and HSC transplantation. NR treatment induced the mitochondrial unfolded protein response in aged MuSCs, and mitigated muscle degeneration by decreasing MuSC senescence (Zhang et al., 2016). Treatment with NR was also effective in preventing muscle degeneration in a mouse model of muscular dystrophy. Treatment with NR rejuvenated intestine stem cells (ISCs) from aged mice and reversed an impaired ability to repair gut damage (Igarashi et al., 2019), attenuates NSC senescence and increases the lifespan of aged C57BL/6J mice (Zhang et al., 2016). These findings indicate that the mitochondrial metabolic checkpoint regulation of stem cell aging is a conserved mechanism across tissues and offer a translational path for maintaining tissue homeostasis and treating tissue degenerative diseases.
Future Directions
Recent studies support a model that stem cell aging is driven by epigenetic drift and the resulting dysregulation of gene expression and cellular function (Buisman and de Haan, 2019; Chen and Kerr, 2019; Grigoryan et al., 2018). However, many questions remain unaddressed. What are the epigenetic changes in stem cells during aging? What causes the epigenetic drift in aged stem cells? How does epigenetic drift lead to loss of stem cell function? Mitochondrial stress may provide a piece of the puzzle in understanding the epigenetic regulation of stem cell aging because intermediates from mitochondrial metabolism can act as co-enzymes for epigenetic regulators (Matilainen et al., 2017). Several TCA cycle intermediates such as acetyl-coenzyme A (acetyl-CoA), α-ketoglutarate (α-KG), NAD+, and S-adenosyl-methionine (SAM) from one-carbon metabolism are among the most important metabolites in epigenetic regulation (Matilainen et al., 2017). Future studies are needed to elucidate how mitochondrial stress affects these epigenetic remodeling metabolites, which in turn cause epigenetic drive during stem cell aging.
The understanding of stem cell aging has been mostly powered by studies in mouse models. It is pivotal to understand if the knowledge we gained in mouse studies can be translated to humans. Recent human studies reveal a phenomenon termed clonal hematopoiesis, describing a clonal expansion of blood cells derived from mutated HSCs in aged humans (Jaiswal et al., 2014; Jaiswal et al., 2017). Individuals with clonal hematopoiesis are of higher risk for not only hematologic malignancies but also diseases in distal tissues. It would be of interest to understand if mitochondrial stress drives stem cell aging in humans and contributes to the emergence of clonal hematopoiesis.
Figure 1. The mitochondrial metabolic checkpoint regulates stem cell quiescence, maintenance, and aging.
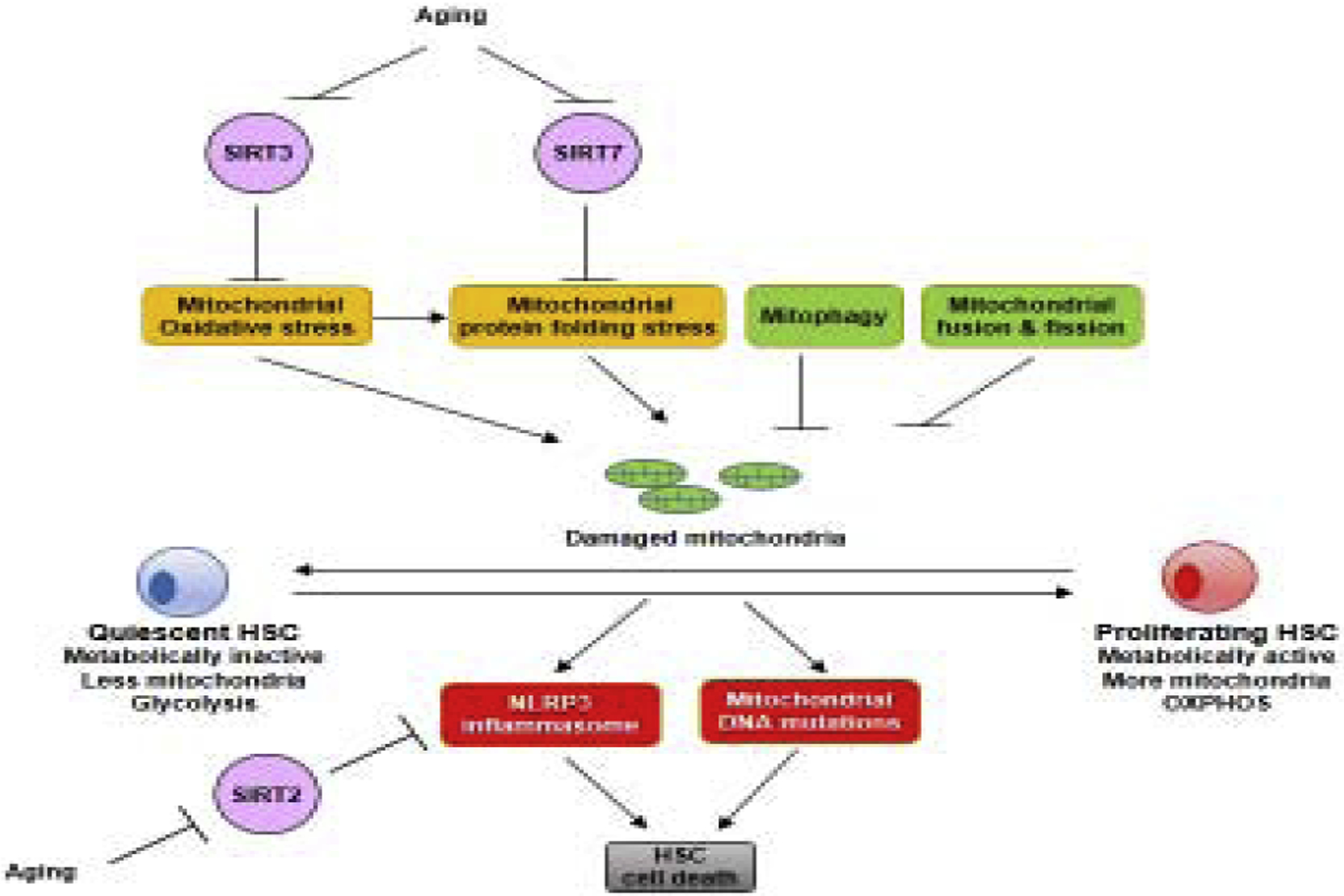
Upon stem cell transition from quiescence to proliferation, mitochondrial biogenesis takes place, which is associated with increased mitochondrial oxidative stress and mitochondrial protein folding stress. SIRT3 and SIRT7 govern mitochondrial stress in stem cells. Damaged mitochondria can also be cleared by mitochondrial fusion and fission, and mitophagy. Accumulation of mitochondrial stress results in stem cell death due to DNA mutations and activation of the NLRP3 inflammasome, which is regulated by SIRT2. During aging, the expression of SIRT2, SIRT3, and SIRT7 reduces in stem cells, resulting in the dysregulation of the mitochondrial metabolic checkpoint and loss of stem cell maintenance.
Highlights.
Mitochondria metabolic checkpoint critically regulates stem cell aging.
Mitochondrial metabolic checkpoint is governed by sirtuins in stem cells.
Mitochondrial metabolic checkpoint has therapeutic implications for aging-associated tissue degeneration.
ACKNOWLEDGMENTS
Supported by NIH R01DK 117481 (D.C.), R01DK101885 (D.C.), R01AG063404 (D.C.), R01AG 063389 (D.C.), National Institute of Food and Agriculture (D.C.), James C.Y. Soong Fellowship (W.-C. M.), Government Scholarship for Study Abroad (GSSA) from Taiwan (W.-C. M.), the ITO Scholarship (R.O.), the Honjo International Scholarship (R.O.), and NIH T32GM098218 (A.W.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- Ahlqvist KJ, Hamalainen RH, Yatsuga S, Uutela M, Terzioglu M, Gotz A, Forsstrom S, Salven P, Angers-Loustau A, Kopra OH, Tyynismaa H, Larsson NG, Wartiovaara K, Prolla T, Trifunovic A, Suomalainen A, 2012. Somatic progenitor cell vulnerability to mitochondrial DNA mutagenesis underlies progeroid phenotypes in Polg mutator mice. Cell metabolism 15, 100–109. [DOI] [PubMed] [Google Scholar]
- Anso E, Weinberg SE, Diebold LP, Thompson BJ, Malinge S, Schumacker PT, Liu X, Zhang Y, Shao Z, Steadman M, Marsh KM, Xu J, Crispino JD, Chandel NS, 2017. The mitochondrial respiratory chain is essential for haematopoietic stem cell function. Nature cell biology 19, 614–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K, Xie S, Qiu X, Mohrin M, Shin J, Liu Y, Zhang D, Scadden DT, Chen D, 2013. SIRT3 reverses aging-associated degeneration. Cell Rep 3, 319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buisman SC, de Haan G, 2019. Epigenetic Changes as a Target in Aging Haematopoietic Stem Cells and Age-Related Malignancies. Cells 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers SM, Shaw CA, Gatza C, Fisk CJ, Donehower LA, Goodell MA, 2007. Aging hematopoietic stem cells decline in function and exhibit epigenetic dysregulation. PLoS biology 5, e201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Liu Y, Liu R, Ikenoue T, Guan KL, Liu Y, Zheng P, 2008. TSC-mTOR maintains quiescence and function of hematopoietic stem cells by repressing mitochondrial biogenesis and reactive oxygen species. J Exp Med 205, 2397–2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Kerr C, 2019. The Epigenetics of Stem Cell Aging Comes of Age. Trends Cell Biol 29, 563–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Chan DC, 2009. Mitochondrial dynamics--fusion, fission, movement, and mitophagy--in neurodegenerative diseases. Human molecular genetics 18, R169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ML, Logan TD, Hochberg ML, Shelat SG, Yu X, Wilding GE, Tan W, Kujoth GC, Prolla TA, Selak MA, Kundu M, Carroll M, Thompson JE, 2009. Erythroid dysplasia, megaloblastic anemia, and impaired lymphopoiesis arising from mitochondrial dysfunction. Blood 114, 4045–4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung TH, Rando TA, 2013. Molecular regulation of stem cell quiescence. Nat Rev Mol Cell Biol 14, 329–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Haan G, Lazare SS, 2018. Aging of hematopoietic stem cells. Blood 131, 479–487. [DOI] [PubMed] [Google Scholar]
- Ding WX, Yin XM, 2012. Mitophagy: mechanisms, pathophysiological roles, and analysis. Biol Chem 393, 547–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Prat L, Martinez-Vicente M, Perdiguero E, Ortet L, Rodriguez-Ubreva J, Rebollo E, Ruiz-Bonilla V, Gutarra S, Ballestar E, Serrano AL, Sandri M, Munoz-Canoves P, 2016. Autophagy maintains stemness by preventing senescence. Nature 529, 37–42. [DOI] [PubMed] [Google Scholar]
- Grant RW, Dixit VD, 2013. Mechanisms of disease: inflammasome activation and the development of type 2 diabetes. Front Immunol 4, 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoryan A, Guidi N, Senger K, Liehr T, Soller K, Marka G, Vollmer A, Markaki Y, Leonhardt H, Buske C, Lipka DB, Plass C, Zheng Y, Mulaw MA, Geiger H, Florian MC, 2018. LaminA/C regulates epigenetic and chromatin architecture changes upon aging of hematopoietic stem cells. Genome Biol 19, 189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Callaway JB, Ting JP, 2015. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nature medicine 21, 677–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M, Chiang HH, Luo H, Zheng Z, Qiao Q, Wang L, Tan M, Ohkubo R, Mu WC, Zhao S, Wu H, Chen D, 2020. An Acetylation Switch of the NLRP3 Inflammasome Regulates Aging-Associated Chronic Inflammation and Insulin Resistance. Cell Metab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinge A, He J, Bartram J, Javier J, Xu J, Fjellman E, Sesaki H, Li T, Yu J, Wunderlich M, Mulloy J, Kofron M, Salomonis N, Grimes HL, Filippi MD, 2020. Asymmetrically Segregated Mitochondria Provide Cellular Memory of Hematopoietic Stem Cell Replicative History and Drive HSC Attrition. Cell Stem Cell 26, 420–430 e426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houtkooper RH, Pirinen E, Auwerx J, 2012. Sirtuins as regulators of metabolism and healthspan. Nature reviews. Molecular cell biology 13, 225–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi M, Miura M, Williams E, Jaksch F, Kadowaki T, Yamauchi T, Guarente L, 2019. NAD(+) supplementation rejuvenates aged gut adult stem cells. Aging Cell 18, e12935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue S, Noda S, Kashima K, Nakada K, Hayashi J, Miyoshi H, 2010. Mitochondrial respiration defects modulate differentiation but not proliferation of hematopoietic stem and progenitor cells. FEBS letters 584, 3402–3409. [DOI] [PubMed] [Google Scholar]
- Ito K, Suda T, 2014. Metabolic requirements for the maintenance of self-renewing stem cells. Nat Rev Mol Cell Biol 15, 243–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Turcotte R, Cui J, Zimmerman SE, Pinho S, Mizoguchi T, Arai F, Runnels JM, Alt C, Teruya-Feldstein J, Mar JC, Singh R, Suda T, Lin CP, Frenette PS, Ito K, 2016. Self-renewal of a purified Tie2+ hematopoietic stem cell population relies on mitochondrial clearance. Science 354, 1156–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG, Lindsley RC, Mermel CH, Burtt N, Chavez A, Higgins JM, Moltchanov V, Kuo FC, Kluk MJ, Henderson B, Kinnunen L, Koistinen HA, Ladenvall C, Getz G, Correa A, Banahan BF, Gabriel S, Kathiresan S, Stringham HM, McCarthy MI, Boehnke M, Tuomilehto J, Haiman C, Groop L, Atzmon G, Wilson JG, Neuberg D, Altshuler D, Ebert BL, 2014. Age-related clonal hematopoiesis associated with adverse outcomes. The New England journal of medicine 371, 2488–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal S, Natarajan P, Silver AJ, Gibson CJ, Bick AG, Shvartz E, McConkey M, Gupta N, Gabriel S, Ardissino D, Baber U, Mehran R, Fuster V, Danesh J, Frossard P, Saleheen D, Melander O, Sukhova GK, Neuberg D, Libby P, Kathiresan S, Ebert BL, 2017. Clonal Hematopoiesis and Risk of Atherosclerotic Cardiovascular Disease. The New England journal of medicine 377, 111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juntilla MM, Patil VD, Calamito M, Joshi RP, Birnbaum MJ, Koretzky GA, 2010. AKT1 and AKT2 maintain hematopoietic stem cell function by regulating reactive oxygen species. Blood 115, 4030–4038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang E, Wang X, Tippner-Hedges R, Ma H, Folmes CD, Gutierrez NM, Lee Y, Van Dyken C, Ahmed R, Li Y, Koski A, Hayama T, Luo S, Harding CO, Amato P, Jensen J, Battaglia D, Lee D, Wu D, Terzic A, Wolf DP, Huang T, Mitalipov S, 2016. Age-Related Accumulation of Somatic Mitochondrial DNA Mutations in Adult-Derived Human iPSCs. Cell stem cell 18, 625–636. [DOI] [PubMed] [Google Scholar]
- Katajisto P, Dohla J, Chaffer CL, Pentinmikko N, Marjanovic N, Iqbal S, Zoncu R, Chen W, Weinberg RA, Sabatini DM, 2015. Stem cells. Asymmetric apportioning of aged mitochondria between daughter cells is required for stemness. Science 348, 340–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khacho M, Clark A, Svoboda DS, Azzi J, MacLaurin JG, Meghaizel C, Sesaki H, Lagace DC, Germain M, Harper ME, Park DS, Slack RS, 2016. Mitochondrial Dynamics Impacts Stem Cell Identity and Fate Decisions by Regulating a Nuclear Transcriptional Program. Cell stem cell 19, 232–247. [DOI] [PubMed] [Google Scholar]
- Kujoth GC, Hiona A, Pugh TD, Someya S, Panzer K, Wohlgemuth SE, Hofer T, Seo AY, Sullivan R, Jobling WA, Morrow JD, Van Remmen H, Sedivy JM, Yamasoba T, Tanokura M, Weindruch R, Leeuwenburgh C, Prolla TA, 2005. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science 309, 481–484. [DOI] [PubMed] [Google Scholar]
- Liang R, Arif T, Kalmykova S, Kasianov A, Lin M, Menon V, Qiu J, Bernitz JM, Moore K, Lin F, Benson DL, Tzavaras N, Mahajan M, Papatsenko D, Ghaffari S, 2020. Restraining Lysosomal Activity Preserves Hematopoietic Stem Cell Quiescence and Potency. Cell Stem Cell 26, 359–376 e357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G, 2013. The hallmarks of aging. Cell 153, 1194–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchsinger LL, de Almeida MJ, Corrigan DJ, Mumau M, Snoeck HW, 2016. Mitofusin 2 maintains haematopoietic stem cells with extensive lymphoid potential. Nature 529, 528–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo H, Chiang HH, Louw M, Susanto A, Chen D, 2017. Nutrient Sensing and the Oxidative Stress Response. Trends Endocrinol Metab 28, 449–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo H, Mu WC, Karki R, Chiang HH, Mohrin M, Shin JJ, Ohkubo R, Ito K, Kanneganti TD, Chen D, 2019. Mitochondrial Stress-Initiated Aberrant Activation of the NLRP3 Inflammasome Regulates the Functional Deterioration of Hematopoietic Stem Cell Aging. Cell reports 26, 945–954.e944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matilainen O, Quiros PM, Auwerx J, 2017. Mitochondria and Epigenetics - Crosstalk in Homeostasis and Stress. Trends in cell biology 27, 453–463. [DOI] [PubMed] [Google Scholar]
- McDonald SA, Greaves LC, Gutierrez-Gonzalez L, Rodriguez-Justo M, Deheragoda M, Leedham SJ, Taylor RW, Lee CY, Preston SL, Lovell M, Hunt T, Elia G, Oukrif D, Harrison R, Novelli MR, Mitchell I, Stoker DL, Turnbull DM, Jankowski JA, Wright NA, 2008. Mechanisms of field cancerization in the human stomach: the expansion and spread of mutated gastric stem cells. Gastroenterology 134, 500–510. [DOI] [PubMed] [Google Scholar]
- Miyamoto K, Araki KY, Naka K, Arai F, Takubo K, Yamazaki S, Matsuoka S, Miyamoto T, Ito K, Ohmura M, Chen C, Hosokawa K, Nakauchi H, Nakayama K, Nakayama KI, Harada M, Motoyama N, Suda T, Hirao A, 2007. Foxo3a is essential for maintenance of the hematopoietic stem cell pool. Cell stem cell 1, 101–112. [DOI] [PubMed] [Google Scholar]
- Mohrin M, Shin J, Liu Y, Brown K, Luo H, Xi Y, Haynes CM, Chen D, 2015. Stem cell aging. A mitochondrial UPR-mediated metabolic checkpoint regulates hematopoietic stem cell aging. Science 347, 1374–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohrin M, Widjaja A, Liu Y, Luo H, Chen D, 2018. The mitochondrial unfolded protein response is activated upon hematopoietic stem cell exit from quiescence. Aging cell 17, e12756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SJ, Kimble J, 2006. Asymmetric and symmetric stem-cell divisions in development and cancer. Nature 441, 1068–1074. [DOI] [PubMed] [Google Scholar]
- Mortensen M, Watson AS, Simon AK, 2011. Lack of autophagy in the hematopoietic system leads to loss of hematopoietic stem cell function and dysregulated myeloid proliferation. Autophagy 7, 1069–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahira K, Haspel JA, Rathinam VA, Lee SJ, Dolinay T, Lam HC, Englert JA, Rabinovitch M, Cernadas M, Kim HP, Fitzgerald KA, Ryter SW, Choi AM, 2011. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol 12, 222–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norddahl GL, Pronk CJ, Wahlestedt M, Sten G, Nygren JM, Ugale A, Sigvardsson M, Bryder D, 2011. Accumulating mitochondrial DNA mutations drive premature hematopoietic aging phenotypes distinct from physiological stem cell aging. Cell stem cell 8, 499–510. [DOI] [PubMed] [Google Scholar]
- Qiu X, Brown K, Hirschey MD, Verdin E, Chen D, 2010. Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab 12, 662–667. [DOI] [PubMed] [Google Scholar]
- Ren R, Ocampo A, Liu GH, Izpisua Belmonte JC, 2017. Regulation of Stem Cell Aging by Metabolism and Epigenetics. Cell Metab 26, 460–474. [DOI] [PubMed] [Google Scholar]
- Rossi DJ, Jamieson CH, Weissman IL, 2008. Stems cells and the pathways to aging and cancer. Cell 132, 681–696. [DOI] [PubMed] [Google Scholar]
- Salemi S, Yousefi S, Constantinescu MA, Fey MF, Simon HU, 2012. Autophagy is required for self-renewal and differentiation of adult human stem cells. Cell research 22, 432–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J, He M, Liu Y, Paredes S, Villanova L, Brown K, Qiu X, Nabavi N, Mohrin M, Wojnoonski K, Li P, Cheng HL, Murphy AJ, Valenzuela DM, Luo H, Kapahi P, Krauss R, Mostoslavsky R, Yancopoulos GD, Alt FW, Chua KF, Chen D, 2013. SIRT7 represses Myc activity to suppress ER stress and prevent fatty liver disease. Cell reports 5, 654–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun N, Yun J, Liu J, Malide D, Liu C, Rovira II, Holmstrom KM, Fergusson MM, Yoo YH, Combs CA, Finkel T, 2015. Measuring In Vivo Mitophagy. Molecular cell 60, 685–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Luo B, Deng Z, Wang B, Liu F, Li J, Shi W, Xie H, Hu X, Li J, 2016. Mitochondrial aerobic respiration is activated during hair follicle stem cell differentiation, and its dysfunction retards hair regeneration. PeerJ 4, e1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor RW, Barron MJ, Borthwick GM, Gospel A, Chinnery PF, Samuels DC, Taylor GA, Plusa SM, Needham SJ, Greaves LC, Kirkwood TB, Turnbull DM, 2003. Mitochondrial DNA mutations in human colonic crypt stem cells. J Clin Invest 112, 1351–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tormos KV, Anso E, Hamanaka RB, Eisenbart J, Joseph J, Kalyanaraman B, Chandel NS, 2011. Mitochondrial complex III ROS regulate adipocyte differentiation. Cell metabolism 14, 537–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tothova Z, Kollipara R, Huntly BJ, Lee BH, Castrillon DH, Cullen DE, McDowell EP, Lazo-Kallanian S, Williams IR, Sears C, Armstrong SA, Passegue E, DePinho RA, Gilliland DG, 2007. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell 128, 325–339. [DOI] [PubMed] [Google Scholar]
- Trifunovic A, Wredenberg A, Falkenberg M, Spelbrink JN, Rovio AT, Bruder CE, Bohlooly YM, Gidlof S, Oldfors A, Wibom R, Tornell J, Jacobs HT, Larsson NG, 2004. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature 429, 417–423. [DOI] [PubMed] [Google Scholar]
- Twig G, Elorza A, Molina AJ, Mohamed H, Wikstrom JD, Walzer G, Stiles L, Haigh SE, Katz S, Las G, Alroy J, Wu M, Py BF, Yuan J, Deeney JT, Corkey BE, Shirihai OS, 2008. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. The EMBO journal 27, 433–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannini N, Campos V, Girotra M, Trachsel V, Rojas-Sutterlin S, Tratwal J, Ragusa S, Stefanidis E, Ryu D, Rainer PY, Nikitin G, Giger S, Li TY, Semilietof A, Oggier A, Yersin Y, Tauzin L, Pirinen E, Cheng WC, Ratajczak J, Canto C, Ehrbar M, Sizzano F, Petrova TV, Vanhecke D, Zhang L, Romero P, Nahimana A, Cherix S, Duchosal MA, Ho PC, Deplancke B, Coukos G, Auwerx J, Lutolf MP, Naveiras O, 2019. The NAD-Booster Nicotinamide Riboside Potently Stimulates Hematopoiesis through Increased Mitochondrial Clearance. Cell stem cell 24, 405–418.e407. [DOI] [PubMed] [Google Scholar]
- Vannini N, Girotra M, Naveiras O, Nikitin G, Campos V, Giger S, Roch A, Auwerx J, Lutolf MP, 2016. Specification of haematopoietic stem cell fate via modulation of mitochondrial activity. Nature communications 7, 13125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Esbensen Y, Kunke D, Suganthan R, Rachek L, Bjoras M, Eide L, 2011. Mitochondrial DNA damage level determines neural stem cell differentiation fate. J Neurosci 31, 9746–9751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Osenbroch P, Skinnes R, Esbensen Y, Bjoras M, Eide L, 2010. Mitochondrial DNA integrity is essential for mitochondrial maturation during differentiation of neural stem cells. Stem cells (Dayton, Ohio) 28, 2195–2204. [DOI] [PubMed] [Google Scholar]
- Zhang H, Ryu D, Wu Y, Gariani K, Wang X, Luan P, D’Amico D, Ropelle ER, Lutolf MP, Aebersold R, Schoonjans K, Menzies KJ, Auwerx J, 2016. NAD(+) repletion improves mitochondrial and stem cell function and enhances life span in mice. Science 352, 1436–1443. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Marsboom G, Toth PT, Rehman J, 2013. Mitochondrial respiration regulates adipogenic differentiation of human mesenchymal stem cells. PloS one 8, e77077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X, Boyer L, Jin M, Mertens J, Kim Y, Ma L, Ma L, Hamm M, Gage FH, Hunter T, 2016. Metabolic reprogramming during neuronal differentiation from aerobic glycolysis to neuronal oxidative phosphorylation. eLife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]


