Abstract
Wet coffee pulp (WCP), produced as waste from coffee production, is a rich source of bioactive compounds, especially caffeine and chlorogenic acid. However, it contains high moisture content, thus it is challenging for further utilization due to degradation and microbial deterioration. Dehydration is, therefore, necessary to minimize degradation and ease storage and transportation. As a waste, the common drying methods should be prioritized to be feasible for industrial application. This study aimed to determine the impact of different drying conditions of the three common drying methods including low temperature and pressure, vacuum and hot air drying on physical, phytochemical and antioxidant properties of WCP to identify the most suitable drying conditions. Browning index, moisture content, total phenolic content (TPC), flavonoids (TFC), proanthocyanidins, and chlorogenic acid as well as the antioxidant properties of the dried coffee pulp were significantly influenced by different tested conditions. Vacuum drying was found to be more suitable for drying the wet coffee pulp as compared to low temperature and pressure and hot air drying methods. Vacuum drying at 110 °C retained the highest TPC (14.4 mg gallic acid equivalents (GAE)/g dry weight (DW)), proanthocyanidins (6.8 mg catechin equivalents (CE)/g DW), TFC (13.2 CE/g DW), caffeine (2.9 mg/g DW) and antioxidant capacity. Chlorogenic acid (3.4 mg/g DW) was 13% lower, but energy consumption was 37% less than vacuum drying at 90 °C. Therefore, vacuum drying (3.75 mmHg) at 110 °C for 4h 05 min was suggested for dehydration of the wet coffee pulp for subsequent recovery and processing.
Keywords: Food science, Food analysis, Coffee pulp, Coffee by-products, Coffea canephora, Robusta, Bioactive compounds, Antioxidant capacity
Food science; Food analysis; Coffee pulp; Coffee by-products; Coffea canephora; Robusta; Bioactive compounds; Antioxidant capacity.
1. Introduction
Coffee is the world's second-most traded commodity after oil (Mussatto et al., 2011). During coffee production, a large quantity of coffee by-products is generated. They are considered waste and are a significant environmental pollutant due to their large quantity and easy degradation. Approximately one ton of the pulp is generated for every two tons of coffee cherries processed (Roussos et al., 1998). Coffee pulp is a rich source of crude protein (10%) and reducing sugars (12.4% dry basis), and thus can stimulate enzyme activities or the development of microorganisms (Pulgarin et al., 1991; Roussos et al., 1998). Coffee pulp is also a rich source of phenolic compounds at between 1.80-8.56% of the dried pulp. Several phenolic compounds have been identified in coffee pulp, such as 5-caffeoylquinic acid, epicatechin, 3,4-dicaffeoylquinic acid, 3,5-dicaffeoylquinic acid, 4,5- dicaffeoylquinic acid, catechin, rutin, protocatechuic acid, ferulic acid (Ramirez-Martinez, 1988), 5-feruloylquinic acid (Clifford and Ramirez-Martinez, 1991); anthocyanidin cyaniding-3-rutinoside (Prata and Oliveira, 2007). Also, coffee pulp is considered as a natural source of antioxidants (Arellano-González et al., 2011), which allow to donate hydrogen to agents or prevent activities of oxidants (Babbar et al., 2011). Because of coffee pulp’s valuable components, it can be considered as a natural source of functional ingredients in foods, cosmetics, pharmaceutical industries (Esquivel and Jiménez, 2012).
WCP contains a high moisture content, which is closely linked with the degradation of phytochemicals. High moisture content is also challenging for storage and transportation. Removal of moisture by drying is therefore an important first step in the process to retain phytochemicals of coffee pulp for subsequent recovery and processing (Wojdyło et al., 2014). Various drying factors can influence phytochemical levels, as they are sensitive to heat, light and oxygen. It is, therefore, important to determine optimal drying conditions for each type of materials (Papoutsis et al., 2017). Conventional drying techniques, such as sun drying and shade drying have been popularly applied for drying food materials, including coffee as well (Shitanda and Wanjala, 2006; Ghosh and Venkatachalapathy, 2014). These techniques are inexpensive, but sensitive compounds can be easily degraded during the drying process due to long exposure to oxygen and/or sunlight (Chan et al., 2009; Vega-Gálvez et al., 2009; Nguyen et al., 2015). Other common drying techniques, such as vacuum drying, hot air drying, freeze drying and low pressure and low temperature drying have also been applied to remove moisture from various plant materials. Hot air drying was suitable to retain polyunsaturated fatty acids and amino acids of Robusta coffee beans while freeze drying was ideal to retain volatile contents (Dong et al., 2017). Moreover, freeze drying was used to dehydrate jackfruit bulbs and to investigate the effect of different heat treatments and freeze drying method on dried jackfruit bulbs’ properties and their valuable phytochemicals (Xu et al., 2016). In the study of Yanyang Xu et al. (2006), strawberries were dried by using two-stage vacuum freeze drying technique and convective air drying method. Although these methods were applied for many materials, they have not been studied for drying WCP. Therefore, the aim of this study was to determine the impact of hot air, vacuum and low temperature and pressure drying techniques on the physical, phytochemical and antioxidant properties of wet Robusta coffee pulp to identify optimal drying conditions.
2. Materials and methods
2.1. Coffee pulp
Robusta wet coffee pulps were collected from Thang Loi Company, 17th KM, 26A Highway, Hoa Dong Commune, Krong Pak District, Dak Lak Provine, Viet Nam. After collection, the pulps were immediately stored at -18 °C. Samples were defrosted at room temperature before drying experiments. After drying, dried samples were ground to a fine powder using an electric blender (Philips Blender Mill) and screened through a mesh sieve (≤1.4 mm particle size) (Endecotts, London, England), and then stored at -18 °C for further analysis.
2.2. Chemicals of experiments
Chemicals for this project were analytical grade. Folin-Ciocalteu's reagent, methanol, sodium nitrite, hydrochloric acid, formic acid, sodium thiosulphate, aluminium chloride, iron (III) chloride, and vanillin were from Merck brand. 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS), 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,4,6-tris(2-pyridyl)-s-triazine (TPTZ), trolox, gallic acid, (+)-catechine, chlorogenic acid, and caffeine were from Sigma-Aldrich Pty Ltd. Anhydrous sodium carbonate and Sodium hydroxide were purchased from Labco – Chemicals, Australia.
2.3. Experimental design
Fresh Robusta wet pulps with initial moisture content of around 80% were dried to constant weight under 3 different common drying methods, which can be feasible to apply in the industry, including hot air drying, vacuum drying and low temperature and pressure drying methods. Drying curves were constructed to determine drying time for each condition (data not shown). Frozen samples were thawed overnight at room temperature before drying experiments. For each drying batch, approximately 50 g of sample was placed on aluminium trays with about 1 cm thickness. After completion of drying, the time required and final weight for each condition were recorded. For hot air drying, samples were dried using a hot air dryer (Memmert UM400) set at 70, 90 or 110 °C until a constant weight. For vacuum drying, samples were dried in a vacuum dryer (Memmert VO200) set at 70, 90 and 110 °C under vacuum (3.75 mmHg) until a constant weight. For low temperature and pressure drying (LTP), samples were dried in a dryer designed by the University of Technology and Education, Ho Chi Minh City, Viet Nam. Drying conditions were set at 40 °C with a vacuum pressure of 0.001–2.5 mmHg.
2.4. Physical properties of dried coffee pulp and energy consumption of drying equipment
Moisture content of wet and dried coffee pulps was determined according to the AOAC official methods of analysis. Briefly, samples were dried using a hot air dryer set at 120 °C for 4 h. Moisture content was worked out based on weight difference.
The colour of the dried samples was determined using a colorimeter (Konica Minolta CR-400/410, Osaka, Japan). The L-, a- and b-values of the dried coffee pulp were recorded. The L-value (0–100) indicates darkness of the samples; the a-value specifies colour range from red (positive a-values) to green (negative a-values); while the b-value shows the colour range from yellow (positive b-value) to blue (negative b-value). Browning index was calculated according to Eq. (1) below (Oliveira et al., 2015):
| (1) |
The water activity of the dried samples was measured using an Aqualab Water Activity Meter (Decagon Devices, Inc., Pullman, WA).
Energy consumption of each drying method was estimated using Eq. (2) (Xu et al., 2006)
| (2) |
where: W was the consumed electrical energy (kWh), P was the electrical power supplied in kW and t was the time spent for drying (h).
2.5. Preparation of extracts for phytochemical and antioxidant analysis of dried coffee pulp
Coffee pulp extracts were prepared based on the method of Nguyen et al. (2016) with some modifications. 0.2 g of dried samples were extracted with a 20 mL mixture of methanol and deionised water (1:1 v/v). For these experiments, extraction was conducted using an ultrasonic equipment (Soniclean, Pty Ltd., Thebarton, Australia) which was set at 40 °C and 150 W for 30 min. The mixture was agitated every 5 min by a Vortex Mixer to enhance better extraction. After extraction, samples were filtered using a grade 1 Whatman paper under gravity filtration and immediately stored at 4 °C for further analysis within a day.
2.6. Phytochemical analysis
2.6.1. Total phenolic content
Total phenolic content (TPC) was determined according to Vuong et al. (2013). 1 mL of diluted sample extract was added to 5 mL Folin-Ciocalteu solution 10% (v/v), 4 mL Na2CO3 7,5% (w/v) was added and mixed well. The solution was then stored for 1 h in a dark room. Absorbance at 765 nm was measured using a UV spectrophotometer (Cary 60 Bio, UV-Vis, Malaysia). The results were calculated according to a standard curve which was created by using gallic acid and presented as mg of gallic acid equivalents per g of dried sample (mg GAE/g DW).
2.6.2. Total flavonoid content
Total flavonoid content (TFC) was determined according to Vuong et al. (2013). 0.5 mL of sample extract was added into 2 mL of H2O, followed by 0.15 mL of 5% (w/v) NaNO2. The solution was then stored at room temperature (RT) for 6 min. 0.15 mL of 10% (w/v) AlCl3, 2 mL 4% (w/v) NaOH and 0.7 mL of H2O were then added. The final solution was mixed well and kept at RT for a further 15 min before the absorbance was measured at 510 nm by a UV-Vis spectrophotometer (Cary 60 Bio, UV-Vis, Malaysia). Catechin was used to create a standard curve and the results were presented as mg of catechin equivalents per gram of dried sample (mg CE/g DW).
2.6.3. Proanthocyanidin content
Proanthocyanidin content was determined by using the method described by Vuong et al. (2013). 0.5 mL of sample extract was mixed with 3 mL of 4% (w/v) vanillin solution, the sample was then incubated in a dark room for 15 min. The absorbance was then measured at 500 nm by a UV-Vis spectrophotometer (Cary 60 Bio, UV-Vis, Malaysia). Catechin was used to create a standard curve and the results were presented as mg of catechin equivalents per gram of dried sample (mg CE/g DW).
2.6.4. Determination of caffeine and chlorogenic acid
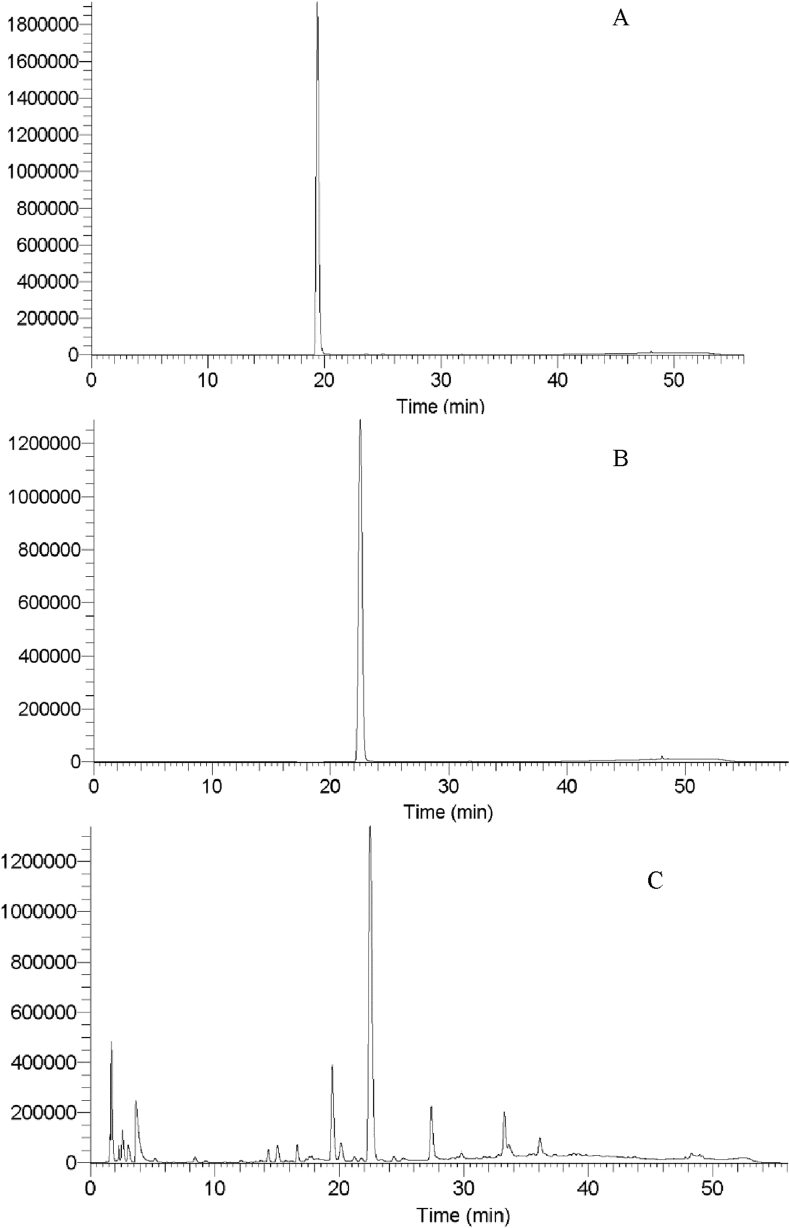
Caffeine and chlorogenic acid were analysed using a Thermo Finnigan HPLC system (Thermo Finnigan Corporation, San Jose, CA, USA) containing a Surveyor MS pump, a Surveyor PDA detector, an auto sampler and the Xcalibur TM 1.3 software. A Luna 5μ Phenyl-hexyl column (250 × 3.00 mm 5 μ micron, Phenomenex, Torrance, CA, USA) was used for separation. The extracts were filtered through 0.45 μm nylon membranes, 25 mm Phenex syringe filters, and were then individually injected into the HPLC system with a 30 μL injection volume. All samples were measured at a wavelength of 210 nm. Two solvents were used including solvent A (0.2% of formic acid in water) and solvent B (0.2% of formic acid in methanol). The flow rate was set at 1 mL/min with gradient as follows: 0–5 min, 0% B; 5–20 min, 20% B; 20–35 min, 50% B; 35–50 min, 80% B; 50–60 min, 0% B. Column oven temperature was set at 35 °C. Compounds were identified by matching retention time with corresponding standards (Figure 1). The external standard solutions were prepared by dissolving standard chemicals in methanol at a concentration of 12.5–1000 μM. Content of caffeine and chlorogenic acid was quantified based on the external standard curves and expressed as mg per gram of dried sample (mg/g DW).
Figure 1.
HPLC chromatograms of chlorogenic acid standard (A), caffeine standard (B) and the extract of sample (C).
2.7. Determination of antioxidant capacity of the coffee pulp extracts
2.7.1. 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) assay
ABTS radical scavenging capacity was determined following the method of Vuong et al. (2013). A stock solution was prepared by mixing equal volumes of a 7.4 mM ABTS and 2.6 mM K2S2O8. The mixture was placed in a dark room for 12–16 h at room temperature. A preparation of working solution was conducted by mixing 1 mL of the prepared stock solution with 60 mL of methanol to obtain an absorbance of 1.1 ± 0.02 at 734 nm. For the ATBS assay, 0.15 mL of the sample extract was mixed with 2.85 mL of working solution and incubated for 2 h. The absorbance of mixture was measured at 734 nm using a UV-Vis spectrophotometer (Cary 60 Bio, UV-Vis, Malaysia). The results were expressed as mg of trolox equivalents per g of sample dry weight (mg TE/g dried sample).
2.7.2. 2,2-diphenyl-1-picryl-hydracyl (DPPH) assay
DDPH radical scavenging capacity was measured as described in Thaipong et al. (2006). A stock solution was prepared by dissolving 0.024 g of DPPH in 100 mL of methanol and stored at 20 °C. A working solution was prepared by diluting 10 mL of DPPH stock solution with 45 mL methanol to obtain an absorbance of 1.1 ± 0.02 units at 515 nm. For the DPPH assay, 0.15 mL of the extract was mixed with 2.85 mL of working solution and was incubated for 3 h. The absorbance of mixture was measured at 515 nm using a UV-Vis spectrophotometer (Cary 60 Bio, UV-Vis, Malaysia). The results were expressed as mg of trolox equivalents per g of sample dry weight (mg TE/g dried sample).
2.7.3. Ferric reducing antioxidant power (FRAP)
FRAP assay was carried out as described by Benzie and Strain (1996). A fresh working FRAP solution were prepared by mixing 300 mM acetate buffer, 10 mM 2,4,6-tris(2-pyridyl)-s-triazine (TPTZ) in 40 mM HCl and 20 mM FeCl3 in the ratio 10:1:1. The solution was warmed at 37 °C before use. For FRAP assay, 2.85 mL of working FRAP solution was added in 0.15 mL of sample, then left at room temperature for 30 min in a dark room before measuring the absorbance at 593 nm. The results were expressed as mg of trolox equivalents per g of sample dry weight (mg TE/g dried sample).
2.8. Statistical analysis
All experiments were conducted in triplicate and the data analyzed using ANOVA in SPSS Statistics 25 software with a P value < 0.05 being considered significant. The comparisons of mean were expressed with ± SD and conducted with LSD Post Hoc Test.
3. Results and discussion
3.1. Effect of different drying conditions on physical properties of coffee pulp, drying time and energy consumption
The initial moisture content of the coffee pulp makes the material susceptible to degradation and results in higher transportation costs. Reducing moisture content is therefore an important step to remove moisture in wet coffee pulp for subsequent recovery and processing. The results (Table 1) showed that when drying to a constant weight, all drying conditions achieved moisture contents less than 6.9% and water activity of less than 0.57. This suggested that dried coffee pulp could be stored a long time because of minimal enzymatic reactions and microorganism growth at these moisture and water activity levels (Friedman, 1996).
Table 1.
Moisture content, water activity, lightness, browning index, drying time and energy consumption of dried coffee pulp under different drying conditions.
| Drying methods | Moisture Content | Water activity | Lightness (L∗) | Browning Index | Drying time | Energy consumption (kWh) |
|---|---|---|---|---|---|---|
| Hot air 70 °C | 6.43 ± 1.71ab | 0.55 ± 0.036abc | 27.01 ± 6.19a | 21.46 ± 7.47c | 7 h 45 min | 10.85 |
| Hot air 90 °C | 5.85 ± 0.62ab | 0.56 ± 0.01ab | 26.53 ± 2.92a | 24.37 ± 4.28c | 4 h 45 min | 6.65 |
| Hot air 110 °C | 3.14 ± 0.77c | 0.53 ± 0.01bc | 24.60 ± 2.18a | 25.09 ± 4.36bc | 3 h 30 min | 4.9 |
| Vacuum 70 °C | 5.89 ± 1.38ab | 0.57 ± 0.00a | 27.30 ± 6.23a | 27.48 ± 10.68bc | 17 h 15 min | 20.7 |
| Vacuum 90 °C | 6.90 ± 0.42a | 0.57 ± 0.01a | 25.93 ± 2.69a | 27.06 ± 7.26bc | 6 h 30 min | 7.8 |
| Vacuum 110 °C | 4.79 ± 0.5bc | 0.53 ± 0.01c | 26.12 ± 6.69a | 30.87 ± 6.08ab | 4 h 05 min | 4.9 |
| LTP | 5.56 ± 0.63ab | 0.56 ± 0.00ab | 28.04 ± 3.94a | 35.39 ± 4.26a | 20 h | 50 |
Data are expressed as means ± standard deviations (n = 3). Means with different superscript letters in the same column differ significantly (p < 0.05).
LTP: Low temperature and pressure drying.
The impact of different drying conditions on lightness and browning of the dried coffee pulp is shown in Table 1. The results indicated that lightness was not significantly affected by difference in drying conditions, however differences in browning was observed. LTP drying and vacuum drying had higher browning index than that of hot air drying at 70 °C and 90 °C. It could be explained that longer drying time and higher drying temperature of different drying conditions made the samples darker. This was due to the oxidation of polyphenolic compounds of coffee pulp. Also, browning could result from either enzymatic or non-enzymatic reactions which occurred during drying processes (Friedman, 1996). In the current study, analysis of the correlation between TPC and browning index (Equation (1)), did not show a strong correlation (R2 of 0.425), indicating that browning colour of the dried coffee pulp was formed by both enzymatic and non-enzymatic browning reactions. There were sugars and protein in coffee pulp (Pulgarin et al., 1991) so that the Maillard reaction, one of factors made samples brown, might occur under drying temperature condition (Lee et al., 1991).
As drying time and energy consumption (Equation (2)) are closely linked with production cost, it is important to consider the time and energy required to sufficiently dry coffee pulp for further utilisation. The initial moisture content of the wet coffee pulp was around 80% and the results in Table 1 showed the time and energy required to dry the coffee pulp to a moisture content less than 6.5% using each drying method. LTP drying required the longest time (20 h) and highest energy (50 kWh), while hot air drying at 110 °C required the shortest time (3.5 h) and least energy (4.9 kWh).
3.2. Effects of drying conditions on phytochemical compounds of coffee pulp
3.2.1. Total phenolic compound
Table 2 indicated that drying conditions significantly affected the levels of TPC in coffee pulp. The samples under vacuum drying conditions at 90 °C had the highest TPC (14.75 mg GAE/g dried sample) but not significant with those at 110 °C (14.36 mg GAE/g dried sample), while hot-air drying methods showed the lowest levels. The decrease in TPC with hot air drying indicated that increased air circulation in hot air drying results in oxidation of some phenolic compounds due to exposure to oxygen and high temperature (Wojdyło et al., 2014). The results also indicated that more TPC was retained when higher drying temperatures was applied for both hot air drying and vacuum drying. This was a result of the longer drying times being required at lower temperature, resulting in less exposure of TPC to heat and oxygen, and thus less degradation of TPC.
Table 2.
Effect of drying conditions on bioactive compounds of dried coffee pulp.
| Drying methods | Bioactive compounds |
||||
|---|---|---|---|---|---|
| TPC |
TFC |
Proanthocyanidins |
Caffeine |
Chlorogenic acid |
|
| (mg GAE/g DW) | (mg CE/g DW) | (mg CE/g DW) | (mg/g DW) | (mg/g DW) | |
| Hot air 70 °C | 2.98 ± 0.18e | 1.48 ± 0.51e | 2.34 ± 0.14c | 2.31 ± 0.39ab | 0.97 ± 0.18c |
| Hot air 90 °C | 3.73 ± 0.31de | 1.77 ± 0.17de | 3.85 ± 0.27bc | 2.09 ± 0.36b | 1.10 ± 0.19c |
| Hot air 110 °C | 9.39 ± 0.87cd | 4.88 ± 0.65cd | 4.94 ± 0.85ab | 2.52 ± 0.30ab | 2.81 ± 0.52b |
| Vacuum 70 °C | 11.74 ± 1.57bc | 7.65 ± 1.41bc | 4.88 ± 0.78ab | 2.05 ± 0.50b | 1.15 ± 0.12c |
| Vacuum 90 °C | 14.75 ± 0.66a | 13.17 ± 3.87a | 6.10 ± 1.00a | 2.92 ± 0.40a | 3.37 ± 0.29a |
| Vacuum 110 °C | 14.36 ± 1.15b | 8.52 ± 1.26b | 6.79 ± 2.06a | 2.97 ± 0.21a | 2.56 ± 0.24b |
| LTP | 11.13 ± 2.37bc | 5.99 ± 1.70bc | 5.43 ± 2.15ab | 2.31 ± 0.51ab | 0.78 ± 0.43c |
TPC: Total phenolic content, TFC: Total flavonoid content, DW: Dry weight. Data are expressed as means ± standard deviations (n = 3). Means with different superscript letters in the same column differ significantly (p < 0.05).
3.2.2. Total flavonoid content
There was a significant difference in TFC between the drying conditions with vacuum drying at 90 °C retaining the highest TFC (13.17 mg CE/g dried sample). Vacuum drying techniques retained significantly more TFC than hot air drying at the same temperature. This indicated that TFC of coffee pulp was degraded when exposed to oxygen during the drying process, which obviously being seen in hot air drying. Their TFC were much lower than those were under vacuum drying and LTP drying. Similar findings were reported by Papoutsis et al. (2017), who found greater retention of TFC in lemon pomace when drying under vacuum compared to hot air drying. Vacuum drying was also found to be more effective for retaining TFC when drying Vitex Agnus-Castus leaves (Vuong et al., 2015).
3.2.3. Proanthocyanidins content
Proanthocyanidin content in coffee pulp was also affected by drying conditions. Vacuum drying at 90 and 110 °C had the highest levels of proanthocyanidins (6.10 and 6.79 mg CE/g DW respectively) whereas hot air drying at 70 °C had the lowest level (2.34 mg CE/g DW). Vacuum drying generally retained more proanthocyanidins than hot air drying at the same temperature. It could be explained that proanthocyanidins, commonly known as condensed tannins, were structurally diverse due to the different hydroxylation patterns of the basic flavan-3-ol units and the linkages between the units (Zhu, 2018). Proanthocyanidins were sensitive to heat, light and oxygen (Yu et al., 2018), therefore under similar temperature, proanthocyanidins had less degradation when exposed to reduced oxygen conditions. These findings concurred with the results reported by Vuong et al. (2015) and Papoutsis et al. (2017), who also found that the highest proanthocyanidins was obtained under vacuum drying conditions.
3.2.4. Caffeine and chlorogenic acid contents
Caffeine and chlorogenic acid are both noteworthy compounds found in coffee pulp. The results (Table 2) showed that caffeine content was not significantly affected by different drying conditions and ranged from 2.05 to 2.97 mg/g dried sample. This was due to the stability of caffeine, which has found to be affected only when temperature exceed 160 °C (Hečimović et al., 2011). In contrast, chlorogenic acid content was significantly affected by drying conditions. Vacuum drying at 90 °C had the highest level of chlorogenic acid (3.4 mg/g), while LTP drying resulting in a significant loss of chlorogenic acid (0.78 mg/g). Coffee beans were a major source of chlorogenic acid, however it was often eliminated during the roasting process (Tajik et al., 2017), indicating that chlorogenic acid was sensitive to heat and other drying conditions.
3.3. Effect of drying conditions on the antioxidant capacity of dried coffee pulp
Oxidation of biomolecules (e.g. lipids, protein, DNA) is involved of a range of age-related conditions including cardiovascular disease, cancer and rheumatoid arthritis. Antioxidants like polyphenols can lessen or prevent this oxidative damage therefore reducing or delaying the incidence of these diseases (Moon and Shibamoto, 2009). Measurement of antioxidant properties may be conducted using a range of different assays, with each assay having its own advantages and limitations. To best evaluate antioxidant properties of an extract, more than one assay should be used (Vuong et al., 2015). In the current study, three assays were applied to determine the impact of different drying conditions on antioxidant properties of coffee pulp with the results are shown in Table 3.
Table 3.
Effect of drying conditions on antioxidant capacity of dried coffee pulp and correlations (R2) with bioactive compounds.
| Drying methods | Antioxidants capacity |
||
|---|---|---|---|
| ATBS (mg TE/g DW) | DPPH (mg TE/g DW) | FRAP (mg TE/g DW) | |
| Hot air 70 °C | 10.90 ± 0.63a | 0.60 ± 0.03d | 3.65 ± 0.42d |
| Hot air 90 °C | 11.88 ± 0.33b | 0.72 ± 0.08d | 5.04 ± 0.52cd |
| Hot air 110 °C | 20.94 ± 0.10c | 1.74 ± 0.13c | 5.94 ± 1.36c |
| Vacuum 70 °C | 21.35 ± 0.01c | 2.23 ± 0.01a | 12.18 ± 1.40b |
| Vacuum 90 °C | 21.39 ± 0.02c | 2.24 ± 0.01a | 16.73 ± 1.34a |
| Vacuum 110 °C | 21.40 ± 0.01c | 2.24 ± 0.01a | 13.27 ± 0.99b |
| LTP |
21.36 ± 0.02c |
2.00 ± 0.15b |
6.18 ± 1.60c |
| Correlations (R2) | |||
| TPC | 0.866 | 0.953 | 0.733 |
| TFC | 0.609 | 0.743 | 0.892 |
| Proanthocyanidins | 0.748 | 0.894 | 0.578 |
ABTS radical scavenging capacity; DPPH radical scavenging capacity; FRAP: Ferric-reducing antioxidant power; TE: Trolox equivalents FRAP: Ferric reducing antioxidant power, DW: Dry weight. The values are expressed as mean ± standard deviation (n = 3). Means with different superscript letters in the same column differ significantly (p < 0.05).
Different drying conditions were found to affect antioxidant capacity of the dried coffee pulp. Results from ABTS assay (Table 3) revealed that vacuum as well as LTP drying had higher radical scavenging capacities than those of hot air drying. The highest ABTS radical scavenging capacity was achieved from the samples under vacuum drying at 110 °C (21.40 mg TE/g DW) but not significant with those were under vacuum drying at 70, 90 °C and LTP drying (21.35, 21.39 and 21.36 mg TE/g DW, respectively). In contrast, hot air drying at 70 °C had the lowest radical scavenging capacity (10.90 mg TE/g DW). Our findings were similar to the results of a previous study, which found that vacuum drying applied on xao tam phan (Paramignya trimera) root, had higher ABTS radical scavenging capacity than hot air drying (Nguyen et al., 2016). Results (Table 3) showed a strong correlation between TPC and ABTS radical scavenging capacity (R2 = 0.866), which indicated that TPC was the major contributor to antioxidant capacity of the dried coffee pulp. It was due to the redox properties of TPC, they reduced the amount of radicals by donating hydrogen (Babbar et al., 2011). The secondary metabolites of TPC including TFC and proanthocyanidins were also found to have a correlation with ABTS radical scavenging capacity (R2 of 0.609 and 0.748, respectively), indicating that they also contributed.
DPPH assays also indicated that drying conditions affected DPPH radical scavenging capacity of the dried coffee pulp (Table 3). Overall, vacuum drying had more DPPH radical scavenging capacity than those of hot air drying as well as LTP drying. The highest DDPH radical scavenging capacity was obtained from the samples under vacuum drying at 90 and 110 °C (2.24 mg TE/g DW) but not significant with those were under vacuum drying at 70 °C (2.23 mg TE/g DW). The results (Table 3) showed that TPC was the major contributor to free radical scavenging capacity of the dried coffee pulp (R2 = 0.953), while their second metabolites TFC and proanthocyanidins had slightly lower correlation with free radical scavenging capacity of the dried coffee pulp (R2 of 0.743 and 0.894, respectively).
Results from FRAP assay (Table 3) further confirmed that drying conditions also affected ferric antioxidant power of the dried coffee pulp. Vacuum drying generally had higher ferric antioxidant power than those of hot air drying and LTP drying. Vacuum drying at 90 °C had the highest ferric antioxidant power (16.73 mg TE/g dried sample) and hot air drying at 70 °C had the lowest ferric antioxidant power (3.65 mg TE/g dried sample). Interestingly, TFC had the strongest correlation with ferric antioxidant power (R2 = 0.892), compared to TPC and proanthocyanidins (R2 of 0.733 and 0.578, respectively). It was reported that these bioactive compounds were linked to antioxidants activities, obviously, in their reducing-radical powers (Siddhuraju and Becker, 2003). It was also explained that the phytochemicals of coffee pulp had capacities to act as hydrogen donors and radical reducing powers (Babbar et al., 2011).
Overall, results from the three assays revealed that drying conditions influenced antioxidant capacity of the dried coffee pulp. TPC was the major contributor to antioxidant capacity and vacuum drying was most effective in retaining antioxidant capacity of the coffee pulp during the drying process.
4. Conclusions
Different drying conditions indeed influenced the physical, phenolic compounds and antioxidant properties of the Robusta coffee. Coffee pulp dried under vacuum drying at 90 °C or 110 °C had the highest levels of TPC, proanthocyanidins and antioxidant capacity, whereas the coffee pulp dried under vacuum drying at 90 °C had the highest levels of TFC and chlorogenic acid. However longer time and more energy were required for vacuum drying at 90 °C (6h30 min and 7.8 kWh, respectively) as compared to vacuum drying at 110 °C (4h 05 min and 4.9 kWh, respectively). This study recommended vacuum drying at 110 °C for 4h 05 min for subsequent recovery of bioactive compounds or further processing.
Declarations
Author contribution statement
Thy M. K. Tran: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Timothy Kirkman, Minh Nguyen: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Quan V. Vuong: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by the Viet Nam International Education Development – Ministry of Education and Training (Project 911) and the University of Newcastle.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors would like to thank the Centre of Experiment and Practice, Nha Trang University, for mechanical support.
Contributor Information
Thy Minh Kieu Tran, Email: Thy.Tran@uon.edu.au.
Quan Van Vuong, Email: vanquan.vuong@newcastle.edu.au.
References
- Arellano-González M.A. Antioxidant activity of fermented and nonfermented coffee (Coffea arabica) pulp extracts. Food Technol. Biotechnol. 2011;49(3):374. [Google Scholar]
- Babbar N. Total phenolic content and antioxidant capacity of extracts obtained from six important fruit residues. Food Res. Int. 2011;44(1):391–396. [Google Scholar]
- Benzie I.F., Strain J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal. Biochem. 1996;239(1):70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Chan E. Effects of different drying methods on the antioxidant properties of leaves and tea of ginger species. Food Chem. 2009;113(1):166–172. [Google Scholar]
- Clifford M., Ramirez-Martinez J. Tannins in wet-processed coffee beans and coffee pulp. Food Chem. 1991;40(2):191–200. [Google Scholar]
- Dong W. Effect of different drying techniques on bioactive components, fatty acid composition, and volatile profile of robusta coffee beans. Food Chem. 2017;234:121–130. doi: 10.1016/j.foodchem.2017.04.156. [DOI] [PubMed] [Google Scholar]
- Esquivel P., Jiménez V.M. Functional properties of coffee and coffee by-products. Food Res. Int. 2012;46(2):488–495. [Google Scholar]
- Friedman M. Food browning and its prevention: an overview. J. Agric. Food Chem. 1996;44(3):631–653. [Google Scholar]
- Ghosh P., Venkatachalapathy N. Processing and drying of coffee–a review. Int. J. Eng. Res. Technol. 2014;3(12):784–794. [Google Scholar]
- Hečimović I. Comparative study of polyphenols and caffeine in different coffee varieties affected by the degree of roasting. Food Chem. 2011;129(3):991–1000. doi: 10.1016/j.foodchem.2011.05.059. [DOI] [PubMed] [Google Scholar]
- Lee D.S. Nonenzymatic browning in dried red pepper products. J. Food Qual. 1991;14(2):153–163. [Google Scholar]
- Moon J.-K., Shibamoto T. Antioxidant assays for plant and food components. J. Agric. Food Chem. 2009;57(5):1655–1666. doi: 10.1021/jf803537k. [DOI] [PubMed] [Google Scholar]
- Mussatto S.I. Production, composition, and application of coffee and its industrial residues. Food Bioprocess Technol. 2011;4(5):661. [Google Scholar]
- Nguyen V.T. Effects of different drying methods on bioactive compound yield and antioxidant capacity of Phyllanthus amarus. Dry. Technol. 2015;33(8):1006–1017. [Google Scholar]
- Nguyen V.T. Phytochemical retention and antioxidant capacity of xao tam phan (Paramignya trimera) root as prepared by different drying methods. Dry. Technol. 2016;34(3):324–334. [Google Scholar]
- Oliveira S.M. Effect of air-drying temperature on the quality and bioactive characteristics of dried G alega Kale (B rassica oleracea L. var. A cephala) J. Food Process. Preserv. 2015;39(6):2485–2496. [Google Scholar]
- Papoutsis K. Effect of vacuum-drying, hot air-drying and freeze-drying on polyphenols and antioxidant capacity of lemon (Citrus limon) pomace aqueous extracts. Int. J. Food Sci. Technol. 2017;52(4):880–887. [Google Scholar]
- Prata E.R.B.A., Oliveira L.S. Fresh coffee husks as potential sources of anthocyanins. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2007;40(9):1555–1560. [Google Scholar]
- Pulgarin C. Utilization of wastes from coffee production. Biofutur. 1991;102:43–50. [Google Scholar]
- Ramirez-Martinez J.R. Phenolic compounds in coffee pulp: quantitative determination by HPLC. J. Sci. Food Agric. 1988;43(2):135–144. [Google Scholar]
- Roussos S. Sulid State Fermentation; 1998. Biotechnological Management of Coffee Pulp; p. 151. [Google Scholar]
- Shitanda D., Wanjala N. Effect of different drying methods on the quality of jute (Corchorus olitorius L.) Dry. Technol. 2006;24(1):95–98. [Google Scholar]
- Siddhuraju P., Becker K. Antioxidant properties of various solvent extracts of total phenolic constituents from three different agroclimatic origins of drumstick tree (Moringa oleifera Lam.) leaves. J. Agric. Food Chem. 2003;51(8):2144–2155. doi: 10.1021/jf020444+. [DOI] [PubMed] [Google Scholar]
- Tajik N. The potential effects of chlorogenic acid, the main phenolic components in coffee, on health: a comprehensive review of the literature. Eur. J. Nutr. 2017;56(7):2215–2244. doi: 10.1007/s00394-017-1379-1. [DOI] [PubMed] [Google Scholar]
- Thaipong K. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006;19(6–7):669–675. [Google Scholar]
- Vega-Gálvez A. Effect of air-drying temperature on physico-chemical properties, antioxidant capacity, colour and total phenolic content of red pepper (Capsicum annuum, L. var. Hungarian) Food Chem. 2009;117(4):647–653. [Google Scholar]
- Vuong Q.V. Effect of extraction conditions on total phenolic compounds and antioxidant activities of Carica papaya leaf aqueous extracts. J. Herbal Med. 2013;3(3):104–111. [Google Scholar]
- Vuong Q.V. Effect of drying conditions on physicochemical and antioxidant properties of V itex agnus-castus leaves. J. Food Process. Preserv. 2015;39(6):2562–2571. [Google Scholar]
- Wojdyło A. Effect of convective and vacuum–microwave drying on the bioactive compounds, color, and antioxidant capacity of sour cherries. Food Bioprocess Technol. 2014;7(3):829–841. [Google Scholar]
- Xu Y. A two-stage vacuum freeze and convective air drying method for strawberries. Dry. Technol. 2006;24(8):1019–1023. [Google Scholar]
- Xu F. Effects of heat treatment on polyphenol oxidase activity and textural properties of jackfruit bulb. J. Food Process. Preserv. 2016;40(5):943–949. [Google Scholar]
- Yu H.-L. The evaluation of proanthocyanidins/chitosan/lecithin microspheres as sustained drug delivery system. BioMed Res. Int. 2018:9073420. doi: 10.1155/2018/9073420. 2018: 9073420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu F. 2018. Proanthocyanidins in cereals and pseudocereals; pp. 1–13. (Critical Reviews in Food Science and Nutrition). [DOI] [PubMed] [Google Scholar]