Abstract
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is currently defined as the worst pandemic disease. SARS-CoV-2 infects human cells via the binding of its S protein to the receptor angiotensin-converting enzyme (ACE2). The use of ACEIs/ARBs (RAAS inhibitors) regulates the renin-angiotensin-aldosterone system (RAAS) and may increase ACE2 expression. Considering the large use of ACEIs/ARBs in hypertensive patients, some professional groups are concerned about whether the use of RAAS inhibitors affects the risk of SARS-CoV-2 infection or the risk of severe illness and mortality in COVID-19 patients. In this review, we summarize preclinical and clinical studies to investigate whether the use of ACEIs/ARBs increases ACE2 expression in animals or patients. We also analyzed whether the use of these drugs affects the risk of SARS-CoV-2 infection, severe illness or mortality based on recent studies. Finally, the review suggests that current evidence does not support the concerns.
Keywords: COVID-19, SARS-CoV-2, RAAS inhibitor, ACE2
Graphical abstract
1. Introduction
Coronavirus disease 2019 (COVID-19), arising from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, is currently defined as the worst pandemic disease, resulting in substantial medical and financial burden [[1], [2], [3]]. SARS-CoV-2 enters human lung cells via the binding of its viral spike (S) protein to the membrane-bound form of angiotensin-converting enzyme 2 (ACE2) [4]. ACE2 plays a critical role in the regulation of the renin-angiotensin-aldosterone system (RAAS) since it can catalyze angiotensin II (Ang II) to generate Ang 1–7, which exerts a protective effect on lung injury [5,6]. Literature reports have found that hypertension is the most common comorbidity in patients infected with SARS-CoV-2, with rates of 30% in a study of 191 adult patients [7] and 16.9% in a study comprising 1590 patients [8]. ACE inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) are the cornerstone of antihypertensive therapy; nevertheless, they may increase the expression of ACE2 and even increase the risk of infection [9]. Except for patients with hypertension, patients with myocardial infarction, heart failure, diabetes and chronic kidney disease may also be administered RAAS blockers in disease management [[10], [11], [12], [13]]. Considering the large number of patients using RAAS inhibitors worldwide, concerns that RAAS blockers increase the risk of COVID-19 have caught the attention of and have been debated among professionals.
Due to the hypothesis that RAAS inhibitors may be harmful in patients with COVID-19, some medical sources have even suggested the discontinuation of ACEIs and ARBs [9]. Nevertheless, the abrupt withdrawal of these drugs may cause a number of adverse risks, such as fluctuations in blood pressure and deterioration of cardiac function [10,14]. Given these hypotheses and concerns, we urgently need to investigate the use of RAAS inhibitors in patients with COVID-19. Recently, some literature reports and preclinical studies have focused on this hypothesis, and current limited evidence has led to the preliminary idea that RAAS inhibitors may not be associated with an increased risk of COVID-19 or a risk of severe ratios and mortality in patients with COVID-19 [15,16]. In this review, the latest progress and current perspective in the interaction between RAAS inhibitors and COVID-19 will be summarized and discussed.
2. The interaction between SARS-CoV-2 and the renin-angiotensin-aldosterone system
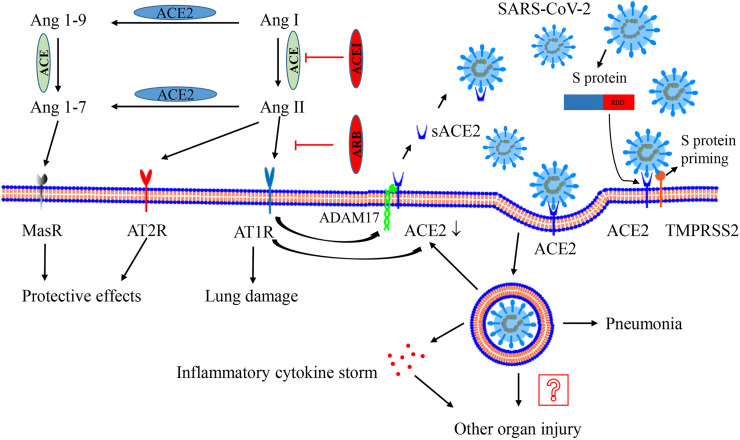
The renin-angiotensin-aldosterone system plays a critical role in the regulation of extracellular volume homeostasis, blood pressure, cardiac function and renal function [10,13,14]. In addition, the system is also associated with cell proliferation, inflammation, fibrosis and severe organ injury, especially acute lung injury [[17], [18], [19]]. The function of the RAAS is mainly regulated by related molecules and enzymes, including Ang I, Ang II, Ang 1–7, Ang 1–9, ACE and ACE2 (Fig. 1 ) [19]. Ang II, which is converted from Ang I via the metabolism of ACE, exerts its effective vasoconstriction and pro-inflammatory function based on Ang II type 1 receptors (AT1Rs) (mainly effect) and exerts its counterbalanced effect by targeting AT2Rs [[20], [21], [22], [23]]. Interestingly, Ang II could also induce cellular internalization of ACE2 through endocytosis and promote its degradation in lysosomes [24]. ACE2, a homologue of ACE, can metabolize Ang II to synthesize Ang 1–7 and can convert Ang I to Ang 1–9, which can be further converted to Ang 1–7 [25]. Ang 1–7 exerts several protective effects, such as antioxidative, anti-inflammatory, and antifibrotic effects, via the binding of the Mas receptor (MasR) [[26], [27], [28]]. Therefore, ACE2 has an important counterbalanced effect on the activation of the Ang II. The increase in ACE2 may attenuate the activation of the RAAS and participate in the improvement of lung injury, which is consistent with the results of a previous study [29]. In brief, the normal Ang II: ACE2 ratio plays an important role in the regulation of lung function or injury. Generally, ACE2 is regarded as a membrane-bound enzyme, and the levels of its soluble form (sACE2) in blood are very low [20,30]. ADAM17 (a disintegrin and metalloprotease 17) is responsible for the cleavage of its membrane anchor. Ang II binds AT1Rs to activate ADAM17, promoting the generation of sACE2 [31]. Although the levels of sACE2 may be increased in plasma or urine in some pathological processes, such as hypertension, the expression levels of membrane ACE2 may not be affected [31,32]. The majority of ACE2 is membrane-bound, and the RAAS has a compensatory balancing effect. General changes in sACE2 levels may not alter the effect of the virus on full-length ACE2 (membrane form). The brief showed in Fig. 1.
Fig. 1.
Interaction between SARS-CoV-2 and the renin–angiotensin–aldosterone system. ACE metabolizes Ang I to generate Ang II, which mainly binds AT1R to activate the system and result in lung injury. ACE2 could metabolizes Ang II to generate Ang 1–7 and convert Ang I to Ang 1–9. Ang 1–9 is furtherly metabolized to generate Ang 1–7. Ang 1–7 exerts the protective effect on lung injury via binding the receptor MasR. AT2R also has a beneficial effect. The activation of AT1R promotes ADAM17 (as a “sheddase”) to cleave the extracellular domain of surface ACE2, generating sACE2 and reducing surface ACE2 expression. Recombinant sACE2 may be a treatment for SARS-CoV-2. After processing of the S-protein by TMPRSS2, SARS-CoV-2 performs its human-cell entry via binding its S-protein to ACE2 and the RBD of S-protein is responsible for the process. After endocytosis of the viral complex, surface ACE2 is further down-regulated, resulting in unopposed Ang II accumulation. Local activation of the renin–angiotensin–aldosterone system may regulate lung injury responses to viral insults. The virus replicates inside the cell, leading to pneumonia and even inflammatory cytokine storms, which may contribute to other organ injury. Abbreviation: ACE, angiotensin-converting enzyme; ACE2, angiotensin-converting enzyme 2; ADAM17, a disintegrin and metalloprotease 17; Ang, angiotensin; AT1R, Ang II type 1 receptor; AT2R, Ang II type 2 receptor; ACEI, ACE inhibitor; ARB, angiotensin receptor blocker; MasR, Mas receptor; TMPRSS2, type II transmembrane serine protease.
Coronavirus entry into human cells depends on the binding of viral S proteins to cellular receptors and on S protein priming by host cell proteases [33,34]. Hoffmann M et al. demonstrated that SARS-CoV-2 infects human cells via the binding of the S protein to the SARS-CoV receptor ACE2 and that type II transmembrane serine protease (TMPRSS2) is responsible for S protein priming [4]. TMPRSS2 entails S protein cleavage at the S1/S2 and S2′ sites, and the fusion of viral and cellular membranes is driven by the S2′ subunit. Other studies found that the SARS-CoV-2 S protein binds to its receptor human ACE2 (hACE2) via its receptor-binding domain (RBD) in key human cells [[35], [36], [37]]. Compared to the RBD of SARS-CoV, SARS-CoV-2 RBD has higher hACE2 binding affinity, supporting its efficient cell entry [35,37]. However, the hACE2 binding affinity of the entire SARS-CoV-2 spike is equal to or lower than that of the SARS-CoV spike, which indicates that SARS-CoV-2 RBD has less exposure than SARS-CoV [35]. The hidden RBD may help the virus evade immune surveillance. In addition, a furin motif allows the spike of SARS-CoV-2 to be preactivated and reduces its dependence on target cell proteases for entry, which is unlike SARS-CoV [35]. All the special characteristics of SARS-CoV-2 may contribute to the rapid spread of the virus, and the difficulty of intervention is enhanced. The brief showed in Fig. 1.
Since the cell entry of SARS-CoV-2 requires the presence of the serine protease TMPRSS2, co-expression of the protein and ACE2 is necessary to acquire infectivity. Several studies have shown that co-expression of ACE2 and TMPRSS2 has been found in human nasal and respiratory sinuses, bronchial epithelium and alveolar epithelial type II cells [38]. The invasion of SARS-CoV-2 leads to typical viral pneumonia, and severe patients ultimately develop severe acute respiratory syndrome [39]. The SARS-CoV-2 infection can also introduce inflammatory “cytokine storm”, which, in turn, is responsible for multiple organ injury [[40], [41], [42]]. In addition to expression in the respiratory system, co-expression of the two proteins has also been documented in the gastrointestinal system, cardiomyocytes, and some other tissues [38]. Another study reported that ACE2 expression was highest in the small intestine, testes, kidneys, heart, thyroid, and adipose tissue and that there was medium expression in the lungs, colon, liver, bladder, and adrenal gland [43]. The widespread expression of ACE2 suggests that the virus may invade multiple organs and cause organ damage. The brief showed in Fig. 1.
In addition, previous studies have supported that SARS-CoV infection reduces the expression of surface ACE2 in mice, which is associated with the worsening of acute lung injury [44]. Since SARS-CoV-2 and SARS-CoV share similar spike proteins, receptors and pathological processes, we speculate that SARS-CoV-2 infection may also decrease ACE2 expression. The decreased ACE2 expression in membrane could inhibit the production of Ang 1–7 and Ang 1–9 [25]. Since the metabolite Ang 1–7, as the end-point of ACE2/Ang 1–7/MasR axis, plays a critical role in maintaining the balance of RAAS [45]. The inhibition of ACE2/Ang 1–7/MasR will be associated with the enhanced activation of Ang II/AT1R, which furtherly promotes the internalization of ACE2 [24] and results in a vicious circle of the imbalance. In brief, SARS-CoV-2 infection may induce and aggravate the imbalance between ACE2/Ang 1–7/MasR and Ang II/AT1R, which promotes the aggravation of viral pneumonia or lung injury. The brief showed in Fig. 1.
3. RAAS inhibitor use and ACE2 expression
Theoretically, the use of RAAS inhibitors could compensatorily lead to an increase in ACE2 expression, which leads to the concern that RAAS inhibitors may increase the risk of COVID-19 and affect the prognosis of these patients. Here, we investigated the effects of ACEIs/ARBs on ACE2 expression according to preclinical studies and clinical data.
First, preclinical studies were searched in the PubMed database, and all the related studies [[46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59]] are listed in Table 1 . Kidoguchi et al. [46] analyzed the expression of ACE2 in adenine-induced chronic renal failure model mice, and the results suggested that azilsartan did not increase ACE2 expression in the kidney compared to the control group. However, de Jong et al. [49] revealed that losartan increased ACE2 mRNA by approximately 2-fold in contralateral (unaffected) kidneys in a unilateral ureteral obstruction (UUO) model. Another diabetic nephropathy model reported that the dose (1 mg/kg/d) of candesartan increased ACE2 expression and activity in the kidney cortex from diabetic mice, and different doses of candesartan also increased ACE2 activity by 25%–50% in the plasma of diabetic mice [51]. Nevertheless, candesartan did not increase ACE2 activity in control mice. In the heart, inserting the human renin and angiotensinogen genes into mice decreased ACE2 expression in cardiac tissue, but azilsartan, not olmesartan, recovered this change [52]. Another ARB, telmisartan, was reported to elevate the expression of cardiac ACE2 in a chronic intermittent hypoxia model [48]. However, Burchill et al. suggested that neither ramipril treatment alone nor in combination with valsartan augmented cardiac ACE2 expression in myocardial infarction mice [53]. Other studies on the effect of RAAS blockers on ACE2 expression in several organs, such as the heart, kidney, brain, intestine and thoracic aorta, are shown in Table 1. According to these preclinical studies, there is no consistent evidence that the use of RAAS inhibitors leads to an increase in ACE2 expression during the pathologic course of the disease (Fig. 2 ). The phenomenon may depend on different disease models, drugs, or doses. Although the expression levels of ACE2 are increased, the changes are also limited. The limited increase in ACE2 levels may not be associated with the increased risk of COVID-19 or not have an effect on the progression of COVID-19. Further observations need to be performed in clinical studies.
Table 1.
Effect of RAAS blockers on ACE2 expression in animal models.
| Source | Animal | Model | Study design | Effect of RAAS blockers on ACE2 |
|---|---|---|---|---|
| Kidoguchi [46],2019 | Mice | Adenine-induced chronic renal failure model | Azilsartan (ARB); 2 mg/kg/day orally for 4 weeks | Compared to the vehicle group, azilsartan didn't increased ACE2 expression in kidney. |
| Abdel-Fattah [47], 2018 | Rat | A cerebral ischemia/reperfusion (I/R) injury model | Three telmisartan (ARB) treatments: 1, 3, and 10 mg/kg/day; orally for 15 days | Telmisartan in the higher doses significantly increased ACE2 expression compared to I/R control values. |
| Wang W [48], 2018 | Mice | A chronic intermittent hypoxia model | Telmisartan (ARB); 10 mg/kg/d for 4 weeks, intragastrically | Telmisartan elevated the expression of cardiac ACE2. |
| de Jong [49], 2017 | Mice | A unilateral ureteral obstruction (UUO) model | Losartan (ARB); 100 mg/L in drinking water; for 7 days | Losartan increased ACE2 mRNA by approximately 2-fold in contralateral (unaffected) kidneys. |
| Yisireyili [50], 2017 | Mice | A stress-induced intestinal inflammation model | Irbesartan (ARB); 3 or 10 mg/kg/day orally for 2 weeks | Irbesartan didn't change ACE2 expression in the intestine of the non-stressed mice, but restored ACE2 expression in stressed mice. |
| Callera [51], 2016 | Mice | A db/db diabetes model | Candesartan (ARB); intermediate, 1 mg/kg/d; high, 5 mg/kg/d; ultra-high, 25 and 75 mg/kg/d; subcutaneous injection for 4 weeks | In kidney cortex: candesartan (75 mg/kg/d) decreased ACE2 expression and activity from control; the dose (1 mg/kg/d) increased ACE2 expression and activity from diabetes mice; in plasma: no changes in ACE2 activity from control; Candesartan increase ACE2 activity by 25%–50% in diabetes. |
| Iwanami [52], 2014 | Mice | Transgenic mice (hRN/hANG-Tg) | Azilsartan (ARB) or olmesartan (ARB); 1 or 5 mg/kg/day orally for 4 weeks | The expression of ACE2 mRNA in heart and kidney were lower in hRN/hANG-Tg control mice than the WT mice; azilsartan not olmesartan recovered ACE2 expression. |
| Burchill [53], 2012 | Rat | A myocardial infarction model | Ramipril (ACEI) (1 mg/kg), valsartan (ARB) (10 mg/kg) or combination; daily, orally for 28 days | Neither treatment alone nor in combination augmented cardiac ACE2 expression. |
| Liu CX [54], 2011 | Rat | A diabetic nephropathy model | Benazepril (ACE1); 10 mg/kg/day for 4 weeks; intragastric intubation | Benazepril increased ACE2 expression and activity by almost 100% in kidney, compared to the no treatment group in diabetic rat; |
| Hamming [55], 2008 | Rat | Health rats treated with low-sodium diet and ACEI | Lisinopril (ACEI) dissolved in the drinking water at a dose of 75 mg/L | Lisinopril did not affect renal ACE2 expression |
| Takeda [56], 2007 | Rat | dahl salt-sensitive hypertensive (DS) rats | Candesartan (ARB); 10 mg/kg/d orally for 8 weeks | A high salt diet decreased ACE2 mRNA in heart from DS rats; candesartan moderately recovered ACE2 mRNA levels in the heart. |
| Agata [57], 2006 | Rat | Stroke-prone spontaneously hypertensive rats | Olmesartan (ARB); 0.5 mg/kg/day orally for 4 weeks | olmesartan significantly increased the cardiac ACE2 expression level compared to that in Wistar Kyoto rats and SHRSP treated with a vehicle |
| Igase [58], 2005 | Rat | A spontaneously hypertensive rat | Olmesartan (ARB); 10 mg/kg/day orally for 14 days | ACE2 mRNA in the thoracic aorta of olmesartan-treated rats was fivefold greater than that in vehicle-treated rats. |
| Ishiyama [59], 2004 | Rat | A myocardial infarction model | Losartan (ARB) (10 mg/kg/day), olmesartan (ARB) (0.1 mg/kg/day) for 28 days; osmotic minipumps | After myocardial infarction, cardiac ACE2 mRNAs did not change. Both losartan and olmesartan augments ACE2 mRNA by approximately 3-fold after myocardial infarction. |
Fig. 2.
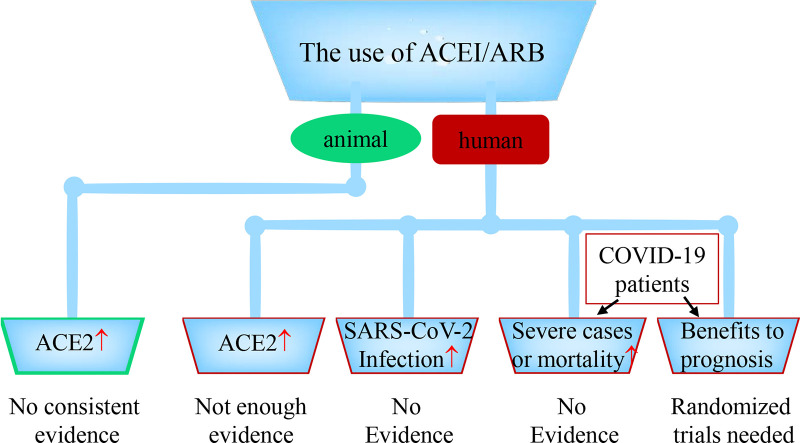
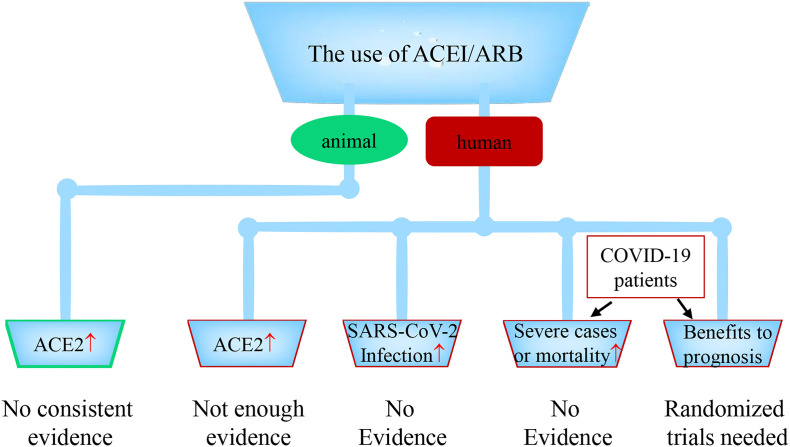
The use of ACEI/ARB, ACE2 expression levels in animals or patients, the risk of SARS-CoV-2 infection, severe cases or mortality. The use of ACEI/ARB increases ACE2 expression or activity in animals: lack consistent evidence. The use of ACEI/ARB increases ACE2 expression or activity in patients: no enough evidence. The use of ACEI/ARB increases the risk of SARS-CoV-2 infection: no evidence. The use of ACEI/ARB increases the risk of severe illness or mortality in COVID-19 patients: no evidence. Whether the use of ACEI/ARB has beneficial or harmful effect on the treatment or prognosis of COVID-19 is still unknown, which need one or more randomized trials to answer the question.
We analyzed five clinical studies [[60], [61], [62], [63], [64]] to investigate the effect of RAAS inhibitors on ACE2 expression (Table 2 ). Wang et al. [60] reported that the use of ACEIs alone and ACEIs+ARBs did not increase urine ACE2 expression in patients with diabetic nephropathy after 12 weeks of drug use. In another study, plasma ACE2 activity was increased in male (10%) and female (20%) diabetes patients who were on ACEI treatment [61]. Additionally, Vuille-dit-Bille et al. [62] concluded that ACEIs but not ARBs increased intestinal mRNA levels of ACE2 by 1-fold in patients. In 2020, SARS-CoV-2 is rapidly spreading around the world, and the concern that RAAS inhibitor use may affect its transmission and pathogenic ability has attracted the attention [65]. Recently, 2 studies on the effects of RAAS inhibitors on ACE2 expression were performed. In one study, plasma ACE2 concentrations in 1485 men and 537 women with heart failure (index cohort) were measured [63]. The results were validated in 1123 men and 575 women (validation cohort). In the index cohort, ACEI or ARB use was not an independent predictor of plasma ACE2 levels [63]. In the validation cohort, both ACEIs (estimate = −0.17, P = 0.002) and ARBs (estimate = −0.15, P = 0.03) were independent predictors of lower plasma ACE2 [63]. Milne et al. [64] reported another clinical study that included 1051 lung tissue samples from the Human Lung Tissue Expression Quantitative Trait Loci Study (Lung eQTL Study) and investigated the gene expression of ACE2 and two relative host cell proteases (TMPRSS2 and ADAM17) that were used as cofactors for virus entry. The results showed that ACEI use was associated with significantly lower expression of ACE2 and TMPRSS2 but was not associated with ADAM17 expression [64]. ARB use was not associated with changed expression levels of these three genes [64]. According to these clinical studies, there is no strong evidence to support the idea that the use of RAAS inhibitors increases ACE2 expression in patients (Fig. 2). Therefore, the hypothesis that RAAS inhibitor use may increase the risk of COVID-19 has hardly been demonstrated.
Table 2.
The effect of RAAS blockers on ACE2 expression in clinical study.
| Source | Participants | Effect of RAAS blockers on ACE2 |
|---|---|---|
| Wang G [60], 2008 | 50 patients with diabetic nephropathy: 26 were being treated by ACEI alone, the other 24 by ACEI and ARB. | The use of ACEIs alone and ACEIs+ARBs did not increase urine ACE2 expression after 12 weeks. |
| Soro-Paavonen [61], 2012 | Quantitative ACE2 activity in serum was measured among 859 type 1 diabetes patients and 204 healthy controls. | ACE2 activity was increased in male (10%) and female (20%) diabetes patients who were on ACEIs treatment. However, ACE2 activity was increased by ARBs use in female patients not male diabetes patients. |
| Vuille-dit-Bille [62], 2015 | 46 patients, of which 9 were under ACEI and 13 ARB treatment. | ACEIs not ARBs increased intestinal mRNA levels of ACE2 by 1-fold in patients. |
| Sama [63], 2020 | ACE2 concentrations were measured in 1485 men and 537 women with heart failure (index cohort). Results were validated in 1123 men and 575 women (validation cohort). | In the index cohort, use of ACEIs, or ARBs was not an independent predictor of plasma ACE2. In the validation cohort, ACEIs (estimate = −0.17, P = 0.002) and ARBs use (estimate = −0.15, P = 0.03) were independent predictors of lower plasma ACE2. |
| Milne [64], 2020 | The gene expressions of ACE2 and two host cell proteases, TMPRSS2 and ADAM17, were evaluated in 1051 lung tissue samples from the Human Lung Tissue Expression Quantitative Trait Loci Study (Lung eQTL Study). | ACEI use was associated with significantly lower ACE2 and TMPRSS2 expression, but was not associated with ADAM17 expression. ARBs were not associated with altered expression of these three genes. |
4. RAAS inhibitor use and risk of SARS-CoV-2 infection
To analyze the interaction between RAAS inhibitors and SARS-CoV-2 infection, we need direct clinical study data. After searching the PubMed databases, we recruited one population-based case-control study [66] and three other retrospective studies [[67], [68], [69]], all of which are shown in Table 3 .
Table 3.
The association between RAAS inhibitors use and the likelihood of SARS-CoV-2 infection.
| Source | Study design and participants | Detail results | Increase of risk |
|---|---|---|---|
| Mancia [66] | A population-based case-control study. 6272 patients with COVID-19 matching 30,759 controls. | Adjusted OR, 0.95 [95% CI, 0.86 to 1.05] for ARBs and 0.96 [95% CI, 0.87 to 1.07] for ACEI | No |
| Reynolds [68] | A multicenter retrospective study; Among 12,594 patients who were tested for Covid-19, 5894 (46.8%) were positive; 1002 had severe illness. 4357 patients co-existed with hypertension, among whom 2573 had a positive test; 634 of these patients had severe illness. | There was no association between any single medication class (e.g. ACEIs, ARBs, or other antihypertensive drugs) and an increased likelihood of a positive COVID-19 test. | No |
| Mehta [69] | A Retrospective cohort study; of 18,472 patients tested for COVID-19, 2285 (12.4%) were taking ACEIs/ARBs and 1735 (9.4%) had the positive COVID-19 test result. | Overlap propensity score-weighted OR, 0.97; 95% CI, 0.81–1.15 for ACEIs/ARBs. | No |
| Dauchet [67] | The study used a clinical epidemiology approach based on the estimation of standardized prevalence ratio (SPR) of consumption of ACEI and ARB in four groups of patients (including 187 COVID-19 positive) and in three French reference samples (the exhaustive North population (n = 1,569,968), a representative sample of the French population (n = 414,046), a random sample of Lille area (n = 1584)). | The SPRs of consumption of ACEI and ARB drugs in COVID-19 patients were similar to the regular consumption of this drug in the reference samples. | No |
Abbreviation: OR, odds ratio.
Mancia et al. [66] performed a case-control study that involved patients with confirmed COVID-19 in the Lombardy region of Italy. The study included 6272 COVID-19 patients and 30,759 controls who were matched based on age, sex, and municipality of residence. In a conditional logistic regression multivariate analysis, neither ACEIs nor ARBs use were associated with the risk of SARS-CoV-2 infection [66]. Reynolds et al. [68] reported a multicenter study involving 12,594 patients. Among these, 5894 (46.8%) were diagnosed with COVID-19, and 1002 had severe illness. The results indicated that there was no association between any single medication class (e.g., ACEIs, ARBs, or other antihypertensive drugs) and an increased likelihood of SARS-CoV-2 infection [68]. In a retrospective cohort study [69] from Ohio and Florida, USA, a total of 18,472 patients were tested for COVID-19. Of these, 2285 (12.4%) were taking ACEIs/ARBs, and 1735 (9.4%) had a positive COVID-19 test result. The overlap propensity score weighting showed that there was no significant association of ACEI and/or ARB use with COVID-19 test positivity (OR, 0.97; 95% CI, 0.81–1.15) [69]. In addition, Dauchet et al. [67] concluded that the standardized prevalence ratio (SPR) of consumption of ACEI and ARB drugs in COVID-19 patients was similar to the regular consumption of this drug in the reference samples.
None of the five studies provide evidence to support the hypothesis that ACEI or ARB use is associated with the risk of SARS-CoV-2 infection (Fig. 2). We suggest that hypertensive patients and other patients taking RAAS inhibitors (e.g., those with chronic heart failure, chronic renal failure and diabetes) [19,70] should not discontinue the use of RAAS inhibitors.
5. RAAS inhibitor use and risk of COVID-19 severe illness or mortality
To investigate the interaction of RAAS inhibitors with the severe illness or mortality of COVID-19, we searched the PubMed database. A total of eleven original retrospective clinical studies [66,68,[71], [72], [73], [74], [75], [76], [77], [78], [79]] were conducted and are shown in Table 4 . In addition, a prospective study based on an ongoing randomized clinical trial (NCT03201185) [80] randomly allocating ramipril or control among patients with successful transcatheter aortic valve replacement was collected.
Table 4.
The association between RAAS inhibitors use and risk of severe or fatal cases with COVID-19.
| Source | Study design and participants | Results | Increase of risk |
|---|---|---|---|
| Previous use or current use at admission | |||
| Mancia [66] | A population-based case-control study. 6272 case patients with COVID-19 matching 30,759 controls. | ARBs or ACEIs use on the risk for severe or fatal cases: adjusted OR, 0.83; 95% CI, 0.63 to 1.10 for ARBs and 0.91; 95% CI, 0.69 to 1.21 for ACEIs | No |
| Reynolds [68] | A multicenter retrospective study; Among 12,594 patients who were tested for Covid-19, 5894 (46.8%) were positive; 1002 had severe illness. | There was no higher risk (by ≥10 percentage points) of severe Covid-19 associated with ACEIs or ARBs use. | No |
| Abajo [73] | A case-population study; 1139 cases with COVID-19 and 11,390 population controls were enrolled. | For COVID-19 requiring admission to hospital, including fatal cases and those admitted to ICU: adjusted OR, 0.94; 95% CI, 0.77–1.15 for RAAS inhibitors. | No |
| Jung S [74] | A nationwide population-based cohort study. Among 5179 confirmed COVID-19 cases, 762 patients were RAAS inhibitor users and 4417 patients were nonusers. | For a higher risk of mortality: adjusted OR, 0.88; 95% CI, 0.53–1.44 for RAAS inhibitors. | No |
| Meng J [72] | A single-center retrospective study; 42 patients were enrolled, including ACEI/ARB group (n = 17) and non-ACEI/ARB group (n = 25). | Patients receiving ACEI or ARB therapy had a lower rate of severe diseases. | No |
| Yang G [76] | A single-center retrospective study; 126 COVID-19 patients with preexisting hypertension were divided into two groups: ARBs/ACEIs group (n = 43) and non-ARBs/ACEIs group (n = 83). | A lower proportion of critical patients (9.3% vs 22.9%; P = 0.061), and a lower death rate (4.7% vs 13.3%; P = 0.216) were observed in ARBs/ACEIs group than non-ARBs/ACEIs group. | No |
| Feng Y [77] | A multicenter retrospective study; 476 patients were divided into three groups (moderate, severe, and critical group). | Compared with severe and critical groups, there were more patients taking ACEI/ARB in moderate group. | No |
| Peng Y [78] | A single-center retrospective study; 112 COVID-19 patients with CVD were divided into two groups (critical group and general group). | No significant difference in the proportion of ACEI/ARB between the critical group and the general group or between non-survivors and survivors. | No |
| Continue to use after admission | |||
| Zhang p [71] | A multicenter retrospective study; 1128 adult patients with hypertension and COVID-19 were enrolled. Model 1: 188 patients taking ACEI/ARB during hospitalization and 940 not; model 2: 1:2 (174 with ACEI/ARB use:348 without) matching; model 3: 1:1 (181 with ACEI/ARB use:181 with other antihypertensive drugs) matching. | ACEI/ARB use on the risk for all-cause mortality: Model 1: adjusted HR, 0.42; 95% CI, 0.19–0.92; P = .03; Model 2: adjusted HR, 0.37; 95%CI, 0.15–0.89; P = .03; Model 3: adjusted HR, 0.29; 95%CI, 0.12–0.69; P = 0.005. | No; possibly reduce risk |
| Li J [75] | A single-center retrospective study; 362 COVID-19 patients with hypertension were enrolled, including 115 patients (31.8%) taking ACEI/ARBs. | The percentage of patients with hypertension taking ACEIs/ARBs did not differ between those with severe and nonsevere infections nor between nonsurvivors and survivors. | No |
| Huang Z [79] | An observational registry study; 50 hospitalized hypertension patients with COVID-19 were grouped into RAS blockers group (n = 20) and non-RAS blockers group (n = 30). | There was no significant difference in clinical severity, clinical course and in-hospital mortality between RAS blockers group and non-RAS blockers group. | No |
Abbreviation: OR, odds ratio; HR, hazard ratio.
First, we analyzed the current or previous use of RAAS inhibitors. Mancia et al. [66] recruited 6272 patients with COVID-19 in Italy and concluded that there was no evidence that the use of ACEIs or ARBs increased the risk of COVID-19 severe or fatal illness. Reynolds et al. [68] reported 5894 COVID-19 patients in New York and suggested that neither ACEIs nor ARBs were associated with a significant increase in the risk of severe illness. In both of the studies described above, the medication data were extracted from medical histories. Abajo et al. [73] collected data for 1139 patients with positive COVID-19 test and 11,390 population controls in Spain and defined exposure to the drugs (ACEIs/ARBs) as current use. They concluded that ACEIs/ARBs did not affect the risk of COVID-19 requiring admission to hospitals, including these fatal cases and critical cases (admitted to the ICU), which indicated that these drugs should not be discontinued or replaced. In another study from South Korea [74], the author identified all RAAS inhibitors that were prescribed within 1 year before the index date. They also concluded that prior use of RAAS inhibitors was not independently associated with mortality among COVID-19 patients. A similar conclusion was also supported by several other studies with small sample sizes from China [72,[76], [77], [78]].
Second, we analyzed the association between the continued use of RAAS inhibitors during hospitalization or observation and severe or fatal illness. P Zhang et al. [71] reported a multicenter retrospective study, and 1128 adult patients with hypertension who were diagnosed with COVID-19 were enrolled. Patients with hypertension were classified as the ACEI/ARB group (n = 188) or non-ACEI/ARB group (n = 940) according to whether they received ACEI/ARB treatment during hospitalization. The results found that the ACEI/ARB group had a lower unadjusted mortality than the non-ACEI/ARB group (3.7% vs. 9.8%; P = 0.01). After adjusting for age, sex, comorbidities and in-hospital medications, the observed risk for all-cause in-hospital mortality was still lower in the ACEI/ARB group than in the non-ACEI/ARB group (adjusted hazard ratio, 0.42; 95% CI, 0.19–0.92; P = 0.03). In a propensity score-matched analysis followed by balancing relative variables, similar results were observed (adjusted hazard ratio, 0.37; 95% CI, 0.15–0.89; P = 0.03). In a further subgroup propensity score-matched analysis, the risk of all-cause mortality was also lower in the ACEI/ARB group than in the group using other antihypertensive drugs (adjusted hazard ratio, 0.30; 95% CI, 0.12–0.70; P = 0.01). The study suggested that the inpatient use of RAAS inhibitors may decrease the risk of all-cause mortality in patients with hypertension and COVID-19. J Li et al. [63] conducted another single-center retrospective study in which 362 COVID-19 patients with hypertension were enrolled, including 115 patients (31.8%) taking ACEI/ARBs at admission with continued use during hospitalization. The results concluded that there were no differences in the percentage of patients taking ACEIs/ARBs between those with severe and nonsevere infections or between nonsurvivors and survivors. In addition, a small retrospective study from China concluded that there were no obvious differences in clinical characteristics, including clinical severity, clinical course and in-hospital mortality, between the RAAS inhibitor group and the non-RAAS inhibitor group [79]. Although all three studies were retrospective and the interpretation of the conclusion was interfered by some underlying confounders, it is impossible to support the hypothesis that inpatient use of RAAS inhibitors is associated with an increased risk for severe illness or mortality.
To evaluate if RAAS inhibitors affect the risk for COVID-19, a prospective study has reached a preliminary conclusion [80]. The prospective study was based on an ongoing randomized control study, which allocated ramipril or control among patients with successful transcatheter aortic valve replacement. The study enrolled 102 patients including 50 ramipril and 52 controls, and these patients took the medicine for at least a month. The results found that ramipril was not associated with the incidence or severity of COVID-19 in the old patients with cardiovascular disease. However, there were only eleven patients diagnosed with COVID-19 in the study, indicating the small size trial had a limited influence on the conclusion.
Taken together, the results of current studies do not support the concerns that the use of RAAS inhibitors increases the risk of SARS-CoV-2 infection and poor prognosis in patients with COVID-19 (Fig. 2). Considering the cornerstone role of RAAS inhibitors in the treatment of hypertension and heart failure, RAAS inhibitors should continue to be prescribed or used in these patients and should not be recommended for suspension or replacement in those with confirmed COVID-19, which is consistent with suggestions from professional scientific societies and experts [15,16,81].
6. RAAS inhibitors: “allies” in the treatment of COVID-19?
P Zhang et al. [71] suggested that the mortality was lower in the ACEIs/ARBs group than the group with other antihypertensive drugs and ACEIs/ARBs use may have beneficial effects on the outcomes of patients with COVID-19. Besides, two meta-analyses suggested that the use of RAAS inhibitors may decrease the risk of mortality in COVID-19 patients [82,83]. Are these RAAS inhibitors “allies” in the treatment of COVID-19? ACEIs use inhibits the generation of Ang II, indirectly promoting Ang II to product Ang 1–9 and Ang 1–7 via ACE2. The activation of Ang II/AT1R is partly inhibited and the balance may gradually shift in favor of lung function. The use of ARBs can directly block the function of AT1R and the activity of Ang II/AT1R axis and, as a compensatory, enhance the activity of AEC2/Ang 1–7/MasR axis, whose end-point is metabolite Ang 1–7. Previous studies have supported that the administration of Ang 1–7 protects against experimental acute lung injury and acute respiratory distress syndrome [[84], [85], [86]]. Besides, the inactivation of AT1R may reduce the cleavage and internalization of the surface ACE2 [24,31,87]. And the maintaining of surface ACE2 in lung will furtherly promote the production of Ang 1–7. Taken together, ACEIs and ARBs may contribute to the attenuation of lung injury or inflammation via the induction of Ang 1–7 in COVID-19 patients. Therefore, ACEIs and ARBs may theoretically be the candidates for the treatment of COVID-19 patients. ARBs, as the direct antagonist of AT1R, should attract more attention. However, we still need more randomized trials to definitively answer the question of whether ACE inhibitors or ARBs pose benefits to patients with COVID-19 (Fig. 2). After checking the U.S. National Library of Medicine, we found that several registered randomized trials (including NCT04330300, NCT04355429, NCT04345406 and NCT04312009) have started to address this question, and the results deserve our attention in the future [[88], [89], [90], [91], [92]]. In addition, ACE2/Ang 1–7/MasR axis may be a promising intervention target to prevent from SARS-CoV-2 induced lung injury. And, whether recombinant Ang 1–7 and the agonist of MasR have a beneficial effect on the treatment of COVID-19 needs furtherly preclinical and clinical studies in the future.
7. Conclusion
Current evidence does not support the concerns that the use of RAAS inhibitors is associated with an increased risk of SARS-CoV-2 infection or poor prognosis. General patients and even COVID-19 patients are advised to continue using RAAS inhibitors, since the inappropriate discontinuation of or changes in medication may lead to fluctuations in blood pressure or the progression of related diseases. Whether the use of RAAS inhibitors poses benefits to the treatment or prognosis of COVID-19 patients furtherly needs more randomized trials in future.
List of abbreviations
- COVID-19
coronavirus disease 2019
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- ACE
angiotensin-converting enzyme
- ACE2
angiotensin-converting enzyme 2
- RAAS
renin-angiotensin-aldosterone system
- Ang II
angiotensin II
- ACEI
ACE inhibitor
- ARB
angiotensin receptor blocker
- AT1R
Ang II type 1 receptor
- AT2R
Ang II type 2 receptor
- MasR
Mas receptor
- ADAM17
a disintegrin and metalloprotease 17
- RBD
receptor-binding domain
- TMPRSS2
type II transmembrane serine protease
Funding
None.
Author contribution
J.Z., M.W. and J.W. wrote the manuscript. Y.X. and M.Z. prepared the figures and tables.
Declaration of competing interest
The authors declare no conflict of interest in relation to this manuscript.
Acknowledgement
We respectfully and sincerely thank all front-line medical staff for hard work and sacrifice.
References
- 1.Lai C.C., Shih T.P., Ko W.C., Tang H.J., Hsueh P.R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int. J. Antimicrob. Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shah A., Kashyap R., Tosh P., Sampathkumar P., O’Horo J.C. Guide to understanding the 2019 novel coronavirus. Mayo Clin. Proc. 2020;95:646–652. doi: 10.1016/j.mayocp.2020.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mahase E. Covid-19: WHO declares pandemic because of “alarming levels” of spread, severity, and inaction. BMJ. 2020;368:m1036. doi: 10.1136/bmj.m1036. [DOI] [PubMed] [Google Scholar]
- 4.Hoffmann M., Kleine-Weber H., Schroeder S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. CELL. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rice G.I., Thomas D.A., Grant P.J., Turner A.J., Hooper N.M. Evaluation of angiotensin-converting enzyme (ACE), its homologue ACE2 and neprilysin in angiotensin peptide metabolism. Biochem. J. 2004;383:45–51. doi: 10.1042/BJ20040634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang M., Gao Y., Zhao W., Yu G., Jin F. ACE-2/ANG1-7 ameliorates ER stress-induced apoptosis in seawater aspiration-induced acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2018;315:L1015–L1027. doi: 10.1152/ajplung.00163.2018. [DOI] [PubMed] [Google Scholar]
- 7.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. LANCET. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guan W.J., Liang W.H., Zhao Y., et al. Comorbidity and its impact on 1590 patients with Covid-19 in China: a nationwide analysis. Eur. Respir. J. 2020;55 doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir. Med. 2020;8:e21. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khan M.S., Fonarow G.C., Ahmed A., et al. Dose of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers and outcomes in heart failure: a meta-analysis. Circ Heart Fail. 2017;10 doi: 10.1161/CIRCHEARTFAILURE.117.003956. [DOI] [PubMed] [Google Scholar]
- 11.McMurray J., Solomon S., Pieper K., et al. The effect of valsartan, captopril, or both on atherosclerotic events after acute myocardial infarction: an analysis of the Valsartan in Acute Myocardial Infarction Trial (VALIANT) J. Am. Coll. Cardiol. 2006;47:726–733. doi: 10.1016/j.jacc.2005.09.055. [DOI] [PubMed] [Google Scholar]
- 12.Wang B., Wang F., Zhang Y., et al. Effects of RAS inhibitors on diabetic retinopathy: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015;3:263–274. doi: 10.1016/S2213-8587(14)70256-6. [DOI] [PubMed] [Google Scholar]
- 13.Xie X., Liu Y., Perkovic V., et al. Renin-angiotensin system inhibitors and kidney and cardiovascular outcomes in patients with CKD: a Bayesian network meta-analysis of randomized clinical trials. Am. J. Kidney Dis. 2016;67:728–741. doi: 10.1053/j.ajkd.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 14.Turnbull F., Neal B., Pfeffer M., et al. Blood pressure-dependent and independent effects of agents that inhibit the renin-angiotensin system. J. Hypertens. 2007;25:951–958. doi: 10.1097/HJH.0b013e3280bad9b4. [DOI] [PubMed] [Google Scholar]
- 15.American College of Cardiology HFSA/ACC/AHA statement addresses concerns: using RAAS antagonists in COVID-19. March 17, 2020. https://www.acc.org/latest-incardiology/articles/2020/03/17/08/59/hfsa-acc-aha-statement-addresses-concerns-re-using-raas-antagonists-in-covid-19
- 16.European Society of Cardiology Position statement of the ESC Council on Hypertension on ACE-inhibitors and angiotensin receptor blockers. March 13, 2020. https://www.escardio.org/Councils/Council-on-Hypertension-(CHT)/News/position-statement-of-the-esc-council-on-hypertension-on-ace-inhibitors-and-ang
- 17.Kreutz R., Algharably E., Azizi M., et al. Hypertension, the renin-angiotensin system, and the risk of lower respiratory tract infections and lung injury: implications for COVID-19. Cardiovasc. Res. 2020 doi: 10.1093/cvr/cvaa097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patel S., Rauf A., Khan H., Abu-Izneid T. Renin-angiotensin-aldosterone (RAAS): the ubiquitous system for homeostasis and pathologies. Biomed. Pharmacother. 2017;94:317–325. doi: 10.1016/j.biopha.2017.07.091. [DOI] [PubMed] [Google Scholar]
- 19.Kobori H., Nangaku M., Navar L.G., Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol. Rev. 2007;59:251–287. doi: 10.1124/pr.59.3.3. [DOI] [PubMed] [Google Scholar]
- 20.Arendse L.B., Danser A., Poglitsch M., et al. Novel therapeutic approaches targeting the renin-angiotensin system and associated peptides in hypertension and heart failure. Pharmacol. Rev. 2019;71:539–570. doi: 10.1124/pr.118.017129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sparks M.A., Stegbauer J., Chen D., et al. Vascular type 1A angiotensin II receptors control BP by regulating renal blood flow and urinary sodium excretion. J. Am. Soc. Nephrol. 2015;26:2953–2962. doi: 10.1681/ASN.2014080816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohshima K., Mogi M., Nakaoka H., et al. Possible role of angiotensin-converting enzyme 2 and activation of angiotensin II type 2 receptor by angiotensin-(1-7) in improvement of vascular remodeling by angiotensin II type 1 receptor blockade. Hypertension. 2014;63:e53–e59. doi: 10.1161/HYPERTENSIONAHA.113.02426. [DOI] [PubMed] [Google Scholar]
- 23.Rincon J., Correia D., Arcaya J.L., et al. Role of angiotensin II type 1 receptor on renal NAD(P)H oxidase, oxidative stress and inflammation in nitric oxide inhibition induced-hypertension. Life Sci. 2015;124:81–90. doi: 10.1016/j.lfs.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deshotels M.R., Xia H., Sriramula S., Lazartigues E., Filipeanu C.M. Angiotensin II mediates angiotensin converting enzyme type 2 internalization and degradation through an angiotensin II type I receptor-dependent mechanism. Hypertension. 2014;64:1368–1375. doi: 10.1161/HYPERTENSIONAHA.114.03743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McKinney C.A., Fattah C., Loughrey C.M., Milligan G., Nicklin S.A. Angiotensin-(1-7) and angiotensin-(1-9): function in cardiac and vascular remodelling. Clin Sci (Lond) 2014;126:815–827. doi: 10.1042/CS20130436. [DOI] [PubMed] [Google Scholar]
- 26.Liu C., Lv X.H., Li H.X., et al. Angiotensin-(1-7) suppresses oxidative stress and improves glucose uptake via Mas receptor in adipocytes. Acta Diabetol. 2012;49:291–299. doi: 10.1007/s00592-011-0348-z. [DOI] [PubMed] [Google Scholar]
- 27.Magalhaes G.S., Rodrigues-Machado M.G., Motta-Santos D., et al. Chronic allergic pulmonary inflammation is aggravated in angiotensin-(1-7) Mas receptor knockout mice. Am J Physiol Lung Cell Mol Physiol. 2016;311:L1141–L1148. doi: 10.1152/ajplung.00029.2016. [DOI] [PubMed] [Google Scholar]
- 28.Sukumaran V., Tsuchimochi H., Tatsumi E., Shirai M., Pearson J.T. Azilsartan ameliorates diabetic cardiomyopathy in young db/db mice through the modulation of ACE-2/ANG 1-7/Mas receptor cascade. Biochem. Pharmacol. 2017;144:90–99. doi: 10.1016/j.bcp.2017.07.022. [DOI] [PubMed] [Google Scholar]
- 29.Imai Y., Kuba K., Rao S., et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Serfozo P., Wysocki J., Gulua G., et al. Ang II (angiotensin II) conversion to angiotensin-(1-7) in the circulation is POP (prolyloligopeptidase)-dependent and ACE2 (angiotensin-converting enzyme 2)-independent. Hypertension. 2020;75:173–182. doi: 10.1161/HYPERTENSIONAHA.119.14071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu J., Sriramula S., Xia H., et al. Clinical relevance and role of neuronal AT1 receptors in ADAM17-mediated ACE2 shedding in neurogenic hypertension. Circ. Res. 2017;121:43–55. doi: 10.1161/CIRCRESAHA.116.310509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Danser A., Epstein M., Batlle D. Renin-angiotensin system blockers and the COVID-19 pandemic: at present there is no evidence to abandon renin-angiotensin system blockers. Hypertension. 2020;75:1382–1385. doi: 10.1161/HYPERTENSIONAHA.120.15082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li F. Structure, function, and evolution of coronavirus spike proteins. Annu Rev Virol. 2016;3:237–261. doi: 10.1146/annurev-virology-110615-042301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perlman S., Netland J. Coronaviruses post-SARS: update on replication and pathogenesis. Nat Rev Microbiol. 2009;7:439–450. doi: 10.1038/nrmicro2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shang J., Wan Y., Luo C., et al. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. U. S. A. 2020;117:11727–11734. doi: 10.1073/pnas.2003138117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shang J., Ye G., Shi K., et al. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020;581:221–224. doi: 10.1038/s41586-020-2179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wrapp D., Wang N., Corbett K.S., et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bertram S., Heurich A., Lavender H., et al. Influenza and SARS-coronavirus activating proteases TMPRSS2 and HAT are expressed at multiple sites in human respiratory and gastrointestinal tracts. PLoS One. 2012;7 doi: 10.1371/journal.pone.0035876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fu L., Wang B., Yuan T., et al. Clinical characteristics of coronavirus disease 2019 (COVID-19) in China: a systematic review and meta-analysis. J. Inf. Secur. 2020;80:656–665. doi: 10.1016/j.jinf.2020.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coperchini F., Chiovato L., Croce L., Magri F., Rotondi M. The cytokine storm in COVID-19: an overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020;53:25–32. doi: 10.1016/j.cytogfr.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ye Q., Wang B., Mao J. The pathogenesis and treatment of the ‘Cytokine Storm’ in COVID-19. J. Inf. Secur. 2020;80:607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chousterman B.G., Swirski F.K., Weber G.F. Cytokine storm and sepsis disease pathogenesis. Semin. Immunopathol. 2017;39:517–528. doi: 10.1007/s00281-017-0639-8. [DOI] [PubMed] [Google Scholar]
- 43.Li M.Y., Li L., Zhang Y., Wang X.S. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty. 2020;9:45. doi: 10.1186/s40249-020-00662-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuba K., Imai Y., Rao S., et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Santos R., Sampaio W.O., Alzamora A.C., et al. The ACE2/angiotensin-(1-7)/MAS axis of the renin-angiotensin system: focus on angiotensin-(1-7) Physiol. Rev. 2018;98:505–553. doi: 10.1152/physrev.00023.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kidoguchi S., Sugano N., Takane K., et al. Azilsartan causes natriuresis due to its sympatholytic action in kidney disease. Hypertens. Res. 2019;42:1507–1517. doi: 10.1038/s41440-019-0271-1. [DOI] [PubMed] [Google Scholar]
- 47.Abdel-Fattah M.M., Messiha B., Mansour A.M. Modulation of brain ACE and ACE2 may be a promising protective strategy against cerebral ischemia/reperfusion injury: an experimental trial in rats. Naunyn Schmiedeberg’s Arch. Pharmacol. 2018;391:1003–1020. doi: 10.1007/s00210-018-1523-3. [DOI] [PubMed] [Google Scholar]
- 48.Wang W., Song A., Zeng Y., et al. Telmisartan protects chronic intermittent hypoxic mice via modulating cardiac renin-angiotensin system activity. BMC Cardiovasc. Disord. 2018;18:133. doi: 10.1186/s12872-018-0875-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de Jong M.A., Mirkovic K., Mencke R., et al. Fibroblast growth factor 23 modifies the pharmacological effects of angiotensin receptor blockade in experimental renal fibrosis. Nephrol. Dial. Transplant. 2017;32:73–80. doi: 10.1093/ndt/gfw105. [DOI] [PubMed] [Google Scholar]
- 50.Yisireyili M., Uchida Y., Yamamoto K., et al. Angiotensin receptor blocker irbesartan reduces stress-induced intestinal inflammation via AT1a signaling and ACE2-dependent mechanism in mice. Brain Behav. Immun. 2018;69:167–179. doi: 10.1016/j.bbi.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 51.Callera G.E., Antunes T.T., Correa J.W., et al. Differential renal effects of candesartan at high and ultra-high doses in diabetic mice-potential role of the ACE2/AT2R/Mas axis. Biosci. Rep. 2016;36 doi: 10.1042/BSR20160344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Iwanami J., Mogi M., Tsukuda K., et al. Role of angiotensin-converting enzyme 2/angiotensin-(1-7)/Mas axis in the hypotensive effect of azilsartan. Hypertens. Res. 2014;37:616–620. doi: 10.1038/hr.2014.49. [DOI] [PubMed] [Google Scholar]
- 53.Burchill L.J., Velkoska E., Dean R.G., Griggs K., Patel S.K., Burrell L.M. Combination renin-angiotensin system blockade and angiotensin-converting enzyme 2 in experimental myocardial infarction: implications for future therapeutic directions. Clin Sci (Lond) 2012;123:649–658. doi: 10.1042/CS20120162. [DOI] [PubMed] [Google Scholar]
- 54.Liu C.X., Hu Q., Wang Y., et al. Angiotensin-converting enzyme (ACE) 2 overexpression ameliorates glomerular injury in a rat model of diabetic nephropathy: a comparison with ACE inhibition. Mol. Med. 2011;17:59–69. doi: 10.2119/molmed.2010.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hamming I., van Goor H., Turner A.J., et al. Differential regulation of renal angiotensin-converting enzyme (ACE) and ACE2 during ACE inhibition and dietary sodium restriction in healthy rats. Exp. Physiol. 2008;93:631–638. doi: 10.1113/expphysiol.2007.041855. [DOI] [PubMed] [Google Scholar]
- 56.Takeda Y., Zhu A., Yoneda T., Usukura M., Takata H., Yamagishi M. Effects of aldosterone and angiotensin II receptor blockade on cardiac angiotensinogen and angiotensin-converting enzyme 2 expression in Dahl salt-sensitive hypertensive rats. Am. J. Hypertens. 2007;20:1119–1124. doi: 10.1016/j.amjhyper.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 57.Agata J., Ura N., Yoshida H., et al. Olmesartan is an angiotensin II receptor blocker with an inhibitory effect on angiotensin-converting enzyme. Hypertens. Res. 2006;29:865–874. doi: 10.1291/hypres.29.865. [DOI] [PubMed] [Google Scholar]
- 58.Igase M., Strawn W.B., Gallagher P.E., Geary R.L., Ferrario C.M. Angiotensin II AT1 receptors regulate ACE2 and angiotensin-(1-7) expression in the aorta of spontaneously hypertensive rats. Am. J. Physiol. Heart Circ. Physiol. 2005;289:H1013–H1019. doi: 10.1152/ajpheart.00068.2005. [DOI] [PubMed] [Google Scholar]
- 59.Ishiyama Y., Gallagher P.E., Averill D.B., Tallant E.A., Brosnihan K.B., Ferrario C.M. Upregulation of angiotensin-converting enzyme 2 after myocardial infarction by blockade of angiotensin II receptors. Hypertension. 2004;43:970–976. doi: 10.1161/01.HYP.0000124667.34652.1a. [DOI] [PubMed] [Google Scholar]
- 60.Wang G., Lai F.M., Lai K.B., et al. Urinary mRNA expression of ACE and ACE2 in human type 2 diabetic nephropathy. Diabetologia. 2008;51:1062–1067. doi: 10.1007/s00125-008-0988-x. [DOI] [PubMed] [Google Scholar]
- 61.Soro-Paavonen A., Gordin D., Forsblom C., et al. Circulating ACE2 activity is increased in patients with type 1 diabetes and vascular complications. J. Hypertens. 2012;30:375–383. doi: 10.1097/HJH.0b013e32834f04b6. [DOI] [PubMed] [Google Scholar]
- 62.Vuille-dit-Bille R.N., Camargo S.M., Emmenegger L., et al. Human intestine luminal ACE2 and amino acid transporter expression increased by ACE-inhibitors. Amino Acids. 2015;47:693–705. doi: 10.1007/s00726-014-1889-6. [DOI] [PubMed] [Google Scholar]
- 63.Sama I.E., Ravera A., Santema B.T., et al. Circulating plasma concentrations of angiotensin-converting enzyme 2 in men and women with heart failure and effects of renin-angiotensin-aldosterone inhibitors. Eur. Heart J. 2020;41:1810–1817. doi: 10.1093/eurheartj/ehaa373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Milne S., Yang C.X., Timens W., Bosse Y., Sin D.D. SARS-CoV-2 receptor ACE2 gene expression and RAAS inhibitors. Lancet Respir. Med. 2020;8:e50–e51. doi: 10.1016/S2213-2600(20)30224-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guzik T.J., Mohiddin S.A., Dimarco A., et al. COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc. Res. 2020 doi: 10.1093/cvr/cvaa106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mancia G., Rea F., Ludergnani M., Apolone G., Corrao G. Renin-angiotensin-aldosterone system blockers and the risk of Covid-19. N. Engl. J. Med. 2020;382:2431–2440. doi: 10.1056/NEJMoa2006923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dauchet L., Lambert M., Gauthier V., et al. ACE inhibitors, AT1 receptor blockers and COVID-19: clinical epidemiology evidences for a continuation of treatments. The ACER-COVID study. medRxiv. 2020:2020–2024. [Google Scholar]
- 68.Reynolds H.R., Adhikari S., Pulgarin C., et al. Renin-angiotensin-aldosterone system inhibitors and risk of Covid-19. N. Engl. J. Med. 2020;382:2441–2448. doi: 10.1056/NEJMoa2008975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mehta N., Kalra A., Nowacki A.S., et al. Association of use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with testing positive for coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Deferrari G., Ravera M., Deferrari L., Vettoretti S., Ratto E., Parodi D. Renal and cardiovascular protection in type 2 diabetes mellitus: angiotensin II receptor blockers. J. Am. Soc. Nephrol. 2002;13(Suppl. 3):S224–S229. doi: 10.1097/01.asn.0000032544.37147.ae. [DOI] [PubMed] [Google Scholar]
- 71.Zhang P., Zhu L., Cai J., et al. Association of Inpatient use of angiotensin converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ. Res. 2020;126:1671–1681. doi: 10.1161/CIRCRESAHA.120.317134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Meng J., Xiao G., Zhang J., et al. Renin-angiotensin system inhibitors improve the clinical outcomes of COVID-19 patients with hypertension. Emerg Microbes Infect. 2020;9:757–760. doi: 10.1080/22221751.2020.1746200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.de Abajo F.J., Rodriguez-Martin S., Lerma V., et al. Use of renin-angiotensin-aldosterone system inhibitors and risk of COVID-19 requiring admission to hospital: a case-population study. Lancet. 2020;395:1705–1714. doi: 10.1016/S0140-6736(20)31030-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jung S.Y., Choi J.C., You S.H., Kim W.Y. Association of renin-angiotensin-aldosterone system inhibitors with COVID-19-related outcomes in Korea: a nationwide population-based cohort study. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li J., Wang X., Chen J., Zhang H., Deng A. Association of renin-angiotensin system inhibitors with severity or risk of death in patients with hypertension hospitalized for coronavirus disease 2019 (COVID-19) infection in Wuhan, China. JAMA Cardiol. 2020;5:825. doi: 10.1001/jamacardio.2020.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yang G., Tan Z., Zhou L., et al. Effects of ARBs and ACEIs on virus infection, inflammatory status and clinical outcomes in COVID-19 patients with hypertension: a single center retrospective study. Hypertension. 2020;76:51–58. doi: 10.1161/HYPERTENSIONAHA.120.15143. [DOI] [PubMed] [Google Scholar]
- 77.Feng Y., Ling Y., Bai T., et al. COVID-19 with different severity: a multi-center study of clinical features. Am. J. Respir. Crit. Care Med. 2020;201:1380–1388. doi: 10.1164/rccm.202002-0445OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Peng YD, Meng K, Guan HQ, et al. [Clinical characteristics and outcomes of 112 cardiovascular disease patients infected by 2019-nCoV]. Zhonghua Xin Xue Guan Bing Za Zhi 2020; 48:E4. [DOI] [PubMed]
- 79.Huang Z., Cao J., Yao Y., et al. The effect of RAS blockers on the clinical characteristics of COVID-19 patients with hypertension. Ann Transl Med. 2020;8:430. doi: 10.21037/atm.2020.03.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Amat-Santos I.J., Santos-Martinez S., Lopez-Otero D., et al. Ramipril in high risk patients with COVID-19. J. Am. Coll. Cardiol. 2020;76:268–276. doi: 10.1016/j.jacc.2020.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jarcho J.A., Ingelfinger J.R., Hamel M.B., D’Agostino R.S., Harrington D.P. Inhibitors of the renin-angiotensin-aldosterone system and Covid-19. N. Engl. J. Med. 2020;382:2462–2464. doi: 10.1056/NEJMe2012924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pirola C.J., Sookoian S. Estimation of RAAS-inhibitor effect on the COVID-19 outcome: a meta-analysis. J. Inf. Secur. 2020 doi: 10.1016/j.jinf.2020.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Guo X., Zhu Y., Hong Y. Decreased mortality of COVID-19 with renin-angiotensin-aldosterone system inhibitors therapy in patients with hypertension: a meta-analysis. Hypertension. 2020;76:e13–e14. doi: 10.1161/HYPERTENSIONAHA.120.15572. [DOI] [PubMed] [Google Scholar]
- 84.Klein N., Gembardt F., Supe S., et al. Angiotensin-(1-7) protects from experimental acute lung injury. Crit. Care Med. 2013;41:e334–e343. doi: 10.1097/CCM.0b013e31828a6688. [DOI] [PubMed] [Google Scholar]
- 85.Supe S., Kohse F., Gembardt F., Kuebler W.M., Walther T. Therapeutic time window for angiotensin-(1-7) in acute lung injury. Br. J. Pharmacol. 2016;173:1618–1628. doi: 10.1111/bph.13462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wosten-van A.R., Lutter R., Specht P.A., et al. Acute respiratory distress syndrome leads to reduced ratio of ACE/ACE2 activities and is prevented by angiotensin-(1-7) or an angiotensin II receptor antagonist. J. Pathol. 2011;225:618–627. doi: 10.1002/path.2987. [DOI] [PubMed] [Google Scholar]
- 87.Deshotels M.R., Xia H., Sriramula S., Lazartigues E., Filipeanu C.M. Angiotensin II mediates angiotensin converting enzyme type 2 internalization and degradation through an angiotensin II type I receptor-dependent mechanism. Hypertension. 2014;64:1368–1375. doi: 10.1161/HYPERTENSIONAHA.114.03743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.U.S. National Library of Medicine. Coronavirus (COVID-19) ACEi/ARB investigation (CORONACION). ClinicalTrials.gov Identifier: NCT04330300 [updated April 13, 2020]. Accessed at https://clinicaltrials.gov/ct2/show/NCT04330300term=NCT04330300&draw=2&rank=1 on May 20, 2020.
- 89.U.S. National Library of Medicine. Efficacy of captopril in Covid-19 patients with severe acute respiratory syndrome (SARS) CoV-2 pneumonia (CAPTOCOVID). ClinicalTrials.gov Identifier: NCT04355429 [updated April 28, 2020]. Accessed at https://clinicaltrials.gov/ct2/show/NCT04355429?term=NCT04355429&draw=2&rank=1 on May 20, 2020.
- 90.U.S. National Library of Medicine. Angiotensin converting enzyme inhibitors in treatment of Covid 19. ClinicalTrials.gov Identifier: NCT04345406 [updated April 14, 2020]. Accessed at https://clinicaltrials.gov/ct2/show/NCT04345406?term=NCT04345406&draw=2&rank=1 on May 20, 2020.
- 91.U.S. National Library of Medicine. Losartan for patients with COVID-19 requiring hospitalization. ClinicalTrials.gov Identifier: NCT04312009 2020 [updated March 23, 2020]. Accessed at https://clinicaltrials.gov/ct2/show/NCT04312009 on May 20, 2020.
- 92.U.S. National Library of Medicine. Angiotensin receptor blockers and angiotensin-converting enzyme inhibitors and adverse outcomes in patients with COVID19 (BRACE-CORONA). ClinicalTrials.gov Identifier: NCT04364893. [updated April 28, 2020]. Accessed at https://clinicaltrials.gov/ct2/show/NCT04364893 on May 20, 2020.