ABSTRACT
Mosquitoes are best known for their proclivity towards biting humans and transmitting bloodborne pathogens, but there are over 3500 species, including both blood-feeding and non-blood-feeding taxa. The diversity of host preference in mosquitoes is exemplified by the feeding habits of mosquitoes in the genus Malaya that feed on ant regurgitation or those from the genus Uranotaenia that favor amphibian hosts. Host preference is also by no means static, but is characterized by behavioral plasticity that allows mosquitoes to switch hosts when their preferred host is unavailable and by learning host cues associated with positive or negative experiences. Here we review the diverse range of host-preference behaviors across the family Culicidae, which includes all mosquitoes, and how adaptations in neural circuitry might affect changes in preference both within the life history of a mosquito and across evolutionary time-scales.
KEY WORDS: Insect, Sensory processing, Learning, Sensory systems, Neurobiology
Summary: This Review discusses the environmental, neurobiological and genetic factors driving extreme host preference diversity and plasticity among the Culicidae, both within the life history of a mosquito and across evolutionary time-scales.
Introduction
Mosquitoes that prefer to bite humans have long been studied in their capacity as disease vectors, commanding significant influence over global ecosystems, epidemiology and economies by their impact on human health and welfare (WHO, 2016). However, over 3500 species of mosquito have been described in the family Culicidae, inhabiting every continent except Antarctica (Knight and Stone, 1977). Preferred hosts vary widely across mosquito species, ranging from humans and other mammals to reptiles, birds and arthropods (Fig. 1) (Harris et al., 1969; Tempelis, 1975). Some species may be specialists or opportunists and some have evolved loss of blood-feeding entirely, including all members of the genera Malaya, Topomyia and Toxorhynchites (Clements, 1999; Day, 2005; Steffan and Evenhuis, 1981). Where blood-feeding has been conserved, host preference does not track the phylogeny of Culicidae, and different species within a genus may prefer different sources of blood. An intriguing issue relates to how mosquitoes choose their hosts.
Fig. 1.
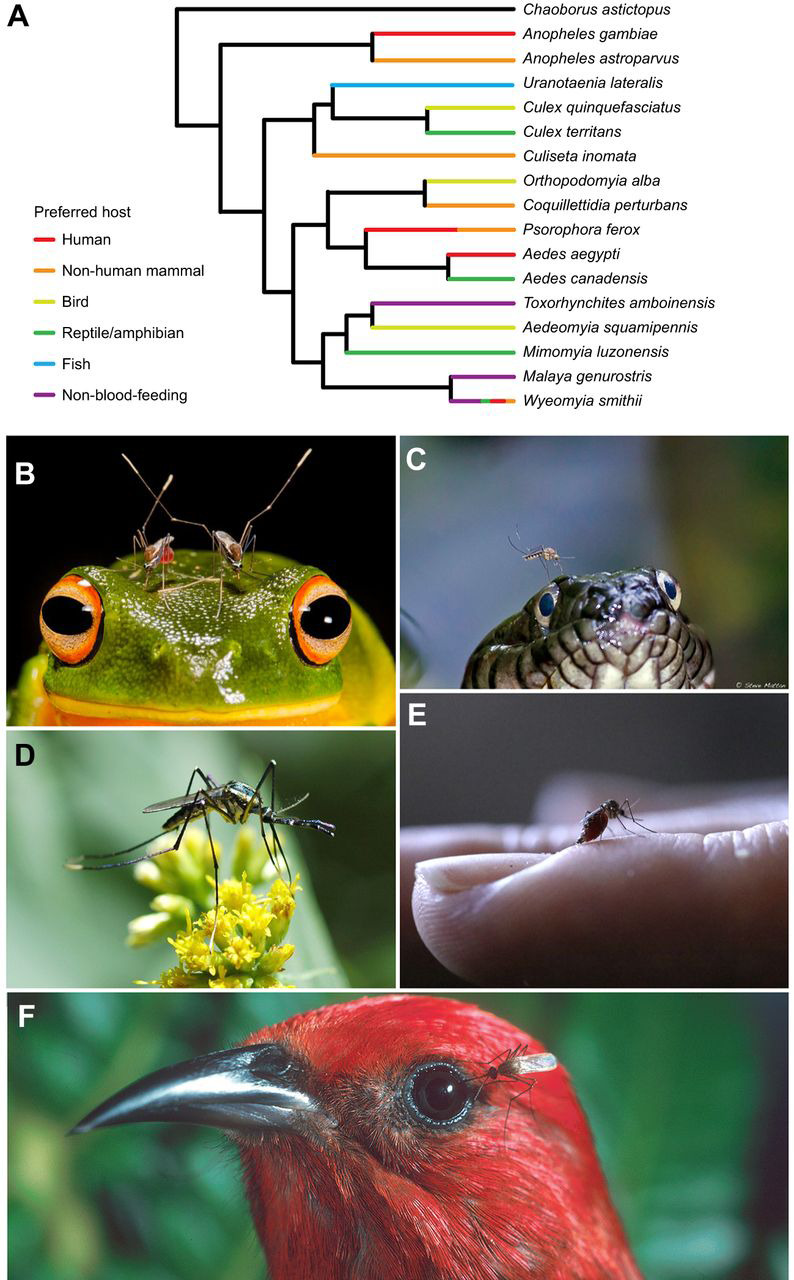
Various host preferences have evolved multiple times in different mosquito lineages. (A) Phylogenetic relationships of selected mosquito species, with midges (Chaoborus astictopus) shown as an outgroup, adapted from Reidenbach et al. (2009). Taxa are color coded by dominant host preference (Braima et al., 2017; Burkett-Cadena and Blosser, 2017; Clements, 1999; Danabalan et al., 2014; Edman et al., 1972; Gillies, 1964; Molaei et al., 2008; Steffan and Evenhuis, 1981; Tempelis, 1975). Within a genus, host preference may vary across species. The pitcher-plant mosquito Wyeomyia smithii is an obligate non-blood-feeder in the northern part of its range, whereas individuals living in the southern part of this species' range may blood feed after the first oviposition (Lounibous et al., 1982; Bradshaw and Holzapfel, 1983). (B–F) Examples of the diversity of hosts preferred by different mosquito species, including frogs (B), snakes (C), flowers/non-blood-feeding (D), humans and other mammals (E) and birds (F). Photo credits: (B) Matthew McIntosh, (C) Steve Mattan, (D) Pennsylvania Department of Natural Resources, (E) J.A.R., (F) Jack Jeffrey.
To answer this question about host preference and behavior, we must examine the mosquito sensory nervous system that is used to detect hosts and the brain that computes and commands behavioral actions. To a great extent, the nervous system is hard-wired, with genetically determined receptors for specific information in the environment, allowing mosquitoes to detect cues from some host organisms and not others (McBride et al., 2014; Rinker et al., 2013). By comparing which receptors are expressed by different mosquito species, we can begin to understand differences in host-seeking behaviors. However, there are significant gaps in our knowledge about the degree to which various types of plasticity and learning contribute to host preferences. One clue comes from epidemiological analyses of disease vectors, including mosquitoes, suggesting that only 20% of a host population can account for 80% of transmission potential (Woolhouse et al., 1997). This implies that, within a host species, a subset of individuals is attracting more mosquitoes than the rest (Kelly, 2001). One explanation for this heterogeneity is that sensory cues emitted from hosts might be highly variable within a species, and mosquito receptors are tuned to respond to only a subset of such cues. Another is that mosquitoes can learn and remember sensory information associated with the best (and worst) hosts and change their behavior based on experience. In this Review, we explore the neural mechanisms and other factors underlying host-seeking and host preferences, both genetically determined as well as those subject to plasticity (Fig. 2).
Fig. 2.
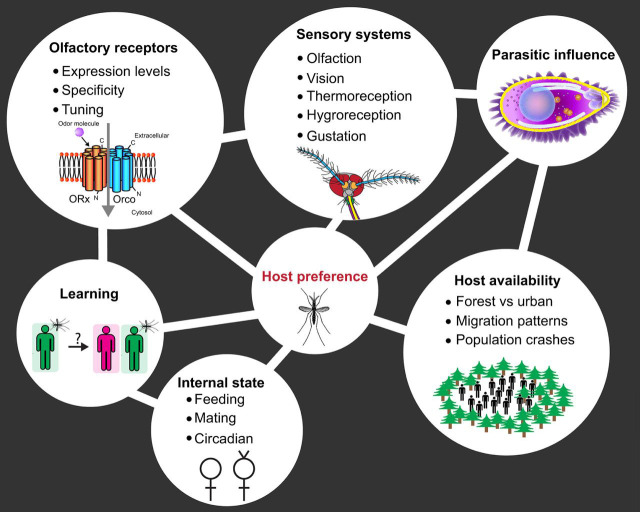
Factors influencing mosquito host-preference behaviors. Mosquitoes use multiple senses including olfaction, vision, thermoreception, hygroreception and gustation to track their hosts. Both peripheral and central nervous system components serving these senses can evolve adaptations for detecting certain hosts. Olfaction has the largest impact on host seeking and expression levels; the specificity and tuning of olfactory receptor proteins can affect host preference. Other factors contributing to host preference include parasitic influence such as from malaria-causing Plasmodium, host availability, internal state, and learning. ORx, olfactory receptor (unspecified); Orco, olfactory co-receptor.
Host preferences in specialists and generalists
Researchers rely on two general methods to determine the hosts preferred by mosquitoes: behavioral observation and blood meal analyses. Behavioral observation can be performed in the field or laboratory, using traps baited with potential host-related scents or by placing traps in tents or huts (Mclver, 1968; Service, 1993; Busula et al., 2015). The first study to show human host preference in the African malaria mosquito Anopheles gambiae in Tanzania (Gillies, 1964) employed an experimental hut enclosed in a mosquito net. Mosquitoes were released to make a choice between two chambers: one containing a sleeping human volunteer and one containing a sleeping ox calf. In other laboratory studies, wind tunnels were used to observe mosquito behavior in response to host odors presented upwind and two-choice olfactometers (see Glossary) or ‘Y-mazes’ were used to calculate preferences (Knols et al., 1994; Eiras and Jepson, 1991; Cooperband et al., 2008; Vinauger et al., 2014). A Y-maze consists of an entrance and two arms with air flowing towards the entrance. In either arm, researchers can present host odors or clean-air controls and record the proportion of mosquitoes that chose one arm over the other. In blood meal analyses, researchers collect mosquitoes either in the field or semi-field and extract gut contents of fed mosquitoes to characterize host identity using forensic methods such as PCR or probing blood with host-derived antibodies (Tempelis, 1975; Takken and Verhulst, 2013).
Glossary.
Aviphilic
A preference for birds as hosts.
CRISPR-Cas9
Genome-editing technology derived from the bacterial immune system.
Dendrite
An input region or branch of a neuron.
De-orphaning
The process of identifying a receptor's endogenous ligand.
Facet
A lens of a compound eye.
Gustation
The chemical sensing of taste.
Hygroreception
The ability to detect changes in water content.
Ionotropic receptor
A receptor protein that directly forms an ion channel.
Lamina
The first insect optic lobe neuropil receiving input from the retina.
Maxillary palp
A head appendage of mosquitoes that is equipped with multiple sensory receptors.
Olfactometer
A maze or apparatus used to measure the detection of, or preference for, odors.
Retinotopy
The mapping of visual information from the retina onto neurons in the brain.
Tastant
A chemical that activates taste receptors.
Valence
The intrinsic attractive or aversive quality of a stimulus.
Zoonotic
Describing a pathogen that can be transmitted from animals to humans.
Observations of mosquito host preferences have revealed both generalist and specialist strategies across species. Blood meal analysis of the tiger mosquito Aedes albopictus revealed a generalist strategy, with females feeding on a wide variety of mammals, including rabbits, deer and dogs, as well as a variety of birds, including passerines, pigeons and quail (Savage et al., 1993). The crabhole mosquito Deinocerites pseudes will also feed on a range of hosts, including reptiles, mammals and amphibians, but, depending on the location, proportions of mosquitoes feeding on reptile versus mammalian hosts varied dramatically, possibly owing to host availability (Tempelis, 1975). A similar analysis of blood meals ingested by the frog-feeding mosquito Culex territans in rural New Jersey characterized this species as specialist, with over 88% feeding on amphibian hosts and 6% feeding on reptiles (Crans, 1970). Fish species are rarely described as mosquito hosts, presumably owing to access issues beneath the water, but one mosquito species, Uranotaenia lateralis, is described as specializing on mudskippers, a type of goby that walks on land (Tempelis, 1975). Another specialist, the black-tailed mosquito Culiseta melanura, prefers avian hosts, with nearly 90% of mosquito blood meals identified as coming from birds such as the American robin and wood thrush, although they are capable of feeding on mammals (Molaei and Andreadis, 2006). Certainly, these specialists prefer one type of host, but can often be observed feeding on non-preferred hosts for a variety of reasons. Although mosquitoes in the An. gambiae complex are renowned for their high affinity to humans, they will nevertheless feed on other mammals when humans are not available (Lefèvre et al., 2009). Mysteriously, the Indo-Pakistan malaria mosquito Anopheles stephensi, and other mosquitoes, have been observed feeding on the hemolymph of other insect larvae such as caterpillars (Harris et al., 1969; George et al., 2014).
Mosquitoes belonging to the genera Toxorhynchites, Malaya and Topomyia do not blood feed and only ingest carbohydrates from plants and other sources. Species of Malaya could have the most intriguing feeding habits among mosquitoes, exploiting a natural behavior of ants that regurgitate when probed by the antennae of conspecifics. A Malaya mosquito will use its proboscis to probe the face and mouthparts of an ant until she regurgitates a droplet of sugary liquid, then the mosquito sucks up the liquid and flies away (MacDonald and Traub, 1960). It is unclear, however, how females of this genus acquire the protein necessary for oviposition if they do not take blood meals. For females in the genus Toxorhynchites, this problem is solved by its larvae predating on the larvae of other mosquito species, thus acquiring the necessary protein before eclosion as adults (Steffan and Evenhuis, 1981). As adults, Toxorhynchites feed solely on flower nectars and other sources of plant carbohydrates and do not seek hosts for blood meals, making them a particularly interesting group for comparison with blood-feeding mosquitoes (Fig. 1D). Phylogenetic analysis suggests that Toxorhynchites and Aedes share a sister relationship, with Culex and Anopheles being more distantly related, and thus that host preference does not necessarily track phylogeny (Fig. 1A) (Zhou et al., 2014). With species belonging to these genera having such diverse host preferences, what differences in their nervous systems might account for these behaviors? Comparative studies of drosophilids, moths and other taxa have led to significant advances in understanding olfactory processing in insects (Vickers et al., 1998; Dekker et al., 2006; Stacconi et al., 2014). With thousands of species representing a diverse repertoire of host-seeking behaviors, mosquitoes constitute an excellent study group for comparative neuroethology that can provide insights into general principles of insect host preferences.
Mosquito sensory system: olfaction
Host-seeking behavior depends on the integration of several sensory systems, including olfaction, vision, thermoreception, hygroreception (see Glossary) and gustation (see Glossary). Before a mosquito can see its host or use any of those latter senses, it first detects chemical cues, and olfaction is thought to contribute the most to host seeking (Takken and Knols, 1999). The olfactory nervous system is highly conserved across insects and even shares similar organization across the animal kingdom between vertebrates and invertebrates.
Like other insects, mosquitoes detect odor chemicals through their olfactory receptors (ORs) located on the dendrites (see Glossary) of neurons within hair-like sensilla on their antennae and, to a lesser extent, on the maxillary palps (see Glossary) (Fig. 3B,C). Odor molecules enter the sensilla through pores in the cuticle, and odorant-binding proteins transport them through the aqueous hemolymph to the ORs (Steinbrecht, 1997; Wang et al., 2010; Leal, 2013). The ORs of mosquitoes and other insects form heterodimers with a co-receptor (Orco) to form a ligand-gated ion channel that can be tightly tuned to bind to one type of odor molecule or broadly tuned to bind to several types (Fig. 3D) (Larsson et al., 2004; Carey et al., 2010). When odor molecules bind to ORs, the ion pore opens, allowing the olfactory receptor neuron (ORN) to depolarize and propagate an action potential (Carraher et al., 2015; Wolff and Strausfeld, 2015). Each ORN generally expresses only a single type of OR, although each sensillum can house two ORNs with different receptors (Fig. 3C) (Ghaninia et al., 2007; Hill et al., 2009; Syed and Leal, 2009; Ye et al., 2016). Maxillary palp sensilla can house an additional gustatory receptor in addition to two ORs (Lu et al., 2007). In rare cases, polycistronic mRNA transcription can lead to multiple ORs being expressed on the same ORN. One such case was discovered in An. gambiae, which possesses a set of six ORs encoded by genes that are arranged in clusters and co-expressed in the same ORNs, which can thus respond to odor blends rather than single compounds (Karner et al., 2015). Receptor numbers can vary across mosquito species, ranging from 79 OR genes in An. gambiae to 110 in the yellow fever mosquito Aedes aegypti and 177 in the southern house mosquito Culex quinquefasciatus (Hill et al., 2002; Bohbot et al., 2007; Leal et al., 2013; Zhou et al., 2014).
Fig. 3.
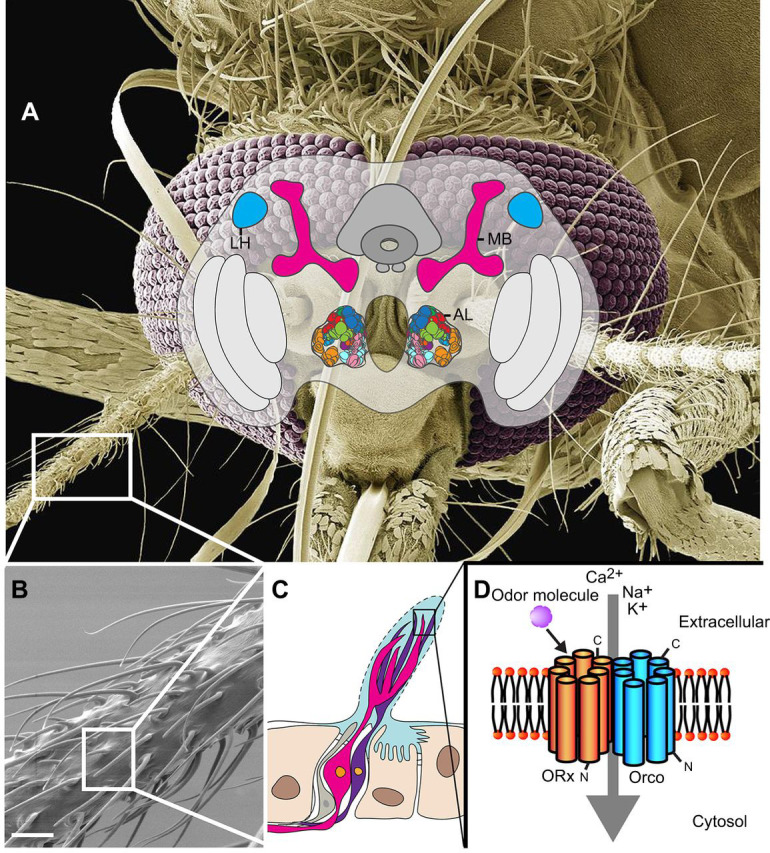
Mosquito olfactory nervous system. (A) Diagram of the mosquito brain and relative position within the head capsule. The antennal lobes (AL) are the primary olfactory centers and comprise ball-shaped glomeruli that are distinct synaptic zones where specific odor identities are encoded. Olfactory information is projected from the antennal lobes to the mushroom bodies (MB), which are necessary for learning and memory and also integrate sensory information from other modalities such as vision and mechanosensation. The lateral horns (LH) also integrate sensory information and mediate the encoding of intensity and valence of stimuli. Scanning electron micrograph (SEM) courtesy of Steve Gschmeissner. (B-D) Mosquito antennae are decorated with hair-like cuticular structures called sensilla (B; SEM courtesy of Walter Leal, scale bar: ∼10 µm) that house the dendrites of olfactory receptor neurons as diagramed in C. Each sensillum is perforated by pores (dashed lines), allowing odor molecules to enter, be transported through aqueous hemolymph (pale blue) and bind olfactory receptors (ORx), as diagramed in D. Odor molecules bind and activate seven-transmembrane domain ORx to open an ion pore in the plasma membrane between the olfactory receptor and the olfactory co-receptor (Orco), allowing cations to flow into the neuron, triggering an action potential.
ORs that bind the vertebrate host odor 1-octen-3-ol are among the most studied in mosquitoes, being highly conserved in this group. 1-Octen-3-ol is a mushroom alcohol that smells earthy, like fungi, but is also found in the breath and sweat of mammals including humans. In An. gambiae, the receptor protein AgOR8 is sensitive to 1-octen-3-ol, as are its orthologs AaOR8 in Ae. aegypti and CqOr118 in Cx. quinquefasciatus (Wang et al., 2010; Bohbot et al., 2013; Hill et al., 2015; Majeed et al., 2016). 1-Octen-3-ol works synergistically with CO2 to elicit attraction behaviors in species such as Ae. aegypti and An. gambiae, whereas it repels Cx. quinquefasciatus (Xu et al., 2015). It is emitted at different concentrations in various hosts such as humans or cows, whereas it has not been detected in odor profiles of birds such as chickens (Cook et al., 2011; Majeed et al., 2016). This compound can also modulate the attraction of mosquitoes to preferred and non-preferred host odors. For example, in one study, Cx. quinquefasciatus landed preferentially on a feeder treated with chicken odor, but did not respond to the same feeder when human-specific concentrations of 1-octen-3-ol were added. However, Ae. aegypti and Anopheles coluzzii (formerly An. gambiae molecular M form; Coetzee et al., 2013) did not prefer feeders treated with chicken odor but did land when chicken odor was supplemented with human-specific concentrations of 1-octen-3-ol (Majeed et al., 2016).
ORNs send their axons through the length of the antenna via the antennal nerve or project from the maxillary palps and terminate in the primary olfactory neuropil of the central brain, called the antennal lobe (Fig. 3A). There, ORNs expressing the same OR converge in a ball-shaped zone called the glomerulus, where they synapse onto projection neurons that then relay olfactory information to higher-order centers. Recording physiological activity in the antennal lobe reveals what is termed an ‘odortypic map’, where odor identity is represented by the pattern of glomeruli that are activated. In a variety of insect species, experiments in which calcium indicators are either applied to or expressed genetically in antennal lobe neurons have allowed researchers to observe which glomeruli are activated when insects are presented with olfactory stimuli (Dupuy et al., 2010; Galizia et al., 1999; Skiri et al., 2004; Wang et al., 2003). At physiological concentrations, a single odorant presented to an insect can stimulate activity in only one or a few glomeruli per antennal lobe, whereas a mixture of odorants can activate many glomeruli in a robust pattern distinct from the pattern observed when a different odor blend is presented. In the fruit fly Drosophila melanogaster, odorants have been mapped to activity in each glomerulus (Wang et al., 2003), whereas such maps have not yet been developed in mosquitoes. However, electrophysiological analysis of ORNs originating in the maxillary palps of An. gambiae and Ae. aegypti has revealed their sensitivity to CO2 and 1-octen-3-ol, chemicals emitted by vertebrate hosts (Lu et al., 2007; Grant and Dickens, 2011). Anterograde backfills of the maxillary palps with fluorescent dyes reveal their projection to a subset of glomeruli located dorsomedially in the antennal lobes of both these species (Anton et al., 2003; Ignell et al., 2005). Future work to map odor-evoked glomerular responses in mosquitoes will broaden our understanding of how behaviorally relevant odors are represented in the mosquito brain, how much activity at the peripheral level translates to activity in the central nervous system, and to what extent this activity can be modulated.
Olfactory information from the antennal lobes is encoded into memory, associated with positive or negative valences (see Glossary), and used to elicit behavioral actions in higher-order centers called ‘mushroom bodies’ (Fig. 3A) and ‘lateral horns’. Projection neurons, the dendrites of which are generally constrained to a single glomerulus in mosquitoes, send bifurcated axons that terminate in both the input region of the mushroom bodies (involved in learning and memory) and the lateral horns (which integrate olfactory information and are implicated in encoding intensity and valence of odor stimuli) (Heisenberg, 2003; Strutz et al., 2014; Schultzhaus et al., 2017). Although these structures were originally thought to serve these roles distinctly, we now know that there is a great deal of communication between the mushroom bodies and lateral horns in mediating both learned and innate behaviors (Schultzhaus et al., 2017). In Drosophila, mushroom body output neurons (MBONs) have been individually identified as mediating specific behavioral outputs involved in approach or avoidance (Owald et al., 2015). Although these have not been studied in mosquitoes, identification of MBONs and the behaviors they mediate will be crucial to understanding the neurobiology of host preference.
Evolution of mosquito ORs
While the first insects most likely lacked ORs, the evolution of these receptors occurred within the group of insects that also evolved winged flight (Missbach et al., 2014). Olfaction is of the utmost importance to flying insects that require rapid detection of airborne odor chemicals, discrimination of gradients, and fast adaptation in order to follow plumes emitted by crucial resources such as food, mates and oviposition sites, and to avoid odors associated with predators and other threats. Like other flying insects, mosquitoes fly in order to search for these resources, depending on their internal state, whether they be satiated or starved, mated or unmated, gravid or not. Female mosquitoes searching for a host tend to fly upwind until they encounter a plume of host odor and/or CO2 and then they employ a strategy of casting flight into the plume and surging flight upwind in order to close the distance between themselves and the source (Cardé and Willis, 2008; van Breugel et al., 2015). In order to detect host odors from a distance, mosquitoes must detect salient odor chemicals at low concentrations, and to effect this they possess many receptors for those odors as well as receptors that are narrowly tuned to host odors to increase signal over environmental noise. Furthermore, it is important that ORs rapidly adapt so the mosquito can determine when it has wandered out of the odor plume into a lower odor gradient and must return towards a higher gradient.
Which characteristics of mosquito ORs evolved divergently to contribute to the different host preferences? As previously mentioned, different species possess varying numbers of OR genes, which can limit or expand the number of odor molecules that are detectable (Zhou et al., 2014). For any given OR, expression levels can increase over evolutionary time owing to selection for sensitivity towards a certain host odor. Alternatively, receptor expression levels can decrease over generations if their ligand molecules become less salient in host odor profiles. Evidence for increased representation of ORs for the molecule nonanal, a grassy-smelling odor chemical emitted by humans and birds in particular, has been reported for the antennae of the aviphilic (see Glossary) Cx. quinquefasciatus (Syed and Leal, 2009). Electrophysiological recordings from sensilla housing ORNs that respond to nonanal indicate that these account for ∼40% of antennal ʻdetectors' in Cx. quinquefasciatus and are extremely sensitive, responding to stimulations using an odor cartridge loaded with only 0.001 ng of nonanal.
Evolved changes in OR gene sequence can alter ligand binding properties and therefore change tuning and sensitivity to odor molecules. Mutations in ligand-binding sites can change which odor molecules can be detected by the receptor, whereas mutations at other sites may alter the specificity or sensitivity of receptors, thus altering the response tuning of ORNs (Andersson et al., 2015). A single point mutation that replaces proline in CquiOR10, a receptor for the oviposition attractant 3-methylindole in Cx. quinquefasciatus, could either abolish or greatly diminish sensitivity to that compound (Xu and Leal, 2013).
Within a mosquito species, host preference can vary across divergent populations and is reflected in differences in OR gene expression levels and allelic variation. For example, when presented with a two-choice test between human or non-human vertebrate scents, strains of Ae. aegypti collected from forest environments displayed preference for the non-human vertebrate, whereas those collected from domestic environments, such as water-storage containers outside homes in a Kenyan village, preferred human scent. Human host preference was associated with differential expression of OR genes, including AaegOr4, which encodes a receptor for the human odorant sulcatone (6-methyl-5-hepten-2-one). Not only were expression levels of AaegOr4 linked to preference for human hosts, but also certain alleles conferred higher sensitivity to sulcatone, and these alleles were present at higher frequency in human-preferring Ae. aegypti colonies (McBride et al., 2014). Another mosquito species that prefers human hosts, An. gambiae, has convergently evolved several ORs responsive to sulcatone (Carey et al., 2010), suggesting that host preference is driven by selection for OR profiles in the peripheral nervous system.
To add to the complexity of peripheral nervous system ‘players’, the evolution of which can contribute to host-preference phenotypes, recent comparative proteomic and transcriptomic studies have revealed differences in the abundance of odorant-binding proteins of closely related mosquitoes with divergent host preferences (Rinker et al., 2013; Price and Fonseca, 2015; Iovinella et al., 2017). One example comes from comparisons of species in the An. gambiae complex. Whereas An. coluzzii specifically prefer human hosts, Anopheles arabiensis are generalists, and Anopheles quadriannulatus strongly prefer non-human hosts. Given a choice between clean air and human odor in an olfactometer, the majority of An. quadriannulatus choose clean air, suggesting they are actually repelled by human odors (Dekker et al., 2001). Although these species share the same suite of genes encoding ORs, they diverge in transcript abundance and are likely to have different antennal expression patterns (Rinker et al., 2013). Furthermore, non-OR genes, such as those encoding odorant-binding proteins, were expressed at different levels of abundance across the three taxa (Iovinella et al., 2017). Similarly, mosquitoes in the Cx. pipiens complex – form pipiens and form molestus – were identified as preferring avian hosts and mammalian hosts, respectively (Kilpatrick et al., 2007; Gomes et al., 2012). A comparison of Cx. pipiens form pipiens and form molestus transcriptomes revealed that odorant-binding proteins were statistically overrepresented in the subset of genes with the highest divergence rates (Price and Fonseca, 2015). Thus, evolutionary changes to the olfactory periphery, even at the molecular level, can significantly impact host preference.
Other sensory cues in host preference
Beyond olfaction, mosquitoes use a variety of other cues to locate and bite specific hosts. For instance, vision is a crucial modality for insect vectors and is integrally tied to olfactory and thermosensory behaviors. It has long been known that mosquitoes are attracted to black objects (Howlett, 1910; Kennedy, 1940; Rao, 1947; Smart and Brown, 1956), and this information has led to the development of black traps (Bidlingmayer, 1994). However, surprisingly little is known about what other visual features mosquitoes find attractive, or why black is so attractive. Although it had been suggested that CO2 – an indicator of nearby vertebrate hosts – ‘gates’ thermosensory behaviors in mosquitoes (Mcmeniman et al., 2014), work by van Breugel et al. (2015) showed that CO2 gates visual search behaviors in Ae. aegypti. These mosquitoes ignore visual objects in the absence of CO2, but become highly attracted to them after encountering the CO2 plume (Fig. 4A). After investigating the visual object, other cues (heat, water vapor) help mediate behaviors such as probing and biting (Howlett, 1910; McMeniman et al., 2014; van Breugel et al., 2015). Nonetheless, the often dramatic differences between hosts in their visual features and sizes, and emitted temperatures and water vapors, provide additional selective pressures on the tuning of these sensory channels for different mosquito species.
Fig. 4.
Integration of different senses contributes to host-seeking behavior. (A) After encountering the odor plume (green arrow), the mosquito visual system is ‘turned on’ to mediate attraction to high-contrast dark objects (dark red circle, which is emitting heat). The trajectory is color coded according to olfactory-visual integration (blue arrowheads) and visual-thermosensory integration (red arrows) as a function of distance from the target. (B) The ommatidia of different mosquito species exhibit similar patterns of opsin expression. (Top) For Ae. aegypti, opsin2 is expressed in the dorsal and ventral portions of the eye, whereas opsin8 is expressed in the central part of the eye. (Bottom) An. gambiae ommatidia exhibit similar patterns of opsin expression to those of Ae. aegypti. Reproduced from Hu et al. (2009), courtesy of Joseph O'Tousa. Scales bars: ∼50 µm. (C) The location (arrow; magnified in inset) of the two ‘peg-in-pit’ sensilla on the Ae. aegypti mosquito antenna that houses the hygrosensory and thermosensory neurons. The ‘peg’ extends out near to the opening of the sensilla. Three neurons within the sensilla extend either into the peg or to the base of the peg. Reproduced from Gingl et al. (2005) and Davis and Sokolove (1975) with permission.
Vision
Despite the importance of vision in mediating mosquito behaviors, little is known about the neural bases of visual behaviors. Despite these knowledge gaps, there is increasing information on the peripheral organization and function of the mosquito eye (Hu et al., 2014; Moon et al., 2014; Rocha et al., 2015). The compound eye comprises ∼500–2000 ommatidia, with each ommatidia comprising eight photoreceptor cells (Land et al., 1999; Hu et al., 2014). Comparing among mosquito species, the number and size of the ommatidia differ, with nocturnal and crepuscular mosquitoes having larger facet (see Glossary) diameters (An. gambiae sensu stricto, An. stephensi, Cx. pipiens molestus), whereas diurnal mosquitoes have smaller facets (Sato, 1957, 1959; Land et al., 1997; Kawada et al., 2006). The facet diameter is also non-uniform, with the largest facets pointing downward (Land et al., 1997; Sato, 1953), in contrast to most insects, which have the largest facets in the anterodorsal region of the eye (Land, 1989; Land et al., 1997). The expression of genes encoding the visual receptor protein rhodopsin is also non-uniform in the mosquito eye and might reflect host specificity (Hu et al., 2009). For instance, in Ae. aegypti and An. gambiae, both the genes opsin2 and opsin8 (which encode UV-sensitive and long wavelength-sensitive opsins, respectively) exhibit similar, non-overlapping patterns of expression, with opsin8 expressed in the central region of the eye (pointing downward), and opsin2 expressed in the dorsal rim and ventral edge of the central region. But there are differences between these two species in the ventral part of the eye that supplies visual information for the area directly underneath the mosquito, and this is likely to play a role in feeding and egg-laying behaviors: in Ae. aegypti, the ventral region is dominated by the UV-sensitive opsin8, whereas for An. gambiae it is opsin2 that is expressed (Fig. 4B). These differences might again reflect adaptations to the different levels and composition of light.
These photoreceptor cells make retinotopic (see Glossary) connections to large monopolar cells in the lamina (see Glossary) that project to downstream regions in the optic lobe (the visual processing center of the mosquito brain) (Land and Horwood, 2005). Given that odors ‘gate’ visually guided search behaviors, this raises the question of how this information is processed in the mosquito brain. Work in other dipterans has provided tantalizing information on object-selective neurons in the lobula plate region of the optic lobe, and the links between olfactory and visual centers. For example, small-field ‘object-selective’ neurons have been identified in the fly lobula plate (Collett, 1972; Keleş and Frye, 2017), although it is unknown how olfactory input influences these neurons. Other neurons in the insect lobula plate have been shown to process wide-field motion (termed optic flow) (Collett, 1972; Maimon et al., 2010; Suver et al., 2012); these neurons are important for the flying insect as it navigates in the environment. In Drosophila, these neurons are modulated by odors, and are thought to aid odor-guided flight (Frye and Dickinson, 2004; Wasserman et al., 2015). One candidate for modulating the object-selective neurons is aminergic modulation. In Ae. aegypti, dopaminergic neurons innervate both olfactory (antennal lobe) and lobula plate regions, and these neuromodulators increase neural responses in both brain areas (Vinauger et al., 2018). Nonetheless, how fine-scale visual features (such as those on a host) are processed by the mosquito visual system, and how mosquitoes can distinguish between different host species, remain open questions.
Thermosensation and hygrosensation
After detecting the appropriate odor and visual information, mosquitoes use other sensory modalities, such as heat and water vapor, to help provide additional cues to discriminate between hosts and make biting decisions (Takken and Verhulst, 2013). An important aspect is that air temperature and water vapor are inextricably linked, which has important implications for how this information is processed by the navigating mosquito. Examining these cues in isolation, thermal information comprises radiative, convective and conductive (through skin, hair) components. For instance, mammals, including humans, produce all three components, and convective vortices shed from the human arm in a wind tunnel can differ more than 1°C from ambient ∼10 cm away from the source (van Breugel et al., 2015). Similarly, water vapor close to the skin surface can range 20–80% from ambient depending on the air temperature (Buettner, 1959). These close-range cues differ between hosts: compared with humans, canines and many ungulates have elevated temperatures (∼38.5°C) and relatively low water vapor from the body surface, although the relative humidity in their breath is ∼95% (Hemingway and Lillehei, 1950). By contrast, ectotherms (amphibians, reptiles) have surface temperatures close to ambient and, in the case of amphibians, the relative humidity close to the skin can be high (60–80%) (Crawshaw, 1980). The interplay between temperature and humidity, and differences in these cues between hosts, provides a rich sensory environment to explore how they impact mosquito behavior.
How is this thermosensory and hygrosensory information transduced and processed by the mosquito nervous system? For Ae. aegypti, thermosensory information is encoded by sensory neurons located in peg-in-pit sensilla on the tip of the antennae (Fig. 4C). This sensilla houses ‘hot’ and ‘cold’ neurons that are sensitive to slight changes (0.1°C, corresponding to changes in radiant heat of ∼1 mW cm−2) in temperature (Gingl et al., 2005) (Fig. 4C). Although these neurons respond to abrupt temperature changes – similar to what might occur in a convective plume – they are most sensitive to, and predictive of, radiative heat (Gingl et al., 2005). In many other insect species, including mosquitoes, heat and water vapor are integrally linked. In Drosophila, the ionotropic receptors (see Glossary) IR40a, IR93a and IR25a are key mediators of humidity and thermosensory preferences (Enjin et al., 2016; Knecht et al., 2016; Frank et al., 2017). These receptors are expressed in thermosensory and hygrosensory neurons on the fly antennae, with IR40a important for hygrosensation, and IR93a and IR25a – expressed in thermosensory neurons – mediating both humidity and temperature preference. It remains unclear whether orthologs exist in mosquitoes, but, as thermosensory neurons located in the mosquito peg-in-pit sensilla are also sensitive to water vapor (Roth and Willis, 1952; Bar-Zeev, 1960; Gingl et al., 2005), this suggests that they possess a similar mechanism for encoding temperature and humidity information. Given the differences in humidity and temperature among host species, it stands to reason that the tuning of the thermosensory and hygrosensory neurons will also show specificity for the environmental cues indicative of an appropriate host.
Gustation
Once the host is located and the mosquito has landed, the mosquito must quickly obtain a blood meal to avoid the defensive behavior of the host; it does this by repeatedly contacting the surface of the host with its labellum – the tip of the mosquito proboscis – before biting (Clements, 1992). Taste receptors located on the mosquito tarsae and labellum play key roles in mediating pre-biting and biting behaviors (Clements, 1992; Sparks et al., 2013). Moreover, surface ligands on the skin can differ dramatically among hosts – for instance, amphibians have enormous quantities of antimicrobial peptides on the skin compared with any equivalent tissue in mammals (Erspamer, 1971). Mosquitoes can be sensitive to compounds located on the skin surface (Weldon and Carroll, 2007), and to topically applied repellents (Curtis et al., 1991; Maia and Moore, 2011; DeGennaro et al., 2013). However, compared with olfaction, our understanding of the molecular mechanisms mediating taste transduction, and how that information is processed in the brain, remains relatively rudimentary (Benton, 2017). Taste receptors include two large gene families: the ‘gustatory receptors’ and a subset of the ‘ionotropic receptors’. Gustatory receptors are extremely divergent between mosquito species, which might reflect differences in taste preferences (Kent et al., 2008). The inability to express the gustatory receptors in heterologous systems, in sharp contrast to ORs, has limited the functional characterization of these receptors and the identification of their cognate ‘tastants’ (see Glossary). Nonetheless, the chemical ecology of identifying potential ligands that activate these receptors, and de-orphaning (see Glossary) and examining the expression patterns of the receptors, provide rich fields for future research.
For insects, one important point is that, in contrast to mammals, taste is integrated with olfaction at both the receptor level and in the brain. For instance, putative gustatory receptors responsive to CO2 have been found in Ae. aegypti (AaGr1 and AaGr3), An. gambiae (AgGr22 and AgGr24) and Cx. pipiens quinquefasciatus (CpGr1 and CpGr3), and, in An. gambiae, ORNs in the proboscis project to the subesophageal zone, rather than the antennal lobe (Riabinina et al., 2016). It remains an open question as to how taste and olfactory information is integrated in the brain to mediate host-specific behaviors.
Plasticity in host preference
As mentioned above, conclusions drawn from blood meal analyses can be skewed by host availability as even specialist mosquitoes can adapt to feeding on non-preferred hosts in response to scarcity of the preferred host. One such example comes from an analysis of blood meals taken by a ‘forest’ population of An. gambiae sensu stricto on an island off the coast of West Africa. Unlike previously studied populations (Gillies, 1964; Besansky et al., 2004), the forest population proportionally fed less on humans and mainly fed on dogs when collected outdoors (Sousa et al., 2001). Mosquitoes collected indoors from the same area primarily fed on humans. It remains uncertain whether the changes in preference are reflected in changes to OR expression or sequence.
Plasticity in mosquito host preference between non-human vertebrates (‘zoophily’) and humans (‘anthropophily’) is a key factor mediating the transmission of zoonotic (see Glossary) vector-borne diseases such as West Nile virus, dengue fever and Japanese encephalitis virus (Bean et al., 2013). On the mid-Atlantic coast of the USA, Cx. pipiens mosquitoes prefer to feed on American robin thrushes during the spring and early summer, increasing their probability of becoming infected with West Nile virus. During the late summer and early autumn, the robins disperse and migrate, coinciding with a shift in Cx. pipiens host preference from birds to humans and a highly correlated increase in human cases of West Nile virus (Kilpatrick et al., 2006).
Apart from host availability, another driver of plasticity in host preference could be the influence of parasites. Parasites of the genus Hepatozoon typically infect the blood of amphibians and reptiles as well as mosquitoes that feed on these ectotherms. Fewer Cx. pipiens infected with developing Hepatozoon were found to feed on snakes than their uninfected counterparts, and the infection status of snake hosts might have had an effect on attraction, although this remains to be verified (Ferguson et al., 2013). Studies have also shown that children infected with the malaria parasite Plasmodium falciparum attract twice as many An. gambiae mosquitoes than uninfected children (Lacroix et al., 2005). In wind-tunnel experiments, mice infected with malaria attracted more An. stephensi than uninfected mice and this increase in attraction was related to changes in host odor profiles, including increases in 3-methyl butanoic acid, 2-methyl butanoic acid, hexanoic acid and tridecane. Furthermore, benzothiazole was found in lower concentrations in the odor profiles of infected mice, and benzothiazole decreases the attraction of mosquitoes to malaria-infected mouse odor (De Moraes et al., 2014). Understanding how parasites alter host odors and mosquito sensory perception could prove helpful in developing deterrents for disease vectors.
Mosquito learning
While host availability can drive plasticity in host preference, mosquitoes are capable of learning and modifying their host-preference behaviors based on prior experience. Meta-analysis of distributions of disease vector host transmission rates suggests that 20% of hosts account for 80% of the transmission potential in the population (Woolhouse et al., 1997). This means that even though a species of mosquito innately prefers one type of host, such as avian hosts for example, they might only be biting a small fraction of that group. One explanation for this heterogeneity is that mosquitoes learn to focus host preference on a subset of hosts after associating their sensory cues with positive blood-feeding experiences (Fig. 5A). Furthermore, a negative feeding experience can occur if hosts are defensive or provide low-quality blood meals, and their cues would become associated with conditioned aversion. In fact, Mwandawiro et al. (2000) found that mosquitoes in the genus Culex were influenced by prior experience when choosing between cow or pig hosts. For some species, those mosquitoes that previously fed on cows preferred cows in subsequent encounters and those that previously fed on pigs likewise chose pig hosts. More recently, Vantaux et al. (2014) demonstrated that previous experience can impact host preference in An. coluzzii. However, unlike Culex mosquitoes that preferred the same host as in prior feedings, after only one experience feeding on a rabbit, An. coluzzii significantly decreased their preference for rabbit on subsequent feedings compared with mosquitoes that had previously fed on humans or guinea pigs. An open question is what component of the blood feeding experience determines whether mosquitoes will be attracted or repelled in subsequent feedings. It is probable that reception of negative experiences is less likely to influence subsequent decisions by mosquito species that have stronger innate preferences, such as specialist feeders.
Fig. 5.
Experience impacts future host choice in mosquitoes. Evidence from epidemiological studies suggests that 20% of a host population can be responsible for 80% of transmission potential from disease vectors such as mosquitoes (Woolhouse et al., 1997). One explanation for this observation is that mosquitoes can ‘learn’ from experience. (A) Sensory cues can differ from one individual to another, such as different humans emitting different odor profiles. If a mosquito bites an individual with a certain odor profile and has a positive experience, it might learn to associate that odor profile with a high-quality blood meal. Subsequently, it will choose to bite those individuals with similar odor profiles, here depicted in different colors. (B) In the laboratory, mosquito learning can be assayed, for example by pairing a conditioned stimulus (CS), such as an odor delivered into a chamber via tubing, with an unconditioned stimulus (US), such as a mechanical shock delivered by placing vials of mosquitoes on a vortex mixer. The CS is presented for 1 min, the US is presented for the last 30 s overlapping with the CS, and the inter-trial interval (ITI) is 2 min. (C) After 24 h, mosquitoes are tested in a y-maze olfactometer. A pump blows clean air into a control arm and the CS odor into the other arm. Mosquitoes are placed in a starting box, fly down the main arm into a central box and then ‘choose’ between the two arms. A preference index is calculated as the number of mosquitoes choosing the tested odor minus the number choosing the control arm, divided by the total number of mosquitoes. If the preference index becomes significantly more negative after training, the mosquitoes are considered to have learned to associate the tested odor with the aversive shock. B and C are adapted from Vinauger et al. (2018).
Several studies have also demonstrated the ability of mosquitoes to learn associatively in classical conditioning experiments in which a conditioned stimulus such as an odor is paired with an unconditioned stimulus, which could be a blood reward (appetitive) or a shock (aversive). In an appetitive conditioning paradigm, Tomberlin et al. (2006) paired unconditioned reward stimuli (blood and sugar) with conditioned odor stimuli (vanilla and strawberry odors). Culex quinquefasciatus learned to associate the odors with the rewards and to probe a pipette coated with reward odor, even in the absence of sugar or blood. Aedes aegypti also exhibit the ability to learn associations between appetitive blood rewards and conditioned odors such as 1-octen-3-ol, d-lactic acid and (Z)-3-hexen-1-ol (Vinauger et al., 2014). Interestingly, some odors could not be learned, such as β-myrcene and benzyl alcohol, suggesting a heterogeneity in encoding different odors into memory, although the neural basis of this difference is not well understood. Appetitive learning in mosquitoes could have consequences for repellent use in humans. Aedes aegypti that were pre-exposed to the common repellent DEET, and trained repetitively with DEET and lactic acid, subsequently showed a decreased aversion to the repellent (Vinauger et al., 2014).
Not only can mosquitoes learn to associate odors with an unconditioned stimulus, but they can also learn visual cues, particularly when both vision and olfaction are integrated, as they would be in a natural host encounter. For example, visual learning has been demonstrated in An. gambiae trained to associate white or checkered circles around their blood feeders with the quality of blood contained within (Chilaka et al., 2012). They could also learn to associate olfactory cues with good or poor quality blood. However, Menda et al. (2013) then examined the effect of stimuli from different modalities being presented together during a classical conditioning paradigm. Although mosquitoes have an innate preference for resting on dark surfaces, the authors showed that Ae. aegypti can learn to avoid this visual cue when the dark surface is paired with an aversive unconditioned stimulus such as an electric shock. When the dark visual stimulus was paired with 1-octen-3-ol, an olfactory stimulus, the association with the aversive shock was strengthened, suggesting a synergy of sensory integration and learning in higher-order brain centers such as the mushroom bodies of mosquitoes.
Vinauger et al. (2018) further explored learning in Ae. aegypti, but this time using aversive training in which the conditioned odor stimulus was paired with a mechanical shock delivered to the entire training chamber (Fig. 5B,C). This allowed tight temporal control of presentation of conditioned and unconditioned stimuli; previously, researchers could not control when a mosquito drank from a blood-feeder or landed on an electrified surface. Again, it was found that mosquitoes could learn the association and, this time, to avoid a conditioned odor such as 1-octen-3-ol. Mosquitoes were also trained aversively against odors collected from chickens and rats, but could only learn to avoid the rat odor. If mosquitoes possess the necessary receptors and can detect odors such as β-myrcene and compounds in the chicken odor profile, why does this olfactory information not get encoded into memory, whereas other odors, such as 1-octen-3-ol, do get encoded?
One process that could be responsible for differential learning of odors is neuromodulation by biogenic amines such as dopamine. Dopamine signaling is necessary for learning and memory in both the antennal lobes and mushroom bodies of insects (Vergoz et al., 2007; Dacks et al., 2012; Waddell, 2013). In Ae. aegypti, disruption of dopamine signaling either pharmacologically or genetically, by knocking down dopamine receptor expression with double-stranded RNA or the CRISPR-Cas9 (see Glossary) system, significantly impairs olfactory learning and memory (Vinauger et al., 2018). Furthermore, electrophysiological recordings suggest that dopamine modulates neural representation of olfactory information in the mosquito antennal lobes, although various odor representations were modulated to different degrees by application of dopamine to the brain. Both the mushroom bodies and antennal lobes are heavily innervated by dopaminergic neurons, although, interestingly, innervation of olfactory glomeruli is heterogeneous.
Concluding remarks
Mosquitoes exhibit a variety of host preferences across species, and within species host preference can be heterogeneous. This heterogeneity might in part be explained by differences in mosquito nervous systems and learning behaviors. A number of questions arise. What is the relationship between the representation of odors shifting differentially in response to dopamine and the heterogeneity of dopaminergic innervation to the antennal lobe glomeruli? Can this heterogeneity in part explain why mosquitoes can learn associations with some odors and not others (Vinauger et al., 2014)? To understand these questions, future studies could examine specific concentrations of dopamine and dopamine receptor expression in glomeruli and map out which odors evoke activity in each of the glomeruli. It is possible that odors that can be learned by mosquitoes are encoded in glomeruli with different levels of dopamine and its receptor compared with glomeruli that encode odors that are not learned. It is also unknown whether patterns of dopaminergic innervation are conserved across different species. Does the glomerulus encoding 1-octen-3-ol in Ae. aegypti have the same relative expression levels of dopamine as the glomerulus encoding 1-octen-3-ol in An. gambiae? Would it be different in a mosquito that does not prefer human hosts? Thus far, studies of mosquito learning and memory have focused on a few species, particularly those that serve as vectors for human diseases, but little effort has been made to compare the encoding of olfactory information into memory across species in order to understand how this process impacts host preferences. Comparative as well as in-depth interrogations of mosquito neurophysiology will be necessary to understand the heterogeneity of their feeding patterns and host preferences.
Another area of mosquito neurophysiology rich in open questions is that of sensory integration in species with various host preferences. Do integration centers in the brain of Cx. territans, which prefer frogs, receive inputs from the same modalities as an anthropophilic species? Perhaps thermoreception is not represented to as great an extent and auditory signals tuned to frog mating calls might instead be more highly represented. Do visual cues from hosts have the same salience to nocturnal and diurnal mosquito species? Comparison of the retina, optic lobe and central complex in species that search for different hosts in different visual ecologies might not only reveal drivers of host preference but would enrich our understanding of insect visual processing in general.
Differences in sensory transduction, neural encoding and plasticity across species almost certainly affect mosquito responses to vector control methods including traps, repellents and nets. Traps may appeal to any sensory modality and researchers have developed chemical, visual and even sound lures to attract and capture or kill mosquitoes (Benelli et al., 2016). However, what attracts one species endemic to South America may not attract another species in India, for example. As discussed above, stimuli that attract some species may also repel others, so control methods must be matched to the sensory and behavioral profiles of target vectors (Potter, 2014; Xu et al., 2015). There is also evidence that experience and learning affect mosquito responses to control agents such as DEET (Stanczyk et al., 2013; Vinauger et al., 2014, 2016). Understanding the neurobiological mechanisms underlying differential learning across mosquitoes might aid in the search for more effective repellents in different parts of the world. This line of research might also uncover new targets for genetic engineering to disrupt genes mediating host-preference behaviors or plasticity that allows behavioral switching from zoophilic to anthropophilic preferences. Using CRISPR-Cas9 gene drives, researchers have already aimed to insert transgenes that reduce mosquito fitness or impart resistance to malaria (Gantz et al., 2015; Hammond et al., 2016; Alphey, 2016). It is possible that this technology may be adapted to dysregulate proteins involved in learning and memory, such as biogenic amines and their receptors, although the ethical and environmental ramifications should be seriously considered and debated (Johnson et al., 2011; Waddell, 2013; Oye et al., 2014; Vinauger et al., 2018).
Mosquitoes as a clade have a rich diversity of hosts, behaviors, life histories and ecologies distributed across six continents, yet they have retained comparable neuroanatomy. This makes them an excellent group to study how brains can evolve to mediate adaptive behaviors such as preference for different hosts. By studying the full diversity of mosquitoes, whether they bite mammals, feed solely on nectar or ant regurgitation, future research should reveal the neural mechanisms that drive some species to feed on humans and on animal reservoirs of diseases.
Acknowledgements
We thank Chloé Lahondère (Virginia Tech) and Clément Vinauger (Virginia Tech) for invaluable discussions and for diagrams of the classical conditioning paradigm. Harry Tichy (University of Vienna) and Walter Leal (UC Davis) provided scanning electron micrographs and helpful conversation. Joseph O’Tusa (University of Notre Dame) provided micrographs of opsin immunohistochemistry. We thank Floris van Breugel (University of Washington) for helpful suggestions and edits to the manuscript and for providing an example trace from his wind-tunnel experiments. Finally, we deeply appreciate the images kindly shared by Steve Gschmeissner, Matthew McIntosh, Steve Mattan, the Pennsylvania Department of Natural Resources and Jack Jeffrey.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Funding
We acknowledge the support of the Air Force Office of Scientific Research under grants FA9550-14-1-0398 and FA9550-16-1-0167, National Science Foundation under grant IOS-1354159, an Endowed Professorship for Excellence in Biology, University of Washington (J.A.R.) and the Human Frontier Science Program under grant HFSP-RGP0022.
References
- Alphey L. (2016). Can CRISPR-Cas9 gene drives curb malaria? Nat. Biotechnol. 34, 149-150. 10.1038/nbt.3473 [DOI] [PubMed] [Google Scholar]
- Andersson M. N., Löfstedt C. and Newcomb R. D. (2015). Insect olfaction and the evolution of receptor tuning. Front. Ecol. Evol. 3, 53 10.3389/fevo.2015.00053 [DOI] [Google Scholar]
- Anton S., van Loon J. J. A., Meijerink J., Smid H. M., Takken W. and Rospars J.-P. (2003). Central projections of olfactory receptor neurons from single antennal and palpal sensilla in mosquitoes. Arthropod. Struct. Dev. 32, 319-327. 10.1016/j.asd.2003.09.002 [DOI] [PubMed] [Google Scholar]
- Bar-Zeev M. (1960). The location of hygroreceptors and moisture receptors in Aedes aegypti (l.). Entomol. Exp. Appl. 3, 251-256. 10.1111/j.1570-7458.1960.tb00455.x [DOI] [Google Scholar]
- Bean A. G. D., Baker M. L., Stewart C. R., Cowled C., Deffrasnes C., Wang L.-F. and Lowenthal J. W. (2013). Studying immunity to zoonotic diseases in the natural host - keeping it real. Nat. Rev. Immunol. 13, 851-861. 10.1038/nri3551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benelli G., Jeffries C. L. and Walker T. (2016). Biological control of mosquito vectors: past, present, and future. Insects 7, 52 10.3390/insects7040052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton R. (2017). The neurobiology of gustation in insect disease vectors: progress and potential. Curr. Opin. Insect Sci. 20, 19-27. 10.1016/j.cois.2017.02.003 [DOI] [PubMed] [Google Scholar]
- Besansky N. J., Hill C. A. and Costantini C. (2004). No accounting for taste: host preference in malaria vectors. Trends Parasitol. 20, 249-251. 10.1016/j.pt.2004.03.007 [DOI] [PubMed] [Google Scholar]
- Bidlingmayer W. L. (1994). How mosquitoes see traps: role of visual responses. J. Am. Mosq. Control Assoc. 10, 272-279. [PubMed] [Google Scholar]
- Bohbot J., Pitts R. J., Kwon H.-W., Rützler M., Robertson H. M. and Zwiebel L. J. (2007). Molecular characterization of the Aedes aegypti odorant receptor gene family. Insect Mol. Biol. 16, 525-537. 10.1111/j.1365-2583.2007.00748.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohbot J. D., Durand N. F., Vinyard B. T. and Dickens J. C. (2013). Functional development of the octenol response in Aedes aegypti. Front. Physiol. 4, 39 10.3389/fphys.2013.00039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw W. E. and Holzapfel C. M. (1983). Life cycle strategies in Wyeomyia smithii: seasonal and geographic adaptations. In Diapause and Life Cycle Strategies in Insects (ed. Brown V. K. and Hodek I.), pp. 167-185. Dordrecht, The Netherlands: Kluwer. [Google Scholar]
- Braima K. A., Muslimin M., M. Ghazali A.-R., Wan-Nor F., Wilson J. J., Jeffery J. and Abdul-Aziz N. M. (2017). Feeding behavior of Mimomyia (Etorleptiomyia) luzonensis (Ludlow, 1905) (Diptera, Culicidae) in Peninsular Malaysia. Acta Trop. 171, 138-140. 10.1016/j.actatropica.2017.03.025 [DOI] [PubMed] [Google Scholar]
- Buettner K. J. K. (1959). Diffusion of water vapor through small areas of human skin in normal environment. J. Appl. Physiol. 14, 269-275. [PubMed] [Google Scholar]
- Burkett-Cadena N. D. and Blosser E. M. (2017). Aedeomyia squamipennis (Diptera: Culicidae) in Florida, USA, a new state and country record. J. Med. Entomol. 54, 788-792. [DOI] [PubMed] [Google Scholar]
- Busula A. O., Takken W., Loy D. E., Hahn B. H., Mukabana W. R. and Verhulst N. O. (2015). Mosquito host preferences affect their response to synthetic and natural odour blends. Malar. J. 14, 133 10.1186/s12936-015-0635-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardé R. T. and Willis M. A. (2008). Navigational strategies used by insects to find distant, wind-borne sources of odor. J. Chem. Ecol. 34, 854-866. 10.1007/s10886-008-9484-5 [DOI] [PubMed] [Google Scholar]
- Carey A. F., Wang G., Su C.-Y., Zwiebel L. J. and Carlson J. R. (2010). Odorant reception in the malaria mosquito Anopheles gambiae. Nature 464, 66-71. 10.1038/nature08834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carraher C., Dalziel J., Jordan M. D., Christie D. L., Newcomb R. D. and Kralicek A. V. (2015). Towards an understanding of the structural basis for insect olfaction by odorant receptors. Insect Biochem. Mol. Biol. 66, 31-41. 10.1016/j.ibmb.2015.09.010 [DOI] [PubMed] [Google Scholar]
- Chilaka N., Perkins E. and Tripet F. (2012). Visual and olfactory associative learning in the malaria vector Anopheles gambiae sensu stricto. Malar. J. 11, 27 10.1186/1475-2875-11-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements A. N. (1992). Biology of Mosquitoes, Volume 1: Development, Nutrition and Reproduction. Oxford: CAB International. [Google Scholar]
- Clements A. (1999). The Biology of Mosquitoes, Volume 2: Sensory Reception and Behavior. Oxford: CAB International. [Google Scholar]
- Coetzee M., Hunt R. H., Wilkerson R., Della Torre A., Coulibaly M. B. and Besansky N. J. (2013). Anopheles coluzzii and Anopheles amharicus, new members of the Anopheles gambiae complex. Zootaxa 3619, 246-274. 10.11646/zootaxa.3619.3.2 [DOI] [PubMed] [Google Scholar]
- Collett T. (1972). Visual neurones in the anterior optic tract of the privet hawk moth. J. Comp. Physiol. 78, 396-433. 10.1007/BF01417943 [DOI] [Google Scholar]
- Cook J. I., Majeed S., Ignell R., Pickett J. A., Birkett M. A. and Logan J. G. (2011). Enantiomeric selectivity in behavioural and electrophysiological responses of Aedes aegypti and Culex quinquefasciatus mosquitoes. Bull. Entomol. Res. 101, 541-550. 10.1017/S0007485311000162 [DOI] [PubMed] [Google Scholar]
- Cooperband M. F., McElfresh J. S., Millar J. G. and Cardé R. T. (2008). Attraction of female Culex quinquefasciatus Say (Diptera: Culicidae) to odors from chicken feces. J. Insect Physiol. 54, 1184-1192. 10.1016/j.jinsphys.2008.05.003 [DOI] [PubMed] [Google Scholar]
- Crans W. J. (1970). The blood feeding habits of Culex territans Walker. Mosq. News 30, 445-447. [Google Scholar]
- Crawshaw L. I. (1980). Temperature regulation in vertebrates. Annu. Rev. Physiol. 42, 473-491. 10.1146/annurev.ph.42.030180.002353 [DOI] [PubMed] [Google Scholar]
- Curtis C., Lines J., Lu B. and Renz A. (1991). Natural and synthetic repellents. In Control of Disease Vector in the Community (ed. Curtis C. F.), pp. 75-92. London: Wolfe Publishing Ltd. [Google Scholar]
- Dacks A. M., Riffell J. A., Martin J. P., Gage S. L. and Nighorn A. J. (2012). Olfactory modulation by dopamine in the context of aversive learning. J. Neurophysiol. 108, 539-550. 10.1152/jn.00159.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danabalan R., Monaghan M. T., Ponsonby D. J. and Linton Y.-M. (2014). Occurrence and host preferences of Anopheles maculipennis group mosquitoes in England and Wales. Med. Vet. Entomol. 28, 169-178. 10.1111/mve.12023 [DOI] [PubMed] [Google Scholar]
- Davis E. E. and Sokolove P. G. (1975). Temperature responses of antennal receptors of the mosquito, Aedes aegypti. J. Comp. Physiol. 96, 223-236. 10.1007/BF00612696 [DOI] [Google Scholar]
- Day J. F. (2005). Host-seeking strategies of mosquito disease vectors. J. Am. Mosq. Control Assoc. 21, 17-22. 10.2987/8756-971X(2005)21[17:HSOMDV]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- De Moraes C. M., Stanczyk N. M., Betz H. S., Pulido H., Sim D. G., Read A. F. and Mescher M. C. (2014). Malaria-induced changes in host odors enhance mosquito attraction. Proc. Natl. Acad. Sci. USA 111, 11079-11084. 10.1073/pnas.1405617111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGennaro M., McBride C. S., Seeholzer L., Nakagawa T., Dennis E. J., Goldman C., Jasinskiene N., James A. A. and Vosshall L. B. (2013). orco mutant mosquitoes lose strong preference for humans and are not repelled by volatile DEET. Nature 498, 487-491. 10.1038/nature12206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker T., Takken W. and Braks M. A. H. (2001). Innate preference for host-odor blends modulates degree of anthropophagy of Anopheles gambiae sensu lato (Diptera: Culicidae). J. Med. Entomol. 38, 868-871. 10.1603/0022-2585-38.6.868 [DOI] [PubMed] [Google Scholar]
- Dekker T., Ibba I., Siju K. P., Stensmyr M. C. and Hansson B. S. (2006). Olfactory shifts parallel superspecialism for toxic fruit in Drosophila melanogaster sibling, D. sechellia. Curr. Biol. 16, 101-109. 10.1016/j.cub.2005.11.075 [DOI] [PubMed] [Google Scholar]
- Dupuy F., Josens R., Giurfa M. and Sandoz J.-C. (2010). Calcium imaging in the ant Camponotus fellah reveals a conserved odour-similarity space in insects and mammals. BMC Neurosci. 11, 28 10.1186/1471-2202-11-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman J. D., Webber L. A. and Kale H. W. II (1972). Host-feeding patterns of Florida mosquitoes II. Culiseta. J. Med. Entomol. 9, 429-434. 10.1093/jmedent/9.5.429 [DOI] [PubMed] [Google Scholar]
- Eiras A. E. and Jepson P. C. (1991). Host location by Aedes aegypti (Diptera: Culicidae): a wind tunnel study of chemical cues. Bull. Entomol. Res. 81, 151-160. 10.1017/S0007485300051221 [DOI] [Google Scholar]
- Enjin A., Zaharieva E. E., Frank D. D., Mansourian S., Suh G. S. B., Gallio M. and Stensmyr M. C. (2016). Humidity sensing in Drosophila. Curr. Biol. 26, 1352-1358. 10.1016/j.cub.2016.03.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erspamer V. (1971). Biogenic amines and active polypeptides of the amphibian skin. Annu. Rev. Pharmacol. 11, 327-350. 10.1146/annurev.pa.11.040171.001551 [DOI] [PubMed] [Google Scholar]
- Ferguson L. V., Kirk Hillier N. and Smith T. G. (2013). Influence of Hepatozoon parasites on host-seeking and host-choice behaviour of the mosquitoes Culex territans and Culex pipiens. Int. J. Parasitol. Parasites Wildl. 2, 69-76. 10.1016/j.ijppaw.2012.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank D. D., Enjin A., Jouandet G. C., Zaharieva E. E., Para A., Stensmyr M. C. and Gallio M. (2017). Early integration of temperature and humidity stimuli in the Drosophila Brain. Curr. Biol. 27, 2381-2388.e4. 10.1016/j.cub.2017.06.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye M. A. and Dickinson M. H. (2004). Motor output reflects the linear superposition of visual and olfactory inputs in Drosophila. J. Exp. Biol. 207, 123-131. 10.1242/jeb.00725 [DOI] [PubMed] [Google Scholar]
- Galizia C. G., Sachse S., Rappert A. and Menzel R. (1999). The glomerular code for odor representation is species specific in the honeybee Apis mellifera. Nat. Neurosci. 2, 473-478. 10.1038/8144 [DOI] [PubMed] [Google Scholar]
- Gantz V. M., Jasinskiene N., Tatarenkova O., Fazekas A., Macias V. M., Bier E. and James A. A. (2015). Highly efficient Cas9-mediated gene drive for population modification of the malaria vector mosquito Anopheles stephensi. Proc. Natl. Acad. Sci. USA 112, E6736-E6743. 10.1073/pnas.1521077112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- George J., Blanford S., Thomas M. B. and Baker T. C. (2014). Malaria mosquitoes host-locate and feed upon caterpillars. PLoS ONE 9, e108894 10.1371/journal.pone.0108894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaninia M., Ignell R. and Hansson B. S. (2007). Functional classification and central nervous projections of olfactory receptor neurons housed in antennal trichoid sensilla of female yellow fever mosquitoes, Aedes aegypti. Eur. J. Neurosci. 26, 1611-1623. 10.1111/j.1460-9568.2007.05786.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies M. T. (1964). Selection for host preference in Anopheles gambiae. Nature 203, 852-854. 10.1038/203852a0 [DOI] [PubMed] [Google Scholar]
- Gingl E., Hinterwirth A. and Tichy H. (2005). Sensory representation of temperature in mosquito warm and cold cells. J. Neurophysiol. 94, 176-185. 10.1152/jn.01164.2004 [DOI] [PubMed] [Google Scholar]
- Gomes B., Parreira R., Sousa C. A., Novo M. T., Almeida A. P. G., Donnelly M. J. and Pinto J. (2012). The Culex pipiens complex in continental Portugal: distribution and genetic structure. J. Am. Mosq. Control Assoc. 28, 75-80. 10.2987/8756-971X-28.4s.75 [DOI] [PubMed] [Google Scholar]
- Grant A. J. and Dickens J. C. (2011). Functional characterization of the octenol receptor neuron on the maxillary palps of the yellow fever mosquito, Aedes aegypti. PLoS ONE 6, e21785 10.1371/journal.pone.0021785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond A., Galizi R., Kyrou K., Simoni A., Siniscalchi C., Katsanos D., Gribble M., Baker D., Marois E., Russell S. et al. (2016). A CRISPR-Cas9 gene drive system targeting female reproduction in the malaria mosquito vector Anopheles gambiae. Nat. Biotechnol. 34, 78 10.1038/nbt.3439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris P., Riordan D. F. and Cooke D. (1969). Mosquitoes feeding on insect larvae. Science 164, 184-185. 10.1126/science.164.3876.184 [DOI] [PubMed] [Google Scholar]
- Heisenberg M. (2003). Mushroom body memoir: from maps to models. Nat. Rev. Neurosci. 4, 266-275. 10.1038/nrn1074 [DOI] [PubMed] [Google Scholar]
- Hemingway A. and Lillehei C. W. (1950). Thermal cutaneous vasomotor response in dogs. Am. J. Physiol. 162, 301-307. [DOI] [PubMed] [Google Scholar]
- Hill C. A., Fox N. A., Pitts J. R., Kent L. B., Tan P. L., Chrystal M. A., Cravchik A., Collins F. H., Robertson H. M. and Zweibel L. J. (2002). G protein-coupled receptors in Anopheles gambiae. Science 298, 176-178. 10.1126/science.1076196 [DOI] [PubMed] [Google Scholar]
- Hill S. R., Hansson B. S. and Ignell R. (2009). Characterization of antennal trichoid sensilla from female southern house mosquito, Culex quinquefasciatus Say. Chem. Senses. 34, 231-252. 10.1093/chemse/bjn080 [DOI] [PubMed] [Google Scholar]
- Hill S. R., Majeed S. and Ignell R. (2015). Molecular basis for odorant receptor tuning: a short C-terminal sequence is necessary and sufficient for selectivity of mosquito Or8. Insect Mol. Biol. 24, 491-501. 10.1111/imb.12176 [DOI] [PubMed] [Google Scholar]
- Howlett F. M. (1910). The influence of temperature upon the biting of mosquitoes. Parasitology 3, 479-484. 10.1017/S0031182000002304 [DOI] [Google Scholar]
- Hu X., England J. H., Lani A. C., Tung J. J., Ward N. J., Adams S. M., Barber K. A., Whaley M. A. and O'Tousa J. E. (2009). Patterned rhodopsin expression in R7 photoreceptors of mosquito retina: implications for species-specific behavior. J. Comp. Neurol. 516, 334-342. 10.1002/cne.22114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X., Leming M. T., Whaley M. A. and O'Tousa J. E. (2014). Rhodopsin coexpression in UV photoreceptors of Aedes aegypti and Anopheles gambiae mosquitoes. J. Exp. Biol. 217, 1003-1008. 10.1242/jeb.096347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignell R., Dekker T., Ghaninia M. and Hansson B. S. (2005). Neuronal architecture of the mosquito deutocerebrum. J. Comp. Neurol. 493, 207-240. 10.1002/cne.20800 [DOI] [PubMed] [Google Scholar]
- Iovinella I., Caputo B., Calzetta M., Zwiebel L. J., Dani F. R. and della Torre A. (2017). Profiles of soluble proteins in chemosensory organs of three members of the afro-tropical Anopheles gambiae complex. Comp. Biochem. Physiol. D Genomics Proteomics 24, 41-50. 10.1016/j.cbd.2017.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson O., Becnel J. and Nichols C. D. (2011). Serotonin receptor activity is necessary for olfactory learning and memory in Drosophila melanogaster. Neuroscience 192, 372-381. 10.1016/j.neuroscience.2011.06.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karner T., Kellner I., Schultze A., Breer H. and Krieger J. (2015). Co-expression of six tightly clustered odorant receptor genes in the antenna of the malaria mosquito Anopheles gambiae. Front. Ecol. Evol. 3, 26 10.3389/fevo.2015.00026 [DOI] [Google Scholar]
- Kawada H., Tatsuta H., Arikawa K. and Takagi M. (2006). Comparative study on the relationship between photoperiodic host-seeking behavioral patterns and the eye parameters of mosquitoes. J. Insect Physiol. 52, 67-75. 10.1016/j.jinsphys.2005.09.004 [DOI] [PubMed] [Google Scholar]
- Keleş M. F. and Frye M. A. (2017). Object-detecting neurons in Drosophila. Curr. Biol. 27, 680-687. 10.1016/j.cub.2017.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly D. W. (2001). Why are some people bitten more than others? Trends Parasitol. 17, 578-581. 10.1016/S1471-4922(01)02116-X [DOI] [PubMed] [Google Scholar]
- Kennedy J. S. (1940). The visual responses of flying mosquitoes. Proc. Zool. Soc. Lond. A109, 221-242. 10.1111/j.1096-3642.1940.tb00831.x [DOI] [Google Scholar]
- Kent L. B., Walden K. K. O. and Robertson H. M. (2008). The Gr family of candidate gustatory and olfactory receptors in the yellow-fever mosquito Aedes aegypti. Chem. Senses 33, 79-93. 10.1093/chemse/bjm067 [DOI] [PubMed] [Google Scholar]
- Kilpatrick A. M., Kramer L. D., Jones M. J., Marra P. P. and Daszak P. (2006). West Nile virus epidemics in North America are driven by shifts in mosquito feeding behavior. PLoS Biol. 4, e82 10.1371/journal.pbio.0040082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick A. M., Kramer L. D., Jones M. J., Marra P. P., Daszak P. and Fonseca D. M. (2007). Genetic influences on mosquito feeding behavior and the emergence of zoonotic pathogens. Am. J. Trop. Med. Hyg. 77, 667-671. [PubMed] [Google Scholar]
- Knecht Z. A., Silbering A. F., Ni L., Klein M., Budelli G., Bell R., Abuin L., Ferrer A. J., Samuel A. D. T., Benton R. et al. (2016). Distinct combinations of variant ionotropic glutamate receptors mediate thermosensation and hygrosensation in Drosophila. Elife 5, e17879 10.7554/eLife.17879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight K. L. and Stone A. (1977). A Catalog of the Mosquitoes of the World (Diptera: Culicidae), 2nd edn. College Park, Maryland: Entomological Society of America. [Google Scholar]
- Knols B. G. J., Jong R. D. and Takken W. (1994). Trapping system for testing olfactory responses of the malaria mosquito Anopheles gambiae in a wind tunnel. Med. Vet. Entomol. 8, 386-388. 10.1111/j.1365-2915.1994.tb00104.x [DOI] [PubMed] [Google Scholar]
- Lacroix R., Mukabana W. R., Gouagna L. C. and Koella J. C. (2005). Malaria infection increases attractiveness of humans to mosquitoes. PLoS Biol. 3, e298 10.1371/journal.pbio.0030298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land M. F. (1989). Variations in the structure and design of compound eyes. In Facets of Vision (ed. Stavenga D. G. and Hardie R. C.), pp. 90-111. Berlin: Springer. [Google Scholar]
- Land M. F. and Horwood J. (2005). Different retina-lamina projections in mosquitoes with fused and open rhabdoms. J. Comp. Physiol. A Neuroethol. Sens. Neural. Behav. Physiol. 191, 639-647. 10.1007/s00359-005-0616-x [DOI] [PubMed] [Google Scholar]
- Land M. F., Gibson G. and Horwood J. (1997). Mosquito eye design: conical rhabdoms are matched to wide aperture lenses. Proc. R. Soc. Lond. B Biol. Sci. 264, 1183-1187. 10.1098/rspb.1997.0163 [DOI] [Google Scholar]
- Land M. F., Gibson G., Horwood J. and Zeil J. (1999). Fundamental differences in the optical structure of the eyes of nocturnal and diurnal mosquitoes. J. Comp. Physiol. A 185, 91-103. 10.1007/s003590050369 [DOI] [Google Scholar]
- Larsson M. C., Domingos A. I., Jones W. D., Chiappe M. E., Amrein H. and Vosshall L. B. (2004). Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron 43, 703-714. 10.1016/j.neuron.2004.08.019 [DOI] [PubMed] [Google Scholar]
- Leal W. S. (2013). Odorant reception in insects: roles of receptors, binding proteins, and degrading enzymes. Annu. Rev. Entomol. 58, 373-391. 10.1146/annurev-ento-120811-153635 [DOI] [PubMed] [Google Scholar]
- Leal W. S., Choo Y.-M., Xu P., da Silva C. S. B. and Ueira-Vieira C. (2013). Differential expression of olfactory genes in the southern house mosquito and insights into unique odorant receptor gene isoforms. Proc. Natl. Acad. Sci. USA 110, 18704-18709. 10.1073/pnas.1316059110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefèvre T., Gouagna L.-C., Dabiré K. R., Elguero E., Fontenille D., Renaud F., Costantini C. and Thomas F. (2009). Beyond nature and nurture: phenotypic plasticity in blood-feeding behavior of Anopheles gambiae s.s. when humans are not readily accessible. Am. J. Trop. Med. Hyg. 81, 1023-1029. 10.4269/ajtmh.2009.09-0124 [DOI] [PubMed] [Google Scholar]
- Lounibos L. P., Dover C. V. and O'Meara G. F. (1982). Fecundity, autogeny, and the larval environment of the pitcher-plant mosquito, Wyeomyia smithii. Oecologia 55, 160-164. 10.1007/BF00384482 [DOI] [PubMed] [Google Scholar]
- Lu T., Qiu Y. T., Wang G., Kwon J. Y., Rutzler M., Kwon H.-W., Pitts R. J., van Loon J. J. A., Takken W., Carlson J. R. et al. (2007). Odor coding in the maxillary Palp of the malaria vector mosquito Anopheles gambiae. Curr. Biol. 17, 1533-1544. 10.1016/j.cub.2007.07.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald W. and Traub R. (1960). Malaysian parasites XXXVII an introduction to the ecology of the mosquitoes of the lowland dipterocarp forest of Selangor, Malaya. Stud. Inst. Med. Res. Fed. Malaya 29, 79-109. [Google Scholar]
- Maia M. F. and Moore S. J. (2011). Plant-based insect repellents: a review of their efficacy, development and testing. Malar. J. 10, S11 10.1186/1475-2875-10-S1-S11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maimon G., Straw A. D. and Dickinson M. H. (2010). Active flight increases the gain of visual motion processing in Drosophila. Nat. Neurosci. 13, 393-399. 10.1038/nn.2492 [DOI] [PubMed] [Google Scholar]
- Majeed S., Hill S. R., Birgersson G. and Ignell R. (2016). Detection and perception of generic host volatiles by mosquitoes modulate host preference: context dependence of (R)-1-octen-3-ol. Open Sci. 3, 160467 10.1098/rsos.160467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride C. S., Baier F., Omondi A. B., Spitzer S. A., Lutomiah J., Sang R., Ignell R. and Vosshall L. B. (2014). Evolution of mosquito preference for humans linked to an odorant receptor. Nature 515, 222-227. 10.1038/nature13964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mclver S. B. (1968). Host preferences and discrimination by the mosquitoes Aedes aegypti and Culex tarsalis (Diptera: Culicidae). J. Med. Entomol. 5, 422-428. 10.1093/jmedent/5.4.422 [DOI] [PubMed] [Google Scholar]
- McMeniman C. J., Corfas R. A., Matthews B. J., Ritchie S. A. and Vosshall L. B. (2014). Multimodal integration of carbon dioxide and other sensory cues drives mosquito attraction to humans. Cell 156, 1060-1071. 10.1016/j.cell.2013.12.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menda G., Uhr J. H., Wyttenbach R. A., Vermeylen F. M., Smith D. M., Harrington L. C. and Hoy R. R. (2013). Associative learning in the dengue vector mosquito, Aedes aegypti: avoidance of a previously attractive odor or surface color that is paired with an aversive stimulus. J. Exp. Biol. 216, 218-223. 10.1242/jeb.074898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missbach C., Dweck H. K. M., Vogel H., Vilcinskas A., Stensmyr M. C., Hansson B. S. and Grosse-Wilde E. (2014). Evolution of insect olfactory receptors. Elife 3, e02115 10.7554/eLife.02115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molaei G. and Andreadis T. G. (2006). Identification of avian- and mammalian-derived bloodmeals in Aedes vexans and Culiseta melanura (Diptera: Culicidae) and its implication for west nile virus transmission in Connecticut, USA. J. Med. Entomol. 43, 1088-1093. 10.1093/jmedent/43.5.1088 [DOI] [PubMed] [Google Scholar]
- Molaei G., Andreadis T. G., Armstrong P. M. and Diuk-Wasser M. (2008). Host-feeding patterns of potential mosquito vectors in Connecticut, USA: molecular analysis of bloodmeals from 23 Species of Aedes, Anopheles, Culex, Coquillettidia, Psorophora, and Uranotaenia. J. Med. Entomol. 45, 1143-1151. 10.1093/jmedent/45.6.1143 [DOI] [PubMed] [Google Scholar]
- Moon Y. M., Metoxen A. J., Leming M. T., Whaley M. A. and O'Tousa J. E. (2014). Rhodopsin management during the light–dark cycle of Anopheles gambiae mosquitoes. J. Insect Physiol. 70, 88-93. 10.1016/j.jinsphys.2014.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mwandawiro C., Boots M., Tuno N., Suwonkerd W., Tsuda Y. and Takagi M. (2000). Heterogeneity in the host preference of Japanese encephalitis vectors in Chiang Mai, northern Thailand. Trans. R. Soc. Trop. Med. Hyg. 94, 238-242. 10.1016/S0035-9203(00)90303-1 [DOI] [PubMed] [Google Scholar]
- Owald D., Felsenberg J., Talbot C. B., Das G., Perisse E., Huetteroth W. and Waddell S. (2015). Activity of defined mushroom body output neurons underlies learned olfactory behavior in Drosophila. Neuron 86, 417-427. 10.1016/j.neuron.2015.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oye K. A., Esvelt K., Appleton E., Catteruccia F., Church G., Kuiken T., Lightfoot S. B.-Y., McNamara J., Smidler A. and Collins J. P. (2014). Regulating gene drives. Science 345, 626-628. 10.1126/science.1254287 [DOI] [PubMed] [Google Scholar]
- Potter C. J. (2014). Stop the biting: targeting a mosquito's sense of smell. Cell 156, 878-881. 10.1016/j.cell.2014.02.003 [DOI] [PubMed] [Google Scholar]
- Price D. C. and Fonseca D. M. (2015). Genetic divergence between populations of feral and domestic forms of a mosquito disease vector assessed by transcriptomics. PeerJ 3, e807 10.7717/peerj.807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao T. R. (1947). Visual responses of mosquitoes artificially rendered flightless. J. Exp. Biol. 24, 64-78. [DOI] [PubMed] [Google Scholar]
- Reidenbach K. R., Cook S., Bertone M. A., Harbach R. E., Wiegmann B. M. and Besansky N. J. (2009). Phylogenetic analysis and temporal diversification of mosquitoes (Diptera: Culicidae) based on nuclear genes and morphology. BMC Evol. Biol. 9, 298 10.1186/1471-2148-9-298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riabinina O., Task D., Marr E., Lin C.-C., Alford R., O'Brochta D. A. and Potter C. J. (2016). Organization of olfactory centres in the malaria mosquito Anopheles gambiae. Nat. Commun. 7, ncomms13010 10.1038/ncomms13010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinker D. C., Zhou X., Pitts R. J., Rokas A. and Zwiebel L. J. (2013). Antennal transcriptome profiles of anopheline mosquitoes reveal human host olfactory specialization in Anopheles gambiae. BMC Genomics 14, 749 10.1186/1471-2164-14-749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha M., Kimler K. J., Leming M. T., Hu X., Whaley M. A. and O'Tousa J. E. (2015). Expression and light-triggered movement of rhodopsins in the larval visual system of mosquitoes. J. Exp. Biol. 218, 1386-1392. 10.1242/jeb.111526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth L. M. and Willis E. R. (1952). Possible hygroreceptors in Aedes aegypti (L.) and Blattella germanica (L.). J. Morphol. 91, 1-14. 10.1002/jmor.1050910102 [DOI] [Google Scholar]
- Sato S. (1953). Structure and development of the compound eye of Aedes (Finlaya) japonicus Theobald. Sci. Rep. Tohoku Imp. Univ. 20, 33-44. [Google Scholar]
- Sato S. (1957). On the dimensional characters of the compound eye of Culex pipiens var. pallens Coquillet. Sci. Rep. Tohoku Imp. Univ. 23, 83-90. [Google Scholar]
- Sato S. (1959). Structure and development of the compound eye of Culex (Lutzia) vorax Edwards. Sci. Rep. Tohoku Imp. Univ. 25, 99-110. [Google Scholar]
- Savage H. M., Niebylski M. L., Smith G. C., Mitchell C. J. and Craig G. B. (1993). Host-feeding patterns of Aedes albopictus (Diptera: Culicidae) at a temperate North American site. J. Med. Entomol. 30, 27-34. 10.1093/jmedent/30.1.27 [DOI] [PubMed] [Google Scholar]
- Schultzhaus J. N., Saleem S., Iftikhar H. and Carney G. E. (2017). The role of the Drosophila lateral horn in olfactory information processing and behavioral response. J. Insect Physiol. 98, 29-37. 10.1016/j.jinsphys.2016.11.007 [DOI] [PubMed] [Google Scholar]
- Service M. W. (1993). Sampling adults by animal bait catches and by animal-baited traps. In Mosquito Ecology, pp. 349-498. Dordrecht: Springer. [Google Scholar]
- Skiri H. T., Galizia C. G. and Mustaparta H. (2004). Representation of primary plant odorants in the antennal lobe of the moth Heliothis virescens using calcium imaging. Chem. Senses 29, 253-267. 10.1093/chemse/bjh026 [DOI] [PubMed] [Google Scholar]
- Smart M. R. and Brown A. W. A. (1956). Studies on the responses of the female Aëdes Mosquito. Part VII.—The effect of skin temperature, hue and moisture on the attractiveness of the human hand. Bull. Entomol. Res. 47, 89-100. 10.1017/S000748530004654X [DOI] [Google Scholar]
- Sousa C. A., Pinto J., Almeida P. G., Ferreira C., do Rosário V. E. and Charlwood J. D. (2001). Dogs as a favored host choice of Anopheles gambiae sensu stricto (Diptera: Culicidae) of São Tomé, West Africa. J. Med. Entomol. 38, 122-125. 10.1603/0022-2585-38.1.122 [DOI] [PubMed] [Google Scholar]
- Sparks J. T., Vinyard B. T. and Dickens J. C. (2013). Gustatory receptor expression in the labella and tarsi of Aedes aegypti. Insect Biochem. Mol. Biol. 43, 1161-1171. 10.1016/j.ibmb.2013.10.005 [DOI] [PubMed] [Google Scholar]
- Stacconi R., Valerio M., Hansson B. S., Rybak J., and Romani R. (2014). Comparative neuroanatomy of the antennal lobes of 2 homopteran species. Chem. Senses 39, 283-294. 10.1093/chemse/bjt114 [DOI] [PubMed] [Google Scholar]
- Stanczyk N. M., Brookfield J. F. Y., Field L. M. and Logan J. G. (2013). Aedes aegypti mosquitoes exhibit decreased repellency by DEET following previous exposure. PLoS ONE 8, e54438 10.1371/journal.pone.0054438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffan W. A. and Evenhuis N. L. (1981). Biology of Toxorhynchites. Annu. Rev. Entomol. 26, 159-181. 10.1146/annurev.en.26.010181.001111 [DOI] [Google Scholar]
- Steinbrecht R. A. (1997). Pore structures in insect olfactory sensilla: a review of data and concepts. Int. J. Insect Morphol. Embryol. 26, 229-245. 10.1016/S0020-7322(97)00024-X [DOI] [Google Scholar]
- Strutz A., Soelter J., Baschwitz A., Farhan A., Grabe V., Rybak J., Knaden M., Schmuker M., Hansson B. S. and Sachse S. (2014). Decoding odor quality and intensity in the Drosophila brain. Elife 3, e04147 10.7554/eLife.04147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suver M. P., Mamiya A. and Dickinson M. H. (2012). Octopamine neurons mediate flight-induced modulation of visual processing in Drosophila. Curr. Biol. 22, 2294-2302. 10.1016/j.cub.2012.10.034 [DOI] [PubMed] [Google Scholar]
- Syed Z. and Leal W. S. (2009). Acute olfactory response of Culex mosquitoes to a human- and bird-derived attractant. Proc. Natl. Acad. Sci. USA 106, 18803-18808. 10.1073/pnas.0906932106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takken W. and Knols B. G. J. (1999). Odor-mediated behavior of afrotropical malaria mosquitoes. Annu. Rev. Entomol. 44, 131-157. 10.1146/annurev.ento.44.1.131 [DOI] [PubMed] [Google Scholar]
- Takken W. and Verhulst N. O. (2013). Host preferences of blood-feeding mosquitoes. Annu. Rev. Entomol. 58, 433-453. 10.1146/annurev-ento-120811-153618 [DOI] [PubMed] [Google Scholar]
- Tempelis C. H. (1975). Host-feeding patterns of mosquitoes, with a review of advances in analysis of blood meals by serology. J. Med. Entomol. 11, 635-653. 10.1093/jmedent/11.6.635 [DOI] [PubMed] [Google Scholar]
- Tomberlin J. K., Rains G. C., Allan S. A., Sanford M. R. and Lewis W. J. (2006). Associative learning of odor with food- or blood-meal by Culex quinquefasciatus Say (Diptera: Culicidae). Naturwissenschaften 93, 551 10.1007/s00114-006-0143-9 [DOI] [PubMed] [Google Scholar]
- van Breugel F., Riffell J., Fairhall A. and Dickinson M. H. (2015). Mosquitoes use vision to associate odor plumes with thermal targets. Curr. Biol. 25, 2123-2129. 10.1016/j.cub.2015.06.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vantaux A., Lefèvre T., Dabiré K. R. and Cohuet A. (2014). Individual experience affects host choice in malaria vector mosquitoes. Parasit. Vectors 7, 249 10.1186/1756-3305-7-249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergoz V., Roussel E., Sandoz J.-C. and Giurfa M. (2007). Aversive learning in honeybees revealed by the olfactory conditioning of the sting extension reflex. PLoS ONE 2, e288 10.1371/journal.pone.0000288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers N., Christensen T. and Hildebrand J. (1998). Combinatorial odor discrimination in the brain: attractive and antagonist odor blends are represented in distinct combinations of uniquely identifiable glomeruli. J. Comp. Neurol. 400, 35-56. [DOI] [PubMed] [Google Scholar]
- Vinauger C., Lutz E. K. and Riffell J. A. (2014). Olfactory learning and memory in the disease vector mosquito Aedes aegypti. J. Exp. Biol. 217, 2321-2330. 10.1242/jeb.101279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinauger C., Lahondère C., Cohuet A., Lazzari C. R. and Riffell J. A. (2016). Learning and memory in disease vector insects. Trends Parasitol. 32, 761-771. 10.1016/j.pt.2016.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinauger C., Lahondère C., Wolff G. H., Locke L. T., Liaw J. E., Parrish J. Z., Akbari O. S., Dickinson M. H. and Riffell J. A (2018). Modulation of host learning in Aedes aegypti mosquitoes. Curr. Biol. 28, 333-344.e8. 10.1016/j.cub.2017.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddell S. (2013). Reinforcement signalling in Drosophila; dopamine does it all after all. Curr. Opin. Neurobiol. 23, 324-329. 10.1016/j.conb.2013.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. W., Wong A. M., Flores J., Vosshall L. B. and Axel R. (2003). Two-photon calcium imaging reveals an odor-evoked map of activity in the fly brain. Cell 112, 271-282. 10.1016/S0092-8674(03)00004-7 [DOI] [PubMed] [Google Scholar]
- Wang G., Carey A. F., Carlson J. R., Zwiebel L. J. and Hildebrand J. G. (2010). Molecular basis of odor coding in the malaria vector mosquito Anopheles gambiae. Proc. Natl. Acad. Sci. USA 107, 4418-4423. 10.1073/pnas.0913392107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman S. M., Aptekar J. W., Lu P., Nguyen J., Wang A. L., Keles M. F., Grygoruk A., Krantz D. E., Larsen C. and Frye M. A. (2015). Olfactory neuromodulation of motion vision circuitry in Drosophila. Curr. Biol. 25, 467-472. 10.1016/j.cub.2014.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weldon P. and Carroll J. F. (2007). Vertebrate chemical defense: secreted and topically acquired deterrents of arthropods. In Insect Repellents: Principles, Methods, and Uses (ed. Debboun M., Frances S. P. and Strickman D.), pp. 47-75. Boca Raton, FL: CRC Press. [Google Scholar]
- WHO (2016). World Malaria Report 2016. Geneva: World Health Organizaion. [Google Scholar]
- Wolff G. H. and Strausfeld N. J. (2015). The insect brain: a commentated primer. In Structure and Evolution of Invertebrate Nervous Systems (ed. Schmidt-Rhaesa A., Harzsch S. and Purschke G.), pp. 597-639. Oxford: Oxford University Press. [Google Scholar]
- Woolhouse M. E. J., Dye C., Etard J.-F., Smith T., Charlwood J. D., Garnett G. P., Hagan P., Hii J. L. K., Ndhlovu P. D., Quinnell R. J. et al. (1997). Heterogeneities in the transmission of infectious agents: implications for the design of control programs. Proc. Natl. Acad. Sci. USA 94, 338-342. 10.1073/pnas.94.1.338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P. and Leal W. S. (2013). Probing insect odorant receptors with their cognate ligands: insights into structural features. Biochem. Biophys. Res. Commun. 435, 477-482. 10.1016/j.bbrc.2013.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P., Zhu F., Buss G. K. and Leal W. S. (2015). 1-Octen-3-ol - the attractant that repels. F1000Res 4, 156 10.12688/f1000research.6646.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z., Liu F. and Liu N. (2016). Olfactory responses of southern house mosquito, Culex quinquefasciatus, to human odorants. Chem. Senses 41, 441-447. 10.1093/chemse/bjw071 [DOI] [PubMed] [Google Scholar]
- Zhou X., Rinker D. C., Pitts R. J., Rokas A. and Zwiebel L. J. (2014). Divergent and conserved elements comprise the chemoreceptive repertoire of the nonblood-feeding mosquito Toxorhynchites amboinensis. Genome Biol. Evol. 6, 2883-2896. 10.1093/gbe/evu231 [DOI] [PMC free article] [PubMed] [Google Scholar]