ABSTRACT
There is accumulating evidence that, from bacteria to mammalian cells, messenger RNAs (mRNAs) are produced in intermittent bursts – a much ‘noisier’ process than traditionally thought. Based on quantitative measurements at individual promoters, diverse phenomenological models have been proposed for transcriptional bursting. Nevertheless, the underlying molecular mechanisms and significance for cellular signalling remain elusive. Here, we review recent progress, address the above issues and illuminate our viewpoints with simulation results. Despite being widely used in modelling and in interpreting experimental data, the traditional two‐state model is far from adequate to describe or infer the molecular basis and stochastic principles of transcription. In bacteria, DNA supercoiling contributes to the bursting of those genes that express at high levels and are topologically constrained in short loops; moreover, low‐affinity cis‐regulatory elements and unstable protein complexes can play a key role in transcriptional regulation. Integrating data on the architecture, kinetics, and transcriptional input–output function is a promising approach to uncovering the underlying dynamic mechanism. For eukaryotes, distinct bursting features described by the multi‐scale and continuum models coincide with those predicted by four theoretically derived principles that govern how the transcription apparatus operates dynamically. This consistency suggests a unified framework for comprehending bursting dynamics at the level of the structural and kinetic basis of transcription. Moreover, the existing models can be unified by a generic model. Remarkably, transcriptional bursting enables regulatory information to be transmitted in a digital manner, with the burst frequency representing the strength of regulatory signals. Such a mode guarantees high fidelity for precise transcriptional regulation and also provides sufficient randomness for realizing cellular heterogeneity.
Keywords: gene expression, MS2, PP7, ratchet model, multi‐scale model, continuum model, WLW model, temporal occupancy rate, frequency code, burst cluster
I. INTRODUCTION
Like a coin with two opposite sides, gene transcription exhibits duality. On the one hand, it is an accurate process. When and at what rate to transcribe a gene is subject to precise regulation, and accuracy is essential for cell fitness and development (Brivanlou & Darnell, 2002; Blake et al., 2003; Gregor et al., 2007; Fuda, Ardehali, & Lis, 2009; Weake & Workman, 2010; Wang, Liu, & Wang, 2012; Senecal et al., 2014). On the other hand, it is a stochastic process. Randomness is an inherent nature of biomolecular interactions, and the resulting heterogeneity is indispensible for cell differentiation and survival (Kussell & Leibler, 2005; Eldar & Elowitz, 2010; Torres‐Padilla & Chambers, 2014; Buettner et al., 2015; Dueck, Eberwine, & Kim, 2016). It is challenging to comprehend such a contradiction, especially given that messenger RNAs (mRNAs) are widely found to be generated in discontinuous bursts (Chubb et al., 2006; Raj et al., 2006; Chubb & Liverpool, 2010; Suter et al., 2011; Sanchez & Golding, 2013; Chong et al., 2014).
Traditional biochemical technologies assayed gene transcription in millions of cells simultaneously, leaving an impression that mRNAs were produced at a continuous and smooth rate. Taking into account the stochasticity of molecular interactions, the transcription rate of a gene (defined as the number of transcripts produced per unit time) was believed to fluctuate slightly around an average (Raj & van oudenaarden, 2008; Lenstra et al., 2016). Thus, mRNAs were thought to be produced with a constant probability per unit time, namely via a Poisson process.
Measurements in individual cells instead revealed that transcription occurs in intermittent bursts and that such bursting is ubiquitous from bacteria to mammalian cells (Golding et al., 2005; Chubb et al., 2006; Raj et al., 2006; Tripathi & Chowdhury, 2008; Suter et al., 2011; Sanchez & Golding, 2013; Chong et al., 2014; Corrigan et al., 2016; Fukaya, Lim, & Levine, 2016; Tantale et al., 2016). By labelling individual mRNAs using single‐molecule fluorescence in situ hybridization (smFISH), it was observed that a number of mRNAs from a gene emerge suddenly and then disappear gradually during a short time window (Raj et al., 2006; Battich, Stoeger, & Pelkmans, 2013; Larson et al., 2013; Buxbaum, Haimovich, & Singer, 2015; Lenstra et al., 2016) (Fig. 1A). To visualize transcription initiation directly, an array of a specific sequence is inserted after the promoter (Bertrand et al., 1998; Lim & Peabody, 2002; Chubb et al., 2006; Hocine et al., 2013). The transcribed sequence forms a stem loop, which binds the bacteriophage coat protein MS2 or PP7. The nascent mRNAs can thus be tracked in real time using time‐lapse fluorescence microscopy. It was found that many RNA polymerases successively escape from the promoter within several minutes, resulting in a convoy of closely spaced polymerases that elongates along the DNA and ultimately releases a burst of mRNAs (Tantale et al., 2016) (Fig. 1B). After the launch of this polymerase convoy, the promoter enters a refractory period without transcriptional activity.
Figure 1.
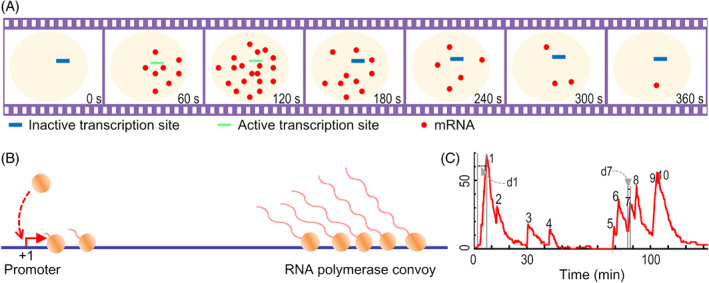
Schematic of transcriptional bursting. (A) A burst of messenger RNAs (mRNAs) is produced in a short time period with the gene active. (B) During each active period, RNA polymerases successively escape from the promoter and elongate as a convoy, leading to a burst. Two convoys are shown separately in generation and elongation. (C) A sample time series of the number of cellular mRNAs. Ten bursts are shown, with the duration of the first and seventh bursts denoted by d1 and d7.
In characterizing the time series of the number of cellular mRNAs, the duration of a burst is defined as its rising period that corresponds to the time window of synthesizing transcripts (Fig. 1C). Usually, the duration of a burst is no more than several minutes. The refractory period between two successive bursts ranges from seconds to tens of minutes or even longer. The magnitude of a burst can be up to dozens of mRNAs. The burst duration and magnitude are collectively termed burst size. Both the burst size and burst frequency (defined as the number of bursts per unit time) are potentially modulated by transcriptional activators.
Transcriptional bursting has been a hot topic of recent reviews (Boeger et al., 2015; Munsky & Neuert, 2015; Munsky, Fox, & Neuert, 2015; Reinius & Sandberg, 2015; Lenstra et al., 2016; Bressloff, 2017; Chubb, 2017; Hnisz et al., 2017; Nicolas, Phillips, & Naef, 2017; Yao, 2017). Here we focus on findings based on advanced quantitative investigations at individual alleles, aiming to connect the existing phenomenological models with the molecular mechanism of how transcription is dynamically orchestrated. We further elucidate that it is the frequency modulation that transmits information, which fulfills the accuracy and randomness requisites for transcriptional regulation.
II. TWO‐STATE MODEL OF TRANSCRIPTIONAL BURSTING
Intuitively, transcriptional bursting can be characterized with a simple two‐state model (Fig. 2A), where the gene promoter switches between a transcriptionally active state (ON) and an inactive state (OFF) (Lionnet & Singer, 2012; Munsky, Neuert, & van Oudenaarden, 2012; Sanchez, Choubey, & Kondev, 2013; Albayrak et al., 2016; Xu et al., 2016). A burst of transcripts is generated in the ON state, whereas the OFF state is non‐permissive for transcription. The two‐state model is usually depicted by three parameters: activation rate k on (reaction rate from the OFF to ON state), inactivation rate k off (reaction rate from ON to OFF), and mRNA synthesis rate from the ON state k m. k on is positively related to the concentration of transcriptional activators. Both k off and k m are constant, i.e. the duration of the ON state is exponentially distributed and mRNA synthesis is a Poisson process.
Figure 2.
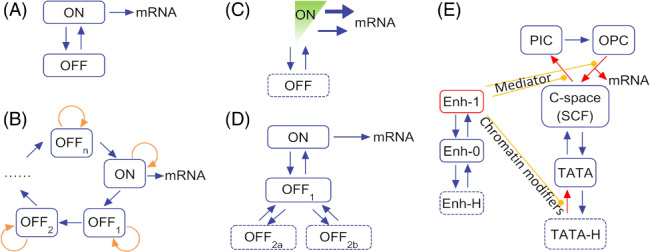
Models for transcriptional bursting. (A) Two‐state model. The gene has two alternative states, ON and OFF, whose lifetimes are usually assumed to obey two different exponential distributions. The mRNAs are produced via a Poisson process. (B) Ratchet model. A series of ordered sub‐OFF states exists. (C) Continuum model. Instead of a Poisson process, transcripts are generated at quasi‐continuous rates. (D) Multi‐scale model. Multiple layers of sub‐OFF states exist. (E) The Wang–Liu–Wang (WLW) model. The core promoter region is described as converting among five states, which are ‘being occupied by histones (TATA‐H)’, ‘naked (TATA)’, ‘being occupied by the C‐space (SCF), PIC or OPC’. The ‘C‐space’ refers to a clamp‐like space formed between the Mediator and the enhancer. The enhancer (Enh) has three states, i.e. being bound by histones (Enh‐H) or an activator (Enh‐1), or naked (Enh‐0). OPC, open complex; PIC, pre‐initiation complex; SCF, scaffold complex; TATA, TATA‐box.
The original two‐state model was proposed to explain cell heterogeneity 25 years ago (Ko, 1992). It is widely used in the analysis of experimental data and theoretical simulation (see also the review by Sanchez et al., 2013). However, its application scope has not been well defined. Theoretically, it cannot be ruled out that the transcriptional dynamics described well by the two‐state model instead originate from other stochastic principles (Pedraza & Paulsson, 2008); indeed, advanced measurements have suggested much more complex models as discussed below. Results rooted in the two‐state model should therefore be interpreted with care.
III. TRANSCRIPTIONAL BURSTING IN BACTERIA
In individual prokaryotic cells, the copy number of transcripts from a promoter is usually no more than 10 (Taniguchi et al., 2010), much lower than that in mammalian cells. Number fluctuation is thus conspicuous. Consequently, to uncover the principles that govern mRNA production necessitates collecting massive quantities of data via tracing the time series of transcription initiation events or changes in MS2/PP7‐labelled mRNAs. While this is realizable, it has not been done sufficiently. Nevertheless, it was confirmed that mRNA production is not a Poisson process but can be well fitted by the two‐state model (Golding et al., 2005; Taniguchi et al., 2010; So et al., 2011; Sanchez & Golding, 2013; Skinner et al., 2013; Chong et al., 2014).
The mechanism for transcriptional bursting in bacteria is largely unclear. However, for those genes that express at high levels and are topologically constrained in short loops (∼10000 base pairs on average), the bursting was revealed to be primarily caused by DNA supercoiling (Chong et al., 2014; Levens & Larson, 2014; Ma & Wang, 2016). An elongating polymerase results in positive supercoiling ahead of it and negative supercoiling behind it. A long interval is required to relieve the positive supercoiling due to limited quantities of DNA gyrase; positive supercoiling thus gradually accumulates, slowing down the elongation of the polymerase. On the other hand, negative supercoiling can be rapidly relieved by topoisomerase 1A and possibly helps relieve the positive supercoiling caused by a posterior polymerase. Consequently, a convoy of polymerases stops elongating, and the transcription initiation is eventually suspended. Once the positive supercoiling is removed, the convoy proceeds rapidly. A newly launched polymerase that is far behind the convoy will lead another burst.
In‐depth exploration of the bursting mechanism requires determination of the pathways and kinetics of molecular interactions among the various regulatory proteins, σ factor, the polymerase, and cis‐regulatory elements (Pedraza & Paulsson, 2008; Friedman & Gelles, 2012; Gourse & Landick, 2012). This is challenging, especially when unstable protein complexes and unknown transient interactions are involved (Friedman & Gelles, 2012). A promising approach is to adopt a hybrid method that combines the data on molecular structure, molecular interaction kinetics, and transcriptional input–output function, as exemplified by a recent study which unraveled the transcriptional dynamics of the glnAp2 promoter (Wang, Liu, & Wang, 2016).
Upstream of the glnAp2 promoter, there are two enhancer sequences and three low‐affinity sequences for the binding of transcriptional activator nitrogen regulatory protein C (NtrC). In contrast with the traditional view that transcription initiation is stimulated by an NtrC hexamer simultaneously bound to the two enhancers, Wang et al. (2016) revealed that the initiation is stimulated by an NtrC hexamer at either enhancer (Fig. 3). Moreover, the main regulatory mode involves short‐lived DNA bridging, via which the proximal enhancer is connected to a low‐affinity site adjacent to the transcription start site (TTS). With the DNA bridging, the NtrC hexamer at the distal enhancer is in the vicinity of the TTS and thus immediately stimulates the polymerases one by one, leading to a burst. This work stresses the importance of low‐affinity sites, unstable protein complexes, and transient molecular interactions in orchestrating transcription.
Figure 3.
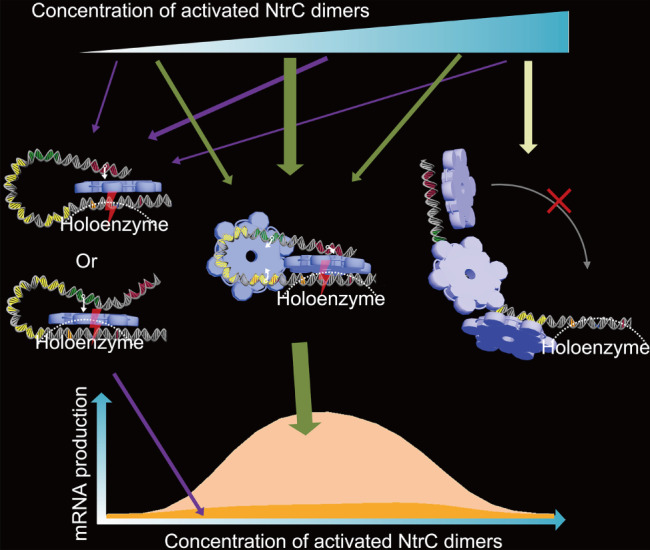
Molecular mechanisms for transcriptional regulation at the glnAp2 promoter. A transcriptional activator nitrogen regulatory protein C (NtrC) hexamer (shown in blue) formed at the remote or proximal enhancer (separately coloured in red and green; the connection between an enhancer and an NtrC hexamer is denoted by white arrows) is able to catalyse the polymerase holoenzyme (outlined by a dashed line) at the core promoter (the −24, −12, and +1 sites are denoted in orange, blue, and red, respectively). For a wide range of NtrC concentrations, these two modes only contribute to a small proportion of messenger RNAs (mRNAs) produced, since it takes a long time for the hexamers to find the holoenzyme. In the third mode, there exists a DNA bridging—an NtrC hexamer connects the proximal enhancer to a low‐affinity site (the three low‐affinity sites are coloured in yellow). This bridging facilitates the hexamer at the remote enhancer to catalyse multiple rounds of transcription initiation. At very high concentrations of NtrC dimers, NtrC oligomers are formed at the low‐affinity sites, rendering the DNA rigid and turning the gene off.
IV. TRANSCRIPTIONAL BURSTING IN EUKARYOTES
In higher eukaryotes, DNA is packaged by nucleosomes that consist of histones; both the DNA and histones are epigenetically modified (Catez, Ueda, & Bustin, 2006; Kouzarides, 2007; Coulon et al., 2013). Transcriptional progression begins with DNA demethylation, histone modification and nucleosome eviction. The transcription apparatus (TA), which is mainly composed of gene‐specific transcriptional activator(s), general transcription factors (TFs) including TFII‐B, ‐D, ‐E, ‐F, and ‐H, the Mediator complex, and RNA polymerase II (Pol II), assembles on the promoter to synthesise transcripts. The destruction of the TA may be followed by its reconstruction, or nucleosome recruitment and epigenetic reprogramming. Thus, the TA together with the chromatin determines transcriptional dynamics in eukaryotic cells.
For some genes, chromatin has a primary role in shaping transcriptional dynamics (Métivier et al., 2003; Métivier, Reid, & Gannon, 2006; Rybakova et al., 2015a). The promoter state, which is determined by how the promoter is chemically modified and how the associated proteins are structurally assembled and chemically modified, evolves sequentially due to successive occurrence of irreversible molecular reactions [e.g. the demethylation of DNA engages a series of reactions that orchestrate the oxidation of cytosine and base excision and repair (Nabel & Kohli, 2011)]. Moreover, the promoter state evolves cyclically, repeating the stages from histone eviction, then TA assembly and destruction, to chromatin recovery (Reid et al., 2003; Hager, McNally, & Misteli, 2009; Stavreva et al., 2009; Wang et al., 2014). Such irreversibility and periodicity were described by a ratchet model (Krasnov et al., 2016; Lemaire et al., 2006; Métivier et al., 2006; Wang et al., 2014) (Fig. 2B). Each cycle of the ratchet includes more than 100 molecular reactions, and most of the reactions involve chromatin modifications. Transcripts are produced during a short ‘ON’ period. Typical genes obeying such dynamics include the oestrogen receptor α‐regulated pS2 gene in human cells and the copper receptor Ace1p‐regulated CUP1 gene in yeast cells (Reid et al., 2003; Karpova et al., 2008; Wang et al., 2014). Once induced simultaneously by oestrogen or copper, a population of the promoters exhibits synchronized transcriptional activity for a relatively long time (Lemaire et al., 2006; Wang et al., 2014; Rybakova et al., 2015a).
For most genes, however, it seems that the promoter largely lacks histones or the chromatin can be easily opened. Extensive investigation revealed that the time series of transcriptional bursting is primarily shaped by the TA, whose gene specificity is defined by the cis‐regulatory elements and promoter sequence (Suter et al., 2011). Additionally, the duration of the ON state is exponentially distributed, while the OFF state cannot be described by one single step. Two novel models have been built to describe this type of promoter. One is the ‘continuum model’ based on a highly expressed housekeeping actin gene (Corrigan et al., 2016). The other is the ‘multi‐scale model’ based on the fully activated HIV‐1 gene (Tantale et al., 2016). High expression means that the promoter is largely evicted from histones, and thus the models accurately reflect the dynamics of the TA.
The continuum model has an OFF state and a special ON state (Corrigan et al., 2016) (Fig. 2C). During the ON period, transcripts are not generated via a Poisson process as assumed by the two‐state model. The time intervals between two successive initiation events range from several seconds to more than ten seconds. These intervals do not obey a single exponential distribution; instead, they obey a large number of exponential distributions whose expected values range quasi‐continuously. The corresponding initiation rate thus spans a nearly continuous spectrum. Two mechanisms were speculated to be at work. The ON state is composed of a large number of sub‐states, each with a distinct initiation rate. These sub‐states are defined by specific binding of transcription factors or epigenetic marks and are closely spaced in time, so that they are hard to distinguish or count. Alternatively, in the ON state the promoter switches between a primed state and a mature state that produces a transcript. The mature state depends on the local and time‐varying concentration of Pol II. Such a continuum feature was reported previously (Bothma et al., 2014).
The multi‐scale model stresses that transcription is initiated in units of bursts and the bursts are separated with multiple timescales (Tantale et al., 2016) (Fig. 2D). Each burst is triggered by a Pol II convoy – a group of Pol IIs (with the number ranging from 1 to 30) successively launches from the promoter within several minutes and then elongates at a rate of ∼4.1 kb/min. With the Mediator at the promoter, the refractory period between two successive bursts obeys an exponential distribution with the expected value of ∼100 s. Such Mediator‐dependent two‐state dynamics have a precondition that the TATA‐box binding protein (TBP, one subunit of TFIID) is at the promoter. Typically, several convoys form and depart in the presence of the TBP. Without the TBP, the promoter enters a non‐permissive period, which does not obey a single exponential distribution; presumably, the non‐permissive periods depend on different extents of promoter methylation or occupancy by histones.
Compared with the simple two‐state model, the ratchet, continuum, and multi‐scale models more accurately describe transcriptional dynamics and provide more insights into transcriptional regulation. However, these models appear to differ substantially from each other. It is unclear to what extent these models are condition specific or reveal the general mechanism of transcription. Interestingly, these models can be unified in an earlier theoretical framework of how the TA operates (Wang et al., 2012).
V. OPERATING PRINCIPLES OF THE EUKARYOTIC TRANSCRIPTION APPARATUS
Transcription is a complex and highly dynamic process (Fuda et al., 2009; Vera et al., 2016; Cuvier & Fierz, 2017; Teves et al., 2018). Thousands of transcription factors have been identified as participating in eukaryotic transcription; the combinatorial interactions among these factors and specific DNA sequences control the activity of Pol II. Yet the kinetics of these interactions are largely beyond technical detection (Gourse & Landick, 2012; Rusk, 2014; Sung et al., 2014; Sepúlveda et al., 2016). Despite such complexity and difficulty, it has been shown that the eukaryotic TA mostly shares a general architecture (Yudkovsky, Ranish, & Hahn, 2000; Hahn, 2004; Thomas & Chiang, 2006; Kornberg, 2007; Fuda et al., 2009). The core promoter of an active gene is often occupied by the scaffold complex (SCF), which is mainly composed of the general transcription factors including TFII‐A, ‐D, ‐E, and ‐H and the Mediator. The transcriptional activator, whose concentration changes with cellular signalling, associates with the enhancer. The enhancer‐bound activator exerts control over transcription initiation via the Mediator. Pol II assembles onto the SCF, followed by formation of the transcription preinitiation complex (PIC), then the open complex (OPC), and the elongation complex, which synthesises a transcript (Kornberg, 2005, 2007; Malik & Roeder, 2005).
Based on the general structural organization and conformational changes of the TA, the ensemble and probability theories were exploited to probe how the TA dynamically operates (Wang et al., 2012). Theoretical analyses and numerical simulation revealed that, for the TA to orchestrate a reliable response to changing activator concentration, transcripts are essentially generated in units of bursts. It was also proposed that the TA operates as dictated by the following four principles.
Transcriptional activators cyclically bind to and dissociate from the enhancer, with the individual binding time no more than several minutes. Some of those cycling activators act to recruit chromatin modifiers, while some control transcription initiation via the Mediator.
There temporarily exists a ‘clamp‐like space (C‐space)’ between the enhancer and the Mediator (Fig. 4A), which functions to detect the activator concentration via a statistical quantity, R TOR. The C‐space is relatively stable; it persists for many rounds of activators cycling in and out. R TOR is the activators' temporal occupancy rate in the C‐space: R TOR = ∑t i /T, where t i is the residence time of the i‐th (i = 1, 2, 3, …, n) round of cycling and T is the total time corresponding to n rounds of cycling. As the activator concentration rises, n becomes larger in probability for fixed T, and so does R TOR. In this way, R TOR robustly encodes the activator concentration (Fig. 4B). Note that the basic unit of R TOR is the individual residence time t i.
The Mediator transfers information from the settled activator to Pol II through allostery. The entry of an activator into the C‐space induces allostery in the Mediator, facilitating Pol II to assemble and initiate transcription. Without the activator, transcription initiation rarely occurs.
With an activator in the C‐space, a convoy of Pol IIs is launched, leading to a burst of transcripts. The time interval between two successive initiation events is far shorter than the activator's residence time. Typically, the interval is of seconds. Such intensive initiations together with the Mediator's allostery guarantee that the basic unit of R TOR is converted to the number of transcripts.
Figure 4.
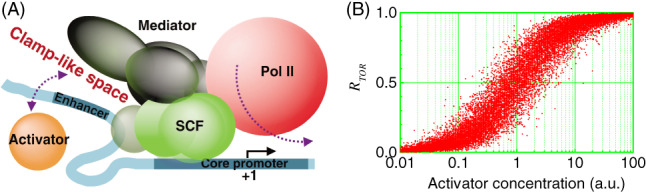
General mechanism of how the transcription apparatus operates in eukaryotes. (A) The Mediator, which is a component of the scaffold complex (SCF), is temporarily tethered in the vicinity of the enhancer, forming a clamp‐like space (C‐space) where activators cycle in and out. With an activator in the C‐space, RNA polymerase II (Pol II) initiates transcription rapidly, leading to a burst of messenger RNAs (mRNAs). (B) With increasing activator concentration, the activators' temporal occupancy rate in the C‐space, R TOR, rises and saturates in probability. The relationship is a statistical mapping, meaning that slight fluctuations in activator concentration (i.e. extrinsic noise) can be filtered out, thus endowing transcriptional regulation with strong robustness. Shown are simulation results under the condition that activators have cycled for five rounds. Reprinted from Wang et al. (2012).
These principles suggest that transcriptional bursts are separated with multiple timescales and that the initiation rate is continuous during each ON period. The first layer that separates the bursts is controlled by the activator's cycling within the C‐space, manifested as activator–Mediator‐dependent two‐state dynamics. The second layer is determined by the stability of the SCF where the C‐space forms, as marked by the TBP's presence or absence. The third and higher layers depend on the extent to which the promoter is epigenetically modified and recaptured by histones. On the other hand, even with an activator in the C‐space, synthesizing a transcript requires many steps from the SCF, PIC, and OPC, to elongation. These steps do not have identical reaction rates. That is, the intervals between two successive initiation events do not obey an exponential distribution. Given fluctuations in local concentrations of Pol II and general transcription factors such as TFIIF, the initiation rate will range over a wide spectrum. In other words, the multi‐scale and continuum models touch on different aspects of gene transcription, which are shared by various genes to different degrees.
The above principles are recapitulated by the Wang–Liu–Wang (WLW) model (Fig. 2E), with which simulation results reproduced different profiles of gene expression with the precision reaching the standard deviation of the transcriptional response (Wang et al., 2012). In this model, transcription is initiated via steps from the SCF, to PIC, to OPC; these steps are tightly controlled by activators via the Mediator. The destruction of the SCF may be followed by histone rebinding. Histones can be easily evicted in the presence of enhancer‐bound activators that recruit chromatin remodelling enzymes and modifiers. Notably, numeric simulation with the WLW model reproduces the multi‐scale and continuum features (Fig. 5). Additionally, the WLW model can be expanded to embody the ratchet model if a sufficient number of steps on chromatin modifications are included (Wang et al., 2014).
Figure 5.
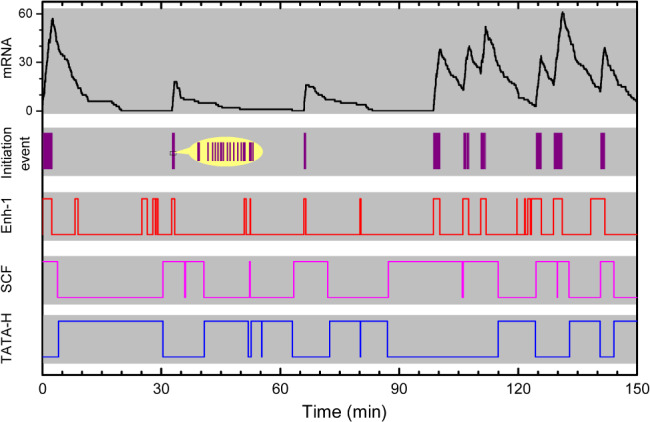
Concurrent multi‐scale and continuum features of transcriptional bursting. Shown are simulation results with the Wang–Liu–Wang (WLW) model. Transcription initiation events are denoted by violet vertical lines, with the inset showing an enlarged view. Enh‐1 denotes whether the enhancer (Enh) is bound by activators, with the upper parts of the line denoting the bound state. SCF and TATA‐H denote whether the TATA‐box in the core promoter region is occupied by the scaffold complex (SCF) and histones (H), respectively. The dynamics of activators cycling in the C‐space, the formation and destruction of the SCF, and occupancy of the core promoter by histones endow the bursts with multiple time scales. The time intervals between two successive initiation events do not obey a single exponential distribution, as shown in the inset.
VI. EFFECTS OF TRANSCRIPTIONAL BURSTING ON EUKARYOTIC CELLULAR SIGNALLING
Given that transcripts are synthesized in episodic bursts, cells must somehow deal with this ‘noisy’ behaviour. This first raises an issue of how the bursting transfers information (Skupsky et al., 2010; Dar et al., 2012; Molina et al., 2013; Corrigan & Chubb, 2014; Senecal et al., 2014). As mentioned above, the statistical quantity R TOR is exploited by the TA to encode activator concentration and guide transcription initiation (Wang et al., 2012); consequently, increasing activator concentration leads to an increase in burst frequency (Fig. 6). That is, the burst frequency represents the strength of regulatory signals. By contrast, burst size is determined by the activators' residence time in the C‐space, irrespective of activator concentration. For a given promoter and constant concentrations of related proteins such as the general transcription factors and Pol II, the burst size obeys a specific distribution. Note that, when detected experimentally, a burst cluster that is composed of two or more closely adjoined bursts could be mistaken as one burst. Because the burst cluster emerges with a larger probability at higher activator concentrations, an inaccurate conclusion might be obtained that the burst size rises with increasing activator concentration (Fig. 6).
Figure 6.
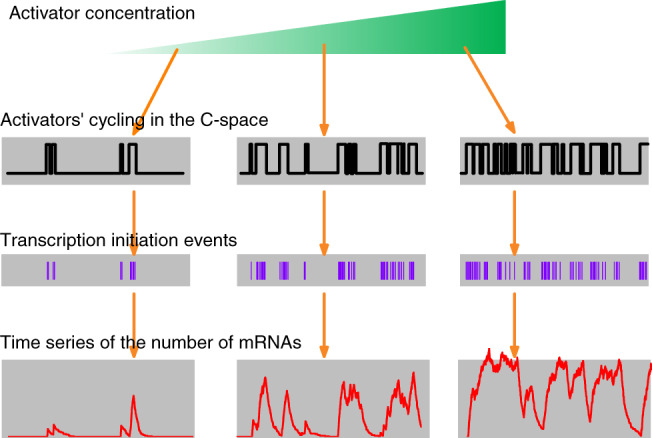
Modulation of transcriptional bursts. Increasing the activator concentration leads to more frequent cycling of activators in the C‐space, with the distribution of residence time unaffected. The residence times shape the occurrence of transcription initiation events, thereby controlling the time series of mRNA number. As a result, the burst frequency, rather than burst size, is subject to modulation by activator concentration. Note that the bursting with high frequency makes it hard to differentiate between two successive bursts.
Advanced measurements at single‐transcript resolution validated these theoretical predictions. For the c‐Fos gene, for example, the burst frequency is modulated by activator concentration, and the burst duration is determined by the duration of activator binding to DNA (Senecal et al., 2014). Additionally, the transcription initiation rate during activator–DNA binding will be affected if the strength of the activator's transactivation domain is altered by mutation. The same conclusions were reached for steroid‐receptor‐induced gene transcription (Larson et al., 2013). Increasing the activated steroid receptor leads to an increase in burst frequency, whereas the duration and magnitude of individual bursts are not affected. Further analyses on enhancer–promoter communications in living Drosophila embryos, mouse, and human cells also confirmed this frequency modulation (Bartman et al., 2016; Fukaya et al., 2016). Collectively, transcriptional bursting is modulated in a digital manner.
Fluctuations due to bursting can be directly utilized by positive feedback loops in gene regulatory networks to induce the bistability of gene expression and thereby cell differentiation (Kussell & Leibler, 2005; Eldar & Elowitz, 2010; Torres‐Padilla & Chambers, 2014; Buettner et al., 2015; Dueck et al., 2016). Nevertheless, a second issue arises concerning how the accuracy of cellular signalling is achieved given the fluctuations. Recent studies showed that, after the release of a transcript from DNA, the mature mRNA tends to reside in the nucleus for a long time (Bahar Halpern et al., 2015; Battich, Stoeger, & Pelkmans, 2015). mRNAs produced in discontinuous bursts thus accumulate in a pool and are slowly released into the cytoplasm. It was thus suggested that the nuclear retention of mRNAs tends to minimize the burst noise.
VII. CONCLUSIONS
(1) Genes are transcribed in episodic bursts. This feature is ubiquitous from bacteria to mammalian cells, regardless of whether they are constitutive or inducible genes.
(2) Transcriptional bursts in eukaryotes take place with multiple timescales, and the transcription initiation rate is continuous during each ON period. Such features are consistent with those dictated by four theoretically derived principles that govern how the TA operates dynamically. This in turn suggests that eukaryotic genes likely share similar dynamic principles for TA operation, as they share the same set of general transcription factors.
(3) The ratchet, multi‐scale, and continuum models emphasize different profiles of transcriptional dynamics. These models are applicable to genes of specific categories and can be unified by the WLW model, which depicts the core steps of regulated transcription and can be accommodated to characterize gene specificity. Additionally, conclusions derived from the traditional two‐state model should be treated cautiously.
(4) Transcriptional bursting in eukaryotes allows digital information conversion, by which regulatory signals modulate the burst frequency rather than burst size. For a given TA, the burst size obeys a specific distribution; experimentally, the burst size might be mistaken as subject to modulation since it is hard to differentiate a burst from a burst cluster.
(5) Transcriptional bursting fulfills the accuracy and stochasticity requirements for the transcriptional response. The modulation of burst frequency is an embodiment of the R TOR code, which represents an accurate encoding of time‐varying activator concentration; moreover, bursting‐induced fluctuations in mRNA number can be smoothed by nuclear retention of mRNAs. On the other hand, such fluctuations can also be enlarged to achieve sufficient heterogeneity.
(6) Low‐affinity DNA binding sites, unstable protein complexes, and DNA supercoiling can play crucial roles in regulating transcription. Investigating transcriptional dynamics necessitates both live imaging methods with high resolution (Skupsky et al., 2010; Suter et al., 2011; Evans et al., 2012; Friedman, Mumm, & Gelles, 2013; Gebhardt et al., 2013; Hocine et al., 2013; Kouno et al., 2013; Lickwar, Mueller, & Lieb, 2013; Yunger et al., 2013; Sidaway‐Lee et al., 2014; Annibale & Gratton, 2015; Camunas‐Soler et al., 2015; Gocheva et al., 2015; Roberts et al., 2015; Rybakova et al., 2015a; Corrigan et al., 2016; Tantale et al., 2016) and quantitative computer simulations with appropriate theories and models (Skupsky et al., 2010; Suter et al., 2011; Wang et al., 2012; Maina et al., 2014; Choubey, Kondev, & Sanchez, 2015; Stefan et al., 2015; Rybakova et al., 2015a, b ; Corrigan et al., 2016; Tantale et al., 2016). Specifically, integrating diverse sets of data makes it possible to present a coherent dynamic picture of gene transcription in bacteria (Wang et al., 2016).
(7) The significance of various bursting patterns from different promoters remains to be addressed. Firstly, unscrambling the bursting patterns paves the way to reveal the dynamics of both the chromatin and the TA. By statistically analysing the Mediator‐ and TBP‐dependent bursting dynamics (such as the burst number, duration, and interval), for example, it can be inferred that the Mediator interacts with the DNA much more transiently than the TBP (Tantale et al., 2016; Teves et al., 2018). Secondly, it is an open issue why different genes adopt different bursting patterns to realize their functions. In other words, are distinct bursting patterns exploited or accommodated to achieve specific signalling capability?
VIII. ACKNOWLEDGEMENTS
This work was supported by the Ministry of Science and Technology of the People's Republic of China [2013CB834104], National Natural Science Foundation of China [31361163003, 81421091, and 11175084], and Fundamental Research Funds for the Central Universities [JUSRP11838].
[The copyright line for this article was changed on 03 August 2018 after original online publication]
Contributor Information
Wei Wang, Email: wangwei@nju.edu.cn.
Feng Liu, Email: fliu@nju.edu.cn.
IX. REFERENCES
- Albayrak, C. , Jordi, C. A. , Zechner, C. , Lin, J. , Bichsel, C. A. , Khammash, M. & Tay, S. (2016). Digital quantification of proteins and mRNA in single mammalian cells. Molecular Cell 61, 914–924. [DOI] [PubMed] [Google Scholar]
- Annibale, P. & Gratton, E. (2015). Single cell visualization of transcription kinetics variance of highly mobile identical genes using 3D nanoimaging. Scientific Reports 5, 9258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahar Halpern, K. , Caspi, I. , Lemze, D. , Levy, M. , Landen, S. , Elinav, E. , Ulitsky, I. & Itzkovitz, S. (2015). Nuclear retention of mRNA in mammalian tissues. Cell Reports 13, 2653–2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartman, C. R. , Hsu, S. C. , Hsiung, C. C. S. , Raj, A. & Blobel, G. A. (2016). Enhancer regulation of transcriptional bursting parameters revealed by forced chromatin looping. Molecular Cell 62, 237–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battich, N. , Stoeger, T. & Pelkmans, L. (2013). Image‐based transcriptomics in thousands of single human cells at single‐molecule resolution. Nature Methods 10, 1127–1133. [DOI] [PubMed] [Google Scholar]
- Battich, N. , Stoeger, T. & Pelkmans, L. (2015). Control of transcript variability in single mammalian cells. Cell 163, 1596–1610. [DOI] [PubMed] [Google Scholar]
- Bertrand, E. , Chartrand, P. , Schaefer, M. , Shenoy, S. M. , Singer, R. H. & Long, R. M. (1998). Localization of ASH1 mRNA particles in living yeast. Molecular Cell 2, 437–445. [DOI] [PubMed] [Google Scholar]
- Blake, W. J. , Kaern, M. , Cantor, C. R. & Collins, J. J. (2003). Noise in eukaryotic gene expression. Nature 422, 633–637. [DOI] [PubMed] [Google Scholar]
- Boeger, H. , Shelansky, R. , Patel, H. & Brown, C. R. (2015). From structural variation of gene molecules to chromatin dynamics and transcriptional bursting. Genes 6, 469–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bothma, J. P. , Garcia, H. G. , Esposito, E. , Schlissel, G. , Gregor, T. & Levine, M. (2014). Dynamic regulation of eve stripe 2 expression reveals transcriptional bursts in living Drosophila embryos. Proceedings of the National Academy of Sciences of the United States of America 111, 10598–10603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressloff, P. C. (2017). Stochastic switching in biology: from genotype to phenotype. Journal of Physics A: Mathematical and Theoretical 50, 133001. [Google Scholar]
- Brivanlou, A. H. & Darnell, J. E. (2002). Signal transduction and the control of gene expression. Science 295, 813–818. [DOI] [PubMed] [Google Scholar]
- Buettner, F. , Natarajan, K. N. , Casale, F. P. , Proserpio, V. , Scialdone, A. , Theis, F. J. , Teichmann, S. A. , Marioni, J. C. & Stegle, O. (2015). Computational analysis of cell‐to‐cell heterogeneity in single‐cell RNA‐sequencing data reveals hidden subpopulations of cells. Nature Biotechnology 33, 155–160. [DOI] [PubMed] [Google Scholar]
- Buxbaum, A. R. , Haimovich, G. & Singer, R. H. (2015). In the right place at the right time: visualizing and understanding mRNA localization. Nature Reviews Molecular Cell Biology 16, 95–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camunas‐Soler, J. , Manosas, M. , Frutos, S. , Tulla‐Puche, J. , Albericio, F. & Ritort, F. (2015). Single‐molecule kinetics and footprinting of DNA bis‐intercalation: the paradigmatic case of thiocoraline. Nucleic Acids Research 43, 2767–2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catez, F. , Ueda, T. & Bustin, M. (2006). Determinants of histone H1 mobility and chromatin binding in living cells. Nature Structural and Molecular Biology 13, 305–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong, S. S. , Chen, C. Y. , Ge, H. & Xie, X. S. (2014). Mechanism of transcriptional bursting in bacteria. Cell 158, 314–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choubey, S. , Kondev, J. & Sanchez, A. (2015). Deciphering transcriptional dynamics in vivo by counting nascent RNA molecules. PLoS Computational Biology 11, e1004345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chubb, J. R. (2017). Symmetry breaking in development and stochastic gene expression. Wiley Interdisciplinary Reviews‐Developmental Biology 6, e284. [DOI] [PubMed] [Google Scholar]
- Chubb, J. R. & Liverpool, T. B. (2010). Bursts and pulses: insights from single cell studies into transcriptional mechanisms. Current Opinion in Genetics & Development 20, 478–484. [DOI] [PubMed] [Google Scholar]
- Chubb, J. R. , Trcek, T. , Shenoy, S. M. & Singer, R. H. (2006). Transcriptional pulsing of a developmental gene. Current Biology 16, 1018–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigan, A. M. & Chubb, J. R. (2014). Regulation of transcriptional bursting by a naturally oscillating signal. Current Biology 24, 205–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigan, A. M. , Tunnacliffe, E. , Cannon, D. & Chubb, J. R. (2016). A continuum model of transcriptional bursting. eLife 5, e13051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulon, A. , Chow, C. C. , Singer, R. H. & Larson, D. R. (2013). Eukaryotic transcriptional dynamics: from single molecules to cell populations. Nature Reviews Genetics 14, 572–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuvier, O. & Fierz, B. (2017). Dynamic chromatin technologies: from individual molecules to epigenomic regulation in cells. Nature Reviews Genetics 18, 457–472. [DOI] [PubMed] [Google Scholar]
- Dar, R. D. , Razooky, B. S. , Singh, A. , Trimeloni, T. V. , McCollum, J. M. , Cox, C. D. , Simpson, M. L. & Weinberger, L. S. (2012). Transcriptional burst frequency and burst size are equally modulated across the human genome. Proceedings of the National Academy of Sciences of the United States of America 109, 17454–17459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dueck, H. , Eberwine, J. & Kim, J. (2016). Variation is function: Are single cell differences functionally important? BioEssays 38, 172–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldar, A. & Elowitz, M. B. (2010). Functional roles for noise in genetic circuits. Nature 467, 167–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans, V. C. , Barker, G. , Heesom, K. J. , Fan, J. , Bessant, C. & Matthews, D. A. (2012). De novo derivation of proteomes from transcriptomes for transcript and protein identification. Nature Methods 9, 1207–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman, L. J. & Gelles, J. (2012). Mechanism of transcription initiation at an activator‐dependent promoter defined by single‐molecule observation. Cell 148, 679–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman, L. J. , Mumm, J. P. & Gelles, J. (2013). RNA polymerase approaches its promoter without long‐range sliding along DNA. Proceedings of the National Academy of Sciences of the United States of America 110, 9740–9745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuda, N. J. , Ardehali, M. B. & Lis, J. T. (2009). Defining mechanisms that regulate RNA polymerase II transcription in vivo. Nature 461, 186–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukaya, T. , Lim, B. & Levine, M. (2016). Enhancer control of transcriptional bursting. Cell 166, 358–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhardt, J. C. M. , Suter, D. M. , Roy, R. , Zhao, Z. Q. W. , Chapman, A. R. , Basu, S. , Maniatis, T. & Xie, X. S. (2013). Single‐molecule imaging of transcription factor binding to DNA in live mammalian cells. Nature Methods 10, 421–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gocheva, V. , Le Gall, A. , Boudvillain, M. , Margeat, E. & Nollmann, M. (2015). Direct observation of the translocation mechanism of transcription termination factor Rho. Nucleic Acids Research 43, 2367–2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golding, I. , Paulsson, J. , Zawilski, S. M. & Cox, E. C. (2005). Real‐time kinetics of gene activity in individual bacteria. Cell 123, 1025–1036. [DOI] [PubMed] [Google Scholar]
- Gourse, R. L. & Landick, R. (2012). CoSMoS unravels mysteries of transcription initiation. Cell 148, 635–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregor, T. , Tank, D. W. , Wieschaus, E. F. & Bialek, W. (2007). Probing the limits to positional information. Cell 130, 153–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hager, G. L. , McNally, J. G. & Misteli, T. (2009). Transcription dynamics. Molecular Cell 35, 741–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn, S. (2004). Structure and mechanism of the RNA polymerase II transcription machinery. Nature Structural Molecular Biology 11, 394–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hnisz, D. , Shrinivas, K. , Young, R. A. , Chakraborty, A. K. & Sharp, P. A. (2017). A phase separation model for transcriptional control. Cell 169, 13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocine, S. , Raymond, P. , Zenklusen, D. , Chao, J. A. & Singer, R. H. (2013). Single‐molecule analysis of gene expression using two‐color RNA labeling in live yeast. Nature Methods 10, 119–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpova, T. S. , Kim, M. J. , Spriet, C. , Nalley, K. , Stasevich, T. J. , Kherrouche, Z. , Heliot, L. & McNally, J. G. (2008). Concurrent fast and slow cycling of a transcriptional activator at an endogenous promoter. Science 319, 466–469. [DOI] [PubMed] [Google Scholar]
- Ko, M. S. H. (1992). Induction mechanism of a single gene molecule: stochastic or deterministic? BioEssays 14, 341–346. [DOI] [PubMed] [Google Scholar]
- Kornberg, R. D. (2005). Mediator and the mechanism of transcriptional activation. Trends in Biochemical Sciences 30, 235–239. [DOI] [PubMed] [Google Scholar]
- Kornberg, R. D. (2007). The molecular basis of eukaryotic transcription. Proceedings of the National Academy of Sciences of the United States of America 104, 12955–12961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouno, T. , de Hoon, M. , Mar, J. C. , Tomaru, Y. , Kawano, M. , Carninci, P. , Suzuki, H. , Hayashizaki, Y. & Shin, J. W. (2013). Temporal dynamics and transcriptional control using single‐cell gene expression analysis. Genome Biology 14, R118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouzarides, T. (2007). Chromatin modifications and their function. Cell 128, 693–705. [DOI] [PubMed] [Google Scholar]
- Krasnov, A. N. , Mazina, M. Y. , Nikolenko, J. V. & Vorobyeva, N. E. (2016). On the way of revealing coactivator complexes cross‐talk during transcriptional activation. Cell & Bioscience 6, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kussell, E. & Leibler, S. (2005). Phenotypic diversity, population growth, and information in fluctuating environments. Science 309, 2075–2078. [DOI] [PubMed] [Google Scholar]
- Larson, D. R. , Fritzsch, C. , Sun, L. , Meng, X. , Lawrence, D. S. & Singer, R. H. (2013). Direct observation of frequency modulated transcription in single cells using light activation. eLife 2, e00750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire, V. , Lee, C. F. , Lei, J. , Métivier, R. & Glass, L. (2006). Sequential recruitment and combinatorial assembling of multiprotein complexes in transcriptional activation. Physical Review Letters 96, 198102. [DOI] [PubMed] [Google Scholar]
- Lenstra, T. L. , Rodriguez, J. , Chen, H. M. & Larson, D. R. (2016). Transcription dynamics in living cells. Annual Review of Biophysics 45, 25–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levens, D. & Larson, D. R. (2014). A new twist on transcriptional bursting. Cell 158, 241–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lickwar, C. R. , Mueller, F. & Lieb, J. D. (2013). Genome‐wide measurement of protein‐DNA binding dynamics using competition ChIP. Nature Protocols 8, 1337–1353. [DOI] [PubMed] [Google Scholar]
- Lim, F. & Peabody, D. S. (2002). RNA recognition site of PP7 coat protein. Nucleic Acids Research 30, 4138–4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lionnet, T. & Singer, R. H. (2012). Transcription goes digital. EMBO Reports 13, 313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, J. & Wang, M. D. (2016). DNA supercoiling during transcription. Biophysical Reviews 8, S75–S87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maina, C. W. , Honkela, A. , Matarese, F. , Grote, K. , Stunnenberg, H. G. , Reid, G. , Lawrence, N. D. & Rattray, M. (2014). Inference of RNA polymerase II transcription dynamics from chromatin immunoprecipitation time course data. PLoS Computational Biology 10, e1003598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik, S. & Roeder, R. G. (2005). Dynamic regulation of pol II transcription by the mammalian Mediator complex. Trends in Biochemical Sciences 30, 256–263. [DOI] [PubMed] [Google Scholar]
- Métivier, R. , Penot, G. , Hubner, M. R. , Reid, G. , Brand, H. , Kos, M. & Gannon, F. (2003). Estrogen receptor‐α directs ordered, cyclical, and combinatorial recruitment of cofactors on a natural target promoter. Cell 115, 751–763. [DOI] [PubMed] [Google Scholar]
- Métivier, R. , Reid, G. & Gannon, F. (2006). Transcription in four dimensions: nuclear receptor‐directed initiation of gene expression. EMBO Reports 7, 161–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina, N. , Suter, D. M. , Cannavo, R. , Zoller, B. , Gotic, I. & Naef, F. (2013). Stimulus‐induced modulation of transcriptional bursting in a single mammalian gene. Proceedings of the National Academy of Sciences of the United States of America 110, 20563–20568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munsky, B. , Fox, Z. & Neuert, G. (2015). Integrating single‐molecule experiments and discrete stochastic models to understand heterogeneous gene transcription dynamics. Methods 85, 12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munsky, B. & Neuert, G. (2015). From analog to digital models of gene regulation. Physical Biology 12, 045004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munsky, B. , Neuert, G. & van Oudenaarden, A. (2012). Using gene expression noise to understand gene regulation. Science 336, 183–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabel, C. S. & Kohli, R. M. (2011). Demystifying DNA demethylation. Science 333, 1229–1230. [DOI] [PubMed] [Google Scholar]
- Nicolas, D. , Phillips, N. E. & Naef, F. (2017). What shapes eukaryotic transcriptional bursting? Molecular BioSystems 13, 1280–1290. [DOI] [PubMed] [Google Scholar]
- Pedraza, J. M. & Paulsson, J. (2008). Effects of molecular memory and bursting on fluctuations in gene expression. Science 319, 339–343. [DOI] [PubMed] [Google Scholar]
- Raj, A. , Peskin, C. S. , Tranchina, D. , Vargas, D. Y. & Tyagi, S. (2006). Stochastic mRNA synthesis in mammalian cells. PLoS Biology 4, e309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj, A. & van Oudenaarden, A. (2008). Nature, nurture, or chance: stochastic gene expression and its consequences. Cell 135, 216–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid, G. , Hubner, M. R. , Métivier, R. , Brand, H. , Denger, S. , Manu, D. , Beaudouin, J. , Ellenberg, J. & Gannon, F. (2003). Cyclic, proteasome‐mediated turnover of unliganded and liganded ERα on responsive promoters is an integral feature of estrogen signaling. Molecular Cell 11, 695–707. [DOI] [PubMed] [Google Scholar]
- Reinius, B. & Sandberg, R. (2015). Random monoallelic expression of autosomal genes: stochastic transcription and allele‐level regulation. Nature Reviews Genetics 16, 653–664. [DOI] [PubMed] [Google Scholar]
- Roberts, T. C. , Hart, J. R. , Kaikkonen, M. U. , Weinberg, M. S. , Vogt, P. K. & Morris, K. V. (2015). Quantification of nascent transcription by bromouridine immunocapture nuclear run‐on RT‐qPCR. Nature Protocols 10, 1198–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusk, N. (2014). Transcription factors without footprints. Nature Methods 11, 988–989. [DOI] [PubMed] [Google Scholar]
- Rybakova, K. N. , Bruggeman, F. J. , Tomaszewska, A. , Mone, M. J. , Carlberg, C. & Westerhoff, H. V. (2015a). Multiplex eukaryotic transcription (in) activation: timing, bursting and cycling of a ratchet clock mechanism. PLoS Computational Biology 11, e1004236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybakova, K. N. , Tomaszewska, A. , van Mourik, S. , Blom, J. , Westerhoff, H. V. , Carlberg, C. & Bruggeman, F. J. (2015b). Tracing the molecular basis of transcriptional dynamics in noisy data by using an experiment‐based mathematical model. Nucleic Acids Research 43, 153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez, A. , Choubey, S. & Kondev, J. (2013). Stochastic models of transcription: from single molecules to single cells. Methods 62, 13–25. [DOI] [PubMed] [Google Scholar]
- Sanchez, A. & Golding, I. (2013). Genetic determinants and cellular constraints in noisy gene expression. Science 342, 1188–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senecal, A. , Munsky, B. , Proux, F. , Ly, N. , Braye, F. E. , Zimmer, C. , Mueller, F. & Darzacq, X. (2014). Transcription factors modulate c‐Fos transcriptional bursts. Cell Reports 8, 75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepúlveda, L. A. , Xu, H. , Zhang, J. , Wang, M. & Golding, I. (2016). Measurement of gene regulation in individual cells reveals rapid switching between promoter states. Science 351, 1218–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidaway‐Lee, K. , Costa, M. J. , Rand, D. A. , Finkenstadt, B. & Penfield, S. (2014). Direct measurement of transcription rates reveals multiple mechanisms for configuration of the Arabidopsis ambient temperature response. Genome Biology 15, R45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner, S. O. , Sepúlveda, L. A. , Xu, H. & Golding, I. (2013). Measuring mRNA copy number in individual Escherichia coli cells using single‐molecule fluorescent in situ hybridization. Nature Protocols 8, 1100–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skupsky, R. , Burnett, J. C. , Foley, J. E. , Schaffer, D. V. & Arkin, A. P. (2010). HIV promoter integration site primarily modulates transcriptional burst size rather than frequency. PLoS Computational Biology 6, e1000952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So, L. H. , Ghosh, A. , Zong, C. , Sepulveda, L. A. , Segev, R. & Golding, I. (2011). General properties of transcriptional time series in Escherichia coli. Nature Genetics 43, 554–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavreva, D. A. , Wiench, M. , John, S. , Conway‐Campbell, B. L. , McKenna, M. A. , Pooley, J. R. , Johnson, T. A. , Voss, T. C. , Lightman, S. L. & Hager, G. L. (2009). Ultradian hormone stimulation induces glucocorticoid receptor‐mediated pulses of gene transcription. Nature Cell Biology 11, 1093–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan, D. , Pinel, C. , Pinhal, S. , Cinquemani, E. , Geiselmann, J. & de Jong, H. (2015). Inference of quantitative models of bacterial promoters from time‐series reporter gene data. PLoS Computational Biology 11, e1004028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung, M. H. , Guertin, M. J. , Baek, S. & Hager, G. L. (2014). DNase footprint signatures are dictated by factor dynamics and DNA sequence. Molecular Cell 56, 275–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter, D. M. , Molina, N. , Gatfield, D. , Schneider, K. , Schibler, U. & Naef, F. (2011). Mammalian genes are transcribed with widely different bursting kinetics. Science 332, 472–474. [DOI] [PubMed] [Google Scholar]
- Taniguchi, Y. , Choi, P. J. , Li, G. W. , Chen, H. , Babu, M. , Hearn, J. , Emili, A. & Xie, X. S. (2010). Quantifying E. coli proteome and transcriptome with single‐molecule sensitivity in single cells. Science 329, 533–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tantale, K. , Mueller, F. , Kozulic‐Pirher, A. , Lesne, A. , Victor, J. M. , Robert, M. C. , Capozi, S. , Chouaib, R. , Backer, V. , Mateos‐Langerak, J. , Darzacq, X. , Zimmer, C. , Basyuk, E. & Bertrand, E. (2016). A single‐molecule view of transcription reveals convoys of RNA polymerases and multi‐scale bursting. Nature Communications 7, 12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teves S. S., An, L. , Bhargava‐Shah, A. , Xie, L. , Darzacq, X. & Tjian, R. (2018). A stable mode of bookmarking by TBP recruits RNA Polymerase II to mitotic chromosomes. eLife 7, e35621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, M. C. & Chiang, C. M. (2006). The general transcription machinery and general cofactors. Critical Reviews in Biochemistry and Molecular Biology 41, 105–178. [DOI] [PubMed] [Google Scholar]
- Torres‐Padilla, M.‐E. & Chambers, I. (2014). Transcription factor heterogeneity in pluripotent stem cells: a stochastic advantage. Development 141, 2173–2181. [DOI] [PubMed] [Google Scholar]
- Tripathi, T. & Chowdhury, D. (2008). Transcriptional bursts: a unified model of machines and mechanisms. EPL 84, 68004. [Google Scholar]
- Vera, M. , Biswas, J. , Senecal, A. , Singer, R. H. & Park, H. Y. (2016). Single‐cell and single‐molecule analysis of gene expression regulation. Annual Review of Genetics 50, 267–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. , Liu, F. , Li, J. & Wang, W. (2014). Reconciling the concurrent fast and slow cycling of proteins on gene promoters. Journal of the Royal Society Interface 11, 20140253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. , Liu, F. & Wang, W. (2012). Dynamic mechanism for the transcription apparatus orchestrating reliable responses to activators. Scientific Reports 2, 422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. , Liu, F. & Wang, W. (2016). Kinetics of transcription initiation directed by multiple cis‐regulatory elements on the glnAp2 promoter. Nucleic Acids Research 44, 10530–10538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weake, V. M. & Workman, J. L. (2010). Inducible gene expression: diverse regulatory mechanisms. Nature Reviews Genetics 11, 426–437. [DOI] [PubMed] [Google Scholar]
- Xu, H. , Skinner, S. O. , Sokac, A. M. & Golding, I. (2016). Stochastic kinetics of nascent RNA. Physical Review Letters 117, 128101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao, J. (2017). Imaging transcriptional regulation of eukaryotic mRNA genes: advances and outlook. Journal of Molecular Biology 429, 14–31. [DOI] [PubMed] [Google Scholar]
- Yudkovsky, N. , Ranish, J. A. & Hahn, S. (2000). A transcription reinitiation intermediate that is stabilized by activator. Nature 408, 225–229. [DOI] [PubMed] [Google Scholar]
- Yunger, S. , Rosenfeld, L. , Garini, Y. & Shav‐Tal, Y. (2013). Quantifying the transcriptional output of single alleles in single living mammalian cells. Nature Protocols 8, 393–408. [DOI] [PMC free article] [PubMed] [Google Scholar]


