Abstract
Helicobacter pylori has several virulance factor i.e. VacA, CagA, BabA, SabA, AlpA, AlpB and etc. VacA has several polymorphic region in the nucleotide sequence such as s,m,i,d and, c. It has been suggested that each variation in these polymorphic region has been influenced the toxicity of VacA toxin. We performed a comprehensive meta-analysis to determine the main role of VacAi1/i2 in development into peptic ulcer and gastric cancer in an Iranian population.
Keywords: Gastric cancer, Helicobacter pylori, Iran, peptic ulcer, vacA
To the Editor,
Helicobacter pylori is the most highlighted and prominent pathogenic bacterium of the human gastric submucosa; it colonizes the stomachs of half of the world's population [1]. Most of the populations living in developing countries are predominantly infected during childhood and the amount of infection in these areas is nearly 100% [2]. With the introduction of H. pylori as a first-class factor for gastric cancer (GC) by the International Agency for Research on Cancer in 1994, extensive attention was paid by gastroenterologists to this bacterium. Helicobacter pylori has been identified as the aetiological cause of chronic gastritis, peptic ulcer diseases (PUD), gastric adenocarcinoma and mucosa-associated lymphoid tissue lymphoma and some extra-gastrointestinal disorders [[3], [4], [5]]. A significant number of H. pylori-infected individuals remain asymptomatic and PUD is observed in only 15%–20% and GC in 1%–2% of individuals, so the question arises as to why the clinical symptoms are observed in only a small part of the population with the rest remaining as asymptomatic carriers. Based on available evidence, genetic characteristics and H. pylori strain virulence factors, host genome and its epigenetic events, and the environmental conditions can play decisive roles in the incidence of H. pylori-related gastrointestinal outcomes [1,[5], [6], [7]].
The most famous H. pylori virulence factors include vacA and cagA. The cagA (cytotoxin-associated gene A) encodes a toxin of 120–140 kDa, which is phosphorylated at the site of its own EPIYA motif (tyrosine residue) by Src kinase family proteins of the host and, subsequent to phosphorylation, cagA-P induces the ‘hummingbird phenotype’ and incidence of GC through alteration in cytoskeletal rearrangements, cell survival and proliferation, and changes in polarity [8]. According to the previous studies, nearly 100% of H. pylori isolates in the Japanese population produce and express cagA [9]. Iran is the fourth country in terms of GC prevalence in Asia, and nearly 69% of H. pylori isolates in Iranian patients harbour (containing, transporting) cagA [10]. The vacA (vacuolating cytotoxin gene A) is another virulence factor, which releases cytochrome c from the mitochondria by entering into the gastric epithelium, induces apoptosis, destroys tight junctions and intercellular connections, and produces vacuole in the host cells [11]. The vacA consists of five polymorphic regions including signal sequence (s1/s2), middle (m1/m2), intermediate (i1/i2), deletion (d1/d2) and c (c1/c2). Only about 50% of the H. pylori strains encode the vacA [12,13]. Also, the combination of each region determines the vacuolating formation strength. For example, the vacA s1m1 strains express a large amount of toxin with high vacuolating formation strength, whereas s1m2 strains produce a moderate amount of toxin and s2m2 strains are not, or are rarely, toxic [14]. There are controversial results regarding the relationship between i1/i2 segments [15,16]. In this study, for the first time in Iran, we conducted a meta-analysis study to assess the possible role and relationship of vacA i1/i2 in the Iranian population.
Potentially supported documents were collected by searching the PubMed, Scopus, Embase, Elton B. Stephens Company (EBSCO), Google scholar, Scientific Information Database (SID), Islamic Science Citation Database (ISC), and magiran databases. The documents were investigated using the keywords ‘Helicobacter pylori’, ‘Peptic Ulcer Disease’, ‘Gastric Cancer’, ‘VacA protein’ and ‘Iran’. Studies were collected up to April 2020, without language and time constraints, and all the original articles (including case–control, cohort, prospective, retrospective), letters to the editor and congress abstracts that were about determining the frequency of vacA i1/i2 genotypes in the Iranian PUD and GC patients were studied; information including the first author, publication year, location, distribution of gender and age, number of H. pylori strains and distribution of vacA i1/i2 genotypes are listed in Table 1.
Table 1.
Characteristics of included study
| Ref. |
Publication year |
Location |
Female/male; age |
H. pylori strains |
vacA i1/2 genotype |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total |
PU |
GC |
||||||||
| i1 | i2 | i1 | i2 | i1 | i2 | |||||
| Bakhti et al. [17] | 2019 | Ardabil | NA; 46.52 | 290 | 112 | 120 | 34 | 23 | 32 | 17 |
| Mottaghi et al. [18] | 2014 | Tabriz | 45/44; NA | 89 | 46 | 43 | 6 | 11 | 21 | 3 |
| Bakhti et al. [19] | 2015 | Ardabil | 77/100; 50 | 177 | 76 | 98 | 34 | 23 | NA | NA |
| Bakhti et al. [SID] | 2014 | Ardabil | NA; NA | 160 | 77 | 90 | NA | NA | 37 | NA |
| Bakhti et al. [SID] | 2014 | Ardabil | NA; NA | 171 | 33 | 33 | 33 | NA | NA | NA |
| Abdi et al. [20] | 2017 | Ardabil | 44/85; 53.57 | 103 | 40 | 27 | NA | NA | 24 | 9 |
| Bakhti et al. [21] | 2015 | Tehran | NA; 45.34 | 217 | 102 | 106 | 34 | 23 | 29 | 9 |
| Douraghi et al. [21] | 2009 | Tehran | 91/116; 44.8 ± 16 | 207 | 159 | 94 | 20 | NA | 30 | NA |
SID: Scientific Information Database.
The relationship of the infection with vacA i1/2 strains and development of GC lesions was calculated using the odds ratio index with 95% CI. The random effects model (Dersimonian and Laird method) was used in cases of high heterogeneity including I2 index >25% and Cochran's Q-test p < 0.005; the statistical analysis was performed using Comprehensive Meta-Analysis (CMA v. 2.0) software. The publication bias was also measured according to Egger's regression method [4].
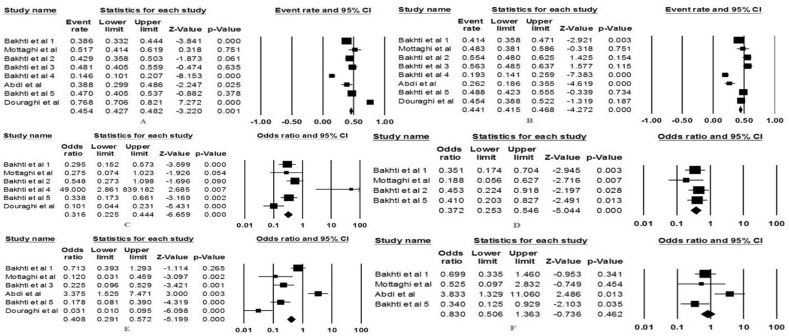
The eight studies that met our criteria were entered into the present analysis [12,[17], [18], [19], [20], [21]]. The studies were conducted in the period 2009–2019, five studies were performed in Ardabil, two in Tehran and one in Tabriz. In this study, the data from 1746 patients with an average age of about 48 ± 4 years were evaluated; about 41.9% of the patients were women and the rest were men. In general, 1414 strains of H. pylori were isolated from the patients in the present study. The frequency of vacA i1 in the present study was about 45.61% and the frequency of vacA i2 was about 43.21%. Based on the results of statistical analysis, we did not observe any significant relationship between infection acquisition with H. pylori vacA i1 or i2 and development of PUD or GC in the Iranian patients (Fig. 1).
Fig. 1.
Forrest plots of the vacA i1/i2 association with Helicobacter pylori-related gastrointestinal diseases. (a) vacA i1 in peptic ulcer disease (PUD) and gastric cancer (GC) development; (b) vacA i2 in PUD and GC development; (c) vacA i1 in PUD development; (d) vacA i2 in PUD development; (e) vacA i1 in GC development; (f) vacA i2 in GC development.
In the next step, we evaluated the role of infection with the H. pylori strains cagA + vacA i1/2 with PUD and GC, although no significant relationship was obtained for this. Moreover, because of insufficient information and the ambiguity of some results, we could not determine the role of cagA+/i1 or i2/s1m1 strains or vacA i1 or i2/s1m1 strains in the formation and development of PUD and GC. According to Egger's regression intercept (bias studies) no publication bias was observed in the included studies. It seems that vacA i1 or i2 has no role in the formation and onset of PUD and GC in the Iranian population.
The i locus is located between regions s and m and plays a functional role in the activity and formation of vacuole, such that the strains containing vacA i1 have the ability of vacuolating formation, while vacA i2 strains are unable to produce vacuolation [22]. Our data on the role of constellation in vacA polymorphic regions and the synergistic effects between vacA i1/2 alleles and cagA in the occurrence of PUD and GC are limited (Fig. 2).
Fig. 2.
The schematic of the vacA role in Helicobacter pylori pathogenesis.
In this study, we showed that infection with vacA i1/2 strains had no effect on the development and severity of PUD and GC in the Iranian population. We also demonstrated that the combination of vacA i1/2 and cagA played no roles in the formation of H. pylori-related gastrointestinal issues.
In a study of strains isolated from Iraqi and Iranian patients with gastric ulcer, the vacA i1 genotype was only associated with gastric ulcer in the Iraqi patients [23]. However, studies in East Asia have found a significant relationship between the vacA i1 allele as well as PUD and GC, such that i1 is considered a biomarker for the H. pylori-related gastrointestinal disease. Bagheri et al. revealed the high abundance of vacA i1 allele in Iranian patients [[24], [25], [26]].
In their study on the Swedish population, Karlsson et al. found no significant relationship between vacA i1 and PUD or GC development [27]. González-Rivera et al. found that by inhibiting the nuclear factor of activated T cells, i1 suppresses interleukin-2 production and prevents the formation of chronic inflammation and PUD [28]. However, Memon et al. observed a significant relationship between i1 genotype and duodenal ulcer [29].
In their meta-analysis, Liu et al. stated that infection with the vacA i1 genotype was significantly related to gastric cancer. They stated that the vacA i1 genotype had a significant relationship with GC, particularly in Asian countries (OR 10.89; 95% CI 4.11–20.88) [30]. Sugimoto et al. also showed that there is a significant relationship between vacA i1 genotype and GC development in Asian countries [14]. However, they noted in their study that a great number of s1, m1 and s1m1 strains contained i1. Hence, it is not yet possible to make a definite decision as to whether the vacA i1 genotype causes GC [14]. Numerous studies have also shown that the frequency of the vacA i1 genotype is high in vacA s1m1 strains (based on the results of many studies, these strains increase the risk of PUD and GC), which could alter the results and lead to false negatives [22,23,31,32]. In their meta-analysis, Pourmohammadi et al. showed that the vacA i1 genotype has no role in the GC development. They stated that cagA and vacA s1m1 have a significant relationship with the GC development [33].
Hence, the role of host genetics and environmental factors in the occurrence of PUD and GC, as well as the high frequency of cagA+ and vacA s1m1 strains in individuals with PUD and GC, cannot be definitely decided through the impact of vacA i1/2 alleles in PUD and GC development because of the diversity of the strains and differences in the frequency of vacA i1/2 in strains of different regions of the world. We need to plan and implement more and larger studies.
Conflict of interest
There is no conflict of interest.
References
- 1.Yousefi B., Mohammadlou M., Abdollahi M., Salek Farrokhi A., Karbalaei M., Keikha M. Epigenetic changes in gastric cancer induction by Helicobacter pylori. J Cell Physiol. 2019;234:21770–21784. doi: 10.1002/jcp.28925. [DOI] [PubMed] [Google Scholar]
- 2.Segal I., Ally R., Sitas F., Walker A. Helicobacter pylori: the African enigma. Gut. 1998;43:300. doi: 10.1136/gut.43.2.300a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takahashi-Kanemitsu A., Knight C.T., Hatakeyama M. Molecular anatomy and pathogenic actions of Helicobacter pylori CagA that underpin gastric carcinogenesis. Cell Mol Immunol. 2020;17:50–63. doi: 10.1038/s41423-019-0339-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Youssefi M., Ghazvini K., Farsiani H., Tafaghoudi M., Keikha M. A systematic review and meta-analysis of outcomes of infection with Helicobacter pylori dupA+ strains in Iranian patients. Gene Rep. 2020:100650. [Google Scholar]
- 5.Keikha M., Eslami M., Yousefi B., Ghasemian A., Karbalaei M. Potential antigen candidates for subunit vaccine development against Helicobacter pylori infection. J Cell Physiol. 2019;234:21460–21470. doi: 10.1002/jcp.28870. [DOI] [PubMed] [Google Scholar]
- 6.Kusters J.G., van Vliet A.H., Kuipers E.J. Pathogenesis of Helicobacter pylori infection. Clin Microbiol Rev. 2006;19:449–490. doi: 10.1128/CMR.00054-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamaoka Y. Mechanisms of disease: Helicobacter pylori virulence factors. Nat Rev Gastroenterol Hepatol. 2010;7:629. doi: 10.1038/nrgastro.2010.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagase L., Hayashi T., Senda T., Hatakeyama M. Dramatic increase in SHP2 binding activity of Helicobacter pylori Western CagA by EPIYA-C duplication: its implications in gastric carcinogenesis. Sci Rep. 2015;5:15749. doi: 10.1038/srep15749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shiota S., Murakawi K., Suzuki R., Fujioka T., Yamaoka Y. Helicobacter pylori infection in Japan. Exp Rev Gastroenterol Hepatol. 2013;7:35–40. doi: 10.1586/egh.12.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nouraie M., Latifi-Navid S., Rezvan H., Radmard A.R., Maghsudlu M., Zaer-Rezaii H. Childhood hygienic practice and family education status determine the prevalence of Helicobacter pylori infection in Iran. Helicobacter. 2009;14:40–46. doi: 10.1111/j.1523-5378.2009.00657.x. [DOI] [PubMed] [Google Scholar]
- 11.Palframan S.L., Kwok T., Gabriel K. Vacuolating cytotoxin A (VacA), a key toxin for Helicobacter pylori pathogenesis. Front Cell Infect Microbiol. 2012;2:92. doi: 10.3389/fcimb.2012.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bakhti S.Z., Latifi-Navid S., Mohammadi S., Zahri S., Bakhti F.S., Feizi F. Relevance of Helicobacter pylori vacA 3ʹ-end region polymorphism to gastric cancer. Helicobacter. 2016;21:305–316. doi: 10.1111/hel.12284. [DOI] [PubMed] [Google Scholar]
- 13.Ito Y., Azuma T., Ito S., Miyaji H., Hirai M., Yamazaki Y. Analysis and typing of the vacA gene from cagA-positive strains of Helicobacter pylori isolated in Japan. J Clin Microbiol. 1997;35:1710–1714. doi: 10.1128/jcm.35.7.1710-1714.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sugimoto M., Zali M.R., Yamaoka Y. The association of vacA genotypes and Helicobacter pylori-related gastroduodenal diseases in the Middle East. Eur J Clin Microbiol Infect Dis. 2009;28:1227–1236. doi: 10.1007/s10096-009-0772-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim J.Y., Kim N., Nam R.H., Suh J.H., Chang H., Lee J.W. Association of polymorphisms in virulence factor of Helicobacter pylori and gastroduodenal diseases in South Korea. J Gastroenterol Hepatol. 2014;29:984–991. doi: 10.1111/jgh.12509. [DOI] [PubMed] [Google Scholar]
- 16.Yakoob J., Abid S., Abbas Z., Jafri W., Ahmad Z., Ahmed R. Distribution of Helicobacter pylori virulence markers in patients with gastroduodenal diseases in Pakistan. BMC Gastroenterol. 2009;9:87. doi: 10.1186/1471-230X-9-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bakhti S.Z., Latifi-Navid S., Zahri S. Unique constellations of five polymorphic sites of Helicobacter pylori vacA and cagA status associated with risk of gastric cancer. Infect Genet Evol. 2020;79:104167. doi: 10.1016/j.meegid.2019.104167. [DOI] [PubMed] [Google Scholar]
- 18.Mottaghi B., Safaralizadeh R., Bonyadi M.J., Latifi-Navid S., Somi M.H., Mahdavi M. Relationship of Helicobacter pylori vacA i1 and i2 alleles with gastric cancer risk, Iran: a brief report. Tehran Univ Med J. 2014;72(9) [Google Scholar]
- 19.Bakhti S.Z., Latifi-Navid S., Zahri S., Yazdanbod A. Relationship between new allelic types of Helicobacter pylori vacA Gene and cagA status and risk of GU or DU in Iran. J Ardabil Univ Med Sci. 2015;15:246–254. [Google Scholar]
- 20.Abdi E., Latifi-Navid S., Zahri S., Yazdanbod A., Safaralizadeh R. Helicobacter pylori genotypes determine risk of non-cardia gastric cancer and intestinal-or diffuse-type GC in Ardabil: a very high-risk area in Northwestern Iran. Microb Pathog. 2017;107:287–292. doi: 10.1016/j.micpath.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 21.Douraghi M., Talebkhan Y., Zeraati H., Ebrahimzadeh F., Nahvijoo A., Morakabati A. Multiple gene status in Helicobacter pylori strains and risk of gastric cancer development. Digestion. 2009;80:200–207. doi: 10.1159/000229774. [DOI] [PubMed] [Google Scholar]
- 22.Rhead J.L., Letley D.P., Mohammadi M., Hussein N., Mohagheghi M.A., Hosseini M.E. A new Helicobacter pylori vacuolating cytotoxin determinant, the intermediate region, is associated with gastric cancer. Gastroenterology. 2007;133:926–936. doi: 10.1053/j.gastro.2007.06.056. [DOI] [PubMed] [Google Scholar]
- 23.Hussein N.R., Mohammadi M., Talebkhan Y., Doraghi M., Letley D.P., Muhammad M.K. Differences in virulence markers between Helicobacter pylori strains from Iraq and those from Iran: potential importance of regional differences in H. pylori-associated disease. J Clin Microbiol. 2008;46:1774–1779. doi: 10.1128/JCM.01737-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jang S., Jones K.R., Olsen C.H., Joo Y.M., Yoo Y.J., Chung I.S. Epidemiological link between gastric disease and polymorphisms in VacA and CagA. J Clin Microbiol. 2010;48:559–567. doi: 10.1128/JCM.01501-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lui S.Y., Chuah S.W., Goh H.L., Lee K.Y., Lee V.S., Ho B. Different CagA and VacA polymorphisms are found in the Chinese versus the Malay and Indian populations: an analysis of Helicobacter pylori virulence genes in Singapore. Proc Sing Healthc. 2010;19:12–18. [Google Scholar]
- 26.Bagheri N., Azadegan-Dehkordi F., Rafieian-Kopaei M., Rahimian G., Asadi-Samani M., Shirzad H. Clinical relevance of Helicobacter pylori virulence factors in Iranian patients with gastrointestinal diseases. Microb Pathog. 2016;100:154–162. doi: 10.1016/j.micpath.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 27.Karlsson A., Ryberg A., Dehnoei M.N., Borch K., Monstein H.J. Association between cagA and vacA genotypes and pathogenesis in a Helicobacter pylori infected population from South-eastern Sweden. BMC Microbiol. 2012;12:129. doi: 10.1186/1471-2180-12-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.González-Rivera C., Algood H.M., Radin J.N., McClain M.S., Cover T.L. The intermediate region of Helicobacter pylori VacA is a determinant of toxin potency in a Jurkat T cell assay. Infect Immun. 2012;80:2578–2588. doi: 10.1128/IAI.00052-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Memon A.A., Hussein N.R., Deyi V.Y., Burette A., Atherton J.C. Vacuolating cytotoxin genotypes are strong markers of gastric cancer and duodenal ulcer-associated Helicobacter pylori strains: a matched case–control study. J Clin Microbiol. 2014;52:2984–2989. doi: 10.1128/JCM.00551-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu X., He B., Cho W.C., Pan Y., Chen J., Ying H. A systematic review on the association between the Helicobacter pylori vacA i genotype and gastric disease. FEBS Open Bio. 2016;6:409–417. doi: 10.1002/2211-5463.12046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Basso D., Zambon C.F., Letley D.P., Stranges A., Marchet A., Rhead J.L. Clinical relevance of Helicobacter pylori cagA and vacA gene polymorphisms. Gastroenterology. 2008;135:91–99. doi: 10.1053/j.gastro.2008.03.041. [DOI] [PubMed] [Google Scholar]
- 32.Ogiwara H., Graham D.Y., Yamaoka Y. vacA i-region subtyping. Gastroenterology. 2008;134:1267. doi: 10.1053/j.gastro.2007.11.062. [DOI] [PubMed] [Google Scholar]
- 33.Pourmohammadi A., Ghotaslou R., Leylabadlo H.E., Nasiri M.J., Dabiri H., Hashemi A. Risk of gastric cancer in association with Helicobacter pylori different virulence factors: a systematic review and meta-analysis. Microb Pathog. 2018;118:214–219. doi: 10.1016/j.micpath.2018.03.004. [DOI] [PubMed] [Google Scholar]