Abstract
Background: It is well known that insulin-like growth factor 2 mRNA-binding protein 2 (IGF2BP2) rs4402960 polymorphism is associated with Type 2 diabetes mellitus, which has a shared genetic background with gestational diabetes mellitus (GDM). Previous studies have yielded controversial results about the link between IGF2BP2 rs4402960 polymorphism and GDM risk. Thus, a meta-analysis was performed to obtain more conclusive results.
Methods: Clinical and genotype data were determined for 305 GDM and 1216 healthy participants recruited. Eligible studies were retrieved in PubMed, Web of science, EMBASE, and Scopus. Odds ratios (ORs) with 95% confidence intervals (CIs) were utilized to evaluate the relationship between IGF2BP2 polymorphisms and GDM susceptibility in five genetic models. The subgroup stratified analysis and trial sequential analysis (TSA) were performed.
Results: In this case–control study, no significant association was revealed between IGF2BP2 polymorphism and GDM (P>0.05). When combined with the previous studies in the meta-analysis, there was no statistical association between IGF2BP2 polymorphism and GDM (allele model: OR = 1.01, 95% CI = 0.86–1.18; dominant model: OR = 1.00, 95% CI = 0.81–1.24; recessive model: OR = 1.08, 95% CI = 0.91–1.29; heterozygous model: OR = 0.99, 95% CI = 0.80–1.24; homozygous model: OR = 1.06, 95% CI = 0.78–1.42). No association was observed in five genetic models in each subgroup. TSA indicated sufficient proof of such null association in the overall population.
Conclusions: This meta-analysis provides sufficient statistical evidence indicating null association between IGF2BP2 rs4402960 polymorphism and GDM risk.
Keywords: Genetic variation, Gestational diabetes mellitus, Meta-analysis, Polymorphisms
Introduction
Gestational diabetes mellitus (GDM) is defined as glucose intolerance that occurs during pregnancy. The GDM is the most prevalent metabolic disorder during pregnancy with prevalence between 9.3% and 25.5%, which is attributed mainly to obesity, older maternal age, and a sedentary lifestyle [1]. GDM results in significant perinatal mortalities and comorbidities. Pregnant women complicated with GDM tend to have increasing risk of obesity, diabetes mellitus (DM), and cardiovascular disease. The underlying factors leading to the development of GDM are difficult to determine, and may involve a combination of diverse environmental, genetic, and epigenetic factors. An increasing amount of evidence points to a connection between genetics and GDM [2].
Insulin-like growth factor 2 mRNA-binding protein 2 (IGF2BP2) is part of the family of mRNA-binding proteins regulating insulin-like growth factor 2 (IGF2) translation. IGF2BP2 is located on chromosome 3q27. A previous animal study showed that IGF2 is involved in pancreatic function [3]. Several studies have reported that the polymorphisms in IGF2BP2 is associated with reduced β-cell function [4,5] and risk of DM [6]. Previous studies revealed that 2.6–70% of women with a history of GDM will develop DM 28 years postpartum [7], and women with a family history of DM may be predisposed to an increased risk of GDM [8]. GDM may share the same risk factors and genetic susceptibilities with DM that indicates that IGF2BP2 gene polymorphism may also be associated with GDM.
To date, researchers investigated the association between three IGF2BP2 polymorphisms (rs4402960, rs1470579, rs11705701) and GDM. There was no evidence of the association between IGF2BP2 rs1470579 or rs11705701 and risk of GDM [9,10]. Recently, the association between IGF2BP2 rs4402960 polymorphism and risk of GDM has been discussed in many studies [11,12]. However, the results were inconsistent. Therefore, we conducted a meta-analysis of studies on the IGF2BP2 polymorphism with the aim of providing a more comprehensive summary of currently available research to evaluate the relationship between the IGF2BP2 rs4402960 polymorphism and GDM risk.
Methods
Study subjects
Between January 2014 and December 2018, 1521 singleton pregnant women were enrolled in the present study in the Department of Obstetrics, at the First Affiliated Hospital of China Medical University. All participants were provided with written informed consent and the study protocol was approved by the Medical Ethics Review Board of China Medical University (Shenyang, Liaoning, China).
Included GDM cases were identified after a glucose challenge test between weeks 24 and 28 of gestation. Participants were not eligible if they had a history of diabetes (including GDM in previous pregnancies). The control group consisted of 1216 middle-aged (18–45 years) non-diabetic Chinese women from the prospective hospital-based cohort [13]. We excluded those who presenting with maternal-related abnormalities, mothers with a history of drug abuse and depression, carriers of a blood-transmitted infectious disease, hypertension, and cardiovascular diseases.
All pregnant women were screened for GDM at 24–28 gestation weeks with a 75 g, 2 h oral glucose tolerance test. GDM was defined according to the International Association of Diabetes and Pregnancy Study Groups [14]. A diagnosis of GDM was made when one or more of the test parameters equaled or exceeded the following cut points: fasting: 5.1 mmol/l, 1-h: 10.0 mmol/l, or 2-h: 8.5 mmol/l. We also measured anthropometric parameters such as weight and height. Body mass index (BMI) was calculated. We also recorded maternal age, gravida, parity, family history of diabetes, and lipid profile.
Genotyping
About 5 ml blood sample was collected from each participant. DNA was extracted with standard proteinase K digestion, followed by phenol–chloroform extraction and ethanol precipitation.
Genotyping of IGF2BP2 rs4402960 was performed with the Taqman allelic discrimination assays using ABI 7500 Real Time PCR (Applied Biosystems, Foster City, CA). All primers and probes were designed by Applied Biosystems (Foster City, CA). The primers sequence were 5′-GGAGCAGTAAGGTAGGATGGACAGTAGATT-3′ (forward) and 5′-AAGATACTGATTGTG TTTGCAAACATGCCC-3′ (reverse). Assay ID is C__2165199_10, and context sequence is AGTAAGGTAGGATGGACAGTAGATT[G/T]AAGATACTGATTGTGTTTGCAAACA. The reaction mix included 10 μl DNA, 25 μl master mix (Applied Biosystems), 2.5 μl probe, and 12.5 μl sterile water in a final volume of 50 μl. PCR conditions comprised an initial denaturing step at 95°C for 10 min, followed by 45 cycles of 92°C for 30 s, with a final extension at 60°C for 1 min. PCR plates were read on an ABI PRISM 7900 instrument (Applied Biosystems). Two authors (G.S. and G.Z.) independently reviewed the genotyping results and input data.
Meta-analysis
Two examiners (J.L. and G.S.) independently searched for the significant studies in databases, including PubMed, Web of science, EMBASE, and Scopus on March 15, 2020. The search terms are the ‘gestational diabetes mellitus’, ‘polymorphism/variant/mutation/’, and ‘IGF2BP2’. Original studies were eligible if they met the following criteria: (I) case-control studies; (II) literatures on associations between IGF2BP2 rs4402960 polymorphism and GDM; (III) results were denoted as odds ratio (OR) with 95% confidence intervals (CI). Original studies were ineligible if they: (I) were reviews, letters, or case reports; (II) did not contain outcomes which can be converted to OR and 95% CI; (III) included control group that was not involved with healthy women; (IV) were laboratory studies with animals. For duplicate publications, we only included the study with the largest sample size for analyses.
Two researchers (J.L. and G.Z.) carried out data extraction independently. If there were disagreements, they discussed and reached a consensus with the help from a third reviewer (TM). The data contained the first author, publication year, ethnicity, control source, genotyping method, genotype distribution in case and control groups. The Newcastle–Ottawa Scale (NOS) was used to assess the quality of all studies included. The NOS quality score ranges from 0 to 9 stars. Two authors independently assessed the quality of the studies included. Disagreements were resolved by discussion.
Statistical analysis
Chi-square test was used to compare Allele/genotype frequencies between GDM and control groups. The strength of association between IGF2BP2 rs4402960 polymorphism and GDM risk was assessed by calculating the OR with 95% CI. We calculated the OR by genotype and allele model comparisons of IGF2BP2 rs4402960 polymorphism between cases and controls. The P value for Hardy–Weinberg equilibrium (HWE) was calculated in the control group using the chi-square test. Heterogeneity was assessed by using the I2 statistics. If there was no heterogeneity (P>0.1 or I2<50%), a fixed-effects model was used to estimate the pooled OR; otherwise, a random-effects model was utilized. Subgroup stratified analysis was performed using genotyping method (TaqMan assay or PCR), ethnicity (Caucasian or Asian), and control source (hospital-based or population-based). Study effects, such as publication bias, were evaluated with Egger’s tests and a P value of < 0.1 was considered statistically significant for asymmetry. Sensitivity analyses were directed to assess the influence of individual study on the overall estimate. Statistical analyses were performed with Stata (version 14.0; StataCorp, College Station, TX). The TSA (version 0.9.5.10, http://www.ctu.dk/tsa/) was conducted to maintain a 95% confidence interval, a 20% relative risk reduction, overall 5% risk of a type I error and 20% of the type II error (a power of 80%) in this study to reduce the risk of type I error.
Results
Case–control study
In total, 305 patients with GDM and 1216 controls participated in the present study. The baselines of characteristics of participants are shown in Table 1. Compared to the control groups, there was no difference in the maternal age, height, weight, pre-pregnancy BMI, gravida, parity, family history of diabetes, and low-density lipoprotein cholesterol (P>0.05). There were significant differences between the two groups with respect to triglyceride, total cholesterol, and high-density lipoprotein cholesterol (P<0.05). There was no difference in genotype frequencies between GDM and controls groups in IGF2BP2. Meanwhile, there was no significant susceptibility for rs4402960 polymorphism with GDM risk within five genetic models (all P>0.05; Table 2).
Table 1. Table 1 Clinical characteristics of pregnant women enrolled in the study.
| GDM (n=305) | Control (n=1216) | P | |
|---|---|---|---|
| Maternal age (years) | 32.13 ± 3.04 | 32.13 ± 3.04 | 0.985 |
| Height (m) | 1.63 ± 0.04 | 1.63 ± 0.05 | 0.303 |
| Weight (kg) | 60.4 ± 7.24 | 59.8 ± 7.63 | 0.195 |
| Pre-pregnancy BMI (kg/m2) | 22.85 ± 3.06 | 22.69 ± 3.08 | 0.417 |
| Gravida | 1 (1–2) | 1 (1–2) | 0.370 |
| Parity | 0 (0–1) | 0 (0–1) | 0.994 |
| Family history of diabetes | 20 (7%) | 64 (5%) | 0.376 |
| Triglyceride (mg/dl)* | 232.53 ± 61.48 | 223.83 ± 62.16 | 0.029 |
| Total cholesterol (mg/dl)* | 241.06 ± 29.44 | 236.87 ± 30.67 | 0.032 |
| High-density lipoprotein cholesterol (mg/dl)* | 67.98 ± 8.93 | 69.18 ± 7.09 | 0.013 |
| Low-density lipoprotein cholesterol (mg/dl)* | 129.35 ± 22.02 | 131.44 ± 22.19 | 0.141 |
BMI, body mass index.
Maternal age, height, weight, and pre-pregnancy BMI were expressed as the mean ± standard deviation.
Gravida and parity were expressed as the median and interquartile ranges (interquartile ranges: the range of values lying between 25th and 75th centiles.
: test from each participant before 19 weeks + 6 days.
Table 2. Genotype frequencies and analysis of IGF2BP2 rs4402960 in Chinese population.
| n (%) | P | Comparison | Type of model | OR (95% CI) | P | ||
|---|---|---|---|---|---|---|---|
| GDM | Control group | ||||||
| GG | 168 (55%) | 608 (50%) | 0.179 | T vs. G allele | Allele | 0.83 (0.68–1.01) | 0.060 |
| GT | 115 (38%) | 488 (40%) | TT+GT vs. GG | Dominant | 0.82 (0.63–1.05) | 0.112 | |
| TT | 22 (7%) | 120 (10%) | TT vs. GG+GT | Recessive | 0.71 (0.44–1.14) | 0.154 | |
| TT vs. GT | Heterozygous | 0.85 (0.65–1.11) | 0.322 | ||||
| TT vs. GG | Homozygous | 0.66 (0.41–1.08) | 0.096 | ||||
Abbreviations: CI, confidence interval; OR, odds ratio.
Literature search
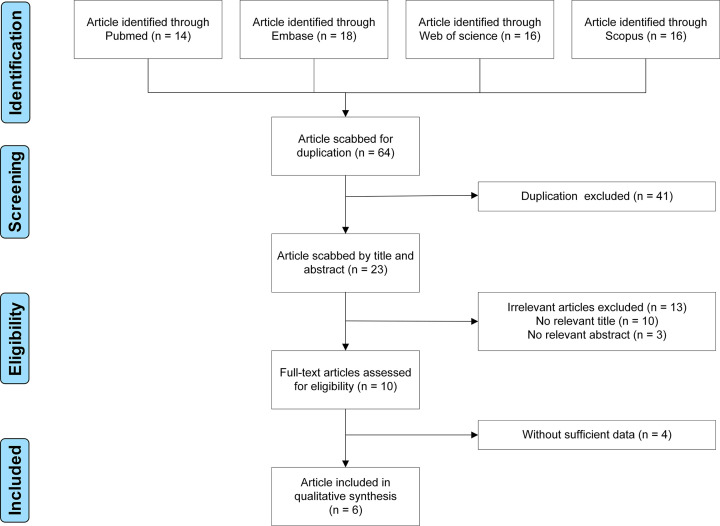
About 64 potentially eligible studies were retrieved. After application of the criteria, six studies were kept (Figure 1). Characteristics of the seven studies, including ours, are shown in Table 3. The published year ranged from 2009 to 2020. The subjects in four were Asians, and Caucasians in three studies. Six were hospital-based design, only one was population-based design. Five studies used TaqMan assay, and two studies used polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP). NOS scores were between six and eight (details are shown in the Supplementary Material). A total of 8346 subjects were involved, including 2720 GDM patients and 5626 healthy women. All P values of HWE were more than 0.05.
Figure 1. Flow chart of study selection.
Table 3. Characteristics of 2720 gestational diabetes mellitus cases and 5626 controls included in this meta-analysis.
| First author | Year | Country | Control source | Genotyping method | NOS Score | Case (n) | Control (n) | HWE (P) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | GG | GT | TT | Total | GG | GT | TT | |||||||
| Lauenborg | 2009 | Denmark | Hospital-based | TaqMan assay | 6 | 274 | 115 | 132 | 27 | 2334 | 1138 | 972 | 224 | 0.433 |
| Cho | 2009 | Korea | Population-based | TaqMan assay | 6 | 857 | 389 | 365 | 103 | 627 | 313 | 257 | 57 | 0.685 |
| Wang | 2011 | China | Hospital-based | TaqMan assay | 8 | 705 | 371 | 278 | 56 | 1025 | 605 | 361 | 59 | 0.596 |
| Chon | 2013 | Korea | Hospital-based | PCR-RFLP | 6 | 94 | 57 | 30 | 7 | 41 | 15 | 24 | 2 | 0.053 |
| Popova | 2017 | Russia | Hospital-based | PCR-RFLP | 7 | 278 | 120 | 134 | 24 | 179 | 77 | 76 | 26 | 0.310 |
| Tarnowski | 2019 | Poland | Hospital-based | TaqMan assay | 7 | 207 | 105 | 76 | 26 | 204 | 89 | 93 | 22 | 0.753 |
| Current study | 2020 | China | Hospital-based | TaqMan assay | 6 | 305 | 168 | 115 | 22 | 1216 | 608 | 488 | 120 | 0.131 |
Abbreviations: HWE, Hardy–Weinberg equilibrium; NOS, Newcastle–Ottawa Scale; PCR-RFLP, polymerase chain reaction-restriction fragment length polymorphism.
Quantitative synthesis
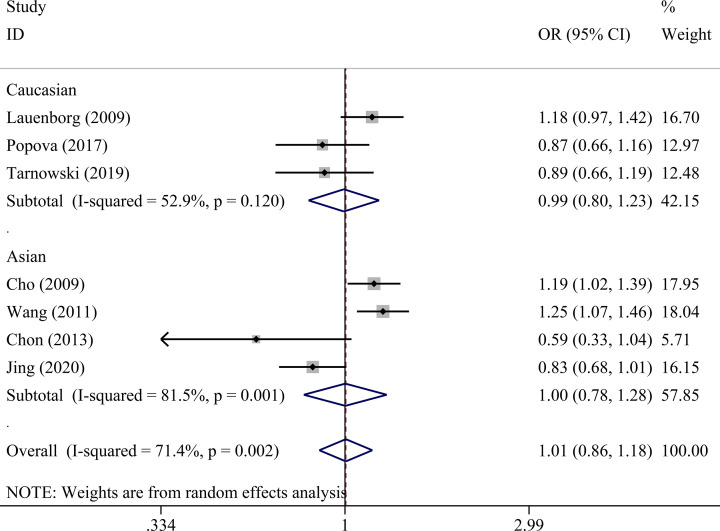
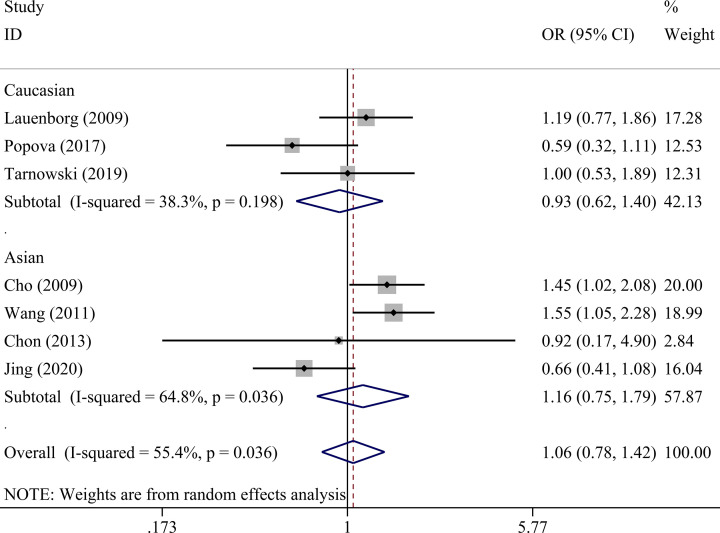
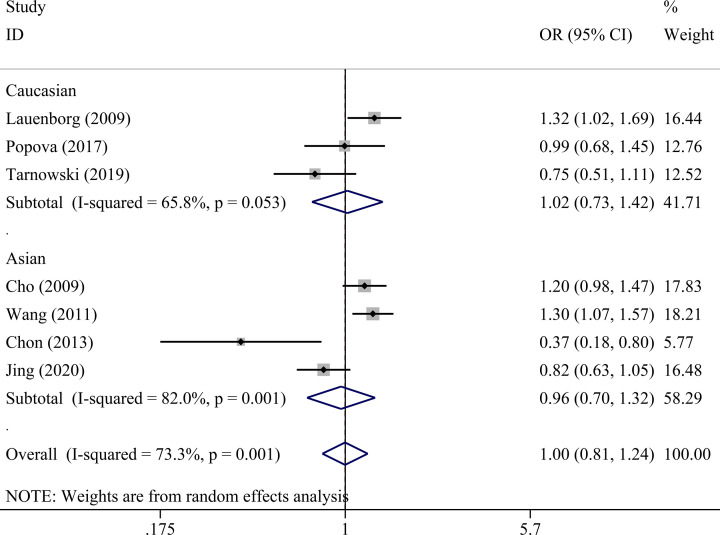
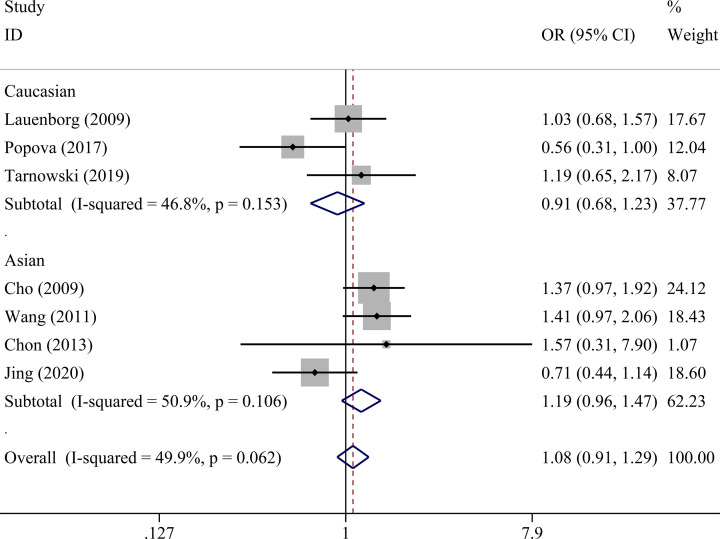
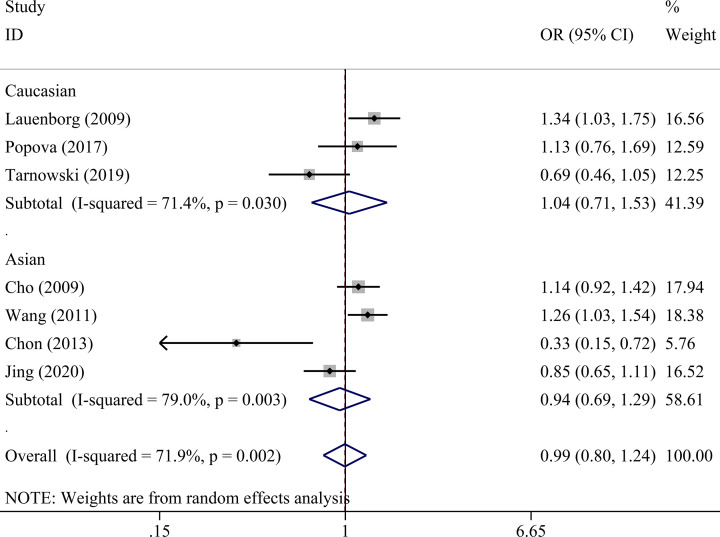
There was no statistically significant association between IGF2BP2 rs4402960 polymorphism and GDM within five genetic models as shown in Table 4: allele (OR = 1.01, 95% CI = 0.86–1.18), dominant (OR = 1.00, 95% CI = 0.81–1.24), recessive (OR = 1.08, 95% CI = 0.91–1.29), heterozygous (OR = 0.99, 95% CI = 0.80–1.24), and homozygous models (OR = 1.06, 95% CI = 0.78–1.42). Subgroup analysis was conducted to reveal some details regarding potential associations between IGF2BP2 rs4402960 polymorphism and GDM risk. No association was observed in five genetic models in each subgroup (Table 5 and Figures 2–6).
Table 4. Meta-analysis of association between IGF2BP2 rs4402960 polymorphisms and risk of gestational diabetes mellitus.
| Comparison | Type of model | Test of association | Test of heterogeneity | Statistical model | Test of publication bias Egger's, P value | Sensitivity analysis | |||
|---|---|---|---|---|---|---|---|---|---|
| Odds ratio (95% CI) | P | I2 (%) | P | Odds ratio (95% CI) min | Odds ratio (95% CI) max | ||||
| T vs. G allele | Allele | 1.01 (0.86–1.18) | 0.950 | 71.4 | 0.002 | Random | 0.072 | 0.96 (0.80–1.14) | 1.06 (0.91–1.23) |
| TT+GT vs. GG | Dominant | 1.00 (0.81–1.24) | 0.986 | 73.3 | 0.001 | Random | 0.072 | 0.94 (0.73–1.20) | 1.07 (0.89–1.28) |
| TT vs. GG+GT | Recessive | 1.08 (0.91–1.29) | 0.368 | 49.9 | 0.062 | Fixed | 0.368 | 0.99 (0.81–1.22) | 1.17 (0.97–1.41) |
| TT vs. GT | Heterozygous | 0.99 (0.80–1.24) | 0.964 | 71.9 | 0.002 | Random | 0.055 | 0.93 (0.72–1.20) | 1.08 (0.90–1.28) |
| TT vs. GG | Homozygous | 1.06 (0.78–1.42) | 0.718 | 55.4 | 0.036 | Random | 0.368 | 0.97 (0.70–1.34) | 1.18 (0.90–1.55) |
Abbreviations: CI, confidence interval; OR, odds ratio.
Table 5. Subgroup analysis of the associations of IGF2BP2 rs4402960 (G>T) polymorphisms with gestational diabetes mellitus.
| Subgroup | N | Allele model (T vs. G allele) | Dominant model (TT+GT vs. GG) | Recessive model (TT vs. GG+GT) | Heterozygous model (TT vs. GT) | Homozygous model (TT vs. GG) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Test of association | Test of heterogeneity | Test of association | Test of heterogeneity | Test of association | Test of heterogeneity | Test of association | Test of heterogeneity | Test of association | Test of heterogeneity | ||||||||||||
| Odds ratio (95% CI) | P | I2 (%) | P | Odds ratio (95% CI) | P | I2 (%) | P | Odds ratio (95% CI) | P | I2 (%) | P | Odds ratio (95% CI) | P | I2 (%) | P | Odds ratio (95% CI) | P | I2 (%) | P | ||
| Genotyping method | |||||||||||||||||||||
| TaqMan assay | 5 | 1.07 (0.91–1.26) | 0.410 | 71.7 | 0.007 | 1.08 (0.87–1.33) | 0.491 | 72.0 | 0.006 | 1.15 (0.96–1.38) | 0.135 | 37.3 | 0.173 | 1.06 (0.87–1.30) | 0.552 | 67.4 | 0.015 | 1.16 (0.86–1.56) | 0.321 | 54.6 | 0.066 |
| PCR-RFLP | 2 | 0.78 (0.55–1.11) | 0.164 | 33.1 | 0.222 | 0.65 (0.25–1.67) | 0.368 | 80.4 | 0.024 | 0.64 (0.37–1.10) | 0.107 | 28.6 | 0.237 | 0.64 (0.19–2.14) | 0.468 | 86.8 | 0.006 | 0.63 (0.35–1.12) | 0.115 | 0 | 0.628 |
| Ethnicity | |||||||||||||||||||||
| Caucasian | 3 | 0.99 (0.80–1.23) | 0.949 | 52.9 | 0.120 | 1.02 (0.73–1.42) | 0.906 | 65.8 | 0.053 | 0.91 (0.68–1.23) | 0.550 | 46.8 | 0.153 | 1.04 (0.71–1.53) | 0.837 | 71.4 | 0.030 | 0.93 (0.62–1.40) | 0.722 | 38.3 | 0.198 |
| Asian | 4 | 1.00 (0.78–1.28) | 0.988 | 81.5 | 0.001 | 0.96 (0.70–1.32) | 0.810 | 82.0 | 0.001 | 1.19 (0.96–1.47) | 0.120 | 50.9 | 0.106 | 0.94 (0.69–1.29) | 0.722 | 79.0 | 0.003 | 1.16 (0.75–1.79) | 0.510 | 64.8 | 0.036 |
| Control source | |||||||||||||||||||||
| Hospital-based | 6 | 0.96 (0.80–1.16) | 0.691 | 73.4 | 0.002 | 0.94 (0.72–1.23) | 0.675 | 76.7 | 0.001 | 0.99 (0.80–1.22) | 0.953 | 48.2 | 0.086 | 0.95 (0.92–1.25) | 0.703 | 76.3 | 0.001 | 0.97 (0.69–1.37) | 0.874 | 54.0 | 0.054 |
| Population-based | 1 | 1.19 (1.02–1.39) | 0.031 | – | – | 1.20 (0.98–1.47) | 0.084 | – | – | 1.37 (0.97–1.92) | 0.073 | – | – | 1.14 (0.92–1.42) | 0.231 | – | – | 1.45 (1.02–2.08) | 0.039 | – | – |
Abbreviations: CI, confidence interval; HWE, Hardy–Weinberg equilibrium; OR, odds ratio; PCR-RFLP, polymerase chain reaction-restriction fragment length polymorphism.
Figure 2. Forest plot for the association between IGF2BP2 rs4402960 polymorphism and GDM risk using ethnicity subgroup analysis in the allele model (T vs. G allele).
Figure 6. Forest plot for the association between IGF2BP2 rs4402960 polymorphism and GDM risk using ethnicity subgroup analysis in the homozygous model (TT vs. GG).
Figure 3. Forest plot for the association between IGF2BP2 rs4402960 polymorphism and GDM risk using ethnicity subgroup analysis in the dominant model (TT+GT vs. GG).
Figure 4. Forest plot for the association between IGF2BP2 rs4402960 polymorphism and GDM risk using ethnicity subgroup analysis in the recessive model (TT vs. GG+GT).
Figure 5. Forest plot for the association between IGF2BP2 rs4402960 polymorphism and GDM risk using ethnicity subgroup analysis in the heterozygous model (TT vs. GT).
Sensitivity analysis and publication bias
There was no change after the deletion of any of the studies involved in the investigation, and no publication bias was detected (Table 4).
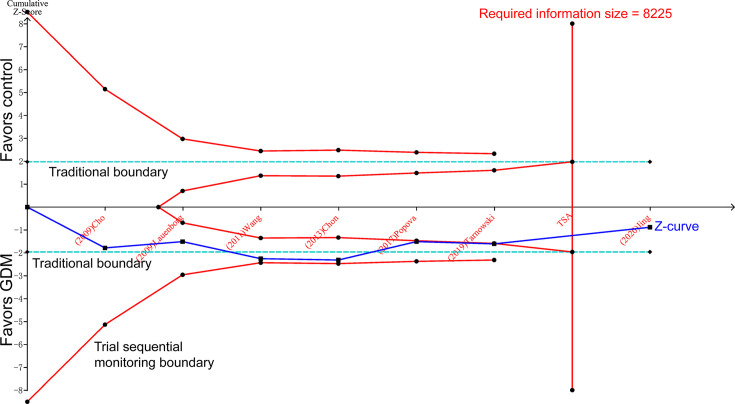
Trial sequential analysis
The cumulative Z-curve passed both the traditional boundary and required information size line, indicating sufficient proof of such association within the overall population (Figure 7).
Figure 7. Trial sequential analysis of GDM risk associated with IGF2BP2 rs4402960 polymorphism in the recessive model.
Discussion
Our case–control study did not reveal any association between IGF2BP2 rs4402960 polymorphism and GDM risk. Further meta-analysis involving seven studies indicated that IGF2BP2 rs4402960 polymorphism was not associated with GDM risk in overall population, Caucasian, or Asian under various genetic models. TSA analysis indicated that the pooled sample size was sufficient to support these null associations.
IGF2BP2 belongs to a mRNA-binding protein family that plays important roles in RNA localization, stability, and translation [15]. IGF2BP2 is highly expressed in pancreatic islets and binds to IGF2, which is an important growth and insulin signaling molecule [15]. Previous studies have shown the involvement of IGF2BP2 gene polymorphism in attenuating the first phase of glucose-stimulated insulin secretion based on hyperglycaemic clamps, and the regulation of pancreatic β-cell function in Type 2 diabetes mellitus (T2DM) patients [16]. Therefore, the IGF2BP2 rs4402960 polymorphism plays a role in pathogenesis of T2DM [17]. T2DM and GDM share a similar genetic background due to the common family history and similar features of impaired glucose tolerance between these two diseases [18]. Several T2DM-associated genetic variants have been confirmed to be associated with GDM, such as TCF7L2 rs12255372 and MTNR1B rs10830963 [18] while the effects of other T2DM-associated genetic variants on GDM are controversial, such as WFS1 rs10010131 and ABCA1 rs1800977 [11,19].
Early studies showed that IGF2BP2 rs4402960 polymorphism was associated with GDM risk [11,12,20]. This finding has been cited in previously published reports [18,21]. However, recent studies revealed there was no association between IGF2BP2 rs4402960 polymorphism with GDM [10,22]. Results reported to date are inconsistent. This could be due to limited statistical power with individual studies having relatively small sample sizes and the analysis of a specific ethnicity. For many of these studies, meta-analysis was usually performed in terms of genetic association studies of complicated diseases. Therefore, to further investigate the influence of IGF2BP2 rs4402960 polymorphism on GDM risk, a meta-analysis of six previously published reports in combination with the present results of the case–control study was conducted. As far as we know, this is the first meta-analysis to date comprehensively quantified the association between the IGF2BP2 and GDM risk.
However, meta-analysis has its potential limitation, resulting in spuriously overestimated (type I errors) or spuriously underestimated (type II errors) effects [23]. As a complement of meta-analysis, TSA was designed. TSA could reduce the risk of type I error by estimation of required information size with an adjusted threshold for statistical significance, and estimate whether further additional trials are needed [24]. If the cumulative Z-curve touches the trial sequential monitoring boundary, the futility boundary, or the required information size line, it shows firm evidence for such study. If not, additional studies are necessary to reach a consistent conclusion [24].
In our TSA results, the cumulative Z-curve crosses the traditional boundary using the Cho, Lauenborg, Wang, and Chon’s studies [11,12,20,25]. However, sample size of these four studies is not large enough. Two former meta-analyses ended up with the false positive conclusion due to the earlier publish time with insufficient sample size [21,26]. Utilizing the data reported in recent studies, the cumulative Z-curve goes back to the zone between two traditional boundaries, then crosses the futility boundary and reaches the required information size line. Until now, there is sufficient evidence to support the null association between IGF2BP2 rs4402960 polymorphism and GDM risk. This trend of cumulative Z-curve explains the TSA advantage, which helps us avoid reaching a premature statistical significance conclusion [23].
Limitation
There were limitations in the present study. First, the sample size in each subgroup was small. Second, there was no study that includes the Blacks or Hispanics.
Conclusion
This meta-analysis provides sufficient statistical evidence indicating null association between IGF2BP2 rs4402960 polymorphism and GDM risk.
Supplementary Material
Acknowledgements
We gratefully acknowledge the assistance of Frederick W.K. Kan, Professor of Department of Biomedical and Molecular Sciences, Queen's University, for refining the manuscript.
Abbreviations
- BMI
body mass index
- CI
confidence interval
- DM
diabetes mellitus
- GDM
gestational diabetes mellitus
- HWE
Hardy–Weinberg equilibrium
- IGF2BP2
insulin-like growth factor 2 mRNA-binding protein 2
- NOS
Newcastle–Ottawa Scale
- OR
odds ratio
- PCR-RFLP
polymerase chain reaction-restriction fragment length polymorphism
- T2DM
Type 2 diabetes mellitus
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China [grant number 81871173].
Author Contribution
J.L., G.S., and T.M. conceived and designed the meta-analysis. J.L., G.Z., and T.M. performed the literature search. G.S. analyzed the data. L.J. and T.M. wrote the paper.
References
- 1.Sacks D.A., Hadden D.R., Maresh M., Deerochanawong C., Dyer A.R., Metzger B.E. et al. (2012) Frequency of gestational diabetes mellitus at collaborating centers based on IADPSG consensus panel-recommended criteria: the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study. Diabetes Care 35, 526–528 10.2337/dc11-1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosik J., Szostak B., Machaj F. and Pawlik A. (2020) The role of genetics and epigenetics in the pathogenesis of gestational diabetes mellitus. Ann. Hum. Genet. 84, 114–124 10.1111/ahg.12356 [DOI] [PubMed] [Google Scholar]
- 3.Gu L., Shigemasa K. and Ohama K. (2004) Increased expression of IGF II mRNA-binding protein 1 mRNA is associated with an advanced clinical stage and poor prognosis in patients with ovarian cancer. Int. J. Oncol. 24, 671–678 [PubMed] [Google Scholar]
- 4.Palmer N.D., Goodarzi M.O., Langefeld C.D., Ziegler J., Norris J.M., Haffner S.M. et al. (2008) Quantitative trait analysis of type 2 diabetes susceptibility loci identified from whole genome association studies in the Insulin Resistance Atherosclerosis Family Study. Diabetes 57, 1093–1100 10.2337/db07-1169 [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez S., Eiriksdottir G., Gaunt T.R., Harris T.B., Launer L.J., Gudnason V. et al. (2010) IGF2BP1, IGF2BP2 and IGF2BP3 genotype, haplotype and genetic model studies in metabolic syndrome traits and diabetes. Growth Hormone IGF Res.: Off. J. Growth Hormone Res. Soc. Int. IGF Res. Soc. 20, 310–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rao P., Wang H., Fang H., Gao Q., Zhang J., Song M. et al. (2016) Association between IGF2BP2 Polymorphisms and Type 2 Diabetes Mellitus: A Case-Control Study and Meta-Analysis. Int. J. Environ. Res. Public Health 13, 574 10.3390/ijerph13060574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim C., Newton K.M. and Knopp R.H. (2002) Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care 25, 1862–1868 10.2337/diacare.25.10.1862 [DOI] [PubMed] [Google Scholar]
- 8.Williams M.A., Qiu C., Dempsey J.C. and Luthy D.A. (2003) Familial aggregation of type 2 diabetes and chronic hypertension in women with gestational diabetes mellitus. J. Reprod. Med. 48, 955–962 [PubMed] [Google Scholar]
- 9.Xie K., Chen T., Zhang Y., Wen J., Cui X., You L. et al. (2019) Association of rs10830962 polymorphism with gestational diabetes mellitus risk in a Chinese population. Sci. Rep. 9, 5357 10.1038/s41598-019-41605-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tarnowski M., Bujak J., Kopytko P., Majcher S., Ustianowski P., Dziedziejko V. et al. (2019) Effect of FTO and IGF2BP2 gene polymorphisms on duration of pregnancy and Apgar scores in women with gestational diabetes. J. Diabetes Res. 39, 151–156 [DOI] [PubMed] [Google Scholar]
- 11.Lauenborg J., Grarup N., Damm P., Borch-Johnsen K., Jorgensen T., Pedersen O. et al. (2009) Common type 2 diabetes risk gene variants associate with gestational diabetes. J. Clin. Endocrinol. Metab. 94, 145–150 10.1210/jc.2008-1336 [DOI] [PubMed] [Google Scholar]
- 12.Cho Y.M., Kim T.H., Lim S., Choi S.H., Shin H.D., Lee H.K. et al. (2009) Type 2 diabetes-associated genetic variants discovered in the recent genome-wide association studies are related to gestational diabetes mellitus in the Korean population. Diabetologia 52, 253–261 10.1007/s00125-008-1196-4 [DOI] [PubMed] [Google Scholar]
- 13.Liu J., Song G., Meng T. and Zhao G. (2020) Epicardial adipose tissue thickness as a potential predictor of gestational diabetes mellitus: a prospective cohort study. BMC Cardiovasc. Disord. 20, 184 10.1186/s12872-020-01480-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weinert L.S. (2010) International Association of Diabetes and Pregnancy Study Groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy: comment to the International Association of Diabetes and Pregnancy Study Groups Consensus Panel. Diabetes Care 33, e97, author reply e98 10.2337/dc10-0544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Christiansen J., Kolte A.M., Hansen T. and Nielsen F.C. (2009) IGF2 mRNA-binding protein 2: biological function and putative role in type 2 diabetes. J. Mol. Endocrinol. 43, 187–195 10.1677/JME-09-0016 [DOI] [PubMed] [Google Scholar]
- 16.Groenewoud M.J., Dekker J.M., Fritsche A., Reiling E., Nijpels G., Heine R.J. et al. (2008) Variants of CDKAL1 and IGF2BP2 affect first-phase insulin secretion during hyperglycaemic clamps. Diabetologia 51, 1659–1663 10.1007/s00125-008-1083-z [DOI] [PubMed] [Google Scholar]
- 17.Rao P., Wang H., Fang H., Gao Q., Zhang J., Song M. et al. (2016) Association between IGF2BP2 Polymorphisms and Type 2 Diabetes Mellitus: A Case-Control Study and Meta-Analysis. Int. J. Environ. Res. Public Health 13, 574 10.3390/ijerph13060574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu S., Liu Y. and Liao S. (2019) Heterogeneous impact of type 2 diabetes mellitus-related genetic variants on gestational glycemic traits: review and future research needs. Mol. Genet. Genom.: MGG 294, 811–847 10.1007/s00438-019-01552-0 [DOI] [PubMed] [Google Scholar]
- 19.Ghafar M.T.A., Shalaby K.H., Okda H.I. and Rizk F.H.J.M.G. (2020) Association of ABCA1 (C69T) gene polymorphism with dyslipidemia and type 2 diabetes among the Egyptian population. Meta. Gene. 100714 10.1016/j.mgene.2020.100714 [DOI] [Google Scholar]
- 20.Wang Y., Nie M., Li W., Ping F., Hu Y., Ma L. et al. (2011) Association of six single nucleotide polymorphisms with gestational diabetes mellitus in a Chinese population. PLoS ONE 6, e26953 10.1371/journal.pone.0026953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mao H., Li Q. and Gao S. (2012) Meta-analysis of the relationship between common type 2 diabetes risk gene variants with gestational diabetes mellitus. PLoS ONE 7, e45882 10.1371/journal.pone.0045882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Popova P.V., Klyushina A.A., Vasilyeva L.B., Tkachuk A.S., Bolotko Y.A., Gerasimov A.S. et al. (2017) Effect of gene-lifestyle interaction on gestational diabetes risk. Oncotarget 8, 112024–112035 10.18632/oncotarget.22999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wetterslev J., Jakobsen J.C. and Gluud C. (2017) Trial Sequential Analysis in systematic reviews with meta-analysis. BMC Med. Res. Method. 17, 39 10.1186/s12874-017-0315-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wetterslev J., Thorlund K., Brok J. and Gluud C. (2008) Trial sequential analysis may establish when firm evidence is reached in cumulative meta-analysis. J. Clin. Epidemiol. 61, 64–75 10.1016/j.jclinepi.2007.03.013 [DOI] [PubMed] [Google Scholar]
- 25.Chon S.J., Kim S.Y., Cho N.R., Min D.L., Hwang Y.J. and Mamura M. (2013) Association of Variants in PPARγ2, IGF2BP2, and KCNQ1 with a susceptibility to gestational diabetes mellitus in a Korean population. Yonsei Med. J. 54, 352–357 10.3349/ymj.2013.54.2.352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu L., Cui L., Tam W.H., Ma R.C. and Wang C.C. (2016) Genetic variants associated with gestational diabetes mellitus: a meta-analysis and subgroup analysis. Sci. Rep. 6, 30539 10.1038/srep30539 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.