Abstract
Reticuloendotheliosis virus (REV) can cause runting, immunosuppression, acute reticulum cell neoplasia, and chronic lymphoid tumors in a variety of domestic and wild birds. We diagnosed a case of reticuloendotheliosis with obvious tumors in liver and kidney. We isolated and sequenced the virus and performed pathogenicity testing of the REV strain. Immunohistochemistry and PCR confirmed that the diseased layer chickens were infected with REV. The strain, named BJ1503, was successfully isolated from the case by inoculation of tissue homogenates onto chicken embryo fibroblasts. The length of the proviral REV genome is 8,293 nucleotides. The isolate had 99.7% identity with REV-HA9901 (AY842951.1), which was isolated from Jiangsu, China, in 1999. The chickens infected with REV-BJ1503 had depressed weight gain and lymphoid atrophy. Our findings suggest that REV isolate BJ1503 was phylogenetically close to the earlier strain found in China, with minor variations, and the virus was associated with severe production problems.
Keywords: pathogenicity, reticuloendotheliosis virus, sequence analysis, virus isolation
Avian reticuloendotheliosis virus (REV; Retroviridae, Orthoretrovirinae, Gammaretrovirus, Reticuloendotheliosis virus)18 can cause neoplasia, immunosuppression, and runting syndrome in many avian hosts worldwide.20 Representative strains of REV include defective REV-T, non-defective REV-A, chick syncytial virus (CSV), duck infectious anemia virus (DIAV), and spleen necrosis virus (SNV).2,6
Infection by non-defective REV can cause T- and B-cell chronic lymphoid neoplasia as well as a variety of non-neoplastic syndromes, such as runting and anemia.15 The defective strain, REV-T, can cause acute reticulum cell neoplasia.14 Myxosarcomas, fibrosarcomas, and renal adenocarcinomas caused by REV infection have also been reported.19 REV can integrate into fowlpox virus and Marek disease virus (MDV; Gallid alphaherpesvirus 2), potentially causing various degrees of viral contamination in vaccines produced in eggs.2,3 The use of a contaminated vaccine in chickens can result in serious immunosuppression in the flock. Coinfection with REV and other immunosuppressive viruses, such as avian leukosis virus subgroup J or MDV, has become more prevalent and can cause great harm to the health and sustainable development of the poultry industry.4,6,13
REV strains have been isolated from chicken populations located in different areas of China,9,11,12,17 but few studies have investigated the pathogenicity of the new REV isolate. We diagnosed a clinical case, characterized the REV isolate, and established an animal infection model to study the molecular characteristics and pathogenicity of the virus.
A suspected case of reticuloendotheliosis in chickens from Beijing was brought to the Diagnosis and Research Center of Poultry & Livestock Infectious Diseases, China Agricultural University (Beijing, China), for diagnosis. The flock was comprised of 15,000 layers, and the incidence of tumors was 1.5%. The diseased layer chickens were 67 d old. Autopsies were performed on the diseased chickens. Tissues were fixed in 4% paraformaldehyde solution for histologic examination and immunohistochemistry as described previously.1 Portions of the liver were stored at −20°C for PCR testing.
Total DNA was extracted from liver homogenates (TIANamp genomic DNA kit; Tiangen, Beijing, China) according to the manufacturer’s instructions. Primers were designed based on the conserved regions of the LTR gene of REV (RF: 5′-CGATCTACTGAAGGATAT-3′, RL: 5′-GAAATGATCGGTCGGATC-3′). PCR conditions were: 5 min at 95°C, followed by 30 cycles of 45 s at 95°C, 30 s at 50°C, 20 s at 72°C, and a final elongation for 10 min at 72°C. PCR products with expected sizes and the primers were sent for sequencing (TransGen Biotech, Beijing, China).
Virus isolation was performed by inoculating chicken embryo fibroblasts (CEF) with liver homogenates. The liver homogenates were centrifuged at 7,104 × g for 10 min at 4°C, and the supernatant was obtained and filtered to eliminate bacteria. The filtrate was inoculated into CEF incubated in Dulbecco modified Eagle medium (Thermo Scientific, Shanghai, China) containing 2% fetal bovine serum (Hyclone, Logan, UT) and cultured for 6 d in a 5% CO2 incubator at 37°C. After freezing and thawing 3 times, the cells and supernatants containing viral stocks were harvested and blindly passaged serially 3 times into CEF.8 Total DNA of the supernatants was extracted and checked by PCR using the primers mentioned above.
Total genomic DNA was extracted from the isolated virus culture (EasyPure genomic DNA kit; TransGen Biotech). Eight primer pairs were used for PCR amplification of the complete genome of BJ1503 (Table 1). Oligonucleotide primers were designed (v.5.0; Premier Biosoft, San Francisco, CA), based on available REV-HA9901 DNA sequences (GenBank accession AY842951).16 All oligonucleotides were synthesized at Sangon Biotech (Shanghai, China). PCR amplification conditions were as described above. PCR products with expected sizes were sent to TransGen Biotech for sequencing.
Table 1.
Primers used for whole-genome sequencing of avian reticuloendotheliosis virus.
| Location | Forward 5′→3′ | Reverse 5′→3′ |
|---|---|---|
| 58–1507 | GCTTCTGTAATCATGCTTGCT | GGCCCTTGTGCTCCGAACTCT |
| 1112–3035 | GGGACTCTAGACTTTGGGGTG | GCCCGCTGCTCTGAATTTGTG |
| 1743–2681 | TGTTTACGACGGAGGAGAGGG | GTCTGGGCACTCAGGAATCAC |
| 2528–3194 | GGAGCGACCCATTCTGTAGTG | TAGGCTGAGGAGGGTGTATGG |
| 2734–4445 | AAGGGCTACCATCTCGTTCAC | GAGGGGTTCTGCCCAAAT |
| 4277–5666 | CCAATACACCATTGCCTGGAC | TGCCCGCCTGGTCAACTTTAC |
| 5860–7467 | GGTAAAGGTCGCTGGGAAGAC | TTCCACAGGGGGTTGTCGTAC |
| 7371–8247 | TGCCGAACAAGGAGGGATATG | GATTCAGTCCGGATCCCAACC |
Primers were designed according to reticuloendotheliosis virus strain HA9901 (AY842951).
The individual overlapping sequences from PCR products were aligned for preparation of the complete sequence of BJ1503 proviral genome using the SeqMan function in the DNASTAR sequence analysis software (DNASTAR, Madison, WI). The nucleotide sequences of the whole genome and LTR, pol, env, and gag genes of the isolate were aligned with published REV strains using MegAlign in DNASTAR v.5.0 by Clustal W alignment. Molecular evolutionary genetic analysis was carried out using MEGA 5.0 software (DNASTAR).
One-d-old specific pathogen–free (SPF) chickens (n = 40) were divided into 2 groups of 20, the experimental (REV) and control groups. Each chicken from the control group was inoculated subcutaneously with 0.5 mL of saline. In the REV group, each chicken was inoculated subcutaneously with 0.5 mL of BJ1503 containing 105 TCID50. To compare the effects of viral infection on growth retardation and immunosuppression, the growth of the chickens was observed daily. At 2, 3, 4, 5, 6, and 7 wk post-challenge, 3 chickens from each group were weighed and euthanized, and individual body weights and immune organ indices (thymus/body weight, spleen/body weight, bursa of Fabricius/body weight, g/kg) were measured. Samples of liver, spleen, kidney, thymus, and bursa of Fabricius from the chickens were fixed in 10% neutral-buffered formalin for histologic examination. Treatment of laboratory animals was approved by the Beijing Administration Committee of Laboratory Animals under the leadership of the Beijing Association for Science and Technology (approval ID SYXK [Jing] 2013-0013). The protocols for this experiment were performed according to the guidelines of the Animal Welfare and Ethical Censor Committee at China Agricultural University.
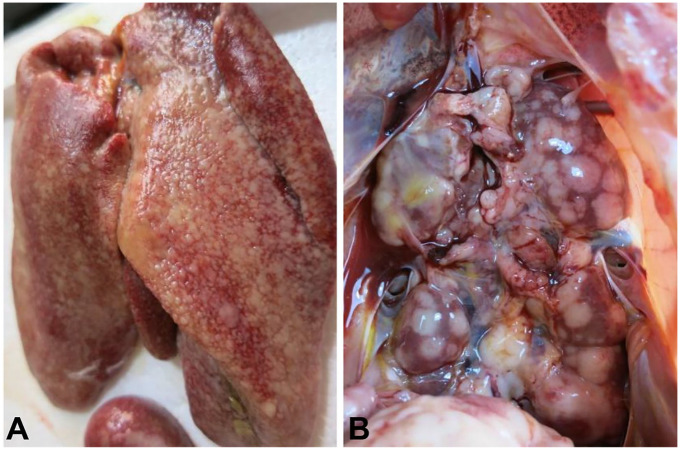
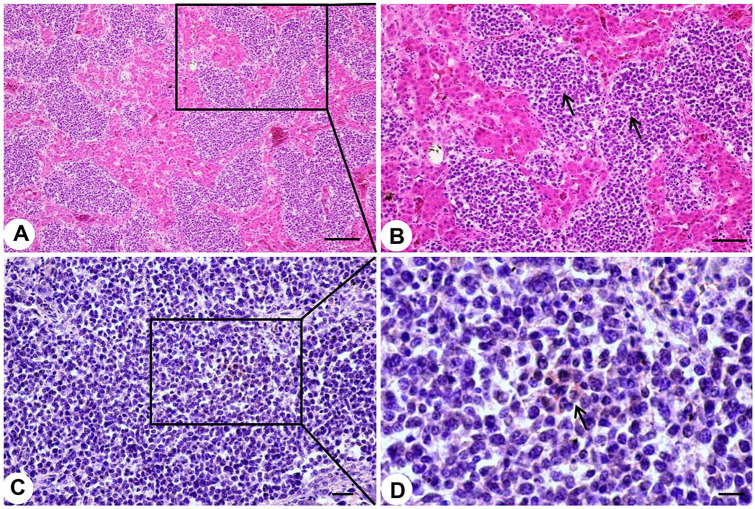
The livers of diseased chickens were enlarged, with many white nodules on the liver surface (Fig. 1A). The kidneys were also enlarged and contained many neoplastic nodules of 0.2–0.8 cm diameter (Fig. 1B). Histologic examination revealed that the livers of the diseased chickens were infiltrated by neoplastic reticulum cells and lymphoblasts (Fig. 2A, 2B). REV antigen was detected by immunohistochemistry in the neoplastic lymphoid cells in the liver (Fig. 2C, 2D).
Figure 1.
Autopsy observations of diseased layer chickens infected by avian reticuloendotheliosis virus. A. Nodules on the surface of a diseased chicken liver. B. Enlarged kidney with neoplastic nodules.
Figure 2.
Typical histopathologic changes and detection of avian reticuloendotheliosis virus (REV) antigens in liver of diseased 67-d-old chickens. A, B. Tumors in liver with reticulum cells and lymphoblasts with mitoses. Arrows in panel B indicate tumor cells. A. Bar = 100 μm. B. Bar = 50 μm. C, D. Positive REV signal in the liver. Arrow in panel D indicates positive REV signal. C. Bar = 20 μm. D. Bar = 10 μm.
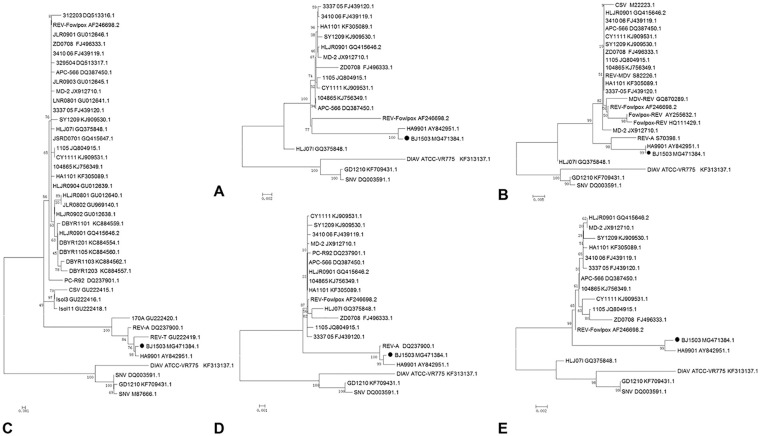
We detected DNA of REV from liver homogenates of the diseased chickens, but not from the vaccines administered to the chickens. The REV strain, named BJ1503, isolated from liver samples of the diseased chickens by CEF culture, was confirmed with PCR using primers specific to the LTR gene. The nucleotide sequence of the BJ1503 provirus genome was obtained and submitted (GenBank accession MG471384). REV BJ1503 was 8,293 nucleotides long. The nucleotide percent identity of BJ1503 to the reference strains was 93.2–99.7%; the nucleotide percent identity was 99.7% with REV-HA9901 isolated from Jiangsu, China, in 1999 (Table 2). The whole genome sequence and LTR, pol, env, and gag phylogenetic analysis showed that BJ1503 had a close genetic relationship with HA9901 (Fig. 3).
Table 2.
The whole-genome nucleotide percent identity of reticuloendotheliosis virus (REV) strain BJ1503 compared to reference REVs.
| Reference strain | GenBank accession | Homology (%) |
|---|---|---|
| HLJ071 | GQ375848 | 97.0 |
| HLJR0901 | GQ415646 | 97.8 |
| MD-2 | JX912710 | 97.8 |
| REV-Fowlpox | AF246698 | 96.6 |
| SNV | DQ003591 | 94.4 |
| SY1209 | KJ909530 | 97.7 |
| ZD0708 | FJ496333 | 97.3 |
| 1105 | JQ804915 | 97.8 |
| HA9901 | AY842951 | 99.7 |
| 3337 05 | FJ439120 | 97.7 |
| 3410 06 | FJ439119 | 97.8 |
| 104865 | KJ756349 | 97.9 |
| APC-566 | DQ387450 | 97.9 |
| ATCC-VR775 | KF313137 | 93.2 |
| CY1111 | KJ909531 | 97.8 |
| GD1210 | KF709431 | 94.5 |
| HA1101 | KF305089 | 97.8 |
Figure 3.
Phylogenetic relationship of avian reticuloendotheliosis virus (REV) strain BJ1503 based on nucleotide sequences. A. The whole genome. B. LTR gene. C. env gene. D. pol gene. E. gag gene. The numbers at the forks indicate bootstrap values (1,000 replicates). Black dots represent the REV strain isolated in our study.
The bodyweight of chickens from the infected group was lower than the control group, especially from 4–6 wk post-infection (Suppl. Fig. 1A). The thymus index of infected chickens was decreased compared to the control group from 3–4 wk post-infection (Suppl. Fig. 1B). The spleen index of infected chickens was higher than the control group most of the time (Suppl. Fig. 1C), and the bursa of Fabricius index from the infected group was lower than control group from 2–4 wk post-infection (Suppl. Fig. 1D).
Compared with the control group (Suppl. Fig. 2A–E), there were obvious pathologic changes in organs from the REV-infected group (Table 3). Examination of the liver demonstrated infiltration by many reticulum cells (Suppl. Fig. 2F). Slight hemorrhage, lymph sinus expansion, lymphocyte infiltration, and mitotic figures were observed in the spleen (Suppl. Fig. 2G). Many tumor cells were present in the renal interstitium, including many reticulum cells (Suppl. Fig. 2H). The thymus had a significant decrease of the cortical area, and lymphocyte necrosis in the medulla (Suppl. Fig. 2I). In the bursa of Fabricius, lymphoid follicle atrophy, a significant decrease in the number of lymphocytes in the medulla of the lymphoid follicles, and mitotic figures could be seen (Suppl. Fig. 2J). No lesions were observed in the control group.
Table 3.
Histologic lesions in specific pathogen–free chickens inoculated with reticuloendotheliosis virus.
| Tissue | Week of sampling
(post-inoculation) |
|||||
|---|---|---|---|---|---|---|
| 2 | 3 | 4 | 5 | 6 | 7 | |
| Liver | 1 of 3 | 2 of 3 | 3 of 3 | 3 of 3 | 2 of 3 | 2 of 3 |
| Spleen | 2 of 3 | 2 of 3 | 2 of 3 | 2 of 3 | 2of 3 | 1 of 3 |
| Kidney | 1 of 3 | 2 of 3 | 3 of 3 | 3 of 3 | 2 of 3 | 2 of 3 |
| Thymus | 2 of 3 | 2 of 3 | 3 of 3 | 2 of 3 | 2 of 3 | 1 of 3 |
| Bursa | 2 of 3 | 3 of 3 | 3 of 3 | 2 of 3 | 2 of 3 | 1 of 3 |
One-d-old specific pathogen–free chickens were inoculated with reticuloendotheliosis virus strain BJ1503 (105 TCID50) subcutaneously.
In our model of BJ1503 infection, the bodyweight in the infected group increased slowly compared with the control group. In agreement with other reports,12 the thymus and bursa of Fabricius indices decreased, indicating atrophy of these immune organs, which may have a serious impact on immune function. Histologic examination indicated that REV BJ1503 could induce proliferation of reticulum cells, lymphosarcoma, and atrophy of thymus and bursa in 1-d-old SPF chickens. However, compared with the clinical case, no obvious tumor nodules in various tissues were found in the experimentally infected chickens. This difference could be interpreted in light of previous research, in which REV maintained over long periods in CEF culture would lack oncogenic potential,7,10 or the environment of the SPF chickens was sterile, such that the chickens were able to build up resistance to REV-BJ1503. It is also possible that the growth period was not long enough for the chickens to produce visible tumor nodules.5,16 Although obvious tumors were not observed in our animal infection model, the new REV strain has led to severe production problems in the poultry industry, including increased tumor incidence and mortality.
Supplemental Material
Supplemental material, Supplemental_material for Isolation and pathogenicity testing of avian reticuloendotheliosis virus from layer chickens in China by Ahui Xu, Caiyun Huo, Qi Zhong, Meiyu Xu, Yurong Yang, Haiyan Tian, Guozhong Zhang and Yanxin Hu in Journal of Veterinary Diagnostic Investigation
Acknowledgments
We thank LetPub (www.letpub.com) for providing linguistic assistance during the preparation of this manuscript.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This work was supported by Chinese Universities Scientific Fund (grant 2017DY003) and Henan Science and Technology Open Cooperation Project (152106000056).
ORCID iD: Yanxin Hu  https://orcid.org/0000-0001-7009-8451
https://orcid.org/0000-0001-7009-8451
Supplementary material: Supplementary material for this article is available online.
References
- 1. Cheng J, et al. Pathogenicity differences between QX-like and Mass-type infectious bronchitis viruses. Vet Microbiol 2018;213:129–135. [DOI] [PubMed] [Google Scholar]
- 2. Fadly AM, Witter RL. Comparative evaluation of in vitro and in vivo assays for the detection of reticuloendotheliosis virus as a contaminant in a live virus vaccine of poultry. Avian Dis 1997;41:695–701. [PubMed] [Google Scholar]
- 3. Fadly AM, et al. An outbreak of lymphomas in commercial broiler breeder chickens vaccinated with a fowlpox vaccine contaminated with reticuloendotheliosis virus. Avian Pathol 1996;25:35–47. [DOI] [PubMed] [Google Scholar]
- 4. Garcia M, et al. Molecular characterization of reticuloendotheliosis virus insertions in the genome of field and vaccine strains of fowl poxvirus. Avian Dis 2003;47:343–354. [DOI] [PubMed] [Google Scholar]
- 5. Haque MA, Saif MTA. A review of MEMS-based microscale and nanoscale tensile and bending testing. Exp Mechanics 2003;43:248–255. [Google Scholar]
- 6. Hertig C, et al. Field and vaccine strains of fowlpox virus carry integrated sequences from the avian retrovirus, reticuloendotheliosis virus. Virology 1997;235:367–376. [DOI] [PubMed] [Google Scholar]
- 7. Hoelzer JD, et al. Transformation by reticuloendotheliosis virus—development of a focus assay and isolation of a nontransforming virus. Virology 1979;93:20–30. [DOI] [PubMed] [Google Scholar]
- 8. Jiang L, et al. First isolation of reticuloendotheliosis virus from mallards in China. Arch Virol 2014;159:2051–2057. [DOI] [PubMed] [Google Scholar]
- 9. Jiang L, et al. Molecular characterization and phylogenetic analysis of the reticuloendotheliosis virus isolated from wild birds in Northeast China. Vet Microbiol 2013;166:68–75. [DOI] [PubMed] [Google Scholar]
- 10. Koyama H, et al. The relationship between feathering abnormalities (“nakanuke”) and tumour production in chickens inoculated with reticuloendotheliosis virus. Avian Pathol 1980;9:331–340. [DOI] [PubMed] [Google Scholar]
- 11. Li JP, et al. Isolation, identification, and whole genome sequencing of reticuloendotheliosis virus from a vaccine against Marek’s disease. Poultry Sci 2015;94:643–649. [DOI] [PubMed] [Google Scholar]
- 12. Li Y, et al. Genome analysis and pathogenicity of reticuloendotheliosis virus isolated from a contaminated vaccine seed against infectious bursal disease virus: first report in China. J Gen Virol 2016;97:2809–2815. [DOI] [PubMed] [Google Scholar]
- 13. Moore KM, et al. Reticuloendotheliosis virus (REV) long terminal repeats incorporated in the genomes of commercial fowl poxvirus vaccines and pigeon poxviruses without indication of the presence of infectious REV. Avian Dis 2000;44:827–841. [PubMed] [Google Scholar]
- 14. Robinson FR, Twiehaus MJ. Isolation of the avian reticuloendothelial virus (strain T). Avian Dis 1974;18:278–288. [PubMed] [Google Scholar]
- 15. Wang G, et al. New pathogenetic characters of reticuloendotheliosis virus isolated from Chinese partridge in specific-pathogen-free chickens. Microb Pathog 2012;53:57–63. [DOI] [PubMed] [Google Scholar]
- 16. Wang Y, et al. Sequence analysis for the complete proviral genome of reticuloendotheliosis virus Chinese strain HA9901. Sci China C Life Sci 2006;49:149–157. [DOI] [PubMed] [Google Scholar]
- 17. Wei K, et al. Probable congenital transmission of reticuloendotheliosis virus caused by vaccination with contaminated vaccines. PLoS One 2012;7:e43422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Witter RL, Glass SE. Reticuloendotheliosis in breeder turkeys. Avian Dis 1984;28:742–750. [PubMed] [Google Scholar]
- 19. Witter RL, et al. Tolerance, viral shedding, and neoplasia in chickens infected with non-defective reticuloendotheliosis viruses. Avian Dis 1981;25:374–394. [PubMed] [Google Scholar]
- 20. Zavala G, et al. Enzootic reticuloendotheliosis in the endangered Attwater’s and greater prairie chickens. Avian Dis 2006;50:520–525. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplemental_material for Isolation and pathogenicity testing of avian reticuloendotheliosis virus from layer chickens in China by Ahui Xu, Caiyun Huo, Qi Zhong, Meiyu Xu, Yurong Yang, Haiyan Tian, Guozhong Zhang and Yanxin Hu in Journal of Veterinary Diagnostic Investigation