Abstract
Aims/Introduction
Uric acid is synthesized by oxidation of hypoxanthine and xanthine using a catalyzing enzyme, xanthine oxidoreductase (XOR), which can be a source of reactive oxygen species. Plasma XOR activity is a metabolic biomarker associated with obesity, hyperuricemia, liver dysfunction and insulin resistance. However, it has recently been reported that XOR activity in fat tissue is low in humans, unlike in rodents, and that hypoxanthine is secreted from human fat tissue.
Materials and Methods
The associations of obesity with hypoxanthine, xanthine and plasma XOR activity were investigated in 484 participants (men/women: 224/260) of the Tanno‐Sobetsu Study.
Results
Levels of hypoxanthine, xanthine and plasma XOR activity were significantly higher in men than in women. In 59 participants with hyperuricemia, 11 (men/women: 11/0) participants were being treated with an XOR inhibitor and had a significantly higher level of xanthine, but not hypoxanthine, than that in participants without treatment. In all of the participants, hypoxanthine concentration in smokers was significantly higher than that in non‐smokers. Stepwise and multivariate regression analyses showed that body mass index, smoking habit and xanthine were independent predictors of hypoxanthine after adjustment of age, sex and use of antihyperuricemic drugs. Whereas, alanine transaminase, hypoxanthine and plasma XOR activity were independent predictors for xanthine, and alanine transaminase, triglycerides and xanthine were independent predictors for plasma XOR activity.
Conclusions
The concentration of hypoxanthine, but not that of xanthine, is independently associated with obesity and smoking habit, indicating differential regulation of hypoxanthine and xanthine in a general population.
Keywords: Purine metabolism, Salvage pathway, Xanthine oxidoreductase
In the present study, we investigated the associations of obesity with hypoxanthine, xanthine and plasma xanthine oxidoreductase activity in 484 participants from the general population. The concentration of hypoxanthine was independently associated with body mass index and smoking habit, whereas the level of xanthine was associated with parameters of mainly purine metabolism in the liver, including alanine transaminase, hypoxanthine and plasma xanthine oxidoreductase activity, indicating differential regulation of hypoxanthine and xanthine in a general population. In obesity, human adipose tissue would be a source of hypoxanthine as a substrate of xanthine oxidoreductase in the purine metabolism pathway.

Introduction
Hyperuricemia is closely associated with obesity and metabolic disturbances, such as insulin resistance, dyslipidemia, hypertension and cardiovascular diseases1, 2, 3. In the purine metabolism pathway, uric acid is synthesized by oxidation of hypoxanthine and xanthine using a catalyzing enzyme, xanthine oxidoreductase (XOR)4. XOR is inducted as xanthine dehydrogenase, which catalyzes the reduction of oxidized nicotinamide adenine dinucleotide (NAD+) to reduced nicotinamide adenine dinucleotide (NADH), and is post‐translationally converted to xanthine oxidase, which produces hydrogen peroxide and superoxide by using oxygen4. Therefore, XOR can be an important source of reactive oxygen species, and it contributes to the development of oxidative stress‐associated tissue disturbances5.
Plasma hypoxanthine is an extracellular molecule that reflects intracellular energy metabolism6, leading to a marker of hypoxia in tissue7, 8 and free radical formation after reperfusion9. Therefore, plasma hypoxanthine is used as a tool for the diagnosis of hypoxia‐related diseases, including cardiovascular disease, respiratory disease and hemolytic disorders10. Hypoxanthine is released from cells under a condition of hypoxia into the blood circulation11, 12, 13, and is finally incorporated into the liver and metabolized to uric acid14. As production of hypoxanthine in several tissues, including fat tissue, contributes to the production of uric acid through XOR, it has been suggested that the disturbance of purine catabolism in several tissues other than the liver as a main organ is, at least in part, related to free radical formation as well as hyperuricemia15.
As activity of plasma XOR is much lower in humans than in animals16, it has been difficult to accurately measure the activity in humans. An accurate and sensitive assay for activity of plasma XOR in humans has been newly developed using liquid chromatography and triple quadrupole mass spectrometry to measure [13C2, 15N2]‐uric acid using [13C2, 15N2]‐xanthine as a substrate17. Using this assay, we and others have shown that activity of plasma XOR is a novel metabolic biomarker associated with obesity, insulin resistance, hyperuricemia, liver dysfunction and adipokines18, 19, 20, 21. Furthermore, we showed unexpected high activity of plasma XOR in some hyperuricemic patients by using an XOR inhibitor19 and in some female relatively hypouricemic patients 22.
XOR is strongly expressed in adipose tissue of murine models, and formation of uric acid can be increased in obesity‐related insulin resistance23. In our previous study carried out with 627 participants, activity of plasma XOR was found to be independently associated with body mass index (BMI) and uric acid in a general population19. However, it has recently been reported that activity of XOR in human fat tissue is much lower than that in mouse fat tissue and that in the mouse liver15. Furthermore, hypoxanthine is secreted from human fat tissue, particularly under a hypoxia condition15. In addition, a previous study showed that plasma hypoxanthine levels were significantly lower in lean individuals (n = 16) than in obese individuals (n = 7)13. These findings suggest that human fat tissue might be a source of hypoxanthine as a substrate of XOR, but not a source of XOR itself, in the purine catabolism pathway. In the present study, we investigated the significance and association of hypoxanthine, xanthine and plasma XOR activity in a general population.
Methods
Study participants
In our population‐based cohort, the Tanno‐Sobetsu Study, 605 Japanese individuals (men/women: 280/325) of Sobetsu Town underwent annual examinations in 2017. Among them, 121 individuals (men/women: 56/65) were excluded, as the time until blood processing of centrifugal plasma separation was >3 h, which might affect concentrations of hypoxanthine and xanthine by leakage from erythrocytes24, 25, 26. A total of 484 individuals (men/women: 224/260) were enrolled in the present study. This study was approved by the ethical committee of Sapporo Medical University, and was carried out under the principles of the Declaration of Helsinki. Written informed consent was received from all of the participants.
Measurements
Medical health checkups, including measurements of biochemical parameters and blood pressure, and calculation of BMI, estimated glomerular filtration rate (eGFR) and homeostasis model assessment of insulin resistance (HOMA‐IR) were carried out as previously described19. Hemoglobin A1c (HbA1c) level was presented as the National Glycohemoglobin Standardization Program equivalent value.
Hypertension was diagnosed as the use of drugs for hypertension, systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg. Diabetes mellitus was diagnosed as the use of drugs for diabetes mellitus or a combination of fasting glucose ≥126 mg/dL and HbA1c ≥6.5%. Dyslipidemia was diagnosed as the use of drugs for dyslipidemia, low‐density lipoprotein cholesterol ≥140 mg/dL, triglycerides ≥150 mg/dL or high‐density lipoprotein (HDL) cholesterol <40 mg/dL. Hyperuricemia was diagnosed as the use of drugs for hyperuricemia or uric acid >7 mg/dL.
Concentrations of hypoxanthine and xanthine
Blood samples were obtained using a collection tube coated with ethylenediaminetetraacetic acid‐2K, and they were kept at 4°C until centrifugation. For plasma separation, the samples were centrifuged at 2,000 g for 10 min at 4°C. Plasma concentrations of xanthine and hypoxanthine were determined as previously reported26. In brief, plasma samples were added to methanol containing [13C2, 15N2] xanthine and [13C3, 15N] hypoxanthine as internal controls, and were centrifuged at 3,000 g for 15 min at 4°C. The supernatant (40 μL) was diluted with 160 μL distilled water, and concentrations of xanthine and hypoxanthine were measured using liquid chromatography and triple quadrupole mass spectrometry (Nexera SCIEX QTRAP 4500; SHIMADZU, Kyoto, Japan).
Measurement of plasma XOR activity
Activity of XOR in plasma was determined by liquid chromatography and triple quadrupole mass spectrometry to detect [13C2, 15N2]‐uric acid using [13C2, 15N2]‐xanthine as a substrate as previously reported17, 19. The lower detection limit was 6.67 pmol/h/mL plasma, and coefficients of variation in intra‐assay and interassay were 6.5 and 9.1%, respectively17.
Statistical analysis
Numeric values are shown as the mean ± standard deviation for normal distributions or medians (interquartile ranges) for skewed variables. The distribution normality for each parameter was analyzed using the Shapiro–Wilk W‐test. Skewed parameters were logarithmically transformed for regression analyses. Intergroup differences in percentages of demographic values were analyzed by the χ2‐test. Comparison between two groups was analyzed using Student’s t‐test for parametric parameters, and the Mann–Whitney U‐test for non‐parametric parameters. One‐way analysis of variance and the Tukey–Kramer post‐hoc test for parametric parameters, and the Kruskal–Wallis test and the Steel–Dwass post‐hoc test for non‐parametric parameters were carried out for testing significant differences in variables between multiple groups. The correlation between two values was carried out using Pearson’s correlation coefficient. Stepwise and subsequent multivariate regression analyses were carried out to show independent parameters of hypoxanthine, xanthine, plasma XOR activity and uric acid using age, sex, use of antihyperuricemic drugs and variables with a significant correlation as independent predictors after consideration of multicollinearity based on the Akaike information criterion, showing the percentage of variance in the object variables that the chosen independent values explained (R 2), unstandardized regression coefficient and standard error of regression coefficient, the t‐ratio calculated as the ratio of regression coefficient and standard error, and the standardized regression coefficient (β). A P < 0.05 was considered to be statistically significant. All statistical analyses were determined by using JMP for Macintosh (SAS Institute, Cary, NC, USA).
Results
Basal characteristics of the participants
The characteristics of the 484 recruited participants (men/women: 224/260, mean age 65 ± 15 years) are shown in Table 1. Hypertension, dyslipidemia, diabetes mellitus and hyperuricemia were found in 276 (57%), 265 (54.8%), 59 (12.2%) and 59 (12.2%) participants, respectively. Male participants had significantly larger waist circumference and BMI; significantly higher frequencies of hyperuricemia, and drinking and smoking habits; higher levels of aspartate transaminase (AST), alanine aminotransferase (ALT), γ‐glutamyl transpeptidase (γGTP), blood urea nitrogen (BUN), creatinine, uric acid, triglycerides, fasting glucose, hypoxanthine, xanthine and activity of plasma XOR; and lower levels of total cholesterol, low‐density lipoprotein cholesterol and HDL cholesterol than did female participants. There was no significant sex difference in age, blood pressure, eGFR, HbA1c, insulin or HOMA‐IR.
Table 1.
Participant characteristics
| Total (n = 484) | Men (n = 224) | Women (n = 260) | P | |
|---|---|---|---|---|
| Age (years) | 65 ± 15 | 65 ± 15 | 65 ± 15 | 0.910 |
| Body mass index (kg/m2) | 23.4 ± 3.9 | 24.0 ± 3.7 | 22.9 ± 4.1 | 0.001 |
| Waist circumference (cm) | 85.1 ± 11.1 | 86.8 ± 10.6 | 83.6 ± 11.2 | 0.002 |
| Systolic blood pressure (mmHg) | 137 ± 21 | 137 ± 18 | 138 ± 23 | 0.536 |
| Diastolic blood pressure (mmHg) | 77 ± 11 | 77 ± 11 | 76 ± 11 | 0.144 |
| Smoking habit | 75 (15.5) | 50 (22.3) | 25 (9.6) | <0.001 |
| Alcohol drinking habit | 200 (41.3) | 129 (57.6) | 71 (27.3) | <0.001 |
| Disease | ||||
| Hypertension | 276 (57.0) | 129 (57.6) | 147 (56.5) | 0.816 |
| Diabetes mellitus | 59 (12.2) | 34 (15.2) | 25 (9.6) | 0.062 |
| Dyslipidemia | 265 (54.8) | 120 (53.6) | 145 (55.8) | 0.628 |
| Hyperuricemia | 59 (12.2) | 51 (22.8) | 8 (3.1) | <0.001 |
| Medications | ||||
| Antihypertensive drugs | 171 (35.3) | 80 (35.7) | 91 (35.0) | 0.870 |
| Antidiabetic drugs | 47 (9.7) | 27 (12.1) | 20 (7.7) | 0.106 |
| Antidyslipidemic drugs | 103 (21.3) | 39 (17.4) | 64 (24.6) | 0.054 |
| Antihyperuricemic drugs | 14 (2.9) | 12 (5.4) | 2 (0.8) | 0.003 |
| Biochemical data | ||||
| AST (IU/L) | 24 (21–28) | 25 (21–30) | 23 (20–26) | <0.001 |
| ALT (IU/L) | 19 (15–25) | 23 (17–30) | 17 (14–22) | <0.001 |
| γGTP (IU/L) | 22 (16–34) | 28 (20–42) | 18 (15–26) | <0.001 |
| Blood urea nitrogen (mg/dL) | 16 ± 5 | 17 ± 5 | 15 ± 4 | <0.001 |
| Creatinine (mg/dL) | 0.8 (0.7–0.9) | 0.9 (0.8–1.0) | 0.7 (0.6–0.8) | <0.001 |
| eGFR (mL/min/1.73 m2) | 67 ± 14 | 69 ± 16 | 66 ± 13 | 0.052 |
| Uric acid (mg/dL) | 5.3 ± 1.3 | 6.0 ± 1.2 | 4.8 ± 1.1 | <0.001 |
| Total cholesterol (mg/dL) | 207 ± 36 | 199 ± 34 | 214 ± 36 | <0.001 |
| LDL cholesterol (mg/dL) | 119 ± 29 | 115 ± 29 | 123 ± 29 | 0.002 |
| HDL cholesterol (mg/dL) | 65 ± 19 | 59 ± 16 | 71 ± 20 | <0.001 |
| Triglycerides (mg/dL) | 91 (67–122) | 95 (68–134) | 88 (64–109) | 0.006 |
| Fasting glucose (mg/dL) | 95 (88–104) | 98 (90–108) | 93 (87–101) | <0.001 |
| Insulin (µU/mL) | 4.9 (3.3–7.0) | 4.9 (3.3–7.5) | 4.9 (3.4–6.9) | 0.707 |
| HOMA‐IR | 0.39 (0.29–0.51) | 0.40 (0.30–0.52) | 0.38 (0.28–0.48) | 0.110 |
| HbA1c (%) | 5.6 (5.3–5.9) | 5.6 (5.4–6.0) | 5.6 (5.3–5.8) | 0.082 |
| Hypoxanthine (µmol/L) | 3.1 ± 1.6 | 3.3 ± 1.7 | 2.9 ± 1.4 | 0.003 |
| Xanthine (µmol/L) | 0.9 (0.7–1.1) | 0.9 (0.7–1.3) | 0.8 (0.7–1.0) | <0.001 |
| XOR activity (pmol/h/mL plasma) | 38.0 (25.2–67.8) | 46.4 (29.6–88.6) | 32.4 (22.6–54.8) | <0.001 |
Variables are expressed as number (%), mean ± standard deviation or median (interquartile range).
γGTP, γ‐glutamyl transpeptidase; ALT, alanine transaminase; AST, aspartate transaminase; eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1c; HOMA‐IR, homeostasis model assessment of insulin resistance; LDL, low‐density lipoprotein; XOR, xanthine oxidoreductase.
Participants treated with an XOR inhibitor
Of 59 participants (men/women: 51/8) with hyperuricemia, 14 (men/women: 12/2) participants were being treated with antihyperuricemic drugs, including XOR inhibitors (men/women: 11/0) and benzbromarone (men/women: 1/2). Characteristics of the 11 men being treated with an XOR inhibitor (febuxostat/allopurinol: 3/8) are shown in Table S1. Among them, plasma XOR activities in three patients (participants #9–11) were more than the upper quartile of the activity (88.6 pmol/h/mL plasma) in male participants, and the three patients had liver dysfunction or a smoking habit, and were being treated for diabetes mellitus and/or dyslipidemia.
When male participants were divided by the absence and presence of hyperuricemia treated with or not treated with an XOR inhibitor, the level of uric acid in hyperuricemic patients treated with an XOR inhibitor (n = 11) was significantly lower than that in hyperuricemic patients without treatment (n = 40), but was still significantly higher than that in participants without hyperuricemia (n = 173; Figure 1a). There was no significant intergroup difference in levels of plasma XOR activity (Figure 1b) or hypoxanthine (Figure 1c). Male participants with hyperuricemia who were being treated with an XOR inhibitor had a significantly higher level of xanthine than did that in participants without treatment and that in participants without hyperuricemia (Figure 1d).
Figure 1.
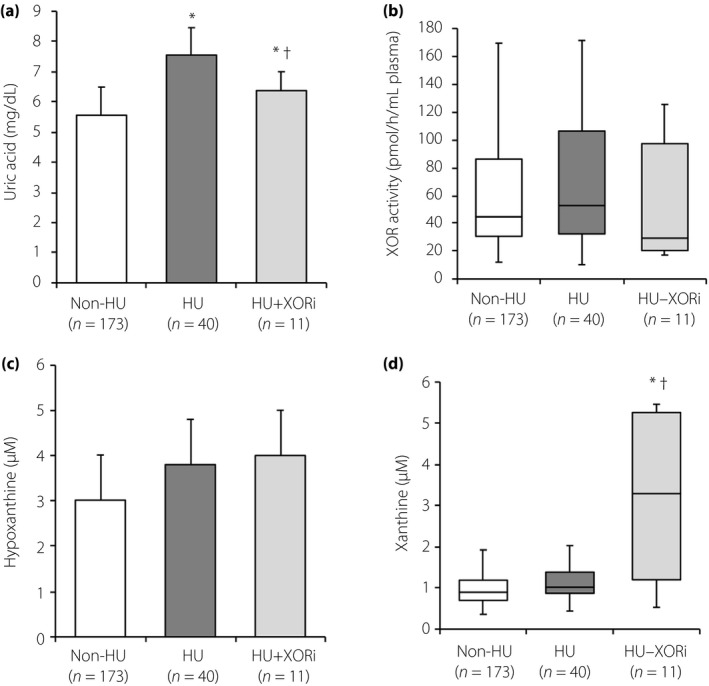
Comparisons of purine metabolism‐related parameters in male subjects treated with and without an xanthine oxidoreductase inhibitor (XORi). (a–d) Comparisons of (a) uric acid, (b) plasma xanthine oxidoreductase (XOR) activity, (c) hypoxanthine and (d) xanthine in male participants without hyperuricemia (Non‐HU, n = 173) and male patients with hyperuricemia in the absence (HU, n = 40) and the presence (HU‐XORi, n = 11) of treatment with an XOR inhibitor. *P < 0.05 versus non‐HU, † P < 0.05 versus HU.
In women, uric acid level in participants with hyperuricemia (n = 8) was significantly higher than that in participants without hyperuricemia (n = 252; Figure S1a). There was no significant difference between participants with and those without hyperuricemia in levels of plasma XOR activity (Figure S1b), hypoxanthine (Figure S1c) or xanthine (Figure S1d).
Comparisons of levels of hypoxanthine, xanthine and plasma XOR activity by habits of smoking and alcohol drinking
The concentration of hypoxanthine (Figure S2a), but not the concentration of xanthine (Figure S2b) or plasma XOR activity (Figure S2c), in smokers was significantly higher than that in non‐smokers. Uric acid level was significantly higher in smokers than in non‐smokers (Figure S2d). No significant difference was found between levels of hypoxanthine (Figure S2e), xanthine (Figure S2f) or plasma XOR activity (Figure S2g) in participants with and those without an alcohol drinking habit. Uric acid level was significantly higher in participants with an alcohol drinking habit than in those without the habit (Figure S2h).
Associations of hypoxanthine level with clinical variables
As shown in Table S2, the concentration of hypoxanthine was positively correlated with BMI (Figure 2a), waist circumference and levels of γGTP, ALT, eGFR, triglycerides, uric acid, HOMA‐IR and xanthine (Figure 2b), and was negatively correlated with age and levels of HDL cholesterol and BUN. Similar correlations of hypoxanthine with BMI, waist circumference and xanthine were found when sex was separately tested (Table S2).
Figure 2.
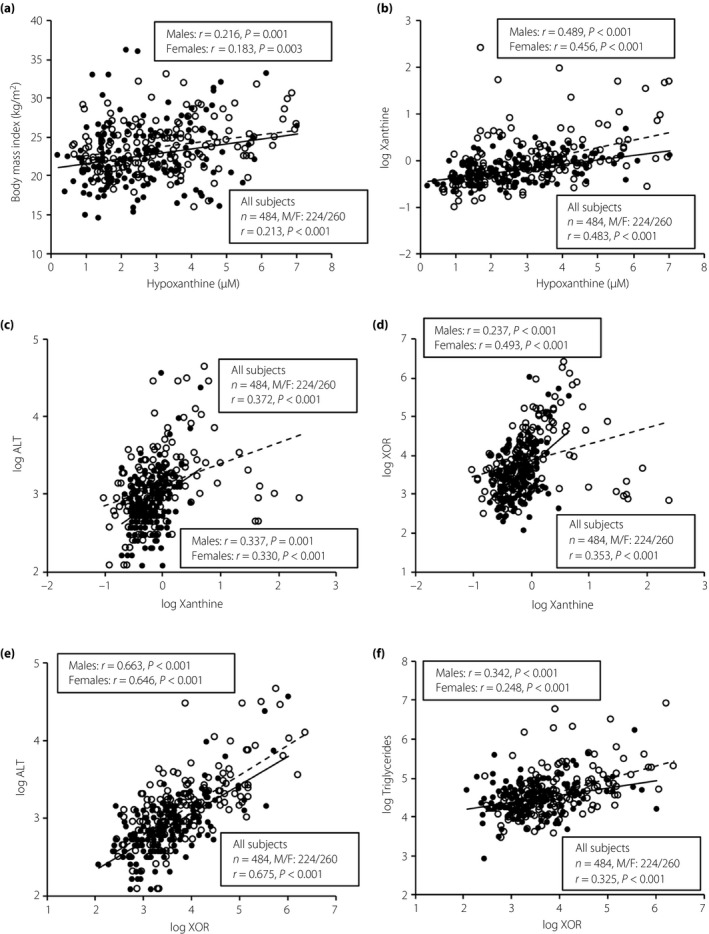
Correlations of hypoxanthine, xanthine and plasma xanthine oxidoreductase (XOR) activity with metabolic parameters. (a) Body mass index and (b) logarithmically transformed (log) xanthine were plotted against the plasma level of hypoxanthine in each participant (n = 484). (c) Log alanine aminotransferase (ALT) and (d) log plasma XOR activity were plotted against log xanthine in each participant. (e) Log ALT and (f) log triglycerides were plotted against log XOR in each participant. Open circles and broken regression line, men (n = 224); closed circles and solid regression line, women (n = 260). M/F, male/female.
Stepwise multivariate regression analyses for hypoxanthine using age, sex, use of antihyperuricemic drugs, smoking habit and the correlated parameters as possible predictors showed that BMI (β = 0.106, P = 0.008), smoking habit (β = 0.118, P = 0.005) and xanthine (β = 0.468, P < 0.001) were independently associated with hypoxanthine after adjustment of age, sex and use of antihyperuricemic drugs (R 2 = 0.307; Table 2).
Table 2.
Multivariate regression analyses for hypoxanthine, xanthine, xanthine oxidoreductase activity and uric acid
| Regression coefficient | SE | Standardized regression coefficient (β) | t | P | |
|---|---|---|---|---|---|
| Hypoxanthine | |||||
| Age | −0.018 | 0.004 | −0.175 | −4.27 | <0.001 |
| Sex (male) | 0.001 | 0.063 | 0.001 | 0.02 | 0.988 |
| Use of antihyperuricemic drugs | −0.098 | 0.185 | −0.021 | −0.53 | 0.595 |
| Body mass index | 0.042 | 0.016 | 0.106 | 2.66 | 0.008 |
| Smoking habit | 0.250 | 0.088 | 0.118 | 2.83 | 0.005 |
| log Xanthine | 1.501 | 0.134 | 0.468 | 11.22 | <0.001 |
| R 2 = 0.307 | |||||
| log Xanthine | |||||
| Age | 0.003 | 0.001 | 0.105 | 2.90 | 0.004 |
| Sex (Male) | 0.017 | 0.018 | 0.034 | 0.92 | 0.357 |
| Use of antihyperuricemic drugs | 0.275 | 0.051 | 0.192 | 5.37 | <0.001 |
| log ALT | 0.173 | 0.052 | 0.163 | 3.30 | 0.001 |
| Hypoxanthine | 0.141 | 0.011 | 0.456 | 12.59 | <0.001 |
| log XOR | 0.130 | 0.029 | 0.215 | 4.51 | <0.001 |
| R 2 = 0.416 | |||||
| log XOR | |||||
| Age | 0.003 | 0.002 | 0.052 | 1.58 | 0.115 |
| Sex (Male) | −0.003 | 0.028 | −0.004 | −0.11 | 0.912 |
| Use of antihyperuricemic drugs | −0.219 | 0.081 | −0.092 | −2.69 | 0.007 |
| log ALT | 1.071 | 0.066 | 0.607 | 16.19 | <0.001 |
| log Triglycerides | 0.179 | 0.052 | 0.119 | 3.41 | 0.001 |
| log Xanthine | 0.198 | 0.061 | 0.119 | 3.22 | 0.001 |
| R 2 = 0.489 | |||||
| Uric acid | |||||
| Age | −0.021 | 0.004 | −0.243 | −5.42 | <0.001 |
| Sex (Male) | 0.505 | 0.050 | 0.396 | 10.09 | <0.001 |
| Use of antihyperuricemic drugs | −0.025 | 0.143 | −0.007 | −0.17 | 0.862 |
| Body mass index | 0.030 | 0.013 | 0.093 | 2.38 | 0.018 |
| log ALT | 0.498 | 0.118 | 0.176 | 4.21 | <0.001 |
| eGFR | −0.031 | 0.004 | −0.352 | −7.66 | <0.001 |
| log Triglycerides | 0.277 | 0.094 | 0.115 | 2.94 | 0.003 |
| R 2 = 0.358 | |||||
ALT, alanine transaminase; eGFR, estimated glomerular filtration rate; SE, standard error; XOR, xanthine oxidoreductase.
Associations of xanthine level with clinical variables
As shown in Table S3, the concentration of xanthine was positively correlated with BMI, waist circumference, diastolic blood pressure, and levels of ALT (Figure 2c), AST, γGTP, creatinine, triglycerides, uric acid, fating glucose, HbA1c and hypoxanthine, and activity of plasma XOR (Figure 2d), and was negatively correlated with level of HDL cholesterol. Similar correlations between the parameters, except diastolic blood pressure, creatinine, HbA1c and HDL cholesterol, were found when sex was separately tested (Table S3).
Stepwise multivariate regression analyses for xanthine using age, sex, use of antihyperuricemic drugs and the correlated parameters as possible determinants showed that use of antihyperuricemic drugs (β = 0.192, P < 0.001), ALT (β = 0.163, P = 0.001), hypoxanthine (β = 0.456, P < 0.001) and plasma XOR activity (β = 0.215, P < 0.001) were independently associated with xanthine after adjustment of age and sex (R 2 = 0.416; Table 2).
Associations of plasma XOR activity with clinical variables
As shown in Table S4, plasma XOR activity was positively correlated with waist circumference, BMI, systolic and diastolic blood pressures, and levels of AST, ALT (Figure 2e), γGTP, uric acid, eGFR, triglycerides (Figure 2f), fating glucose, insulin, HOMA‐IR, HbA1c and xanthine, and was negatively correlated with HDL cholesterol level. Similar correlations between the variables, except systolic and diastolic blood pressures, eGFR, HDL cholesterol, fasting glucose, insulin, HOMA‐IR and HbA1c, were found when sex was separately tested (Table S4).
Stepwise multivariate regression analyses for activity of XOR in plasma using age, sex, use of antihyperuricemic drugs and the correlated parameters as possible determinants showed that use of antihyperuricemic drugs (β = −0.092, P = 0.007), ALT (β = 0.607, P < 0.001), triglycerides (β = 0.119, P = 0.001) and xanthine (β = 0.119, P = 0.001) were independently associated with plasma XOR activity after adjustment of age and sex (R 2 = 0.489; Table 2).
Associations of uric acid with clinical variables
As shown in Table S5, the concentration of uric acid was positively correlated with BMI, waist circumference, diastolic blood pressure and levels of AST, ALT, γGTP, BUN, creatinine, triglycerides, hypoxanthine, xanthine and activity of plasma XOR, and was negatively correlated with levels of eGFR and HDL cholesterol. Similar correlations between the variables, except diastolic blood pressures, BUN, eGFR, HDL cholesterol and hypoxanthine, were found when sex was separately tested (Table S5).
Stepwise multivariate regression analyses for uric acid using age, sex, use of antihyperuricemic drugs, habits of smoking and alcohol drinking, and the correlated parameters as possible predictors showed that BMI (β = 0.093, P = 0.018), ALT (β = 0.176, P < 0.001), eGFR (β = −0.352, P < 0.001) and triglycerides (β = 0.115, P = 0.003) were independently associated with uric acid after adjustment of age, sex and use of antihyperuricemic drugs (R 2 = 0.358; Table 2).
Discussion
The present study showed differential regulation of circulating hypoxanthine and xanthine in a general population. The concentration of hypoxanthine, but not that of xanthine, was independently associated with BMI (β = 0.106, P = 0.008) and smoking habit (β = 0.118, P = 0.005) after adjustment of age, sex and use of antihyperuricemic drugs (Table 2). In contrast, the level of xanthine was independently associated with ALT as a liver enzyme, hypoxanthine as an upstream metabolite of xanthine, and activity of plasma XOR, an enzyme of purine metabolism using xanthine as a substrate, after adjustment of age, sex and the use of antihyperuricemic drugs (Table 2). BMI was not selected as an independent determinant of xanthine or activity of plasma XOR in the present study. Furthermore, men who were being treated with an XOR inhibitor had a significantly higher level of xanthine, but not hypoxanthine, than did that in participants without treatment (Figure 1c, d).
Regarding the association of hypoxanthine with BMI, a similar study using a small number of participants showed that the plasma level of hypoxanthine was significantly higher in participants with obesity (n = 7) than in lean participants (n = 16)13. It has been reported that the tissue content of hypoxanthine in human adipose tissue is higher than the contents of xanthine and uric acid, and that human fat tissue mainly produces hypoxanthine among purine catabolism‐related metabolites15. In a condition of hypoxia, adenosine triphosphate is degraded to hypoxanthine through adenosine diphosphate, adenosine monophosphate, adenosine and inosine25, and hypoxanthine, a nucleobase, is secreted from cells through several transporters, including equilibrative nucleobase transporter 1 and equilibrative nucleoside transporters26, 27, 28, 29. It has also been shown that hypoxia increases the production of hypoxanthine in human adipocytes15. In an obese condition, the amount of adipose tissue, especially in visceral fat, generally increases with an increase in adipocyte size rather than an increase in the number of adipocytes30, 31, and oxygen partial pressure in fat tissues is low in obesity in both mice and humans32, 33, 34, 35, 36, 37. Therefore, hypertrophic visceral adipocytes might be more affected by local hypoxia, leading to hypoxanthine overproduction. An increase in whole body fat mass and local hypoxia in obese fat tissue might boost the production of hypoxanthine from fat tissue.
In the present study, hypoxanthine concentration was found to be independently associated with current habitual smoking, possibly caused by local hypoxia in several lung cells. It has also been reported that nicotine degrades a purine salvage enzyme, hypoxanthine‐guanine phosphoribosyltransferase (HGPRT)38, and that smoking lowers the activity of HGPRT39, suggesting an increase in plasma hypoxanthine levels by inhibition of salvage pathway. In contrast, unlike in a previous study19, there was no significant difference in plasma activities of XOR between participants with and those without a smoking habit in the present study. The discrepant results might be caused by the number of cigarettes smoked and the time of cessation from the cigarette smoked in the recruited participants. Hypoxia might affect hypoxanthine more than XOR activity. The induction of hypoxanthine as a substrate of XOR in the purine metabolism pathway might contribute to the activation of plasma XOR activity and the increase in oxidative stress, eventually leading to uric acid production as an anti‐oxidant molecule.
Men with hyperuricemia who were being treated with an XOR inhibitor did not have a significantly lower plasma XOR activity than that in participants without treatment (Figure 1b). The main reason was that some of the patients (participants #9–11) had high plasma XOR activities, despite treatment with an XOR inhibitor (Table S1). Those participants, who had liver dysfunction or a smoking habit and were being treated for diabetes mellitus and/or dyslipidemia, might be resistant to an XOR inhibitor, as previously reported19.
Both xanthine and hypoxanthine are oxypurines and precursors of uric acid, which are oxidized by XOR, in purine catabolism4. Therefore, XOR inhibitors are thought to increase the upstream metabolites. A previous study showed enhanced purine salvage during administration of allopurinol, an XOR inhibitor, in humans40. The present study showed that men who were being treated with an XOR inhibitor had a significantly higher level of xanthine, but not hypoxanthine, than that in men without treatment. Hypoxanthine is simultaneously metabolized to a purine nucleotide, inosine monophosphate, by HGPRT in the salvage pathway of purine metabolism, which recycles the basic materials for reconstitution of DNA, RNA and purine nucleotides, including adenosine triphosphate, without adenosine triphosphate expenditure, and cooperates with the de novo pathway of purine metabolism4, 6, 41. Normally, approximately 90% of hypoxanthine is reutilized and converted to inosine monophosphate through the salvage pathway25. In hereditary xanthinuria caused by XOR deficiency, hypoxanthinuria is not observed because of the salvage pathway of purine metabolism by HGPRT42, 43. The lack of an increase in hypoxanthine by XOR inhibitors in the present study was probably due to activation of the salvage pathway.
Taken together, the results show the following important effects of XOR inhibitors: (i) a decrease in uric acid for preventing gout; (ii) reduction of XOR activity for subsequent inhibition of reactive oxygen species, including hydrogen peroxide and superoxide; and (iii) energy supply from hypoxanthine through the salvage pathway. Interventional studies using a large number of patients treated with and not treated with an XOR inhibitor are required to clarify the significance of measurements of hypoxanthine, xanthine and plasma XOR activity.
The present study had some limitations. First, whether the results can be generalized to other ethnicities is unclear, as the recruited participants were only Japanese people. Second, the number of cigarettes smoked, passive smoking and regular exercise, which might affect oxypurines and activity of XOR13, 19, were not investigated in the present study. Third, the origins of hypoxanthine, xanthine and uric acid would be from not only tissues including adipose tissue and the liver, but also reaction in plasma. Appropriate assessments of reactions in plasma of oxypurines need to be considered in future analysis. Finally, the time until plasma separation, which might affect plasma XOR activity and concentrations of xanthine and hypoxanthine by leakage from erythrocytes24, 25, varied, as blood samples were obtained in the rural town of Sobetsu, and were transported to and centrifuged in a central laboratory. A previous study using blood samples in a tube coated with ethylenediaminetetraacetic acid‐2K showed that 3 h, but not 6 h, until plasma separation did not cause a significant increase in the level of hypoxanthine or xanthine26. Therefore, participants for whom the time until blood processing was >3 h were excluded from the present study. It has recently been proposed that using a commercially available tube (PAXgene Blood DNA tube; Becton, Dickinson and Company, Franklin Lakes, NJ, USA) for blood collection enables accurate measurements of hypoxanthine and xanthine regardless of the time until plasma separation26.
In conclusion, the concentration of hypoxanthine is independently associated with BMI and smoking habit, whereas the level of xanthine is associated with parameters of mainly purine metabolism in the liver, including ALT, hypoxanthine and plasma XOR activity, indicating differential regulation of hypoxanthine and xanthine in a general population. In obesity, human adipose tissue would be a source of hypoxanthine as a substrate of XOR in the purine metabolism pathway. The reduction of adiposity and cessation of smoking might be novel therapeutic strategies for adipose‐derived hypoxanthine‐mediated metabolic disorders.
Disclosure
T Murase and T Nakamura at Sanwa Kagaku Kenkyusho Co., Ltd. measured levels of hypoxanthine, xanthine and plasma activity of XOR. The authors declare no conflict of interest.
Supporting information
Figure S1 | Comparisons of purine metabolism‐related parameters in female participants with and without hyperuricemia.
Figure S2 | Comparisons of hypoxanthine, xanthine, xanthine oxidoreductase (XOR) activity and uric acid with habits of smoking and alcohol drinking.
Table S1 | Characteristics of 11 participants treated with an xanthine oxidoreductase inhibitor.
Table S2 | Correlation analysis for hypoxanthine.
Table S3 | Correlation analysis for log xanthine.
Table S4 | Correlation analysis for log xanthine oxidoreductase.
Table S5 | Correlation analysis for uric acid.
Acknowledgments
MF has been supported by grants from Japan Society for the Promotion of Science (JSPS) and Gout and Uric Acid Foundation of Japan.
J Diabetes Investig 2020; 11: 878–887
References
- 1. Matsuura F, Yamashita S, Nakamura T, et al Effect of visceral fat accumulation on uric acid metabolism in male obese subjects: visceral fat obesity is linked more closely to overproduction of uric acid than subcutaneous fat obesity. Metabolism 1998; 47: 929–933. [DOI] [PubMed] [Google Scholar]
- 2. Kim TH, Lee SS, Yoo JH, et al The relationship between the regional abdominal adipose tissue distribution and the serum uric acid levels in people with type 2 diabetes mellitus. Diabetol Metab Syndr 2012; 4: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Matsuzawa Y, Funahashi T, Nakamura T. The concept of metabolic syndrome: contribution of visceral fat accumulation and its molecular mechanism. J Atheroscler Thromb 2011; 18: 629–639. [DOI] [PubMed] [Google Scholar]
- 4. Nishino T, Okamoto K. Mechanistic insights into xanthine oxidoreductase from development studies of candidate drugs to treat hyperuricemia and gout. J Biol Inorg Chem 2015; 20: 195–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Battelli MG, Bolognesi A, Polito L. Pathophysiology of circulating xanthine oxidoreductase: new emerging roles for a multi‐tasking enzyme. Biochim Biophys Acta 2014; 1842: 1502–1517. [DOI] [PubMed] [Google Scholar]
- 6. Murray AW. The biological significance of purine salvage. Annu Rev Biochem 1971; 40: 811–826. [DOI] [PubMed] [Google Scholar]
- 7. Saugstad OD. Hypoxanthine as an indicator of tissue hypoxia. A study of plasma, cerebro‐spinal and brain tissue concentrations. J Oslo City Hosp 1977; 27: 29–40. [PubMed] [Google Scholar]
- 8. Saugstad OD, Kroese A, Myhre HO, et al Alteration of plasma hypoxanthine concentration during ischaemia in the forelimb of the pig. Scand J Clin Lab Invest 1977; 37: 517–520. [DOI] [PubMed] [Google Scholar]
- 9. Sahlin K, Ekberg K, Cizinsky S. Changes in plasma hypoxanthine and free radical markers during exercise in man. Acta Physiol Scand 1991; 142: 275–281. [DOI] [PubMed] [Google Scholar]
- 10. Michiels C. Physiological and pathological responses to hypoxia. Am J Pathol 2004; 164: 1875–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bangsbo J, Sjodin B, Hellsten‐Westing Y. Exchange of hypoxanthine in muscle during intense exercise in man. Acta Physiol Scand 1992; 146: 549–550. [DOI] [PubMed] [Google Scholar]
- 12. Hellsten‐Westing Y, Balsom PD, Norman B, et al The effect of high‐intensity training on purine metabolism in man. Acta Physiol Scand 1993; 149: 405–412. [DOI] [PubMed] [Google Scholar]
- 13. Saiki S, Sato T, Kohzuki M, et al Changes in serum hypoxanthine levels by exercise in obese subjects. Metabolism 2001; 50: 627–630. [DOI] [PubMed] [Google Scholar]
- 14. Hellsten‐Westing Y, Kaijser L, Ekblom B, et al Exchange of purines in human liver and skeletal muscle with short‐term exhaustive exercise. Am J Physiol 1994; 266: R81–86. [DOI] [PubMed] [Google Scholar]
- 15. Nagao H, Nishizawa H, Tanaka Y, et al Hypoxanthine Secretion from Human Adipose Tissue and its Increase in Hypoxia. Obesity 2018; 26: 1168–1178. [DOI] [PubMed] [Google Scholar]
- 16. Parks DA, Granger DN. Xanthine oxidase: biochemistry, distribution and physiology. Acta Physiol Scand Suppl 1986; 548: 87–99. [PubMed] [Google Scholar]
- 17. Murase T, Nampei M, Oka M, et al A highly sensitive assay of human plasma xanthine oxidoreductase activity using stable isotope‐labeled xanthine and LC/TQMS. J Chromatogr B Analyt Technol Biomed Life Sci 2016; 1039: 51–58. [DOI] [PubMed] [Google Scholar]
- 18. Washio KW, Kusunoki Y, Murase T, et al Xanthine oxidoreductase activity is correlated with insulin resistance and subclinical inflammation in young humans. Metabolism 2017; 70: 51–56. [DOI] [PubMed] [Google Scholar]
- 19. Furuhashi M, Matsumoto M, Tanaka M, et al Plasma xanthine oxidoreductase activity as a novel biomarker of metabolic disorders in a general population. Circ J 2018; 82: 1892–1899. [DOI] [PubMed] [Google Scholar]
- 20. Furuhashi M, Matsumoto M, Murase T, et al Independent links between plasma xanthine oxidoreductase activity and levels of adipokines. J Diabetes Investig 2019; 10: 1059–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Furuhashi M, Koyama M, Matsumoto M, et al Annual change in plasma xanthine oxidoreductase activity is associated with changes in liver enzymes and body weight. Endocr J 2019; 66: 777–786. [DOI] [PubMed] [Google Scholar]
- 22. Furuhashi M, Mori K, Tanaka M, et al Unexpected high plasma xanthine oxidoreductase activity in female subjects with low levels of uric acid. Endocr J 2018; 65: 1083–1092. [DOI] [PubMed] [Google Scholar]
- 23. Tsushima Y, Nishizawa H, Tochino Y, et al Uric acid secretion from adipose tissue and its increase in obesity. J Biol Chem 2013; 288: 27138–27149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jorgensen S, Poulsen HE. On accumulation of hypoxanthine plus xanthine in withdrawn human blood. Acta Pharmacol Toxicol 1955; 11: 287–294. [DOI] [PubMed] [Google Scholar]
- 25. Saugstad OD. Hypoxanthine as an indicator of hypoxia: its role in health and disease through free radical production. Pediatr Res 1988; 23: 143–150. [DOI] [PubMed] [Google Scholar]
- 26. Nakamura T, Murase T, Satoh E, et al Establishment of the process in blood sampling and sample handling as a biomarker of hypoxia‐inducible diseases; plasma hypoxanthine and xanthine measurement. J Mol Biomark Diagn 2018; 9. [Google Scholar]
- 27. Yao SY, Ng AM, Cass CE, et al Nucleobase transport by human equilibrative nucleoside transporter 1 (hENT1). J Biol Chem 2011; 286: 32552–32562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yao SY, Ng AM, Vickers MF, et al Functional and molecular characterization of nucleobase transport by recombinant human and rat equilibrative nucleoside transporters 1 and 2. Chimeric constructs reveal a role for the ENT2 helix 5–6 region in nucleobase translocation. J Biol Chem 2002; 277: 24938–24948. [DOI] [PubMed] [Google Scholar]
- 29. Furukawa J, Inoue K, Maeda J, et al Functional identification of SLC43A3 as an equilibrative nucleobase transporter involved in purine salvage in mammals. Sci Rep 2015; 5: 15057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tchoukalova YD, Votruba SB, Tchkonia T, et al Regional differences in cellular mechanisms of adipose tissue gain with overfeeding. Proc Natl Acad Sci USA 2010; 107: 18226–18231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tchkonia T, Thomou T, Zhu Y, et al Mechanisms and metabolic implications of regional differences among fat depots. Cell Metab 2013; 17: 644–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kabon B, Nagele A, Reddy D, et al Obesity decreases perioperative tissue oxygenation. Anesthesiology 2004; 100: 274–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hosogai N, Fukuhara A, Oshima K, et al Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes 2007; 56: 901–911. [DOI] [PubMed] [Google Scholar]
- 34. Nishimura S, Manabe I, Nagasaki M, et al Adipogenesis in obesity requires close interplay between differentiating adipocytes, stromal cells, and blood vessels. Diabetes 2007; 56: 1517–1526. [DOI] [PubMed] [Google Scholar]
- 35. Wang B, Wood IS, Trayhurn P. Dysregulation of the expression and secretion of inflammation‐related adipokines by hypoxia in human adipocytes. Pflugers Arch 2007; 455: 479–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ye J, Gao Z, Yin J, et al Hypoxia is a potential risk factor for chronic inflammation and adiponectin reduction in adipose tissue of ob/ob and dietary obese mice. Am J Physiol Endocrinol Metab 2007; 293: E1118–1128. [DOI] [PubMed] [Google Scholar]
- 37. Rausch ME, Weisberg S, Vardhana P, et al Obesity in C57BL/6J mice is characterized by adipose tissue hypoxia and cytotoxic T‐cell infiltration. Int J Obes 2008; 32: 451–463. [DOI] [PubMed] [Google Scholar]
- 38. Hang B, Sarker AH, Havel C, et al Thirdhand smoke causes DNA damage in human cells. Mutagenesis 2013; 28: 381–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chang SJ, Chen SM, Chiang SL, et al Association between cigarette smoking and hypoxanthine guanine phosphoribosyltransferase activity. Kaohsiung J Med Sci 2005; 21: 495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Edwards NL, Recker D, Airozo D, et al Enhanced purine salvage during allopurinol therapy: an important pharmacologic property in humans. J Lab Clin Med 1981; 98: 673–683. [PubMed] [Google Scholar]
- 41. Townsend MH, Robison RA, O'Neill KL. A review of HPRT and its emerging role in cancer. Med Oncol 2018; 35: 89. [DOI] [PubMed] [Google Scholar]
- 42. Mateos FA, Puig JG, Jimenez ML, et al Hereditary xanthinuria. Evidence for enhanced hypoxanthine salvage. J Clin Invest 1987; 79: 847–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kojima T, Nishina T, Kitamura M, et al Biochemical studies on the purine metabolism of four cases with hereditary xanthinuria. Clin Chim Acta 1984; 137: 189–198. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 | Comparisons of purine metabolism‐related parameters in female participants with and without hyperuricemia.
Figure S2 | Comparisons of hypoxanthine, xanthine, xanthine oxidoreductase (XOR) activity and uric acid with habits of smoking and alcohol drinking.
Table S1 | Characteristics of 11 participants treated with an xanthine oxidoreductase inhibitor.
Table S2 | Correlation analysis for hypoxanthine.
Table S3 | Correlation analysis for log xanthine.
Table S4 | Correlation analysis for log xanthine oxidoreductase.
Table S5 | Correlation analysis for uric acid.


