Abstract
Background
The accurate detection of SARS-CoV-2 through respiratory sampling is critical for the prevention of further transmission and the timely initiation of treatment for COVID-19. There is a diverse range of SARS-CoV-2 detection rates in reported studies, with uncertainty as to the optimal sampling strategy for COVID-19 diagnosis and monitoring.
Methods
We performed a systematic review and meta-analysis of studies comparing respiratory sampling strategies for the detection of SARS-CoV-2 RNA. The inclusion criteria were studies that assessed at least two respiratory sampling sites (oropharyngeal swab, nasopharyngeal swab, and sputum) in participants with COVID-19. The percentage positive tests were compared between sampling modalities by constructing a Z-test assuming independence and using the standard errors obtained from the random effects meta-analysis.
Findings
From 1039 total studies, we identified 11 studies that met our inclusion criteria, with SARS-CoV-2 testing results from a total of 3442 respiratory tract specimens. Compared to nasopharyngeal swab sampling, sputum testing resulted in significantly higher rates of SARS-CoV-2 RNA detection while oropharyngeal swab testing had lower rates of viral RNA detection. Earlier sampling after symptom onset was associated with improved detection rates, but the differences in SARS-CoV-2 RNA detection by sampling method was consistent regardless of the duration of symptoms.
Interpretation
The results support sputum sampling as a valuable method of COVID-19 diagnosis and monitoring, and highlight the importance of early testing after symptom onset to increase the rates of COVID-19 diagnosis.
Funding
This study was funded in part by the NIH grants U01AI106701 and by the Harvard University for AIDS Research (NIAID 5P30AI060354).
Keywords: COVID-19, SARS-CoV-2, Diagnosis, Nasopharyngeal, Oropharyngeal, Sputum
Research in context.
Evidence before this study
The accurate detection of SARS-CoV-2 through respiratory sampling is critical for the prevention of further transmission and the timely initiation of treatment for COVID-19.
A number of studies have compared the use of nasopharyngeal swab, oropharyngeal swab, or sputum in the detection of SARS-CoV-2 RNA. However, there is a diverse range in the reported SARS-CoV-2 detection rates with each of the sampling methods, leading to uncertainty about the optimal diagnostic modality. We performed a systematic review and meta-analysis of studies comparing respiratory sampling strategies for the detection of SARS-CoV-2 RNA. A computerised search was implemented in PubMed, MedRxiv and BioRxiv through April 30, 2020. The inclusion criteria were studies that assessed at least two respiratory sampling sites (oropharyngeal swab, nasopharyngeal swab, and sputum) in participants with COVID-19.
Added value of this study
To our knowledge, this is the first systematic review and meta-analysis on the optimal respiratory sampling for COVID-19 diagnosis and monitoring. We combined data from 11 papers that in total reported SARS-CoV-2 RNA testing from a total of 1299 nasopharyngeal swabs, 1083 oropharyngeal swabs, and 1060 sputum samples. Compared to nasopharyngeal swab sampling, sputum testing resulted in significantly higher rates of SARS-CoV-2 RNA detection while oropharyngeal swab testing had lower rates of viral RNA detection. Earlier sampling after symptom onset was associated with improved detection rates, but the differences in SARS-CoV-2 RNA detection by sampling method was consistent regardless of the duration of symptoms.
Implications of all the available evidence
These findings provide guidance on the optimal methods for the diagnosis and monitoring of COVID-19 patients. The results support the use of sputum testing as a valuable method for SARS-CoV-2 RNA detection and highlight the importance of early testing after symptom onset to increase the rates of COVID-19 diagnosis. For patients who are unable to provide sputum samples, nasopharyngeal swabs were superior to oropharyngeal swabs in the detection of SARS-CoV-2 RNA.
Alt-text: Unlabelled box
1. Introduction
The most common route of SARS-CoV-2 transmission is through exposure to respiratory secretions of close contacts [1] as the respiratory tract represents the major area of viral shedding [2]. The accurate diagnosis of coronavirus disease 2019 (COVID-19) infection through respiratory sampling is critical for the prevention of further transmission, clinical trial inclusion criteria and the timely initiation of treatment. In addition to its importance in diagnosis, respiratory sampling plays a central role in determining the duration of viral shedding, with implications for clinical management of potentially infectious patients, decisions on the duration of social isolation, and our understanding of viral transmission and pathogenesis [3].
Nasopharyngeal swabs are one of the most commonly used methods of respiratory secretion sampling for the detection of SARS-CoV-2 viral RNA. However, the use of nasopharyngeal swabs have a number of drawbacks, including that high-quality swab samples are technically challenging to obtain [4], nasopharyngeal swabbing increases the risk to healthcare providers due to the frequent induction of reflex sneezing/coughing, and the disruption of the supply of swabs, transport media, and personal protective equipment (PPE). For all of these reasons, there is intense interest in the comparison of nasopharyngeal swab and alternative sampling methods for the detection of SARS-CoV-2 RNA at respiratory sites. In Asia and other parts of the world, oropharyngeal swabs are a common method of COVID-19 diagnosis [2,3] and there is also interest in the study of sputum as an effective, and less invasive method of COVID-19 diagnosis.
In the published literature, there has been a wide variance in the reported SARS-CoV-2 detection rates with each of the diagnostic methods. In COVID-19 diagnosed individuals, the reported SARS-CoV-2 detection rate has ranged from 25% to >70% of collected nasopharyngeal swabs [5,6], 32% to 65% for oropharyngeal swabs [2,5], and 48% to >90% for sputum [7,8]. This has led to significant uncertainty and confusion in the field as to the reason behind the disparate testing results and the optimal diagnostic sampling strategy for diagnosis and monitoring of COVID-19 patients. In this study, we performed a systematic review of the literature and meta-analysis to compare the ability of nasopharyngeal swabs, oropharyngeal swabs, and sputum to detect SARS-CoV-2 RNA.
2. Materials and methods
2.1. Search strategy and selection criteria
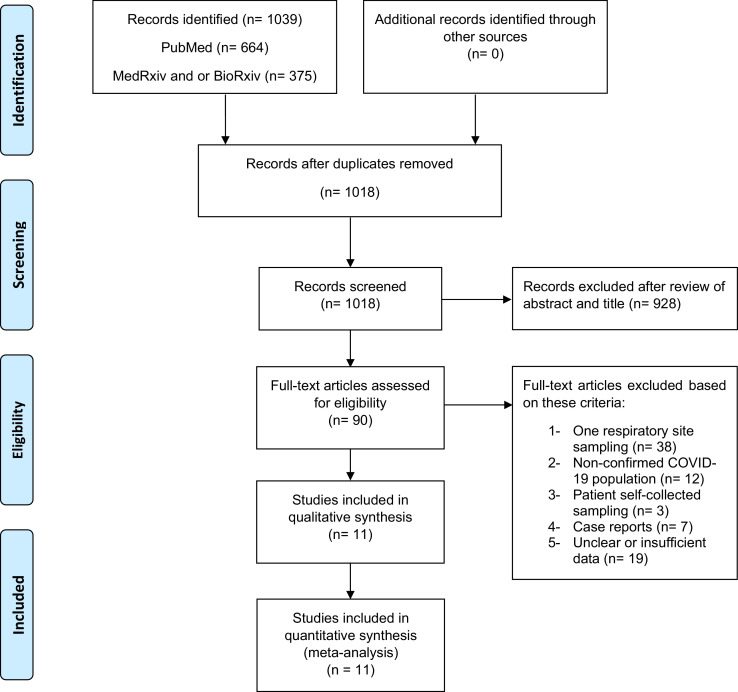
In this systematic review and meta-analysis, we used the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) to review literatures and report our results (Fig. 1). Preprint servers were included in our search given their role in the dissemination of COVID-19 research reports. A computerised search was implemented in PubMed, MedRxiv and BioRxiv using a search term, “((COVID OR COVID-19 OR SARS) AND (throat OR nasal OR nasopharyngeal OR oropharyngeal OR oral OR saliva OR sputum OR PCR))”. The search was completed through April 30, 2020. In addition, we reviewed the reference sections of relevant articles. We included studies that assessed at least two respiratory sites of sampling for individuals with confirmed COVID-19 and excluded studies with patient self-collected samples. When numerical results were not reported or further clarification was needed, we contacted the corresponding authors for additional information.
Fig. 1.
Study profile.
Two authors (AM, EE) screened the citations and three authors (AM, EE, YL) independently extracted data from the included studies. In the 11 studies included in this meta-analysis, we extracted summary estimates from tables or texts from four studies. For the other nine studies, we were able to obtain individual-level data from the manuscripts or study authors.
2.2. Statistical analysis
The estimated percentages of positive SARS-CoV-2 qPCR tests and the 95% confidence intervals (95% CI) were calculated for each sampling strategy using random-effects meta-analyses for binomial data using Stata Metaprop package [9]. We also performed an analysis stratified by the duration of symptoms prior to the testing, with results categorized as 0–7 days, 8–14 days, >14 days from symptom onset. We compared the proportion of positive tests between sampling sites by constructing a Z-test assuming independence and using the standard errors obtained from the random effects meta-analysis. For analyses involving studies with small sample size and sensitivity value extremely high (towards 1) or low (towards 0), we incorporated Freeman-Tukey Double Arcsine Transformation method to stabilize the variances for the by-study confidence intervals.
We used inconsistency index (I2) test to assess the heterogeneity between each study. Meta-analyses were performed using Stata 13.1 (StataCorp). GraphPad 8.5 (Prism) was used to demonstrate sensitivities at different time points from different sites of respiratory tracts. No adjustments for multiple comparisons were performed. Sensitivity analyses were performed that excluded preprints or included only studies that incorporated sputum sampling.
3. Results
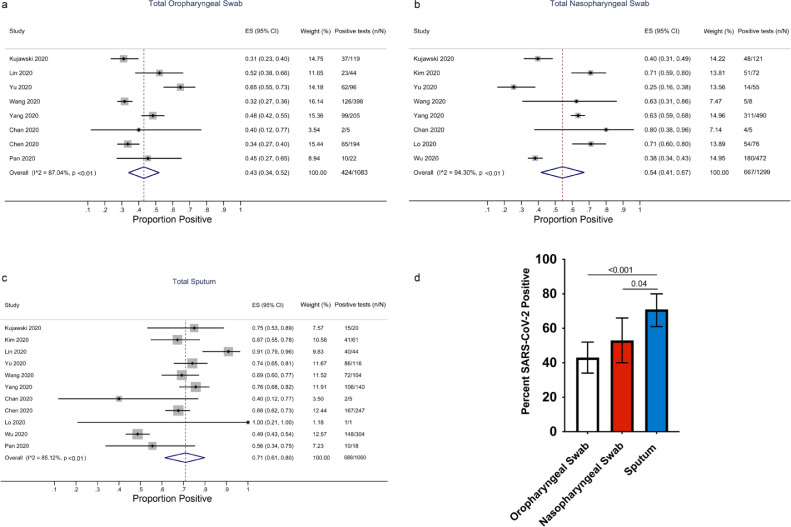
From the 1039 studies identified in our search, we excluded 21 duplicates and after screening the abstracts of the remaining articles, 90 full-text articles were obtained for further review (Fig. 1). Based on our selection criteria, 79 of those studies were excluded and 11 studies met our inclusion criteria [1,2,[5], [6], [7],[10], [11], [12], [13], [14], [15]] (Table 1). Of those, nine studies were from China. In total, 757 COVID-19 confirmed patients with 3442 respiratory samples were included in this analysis, including results from 1083 oropharyngeal swabs, 1299 nasopharyngeal swabs and 1060 sputum samples. By sampling method, the estimated percentage of positive samples was 43% (95% CI: 34–52%) for oropharyngeal swabs, 54% (95% CI: 41–67%) for nasopharyngeal swabs and 71% (95% CI: 61–80%) for sputum (Fig. 2a–c). The rate of SARS-CoV-2 detection was significantly higher in sputum than either oropharyngeal swabs or nasopharyngeal swabs (Fig. 2d).
Table 1.
Studies included in the Meta-Analysis.
| Country | Age Median (Range) | Sex (M%) | Patients (N) | Total samples | |
|---|---|---|---|---|---|
| Kujawski et al. (2020) [10] | US | 53 (2–68) | 68 | 12 | 219 |
| Kim et al. (2020) [6] | Korea | 40 (20–73) | 54 | 28 | 133 |
| Lin et al. (2020) [11] | China | 57 (38–84) | 52 | 52 | 88 |
| Yu et al. (2020) [5] | China | 40 (32–63) | 50 | 76 | 267 |
| Wang et al. (2020) [2] | China | 44 (5–67) | 68 | 205 | 525 |
| Yang et al. (2020) [12] | China | 52 (2–86) | 60 | 213 | 864 |
| Chan et al. (2020) [1] | China | 63 (10–66) | 50 | 5 | 15 |
| Chen et al. (2020) [13] | China | 36 (2–65) | 64 | 22 | 440 |
| Lo et al. (2020) [14] | China | 54 (27–64) | 30 | 10 | 85 |
| Wu et al. (2020) [7] | China | 67 ± 9a | 55 | 132 | 776 |
| Pan et al. (2020) [15] | China | .. | .. | 2 | 40 |
Mean age ± SD
Fig. 2.
Rates of SARS-CoV-2 detection by three methods of sampling. Forest plots of detection rates for oropharyngeal swabs (a), nasopharyngeal swabs (b), and sputum (c) and in a pooled analysis (d). The error bars in (d) are 95% confidence intervals (95% CIs). P-values were calculated by the Z-test.
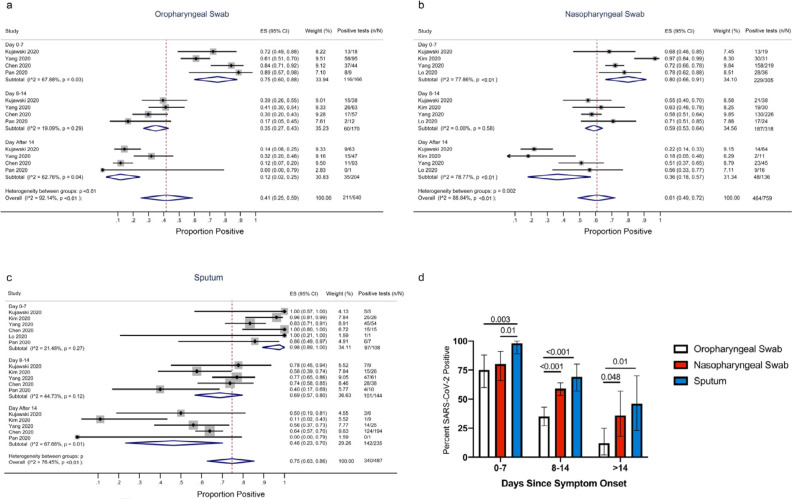
Among the 11 studies, 6 provided details on the time of sampling after symptom onset, including results from 540 oropharyngeal swabs, 759 nasopharyngeal swabs, and 487 sputum samples. For all sampling methods, rates of SARS-CoV-2 detection was highest early after symptom onset. For oropharyngeal swab sampling, the estimated percentage of positive tests were 75% (95% CI: 60–88%) between days 0–7, 35% (95% CI: 27–43%) between days 8–14 and 12% (95% CI: 2–25%) after 14 days from symptom onset (Fig. 3a). For nasopharyngeal swabs, the estimated percentage positive was 80% (95% CI: 66–91%), 59% (95% CI: 53–64%) and 36% (95% CI: 18–57%) at 0–7 days, 8–14 days and >14 days after symptom onset, respectively (Fig. 3b). For sputum, the estimated percentage positive was 98% (95% CI: 89–100%), 69% (95% CI: 57–80%), and 46% (95% CI: 23–70%) at 0–7 days, 8–14 days, and >14 days after symptom onset, respectively (Fig. 3c). For every time period, sputum had the highest percentage of positive results while oropharyngeal swabs had the lowest (Fig. 3d). In the overall pooled analysis, we detected significant heterogeneity between studies (Z-test P < 0.001) for all detection methods, Fig. 2a–c). Much of the heterogeneity between studies could be accounted for by differences in the participant populations, specifically the timing of symptom onset. In the analysis stratified by days since symptom onset, we observed substantially lower rates of heterogeneity between studies (Fig. 3a–c). We also performed a sensitivity analysis excluding preprint studies [12] and demonstrated similar results (Supplementary Figs. S1 and S2). We also performed a sensitivity analysis of only studies that included sputum sampling, and the results again showed that sputum sampling had the highest rates of positive results (Supplementary Fig. S3).
Fig. 3.
Rates of SARS-CoV-2 detection by three methods of sampling and time since symptom onset. Forest plots of detection rates for oropharyngeal swabs (a), nasopharyngeal swabs (b), and sputum (c) are categorized by days since symptom onset (0–7, 8–14, >14 days) and in a pooled analysis (d). The error bars in (d) are 95% confidence intervals (95% CIs). P-values were calculated by the Z-test.
4. Discussion
In this systematic review and meta-analysis, we combined data from 11 papers that in total reported SARS-CoV-2 RNA testing from 1299 nasopharyngeal swabs, 1083 oropharyngeal swabs, and 1060 sputum samples. The results demonstrate that the rate of sample positivity was highest in sputum specimens and lowest in oropharyngeal swabs. We detected heterogeneity between the reported results by study, much of which could be accounted for by differences in the timing of respiratory sampling. For all three sampling modalities, we found that the likelihood of a positive result declined with longer time since symptom onset. Regardless of the time frame studied, sputum consistently had the highest positive rates while oropharyngeal swabs had the lowest.
Like SARS-CoV-1, the morbidity and mortality of SARS-CoV-2 largely stem from lower respiratory tract disease [16,17]. Prior studies of individuals with more severe disease have noted a higher rate of SARS-CoV-2 detection and viral loads in lower respiratory tract samples, such as bronchoalveolar lavage fluid and endotracheal aspirates, compared to upper respiratory tract specimens [2,3]. These results are concordant with the far higher density of the SARS-CoV-2 viral target, the human angiotensin-converting enzyme 2 (ACE2) receptor, in pneumocytes and in lower airway epithelial cells compared to epithelial cells in the upper airway [18,19]. The higher SARS-CoV-2 detection rates in sputum samples could relate to at least partial sampling of the lower respiratory tract, although comparisons of SARS-CoV-2 RNA detection in concurrently collected sputum and saliva would help address this question. The results suggest that sampling of only upper respiratory tract samples, e.g., using nasopharyngeal swabs, may lead to missed diagnosis of COVID-19 and an inaccurate assumption of SARS-CoV-2 viral clearance that dictates the duration of social isolation and return to work policies.
Nasopharyngeal swabs remain the reference test for COVID-19 diagnosis in many parts of the world, but their use has a number of drawbacks. First, nasopharyngeal swabs can be technically challenging to obtain [4] and variable sample quality remains a problem. Second, nasopharyngeal swabbing causes discomfort and frequent reflex sneezing or coughing, and thus requires high-level personal protective equipment for healthcare workers, which are in short supply. Finally, there is a global shortage of nasopharyngeal swabs and transport medium that necessitates the search for potential alternative methods for the sampling of respiratory secretions. In addition to our finding of improved detection of SARS-CoV-2 RNA, the use of sputum sampling removes the need for difficult to obtain swabs, improves patient acceptance, and increases the chances of sample self-collection by the patient, which would decrease the risk to healthcare workers. Furthermore, the higher sensitivity of sputum for the detection of COVID-19 is also supported by previously reported studies on the detection of non-COVID-19 respiratory viruses [20], [21], [22].
One limitation of this meta-analysis is that these studies mainly enrolled hospitalized patients and it is unclear how the results may differ for individuals with asymptomatic infection or only mild symptoms. However, there are reports that rates of nasopharyngeal, oropharyngeal, and sputum positivity may not be substantially altered in those with mild symptoms versus those with severe disease [12]. The majority of studies included in this meta-analysis originated from China and additional studies are needed to assess generalizability. We also noted differences between studies in the qPCR assay and patient selection (Supplemental Tables 1 and 2) that could have accounted for some of the heterogeneity, although results were generally consistent within most studies and we were able to reduce the detected heterogeneity of the studies by comparing results by timing of symptom onset. A potential limitation with the use of sputum is that not all COVID-19 patients are able to expectorate sputum, which may be reflected by the lower number of tested sputum samples compared to nasopharyngeal or oropharyngeal swabs. However, for those who are unable to produce sputum, nasopharyngeal testing may continue to play an important role in the diagnosis or monitoring of COVID-19 patients. Recent reports have also suggested that saliva, mid-turbinate and anterior nasal swab sampling may represent simpler alternatives to nasopharyngeal swabs [23], [24], [25]. Our results support the need for carefully designed prospective studies comparing samples from different respiratory tract sites while controlling for potential confounders, including timing of collection, temperature variability, swab types, and laboratory processing procedures.
In summary, this systematic review and meta-analysis demonstrates that compared to nasopharyngeal swab sampling, sputum testing resulted in significantly higher rates of SARS-CoV-2 RNA detection while oropharyngeal swab testing had lower rates of viral RNA detection. Earlier sampling after symptom onset was associated with improved detection rates., but the differences in SARS-CoV-2 RNA detection was consistent between sampling strategies regardless of the duration of symptoms.
Contributors
AM, EE, YL, and JL performed the literature review and data abstraction. AM, EE, YL, RB, and JL were involved in the statistical analysis. All authors contributed to the writing and editing of the manuscript.
Funding sources
This study was funded in part by the NIH grants U01AI106701 and by the Harvard University for AIDS Research (NIAID 5P30AI060354). The funders had no role in study design, data extraction, data analysis, data interpretation, or writing of this study.
Declarations of Competing Interest
The other authors have nothing to disclose.
Acknowledgements
We would like to thank Dr. Myoung-don Oh for providing additional data from his manuscript and the authors of contributing studies and MedRxiv for making the findings available through publicly accessible pre-prints.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2020.102903.
Appendix. Supplementary materials
References
- 1.Chan J.F., Yuan S., Kok K.H. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang W., Xu Y., Gao R. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323(18):1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.To K.K., Tsang O.T., Leung W.S. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020;20(5):565–574. doi: 10.1016/S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marty F.M., Chen K., Verrill K.A. How to Obtain a Nasopharyngeal Swab Specimen. N Engl J Med. 2020;382(22):e76. doi: 10.1056/NEJMvcm2010260. [DOI] [PubMed] [Google Scholar]
- 5.Yu F., Yan L., Wang N. Quantitative detection and viral load analysis of SARS-CoV-2 in infected patients. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim E.S., Chin B.S., Kang C.K. Clinical course and outcomes of patients with severe acute respiratory syndrome Coronavirus 2 infection: a preliminary report of the first 28 patients from the Korean Cohort Study on COVID-19. J Korean Med Sci. 2020;35(13):e142. doi: 10.3346/jkms.2020.35.e142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu J., Liu J., Li S. Detection and analysis of nucleic acid in various biological samples of COVID-19 patients. Travel Med Infect Dis. 2020 doi: 10.1016/j.tmaid.2020.101673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan J.F., Yip C.C., To K.K. Improved Molecular Diagnosis of COVID-19 by the Novel, Highly Sensitive and Specific COVID-19-RdRp/Hel Real-Time Reverse Transcription-PCR Assay Validated In Vitro and with Clinical Specimens. J Clin Microbiol. 2020;58(5) doi: 10.1128/JCM.00310-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nyaga V.N., Arbyn M., Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health. 2014;72(1):39. doi: 10.1186/2049-3258-72-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The COVID-19 Investigation Team Clinical and virologic characteristics of the first 12 patients with coronavirus disease 2019 (COVID-19) in the United States. Nat. Med. 2020;26(6):861–868. doi: 10.1038/s41591-020-0877-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin C., Xiang J., Yan M., Li H., Huang S., Shen C. Comparison of throat swabs and sputum specimens for viral nucleic acid detection in 52 cases of novel coronavirus (SARS-Cov-2)-infected pneumonia (COVID-19) Clin Chem Lab Med. 2020;58(7):1089–1094. doi: 10.1515/cclm-2020-0187. [DOI] [PubMed] [Google Scholar]
- 12.Yang Y., Yang M., Shen C., et al. Evaluating the accuracy of different respiratory specimens in the laboratory diagnosis and monitoring the viral shedding of 2019-nCoV infections. medRxiv2020: 2020.02.11.20021493.
- 13.Chen C., Gao G., Xu Y. SARS-CoV-2-positive sputum and feces after conversion of pharyngeal samples in patients with COVID-19. Ann Intern Med. 2020;172(12):832–834. doi: 10.7326/M20-0991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lo I.L., Lio C.F., Cheong H.H. Evaluation of SARS-CoV-2 RNA shedding in clinical specimens and clinical characteristics of 10 patients with COVID-19 in Macau. Int J Biol Sci. 2020;16(10):1698–1707. doi: 10.7150/ijbs.45357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pan Y., Zhang D., Yang P., Poon L.L.M., Wang Q. Viral load of SARS-CoV-2 in clinical samples. Lancet Infect Dis. 2020;20(4):411–412. doi: 10.1016/S1473-3099(20)30113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ding Y., Wang H., Shen H. The clinical pathology of severe acute respiratory syndrome (SARS): a report from China. J Pathol. 2003;200(3):282–289. doi: 10.1002/path.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Menter T., Haslbauer J.D., Nienhold R. Post-mortem examination of COVID19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings of lungs and other organs suggesting vascular dysfunction. Histopathology. 2020 doi: 10.1111/his.14134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bosch B.J., Smits S.L., Haagmans B.L. Membrane ectopeptidases targeted by human coronaviruses. Curr Opin Virol. 2014;6:55–60. doi: 10.1016/j.coviro.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamming I., Timens W., Bulthuis M.L., Lely A.T., Navis G., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jeong J.H., Kim K.H., Jeong S.H., Park J.W., Lee S.M., Seo Y.H. Comparison of sputum and nasopharyngeal swabs for detection of respiratory viruses. J Med Virol. 2014;86(12):2122–2127. doi: 10.1002/jmv.23937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Falsey A.R., Formica M.A., Walsh E.E. Yield of sputum for viral detection by reverse transcriptase PCR in adults hospitalized with respiratory illness. J Clin Microbiol. 2012;50(1):21–24. doi: 10.1128/JCM.05841-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Branche A.R., Walsh E.E., Formica M.A., Falsey A.R. Detection of respiratory viruses in sputum from adults by use of automated multiplex PCR. J Clin Microbiol. 2014;52(10):3590–3596. doi: 10.1128/JCM.01523-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wyllie A.L., Fournier J., Casanovas-Massana A., et al. Saliva is more sensitive for SARS-CoV-2 detection in COVID-19 patients than nasopharyngeal swabs. medRxiv2020: 2020.04.16.20067835.
- 24.Tu Y.P., Jennings R., Hart B. Swabs collected by patients or health care workers for SARS-CoV-2 testing. N Engl J Med. 2020 doi: 10.1056/NEJMc2016321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pere H., Podglajen I., Wack M. Nasal swab sampling for SARS-CoV-2: a convenient alternative in times of nasopharyngeal swab shortage. J Clin Microbiol. 2020;58(6) doi: 10.1128/JCM.00721-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.