Abstract
Fungal pathogen Candida albicans has a complex cell wall consisting of an outer layer of mannans and an inner layer of β-glucans and chitin. The fungal cell wall is the primary target for antifungals and is recognized by host immune cells. Environmental conditions such as carbon sources, pH, temperature, and oxygen tension can modulate the fungal cell wall architecture. Cellular signaling pathways, including the mitogen-activated protein kinase (MAPK) pathways, are responsible for sensing environmental cues and mediating cell wall alterations. Although iron has recently been shown to affect β-1,3-glucan exposure on the cell wall, we report here that iron changes the composition of all major C. albicans cell wall components. Specifically, high iron decreased the levels of mannans (including phosphomannans) and chitin; and increased β-1,3-glucan levels. These changes increased the resistance of C. albicans to cell wall-perturbing antifungals. Moreover, high iron cells exhibited adequate mitochondrial functioning; leading to a reduction in accumulation of lactate that signals through the transcription factor Crz1 to induce β-1,3-glucan masking in C. albicans. We show here that iron-induced changes in β-1,3-glucan exposure are lactate-dependent; and high iron causes β-1,3-glucan exposure by preventing lactate-induced, Crz1-mediated inhibition of activation of the fungal MAPK Cek1. Furthermore, despite exhibiting enhanced antifungal resistance, high iron C. albicans cells had reduced survival upon phagocytosis by macrophages. Our results underscore the role of iron as an environmental signal in multiple signaling pathways that alter cell wall architecture in C. albicans, thereby affecting its survival upon exposure to antifungals and host immune response.
Keywords: Candida albicans, iron, cell wall, lactic acid, mitochondrial membrane potential, ATP, mitogen-activated protein kinase (MAPK), signaling, cytokine, phagocytosis
Iron is an essential transition metal that is required for several cellular processes including synthesis of heme and iron-sulfur (Fe-S) cluster-containing proteins, amino acids, DNA, lipids, and sterols (1, 2). In excess, however, iron can generate reactive oxygen species (ROS) as a biproduct of metabolic processes via Haber–Weiss/Fenton reaction (3). Therefore, despite its importance in cellular functions, iron has reduced bioavailability in the mammalian host (4). This serves the dual purpose of restricting growth of microbial pathogens by withholding an essential nutrient (nutritional immunity) (5) and protection from toxicity caused by iron-mediated oxidative damage.
Opportunistic fungal pathogen Candida albicans typically lives as a benign commensal on mucosal and cutaneous surfaces in humans. Iron plays an important role in its transition between virulent and commensal lifestyles (6, 7). Virulence of C. albicans can be traced to several properties. These include: adhesion to host cells, biofilm formation, and morphological transition from yeast-to-hyphae (8). Fungal cell wall and membrane harbor various structural components and proteins that facilitate C. albicans virulence (8); although iron has been shown to affect all major virulence traits of C. albicans (9, 10). Hence it is not surprising that iron-mediated changes to C. albicans secretome as well as cell wall proteome and cell membrane structure and composition have been previously reported (11–13). C. albicans cell wall consists of an outer layer of mannans, covalently associated with N-, O-, and ether-linked glycosylated cell wall proteins (mannoproteins), comprising 35–40% of the cell wall and an inner layer of β-glucans and chitin. β-Glucans are the major components of the inner cell wall (50–60% of the cell wall) and consist of a core structure that is largely made of β-1,3-glucans, along with a minor amount of β-1,6-glucan (14). Changes to C. albicans cell wall architecture have been observed in response to different environmental conditions. Acidic environments such as lower pH or lactate reduce mannan levels in the outer cell wall layer, whereas limitation of zinc, another transition metal like iron, increases mannan levels (15–17). On the other hand, acidic conditions reduce the thickness of the inner cell wall (18) by decreasing β-1,3-glucan levels (15). However, zinc limitation altered chitin exposure on the cell surface, without affecting β-1,3-glucan levels (17). Iron was been recently shown to affect exposure of β-1,3-glucan; however, overall effects of iron on the complex C. albicans cell wall structure remain unknown.
Cell wall remodeling affects the susceptibility of C. albicans toward various antifungals, including cell wall-perturbing (CWP) agents (18–21). CWP agents inhibit fungal growth by targeting cell wall components to disrupt cell wall integrity. Tunicamycin binds to an enzyme UDP-GlcNAc:dolichyl-phosphate N-acetylglucosamine phosphotransferase, which catalyzes the primary step of N-linked mannan synthesis (22). Interestingly, antifungal activity of tunicamycin depends on the Cek1 MAPK pathway (23) that we have previously shown to be responsive to environmental iron (10). Furthermore, zymolyase, a mixture of β-1,3-glucanases and proteases, lyses fungal cell by inhibiting β-1,3-glucan synthesis. In Saccharomyces cerevisiae, the activation of Slt2p (a homolog of C. albicans cell wall integrity MAPK Mkc1) makes cells resistant to glucanase digestion (24), whereas high iron increases Slt2p-activation in nonalbicans Candida species (25). Also, Mkc1 and Cek1 MAPK pathways play a cooperative role in regulation of cell wall architecture in C. albicans (26). Changes to C. albicans cell wall in response in host iron can therefore be consequential for the outcome of antifungal therapy.
In addition, cell wall components are also crucial for host-pathogen interaction. These components act as pathogen-associated molecular patterns (PAMPs) and stimulate immune signaling, when recognized by host pattern recognition receptors (14). β-1,3-Glucan and chitin are normally shielded by the superficial mannans to limit pathogen recognition (14). Therefore, changes in cell wall mannans can contribute to exposure of underlying β-1,3-glucan and chitin. Various environmental cues including iron can induce β-1,3-glucan masking to limit recognition of fungal cells and stimulation of cytokine production by host immune cells. However, MAPK Cek1 activation by phosphorylation (Cek1-P) is crucial for β-1,3-glucan unmasking or exposure. Despite this, numerous studies focusing on the effect of environmental conditions on β-1,3-glucan masking have not examined the status of Cek1 activation under those respective conditions and how it may contribute to β-1,3-glucan exposure levels.
Here we reveal for the first time that changes in environmental iron modulate the levels of all major cell wall components of C. albicans. High iron-mediated changes to the cell wall, in turn, led to an enhanced resistance to CWP agents. We further define a novel mechanism by which iron influences β-1,3-glucan exposure. We show that iron communicates with a noncanonical pathway involving lactic acid signaling through Crz1; whereby high iron limits intracellular lactate accumulation to reduce Crz1-mediated inhibition of MAPK Cek1-activation, leading to β-1,3-glucan exposure.
Results
Iron induces cell wall remodeling in C. albicans
To examine whether iron can modulate fungal cell wall components, C. albicans cells were grown in YNB-based minimal medium without added iron or with 100 μm added iron, representing comparatively limited iron medium (LIM) and higher or replete iron medium (RIM). We next compared the intracellular iron levels in C. albicans cells grown in these respective medium with intracellular iron levels of cells grown in yeast-extract peptone dextrose (YPD) medium, the most commonly used medium for fungal growth. C. albicans cells grown in our LIM had iron levels similar to YPD-grown cells, whereas cells grown in RIM showed a 97.83% increase in intracellular iron content, compared with YPD-grown cells (Fig. S1A). All three growth conditions maintained similar growth rates (Fig. S1B).
LIM- and RIM-grown C. albicans cells were then used to measure the levels of all key components of the cell wall. The cells were stained with concanavalin A (ConA, for mannan), aniline blue (AB, for β-1,3-glucan), or CFW (for chitin), and mean fluorescence intensities (MFI) were measured. We also determined the levels of acid-labile mannans (phosphomannans) by measuring the levels of alcian blue binding to the cell surface. In response to changes in environmental iron, major differences in the levels of all key cell wall components were observed (Fig. 1). RIM-grown cells with higher intracellular iron (Fig. S1A) showed significantly lower fluorescence levels for ConA binding, indicating the presence of a much thinner layer of mannans, when compared with LIM cells (Fig. 1A). Similarly, the incidence of phosphomannans was significantly lower in RIM cells (Fig. S2). The levels of β-1,3-glucan, the dominant structure of the inner cell wall, were significantly higher in RIM cells (Fig. 1B); however, like mannans, levels of chitin were significantly lower in RIM-grown cells, compared with LIM cells (Fig. 1C).
Figure 1.
Iron influences cell wall architecture. Fluorescent micrographs for WT C. albicans (CAI4), grown in LIM or RIM, stained for mannan (ConA) (A), β-1,3-glucan (AB) (B), and chitin (CFW) (C). Micrographs (scale bar = 5 μm) are representative of three independent replicate experiments. MFI for n > 150 cells for each growth condition are represented as MFI ± S.D. and error bars indicate 95% CI. Data were analyzed using Student's t test. *, p ≤ 0.05.
Because β-1,3-glucan levels in the β-glucan layer that is directly above the chitin layer were increased under high iron conditions, we also examined whether this led to a masking of chitin. Using wheat germ agglutinin (WGA) stain, a lectin that specifically binds to surface-exposed chitin, we determined that high iron RIM cells showed significantly lower exposure of chitin, compared with cells grown in LIM (Fig. S3). Thus, iron modulates all major components of the fungal cell wall, and increase in intracellular iron potentially induces reduction in synthesis of mannan and chitin, along with reduced chitin exposure, whereas increasing β-1,3-glucan synthesis.
Finally, to corroborate iron-mediated cell wall changes at a cellular structure level, we performed transmission EM to examine the ultrastructure of the cell wall of LIM and RIM cells. Micrographs of RIM cells showed a significantly thinner outer layer of mannans with a scattered fibrillar arrangement and a thicker inner layer (Fig. 2). Thus, iron changed the levels of individual cell wall components to drastically affect the cell wall at the structural level.
Figure 2.
Iron alters ultrastructure of cell wall. A, electron micrographs for ultrastructure of the cell walls of log-phase WT C. albicans (CAI4) cells, grown in LIM or RIM. Micrographs scale bar = 100 nm. B, quantification of the thickness of the mannoprotein fibril length (outer cell wall layer) and inner cell wall layer. Mean ± S.D. from 10 individual cells are presented (cell periphery of each cell was measured at 30 different points) and error bars indicate 95% CI. Data were analyzed using Student's t test. **, p ≤ 0.005 and *, p ≤ 0.05.
High iron makes C. albicans cells resistant to cell wall perturbing agents
We hypothesized that changes in C. albicans cell wall structure may influence its susceptibility to CWP agents. We thus compared the sensitivity of RIM and LIM cells to tunicamycin (inhibitor of N-linked mannosylation), zymolyase (β-1,3-glucanase), and CFW (that interferes with cross-linking of chitin). Our spot assay results revealed that compared with LIM cells, RIM cells were more resistant to tunicamycin (Fig. 3A) and CFW (Fig. 3C), and similarly, also more resistant to zymolyase, with a significant increase (14.24 ± 2.76%) in cell growth (Fig. 3B). Taken together with the effect of iron on the C. albicans cell wall (Fig. 1), these results show that high iron-induced changes to the cell wall made these cells more resistant to CWP antifungals.
Figure 3.
Iron impacts antifungal resistance. Resistance of WT C. albicans (CAI4) cells grown in LIM or RIM to cell wall perturbing agents were tested either on respective YNB-based solid media against (A) tunicamycin (1.5 μg ml−1) and (C) CFW (100 μg ml−1) by spot dilution assay after 48 h or in liquid medium against (B) zymolyase (30 units) after 90 min at 30 °C. Zymolyase sensitivity data are expressed as mean ± S.D. and presented as % growth of C. albicans (at OD600) between treated and untreated controls for LIM and RIM. Error bars indicate 95% CI. Data were analyzed by using Student's t test. *, p ≤ 0.05. All sensitivity results (spot or liquid medium assays) are representative of three independent experiments.
High iron induces β-1,3-glucan exposure by limiting intracellular l-lactate accumulation
The observed changes in cell wall components (Fig. 1), particularly mannans, are known to cause exposure of β-1,3-glucan on the cell surface (27, 28). We next tested the effect of iron on β-1,3-glucan exposure, using a monoclonal anti-β-1,3-glucan antibody and Cy3 labeling that allows for detection of exposed β-1,3-glucan in fungal cells. RIM cells displayed significantly higher levels of exposed β-1,3-glucans (1.7-fold increase), compared with LIM cells (Fig. 4A). This suggests that high iron conditions induce exposure of the β-1,3-glucan layer, most likely due to the thinner and scattered outer layer of mannan fibrils observed under high iron (Fig. 1A and 2).
Figure 4.
High iron reduces mitochondrial dysfunction and l-lactate production to induce β-1,3-glucan exposure. A, fluorescent micrographs for WT C. albicans (CAI4), grown in LIM or RIM, stained with anti-β-1,3-glucan antibody and Cy3-labeled secondary antibody for exposed β-1,3-glucan. Micrographs scale bar = 5 μm. MFI for n > 150 cells from three independent experiments for each growth condition represented as MFI ± S.D. and error bars indicate 95% CI. Data were analyzed using Student's t test. *, p ≤ 0.05. C. albicans (CAI4) cells grown in comparatively lower and higher iron conditions were examined for mitochondrial activity. B, cells were stained with JC-1, and mitochondrial membrane potential was analyzed by fluorescence microscopy and expressed as ratio of JC-1 red/green fluorescence. C, ATP was measured by the luminescence method in relative light units and normalized by total protein concentration (μg). D, cell supernatants and cell pellets were used for determination of extracellular and intracellular l-lactate concentration, respectively, by a fluorescence-based method. E, for intracellular ROS detection, cells were stained with 10 μmol liter−1 of DCFH-DA in the dark, for 30 min with gentle agitation (120 rpm) and then imaged by confocal microscope at ×100 magnification. MFI for n > 150 cells for each growth condition are represented as MFI ± S.D. and error bars indicate 95% CI. Data were analyzed using one-way ANOVA. ***, p ≤ 0.0005 and *, p < 0.05. F, fluorescent micrographs for WT C. albicans (CAI4), grown in LIM or RIM medium in presence of glucose (Glu) or lactate (Lac), stained with anti-β-1,3-glucan antibody and Cy3-labeled secondary antibody for exposed β-1,3-glucan. Micrographs scale bar = 5 μm. MFI for n > 150 cells from three independent experiments for each growth condition represented as MFI ± S.D. and error bars indicate 95% CI. Data were analyzed using Student's t test. *, p ≤ 0.05; ns, no significance.
A previous study showed triggering of β-1,3-glucan masking (thus reducing its exposure) through a lactate-mediated signaling pathway, when C. albicans cells were grown in presence of lactate as carbon source (16). In this study, we used glucose as the sole carbon source. However, cells with comparatively lower iron can exhibit a reduction in mitochondrial function (29) because many of the proteins involved in oxidative phosphorylation are Fe-S cluster or heme iron-containing proteins. This, in turn, can shift cellular metabolism in these cells toward a fermentation-like process leading to enhanced lactic acid accumulation (30); whereas RIM cells with higher iron would instead show a reduction in lactate accumulation. We thus hypothesized that our glucose-grown RIM cells might have enhanced mitochondrial functioning that can limit lactate-mediated β-1,3-glucan masking. To examine this, we first tested mitochondrial function by measuring mitochondrial membrane potential and ATP levels in our glucose-grown C. albicans cells. High iron cells showed a 1.34-fold increase in mitochondrial membrane potential (Fig. 4B) and a 1.6-fold increase in cellular ATP levels (Fig. 4C), indicating enhanced mitochondrial activity. To confirm if this, in turn, led to reduction in l-lactate (a dominant form of lactic acid) accumulation intracellularly, we also measured the levels of intracellular l-lactate, as well as extracellular levels (to account for release from the cells, if any). l-Lactate concentration was significantly lower under high iron RIM cells, compared with LIM-grown cells. Intracellular and extracellular concentrations were 2.7-fold (82.45 versus 226.5 μg/ml) and 28.8-fold (1.64 versus 47.35 μg/ml) lower, respectively, in RIM cells, compared with LIM cells (Fig. 4D). Additionally, changes in environmental iron can also alter cellular ROS production. We examined ROS levels in low and high iron C. albicans cells. As expected, we found that ROS production was higher in high iron RIM cells, as compared LIM-grown cells (Fig. 4E).
We next sought to conform if reduction in l-lactate accumulation is indeed a contributing factor in the effect of high iron on enhanced β-1,3-glucan exposure. To understand this, we tested the effect of iron on β-1,3-glucan exposure in the presence of glucose or lactate as a sole carbon source, in parallel experiments. Interestingly, levels of β-1,3-glucan exposure were similar in lactate-grown LIM and RIM cells, with a clear loss of enhanced β-1,3-glucan exposure that was observed under high iron conditions (Fig. 4F), when glucose served as the sole carbon source (Fig. 4, A and F). As expected, both LIM and RIM cells grown on lactate also showed much higher levels of intracellular l-lactate (919.7 ± 134.7 and 791.7 ± 147.4, respectively; Fig. S4). Thus, enhanced intracellular l-lactate accumulation in LIM-grown cells with glucose as the sole carbon source (Fig. 4D), as well as exogenous lactate regardless of iron levels, both led to β-1,3-glucan masking (Fig. 4, A and F, respectively). This showed that lactate dominates over the effect of high iron on β-1,3-glucan exposure, and thus high iron-induced exposure in glucose-grown cells is a result of reduction in intracellular l-lactate accumulation.
Effect of iron on changes in β-1,3-glucan exposure require Crz1
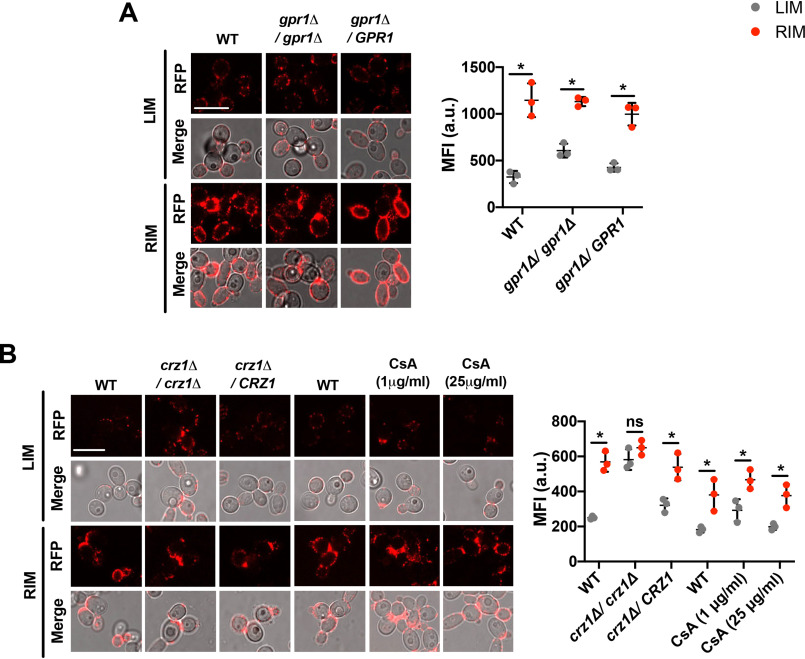
A role for lactate sensor (Gpr1) and transcriptional factor Crz1 in exogenous lactate-induced β-1,3-glucan masking has been demonstrated before (16). High iron in glucose-grown RIM C. albicans cells led to a decrease in intracellular as well as extracellular lactate, whereas glucose-grown LIM cells showed comparatively higher levels of both intra- and extracellular lactate (Fig. 4D). Hence, we next tested if the released extracellular l-lactate in LIM cells signals through Gpr1 to induce the observed β-1,3-glucan masking. We first examined β-1,3-glucan exposure levels in glucose-grown C. albicans gpr1Δ/gpr1Δ LIM and RIM cells, along with their respective parental WT and re-constituted strains (strains with reintegration of a single copy of the respective gene into the genome of the corresponding null mutant). Glucose-grown C. albicans cells lacking Gpr1 retained the effect of iron, with significant β-1,3-glucan masking observed in LIM cells (Fig. 5A). This showed that the effect of iron on levels of β-1,3-glucan exposure, in glucose-grown cells, is independent of Gpr1, and therefore, solely mediated by intracellular l-lactate. Furthermore, to examine the role of Crz1 on the effect of iron on β-1,3-glucan masking, we measured β-1,3-glucan exposure levels in glucose-grown crz1Δ/crz1Δ LIM and RIM cells, along with their respective parental WT and re-constituted strains. β-1,3-Glucan masking was blocked, resulting in β-1,3-glucan exposure, in glucose-grown LIM cells lacking Crz1; whereas the iron effect was restored to WT levels in the re-constituted strain (Fig. 5B). This suggests that enhanced intracellular l-lactate accumulation in LIM cells (Fig. 4D) mediates decrease in β-1,3-glucan exposure levels (causing masking instead), through Crz1 activation, independent of the lactate sensor Gpr1.
Figure 5.
Effect of iron on lactate-mediated changes to β-1,3-glucan exposure levels are dependent on Crz1, but independent of Gpr1 and calcineurin. A, fluorescent microscopic analysis of β-1,3-glucan exposure was performed in glucose-grown (GPR1 WT, gpr1Δ/gpr1Δ, gpr1Δ/GPR1) C. albicans cells grown in LIM or RIM medium. Micrographs scale bar = 5 μm. MFI for n > 150 cells from three independent experiments for each growth condition are represented as MFI ± S.D. and error bars indicate 95% CI. Data were analyzed using Student's t test. *, p < 0.05. B, fluorescent microscopic analysis of β-1,3-glucan exposure in glucose-grown CRZ1 WT, crz1Δ/crz1Δ, and crz1Δ/CRZ1 strains; and WT (CAI4) C. albicans cells were treated with 1 μg ml−1 (for 12 h) or 25 μg ml−1 (for 8 h) CsA in LIM or RIM. Micrographs scale bar = 5 μm. MFI for n > 150 cells from three independent experiments for each growth condition are represented as MFI ± S.D. and error bars indicate 95% CI. Data were analyzed using Student's t test. *, p < 0.05 and ns = no significance.
Also, in C. albicans, the calcineurin pathway activates Crz1 in response to calcium and cell wall stress (31). Using cyclosporine A (CsA), an inhibitor of the calcineurin pathway (32), we next examined β-1,3-glucan exposure levels in LIM or RIM WT C. albicans cells treated with 1 or 25 μg ml−1 CsA. l-Lactate–mediated β-1,3-glucan masking was not perturbed in CsA-treated LIM cells (Fig. 5B). Overall, changes in iron levels influence β-1,3-glucan masking via activation of transcriptional factor Crz1, independent of the calcineurin pathway.
High iron-induced β-1,3-glucan exposure is mediated by higher levels of Cek1 phosphorylation
Crz1 is necessary in mediating β-1,3-glucan masking caused by intracellular lactate accumulation in glucose-grown LIM C. albicans cells (Fig. 4D). On the other hand, high iron induces Cek1 activation by phosphorylation (Cek1-P) (10) that in turn is known to cause β-1,3-glucan exposure in C. albicans (33). We thus hypothesized that accumulation of higher levels of intracellular l-lactate in glucose-grown LIM cells might induce β-1,3-glucan masking by Crz1-mediated inhibition of Cek1-P. We first compared Cek1-P levels in glucose- and lactate-grown WT LIM or RIM cells. Indeed, only glucose-grown high iron RIM cells showed maximal Cek1-P, whereas Cek1-P was repressed in respective glucose-grown LIM cells or lactate-grown LIM or RIM cells (Fig. 6A), confirming lactate-mediated inhibition of Cek1-P. We next tested the role of Crz1 in mediating Cek1-P inhibition. Glucose-grown LIM cells lacking Crz1 showed de-repression of Cek1-P inhibition (Fig. 6B), suggested Crz1 inhibits Cek1-P. Together these results show that high iron-induced reduction in l-lactate accumulation decreases β-1,3-glucan masking (thus causing exposure) by limiting lactate-induced Crz1-mediated inhibition of Cek1 activation.
Figure 6.

Lactate and Crz1 signaling inhibit Cek1 phosphorylation. Cek1-P (molecular mass ≈ 42 kDa) was detected in C. albicans WT (CAI4) cells grown in the presence of glucose (Glu) or lactate (Lac) in low iron (LIM) or high iron (RIM) medium (A), and in C. albicans CRZ1 WT and crz1Δ/crz1Δ cells grown in the presence of glucose (Glu) in LIM or RIM medium (B). Normalized protein content (30 µg) was loaded in each well. The amount of total Hog1 protein (molecular mass ≈ 43 kDa) was probed as a loading control (lower panels of A and B). Proteins extracted from glucose-grown cek1Δ/cek1Δ cells (for Cek1-P detection) and hog1Δ/hog1Δ cells (for Hog1 protein detection) in LIM medium were used as negative controls. Molecular weight markers are indicated. Splicing of blots is indicated by vertical black lines. The blots represent the results of two independent experiments.
High iron-induced β-1,3-glucan exposure enhances C. albicans phagocytosis and stimulation of cytokine production
To examine if high iron-induced β-1,3-glucan exposure affects host immune response against C. albicans, we exposed primary murine bone marrow-derived macrophages (BMDMs) to glucose-grown LIM or RIM cells of WT C. albicans. Macrophage mediated phagocytosis of WT RIM-grown high iron. C. albicans cells (with higher β-1,3-glucan exposure; Fig. 4A) were significantly enhanced, with a 3.5-fold increase in phagocytic index (Fig. 7A); whereas a 3.4-fold lower survival upon phagocytosis was observed (Fig. 7B) for these fungal cells, compared with WT-LIM cells. Glucose-grown LIM cells of a C. albicans clinical strain that showed β-1,3-glucan exposure despite growth in low iron (Fig. S5) was used as a control representing C. albicans cells with low iron and exposed β-1,3-glucan. This LIM-grown clinical strain showed phagocytosis and survival similar to high iron WT-RIM cells (Fig. 7, A and B), confirming that this effect is mediated by β-1,3-glucan exposure levels.
Figure 7.
High iron enhances the host immune response. A, primary murine BMDMs were infected with WT C. albicans cells grown in LIM or RIM (along with a LIM-grown clinical strain as a control representing C. albicans cells with higher exposure of β-1,3-glucan exposure even under low iron) at an m.o.i. of 3 for 1 h at 37 °C under 5% CO2. Micrographs (A, left) showed external C. albicans cells stained with CFW (blue color) and phagocytosed C. albicans cells (unstained; shown by yellow arrows). Images represent the data derived from three independent experiments in triplicates. Micrographs scale bar = 25 μm. Phagocytic index (A, right) and survival percentage of phagocytosed C. albicans (B) were obtained from three independent experiments. The results are represented as mean ± S.D. and error bars indicate 95% CI. Data were analyzed using Student's t test. ***, p ≤ 0.0005 and **, p ≤ 0.005. C, inflammatory cytokines (IFN-γ, IL-1α, and TNF-α) and chemokine (MIG) responses from IFN-γ (50 ng ml−1) and LPS (100 ng ml−1) activated murine BMDMs to WT C. albicans cells grown in LIM or RIM medium after 6 h of co-incubation at an m.o.i. of 5, at 37 °C under 5% CO2. Mean ± S.D. for two independent experiments are presented and error bars indicate 95% CI. Data were analyzed using Student's t test. *, p < 0.05.
Furthermore, WT-RIM cells stimulated significantly higher levels of pro-inflammatory cytokine (interferon γ, IFN-γ; 1.7-fold, interleukin 1α, IL-1α; 4.6-fold, tumor necrosis factor α, TNF-α; 1.5-fold), and chemokine (monokine induced by γ interferon, MIG; 6.1-fold) production from BMDMs, as compared with WT-LIM cells (Fig. 7C). Therefore, high iron conditions promote C. albicans cells to potentially become less virulent, in terms of increased susceptibility to phagocytosis, reduced survival within phagosomes, and enhanced immune activation of macrophages.
Discussion
Iron drastically changes the fungal cell wall to affect antifungal susceptibility
High iron decreased the levels of total mannans including phosphomannans in the outer cell wall; whereas increasing and decreasing the levels of β-1,3-glucan and chitin, respectively, in the inner wall (Figs. 1 and S2). Furthermore, we show that increase in iron also causes masking of chitin on the cell surface (Fig. S3). Increase in β-1,3-glucan levels are most likely responsible for this masking because chitin is linked to β-1,3-glucan to form the core structure of the cell wall. A recent study similarly showed at the structural level that high iron decreased the thickness of the mannan-containing outer cell wall (34). Differences in growth rates can also alter cell wall composition. However, our unique growth conditions allowed us to achieve significant differences in intracellular iron levels between LIM and RIM cells, without affecting growth rates (Fig. S1, A and B). Thus, iron alone, unlike other environmental signals, can drastically alter the levels of all major C. albicans cell wall constituents.
Compositional changes to the cell wall, in turn, had dramatic effects on antifungal susceptibility. A majority of these changes can be explained by previously defined iron-mediated transcriptional events. The expression of MNT4, the mannosyltransferase gene responsible for N-mannan branching (35), is down-regulated under high iron (9). Reduction in N-mannan branching leads to a thinner mannan layer, as observed under high iron (Fig. 1A), whereas tunicamycin inhibits N-glycosylation. Thus tunicamycin treatment under high iron would limit the damage due to reduction in binding targets (Figs. 1A and 2), thus causing greater resistance. On the other hand, PHR2 and BGL2 (involved in cross-linking between β-1,3- and β-1,6-glucans) (36, 37) show an enhanced expression under high iron (9), thereby causing a high iron-mediated increase in β-1,3-glucan levels (Fig. 1B). As a result, zymolyase, an enzyme that challenges the integrity of the β-1,3-glucan layer, did not affect the growth of high iron cells that have a more robust β-1,3-glucan layer while reducing the growth of LIM cells with a thinner layer of β-1,3-glucan (Fig. 3B). In addition, an increase in β-1,3-glucan levels would limit the stimulation of over-compensatory chitin synthesis that occurs with reduction in β-1,3-glucan levels (38). This phenomenon explains the decrease in cell wall chitin content (Fig. 1C) and reduced sensitivity to chitin-binding CWF (Fig. 3C) under high iron.
Iron induces unique biochemical pathways to trigger β-1,3-glucan masking
Fungal cell wall synthesis is a metabolically demanding process. Growth under low iron or oxygen tension (hypoxia), or on nonfermentable carbon sources like lactate, directly impedes cellular metabolism. Hence it is not surprising that all of these conditions have been shown to affect C. albicans cell wall architecture (16, 34, 39). Pradhan et al. (34) compared and contrasted these three stimuli in terms of the signaling mechanisms involved in their effect on β-1,3-glucan masking. Hypoxia and lactate as sole carbon source caused glucan masking in a cAMP-PKA–dependent manner, whereas low iron-mediated masking depended on the PKA pathway, yet independent of cAMP. Hypoxia-induced masking was also dependent on mitochondrial SOD2 but the authors show that iron-induced masking was independent of SOD1-6. Furthermore, the masking effect of growth on lactate depended on lactate sensor Gpr1 and on Crz1. As expected, iron-mediated masking depended on iron transporter Ftr1 and iron homeostasis regulator Sef.
In this study, we present a novel biochemical mechanism for iron-mediated changes in β-1,3-glucan exposure levels (Fig. 8). This mechanism depends on the fundamental aspects of the effect of iron on cellular metabolism. Restriction of cellular iron alters metabolism and encourages utilization of glucose via fermentative mechanisms rather than tricarboxylic acid (TCA) cycle and oxidative phosphorylation (40). Also, biogenesis of Fe-S containing proteins that are components of both tricarboxylic acid cycle and the electron transport chain are down-regulated under low-iron conditions in C. albicans (9). These events under a low iron environment can modulate metabolic activity leading to production of lactate (41). Similarly, previous studies demonstrated that iron deficiency promoted lactate production in higher eukaryotes like rats (42) and humans (30). On the other hand, as observed here, high iron led to an increase in mitochondrial membrane potential and a reduction in accumulation of l-lactate in C. albicans cells (Fig. 4, B and C). Taken together with the fact that lactic acid induces β-1,3-glucan masking (16), led us to believe that the reduced masking and hence higher exposure of β-1,3-glucan under high iron is mediated by reduction in accumulation of intracellular lactic acid. This was reaffirmed by the fact that substituting glucose with lactate as the sole carbon source essentially caused β-1,3-glucan masking, regardless of the iron levels. Although exogenous lactate triggers β-1,3-glucan masking through Gpr1 and Crz1 (16), iron-induced changes to β-1,3-glucan exposure levels mediated by intracellular l-lactate were independent of Gpr1, yet Crz1-dependent (Fig. 5).
Figure 8.
Model for iron-mediated changes to β-1,3-glucan exposure levels in C. albicans. High iron levels promote β-1,3-glucan exposure by limiting lactate-induced Crz1-mediated inhibition of Cek1-activation.
It is, however, imperative to note that growth on lactate as the sole carbon source and low iron-induced intracellular l-lactate accumulation represent distinct metabolic states. Lactate-grown cells utilize an alternative respiration pathway involving an external respiratory protein, NADH-dehydrogenase (alternate complex 1), which is unable to translocate protons to the mitochondria, thereby affecting energy production. In response, these cells up-regulate cytochrome Cyb2, a conserved heme and flavin mononucleotide containing fungal protein located in mitochondrial inner membrane space (43). This protein is a lactate dehydrogenase that converts l-lactate to pyruvate and maintains mitochondrial membrane potential for energy production (44). Growth on low iron instead leads to a decrease in mitochondrial membrane potential due to a potential reduction in synthesis of Fe-S containing proteins, such as, complex 1 and cytochrome bc1. As mentioned previously, this will trigger energy generation by fermentative pathways leading to lactic acid production. More importantly, Cyb2 that is required for lactate utilization, is an iron containing heme protein, and is therefore down-regulated under low iron (9). This will cause lactic acid accumulation, as observed, under low iron. Interestingly, because hypoxia-induced β-1,3-glucan masking depends on mitochondrial ROS signals, it is safe to assume that low iron, hypoxia, and lactate as sole carbon source utilize three distinct mechanisms for β-1,3-glucan masking, all of which involve the mitochondria.
High iron uniquely prevents Crz1-mediated inhibition of Cek1 MAPK activation to expose fungal β-1,3-glucans
We have previously shown that high environmental iron signals into the Cek1 pathway, leading to its activation via phosphorylation (10). This induction of Cek1-P is necessary for glucan exposure (33). We present here a novel mechanism for how iron changes β-1,3-glucan exposure, wherein, environmental iron as a signal links the Cek1 MAPK pathway to intracellular l-lactate–mediated Crz1 signaling. Reduction in intracellular l-lactate accumulation under high iron prevents Crz1-mediated inhibition of Cek1-P, thereby causing β-1,3-glucan exposure. To our knowledge, this is the first study showing Crz1-mediated inhibition of Cek1-P. The parent pathway (calcineurin pathway) of Crz1 has been shown to inhibit Fus3 (a Cek1 homolog) in S. cerevisiae (45). Thus, the cross-talk between the calcineurin pathway and MAPK Cek1 seems to be conserved between C. albicans and S. cerevisiae.
Opposing roles for iron levels in fungal pathogenesis
Host niches vary greatly in terms of iron availability. Iron levels can range from extremely low availability approaching 10−12 μm in blood (46) to up to 400 μm in the gut (47), as represented by our limited and replete iron medium, respectively. This range in iron availability can have major effects on host-pathogen interactions. Fungal cells mask their immunogenic β-1,3-glucan to escape immune surveillance (48), thereby evading phagocytosis and reducing stimulation of pro-inflammatory cytokines; whereas β-1,3-glucan exposure enhances the fungal recognition (49).
High iron-induced β-1,3-glucan exposure showed similar effects with a significant increase in key pro-inflammatory cytokines (Fig. 7C), such as IL-1α, TNF-α, and IFN-γ. IL-1α is known to intensify neutrophil crowding to clear large microbes such as C. albicans (50), whereas TNF-α and IFN-γ play important roles in promoting neutrophils functions (51). In contrast, fungal cells in mice treated with the iron chelator deferasirox showed lower neutrophil recruitment in the tongue tissue during oropharyngeal candidiasis (52). Thus, greater β-1,3-glucan exposure in RIM C. albicans cells increases recognition by phagocytic cells, thereby decreasing subsequent survival by reducing the ability of these cells to evade phagocytosis. In addition, ROS generated by macrophages causes fungal killing (53), whereas C. albicans SOD4 and SOD5 are crucial in mitigating this effect (54). Interestingly, down-regulation of SOD4 under high iron (55), together with higher ROS observed in high iron RIM-grown C. albicans cells (Fig. 4E) most definitely further contributed toward the observed reduction in survival of RIM cells upon phagocytosis (Fig. 7B). Also, reduction in lactate accumulation under high iron can limit function of the glyoxylate cycle (56) that is crucial for fungal survival within the macrophages (57).
From a nutritional immunity perspective, it is known that increased availability of iron as a nutrient can help promote microbial growth and thus enhance C. albicans infection in vivo (52, 58). As we have shown, high iron conditions made C. albicans cells more resistant to antifungal agents (Fig. 3). However, because high iron makes cells more susceptible to phagocytosis by macrophages (Fig. 7A), we highlight here the opposing effects of iron in host-pathogens interactions. High iron, on one hand can enhance infection and antifungal resistance. In contrast, as shown in this study, it also allows for reduced virulence traits, specifically in terms of increased susceptibility to host immune attack (Fig. 7A) by maximizing the exposure of immunogenic PAMPs (Fig. 4A) and increasing ROS levels (Fig. 4E).
Experimental procedures
Yeast strains, media, and culture conditions
C. albicans CAI4 strain was used as the WT unless otherwise stated. The genotype of the mutants and their respective parental strains are listed in Table 1. YNB media lacking iron, copper, and ammonium chloride (4027-112; MP Biomedicals), with 2% glucose (or 2% lactate, when stated) and other added supplements was used as LIM, and addition of 100 μm FeCl3·6H2O in LIM medium was used as RIM, as described previously (10). For all experiments, exponential phase cells obtained from two subsequent overnight cultures were used. For the preparation of two overnight cultures, one overnight culture was grown in LIM and RIM, and re-diluted 100-fold into fresh respective medium for another round of overnight growth. Cells from the second overnight culture were diluted to 0.3 optical density (OD) at 600 nm in 10 ml of fresh respective media and grown for 4 h at 30˚C at 210 rpm, unless stated otherwise. For antifungal sensitivity assays, uridine (50 μg ml−1) containing YNB agar plates were used.
Table 1.
List of C. albicans strains used in this study
| Strains | Genotype | Sources |
|---|---|---|
| CAI4 (WT) | ura3Δ::imm434/URA3 | Ref. 65 |
| CRZ1-WT (BWP17) | ura3Δ::λimm434/ura3Δ::λimm434his1::hisG/his1::hisGarg4::hisG/arg4::hisG | Ref. 66 |
| crz1Δ/crz1Δ | ura3Δ::λimm434/ura3Δ::λimm434his1::hisG::HIS1/his1::hisG arg4::hisG/arg4::hisG crz1::UAU1/crz1::ARG4 | Ref. 31 |
| crz1Δ/CRZ1 | ura3Δ::λimm434/ura3Δ::λimm434his1::hisG::HIS1/his1::hisG arg4::hisG/arg4::hisG crz1::UAU1/CRZ1::ARG4 | Ref. 31 |
| GPR1-WT (YTC028) | Δura3::imm434/Δura3::imm434 TRP1/TRP1::URA3 | Ref. 67 |
| gpr1Δ/gpr1Δ | Δura3::imm434/Δura3::imm434gpr1::hisG/gpr1::hisG TRP1/TRP1::URA3 | Ref. 67 |
| gpr1Δ/GPR1 | Δura3::imm434/Δura3::imm434GPR1/gpr1::hisG TRP1/TRP1::URA3 | Ref. 67 |
| cek1Δ/cek1Δ | Δura3::imm434/Δura3::imm434cek1Δ::hisG-URAhisG/cek1Δ::hisG | Ref. 68 |
| hog1Δ/hog1Δ | Δura3::imm434/Δura3::imm434his1::hisG/his1::hisGhog1::loxP-ura3-loxP/hog1::loxP-HIS1-loxP CIp20 (URA3 HISI) | Ref. 69 |
Growth rate assay
Exponential phase WT C. albicans (CAI4) cells grown in LIM, RIM, or YPD (242810, Difco) medium were taken as the start culture for growth assays in the respective medium. A total 200 μl volume of media and culture with a start of OD600 = 0.1 was placed in each well of a 96-well transparent plate. The plate was placed in a microplate reader (BioTek) and C. albicans growth in each well was determined by measurement of OD600 followed by 24 h incubation at 30˚C with gentle shaking.
Determination of intracellular iron content
Intracellular iron concentrations were determined by modified ferrozine-based colorimetric method (59). C. albicans WT exponential phase cells grown in 100 ml of LIM, RIM, or YPD were harvested, washed with iron-free water, and resuspended in 600 μl of 0.5 m NaOH. After addition of 300 mg of iron-free glass beads (G8772-100G; Sigma) cells were disrupted using bead beater (MP Biomedicals) for 3 homogenizations at 6,500 × g for 45 s per homogenization with a 15-s pause between homogenizations. For removal of beads and cell debris, centrifugation was performed at 5,000 × g for 3 min and 200 μl of the cell supernatant (as sample) or 0.5 m NaOH (negative control) was used for detection. For standard, 1:2 serial dilution (300 to 4.68 μm) of FeCl3, 0.5 m NaOH was used. Iron-releasing agent (equal volumes of 1.4 m HCl and 4.5% (w/v) KMnO4) was added to the samples and incubated for 2 h at 60 °C and 300 rpm and then cooled for 5 min with open lids. Finally, freshly prepared 30 μl detection mix (6.5 mm ferrozine, 6.5 mm neocuproine, 2.5 m ammonium acetate, 1 m ascorbic acid in iron-free water) was added and samples were incubated for 30 min at room temperature. The iron content was determined by measurement of the OD568 normalized against the cell number. The experiment was performed in three biological replicates.
Staining and quantification of cell wall components
To determine the cell wall components, 2.5 × 106 exponential cells of WT C. albicans grown in LIM or RIM were fixed with 4% paraformaldehyde for 30 min in PBS. After three washes with PBS, fixed cells were stained with 50 μg ml−1 ConA (C11252; Invitrogen) for mannan, 0.1 mg ml−1 of AB (100-1; Bioscience supplies) for total β-1,3-glucan, 5 μg ml−1 of CFW (F3543-1G; Sigma-Aldrich) for total chitin, and 100 μg ml−1 of WGA (W11261; Invitrogen) for exposed chitin, followed by incubation at room temperature for 45, 30, 10, and 45 min, respectively. Fluorescence intensities of stained cells were quantified by a fluorescence microscope (Zeiss) using GFP (for ConA and WGA) and 4′,6-diamidino-2-phenylindole filters (for AB and CFW) at ×100 magnification. Staining of exposed β-1,3-glucan in fixed cells was performed using primary anti-β-1,3-glucan antibody (Bioscience Supplies, Australia) and secondary goat anti-mouse antibody conjugated to Cy3 (Jackson ImmunoResearch) as described previously (49). Images were taken by confocal microscope using an argon laser (520 or 530 nm lines) in combination with FITC filters at ×100 magnification. MFI of stained cells were measured using ImageJ software. The levels of phosphomannans were determined by measuring the binding of 3 mg ml−1 of cationic dye alcian blue (400460100; Sigma-Aldrich) to 2.5 × 107 exponential cells of C. albicans and calculated as described previously (21).
Transmission EM
C. albicans WT yeast cells were grown to log phase as described above and high pressure frozen with a Leica EMPact high pressure freezer using 1.2 mm wide × 200 μm deep high pressure freezer carriers. Samples were transferred under liquid nitrogen to a Leica automated freeze substitution unit and freeze substituted in 1% osmium tetroxide containing 1% water in acetone. Yeast were freeze substituted at −90 °C for 72 h, warmed to −20 °C at a rate of 10 °C h−1, held at −20 °C for 12 h, and then warmed to 20 °C at a rate of 10 °C h−1. Samples were washed in 100% acetone, gradually infiltrated with Quetol 651/NSA resin, and then polymerized at 55 °C for 24 h. Ultrathin sections were cut on a Leica UC7 ultramicrotome, and sections were collected onto 200 mesh formvar/carbon-coated copper grids and post-stained with 2% uranyl acetate in 50% methanol and Reynolds' lead citrate. Yeast cell walls were imaged on a Zeiss Libra 120 transmission electron microscope operated at 120 kV, and images were acquired with a Gatan Ultrascan 1000 CCD camera.
Antifungal sensitivity
Antifungal susceptibility of exponential yeast cells of C. albicans WT grown in LIM or RIM to tunicamycin and CWF were assessed on respective YNB agar plates containing 1.5 or 100 μg ml−1 of the respective drug concentrations, by spot dilution assay, as described before (60). Zymolyase (E1004; Genesee Scientific) was resuspended in storage buffer and contained 50% glycerol (for osmotic support) and used for sensitivity assay. C. albicans growth was measured in the presence or absence of 30 units of zymolyase in the respective liquid media in 96-well microtiter plates (Corning), for 90 min at 30 ˚C with shaking in a microplate reader (BioTek) as described previously (60). The differences in cell growth between LIM and RIM were calculated using the following formula: % growth of C. albicans = (OD600 of zymolase-treated group/OD600 of untreated group) × 100.
Mitochondrial activity
Mitochondrial membrane potential in exponential phase WT C. albicans cells grown in LIM or RIM was determined using the cyanine dye JC-1 (T4069-5MG; Sigma), as described previously (61). The red and green fluorescence of JC-1 was monitored using a fluorescence microscope (Zeiss) with RFP and GFP filters.
For intracellular ATP levels detection, fresh 1 overnight cultures of YPD grown cells were prepared at 30 ˚C with gentle shaking and the next day cultures were inoculated with an initial OD600 = 0.3 in fresh YPD medium with or without bathophenanthriline disulfonic acid (200 μM) for lower and higher iron conditions, respectively, incubated for 4 h in similar conditions. ATP levels in active C. albicans cells were measured by using a BacTiter-Glo™ kit (Promega) following the manufacturer's instructions. Luminescence was recorded on a Biotek 96 Microplate reader using luminescence program. The equal number of cells were used to determine total protein concentration using the bicinchoninic acid (Thermo Scientific) assay. Results are presented in relative luminescence unit as an average of ATP normalized to total protein concentration analyzed from triplicates obtained from three different experiments.
l-Lactate assay
Extracellular and intracellular l-lactate levels in exponential phase WT C. albicans cells grown in LIM or RIM were measured by a l-lactate assay kit (Cayman Chemical), as per the manufacturer's instructions. The fluorescence of l-lactate standards between the range of 0 to 1000 μm and samples were measured using excitation/emission wavelengths 530/585 nm by BioTek multimode synergy HTX system using Gen5 software. l-Lactate concentrations were determined from l-lactate standard curves as suggested by the manufacturer.
ROS production
Intracellular levels of ROS were assayed by a fluorescence microscopy with 2ʹ,7ʹ-dichlorofluorescin diacetate (DCFH-DA) staining, as described previously (61). Briefly, WT exponential phase C. albicans cells (1 × 107 cells ml−1) grown in LIM or RIM were resuspended in the respective medium, and 10 μmol of liter−1 of DCFH-DA (Invitrogen) was added. The cells were incubated in the dark for 30 min with gentle agitation. Then, the cells were collected and washed three times with PBS. Fluorescence images were taken using confocal microscope using an argon laser (510 or 520 nm lines) in combination with FITC filters at ×100 magnification. The fluorescence intensities were quantified using ImageJ software.
Macrophage-mediated phagocytosis of C. albicans and cytokine production
BMDM isolation
Murine BMDM were generated from 4- to 6-week–old C57BL/6 female mice (Jackson Laboratory, Bar Harbor, ME) and differentiated as described previously (62). BMDM cells were used at day 7 for all experiments. Trypsinized 0.5 × 106 cells were seeded on a 6-well–plate (Corning Inc.) on coverslips for phagocytosis experiments and on wells for C. albicans survival and cytokine stimulation assays, followed by incubation at 37 °C in 5% CO2 1 day prior to the experiment.
BMDM phagocytosis and fungicidal activity
BMDM cells were co-incubated with exponential phase C. albicans cells at a multiplicity of infection (m.o.i.) of 3:1 in RPMI 1640 with 2.05 mm l-glutamine (HyClone) (final volume, 1 ml) for 1 h at 37 °C under 5% CO2. After incubation, media was removed from each well and BMDM cells were washed three times with PBS gently. To stain nonphagocytosed C. albicans cells, 10 μg ml−1 of CFW was added in each well for 1 min at room temperature. Afterward, extra CFW was removed by gentle washing with ice-cold PBS and immediately cells were fixed with 4% paraformaldehyde for 20 min. After two washes with PBS, coverslips were placed on glass slide and images were taken using the EVOS-FL Fluorescence-Imaging system (Life Technologies). Phagocytic index was calculated using the formula: [(total number of engulfed candida/total number of counted macrophages) × (number of macrophages containing engulfed cells/total number of counted macrophages)] × 100.
Murine BMDM fungicidal activity toward exponential phase C. albicans cells was assessed at m.o.i. of 3:1 as mentioned above and quantified in colony-forming units as described previously (63). Colony-forming units of C. albicans grown in the same condition without macrophages used as control. The percent survival of Candida is defined as (cfu of treatment group/cfu of control group) × 100.
Cytokine analysis
On day 5, after isolation of murine, BMDM, 105 cells per well, were seeded into 24-well–plates. On day 6, macrophages were treated with 50 ng ml−1 of IFN-γ (714006; Biolegend) and 100 ng ml−1 of LPS (LPS25; EMD Millipore) for 6 h. Day 7, LIM- or RIM-grown C. albicans cells were added to each well at m.o.i. of 5:1 macrophages. At time 0 and after 24 h post-infection, 100 μl of supernatant was collected from each well and stored at −80 °C. The concentrations of cytokines and chemokines were quantified in supernatants using the mouse cytokine/chemokine 31-Plex (MD31) luminex array (Eve Technologies, Calgary, Alberta).
Western blotting
Exponential phase cells of WT C. albicans and crz1 null mutant were grown in LIM or RIM either in the presence of 2% glucose or 2% l-lactate as described above (in the first section of “Experimental procedures”), and were processed for immunoblotting to detect for Cek1-P and Hog1 protein (as a loading control) as described previously (64). Total protein concentrations were determined using the RC DCTM protein assay kit (Bio-Rad) and normalized protein content (30 µg) was loaded in each well for SDS-PAGE. α-Phospho-p42/44 MAPK ERK1/2 Thr-202/Tyr-204 rabbit mAb (Cell Signaling Technology) was used for Cek1-P detection and Hog1 antibody (D3: sc-165978 from Santa Cruz Biotechnology, Inc.) was used for detection of Hog1 protein, as the primary antibody. Goat α-rabbit IgG-HRP (Jackson ImmunoResearch Laboratories, Inc.) was used for detection of Cek1-P and goat α-mouse IgG-HRP (Santa Cruz Biotechnology, Inc.) was used for detection of Hog1 protein, as the secondary antibody. Protein extracted from exponential cells of glucose-grown C. albicans cek1 null mutant and hog1 null mutant was used as negative control for Cek1-P and Hog1 protein, respectively, whereas Hog1 protein represents the loading control.
Ethics statement
Animal related experiments were performed as per the guidelines of the Animal Care and Use Protocol (ACUP 4623) reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) at Temple University to ensure proper care and handling of laboratory animals.
Statistical analysis
Statistical analysis was performed by unpaired t test or one-way ANOVA with Tukey multiple comparison test, when two or more than two experimental groups were evaluated using GraphPad Prism software version 7 (GraphPad Software, USA). Error bars represent 95% confidence interval (CI).
Data availability
All of the data are contained within the main paper and supporting information.
Supplementary Material
Acknowledgments
We acknowledge Shannon Modla (Delaware Biotechnology Institute) for transmission electron microscopy technique and the assistance of Bettina Buttaro (Temple University) for help with confocal microscopy. We are grateful to Malcolm Whiteway (Concordia University) for CAI4 and cek1Δ/cek1Δ strains, Janet Quinn (Newcastle University) for hog1Δ/hog1Δ strain, as well as Joseph Heitman and Wiley Schell (Duke University) for YTC 028 and BWP17 and mutant strains (gpr1Δ/gpr1Δ, gpr1Δ/GPR1, crz1Δ/crz1Δ, and crz1Δ/CRZ1).
This article contains supporting information.
Author contributions—A. Tripathi, E. L., A. Tsygankov, and S. P. conceptualization; A. Tripathi and S. P. software; A. Tripathi, A. Tsygankov, and S. P. formal analysis; A. Tripathi and S. P. validation; A. Tripathi and S. P. investigation; A. Tripathi, E. L., A Tsygankov, and S. P. methodology; A. Tripathi writing-original draft; E. L., A. Tsygankov, and S. P. resources; S. P. supervision; S. P. funding acquisition; S. P. project administration; S. P. writing-review and editing.
Funding and additional information—This work was supported by NIDCR, National Institutes of Health Grant R03DE026451 (to S. P.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of interest—The authors have no conflict of interest.
- ROS
- reactive oxygen species
- AB
- aniline blue
- BMDM
- bone marrow-derived macrophage
- Cek1-P
- phosphorylated mitogen-activated protein kinase Cek1
- cfu
- colony-forming unit
- CFW
- calcofluor white
- CI
- confidence interval
- ConA
- concanavalin A
- CsA
- cyclosporine A
- CWP
- cell wall perturbing
- DCFH-DA
- 2ʹ,7ʹ-dichlorofluorescin diacetate
- IFN-γ
- interferon γ
- IL-1α
- interleukin 1α
- LIM
- limited iron media
- LPS
- lipopolysaccharides
- MAPK
- mitogen-activated protein kinase
- MFI
- mean fluorescence intensity
- MIG
- monokine induced by γ interferon
- m.o.i.
- multiplicity of infection
- PAMP
- pathogen-associated molecular pattern molecules
- PKA
- protein kinase A
- RIM
- replete iron media
- SOD
- superoxide dismutase
- TNF-α
- tumor necrosis factor α
- WGA
- wheat germ agglutinin
- YNB
- yeast nitrogen base
- YPD
- yeast-extract peptone dextrose
- a.u.
- absorbance unit.
References
- 1. Schaible U. E., and Kaufmann S. H. (2004) Iron and microbial infection. Nat. Rev. Microbiol. 2, 946–953 10.1038/nrmicro1046 [DOI] [PubMed] [Google Scholar]
- 2. Misslinger M., Lechner B. E., Bacher K., and Haas H. (2018) Iron-sensing is governed by mitochondrial, not by cytosolic iron-sulfur cluster biogenesis in Aspergillus fumigatus. Metallomics 10, 1687–1700 10.1039/c8mt00263k [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Halliwell B., and Gutteridge J. M. (1984) Role of iron in oxygen radical reactions. Methods Enzymol. 105, 47–56 10.1016/s0076-6879(84)05007-2 [DOI] [PubMed] [Google Scholar]
- 4. Sutak R., Lesuisse E., Tachezy J., and Richardson D. R. (2008) Crusade for iron: iron uptake in unicellular eukaryotes and its significance for virulence. Trends Microbiol 16, 261–268 10.1016/j.tim.2008.03.005 [DOI] [PubMed] [Google Scholar]
- 5. Núñez G., Sakamoto K., and Soares M. P. (2018) Innate nutritional immunity. J. Immunol. 201, 11–18 10.4049/jimmunol.1800325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen C., Pande K., French S. D., Tuch B. B., and Noble S. M. (2011) An iron homeostasis regulatory circuit with reciprocal roles in Candida albicans commensalism and pathogenesis. Cell Host Microbe 10, 118–135 10.1016/j.chom.2011.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen C., and Noble S. M. (2012) Post-transcriptional regulation of the Sef1 transcription factor controls the virulence of Candida albicans in its mammalian host. PLoS Pathog. 8, e1002956 10.1371/journal.ppat.1002956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fourie R., Kuloyo O. O., Mochochoko B. M., Albertyn J., and Pohl C. H. (2018) Iron at the centre of Candida albicans interactions. Front. Cell Infect. Microbiol. 8, 185 10.3389/fcimb.2018.00185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lan C. Y., Rodarte G., Murillo L. A., Jones T., Davis R. W., Dungan J., Newport G., and Agabian N. (2004) Regulatory networks affected by iron availability in Candida albicans. Mol. Microbiol. 53, 1451–1469 10.1111/j.1365-2958.2004.04214.x [DOI] [PubMed] [Google Scholar]
- 10. Puri S., Lai W. K., Rizzo J. M., Buck M. J., and Edgerton M. (2014) Iron-responsive chromatin remodelling and MAPK signalling enhance adhesion in Candida albicans. Mol. Microbiol. 93, 291–305 10.1111/mmi.12659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Prasad T., Chandra A., Mukhopadhyay C. K., and Prasad R. (2006) Unexpected link between iron and drug resistance of Candida spp.: iron depletion enhances membrane fluidity and drug diffusion, leading to drug-susceptible cells. Antimicrob. Agents Chemoth. 50, 3597–3606 10.1128/AAC.00653-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sorgo A. G., Brul S., de Koster C. G., de Koning L. J., and Klis F. M. (2013) Iron restriction-induced adaptations in the wall proteome of Candida albicans. Microbiology 159, 1673–1682 10.1099/mic.0.065599-0 [DOI] [PubMed] [Google Scholar]
- 13. Klis F. M., and Brul S. (2015) Adaptations of the secretome of Candida albicans in response to host-related environmental conditions. Eukaryot. Cell 14, 1165–1172 10.1128/EC.00142-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Arana D. M., Prieto D., Roman E., Nombela C., Alonso-Monge R., and Pla J. (2009) The role of the cell wall in fungal pathogenesis. Microb. Biotechnol. 2, 308–320 10.1111/j.1751-7915.2008.00070.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sherrington S. L., Sorsby E., Mahtey N., Kumwenda P., Lenardon M. D., Brown I., Ballou E. R., MacCallum D. M., and Hall R. A. (2017) Adaptation of Candida albicans to environmental pH induces cell wall remodelling and enhances innate immune recognition. PLoS Pathog. 13, e1006403 10.1371/journal.ppat.1006403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ballou E. R., Avelar G. M., Childers D. S., Mackie J., Bain J. M., Wagener J., Kastora S. L., Panea M. D., Hardison S. E., Walker L. A., Erwig L. P., Munro C. A., Gow N. A., Brown G. D., MacCallum D. M., et al. (2016) Lactate signalling regulates fungal β-glucan masking and immune evasion. Nat. Microbiol. 2, 16238 10.1038/nmicrobiol.2016.238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Malavia D., Lehtovirta-Morley L. E., Alamir O., Weiß E., Gow N. A. R., Hube B., and Wilson D. (2017) Zinc limitation induces a hyper-adherent goliath phenotype in Candida albicans. Front. Microbiol. 8, 2238 10.3389/fmicb.2017.02238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ene I. V., Adya A. K., Wehmeier S., Brand A. C., MacCallum D. M., Gow N. A., and Brown A. J. (2012) Host carbon sources modulate cell wall architecture, drug resistance and virulence in a fungal pathogen. Cell Microbiol. 14, 1319–1335 10.1111/j.1462-5822.2012.01813.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ostrosky-Zeichner L., Oude Lashof A. M., Kullberg B. J., and Rex J. H. (2003) Voriconazole salvage treatment of invasive candidiasis. Eur. J. Clin. Microbiol. Infect. Dis. 22, 651–655 10.1007/s10096-003-1014-3 [DOI] [PubMed] [Google Scholar]
- 20. Lesage G., and Bussey H. (2006) Cell wall assembly in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 70, 317–343 10.1128/MMBR.00038-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Harris M., Mora-Montes H. M., Gow N. A. R., and Coote P. J. (2009) Loss of mannosylphosphate from Candida albicans cell wall proteins results in enhanced resistance to the inhibitory effect of a cationic antimicrobial peptide via reduced peptide binding to the cell surface. Microbiology 155, 1058–1070 10.1099/mic.0.026120-0 [DOI] [PubMed] [Google Scholar]
- 22. Yoo J., Mashalidis E. H., Kuk A. C. Y., Yamamoto K., Kaeser B., Ichikawa S., and Lee S. Y. (2018) GlcNAc-1-P-transferase-tunicamycin complex structure reveals basis for inhibition of N-glycosylation. Nat. Struct. Mol. Biol. 25, 217–224 10.1038/s41594-018-0031-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Roman E., Cottier F., Ernst J. F., and Pla J. (2009) Msb2 signaling mucin controls activation of Cek1 mitogen-activated protein kinase in Candida albicans. Eukaryot. Cell 8, 1235–1249 10.1128/EC.00081-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Boorsma A., de Nobel H., ter Riet B., Bargmann B., Brul S., Hellingwerf K. J., and Klis F. M. (2004) Characterization of the transcriptional response to cell wall stress in Saccharomyces cerevisiae. Yeast 21, 413–427 10.1002/yea.1109 [DOI] [PubMed] [Google Scholar]
- 25. Srivastava V. K., Suneetha K. J., and Kaur R. (2015) The mitogen-activated protein kinase CgHog1 is required for iron homeostasis, adherence and virulence in Candida glabrata. FEBS J. 282, 2142–2166 10.1111/febs.13264 [DOI] [PubMed] [Google Scholar]
- 26. Monge R. A., Roman E., Nombela C., and Pla J. (2006) The MAP kinase signal transduction network in Candida albicans. Microbiology 152, 905–912 10.1099/mic.0.28616-0 [DOI] [PubMed] [Google Scholar]
- 27. Hall R. A., and Gow N. A. (2013) Mannosylation in Candida albicans: role in cell wall function and immune recognition. Mol. Microbiol. 90, 1147–1161 10.1111/mmi.12426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Graus M. S., Wester M. J., Lowman D. W., Williams D. L., Kruppa M. D., Martinez C. M., Young J. M., Pappas H. C., Lidke K. A., and Neumann A. K. (2018) Mannan molecular substructures control nanoscale glucan exposure in Candida. Cell Rep. 24, 2432–2442.e2435 10.1016/j.celrep.2018.07.088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Grahl N., Demers E. G., Lindsay A. K., Harty C. E., Willger S. D., Piispanen A. E., and Hogan D. A. (2015) Mitochondrial activity and Cyr1 are key regulators of Ras1 activation of C. albicans virulence pathways. PLoS Pathog. 11, e1005133 10.1371/journal.ppat.1005133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Finch C. A., Gollnick P. D., Hlastala M. P., Miller L. R., Dillmann E., and Mackler B. (1979) Lactic acidosis as a result of iron deficiency. J. Clin. Invest. 64, 129–137 10.1172/JCI109431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Karababa M., Valentino E., Pardini G., Coste A. T., Bille J., and Sanglard D. (2006) CRZ1, a target of the calcineurin pathway in Candida albicans. Mol. Microbiol. 59, 1429–1451 10.1111/j.1365-2958.2005.05037.x [DOI] [PubMed] [Google Scholar]
- 32. Bruno V. M., and Mitchell A. P. (2005) Regulation of azole drug susceptibility by Candida albicans protein kinase CK2. Mol. Microbiol. 56, 559–573 10.1111/j.1365-2958.2005.04562.x [DOI] [PubMed] [Google Scholar]
- 33. Chen T., Wagner A. S., Tams R. N., Eyer J. E., Kauffman S. J., Gann E. R., Fernandez E. J., and Reynolds T. B. (2019) Lrg1 regulates β(1,3)-glucan masking in Candida albicans through the Cek1 MAP kinase pathway. mBio 10 10.1128/mBio.01767-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pradhan A., Avelar G. M., Bain J. M., Childers D., Pelletier C., Larcombe D. E., Shekhova E., Netea M. G., Brown G. D., Erwig L., Gow N. A. R., and Brown A. J. P. (2019) Non-canonical signalling mediates changes in fungal cell wall PAMPs that drive immune evasion. Nat. Commun. 10, 5315 10.1038/s41467-019-13298-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mora-Montes H. M., Bates S., Netea M. G., Castillo L., Brand A., Buurman E. T., Diaz-Jimenez D. F., Jan Kullberg B., Brown A. J., Odds F. C., and Gow N. A. (2010) A multifunctional mannosyltransferase family in Candida albicans determines cell wall mannan structure and host-fungus interactions. J. Biol. Chem. 285, 12087–12095 10.1074/jbc.M109.081513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Goldman R. C., Sullivan P. A., Zakula D., and Capobianco J. O. (1995) Kinetics of β-1,3 glucan interaction at the donor and acceptor sites of the fungal glucosyltransferase encoded by the BGL2 gene. Eur. J. Biochem. 227, 372–378 10.1111/j.1432-1033.1995.tb20399.x [DOI] [PubMed] [Google Scholar]
- 37. Mühlschlegel F. A., and Fonzi W. A. (1997) PHR2 of Candida albicans encodes a functional homolog of the pH-regulated gene PHR1 with an inverted pattern of pH-dependent expression. Mol. Cell. Biol. 17, 5960–5967 10.1128/mcb.17.10.5960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yang F., Zhang L., Wakabayashi H., Myers J., Jiang Y., Cao Y., Jimenez-Ortigosa C., Perlin D. S., and Rustchenko E. (2017) Tolerance to caspofungin in Candida albicans is associated with at least three distinctive mechanisms that govern expression of FKS genes and cell wall remodeling. Antimicrob. Agents Chemother. 61 10.1128/AAC.00071-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pradhan A., Avelar G. M., Bain J. M., Childers D. S., Larcombe D. E., Netea M. G., Shekhova E., Munro C. A., Brown G. D., Erwig L. P., Gow N. A. R., and Brown A. J. P. (2018) Hypoxia promotes immune evasion by triggering β-glucan masking on the Candida albicans cell surface via mitochondrial and cAMP-protein kinase A signaling. mBio 9 10.1128/mBio.01318-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Philpott C. C., Leidgens S., and Frey A. G. (2012) Metabolic remodeling in iron-deficient fungi. Biochim. Biophys. Acta 1823, 1509–1520 10.1016/j.bbamcr.2012.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dostal A., Lacroix C., Bircher L., Pham V. T., Follador R., Zimmermann M. B., and Chassard C. (2015) Iron modulates butyrate production by a child gut microbiota in vitro. mBio 6, e01453–15 10.1128/mBio.01453-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ohira Y., Chen C. S., Hegenauer J., and Saltman P. (1983) Adaptations of lactate metabolism in iron-deficient rats. Proc. Soc. Exp. Biol. Med. 173, 213–216 10.3181/00379727-173-41633 [DOI] [PubMed] [Google Scholar]
- 43. Waller R. F., Jabbour C., Chan N. C., Celik N., Likic V. A., Mulhern T. D., and Lithgow T. (2009) Evidence of a reduced and modified mitochondrial protein import apparatus in microsporidian mitosomes. Eukaryot. Cell 8, 19–26 10.1128/EC.00313-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Grad L. I., Sayles L. C., and Lemire B. D. (2005) Introduction of an additional pathway for lactate oxidation in the treatment of lactic acidosis and mitochondrial dysfunction in Caenorhabditis elegans. Proc. Natl. Acad. Sci. U.S.A. 102, 18367–18372 10.1073/pnas.0506939102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ly N., and Cyert M. S. (2017) Calcineurin, the Ca2+-dependent phosphatase, regulates Rga2, a Cdc42 GTPase-activating protein, to modulate pheromone signaling. Mol. Biol. Cell 28, 576–586 10.1091/mbc.E16-06-0432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Agoro R., and Mura C. (2019) Iron supplementation therapy, a friend and foe of mycobacterial infections?. Pharmaceuticals 12, 75 10.3390/ph12020075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yilmaz B., and Li H. (2018) Gut microbiota and iron: the crucial actors in health and disease. Pharmaceuticals 11, 98 10.3390/ph11040098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Marcos C. M., de Oliveira H. C., de Melo W. C., da Silva J. F., Assato P. A., Scorzoni L., Rossi S. A., de Paula E. S. A. C., Mendes-Giannini M. J., and Fusco-Almeida A. M. (2016) Anti-immune strategies of pathogenic fungi. Front. Cell Infect. Microbiol. 6, 142 10.3389/fcimb.2016.00142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Davis S. E., Hopke A., Minkin S. C. Jr., Montedonico A. E., Wheeler R. T., and Reynolds T. B. (2014) Masking of β(1-3)-glucan in the cell wall of Candida albicans from detection by innate immune cells depends on phosphatidylserine. Infect. Immun. 82, 4405–4413 10.1128/IAI.01612-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Warnatsch A., Tsourouktsoglou T. D., Branzk N., Wang Q., Reincke S., Herbst S., Gutierrez M., and Papayannopoulos V. (2017) Reactive oxygen species localization: programs inflammation to clear microbes of different size. Immunity 46, 421–432 10.1016/j.immuni.2017.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Djeu J. Y., Blanchard D. K., Halkias D., and Friedman H. (1986) Growth inhibition of Candida albicans by human polymorphonuclear neutrophils: activation by interferon-γ and tumor necrosis factor. J. Immunol. 137, 2980–2984 [PubMed] [Google Scholar]
- 52. Puri S., Kumar R., Rojas I. G., Salvatori O., and Edgerton M. (2019) Iron chelator deferasirox reduces Candida albicans invasion of oral epithelial cells and infection levels in murine oropharyngeal candidiasis. Antimicrob. Agents Chemother. 63, e02152–18 10.1128/AAC.02152-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lorenz M. C., Bender J. A., and Fink G. R. (2004) Transcriptional response of Candida albicans upon internalization by macrophages. Eukaryot. Cell 3, 1076–1087 10.1128/EC.3.5.1076-1087.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Frohner I. E., Bourgeois C., Yatsyk K., Majer O., and Kuchler K. (2009) Candida albicans cell surface superoxide dismutases degrade host-derived reactive oxygen species to escape innate immune surveillance. Mol. Microbiol. 71, 240–252 10.1111/j.1365-2958.2008.06528.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Schatzman S. S., Peterson R. L., Teka M., He B., Cabelli D. E., Cormack B. P., and Culotta V. C. (2020) Copper-only superoxide dismutase enzymes and iron starvation stress in Candida fungal pathogens. J. Biol. Chem. 295, 570–583 10.1074/jbc.RA119.011084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ueno K., Matsumoto Y., Uno J., Sasamoto K., Sekimizu K., Kinjo Y., and Chibana H. (2011) Intestinal resident yeast Candida glabrata requires Cyb2p-mediated lactate assimilation to adapt in mouse intestine. PLoS One 6, e24759 10.1371/journal.pone.0024759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lorenz M. C., and Fink G. R. (2002) Life and death in a macrophage: role of the glyoxylate cycle in virulence. Eukaryot. Cell 1, 657–662 10.1128/ec.1.5.657-662.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Savage K. A., del Carmen Parquet M., Allan D. S., Davidson R. J., Holbein B. E., Lilly E. A., and Fidel P. L. Jr. (2018) Iron restriction to clinical isolates of Candida albicans by the novel chelator DIBI inhibits growth and increases sensitivity to azoles in vitro and in vivo in a murine model of experimental vaginitis. Antimicrob. Agents Chemother. 62, e02576–17 10.1128/AAC.02576-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gerwien F., Safyan A., Wisgott S., Hille F., Kaemmer P., Linde J., Brunke S., Kasper L., and Hube B. (2016) A novel hybrid iron regulation network combines features from pathogenic and nonpathogenic yeasts. mBio 7, e01782–16 10.1128/mBio.01782-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Alfatah M., Bari V. K., Nahar A. S., Bijlani S., and Ganesan K. (2017) Critical role for CaFEN1 and CaFEN12 of Candida albicans in cell wall integrity and biofilm formation. Sci. Rep. 7, 40281 10.1038/srep40281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sun L., Liao K., Hang C., and Wang D. (2017) Honokiol induces reactive oxygen species-mediated apoptosis in Candida albicans through mitochondrial dysfunction. PLoS One 12, e0172228 10.1371/journal.pone.0172228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tursi S. A., Lee E. Y., Medeiros N. J., Lee M. H., Nicastro L. K., Buttaro B., Gallucci S., Wilson R. P., Wong G. C. L., and Tükel Ç. (2017) Bacterial amyloid curli acts as a carrier for DNA to elicit an autoimmune response via TLR2 and TLR9. PLoS Pathog. 13, e1006315 10.1371/journal.ppat.1006315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Du C., and Calderone R. A. (2009) Phagocytosis and killing assays for Candida species. Methods Mol. Biol. 499, 17–26 10.1007/978-1-60327-151-6_3 [DOI] [PubMed] [Google Scholar]
- 64. Puri S., Kumar R., Chadha S., Tati S., Conti H. R., Hube B., Cullen P. J., and Edgerton M. (2012) Secreted aspartic protease cleavage of Candida albicans Msb2 activates Cek1 MAPK signaling affecting biofilm formation and oropharyngeal candidiasis. PLoS One 7, e46020 10.1371/journal.pone.0046020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Fonzi W. A., and Irwin M. Y. (1993) Isogenic strain construction and gene mapping in Candida albicans. Genetics 134, 717–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wilson R. B., Davis D., and Mitchell A. P. (1999) Rapid hypothesis testing with Candida albicans through gene disruption with short homology regions. J. Bacteriol. 181, 1868–1874 10.1128/JB.181.6.1868-1874.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Miwa T., Takagi Y., Shinozaki M., Yun C. W., Schell W. A., Perfect J. R., Kumagai H., and Tamaki H. (2004) Gpr1, a putative G-protein-coupled receptor, regulates morphogenesis and hypha formation in the pathogenic fungus Candida albicans. Eukaryot. Cell 3, 919–931 10.1128/EC.3.4.919-931.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Csank C., Schroppel K., Leberer E., Harcus D., Mohamed O., Meloche S., Thomas D. Y., and Whiteway M. (1998) Roles of the Candida albicans mitogen-activated protein kinase homolog, Cek1p, in hyphal development and systemic candidiasis. Infect. Immun. 66, 2713–2721 10.1128/IAI.66.6.2713-2721.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Smith D. A., Nicholls S., Morgan B. A., Brown A. J., and Quinn J. (2004) A conserved stress-activated protein kinase regulates a core stress response in the human pathogen Candida albicans. Mol. Biol. Cell 15, 4179–4190 10.1091/mbc.e04-03-0181 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All of the data are contained within the main paper and supporting information.