Abstract
BACKGROUND
Transfusion is associated with organ failure and nosocomial infection in trauma patients, which may be mediated by soluble bioactive substances in blood products, including extracellular vesicles (EVs). We hypothesize that removing EVs, by washing or filtering of blood products, reduces organ failure and improves host immune response.
MATERIALS AND METHODS
Blood products were prepared from syngeneic rat blood. EVs were removed from RBCs and platelets by washing. Plasma was filtered through a 0.22‐μm filter. Rats were traumatized by crush injury to the intestines and liver, and a femur was fractured. Rats were hemorrhaged until a mean arterial pressure of 40 mm Hg and randomized to receive resuscitation with standard or washed/filtered blood products, in a 1:1:1 ratio. Sham controls were not resuscitated. Ex vivo whole blood stimulation tests were performed and histopathology was done.
RESULTS
Washing of blood products improved quality metrics compared to standard products. Also, EV levels reduced by 12% to 77%. The coagulation status, as assessed by thromboelastometry, was deranged in both groups and normalized during transfusion, without significant differences. Use of washed/filtered products did not reduce organ failure, as assessed by histopathologic score and biochemical measurements. Immune response ex vivo was decreased following transfusion compared to sham but did not differ between transfusion groups.
CONCLUSION
Filtering or washing of blood products improved biochemical properties and reduced EV counts, while maintaining coagulation abilities. However, in this trauma and transfusion model, the use of optimized blood components did not attenuate organ injury or immune suppression.
ABBREVIATIONS
- AST
aspartate transaminase
- CINC‐3
cytokine‐induced neutrophil chemoattractant 3
- EVs
extracellular vesicles
- FFP
fresh frozen plasma
- LPS
lipopolysaccharide
- MAP
mean arterial pressure
- MOF
multiple organ failure
- PLT
platelet
- ROTEM
rotational thromboelastometry
- SAGM
saline‐adenine‐glucose‐mannitol
Typically, bleeding trauma patients develop a hypocoagulopathy, referred to as trauma‐induced coagulopathy.1, 2 Balanced resuscitation with blood products in ratios similar to whole blood is the mainstay to treat trauma‐induced coagulopathy and prevent exsanguination. However, transfusion is associated with adverse effects, including multiple organ failure (MOF)3, 4, 5, 6 and nosocomial infection.7 The mechanisms by which transfusion exerts these effects probably include modulation of the host coagulation and immune response. Several studies showed an association between trauma‐induced coagulopathy and the development of MOF.3, 4, 5 Also, following trauma, both pro‐ and anti‐inflammatory profiles have been reported.7 A proinflammatory response can contribute to MOF,8, 9 while an immunosuppressive response following blood transfusion in trauma patients is thought to predispose patients to postinjury infection.10
During storage, a variety of biochemical changes occur in blood products, collectively referred to as storage lesion.11, 12, 13 These biochemical changes include accumulation of bioactive substances, such as lysophosphatidylcholines,14 soluble CD40‐ligand15 and extracellular vesicles (EVs),13, 16 and have the ability to exert various proinflammatory processes, for example, priming of neutrophils.17, 18
EVs, typically ranging from 50 to 500 nm,19 are present in all blood components and are important contributors to their storage lesion.20, 21, 22 These EVs may contribute to coagulation in trauma, as low levels have been associated with coagulopathy in trauma.23 However, EVs in blood products also activate the endothelium,24 which may derange host response and contribute to organ failure. EVs also mediate proinflammatory and/or immune suppressive processes in trauma.25 Washing of cells or filtration of plasma may improve blood product quality by reducing EV concentration,26, 27, 28 as well as the amount of other bioactive agents, including lysophosphatidylcholines29 and soluble CD40‐ligand 30and thereby decrease transfusion‐related adverse events. In line with this, in murine models, washing of cells led to an abrogation of lung injury31, 32 and systemic inflammation33 induced by RBCs or platelets. Clinical studies show differential results. Whereas washing of products seemed to reduce systemic inflammation in children undergoing cardiac surgery,34 this effect was not seen in adults.27 A Cochrane review investigating the effect of transfusion of washed RBCs in preterm infants also did not show any benefit.35 However, these studies evaluated RBC transfusions only. As organ failure associated with transfusion is dose dependent,3 modification of different blood products during multiple transfusion may have a greater impact on outcome.
The aim of this study was to investigate whether reducing the amount of EVs in transfusion products, by washing RBCs and platelets or filtering plasma, decreases the inflammatory response and occurrence of MOF in a trauma and multiple‐transfusion model. Also, we hypothesized that washed/filtered products would attenuate an immunosuppressive effect on host immune response, while maintaining oxygen‐carrying and procoagulant capacity.
MATERIALS AND METHODS
Animal study design
The animal care and use committee of our tertiary hospital approved this study. Animal procedures were carried out in compliance with Institutional Standards for Use of Laboratory Animals. A pilot (n = 3) for the induction of trauma and bleeding was performed to set up the model, resulting in metabolic acidosis, as well as a hypocoagulopathic profile as assessed by rotational thromboelastometry (ROTEM) traces. A subsequent pilot (n = 3) was performed to establish the transfusion protocol that resulted in a correction of the coagulopathy and pH levels.
Preparation of blood products
Rat blood products were obtained from syngeneic Sprague‐Dawley rats (Envigo). For each rat in the experiment, a donor rat was available. Following anesthesia, using isoflurane, blood was drawn by heart puncture into a syringe containing 1.25 mL of citrate‐phosphate‐dextrose (Fresenius HemoCare GmbH). Blood was pooled for component preparation. Blood was handled as we have reported before31, 32 and stored according to national standards for human blood (Sanquin Blood Supply Foundation). In short, blood was centrifugated for 10 minutes at 1892g and 20°C. Plasma was separated and the buffy coat was removed from the packed erythrocytes. Saline‐adenine‐glucose‐mannitol (SAGM; Fresenius Hemocare) was added to the erythrocytes up to a hematocrit of 60%.32 The final products were stored at 4°C for 14 days (equaling storage of human RBCs for 35 days).32 For the preparation of standard rat fresh frozen plasma (FFP) products, the plasma separated from the RBCs was frozen at −80 °C and stored for 14 days. For the preparation of standard rat platelet (PLT) products, another batch of animals was used. Whole blood was again collected by heart puncture and centrifugated for 10 minutes at 1892g and 20°C. The plasma was removed and the buffy coat was separated from the RBCs. The buffy coat was diluted with pooled plasma to a hematocrit of approximately 20% and again centrifugated for 10 minutes at 288g and 20°C to separate the majority of the remaining erythrocytes and leucocytes from the platelets.36 The supernatant, containing platelet‐rich plasma, was transferred into culture flasks at 22°C under a 5% CO2/95% air mixture for 5 days and was put on a PLT incubator (Helmer) shaking horizontally with 1 cycle/second.31
Optimization of blood products
Both standard and optimized RBCs were kept at room temperature approximately 1 hour before transfusion. To optimize the RBC products, they were washed after 14 days of storage. To obtain soluble factors from the product as much as possible, 0,9% saline was added before centrifugation. The erythrocyte‐saline mixture was centrifugated for 15 minutes at 1500g and 4 °C. The supernatant was removed and the erythrocytes were washed using SAGM (1:1) and centrifuged for 15 minutes at 1250g and 4°C. The supernatant was removed, and new SAGM was added to the erythrocytes up to the original volume of the rat RBC product. This method was adopted, as it was shown to augment lung injury.32
FFP products were thawed for 45 to 60 minutes in ice water. After thawing, plasma was kept at room temperature for at least 10 minutes. To optimize the FFP products, the plasma was filtered using a 0.22‐μm filter. We chose this filter, as earlier studies showed a reduction in EVs, when a 0.2‐μm pore size was used to filter plasma.28
The rat PLT products were washed after 5 days of storage. The standard PLT products stayed on the PLT incubator until right before transfusion. To optimize PLT products, they were centrifuged for 15 minutes at 1250g and 22°C. The supernatant of the product was removed. The PLTs were then washed using a leukocyte‐reduced apheresis platelet concentrate (Intersol, Fresenius‐Kabi), which was added up to the original volume and centrifuged for 15 minutes at 1000g and 22°C. The supernatant was removed, and Intersol was added up to the original volume of the PLT product. This method was used, as it was shown to mitigate lung injury.31
Biochemical changes in blood products
Blood products were sampled just before transfusion and analyzed for pH, glucose, electrolytes, and lactate with a blood gas analyzer (Rapidlab 865, Siemens Medical Solutions Diagnostics). In the PLT products, the modified Kunicki morphology score was calculated.37
Extracellular vesicle measurements
EVs were measured in supernatant of all standard and optimized blood products. A flow cytometer (Apogee A60‐Micro, Apogee Flow Systems), equipped with a 200‐mW 405‐nm laser, was used to detect forward‐scattered light, side‐scattered light, and fluorescence of EVs. This flow cytometer was used, as it is able to detect smaller particles in comparison to conventional flow cytometers.38 To remove cells, blood products were centrifuged twice (1750g, 10 min, 20°C). After being stored in −80°C, supernatant of each blood product was thawed at 37°C and diluted to provide approximately 5.000 events per second to prevent swarm detection of EVs.39 Twenty μL of prediluted sample was stained with the following lineage‐specific monoclonal antibodies to identify the cell of origin of EVs: integrin β3 (sc‐53351, Santa Cruz Biotechnology)40, 41 for PLT‐derived EVs, erythroid cell antibody42 for RBC‐derived EVs (BioLegend), and anti‐CD45 (NB100‐64895APC, Novus Biologicals)43 for WBC‐derived EVs. Rosetta Calibration (Exometry) was used determine the size of EVs44 according to Mie theory.45 Data acquisition was performed with Apogee software, and data were processed with FLOWJO v.10.3 (FlowJo LLC).
Experimental protocol
Male Sprague‐Dawley rats were approximately 12 weeks of age, with an average weight around 350 g. Animals lived in standard cages under normal conditions of a 12:12‐hour light:dark cycle and received standard lab chow and water ad libitum. The experiments were performed every other day and started around 08 a.m.
After sedation and analgesia with ketamine 100 mg/mL (Eurovet), dexmedetomidine 0,5 mg/mL (Orion Pharma) and atropine 0,5 mg/mL (Centrafarm), a tracheotomy was performed in the rats and they were put on a mechanical ventilator (Servo 900C, Siemens). Rats were ventilated in a pressure‐controlled mode, with 10 cm H2O peak inspiratory pressure and 5 cm H2O positive end expiratory pressure, and FiO2 was set at 30%. Recruitment maneuvers were performed every 60 minutes by increasing peak inspiratory pressure to 30 cm H2O during 5 breaths. Respiratory rate was set at 35 breaths per minute.
Throughout the experiment, rats received ketamine as maintenance anesthesia, and they were monitored using intra‐arterial blood pressure monitoring. Urine production was recorded and collected using a suprapubic catheter. Polytrauma was induced by crush injury of the small intestines and the left and medial liver lobes using a metal clamp covered with silicone tubing for 3 seconds.46 The right femur was fractured using a blunt guillotine, in which a 650 g of steel weight dropped on the right femur which was resting on two supporting pillars, one under the hip and one under the knee (see Fig. S1, available as supporting information in the online version of this paper). The rats then underwent controlled hemorrhage, by withdrawal of blood from a catheter in the carotid artery, until mean arterial pressure (MAP) was 40 mm Hg. This equated to approximately 30% of their estimated circulating blood volume, thereby mimicking the clinical scenario of acute massive bleeding leading to a shock state with severe hypotension, and activation of the host immune response.
After approximately 45 minutes in a shocked state, animals were transfused with blood products in a 1:1:1 ratio of RBC:FFP:PLTs until an MAP of 65 mm Hg was reached. To achieve the 1:1:1 ratio, all blood products were pooled together and transfused at a rate of 8 mL/h. Animals did not receive any further fluids. Four hours after transfusion, rats were sacrificed by exsanguination, as earlier models of hemorrhage and resuscitation showed signs of MOF at this time point.47
Randomization
Animals were randomized (n = 8 per group) to receive no transfusion, transfusion with standard blood products, or transfusion with optimized products. Because the administration of crystalloids is also associated with MOF,3 rats receiving no transfusion did not receive resuscitation of any kind. A fourth group consisted of healthy rats that were used as donors for blood product preparation and as controls in the histopathologic organ examination.
Blood and tissue sampling
Blood samples were collected prior to trauma (T = −1), at the end of the shock period; just after resuscitation was initiated (T = 0); and at 1 (T = 1), 2 (T = 2), and 4 (T = 4) hours (see Fig. 1). Plasma levels of aspartate transaminase (AST), alanine transaminase and lactate dehydrogenase were measured using standard enzymatic reactions and spectrophotometric assays. Creatinine was measured by colorimetric determination using creatinase. ROTEM (ROTEM Delta, Werfen) EXTEM measurements were also performed, but only in the transfused rats, since no extra blood could be drawn in the nonresuscitated rats due to severe shock.
Figure 1.
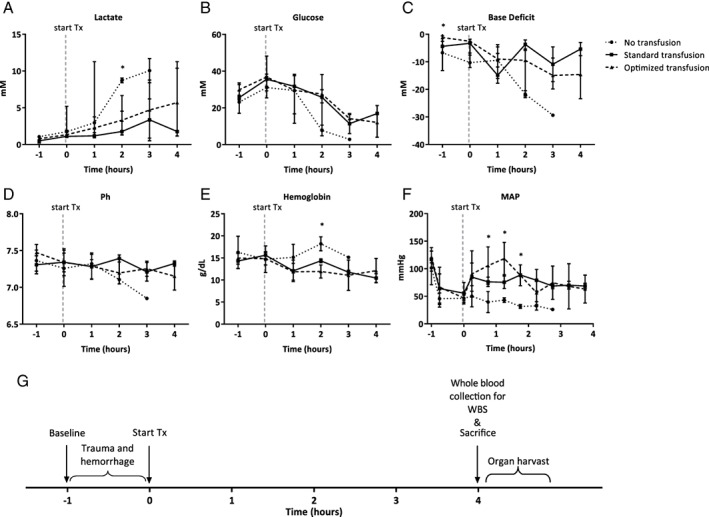
Parameters of shock in different treatment groups over time. (A) Blood lactate levels expressed as medians. (B) Blood glucose levels expressed as medians. (C) Base deficit levels expressed as medians. (D) Blood pH levels expressed as means. (E) Hemoglobin levels in g/dL expressed as means. (F) Mean arterial pressure in mm Hg expressed as means. (G) Timeline of the experiment. Every hour blood samples were collected for biochemical analysis, blood gas analysis, and ROTEM. WBS = whole blood stimulations. * = p < 0.05.
Every hour, arterial blood gas analysis was performed (Rapidlab 865 blood gas analyzer, Siemens Medical Solutions Diagnostics).
After sacrifice by exsanguination, the trachea and lungs were taken out en bloc. Bronchoalveolar lavage fluid (BALF) of the left lung was performed by instilling 2 mL of sterile 0.9% saline in the trachea three times, using a syringe. Immediately after instillation over 10 to 15 seconds, the fluid was aspirated with the same syringe.
Organs were removed after exsanguination, fixed in 10% buffered formalin, embedded in paraffin and cut into 4‐μm‐thick slides for histopathologic examination. Organs of the donor rats were harvested as healthy controls to the other groups (n = 8).
Immune response
Ex vivo whole blood stimulation tests with lipopolysaccharide (LPS; from Escherichia coli, lot number 064M4125V) were performed by adding 250 μL of rat whole blood to 250 μL of prepared stimulation solution containing 0.5 ng/mL of LPS.25 Samples were then incubated for 24 hours at 37°C and 5% CO2. After 24 hours, samples were centrifuged at 600g for 10 minutes at 4°C. The supernatant was collected and stored at −80°C for later analysis of levels of IL‐6 and cytokine‐induced neutrophil chemoattractant 3 (CINC‐3; R&D Systems). These cytokines were measured, as they are usually elevated and frequently used in trauma and transfusion models.31, 32, 48
Histopathologic examination of organ injury
For histopathologic examination, hematoxylin and eosin staining was performed. A pathologist blinded to the treatment groups examined the tissues and graded the severity of injury to the organ on a scale of 0 to 3 (0 = absent, 1 = mild, 2 = moderate, 3 = severe), based on previously published validated scoring systems.49, 50 Severity of liver injury was assessed by scoring the presence of necrosis, hemorrhage, portal inflammation, and neutrophil infiltration. Kidney injury was scored on the presence of epithelial necrosis or luminal necrotic debris in the cortical tubules, tubular dilation, neutrophil extravasation, and hemorrhage. Lung injury was assessed by scoring the presence of lung edema, interstitial inflammatory cell infiltration, endothelitis and hemorrhage. Injury to the small intestine was scored on the presence of diffuse swelling of the villi, neutrophil infiltration in the submucosa, necrosis, and hemorrhage.
Statistical analyses
Acute lung injury is an important manifestation of MOF in trauma patients.51, 52 In a previous rat transfusion model, washing of RBC products reduced IL‐6 levels, with a mean difference of of 8 ng/g (standard deviation, 4 ng/g). Therefore, to obtain a power of 80%, assuming a 5% significance level and using a two‐sided unpaired t test, a total of six rats should be randomized to each group. As we expected a mortality rate within the groups of around 20%, we included eight rats in each group.
Normality for continuous variables was tested by visual inspection of histograms and by Kolmogorov‐Smirnov test. Results are expressed as either mean ± standard deviation or as median ± interquartile range depending on the distribution of the variables. To test for differences between standard and optimized blood products, the Student t test or Mann‐Whitney U test was used. For comparisons between the experimental groups, a one‐way analysis of variance or Kruskal‐Wallis test was used, both with a Dunnett's posttest for multiple comparisons. A p value of less than 0.05 was considered to be statistically significant. Statistics were done with computer sofware (SPSS Statistics 24, IBM).
RESULTS
At baseline (i.e., after tracheotomy but before trauma), animals in the optimized transfusion group had a lower base deficit compared to the other groups (see Table 1). Base deficit was not statistically different directly following trauma and transfusion between groups. The nonresuscitated animals did not survive the follow‐up period; 3 out of 8 animals survived until 3 hours follow‐up, the others died after 2 hours. The resuscitated animals survived throughout the experiment. All animals were severely injured and in shock, reflected by low blood pressure as well as an increased base deficit (see Fig. 1). Following resuscitation, MAP increased in both groups receiving transfusion. The group receiving no resuscitation remained on a MAP of 40 mm Hg. The volume of products needed to reach a MAP of 65 mm Hg did not differ between groups (14.7 ± 2.8 mL/kg in the standard transfusion group vs. 14.6 ± 2.7 mL/kg in the optimized group; p = NS). Hemoglobin level did not drop in the group receiving no resuscitation, which may be due to severe fluid depletion, as animals did not receive any fluids and were put on a mechanical ventilator. In line with this, their hematocrit levels increased during the experiment (data not shown).
Table 1.
Animal characteristics prior to and directly after trauma
| No transfusion | Standard products | Optimized products | ||||
|---|---|---|---|---|---|---|
| Baseline | Hypovolemic shock* | Baseline | Hypovolemic shock* | Baseline | Hypovolemic shock* | |
| Clinical parameters | ||||||
| Weight (g) | 404.0 (401.5–405.5) | 406.0 (398.0–416.0) | 395.0 (393.3–401.3)† ‡ | |||
| Chemical lab | ||||||
| Hemoglobin level (mmol/L) | 10.1 (8.5–11.7) | 9.1 (7.6–10.7) | 9.1 (7.8–9.7) | 9.9 (9.3–10.1) | 9.4 (8.6–10.0) | 9.2 (8.3–9.8) |
| Hematocrit (%) | NR | 0.39 (0.36–0.42) | 0.47 (0.31–0.56) | 0.50 (0.38–0.56) | 0.52 (0.49–0.64) | 0.56 (0.51–0.62) |
| Creatinine (μmol/L) | 30.5 (26.0–35.0) | 37.0 (33.0–64.0) | 24.0 (20.0–26.0)† | 34.0 (30.0–62.0)§ | 28.5 (26.3–39.8)‡ | 42.0 (35.5–51.5)§ |
| AST (U/L) | 51 (51–51) | 61 (48–97) | 56 (53–79) | 110 (94–113)§ | 73 (57–98) | 105 (89–122) |
| ALT (U/L) | 37 (35–39) | 36 (36–76) | 46 (38–54) | 84 (67–89)§ | 53 (52–63) | 84 (69–114)§ |
| LDH (mmol/L) | 80 (80–80) | 221 (137–430) | 237 (172–301) | 505 (393–641)§ | 316 (188–499) | 513 (425–728) |
| Blood gas | ||||||
| pH | 7.42 (7.20–7.47) | 7.40 (7.01–7.43) | 7.28 (7.24–7.38) | 7.36 (7.13–7.45) | 7.47 (7.39–7.52) | 7.36 (7.23–7.39) |
| pCO2 (mm Hg) | 38.2 (14.0–57.1) | 36.9 (22.4–104.3) | 44.6 (31.9–64.1) | 35.7 (37.7–70.4) | 30.9 (24.0–42.9) | 40.2 (32.2–52.9) |
| HCO3 – (mmol/L) | 21.9 (10.1–24.9) | 20.5 (14.4–24.7) | 21.4 (17.8–27.6) | 21.2 (18.8–22.5) | 22.4 (18.7–25.6) | 21.9 (18.1–23.5) |
| BD (mmol/L) | −6.7 (−13.2 – −0.6) | −10.3 (−12.1 – −2.6) | −4.4 (−6.8 – −2.6) | −3.3 (−7.6 – −1.8) | −1.2 (−2.6–0.8)‡ | −2.6 (−6.8 – −2.2)§ |
| Lactate (mmol/L) | 1.06 (0.21–1.14) | 1.74 (1.06–5.19) | 0.50 (0.31–0.58) | 1.11 (0.89–1.64)§ | 0.78 (0.74–1.04) | 1.33 (1.07–1.93)§ |
| Hemodynamics | ||||||
| MAP (mm Hg) | 105 (69–130) | 48 (38–53)§ | 125 (98–136) | 50 (41–67)§ | 120 (114–128) | 50 (41–51)§ |
Values are expressed as median and IQR.
Values are recorded after trauma and hemorrhage, just after transfusion therapy was given in the resuscitation groups.
p < 0.05 versus no transfusion group.
p < 0.05 versus standard transfusion group.
p < 0.05 versus baseline value in same group.
ALT = alanine transaminase; AST = aspartate transaminase; BD = base deficit; LDH = lactic dehydrogenase; MAP = mean arterial pressure; NR = not recorded.
Effect of filtering or washing on biochemical properties of blood products
Washing of cellular products resulted in an increase in pH and sodium levels, while potassium and lactate levels dropped significantly (see Table 2). Glucose levels of the products increased after washing. Filtration of the plasma products did not lead to any significant differences in biochemical properties compared to the nonfiltered product.
Table 2.
Biochemical properties of standard and optimized blood products
| RBCs | Fresh frozen plasma | Platelets | ||||
|---|---|---|---|---|---|---|
| Standard product | Optimized product | Standard product | Optimized product | Standard product | Optimized product | |
| pH | 6.6 (0.0) | 7.1 (0.0)* | 7.52 (0.01) | NR | 6.74 (0) | 6.98 (0)* |
| Lactate (mmol/L) | 21.2 (1.7) | 1.6 (0.1)* | 2.2 (1.2) | 1.5 (0.1) | NR | NR |
| Na+ (mmol/L) | 132.5 (0.1) | 143.5 (0.6)* | 145.2 (3.5) | 140.4 (0.9) | 130.5 (0.7) | 147.0 (1.4)* |
| K+ (mmol/L) | 31.2 (2.1) | 1.4 (0.1)* | 4.0 (0.9) | 4.7 (0.0) | 9.0 (0) | 5.8 (0.1)* |
| Ca2+ (mmol/L) | 0.21 (0) | 0.23 (0) | NR | NR | 0.25 (0) | 0.25 (0) |
| Cl− (mmol/L) | 114.0 (0.7) | 137.0 (12.7) | 78.0 (5.2) | 82.3 (0.6) | NR | NR |
| Glucose (mmol/L) | 19.4 (0.1) | 39.4 (0.6)* | 30.1 (1.8) | 26.6 (0.4) | 5.1 (0) | 1.1 (0)* |
| Morphology, Kunicki score | NA | NA | NA | NA | 224 (215–224) | 217 (216–217) |
| Platelet‐derived EVs (x106 EVs/mL) | 100 (165) | 85.5 (145) | 32.8 (19.2) | 14.4 (14.1) | 289 (193) | 50.2 (29.5) |
| Leucocyte‐derived EVs (x106 EVs/mL) | 7.4 (1.7) | 2.7 (1.6)* | 22.3 (9.6) | 10.3 (1.1) | 187 (186) | 65.7 (65.5) |
| RBC‐derived EVs (x106 EVs/mL) | 135 (119) | 125 (107) | 19.2 (3.1) | 5.6 (1.5)* | 116 (33.2) | 19.7 (13.9)* |
| Total EVs (x106 EVs/mL) | 243 (64.7) | 214 (39.8) | 74.3 (31.4) | 30.3 (16.4) | 593 (403) | 136 (106) |
Values are expressed as mean and SD.
EVs = extracellular vesicles; NA = not applicable; NR = not recorded.
P < 0.05 vs. standard product.
Numbers of microparticles reduced by filtering or washing blood products
RBC products showed low levels of platelet‐derived EVs and WBC‐derived EVs, but high levels of erythrocyte‐derived EVs. Washing led to a reduction of 12% in the total number of EVs. FFP showed relatively low numbers of EVs/mL, which decreased 59% following filtration of plasma products. The PLT product showed the highest amount of EVs in the product. A reduction of 77% was found in the number of EVs in the PLT products (see Table 2). In particular, the number of vesicles 200 nM or more were reduced in FFP and PLT products after washing or filtering. However, to some extent, smaller EVs were also reduced (see Fig. S2, available as supporting information in the online version of this paper). Washing of the RBC products did not reduce EVs much.
Effect of filtering or washing of blood products on coagulation capacity
ROTEM was performed in the transfusion groups but was not feasible in the nontransfused control group. Following trauma and initiation of transfusion, measurements were taken in both transfusion groups. Trauma resulted in hypocoagulopathic traces, as reflected by a decrease in maximum clot firmness and alfa angle when compared to baseline. Resuscitation improved the coagulation status of the rats (see Fig. 2A–D). There were no significant differences in coagulation profile between rats treated with optimized blood products compared to standard blood products.
Figure 2.
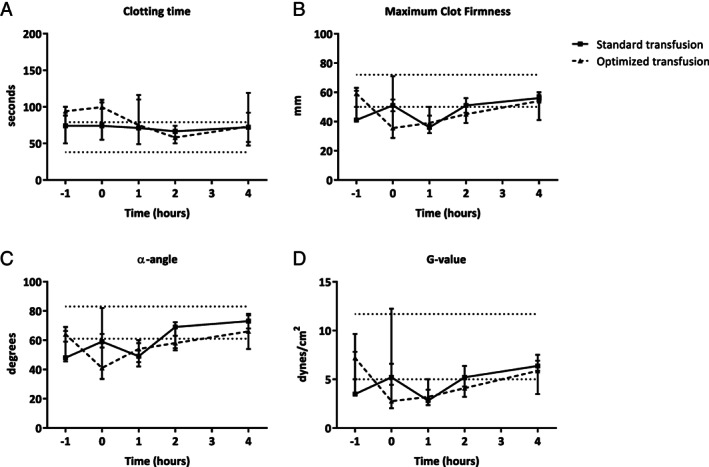
Coagulation parameters over time as measured by rotation thromboelastometry. Values are presented as median values. (A) Clotting time (CT) measured in seconds (dotted lines are reference values*: 38–79 seconds). (B) Maximum clot firmness (MCF) measured in mm (dotted lines are reference values*: 50–72 mm). (C) α‐angle measured in degrees (dotted lines are reference values*: 61–83 degrees). (D) G‐value measured in dynes/cm2 (dotted lines are reference values*: 5–11.7 dynes/cm2).4 * = Based on human reference values.
Effect of filtering or washing of blood products on organ dysfunction
Compared to healthy controls, the trauma model resulted in organ injury when assessed by histopathology, including kidney injury (p = 0.034) and lung injury (p = 0.031), whereas a trend was found in the induction of gut injury (p = 0.089). Liver injury was not increased when compared to healthy controls. The total organ injury score was significantly higher in shocked rats when compared to healthy rat (p = 0.022). Pulmonary edema was increased in the rats receiving transfusion compared to sham controls and healthy rats (see Fig. 3B). However, the amount of organ injury did not differ between the transfusion groups (see Fig. 3A). Also, trauma and transfusion resulted in increased levels of creatinine, AST and alanine transaminase when compared to baseline (see Table 1). The rats receiving optimized blood products had significantly lower AST levels compared to the rats receiving standard blood products (p = 0.009), but this difference was gone at the 4‐hour time point (p = 0.085). In the other organ systems, no significant differences between the transfusion groups were found (see Fig. 4A–D).
Figure 3.
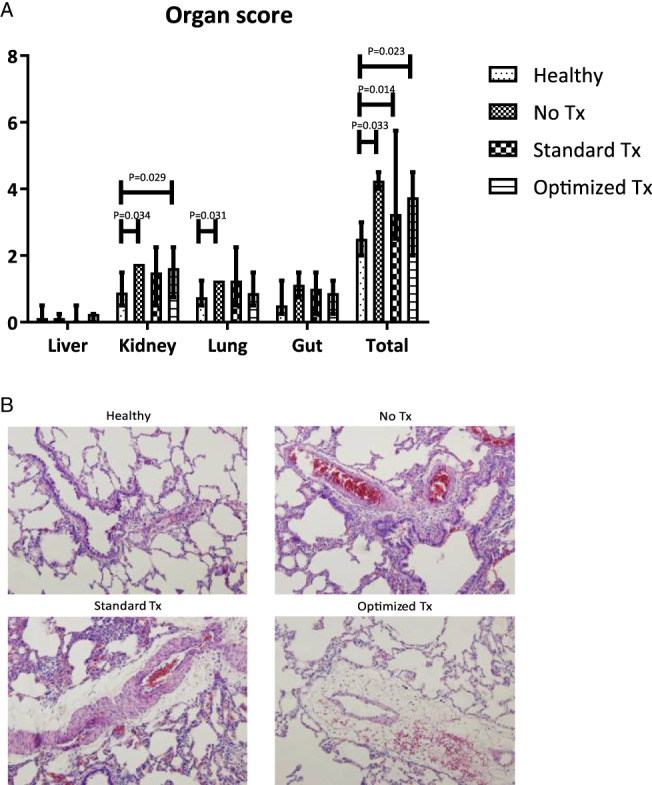
Histopathologic examination of organ injury. (A) Organ injury score based on histopathologic examination, presented as median and interquartile ranges. (B) Representative microphotographs of lung tissue (magnification 100×). [Color figure can be viewed at wileyonlinelibrary.com]
Figure 4.
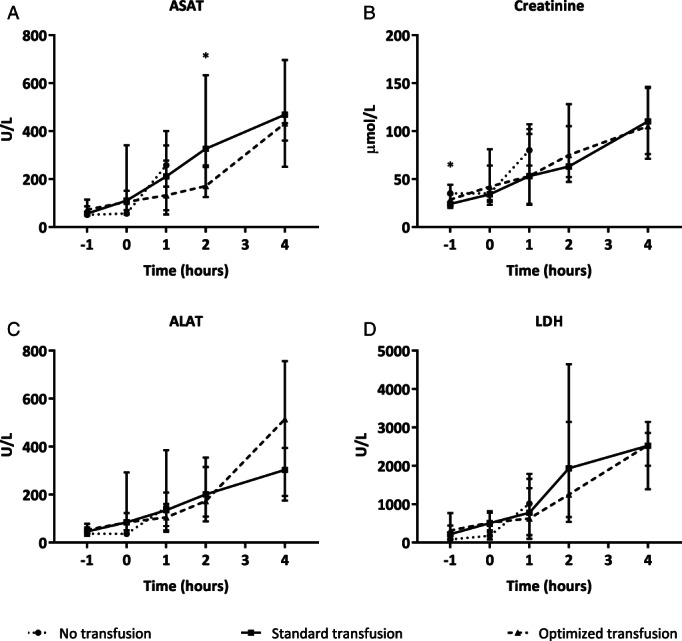
Markers of organ injury over time. Values are given in median and interquartile ranges. (A) AST levels measured in arterial blood samples (p value determined by Mann‐Whitney U test). (B) Creatinine levels measured in arterial blood samples. (C) ALT levels measured in arterial blood samples. (D) Lactate dehydrogenase (LDH) levels measured in arterial blood samples.* = p < 0.05.
Effect of filtering or washing of blood products on host response
Trauma and shock resulted in increased systemic levels of IL‐6 in plasma over time in all groups. Also, bronchoalveolar lavage fluid samples showed increased levels of IL‐6. There was no difference in IL‐6 levels between transfusion groups, however (see Fig. 5A–B).
Figure 5.
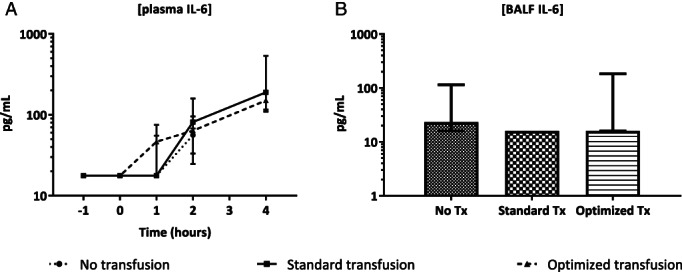
Cytokine levels in plasma over time and in lungs at sacrifice. (A) Plasma IL‐6 levels over time in the different groups. (B) IL‐6 levels in bronchoalveolar lavage fluid taken at exsanguination in the different groups. Values are given in median and interquartile ranges.
In ex vivo whole blood stimulation experiments, rats receiving no transfusion showed an intact immune response, as assessed by increased IL‐6 levels in response to LPS stimulation, which was abrogated in the transfused rats (p = 0.007). CINC‐3 levels were nonsignificantly decreased in the transfused rats (p = 0.280). However, IL‐6 and CINC‐3 levels did not differ significantly between the group transfused with standard products compared to the optimized products (see Fig. 6A–B).
Figure 6.
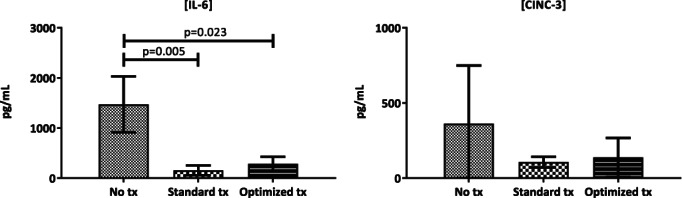
Whole blood stimulations with LPS in different treatment groups. Cytokine levels after whole blood stimulation of samples taken at exsanguination and stimulated with LPS. Values are given in mean and standard deviation.
DISCUSSION
Hemorrhagic shock and multiple transfusion in trauma patients often results in coagulopathy and organ damage.6 Our model reflected these derangements. Also, the storage lesion in the rat blood products reflected the storage lesion of expired human blood.31, 32, 53 Washing or filtering of blood products improved quality metrics of the blood products but did not attenuate the occurrence of organ failure, nor did it improve host response to bacterial antigen LPS in our model.
Improvement of products by reducing soluble mediators is gaining attention as an intervention to decrease transfusion‐related adverse events. Although concerns have been raised both about decreasing the oxygen‐delivering capacity of the products54 and about feasibility,55 a trial with washed RBCs in cardiac surgery patients is currently ongoing.56 Biochemical changes in blood products during storage17 include accumulation of procoagulant and inflammatory EVs,21, 57 which can be altered by washing of these products.26, 58 In vitro, electrolyte balance seems to stabilize and microaggregates are removed from the products.59, 60, 61 This is in line with our results because electrolyte balance and lactate levels stabilized in our washed cellular products and the numbers of EVs dropped markedly in all blood products after washing or filtering. However, the effect of RBC washing on the amount of EVs was modest. A possible explanation could be that washing may have contributed to increased membrane instability and EV formation. This is supported by the fact that we measured so‐called ghost erythrocytes in our washed RBC products, which are 6‐ to 8‐μm empty cell fragments.62
Importantly, in vivo coagulation ability as measured by ROTEM was not adversely affected by washing or filtering of blood products. Several studies have shown the procoagulant potential of EVs in blood products in vitro.21, 63 Earlier studies in rats have shown that transfusion of washed RBC products leads to a prolongation of the clotting time and a reduction in clot strength, compared to transfusion of standard RBCs.64 It is thought that washing of RBC products with normal saline removes residual plasma proteins, leading to a reduction in the hemostatic properties of RBCs.61 In our study however, removal of these EVs did not alter the in vivo coagulation properties in our model. Both transfusion groups received FFP and platelets next to RBCs, possibly eliminating any effect of washed RBCs on the coagulation status of the rats. Also, lactate levels were similar in the transfusion groups, which may reflect an equal ability of washed RBCs to deliver oxygen to tissues when compared to standard RBCs.
Despite improved biochemical properties of the blood products and a reduction in the amount of EVs, we found little effect of washing or filtering on organ functioning. The only exception was that AST levels were decreased after transfusion with optimized products compared to standard products. This may be in line with a study in a rat hemorrhage model, where it was shown that liver injury was lower in animals receiving fresh blood compared to stored blood.65 The limited benefit of washing of products in our study reflects findings in a porcine model, in which washing of RBC products did not reduce organ injury compared to standard transfusion products.66 These previous studies focused on transfusion of RBC blood products only. In a setting of transfusion of multiple modified blood products, the possible mitigating effect of washing of products on organ damage would be expected to be greater. Our results, however, suggest that optimizing blood products in the setting of multiple transfusion also does not limit organ damage.
Furthermore, washing or filtering of blood products did not affect host immune response. Our model was characterized by a systemic proinflammatory response, as reflected by increased plasma and bronchoalveolar lavage fluid IL‐6 levels, as shown before in vitro.67 There were no differences between transfusion groups on IL‐6 levels in our study. Previous studies have shown contrasting results of washing of RBCs, with both a decrease in cytokine production as well as no effect at all.18, 31, 32, 33
Concurrent with systemic inflammation, a simultaneous immune depression of the innate immune cells may occur after transfusion.7, 67 In line with this, we observed a decreased cytokine response to stimulation with LPS in transfused rats compared to the rats receiving no transfusion. However, this immunosuppressive effect of transfusion was not altered by washing or filtering of the blood products. An explanation may be that the cellular component of products are responsible for the immunomodulatory effect. This is underlined by an in vitro study in which the cellular component of autologous collected blood induced anti‐inflammatory cytokine production, whereas no effect was found after the cells from the salvaged blood were removed.68 Also, residual WBCs may play a role in the occurrence of transfusion‐related immunomodulation.69
The study had some limitations. The time frame of 4 hours of follow‐up may not have been sufficient for an intervention to modulate a difference in organ damage. Also, the measurement of EVs using flow cytometry may have failed to detect the smallest EVs and may have underestimated the amount of vesicles in the products. Furthermore, apart from EVs, we did not measure any other bioactive mediators in the blood products. However, washing and filtering protocols have previously shown to reduce lysophosphatidylcholines and CD40‐ligand. A strong aspect of our study is that this is the first preclinical study investigating transfusion of several modified blood products (RBCs, PLTs, and FFP) simultaneously.
CONCLUSION
Filtering or washing of blood products reduces aspects of storage lesion, without affecting the hemostatic capacity of the products but does not result in reduced organ injury, nor does it improve the immunosuppressed host response in our model. These results suggest that washing or filtering of blood products may have no relevant clinical effects.
CONFLICT OF INTEREST
The authors have disclosed no conflicts of interest.
Supporting information
Fig. S1. Blunt guillotine used for the femur fracture of all traumatized rats. Tubes are 23 cm in height. A marking is placed at 14 cm (height from which the weight should be dropped). Weight of the cross bar is 650 grams. Distance between the supporting pillars for the femur is 2 cm.
Fig. S2. Size characterization of extracellular vesicles based on cell of origin
Presented at: Annual meeting American Association of Blood Banks 2017, San Diego, CA, USA.
REFERENCES
- 1. Brohi K, Cohen MJ, Davenport RA. Acute coagulopathy of trauma: mechanism, identification and effect. Curr Opin Crit Care 2007;13:680‐5. [DOI] [PubMed] [Google Scholar]
- 2. Davenport RA, Brohi K. Cause of trauma‐induced coagulopathy. Curr Opin Anaesthesiol 2016;29:212‐9. [DOI] [PubMed] [Google Scholar]
- 3. Balvers K, Wirtz MR, van Dieren S, et al. Risk factors for trauma‐induced coagulopathy‐ and transfusion‐associated multiple organ failure in severely injured trauma patients. Front Med 2015;2:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Muller MC, Balvers K, Binnekade JM, et al. Thromboelastometry and organ failure in trauma patients: a prospective cohort study. Crit Care 2014;18:687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Peltan ID, Vande Vusse LK, Maier RV, et al. An international normalized ratio–based definition of acute traumatic coagulopathy is associated with mortality, venous thromboembolism, and multiple organ failure after injury. Crit Care Med 2015;43:1429‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ciesla DJ, Moore EE, Johnson JL, et al. A 12‐year prospective study of postinjury multiple organ failure: has anything changed? Arch Surg 2005;140:432‐8 discussion 438–40. [DOI] [PubMed] [Google Scholar]
- 7. Muszynski JA, Spinella PC, Cholette JM, et al. Transfusion‐related immunomodulation: review of the literature and implications for pediatric critical illness. Transfusion 2017;57(1):195‐206. doi: 10.1111/trf.13855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hazeldine J, Hampson P, Lord JM. The impact of trauma on neutrophil function. Injury 2014;45:1824‐33. [DOI] [PubMed] [Google Scholar]
- 9. Cabrera CP, Manson J, Shepherd JM, et al. Signatures of inflammation and impending multiple organ dysfunction in the hyperacute phase of trauma: a prospective cohort study. PLoS Med 2017;14:e1002352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Leliefeld PH, Wessels CM, Leenen LP, et al. The role of neutrophils in immune dysfunction during severe inflammation. Crit Care 2016;20:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Spieth PM, Zhang H. Storage injury and blood transfusions in trauma patients. Curr Opin Anaestesiol 2018;31(2):234‐237. doi: 10.1097/ACO.0000000000000565 [DOI] [PubMed] [Google Scholar]
- 12. Singh S, Shams Hakimi C, Jeppsson A, et al. Platelet storage lesion in interim platelet unit concentrates: a comparison with buffy‐coat and apheresis concentrates. Transfus Apher Sci 2017;56:870‐4. [DOI] [PubMed] [Google Scholar]
- 13. Spinella PC, Frazier E, Pidcoke HF, et al. All plasma products are not created equal: characterizing differences between plasma products. J Trauma Acute Care Surg 2015;78(6 Suppl 1):S18‐25. [DOI] [PubMed] [Google Scholar]
- 14. Silliman CC, Burke T, Kelher MR. The accumulation of lipids and proteins during red blood cell storage: the roles of leucoreduction and experimental filtration. Blood Transfus 2017;15:131‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wenzel F, Gunther W, Baertl A, et al. Platelet transfusion alters CD40L blood level and release capacity in patients suffering from thrombocytopenia. Transfusion 2012;52:1213‐20. [DOI] [PubMed] [Google Scholar]
- 16. Sparrow RL. Red blood cell storage duration and trauma. Transfus Med Rev 2015;29:120‐6. [DOI] [PubMed] [Google Scholar]
- 17. Kor DJ, Van Buskirk CM, Gajic O. Red blood cell storage lesion. Bosn J Basic Med Sci 2009;9:21‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cardo LJ, Wilder D, Salata J. Neutrophil priming, caused by cell membranes and microvesicles in packed red blood cell units, is abrogated by leukocyte depletion at collection. Transfus Apher Sci 2008;38:117‐25. [DOI] [PubMed] [Google Scholar]
- 19. van Niel G, D'Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol 2018;19:213‐28. [DOI] [PubMed] [Google Scholar]
- 20. Burnouf T, Chou ML, Goubran H, et al. An overview of the role of microparticles/microvesicles in blood components: are they clinically beneficial or harmful? Transfus Apher Sci 2015;53:137‐45. [DOI] [PubMed] [Google Scholar]
- 21. Bouchard BA, Orfeo T, Keith HN, et al. Microparticles formed during storage of red blood cell units support thrombin generation. J Trauma Acute Care Surg 2018;84:598‐605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Seghatchian J, Amiral J. Unresolved clinical aspects and safety hazards of blood derived‐EV/MV in stored blood components: from personal memory lanes to newer perspectives on the roles of EV/MV in various biological phenomena. Transfus Apher Sci 2016;55:10‐22. [DOI] [PubMed] [Google Scholar]
- 23. Matijevic N, Wang YW, Wade CE, et al. Cellular microparticle and thrombogram phenotypes in the Prospective Observational Multicenter Major Trauma Transfusion (PROMMTT) study: correlation with coagulopathy. Thromb Res 2014;134:652‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Straat M, van Hezel ME, Boing A, et al. Monocyte‐mediated activation of endothelial cells occurs only after binding to extracellular vesicles from red blood cell products, a process mediated by beta‐integrin. Transfusion 2016;56:3012‐20. [DOI] [PubMed] [Google Scholar]
- 25. Balvers K, Curry N, Kleinveld DJ, et al. Endogenous microparticles drive the pro‐inflammatory host immune response in severely injured trauma patients. Shock 2015;43:317‐21. [DOI] [PubMed] [Google Scholar]
- 26. Loh YS, Tan S, Kwok M, et al. Reduction of biological response modifiers in the supernatant of washed paediatric red blood cells. Vox Sang 2016;111:365‐73. [DOI] [PubMed] [Google Scholar]
- 27. Wozniak MJ, Sullo N, Qureshi S, et al. Randomized trial of red cell washing for the prevention of transfusion‐associated organ injury in cardiac surgery. Br J Anaesth 2017;118:689‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Matijevic N, Wang YW, Kostousov V, et al. Decline in platelet microparticles contributes to reduced hemostatic potential of stored plasma. Thromb Res 2011;128:35‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Spinelli SL, Lannan KL, Casey AE, et al. Isoprostane and isofuran lipid mediators accumulate in stored red blood cells and influence platelet function in vitro. Transfusion 2014;54:1569‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Silliman CC, Kelher MR, Khan SY, et al. Experimental prestorage filtration removes antibodies and decreases lipids in RBC supernatants mitigating TRALI in vivo. Blood 2014;123:3488‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vlaar AP, Hofstra JJ, Kulik W, et al. Supernatant of stored platelets causes lung inflammation and coagulopathy in a novel in vivo transfusion model. Blood 2010;116:1360‐8. [DOI] [PubMed] [Google Scholar]
- 32. Vlaar AP, Hofstra JJ, Levi M, et al. Supernatant of aged erythrocytes causes lung inflammation and coagulopathy in a “two‐hit” in vivo syngeneic transfusion model. Anesthesiology 2010;113:92‐103. [DOI] [PubMed] [Google Scholar]
- 33. Belizaire RM, Makley AT, Campion EM, et al. Resuscitation with washed aged packed red blood cell units decreases the proinflammatory response in mice after hemorrhage. J Trauma Acute Care Surg 2012;73:S128‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cholette JM, Henrichs KF, Alfieris GM, et al. Washing red blood cells and platelets transfused in cardiac surgery reduces postoperative inflammation and number of transfusions: results of a prospective, randomized, controlled clinical trial. Pediatr Crit Care Med 2012;13:290‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Keir AK, Wilkinson D, Andersen C, et al. Washed versus unwashed red blood cells for transfusion for the prevention of morbidity and mortality in preterm infants. Cochrane Database Syst Rev 2016;1:CD011484. doi:10.1002/14651858.CD011484.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dhurat R, Sukesh M. Principles and methods of preparation of platelet‐rich plasma: a review and author's perspective. J Cutan Aesthet Surg 2014;7:189‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Levin E, Culibrk B, Gyongyossy‐Issa MI, et al. Implementation of buffy coat platelet component production: comparison to platelet‐rich plasma platelet production. Transfusion 2008;48:2331‐7. [DOI] [PubMed] [Google Scholar]
- 38. van der Pol E, Coumans FA, Grootemaat AE, et al. Particle size distribution of exosomes and microvesicles determined by transmission electron microscopy, flow cytometry, nanoparticle tracking analysis, and resistive pulse sensing. J Thromb Haemost 2014;12:1182‐92. [DOI] [PubMed] [Google Scholar]
- 39. de Rond L, van der Pol E, Hau CM, et al. Comparison of generic fluorescent markers for detection of extracellular vesicles by flow cytometry. Clin Chem 2018;64:680‐9. [DOI] [PubMed] [Google Scholar]
- 40. Shan LY, Li JZ, Zu LY, et al. Platelet‐derived microparticles are implicated in remote ischemia conditioning in a rat model of cerebral infarction. CNS Neurosci Ther 2013;19:917‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gao C, Li R, Liu Y, et al. Rho‐kinase‐dependent F‐actin rearrangement is involved in the release of endothelial microparticles during IFN‐alpha‐induced endothelial cell apoptosis. J Trauma Acute Care Surg 2012;73:1152‐60. 10.1097/TA.0b013e318265d04b. [DOI] [PubMed] [Google Scholar]
- 42. da SilveiraCavalcante L, Acker JP, Holovati JL. Differences in rat and human erythrocytes following blood component manufacturing: the effect of additive solutions. Transfus Med Hemother 2015;42:150‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tual‐Chalot S, Guibert C, Muller B, et al. Circulating microparticles from pulmonary hypertensive rats induce endothelial dysfunction. Am J Respir Crit Care Med 2010;182:261‐8. [DOI] [PubMed] [Google Scholar]
- 44. Varga Z, van der Pol E, Palmai M, et al. Hollow organosilica beads as reference particles for optical detection of extracellular vesicles. J Thromb Haemost 2018;16(8):1646‐1655. [DOI] [PubMed] [Google Scholar]
- 45. van der Pol E, Sturk A, van Leeuwen T, et al. Standardization of extracellular vesicle measurements by flow cytometry through vesicle diameter approximation. J Thromb Haemost 2018;16:1236‐45. [DOI] [PubMed] [Google Scholar]
- 46. Darlington DN, Craig T, Gonzales MD, et al. Acute coagulopathy of trauma in the rat. Shock 2013;39:440‐6. [DOI] [PubMed] [Google Scholar]
- 47. Dai ZL, Wu J, Meng C, et al. Ringer's malate solution protects against the multiple organ injury and dysfunction caused by hemorrhagic shock in rats. Shock 2012;38:268‐74. [DOI] [PubMed] [Google Scholar]
- 48. Wu X, Schwacha MG, Dubick MA, et al. Trauma‐related acute lung injury develops rapidly irrespective of resuscitation strategy in the rat. Shock 2016;46:108‐14. [DOI] [PubMed] [Google Scholar]
- 49. Straat M, Tuip A, Klei TRL, et al. Endotoxemia results in trapping of transfused red blood cells in lungs with associated lung injury. Shock 2017;48:484‐9. [DOI] [PubMed] [Google Scholar]
- 50. Lee CC, Lee RP, Subeq YM, et al. Fluvastatin attenuates severe hemorrhagic shock‐induced organ damage in rats. Resuscitation 2009;80:372‐8. [DOI] [PubMed] [Google Scholar]
- 51. Park PK, Cannon JW, Ye W, et al. Transfusion strategies and development of acute respiratory distress syndrome in combat casualty care. J Trauma Acute Care Surg 2013;75:S238‐46. [DOI] [PubMed] [Google Scholar]
- 52. Sharpe JP, Weinberg JA, Magnotti LJ, et al. Does plasma transfusion portend pulmonary dysfunction? A tale of two ratios. J Trauma Acute Care Surg 2013;75:32‐6 discussion 6. [DOI] [PubMed] [Google Scholar]
- 53. d'Almeida MS, Jagger J, Duggan M, et al. A comparison of biochemical and functional alterations of rat and human erythrocytes stored in CPDA‐1 for 29 days: implications for animal models of transfusion. Transfus Med 2000;10:291‐303. [DOI] [PubMed] [Google Scholar]
- 54. Weisbach V, Riego W, Strasser E, et al. The in vitro quality of washed, prestorage leucocyte‐depleted red blood cell concentrates. Vox Sang 2004;87:19‐26. [DOI] [PubMed] [Google Scholar]
- 55. Silliman CC, Moore EE, Johnson JL, et al. Transfusion of the injured patient: proceed with caution. Shock 2004;21:291‐9. [DOI] [PubMed] [Google Scholar]
- 56. Warner MA, Welsby IJ, Norris PJ, et al. Point‐of‐care washing of allogeneic red blood cells for the prevention of transfusion‐related respiratory complications (WAR‐PRC): a protocol for a multicenter randomised clinical trial in patients undergoing cardiac surgery. BMJ Open 2017;7:e016398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Radwanski K, Garraud O, Cognasse F, et al. The effects of red blood cell preparation method on in vitro markers of red blood cell aging and inflammatory response. Transfusion 2013;53:3128‐38. [DOI] [PubMed] [Google Scholar]
- 58. Bennett‐Guerrero E, Kirby BS, Zhu H, et al. Randomized study of washing 40‐ to 42‐day‐stored red blood cells. Transfusion 2014;54:2544‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Westphal‐Varghese B, Erren M, Westphal M, et al. Processing of stored packed red blood cells using autotransfusion devices decreases potassium and microaggregates: a prospective, randomized, single‐blinded in vitro study. Transfus Med 2007;17:89‐95. [DOI] [PubMed] [Google Scholar]
- 60. Gruber M, Breu A, Frauendorf M, et al. Washing of banked blood by three different blood salvage devices. Transfusion 2013;53:1001‐9. [DOI] [PubMed] [Google Scholar]
- 61. Schmidt AERM, Kirkley SA, Blumberg N. Proven and potential clinical benefits of washing red blood cells before transfusion: current perspectives. Int J Clin Transfus Med 2016;4:79‐88. [Google Scholar]
- 62. Arraud N, Linares R, Tan S, et al. Extracellular vesicles from blood plasma: determination of their morphology, size, phenotype and concentration. J Thromb Haemost 2014;12:614‐27. [DOI] [PubMed] [Google Scholar]
- 63. Aleshnick M, Foley JH, Keating FK, et al. Procoagulant activity in stored units of red blood cells. Biochem Biophys Res Commun 2016;474:680‐5. [DOI] [PubMed] [Google Scholar]
- 64. Torres LN, Sondeen JL, Dubick MA, et al. Systemic and microvascular effects of resuscitation with blood products after severe hemorrhage in rats. J Trauma Acute Care Surg 2014;77:716‐23. [DOI] [PubMed] [Google Scholar]
- 65. Matot I, Katz M, Pappo O, et al. Resuscitation with aged blood exacerbates liver injury in a hemorrhagic rat model. Crit Care Med 2013;41:842‐9. [DOI] [PubMed] [Google Scholar]
- 66. Wozniak MJ, Qureshi S, Sullo N, et al. A comparison of red cell rejuvenation versus mechanical washing for the prevention of transfusion‐associated organ injury in swine. Anesthesiology 2018;128:375‐85. [DOI] [PubMed] [Google Scholar]
- 67. Schneider SO, Rensing H, Graber S, et al. Impact of platelets and fresh frozen plasma in contrast to red cell concentrate on unstimulated and stimulated cytokine release in an in vitro model of transfusion. Scand J Immunol 2009;70:101‐5. [DOI] [PubMed] [Google Scholar]
- 68. Schneider SO, Rensing H, Hartmann L, et al. Impact of intraoperatively salvaged and washed blood on stimulated cytokine release in vitro. Transfusion 2014;54:2782‐90. [DOI] [PubMed] [Google Scholar]
- 69. Remy KE, Hall MW, Cholette J, et al. Mechanisms of red blood cell transfusion‐related immunomodulation. Transfusion 2018;58:804‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Blunt guillotine used for the femur fracture of all traumatized rats. Tubes are 23 cm in height. A marking is placed at 14 cm (height from which the weight should be dropped). Weight of the cross bar is 650 grams. Distance between the supporting pillars for the femur is 2 cm.
Fig. S2. Size characterization of extracellular vesicles based on cell of origin


