Abstract
Background
Reports of hepatitis B virus (HBV) reactivation in solid tumors are very limited, and their frequencies and risk factors were previously unknown.
Aim
To evaluate the prevalence and risk factors of HBV reactivation in patients with solid tumors with resolved HBV infection.
Methods
All 1088 patients with solid tumors were assessed for eligibility; 251 patients had resolved HBV infection (negative for HBs antigen and positive for anti‐HBc antibody and/or positive for anti‐HBs antibody), and HBV‐DNA was assessed for 243 of these patients in whom we analyzed the prevalence of HBV reactivation. Risk factors for HBV reactivation were exploratorily evaluated by analysis of a case–control study.
Results
The prevalence of HBV‐DNA reactivation was 2.1% (95% confidence interval [CI], 0.3–3.9%). We did not observe any exacerbation of HBV‐DNA by early intervention. A low anti‐HBs antibody titer (<10.0 mIU/mL) and high average daily dexamethasone dose (>1.0 mg/day) were high risk factors, with odds ratios of 5.94 (95% CI, 1.15–30.6, P = 0.03) and 8.69 (95% CI, 1.27–58.8, P = 0.02), respectively.
Conclusion
HBV reactivation in solid tumor patients was relatively rare. Therefore, risk factors that can identify targets for HBV screening must be determined in future studies.
Keywords: de novo hepatitis, HBs antibody, resolved HBV infection, solid tumor, steroid
1. INTRODUCTION
De novo hepatitis B, in which the hepatitis B virus (HBV) is reactivated and hepatitis develops due to chemotherapy or immunosuppressive therapy performed in patients with HBV, is a serious problem. Individuals with resolved HBV infection are negative for HBs antigen (HBsAg) but positive for anti‐HBc antibody (HBcAb) and/or anti‐HBs antibodies (HBsAb). Therefore, they are considered clinically cured. However, that HBV remains in hepatocytes can be reactivated by immunosuppression of the host with chemotherapy. Anti‐CD20 antibodies and the administration of steroids for hematopoietic tumors are high risk factors for de novo hepatitis B.1 This condition has even higher rates of fulmination and mortality than normal hepatitis B infection.2 It is important to perform HBV screening in such patients for the prevention and early diagnosis of de novo hepatitis B.
The frequency and severity of de novo hepatitis B in patients with solid tumors have not been clarified because of the small number of relevant reports.3 Scientific societies express different views on HBV screening for solid tumors. According to the American Society of Clinical Oncology (ASCO) guidelines, the usefulness of HBV screening for solid tumors is unclear.4 In the European Association for the Study of the Liver (EASL) guidelines, however, HBV screening for all patients with solid tumors is recommended.5 The cost of HBV‐DNA monitoring is also a problem.6 A consensus has not been reached regarding appropriate screening and monitoring.
In clinical practice, the HBV screening rate in Canada, the United States and Australia is as low as 14% to 19%.6, 7, 8 In Japan, which is one of the HBV‐endemic areas, 23.2% of the population has resolved HBV infection,9 but the HBV screening rate is only 20.4%.10
In patients with solid tumors with resolved HBV infection, it is necessary to identify a group with high risk and increase their screening rate to prevent de novo hepatitis B. Since 2012, our hospital has been conducting HBV screening in accordance with the Japanese de novo hepatitis B guidelines.11 We conducted a retrospective study to evaluate the reactivation prevalence and risk factors in patients with resolved HBV infection.
2. MATERIALS AND METHODS
2.1. Study population and design
This retrospective study was conducted at the Kobe City Medical Center General Hospital. At the hospital, although the measurement rate of HBcAb and HBsAb for solid tumor patients with systemic chemotherapy was 90.0%, the measurement rate of HBV‐DNA for patients with resolved HBV infection was 96.8%. Patients who were eligible for enrolment had solid tumors, were over 20 years old, and had started systemic chemotherapy between September 2012 and October 2014. We analyzed data from patients who had resolved HBV infection (negative for HBsAg and positive for HBcAb and/or positive for HBsAb) in October 2015.
At the hospital, HBV screening was conducted according to the Japanese de novo hepatitis B guidelines. In HBsAg‐negative patients (<0.05 IU/mL), we measured HBcAb and HBsAb. In HBcAb‐positive (sample/cutoff [S/CO] ratio ≥1.0) and HBsAb‐positive (≥10.0 mIU/mL) patients, HBV‐DNA was measured. If HBV‐DNA was positive, we consulted a hepatologist. If HBV‐DNA was negative, the HBV‐DNA was monitored once every 1–2 months. If HBV‐DNA became positive during monitoring, we promptly consulted a hepatologist.
Figure 1 shows the flow diagram from assessment for eligibility to enrollment. HBsAg levels were measured in all 1088 patients with solid tumors. Nineteen patients were positive. In 90 patients, neither HBcAb nor HBsAb was measured. Among 979 patients who were negative for HBsAg and who underwent measurement of HBcAb and HBsAb, 251 were positive for HBcAb and/or HBsAb. This group consisted of patients with resolved HBV infection, and HBV‐DNA was measured in 243 of these patients. We analyzed the prevalence and risk factors of HBV reactivation in these 243 patients.
Figure 1.
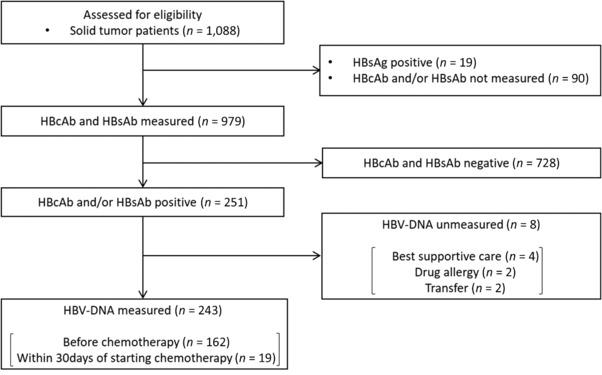
Flow diagram of patient selection. This figure shows the flow diagram from assessment for eligibility to enrollment. HBV, hepatitis B virus; HBsAg, HBs antigen; HBcAb, anti‐HBc antibody; HBsAb, anti‐HBs antibody
2.2. Outcome and risk factor
The primary outcome of this study was determining the prevalence of HBV reactivation, which was measured as the percentage of patients with resolved HBV infection in whom HBV‐DNA was reactivated. Risk factors for HBV reactivation were explored through analysis of a case–control study. Of 243 patients with resolved HBV infection, individuals in whom HBV‐DNA was reactivated were considered patient cases and those in whom it was not reactivated were controls. Plasma HBV‐DNA levels were determined with the TaqMan real‐time polymerase chain reaction (RT‐PCR) assay. Patients with ≥1.3 log IU/mL or unquantifiable patients with <1.3 log IU/mL (detection of amplification reaction signal) were classified as positive for HBV‐DNA. A positive conversion of HBV‐DNA was defined as HBV reactivation. In daily clinical practice, dexamethasone is intermittently administered for 1–5 days during each cycle of chemotherapy. To objectively evaluate the dexamethasone dose, the average daily dose was calculated as the total dexamethasone dose divided by the length of the chemotherapy duration in days.
2.3. Statistical analysis
The HBV reactivation prevalence was estimated as the number of HBV reactivation cases divided by the total number of patients with resolved HBV infection (negative for HBsAg and positive for HBcAb and/or positive for HBsAb). The 95% confidence interval (95% CI) of the HBV reactivation prevalence was calculated by the score test.
As a secondary endpoint, risk factors for HBV reactivation were evaluated by Pearson's χ 2 test. The significance level was set at P ≤ 0.05. In addition, 95% CIs were estimated by point estimation of the odds ratio and the score test. If the cell frequency was 0 in the two‐way table, 0.5 was added to each cell, and, Pearson's χ 2 test was performed with a significance level of 5% on both sides.
In this study, because the outcome event was very rare, confounders were not adjusted for. In addition, because this study is exploratory, we did not adjust the multiplicity in the assessment of risk factors either. All analysis of the background factors was performed using SPSS 24.0 (SPSS Inc., Chicago, IL, USA) and SAS 9.4 (SAS Institute Inc., Cary, NC, USA).
2.4. Ethics
The study was done in accordance with the Declaration of Helsinki and the regulations of the Japanese Ministry of Health, Labor and Welfare. The study protocol was approved by the ethics committee of Kobe City Medical Center General Hospital.
In this study, only data from daily clinical practice were collected and evaluated. Based on the Guidelines for Epidemiological Research,12 we obtained consent by disclosing research information for opting‐out.
3. RESULTS
3.1. Background of patients with resolved HBV infection
Table 1 shows the baseline characteristics of the 243 patients with resolved HBV infection who underwent HBV‐DNA monitoring. The median time from starting chemotherapy to the end of observation was 26.2 months. The median HBcAb titer was 8.0 S/CO, and the median HBsAb titer was 53.3 mIU/mL. A total of 201 patients (82.7%) received dexamethasone, and the median average daily dexamethasone dose was 0.71 mg/day.
Table 1.
Characteristics of 243 patients with resolved HBV infection
| Characteristic | Total patients |
|---|---|
| All patients | 243 (100.0%) |
| Age | |
| Median (range) | 69 years (25–87) |
| Sex | |
| Male | 144 (59.3%) |
| Type of cancer | |
| Respiratory | 82 (33.7%) |
| Gastrointestinal | 67 (27.6%) |
| Gynecological | 34 (14.0%) |
| Urological | 31 (12.8%) |
| Breast | 15 (6.2%) |
| Othersa | 14 (5.8%) |
| Chemotherapy regimen | |
| Platinum containing | 161 (66.3%) |
| Anthracycline containing | 25 (10.3%) |
| Observation period | |
| Median (range) | 26.2 months (12.2–37.4) |
| HBcAb titer | |
| Median (range) | 8.0 S/CO (0.0–53.6) |
| HBsAb titerb | |
| Median (range) | 53.3 mIU/mL (0.0 to ≥1000) |
| Dexamethasone | |
| Patients | 201 (82.7%) |
| Total dose | |
| Median (range) | 95.7 mg (0–897.2) |
| Average dosec | |
| Median (range) | 0.71 mg/day (0–8.25) |
| >1.0 mg/day | 79 (32.5%) |
Abbreviations: HBcAb, anti‐HBc antibody; HBsAb, anti‐HBs antibody; HBV, hepatitis B virus; S/CO, sample/cutoff ratio.
Head and neck, 12 (4.9%); dermatological, 1 (0.4%); brain, 1 (0.4%).
HBsAb titer ≥1000 was calculated as 1000.
The average daily dexamethasone dose was calculated as the total dexamethasone dose divided by the length of the chemotherapy duration in days.
Of the 243 patients who underwent HBV‐DNA measurement, HBV‐DNA was measured before starting chemotherapy in 162 patients (66.7%) and within 30 days of starting chemotherapy in 19 patients (7.8%).
3.2. Prevalence and details of HBV‐DNA reactivation patients
HBV reactivation was found in five patients and the prevalence of the HBV‐DNA reactivation was 2.1% (95% CI, 0.3–3.9%). In these patients (patients 1–5), we clearly observed the positive conversion of HBV‐DNA during chemotherapy (Table 2). In addition, two other patients were positive for HBV‐DNA. One patient was positive before starting chemotherapy (patient 6), and another patient was positive 3 months after starting chemotherapy. No measured data were available before starting chemotherapy for one patient (patient 7). The prevalence of HBV‐DNA positivity was 2.9% (seven patients).
Table 2.
Details of seven HBV‐DNA‐positive patients
| Interventions | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient No. | Age | Sex | Type of cancer | Regimen | Period until activation (months) | HBV‐DNA titer (peak) (log IU/mL) | NAA | CT discontinuation | Clinical course | Total DEX dose (mg) | DEX average dose per day (mg/day) |
| 1 | 63 | F | Gyne | PAC +CBDCA | 4 | <1.3 | − | No | Better | 140.4 | 1.5 |
| 2 | 72 | M | Resp | IRI +CDDP | 7 | <1.3 | − | Yes | Stable | 66 | 2.6 |
| 3 | 74 | M | Resp | BEV +PEM +CDDP | 6 | <1.3 | − | No | Stable | 159.9 | 2.9 |
| 4 | 76 | M | Gastr | Cape +L‐OHP | 12 | 1.4 | + | Yes | Better | 26.4 | 0.3 |
| 5 | 64 | M | Resp | ETP +CDDP | 1 | 1.4 | + | Yes | Better | 112.2 | 3.0 |
| 6 | 69 | F | Gyne | PAC +CBDCA | 0a | <1.3 | − | Yes | Better | 430.2 | 1.3 |
| 7 | 81 | M | Resp | GEM +CDDP | 3b | < 1.3 | − | No | Stable | 156.4 | 1.5 |
Abbreviations: BEV, bevacizumab; Cape, capecitabine; CBDCA, carboplatin; CDDP, cisplatin; CT, chemotherapy; DEX, dexamethasone; ETP, etoposide; Gastr, gastrointestinal; GEM, gemcitabine; Gyne, gynecological; IRI, irinotecan; L‐OHP, oxaliplatin; NAA, nucleic acid analogues; PAC, paclitaxel; PEM, pemetrexed; Resp, respiratory.
Positive for HBV‐DNA before chemotherapy.
No data on HBV‐DNA before chemotherapy.
In the clinical course, “Stable” and “Better” mean no changed and decreased HBV‐DNA levels, respectively. The average DEX dose per day was calculated as the total dexamethasone dose divided by the length of the chemotherapy duration in days.
Two HBV‐DNA‐positive patients (patients 4 and 5) had quantifiable levels (≥1.3 log IU/mL) of HBV‐DNA. In these patients, chemotherapy was discontinued and nucleic acid analogs were administered. We observed HBV‐DNA negative conversion. The other five HBV‐DNA‐positive patients (patients 1, 2, 3, 6 and 7) had unquantifiable levels (<1.3 log IU/mL). In these patients, we did not administer nucleic acid analogs to all the patients, and chemotherapy was continued in three patients (patients 1, 3 and 7). We did not observe any exacerbation of HBV‐DNA.
The period from starting chemotherapy to HBV‐DNA reactivation ranged from 1 to 12 months. Patient 5 turned positive 1 month after starting chemotherapy, and patient 4 turned positive 8 months after ending chemotherapy.
3.3. Risk factors for HBV reactivation
Figure 2 shows the results of the examination of risk factors for HBV reactivation by the χ 2 test. A low HBsAb titer (<10.0 mIU/mL) and high average daily dexamethasone dose (>1.0 mg/day) were high risk factors, with odds ratios of 5.94 (95% CI, 1.15–30.6, P = 0.03) and 8.69 (95% CI, 1.27–58.8, P = 0.02), respectively. When the 243 patients were grouped according to these risk factors, the HBV reactivation prevalence for high risk (n = 13, with both factors present), moderate risk (n = 104, with one factor present) and low risk (n = 126, with neither factor present) cases were 15.4% (2/13), 2.9% (3/104) and 0.0% (0/126), respectively.
Figure 2.
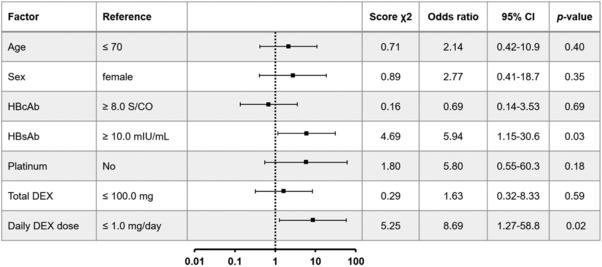
Odds ratio for HBV reactivation. The forest plot shows the odds ratios and 95% CIs of clinical parameters calculated by the χ 2 test for HBV reactivation. The proportion of patients in each reference group were 58.4% (≤70 years old, 48.6% (female), 51.0% (HBcAb ≥8.0 S/CO), 73.3% (HBsAb ≥15.0 mIU/mL), 33.7% (non‐platinum regimen), 51.9% (total DEX ≤100 mg) and 32.5% (daily DEX dose ≤1.0 mg/day). HBsAg, HBs antigen; HBcAb, anti‐HBc antibody; platinum, chemotherapy regimen containing platinum; DEX, dexamethasone; total DEX, the total DEX dose; daily DEX dose, calculated as the total dexamethasone dose divided by the length of the chemotherapy duration in days
4. DISCUSSION
Reports of HBV reactivation and de novo hepatitis B in solid tumors are very limited, and their frequencies and risk factors were previously unknown.13 This study enrolled 243 patients. To the best of our knowledge, this is the largest study to evaluate the risk factors in detail of de novo hepatitis B in patients with solid tumors with resolved HBV. The enrolled patients had various types of tumors, including respiratory, gastrointestinal, gynecological, breast and other cancers. The prevalence of HBV‐DNA reactivation was 2.1%, and the prevalence of HBV‐DNA positivity was 2.9%. HBV reactivation in solid tumor patients was relatively rare. Therefore, identifying risk factors was necessary to identify high‐risk cases. We showed that the HBsAb titer (<10 mIU/mL) and average daily dexamethasone dose (>1.0 mg/day) were significantly associated with HBV reactivation. Between high‐ and low‐risk cases, a large difference in HBV reactivation prevalence was observed.
Similar results have been reported in studies on HBV reactivation in patients with resolved HBV infection. Kusumoto et al. conducted a multivariate analysis of HBV reactivation in untreated CD20‐positive B‐cell lymphoma. They found that age, type of lymphoma, HBsAb titer and the HBV‐DNA quantification value were risk factors.14 Fukuda et al. examined the risk factors of HBV reactivation in patients with rheumatoid arthritis. They found that age, HBsAb titer and the administration of prednisolone were associated with HBV reactivation.15 A low HBsAb titer was commonly identified as a risk factor in several studies. In a meta‐analysis, Paul et al. reported that negative HBsAb was associated with HBV reactivation in patients with hematologic cancer.16 A low HBsAb titer may be due to a fragile immune response against HBV. Regarding the chemotherapy regimen, all patients with HBV reactivation had received chemotherapy containing platinum, which was not associated with HBV reactivation in this study. In the aforementioned study, there was no detectable association between immunosuppressants, such as methotrexate, and HBV reactivation in patients with rheumatoid arthritis.15 In general, the intensity of chemotherapy for solid tumors may be lower than that for hematologic cancer. In this study, dexamethasone (≥1.0 mg/day) was a risk factor. Preclinical studies have reported that steroids directly stimulate HBV replication and gene expression.17, 18 Based on these findings, we speculate that the steroid dose is more likely than the chemotherapy regimen to be a factor in HBV reactivation in patients with solid tumors. For chemotherapy regimens with a high or moderate emetic risk such as platinum‐containing therapeutics, intermittent administration of 4–8 mg dexamethasone for 3–5 days during each cycle of the chemotherapy is recommended in each country's guidelines.19, 20, 21 In other words, highly and moderately emetic chemotherapies are considered to be high risk for HBV reactivation from the results of this study.
In this study, the prevalence of HBV reactivation was extremely low in patients who did not have the above risk factors. However, some patients may be overlooked during HBV screening. Day et al. reported that HBV screening for all patients before chemotherapy according to current guidelines was not cost‐effective for patients with solid tumors.6 Therefore, targeted screening and monitoring should be considered. Reactivation was confirmed in a certain proportion of patients with low HBsAb titers and high doses of steroids. However, this study showed that appropriate screening and monitoring prevent the onset and severity of hepatitis.
This study had several limitations. First, information on HBV vaccination history was not obtained. In general, only HBsAb‐positive patients are considered positive due to vaccination and are not at risk for HBV reactivation. However, because HBV reactivation has been reported in even these patients,22, 23 we included such patients in HBV screening. HBV vaccination has not been incorporated into universal vaccination programs in Japan, and there are thought to be very few patients with a history of HBV vaccination in Japan. Second, it was not possible to screen some patients before starting chemotherapy. Therefore, we cannot exclude the possibility that some patients became HBV positive before screening and subsequently became HBV negative. However, the period during which screening was delayed was short, and we believe that the likelihood of such patients progressing without any intervention was very low. Third, confounding factors may have influenced HBV reactivation, because this was a retrospective observational study and the incidence of HBV reactivation was small.
In conclusion, five patients (2.1%) showed reactivation of HBV‐DNA, and no patients developed hepatitis due to early intervention. We showed that HBsAb titer and average daily steroid dose were associated with HBV reactivation. In the future, risk factors that can identify targets for HBV screening must be determined by prospective studies.
CONFLICT OF INTEREST
All authors have declared no conflict of interest.
ACKNOWLEDGMENTS
We would like to thank the members of the Pharmacy Department, Kobe City Medical Center General Hospital, for their survey of the patients who needed HBV screening.
FUNDING
The work had no specific funding.
Kotake T, Satake H, Okita Y, et al. Prevalence and risk factors of hepatitis B virus reactivation in patients with solid tumors with resolved HBV infection. Asia‐Pac J Clin Oncol. 2019;15:63–68. 10.1111/ajco.13050
Present address: Takeshi Kotake, Breast Surgery Department, Kyoto University Hospital, Kyoto, Japan
REFERENCES
- 1. Umemura T, Tanaka E, Kiyosawa K, Kumada H. Mortality secondary to fulminant hepatic failure in patients with prior resolution of hepatitis B virus infection in Japan. Clin infect Dis. 2008;47(5):e52–e56. [DOI] [PubMed] [Google Scholar]
- 2. Oketani M, Ido A, Uto H, Tsubouchi H. Prevention of hepatitis B virus reactivation in patients receiving immunosuppressive therapy or chemotherapy. Hepatol Res. 2012;42(7):627–636. [DOI] [PubMed] [Google Scholar]
- 3. Yeo W, Chan TC, Leung NW, et al. Hepatitis B virus reactivation in lymphoma patients with prior resolved hepatitis B undergoing anticancer therapy with or without rituximab. J Clin Oncol. 2009;27(4):605–611. [DOI] [PubMed] [Google Scholar]
- 4. Hwang JP, Somerfield MR, Alston‐Johnson DE, et al. Hepatitis B virus screening for patients with cancer before therapy: american Society of Clinical Oncology Provisional Clinical Opinion Update. J Clin Oncol. 2015;11(4):e487–e489. [DOI] [PubMed] [Google Scholar]
- 5. EASL clinical practice guidelines: management of chronic hepatitis B virus infection. J Hepatol. 2012;57(1):167–185. [DOI] [PubMed] [Google Scholar]
- 6. Day FL, Karnon J, Rischin D. Cost‐effectiveness of universal hepatitis B virus screening in patients beginning chemotherapy for solid tumors. J Clin Oncol. 2011;29(24):3270–3277. [DOI] [PubMed] [Google Scholar]
- 7. Lee R, Vu K, Bell CM, Hicks LK. Screening for hepatitis B surface antigen before chemotherapy: current practice and opportunities for improvement. Curr Oncol (Toronto, Ont). 2010;17(6):32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang Y, Luo XM, Yang D, et al. Testing for hepatitis B infection in prospective chemotherapy patients: a retrospective study. World J Gastroenterol. 2013;19(6):923–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tanaka J, Kumagai J, Katayama K, et al. Sex‐ and age‐specific carriers of hepatitis B and C viruses in Japan estimated by the prevalence in the 3,485,648 first‐time blood donors during 1995–2000. Intervirology. 2004;47(1):32–40. [DOI] [PubMed] [Google Scholar]
- 10. Ikeda M, Yamamoto H, Kaneko M, et al. Screening rate for hepatitis B virus infection in patients undergoing chemotherapy in Japan. Int J Clin Oncol. 2016;21(6):1162–1166. [DOI] [PubMed] [Google Scholar]
- 11. Tsubouchi H, KH, Kiyosawa K. Prevention of immunosuppressive therapy or chemotherapy‐induced reactivation of hepatitis B virus infection‐Joint report of the Intractable Liver Diseases Study Group of Japan and the Japanese Study Group of the Standard Antiviral Therapy for Viral Hepatitis. Acta Hepatol Jpn. 2009;50:5. [Google Scholar]
- 12. Ministry of Education C, Sports, Science and Technology and Ministry of Health, Labour and Welfare in Japan. Ethical Guideline for Epidemiological Research. 2002. https://www.niph.go.jp/wadai/ekigakurinri/guidelines.pdf.
- 13. Voican CS, Mir O, Loulergue P, et al. Hepatitis B virus reactivation in patients with solid tumors receiving systemic anticancer treatment. Ann Oncol. 2016;27(12):2172–2184. [DOI] [PubMed] [Google Scholar]
- 14. Kusumoto S, Tanaka Y, Suzuki R, et al. Monitoring of hepatitis B Virus (HBV) DNA and risk of HBV reactivation in B‐cell lymphoma: a prospective observational study. Clin Infect Dis. 2015;61(5):719–729. [DOI] [PubMed] [Google Scholar]
- 15. Fukuda W, Hanyu T, Katayama M, et al. Incidence of hepatitis B virus reactivation in patients with resolved infection on immunosuppressive therapy for rheumatic disease: a multicentre, prospective, observational study in Japan. Ann Rheum Dis. 2016;76(6):1051–1056. [DOI] [PubMed] [Google Scholar]
- 16. Paul S, Dickstein A, Saxena A, et al. Role of surface antibody in hepatitis B reactivation in patients with resolved infection and hematologic malignancy: a meta‐analysis. Hepatology. 2017;66(2):379–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tur‐Kaspa R, Burk RD, Shaul Y, Shafritz DA. Hepatitis B virus DNA contains a glucocorticoid‐responsive element. Proc Natl Acad Sci U S A. 1986;83(6):1627–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chou CK, Wang LH, Lin HM, Chi CW. Glucocorticoid stimulates hepatitis B viral gene expression in cultured human hepatoma cells. Hepatology. 1992;16(1):13–18. [DOI] [PubMed] [Google Scholar]
- 19. Roila F, Molassiotis A, Herrstedt J, et al. MASCC and ESMO guideline update for the prevention of chemotherapy‐ and radiotherapy‐induced nausea and vomiting and of nausea and vomiting in advanced cancer patients. Ann Oncol. 2016;27(suppl 5):v119–v133. [DOI] [PubMed] [Google Scholar]
- 20. Takeuchi H, Saeki T, Aiba K, et al. Japanese Society of Clinical Oncology clinical practice guidelines 2010 for antiemesis in oncology: executive summary. Int J Clin Oncol. 2016;21(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hesketh PJ, Bohlke K, Lyman GH, et al. Antiemetics: american Society of Clinical Oncology Focused Guideline Update. J Clin Oncol. 2016;34(4):381–386. [DOI] [PubMed] [Google Scholar]
- 22. Awerkiew S, Daumer M, Reiser M, et al. Reactivation of an occult hepatitis B virus escape mutant in an anti‐HBs positive, anti‐HBc negative lymphoma patient. J Clin Virol. 2007;38(1):83–86. [DOI] [PubMed] [Google Scholar]
- 23. Ferreira R, Carvalheiro J, Torres J, et al. Fatal hepatitis B reactivation treated with entecavir in an isolated anti‐HBs positive lymphoma patient: a case report and literature review. Saudi J Gastroenterol. 2012;18(4):277–281. [DOI] [PMC free article] [PubMed] [Google Scholar]


