Abstract
Tenacity--persistence in the face of challenge--has received increasing attention, particularly because it contributes to better academic achievement, career opportunities and health outcomes. We review evidence from non-human primate neuroanatomy and structural and functional neuroimaging in humans suggesting that the anterior mid cingulate cortex (aMCC) is an important network hub in the brain that performs the cost/benefit computations necessary for tenacity. Specifically, we propose that its position as a structural and functional hub allows the aMCC to integrate signals from diverse brain systems to predict energy requirements that are needed for attention allocation, encoding of new information, and physical movement, all in the service of goal attainment. We review and integrate research findings from studies of attention, reward, memory, affect, multimodal sensory integration, and motor control to support this hypothesis. We close by discussing the implications of our framework for educational achievement, exercise and eating disorders, successful aging, and neuropsychiatric disorders such as depression and dementia.
Keywords: tenacity, anterior mid-cingulate cortex, energy regulation
When faced with a difficult challenge, such as mastering complex equations or training for a marathon, many individuals will find the effort too costly, and withdraw. Others, however, will marshal their resources, and persist in their efforts against the same challenges, even in the absence of immediate reward. This individual difference has received a great deal of attention in recent years, as growing research indicates that individuals who persevere in the face of challenging situations show better life outcomes in the domains of health, academic achievement, and career success (Duckworth and Quinn, 2009; Duckworth and Gross, 2014).
This tendency to carry on despite difficulty has been called by multiple names. Recent studies of life achievement describe it as ‘grit2019, defined as ‘passion and perseverance toward especially long-term goals’ (Duckworth and Quinn, 2009; Duckworth and Gross, 2014). Others have used the term ‘persistence’, (defined as ‘the ability to generate and maintain arousal internally’)(Cloninger et al., 1993; Holroyd and Umemoto, 2016), ‘perseverance’ (defined as ‘the maintenance of effort over time’(Patzelt et al., 2019), or defined it against its opposite, ‘apathy’ (Le Heron et al., 2019). Here we use the term tenacity to describe both a persistent pattern of behavior, and a fundamental bias in effort computations (as described by (Shenhav and Botvinick, 2013; Shenhav et al., 2013; Shenhav et al., 2017)), by which the costs of effort are devalued, and the value of long-term rewards is emphasized.
Multiple prominent theories of effort and motivation posit an important role for the anterior portion of the cingulate cortex in mediating individual differences in personality which promote tenacious behavior (Holroyd, 2016; Holroyd and Umemoto, 2016; Le Heron et al., 2017; Le Heron et al., 2019; Patzelt et al., 2019).
In this paper, we will review evidence supporting this view, focusing on how anterior mid-cingulate cortex (aMCC) plays a central role in establishing tenacity. Due to its position at the intersection of multiple intrinsic networks, the aMCC can integrate signals related to interoception, allostasis, executive function, motor planning, and sensory integration. We will argue that this uniquely connected position allows aMCC to weigh predicted energy requirements against predicted rewards and allocate physiological and attentional resources to achieve desired goals.
To support this view, we will first present evidence from neuroanatomy, tractography, and intrinsic functional connectivity imaging indicating that the aMCC is a major hub, sitting at the intersection of multiple intrinsic brain networks, and among the most broadly connected regions of the brain. Next, we will review functional evidence of a variety of task domains to show that the aMCC is a domain-general ‘hot-spot’ in the brain, implicated in a wide variety of tasks. We will then consider how variability in aMCC function may account for the difference between tenacity and withdrawal in the face of challenge. Finally, we will review studies of aMCC structure and function in cases of tenacity as well as apathy or withdrawal, and consider the implications of our hypothesis for educational achievement, successful aging, exercise and obesity, and neuropsychiatric disorders such as depression and dementia
Anterior Mid-Cingulate Cortex as a Structural and Functional hub: Neuroanatomy and Connectivity
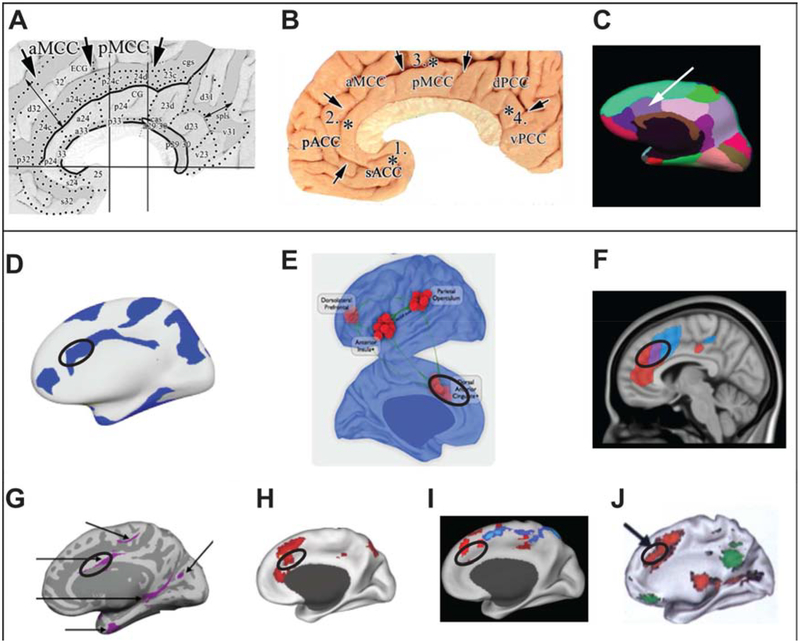
Since 1907 when the anterior division of the mid-cingulate cortex was first described (Smith, 1907; see (Vogt, 2016)), our understanding of the aMCC organization has significantly advanced. In humans, the agranular aMCC is located dorsal to the genu of the corpus callosum, extending rostrally to the border of the superior frontal gyrus, encompassing areas 32’ and a24c’ and areas in the rostral and caudal cingulate premotor areas (rCMA/ rCPMA) (Picard and Strick, 2001; Palomero-Gallagher et al., 2008; Vogt, 2016) (see Figures 1A, B, C; see also Figure 1 and 3 in (Vogt, 2016). The cytoarchitecture and neurocytology of aMCC point to fundamental differences between the aMCC and anterior cingulate cortex (ACC) (see Figures 3, 4 in (Vogt, 2016)). However, many neuroimaging studies often employ other terminologies such as dorsal ACC to refer to aMCC. In this article, all figure labels refer to aMCC. This may not be the term used in the papers we discuss but using this term we aim to accurately refer to the neuroanatomical region reported in the original text.
Figure 1.
Neuroanatomy and connectivity of the aMCC. Four Cingulate Regions & Subregions proposed by Vogt (A)(Vogt, 2016) and (B)(Vogt, 2005); Freesurfer cortical parcellation of the aMCC (white arrow) (C) (Desikan et al., 2006); aMCC (black circle) as a member of the brain’s ‘rich club’ hubs (D)(van den Heuvel and Sporns, 2013a); aMCC (black circle, labeled as ‘dACC’ by the authors) as a key region of the multimodal integration network (E)(Sepulcre et al., 2012); aMCC (black circle) sits at the nexus (purple) of two salience subsystems; the dorsal salience subsystem (blue) associated with executive function and the ventral salience subsystem (red) associated with visceroautonomic processing (F)(Touroutoglou et al., 2012); aMCC (black circle) as a key region of the large-scale allostatic/interoceptive system (G)(Kleckner et al., 2017), frontoparietal control system (H)(Vincent et al., 2008), ventral attention system (I)(Fox et al., 2006), and cinguloopercular network (J)(Dosenbach et al., 2007).
Invasive tract tracing studies in macaques (the monkey aMCC comprises areas a24a’, a24b’ and a24c’ but lacks area 32’; see Figure 3 in Vogt, 2016) (Bates and Goldam-Rakic, 1993; Picard and Strick, 2001; Morecraft et al., 2012; Procyk et al., 2016) and diffusion weighted tractography in humans (see Figure 5 in (Beckmann et al., 2009) have both demonstrated a distinct aMCC structural connectivity pattern from the rest of the cingulate cortex. The aMCC has strong connections with frontoparietal and temporal regions (i.e., dorsal prefrontal cortex, medial and lateral orbitofrontal cortex, anterior and posterior insula, caudal parietal cortex, rostral superior temporal gyrus), motor regions (i.e., premotor, supplementary motor cortices) as well as subcortical structures (i.e., thalamus, dorsal striatum, amygdala, hypothalamus, and periaqueductal gray) (Mesulam and Mufson, 1982; Bates and Goldam-Rakic, 1993; Morecraft and Van Hoesen, 1998; Picard and Strick, 2001; Morecraft et al., 2012; Procyk et al., 2016). This pattern of aMCC structural connectivity led Morecraft and Van Hoesen (Morecraft and Van Hoesen, 1998) to propose that the aMCC may serve as a cortical entry point for limbic influence on the voluntary motor system. The aMCC includes also dopaminergic, noradrenergic and serotonergic terminals (Williams and Goldman-Rakic, 1998; Bar et al., 2016). These dense monoamine innervations suggest a crucial role in motivational functions for aMCC (Vogt, 2016).
Recent advances in human brain connectomics have further identified aMCC as a major structural hub central to maximizing communication between multiple areas (Hagmann et al., 2008; van den Heuvel and Sporns, 2011; Nijhuis et al., 2013; van den Heuvel and Sporns, 2013b, a) (see Figure 1D). Compared to other brain regions, hub regions, such as the aMCC, are substantially more structurally connected with the rest of the brain (Hagmann et al., 2008; van den Heuvel et al., 2012) and are metabolically more active (Collin et al., 2014). Network analysis studies of intrinsic brain connectivity have also identified the aMCC as a functional hub on the basis of its spontaneous activity (Margulies et al., 2007). It has been demonstrated that aMCC is as a key region in a multimodal network that integrates information originating from primary sensory regions (e.g., visual, auditory, and somatosensory) (Sepulcre et al., 2012) (see Figure 1E). Furthermore, neuroimaging studies reveal that the aMCC belongs to at least five, partially overlapping, intrinsic brain networks. Some of these have been associated with visceromotor functions conventionally referred to as the “salience” (Seeley et al., 2007; Touroutoglou et al., 2012) and “allostatic-interoceptive” networks (Kleckner et al., 2017) (see Figures 1F, G) and others with executive function, attention, and motor control conventionally referred to as “frontoparietal control” (Vincent et al., 2008), “ventral attention” (Fox et al., 2006), and “cingulo-operculum control” networks (Dosenbach et al., 2007; Nelson et al., 2010) (see Figures 1H, I, J). Collectively, the findings above point to the possibility that the aMCC serves as a structural and functional hub of communication, that synchronizes information from otherwise segregated systems (see Figure 1F). We propose that the aMCC’s connections allow it to integrate information from multiple brain networks to drive goal-directed behaviors. In other words, its position allows it to participate in the willed control of our behavior (Paus, 2001; Parvizi et al., 2013).
Anterior Mid-Cingulate Cortex as a “Hot Spot” in the Brain: Task-related fMRI
Due to its hub centrality, the aMCC is well situated to receive a wide range of signals from other brain regions. Consistent with its diverse inputs, the aMCC region has been implicated in a large and diverse set of distinct tasks (see Table 1 for examples of evidence supporting the ubiquity of aMCC activations across tasks), including those involving motor function and executive function, memory, emotion, pain, and somatosensation (Beckmann et al., 2009; Shackman et al., 2011; Bahlmann et al., 2015; Wager et al., 2016). Meta-analytic evidence further indicates that the aMCC is among the most consistently reported areas of activation in functional MRI (fMRI) studies over the past 20 years (Yarkoni et al., 2011); for a meta-analysis of 1,114 studies see (Nelson et al., 2010); for a meta-analysis of 5,633 studies see (Clark-Polner et al., 2016)) (see Figures 2A and B). Compared to other activation foci, the aMCC also exhibits the highest degree of functional diversity (Anderson et al., 2013; Bertolero et al., 2015) and flexibility (Yeo et al., 2015; Betzel et al., 2017) (see Figures 2C, D). Furthermore, due to its participation in a diverse range of tasks, the aMCC is included in a set of co-activating regions collectively known as the “multiple demand network” (Duncan, 2010)(see Figure 2E).
Table 1.
Examples of neuroscience evidence that aMCC participates in a wide range of tasks.
| Observation | Task domain | Citations | Example Figure |
|---|---|---|---|
| aMCC is robustly engaged by pain and negative affect. | Pain, Negative affect | (Derbyshire et al., 2004; Vogt, 2005; Shackman et al., 2011; Yarkoni et al., 2011; Lindquist et al., 2012; Lieberman and Eisenberger, 2015) | See Fig.2 in Derbyshire et al., 2004 |
| aMCC functions as a ‘neural alarm’, directing attention toward potential conflicts with enduring survival goals. For example, aMCC is implicated in the experience of hunger, thirst, and breathlessness. | Pain, Negative affect | (Lieberman and Eisenberger, 2015) | See Fig. 6 in Lieberman & Eisenberger, 2015 |
| aMCC activation is associated with a variety of emotions, such as fear, disgust, anger and sadness. | Pain, Negative affect | (Lindquist et al., 2012; Touroutoglou et al., 2015; Raz et al., 2016) | See Fig. 4 in Lindquist et al., 2012 |
| aMCC is anticipating and predicting pending noxious stimulation, so as to prepare avoidance responses. For example, aMCC activity increases as a function of the proximity of a tarantula to the participants’ foot. | Pain, Negative affect | (Mobbs et al., 2010; Vogt, 2016) | See Fig. 2 in Mobbs et al., 2010 |
| aMCC activity in difficult tasks is linked to negative affect during task. For example, aMCC response to error processing tracks within-subject changes in felt frustration. | Pain, Negative affect | (McGuire and Botvinick, 2010; Spunt et al., 2012) | See Fig. 2 in Spunt et al., 2012 |
| The aMCC is engaged by positive experiences, particularly in reward-based decision-making tasks. The aMCC tracks both the magnitude and the probability of predicted rewards. | Reward decision making | (Kouneiher et al., 2009; Lindquist et al., 2012; Bahlmann et al., 2015) | See Fig. 3A in Kouneiher et al., 2009 |
| aMCC integrates reward with motor responses. For example, a reduction in an anticipating reward significantly increases the firing rate of aMCC neurons in a way that is directly linked with the movement ultimately made. | Reward decision making | (Williams et al., 2004) | See Fig. 1 in Williams et al., 2004 |
| The aMCC is sensitive to both increases and decreases in reward. Its signal during reward- decision making approximates an underlying U-shaped function, indicative of signal related to arousal or salience processing. | Reward decision making | (Bush et al., 2002; Rushworth and Behrens, 2008; Bartra et al., 2013) | See Fig. 10 in Bartra et al., 2013 |
| aMCC is engaged by the degree of difficulty in demanding tasks. For example, greater aMCC activity is associated with increased working memory load, more challenging mental arithmetic, memory retrieval over longer delays and more precise visual discrimination. | Effort Cognitive and Motor Control | (Duncan and Owen, 2000; Davis et al., 2005; Cole and Schneider, 2007; Duncan, 2010; Boehler et al., 2011; Duncan, 2013; Engstrom et al., 2013; Fedorenko et al., 2013; Power and Petersen, 2013; Dhanjal and Wise, 2014; Hoffstaedter et al., 2014) | See Fig. 2 in Fedorenko et al. 2013 |
| aMCC plays a role in predicting effort requirements. For example, its activity in learning tasks is modulated by previous trials in a way that speeds responses to trials of equivalent difficulty, and slows them when difficulty levels change. | Effort, Cognitive and Motor Control | (Modirrousta and Fellows, 2008; Sheth et al., 2012) | See Fig. 1 in Sheth et al. 2012 |
| aMCC activates when requirements change, errors are detected, available options are in conflict, novel tasks are encountered or alternative course of actions are being considered. | Effort, Cognitive and Motor Control | (Raichle et al., 1994; Bush et al., 1998; Barch et al., 2001; Ullsperger and von Cramon, 2001; Botvinick et al., 2004; Jessup et al., 2010; Nee et al., 2011; Kolling et al., 2014). | See Fig. 9 in Nee et al., 2011 |
| aMCC signal increases in response to prediction errors. | Effort, Cognitive and Motor Control | (Jocham et al., 2009; Sheth et al., 2012; Kolling et al., 2016) | See Fig. 5 in Jocham et al., 2009 |
| aMCC is engaged in social processing. For example, aMCC activations are observed during the Ultimatum Game, a social interaction task that particularly requires predicting and monitoring the effects of decisions on the behavior of others. | Social Cognition tasks | (Kirk et al., 2011; Apps et al., 2013) | See Fig. 2 in Kirk et al., 2011 |
| The aMCC is a core node of the central autonomic network that calibrates bodily reactions to match anticipated outcomes. | Autonomic reactivity tasks | (Beissner et al., 2013) (Critchley et al., 2000; Critchley et al., 2003; Critchley, 2009; Wager et al., 2009; Hermans et al., 2011; Gianaros and Wager, 2015) | See Fig. 1 in Beissner et al. 2013 |
| aMCC integrates pain, arousal, motivation and cognitive control. | Integrative function | (Shackman et al., 2011; Bahlmann et al., 2015). | See Fig. 2 in Shackman et al., 2011 |
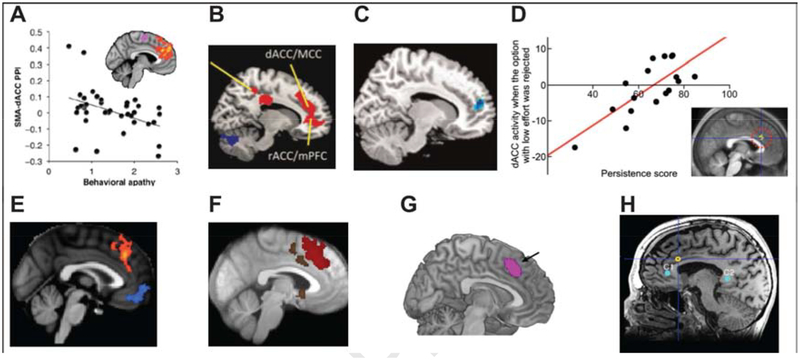
Figure 2.
The role of aMCC in tenacious behaviors. Stronger functional connectivity between aMCC (labeled as ‘dACC’ by the authors) and supplementary motor is linked to lower levels of apathy (A) (Bonnelle et al., 2016); stronger functional connectivity between aMCC and ventral striatum is associated with grit (B) (Myers et al., 2016); spontaneous aMCC activity predicts grit (C)(Wang et al., 2017b); greater aMCC (labeled as ‘dACC’ by the authors) activity is associated with higher levels of persistence (D)(Kurniawan et al., 2010); aMCC signal is associated with willingness to exert more effort (E)(Bonnelle et al., 2016); aMCC activity increases during effort magnitude estimation (F)(Scholl et al., 2015); aMCC signal tracks the subjective value of effort exerted (G)(Chong et al., 2017); aMCC stimulation (yellow circle) increases the will to persevere (H)(Parvizi et al., 2013).
The common engagement of the aMCC by diverse tasks suggests that the region may play an integrative role across multiple brain systems. A number of convergent functions have been suggested, including integration of information about pain and negative affect in guiding behavior (Shackman et al., 2011), integration of signals related to motivation with cognitive control (Bahlmann et al., 2015), the computation of optimal levels of cognitive control or effort (Botvinick and Braver, 2015; Shenhav et al., 2016; Shenhav et al., 2017), the processing of prediction error in effortful tasks (Vassena et al., 2017a), and the selection of and persistence in pursuit of high-level goals (Holroyd and Yeung, 2012; Holroyd and Umemoto, 2016) (See Box 1).
Box 1: Theories of anterior Mid-Cingulate Cortex Function.
Early theories of aMCC function focused on the aMCC’s role in selecting actions, as in cases where two behaviors are in conflict (Botvinick et al., 2001; van Veen et al., 2001; Botvinick et al., 2004), or when errors must be monitored (Brown and Braver, 2005; Holroyd and Coles, 2008); see (Vassena et al., 2017a) for review). This led to the view that the aMCC’s role is the deployment of cognitive control to aid in action selection (see (Vassena et al., 2017a)). However, these theories cannot account for evidence of increased aMCC engagement in situations when participants have only one response option, but motivational conditions are altered through changes in reward or task demands (Vassena et al., 2014).
We hypothesize that the aMCC performs all of these functions in the service of a broader common goal: efficient energy regulation. On this view, aMCC activation is ubiquitous because energy regulation computations are ubiquitous, accompanying the consideration of every action or personally relevant event (see (Barrett and Simmons, 2015; Barrett, 2017b, a)) (see also Box 1). Our hypothesis is that the aMCC’s position as a hub at the nexus of motor, visceromotor and attention systems allows it to integrate information related to interoception, executive function, sensory processing and motor control to anticipate expected physiological demands and then to effectively deploy the body’s energy resources to achieve goals. This domain general function would require multiple component computations, which have been observed as aMCC engagement in a wide variety of tasks.
An important component of efficient energy computations, observed in multiple task-evoked fMRI studies of aMCC (Jocham et al., 2009; Sheth et al., 2012; Kolling et al., 2016) is the prediction of behavioral outcomes, so that the value of potential actions can be assessed. Consistent with the predictive function of aMCC (see also Box 1), there is substantial evidence of aMCC activation in situations where pain or reward is anticipated, or novel unexpected events occur (Raichle et al., 1994; Kouneiher et al., 2009; Mobbs et al., 2010). The aMCC activations during the experience of reward or punishment (Kouneiher et al., 2009; Lindquist et al., 2012; Bahlmann et al., 2015) can thus be understood as testing expected hedonic values against experience. Activations during social behavior (Kirk et al., 2011; Apps et al., 2013) may represent comparisons between the anticipated and actual behavior of others. Similarly, the aMCC is active during task selection (Kolling et al., 2014) as predicted outcomes are compared; during task performance (Modirrousta and Fellows, 2008; Sheth et al., 2012) as outcomes are tested against predictions; and during effortful tasks (Boehler et al., 2011; Engstrom et al., 2013; Fedorenko et al., 2013) as predictions of available resources are updated and additional cognitive and physiological resources are deployed to meet the task demands.
In addition to potential rewards, assessment of the energetic costs of task performance is crucial to efficient energy regulation. The aMCC appears to encode not only the value of a reward but also the cost of the effort required to obtain it (Harris and Lim, 2016). This effort may take the form of increased cognitive control, which is frequently experienced as aversive (Dreisbach and Fischer, 2012; Schouppe et al., 2012; Kurzban et al., 2013; Inzlicht et al., 2015; Saunders et al., 2015). Indeed, activity of the aMCC region during difficult tasks is positively correlated with a subjective sense of frustration (Spunt et al., 2012), as well as desire to avoid the task (McGuire and Botvinick, 2010).
Additionally, some evidence suggests that the aMCC also represents physiological costs of action by monitoring the internal state of the body. Multiple studies have implicated the aMCC in tasks involving interoception and pain, hunger, thirst, and breathlessness, leading Lieberman and Eisenberger (2015) to describe the aMCC as a ‘neural alarm’, that serves to direct attention toward potential conflicts with enduring survival goals.
When expectations of the cost and benefit of goal pursuit are violated, it is crucial for efficient energy regulation that resource allocation be quickly recalibrated. Multiple theories suggest a role for aMCC in preparing control systems for future demands by adjusting to prediction errors (Alexander and Brown, 2011; Barrett and Simmons, 2015; Alexander and Brown, 2017). For example, its activity in learning tasks is modulated by previous trials in a way that speeds responses to trials of equivalent difficulty, and slows them when difficulty levels change (Modirrousta and Fellows, 2008; Sheth et al., 2012). In another example, the aMCC responses to pain are modulated by the belief that the pain can be controlled (Salomons et al., 2004), suggesting that this region may play a role in anticipating and predicting pending noxious stimulation, so as to prepare avoidance responses (Vogt, 2016).
Following recalibration of energetic computations, it may be necessary to deploy additional energy resources to support task demands, or to limit resources in order to suppress costly behavior. Consistent with this view, some studies (Beissner et al., 2013) indicate that the aMCC may also serve to modulate the internal state of the body to prepare for action, through alterations in blood pressure, heart rate, and hormonal responses. A potential mechanism through which this could be achieved is by the regulation of arousal to prepare for action. The aMCC is well equipped to modulate states of arousal through its connections to mid-brain nuclei (Bar et al., 2016), and substantial research indicates that the aMCC indeed exerts regulatory control over various autonomic processes (Beissner et al., 2013). For example, cognitive and social stressors known to evoke autonomic stress responses robustly engage the aMCC (Wager et al., 2009; Gianaros and Wager, 2015) and increased blood flow in the aMCC is correlated with increases in blood pressure and heart rate variability, evoked by both mental (working memory) and physical (isometric exercise) exertion (Critchley et al., 2000; Critchley et al., 2003). Acute stressors of the sort that evoke stress hormone release also activate the aMCC (Gianaros and Wager, 2015), and the degree of stress-evoked activation predicts the magnitude of the hormonal stress response (Hermans et al., 2011).
Thus, due to its position as a network hub, the aMCC can synchronize information from diverse systems in order to guide behavior towards efficient energy balance. However, the effectiveness of aMCC computations are likely to vary between individuals, particularly in situations when the cost of effort is high, and rewards are uncertain or deferred. Under these circumstances, some will demonstrate tenacity and persist while others may simply quit. In the following section, we will consider how variability in aMCC function could underlie individual differences of this sort in tenacity.
Holroyd & Yeung (2012) attempted to reconcile evidence for the aMCC role in motivation and decision-making, with their Hierarchical Learning Model, which holds that the primary role of aMCC is the selection and maintenance of ‘options’, or high-level abstract behavioral plans generated from nested reinforcement learning (Holroyd and Yeung, 2012). Thus, the aMCC will be particularly active when the value of competing options must be compared, or when habitual responses must be suppressed in service of a larger goal. It will also be engaged when behavioral plans must be re-evaluated in the face of changing demands or reward conditions. On this view, then, the aMCC serves to direct behavior towards goals computed to be most valuable by regulating systems involved in cognitive control and task execution (Holroyd and Yeung, 2012).
Others have emphasized the notion that the aMCC computes not only the value of competing options, but also the hedonic costs of exerting effort and cognitive control to pursue selected goals. Thus, on this view, aMCC activation during action selection primarily represents the computation of the resources required to complete the task (Botvinick, 2007; Botvinick and Braver, 2015). In their Expected Value of Control model, Shenhav and colleagues (Shenhav et al., 2013; Shenhav et al., 2017) argue that the role of the aMCC is to track the cost of both mental and physical effort exerted during task performance, predict the cost of effort required for all available options, and then integrate that information with expectations of reward to determine if the exertion of cognitive control is worthwhile (Shenhav et al., 2013; Shenhav et al., 2017). This theory can thus account for aMCC activation in response to effort, changes in reward, action selection and cognitive control as expressions of the same core function.
Value computations of this sort, however, depend critically on the accuracy of estimates of future behavioral outcomes. Thus, some theories hold that the core, domain-general function of aMCC is preparing control systems for future demands by predicting the results of possible actions, and comparing these predictions to experience (Alexander and Brown, 2011, 2017). According to these models, mismatches between predicted and experienced outcomes generate a ‘prediction error’ signal, which can be used to update future predictions. On this view, aMCC activity in response to increased effort represents the processing of error in the prediction of expected task demands (Vassena et al., 2017b). Consistent with predictive models of aMCC function, there is substantial evidence of aMCC activation in situations where there is no choice to be made, or behavior to perform, such as when pain or reward is simply anticipated, or novel events occur (Vassena et al., 2014; Vassena et al., 2017b).
Other predictive models have argued that the aMCC’s serves not only to predict external events, but also internal states of the body, allowing for the maintenance of allostasis (i.e. efficient energy regulation through prediction of future energy needs) (Barrett and Simmons, 2015) [see also (Barrett, 2017b, a)]. To effectively calculate the expected value of a behavior, it is crucial to anticipate the costs in terms of physiological resources, and to deploy additional resources through arousal regulation when necessary. To maintain goal-directed behavior, these interoceptive predictions must be compared to experience, so that prediction error can be used to adjust energy resources to meet task needs. Chanes and Barret (2016) have also speculated that aMCC with its extensive connections is well suited to provide an integrated workspace for unified conscious experience due to its ability to represent information across different modalities based on allostatic relevance.
The Role of Anterior Mid-Cingulate Cortex in Tenacity
Together, the various aMCC computations described in the previous section serve to regulate the amount of effort directed toward any potential behavior. The function of such effort regulation has been debated, with most theories falling into two broad categories (see(Shenhav et al., 2017)). Some accounts hold that effort serves to manage intrinsic costs to finite resources such as metabolic resources (Gailliot and Baumeister, 2007; Gailliot et al., 2007; Holroyd, 2016), short-term memory(Elmore et al., 2011), or processing capacity (Wickens, 1984; Feng et al., 2014). Other accounts emphasize the management of opportunity costs (Kurzban et al., 2013); see also (Shenhav et al., 2017), arguing that effort regulation ensures that cognitive control will be exerted only towards the highest value option currently available.
Our view is that the overarching function of effort computations (and indeed, a fundamental goal of the nervous system – see (Kleckner et al., 2017)) is the maintenance of allostasis. While we posit that the allocation of effort towards any particular behavior is a result of computations which serve to minimize opportunity costs, we also hold that information about available resources and distance from energy balance are important factors entering into these computations, as the estimated value of prospective rewards and costs of prospective effort are in part determined by the current and predicted energy state of the individual.
This account suggests that individual differences in effort computations could make the difference between persistence and withdrawal during demanding tasks. An individual might continue to exert effort on a demanding task because she estimates the value of an expected reward as greater than others do, because he perceives the cost of action as lower than others do, or because she perceives the body’s available resources to be relatively greater. Indeed, while high levels of effort are generally experienced as aversive, some individuals rate effort as more aversive than others (McGuire and Botvinick, 2010; Dreisbach and Fischer, 2012; Schouppe et al., 2012; Spunt et al., 2012; Kurzban et al., 2013; Inzlicht et al., 2015; Saunders et al., 2015).
We propose that tenacity can be understood as a kind of bias in aMCC computations: a tendency to maintain the representation of expected rewards, devalue the cost of effort and to judge one’s available physiological resources as meeting or exceeding task demands even in the face of negative affect. There are three sources of evidence that the aMCC is involved in tenacity: lesion studies, neuroimaging studies, and stimulation studies. We review each in turn.
Lesion Studies:
In animal research, lesion studies in rodents and primates have long implicated a region homologous to MCC to be critical for integration of reward and effort costs to motivate behavior (Walton et al., 2007; Kampe et al., 2009; Holroyd and McClure, 2015; Salamone et al., 2016) (see also (Le Heron et al., 2019) for animal studies showing the role of MCC in motivation). Indeed, in rats, inactivation of ACC (a region homologous to aMCC in humans) causes animals to reduce willingness to expend mental effort (Hosking et al., 2014) and decrease the energy/time expenditure required to obtain a proportional reward (Wang et al., 2017a). In monkeys, lesion studies also (Chudasama et al., 2013) demonstrate a critical role for ACC/MCC in sustaining effective choice behavior in the face of changing biological needs. Consistent with these findings, we recently demonstrated that the monkey aMCC is part of an intrinsic connectivity network that subserves arousal regulation (Touroutoglou et al., 2016).
Clinical research in humans has also indicated that damage to the aMCC or its connections can produce profound motivational changes. Reports of damage to the cingulate influencing motivation go back to Papez (1937, see (Szczepanski and Knight, 2014)], who observed that tumors pressing on the cingulate cortex led to “indifference to the environment, change in personality or character, [and a] stuporous or comatose state”. More recent studies have connected damage to the aMCC region with motivational impairment, apathy and inability to plan for long term goals (Devinsky et al., 1995; van Reekum et al., 2005; Szczepanski and Knight, 2014; Ducharme et al., 2017).
Naccache et al. (2005) examined the motivational disruptions associated with cingulate damage in a revealing case study of an individual with a lesion encompassing aMCC extending to dorsomedial prefrontal cortex (Naccache et al., 2005). The patient was presented with a number of tasks requiring executive function designed such that the difficulty of the tasks could be systematically varied. While the patient was able to perform these tasks in a relatively unimpaired manner, he reported no subjective sense of effort, or change in perceived effort as the difficulty increased. Furthermore, he showed no physiological evidence of increased effort, unlike controls, whose skin conductance increased along with difficulty.
Neuroimaging Studies:
Multiple neuroimaging studies have indicated that variability in aMCC function may account for the difference between tenacity and withdrawal in the face of challenge.
A major potential source of individual variability in tenacity is the strength of communication between the aMCC and other regions involved in signaling reward, autonomic processing or exercising cognitive control. It has been shown that the strength of intrinsic functional connectivity between aMCC and striatal regions predicted survey measures of both ‘grit’ and ‘growth mindset’ (the belief that cognitive abilities are malleable, and can be improved through hard work) (Myers et al., 2016). Furthermore, stronger structural and functional connectivity between aMCC and supplementary motor area predicts lower levels of apathy (apathy defined as lack of motivation to initiate behavior or respond to environmental stimuli) (Bonnelle et al., 2016) (see Figures 2A and 2B).
More direct evidence comes from a recent study examining the neural substrates of grit, demonstrating that individual’s grit is related to the spontaneous activity of aMCC (Wang et al., 2017b)(see Figure 2C). The magnitude of activity within the aMCC during effort judgments has also been shown to predict persistence (see Figure 2D). Indeed, aMCC activity during trials where participants chose a more difficult task over an easier one was positively associated with trait-level persistence (Kurniawan et al., 2010). In another study, Bonnelle et al. (2016) further reported that greater activity in the aMCC during cost-benefit weighing was associated with willingness to exert more effort (Bonnelle et al., 2016) (see Figure 2E). Relatedly, Scholl and colleagues (2015) reported that aMCC activity was greater during the choice of high effort vs. low effort options (Scholl et al., 2015) (see Figure 2F). Consistent with these findings, Spielberg and colleagues place the aMCC in a network involved in maintaining goal pursuit (Spielberg et al., 2012) (see their Figure 3c).
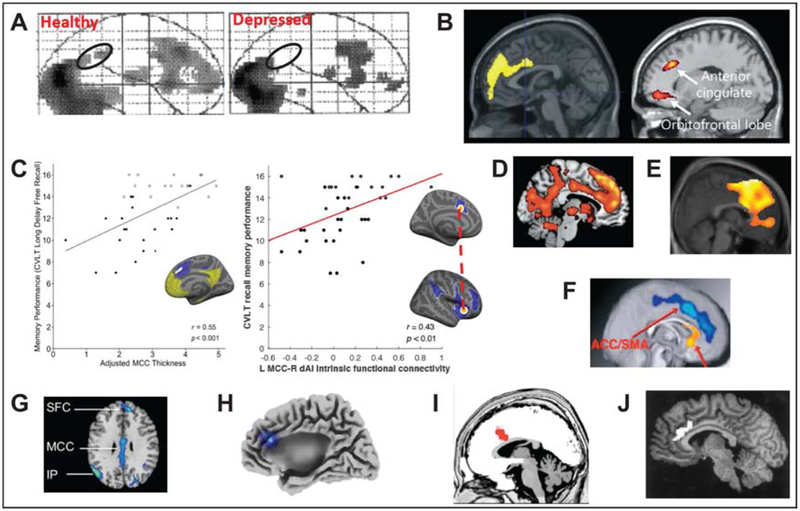
Figure 3.
Implications of the aMCC role in tenacity. Compared to healthy controls, patients with depression show reduced regional cerebral blood flow in the aMCC (black circle) during an effortful task (A)(Elliott et al., 1997); apathy scores correlate with altered glucose metabolism in aMCC in early dementia including Alzheimer’s disease, frontotemporal dementia (left, (Schroeter et al., 2011) and Parkinson’s disease (right, labeled as ‘ACC’ by the authors (Huang et al., 2013) (B); aMCC cortical thickness (left, aMCC indicated with white)(Sun et al., 2016) and intrinsic connectivity to anterior insula (right (Zhang et al., 2019)) both predict successful memory performance in superagers and typical older adults (C); aMCC signal increases during effortful memory retrieval in older adults (D)(Dhanjal and Wise, 2014); high exercise intensity is linked to metabolic changes in aMCC (E)(Kemppainen et al., 2005); gray matter volume in frontal regions including the aMCC was increased (blue) for aerobic exercisers relative to nonaerobic controls (F)(Colcombe et al., 2006); obese adolescents show aMCC weaker activation in response to foods compared to lean adolescents (G) (Carnell et al., 2017); transcranial pink noise stimulation of aMCC decreases self-reported desire to eat in women with obesity (H)(Leong et al., 2018); aMCC activity during task increases in individuals who follow the task instructions closely (I)(Mulert et al., 2005); aMCC regional blood flow is associated with faster reaction times in a somatosensory reaction time task (J)(Naito et al., 2000).
Other task-related studies have indicated that the aMCC serves to integrate information about cost and benefit to sustain motivated behavior. Chong et al. (2017) reported that aMCC activity was associated with a preference to make an effort in return for reward, regardless of whether the task required mental or physical effort exertion (Chong et al., 2017) (see Figure 2G).
Additionally, at least one study has related aMCC anatomy to a tenacious mindset. Van Schuerbeek and colleagues (Van Schuerbeek et al., 2011) reported that greater gray matter volume in a cluster including the aMCC and pMCC predicted higher levels of persistence as assessed by personality inventory (see Figure 4 in (Van Schuerbeek et al., 2011)).
Taken together, these findings indicate that the aMCC plays an important role in judgments of the subjective value of effort that can influence the choice between responding with tenacity or withdrawal.
Stimulation Studies:
Perhaps the strongest evidence for a role of aMCC in tenacity can be found in studies of experimental stimulation of the aMCC. Multiple studies have reported goal-oriented behaviors in response to aMCC stimulation (Escobedo and Cravioto, 1973; Talairach et al., 1973; Bancaud et al., 1976; Kremer et al., 2001; Chassagnon et al., 2008; Caruana et al., 2018). In a recent well-designed study, Caruana and colleagues analyzed the effect of electrical stimulation applied to1789 cingulate sites, in 329 patients and provided causal data related to role of aMCC in motivation. Consistent with the role of aMCC in tenacity, the authors found that the stimulation of aMCC elicits a variety of goal-oriented behaviors [(see Figure 3 in Caruana et al. (2018)]. The results lead the authors to suggest that “aMCC might provide the motivational drive to perform actions, playing an excitatory and/or inhibitory role on these motor circuits”. Interestingly, they authors further demonstrated that aMCC controls these goal-oriented behaviors according to a dorsoventral ‘actotopic’ organization: stimulation of dorsal aMCC evoked movements directed towards the body (e.g., rubbing the eyes); stimulation of middle aMCC elicited actions in the peripersonal space (e.g. exploratory gaze movements); and stimulation of ventral aMCC led to whole body movements towards extrapersonal space (e.g. the impulse to get up as some patients described it: ‘I felt I was willing to go away’). Taken together, it is possible that the aMCC function in motivation may be embodied, as suggested by Apps (2018) organized within a space- or body-framed topography.
In another study that used a structured task assessing effort perception, Zenon and colleagues (2015) reported that transcranial magnetic stimulation of supplementary motor area (an area strongly connected to aMCC) significantly reduced the perceived aversiveness of a physically demanding task. This resulted in a greater willingness to exert effort, even for a reduced reward (Zenon et al., 2015). However, given the close proximity of supplementary motor area and aMCC, it is difficult to assess the degree of aMCC contribution to this effect.
A more compelling example is provided by Parvizi and colleagues (Parvizi et al., 2013), who employed direct electrical stimulation of the aMCC, producing what they described as an increase in the ‘will to persevere (Parvizi et al., 2013) (see Figure 2H). Patients generally described the experience of aMCC stimulation as evoking the feeling of preparing for a difficult challenge. As one patient put it: “I started getting this feeling like … I was driving into a storm. […] like, you’re headed towards a storm […] and you’ve got to get across the hill and all of a sudden you’re sitting there going how am I going to get over that, through that?”
Implications of the aMCC Role in Tenacity for Achievement, Aging, Health, and Illness
Tenacity will influence performance particularly wherever there is challenge. In this section, we will consider evidence that aMCC structure and function predicts life and health outcomes across multiple domains. We will limit our focus on the extreme ends of aMCC variability. We will first consider the role of aMCC dysfunction in apathy (or absence of motivation), specifically in cases of depression and neurodegenerative disease. We will then review findings indicating that greater aMCC integrity predicts tenacity that is associated with positive life outcomes in aging, health, and academics.
Depression:
Apathy, defined by DSM-5 as a pronounced lack of motivation, goal-directed behavior and emotional responsiveness, is among the most commonly reported symptoms of depression (Pizzagalli, 2014). Numerous studies demonstrate that patients with depression are less willing to expend effort for rewards than controls (Treadway et al., 2012). Given the role of aMCC in effort processing, as Holroyd (2012) and others have argued, it seems likely that the aMCC is compromised in depressed individuals. Indeed, a substantial body of evidence also implicates aMCC dysfunction in depression (Chen et al., 2007; Steele et al., 2007; Crossley et al., 2014) (see also (Holroyd and Umemoto, 2016; Vogt, 2016)). It should be noted, however, that depression is a heterogeneous clinical syndrome and as such, distinct dimensions of symptomology are associated with alteration of brain function in distinct regions (Drysdale et al., 2017). Indeed, in addition to apathy, depression is defined by a pronounced anhedonia and negative attentional bias which have been alleviated by deep brain stimulation of other brain regions, notably the subgenual ACC(Holtzheimer and Mayberg, 2012).
Dysfunction of the aMCC seems particularly relevant to motivational problems, rather than hedonic components of depression. It has been shown that the degree of reduction in aMCC volume predicts severity of apathetic symptoms in depression (Lavretsky et al., 2007). Furthermore, depressed individuals exhibit reduced activation of aMCC during a complex planning task (Elliott et al., 1997) (see Figure 3A). Disruption of aMCC function could mediate apathy through disruption of reward processing (Holroyd and Umemoto, 2016), as aMCC response to reward learning is reduced in depression (Kumar et al., 2008). Apathy may also be related to impaired ability of aMCC to effectively adjust to prediction errors. In one study employing a gambling task (Steele et al., 2007), healthy individuals responded to negative feedback with aMCC activation and improved reaction times, while depressed individuals showed neither aMCC engagement nor behavioral improvement following errors (Steele et al., 2007). Collectively, these studies are consistent with the proposal that depression disrupts the aMCC’s ability to act as the central hub (Pizzagalli, 2014), thus compromising motivation (Holroyd and Umemoto, 2016).
Neurodegenerative Disease:
Apathy has also been observed in a wide range of neurological disorders, including Alzheimer’s disease (Mega et al., 1996), behavioral variant of frontotemporal dementia (bv-FTD) (Ducharme et al., 2017) and Parkinson’s disease (Aarsland et al., 2009). In a recent review, Le Heron and colleagues argue that despite the diversity among these disorders in causes and behavioral symptoms, all of these disorders share a common neural feature: the disruption of an interconnected group of brain regions involved in reward processing and cognitive control, with the aMCC at its core (see Figure 4 in (Le Heron et al., 2017).
Indeed, in Alzheimer’s disease, the magnitude of abnormal aMCC function (Migneco et al., 2001; Schroeter et al., 2011; Le Heron et al., 2017) is linked to apathetic symptoms (see Figure 3B). In bvFTD, apathy has been associated with atrophy, hypometabolism and/or hypoperfusion in aMCC and its connected regions, including dorsolateral prefrontal cortex, orbitofrontal cortex, and the medial and ventromedial superior frontal gyri (Franceschi et al., 2005; Schroeter et al., 2011; Ducharme et al., 2017; Le Heron et al., 2017). Similarly, altered metabolism in aMCC region is associated with the degree of apathy in Parkinson’s disease (Huang et al., 2013; Le Heron et al., 2017).
Superaging:
Emerging research suggests that exceptional cases of healthy aging may be associated with increased tenacity. While in the majority of elderly people, episodic memory function declines with age (Grady and Craik, 2000; Cansino, 2009) accompanied by a number of age-related neurobiological changes, including structural atrophy (Brickman et al., 2007; Bakkour et al., 2013), loss of intrinsic network coherence (Andrews-Hanna et al., 2007; Wang et al., 2010), and altered brain activity during memory (Grady et al., 2006; Maillet and Rajah, 2014), there are exceptions. Multiple recent studies have identified a remarkable subgroup of elderly people, known as ‘superagers’, whose performance on some cognitive measures is equivalent to middle aged (Gefen et al., 2014) and even young (Sun et al., 2016) adults, despite their advanced age.
Many of the most pronounced neurobiological differences between superagers and typical older adults involve the structure and function of the aMCC. Anatomically, we showed that the cortical thickness of aMCC in superagers is actually equivalent to young controls (Sun et al., 2016), exceeding the thickness of the middle-aged (Harrison et al., 2012). Recently, we further demonstrated that superagers not only have greater cortical thickness in aMCC but also exhibit greater intrinsic functional connectivity between aMCC and major nodes of the salience network when compared to typical older adults (Zhang et al., 2019). Importantly, the thickness and network connectivity of the aMCC can predict successful memory performance in older adults (see Figure 3C). Thus, superagers show a more ‘youthful’ pattern of memory function, aMCC structure and network connectivity than most elderly people.
Given that cognitive effort is especially costly for older adults (Westbrook et al., 2013), does this preserved aMCC function and cognitive ability in superagers indicate a more ‘tenacious’ attitude towards challenging tasks? As aMCC activity is associated with exertion of effort in response to multiple cognitive demands (Fedorenko et al., 2013), persistence in the face of difficulty should be associated with greater aMCC activity during memory performance. Indeed, during difficult trials on a memory task, elderly people show greater aMCC activity than during easier trials (see Figure 3D) (Dhanjal and Wise, 2014).
Exercise and Obesity and Eating Disorders:
Another area where an understanding of the neural basis of tenacity could have profound implications for public health is the study of exercise and obesity. Maintenance of an exercise regimen requires tenacity, and indeed individual differences in grit predict adherence to physical exercise (Reed et al., 2013). Studies of brain metabolism during exercise further indicate that aMCC metabolism relates to exercise intensity, and also that this coupling is significantly stronger in individuals with greater exercise capacity (who most likely exercise regularly) (Kemppainen et al., 2005)(see Figure 3E). Thus, aMCC may be better able to accurately assess the metabolic costs of exertion in tenacious individuals. Additionally, some evidence suggests that regular exercise may actually increase aMCC volume (Colcombe et al., 2006) (see Figure 3F), suggesting the exciting possibility that it may be possible to physically ‘train up’ aMCC function, and in turn, tenacity.
Tenacity may also influence weight loss outcomes through the ability to defer rewards, as well as the willingness to engage in effortful exercise. As such variability in aMCC function may be an important predictor of success in dieting. Choosing healthy foods over more calorically dense options is associated with greater aMCC activity (Harding et al., 2017), and a greater aMCC response to calorically dense foods is associated with reduced obesity risk (Carnell et al., 2017) (see Figure 3G). Thus, increased aMCC activation in response to food seems to be related to the deployment of cognitive control. Notably, formerly obese individuals who have successfully maintained weight loss show substantially enhanced aMCC (superior frontal/cingulate by authors) activation in response to food cues (McCaffery et al., 2009), indicating that aMCC responses to food stimuli is associated with a greater ability to self-regulate appetite.
If aMCC activation in response to food-associated cues indicates the deployment of cognitive control resources, then treatments that augment aMCC activity could potentially result in more effective self-regulation of cravings. In fact, at least one attempt to experimentally bolster aMCC processing has shown some success in influencing appetite. Leong et al. (2018) showed in women with obesity that transcranial pink noise stimulation targeted at the aMCC region results in a reduction in self-reported appetite on a ‘desire to eat’ scale (Leong et al., 2018) (see Figure 3H).
We note, however, that increased fixation to goals achieved by aMCC may be linked to abnormal self-monitoring problems that are commonly seen in the pathophysiology of eating disorders (EDs). Indeed, a recent review of fMRI studies demonstrated that anorexia nervosa (AN), bulimia nervosa (BN), and binge eating disorder (BED are associated with altered activation and connectivity in aMCC (Gaudio et al., 2016; Steward et al., 2018). It has been shown that patients with BN and AN tend to exhibit greater functional connectivity of MCC (dACC by the authors) and precuneus (Lee et al., 2014), and this connectivity predicts higher levels of body shape concerns seen in these patients. Consistent with these findings, Geisler et al. used a probabilistic reversal learning task and showed increased activation of aMCC during negative feedback in a group of patients with AN. Importantly, this activation correlated with higher levels of perfectionism (Geisler et al., 2017). As the authors suggest, hyperfunctioning of aMCC may be related to increased fixation to goals (e.g. skinny body) often associated with AN. Interestingly, the abnormal functioning of the aMCC (and its connected regions) seems to improve in recovered patients with ED (for a review see (Steward et al., 2018)).
Taken together, these findings suggest the possibility of neuromodulation of the aMCC as a treatment for obesity and eating disorders, although further research is necessary to assess whether the effects of neuromodulation would lead to lasting behavioral change.
Academic Achievement and Professional Success:
In addition to conveying resilience to negative health outcomes, substantial and growing evidence indicates that grit and persistence predict achievement in multiple areas, such as educational achievement and professional success (Duckworth and Gross, 2014). Both grit and persistence have been associated with aMCC function; spontaneous aMCC activity predicts grit (Wang et al., 2017b), and greater aMCC volume predicts persistence (Van Schuerbeek et al., 2011). This suggests that aMCC may contribute to the increased achievement associated with tenacity.
Indeed, growing evidence connects tenacity, aMCC activity and better performance. Mulert et al. (2005) has demonstrated that when individuals follow task instructions, there is a close relationship between conscious effort and aMCC activity (Mulert et al., 2005)(see Figure 3I). Across three different tasks, Naito et al. (2000) also found a significant positive correlation between blood flow in aMCC and speeded reactions (Naito et al., 2000) (see Figure 3J). More direct evidence comes from a recent study that demonstrated a link between aMCC function and academic performance, noting that spontaneous activity in this region is correlated with both grit scores (see Figure 2C) and academic performance (Wang et al., 2017b). Critically, mediation analysis showed that the grit-academics relationship is mediated by the aMCC activity, indicating that superior aMCC function explains greater academic achievement in tenacious individuals.
Conclusion and Future Directions:
Tenacity is a powerful predictor of health and achievement and research on its neural basis could offer greater understanding of the qualities that promote exceptional achievement. The preceding evidence suggests a central role for the aMCC in subserving tenacity. Positioned at the intersection of systems involved in autonomic processing, interoception, executive function, motor planning, and sensory integration, the aMCC receives the information necessary to perform domain-general computational functions to mobilize physiological and cognitive resources to meet task needs. Such processes may include the (a) prediction of behavioral outcomes, (b) assessment of the energetic costs of task performance, (c) monitoring the internal state of the body, (d) adjusting to prediction errors, and (e) modulation of the internal state of the body to prepare for action. We propose that tenacity can be understood as a kind of bias in these aMCC computations: a tendency to maintain the representation of expected rewards and to judge one’s available physiological resources as meeting or exceeding task demands. Consistent with this view, we presented emerging evidence indicating that greater aMCC structure and function are linked to tenacious behavior in many domains of life and health. In contrast, the disruption of aMCC has been associated with apathy and other motivational problems implicated in many neuropsychiatric disorders (Le Heron et al., 2017). Thus, understanding how the aMCC can contribute to achieving goals can also potentially provide therapeutic insight into health difficulties ranging from obesity and eating disorders to depression and dementia.
One intriguing possibility is that the structure and function of aMCC could be altered with sufficient behavioral training. Indeed, as a flexible hub, the MCC may be better equipped than other brain regions to reshape its connectivity in response to learning. It has been proposed that protein receptors important for plasticity (such as CaMKII, NR2B, NMDA) are highly expressed in limbic circuits including aMCC (Wei et al., 1999; Palomero-Gallagher et al., 2008; Garcia-Cabezas et al., 2017; Burt et al., 2018; Palomero-Gallagher and Zilles, 2019). Interestingly, Garcia-Cabezas et al. (2017) recently showed that in adult monkeys, markers of synaptic plasticity are high in MCC (and other limbic regions) while markers of stability (as expressed by cellular factors that inhibit synaptic plasticity) are low. These findings suggest that training related improvement in behaviors requiring tenacity may be mediated through the aMCC. We have seen evidence that aMCC can be ‘trained up” in the domain of exercise (Colcombe et al., 2006). Future studies (see Outstanding Questions Box) might focus on the development of interventions in aMCC activity and connectivity in other domains. Such interventions may have a broad therapeutic value (Downar et al., 2016; Le Heron et al., 2017).
Outstanding Questions Box.
If preserved aMCC anatomy is associated with successful aging, does early aMCC development predict later life success?
Does the ability of aMCC to efficiently regulate energy resources in response to laboratory challenges also translate to resilience to stressful life events such as grief, professional failure, or divorce?
Can you make a person be tenacious? Can tenacity be trained behaviorally in school or later in life? If for example regular exercise influences aMCC volume, could exercise also influence tenacity?
Can we alter the level of tenacity via noninvasive stimulation of the aMCC in a way that leads to lasting behavioral changes? Which neuromodulation techniques can specifically stimulate the aMCC?
Given that the aMCC is a linchpin area receiving inputs from multiple brain networks, does tenacity imply a more functionally connected brain?
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: none
References
- Aarsland D, Marsh L, Schrag A (2009) Neuropsychiatric symptoms in Parkinson’s disease. Movement Disorders 24: 2175–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander WH, Brown JW (2011) Medial prefrontal cortex as an action-outcome predictor. Nat Neurosci 14:1338–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander WH, Brown JW (2017) The Role of the Anterior Cingulate Cortex in Prediction Error and Signaling Surprise. Top Cogn Sci. [DOI] [PubMed] [Google Scholar]
- Anderson ML, Kinnison J, Pessoa L (2013) Describing functional diversity of brain regions and brain networks. Neuroimage 73:50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, Buckner RL (2007) Disruption of large-scale brain systems in advanced aging. Neuron 56:924–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apps MA, Lockwood PL, Balsters JH (2013) The role of the midcingulate cortex in monitoring others’ decisions. Front Neurosci 7:251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apps MAJ (2018) Stimulating cingulate: distinct behaviours arise from discrete zones. Brain 141:2827–2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahlmann J, Aarts E, D’Esposito M (2015) Influence of motivation on control hierarchy in the human frontal cortex. J Neurosci 35:3207–3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakkour A, Morris JC, Wolk DA, Dickerson BC (2013) The effects of aging and Alzheimer’s disease on cerebral cortical anatomy: specificity and differential relationships with cognition. Neuroimage 76:332–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bancaud J, Talairach J, Geier S, Bonis A, Trottier S, Manrique M (1976) [Behavioral manifestations induced by electric stimulation of the anterior cingulate gyrus in man]. Rev Neurol (Paris) 132:705–724. [PubMed] [Google Scholar]
- Bar KJ, de la Cruz F, Schumann A, Koehler S, Sauer H, Critchley H, Wagner G (2016) Functional connectivity and network analysis of midbrain and brainstem nuclei. Neuroimage 134:53–63. [DOI] [PubMed] [Google Scholar]
- Barch DM, Braver TS, Akbudak E, Conturo T, Ollinger J, Snyder A (2001) Anterior cingulate cortex and response conflict: effects of response modality and processing domain. Cereb Cortex 11:837–848. [DOI] [PubMed] [Google Scholar]
- Barrett LF (2017a) How emotions are made: The secret life of the brain.. New York: Houghton Mifflin Harcourt. [Google Scholar]
- Barrett LF (2017b) The theory of constructed emotion: an active inference account of interoception and categorization. Soc Cogn Affect Neurosci 12:1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, Simmons WK (2015) Interoceptive predictions in the brain. Nat Rev Neurosci 16:419–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartra O, McGuire JT, Kable JW (2013) The valuation system: a coordinate-based meta-analysis of BOLD fMRI experiments examining neural correlates of subjective value. Neuroimage 76:412–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates JF, Goldam-Rakic PS (1993) Prefrontal Connections of Medial Motor Areas in the Rhesus Monkey. E JOURNAL OF COMPARATIVE NEUROLOGY 336:211–228. [DOI] [PubMed] [Google Scholar]
- Beckmann M, Johansen-Berg H, Rushworth MF (2009) Connectivity-based parcellation of human cingulate cortex and its relation to functional specialization. J Neurosci 29:1175–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beissner F, Meissner K, Bar KJ, Napadow V (2013) The autonomic brain: an activation likelihood estimation meta-analysis for central processing of autonomic function. J Neurosci 33:10503–10511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolero MA, Yeo BT, D’Esposito M (2015) The modular and integrative functional architecture of the human brain. Proc Natl Acad Sci U S A 112:E6798–6807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betzel RF, Satterthwaite TD, Gold JI, Bassett DS (2017) Positive affect, surprise, and fatigue are correlates of network flexibility. Sci Rep 7:520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehler CN, Hopf JM, Krebs RM, Stoppel CM, Schoenfeld MA, Heinze HJ, Noesselt T (2011) Task-load-dependent activation of dopaminergic midbrain areas in the absence of reward. J Neurosci 31:4955–4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnelle V, Manohar S, Behrens T, Husain M (2016) Individual Differences in Premotor Brain Systems Underlie Behavioral Apathy. Cereb Cortex 26:807–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick MM (2007) Conflict monitoring and decision making: reconciling two perspectives on anterior cingulate function. Cogn Affect Behav Neurosci 7:356–366. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver T (2015) Motivation and cognitive control: from behavior to neural mechanism. Annu Rev Psychol 66:83–113. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS (2004) Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn Sci 8:539–546. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD (2001) Conflict monitoring and cognitive control. Psychol Rev 108:624–652. [DOI] [PubMed] [Google Scholar]
- Brickman AM, Habeck C, Zarahn E, Flynn J, Stern Y (2007) Structural MRI covariance patterns associated with normal aging and neuropsychological functioning. Neurobiol Aging 28:284–295. [DOI] [PubMed] [Google Scholar]
- Brown JW, Braver TS (2005) Learned predictions of error likelihood in the anterior cingulate cortex. Science 307:1118–1121. [DOI] [PubMed] [Google Scholar]
- Burt JB, Demirtas M, Eckner WJ, Navejar NM, Ji JL, Martin WJ, Bernacchia A, Anticevic A, Murray JD (2018) Hierarchy of transcriptomic specialization across human cortex captured by structural neuroimaging topography. Nat Neurosci 21:1251–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Whalen PJ, Rosen BR, Jenike MA, McInerney SC, Rauch SL (1998) The counting Stroop: an interference task specialized for functional neuroimaging--validation study with functional MRI. Hum Brain Mapp 6:Hum Brain Mapp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Vogt BA, Holmes J, Dale AM, Greve D, Jenike MA, Rosen BR (2002) Dorsal anterior cingulate cortex: a role in reward-based decision making. Proc Natl Acad Sci U S A 99:523–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cansino S (2009) Episodic memory decay along the adult lifespan: a review of behavioral and neurophysiological evidence. Int J Psychophysiol 71:64–69. [DOI] [PubMed] [Google Scholar]
- Carnell S, Benson L, Chang KV, Wang Z, Huo Y, Geliebter A, Peterson BS (2017) Neural correlates of familial obesity risk and overweight in adolescence. Neuroimage 159:236–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruana F, Gerbella M, Avanzini P, Gozzo F, Pelliccia V, Mai R, Abdollahi RO, Cardinale F, Sartori I, Lo Russo G, Rizzolatti G (2018) Motor and emotional behaviours elicited by electrical stimulation of the human cingulate cortex. Brain 141:3035–3051. [DOI] [PubMed] [Google Scholar]
- Chanes L, Barrett LF (2016) Redefining the Role of Limbic Areas in Cortical Processing. Trends Cogn Sci 20:96–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassagnon S, Minotti L, Kremer S, Hoffmann D, Kahane P (2008) Somatosensory, motor, and reaching/grasping responses to direct electrical stimulation of the human cingulate motor areas. J Neurosurg 109:593–604. [DOI] [PubMed] [Google Scholar]
- Chen CH, Ridler K, Suckling J, Williams S, Fu CH, Merlo-Pich E, Bullmore E (2007) Brain imaging correlates of depressive symptom severity and predictors of symptom improvement after antidepressant treatment. Biol Psychiatry 62:407–414. [DOI] [PubMed] [Google Scholar]
- Chong TT, Apps M, Giehl K, Sillence A, Grima LL, Husain M (2017) Neurocomputational mechanisms underlying subjective valuation of effort costs. PLoS Biol 15:e1002598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudasama Y, Daniels TE, Gorrin DP, Rhodes SE, Rudebeck PH, Murray EA (2013) The role of the anterior cingulate cortex in choices based on reward value and reward contingency. Cereb Cortex 23:2884–2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark-Polner E, Wager TD, Satpute AB, Barrett LF (2016) Neural fingerprinting: Meta-analysis, variation, and the search for brain-based essences in the science of emotion In: Handbook of emotions, 4th Edition (Barrett LF, Lewis M, Haviland-Jones JM, eds). New York: Guilford. [Google Scholar]
- Cloninger CR, Svrakic DM, Przybeck TR (1993) A psychobiological model of temperament and character. Arch Gen Psychiatry 50:975–990. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Erickson KI, Scalf PE, Kim JS, Prakash R, McAuley E, Elavsky S, Marquez DX, Hu L, Kramer AF (2006) Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci 61:1166–1170. [DOI] [PubMed] [Google Scholar]
- Cole MW, Schneider W (2007) The cognitive control network: Integrated cortical regions with dissociable functions. Neuroimage 37:343–360. [DOI] [PubMed] [Google Scholar]
- Collin G, Sporns O, Mandl RC, van den Heuvel MP (2014) Structural and functional aspects relating to cost and benefit of rich club organization in the human cerebral cortex. Cereb Cortex 24:2258–2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD (2009) Psychophysiology of neural, cognitive and affective integration: fMRI and autonomic indicants. Int J Psychophysiol 73:88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Corfield DR, Chandler MP, Mathias CJ, Dolan RJ (2000) Cerebral correlates of autonomic cardiovascular arousal: a functional neuroimaging investigation in humans. J Physiol 523 Pt 1:259–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Josephs O, O’Doherty J, Zanini S, Dewar BK, Cipolotti L, Shallice T, Dolan RJ (2003) Human cingulate cortex and autonomic control: converging neuroimaging and clinical evidence. Brain 126:2139–2152. [DOI] [PubMed] [Google Scholar]
- Crossley NA, Mechelli A, Scott J, Carletti F, Fox PT, McGuire P, Bullmore ET (2014) The hubs of the human connectome are generally implicated in the anatomy of brain disorders. Brain 137:2382–2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis KD, Taylor KS, Hutchison WD, Dostrovsky JO, McAndrews MP, Richter EO, Lozano AM (2005) Human anterior cingulate cortex neurons encode cognitive and emotional demands. J Neurosci 25:8402–8406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derbyshire SW, Whalley MG, Stenger VA, Oakley DA (2004) Cerebral activation during hypnotically induced and imagined pain. Neuroimage 23:392–401. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ (2006) An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31:968–980. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA (1995) Contributions of anterior cingulate cortex to behaviour. Brain 118 (Pt 1):279–306. [DOI] [PubMed] [Google Scholar]
- Dhanjal NS, Wise RJ (2014) Frontoparietal cognitive control of verbal memory recall in Alzheimer’s disease. Ann Neurol 76:241–251. [DOI] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA, Fox MD, Snyder AZ, Vincent JL, Raichle ME, Schlaggar BL, Petersen SE (2007) Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci U S A 104:11073–11078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downar J, Blumberger DM, Daskalakis ZJ (2016) The Neural Crossroads of Psychiatric Illness: An Emerging Target for Brain Stimulation. Trends Cogn Sci 20:107–120. [DOI] [PubMed] [Google Scholar]
- Dreisbach G, Fischer R (2012) The role of affect and reward in the conflict-triggered adjustment of cognitive control. Front Hum Neurosci 6:342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drysdale AT et al. (2017) Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med 23:28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducharme S, Price BH, Dickerson BC (2017) Apathy: a neurocircuitry model based on frontotemporal dementia. J Neurol Neurosurg Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth AL, Quinn PD (2009) Development and validation of the short grit scale (grit-s). J Pers Assess 91:166–174. [DOI] [PubMed] [Google Scholar]
- Duckworth AL, Gross JJ (2014) Self-Control and Grit: Related but Separable Determinants of Success. Curr Dir Psychol Sci 23:319–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J (2010) The multiple-demand (MD) system of the primate brain: mental programs for intelligent behaviour. Trends Cogn Sci 14:172–179. [DOI] [PubMed] [Google Scholar]
- Duncan J (2013) The structure of cognition: attentional episodes in mind and brain. Neuron 80:35–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J, Owen AM (2000) Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci 23:475–483. [DOI] [PubMed] [Google Scholar]
- Elliott R, Baker SC, Rogers RD, O’Leary DA, Paykel ES, Frith CD, Dolan RJ, Sahakian BJ (1997) Prefrontal dysfunction in depressed patients performing a complex planning task: a study using positron emission tomography. Psychol Med 27:931–942. [DOI] [PubMed] [Google Scholar]
- Elmore LC, Ma WJ, Magnotti JF, Leising KJ, Passaro AD, Katz JS, Wright AA (2011) Visual short-term memory compared in rhesus monkeys and humans. Curr Biol 21:975–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engstrom M, Landtblom AM, Karlsson T (2013) Brain and effort: brain activation and effort-related working memory in healthy participants and patients with working memory deficits. Front Hum Neurosci 7:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobedo M, Cravioto J (1973) Studies on the malabsorption syndromes. Inhibition of (Na+-K+) ATPase of small intestine microvilli by pyrrolidone carboxylic acid. Clin Chim Acta 49:147–151. [DOI] [PubMed] [Google Scholar]
- Fedorenko E, Duncan J, Kanwisher N (2013) Broad domain generality in focal regions of frontal and parietal cortex. Proc Natl Acad Sci U S A 110:16616–16621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng SF, Schwemmer M, Gershman SJ, Cohen JD (2014) Multitasking versus multiplexing: Toward a normative account of limitations in the simultaneous execution of control-demanding behaviors. Cogn Affect Behav Neurosci 14:129–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME (2006) Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci U S A 103:10046–10051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi M, Anchisi D, Pelati O, Zuffi M, Matarrese M, Moresco RM, Fazio F, Perani D (2005) Glucose metabolism and serotonin receptors in the frontotemporal lobe degeneration. Ann Neurol 57:216–225. [DOI] [PubMed] [Google Scholar]
- Gailliot MT, Baumeister RF (2007) The physiology of willpower: linking blood glucose to self-control. Pers Soc Psychol Rev 11:303–327. [DOI] [PubMed] [Google Scholar]
- Gailliot MT, Baumeister RF, DeWall CN, Maner JK, Plant EA, Tice DM, Brewer LE, Schmeichel BJ (2007) Self-control relies on glucose as a limited energy source: willpower is more than a metaphor. J Pers Soc Psychol 92:325–336. [DOI] [PubMed] [Google Scholar]
- Garcia-Cabezas MA, Joyce MKP, John YJ, Zikopoulos B, Barbas H (2017) Mirror trends of plasticity and stability indicators in primate prefrontal cortex. Eur J Neurosci 46:2392–2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudio S, Wiemerslage L, Brooks SJ, Schioth HB (2016) A systematic review of resting-state functional-MRI studies in anorexia nervosa: Evidence for functional connectivity impairment in cognitive control and visuospatial and body-signal integration. Neurosci Biobehav Rev 71:578–589. [DOI] [PubMed] [Google Scholar]
- Gefen T, Shaw E, Whitney K, Martersteck A, Stratton J, Rademaker A, Weintraub S, Mesulam MM, Rogalski E (2014) Longitudinal neuropsychological performance of cognitive SuperAgers. J Am Geriatr Soc 62:1598–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler D, Ritschel F, King JA, Bernardoni F, Seidel M, Boehm I, Runge F, Goschke T, Roessner V, Smolka MN, Ehrlich S (2017) Increased anterior cingulate cortex response precedes behavioural adaptation in anorexia nervosa. Sci Rep 7:42066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Wager TD (2015) Brain-Body Pathways Linking Psychological Stress and Physical Health. Curr Dir Psychol Sci 24:313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL, Craik FI (2000) Changes in memory processing with age. Curr Opin Neurobiol 10:224–231. [DOI] [PubMed] [Google Scholar]
- Grady CL, Springer MV, Hongwanishkul D, McIntosh AR, Winocur G (2006) Age-related changes in brain activity across the adult lifespan. J Cogn Neurosci 18:227–241. [DOI] [PubMed] [Google Scholar]
- Hagmann P, Cammoun L, Gigandet X, Meuli R, Honey CJ, Wedeen VJ, Sporns O (2008) Mapping the structural core of human cerebral cortex. PLoS Biol 6:e159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding IH, Andrews ZB, Mata F, Orlandea S, Martinez-Zalacain I, Soriano-Mas C, Stice E, Verdejo-Garcia A (2017) Brain substrates of unhealthy versus healthy food choices: influence of homeostatic status and body mass index. Int J Obes (Lond). [DOI] [PubMed] [Google Scholar]
- Harris A, Lim SL (2016) Temporal Dynamics of Sensorimotor Networks in Effort-Based Cost-Benefit Valuation: Early Emergence and Late Net Value Integration. J Neurosci 36:7167–7183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison TM, Weintraub S, Mesulam MM, Rogalski E (2012) Superior memory and higher cortical volumes in unusually successful cognitive aging. J Int Neuropsychol Soc 18:1081–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans EJ, van Marle HJ, Ossewaarde L, Henckens MJ, Qin S, van Kesteren MT, Schoots VC, Cousijn H, Rijpkema M, Oostenveld R, Fernandez G (2011) Stress-related noradrenergic activity prompts large-scale neural network reconfiguration. Science 334:1151–1153. [DOI] [PubMed] [Google Scholar]
- Hoffstaedter F, Grefkes C, Caspers S, Roski C, Palomero-Gallagher N, Laird AR, Fox PT, Eickhoff SB (2014) The role of anterior midcingulate cortex in cognitive motor control: evidence from functional connectivity analyses. Hum Brain Mapp 35:2741–2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holroyd CB (2016) The waste disposal problem of effortful control In: Motivation and Cognitive Control (Braver T, ed), pp 235–260. New York: NY: Psychology Press. [Google Scholar]
- Holroyd CB, Coles MG (2008) Dorsal anterior cingulate cortex integrates reinforcement history to guide voluntary behavior. Cortex 44:548–559. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Yeung N (2012) Motivation of extended behaviors by anterior cingulate cortex. Trends Cogn Sci 16:122–128. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, McClure SM (2015) Hierarchical control over effortful behavior by rodent medial frontal cortex: A computational model. Psychol Rev 122:54–83. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Umemoto A (2016) The research domain criteria framework: The case for anterior cingulate cortex. Neurosci Biobehav Rev 71:418–443. [DOI] [PubMed] [Google Scholar]
- Holtzheimer PE, Mayberg HS (2012) Neuromodulation for treatment-resistant depression. F1000 Med Rep 4:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosking JG, Cocker PJ, Winstanley CA (2014) Dissociable contributions of anterior cingulate cortex and basolateral amygdala on a rodent cost/benefit decision-making task of cognitive effort. Neuropsychopharmacology 39:1558–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Ravdin LD, Nirenberg MJ, Piboolnurak P, Severt L, Maniscalco JS, Solnes L, Dorfman BJ, Henchcliffe C (2013) Neuroimaging markers of motor and nonmotor features of Parkinson’s disease: an 18f fluorodeoxyglucose positron emission computed tomography study. Dement Geriatr Cogn Disord 35:183–196. [DOI] [PubMed] [Google Scholar]
- Inzlicht M, Bartholow BD, Hirsh JB (2015) Emotional foundations of cognitive control. Trends Cogn Sci 19:126–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessup RK, Busemeyer JR, Brown JW (2010) Error effects in anterior cingulate cortex reverse when error likelihood is high. J Neurosci 30:3467–3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jocham G, Neumann J, Klein TA, Danielmeier C, Ullsperger M (2009) Adaptive coding of action values in the human rostral cingulate zone. J Neurosci 29:7489–7496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampe J, Tschop MH, Hollis JH, Oldfield BJ (2009) An anatomic basis for the communication of hypothalamic, cortical and mesolimbic circuitry in the regulation of energy balance. Eur J Neurosci 30:415–430. [DOI] [PubMed] [Google Scholar]
- Kemppainen J, Aalto S, Fujimoto T, Kalliokoski KK, Langsjo J, Oikonen V, Rinne J, Nuutila P, Knuuti J (2005) High intensity exercise decreases global brain glucose uptake in humans. J Physiol 568:323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk U, Downar J, Montague PR (2011) Interoception drives increased rational decision-making in meditators playing the ultimatum game. Front Neurosci 5:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner IR, Zhang J, Touroutoglou A, Chanes L, Xia C, Simmons WK, Quigley KS, Dickerson BC, Barrett LF (2017) Evidence for a Large-Scale Brain System Supporting Allostasis and Interoception in Humans. Nat Hum Behav 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolling N, Wittmann M, Rushworth MF (2014) Multiple neural mechanisms of decision making and their competition under changing risk pressure. Neuron 81:1190–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolling N, Wittmann MK, Behrens TE, Boorman ED, Mars RB, Rushworth MF (2016) Value, search, persistence and model updating in anterior cingulate cortex. Nat Neurosci 19:1280–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouneiher F, Charron S, Koechlin E (2009) Motivation and cognitive control in the human prefrontal cortex. Nat Neurosci 12:939–945. [DOI] [PubMed] [Google Scholar]
- Kremer S, Chassagnon S, Hoffmann D, Benabid AL, Kahane P (2001) The cingulate hidden hand. J Neurol Neurosurg Psychiatry 70:264–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Waiter G, Ahearn T, Milders M, Reid I, Steele JD (2008) Abnormal temporal difference reward-learning signals in major depression. Brain 131:2084–2093. [DOI] [PubMed] [Google Scholar]
- Kurniawan IT, Seymour B, Talmi D, Yoshida W, Chater N, Dolan RJ (2010) Choosing to make an effort: the role of striatum in signaling physical effort of a chosen action. J Neurophysiol 104:313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurzban R, Duckworth AL, Kable JW, Myers J (2013) An opportunity cost model of subjective effort and task performance. Behav Brain Sci 36:661–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavretsky H, Ballmaier M, Pham D, Toga A, Kumar A (2007) Neuroanatomical characteristics of geriatric apathy and depression: a magnetic resonance imaging study. Am J Geriatr Psychiatry 15:386–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Heron C, Apps MAJ, Husain M (2017) The anatomy of apathy: A neurocognitive framework for amotivated behaviour. Neuropsychologia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Heron C, Holroyd CB, Salamone J, Husain M (2019) Brain mechanisms underlying apathy. J Neurol Neurosurg Psychiatry 90:302–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Ran Kim K, Ku J, Lee JH, Namkoong K, Jung YC (2014) Resting-state synchrony between anterior cingulate cortex and precuneus relates to body shape concern in anorexia nervosa and bulimia nervosa. Psychiatry Res 221:43–48. [DOI] [PubMed] [Google Scholar]
- Leong SL, De Ridder D, Vanneste S, Sutherland W, Ross S, Manning P (2018) High definition transcranial pink noise stimulation of anterior cingulate cortex on food craving: An explorative study. Appetite 120:673–678. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Eisenberger NI (2015) The dorsal anterior cingulate cortex is selective for pain: Results from large-scale reverse inference. Proc Natl Acad Sci U S A 112:15250–15255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist KA, Wager TD, Kober H, Bliss-Moreau E, Barrett LF (2012) The brain basis of emotion: a meta-analytic review. Behav Brain Sci 35:121–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maillet D, Rajah MN (2014) Age-related differences in brain activity in the subsequent memory paradigm: a meta-analysis. Neurosci Biobehav Rev 45:246–257. [DOI] [PubMed] [Google Scholar]
- Margulies DS, Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP (2007) Mapping the functional connectivity of anterior cingulate cortex. Neuroimage 37:579–588. [DOI] [PubMed] [Google Scholar]
- McCaffery JM, Haley AP, Sweet LH, Phelan S, Raynor HA, Del Parigi A, Cohen R, Wing RR (2009) Differential functional magnetic resonance imaging response to food pictures in successful weight-loss maintainers relative to normal-weight and obese controls. Am J Clin Nutr 90:928–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire JT, Botvinick MM (2010) Prefrontal cortex, cognitive control, and the registration of decision costs. Proc Natl Acad Sci U S A 107:7922–7926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mega MS, Cummings JL, Fiorello T, Gombein J (1996) The spectrum of behavioral changes in Alzheimer’s disease. Neurology 46:130–135. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ (1982) Insula of the Old World Monkey. III: Efferent Cortical Output and Comments on Function. 212:38–52. [DOI] [PubMed] [Google Scholar]
- Migneco O, Benoit M, Koulibaly PM, Dygai I, Bertogliati C, Desvignes P, Robert PH, Malandain G, Bussiere F, Darcourt J (2001) Perfusion brain SPECT and statistical parametric mapping analysis indicate that apathy is a cingulate syndrome: a study in Alzheimer’s disease and nondemented patients. Neuroimage 13:896–902. [DOI] [PubMed] [Google Scholar]
- Mobbs D, Yu R, Rowe JB, Eich H, FeldmanHall O, Dalgleish T (2010) Neural activity associated with monitoring the oscillating threat value of a tarantula. Proc Natl Acad Sci U S A 107:20582–20586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modirrousta M, Fellows LK (2008) Dorsal medial prefrontal cortex plays a necessary role in rapid error prediction in humans. J Neurosci 28:14000–14005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morecraft RJ, Van Hoesen GW (1998) Convergence of limbic input to the cingulate motor cortex in the rhesus monkey. Brain Res Bull 45:209–232. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, Stilwell-Morecraft KS, Cipolloni PB, Ge J, McNeal DW, Pandya DN (2012) Cytoarchitecture and cortical connections of the anterior cingulate and adjacent somatomotor fields in the rhesus monkey. Brain Res Bull 87:457–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulert C, Menzinger E, Leicht G, Pogarell O, Hegerl U (2005) Evidence for a close relationship between conscious effort and anterior cingulate cortex activity. Int J Psychophysiol 56:65–80. [DOI] [PubMed] [Google Scholar]
- Myers CA, Wang C, Black JM, Bugescu N, Hoeft F (2016) The matter of motivation: Striatal resting-state connectivity is dissociable between grit and growth mindset. Soc Cogn Affect Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naccache L, Dehaene S, Cohen L, Habert MO, Guichart-Gomez E, Galanaud D, Willer JC (2005) Effortless control: executive attention and conscious feeling of mental effort are dissociable. Neuropsychologia 43:1318–1328. [DOI] [PubMed] [Google Scholar]
- Naito E, Kinomura S, Geyer S, Kawashima R, Roland PE, Zilles K (2000) Fast reaction to different sensory modalities activates common fields in the motor areas, but the anterior cingulate cortex is involved in the speed of reaction. J Neurophysiol 83:1701–1709. [DOI] [PubMed] [Google Scholar]
- Nee DE, Kastner S, Brown JW (2011) Functional heterogeneity of conflict, error, task-switching, and unexpectedness effects within medial prefrontal cortex. Neuroimage 54:528–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SM, Dosenbach NUF, Cohen AL, Wheeler ME, Schlaggar BL, Petersen SE (2010) Role of the anterior insula in task-level control and focal attention. Brain Structure and Function 214:669–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijhuis EH, van Cappellen van Walsum AM, Norris DG (2013) Topographic hub maps of the human structural neocortical network. PLoS One 8:e65511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomero-Gallagher N, Zilles K (2019) Cortical layers: Cyto-, myelo-, receptor-and synaptic architecture in human cortical areas. Neuroimage 197:716–741.28811255 [Google Scholar]
- Palomero-Gallagher N, Mohlberg H, Zilles K, Vogt B (2008) Cytology and receptor architecture of human anterior cingulate cortex. J Comp Neurol 508:906–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvizi J, Rangarajan V, Shirer WR, Desai N, Greicius Michael D (2013) The Will to Persevere Induced by Electrical Stimulation of the Human Cingulate Gyrus. Neuron 80:1359–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patzelt EH, Kool W, Millner AJ, Gershman SJ (2019) The transdiagnostic structure of mental effort avoidance. Sci Rep 9:1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T (2001) Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nat Rev Neurosci 2:417–424. [DOI] [PubMed] [Google Scholar]
- Picard N, Strick PL (2001) Imaging the premotor areas. Current Opinion in Neurobiology 11:663–672. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA (2014) Depression, stress, and anhedonia: toward a synthesis and integrated model. Annu Rev Clin Psychol 10:393–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Petersen SE (2013) Control-related systems in the human brain. Curr Opin Neurobiol 23:223–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Procyk E, Wilson CR, Stoll FM, Faraut MC, Petrides M, Amiez C (2016) Midcingulate Motor Map and Feedback Detection: Converging Data from Humans and Monkeys. Cereb Cortex 26:467–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, Fiez JA, Videen TO, MacLeod AK, Pardo JV, Fox PT, Petersen SE (1994) Practice-related Changes in Human Brain Functional Anatomy during Nonmotor Learnin. Cereb Cortex 4 1047–1321. [DOI] [PubMed] [Google Scholar]
- Raz G, Touroutoglou A, Wilson-Mendenhall C, Gilam G, Lin T, Gonen T, Jacob Y, Atzil S, Admon R, Bleich-Cohen M, Maron-Katz A, Hendler T, Barrett LF (2016) Functional connectivity dynamics during film viewing reveal common networks for different emotional experiences. Cogn Affect Behav Neurosci 16:709–723. [DOI] [PubMed] [Google Scholar]
- Reed J, Pritschet BL, Cutton DM (2013) Grit, conscientiousness, and the transtheoretical model of change for exercise behavior. J Health Psychol 18:612–619. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Behrens TE (2008) Choice, uncertainty and value in prefrontal and cingulate cortex. Nat Neurosci 11:389–397. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Yohn SE, Lopez-Cruz L, San Miguel N, Correa M (2016) Activational and effort-related aspects of motivation: neural mechanisms and implications for psychopathology. Brain 139:1325–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomons TV, Johnstone T, Backonja MM, Davidson RJ (2004) Perceived controllability modulates the neural response to pain. J Neurosci 24:7199–7203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders B, Milyavskaya M, Inzlicht M (2015) What does cognitive control feel like? Effective and ineffective cognitive control is associated with divergent phenomenology. Psychophysiology 52:1205–1217. [DOI] [PubMed] [Google Scholar]
- Scholl J, Kolling N, Nelissen N, Wittmann MK, Harmer CJ, Rushworth MF (2015) The Good, the Bad, and the Irrelevant: Neural Mechanisms of Learning Real and Hypothetical Rewards and Effort. J Neurosci 35:11233–11251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schouppe N, De Houwer J, Ridderinkhof KR, Notebaert W (2012) Conflict: run! Reduced Stroop interference with avoidance responses. Q J Exp Psychol (Hove) 65:1052–1058. [DOI] [PubMed] [Google Scholar]
- Schroeter ML, Vogt B, Frisch S, Becker G, Seese A, Barthel H, Mueller K, Villringer A, Sabri O (2011) Dissociating behavioral disorders in early dementia—An FDG-PET study. Psychiatry Research: Neuroimaging 194:235–244. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD (2007) Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 27:2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepulcre J, Sabuncu MR, Yeo TB, Liu H, Johnson KA (2012) Stepwise connectivity of the modal cortex reveals the multimodal organization of the human brain. J Neurosci 32:10649–10661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ (2011) The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat Rev Neurosci 12:154–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenhav A, Botvinick MM (2013) Motivated action: new light on prefrontal-neuromodulatory circuits. Curr Biol 23:R161–163. [DOI] [PubMed] [Google Scholar]
- Shenhav A, Botvinick MM, Cohen JD (2013) The expected value of control: an integrative theory of anterior cingulate cortex function. Neuron 79:217–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenhav A, Cohen JD, Botvinick MM (2016) Dorsal anterior cingulate cortex and the value of control. Nat Neurosci 19:1286–1291. [DOI] [PubMed] [Google Scholar]
- Shenhav A, Musslick S, Lieder F, Kool W, Griffiths TL, Cohen JD, Botvinick MM (2017) Toward a Rational and Mechanistic Account of Mental Effort. [DOI] [PubMed] [Google Scholar]
- Sheth SA, Mian MK, Patel SR, Asaad WF, Williams ZM, Dougherty DD, Bush G, Eskandar EN (2012) Human dorsal anterior cingulate cortex neurons mediate ongoing behavioural adaptation. Nature 488:218–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberg JM, Miller GA, Warren SL, Engels AS, Crocker LD, Banich MT, Sutton BP, Heller W (2012) A brain network instantiating approach and avoidance motivation. Psychophysiology 49:1200–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spunt RP, Lieberman MD, Cohen JR, Eisenberger NI (2012) The Phenomenology of Error Processing: The Dorsal ACC Response to Stop-signal Errors Tracks Reports of Negative Affect. Journal of Cognitive Neuroscience 24:1753–1765. [DOI] [PubMed] [Google Scholar]
- Steele JD, Kumar P, Ebmeier KP (2007) Blunted response to feedback information in depressive illness. Brain 130:2367–2374. [DOI] [PubMed] [Google Scholar]
- Steward T, Menchon JM, Jimenez-Murcia S, Soriano-Mas C, Fernandez-Aranda F (2018) Neural Network Alterations Across Eating Disorders: A Narrative Review of fMRI Studies. Curr Neuropharmacol 16:1150–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun FW, Stepanovic MR, Andreano J, Barrett LF, Touroutoglou A, Dickerson BC (2016) Youthful Brains in Older Adults: Preserved Neuroanatomy in the Default Mode and Salience Networks Contributes to Youthful Memory in Superaging. J Neurosci 36:9659–9668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczepanski SM, Knight RT (2014) Insights into human behavior from lesions to the prefrontal cortex. Neuron 83:1002–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Bancaud J, Geier S, Bordas-Ferrer M, Bonis A, Szikla G, Rusu M (1973) The cingulate gyrus and human behaviour. Electroencephalogr Clin Neurophysiol 34:45–52. [DOI] [PubMed] [Google Scholar]
- Touroutoglou A, Hollenbeck M, Dickerson BC, Barrett FL (2012) Dissociable large-scale networks anchored in the right anterior insula subserve affective experience and attention. NeuroImage 60:1947–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touroutoglou A, Lindquist KA, Dickerson BC, Barrett LF (2015) Intrinsic connectivity in the human brain does not reveal networks for ‘basic’ emotions. Soc Cogn Affect Neurosci 10:1257–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touroutoglou A, Bliss-Moreau E, Zhang J, Mantini D, Vanduffel W, Dickerson BC, Barrett LF (2016) A ventral salience network in the macaque brain. Neuroimage 132:190–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Bossaller NA, Shelton RC, Zald DH (2012) Effort-based decision-making in major depressive disorder: a translational model of motivational anhedonia. J Abnorm Psychol 121:553–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullsperger M, von Cramon DY (2001) Subprocesses of performance monitoring: a dissociation of error processing and response competition revealed by event-related fMRI and ERPs. Neuroimage 14:1387–1401. [DOI] [PubMed] [Google Scholar]
- van den Heuvel MP, Sporns O (2011) Rich-club organization of the human connectome. J Neurosci 31:15775–15786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Sporns O (2013a) An anatomical substrate for integration among functional networks in human cortex. J Neurosci 33:14489–14500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Sporns O (2013b) Network hubs in the human brain. Trends Cogn Sci 17:683–696. [DOI] [PubMed] [Google Scholar]
- van den Heuvel MP, Kahn RS, Goni J, Sporns O (2012) High-cost, high-capacity backbone for global brain communication. Proc Natl Acad Sci U S A 109:11372–11377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Reekum R, Stuss DT, Ostrander L (2005) Apathy: why care? J Neuropsychiatry Clin Neurosci 17:7–19. [DOI] [PubMed] [Google Scholar]
- Van Schuerbeek P, Baeken C, De Raedt R, De Mey J, Luypaert R (2011) Individual differences in local gray and white matter volumes reflect differences in temperament and character: a voxel-based morphometry study in healthy young females. Brain Res 1371:32–42. [DOI] [PubMed] [Google Scholar]
- van Veen V, Cohen JD, Botvinick MM, Stenger VA, Carter CS (2001) Anterior cingulate cortex, conflict monitoring, and levels of processing. Neuroimage 14:1302–1308. [DOI] [PubMed] [Google Scholar]
- Vassena E, Holroyd CB, Alexander WH (2017a) Computational Models of Anterior Cingulate Cortex: At the Crossroads between Prediction and Effort. Front Neurosci 11:316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassena E, Deraeve J, Alexander WH (2017b) Predicting Motivation: Computational Models of PFC Can Explain Neural Coding of Motivation and Effort-based Decision-making in Health and Disease. J Cogn Neurosci 29:1633–1645. [DOI] [PubMed] [Google Scholar]
- Vassena E, Silvetti M, Boehler CN, Achten E, Fias W, Verguts T (2014) Overlapping neural systems represent cognitive effort and reward anticipation. PLoS One 9:e91008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL (2008) Evidence for a Frontoparietal Control System Revealed by Intrinsic Functional Connectivity. Journal of Neurophysiology 100:3328–3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA (2005) Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci 6:533–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA (2016) Midcingulate cortex: Structure, connections, homologies, functions and diseases. J Chem Neuroanat 74:28–46. [DOI] [PubMed] [Google Scholar]
- Wager TD, van Ast VA, Hughes BL, Davidson ML, Lindquist MA, Ochsner KN (2009) Brain mediators of cardiovascular responses to social threat, part II: Prefrontal-subcortical pathways and relationship with anxiety. Neuroimage 47:836–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Atlas LY, Botvinick MM, Chang LJ, Coghill RC, Davis KD, Iannetti GD, Poldrack RA, Shackman AJ, Yarkoni T (2016) Pain in the ACC? Proc Natl Acad Sci U S A 113:E2474–2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton ME, Rudebeck PH, Bannerman DM, Rushworth MF (2007) Calculating the cost of acting in frontal cortex. Ann N Y Acad Sci 1104:340–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Laviolette P, O’Keefe K, Putcha D, Bakkour A, Van Dijk KR, Pihlajamaki M, Dickerson BC, Sperling RA (2010) Intrinsic connectivity between the hippocampus and posteromedial cortex predicts memory performance in cognitively intact older individuals. Neuroimage 51:910–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Hu SH, Shi Y, Li BM (2017a) The roles of the anterior cingulate cortex and its dopamine receptors in self-paced cost-benefit decision making in rats. Learn Behav 45:89–99. [DOI] [PubMed] [Google Scholar]
- Wang S, Zhou M, Chen T, Yang X, Chen G, Wang M, Gong Q (2017b) Grit and the brain: spontaneous activity of the dorsomedial prefrontal cortex mediates the relationship between the trait grit and academic performance. Soc Cogn Affect Neurosci 12:452–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei F, Li P, Zhuo M (1999) Loss of synaptic depression in mammalian anterior cingulate cortex after amputation. J Neurosci 19:9346–9354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westbrook A, Kester D, Braver TS (2013) What is the subjective cost of cognitive effort? Load, trait, and aging effects revealed by economic preference. PLoS One 8:e68210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickens DD (1984) Processing resources in attention In: Varieties of Attention (Parasuraman R, Davies DR, Beatty J, eds), pp 63–102. New York: New York: Academic. [Google Scholar]
- Williams SM, Goldman-Rakic PS (1998) Widespread origin of the primate mesofrontal dopamine system. Cereb Cortex 8:321–345. [DOI] [PubMed] [Google Scholar]
- Williams ZM, Bush G, Rauch SL, Cosgrove GR, Eskandar EN (2004) Human anterior cingulate neurons and the integration of monetary reward with motor responses. Nat Neurosci 7:1370–1375. [DOI] [PubMed] [Google Scholar]
- Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD (2011) Large-scale automated synthesis of human functional neuroimaging data. Nat Methods 8:665–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo BT, Krienen FM, Eickhoff SB, Yaakub SN, Fox PT, Buckner RL, Asplund CL, Chee MW (2015) Functional Specialization and Flexibility in Human Association Cortex. Cereb Cortex 25:3654–3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenon A, Sidibe M, Olivier E (2015) Disrupting the supplementary motor area makes physical effort appear less effortful. J Neurosci 35:8737–8744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Andreano JM, Dickerson BC, Touroutoglou A, Barrett LF (2019) Stronger Functional Connectivity in the Default Mode and Salience Networks Is Associated With Youthful Memory in Superaging. Cereb Cortex. [DOI] [PMC free article] [PubMed] [Google Scholar]