Abstract
Introduction:
When the Centers for Medicare and Medicaid Services announced coverage for low dose CT lung cancer screening, they also mandated that imaging centers offer smoking cessation services. We designed the Optimizing Lung Screening (OaSiS) trial to evaluate strategies to implement the Public Health Service Guidelines for Treating Tobacco Use and Dependence during CT screening for lung cancer.
Methods and Design:
OaSiS was implemented using a pragmatic effectiveness-implementation hybrid design in 26 imaging clinics across the United States affiliated with the National Cancer Institute’s National Community Oncology Research Program (NCORP). The 26 sites selected for participation in the OaSiS trial were randomized to receive either a compendium of implementation strategies to add or enhance smoking cessation services during lung screening or to usual care. Usual care sites were given the option to receive the full compendium of implementation strategies at the conclusion of data collection. We have evaluated both the effectiveness of the implementation strategies to improve smoking cessation at six months among patients undergoing LDCT screening as well as the adoption and sustainability of evidence-based tobacco cessation strategies in imaging clinics.
Discussion:
The OaSiS trial was designed to identify opportunities for implementing evidence-based smoking cessation into LDCT lung cancer screening imaging facilities and to establish the effectiveness of these services. We report our study design and evaluation, including strengths of the pragmatic design and the inclusion of a diverse range of screening programs. Establishing these tobacco cessation services will be critical to reducing smoking related morbidity and mortality.
Keywords: tobacco cessation, lung cancer screening, PHS guidelines, low dose computed tomography, smoking cessation, NCORP
1. INTRODUCTION
In 2011, the National Lung Screening Trial reported that screening with three annual low-dose chest computed tomography (LDCT) scans could reduce lung cancer mortality by 20% compared to patients undergoing three annual single-view posteroanterior chest radiographs [1, 2]. Medical societies and the United States Preventive Services Task Force have endorsed annual LDCT screening in adults age 55 – 80 who have a 30 pack-year smoking history, currently smoke or have quit smoking within the past 15 years, and are asymptomatic for lung cancer [2]. In 2014, The Centers for Medicare and Medicaid Services (CMS) added annual LDCT lung cancer screening as a covered service benefit for persons at high risk for lung cancer who meet these eligibility criteria, with the exception of limiting the age range to 55 – 77 years (instead of 80 years) [3].
In order to receive payment, CMS requires multiple qualifications of the imaging center and the interpreting radiologist, including that the LDCT imaging center “make available smoking cessation interventions for current smokers.” What is not specified in the mandate is what qualifies as “available” or what must be included in the “interventions.” Services could be as limited as ask, advise, and refer, or as resource intensive as multi-session individual counseling.
Multiple factors affect the types of cessation services LDCT imaging centers can provide. Health care delivery system factors may include: the referral and follow-up procedures for lung cancer screening (LCS); provider willingness and capacity to offer cessation support; space for confidential conversations about smoking cessation; reinforcement for cessation by the primary care provider before and/or after the screening visit; availability of centralized cessation services within a hospital and/or community-based services, etc. [4]. Patient factors may also affect uptake of these services, such as receptivity to cessation support, time constraints imposed by a routine imaging visit, self-efficacy to quit, prior quit attempts, and familial support for quitting.
The Public Health Service Guidelines for Treating Tobacco Use and Dependence offer evidence-based health system, provider, and patient strategies for tobacco cessation which are cost-effective and can impact a large number of smokers. These strategies, often referred to as the 5As, include the following: Ask patients about tobacco use at every visit; Advise all tobacco users to quit; Assess readiness to quit; Assist patients who are willing to quit; and Arrange follow-up contact [5]. While clinics routinely ask patients to quit smoking, they often fail to advise them to quit, assess their readiness to quit, assist them in quitting and arrange follow-up contact [6, 7].
We designed the Optimizing Lung Screening (OaSiS) trial to evaluate strategies to implement the PHS Guidelines during routine CT screening for lung cancer and the effectiveness of these strategies in lung screening patients [8]. To ensure the 5As were implemented in the imaging setting, our protocol was designed to help screening clinics incorporate the following evidence-based elements of effective tobacco cessation programs: 1) implement a current tobacco user identification system within the imaging setting for all patients undergoing lung screening; 2) educate clinic staff about strategies and resources to promote cessation; 3) dedicate specific personnel responsible for organizing each clinic’s efforts to promote cessation; and 4) encourage the use of effective, evidence-based treatments for tobacco cessation that are feasible for patients undergoing lung screening. Combining systems- and provider-level services is more effective in promoting cessation than either approach alone [5]. This study was funded as part of the National Cancer Institute’s (NCI) Smoking Cessation at Lung Examination (SCALE) Collaboration to conduct research on smoking cessation treatment among patients who are screened for lung cancer [9].
1.1. Implementation Framework
Implementation science focuses on the translation of scientific discovery into “real world” clinical and community-based settings [10]. Implementation research is intended to close the gap between efficacious interventions (such as the PHS Guidelines) and real-world health care (such as community-based LDCT lung cancer screening imaging facilities) [11]. Importantly, implementation science emphasizes adaptation to the “local context” to embed evidence-based interventions in clinical settings [12]. Our implementation strategy is multi-faceted and targets the health care delivery system, including providers and the imaging clinics.
The implementation strategy builds upon constructs from organizational change theories (e.g., Diffusion of Innovations). These constructs include organizational climate and culture (compatibility of tobacco cessation services within the clinical mission of lung cancer screening) [12]. We expect implementation of the PHS Guidelines to depend on the simplicity of the services (e.g., nicotine replacement therapy recommendations versus intensive onsite counseling), the ability to try and adapt strategies to the local needs and resources, and the resources and support for different approaches (creating systems for feedback on success of cessation services in promoting quitting among screening patients) [13–16].
1.2. Implementation Strategies
Figure 1 provides an overview of the four implementation strategies used to encourage the adoption PHS Guidelines in imaging clinics: Webinars, Site Visit and Strategic Planning, Performance Coaching using Audit and Feedback, and Peer Learning Calls.
Figure 1:
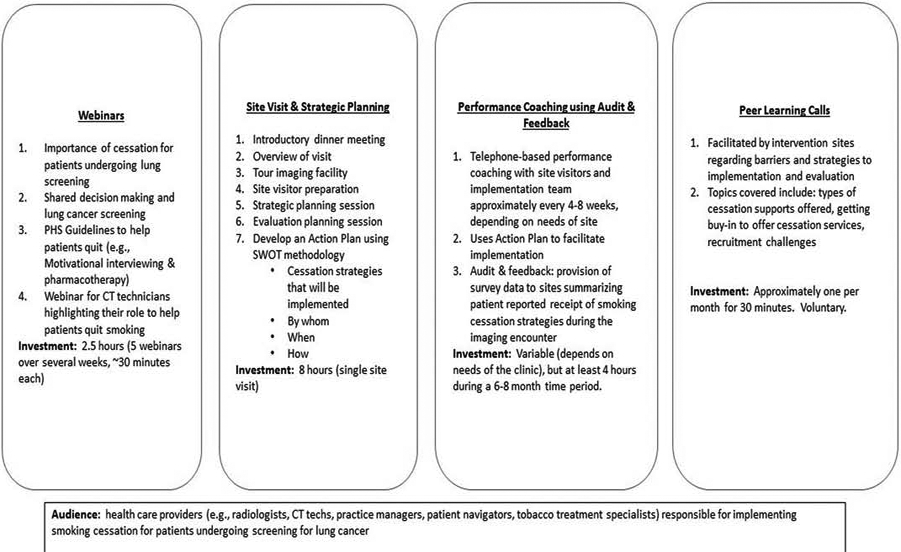
Implementation Strategies to Promote Smoking Cessation Services for Patients Undergoing Screening for Lung Cancer
Webinars:
Personnel from all intervention clinics were invited to watch four webinars (each <20–40 minutes) on the importance of tobacco cessation for patients undergoing lung screening, evidence-based strategies to assist patients with quitting, brief intervention for behavior change (motivational interviewing and pharmacotherapy), and a specially tailored webinar for CT technologists that integrates elements of the three previous webinars. All webinars were conducted live and trainers were available to answer questions. We also recorded all webinars to make them available to individuals who could not attend the scheduled live session. After completion of the four webinars, a site visit and strategic planning session were scheduled.
Site Visit and Strategic Planning.
Three to four members of the research team (including at least one physician, one social scientist, and a project manager) visited each imaging clinic participating in the intervention arm. The site visit commenced with an introductory dinner with members of the NCORP research team, as well as representatives from radiology and the imaging clinic, key institutional leaders, and members of the Wake Forest research team. This was often the first time that staff from different parts of the same health system had met one another and served as a critical ice-breaker and foundation for the full site visit. The site visit continued the following morning with an overview of the study purpose and timeline, a tour of the imaging clinic where the cessation support services would be implemented, and a strategic planning session, where key decision makers at the health system and potential cessation implementers were invited to engage.
Strategic planning was facilitated by the OaSiS research team. The strategic planning facilitators explained the purpose of the strategic planning session, helped clinics identify strengths within their organization that could facilitate routine cessation support for patients undergoing screening for lung cancer (e.g., dedicated staff for smoking cessation), identified potential barriers to implementation (e.g., lack of training of clinicians or other personnel in smoking cessation), and identified opportunities external to the organization that could affect implementation (e.g., availability of free/low cost resources that promote cessation). Sites were encouraged to select strategies based on the likelihood of adoption and sustainability after conclusion of the trial.
Each clinic identified strategies that could be implemented immediately with limited resources (short-term outcomes) and those that required adaptation or post-training support (long-term outcomes). The research team encouraged the clinic personnel to prioritize strategies that focused on “assisting” patients with cessation and “arranging” follow-up care for patients who want to quit. We prompted clinics with a variety of evidence-based cessation strategies, some that required very limited resources and are very easily implemented with minimal interruptions to clinic flow. The strategic planning session resulted in an Action Plan. The action plan identified which cessation support strategies would be adopted and when, who was responsible for implementing them, and identified potential barriers and solutions to implementation. Importantly, this strategic planning session created a public accountability for implementation.
After the strategic planning session was completed, the NCORP staff and the OaSiS team discussed the planned data collection to be conducted by the NCORP staff. Following the site visit, the research team provided (electronically) the written Action Plan to the tobacco cessation champion and NCORP personnel, which incorporated the decisions made during the site visit. Site personnel had an opportunity to revise and return the edited version to the research team. Approximately 4–6 weeks after the site visit, the OaSiS team and the site implementation team initiated the first performance coaching telephone call.
Performance Coaching:
Performance coaching lasted for approximately 4–8 months during the implementation phase of the trial and utilized the Action Plan that was developed as part of the strategic planning process. Coaches were those research personnel who facilitated the site visit. Coaching has predominantly been used in performance management as a strategy for offering ongoing support and feedback to employees [17, 18] and is an adaptable methodology aligned with the unique needs of lung cancer screening sites. Coaches provided support and feedback to help clinics implement smoking cessation services identified in their action plan [17]. There were four main dimensions to coaching: (1) provided direction, which included clear articulation of the goals and values of implementing tobacco cessation services during LDCT screening; (2) offered support during implementation (identifying gaps in implementation and helping identify improvements) in conjunction with the Action Plan; (3) engaged personnel within the lung cancer screening process in problem-solving; and (4) identified and brainstormed strategies to remove barriers to implementation. Coaching included phone conferences among site personnel and coaching teams approximately once every 4–8 weeks after the site visit. Project managers and coaching teams were responsive to concerns raised by the sites in-between coaching calls. Interactions with sites regarding implementation (electronic, phone, in-person) were documented in a coaching tracking log.
Provision of a Toolkit:
Each intervention site received a starter pack of at least 50 “toolkits” to give current smokers undergoing lung screening (independent of whether or not they enrolled in the study). The toolkit included patient-focused, low-cost health promotion materials (e.g., bag clips and glasses wipes with QuitLine number, refrigerator magnets with quitting promotional messaging) and substitutes for the “hand-to-mouth” habit that would otherwise be satisfied with cigarettes (e.g., chewing gum, lollipops). Sites were given the option to personalize their toolkits, but the kits were designed to be very low cost and sustainable. Information on re-ordering supplies was made available to all intervention clinics.
Audit & Feedback:
Sites were provided with descriptive data after at least 10 patients had completed a survey 14 days after their lung imaging appointment. The 14-day survey included a comprehensive list of the smoking cessation support strategies that could be offered to patients during the routine screening encounter. We facilitated a discussion that focused on the strategies the site chose to adopt and those that patients reported they received. These data provided early feedback to the sites from the patients’ perspectives on adoption and implementation of cessation services.
Peer Learning Calls:
Sites were also invited to participate in brief (<30 minute) peer learning calls that were led by 1 or 2 sites on lessons learned pertaining to implementation of smoking cessation services, recruitment, and/or data collection. The intent was for sites to learn from one another regarding the challenges and strategies to overcome them pertaining to the OaSiS trial. Peer learning calls happened approximately every other month and were voluntary. The calls were moderated by the project management team.
2. METHODS
2.1. Study Overview
The Optimizing Lung Screening (OaSiS) trial (NCT03291587) used a pragmatic effectiveness-implementation hybrid design (Type II) of imaging clinics across the United States affiliated with the Wake Forest National Cancer Institute’s National Community Oncology Research Program (NCORP) Research Base [19]. Twenty six sites were invited to participate with the goal of retaining 22 for the duration of the trial. The sites participating in the OaSiS trial were randomized to receive either the compendium of implementation strategies previously described or to usual care. Usual care sites were offered the all implementation strategies at the conclusion of data collection. Sites and their personnel were unblinded to the randomization arm. Patients seeking care in these clinics were notified at the time of their appointment that their clinic was participating in the trial; however, they were unaware whether their site was an intervention or usual care site.
The trial was designed to simultaneously evaluate the effectiveness of a compendium of implementation strategies to reduce 7-day smoking prevalence measured at six month post-LDCT and to evaluate the reach, adoption, adaptation, and sustainability of evidence-based smoking cessation services offered in imaging clinics.
2.2. Imaging Clinic Eligibility, Recruitment, and Measurement:
Setting:
NCORP is an NCI-supported national network that brings cancer clinical trials and cancer care delivery research to the community. NCORP includes 46 community sites of which 12 are designated as minority/underserved community sites; there are 1000+ locations affiliated with the community sites.
Eligibility:
An email was sent to all NCORP community sites within the Wake Forest NCORP Research Base network describing the study and eligibility criteria. Community sites were asked express their interest in participating and to nominate imaging clinics for participation; only one clinic from each community site would be randomly selected to participate in the trial. NCORP sites were eligible to participate in this trial if they had an imaging clinic that screened at least 50 patients for lung cancer within the preceding six months, and if they agreed to be randomized and participate in all aspects of the intervention and evaluation. Sites also agreed to have NCORP research personnel serve as the study liaison for evaluation purposes and to identify another individual to serve as a tobacco cessation program champion. The major qualifications of the champion included a commitment to smoking cessation, communication skills, and the capacity to affect change within the imaging facility. Champions were individuals such as a lung cancer screening coordinator or navigator, nurse or advanced practice professional on site who provides cessation services, or physician leaders of the imaging program.
Recruitment:
Twenty eight interested community sites completed a brief eligibility survey that assessed lung cancer screening volume, the racial/ethnic composition of lung cancer screening patients, availability of tobacco cessation support services (e.g., medication, counseling, QuitLine) within an imaging clinic affiliated with the community site. Some community sites nominated more than one clinic. Twenty-six community sites in twenty states were selected to participate, including all 6 minority sites that expressed interest. Our goal and power calculations were based on 22 sites completing the trial with each site accruing 50 baseline participants to the trial. One non-minority site refused participation due to health system transitions prior to randomization; a replacement community site was selected. Clinics were matched based on lung screening volume and racial/ethnic diversity, and then they were randomly assigned to the treatment or usual care arm. One intervention and one usual care clinic were unable to accrue patients to the trial and dropped out of the study in Year 3. Twenty-four sites completed all aspects of the trial, exceeding our original goal of 22 sites.
Measurement:
NCORP site personnel completed a single brief organizational assessment at baseline and in Year 4 of the study for each participating imaging clinic. See Table 1.
Table 1:
Measurement of Sites Participating in the OaSiS Trial
| • Baseline (RedCap Survey), n=26: Rural vs. Urban; Health System Ownership (e.g., independent; hospital system, private vs. public); Imaging Clinic Staffing (FTEs); Payer Mix ; (e.g.,% Medicaid; Medicare; Commercial Insurance; Uninsured); Self vs. Provider Referral for Lung Screening; Length of Time Offering Lung Screening; # of LDCT Screened Patients in Prior 6 months; Percentage of Screened Patients who are Smokers; Presence of Central Coordinator/Navigator for Shared Decision making; Smoking Cessation Services Offered (during and after lung screening) |
| • Year 4 (RedCap Survey), n=24: Length of Time Offering Lung Screening; # of LDCT Screened Patients in Prior 12 months; Percentage of Screened Patients who are Smokers; Payer Mix (e.g.% Medicaid; Medicare; Commercial Insurance; Uninsured); Self vs. Provider Referral for Lung Screening; presence of a multidisciplinary thoracic oncology program; Requirement for shared decision making; Presence of Central Coordinator/Navigator for Shared Decision making; Smoking cessation services offered (before, during and after lung screening); |
2.5. Key Informant Eligibility, Recruitment and Measurement:
Eligibility:
Key informants, who would participate in baseline semi-structured interviews, were eligible if they were age 18 or older, a member of the lung cancer screening team or someone responsible for implementing or supporting smoking cessation support for patients receiving lung cancer screening (e.g., centralized tobacco treatment specialist). They were included in the protocol to understand the facilitators and barriers to implementing cessation support in the lung imaging setting.
Recruitment:
NCORP personnel worked with the research team to identify at least two key informants according to the eligibility criteria. Key informants included program champions, imaging facility program directors (e.g., CT practice manager), health care providers (e.g., physicians, nurses, CT technicians), and centralized tobacco treatment specialists. Written informed consent was obtained from each key informant.
Measurement:
Interviews were conducted by the research team via telephone, audio recorded, and transcribed verbatim. Each key informant received a $25 gift card for participation. Table 2 provides a summary of the constructs measured at baseline and Year 4.
Table 2:
Constructs in the Semi-Structured Interviews of Key Informants Participating in the OaSiS Trial
| • Baseline (n=60): Open-ended questions about availability of smoking cessation services; organizational readiness/priority/advantage of implementing cessation services; resources needed for implementation; and feasibility and appropriateness of varied cessation services in the imaging setting (quantitative) |
| • Follow-Up (Target Accrual, n=48): Open-ended questions about availability of smoking cessation services; organizational readiness/priority/advantage of implementing cessation services; resources needed for implementation; and feasibility and appropriateness of varied cessation services in the imaging setting (quantitative); sustainability of smoking cessation services implemented during the trial in terms of organizational capacity, strategic planning and ability to adapt services to changes in the health system. Follow-up key informant interviews are currently ongoing. |
2.6. Lung Screening Patients: Eligibility, Recruitment, and Measurement:
Eligibility:
All self-reported current smokers (defined as smoking ‘every day’ or ‘some days’) aged 55–77, who met the CMS eligibility criteria for lung cancer screening and who showed up for their screening appointment, were eligible to participate in the study. Patient exclusion criteria included: current use (previous 30 days) of a tobacco dependence treatment including bupropion, varenicline, or nicotine replacement because the person was trying to quit. The use of bupropion for depression did not exclude the patient from participating nor did the occasional use of nicotine replacement therapy or e-cigarettes to avoid smoking in public spaces. Those with a physical or cognitive impairment that would prevent a person from engaging in survey research (such as blindness, deafness, or dementia) and non-English speaking patients were also excluded from the trial. Of the 1,503 screened for eligibility to date, 78 (5.2%) were ineligible due to actively trying to quit or due to an impairment that would prevent them for participating in research. Despite patients expressing a strong desire to quit, few were actively trying to do so.
Recruitment:
Patients scheduled to undergo LCS were screened for study eligibility by NCORP personnel via telephone prior to their lung screening appointment or in-person on the day of their appointment. Eligible patients who expressed interest in the study were approached immediately prior to their LDCT by NCORP research personnel to review the informed consent document and to participate. A signed informed consent form was obtained. All patients who presented for lung cancer screening throughout the duration of the trial, regardless of whether or not they were enrolled, were notified that the study was ongoing, but they were not made aware whether the imaging clinic was in the intervention or usual care arm of the trial.
Measurement:
Patient participants completed surveys administered by NCORP personnel at four time points: The baseline survey was conducted in person immediately preceding the patient’s LDCT appointment, and three follow-up surveys were administered by telephone (14 days, 3 months, and 6 months after baseline). Table 1 summarizes the measures at each survey. Surveys took approximately 15 minutes or less to complete. See Table 3.
Table 3:
Measurement of Lung Cancer Screening Participants in the OaSiS Trial (Baseline Accrual Goal, n=1,200; 50 participants for each of the 24 participating sites). To date, 1,053 participants have enrolled in the study (88%).
| Baseline (In-Person): Demographics (e.,g, sex, age, race, ethnicity, education, marital status, income, insurance); Health Status; Prior History of Cancer; Perceived Lung Cancer Risk and Worry; Perceived Benefit of Quitting; Quit Behavior (Readiness and Confidence to Quit, Prior Quit attempts); Self-Reported Smoking Status (Past 7 and 30 days; Nicotine Dependence (Fagerstrom); Number of Pack Years Smoked; Number of Cigarettes per Day; Other Tobacco Products Used (e.g., e-cigarettes) |
| • 14 Days (Telephone): Scope and Satisfaction with Smoking Cessation Services Received during and after LDCT (e.g., asked about tobacco, assessed readiness to quit, cessation support services received; follow-up cessation services provided after LDCT screening); Receipt and Understanding of Lung Screening Results; Smoking Status (Past 7 and 30 days) |
| • 3 Months (Telephone): Scope and Satisfaction with Smoking Cessation Services Received during and after LDCT (e.g., asked about tobacco, assessed readiness to quit, cessation support services received; follow-up cessation services provided after LDCT screening); Lung Cancer Risk and Worry; Perceived Benefits of Quitting; LungRads Score; Lung Screening Impact on Tobacco Use Behavior; Smoking Status (Past 7 and 30 days; Nicotine Dependence); Number of Pack Years Smoked; Number of Cigarettes per Day; Other Tobacco Products Used (e.g., e-cigarettes) |
| • 6 Months (Telephone): Lung Cancer Risk and Worry; Perceived Benefits of Quitting; Changes in Demographics (e.g., marital status); Lung Screening Impact on Tobacco Use Behavior; Smoking Status (Past 7 and 30 days; Nicotine Dependence |
| • Biochemical Validation of Self-Reported Smoking Status: Mailed Salivary Cotinine Kit |
Study participants were offered a $10 gift card for participation in each survey. To minimize attrition, NCORP staff mailed reminders to participants about 1–2 weeks prior to the 3- and 6-month follow-up surveys. At least three attempts were made to reach each participant by phone. As a final step, the team could mail a survey to non-respondents with an enclosed incentive (gift card).
Salivary cotinine:
Patients who self-reported not smoking at the 6-month telephone follow-up were sent a mailed saliva collection kit to biochemically validate self-reported tobacco use. The saliva kits were mailed out centrally by the Wake Forest NCORP Research Base. The mailed salivary collection kit includes a salivette (a plastic vial that contains a small cotton roll, like those used by dentists) along with written instructions on how to obtain the salivary sample. Saliva samples were collected from participants using SalivaBio Oral Swab saliva collection kits available from Salimetrics, Inc. (Carlsbad, CA). Saliva specimens were mailed to the NCORP Research Base Core Lab, centrifuged to collect the saliva from the swabs, the volume measured, and transferred to smaller freezer tubes for storage of the specimen at −80°C. Cotinine levels were determined by ELISA using the high sensitivity Salivary Cotinine quantitative Enzyme Immuno Assay kit provided by Salimetrics, Inc. Saliva samples were analyzed in duplicate. When the determined level of a sample is above the upper range of the ELISA (200 ng/mL), the assays are repeated with a higher sample dilution to obtain cotinine levels within the standard range of the assay. Participants who returned the samples received a $20 gift card. While this method was the most feasible solution for biochemically validating patients self-reported tobacco use behavior, it is impossible to confirm the identity of the individual who provided the sample – a limitation of the study.
2.6. Primary Outcome and Statistical Analysis Plan
The primary outcome of the study is biochemically-validated past 7-day tobacco use assessed at 6 months post screening visit. Original power calculations were based on 22 of 26 sites completing the trial with each site accruing 50 patient participants (n=1,100) and a 6-month retention rate of 76% (n=832). Because we retained 24 instead of 26 clinics for the duration of the study, we have exceeded our baseline patient accrual goal. Six month data collection is ongoing.
Sample size estimates were based on the following data. Approximately 48% of patients who present for lung cancer screening are current smokers, and the percentage of persons who successfully quit smoking in the previous year ranges from 4.9% (among 45–65 year olds) to 7.4% (among persons 65 and older) [20, 21] Receipt of counseling to promote cessation (vs. no counseling) increases abstinence 1.3 to 2.3 fold (low versus high intensity counseling), while pharmacotherapy (vs. placebo) increases the odds of abstinence 2.3–3.1 fold (patch vs. varenicline) [5]. With this sample size, we had 80% power to detect a difference in the primary outcome (7-day abstinence) between groups. This calculation assumes that the control group abstinence rate was 10% and the intervention group rate was 20% using a 2-sided Z-test for comparing proportions with alpha=0.05 (2-sided). For this calculation, in order to account for the cluster randomized design we used an intra-class correlation value of 0.03 [22]. This calculation should be conservative since the control group abstinence rate may be lower than 10% which would mean that the intervention effect could also be less and still lead to statistical (and clinical) significance.
In order to compare groups, a multi-level logistic regression model approach will be used [23]. This model allows for both fixed and random effects to be included. The initial model for the primary analysis included two fixed effects: community site (used in the randomization) and intervention (yes, no). Additionally, we included one random effect, imaging clinic. The model can be written as Yijk = μ+ γk+αj+ βk(j) + εi(jk), where Yijk is the outcome (i.e. 6-month smoking abstinence) measured on the ith participant, under the jth intervention in the kth clinic; μ is the grand mean; γk is the cluster (site) for clinic k; αj is the fixed treatment effect for group j; βk(j) is the random effect of the kth clinic nested within the exposure group; and εi(jk) is the error term for the ith participant nested within the treatment group and clinic. Other fixed effects can be added at the patient level (e.g., age, gender, race). The random clinic effect allows the possibility of correlated observations (participants) within clinics.
2.7: Qualitative Analysis:
Analysis of key informant interviews included: (1) professional transcription and verification; (2) entering transcriptions into Atlas Ti software for data management; (3) development of a common coding system and data dictionary among two members of the team with experience conducting qualitative data analysis; and (4) an iterative comparison of transcripts for codes and themes relevant for implementing smoking cessation services in LDCT lung cancer screening programs.
Key metrics and qualitative data analysis will focus on the following implementation constructs at baseline and follow-up: adoption, fidelity, and adaptation, and sustainability. We hypothesize that adoption depends upon the confidence and ability of clinic personnel to implement the PHS Guidelines; the relative advantage of different strategies compared to one another, in terms of personnel investment, time, other demands; compatibility of the strategies with mission of the LDCT screening program, and simplicity to implement. At follow-up, we focus the qualitative analysis on fidelity – the extent to which screening sites implemented the strategies in accordance with the Guidelines and adaptations that had to be made (role function, workflow considerations) to adopt the PHS Guidelines. The follow-up interview will also focus on sustainability of the strategies following the completion of the OaSiS trial and the critical features that were needed to ensure their long-term embeddedness within the imaging clinic.
Pragmatic Explanatory Continuum Indicator Summary (PRECIS-2):
Our multidisciplinary team (including 4 physicians and 4 social and behavioral scientists) independently completed the PRECIS-2 evaluation to assess the pragmatic nature of the trial in nine domains: eligibility (who is selected to participate in the trial), recruitment (how are participants recruited), setting (where is the trial being done), organization (what expertise and resources are needed to deliver the intervention, flexibility in delivery (how should the intervention be delivered, flexibility in adherence (what measures are in place to ensure participants adhere to the intervention), follow-up (how closely are participants followed), primary outcome (how relevant is it to the participants) and primary analysis [24–27](to what extent are all data included). Each domain was evaluated on a 1 (very explanatory) to 5 (very pragmatic) Likert scale and from the perspective of the imaging clinic and patient participant. Summary scores are depicted in Figure 2. The study was deemed rather or very pragmatic, with a median score of 4 or higher on 6 of 9 domains (eligibility, setting, flexibility in both delivery and adherence, primary outcome and primary analysis). The least pragmatic aspect of the trial is follow-up.
Figure 2:
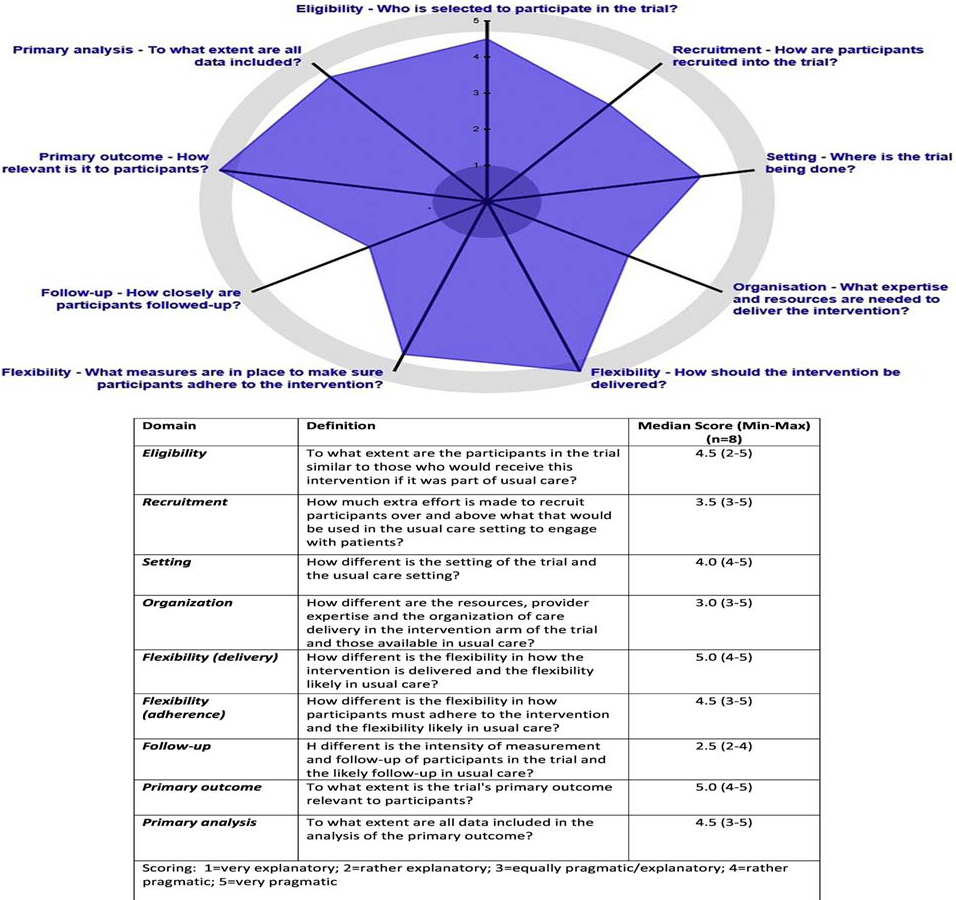
Multidisciplinary Team Ratings (n=8) of the Pragmatic Explanatory Continuum Indicator Summary (PRECIS-2) for the Optimizing Lung Screening Study (OaSiS) Effectiveness-Implementation Hybrid Trial (Type II)
DISCUSSION
OaSiS is in its fourth of five years. Enrollment included 26 clinics of which 24 have remained in the trial. All clinics have completed baseline and follow-up organizational surveys. Sixty health system personnel completed 60 baseline key informant interviews and follow-up interviews are ongoing, with goal of accruing 48 key informants. 1,053 patient participants have enrolled reflecting 88% of our goal. All data collection will be completed within six months. Collaborating with the Wake Forest NCORP Research Base and the NCORP community sites has been instrumental in outstanding accrual. We expect to comfortably exceed 90% of our patient participant accrual goal. With robust, multi-level data, we will be able to answer the major question of the NCI SCALE initiative [9]: How to support lung cancer screening patients to quit smoking whereby optimizing the screening encounter to improve patient outcomes.
An estimated 8.7 million US adults are eligible for low-dose computed tomography (LDCT) lung cancer screening, and up to half of patients presenting for lung cancer screening are current smokers [1]. Because tobacco use is a leading cause of death and quitting smoking is the most powerful way to decrease lung cancer mortality, there is a critical need to implement effective smoking cessation within LDCT chest screening. Quitting, even later in life, increases life expectancy [28], decreases risk of competing causes of death [29, 30], improves survival among lung cancer patients [31], and maximizes the cost-effectiveness of lung cancer screening [32, 33]. Cessation services can also be used to correct misconceptions about lung cancer screening that may inadvertently lower the likelihood of quitting [34].
The lung cancer screening environment distinct from primary care practices, the traditional locale for smoking cessation [35–38]. Accordingly, prior research “cannot be applied without consideration of the setting” (RFA-CA-15–011). While organizational priority of integrating smoking cessation within lung cancer screening is high, implementation of the strategies that “assist” patients with quitting and ensure follow-up care is limited [4]. The OaSiS trial was designed to identify opportunities for implementing evidence-based smoking cessation into LDCT lung cancer screening imaging facilities and to evaluate the effectiveness of evidence-based smoking cessation support in community-based imaging settings.
There may be several barriers to the effectiveness and implementation of evidence-based smoking cessation support in the imaging setting. Patients come to the imaging center for screening and may not be primed for quitting smoking, leading to lower motivation to accept cessation support. Patient motivation may be further weakened if a negative LDCT screen creates a false sense of security about personal risk of lung cancer [6, 34]. Additionally, patients may never see a physician during their visit, and CT technicians often do not feel empowered to counsel or support patients to quit smoking. Upon rare opportunity when patients interact with physicians, providers will likely perceive cessation as secondary to screening and satisfy the CMS mandate through an “ask, advise, and refer” mechanism. Imaging center staff may feel that smoking cessation assessment or advice is out of their scope or comfort zone. This “fulfillment” of the mandate may be no more than checking a box, and patients might not follow up with referrals [4, 39]. Finally, lung cancer screening is evolving rapidly in varied clinical contexts (e.g., radiology, pulmonology, free standing clinics vs. hospital systems). Different clinical contexts may have unique cultures and clinical operations systems making a ‘one size fits all’ approach to implementing tobacco cessation services unlikely.
The rationale for study design was based on the following. First, we have evidence-based guidelines to promote cessation that have been tested in various clinical venues and have demonstrated success via meta-analyses. We cannot assume, however, that the PHS Guidelines will be efficacious in the context of LDCT lung cancer screening. Second, the strategies that accompany these guidelines are likely to be adopted if they are easy to implement (e.g., they are time and cost neutral, they are well-received by the implementers, they fit within the mission of the organization) and there is organizational support for implementation. Third, strategies may be adapted to simplify implementation without sacrificing the PHS Guidelines integrity.
We have carefully considered the opportunities and challenges inherent in the OaSiS trial and cluster randomized trials, more generally. A summary of those is listed below.
1. Partnership with NCORP:
Working with NCORP, a cooperative group structure, allowed us to recruit 26 community-based imaging sites in 20 states and to enhance the diversity of the types of clinics and patients we enrolled in this trial. The network contains over 1,000+ participating locations affiliated with 46 community sites of which 12 are minority/underserved sites. Sites have an existing infrastructure and an expectation that they will engage in cancer care delivery research as part of their NCI designation and funding and were therefore externally incentivized to participate in the OaSiS trial. Additionally, NCORP utilizes a centralized institutional review board structure decreasing the need for site-by-site comprehensive IRB review (many sites, but not all, “recognize” the NCI Central Institutional Review Board (CIRB) for ethical review). Moreover, the infrastructure for recruitment of participants and data collection with support from the Wake Forest NCORP Research Base minimized the research team investment in costs generally associated with data collection, quality control, and training multi-site studies.
Nonetheless, there were some challenges associated with the NCORP partnership including length of time to study activation due to preparation of the protocol and IRB revisions requested by the CIRB. In addition, a few NCORP sites experienced challenges engaging imaging clinics; the NCORP infrastructure was created to support oncology treatment trials, and therefore, a partnership with diagnostic radiology was new which necessitated forging new relationships and regulatory considerations. There was also a learning curve in helping NCORP personnel at several sites understand the distinctions between randomized patient-level oncology trials and cluster randomized pragmatic trials that intervene with providers and health systems. Finally, due to staff turnover at the local level, two sites were unable to accrue and ultimately withdrew from the study.
2. Trade-off between fidelity and adaptation.
A common challenge to all cluster randomized trials is the need to apply interventions with fidelity across diverse settings. At times, there may be the need to allow adaptations of the interventions given unique resources and support at each site. An example of this in the OaSiS trial is the provision of brief motivational counseling and pharmacotherapy support. This is a well-established and efficacious approach to treating nicotine addiction. In some sites, personnel were available to offer this support by a patient navigator at the time of their lung screening appointment. However, in other sites, this support was offered by creating an automated, proactive referral to evidence-based cessation interventions utilizing only local resources that were sustainable after the research. These nuances in site-specific adoption of the PHS Guidelines create unique challenges regarding measuring fidelity, adaptation, and effectiveness when there are varied interventions used in multiple sites.
3. Communication and coordination across multiple sites and with a large, multi-disciplinary team.
In a study with 26 sites across 20 states, it was necessary to create an organizational structure and communication strategy to ensure optimal implementation. NCORP provides an exceptional framework for communication regarding all aspects related to evaluation. Local personnel are often seasoned researchers with experience partnering with trialists from around the country. From an implementation perspective, however, it was necessary to create teams of individuals who assumed responsibility for each intervention site. We chose to have a 3–4 person research team for each site that included a project manager and at least two faculty (one clinical and one behavioral scientist). Team communication, facilitated largely by the project management team, served as a critical nexus for communication both within sites, across sites, and for the multidisciplinary research team. Having a single point of contact for the team was essential to the overall operations of this large, multi-site trial.
Future Directions:
Using the data from this hybrid trial, we will develop a scalable toolkit that can be shared with other imaging settings outside the OaSiS trial. The goal of the toolkit is to support the successful implementation of evidence-based tobacco cessation strategies into clinical settings with LDCT lung cancer screening programs, in the absence of a research study. The toolkit will be developed by the research team and external advisory board (EAB) members using the action plans, performance coaching notes, and key informant interviews. We will explore which smoking cessation strategies were most often adopted and under what health system and imaging setting contexts, as well as those perceived to be the most feasible, appropriate, and efficacious by imaging clinic personnel. The research team will meet bi-weekly during a 6–8 month period to develop and refine each section of the comprehensive toolkit with support from members of our EAB Sections will be brief and written in clear, simple language with step-by-step planning templates for imaging clinics to follow. After each section is developed and refined, the document will be reviewed by the EAB and program champions from the intervention clinics, as well as national stakeholders from professional medical societies (e.g., ASCO) and non-profit agencies committed to cancer prevention and smoking cessation (e.g., ACS, American Lung, Legacy). This will be an iterative process with multiple opportunities for feedback.
Anticipated topics within the toolkit include: How to use the toolkit.; Why smoking cessation is important for LDCT patients; Evidence-based approaches to promote quitting.; Assessing clinic’s readiness to implement evidence-base strategies; What does it take to implement the PHS Guidelines in imaging settings; Resources needed for implementation; Roadblocks/detours/delays and overcoming barriers; How to adapt the PHS Guidelines to meet the clinics’ need; Identifying and using existing health system resources; Sustaining what you start.
Conclusion:
The external validity and scalability of our results will be strengthened by our pragmatic design and inclusion of a diverse range of screening programs. We expect the OASIS trial to yield rich information about the barriers and facilitators of implementing and sustaining tobacco cessation services within varied lung cancer screening sites, as well as the effectiveness of the these strategies for promoting smoking cessation among LDCT screening patients.
Acknowledgements
We would like to acknowledge the partners at NCORP community sites for their willingness and engagement in the OaSiS trial.
Funding
Funding support from the National Cancer Institute under award numbers R01CA207158, UM1CA189824, and P30CA012197. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
All authors of the manuscript report no conflicts of interest.
References
- [1].Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, Gareen IF, Gatsonis C, Marcus PM, Sicks JD, Reduced lung-cancer mortality with low-dose computed tomographic screening, N Engl J Med 365(5) (2011) 395–409. 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Moyer VA, Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement, Ann Intern Med 160(5) (2014) 330–8. 10.7326/M13-2771. [DOI] [PubMed] [Google Scholar]
- [3].The Centers for Medicare & Medicaid Services, Decision Memo for Screening for Lung Cancer with Low Dose Computed Tomography (LDCT) (CAG-00439N). [Google Scholar]
- [4].Ostroff JS, Copeland A, Borderud SP, Li Y, Shelley DR, Henschke CI, Readiness of Lung Cancer Screening Sites to Deliver Smoking Cessation Treatment: Current Practices, Organizational Priority, and Perceived Barriers, Nicotine Tob Res 18(5) (2016) 1067–75. 10.1093/ntr/ntv177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Fiore MC, Jaen CR, Baker TB, Bailey WC, Bennett G, Benowitz NL, Christiansen BA, Connell M, Curry SJ, Dorfman SF, Fraser D, Froelicher ES, Goldstein MG, Hasselblad V, Healton CG, Heishman S, Henderson PN, Heyman RB, Husten C, Koh HK, Kottke TE, Lando HA, Leitzke C, Mecklenburg RE, Mermelstein RJ, Morgan G, Mullen PD, Murray EW, Orleans CT, Piper ME, Robinson L, Stitzer ML, Theobald W, Tommasello AC, Villejo L, Wewers ME, Williams C, Treati CPG, A Clinical Practice Guideline for Treating Tobacco Use and Dependence: 2008 Update - A US Public Health Service report, American Journal of Preventive Medicine 35(2) (2008) 158–176. 10.1016/j.amepre.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Park ER, Gareen IF, Japuntich S, Lennes I, Hyland K, DeMello S, Sicks JD, Rigotti NA, Primary Care Provider-Delivered Smoking Cessation Interventions and Smoking Cessation Among Participants in the National Lung Screening Trial, JAMA Intern Med 175(9) (2015) 1509–16. 10.1001/jamainternmed.2015.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].U.S. Department of Health and Human Services, Smoking Cessation: A Report of the Surgeon General, U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, Atlanta, GA: U.S., 2020. [Google Scholar]
- [8].National Institute of Health. United States National Library of Medicine, Implementation of Smoking Cessation Within NCI NCORP Community Sites (OaSiS). NCT03291587. (Accessed 10/21/2019).
- [9].National Cancer Institute Division of Cancer Control & Population Sciences Behavioral Research Program, Smoking Cessation at Lung Examination: The SCALE Collaboration. https://cancercontrol.cancer.gov/brp/tcrb/scale-collaboration.html. (Accessed 8/28/2015).
- [10].Colditz GA, The promise and challenges of dissemination and implementation research, Dissemination and Implementation Research in Health, Oxford University Press, New York, 2012. [Google Scholar]
- [11].Brownson RC, Jones E, Bridging the gap: translating research into policy and practice, Preventive medicine 49(4) (2009) 313–5. 10.1016/j.ypmed.2009.06.00810.1016/j.ypmed.2009.06.008. [DOI] [PubMed] [Google Scholar]
- [12].Glasgow RE, Vogt TM, Boles SM, Evaluating the public health impact of health promotion interventions: the RE-AIM framework, American journal of public health 89(9) (1999) 1322–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bradley EH, Curry LA, Ramanadhan S, Rowe L, Nembhard IM, Krumholz HM, Research in action: using positive deviance to improve quality of health care, Implement Sci 4 (2009) 25 10.1186/1748-5908-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bradley EH, Pallas S, Bashyal C, Berman P, Curry L, Developing Strategies for Improving Health Care Delivery : Guide to Concepts, Determinants, Measurement, and Intervention Design, (2010). http://hdl.handle.net/10986/13610 [Google Scholar]
- [15].Greenhalgh T, Robert G, Macfarlane F, Bate P, Kyriakidou O, Diffusion of innovations in service organizations: systematic review and recommendations, The Milbank quarterly 82(4) (2004) 581–629. 10.1111/j.0887-378X.2004.00325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Greenhalgh T, Robert G, Macfarlane F, Bate P, Kyriakidou O, Peacock R, Storylines of research in diffusion of innovation: a meta-narrative approach to systematic review, Social science & medicine (1982) 61(2) (2005) 417–30. 10.1016/j.socscimed.2004.12.001. [DOI] [PubMed] [Google Scholar]
- [17].Johnson A, What is Coaching? https://hr.mit.edu/learning-topics/leading/articles/what-is-coaching. (Accessed 9/28/15). [Google Scholar]
- [18].Kowalski K, Casper C, The coaching process: an effective tool for professional development, Nursing administration quarterly 31(2) (2007) 171–9. 10.1097/01.NAQ.0000264867.73873.1a. [DOI] [PubMed] [Google Scholar]
- [19].Curran GM, Bauer M, Mittman B, Pyne JM, Stetler C, Effectiveness-implementation hybrid designs: combining elements of clinical effectiveness and implementation research to enhance public health impact, Medical care 50(3) (2012) 217–26. 10.1097/MLR.0b013e3182408812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Pinsky PF, Church TR, Izmirlian G, Kramer BS, The National Lung Screening Trial: results stratified by demographics, smoking history, and lung cancer histology, Cancer 119(22) (2013) 3976–83. 10.1002/cncr.28326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Schauer GL, Malarcher AM, Asman KJ, Trends in the Average Age of Quitting Among U.S. Adult Cigarette Smokers, Am J Prev Med 49(6) (2015) 939–44. 10.1016/j.amepre.2015.06.028. [DOI] [PubMed] [Google Scholar]
- [22].Donner A, Klar N, Design and Analysis of Cluster Randomization Trials in Health Research, Wiley; 2000. [Google Scholar]
- [23].Omar RZ, Thompson SG, Analysis of a cluster randomized trial with binary outcome data using a multi-level model, Statistics in medicine 19(19) (2000) 2675–88. [DOI] [PubMed] [Google Scholar]
- [24].Loudon K, Treweek S, Sullivan F, Donnan P, Thorpe KE, Zwarenstein M, The PRECIS-2 tool: designing trials that are fit for purpose, BMJ 350 (2015) h2147 10.1136/bmj.h2147 [DOI] [PubMed] [Google Scholar]
- [25].Forbes G, Loudon K, Treweek S, Taylor SJC, Eldridge S, Understanding the applicability of results from primary care trials: lessons learned from applying PRECIS-2, J Clin Epidemiol 90 (2017) 119–126. 10.1016/j.jclinepi.2017.06.007 [DOI] [PubMed] [Google Scholar]
- [26].Loudon K, Zwarenstein M, Sullivan FM, Donnan PT, Gagyor I, Hobbelen H, Althabe F, Krishnan JA, Treweek S, The PRECIS-2 tool has good interrater reliability and modest discriminant validity, J Clin Epidemiol 88 (2017) 113–121. 10.1016/j.jclinepi.2017.06.001 [DOI] [PubMed] [Google Scholar]
- [27].Zwarenstein M, Treweek S, Loudon K, PRECIS-2 helps researchers design more applicable RCTs while CONSORT Extension for Pragmatic Trials helps knowledge users decide whether to apply them, J Clin Epidemiol 84 (2017) 27–29. 10.1016/j.jclinepi.2016.10.010 [DOI] [PubMed] [Google Scholar]
- [28].Taylor DH Jr., Hasselblad V, Henley SJ, Thun MJ, Sloan FA, Benefits of smoking cessation for longevity, American journal of public health 92(6) (2002) 990–6. 10.2105/ajph.92.6.990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hermanson B, Omenn GS, Kronmal RA, Gersh BJ, Beneficial six-year outcome of smoking cessation in older men and women with coronary artery disease. Results from the CASS registry, N Engl J Med 319(21) (1988) 1365–9. 10.1056/NEJM198811243192101. [DOI] [PubMed] [Google Scholar]
- [30].Gellert C, Schottker B, Brenner H, Smoking and all-cause mortality in older people: systematic review and meta-analysis, Arch Intern Med 172(11) (2012) 837–44. 10.1001/archinternmed.2012.1397. [DOI] [PubMed] [Google Scholar]
- [31].Parsons A, Daley A, Begh R, Aveyard P, Influence of smoking cessation after diagnosis of early stage lung cancer on prognosis: systematic review of observational studies with meta-analysis, Bmj 340 (2010) b5569 10.1136/bmj.b5569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Black WC, Gareen IF, Soneji SS, Sicks JD, Keeler EB, Aberle DR, Naeim A, Church TR, Silvestri GA, Gorelick J, Gatsonis C, Cost-effectiveness of CT screening in the National Lung Screening Trial, N Engl J Med 371(19) (2014) 1793–802. 10.1056/NEJMoa1312547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].McMahon PM, Kong CY, Bouzan C, Weinstein MC, Cipriano LE, Tramontano AC, Johnson BE, Weeks JC, Gazelle GS, Cost-effectiveness of computed tomography screening for lung cancer in the United States, Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 6(11) (2011) 1841–8. 10.1097/JTO.0b013e31822e59b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Zeliadt SB, Heffner JL, Sayre G, Klein DE, Simons C, Williams J, Reinke LF, Au DH, Attitudes and Perceptions About Smoking Cessation in the Context of Lung Cancer Screening, JAMA Intern Med 175(9) (2015) 1530–7. 10.1001/jamainternmed.2015.3558. [DOI] [PubMed] [Google Scholar]
- [35].Tong EK, Strouse R, Hall J, Kovac M, Schroeder SA, National survey of US health professionals’ smoking prevalence, cessation practices, and beliefs, Nicotine Tob Res 12(7) (2010) 724–33. 10.1093/ntr/ntq071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Okuyemi KS, Reitzel LR, Fagan P, Interventions to Reduce Tobacco-Related Health Disparities, Nicotine Tob Res 17(8) (2015) 887–91. 10.1093/ntr/ntv096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Foley KL, Pockey JR, Helme DW, Song EY, Stewart K, Jones C, Spangler JG, Sutfin EL, Integrating evidence-based tobacco cessation interventions in free medical clinics: opportunities and challenges, Health Promot Pract 13(5) (2012) 687–95. 10.1177/1524839911433465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Pockey JR, Song EY, Sutfin EL, Spangler JG, Jones C, Helme DW, Foley KL, The need for tobacco cessation in a free clinic population, Addict Behav 37(12) (2012) 1299–302. 10.1016/j.addbeh.2012.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Fiore MC, Goplerud E, Schroeder SA, The Joint Commission’s new tobacco-cessation measures--will hospitals do the right thing?, N Engl J Med 366(13) (2012) 1172–4. 10.1056/NEJMp1115176. [DOI] [PMC free article] [PubMed] [Google Scholar]


