Abstract
Human hair is naturally colored by melanin pigments, which afford myriad colors from black, to brown, to red depending on the chemical structures and specific blends. In recent decades, synthetic efforts have centered on dopamine oxidation to polydopamine, an effective eumelanin similar to the one found in humans. To date, only a few attempts at polydopamine deposition on human hair have been reported, and their translation to widespread usage and potential commercialization is still hampered by the harsh conditions employed. We reasoned that novel, mild, biocompatible approaches could be developed to establish a metal-free route to tunable, nature-inspired, long-lasting coloration of human hair. Herein, we describe synthetic and formulation routes to achieving this goal and show efficacy on a variety of human hair samples via multiple spectroscopic and imaging techniques. Owing to the mild and inexpensive conditions employed, this novel approach has the potential to replace classical harsh hair dyeing conditions that have raised concerns for several decades due to their potential toxicity.
Short abstract
Synthetic melanin nanoparticle coatings on hair surfaces successfully reverse the appearance of melanin loss and hair whitening and provides a novel biomimetic and nontoxic approach to hair dyeing.
Introduction
Human hair is comprised mainly of protein, at 65–95% by weight. Keratin, the most abundant component, is a group of insoluble protein complexes which impart elasticity, suppleness, and resistance to the fibers.1 Melanin, nature’s hair pigment, is mainly distributed in the middle layer of the hair shaft or cortex and is embedded between keratin fibers, where it makes up only 1–3% of human hair by weight. These nanometer-scale granular pigments (200–800 nm) generate the naturally beautiful colors found in human hair. Colors arise from the distribution, concentration, and blending of two types of melanin: brown and black eumelanins, and less commonly, red pheomelanins.2−5 It follows then that the reduction or disappearance of melanin from hair fibers is the phenomenon that leads to color loss and consequent hair greying and eventually whitening.3
Hair whitening is mainly caused by aging, although the regulation of hair pigmentation and pigment concentration can be affected by numerous factors including metabolism, hair-cycle changes, body distribution of melanins, gender differences, and the use of medicines (e.g., chemotherapy), or by specific genetic disorders.3,6,7 Taking these factors into account, the average age for white hair onset is mid-30s, with 50% of people exhibiting 50% gray hair by the time they are 50 years of age.3,8 The first known example of natural hair dye dates back to the ancient Egyptians when henna plant pigments were used for hair darkening and color reinforcement.9 Dye technologies are at the very origin of the chemical industry, with the first artificial “long lasting” hair dye synthesized by L’Oréal founder Eugène Schueller in the early 1900s.9 Since then, hair dyes capable of providing a long lasting and convincing gray to black transition have become popular across cultures and nationalities, with additional colors, including those beyond one’s genetic predisposition, desirable. Because of such widespread use, hair dye industries are now among the most profitable in the cosmetics sector.10 As a matter of fact, studies suggest that over 50% of the population in developed countries has dyed their hair at least once in their life.5,11−14 Despite several studies reporting the potential carcinogenicity of certain conventional hair dye components, frequent development of allergies in clients and colorists, and dye-induced hair damage, the use of small molecule-based dyes in modern society continues to expand, and the industry has made only few minor advances in its chemistry.4,12,14−19
With the rapid expansion of nanotechnology, a field at the intersection of chemistry and materials science, novel creative approaches can now be exploited for the design of new hair dyes. This approach is even more interesting with the discovery that ancient hair dyeing methods also relied on nanostructure deposition.20 While the synthesis of gold nanoparticles in human hair has been proposed as an effective way to darken white hair,21 the long reaction time required by this protocol (16 days) hampers its application as an effective hair dyeing method. A much faster approach involves the use of graphene-based sheets for coloration. Hair coated with this material showed excellent antistatic performance and heat dissipation properties. However, the method was similarly expensive and only produced a single color, black.22 Although both of these nanomaterial-based methods provide paths toward the development of innovative hair dyeing protocols, a very interesting approach to darken hair and a desirable alternative to current formulations would be the use of melanin to reestablish the color of the hair fibers. In a recent example, biotechnology was used to produce a melanin intermediate, and its spontaneous oxidation was proposed as a novel hair dye.23 Owing to myriad examples in the literature describing the synthesis and applications of a synthetic version of the naturally occurring nanosized melanin pigment particles, a scalable and inexpensive approach is now accessible.24 However, human hair dyeing using synthetic melanin has been explored only very recently, and the reported protocols required high concentrations of potentially toxic heavy metals such as copper and iron as chelators,25−27 as well as very strong oxidative conditions,28 which may not be suitable for widespread use in an at-home or in salon applications.29
Herein, we demonstrate the efficient deposition of synthetic melanin on human hair without the need for metal chelators or strong oxidants to generate not only black/brown, but also orange/gold colorations from blond hair. We demonstrate that different colors can be achieved by tuning reaction conditions such as temperature, and that effective hair dyeing can be achieved using milder conditions compared to those previously employed for polydopamine coatings. Moreover, these conditions are similar or even milder than those employed in conventional hair dyeing protocols. In addition, blond and naturally red, brown, and gray hair, as well as hair previously dyed with a very bright commercial dye, were successfully colored to dark brown/black using this method. These results suggest that this novel, inexpensive, mild and versatile approach to generate nature-inspired hair pigmentation opens new opportunities for melanin-based hair dyes, so far limited by the reaction conditions employed, and has the potential to replace classical, harsh hair dyes currently common in the cosmetic industry.
Results and Discussion
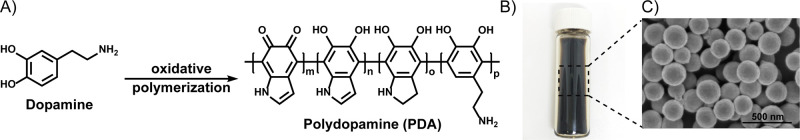
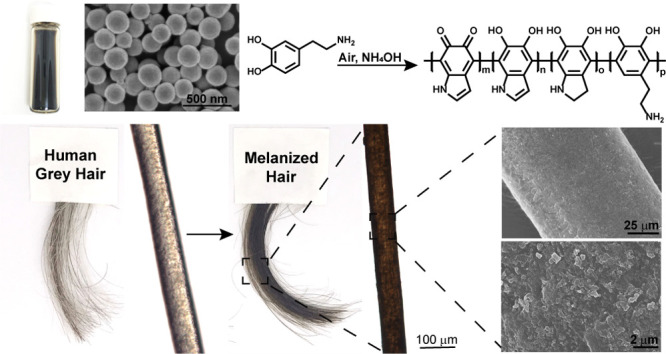
In humans, the biosynthesis of melanin, a rather heterogeneous and polydisperse polymer mainly composed of catechol derivatives, begins with the enzymatic oxidation of l-tyrosine to 3,4-dihydroxyphenylalanine (l-DOPA). This process occurs inside melanocytes in specialized organelles called melanosomes.4,30,31 The resulting dark melanin granules are transferred to hair and the epidermis from the melanocytes, by a process in which melanosomes are endocytosed by epithelial cells.31 Because of the extraordinary properties of this class of polymer, several efforts focus on the development of melanin-inspired materials.32 As a result, several synthetic strategies are now available for the preparation of this class of biopolymer.24 In particular, to date, polydopamine-based materials are most commonly considered as a synthetic mimic of natural eumelanin, the darkest natural pigment of this class of biopolymer, which is abundant in human hair and skin.33 Synthetic versions of eumelanin can be obtained via oxidation of commercially available dopamine (DA) hydrochloride as well as analogues, by various synthetic protocols (Figure 1).24,34,35
Figure 1.
Oxidation of dopamine (DA) to polydopamine (PDA). (A) Oxidative polymerization of DA to PDA and (B) resulting dark brown/black synthetic melanin nanoparticles as determined by (C) scanning electron microscopy (SEM) imaging.
Synthetic approaches using the enzymes laccase and horseradish peroxidase, strong oxidants such as ammonium persulfate, sodium periodate and potassium permanganate, and metal catalysis/hydrogen peroxide, to produce a Fenton-like reaction have been widely explored and optimized for the polymerization of dopamine.24 Very recently, some of these oxidation reactions have been used to deposit polydopamine on human hair.25,26 Reactions employing CuSO4/H2O2 were particularly successful as this method catalyzes both polydopamine deposition and hair binding via metal interaction.26 However, as previously anticipated, these harsh conditions limit the translation of synthetic melanin into a competitive hair dye. For biocompatibility, the auto-oxidation of dopamine in air is the most interesting and gentle approach for generating polydopamine (PDA) coatings or nanoparticles. This oxidation is spontaneous when carried out under alkaline conditions (pH > 7.5) using naturally ambient oxygen, making this method mild, inexpensive, and scalable.24,36 When added to an alkaline solution, the polymerization of DA begins immediately and is accompanied by a color change from clear and colorless, to pale brown, and finally to dark brown and black (PDA). Hence, we envisioned dopamine self-oxidation as the most promising approach for melanin deposition on human hair since the use of air as an oxidant makes this method not only mild, but also extremely inexpensive and scalable. While Tris buffer (pH 8.5) and NaOH are reagents used extensively for successful dopamine oxidation,24,36 ammonium hydroxide is an attractive choice as this base is commonly used in current hair dyeing protocols.4,37 Although some modern hair dye formulations are ammonia free, where ammonia is generally replaced by less volatile/odorous ethanolamine and its derivatives, these alternatives can still create adverse reactions.38 The use of bases in conventional hair dyeing protocols is generally needed to swell the hair cuticle, thereby allowing dye penetration into the hair. In this work, 3% ammonium hydroxide was selected as the initial effective concentration, which is comparable to professional permanent hair dyes.39 To evaluate the performance of our method, we compared it to a previously established protocol using CuSO4/H2O2 additives as a metal- and peroxide-based approach to polydopamine deposition.26 A reaction time of 2 h was selected as this is a feasible and acceptable time for hair dyeing in common practice. The concentration of dopamine was chosen as 5 mg/mL as it corresponds to the low end of other synthetic protocols.26
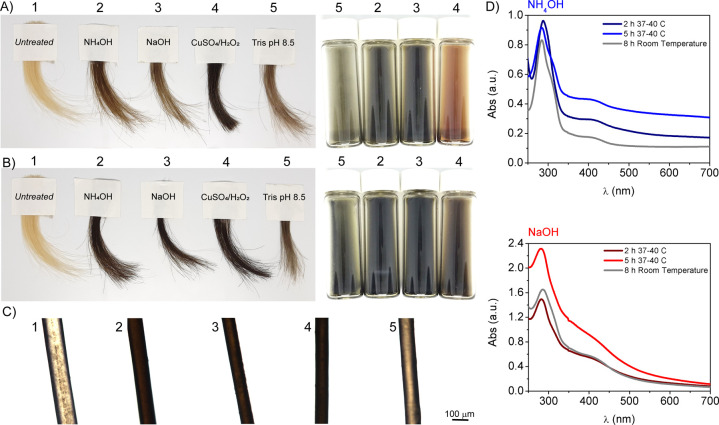
To demonstrate the reproducibility of this method, we purchased blond human hair samples from two different vendors (see Methods for a description of sources). After a 2 h reaction time, both hair samples treated with different alkaline conditions (3% NH4OH, 0.05 N NaOH, and Tris buffer pH 8.5) showed moderate color change (mild darkening) and did not yield a uniform color (Figure 2). This result was confirmed using blond hair samples purchased from a second vendor (Figure S1). As mentioned above, previous studies have used metal ions to chelate polydopamine to human hair at room temperature, and this was reproduced for comparison to our own study (Figure 2).26 To eliminate the need for heavy metals such as copper and iron,39,40 which are generally considered toxic additives,27 we decided to employ higher reaction temperatures to speed the reaction. These conditions have been shown to enhance the deposition of melanin coatings in other applications41 and are used regularly in a salon setting or at home using a hair dryer. Hence, to increase the rate of the process from days to hours, and to obtain a more uniform melanin deposition, we investigated the effect of reaction temperature on hair dyeing. As an initial test, we found that successful reactions were performed in solution at physiological temperature (37–40 °C). UV–Vis spectroscopy was used to monitor the formation of polydopamine in solution at different reaction times and for different reaction conditions at both room temperature (RT) as well as at 37–40 °C (Figure 2 and Figure S2). These spectra clearly show an increased intensity of the band around 400 nm for all polymerizations carried out at higher temperature, thereby confirming that the polymerization rate can be enhanced by increasing the temperature. The color of the obtained solutions clearly supports this result as the reaction performed at 37–40 °C yielded visibly darker colors at shorter time periods (Figure S3). Higher temperatures yielded darker and more uniform hair colors (Figure 2B and Figure S1B), mimicking results obtained using the metal-containing protocol (sample 4 in Figure 2). Different shades of brown color can be better visualized in the optical microscopy images (Figures 2C and 1C). Among the conditions tested, hair samples treated with ammonium or sodium hydroxide showed the darkest colors, thus indicating the efficiency of the dopamine oxidation in basic environments. In particular, success when using the bases was encouraging owing to their current role in commercial products. Moreover, these first results suggest that hair darkening can be achieved avoiding the use of H2O2, which is generally included in permanent hair dye formulations at concentrations of approximately 6%.42
Figure 2.
Higher temperature enhances polydopamine deposition and hair darkening. (A, B) Photographs of hair samples before (1) and after dyeing with polydopamine using 3% NH4OH (2), 0.05 N NaOH (3), 10 mM CuSO4/15 mM H2O2 (4), Tris buffer pH 8.5 (5), as well as color of the resulting polydopamine solutions. Dopamine polymerizations were carried out for 2 h at (A) room temperature and (B) 37–40 °C. (C) Optical microscopy images of the hair samples dyed at 37–40 °C are also reported. (D) UV–Vis spectra of solutions obtained after 2 h dopamine polymerization using 3% NH4OH and 0.05 N NaOH at either room temperature or at 37–40 °C. In every case, hair samples were washed five times with water and three times with a 10% shampoo solution prior to imaging/analysis (see Methods for protocols and for hair sample descriptions from alternative vendors).
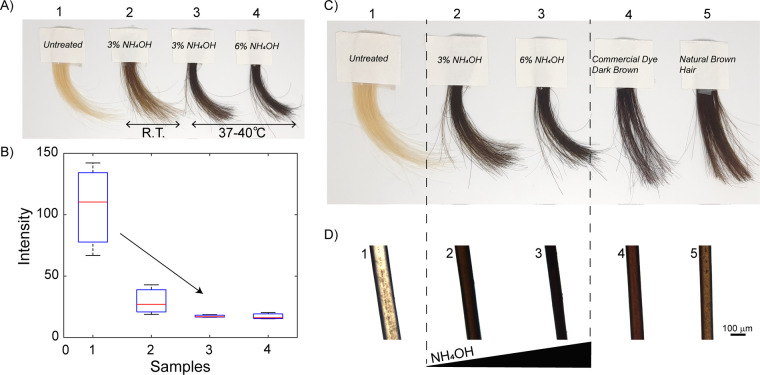
Following initial studies, temperature and ammonium concentrations were adjusted to optimize conditions and obtain various hair colors (Figure 3). Hair darkening can be observed both by visual inspection (Figure 3A), as well as by color intensity measurements (Figure 3B) and analysis of RGB color components (Figure S4). Higher concentrations of ammonium hydroxide in the reaction mixture (6% vs 3%) correlate with slightly darker brown/black colors that resemble natural dark brown hair as well as hair dyed with dark brown commercially available hair dye (Figure 3C,D). We note that 6% ammonium hydroxide is still within the tolerated ammonium hydroxide concentrations, which in the case of professional use, can reach 10%.39 These results indicate that synthetic melanin can be used successfully as a simple, biomimetic, two-ingredient hair dye without the need for metals or harsh reagents. We employ natural hair pigment mimics and utilize a commercial reagent, ammonia, while neglecting the need for complex chemical mixtures, which have been shown to be potential carcinogenic and certainly allergenic in some cases.
Figure 3.
Hair color can be tuned by changing temperature and NH4OH concentration. (A) Hair darkening using NH4OH at room temperature (R.T., 3% NH4OH) and at 37–40 °C (3% and 6% NH4OH) as compared to untreated blond hair (1). (B) Image analysis (color intensity) of photograph (A), showing hair darkening, from blond (1), to dark blond (2), and dark brown (3, 4). (C) Comparison between the color of untreated blond hair (1), hair dyed using 3% NH4OH (2), 6% NH4OH (3), as well as hair dyed using a commercial dark brown hair dye (4) and naturally pigmented dark brown hair (5). (D) Corresponding optical micrographs of individual hair fibers. RGB color analysis of hair samples in photograph A) are included in Supporting Information.
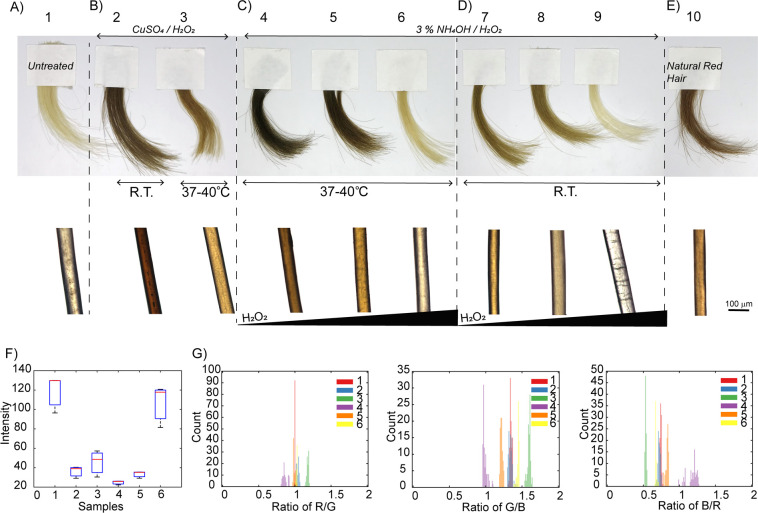
Comparison studies performed at room temperature using CuSO4/H2O2 (10 mM CuSO4, and 15 mM H2O2) showed a dark-brown coloration (Figure 2). During experimentation, we discovered that these reaction solutions immediately turned red and that both the use of different reaction temperatures as well as H2O2 concentrations (Figure S3) could yield variable brown and red shades. In particular, for longer reaction times, the use of a higher concentration of H2O2 resulted in richer red-orange colors. When hair samples were dyed using this protocol, higher concentrations of H2O2 resulted in a bright orange color (Figure 4 and Figure S5 and S6) resembling that of natural red hair (Figure 4E). Although addition of copper into the reaction mixture produced the brightest orange color (Figure 4B,F,G), coupling H2O2 to the NH4OH-based protocol also resulted in either dark (37–40 °C, Figure 4C and Figure S7) or light gold shades (room temperature, Figure 4D and Figure S7), as compared to the darker brown colors previously obtained (Figure 3). These results suggest that, together with temperature and base concentrations, H2O2 can be added to the formulations up to 3% to obtain a wider range of shades. In particular, H2O2 concentrations (<3%, 1 M) lower than those generally employed in permanent hair dyeing protocols (up to 6%, 2 M),5,43 can shift the classic dark brown/black eumelanin color toward clearer and warmer shades.
Figure 4.
Effect of H2O2 and temperature on hair shades from red, to dark or light gold hues. (A) Photograph of blond hair and hair dyed with polydopamine using (B) 10 mM CuSO4/1 M H2O2 at room temperature (2) and at 37–40 °C (3). (C, D) Effect of H2O2 (50 mM (4, 7), 100 mM (5, 8) and 1 M (6, 9)) on hair dyed with polydopamine and 3% NH4OH solutions. The reactions were performed at (C) 37–40 °C (4, 5, 6) and at (D) room temperature (7, 8, 9). (E) Photograph of natural red hair (10). Optical images of the corresponding single hair fibers are shown in the lower panel. (F) Image analysis (color intensity) and (G) RGB color ratios of photographs of hair 1–6. Color analysis of samples dyed at room temperature (7, 8, 9) are included in Supporting Information. All dyeing reactions were carried out for 2 h, and hair samples were washed five times with water and three times with a 10% shampoo solution prior to imaging/analysis.
Initially, as noted above, all experiments were carried out using a washing step after the dye application: five times with water and three with shampoo (see Methods for description). Next, we evaluated the color persistence after a multiple wash protocol. Here, an important distinction needs to be made between semipermanent and permanent hair dyes. While the former washes away within 4–6 weeks of application in the case of washing every other day,5,42 the latter is persistent for 6 weeks with washing every other day. On the basis of a washing schedule of every 2 days for 5 weeks, we designed a follow up study.
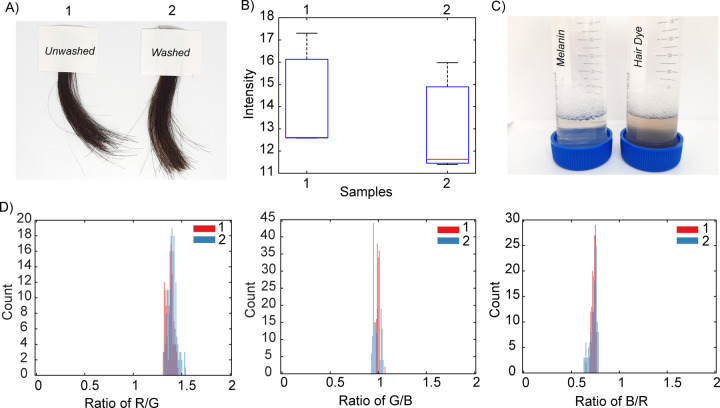
This test for color persistence involved 5 wash cycles with water, followed by 18 with shampoo. Specifically, after washing five times with water, we applied a 10% shampoo solution to wash the hair samples, and this procedure was repeated 18 separate times (Methods for the exact protocol). Subsequently, retention of hair color was evaluated by photographic imaging (Figure 5A) with color intensity/RGB analysis (Figure 5B,D and Figure S8). In the initial test, we used hair dyed using dopamine (5 mg/mL) and 6% NH4OH for 2 h at 37–40 °C (i.e., conditions used in Figure 3C, sample 3). These conditions were chosen because, among all the different colors obtained, this one best mimics the color of hair dyed with a commercial, dark-brown hair dye (Figure 3C). The synthetic, melanin-based dye resists these washes without any evidence of color change or fading, and the color persistence is similar to that of hair dyed with the permanent, commercially available hair dye (Figure S9). We note that while the soiled solution resulting from washing hair dyed with the commercial dye turned brown, the solution derived from washes of the melanin-dyed hair was colorless (Figure 5C). These results confirm that our methodology provides a permanent hair dye that persists at least as well as commercial products.
Figure 5.
Retention of hair color after 18 washes. (A) Photographs of hair dyed with polydopamine using 6% NH4OH for 2 h at 37–40 °C before and after 18 washes with a 10% shampoo solution and (B) color intensity analysis. (C) Comparison between the color of the soiled solution from synthetic melanin-dyed hair and hair dyed using a commercial dark brown hair dye. (D) RGB color ratios (photograph A) of hair before and after washes.
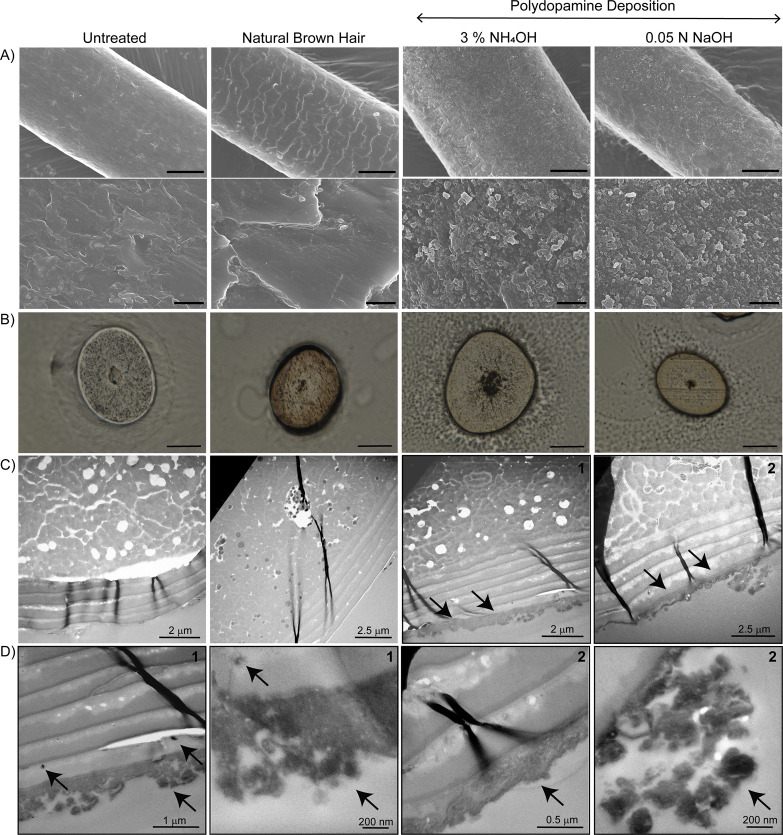
Although dyeing with conventional permanent hair dyes leads to long lasting colors, the harsh conditions employed and the penetration of the small molecule dyes into the cortex make these methods invasive. Hence, with the next series of experiments, we aimed to investigate the mechanism of melanin deposition, its localization within the hair, and the morphology of the resulting colored fibers. First, changes in chemical composition at the hair surface were evaluated by FTIR-ATR spectroscopy. This technique mainly probes the surface of hair since the evanescent wave penetrates only a couple of micrometers in depth. Therefore, changes in the typical hair spectra are indicative of changes in the chemical composition of the hair surface. IR spectra of hair samples derived from two different vendors and dyed with polydopamine under different reaction conditions (Figure S10A,B) revealed only very minor changes, located in the amide I and amide II bands regions (1690–1600 cm–1 and 1575–1480 cm–1, respectively), which derives from overlapping with typical polydopamine bands (Figure S10C).24 While these results confirm the presence of polydopamine on the hair surface, insights into the dyeing mechanism can be obtained using multiple imaging techniques (Figure 6). Specifically, SEM analysis suggested that all oxidations carried out under basic conditions (Tris buffer, NH4OH and NaOH) result in the formation of nanostructures that can be observed before and after (Figure S11) purification of the polydopamine solutions.
Figure 6.
Oxidation in alkaline conditions results in PDA nanoparticles coating the hair surface as determined by SEM, optical microscopy, and TEM imaging. (A) SEM images of untreated blond hair, natural dark-brown hair, and blond hair, dyed with dopamine (3% NH4OH and 0.05 N NaOH), scale bars 25 μm (top row) and 2 μm (second row). (B) Optical microscopy (scale bars 40 μm) and (C) TEM images of hair cross sections. Black arrows and (D) higher magnification TEM images highlight the PDA coatings obtained using NH4OH (1) and NaOH (2) as oxidants.
Oxidation performed at room temperature or using CuSO4/H2O2 did not produce any of these structures. These results support the finding that (i) polydopamine deposition carried out with and without the use of metals occurs via two different mechanisms and that (ii) the formation of melanin nanoparticles obtained using alkaline conditions can be catalyzed by heat. As a result, while hair samples treated with NH4OH and NaOH were coated by these nanoparticles (Figure 6A and Figure S12), hair treated with CuSO4/H2O2 underwent film deposition (Figure S12). Optical microscopy analysis of cross sections of hair treated with NH4OH and NaOH highlighted both a darker color as well as a darker profile (Figure 6B) as compared to untreated hair. TEM imaging confirmed that this darker profile derives from the nanostructured melanin coatings (Figure 6C,D, black arrows highlight the presence of the synthetic melanin layer). Although a few dark nanostructures resembling those found in natural dark-brown hair were observed in the cuticle layers of melanin-coated hair, TEM images confirmed that the dyeing mechanism mainly occurs via nanoparticle deposition without deep penetration into the hair cortex. All together, these results indicate that the proposed protocol allows successful hair dyeing in a permanent manner without compromising the inner hair composition and structure, and more importantly, without altering the mechanical properties of the hair (Figure S13 and Table S1).
Finally, the goal of the last experiment was to demonstrate that polydopamine deposition can be applied to very diverse hair samples. We aimed to determine if this approach could be considered a universal hair dyeing method. We collected hair samples of four very different colors (natural red, brown, gray, and dyed purple) from human donors of different ages and ethnicities. We carried out dopamine polymerization on these human hair samples at 37–40 °C for 2 h using 3% NH4OH. Photographs of hair and optical microscopy images of hair before and after dyeing (Figure 7) reveal successful hair darkening for all of the tested samples. The natural brown and red hair (Figure 7A) had not been chemically treated (dyed or bleached) before. The bright purple hair sample (Figure 7B) had been previously bleached and was dyed with a commercial purple hair dye in the 2 weeks before the experiment. The successful darkening of such different colors and diverse hair samples highlights the efficacy and versatility of this method. Furthermore, the ability to cover naturally gray hair (Figure 7C) and even to tune the color toward their original warmer brown shades (sample 9, Figure 7C) by employing H2O2-containing protocols (Figure 4) confirms the potential of synthetic melanin as a viable hair dye.
Figure 7.
Polydopamine deposition is compatible with a wide range of hair substrates. (A) Natural virgin red (1) and brown (3) hair and (B) hair dyed with a purple hair dye (5) were used as hair substrates. Dopamine oxidation was carried out using 3% NH4OH as shown in Figure 2. (C) Virgin gray hair (7) was dyed using 3% NH4OH (8) and 3% NH4OH/100 mM H2O2 (as for sample 5 in Figure 4). In every case, reactions were carried out at 37–40 °C for 2 h and hair samples were washed five times with water and three times with a 10% shampoo solution prior to imaging/analysis.
Conclusion
Despite concerns regarding the possible toxicity of commercially available hair dyes, their usage continues to grow, and the lack of modern approaches makes this branch of cosmetics a very interesting target for novel and rapidly rising nanomaterial-based approaches. In this work, we demonstrate, for the first time, the deposition of synthetic melanin onto human hair without the need for metals and using similar or milder conditions as compared to generally employed methods used for commercially available hair dyes. This innovative technique allows hair darkening within 2 h at physiological temperature (37–40 °C). Increasing concentration of base resulted in a darker color, whereas the addition of H2O2 yielded warmer and orange/gold, natural-looking shades. The resulting colors were comparable to those of hair dyed with commercially available products, and more importantly, they resembled natural hair colors. Morphological studies suggest that synthetic melanin was deposited onto the hair surface in a nanoparticulate form. This colored layer was found to be resistant to at least 18 washes and did not alter the mechanical properties of the hair. These combined results point out the relevance of this novel, mild and effective method and the potential of biomaterials-based approaches in hair and cosmetics, and most importantly, we specifically demonstrate performance arising by engineering systems to perform like natural materials, in this case, employing synthetic melanin as an additive precisely where melanin is naturally used.
Methods
Dopamine hydrochloride was obtained from Frontier Scientific, and sodium hydroxide and ammonium hydroxide 28–30% (w/v) solution were purchased from Sigma-Aldrich. 30 % H2O2 (w/v) stock solution was purchased from Fisher Scientific. To demonstrate the reproducibility of this method, blond human hair samples were purchased from two different vendors. While most of the experiments were carried out using either blond or dark-brown hair obtained from Jerome Krause Fashion Hair (Evanston, IL), some experiments were repeated using hair samples purchased from a second vendor (Emosa # 613 blond and #2 dark brown) and are reported in the Supporting Information. Natural brown, red, and gray hair that had not been dyed or bleached before, as well as purple-dyed hair were kindly donated. UV–Vis spectroscopy measurements were performed using an Agilent Cary 100 UV–Vis spectrometer using quartz cuvettes. Scanning electron microscopy (SEM) images were acquired on a Hitachi S4800-II cFEG SEM and a Hitachi SU8030, and transmission electron microscopy (TEM) images were acquired on a Hitachi 2300 (scanning TEM) and a JEOL ARM 300 F. Hair samples were imaged using a Leica BM6B widefield optical microscope. FTIR-ATR spectroscopy of both polydopamine and hair samples was performed using a Nexus 870 spectrometer (Thermo Nicolet), and hair mechanical properties were determined using an A. Sintech 20G tensile test machine. No unexpected or unusually high safety hazards were encountered in this work.
Hair Dyeing
Hair dyeing was carried out using 5 mg/mL monomer (dopamine HCl) in water, and hair samples were approximately 2 cm long. The volume of the solution was selected in order to cover the hair sample completely (generally 1 or 2 mL depending on hair size). Either alkaline (Tris buffer pH 8.5 10 mM, 3% or 6% NH4OH and 0.05 N NaOH) or oxidizing conditions (10 mM CuSO4 and 100/50/15 mM or 1 M H2O2) were used for this process. The reaction solutions were stirred either at room temperature or at 37–40 °C. After 2 h, hair samples were washed five times with water.
Hair Washing
Hair samples were washed with a 10% shampoo solution (Ceramol, Unifarco Biomedical) three times and finally rinsed with water prior to imaging/analysis. During each wash, hair was immersed in the shampoo solution and vortexed for 30 s. To test the persistence of the color after multiple washes, hair samples were washed an additional 15 times (in total, 5 times with water and 18 with shampoo). Hair color was compared before and after washing.
UV–Vis Spectroscopy of Polydopamine Solutions
UV–Vis time-dependent spectra were recorded by withdrawing 10 μL of polydopamine solution from each sample at different time intervals. The samples were then diluted in 1 mL of water and analyzed.
SEM of Polydopamine
After a 2 h reaction, polydopamine solutions obtained using the above-reported conditions were drop casted and evaporated onto a silicon wafer substrate at room temperature. The rest of the sample was centrifuged, and the dark-brown precipitate was resuspended in water. This process was repeated three times. All samples were coated with 6 nm osmium and imaged using a Hitachi SU8030 cFEG SEM and a Hitachi SU8030 at 10 kV and 6 kV.
SEM of Hair Samples
Hair samples were adhered onto aluminum SEM stubs by pressing lightly onto carbon tape using a clean glove. The samples were coated with 10 nm osmium and imaged using a Hitachi SU8030 cFEG SEM at 10 kV.
Preparation of Hair Samples for TEM
Hair samples were placed in silicon molds in Embed812 resin and polymerized at 65 °C for 48 h. Ultrathin sections of ca. 80 nm thickness were obtained with an ultramicrotome (Ultracut-S, Leica) and a diamond knife (Diatome). Sections were placed on copper mesh grids or on slotted copper grids with a Formvar/carbon film (EMS).
Preparation of Hair Cross Sections for Optical Microscopy
Hair was embedded with optimal cutting temperature (OCT) medium, and 10 μm sections were cryosectioned at −20 °C and deposited onto glass microscope slides.
Mechanical Properties of Hair
Hair samples with a diameter between 75 and 105 μm were used for this study. Stress–strain curves are reported as an average of five different measurements.
RGB Color Analysis of Hair Photographs
MatLab software was used to determine RGB color components and intensities in order to differentiate the color of hair dyed using different conditions as well as to investigate color fading after multiple washes.
Acknowledgments
We thank A. L. Wheatley, A. Masters, N. Zang, and M. A. Fattah for the grey, red, brown, and purple hair samples used in this study, as well as Jerome Krause Fashion Hair in Evanston, IL, for their kind help in choosing appropriate blond hair samples. We acknowledge Dr. R. Bleher for assistance with TEM hair sample preparation and M. E. Seniw for support with the tensile experiment. C.B. acknowledges the Swiss National Science Foundation (SNSF) for fellowship funding. This research was supported by a MURI through the Air Force Office of Scientific Research (FA 9550-18-1-0142). This work made use of the Northwestern University Mouse Histology and Phenotyping Laboratory (NCI P30-CA060553 awarded to the Robert H. Lurie Comprehensive Cancer Center) and the BioCryo facility of Northwestern University’s NUANCE Center, which has received support from the Soft and Hybrid Nanotechnology Experimental (SHyNE) Resource (NSF ECCS-1542205); the MRSEC program (NSF DMR-1720139) at the Materials Research Center; the International Institute for Nanotechnology (IIN); and the State of Illinois, through the IIN. It also made use of the Biological Imaging Facility at Northwestern University, graciously supported by the Chemistry of Life Processes Institute, the NU Office for Research and the Rice Foundation, and the Central Laboratory for Materials Mechanical Properties, supported by the IMRSEC program of the National Science Foundation (DMR-1720139) at the Northwestern University Materials Research Science and Engineering Center.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acscentsci.0c00068.
This material includes pictures, optical micrographs, and RGB color analysis of hair treated using the conditions described in the manuscript. SEM images of nanoparticles and hair coated with nanoparticles are also reported. Results of experiments performed using blond hair purchased from a second vendor (Emosa), as well as FT-IR spectra of coated hair and mechanical properties of hair before and after treatment are included (PDF)
Author Contributions
C.B. developed the idea, designed the experiments, and analyzed the data. N.C.M. performed SEM imaging of hair samples, K.G. acquired TEM and performed RGB color analysis, and X.Z. acquired SEM of nanoparticles. V.C. and M.M. contributed to the realization of the first experiment, and N.C.G. directed the research.
The authors declare no competing financial interest.
Supplementary Material
References
- Velasco M. V. R.; Dias T. C. D.; de Freitas A. Z.; Vieira N. D.; Pinto C. A. S. D.; Kaneko T. M.; Baby A. R. Hair fiber characteristics and methods to evaluate hair physical and mechanical properties. Braz. J. Pharm. Sci. 2009, 45 (1), 153–162. 10.1590/S1984-82502009000100019. [DOI] [Google Scholar]
- Ghiani S.; Baroni S.; Burgio D.; Digilio G.; Fukuhara M.; Martino P.; Monda K.; Nervi C.; Kiyomine A.; Aime S. Characterization of human hair melanin and its degradation products by means of magnetic resonance techniques. Magn. Reson. Chem. 2008, 46 (5), 471–479. 10.1002/mrc.2202. [DOI] [PubMed] [Google Scholar]
- Van Neste D.; Tobin D. J. Hair cycle and hair pigmentation: dynamic interactions and changes associated with aging. Micron 2004, 35 (3), 193–200. 10.1016/j.micron.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Morel O. J. X.; Christie R. M. Current Trends in the Chemistry of Permanent Hair Dyeing. Chem. Rev. 2011, 111 (4), 2537–2561. 10.1021/cr1000145. [DOI] [PubMed] [Google Scholar]
- Guerra-Tapia A.; Gonzalez-Guerra E. Hair cosmetics: dyes. Actas Dermo-Sifiliogr. 2014, 105 (9), 833–839. 10.1016/j.ad.2014.02.004. [DOI] [PubMed] [Google Scholar]
- Nishimura E. K.; Granter S. R.; Fisher D. E. Mechanisms of hair graying: Incomplete melanocyte stem cell maintenance in the niche. Science 2005, 307 (5710), 720–724. 10.1126/science.1099593. [DOI] [PubMed] [Google Scholar]
- Ricci F.; De Simone C.; Del Regno L.; Peris K. Drug-induced hair colour changes. Eur. J. Dermatol. 2016, 26 (6), 531–536. 10.1684/ejd.2016.2844. [DOI] [PubMed] [Google Scholar]
- Keogh E. V.; Walsh R. J. Rate of Greying of Human Hair. Nature 1965, 207 (4999), 877–878. 10.1038/207877a0. [DOI] [PubMed] [Google Scholar]
- Quiroga M. I. History of cosmetics. Dia Med. 1953, 25 (50), 1300–1306. [PubMed] [Google Scholar]
- Kumar S. Exploratory analysis of global cosmetic industry: major players, technology and market trends. Technovation 2005, 25 (11), 1263–1272. 10.1016/j.technovation.2004.07.003. [DOI] [Google Scholar]
- Patel D.; Narayana S.; Krishnaswamy B. Trends in use of hair dye: a cross-sectional study. Int. J. Trichology 2013, 5 (3), 140–143. 10.4103/0974-7753.125610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosted H.; Hesse U.; Menne T.; Andersen K. E.; Johansen J. D. Contact dermatitis to hair dyes in a Danish adult population: an interview-based study. Br. J. Dermatol. 2005, 153 (1), 132–135. 10.1111/j.1365-2133.2005.06588.x. [DOI] [PubMed] [Google Scholar]
- Bernard A.; Houssin A.; Ficheux A. S.; Wesolek N.; Nedelec A. S.; Bourgeois P.; Hornez N.; Batardiere A.; Misery L.; Roudot A. C. Consumption of hair dye products by the French women population: Usage pattern and exposure assessment. Food Chem. Toxicol. 2016, 88, 123–132. 10.1016/j.fct.2016.01.002. [DOI] [PubMed] [Google Scholar]
- McFadden J. P.; White I. R.; Frosch P. J.; Sosted H.; Johansen J. D.; Menne T. Allergy to hair dye - Its incidence is rising, as more and younger people dye their hair. Brit Med. J. 2007, 334 (7587), 220–220. 10.1136/bmj.39042.643206.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanoni T. B.; Pedrosa T. N.; Catarino C. M.; Spiekstra S. W.; de Oliveira D. P.; Den Hartog G.; Bast A.; Hagemann G.; Gibbs S.; de Moraes Barros S. B.; Maria-Engler S. S. Allergens of permanent hair dyes induces epidermal damage, skin barrier loss and IL-1 alpha increase in epidermal in vitro model. Food Chem. Toxicol. 2018, 112, 265–272. 10.1016/j.fct.2017.12.033. [DOI] [PubMed] [Google Scholar]
- Gago-Dominguez M.; Castelao J. E.; Yuan J. M.; Yu M. C.; Ross R. K. Use of permanent hair dyes and bladder-cancer risk. Int. J. Cancer 2001, 91 (4), 575–579. . [DOI] [PubMed] [Google Scholar]
- Chung K. T. Azo dyes and human health: A review. J. Environ. Sci. Health C Environ. Carcinog Ecotoxicol Rev. 2016, 34 (4), 233–261. 10.1080/10590501.2016.1236602. [DOI] [PubMed] [Google Scholar]
- Takkouche B.; Etminan M.; Montes-Martinez A. Personal use of hair dyes and risk of cancer - A meta-analysis. Jama-J. Am. Med. Assoc 2005, 293 (20), 2516–2525. 10.1001/jama.293.20.2516. [DOI] [PubMed] [Google Scholar]
- Eberle C. E.; Sandler D. P.; Taylor K. W.; White A. J. Hair dye and chemical straightener use and breast cancer risk in a large US population of black and white women. Int. J. Cancer 2019, 0. 10.1002/ijc.32738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P.; Welcomme E.; Hallegot P.; Zaluzec N. J.; Deeb C.; Castaing J.; Veyssiere P.; Breniaux R.; Leveque J. L.; Tsoucaris G. Early use of PbS nanotechnology for an ancient hair dyeing formula. Nano Lett. 2006, 6 (10), 2215–2219. 10.1021/nl061493u. [DOI] [PubMed] [Google Scholar]
- Haveli S. D.; Walter P.; Patriarche G.; Ayache J.; Castaing J.; Van Elslande E.; Tsoucaris G.; Wang P. A.; Kagan H. B. Hair Fiber as a Nanoreactor in Controlled Synthesis of Fluorescent Gold Nanoparticles. Nano Lett. 2012, 12 (12), 6212–6217. 10.1021/nl303107w. [DOI] [PubMed] [Google Scholar]
- Luo C.; Zhou L. Y.; Chiou K.; Huang J. X. Multifunctional Graphene Hair Dye. Chem-Us 2018, 4 (4), 784–794. 10.1016/j.chempr.2018.02.021. [DOI] [Google Scholar]
- Panzella L.; Ebato A.; Napolitano A.; Koike K. The Late Stages of Melanogenesis: Exploring the Chemical Facets and the Application Opportunities. Int. J. Mol. Sci. 2018, 19 (6), 1753. 10.3390/ijms19061753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. L.; Ai K. L.; Lu L. H. Polydopamine and Its Derivative Materials: Synthesis and Promising Applications in Energy, Environmental, and Biomedical Fields. Chem. Rev. 2014, 114 (9), 5057–5115. 10.1021/cr400407a. [DOI] [PubMed] [Google Scholar]
- Im K. M.; Kim T. W.; Jeon J. R. Metal-Chelation-Assisted Deposition of Polydopamine on Human Hair: A Ready-to-Use Eumelanin-Based Hair Dyeing Methodology. ACS Biomater. Sci. Eng. 2017, 3 (4), 628–636. 10.1021/acsbiomaterials.7b00031. [DOI] [PubMed] [Google Scholar]
- Gao Z. F.; Wang X. Y.; Gao J. B.; Xia F. Rapid preparation of polydopamine coating as a multifunctional hair dye. RSC Adv. 2019, 9 (35), 20492–20496. 10.1039/C9RA03177D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowska S.; Brzoska M. M. Metals in cosmetics: implications for human health. J. Appl. Toxicol. 2015, 35 (6), 551–572. 10.1002/jat.3129. [DOI] [PubMed] [Google Scholar]
- Dong Y. Y.; Qiu Y.; Gao D.; Zhang K. L.; Zhou K.; Yin H. G.; Yi G. Y.; Li J.; Xia Z. N.; Fu Q. F. Melanin-mimetic multicolor and low-toxicity hair dye. RSC Adv. 2019, 9 (58), 33617–33624. 10.1039/C9RA07466J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lent E. M.; Crouse L. C. B.; Eck W. S. Acute and subacute oral toxicity of periodate salts in rats. Regul. Toxicol. Pharmacol. 2017, 83, 23–37. 10.1016/j.yrtph.2016.11.014. [DOI] [PubMed] [Google Scholar]
- Korner A.; Pawelek J. Mammalian tyrosinase catalyzes three reactions in the biosynthesis of melanin. Science 1982, 217 (4565), 1163–1165. 10.1126/science.6810464. [DOI] [PubMed] [Google Scholar]
- Riley P. A. Melanin. Int. J. Biochem. Cell Biol. 1997, 29 (11), 1235–1239. 10.1016/S1357-2725(97)00013-7. [DOI] [PubMed] [Google Scholar]
- d’Ischia M.; Napolitano A.; Pezzella A.; Meredith P.; Buehler M. Melanin Biopolymers: Tailoring Chemical Complexity for Materials Design. Angew. Chem. 2019, 59, 2–12. 10.1002/ange.201914276. [DOI] [PubMed] [Google Scholar]
- d’Ischia M.; Napolitano A.; Ball V.; Chen C. T.; Buehler M. J. Polydopamine and eumelanin: from structure-property relationships to a unified tailoring strategy. Acc. Chem. Res. 2014, 47 (12), 3541–3550. 10.1021/ar500273y. [DOI] [PubMed] [Google Scholar]
- Liebscher J.; Mrowczynski R.; Scheidt H. A.; Filip C.; Hadade N. D.; Turcu R.; Bende A.; Beck S. Structure of polydopamine: a never-ending story?. Langmuir 2013, 29 (33), 10539–10548. 10.1021/la4020288. [DOI] [PubMed] [Google Scholar]
- Ryu J. H.; Messersmith P. B.; Lee H. Polydopamine Surface Chemistry: A Decade of Discovery. ACS Appl. Mater. Interfaces 2018, 10 (9), 7523–7540. 10.1021/acsami.7b19865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju K. Y.; Lee Y.; Lee S.; Park S. B.; Lee J. K. Bioinspired Polymerization of Dopamine to Generate Melanin-Like Nanoparticles Having an Excellent Free-Radical-Scavenging Property. Biomacromolecules 2011, 12 (3), 625–632. 10.1021/bm101281b. [DOI] [PubMed] [Google Scholar]
- Huang Y. R.; Li Y. W.; Hu Z. Y.; Yue X. J.; Proetto M. T.; Jones Y.; Gianneschi N. C. Mimicking Melanosomes: Polydopamine Nanoparticles as Artificial Microparasols. ACS Cent. Sci. 2017, 3 (6), 564–569. 10.1021/acscentsci.6b00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo J. A.; Bae I. H.; Jang W. H.; Kim J. H.; Bak S. Y.; Han S. H.; Park Y. H.; Lim K. M. Hydrogen peroxide and monoethanolamine are the key causative ingredients for hair dye-induced dermatitis and hair loss. J. Dermatol. Sci. 2012, 66 (1), 12–19. 10.1016/j.jdermsci.2011.12.015. [DOI] [PubMed] [Google Scholar]
- Johnson W.; Boyer I.. Safety Assessment of Ammonia and Ammonium Hydroxide as Used in Cosmetics, Cosmetic Ingredient Review 2017. [Google Scholar]; (https://www.cir-safety.org/sites/default/files/ammoni122017rep.pdf)
- Zhu J. Y.; Tsehaye M. T.; Wang J.; Uliana A.; Tian M. M.; Yuan S. S.; Li J.; Zhang Y. T.; Volodin A.; Van der Bruggen B. A rapid deposition of polydopamine coatings induced by iron (III) chloride/hydrogen peroxide for loose nanofiltration. J. Colloid Interface Sci. 2018, 523, 86–97. 10.1016/j.jcis.2018.03.072. [DOI] [PubMed] [Google Scholar]
- Zhang C.; Ou Y.; Lei W. X.; Wan L. S.; Ji J.; Xu Z. K. CuSO4/H2O2-Induced Rapid Deposition of Polydopamine Coatings with High Uniformity and Enhanced Stability. Angew. Chem., Int. Ed. 2016, 55 (9), 3054–3057. 10.1002/anie.201510724. [DOI] [PubMed] [Google Scholar]
- Zhou P.; Deng Y.; Lyu B.; Zhang R. R.; Zhang H.; Ma H. W.; Lyu Y. L.; Wei S. C. Rapidly-Deposited Polydopamine Coating via High Temperature and Vigorous Stirring: Formation, Characterization and Biofunctional Evaluation. PLoS One 2014, 9 (11), e113087. 10.1371/journal.pone.0113087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison S.; Sinclair R. Hair colouring, permanent styling and hair structure. J. Cosmet Dermatol 2003, 2 (3–4), 180–185. 10.1111/j.1473-2130.2004.00064.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.