Abstract
Introduction
Helicobacter pylori (H. pylori) is a Gram-negative bacteria that is harbored in the stomach and linked to chronic gastritis, peptic ulcer disease, and gastric malignancy. Most Helicobacter infections are acquired during early infancy. This study aimed to establish the prevalence of H. pylori infection in Jordanian children using the 13C-urea breath test.
Materials and methods
We prospectively enrolled children between the ages of 4 and 17 years from April 2019 to July 2019. Enrolled children were patients with nongastrointestinal complaints at the pediatric clinics of two hospitals and at community centers caring for healthy children in Irbid, Jordan. Questionnaires obtaining data on sociodemographics, clinical symptomatology, and hygienic risk factors were completed. Recruited children underwent a urea breath test (UBT).
Results
Of 340 children who were recruited, 328 (96.5%) were included in the final analysis. The mean age (±standard deviation) was 9.56 (±3.98) years (range, 4.0–17 years), and 168 (51.2%) were males. Only 48 children (14.6%) tested positive. There were no gender differences. Living in an urban area and a family history of previous H. pylori infection were risk factors for the acquisition of infection (P = 0.007 and 0.001, respectively). Although gastrointestinal symptoms were more common in H. pylori-infected children, only hiccups and constipation were statistically significant (P = 0.035 and 0.038, respectively).
Conclusion
H. pylori infects at least 15% of Jordanian children, suggesting a significant drop in infection rates in this group. Larger-scale studies combined with clinical evaluations will be important for further understanding the reasons for the observed decrease in H. pylori infections in Jordanian children.
Keywords: Health sciences, Gastrointestinal system, Infectious disease, Pediatrics, Laboratory medicine, 13C-urea breath test, Infection, Children, Helicobacter pylori, Jordan
Health Sciences; Gastrointestinal System; Infectious Disease; Pediatrics; Laboratory Medicine; 13C-Urea Breath Test, Infection, Children, Helicobacter pylori, Jordan.
1. Introduction
Helicobacter pylori (H. pylori) is a Gram-negative microaerophilic bacillus bacterium that infects the stomach epithelium [1]. H. pylori organisms possess specialized characteristics that allow them to withstand the very acidic environment of the stomach. For example, flagella facilitate H. pylori mobility to the mucus layer at the surface of the gastric mucosa, contributing to bacterial colonization, inflammation, and immune evasion [2]. In addition, H. pylori produces urease, an enzyme that promotes bacterial colonization and is used in the clinic as a biomarker of H. pylori infection as part of the rapid urease test [2]. H. pylori infection contributes to many gastroduodenal diseases, including peptic ulcers (gastric and duodenal), chronic gastritis, gastric mucosa-associated lymphoid tissue lymphoma, and gastric cancer [3]. Although much is known about the association of H. pylori infection with gastrointestinal symptoms in adults, its role in children is less clear [4, 5, 6, 7]. Several studies have shown that the worldwide prevalence of H. pylori infection is high, and some have estimated that 50% or more of the world's population is infected [7, 8]; moreover, the infection rate/prevalence may reach as high as 70% in developing countries [7]. H. pylori infection is largely acquired at an early age and persists to adulthood, as spontaneous clearance is unusual [9]. Factors such as age, gender, ethnicity, and a variety of socioeconomic indicators are associated with the prevalence of H. pylori infection [10, 11, 12, 13]. In addition to overcrowded settings, low socioeconomic status is one of the main factors that puts children at risk of acquiring the infection [14, 15]. This association is evident in those living in developing countries, whereas socioeconomic status in developed countries does not have a notable role in H. pylori infection [16, 17]. Risk of infection by H. pylori reflects exposure during the early years of life [18]; the exact route of transmission is still unknown, despite multiple attempts to uncover the mechanism [19]. Because crowding is a main risk factor for infection and H. pylori infection occurs mainly within families, it has been hypothesized that person-to-person spread is the most likely mode of transmission [20]. Fecal-oral, oral-oral, and gastro-oral transmission routes are also probable, particularly since H. pylori can be isolated from feces, saliva, and vomitus respectively [21, 22, 23]. Given the role of H. pylori infection in gastrointestinal disease, a precise diagnosis of H. pylori infection is critical for treating various gastrointestinal symptoms and preventing serious complications [24].
Diagnostic testing for H. pylori can be either invasive (requiring endoscopy) or non-invasive. Non-invasive testing itself can be either active or passive [3]. An example of the latter is serology testing for antibodies against H. pylori. A positive result can indicate a previously eradicated infection or an existent one but cannot differentiate between the two; this can lead to a higher false positive rate [25]. Use of serology for diagnosis is therefore not recommended in areas where infection is known to be common or for the diagnosis of children [26]. Examples of active testing for H. pylori include the urea breath test (UBT) and stool antigen test [19], both of which are helpful for initial diagnosis and for evidence of eradication. The UBT has a sensitivity and specificity of over 90%, although these results may be inaccurate in patients taking certain medications (proton pump inhibitors or antibiotics) or in those who have undergone previous gastric surgeries [27].
The only study measuring the prevalence of H. pylori infection among schoolchildren in Northern Jordan using serology was performed in 2006 [28]. This study found a concomitant overall seroprevalence rate of 55.5% and concluded that the prevalence of H. pylori infection in children was high. A study in Iraq within the same time period estimated the seroprevalence of H. pylori infection at 27% in young Iraqi children, reaching 58% in children between 2 and 18 years of age [29]. A study from Saudia Arabia reported that H. pylori infected almost one-third of Saudi children under the age of 10 years [30]. A recent study from Egypt reported the overall rate of H. pylori infection in symptomatic children who visited a gastroenterology clinic to be 64.6%. This study depended on the detection of H. pylori antigen in stool [31]. In Lebanon, Naous et al. reported that almost one-fifth of Lebanese children harbor H. pylori, which was evident by the detection of bacterial antigen in stool [32].
A new nationwide study of H. pylori infection in healthy Jordanian adults by Obeidat and Roess [33] reported a seroprevalence of 89%. Despite this high rate, Jordan is considered a low-risk area for gastric cancer, with an age-standardized rate (ASR) of gastric malignancies at 3.9 per 100,000 population [34]. As serological testing is unable to distinguish between active infection and previous exposure, we performed the present study using the UBT. Our goal was to establish a better estimate of the prevalence of H. pylori infection in children living in North Jordan and identify risk factors for infection.
2. Materials and methods
2.1. Patients
We prospectively enrolled children between the ages of 4 and 17 years from April 2019 to July 2019. Enrolled patients were attendees at pediatric clinics at King Abdullah University Hospital and Princess Rahma Teaching Hospital and at community centers caring for healthy children in Irbid, Jordan. Irbid is the main agricultural city in North Jordan with a population of approximately 1,770,158 citizens living in 9 counties and 18 municipalities. It is uniquely characterized by a mixture of urban and rural sub-communities, which were represented by the study participants. Children were included following parental consent.
2.2. Inclusion criteria
-
1.
Children between 4 and 17 years of age
-
2.
Children not currently on medications
-
3.
Children presenting with nongastrointestinal complaints
-
4.
Children who agreed to participate in the study
2.3. Exclusion criteria
-
1.
Children who refused to drink the reagent
-
2.
Children who are currently on medications that might affect the results of the test (acid suppressant therapy, antibiotics, etc.)
-
3.
Children presenting with gastrointestinal complaints
-
4.
Children with a history of a previous abdominal surgery
-
5.
Children with developmental issues preventing them from comprehending the test
-
6.
Children younger than 4 years of age
-
7.
Children/parents who refused to participate
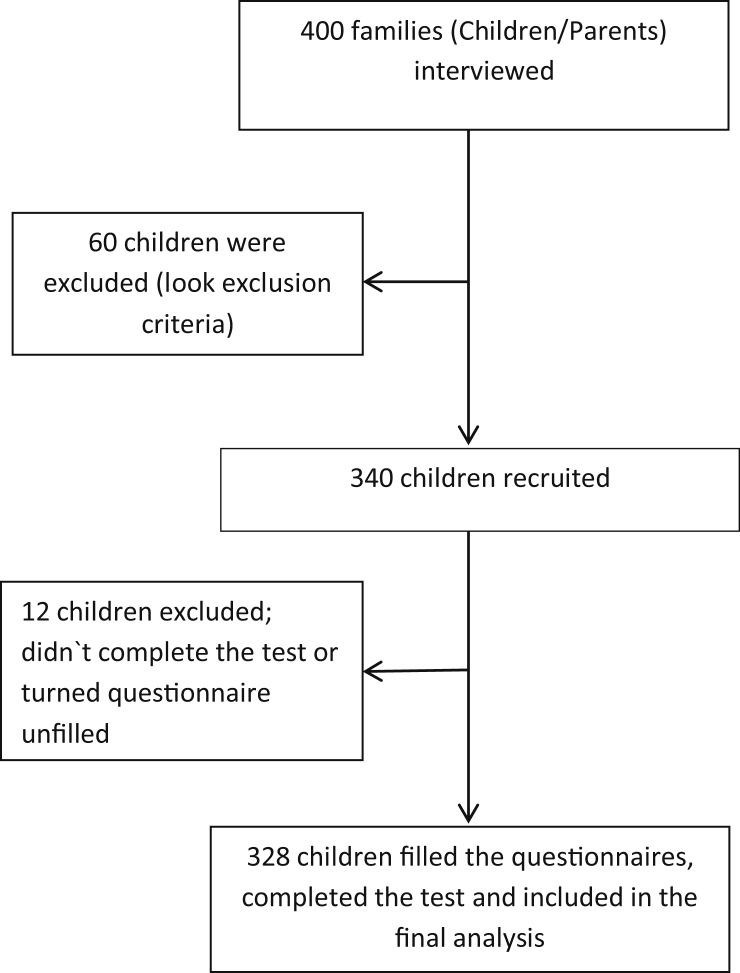
Participating parents and children were interviewed. Participants were asked to fill out a questionnaire about the child's age, sex, family size, housing (size and drinking water source), income, level of education, and the medical history of the family and child. Information regarding the eating habits of the child and hygiene-related behaviors were also collected. Parents willing to know the results of their child's test were asked to provide their contact number (Figure 1).
Figure 1.
Study recruitment process.
2.4. Urea breath test
Recruited children were asked to undergo a UBT. Study participants were first asked to provide a baseline sample of exhaled air at 0 min (test bag). Patients were then asked to drink 75 mg of a 13C-labeled urea substrate (mixed with water according to the manufacturer's recommendations). Thirty minutes later, patients filled a bag with exhaled air. The two samples were then processed simultaneously using a 12/13-CO2 breath test analyzer (IR-Force 2000, Beijing Richen-force and Technology Co., LTD, Chaoyang District, Beijing, P.R. China). The test was considered positive if the delta over the baseline value was >4.0%. The test was run by a trained member of the research team.
2.5. Statistics
Data were entered into a spreadsheet. Statistical analyses were performed using appropriate software. For categorical variables, data were presented as frequency distributions; for continuous variables, data were presented as mean ± standard error of the mean. A significance level of 0.05% was used. To assess associations between categorical variables, a Pearson χ2 test was used, whereas for continuous normally distributed variables, a Student's t-test and ANOVA were used. Stepwise linear regression analysis was performed to include the important independent variable only. Risk factors that were significant in the univariate analysis were used in the multiple logistic regression models. All tests were considered statistically significant at P values less than 0.05.
2.6. Ethical approval
This study was approved by the institutional review board of the Jordan University of Science and Technology (no. 36/117/2018) and the ethics committee of MOH (no. MOH REC 1900049).
3. Results
Of the 340 children recruited for the study, 328 (96.5%) were included in the final analysis. Twelve children were excluded due to either submitting an incomplete questionnaire or not completing the test. The average age was 9.561 ± 3. 955 years and 168 (51.2%) were males (Table 1). Based on UBTs, we determined that the prevalence of H. pylori infection in this cohort was 14.6% (48/328). Although males were more commonly affected compared with females (54.2% vs. 45.8%), this trend was not statistically significant (Table 1).
Table 1.
Prevalence of sociodemographics and familial and hygienic risk factors, according to the results of the 13-C urea breath test.
| Predictor of infection | Negative -ve |
Positive + ve |
P value | ||
|---|---|---|---|---|---|
| Frequency | Percentage 95% CI | Frequency | Percentage 95% CI | ||
| Age | |||||
| 4–5 | 48 | 17.1 (16.0–18.8) |
8 | 16.7 (6.6–26.8) |
NS† |
| 6–11 | 124 | 44.3 (42.1–46.5) |
25 | 52.1 (38.5–65.7) |
|
| 12–17 | 108 | 38.6 (36.4–40.8) |
15 | 31.3 (18.7–43.9) |
|
| Gender | |||||
| Male | 142 | 50.9 (48.6–53.2) |
26 | 54.2 40.7–68.4) |
NS† |
| Female | 137 | 49.1 (46.8–51.4) |
22 | 45.8 (32.3–59.3) |
|
| School | |||||
| No School | 33 | 12 (10.4–13.6) |
5 | 10.4 (2.1–18.7) |
NS† |
| Public | 138 | 50.2 (47.8–52.6) |
26 | 54.2 (40.7–67.7) |
|
| Private | 52 | 18.9 (17.0–20.8) |
13 | 27.1 (15.0–39.2) |
|
| UNRWA | 52 | 18.9 (17.0–20.8) |
4 | 8.3 (0.8–15.8) |
|
| Grade | |||||
| Primary | 172 | 70.8 (67.9–73.7) |
35 | 77.8 (66.0–89.6) |
NS† |
| Secondary | 71 | 29.2 (26.3–32.1) |
10 | 22.2 (10.4–34.0) |
|
| City | |||||
| Urban Area | 154 | 55 (53.2–57.6) |
38 | 79.2 (68.2–90.2) |
0.007 |
| Rural Area | 126 | 44.6 (42.4–46.8) |
10 | 20.8 (9.8–31.8) |
|
| Water Source | |||||
| Filtered + Bottled Water | 190 | 67.9 (65.8–70.1) |
27 | 56.3 (42.8–69.8) |
NS† |
| Municipal + Well Water | 90 | 32.1 (30.0–34.2) |
21 | 43.8 (30.3–57.3) |
|
| Monthly Financial Income | |||||
| >700 US $ | 165 | 58.9 (56.7–61.1) |
32 | 66.7 (53.9–79.5) |
NS† |
| >700 US $ | 115 | 41 (38.9–43.3) |
16 | 33.4 (20.6–46.2) |
|
| Mother Educational Level | |||||
| School | 152 | 54.3 (52.1–56.6) |
22 | 45.8 (32.3–59.3) |
NS† |
| Post School | 128 | 45.7 (43.4–48.0) |
26 | 54.2 (40.7–67.7) |
|
| Father Educational Level | |||||
| School | 167 | 59.6 (57.4–61.8) |
32 | 66.7 (53.9–79.5) |
NS† |
| Post School | 113 | 40.4 (38.2–42.6) |
16 | 33.3 (20.5–46.1) |
|
| Any Smoker at Home | |||||
| No | 107 | 38.2 (36.0–40.4) |
16 | 33.3 (20.5–46.1) |
NS† |
| Yes |
173 |
61.8 (59.6–64.0) |
32 |
66.7 (53.9–79.5) |
|
|
Family History: | |||||
| Heartburn | |||||
| No | 176 | 62.9 (60.7–65.1) |
27 |
56.3 (42.8–69.8) |
NS† |
| Yes | 104 | 37.1 (34.9–39.3) |
21 |
43.8 (30.3–57.3) |
|
| Chronic Stomach Pain | |||||
| No | 176 | 62.9 (60.7–65.1) |
26 | 54.2 (40.8–67.8) |
NS† |
| Yes | 104 | 37.1 (34.9–39.3) |
22 | 45.8 (32.3–59.3) |
|
| H. pylori/Tt with Triple Therapy in Family Member | |||||
| No | 248 | 88.6 (87.2–90.0) |
33 | 68.8 (56.2–81.4) |
0.001 |
| Yes | 32 | 11.4 (10.0–12.8) |
15 | 31.2 (18.6–43.8) |
|
| Gastric/Intestinal Ulcer | |||||
| No | 245 | 87.5 (86.0–90.0) |
41 | 85.4 (75.8–95.0) |
NS† |
| Yes | 35 | 12.5 (11.0–14.0) |
7 | 14.6 (5.0–24.2) |
|
| Gastric Cancer | |||||
| No | 265 | 94.6 (93.6–99.9) |
47 | 97.9 (94.0–101.8) |
NS† |
| Yes | 15 | 5.4 (0.1–0.7) |
1 | 2.1 (1.8–6.0) |
|
| Intestinal Cancer | |||||
| No | 279 | 99.6 (98.6–99.4) |
48 | 100 (99.9–100.0) |
NS† |
| Yes |
1 |
0.4 (0.1–0.7) |
0 |
0 (–0.01–0.01) |
|
|
Hygienic Habits | |||||
| Buying Food from Hawkers around Home/School | |||||
| No | 110 | 45.5 (42.3–48.7) |
21 | 47.7 (33.4–62.0) |
NS† |
| Yes | 132 | 54.5 (51.2–57.7) |
23 | 52.3 (34.0–66.6) |
|
| Using School Toilets | |||||
| No | 124 | 52.3 (48.9–55.7) |
11 | 28.2 (14.3–42.1) |
0.004 |
| Yes | 113 | 47.7 (44.3–51.1) |
28 | 71.8 (57.9–85.7) |
|
| Drinking Tap Water at School | |||||
| No | 207 | 87.7 (85.5–89.9) |
34 | 87.2 (76.9–97.5) |
NS† |
| Yes | 29 | 12.3 (10.1–14.5) |
5 | 12.8 (2.5–23.1) |
|
| Hands Washed after Coming Back Home (Back from School) | |||||
| No | 36 | 14.7 (12.4–17.0) |
14 | 31.8 (18.4–45.2) |
0.008 |
| Yes | 209 | 85.3 (83.0–87.6) |
30 | 68.2 (54.8–81.6) |
|
| Hands Washed before Meal | |||||
| No | 60 | 21.7 (19.8–23.6) |
7 | 14.6 (5.0–24.2) |
NS† |
| Yes | 217 | 78.3 (76.4–80.2) |
41 | 85.4 (75.8–95.0) |
|
| Hands Washed after Meal | |||||
| No | 20 | 7.2 (6.0–8.4) |
5 | 10.4 (2.1–18.7) |
NS† |
| Yes | 256 | 92.8 (91.6–94.0) |
43 | 89.6 (81.3–98.0) |
|
| Hands Washed after Using Toilet | |||||
| No | 17 | 6.1 (5.0–7.2) |
6 | 12.8 (3.6–22.0) |
NS† |
| Yes | 260 | 93.9 (92.5–94.8) |
41 | 87.2 (78.0–96.4) |
|
| Hands Washed after Contact with Animals | |||||
| No | 63 | 24.8 (22.3–27.3) |
12 | 28.6 (15.3–41.9) |
NS† |
| Yes | 191 | 75.2 (72.3–77.7) |
30 | 71.4 (58.1–84.7) |
|
| Sharing Cup/Dish/Towel | |||||
| No | 111 | 40.2 (37.9–42.5) |
18 | 37.5 (24.3–50.7) |
NS† |
| Yes | 165 | 59.8 (57.5–62.1) |
30 | 62.5 (49.3–75.7) |
|
| Eating in One Dish with Family | |||||
| No | 96 | 34.7 (37.9–42.5) |
18 | 37.5 (24.3–50.7) |
NS† |
| Yes | 181 | 65.3 (63.1–67.5) |
30 | 62.5 (49.3–75.7) |
|
Numbers might not add up due to some missing data.
†NS, not statistically significant.
3.1. Risk factors
We found that residing in the urban side of the governorate was associated with higher rates of H. pylori infection compared with non-urban areas (79.2% vs. 20.8%; P = 0.007). A family history of H. pylori infection or previous treatment for H. pylori infection within the family was a risk factor for acquiring the infection (P = 0.001). Parental education levels, family history of chronic gastrointestinal symptoms, or gastric cancers were not associated with increased risk of H. pylori infection in our cohort. Additionally, we found that the source of drinking water was not a statistically significant risk factor (Table 1). Personal hygiene, using the school washroom, and not washing hands upon arriving home from school were also risk factors for H. pylori infection (P = 0.004 and 0.008, respectively) (Table 1).
3.2. Symptomatology
Although heartburn, epigastric pain, recurrent chest pain, early satiety, and halitosis were reported more frequently by children infected with H. pylori in this study, these symptoms did not reach statistical significance. However, excessive hiccups and constipation were significantly more common in infected children (P = 0.038 and 0.041, respectively). Finally, reported atopic manifestations were not significantly negatively associated with H. pylori infection in children in this study (Table 2).
Table 2.
Comparison of symptoms’ prevalence according to H. pylori infection status.
| Predictor of infection | Negative -ve |
Positive + ve |
P value | ||
|---|---|---|---|---|---|
| Frequency | Percentage 95% CI |
Frequency | Percentage 95% CI |
||
| Heart Burn | |||||
| No | 262 | 93.6 (92.5–94.7) |
44 | 91.7 (84.2–99.2) |
NS† |
| Yes | 18 | 6.4 (5.3–7.5) |
4 | 8.3 (0.8–15.8) |
|
| Epigastric Pain | |||||
| No | 221 | 78.9 (77.1–80.7) |
35 | 72.9 (60.8–85.0) |
NS† |
| Yes | 59 | 21.1 (19.3–23.0) |
13 | 27.1 (15.0–39.2) |
|
| Recurrent Chest Pain | |||||
| No | 264 | 94.3 (93.3–95.3) |
44 | 91.7 (84.2–99.2) |
NS† |
| Yes | 16 | 5.7 (4.7–6.7) |
4 | 8.3 (0.8–15.8) |
|
| Flatulence | |||||
| No | 237 | 84.6 (83.0–86.2) |
45 | 93.8 (87.2–100.4) |
NS† |
| Yes | 43 | 15.4 (13.8–17.0) |
3 | 6.3 (–0.3–9.7) |
|
| Nausea | |||||
| No | 255 | 91.1 (89.8–92.4) |
46 | 95.8 (90.3–101.3) |
NS† |
| Yes | 25 | 8.9 (7.6–10.2) |
2 | 4.2 (–1.3–9.7) |
|
| Epigastric Satiety | |||||
| No | 213 | 76.1 (74.2–78.1) |
35 | 72.9 (60.8–85.0) |
NS† |
| Yes | 67 | 23.9 (22.0–25.8) |
13 | 27.1 (15.0–39.2) |
|
| Vomiting | |||||
| No | 267 | 95.4 (94.5–96.3) |
47 | 97.9 (94.0–101.8) |
NS† |
| Yes | 13 | 4.6 (3.7–5.5) |
1 | 2.1 (–1.8–6.0) |
|
| Halitosis | |||||
| No | 225 | 80.4 (78.6–82.2) |
34 | 70.8 (58.4–83.2) |
NS† |
| Yes | 55 | 19.6 (17.8–21.4) |
14 | 29.2 (16.8–41.6) |
|
| Sore Throat | |||||
| No | 233 | 83.2 (81.5–84.9) |
39 | 81.3 (70.7–91.9) |
NS† |
| Yes | 47 | 16.8 (15.1–18.5) |
9 | 18.8 (8.2–24.4) |
|
| Chronic Cough | |||||
| No | 259 | 92.5 (91.3–93.7) |
46 | 95.8 (90.3–101.3) |
NS† |
| Yes | 21 | 7.5 (6.3–8.7) |
2 | 4.2 (–1.3–9.7) |
|
| Excessive Hiccup | |||||
| No | 270 | 96.4 (95.6–97.2) |
43 | 89.6 (81.3–98.0) |
NS† |
| Yes | 10 | 3.6 (2.8–4.4) |
5 | 10.4 (2.1–18.7) |
|
| Recurrent Bronchitis | |||||
| No | 258 | 92.1 (90.9–93.3) |
43 | 89.6 (81.3–98.0) |
NS† |
| Yes | 22 | 7.9 (9.7–9.1) |
5 | 10.4 (2.1–18.7) |
|
| Recurrent Burp | |||||
| No | 267 | 95.4 (90.9–93.3) |
47 | 97.9 (94.0–101.8) |
NS† |
| Yes | 13 | 4.6 (6.7–9.1) |
1 | 2.1 (–1.8–6.0) |
|
| Constipation | |||||
| No | 264 | 94.3 (93.3–95.3) |
41 | 85.4 (75.8–95.0) |
0.035 |
| Yes | 16 | 5.7(5.2–7.4) | 7 | 14.6 (5.0–24.2) |
|
| Diarrhea | |||||
| No | 272 | 97.1 (96.3–97.9) |
47 | 97.3 (92.9–101.7) |
NS† |
| Yes | 8 | 2.9 (2.1–3.7) |
1 | 2.7 (–1.8–6.4) |
|
| Allergy Eye/Skin | |||||
| No | 254 | 90.7(89.4–92.0) | 45 | 93.8 (87.2–100.4) |
NS† |
| Yes | 26 | 9.3(8.0–10.6) | 3 | 6.3 (–0.3–13.0) |
|
| Allergy/Asthma | |||||
| No | 261 | 93.2 (92.1–94.3) |
45 | 93.8 (87.2–100.4) |
NS† |
| Yes | 19 | 6.8 (5.7–7.9) |
3 | 6.3 (–0.3–12.9) |
|
Numbers may not add up due to missing data.
NS†, not statistically significant.
4. Discussion
H. pylori is an important pathogen with an established association with gastritis, peptic ulcer disease, and gastric malignancy [3]. Initial H. pylori infection likely occurs during early childhood, with the bacteria inhabiting the stomach [13]. The UBT is an accurate test for detection of H. pylori infection with high specificity and sensitivity. Importantly, compared to serology testing, the UBT has a higher specificity, and particularly in children, the non-invasive aspect of the test is more appealing [35].
This is the first study from Jordan that assesses the most well-known risk factors of H. pylori infection in children using the UBT as the testing modality. Our results demonstrate that the prevalence of H. pylori infection in this cohort is 14.6%. This number is much lower than previous estimates at 55% from 13 years ago [28], as well as what is reported from neighboring countries [29, 30, 31] and developing countries [36, 37, 38, 39]. We believe the differences between our results and those of previous reports from Jordan are multifactorial. First, the method of detection was different; Bani Hani et al. [28] used serological testing, whereas our study used the UBT, which is more specific for H. pylori infection. In a recent systematic review by Hooi and colleagues [40], a drop in the reported rates of H. pylori infections—regardless of the diagnostic methodology—was observed in developed and some developing countries. The authors concluded that this was due to improved sanitation and hygienic practices. In Jordan, we believe the sanitation conditions have improved significantly over the last decade. Currently, 97 % of the Jordanian population has access to safely managed sanitation services, whereas 99% have access to safely managed drinking water [41]. This effect was not seen in the adult population [34], which is expected, as most H. pylori infections are acquired during childhood. We expect that such an effect will require a longer duration of study.
Moreover, there is widespread use of unprescribed antibiotics in Jordan. Almaayta and colleagues [42] reported that of 202 pharmacies visited, 150 dispensed antibiotics without a prescription, mainly for the treatment of sore throat, urinary symptoms, and diarrhea. Another study reported that one third of antibiotics dispensed at local pharmacies were unprescribed [43]. A cross-sectional study from Irbid done by Yusef and colleagues reported that almost 40% of the participants who received antibiotics did not have a prescription [44]. Although patients receiving antibiotics were excluded from our study, previous exposure to antibiotics could have led to increased clearance or suppression of H. pylori, resulting in a negative UBT and lowering the observed prevalence. Although the questionnaire asked about the recent use of antibiotics, no differences were found between infected and uninfected children. This finding might reflect a recall bias as well as uncertainty about the specific antibiotic used, as we didn't ask specifically about the type of antibiotics. On the other hand, the strict exclusion of children presenting with gastrointestinal symptoms might also contribute to the low prevalence of H. pylori in our study population.
It is believed that the risk factors for childhood infection with H. pylori vary among distinct populations. Identified risk factors in some populations may not be risk factors for others of a different ethnicity or living in different household conditions or locations [18]. Overall, socioeconomic status is the main risk factor in developing countries [18].
H. pylori infection was more common in children from families with a lower economic status, and the infection rate decreased with increased family income; however, it did not reach statistical significance (P > 0.05). This is consistent with a new report on the seroprevalence of H. pylori in Jordanian adults, in whom higher rates of positive infection were associated with lower income; these results also did not reach statistical significance [33].
The association of male or female sex with H. pylori infection is debated. A systematic review by Zamani et al. [8] found there were no differences in H. pylori infection between males and females. In contrast, Ibrahim and colleagues [45] reported a male predominance. In addition to the two seroprevalence studies from Jordan [28, 33], our study also showed male predominance, but this observation was not statistically significant.
Parental education level and number of siblings were reported as risk factors for H. pylori infection by previous groups [46]. Our study did not confirm this relationship, consistent with previous studies by Chi et al. [16] and Roma et al. [17] in Taiwanese and Greek children, respectively. Additionally, unhygienic behavior, including not washing hands after school and eating unwashed fruits and vegetables, were risk factors for H. pylori infection, consistent with recent reports from Poland [47]. On the other hand, living in an urban area was a risk factor for the acquisition of H. pylori infection in our study. This finding is consistent with those of previous reports from Vietnam [48], Nepal [49], and Mexico [50].
Attempts to establish the exact route of H. pylori transmission have not been conclusive. It has been hypothesized that person-to-person spread as well as fecal-oral, oral-oral, and gastro-oral are the likely modes of transmission. In our study, the drinking water source was not associated with increased risk of infection with H. pylori, which was also reported by the recent nationwide seroprevalence study from Jordan [33]. These results likely reflect improvements in sanitation and wide accessibility to clean water [41].
Manifestations of H. pylori infection in children have been debated [4, 5, 6, 7, 49]. In our cohort, we found no association between the majority of gastrointestinal symptoms and being infected with H. pylori. Although a weak correlation was previously reported in Polish children [51], our findings were limited by the fact that recall bias cannot be excluded, as no proper medical evaluation was performed and medical records were not reviewed. On the other hand, our selection criteria of excluding patients presenting primarily with gastrointestinal complaints might further suppress this correlation, if present. The association of constipation with H. pylori infection may reflect a common presence rather than a real association.
This study is the first of its type in our population and the first to report a significant decline in the prevalence of H. pylori in children of a developing country. However, it carries inherent weaknesses: the study participants were not evaluated medically, infections were not confirmed by further testing, and the study results might have been affected by recall bias. A future study that includes a larger number of children is needed to confirm our results.
Declarations
Author contribution statement
E. Altamimi: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
N. Alsharkhat: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
A. AlJawarneh, A. Assi, S. Alawneh and M. Al-Ahmad: Performed the experiments; Contributed reagents, materials, analysis tools or data.
M. Hamad: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This project was funded by the research grant number (20180403), Deanship of Scientific Research, Jordan University of Science and Technology.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors want to thank Dr. Abdelwahab Aleshawi for help performing statistical analyses.
References
- 1.Warren J., Marshall B. Unidentified curved bacillus on gastric epithelium in chronic active gastritis. Lancet. 1983;1:1273–1275. [PubMed] [Google Scholar]
- 2.Camilo V., Sugiyama T., Touati E. Pathogenesis of Helicobacter pylori infection. Helicobacter. 2017;22(Suppl 1) doi: 10.1111/hel.12405. [DOI] [PubMed] [Google Scholar]
- 3.Ernst P.B., Gold B.D. Helicobacter pylori in childhood: new insights into the immunopathogenesis of gastric disease and implications for managing infection in children. J. Pediatr. Gastroenterol. Nutr. 1999;28:462–473. doi: 10.1097/00005176-199905000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Wotherspoon A.C., Ortiz-Hidalgo C., Falzon M.R. Helicobacter pylori-associated gastritis and primary B-cell gastric lymphoma. Lancet. 1991;338(8776):1175–1176. doi: 10.1016/0140-6736(91)92035-z. [DOI] [PubMed] [Google Scholar]
- 5.McColl K.E. Clinical practice. Helicobacter pylori infection. N. Engl. J. Med. 2010;362(17):1597–1604. doi: 10.1056/NEJMcp1001110. [DOI] [PubMed] [Google Scholar]
- 6.Wroblewski L.E., Peek R.M., Jr., Wilson K.T. Helicobacter pylori and gastric cancer: factors that modulate disease risk. Clin. Microbiol. Rev. 2010;23(4):713–739. doi: 10.1128/CMR.00011-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suerbaum S., Michetti P. Helicobacter pylori infection. N. Engl. J. Med. 2002;347(15):1175–1186. doi: 10.1056/NEJMra020542. [DOI] [PubMed] [Google Scholar]
- 8.Zamani M., Ebrahimtabar F., Zamani V. Systematic review with meta-analysis: the worldwide prevalence of Helicobacter pylori infection. Aliment. Pharmacol. Ther. 2018;47(7):868–876. doi: 10.1111/apt.14561. Epub 2018 Feb 12. [DOI] [PubMed] [Google Scholar]
- 9.Poddar U. Helicobacter pylori: a perspective in low- and middle-income countries. Paediatr. Int. Child Health. 2019;39(1):13–17. doi: 10.1080/20469047.2018.1490100. [DOI] [PubMed] [Google Scholar]
- 10.Eshraghian A. Epidemiology of Helicobacter pylori infection among the healthy population in Iran and countries of the Eastern Mediterranean Region: a systematic review of prevalence and risk factors. World J. Gastroenterol. 2014;20(46):17618–17625. doi: 10.3748/wjg.v20.i46.17618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eusebi L.H., Zagari R.M., Bazzoli F. Epidemiology of Helicobacter pylori infection. Helicobacter. 2014;19(Suppl 1):1–5. doi: 10.1111/hel.12165. [DOI] [PubMed] [Google Scholar]
- 12.Laszewicz W., Iwanczak F., Iwanczak B. Seroprevalence of Helicobacter pylori infection in Polish children and adults depending on socioeconomic status and living conditions. Adv. Med. Sci. 2014;59:147-150. doi: 10.1016/j.advms.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Porras C., Nodora J., Sexton R. Epidemiology of Helicobacter pylori infection in six Latin American countries (SWOG Trial S0701) Cancer Causes Control. 2013;24:209-215. doi: 10.1007/s10552-012-0117-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Opekun A.R., Gilger M.A., Denyes S.M. Helicobacter pylori infection in children of Texas. J. Pediatr. Gastroenterol. Nutr. 2000;31:405–410. doi: 10.1097/00005176-200010000-00014. [DOI] [PubMed] [Google Scholar]
- 15.Wizla-Derambure N., Michaud L., Ategbo S. Familial and community environmental risk factors for Helicobacter pylori infection in children and adolescents. J. Pediatr. Gastroenterol. Nutr. 2001;33:58–63. doi: 10.1097/00005176-200107000-00010. [DOI] [PubMed] [Google Scholar]
- 16.Chi H., Bair M.J., Wu M.S. Prevalence of Helicobacter pylori infection in high-school students on Lanyu island, Taiwan: risk factor analysis and effect on growth. J. Formos. Med. Assoc. 2009;108(12):929–936. doi: 10.1016/S0929-6646(10)60005-8. [DOI] [PubMed] [Google Scholar]
- 17.Roma E., Panayiotou J., Pachoula J. Intrafamilial spread of Helico-bacter pylori infection in Greece. J. Clin. Gastroenterol. 2009 Sep;43(8):711–715. doi: 10.1097/MCG.0b013e318192fd8a. [DOI] [PubMed] [Google Scholar]
- 18.Ozbey G., Hanafiah A. Epidemiology, diagnosis, and risk factors of Helicobacter pylori infection in children. Euroasian J. Hepato-Gastroenterol. 2017;7(1):34–39. doi: 10.5005/jp-journals-10018-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rajindrajith S., Devanarayana N.M., de Silva H.J. Helicobacter pylori infection in children. Saudi J. Gastroenterol. 2009;15(2):86–94. doi: 10.4103/1319-3767.48964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kivi M., Johansson A.L., Reilly M. Helicobacter pylori status in family members as risk factors for infection in children. Epidemiol. Infect. 2005;133:645–652. doi: 10.1017/s0950268805003900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goosen C., Theron J., Ntsala M. Evaluation of a novel heminested PCR assay based on the phosphoglucosamine mutase gene for detection of Helicobacter pylori in saliva and dental plaque. J. Clin. Microbiol. 2002;40:205–209. doi: 10.1128/JCM.40.1.205-209.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leung W.K., Siu K.L., Kwok C.K. Isolation of Helicobacter pylori from vomitus of children and its implication in gastro-oral transmission. Am. J. Gastroenterol. 1999;94:2881–2884. doi: 10.1111/j.1572-0241.1999.01431.x. [DOI] [PubMed] [Google Scholar]
- 23.Personnet J., Shmuely H., Haggeerty T. Fecal and oral shedding of Helicobacter pylori from healthy infected adults. J. Am. Med. Assoc. 1999;282:2240–2245. doi: 10.1001/jama.282.23.2240. [DOI] [PubMed] [Google Scholar]
- 24.Wang Yao-Kuang, Kuo Fu-Chen. Diagnosis of Helicobacter pylori infection: current options and developments. World J. Gastroenterol. 2015;21(40):11221–11235. doi: 10.3748/wjg.v21.i40.11221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ricci C., Holton J., Vaira D. Diagnosis of Helicobacter pylori: invasive and non-invasive tests. Best Pract. Res. Clin. Gastroenterol. 2007;21(2):299–313. doi: 10.1016/j.bpg.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 26.Miernyk K.M., Bruden D.L., Bruce M.G. Dynamics of Helicobacter pylori –specific immunoglobulin G for 2 years after successful eradication of Helicobacter pylori infection in an American Indian and Alaska Native population. Clin. Vaccine Immunol. 2007;14:85-86. doi: 10.1128/CVI.00253-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lopes A.I., Vale F.F., Oleasto M. Helicobacter pylori – recent development in investigation. World J. Gastroenterol. 2014;20:9299–9313. doi: 10.3748/wjg.v20.i28.9299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bani-Hani K.E., Shatnawi N.J., El Qaderi S. Prevalence and risk factors of Helicobacter pylori infection in healthy schoolchildren. Chin. J. Dig. Dis. 2006;7(1):55–60. doi: 10.1111/j.1443-9573.2006.00245.x. [DOI] [PubMed] [Google Scholar]
- 29.Hussein N.R., Robinson K., Atherton J.C. A study of age specific Helicobacter pylori seropositivity rates in Iraq. Helicobacter. 2008;13:306–307. doi: 10.1111/j.1523-5378.2008.00618.x. [DOI] [PubMed] [Google Scholar]
- 30.Marie M.A. Seroprevalence of Helicobacter pylori infection in large series of patients in an urban area of Saudi Arabia. Korean J. Gastroenterol. 2008;52:226–229. [PubMed] [Google Scholar]
- 31.Galal Y.S., Ghobrial C.M., Labib J.R. Helicobacter pylori among symptomatic Egyptian children: prevalence, risk factors, and effect on growth. J. Egypt. Publ. Health Assoc. 2019;94:17. doi: 10.1186/s42506-019-0017-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Naous A., Al-Tannir M., Naja Z. Fecoprevalence and determinants of Helicobacter pylori infection among asymptomatic children in Lebanon. J. Med. Liban. 2007;55(3):138–144. [PubMed] [Google Scholar]
- 33.Obaidat M.M., Roess A.A. First nationwide seroepidemiology and risk factors report of Helicobater pylori in Jordan. Helicobacter. 2019;24(3) doi: 10.1111/hel.12572. [DOI] [PubMed] [Google Scholar]
- 34.Awad H.A., Hajeer M.H., Abulihya M.W., Al-Chalabi M.A., Al Khader A.A. Epidemiologic characteristics of gastric malignancies among Jordan University Hospital patients. Saudi Med. J. 2017;38(9):965–967. doi: 10.15537/smj.2017.9.19371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ling D. Carbon-13 urea breath test for Helicobacter pylori infection in patients with uninvestigated ulcer-like dyspepsia: an evidence-based analysis. Ont Health Technol Assessment Series. 2013 October;13(19):1–30. http://www.hqontario.ca/en/documents/eds/2013/full-report-urea-breath-test.pdf [Internet] Available from: [PMC free article] [PubMed] [Google Scholar]
- 36.Ozen A., Ertem D., Pehlivanoglu E. Natural history and symptomatology of Helicobacter pylori in children and factors determining the epidemiology of infection. J. Pediatr. Gastroenterol. Nutr. 2006;42:398–404. doi: 10.1097/01.mpg.0000215307.48169.7b. [DOI] [PubMed] [Google Scholar]
- 37.Mahalanabis D., Rahaman M.M., Sarkar S.A. Helicobacter pylori infection in the young in Bangladesh: prevalence, socioeconomic and nutritional aspects. Int. J. Epidemiol. 1996;25:894–898. doi: 10.1093/ije/25.4.894. [DOI] [PubMed] [Google Scholar]
- 38.Thankachan P., Muthayya S., Sierksma A. Helicobacter pylori infection does not influence the efficacy of iron and vitamin B12 fortification in marginally nourished Indian children. Eur. J. Clin. Nutr. 2010;64:1101–1107. doi: 10.1038/ejcn.2010.126. [DOI] [PubMed] [Google Scholar]
- 39.Santos I.S., Boccio J., Davidsson L. Helicobacter pylori is not associated with anemia in Latin America: results from Argentina, Brazil, Bolivia, Cuba, Mexico, Venezuela. Publ. Health Nutr. 2009;12:1862–1870. doi: 10.1017/S1368980009004789. [DOI] [PubMed] [Google Scholar]
- 40.Hooi J., Lai W.Y., Ng W.K. Global prevalence of Helicobacter pylori infection: a systematic review and meta-analysis. Gastroenterology. 2017;153:420-429. doi: 10.1053/j.gastro.2017.04.022. [DOI] [PubMed] [Google Scholar]
- 41.Progress on Household Drinking Water, Sanitation and hygiene 2000-2017. Special Focus on Inequalities. United Nations Children’s Fund (UNICEF) and World Health Organization (WHO); New York: 2019. [Google Scholar]
- 42.Almaayta A., Mukattash T.L., Hajaj J. Dispensing of non-prescribed antibiotics in Jordan. Patient preference and adherence. Patient Prefer. Adherence. 2015 Sep 30;9:1389–1395. doi: 10.2147/PPA.S91649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haddadin R.N., Alsous M., Wazaify M. Evaluation of antibiotic dispensing practice in community pharmacies in Jordan: a cross sectional study. PloS One. 2019;14(4) doi: 10.1371/journal.pone.0216115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yusef D., Baba A.I., Bashaireh A.Z. Knowledge, practices & attitude toward antibiotics use and bacterial resistance in Jordan: a cross-sectional study. Infect Dis Health. 2018 Mar;23(1):33–40. doi: 10.1016/j.idh.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 45.Ibrahim A., Morais S., Ferro A. Sex-differences in the prevalence of Helicobacter pylori infection in pediatric and adult populations: systematic review and meta-analysis of 244 studies. Dig. Liver Dis. 2017;49:742-749. doi: 10.1016/j.dld.2017.03.019. [DOI] [PubMed] [Google Scholar]
- 46.Jafri W., Yakoob J., Abid S. Helicobacter pylori infection in children: population-based age specific prevalence and risk factors in a developing country. Acta Paediatr. 2010;99(2):279–282. doi: 10.1111/j.1651-2227.2009.01542.x. [DOI] [PubMed] [Google Scholar]
- 47.Szaflarska-Popławska A., Soroczyńska- Wrzyszcz A. Prevalence of Helicobacter pylori infection among junior high school students in Grudziadz, Poland. Helicobacter. 2019;24 doi: 10.1111/hel.12552. [DOI] [PubMed] [Google Scholar]
- 48.Hoang T.T.1, Bengtsson C., Phung D.C. Seroprevalence of Helicobacter pylori infection in urban and rural Vietnam. Clin. Diagn. Lab. Immunol. 2005 Jan;12(1):81–85. doi: 10.1128/CDLI.12.1.81-85.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kawasaki M., Kawasaki T., Ogaki T. Seroprevalence of Helicobacter pylori infection in Nepal: low prevalence in an isolated rural village. Eur. J. Gastroenterol. 1998;10:47–49. doi: 10.1097/00042737-199801000-00009. [DOI] [PubMed] [Google Scholar]
- 50.Leal-Herrera Y., Torres J., Perez-Perez G. Serologic IgG response to urease in Helicobacter pylori-infected persons from Mexico. Am. J. Trop. Med. Hyg. 1999;60:587–592. doi: 10.4269/ajtmh.1999.60.587. [DOI] [PubMed] [Google Scholar]
- 51.Kalach N., Bontems P., Raymond J. Helicobacter pylori infection in children. Helicobacter. 2017;22(Suppl 1) doi: 10.1111/hel.12414. [DOI] [PubMed] [Google Scholar]