Abstract
Background
Our objective was to quantify the risk of acquiring malaria among progeny of women with malaria during pregnancy.
Methods
We searched MEDLINE, EMBASE, CINAHL, and the Cochrane Library for eligible prospective studies. The primary predictor was malaria during pregnancy defined as placental malaria, parasitemia, clinical malaria, or pregnancy-associated malaria. Primary outcomes were parasitemia or clinically defined malaria of young children. We performed meta-analyses to pool adjusted risk estimates using a random-effects model.
Results
Nineteen of 2053 eligible studies met inclusion criteria for the systemic review. Eleven of these studies were quantitative and were included in the meta-analyses. The pooled adjusted odds ratio (aOR) or adjusted hazard ratio (aHR) of malaria during pregnancy for detection of parasitemia in young children were 1.94 (95% confidence interval [CI], 0.93–4.07; P = .08) and 1.46 (95% CI, 1.07–2.00; P < .001), respectively. The pooled aOR or aHR for clinically defined malaria in young children were 2.82 (95% CI, 1.82–4.38; P < .001) and 1.31 (95% CI, 0.96–1.79; P = .09), respectively.
Conclusions
Our results confirmed that malaria during pregnancy significantly increased the overall risk of malaria in young children via indeterminate mechanisms and emphasize the urgent need to implement safe and highly effective strategies to prevent malaria during pregnancy.
Keywords: malaria, parasitemia, pregnancy, placenta, infant
Meta-analyses of 11 eligible studies were performed to estimate the adjusted risk of acquiring malaria among offspring of women with malaria during pregnancy. The results confirmed that malaria during pregnancy significantly increased the overall risk of malaria in young children.
Malaria is a parasitic, vector-borne tropical and subtropical disease that affects individuals residing in 87 countries [1]. Plasmodium falciparum is the most common cause of human malaria with potentially severe complications that disproportionately affect pregnant women and young children [2, 3]. In 2017, malaria led to an estimated 219 million cases and 435 000 deaths worldwide, with children under 5 years accounting for 61% of all deaths [1]. Approximately 125 million women living in malaria-endemic areas become pregnant each year [4]. Primigravid women are most susceptible to malaria because they do not possess natural immunity to the Var2CSA variant of PfEMP1 exposed on the surface of erythrocytes infected with P falciparum strains that selectively sequester in the placenta and adhere to chondroitin sulfate A [5]. It is postulated that in the absence of pregnancy-specific interventions, approximately 45% of pregnant women in Africa would be exposed to malaria, almost half of whom would develop placental malaria (PM) [6].
Malaria during pregnancy is associated with abortion, congenital malaria, infant anemia, intrauterine growth restriction, low birth weight, preterm delivery, and stillbirth [7]. Systematic reviews and meta-analyses have demonstrated that malaria prevention during pregnancy is associated with decreased risk of low birth weight and neonatal mortality [8, 9]. The precise mechanisms by which pregnancy-associated malaria affects fetuses and infants are unknown. In addition to direct invasion and infection of fetuses that occurs rarely, there is growing evidence to suggest that malaria infection during pregnancy can modulate fetal immune responses and influence the subsequent risk for young children of acquiring malaria. In multigravid women particularly, malaria may induce fetal immune priming that leads to increased susceptibility of infants to malaria [10]. However, several cohort studies that have studied the impact of pregnancy-associated malaria on young children have demonstrated varying outcomes hypothesized to be related to timing of maternal infection, gravidity, presence of PM, or common exposure of mother-infant dyads to infectious mosquitoes [11–14].
Knowledge about the effects of malaria during pregnancy on young children’s risk for acquiring malaria is urgently needed to inform evidence-based interventions and to mitigate the attributable morbidity and mortality in early childhood [15, 16]. In this systematic review and meta-analysis, we investigated the direct relationship between malaria during pregnancy and the risk posed to offspring of acquiring malaria in early childhood, and we also evaluated the quality of the evidence.
METHODS
We performed a systematic review and meta-analyses according to standardized guidelines of PRISMA and MOOSE [17, 18].
Literature Search and Study Selection
We searched the relevant medical literature using MEDLINE, EMBASE, CINAHL, and the Cochrane Library from inception through August 28, 2017 without restrictions for language or study design. We developed the search strategy based on synonymous and analogous associations with predictors and outcomes. The following thesaurus search terms (eg, Medical Subject Heading terms) were used: malaria AND pregnancy AND (child OR infant). Each thesaurus term was “exploded” to retrieve related subheadings. To find additional relevant publications, we considered all citations of relevant studies including literature reviews.
Cohort and case-control studies were eligible for inclusion provided they met the following criteria: (1) infection with P falciparum was confirmed in mothers during pregnancy or at delivery—types of maternal infection included PM, parasitemia, clinical malaria, or the all-inclusive term, pregnancy-associated malaria (PAM); (2) young children were evaluated for evidence of P falciparum parasitemia and clinical manifestations of malaria for at least the first 3 months of life; (3) infection with P falciparum in pregnant women and children was confirmed with a positive blood smear, commercial rapid diagnostic test (RDT), or polymerase chain reaction (PCR) assay; and (4) the direct relationship between malaria during pregnancy and the child’s malaria infection was assessed using linked mother-infant data. All eligible studies that met the inclusion criteria were included in the systematic review. Only studies that reported adjusted odds ratios (aORs) or adjusted hazard ratios (aHRs) were included in the meta-analyses to measure adjusted pooled effect sizes.
We excluded review articles, animal studies, and data derived from cross-sectional epidemiological analyses. Furthermore, we did not analyze data from asymptomatic pregnant women or infants from a single article [19] because the sparse data precluded meaningful analysis. Two papers that reported relative risk (RR) or relative rate ratio to measure effect sizes were ineligible because it was not possible to integrate these measures with OR based on the high prevalence of malaria in study areas [20, 21]. We did not include one study that reported HR derived from repeated measures of sequential episodes of parasitemia or clinical malaria because it could not accurately be pooled with HRs from the other eligible studies [14]. One study provided data about maternal malaria infections for each trimester of pregnancy; we used data only for the third trimester [13].
S.P. and I.C.M. independently searched the literature and selected eligible articles. Statistical agreement between the reviewers was measured with the Cohen’s kappa coefficient.
Data Extraction
S.P. and O.M. independently extracted data from each article. Differences between reviewers were resolved through arbitration by J.F.F. Information about authors, study site, sample size, follow-up period, exposure, outcome, measure of effect, unadjusted and adjusted effect sizes, and factors that required adjustment were systematically collated. The primary predictor of interest was malaria infection during pregnancy as determined by parasitological or clinical measures. We accepted the definitions of the types of maternal infection as described in the articles: PM required the presence of malaria parasites or pigment in a placental blood smear or biopsy; parasitemia required the presence of P falciparum in peripheral blood; clinical malaria was defined by the presence of fever with symptoms described in the relevant articles with evidence of malaria parasites in their peripheral blood; PAM included clinical signs as well as evidence of malaria parasites in peripheral blood or the placenta. For outcomes of interest in young children, parasitemia was defined as the presence of malaria parasites in peripheral blood and clinical malaria was defined as fever and other symptoms associated with parasitemia. Malaria parasitemia was detected by blood smear microscopy, commercial RDTs, or PCR assays.
Quality Assessment
S.P. and C.E.N. independently assessed the quality of evidence describing the relationship between malaria infection during pregnancy and the risk of malaria in the offspring using the modified Newcastle-Ottawa Quality Scale (NOS) in order to eliminate bias [22]. This scale assesses the following star-awarded criteria: (1) Selection - representativeness of the exposed cohort (truly or somewhat [eg, collections of participants from at least 3 or more communities] = 1 star), selection of the nonexposed cohort (drawn from the same community as the exposed cohort = 1 star), ascertainment of exposure (laboratory-based determination = 1 star), and demonstration that the outcome of interest was not present at the start of the study (confirmation of the absence of congenital malaria = 1 star); (2) Comparability - comparability of cohorts on the basis of the design or analysis (adjustment for bed net use = 1 star; adjustment for birth season = 1 star); (3) Outcome - assessment of outcome (independent blind assessment or linked records = 1 star), sufficient duration of follow-up (≥3 months = 1 star), adequate follow-up of cohorts (≤30% of subjects lost to follow-up = 1 star). The maximum possible NOS rating was 9 stars.
Statistical Analysis
To measure the pooled aORs or aHRs, we divided the studies into 4 subgroups depending on the predictors during pregnancy that were listed in the article: PM, maternal parasitemia, maternal clinical malaria, or PAM. We then pooled the adjusted effect sizes of the predictor variable in each of the 4 subgroup analyses to determine their relative impact on the outcome variables in young children: parasitemia or clinically defined malaria. We performed meta-analyses using the inverse variance method, which assigns weight to each study according to the respective standard error for each of aOR or aHR rather than according to sample size alone. We then generated forest plots to display each adjusted estimate (aOR or aHR) and 95% confidence intervals (CIs). A two-tailed P < .05 was considered statistically significant.
We used prespecified random-effects meta-regression models and accounted for substantial heterogeneity between studies. This model estimates the between-study variances of effect sizes using the DerSimonian and Laird method [23], and it is added to within-study error to calculate total variance in the included studies. We then confirmed the heterogeneity between studies with Cochran’s Q statistic (χ 2 test) and Higgins’ I2 statistic, 100% × (Q – df)/Q where Q is Cochran’s heterogeneity statistic and df is the degrees of freedom [24]. Higgins’ I2 statistic describes the ratio of heterogeneity to total variance, and the levels of 25%, 50%, and 75% indicate low, moderate, and high heterogeneity [24]. We defined substantial heterogeneity between studies as Higgins’ I2 statistic ≥50%.
We assessed publication bias using funnel plots and tested funnel plot asymmetry using Begg and Mazumdar’s rank correlation test [25] and Egger’s linear regression test [26]. We performed statistical analyses using Review Manager version 5.3.5 (Nordic Cochrane Centre, Copenhagen, Denmark) and STATA version 15.1 (StataCorp, College Station, TX).
RESULTS
Selection of Studies
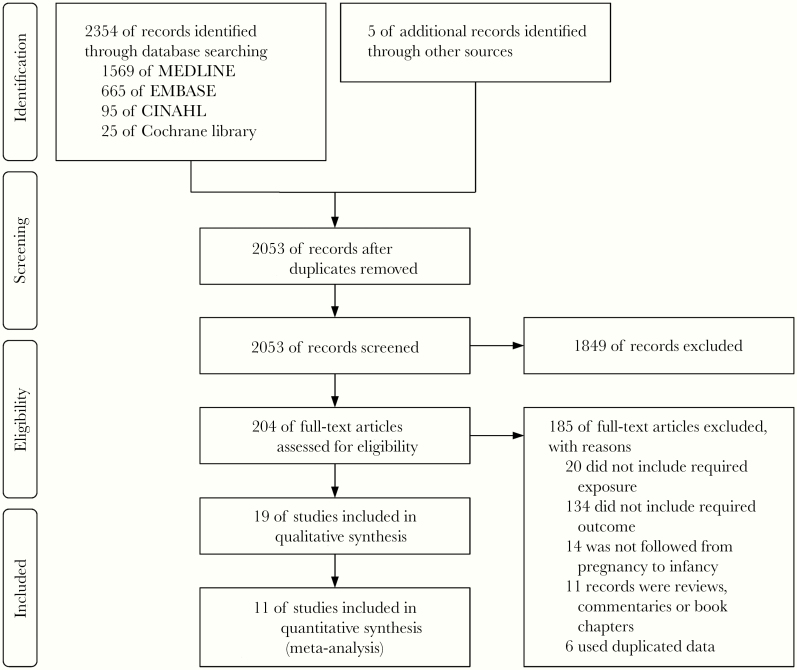
A search of the medical literature using the defined thesaurus terms identified 2053 unique relevant articles. The initial screen found that 204 articles were eligible (Figure 1). We then conducted full-text reviews of these articles and excluded 180 reports because predictors, outcomes, follow-up periods, and/or study designs did not meet our inclusion criteria. Of the 24 remaining articles, 6 articles [27–32] were excluded because they are secondary analyses of datasets already included [19, 20, 33, 34]. We included 2 articles obtained from the Mother-Offspring Malaria Study Project because these reports used different predictors and outcomes [12, 35]. Ultimately, 19 articles met our inclusion criteria for the systematic review, and 11 of these had the requisite data for meta-analyses (Table 1): 9 studies reported aORs [11–14, 19, 33–36] and 4 studies reported aHRs [13, 20, 34, 37]; 2 of these studies reported both aORs and aHRs [13, 34]. Studies with different estimates of risk (aOR vs aHR) could not be analyzed together because HR is a measure of time-to-event outcomes, whereas OR is a measure of dichotomous outcomes regardless of timing. Eight articles were excluded from the meta-analyses because these studies only provided estimates of numbers of episodes [38], proportions [39], prevalence [10], RR [40], rate ratio [41], unadjusted measures [42], or results based on different classifications of the predictor [43] or outcome [44] variables that precluded pooling the effect sizes. Interrater agreement for selection of articles was near perfect (Cohen’s kappa coefficient = 0.88). The Higgins’ I2 statistic was >50%, which indicated substantial heterogeneity between studies and stipulated the need for a random-effects meta-regression approach (Figures 2 and 3).
Figure 1.
Study selection.
Table 1.
Studies Included in the Systematic Review and Meta-Analysesa
| Study | Study Site | Study Design | n | Follow- Up Period | Maternal Malaria Type | Outcome of Offspring | Measure of Effect | Unadjusted Effect | Adjusted Effect | Factors Adjusted for | NOS, Stars |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Studies Included in Systematic Review and Meta-Analyses | |||||||||||
| Asante et al [14] | Ghana | Cohort | 1855 | 12 mo | PM | First parasitemia | HR (multigravidae) | 1.06 (0.91–1.23) | 1.02 (0.88–1.19) | Wealth index, thatched roof, residence area, and distance from health facility | 8 |
| PM | First clinical malaria | HR (multigravidae) | 1.08 (0.91–1.27) | 1.01 (0.85–1.20) | Wealth index, thatched roof, residence area, and distance from health facility | ||||||
| PM | All episodes of parasitemia | HR (multigravidae) | 1.03 (0.89–1.19) | 0.98 (0.85–1.12) | Wealth index, thatched roof, residence area, and distance from health facility, infant ITN use, malaria exposure score | ||||||
| PM | All episodes of clinical malaria | HR (multigravidae) | 1.00 (0.86–1.17) | 0.94 (0.81–1.10) | Wealth index, thatched roof, residence area, and distance from health facility, infant ITN use, malaria exposure score | ||||||
| Bardají et al [37] | Mozam bique | RCT | 997 | 12 mo | PM (acute) | Clinical malaria | OR | 4.20 (1.94–9.12) | 4.63 (2.10–10.24) | HIV status, malaria during pregnancy, and maternal mid-upper arm circumference | 7 |
| PM (chronic) | Clinical malaria | OR | 3.82 (2.06–7.08) | 3.95 (2.07–7.55) | HIV status, malaria during pregnancy, and maternal mid-upper arm circumference | ||||||
| Clinical malaria | Clinical malaria | OR | 2.40 (1.45–3.98) | 1.96 (1.13–3.41) | HIV status, PM, and maternal mid-upper arm circumference | ||||||
| Borgella et al [13] | Benin | Cohort | 194 | 12 mo | PAM (1st trimester) | Parasitemia | OR | 1.33 (0.36–4.94) | 1.12 (0.23–5.45) | PM, age, distance from lake, bednet use, and birth season | 9 |
| PAM (1st trimester) | Clinical malaria | OR | 1.19 (0.35–4.08) | 0.83 (0.13–5.18) | PM, age, distance from lake, bednet use, and birth season | ||||||
| PAM (2nd trimester) | Parasitemia | OR | 1.14 (0.58–2.23) | 0.87 (0.35–2.09) | PM, age, distance from lake, bednet use, and birth season | ||||||
| PAM (2nd trimester) | Clinical malaria | OR | 1.24 (0.61–2.52) | 0.81 (0.31–2.12) | PM, age, distance from lake, bednet use, and birth season | ||||||
| PAM (3rd trimester) | Parasitemia | OR | 2.77 (1.49–5.15) | 4.16 (1.64–10.54) | PM, age, distance from lake, bednet use, and birth season | ||||||
| PAM (3rd trimester) | Clinical malaria | OR | 2.80 (1.45–5.42) | 4.61 (1.70–12.45) | PM, age, distance from lake, bednet use, and birth season | ||||||
| PM | Parasitemia | OR | 1.58 (0.75–3.32) | 0.72 (0.25–2.11) | PAM, age, distance from lake, bednet use, and birth season | ||||||
| PM | Clinical malaria | OR | 1.53 (0.70–3.34) | 0.59 (0.18–1.88) | PAM, age, distance from lake, bednet use, and birth season | ||||||
| PAM (1st trimester) | First parasitemia | HR | 1.30 (0.57–2.92) | 1.00 (0.42–2.39) | PM, age, distance from lake, bednet use, and birth season | ||||||
| PAM (1st trimester) | First clinical malaria | HR | 1.44 (0.49–4.19) | 0.97 (0.32–2.92) | PM, age, distance from lake, bednet use, and birth season | ||||||
| PAM (2nd trimester) | First parasitemia | HR | 1.27 (0.73–2.19) | 1.14 (0.62–2.12) | PM, age, distance from lake, bednet use, and birth season | ||||||
| PAM (2nd trimester) | First clinical malaria | HR | 1.35 (0.74–2.47) | 1.15 (0.58–2.28) | PM, age, distance from lake, bednet use, and birth season | ||||||
| PAM (3rd trimester) | First parasitemia | HR | 2.23 (1.36–3.65) | 2.95 (1.58–5.50) | PM, age, distance from lake, bednet use, and birth season | ||||||
| PAM (3rd trimester) | First clinical malaria | HR | 2.26 (1.29–3.96) | 3.19 (1.59–6.38) | PM, age, distance from lake, bednet use, and birth season | ||||||
| PM | First parasitemia | HR | 1.41 (0.79–2.52) | 0.68 (0.34–1.38) | PAM, age, distance from lake, bednet use, and birth season | ||||||
| PM | First clinical malaria | HR | 1.33 (0.69–2.54) | 0.60 (0.28–1.32) | PAM, age, distance from lake, bednet use, and birth season | ||||||
| Boudová et al [20] | Malawi | RCT | 473 | 1 y | PM | Parasitemia | OR | 2.7(1.1–6.7) | 2.5 (1.0–6.3) | Age, GA, and clinical trial arm | 6 |
| PM | Clinical malaria | OR | 4.1 (1.3–13.1) | 3.9 (1.2–13.0) | Age, GA, and clinical trial arm | ||||||
| Parasitemia | Parasitemia | OR | 1.5 (0.5–4.4) | 1.5 (0.5–4.4) | Age, GA, and clinical trial arm | ||||||
| Parasitemia | Clinical malaria | OR | 1.5 (0.3–7.3) | 1.4 (0.3–7.0) | Age, GA, and clinical trial arm | ||||||
| 2 y | PM | Parasitemia | OR | 2.6 (1.0–6.6) | 2.8 (1.1–7.5) | Age, GA, and clinical trial arm | |||||
| PM | Clinical malaria | OR | 2.8 (1.0–7.9) | 3.2 (1.1–9.4) | Age, GA, and clinical trial arm | ||||||
| Parasitemia | Parasitemia | OR | 1.4 (0.5–3.8) | 1.5 (0.6–4.1) | Age, GA, and clinical trial arm | ||||||
| Parasitemia | Clinical malaria | OR | 1.3 (0.4–4.4) | 1.5 (0.4–4.9) | Age, GA, and clinical trial arm | ||||||
| PM | Cum. parasitemia | RRR | 1.6 (1.0–2.6) | NA | NA | ||||||
| PM | Cum. clinical malaria | RRR | 2.3 (1.1–4.8) | NA | NA | ||||||
| Parasitemia | Cum. parasitemia | RRR | 1.4 (0.8–2.4) | NA | NA | ||||||
| Parasitemia | Cum. clinical malaria | RRR | 2.1 (1.0– 4.5) | NA | NA | ||||||
| Gonçalves et al [12] | Tanzania | Cohort | 882 | 4 y | PM (1st delivery) | First clinical malaria | HR | NA | 1.12 (0.44–2.82) | Hgb genotype, bednet use, sex, village of residence, and season | 9 |
| 4 y | PM (1st delivery) | Parasite density | Coefficient | NA | 0.16 (−0.20 to 0.52) | Hgb genotype, bednet use, sex, village of residence, and season | |||||
| Le Port et al [33] | Benin | Cohort | 545 | 18 mo | PM | First parasitemia | HR | 1.59 (1.17–2.16) | 1.57 (1.16–2.14) | Exposure prediction | 9 |
| Mutabingwa et al [35] | Tanzania | Cohort | 453 | 54 wk | PM | First parasitemia | HR | 1.33 (0.97–1.83) | 1.41 (1.01–1.99) | Residence area, birth season, gravidity, and bednet use | 9 |
| Ndibazza et al [19] | Uganda | RCT | 2289 | 5 y | PAM | All episodes of clinical malaria | HR | NA | 1.23 (1.01–1.51) | Age, parity, education, bednet ownership, SES, HIV status, and residence area | 8 |
| PAM | All episodes of asymptomatic malaria | HR | NA | 1.27 (0.83–1.97) | Age, parity, education, bednet ownership, SES, HIV status, and residence area | ||||||
| Schwarz et al [36] | Gabon | RCT | 527 | 30 mo | PM | First clinical malaria | HR | 2.3 (1.3–3.9) | 2.1 (1.2–3.7) | Gravidity, residence area, birth season, preventive treatment, and bednet use | 7 |
| Sylvester et al [34] | Tanzania | Cohort | 206 | 24 mo | PM | Clinical malaria | OR | 4.632 (2.248–9.541) | 4.791 (2.21–10.38) | Gravidity, birth weight, age, and birth season | 9 |
| Tassi Yunga et al [11] | Cameroon | Cohort | 72 | 12 mo | PM (PM + High) | First parasitemia | HR | 1.5 (0.8–2.8) | 1.5 (0.7–3.1) | Residence area, gravidity, birth season, Hgb genotype, and cord MSP1 IgG level | 7 |
| PM (PM + Low) | First parasitemia | HR | 2.6 (1.3–4.8) | 2.8 (1.3–6.0) | Residence area, gravidity, birth season, Hgb genotype, and cord MSP1 IgG level | ||||||
| Studies Included in Systematic Review but Not Meta-Analyses | |||||||||||
| Awine et al [41] | Ghana | RCT | 686 | 12 mo | PM | Clinical malaria | Rate ratio | NA | 0.86 (0.54–1.37) | Age, parasitemia status at enrolment, Hgb, birth season, sex, SES, residence area, irrigated area, and ITN use | 8 |
| Bonner et al [39] | Kenya | Case Control | 100 | 12 mo | PM | Parasitemia | Proportion | 15.6% in PM+ and 14.7% in PM− | NA | NA | 6 |
| Clinical malaria | Proportion | 3.6% in PM+ and 1.4% in PM− | NA | NA | |||||||
| Cot et al [44] | Cameroon | Cohort | 79 | 2 y | PM | High, medium, and low density parasitemia | OR | NA | 2.3 (0.38–14), 2.6 (0.46–15), and 0.4 (0.05–2.90) | 2H3 chondroitin sulfate, gravidity, and residence area | 6 |
| PM | High, medium, and low density parasitemia | OR | NA | 2.6 (0.39–17), 2.8 (0.50–16), and 0.4 (0.05–3.4) | Palo Alto chondroitin sulfate, gravidity, and residence area | ||||||
| De Beaudrap et al [40] | Uganda | RCT | 832 | 12 mo | Parasitemia | First parasitemia | RR | NA | 2.97 (1.37–6.42) | Education, age, gravidity, residence area, season, HIV status, and bednet use | 8 |
| PM | First parasitemia | RR | NA | 10.42 (2.64–41.10) | Education, age, gravidity, residence area, season, HIV status, and bednet use | ||||||
| Le Hesran et al [43] | Cameroon | Cohort | 197 | 2 y | PM | Parasitemia | Prevalence | Higher in PM+ between 5 and 8 mo | NA | NA | 6 |
| PM | Clinical malaria | Prevalence | 46.5% in PM+ and 38.5% in PM− | NA | NA | ||||||
| Malhotra et al [10] | Kenya | Cohort | 586 | 3 y | PAM | Parasitemia | RR | Exposed/sensitized: 1.4 (0.97–2.07), not exposed: 1.61 (1.10–2.43) | NA | NA | 9 |
| Mutabingwa et al [38] | Tanzania | CT | 327 | 1 y | Parasitemia | Parasitemia | N (episodes) | 3–5 vs 6–7 median no. of parasitemia episodes in infants of mothers who had no parasitemia vs 2+ episodes of parasitemia, respiratory | NA | NA | 7 |
| Slutsker et al [42] | Malawi | RCT | 3915 | 3 mo | Parasitemia | Parasitemia | OR | 1.1 (0.7–1.9) | NA | NA | 6 |
| PM | Parasitemia | OR | 1.2 (0.9–1.4) | NA | NA |
Abbreviations: Cum., cumulative; CT, clinical trial (not randomized); GA, gestational age at delivery; Hgb, hemoglobin; HIV, human immunodeficiency virus; HR, hazard ratio; IgG, immunoglobulin G; ITN, insecticide-treated bed net; mo, months; n, number; NA, not applicable; NOS, Newcastle-Ottawa quality scale; OR, odds ratio; PAM, pregnancy-associated malaria; PM, placental malaria; PM+/−, PM positive/negative group; RCT, randomized controlled trial; RR, relative risk; RRR, relative rate ratio; SES, socioeconomic status; wk, weeks; y, years.
aAge and HIV status refer to maternal age and HIV status.
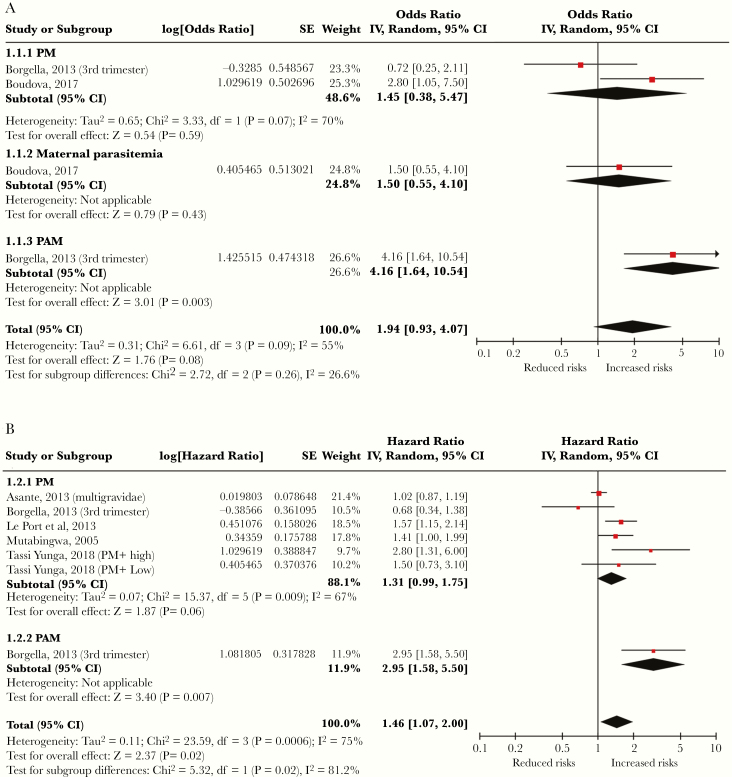
Figure 2.
Forest plots summarizing the pooled analyses of adjusted (A) odds ratios and (B) hazard ratios from studies assessing the relationship between malaria infection during pregnancy and parasitemia of young children. CI, confidence interval; IV, intravenous; PM, placental malaria.
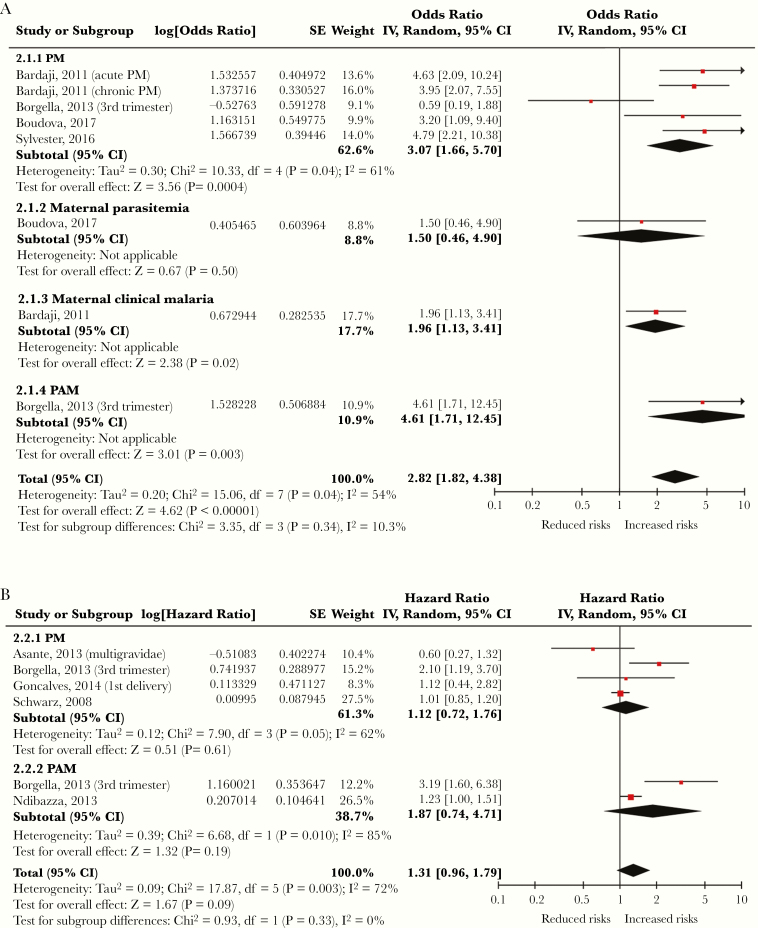
Figure 3.
Forest plots summarizing the pooled analyses of adjusted (A) odds ratios and (B) hazard ratios from studies assessing the relationship between malaria infection during pregnancy and clinically defined malaria of young children. CI, confidence interval; IV, intravenous; PM, placental malaria.
Malaria During Pregnancy Increased the Risk of Parasitemia in Young Children
Six eligible articles were used to estimate the pooled effects of malaria infection during pregnancy on P falciparum parasitemia of young children (Table 1). Subgroup analyses and total effect sizes are shown in Figure 2 [11, 13, 14, 20, 33, 35]. Based on the available data, the pooled aOR was 1.94 (95% CI, 0.93–4.07, P = .08) and the pooled aHR was 1.46 (95% CI, 1.07–2.00, P < .001). We identified a publication bias in the meta-analysis for estimating the pooled aOR of parasitemia (P value of Begg and Mazumdar’s test = .09; P value of Egger’s test = .023), but we did not identify bias for estimating pooled aHR (P value of Begg and Mazumdar’s test = .55; P value of Egger’s test = .14), indicating that the effect size calculation for aHR was more reliable.
Malaria During Pregnancy Increased the Risk of Clinical Malaria in Young Children
Eight articles reported the effects of malaria infection during pregnancy on clinical malaria of young children (Table 1). Subgroup analyses and total effect sizes are shown in Figure 3 [12–14, 19, 20, 34, 36, 37]. The pooled aOR was 2.82 (95% CI, 1.82–4.38; P < .001), and the pooled aHR was 1.31 (95% CI, 0.96–1.79; P = .09). No publication bias was identified in these analyses (P values of Begg and Mazumdar’s test for OR and HR = .27 and .71, respectively; P values of Egger’s test for OR and HR = .61 and .43, respectively).
DISCUSSION
The results of the meta-analyses suggest that malaria infection during pregnancy is associated with a significantly increased risk of P falciparum parasitemia (aHR, 1.46; 95% CI, 1.07–2.00; P < .001) and clinically defined malaria (aOR, 2.82; 95% CI, 1.82–4.38; P < .001) in young children. Our findings are supported by the additional 8 studies we identified in the systematic review but that were excluded from the meta-analyses [10, 38–44]. To our knowledge, only 2 previous publications have reviewed the medical literature on this topic [45, 46]. The report by Moya-Alvarez et al [45] concluded that PAM is associated with congenital malaria and early development of infant malaria. However, that report may have been biased in study selection, interpretation, and conclusions due to methodological limitations and nonstandardized analysis [47]. The systematic review by Kakuru et al [46] did find an association between malaria in pregnancy and malaria in infancy in some studies, but they did not perform a meta-analysis. To avoid similar potential deficiencies in our analyses, we used explicitly prespecified methods that complied with evidence-based standards (PRISMA and MOOSE) to enhance quality and transparency. For the meta-analyses, we adjusted the pooled effects for differences in sample size, study heterogeneity, and variance, and we assessed the studies for publication bias and study quality.
Although our meta-analyses concluded that malaria during pregnancy increases a young child’s risk of acquiring malaria, the precise mechanism of elevated risk remains unclear. It has been postulated that certain infants exposed to P falciparum antigens in utero undergo prenatal immune priming that can lead to an immune tolerant phenotype after birth, and the altered immunological state could potentially be associated with an increased risk of acquiring malaria [10]. In addition, shared environmental and entomological exposures may partly explain the similar risks of malaria experienced by mothers and their offspring. However, the relatively lower risk for infants of primigravid women compared with higher risk for infants of multigravid women suggests that other mechanisms are also implicated [35].
It is interesting to note that although there were trends toward significance in the associations between malaria during pregnancy and the odds of parasitemia (pooled aOR = 1.94, P = .08) or hazard of clinical malaria (pooled aHR = 1.31, P = .09) in young children, they did not achieve statistical significance. On the other hand, the associations between malaria during pregnancy and hazard of parasitemia (aHR = 1.46) or odds of clinical malaria (aOR = 2.82) were highly significant (each P < .001). These discrepancies may be partially explained by publication bias that we detected in the analysis of aOR for parasitemia in children, the small number of eligible studies leading to low power (type 2 error), and potentially additional confounders that may not have been measured.
Similarly, in our subgroup analyses, we found that PM significantly increased the odds of clinical malaria, but not P falciparum parasitemia, in young children. A possible explanation for this discrepancy is that fewer studies with parasitemia as the outcome were available for inclusion, thereby limiting the power of the analysis. Alternatively, it is possible that differential expansion and differentiation of infants’ regulatory T cells, effector memory cells, and dendritic cells in the presence of PM may lead to increased levels of suppressive cytokines and/or enhanced effector functions that inhibit parasite replication [48]. We could not evaluate associations between other subgroups of maternal malaria infection (parasitemia, clinical malaria, and PAM) and risk of malaria in young children because the analyses were limited by a small number of studies.
Two studies that investigated the effect of timing of malaria infection during pregnancy on the risk of malaria in the progeny confirmed that a temporal association existed [13, 40]. It is known that B- and T-cell repertoire diversity and complexity in human fetuses mature as the pregnancy advances [49]. Therefore, the responses of the fetal immune system to malaria antigens may differ depending on the timing of the maternal malaria infection. More immuno-epidemiological studies are needed to elucidate how temporal or other factors related to maternal malaria infections contribute to the risk of malaria in young children.
There is strong evidence that a woman’s susceptibility to malaria is greatest in her first pregnancy, and her risk of malaria infection decreases with successive pregnancies, likely due to the development of naturally acquired immunity that blocks parasite adherence in the placenta [5]. In contrast to this observation, the progeny of primigravid women have the lowest risk of acquiring malaria, particularly if PM is absent [14, 35], compared with the young children of multigravid women [19]. We were not able to perform subgroup meta-analyses to examine whether malaria infection of young children is modified by the interaction between malaria infection during pregnancy and gravidity because of the small number of studies and the heterogenous classification of predictor variables, definitions of outcomes, and statistical tests [12, 14, 35]. Mutabingwa et al [35] reported that infants of primigravid women had significantly lower odds of parasitemia if they had PM (aOR, 0.21; 95% CI, 0.09–0.47) or did not have PM (aOR, 0.67; 95% CI, 0.50–0.92) compared with multigravid women who did not have PM; Gonçalves et al [12] demonstrated that infants of multigravid women with PM had significantly higher hazard of moderately severe or severe malaria (aHR, 1.67; 95% CI, 1.25–2.22) compared with primigravid women with no PM. However, these 2 reports [12, 35] derived data from the same Tanzanian cohort. Asante et al [14] found that infants of primigravid women had a significantly lower hazard of clinical malaria (aHR, 0.60; 95% CI, 0.43–0.84) and parasitemia (aHR, 0.64; 95% CI, 0.48–0.86) if they had no PM compared with multigravid women who also had no PM. Carefully designed studies evaluating the influence of complex interactions between maternal malaria and gravidity on childhood malaria are needed.
A strength of our study is that the analyses were performed using large-scale data derived from 7611 subjects residing in several sub-Saharan African countries. However, our study has several limitations: we did not perform meta-analyses for all predictor subgroups because of insufficient numbers of eligible studies and inconsistent stratification methods among articles; we detected some publication bias that invariably affected the results; many contributing studies may be confounded by shared maternal and infant exposure to infectious mosquitoes, which may not be completely accounted for by adjustment for bed net use, seasonality, and distance to lakeshores; we did not investigate the influence of the precise timing of maternal malaria infection, which was highly variable, or whether there was a potential dose-response effect of maternal parasite densities because of limited data; we did not include duration of follow-up or timing of testing of infants as a covariate in the analyses due to insufficient numbers of studies; and diagnostic tests for malaria (microscopy, RDT, and PCR assays) had different sensitivities. Finally, we did not specifically evaluate the impact of maternal intermittent preventive treatment in pregnancy because of inconsistent reporting although most of the studies incorporated this practice as standard of care.
CONCLUSIONS
In conclusion, the results of our systematic review and meta-analyses showed that malaria infection during pregnancy increases the overall risk of malaria in young children. Despite the heterogeneity of specific predictors, the increased risk of malaria in young children was reasonably consistent. Our findings also revealed knowledge gaps about how risk-modifying factors in pregnancy interact to affect offspring, including the impact of the precise timing of malaria infection, gravidity, parasite density, PM, and intermittent preventive treatment in pregnancy. Therefore, to design highly effective interventions, carefully designed future studies that control for potential confounding by shared exposure of mothers and infants to infectious mosquitoes are urgently needed to elucidate the precise mechanisms by which malaria during pregnancy impacts young children.
Notes
Financial support. This work was funded by a Centers of Biomedical Research Excellence award for Reproductive Health at Women & Infants Hospital of Rhode Island from the National Institute of General Medical Sciences at the National Institutes of Health (P20GM121298-01). An award from the National Institute of Allergy and Infectious Diseases at the National Institutes of Health (K08AI100997; to I. C. M.) also funded this work.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. World Health Organization. World malaria report 2018. Available at: http://www.who.int/malaria/publications/world_malaria_report_2014/wmr-2014-no-profiles.pdf?ua=1. Accessed 4 February 2019.
- 2. Nduka FO, Egbu A, Okafor C, Nwaugo VO. Prevalence of malaria parasites and anaemia in pregnant and non pregnant women in Aba and Okigwe towns of southeast Nigeria. Anim Res Int 2006; 3:508–12. [Google Scholar]
- 3. Cowman AF, Healer J, Marapana D, Marsh K. Malaria: biology and disease. Cell 2016; 167:610–24. [DOI] [PubMed] [Google Scholar]
- 4. Dellicour S, Tatem AJ, Guerra CA, Snow RW, ter Kuile FO. Quantifying the number of pregnancies at risk of malaria in 2007: a demographic study. PLoS Med 2010; 7:e1000221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fried M, Duffy PE. Malaria during pregnancy. Cold Spring Harb Perspect Med 2017; 7:a025551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Walker PG, ter Kuile FO, Garske T, Menendez C, Ghani AC. Estimated risk of placental infection and low birthweight attributable to Plasmodium falciparum malaria in Africa in 2010: a modelling study. Lancet Glob Health 2014; 2:e460–7. [DOI] [PubMed] [Google Scholar]
- 7. Rogerson SJ, Desai M, Mayor A, Sicuri E, Taylor SM, van Eijk AM. Burden, pathology, and costs of malaria in pregnancy: new developments for an old problem. Lancet Infect Dis 2018; 18:e107–18. [DOI] [PubMed] [Google Scholar]
- 8. McClure EM, Goldenberg RL, Dent AE, Meshnick SR. A systematic review of the impact of malaria prevention in pregnancy on low birth weight and maternal anemia. Int J Gynaecol Obstet 2013; 121:103–9. [DOI] [PubMed] [Google Scholar]
- 9. Eisele TP, Larsen DA, Anglewicz PA, et al. Malaria prevention in pregnancy, birthweight, and neonatal mortality: a meta-analysis of 32 national cross-sectional datasets in Africa. Lancet Infect Dis 2012; 12:942–9. [DOI] [PubMed] [Google Scholar]
- 10. Malhotra I, Dent A, Mungai P, et al. Can prenatal malaria exposure produce an immune tolerant phenotype? A prospective birth cohort study in Kenya. PLoS Med 2009; 6:e1000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tassi Yunga S, Fouda GG, Sama G, Ngu JB, Leke RGF, Taylor DW. Increased susceptibility to Plasmodium falciparum in infants is associated with low, not high, placental malaria parasitemia. Sci Rep 2018; 8:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gonçalves BP, Huang CY, Morrison R, et al. Parasite burden and severity of malaria in Tanzanian children. N Engl J Med 2014; 370:1799–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Borgella S, Fievet N, Huynh BT, et al. Impact of pregnancy-associated malaria on infant malaria infection in southern Benin. PLoS One 2013; 8:e80624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Asante KP, Owusu-Agyei S, Cairns M, et al. Placental malaria and the risk of malaria in infants in a high malaria transmission area in Ghana: a prospective cohort study. J Infect Dis 2013; 208:1504–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Murray CJ, Ortblad KF, Guinovart C, et al. Global, regional, and national incidence and mortality for HIV, tuberculosis, and malaria during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014; 384:1005–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kurtis JD, Raj DK, Michelow IC, et al. Maternally-derived antibodies to schizont egress antigen-1 and protection of infants from severe malaria. Clin Infect Dis 2019; 68:1718–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000; 283:2008–12. [DOI] [PubMed] [Google Scholar]
- 19. Ndibazza J, Webb EL, Lule S, et al. Associations between maternal helminth and malaria infections in pregnancy and clinical malaria in the offspring: a birth cohort in Entebbe, Uganda. J Infect Dis 2013; 208:2007–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Boudová S, Divala T, Mungwira R, Mawindo P, Tomoka T, Laufer MK. Placental but not peripheral Plasmodium falciparum infection during pregnancy is associated with increased risk of malaria in infancy. J Infect Dis 2017; 216:732–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dohoo I, Martin S, Stryhn H.. Veterinary Epidemiologic Research. Charlottetown, Prince Edward island, Canada: VER Inc; 2009. [Google Scholar]
- 22. Wells G, Shea B, O’connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 23 May 2018.
- 23. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7:177–88. [DOI] [PubMed] [Google Scholar]
- 24. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003; 327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994; 50:1088–101. [PubMed] [Google Scholar]
- 26. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bouaziz O, Courtin D, Cottrell G, Milet J, Nuel G, Garcia A. Is placental malaria a long-term risk factor for mild malaria attack in infancy? Revisiting a paradigm. Clin Infect Dis 2018; 66:930–5. [DOI] [PubMed] [Google Scholar]
- 28. Le Port A, Watier L, Cottrell G, et al. Malaria infections in infants during the first 12 months of life: role of placental malaria and environmental factors. Trop Med Int Health 2011; 16(Suppl I):88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Le Port A, Watier L, Cottrell G, et al. Infections in infants during the first 12 months of life: role of placental malaria and environmental factors. PLoS One 2011; 6:e27516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Boudova S, Divala T, Mungwira R, et al. The effect of malaria during pregnancy on infant susceptibility to malaria. Am J Trop Med Hyg 2015; 93:386–7. [Google Scholar]
- 31. Ndibazza J, Webb EL, Lule SA, et al. The effect of maternal malaria and helminth infections on childhood malaria: a birth cohort in Entebbe, Uganda. Am J Trop Med Hyg 2012; 87:264–5.22855756 [Google Scholar]
- 32. Sylvester B, Gasarasi DB, Aboud S, et al. Hyperparasitaemia during clinical malaria episodes in infants aged 0-24 months and its association with in utero exposure to Plasmodium falciparum. BMC Res Notes 2018; 11:232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Le Port A, Cottrell G, Chandre F, Cot M, Massougbodji A, Garcia A. Importance of adequate local spatiotemporal transmission measures in malaria cohort studies: application to the relation between placental malaria and first malaria infection in infants. Am J Epidemiol 2013; 178:136–43. [DOI] [PubMed] [Google Scholar]
- 34. Sylvester B, Gasarasi DB, Aboud S, et al. Prenatal exposure to Plasmodium falciparum increases frequency and shortens time from birth to first clinical malaria episodes during the first two years of life: prospective birth cohort study. Malar J 2016; 15:379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mutabingwa TK, Bolla MC, Li JL, et al. Maternal malaria and gravidity interact to modify infant susceptibility to malaria. PLoS Med 2005; 2:e407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schwarz NG, Adegnika AA, Breitling LP, et al. Placental malaria increases malaria risk in the first 30 months of life. Clin Infect Dis 2008; 47:1017–25. [DOI] [PubMed] [Google Scholar]
- 37. Bardají A, Sigauque B, Sanz S, et al. Impact of malaria at the end of pregnancy on infant mortality and morbidity. J Infect Dis 2011; 203:691–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mutabingwa TK, de Geus A, Meuwissen JH, Malle LN. Malaria chemosuppression during pregnancy. VI. Some epidemiological aspects of malaria in infants. Trop Geogr Med 1994; 46:1–7. [PubMed] [Google Scholar]
- 39. Bonner PC, Zhou Z, Mirel LB, et al. Placental malaria diminishes development of antibody responses to Plasmodium falciparum epitopes in infants residing in an area of western Kenya where P. falciparum is endemic. Clin Diagn Lab Immunol 2005; 12:375–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. De Beaudrap P, Turyakira E, Nabasumba C, et al. Timing of malaria in pregnancy and impact on infant growth and morbidity: a cohort study in Uganda. Malar J 2016; 15:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Awine T, Belko MM, Oduro AR, et al. The risk of malaria in Ghanaian infants born to women managed in pregnancy with intermittent screening and treatment for malaria or intermittent preventive treatment with sulfadoxine/pyrimethamine. Malar J 2016; 15:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Slutsker L, Khoromana CO, Hightower AW, et al. Malaria infection in infancy in rural Malawi. Am J Trop Med Hyg 1996; 55:71–6. [DOI] [PubMed] [Google Scholar]
- 43. Le Hesran JY, Cot M, Personne P, et al. Maternal placental infection with Plasmodium falciparum and malaria morbidity during the first 2 years of life. Am J Epidemiol 1997; 146:826–31. [DOI] [PubMed] [Google Scholar]
- 44. Cot M, Le Hesran JY, Staalsoe T, Fievet N, Hviid L, Deloron P. Maternally transmitted antibodies to pregnancy-associated variant antigens on the surface of erythrocytes infected with Plasmodium falciparum: relation to child susceptibility to malaria. Am J Epidemiol 2003; 157:203–9. [DOI] [PubMed] [Google Scholar]
- 45. Moya-Alvarez V, Abellana R, Cot M. Pregnancy-associated malaria and malaria in infants: an old problem with present consequences. Malar J 2014; 13:271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kakuru A, Staedke SG, Dorsey G, Rogerson S, Chandramohan D. Impact of Plasmodium falciparum malaria and intermittent preventive treatment of malaria in pregnancy on the risk of malaria in infants: a systematic review. Malar J 2019; 18:304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pae CU. Why systematic review rather than narrative review? Psychiatry Investig 2015; 12:417–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Prahl M, Jagannathan P, McIntyre TI, et al. Timing of in utero malaria exposure influences fetal CD4 T cell regulatory versus effector differentiation. Malar J 2016; 15:497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rechavi E, Lev A, Lee YN, et al. Timely and spatially regulated maturation of B and T cell repertoire during human fetal development. Sci Transl Med 2015; 7:276ra25. [DOI] [PubMed] [Google Scholar]