Graphical abstract
Chemical compounds reviewed in this article: Nitric oxide (Pubchem CID: 145068), Prostacyclin (Pubchem CID: 5282411), A-779 (Pubchem CID: 10169886), L-NAME (Pubchem CID: 39836), Indomethacin (Pubchem CID: 3715), Sodium nitroprusside (Pubchem CID: 11953895), Resveratrol (Pubchem CID: 445154), HOE-140 (Pubchem CID: 6918173), Nimesulide (Pubchem CID: 4495), Carboprostacyclin (Pubchem CID: 6436393)
Keywords: The contact activation system, The kallikrein/kinin system, The renin-angiotensin system, Mas, Prostacyclin, SIRT1
Abstract
The risk of thrombosis, a globally growing challenge and a major cause of death, is influenced by various factors in the intravascular coagulation, vessel wall, and cellular systems. Among the contributors to thrombosis, the contact activation system and the kallikrein/kinin system, two overlapping plasma proteolytic systems that are often considered as synonymous, regulate thrombosis from different aspects. On one hand, components of the contact activation system such as factor XII initiates activation of the coagulation proteins promoting thrombus formation on artificial surfaces through factor XI- and possibly prekallikrein-mediated intrinsic coagulation. On the other hand, physiological activation of plasma prekallikrein in the kallikrein/kinin system on endothelial cells liberates bradykinin from associated high-molecular-weight kininogen to stimulate the constitutive bradykinin B2 receptor to generate nitric oxide and prostacyclin to induce vasodilation and counterbalance angiotensin II signaling from the renin-angiotensin system which stimulates vasoconstriction. In addition to vascular tone regulation, this interaction between the kallikrein/kinin and renin-angiotensin systems has a thrombo-regulatory role independent of the contact pathway. At the level of the G-protein coupled receptors of these systems, defective bradykinin signaling due to attenuated bradykinin formation and/or decreased B2 receptor expression, as seen in murine prekallikrein and B2 receptor null mice, respectively, leads to compensatory overexpressed Mas, the receptor for angiotensin-(1−7) of the renin-angiotensin system. Mas stimulation and/or its increased expression contributes to maintaining a healthy vascular homeostasis by generating graded elevation of plasma prostacyclin which reduces thrombosis through two independent pathways: (1) increasing the vasoprotective transcription factor Sirtuin 1 to suppress tissue factor expression, and (2) inhibiting platelet activation. This review will summarize the recent advances in this field that support these understandings. Appreciating these subtle mechanisms help to develop novel anti-thrombotic strategies by targeting the vascular receptors in the renin-angiotensin and the kallikrein/kinin systems to maintain healthy vascular homeostasis.
1. Introduction
Arterial thrombus formation is a pathological process involving multiple components of the hemostatic system including platelets and other blood cells, blood coagulation and fibrinolytic proteins, and the vessel wall [1,2]. The plasma contact activation and kallikrein/kinin systems were initially proposed as part of the hemostatic pathways based on their influence on clinical blood coagulation laboratory testing, but now are recognized as being dispensable for normal hemostasis [3]. This assessment is supported by clinical observations that total deficiencies of proteins [factor XII (FXII) [4], prekallikrein [5,6] or high-molecular-weight kininogen (HMW-kininogen) [7,8]] from the contact activation and kallikrein/kinin systems in humans influence thrombosis risk but do not lead to bleeding states [3]. There is an increased interest in components from these systems such as FXII, prekallikrein, and HMW-kininogen as potential safe anti-thrombotic targets [9] because their gene-deleted mice have reduced risk to induced thrombosis. The presumed mechanism for thrombosis reduction is that there is less contact activation of FXII by direct loss of FXII or indirect decrease in its activation due to prekallikrein or HMW-kininogen deficiency, both leading to reduced factor XI (FXI) or factor IX (FIX) activation and, in turn, thrombin generation. However, our investigations indicate that in prekallikrein (klkb1−/−) and bradykinin B2 receptor (BDKRB2) (bdkrb2−/−) deficient mice, there are other mechanisms in addition to reduced contact activation that influence thrombosis risk. In klkb1−/− and bdkrb2−/− mice, the absence of prekallikrein and BDKRB2 leads to increased expression of the vascular receptors of the renin-angiotensin system, Mas and the angiotensin II receptor type 2 (AT2R), that lead to elevated nitric oxide (NO) and prostacyclin (PGI2) that influence thrombus formation [5,10]. The thrombo-protective effect of PGI2 involves both the well-established platelet inhibition and a newly appreciated vasoprotection mechanism mediated by transcription factor sirtuin 1 (silent mating type information regulation 2 homolog 1, SIRT1). This latter mechanism will be the focus of this discussion.
This review will sequentially delineate these pathways by discussing the anti-thrombotic potential of Mas receptor in the context of the kallikrein/kinin system and renin-angiotensin system interactions, the roles of SIRT1 in Mas-mediated vasoprotection against thrombosis, and finally the findings in the murine models of prekallikrein and BDKRB2 deletions.
2. Vascular components of the kallikrein/kinin and renin-angiotensin systems
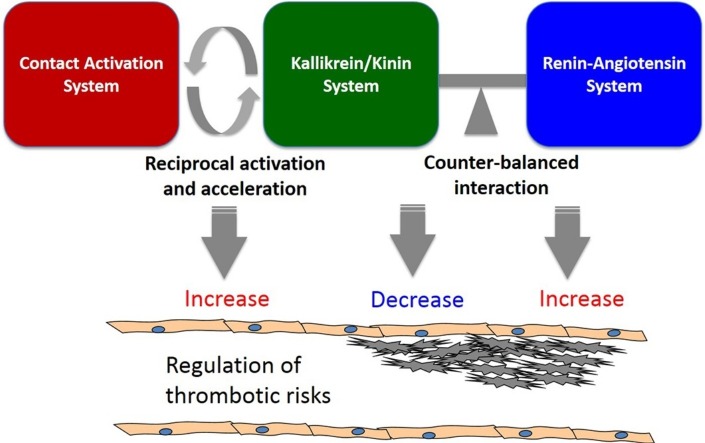
Although recognized as a plasma proteolytic system, the kallikrein/kinin system influences vascular homeostasis. The plasma protein components of this system [including zymogen prekallikrein or enzyme plasma kallikrein, HMW-kininogen and its cleaved form (cleaved HMW-kininogen), and bradykinin which is the major cleavage product of HMW-kininogen by plasma kallikrein] interact with receptors on the vessel wall. HMW-kininogen has at least 3 binding partners and putative receptors on the vessel wall, the urokinase plasminogen activator receptor (uPAR), gC1q receptor (gC1qR), and cytokeratin 1 (CK1) [11]. Prekallikrein and plasma kallikrein binds to cells via HMW-kininogen or directly to as yet unknown sites. FXI and its active form FXIa compete prekallikrein binding to HMW-kininogen on cells, but since the plasma concentration of prekallikrein is an order of magnitude higher than FXI, prekallikrein is the dominate ligand. Additionally, FXII also binds to and competes HMW-kininogen binding to uPAR, gC1qR, and CK1. The product of plasma kallikrein cleavage of HMW-kininogen, bradykinin, binds to two G-protein coupled receptors (GPCRs), the BDKRB2 that is constitutively present on the vessel wall and the bradykinin B1 receptor (BDKRB1) that arises in inflammatory states. This review will focus on the anti-thrombotic property of vascular beds and examine the pathways related to contact pathway protein interactions with the vessel wall. The vessel wall maintains several anti-thrombotic mechanisms to keep the circulation system in a quiescent state [12]. These include but are no means complete endothelial-derived NO [13] and PGI2 [14,15], anticoagulant glycosaminoglycans [16], transmembrane protein thrombomodulin [17], and tissue plasminogen activator (tPA) [18]. It is in the context of NO and PGI2 production and tPA liberation that the GPCRs of the kallikrein/kinin and renin-angiotensin systems are part of the intravascular regulators of thrombotic risks [19] (Fig. 1 ).
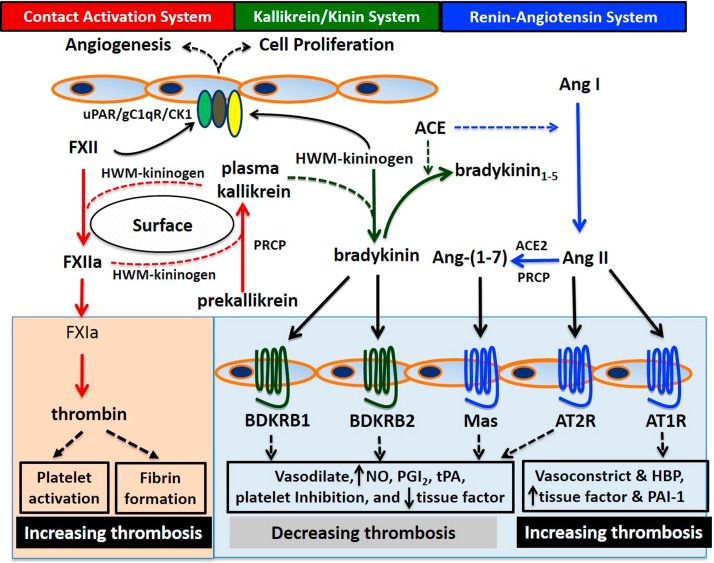
Fig. 1.
The regulation of thrombotic risks by the contact activation system, the kallikrein/kinin system and the renin-angiotensin system. The kallikrein/kinin system interacts with both the contact activation and renin-angiotensin systems at multiple levels. On one hand, pathological activation of FXII into FXIIa on contact surface is accelerated by components from the kallikrein/kinin system such as prekallikrein and HMW-kininogen in a reciprocal manner. In this process prekallikrein is activated into plasma kallikrein which cleaves HMW-kininogen to liberate bradykinin to promote inflammation. FXIIa initiates the intrinsic coagulation cascade through FXIa leading to thrombin generation, which promotes thrombosis by activating platelets and generating fibrin. In this context the kallikrein/kinin system increases thrombotic risks (light orange box on the left). On the other hand, the kallikrein/kinin system influences vascular homeostasis and counteracts with the renin-angiotensin system at the level of vascular receptors. HMW-kininogen binds to a triple-receptor complex including uPAR/gC1qR/CK1 on endothelial cells to stimulate angiogenesis and cell proliferation. Physiological activation of prekallikrein by PRCP on endothelial cells also release bradykinin, whose local delivery to BDKRB1 or BDKRB2 causes vasodilation, produces NO, PGI2 and tPA, and reduces thrombosis by inhibiting platelet activation and tissue factor expression. In addition, the kallikrein/kinin system counterbalances with the renin-angiotensin system at multiple layers. ACE degrades bradykinin but also generates Ang II from Ang I. Ang II and its breakdown product by ACE2 or PRCP, Ang-(1−7), bind to AT2R and Mas, respectively, to induce a similar anti-thrombotic activity as BDKRB1 and BDKRB2. However, binding to Ang II to its dominant receptor AT1R causes vasoconstriction, elevates blood pressure, and increases thrombosis by producing tissue factor and PAI-1. In this context, the kallikrein/kinin system decreases thrombotic risks independent of the contact activation system but through interactions with the renin-angiotensin system (blue box on the right). uPAR: the urokinase plasminogen activator receptor; gC1qR: gC1q receptor; CK1: cytokeratin 1; FXII: coagulation factor XII; FXIIa: activated factor XII; PRCP: prolycarboxypeptidase; HMW-kininogen: high-molecular-weight kininogen; BDKRB2: bradykinin B2 receptor; tPA: tissue-type plasminogen activator; ACE: angiotensin converting enzyme; ACE2: angiotensin converting enzyme2; Ang II: angiotensin II; Ang-(1−7): angiotensin-(1−7); AT2R: angiotensin II receptor type 2; AT1R: angiotensin II receptor type 1; NO: nitric oxide; PGI2: prostacyclin; PAI-1: plasminogen activator inhibitor-1; HBP: high blood pressure.
Although the contact activation and kallikrein/kinin systems are considered the same, each has a different role in vascular homeostasis. Activation of the contact activation system is a pathophysiological process initiated by the exposure of FXII to negatively charged surfaces such as polyphosphates, exosomes, denatured or foreign proteins, e.g., bacterial cell wall, or artificial medical devices. Autoactivation of FXII into FXIIa converts prekallikrein to its active form plasma kallikrein, which further amplifies the FXII activation in a reciprocal manner [20]. HMW-kininogen is a cofactor for FXII and prekallikrein activation by accelerating their reciprocal activation. The subsequent cleavage of FXI by FXIIa triggers a proteolytic cascade of coagulation factors, leading to thrombin generation, which promotes thrombus formation by activating platelets and generating fibrin. Recently, plasma kallikrein has also been shown to activate FIX independent of FXIa, another pathway by which contact activation may influence thrombin formation [21,22]. In addition to activation in pathologic states, the kallikrein/kinin system is physiologically activated by prolylcarboxypeptidase (PRCP), an endothelial cell membrane serine protease that activates prekallikrein independent of FXII [[23], [24], [25]]. Plasma kallikrein, generated either by FXIIa or PRCP, cleaves HMW-kininogen to liberate bradykinin, a vasoactive nonapeptide with a short half-life in the blood. As already stated above formed bradykinin has two vascular receptors: the constitutively expressed BDKRB2 and the BDKRB1 that is expressed during inflammatory condition [26]. As the end product of proteolytic activation of the kallikrein/kinin system, it is the local delivery of bradykinin to its receptors that stimulates endothelial production of NO, PGI2 and tPA [[27], [28], [29], [30]]. It is by this means that the kallikrein/kinin pathway additionally influences thrombosis independent of the coagulation and fibrinolytic systems initiated by contact activation. This recently appreciated pathway is the focus of this presentation (Fig. 1).
The kallikrein/kinin system has multiple interactions with and counterbalances the renin-angiotensin system [31,32] (Fig. 1). One example is angiotensin converting enzyme (ACE). ACE, also known as kininase 1, not only generates angiotensin II (Ang II) from angiotensin I, but also is the major degrading enzyme of bradykinin into a pentapeptide Arg-Pro-Pro-Gly-Phe (bradykinin1−5). At micromolar concentrations, bradykinin1−5 is a direct thrombin inhibitor [33]. Another example is PRCP, a physiological prekallikrein activator, which, along with ACE2, degrades Ang II into angiotensin-(1−7) [Ang-(1−7)] [34]. Activation of the renin-angiotensin system is generally considered prothrombotic [35]. In opposition to bradykinin’s anti-thrombotic effect, Ang II increases the risk of thrombosis by stimulating vascular tissue factor expression and plasminogen activator inhibitor-1 (PAI-1) release through its dominant receptor, angiotensin II receptor type 1 (AT1R) [36,37]. Ang II also binds to another receptor, angiotensin II receptor type 2 (AT2R) with equal affinity to AT1R. If the AT2R is expressed in larger amounts than the AT1R, signals from Ang II binding to the AT2R are amplified leading to NO and PGI2 production [38] to reduce thrombosis risk. Additionally, the receptor for Ang-(1−7) is Mas, whose activation also stimulates NO and PGI2 production to inhibit thrombus formation [39]. Thus, there are four GPCRs whose activation stimulates NO and PGI2 production: BDKRB2, BDKRB1, AT2R, and Mas. Their role is to counterbalance the vasoconstrictive and prothrombotic activity of Ang II binding to the AT1R. In addition, the five GPCRs from the kallikrein/kinin and renin-angiotensin systems form functional homodimers [e.g., AT1R-AT1R [40]] and heterodimers [AT1R-BDKRB2 [41,42], AT2R-BDKRB2 [43], BDKRB2-Mas [44], AT2R-Mas [45] etc.] which influence the downstream signaling events by increasing or decreasing availability of the companion receptor in physiologic and pathophysiologic circumstances (Fig. 1).
The aforementioned examples are just a few among many interaction points between the kallikrein/kinin system and the renin-angiotensin system. The counterbalancing influences of each of these systems on health and diseases have been documented and evidenced in vitro and in vivo in studies on gene-deleted animal models [32]. This review will specifically discuss the counterbalance between the bradykinin/BDKRB2 axis and the Ang-(1−7)/Mas axis in the regulation of vascular homeostasis and thrombotic risks.
Table 1.
List of non-standard abbreviations.
| Abbreviation (gene name) | Full name |
|---|---|
| FXII (F12) | coagulation factor XII |
| FXIIa | activated factor XII |
| HMW-kininogen | high-molecular-weight kininogen |
| BDKRB2 (Bdkrb2) | bradykinin B2 receptor |
| BDKRB1 | bradykinin B1 receptor |
| PRCP | prolylcarboxypeptidase |
| ACE | angiotensin converting enzyme |
| ACE2 | angiotensin converting enzyme 2 |
| AT1R | angiotensin II receptor type 1 |
| AT2R | angiotensin II receptor type 2 |
| Ang II | angiotensin II |
| Ang-(1−7) | angiotensin-(1−7) |
| uPAR | urokinase plasminogen activator receptor |
| gC1qR | gC1q receptor |
| CK1 | cytokeratin 1 |
| tPA | tissue-type plasminogen activator |
| PAI-1 | plasminogen activator inhibitor-1 |
| GPCR | G-protein coupled receptor |
| PPAR | peroxisome proliferator-activated receptor |
| SIRT1 | sirtuin 1, silent mating type information regulation 2 homolog 1 |
| COX-2 | cyclooxygenase-2 |
| NF-κB | nuclear factor-kappa B |
| KLF4 | krüppel-like factor 4 |
3. Roles of Mas receptor, PGI2, and SIRT1 in thrombosis
3.1. Mas receptor and thrombosis
Accumulating evidence demonstrates that the Ang-(1−7)/Mas axis has potent anti-thrombotic effect [39,46]. Mas is the newest member of the GPCRs of the renin-angiotensin system [47] and is widely expressed in vascular cellular components including endothelial cells [48], vascular smooth muscle cells [49] and even platelets [50]. Intravenous infusion of Ang-(1−7) reduced thrombus formation through a mechanism that is believed to be mediated by Mas-derived NO and PGI2 [39]. The anti-thrombotic effects of Ang-(1−7) are abolished by Mas antagonist A-779 or combined pre-treatment with NO synthase inhibitor L-NAME and PGI2 synthase inhibitor indomethacin [51]. In addition, the anti-thrombotic effect of Ang-(1−7) involves platelet Mas receptors, whose activation increases NO production in platelets [50] and potentiates the anti-aggregatory effects of NO donors such as sodium nitroprusside [52]. Mas activation in endothelial cells initiates a signaling pathway involving PI3K/AKT/eNOS leading to NO production [53]. Mas stimulated PGI2 generation is through a signaling pathway including sequential activation of Ca2+/calmodulin-dependent protein kinase II (CaMKII) and mitogen-activated protein kinase (MAPK) that enhances the activity of cytosolic phospholipase A2 (cPLA2) to release arachidonic acid for PGI2 synthesis [54].
3.2. PGI2 and thrombosis
As the key effector for Mas mediated thrombosis protection, PGI2 is the dominant eicosanoid released by blood vessels. PGI2 has two receptors, the cell surface IP receptor and the cytosolic peroxisome proliferator-activated receptor β/δ (PPARβ/δ). The classical understanding of the anti-thrombotic effect of PGI2 is based on its acute effects on suppressing calcium signaling and counteracting platelet-derived thromboxane A2 [55]. The surface IP receptor is coupled to adenylate cyclase, whose activation converts ATP into cyclic AMP (cAMP). Elevation of cAMP directly activates protein kinase A (PKA) [56] and exchange protein directly activated by cAMP (EPAC) [57]. The PKA pathway has a spectrum of substrates, whose phosphorylation leads to inhibition of intracellular calcium mobilization through multiple mechanisms such as blockade of calcium channels [58]. The EPAC pathway also inhibits calcium signaling through unknown mechanisms involving Ras-related protein 1 (Rap1) and Rap2 [59]. Inhibition of intracellular calcium mobilization in platelets and vascular smooth muscle cells leads to attenuated aggregation response [60] and vessel dilation, respectively, both of which contribute to the anti-thrombotic effects of PGI2. Like the surface IP receptor, the intracellular receptor PPARβ/δ has also been reported to regulate signaling events during platelet activation and vessel dilation [[61], [62], [63]].
In addition to its acute effect, PGI2 also exerts chronic genomic effects to influence vascular homeostasis. First, PGI2 binds to PPARβ/δ, which forms heterodimers with retinoid X receptor (RXR), regulating the transcriptional profile of vascular genes, such as those related to angiogenesis and thrombogenicity [[64], [65], [66], [67], [68]]. Second, the cAMP/PKA pathway downstream of the IP receptor activates cAMP response element-binding protein (CREB), which controls the promoter activity of vascular genes such as PAI-1, vascular endothelial growth factor (VEGF) [69] and NADPH oxidase 4 (Nox4) [70].
PGI2 is produced from arachidonic acid sequentially by cyclooxygenase and prostacyclin synthase [55]. Clinical and animal studies show that genetic deletion or chronic pharmacological inhibition of vascular cyclooxygenase-2 (COX-2) is associated with adverse cardiovascular events due to reduced PGI2 production [71,72]. Emerging data demonstrate that the unconventional role of COX-2-derived PGI2 in the regulation of vasoprotective transcription factors presents an important anti-thrombotic mechanism. Ghosh et al. first showed that metabolizing of endocannabinoids 2-arachidonyl glycerol into PGI2 by endothelial COX-2 and prostacyclin synthase activates the transcriptional activity of PPARδ, which suppresses the expression of tissue factor [68], a critical cofactor to factor VIIa of the extrinsic coagulation cascade, the main physiologic hemostatic pathway. COX-2 inhibitors increase, whereas PPARδ activators decrease, the expression of tissue factor in lipopolysaccharide-treated endothelial cells and animals. A later study by Barbieri et al. showed that the absence of vascular COX-2, instead of platelet COX-2, is responsible for the enhanced thrombus formation in COX-2 depleted mice [67]. She further showed that COX-2 deficiency causes decreased expression of PPARδ and SIRT1, which leads to elevated vascular tissue factor activity, therefore increasing the risk of thrombosis. Treatment with an IP receptor or PPARδ antagonist leads to reduced SIRT1 expression, increased tissue factor activity, and enhanced thrombus formation [67], suggesting both PGI2 receptors in the vessels regulate downstream SIRT1 expression. A more recent study using endothelial-specific COX-2 knockout mice confirmed that endothelial COX-2 provides anti-thrombotic effects independent of plasma PGI2 level [14], indicating the effect of local produced PGI2 on the vasculature.
3.3. SIRT1 and thrombosis
SIRT1 belongs to a family of class III histone deacetylases that catalyze the deacetylation of lysine residues on histones and other proteins. The enzymatic activity of sirtuins depends on nicotinamide adenine dinucleotide (NAD+). Sirtuins have important roles in the context of aging and metabolic diseases [73]. There are 7 mammalian orthologs, SIRT1−7, whose activation has been shown to provide cardiovascular benefits such as reducing endothelial dysfunction and thrombosis [74]. Breitenstein et al. showed that pharmacological inhibition or siRNA knockdown of SIRT1 promotes nuclear translocation and DNA binding of the p65 subunit of nuclear factor-kappa B (NF-κB), which increases tissue factor expression through enhanced promoter activity, and accelerates in vivo thrombus formation [75]. The mechanism is mediated by direct acetylation of Lys310 of the NF-κB/p65 subunit. The study by Wu et al. showed that after particulate exposure SIRT1 suppresses coagulopathy in the lung by upregulating the expression of Krüppel-like factor 2 (KLF2), an important transcription factor that regulates thrombomodulin expression [76]. Murine SIRT1 deficiency leads to increased NF-κB acetylation and activation, reduced KLF2 and thrombomodulin protein expression, and elevated fibrin generation in the lung along with reduced expression of tissue factor pathway inhibitor (TFPI) and increased PAI-1 activity. A recent study by Wu et al. showed that SIRT1 dependent deacetylation of transcription factor forkhead box protein O1 (FOXO-1) enhances autophagy, therefore reducing oxidized low-density lipoprotein (ox-LDL)-induced endothelial secretion of Von Willebrand factor and P-selectin, two critical proteins for thrombus formation [77]. In addition, the role of SIRT1 has also been implicated in deep vein thrombosis [78]. Resveratrol, a SIRT1 activator, suppresses the production of proinflammatory cytokines such as tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) through decreased acetylation of NF-κB/p65 subunit and thus inhibits stenosis-induced deep vein thrombosis in a rat model. In addition, SIRT3 has also been reported to prevent arterial thrombus formation through regulating neutrophil extracellular traps formation and plasma tissue factor activity [79].
4. Evidence from the prekallikrein and BDKRB2 deficient mice
The aforementioned regulatory components controlling thrombus formation constitute a novel pathway via the interactions between the kallikrein/kinin and renin-angiotensin systems, the Mas receptor, and PGI2 to regulate thrombotic risk. This pathway was not obvious, but was revealed by detailed mechanistic investigations on two murine models: prekallikrein knockout (klkb1−/−) and BDKRB2 knockout (bdkrb2−/−) mice, both of which have reduced arterial thrombotic risk [5,10,80]. This pathway is a non-canonical mechanism by which the kallikrein/kinin system influences thrombotic risk independent of the contact activation system which is based upon contact activation of FXII leading to thrombin generation through FXIa. In this non-canonical pathway that leads to reduction in thrombosis risk, GPCRs from the renin-angiotensin system produce elevated PGI2 levels that 1) suppresses vessel wall tissue factor through elevated SIRT1 and 2) produces increased platelet inhibition. Both processes correlate with ambient prostacyclin levels. The following paragraphs discuss this pathway through the characterization of these murine gene-deletion models (Fig. 2 ).
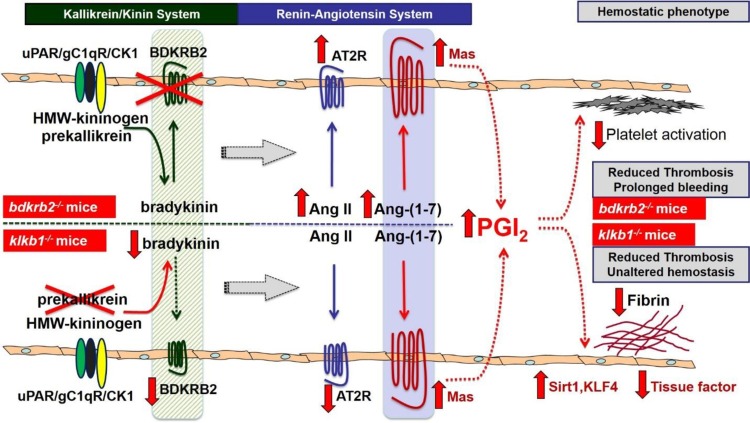
Fig. 2.
Novel mechanisms of thrombosis reduction in bdkrb2−/− mice and klkb1−/− mice. In bdkrb2−/− mice (top portion) BDKRB2 depletion leads to complete absent bradykinin/BDKRB2 signaling, whereas in klkb1−/− mice (bottom portion) prekallikrein depletion causes attenuated bradykinin formation and reduced BDKRB2 expression. Both mice have defective bradykinin/BDKRB2 signaling resulting in compensatory overexpressed Mas receptor. In bdkrb2−/− mice, AT2R receptor is also overexpressed along with elevated plasma Ang II and Ang-(1−7) levels due to heightened ACE activity. In klkb1−/− mice, AT2R is decreased with unchanged plasma Ang-(1−7) level. Therefore, the Ang-(1−7)/Mas axis is enhanced in both mice to produce graded elevation of plasma PGI2 depending on the extend of bradykinin/BDKRB2 deficiency. A 2∼3-folder higher level of PGI2 due to complete absent bradykinin/BDKRB2 signaling in bdkrb2−/− mice causes a platelet activation defect associated with prolonged bleeding, whereas a 0.5∼1-fold higher PGI2 due to modest deficiency of the bradykinin/BDKRB2 signaling in klkb1−/− mice leads to increased SIRT1 and KLF4 expression and reduced vascular tissue factor expression and fibrin formation without reducing platelet function and hemostasis. Both mechanisms independently contribute to thrombosis reduction. uPAR: the urokinase plasminogen activator receptor; gC1qR: gC1q receptor; CK1: cytokeratin 1; HMW-kininogen: high-molecular-weight kininogen; BDKRB2: bradykinin B2 receptor; Ang II: angiotensin II; Ang-(1−7): angiotensin-(1−7); AT2R: angiotensin II receptor type 2; PGI2: prostacyclin; SIRT1: sirtuin 1; KFL4: Krüppel-like factor 4.
4.1. A thrombo-protective mechanism independent of the contact activation system
Klkb1−/− and bdkrb2−/− mice have delayed occlusion in the carotid artery on ferric chloride- and rose bengal-induced thrombosis models [5,10,80]. The prolonged occlusion time in bdkrb2−/− mice is recapitulated in wild-type mice treated with HOE-140 [80], a BDKRB2 antagonist, indicating a mechanism involving defective bradykinin signaling. Although klkb1−/− mice have expected attenuated plasma contact activation as indicated by prolonged activated partial thromboplastin time (aPTT), reduced thrombin generation induced by the contact pathway, and decreased edema in the lung upon collagen/epinephrine insult, these mice, unlike FXII deficient (f12−/−) mice [81,82], do not have a survival advantage over wild-type mice when challenged on a pulmonary embolism model induced by collagen/epinephrine or long chain polyphosphate, potent activators of contact pathway [83]. In addition, reconstitution with purified prekallikrein in klkb1−/− mice was unable to correct their thrombosis delay. These combined observations suggest that the thrombo-protective mechanisms in these two mouse strains are independent of the contact activation system.
4.2. Ang-(1−7)/Mas axis compensates bradykinin/BDKRB2 axis
Klkb1−/− mice have significantly reduced plasma bradykinin (∼40 % of wild-type) and renal BDKRB2 mRNA (∼20 % of wild-type) [5]. Thus, both klkb1−/− and bdkrb2−/− mice have compromised constitutive bradykinin signaling through BDKRB2 due to attenuated bradykinin generation from plasma kallikrein [5] and/or absence of BDKRB2 receptor, respectively [51]. In bdkrb2−/− mice accumulation of unmetabolized bradykinin presumably heightens ACE activity which generates elevated plasma Ang II and its breakdown product Ang-(1−7) [10]. In addition, the mRNA and protein expression of the receptors for Ang II and Ang-(1−7), AT2R and Mas, respectively, are increased in bdkrb2−/− mice. In klkb1−/− mice the attenuated plasma bradykinin level leads to reduced renal ACE and AT2R mRNA, but increased Mas mRNA and protein with unchanged Ang-(1−7) levels. As described above both the bradykinin/BDKRB2 axis of the kallikrein/kinin system and Ang-(1−7)/Mas axis of the renin-angiotensin system have similar effects on vascular homeostasis [19,39]. In addition, the BDKRB2 and Mas crosstalk by forming functional heterodimers that influence the downstream signaling events [44]. Therefore, inactivation of bradykinin/BDKRB2 axis results in a compensatory counter-activation of Ang-(1−7)/Mas axis to maintain physiological homeostasis [31]. Indeed, both klkb1−/− and bdkrb2−/− mice have enhanced activity of Ang-(1−7)/Mas axis producing elevated plasma PGI2 level as determined by 6-keto-PGF1α. Moreover, the Mas antagonist A-779 prevents the elevation of plasma PGI2 level. Importantly, the phenotype of delayed vessel occlusion in klkb1−/− and bdkrb2−/− mice is also corrected by A-779 treatment, further confirming the role of Mas in thrombosis protection.
4.3. Anti-thrombotic effects mediated by Mas-derived PGI2
Bradykinin/BDKRB2 axis signaling is completely absent in bdkrb2−/− mice but only attenuated in klkb1−/− mice. In accordance with this difference, bdkrb2−/− mice have significantly higher plasma level of 6-keto-PGF1α (259 ± 42 pg/mL) [10] than klkb1−/− mice (129 ± 12 pg/mL) [5], although both are higher than wild-type mice (75 ± 10 pg/mL). Treatment with a COX-2 inhibitor nimesulide significantly shortens the occlusion time both in klkb1−/− and in bdkrb2−/− mice. However, the mechanisms by which the elevated PGI2 inhibits thrombosis are different in these two mouse strains. In bdkrb2−/− mice where plasma 6-keto-PGF1α is 2.5-fold higher than in wild-type, a platelet function defect specific to glycoprotein VI (GPVI) and prolonged tail bleeding time are observed. Bdkrb2−/− platelets have reduced integrin activation and P-selectin exposure in response to collagen-related peptide or convulxin. In addition, they also have decreased spreading on collagen, fibrinogen and GFOGER, a peptide agonist for β1 integrin. Treatment with A-779 or nimesulide in vivo corrects the defective platelet spreading, integrin activation and P-selectin exposure, and shortens the tail bleeding time in bdkrb2−/− mice [10,80]. Mechanistically, the platelet GPVI defect is associated with reduced phosphorylation of spleen tyrosine kinase (Syk), which is recapitulated by in vitro treatment with a prostacyclin analog carboprostacyclin, but not with a NO donor DEA NONOate. These data suggest the acquired platelet defect is due to elevated PGI2 instead of NO in bdkrb2−/− mice. Taken together these data in bdkrb2−/− mice have established a clear pathway through which overexpressed Mas-derived PGI2 inhibits platelet functions. However, in klkb1−/− mice where plasma PGF1α is only 0.7-fold higher than wild-type, the anti-thrombotic effects of PGI2 is mediated not by platelet inhibition but by an unconventional mechanism through PGI2/SIRT1/tissue factor pathway.
4.4. Mas/PGI2/SIRT1 pathway inhibits tissue factor expression
Since klkb1−/− mice have a normal tail bleeding time and normal platelet functions in response to major agonists including thrombin, ADP and collagen, we sought a mechanism whereby the inhibited thrombus formation mediated by some elevation of PGI2 is independent of a platelet defect. In accordance with an established role of PGI2 in SIRT1 expression [67], we observed that klkb1−/− mice have increased aortic mRNA and protein expression of SIRT1 and Krüppel-like factor 4 (KLF4), whose expressions are inhibited by in vivo treatment with nimesulide [5]. Both SIRT1 and KLF4 inhibit NF-κB activation and tissue factor expression by direct deacetylation of p65 subunit or interacting with its cofactor p300 [75,84]. As a result of increased SIRT1 and KLF4, klkb1−/− mice have reduced vascular tissue factor mRNA and antigen, which are reversed by nimesulide treatment. In addition, klkb1−/− mice also have reduced tissue factor activity in the vessel wall as determined by chromogenic assay. Reduction of vascular tissue factor antigens is reflected on the decreased endogenous blood-borne tissue factor activity, which is mainly tissue factor-bearing microparticles derived from platelets, leukocytes and vascular cells [[85], [86], [87]]. In the presence of rHA-infestin-4, a FXIIa inhibitor blocking contact activation [88], and without adding exogenous tissue factor, plasma from klkb1−/− mice have significantly reduced thrombin generation induced by endogenous tissue factor compared to wild-type mice. These findings in klkb1−/− mice reveal a novel mechanism by which inhibited vascular tissue factor expression by Mas/PGI2/SIRT1 pathway alone is sufficient to reduce thrombotic risk without compromising hemostasis. It should be noted that there is also increased SIRT1 and KLF4 mRNA in the aorta of bdkrb2−/− mice in addition to their platelet function defect, suggesting the Mas/PGI2/SIRT1 pathway also is operative in the vessels of these mice. This proposed anti-thrombotic pathway in bdkrb2−/− mice has not been fully evaluated yet.
5. Conclusion
In summary, the combined research in klkb1−/− and bdkrb2−/− mice has revealed a novel pathway for thrombosis regulation. This pathway is a previously unappreciated mechanism by which Ang-(1−7)/Mas axis compensates for the defective bradykinin/BDKRB2 axis in vivo to produce a graded elevation of PGI2, leading to graded protection against thrombosis. On one hand, complete bradykinin/BDKRB2 signaling deficiency causes increased Ang II and Ang-(1−7) and overexpressed AT2R and Mas to generate 2∼3-folder higher level of PGI2, which produces an acquired platelet function defect specific to GPVI inhibition. On the other hand, a modest deficiency of bradykinin/BDKRB2 signaling caused by reduced BDKRB2 expression and diminished bradykinin generation due to lack of plasma kallikrein, leads to overexpressed Mas without altering Ang-(1−7), generating 0.5∼1-fold higher PGI2, which elevates the expression of vasoprotective transcription factors such as SIRT1 and KLF4 to inhibit vascular tissue factor expression. Both pathways exert potent anti-thrombotic effects although the former one is associated with prolonged bleeding time due to platelet inhibition while the later one does not inhibit platelet function, but has vasoprotective effects (Fig. 3 ). The aforementioned observations and mechanisms are just the beginning of an emerging area in the research of thrombosis regulation. Ongoing studies aim to dissect this system in a stepwise manner to elaborate the detailed molecular mechanisms. Understanding these mechanisms will help develop novel anti-thrombotic strategies. Targeting the vascular receptors in the renin-angiotensin and the kallikrein/kinin systems has the potential to prevent thrombosis without increasing bleeding risk and maintain healthy vascular homeostasis to reduce the likelihood of vascular diseases such as myocardial infarction and stroke and to understand emerging disorders such as COVID-19 and their coagulopathies.
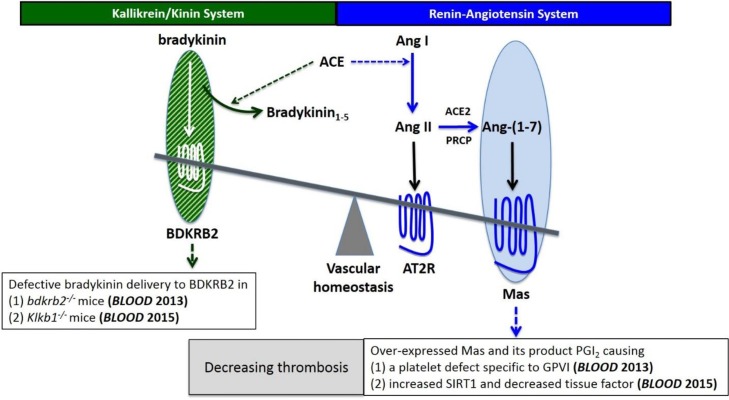
Fig. 3.
Balanced interaction between the bradykinin/BDKRB2 axis and the Ang-(1−7)/Mas axis in the regulation of thrombosis. Overexpressed Mas receptor compensates the defective bradykinin/BDKRB2 signaling to maintain healthy vascular homeostasis, which leads to elevated plasma PGI2 level to reduce thrombotic risks by two independent mechanisms: (1) inhibiting platelet GPVI and thus platelet activation and (2) increasing SIRT1 and decreasing vascular tissue factor. BDKRB2: bradykinin B2 receptor; Ang II: angiotensin II; Ang-(1−7): angiotensin-(1−7); AT2R: angiotensin II receptor type 2; PGI2: prostacyclin; SIRT1: sirtuin 1; GPVI: glycoprotein VI.
Declaration of Competing Interest
The authors declare no conflicts of interest.
Acknowledgements
This work was supported in part by National Key R&D Program of China (No. 2018YFE0113600), Natural Science Foundation of China (No. 81802932), a Global Research Award of American Society of Hematology, a grant from the Key Laboratory for Drug Target Researches and Pharmacodynamic Evaluation of Hubei Province, China, and grants HL052779, HL112666, AI130131, HL144113, HL143402, and CA223301 from the National Institutes of Health, US.
References
- 1.Furie B., Furie B.C. Mechanisms of thrombus formation. N. Engl. J. Med. 2008;359(9):938–949. doi: 10.1056/NEJMra0801082. [DOI] [PubMed] [Google Scholar]
- 2.Jackson S.P. Arterial thrombosis--insidious, unpredictable and deadly. Nat. Med. 2011;17(11):1423–1436. doi: 10.1038/nm.2515. [DOI] [PubMed] [Google Scholar]
- 3.Schmaier A.H. Antithrombotic potential of the contact activation pathway. Curr. Opin. Hematol. 2016;23(5):445–452. doi: 10.1097/MOH.0000000000000271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kleinschnitz C., Stoll G., Bendszus M., Schuh K., Pauer H.U., Burfeind P., et al. Targeting coagulation factor XII provides protection from pathological thrombosis in cerebral ischemia without interfering with hemostasis. J. Exp. Med. 2006;203(3):513–518. doi: 10.1084/jem.20052458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stavrou E.X., Fang C., Merkulova A., Alhalabi O., Grobe N., Antoniak S., et al. Reduced thrombosis in Klkb1-/- mice is mediated by increased Mas receptor, prostacyclin, Sirt1, and KLF4 and decreased tissue factor. Blood. 2015;125(4):710–719. doi: 10.1182/blood-2014-01-550285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bird J.E., Smith P.L., Wang X., Schumacher W.A., Barbera F., Revelli J.P., et al. Effects of plasma kallikrein deficiency on haemostasis and thrombosis in mice: murine ortholog of the Fletcher trait. Thromb. Haemost. 2012;107(6):1141–1150. doi: 10.1160/TH-11-10-0682. [DOI] [PubMed] [Google Scholar]
- 7.Merkulov S., Zhang W.M., Komar A.A., Schmaier A.H., Barnes E., Zhou Y., et al. Deletion of murine kininogen gene 1 (mKng1) causes loss of plasma kininogen and delays thrombosis. Blood. 2008;111(3):1274–1281. doi: 10.1182/blood-2007-06-092338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Langhauser F., Gob E., Kraft P., Geis C., Schmitt J., Brede M., et al. Kininogen deficiency protects from ischemic neurodegeneration in mice by reducing thrombosis, blood-brain barrier damage, and inflammation. Blood. 2012;120(19):4082–4092. doi: 10.1182/blood-2012-06-440057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bickmann J.K., Baglin T., Meijers J.C.M., Renne T. Novel targets for anticoagulants lacking bleeding risk. Curr. Opin. Hematol. 2017;24(5):419–426. doi: 10.1097/MOH.0000000000000367. [DOI] [PubMed] [Google Scholar]
- 10.Fang C., Stavrou E., Schmaier A.A., Grobe N., Morris M., Chen A., et al. Angiotensin 1-7 and Mas decrease thrombosis in Bdkrb2-/- mice by increasing NO and prostacyclin to reduce platelet spreading and glycoprotein VI activation. Blood. 2013;121(15):3023–3032. doi: 10.1182/blood-2012-09-459156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.LaRusch G.A., Mahdi F., Shariat-Madar Z., Adams G., Sitrin R.G., Zhang W.M., et al. Factor XII stimulates ERK1/2 and Akt through uPAR, integrins, and the EGFR to initiate angiogenesis. Blood. 2010;115(24):5111–5120. doi: 10.1182/blood-2009-08-236430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bochenek M.L., Schafer K. Role of endothelial cells in acute and chronic thrombosis. Hamostaseologie. 2019;39(2):128–139. doi: 10.1055/s-0038-1675614. [DOI] [PubMed] [Google Scholar]
- 13.Moore C., Tymvios C., Emerson M. Functional regulation of vascular and platelet activity during thrombosis by nitric oxide and endothelial nitric oxide synthase. Thromb. Haemost. 2010;104(2):342–349. doi: 10.1160/TH09-11-0764. [DOI] [PubMed] [Google Scholar]
- 14.Mitchell J.A., Shala F., Elghazouli Y., Warner T.D., Gaston-Massuet C., Crescente M., et al. Cell-specific gene deletion reveals the antithrombotic function of COX1 and explains the vascular COX1/prostacyclin paradox. Circ. Res. 2019;125(9):847–854. doi: 10.1161/CIRCRESAHA.119.314927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bombeli T., Mueller M., Haeberli A. Anticoagulant properties of the vascular endothelium. Thromb. Haemost. 1997;77(3):408–423. [PubMed] [Google Scholar]
- 16.Sobczak A.I.S., Pitt S.J., Stewart A.J. Glycosaminoglycan neutralization in coagulation control. Arterioscler. Thromb. Vasc. Biol. 2018;38(6):1258–1270. doi: 10.1161/ATVBAHA.118.311102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loghmani H., Conway E.M. Exploring traditional and nontraditional roles for thrombomodulin. Blood. 2018;132(2):148–158. doi: 10.1182/blood-2017-12-768994. [DOI] [PubMed] [Google Scholar]
- 18.Oliver J.J., Webb D.J., Newby D.E. Stimulated tissue plasminogen activator release as a marker of endothelial function in humans. Arterioscler. Thromb. Vasc. Biol. 2005;25(12):2470–2479. doi: 10.1161/01.ATV.0000189309.05924.88. [DOI] [PubMed] [Google Scholar]
- 19.Schmaier A.H. The contact activation and kallikrein/kinin systems: pathophysiologic and physiologic activities. J. Thromb. Haemost. 2016;14(1):28–39. doi: 10.1111/jth.13194. [DOI] [PubMed] [Google Scholar]
- 20.Schmaier A.H., Stavrou E.X. Factor XII - what’s important but not commonly thought about. Res. Pract. Thromb. Haemost. 2019;3(4):599–606. doi: 10.1002/rth2.12235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Noubouossie D.F., Henderson M.W., Mooberry M., Ilich A., Ellsworth P., Piegore M., et al. Red blood cell microvesicles activate the contact system, leading to factor IX activation via 2 independent pathways. Blood. 2020;135(10):755–765. doi: 10.1182/blood.2019001643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Puy C., Wong Z.C., Tucker E.I., Gruber A., Gailani D., Smith S.A., et al. FXII promotes coagulation in a FXI and FIX independent manner. Blood. 2012;120(21) doi: 10.1182/blood.V120.21.3362.3362. 3362-3362. [DOI] [Google Scholar]
- 23.Shariat-Madar Z., Mahdi F., Schmaier A.H. Recombinant prolylcarboxypeptidase activates plasma prekallikrein. Blood. 2004;103(12):4554–4561. doi: 10.1182/blood-2003-07-2510. [DOI] [PubMed] [Google Scholar]
- 24.Schmaier A.H. Assembly, activation, and physiologic influence of the plasma kallikrein/kinin system. Int. Immunopharmacol. 2008;8(2):161–165. doi: 10.1016/j.intimp.2007.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shariat-Madar Z., Mahdi F., Schmaier A.H. Identification and characterization of prolylcarboxypeptidase as an endothelial cell prekallikrein activator. J. Biol. Chem. 2002;277(20):17962–17969. doi: 10.1074/jbc.M106101200. [DOI] [PubMed] [Google Scholar]
- 26.Qadri F., Bader M. Kinin B1 receptors as a therapeutic target for inflammation. Expert Opin. Ther. Targets. 2018;22(1):31–44. doi: 10.1080/14728222.2018.1409724. [DOI] [PubMed] [Google Scholar]
- 27.Colman R.W., Schmaier A.H. Contact system: a vascular biology modulator with anticoagulant, profibrinolytic, antiadhesive, and proinflammatory attributes. Blood. 1997;90(10):3819–3843. [PubMed] [Google Scholar]
- 28.Smith D., Gilbert M., Owen W.G. Tissue plasminogen activator release in vivo in response to vasoactive agents. Blood. 1985;66(4):835–839. [PubMed] [Google Scholar]
- 29.Hong S.L. Effect of bradykinin and thrombin on prostacyclin synthesis in endothelial cells from calf and pig aorta and human umbilical cord vein. Thromb. Res. 1980;18(6):787–795. doi: 10.1016/0049-3848(80)90201-7. [DOI] [PubMed] [Google Scholar]
- 30.Su J.B. Role of bradykinin in the regulation of endothelial nitric oxide synthase expression by cardiovascular drugs. Curr. Pharm. Des. 2017;23(40):6215–6222. doi: 10.2174/1381612823666170622112253. [DOI] [PubMed] [Google Scholar]
- 31.Schmaier A.H. The kallikrein-kinin and the renin-angiotensin systems have a multilayered interaction. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003;285(1):R1–R13. doi: 10.1152/ajpregu.00535.2002. [DOI] [PubMed] [Google Scholar]
- 32.Schmaier A.H. The plasma kallikrein-kinin system counterbalances the renin-angiotensin system. J. Clin. Invest. 2002;109(8):1007–1009. doi: 10.1172/JCI15490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hasan A.A., Amenta S., Schmaier A.H. Bradykinin and its metabolite, Arg-Pro-Pro-Gly-Phe, are selective inhibitors of α-thrombin-induced platelet activation. Circulation. 1996;94(3):517–528. doi: 10.1161/01.cir.94.3.517. [DOI] [PubMed] [Google Scholar]
- 34.Vickers C., Hales P., Kaushik V., Dick L., Gavin J., Tang J., et al. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J. Biol. Chem. 2002;277(17):14838–14843. doi: 10.1074/jbc.M200581200. [DOI] [PubMed] [Google Scholar]
- 35.Brown N.J., Vaughan D.E. Prothrombotic effects of angiotensin. Adv. Intern. Med. 2000;45:419–429. [PubMed] [Google Scholar]
- 36.Nishimura H., Tsuji H., Masuda H., Nakagawa K., Nakahara Y., Kitamura H., et al. Angiotensin II increases plasminogen activator inhibitor-1 and tissue factor mRNA expression without changing that of tissue type plasminogen activator or tissue factor pathway inhibitor in cultured rat aortic endothelial cells. Thromb. Haemost. 1997;77(6):1189–1195. [PubMed] [Google Scholar]
- 37.Feener E.P., Northrup J.M., Aiello L.P., King G.L. Angiotensin II induces plasminogen activator inhibitor-1 and -2 expression in vascular endothelial and smooth muscle cells. J. Clin. Invest. 1995;95(3):1353–1362. doi: 10.1172/JCI117786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patel S.N., Fatima N., Ali R., Hussain T. Emerging role of angiotensin AT2 receptor in anti-inflammation: an update. Curr. Pharm. Des. 2020 doi: 10.2174/1381612826666200115092015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fraga-Silva R.A., Da Silva D.G., Montecucco F., Mach F., Stergiopulos N., da Silva R.F., et al. The angiotensin-converting enzyme 2/angiotensin-(1-7)/Mas receptor axis: a potential target for treating thrombotic diseases. Thromb. Haemost. 2012;108(6):1089–1096. doi: 10.1160/TH12-06-0396. [DOI] [PubMed] [Google Scholar]
- 40.Takezako T., Unal H., Karnik S.S., Node K. Current topics in angiotensin II type 1 receptor research: focus on inverse agonism, receptor dimerization and biased agonism. Pharmacol. Res. 2017;123:40–50. doi: 10.1016/j.phrs.2017.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Quitterer U., AbdAlla S. Vasopressor meets vasodepressor: the AT1-B2 receptor heterodimer. Biochem. Pharmacol. 2014;88(3):284–290. doi: 10.1016/j.bcp.2014.01.019. [DOI] [PubMed] [Google Scholar]
- 42.Quitterer U., Fu X., Pohl A., Bayoumy K.M., Langer A., AbdAlla S. Beta-Arrestin1 prevents preeclampsia by downregulation of mechanosensitive AT1-B2 receptor heteromers. Cell. 2019;176(1–2):318–333. doi: 10.1016/j.cell.2018.10.050. e319. [DOI] [PubMed] [Google Scholar]
- 43.Abadir P.M., Periasamy A., Carey R.M., Siragy H.M. Angiotensin II type 2 receptor-bradykinin B2 receptor functional heterodimerization. Hypertension. 2006;48(2):316–322. doi: 10.1161/01.HYP.0000228997.88162.a8. [DOI] [PubMed] [Google Scholar]
- 44.Cerrato B.D., Carretero O.A., Janic B., Grecco H.E., Gironacci M.M. Heteromerization between the bradykinin B2 receptor and the angiotensin-(1-7) mas receptor: functional consequences. Hypertension. 2016;68(4):1039–1048. doi: 10.1161/HYPERTENSIONAHA.116.07874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patel S., Hussain T. Dimerization of AT2 and mas receptors in control of blood pressure. Curr. Hypertens. Rep. 2018;20(5):41. doi: 10.1007/s11906-018-0845-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fraga-Silva R.A., Sorg B.S., Wankhede M., Dedeugd C., Jun J.Y., Baker M.B., et al. ACE2 activation promotes antithrombotic activity. Mol. Med. 2010;16(5–6):210–215. doi: 10.2119/molmed.2009.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bader M., Alenina N., Andrade-Navarro M.A., Santos R.A. MAS and its related G protein-coupled receptors. Mrgprs. Pharmacol Rev. 2014;66(4):1080–1105. doi: 10.1124/pr.113.008136. [DOI] [PubMed] [Google Scholar]
- 48.Santos R.A., Brosnihan K.B., Jacobsen D.W., DiCorleto P.E., Ferrario C.M. Production of angiotensin-(1-7) by human vascular endothelium. Hypertension. 1992;19(Suppl. 2):II56–II61. doi: 10.1161/01.hyp.19.2_suppl.ii56. [DOI] [PubMed] [Google Scholar]
- 49.Zhang F., Ren X., Zhao M., Zhou B., Han Y. Angiotensin-(1-7) abrogates angiotensin II-induced proliferation, migration and inflammation in VSMCs through inactivation of ROS-mediated PI3K/Akt and MAPK/ERK signaling pathways. Sci. Rep. 2016;6:34621. doi: 10.1038/srep34621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fraga-Silva R.A., Pinheiro S.V., Goncalves A.C., Alenina N., Bader M., Santos R.A. The antithrombotic effect of angiotensin-(1-7) involves mas-mediated NO release from platelets. Mol Med. 2008;14(1–2):28–35. doi: 10.2119/2007-00073.Fraga-Silva. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kucharewicz I., Pawlak R., Matys T., Pawlak D., Buczko W. Antithrombotic effect of captopril and losartan is mediated by angiotensin-(1-7) Hypertension. 2002;40(5):774–779. doi: 10.1161/01.hyp.0000035396.27909.40. [DOI] [PubMed] [Google Scholar]
- 52.Rajendran S., Chirkov Y.Y., Campbell D.J., Horowitz J.D. Angiotensin-(1-7) enhances anti-aggregatory effects of the nitric oxide donor sodium nitroprusside. J. Cardiovasc. Pharmacol. 2005;46(4):459–463. doi: 10.1097/01.fjc.0000176729.51819.a6. [DOI] [PubMed] [Google Scholar]
- 53.Sampaio W.O., Souza dos Santos R.A., Faria-Silva R., da Mata Machado L.T., Schiffrin E.L., Touyz R.M. Angiotensin-(1-7) through receptor Mas mediates endothelial nitric oxide synthase activation via Akt-dependent pathways. Hypertension. 2007;49(1):185–192. doi: 10.1161/01.HYP.0000251865.35728.2f. [DOI] [PubMed] [Google Scholar]
- 54.Muthalif M.M., Benter I.F., Uddin M.R., Harper J.L., Malik K.U. Signal transduction mechanisms involved in angiotensin-(1-7)-stimulated arachidonic acid release and prostanoid synthesis in rabbit aortic smooth muscle cells. J. Pharmacol. Exp. Ther. 1998;284(1):388–398. [PubMed] [Google Scholar]
- 55.Mitchell J.A., Kirkby N.S. Eicosanoids, prostacyclin and cyclooxygenase in the cardiovascular system. Br. J. Pharmacol. 2019;176(8):1038–1050. doi: 10.1111/bph.14167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Torres-Quesada O., Mayrhofer J.E., Stefan E. The many faces of compartmentalized PKA signalosomes. Cell. Signal. 2017;37:1–11. doi: 10.1016/j.cellsig.2017.05.012. [DOI] [PubMed] [Google Scholar]
- 57.Wang P., Liu Z., Chen H., Ye N., Cheng X., Zhou J. Exchange proteins directly activated by cAMP (EPACs): emerging therapeutic targets. Bioorg. Med. Chem. Lett. 2017;27(8):1633–1639. doi: 10.1016/j.bmcl.2017.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cuinas A., Garcia-Morales V., Vina D., Gil-Longo J., Campos-Toimil M. Activation of PKA and Epac proteins by cyclic AMP depletes intracellular calcium stores and reduces calcium availability for vasoconstriction. Life Sci. 2016;155:102–109. doi: 10.1016/j.lfs.2016.03.059. [DOI] [PubMed] [Google Scholar]
- 59.Lezoualc’h F., Fazal L., Laudette M., Conte C. Cyclic AMP sensor EPAC proteins and their role in cardiovascular function and disease. Circ. Res. 2016;118(5):881–897. doi: 10.1161/CIRCRESAHA.115.306529. [DOI] [PubMed] [Google Scholar]
- 60.Davlouros P., Xanthopoulou I., Mparampoutis N., Giannopoulos G., Deftereos S., Alexopoulos D. Role of calcium in platelet activation: novel insights and pharmacological implications. Med Chem. 2016;12(2):131–138. doi: 10.2174/157340641202160208195923. [DOI] [PubMed] [Google Scholar]
- 61.Ali F.Y., Hall M.G., Desvergne B., Warner T.D., Mitchell J.A. PPARβ/δ agonists modulate platelet function via a mechanism involving PPAR receptors and specific association/repression of PKCα--brief report. Arterioscler. Thromb. Vasc. Biol. 2009;29(11):1871–1873. doi: 10.1161/ATVBAHA.109.193367. [DOI] [PubMed] [Google Scholar]
- 62.Li Y.J., Zhu H.X., Zhang D., Li H.C., Ma P., Huang L.Y. Novel endogenous negative modulators of platelet function as potential anti-thrombotic targets. Eur. Rev. Med. Pharmacol. Sci. 2017;21(13):3146–3158. [PubMed] [Google Scholar]
- 63.Kirkby N.S., Sampaio W., Etelvino G., Alves D.T., Anders K.L., Temponi R., et al. Cyclooxygenase-2 selectively controls renal blood flow through a novel PPARβ/δ-dependent vasodilator pathway. Hypertension. 2018;71(2):297–305. doi: 10.1161/HYPERTENSIONAHA.117.09906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kojonazarov B., Luitel H., Sydykov A., Dahal B.K., Paul-Clark M.J., Bonvini S., et al. The peroxisome proliferator-activated receptor β/δ agonist GW0742 has direct protective effects on right heart hypertrophy. Pulm. Circ. 2013;3(4):926–935. doi: 10.1086/674755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.He T., Lu T., d’Uscio L.V., Lam C.F., Lee H.C., Katusic Z.S. Angiogenic function of prostacyclin biosynthesis in human endothelial progenitor cells. Circ. Res. 2008;103(1):80–88. doi: 10.1161/CIRCRESAHA.108.176057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mirza A.Z., Althagafi I.I., Shamshad H. Role of PPAR receptor in different diseases and their ligands: physiological importance and clinical implications. Eur. J. Med. Chem. 2019;166:502–513. doi: 10.1016/j.ejmech.2019.01.067. [DOI] [PubMed] [Google Scholar]
- 67.Barbieri S.S., Amadio P., Gianellini S., Tarantino E., Zacchi E., Veglia F., et al. Cyclooxygenase-2-derived prostacyclin regulates arterial thrombus formation by suppressing tissue factor in a sirtuin-1-dependent-manner. Circulation. 2012;126(11):1373–1384. doi: 10.1161/CIRCULATIONAHA.112.097295. [DOI] [PubMed] [Google Scholar]
- 68.Ghosh M., Wang H., Ai Y., Romeo E., Luyendyk J.P., Peters J.M., et al. COX-2 suppresses tissue factor expression via endocannabinoid-directed PPARδ activation. J. Exp. Med. 2007;204(9):2053–2061. doi: 10.1084/jem.20070828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Atsuta H., Uchiyama T., Kanai H., Iso T., Tanaka T., Suga T., et al. Effects of a stable prostacyclin analogue beraprost sodium on VEGF and PAI-1 gene expression in vascular smooth muscle cells. Int. J. Cardiol. 2009;132(3):411–418. doi: 10.1016/j.ijcard.2007.12.119. [DOI] [PubMed] [Google Scholar]
- 70.Peshavariya H.M., Liu G.S., Chang C.W., Jiang F., Chan E.C., Dusting G.J. Prostacyclin signaling boosts NADPH oxidase 4 in the endothelium promoting cytoprotection and angiogenesis. Antioxid. Redox Signal. 2014;20(17):2710–2725. doi: 10.1089/ars.2013.5374. [DOI] [PubMed] [Google Scholar]
- 71.Yu Y., Ricciotti E., Scalia R., Tang S.Y., Grant G., Yu Z., et al. Vascular COX-2 modulates blood pressure and thrombosis in mice. Sci. Transl. Med. 2012;4(132) doi: 10.1126/scitranslmed.3003787. 132ra154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Grosser T., Fries S., FitzGerald G.A. Biological basis for the cardiovascular consequences of COX-2 inhibition: therapeutic challenges and opportunities. J. Clin. Invest. 2006;116(1):4–15. doi: 10.1172/JCI27291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kane A.E., Sinclair D.A. Sirtuins and NAD(+) in the Development and Treatment of Metabolic and Cardiovascular Diseases. Circ. Res. 2018;123(7):868–885. doi: 10.1161/CIRCRESAHA.118.312498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Winnik S., Auwerx J., Sinclair D.A., Matter C.M. Protective effects of sirtuins in cardiovascular diseases: from bench to bedside. Eur. Heart J. 2015;36(48):3404–3412. doi: 10.1093/eurheartj/ehv290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Breitenstein A., Stein S., Holy E.W., Camici G.G., Lohmann C., Akhmedov A., et al. Sirt1 inhibition promotes in vivo arterial thrombosis and tissue factor expression in stimulated cells. Cardiovasc. Res. 2011;89(2):464–472. doi: 10.1093/cvr/cvq339. [DOI] [PubMed] [Google Scholar]
- 76.Wu Z., Liu M.C., Liang M., Fu J. Sirt1 protects against thrombomodulin down-regulation and lung coagulation after particulate matter exposure. Blood. 2012;119(10):2422–2429. doi: 10.1182/blood-2011-04-350413. [DOI] [PubMed] [Google Scholar]
- 77.Wu Q., Hu Y., Jiang M., Wang F., Gong G. Effect of autophagy regulated by Sirt1/FoxO1 pathway on the release of factors promoting thrombosis from vascular endothelial cells. Int. J. Mol. Sci. 2019;20(17) doi: 10.3390/ijms20174132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yao X., Chen W., Liu J., Liu H., Zhan J.Y., Guan S., et al. Deep vein thrombosis is modulated by inflammation regulated via sirtuin 1/NF-kappaB signalling pathway in a rat model. Thromb. Haemost. 2019;119(3):421–430. doi: 10.1055/s-0038-1676987. [DOI] [PubMed] [Google Scholar]
- 79.Gaul D.S., Weber J., van Tits L.J., Sluka S., Pasterk L., Reiner M.F., et al. Loss of Sirt3 accelerates arterial thrombosis by increasing formation of neutrophil extracellular traps and plasma tissue factor activity. Cardiovasc. Res. 2018;114(8):1178–1188. doi: 10.1093/cvr/cvy036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shariat-Madar Z., Mahdi F., Warnock M., Homeister J.W., Srikanth S., Krijanovski Y., et al. Bradykinin B2 receptor knockout mice are protected from thrombosis by increased nitric oxide and prostacyclin. Blood. 2006;108(1):192–199. doi: 10.1182/blood-2006-01-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Renne T., Pozgajova M., Gruner S., Schuh K., Pauer H.U., Burfeind P., et al. Defective thrombus formation in mice lacking coagulation factor XII. J. Exp. Med. 2005;202(2):271–281. doi: 10.1084/jem.20050664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Muller F., Mutch N.J., Schenk W.A., Smith S.A., Esterl L., Spronk H.M., et al. Platelet polyphosphates are proinflammatory and procoagulant mediators in vivo. Cell. 2009;139(6):1143–1156. doi: 10.1016/j.cell.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mailer R.K.W., Hanel L., Allende M., Renne T. Polyphosphate as a Target for Interference With Inflammation and Thrombosis. Front. Med. (Lausanne) 2019;6(76) doi: 10.3389/fmed.2019.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhou G., Hamik A., Nayak L., Tian H., Shi H., Lu Y., et al. Endothelial Kruppel-like factor 4 protects against atherothrombosis in mice. J. Clin. Invest. 2012;122(12):4727–4731. doi: 10.1172/JCI66056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nomura S. Microparticle and atherothrombotic diseases. J. Atheroscler. Thromb. 2016;23(1):1–9. doi: 10.5551/jat.32326. [DOI] [PubMed] [Google Scholar]
- 86.Kushak R.I., Nestoridi E., Lambert J., Selig M.K., Ingelfinger J.R., Grabowski E.F. Detached endothelial cells and microparticles as sources of tissue factor activity. Thromb. Res. 2005;116(5):409–419. doi: 10.1016/j.thromres.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 87.Freyssinet J.M., Toti F. Formation of procoagulant microparticles and properties. Thromb. Res. 2010;125(Suppl. 1):S46–S48. doi: 10.1016/j.thromres.2010.01.036. [DOI] [PubMed] [Google Scholar]
- 88.Hagedorn I., Schmidbauer S., Pleines I., Kleinschnitz C., Kronthaler U., Stoll G., et al. Factor XIIa inhibitor recombinant human albumin Infestin-4 abolishes occlusive arterial thrombus formation without affecting bleeding. Circulation. 2010;121(13):1510–1517. doi: 10.1161/CIRCULATIONAHA.109.924761. [DOI] [PubMed] [Google Scholar]