Abstract
Background
In the last decades, research focused on gender-related features in patients with tinnitus has often led to controversial results. The complex clinical picture of tinnitus patients often consists of an interdependent relationship between audiological symptoms and co-occurrent psychological disorders, which can complicate the diagnostic evaluation.
Methods
Therefore, we studied 107 patients with tinnitus, investigating their psychological comorbidities in the light of gender differences. All patients were evaluated with ENT/audiological and psychological examination to consider presence/absence, type and gender distribution of psychopathological comorbidities. Patients completed questionnaires on tinnitus distress (Tinnitus Handicap Inventory, THI), anxiety (Beck Anxiety Inventory, BAI), depression (Beck Depression Inventory, BDI), metacognition (Metacognition Questionnaire-30, MCQ-30) and worry (Penn State Worry Questionnaire). The influence of gender on the relationship between tinnitus distress and psychological comorbidities was investigated with simple moderation analyses using the SPSS PROCESS macro.
Results
The total sample included 65 male and 42 female patients (60.7 vs. 39.3%), matched for age and duration of tinnitus. We found no significant differences for tinnitus distress (THI total score, THI subscales) and MCQ-30 subscales, except for the control over thoughts, where men showed significantly higher scores than women (p = 0.045). Also, in our sample women showed significantly higher values for depression (BDI total score, p = 0.019), anxiety (BAI total score, p = 0.010) and worries (PSQW total score, p = 0.015). Moderation analyses revealed a significant influence of gender on the relationship of tinnitus distress with depression: higher scores of tinnitus distress were associated with significantly elevated levels of depression amongst men. No further gender influences could be observed in our sample.
Discussion
In conclusion, our results indicate general gender differences for psychological comorbidities in tinnitus patients, with women reporting more depression, anxiety and worries. Men, on the other hand, showed a higher need to control their thoughts. Additionally, our results indicate that men might have more coping problems with increasing levels of tinnitus distress, leading to increased depressive symptoms. Nevertheless, several gender related aspects in tinnitus patients remain unclear, thus warranting the need future studies in this field.
Keywords: tinnitus, gender, metacognition, depression, anxiety, tinnitus distress
Introduction
Tinnitus is a multifactorial disorder and it involves the perception of sounds, such as ringing or buzzing in the ear or head without detectable external source (Cima et al., 2019). Recent studies reveal that about 30% of adults can experience tinnitus but overall prevalence varied over 8-fold from 5.1 to 42.7% (McCormack et al., 2016). Based on a survey conducted in 2014 on 2,952 individuals, Gallus et al. (2015) found that any tinnitus was reported by 6.2% of Italian adults and severe tinnitus by 1.2%. Moreover, approximately one-third of adults with tinnitus experience it as bothersome and feel impaired in their daily performance (Tunkel et al., 2014). As reported by Cima et al. (2019), bothersome tinnitus might be better described as a negative emotional and auditory experience. Tinnitus can be further distinguished into acute (<3 months), sub-acute (3–6 months) or chronic (>6 months) (Cima et al., 2019).
Etiology of tinnitus seems to be very heterogeneous, though in many cases it occurs after cochlear damages following noise trauma, age, ototoxic drugs, inflammatory diseases or hearing loss. Studies focused on the relation between gender and tinnitus distress often show conflicting results (Meric et al., 1998; Erlandsson and Holgers, 2001; Pinto et al., 2010; Seydel et al., 2013). Psychological comorbidities may either be pre-existent or induced by tinnitus. Core symptoms of tinnitus often mask psychiatric and psychological comorbid symptoms. Apart from phenotypical sex differences, tinnitus patients may also show gender differences in regard to coexisting psychopathologies. Thus, several studies focusing on gender differences in psychological comorbidities among tinnitus patients have been conducted.
Gomaa et al. (2014) reported gender differences for depression, anxiety, and stress among patients with tinnitus. However, they did not conduct sex specific analyses of the association between tinnitus severity and psychiatric distress. Caldirola et al. (2016) investigated the role of worry in 54 patients (29 males, 25 females) with chronic tinnitus and sensorineural hearing loss. Tinnitus-related anxiety, depression symptoms and handicap were significantly associated with the tendency to worry, but they found no associations between tinnitus distress and gender. Udupi et al. (2013) reported similar results in 50 patients (31 males, 19 females) with tinnitus, with tinnitus severity being significantly correlated with depressive symptoms as well as state and trait anxiety. Other factors – including age, gender or hearing status – did not significantly influence tinnitus severity.
Other authors reported gender differences in tinnitus related suffering. In a cross-sectional study, Han et al. (2019) evaluated 134 female and 114 male patients with tinnitus using the THI, BDI, the Korean version of Brief Encounter Psychosocial Instrument (BEPSI-K), and various characteristics of the patients’ tinnitus (including loudness, awareness, annoyance, and effect on life). While the authors found no significant gender difference in tinnitus severity, men showed a stronger association between tinnitus severity and depressive symptoms than women. Additionally, a significant association between stress and tinnitus severity was only found for male participants. Another study assessed gender differences in Positron emission tomography-computed tomography (PET-CT) results in tinnitus patients (Shlamkovich et al., 2016). In their study, 60% of the patients showed an increased uptake in the upper temporal gyrus (UTG) on either side. In their sample, men showed a significant increased uptake in the UTG, both in the subsample of patients with unilateral tinnitus as well as in the total sample.
Previous research has also underlined the presence of higher anxiety and depression levels and suicide attempts in female tinnitus patients. Bashir et al. (2017) found higher scores of depression and anxiety among female patients with tinnitus. While Gallus et al. (2015) found no overall sex differences regarding the prevalence of (chronic) tinnitus, severe levels of tinnitus distress were more frequent in women. Lugo et al. (2019) reported in a cross -sectional study a sex-dependent association of tinnitus with suicide attempts, with severe tinnitus associated with suicide attempts in women but not in men. Furthermore, women reported more tinnitus complaints then men.
To summarize, the evidence base for sex differences in tinnitus comorbidities is still inconclusive. Thus, the aim of the present study was to investigate sex specific patterns of psychological comorbidities, including measure for meta-cognitions, anxiety, depression and worry. Results are analyzed for clinically relevant confounders such as age and tinnitus duration.
Materials and Methods
Sample and Setting
A sample of n = 107 outpatients evaluated at the Tinnitus Center of European Hospital (Rome) between April 2018 and April 2019 was included in this cross-sectional study. To be included in the study, patients (a) had tinnitus, (b) were older than 18 years, (c) spoke Italian fluently, and (d) had no apparent cognitive impairment or major psychiatric/neurological disorders (schizophrenia, Alzheimer’s disease, Parkinson’s disease). The patients were evaluated by an ENT specialist and a psychologist, and they completed the questionnaires as part of the routine clinical practice. Written informed consent was obtained by all patients.
Measures
Tinnitus Sample Case History (TSCH)
The Italian version of the TSCH was used to assess sociodemographic and clinical data. The TSCH was developed by the Tinnitus Research Initiative to facilitate standardized assessment of sociodemographic and clinical data in tinnitus research (Langguth et al., 2007). The questionnaire consists of 35 items on background (i.e., age, gender), tinnitus history (i.e., loudness, pitch, perception at onset, tinnitus location, percentage of awake time aware of tinnitus) and related conditions (i.e., hearing impairment, noise annoyance, vertigo/dizziness, headache, TMJ disorders).
Tinnitus Handicap Inventory (THI)
The THI (Newman et al., 1996) is a widely used self-report questionnaire in tinnitus research, allowing the assessment of the impact of tinnitus in daily life. It consists of 25 items which can be summarized to a total score as well as a functional, emotional, and catastrophic subscale. Based on the total score tinnitus severity can be graded from slight (grade 1) to catastrophic (grade 5). Good reliability (α = 0.94) and validity was reported for the total score of the Italian THI version (Passi et al., 2008). A THI score <36 was considered to indicate a compensated tinnitus, while a score > 36 was considered to indicate a decompensated tinnitus (Salviati et al., 2013; Altissimi et al., 2016).
Beck Anxiety Inventory (BAI)
The BAI (Beck et al., 1988) is a self-administered tool to assess the severity of anxiety over the last 7 days. It consists of 21-items which can be rated on a 4-point scale ranging from 0 (“not at all”) to 3 (“severely”). Based on the total score, anxiety can be classified as minimal (0–7), mild (8–15), moderate (16–25), and severe (26–63).
Beck Depression Inventory (BDI)
The BDI-II (Beck et al., 1996) is a self-report instrument to assess depressive symptoms over the previous 2 weeks, consisting of 21 items. Based on the total score, depression can be classified as minimal (0–13), mild (14–19), moderate (20–28) or severe (29–63).
Metacognition Questionnaire-30 (MCQ-30)
The MCQ-30 (Wells and Cartwright-Hatton, 2004) is a 30-item questionnaire with 5 subscales: (1) positive beliefs about worry (pos); (2) negative beliefs about the controllability of thoughts and danger of worry (neg); (3) cognitive confidence (CC); (4) beliefs about the need to control thoughts (NC); and (5) cognitive self-consciousness (CSC). Higher scores on the total score as well as the subscales indicate higher levels of dysfunctional metacognitions.
Penn State Worry Questionnaire (PSWQ)
The PSWQ (Meyer et al., 1990) is a 16-item self-administered scale designed to measure worry. The items are scored on a 5-point Likert-type scale (1-Not at all typical of me to 5-Very typical of me). Based on the total score, the patients’ worries can be classified as low (16–39), moderate (40–59) and high (60–80).
Audiometry
Pure tone audiometry was carried out into an audiological cabin using a clinical audiometer (Madsen Itera II, GN Otometrics). Normal hearing was defined by threshold <25 dB HL in all frequencies tested between 250 and 8.000 Hz.
Statistical Analyses
Descriptive statistics are presented for men and women separately and group differences were analyzed using independent sample t-tests and χ2-tests. Associations between tinnitus distress and the assessed psychological variables were analyzed with Pearson correlation coefficients and gender differences between all psychological variables using independent sample t-tests. Duration of tinnitus was dichotomized into acute (i.e., <3 months) and sub-acute/chronic tinnitus (>3 months) due to the group sizes. To evaluate the influence of multiple physical comorbidities, the mean number of reported comorbidities (i.e., hearing problems; hyperacusis; headache; vertigo/dizziness; TMD; neck pain; other pain) was correlated with the mean THI, BDI and BAI scores. P-values < 0.05 (two-sided) were considered statistically significant.
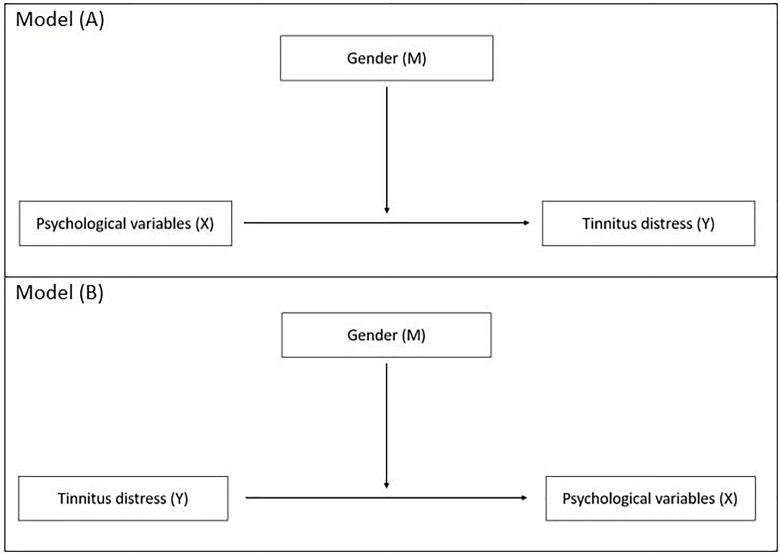
To investigate the potential influence of sex on the relationship of the psychological variables (i.e., MCQ-subscales, BDI total score, BAI total score, PSWQ total score) with tinnitus distress, two moderator analyses were calculated: in model (A) psychological variables were entered as independent variables, sex as moderator variable and tinnitus distress as dependent variable; in model (B) the direction of the association was changed, i.e., tinnitus distress was entered as independent variable, sex as moderator variable and psychological variables as dependent variables. The models are visualized in Figure 1.
FIGURE 1.
Moderator analyses of gender (moderator M) on the relationship of the psychological variables (i.e., depression, anxiety, worries, need for control) with tinnitus distress.
Psychological variables were entered in the models if (a) there were significant sex differences for the variable and (b) they were significantly associated with tinnitus distress. Moderator analyses were calculated using the SPSS PROCESS macro (v3.4) (Hayes, 2013). All moderation analyses were completed utilizing a bootstrapping procedure with 5000 bootstrapped samples. Significance of the indirect effect is determined by examining the 95% confidence interval (CI) of the sampling distribution of the mean. Confidence intervals that do not include zero are considered statistically significant at the 0.05 level. Statistical analyses were performed with IBM SPSS (v22.0).
Results
A total of n = 107 patients was included in the analyses. The mean age was 49.1 (SD: 13.9) years and 60.7% of the sample was male. The majority of patients (75.7%) reported to suffer from their tinnitus for longer than 6 month and most patients (73.8%) described their tinnitus as continuously present. Details on patient characteristics can be found in Table 1. Overall, 19.6% of the patients reported a very mild tinnitus severity (grade I), while 28.0% reported mild (grade II), 29.0% moderate (grade III), 15.9% severe (grade IV), and 7.5% very severe tinnitus distress (grade V). Thus, about half of the patients reported a compensated tinnitus (47.7%), while the other half reported a decompensated tinnitus (52.3%). Based on the pure tone audiometry (250–8.000 Hz), 68 patients had a hearing loss. No gender difference was found regarding hearing loss (p = 0.82).
TABLE 1.
Sociodemographic and clinical data.
| Men (n = 65) |
Women (n = 42) |
|||||
| Mean | (SD) | Mean | (SD) | t-value | p-value | |
| Age | 50.2 | (13.2) | 47.3 | 14.9 | 1.05 | 0.29 |
| Tinnitus loudness (0–100) | 50.6 | (23.1) | 54.8 | (27.2) | 0.76 | 0.45 |
| Awareness of tinnitus a | 81.2 | (28.1) | 77.5 | (28.0) | 0.59 | 0.56 |
| Annoyed by tinnitus a | 51.0 | (34.4) | 47.8 | (30.1) | 0.44 | .66 |
| n | % | n | % | χ2 | p-value | |
| Tinnitus: family history | 15 | 24.6% | 16 | 38.1% | 2.42 | 0.12 |
| Missing | 4 | 6.2% | 1 | 2.4% | ||
| Duration tinnitus | ||||||
| <3 months | 13 | 20.0% | 4 | 9.5% | 2.63 | 0.27 |
| 3–6 months | 5 | 7.7% | 2 | 4.8% | ||
| >6 months | 46 | 70.8% | 35 | 83.3% | ||
| Missing | 1 | 1.5% | 1 | 2.4% | ||
| Tinnitus perception at onset | ||||||
| Gradual | 48 | 73.8% | 23 | 54.8% | 4.15 | 0.04 |
| Abrupt | 8 | 12.3% | 11 | 26.2% | ||
| Missing | 9 | 13.8% | 1 | 2.4% | ||
| Pulsating tinnitus | 10 | 15.4% | 8 | 19.0% | 0.27 | 0.61 |
| Missing | 1 | 1.5% | 1 | 2.4% | ||
| Tinnitus location | ||||||
| right ear | 9 | 13.8% | 6 | 14.3% | 1.31 | 0.73 |
| Left ear | 16 | 24.6% | 7 | 16.7% | ||
| Both ears | 35 | 53.8% | 27 | 64.3% | ||
| Inside the head | 4 | 6.2% | 2 | 4.8% | ||
| Missing | 1 | 1.5% | – | – | ||
| Tinnitus loudness varies from day to day | 42 | 64.6% | 29 | 69.0% | 0.30 | 0.59 |
| Missing | 1 | 1.5% | 1 | 2.4% | ||
| Tinnitus manifestation | 0.01 | 0.92 | ||||
| Intermittent | 14 | 21.5% | 9 | 21.4% | ||
| Constant | 49 | 75.4% | 30 | 71.4% | ||
| Missing | 2 | 3.1% | 3 | 7.1% | ||
| Tinnitus sound | 8.49 | 0.037 | ||||
| Tone | 36 | 55.4% | 13 | 31.0% | ||
| Noise | 12 | 18.5% | 12 | 28.6% | ||
| Crickets | 7 | 10.8% | 3 | 7.1% | ||
| Other | 7 | 10.8% | 11 | 26.2% | ||
| Missing | 3 | 4.6% | 3 | 7.1% | ||
| Objective hearing problem b | 42 | 68.9% | 26 | 66.7% | 0.05 | 0.82 |
| Missing | 4 | 6.2% | 3 | 7.1% | ||
| Subjective hearing problem | 24 | 36.9% | 15 | 35.7% | 0.01 | 0.97 |
| Missing | 2 | 3.1% | 3 | 7.1% | ||
| Hyperacusis | 15 | 23.1% | 19 | 45.2% | 10.45 | 0.005 |
| Missing | 4 | 6.2% | 2 | 4.8% | ||
| Headache | 17 | 26.2% | 23 | 54.8% | 9.24 | 0.002 |
| Missing | 1 | 1.5% | 1 | 2.4% | ||
| Vertigo/dizziness | 10 | 15.4% | 15 | 35.7% | 5.84 | 0.016 |
| Missing | 2 | 3.1% | 1 | 2.4% | ||
| Temporomandibular disorder | 18 | 27.7% | 16 | 38.1% | 1.94 | 0.16 |
| Missing | 2 | 3.1% | 4 | 9.5% | ||
| Neck pain | 33 | 50.8% | 27 | 64.3% | 3.40 | 0.07 |
| Missing | 0 | 0.0% | 3 | 7.1% | ||
| Other pain | 13 | 20.0% | 14 | 33.3% | 2.68 | 0.10 |
| Missing | 5 | 7.7% | 4 | 9.5% | ||
a Percentage of awake time; b pure tone audiometry (250–8.000 Hz). Statistically significant clinical data (P-values < 0.05) are reported in bold.
The mean number of comorbidities was significantly associated with the THI (r = 0.21, p = 0.034), BDI (r = 0.20, p = 0.039) and BAI total score (r = 0.23, p = 0.016). Most patients reported at least one physical comorbidity, with n = 12 (11.2%) having no comorbidities, n = 69 (64.5%) 1–3 comorbidities, and n = 26 (24.3%) four or more comorbidities. The mean number of comorbidities was significantly higher for women than for men (t = 3.1, p = 0.003).
No gender differences were found for tinnitus loudness, duration of tinnitus or tinnitus location, and most other tinnitus characteristics. Yet, women significantly more often reported an abrupt tinnitus onset significantly than men (p = 0.04) and showed significant differences in the sound profile of their tinnitus. There also was a significantly higher prevalence of hyperacusis (p = 0.005), headache (p = 0.002) and vertigo/dizziness (p = 0.016) in women than in men. Patients with headache reported hyperacusis (54.1 vs. 21.0%; p = 0.001) and temporomandibular disorders (51.4 vs. 22.2%; p = 0.003) significantly more often than patients without headache. As for vertigo/dizziness, patients with headache reported a higher prevalence than patients without headache, yet this difference was not statistically significant (33.3 vs. 18.5%; p = 0.086).
As previously reported (Maas et al., 2017; Lopez-Escamez and Amanat, 2020), a higher heritability was found for bilateral tinnitus in men at any age and younger women (<40 years). While young women with a bilateral tinnitus (n = 9) reported a notable higher percentage of tinnitus complaints in their familial history than men (n = 33) with bilateral tinnitus (55.6 vs. 24.2%), this difference was not statistically significant (p = 0.072).
Patients with acute tinnitus showed no significant differences regarding tinnitus distress (p = 0.49), nor with depression (p = 0.22), anxiety (p = 0.12), or worries (p = 0.49) when compared to patients with sub-acute or chronic tinnitus. Yet, both negative beliefs about uncontrollability and danger of worry (p = 0.003) and cognitive self-consciousness (p = 0.030) were significantly higher in patients with acute tinnitus than in patients with sub-acute or chronic tinnitus. No further significant differences were found for the other MCQ subscales.
The Association of Tinnitus Distress With Meta-Cognitions, Anxiety, Depression and Worry
In our sample higher levels of tinnitus distress were significantly associated more depression, anxiety and worries. Regarding metacognitions, higher tinnitus distress was associated with negative beliefs about the controllability of thought, as well as with a higher need to control thoughts. No association was found between tinnitus distress and positive beliefs about worry, cognitive confidence or cognitive self-consciousness. For details see Table 2.
TABLE 2.
Correlations of tinnitus distress and meta-cognitions, anxiety, depression, and worries.
| MCQ pos | MCQ neg | MCQ CC | MCQ NC | MCQ CSC | PSWQ | BDI | BAI | |
| THI total score | 0.13 | 0.38*** | 0.15 | 0.32** | 0.10 | 0.45*** | 0.68*** | 0.47*** |
| MCQ pos | 0.26** | 0.26** | 0.34*** | 0.39*** | 0.11 | −0.02 | 0.02 | |
| MCQ neg | 0.11 | 0.49*** | 0.43*** | 0.61*** | 0.51*** | 0.50*** | ||
| MCQ CC | 0.09 | 0.09 | 0.20* | 0.23* | 0.33** | |||
| MCQ NC | 0.47*** | 0.31** | 0.29** | 0.26** | ||||
| MCQ CSC | 0.36*** | 0.19 | 0.15 | |||||
| PSWQ total score | 0.63*** | 0.64** | ||||||
| BDI | 0.68** |
*p < 0.05, **p < 0.01, ***p < 0.001; THI, Tinnitus Handicap Inventory; MCQ, Metacognition Questionnaire; pos, positive beliefs about worry; neg, negative beliefs about the controllability of thoughts and danger of worry; CC, cognitive confidence; NC, beliefs about the need to control thoughts; CSC, cognitive self-consciousness; PSWQ, Penn State Worry Questionnaire; BDI, Beck Depression Inventory; BAI, Beck Anxiety Inventory.
Gender Differences in Tinnitus Distress, Meta-Cognitions, Depression, Anxiety and Worries
To investigate gender differences for the assessed psychological variables a set of independent sample t-tests was calculated. While no gender effect was found for tinnitus distress, women reported significantly higher scores for anxiety, depression and worries. Regarding meta-cognitions, men reported significantly stronger beliefs about the need to control thoughts than women. For details see Table 3.
TABLE 3.
Gender differences of THI, MCQ, PSWQ, BDI, and BAI.
| Men |
women |
|||||
| Mean | (SD) | Mean | (SD) | t-value | p-value | |
| THI total score | 39.0 | (25.4) | 43.7 | (21.8) | 0.99 | 0.32 |
| THI functioning subscale | 17.8 | (12.0) | 19.9 | (10.6) | 0.91 | 0.36 |
| THI emotional subscale | 12.6 | (9.2) | 14.4 | (8.7) | 0.99 | 0.32 |
| THI catastrophic subscale | 8.6 | (5.5) | 9.5 | (5.0) | 0.87 | 0.39 |
| MCQ pos | 10.8 | (4.2) | 9.7 | (3.2) | 1.42 | 0.16 |
| MCQ neg | 13.0 | (4.1) | 13.2 | (4.3) | 0.26 | 0.80 |
| MCQ CC | 10.9 | (4.2) | 10.9 | (4.8) | 0.02 | 0.98 |
| MCQ NC | 11.3 | (3.5) | 9.9 | (3.1) | 2.03 | 0.045 |
| MCQ CSC | 15.4 | (4.0) | 15.0 | (3.7) | 0.44 | 0.66 |
| PSWQ | 45.2 | (11.3) | 51.0 | (12.5) | 2.47 | 0.015 |
| BDI total | 9.1 | (6.6) | 12.5 | (8.3) | 2.40 | 0.019 |
| BAI total | 8.1 | (7.4) | 12.6 | (10.2) | 2.62 | 0.010 |
SD, standard deviation; THI, Tinnitus Handicap Inventory; MCQ, Metacognition Questionnaire; pos, positive beliefs about worry; neg, negative beliefs about the controllability of thoughts and danger of worry; CC, cognitive confidence; NC, beliefs about the need to control thoughts; CSC, cognitive self-consciousness; PSWQ, Penn State Worry Questionnaire; BDI, Beck Depression Inventory; BAI, Beck Anxiety Inventory; statistically significant values printed bold.
The Moderating Effect of Gender on the Relationship of Tinnitus Distress and Depression, Anxiety, Worries and Need for Control (Meta-Cognition)
In the first model of the moderation analyses, the potential gender influence on the influence of the included psychological variables with the extent of tinnitus distress were tested. While there was a significant association of all psychological variables with tinnitus distress (p < 0.001), no statistically significant moderating effect of gender could be observed (p > 0.05).
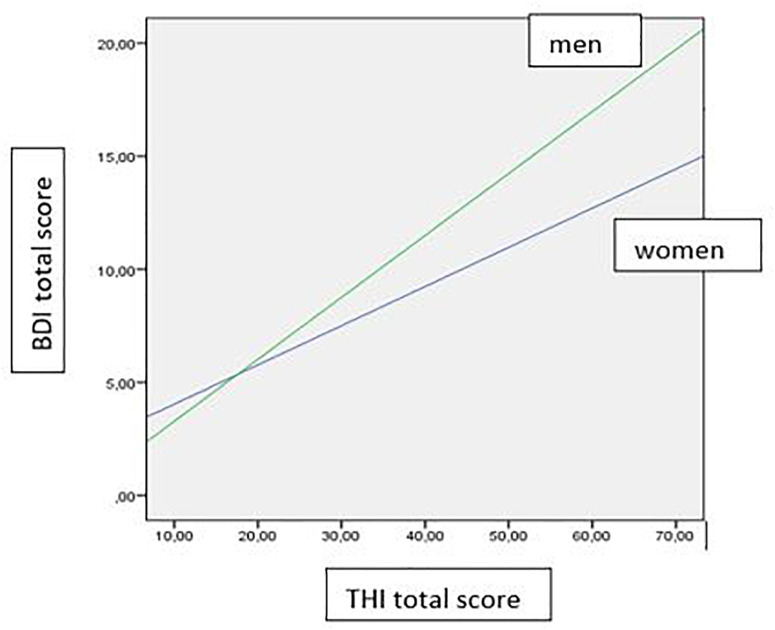
In the second moderation model the reversed effect was tested, thus investigating the moderation effect of gender on the influence of tinnitus distress on the psychological variables. Similar to model (A), there was a significant influence of tinnitus distress on all included psychological variables (p < 0.001). Additionally, gender significantly moderated the influence of tinnitus distress on depression (p = 0.03): while lower levels of tinnitus distress were associated with lower levels depression in men than in women. This effect was reversed with increasing levels of tinnitus distress as shown in Figure 2. For details see Table 4 and Figure 2.
FIGURE 2.
Gender effect on the influence of the THI total score on the BDI total score.
TABLE 4.
Results of the moderator analyses using the PROCESS macro.
| coeff | se | t | p | LLCI | ULCI | |||
| Model (A) | THI R2 = 0.15, MSE = 508.76 p = 0.010 | MCQ NC | 3.12 | 0.80 | 3.91 | <0.001 | 1.54 | 4.70 |
| Sex | 27.80 | 15.00 | 1.87 | 0.07 | −1.77 | 57.73 | ||
| MCQ NC × Sex | −1.92 | 1.38 | −1.39 | 0.17 | −4.65 | 0.81 | ||
| THI R2 = 0.21, MSE = 471.96 p < 0.001 | PSWQ | 1.05 | 0.24 | 4.36 | <0.001 | 0.57 | 1.52 | |
| Sex | 16.13 | 18.06 | 0.89 | 0.37 | −19.69 | 51.95 | ||
| PSWQ × Sex | −0.34 | 0.36 | −0.95 | 0.35 | −1.06 | 0.38 | ||
| THI R2 = 0.47, MSE = 313.96 p < 0.001 | BDI | 2.56 | 0.34 | 7.63 | <0.001 | 1.90 | 3.23 | |
| Sex | 4.34 | 6.23 | 0.70 | 0.49 | −8.02 | 16.71 | ||
| BDI × Sex | −0.68 | 0.47 | −1.43 | 0.16 | −1.61 | 0.26 | ||
| THI R2 = 0.25, MSE = 448.43 p < 0.001 | BAI | 1.77 | 0.36 | 4.98 | <0.001 | 1.06 | 2.48 | |
| Sex | 7.78 | 6.52 | 1.19 | 0.24 | −5.16 | 20.73 | ||
| BAI × Sex | −0.87 | 0.48 | −1.81 | 0.07 | −1.82 | 0.09 | ||
| Model (B) | MCQ NC R2 = 0.17, MSE = 10.07 p < 0.001 | THI | 0.06 | 0.02 | 3.87 | <0.001 | .03 | .09 |
| Sex | −0.08 | 1.32 | 0.06 | .95 | −2.70 | 2.54 | ||
| THI × Sex | −0.04. | 0.03 | 1.30 | .19 | −.09 | .02 | ||
| PSWQ R2 = 0.24, MSE = 114.80 p < 0.001 | THI | 0.21 | 0.05 | 3.94 | <0.001 | 0.10 | 0.31 | |
| Sex | 3.73 | 4.47 | 0.84 | 0.41 | −5.13 | 12.60 | ||
| THI × Sex | 0.02 | 0.09 | 0.26 | 0.80 | −0.16 | 0.21 | ||
| BDI R2 = 0.51, MSE = 28,36 p < 0.001 | THI | 0.17 | 0.03 | 6.61 | <0.001 | 0.12 | 0.23 | |
| Sex | −1.76 | 2.22 | 0.79 | 0.43 | −6.17 | 2.64 | ||
| THI × Sex | 0.10 | 0.05 | 2.18 | .03 | 0.01 | 0.19 | ||
| BAI R2 = 0.27, MSE = 59.24 p < 0.001 | THI | 0.15 | 0.04 | 4.01 | <0.001 | 0.08 | 0.23 | |
| Sex | 1.80 | 3.21 | 0.56 | 0.58 | −4.56 | 8.17 | ||
| THI × Sex | 0.05 | 0.07 | 0.67 | 0.50 | −0.09 | 0.18 |
Coeff, coefficient; se, standard error; LLCI and ULCI, lower and upper levels for confidence interval; MSE, mean squared error; statistically significant values printed bold.
Discussion
The purpose of this study was to investigate gender differences in tinnitus distress and associated psychological comorbidities in patients with tinnitus.
About a quarter of the patients in our sample reported severe or very severe tinnitus distress. In accordance with previous research, increased levels of tinnitus distress were associated with higher levels of depression, anxiety and worries (Durai and Searchfield, 2016; Pattyn et al., 2016; Salazar et al., 2019). Furthermore, we found that higher tinnitus distress was also associated with positive beliefs about worry, negative beliefs about the controllability of thoughts and danger of worry, cognitive confidence, beliefs about the need to control thoughts, and cognitive self-consciousness.
In line with the literature, our results showed that tinnitus was accompanied by hearing loss in 68% of the patients. Similar risk factors responsible for cochlear impairment may be an explanation for this link (Shargorodsky et al., 2010; Martines et al., 2015). In our sample there was no statistically significant gender difference in tinnitus distress, neither in the THI total scale nor in any of the subscales, which is in accordance with previous studies (Han et al., 2019). Yet, there was a distinct gender effect for the assessed psychological comorbidities, with women reporting higher anxiety, depression and worries than men. These gender effects are consistent with previous studies, who also found higher anxiety and depression (Gallus et al., 2015; Pattyn et al., 2016; Bashir et al., 2017; Strumila et al., 2017; Ziai et al., 2017), as well as higher level of distress (Seydel et al., 2013) and increased likelihood for suicide attempts (Lugo et al., 2019) in women.
Comorbidities as hypertension, insomnia and migraine have been previously associated with chronic tinnitus. The role of arterial hypertension as a risk factor for tinnitus is well known from the literature because blood pressure alterations could affect the cochlear microcirculation, yet no gender differences were reported (Figueiredo et al., 2015; Martines et al., 2015; Yang et al., 2015; Figueiredo et al., 2016). Recently, some authors also reported an association between masked hypertension, arterial stiffness and tinnitus (Gun et al., 2019; Gedikli et al., 2020). Additionally, sleep disorders and insomnia are frequently associated with tinnitus (Fioretti et al., 2013; Aazh et al., 2019). As a consequence, the insomnia Cognitive Behavioral Therapy (i-CBT) was proposed as a treatment to reduce insomnia and distressing tinnitus (Marks et al., 2019). Chen et al. (2019) found that patients with non-migraine headache are at significantly greater risk of tinnitus than those without chronic headache. Patients with migraine have also an increased risk to develop cochlear disorders and in particular tinnitus (Hwang et al., 2018). Langguth et al. (2017) described that the laterality and severity of primary headache and tinnitus are significantly related. The co-occurrence of tinnitus and other comorbidities like vertigo, hyperacusis, headache and depression could suggest the presence of a somatoform disorder. In line with the current literature, we found a significantly higher prevalence of headache (p = 0.002) in women than in men. Thus, our findings support the idea to explore different causes of tinnitus to classify multiple subtypes and to find different treatments for patients with tinnitus (Landgrebe et al., 2010; McFerran et al., 2019).
In our sample, there was also a higher prevalence of hyperacusis, headache and vertigo/dizziness in women than in men. This may not be a tinnitus specific finding, since previous studies have found an increased likelihood for hyperacusis (Paulin et al., 2016), headache (Buse et al., 2013) and vertigo or dizziness (Kurre et al., 2012) in women. As proposed by other authors, these findings are supported by the hypothesis that in women homeostasis of labyrinthic fluids may be altered by hormone alterations in the menstrual cycle, pregnancy and menopause causing balance and hearing disorders (Ishii et al., 2009; Reiss and Reiss, 2014). Yet, in our study there was no increased prevalence of temporomandibular disorders, which is in contradiction to previously described prevalence rates (Vielsmeier et al., 2012; Algieri et al., 2017; Edvall et al., 2019). Data from literature reports a greater heritability for bilateral tinnitus in men and young women (Maas et al., 2017; Lopez-Escamez and Amanat, 2020). In our sample, there was a notably higher prevalence of a familial history of tinnitus in younger women (<40 years) than in men. Yet, this difference was not statistically significant. Apart from the psychological comorbidities and clinical features, there was also a gender effect regarding the patients meta cognitions, which–to our knowledge – has not been investigated in patients with tinnitus so far. The concept of meta cognitions describes how people reflect their own cognitive processes. In our sample, men reported significantly stronger beliefs about the need to control thoughts than women. So far, no studies have described a general gender difference regarding metacognitions (e.g., Quattropani et al., 2014). Future studies could confirm our results giving the opportunity to offer tailored therapy, like Metacognition Therapy (Wells, 2009), to treat the worry and the need to control thoughts.
The mediation analyses showed no significant interaction of gender on the relationship of the psychological comorbidities or meta-cognitions on tinnitus distress. Yet, there was an inversed relationship: while in our sample men had lower depression scores than women, we also found that with increasing tinnitus distress, the level of depression increased significantly stronger in men than in women. This partially reproduced the findings of Han et al. (2019), who found that tinnitus severity did not significantly differ between the gender groups but partial correlations between tinnitus severity and depressive symptoms were stronger in males than in females. One explanation for our results could be, that men might have more problems to cope with higher levels of tinnitus distress and thus may react more depressed. Since this is a cross-sectional study, of course we cannot make any causal assumptions, but our results emphasize the importance of psychological comorbidities as predictors of tinnitus severity in clinical management.
This study has some limits. First, the included sample is comparably small and multicenter studies with larger sample would contribute to clarify the results found in our study. Second, concerns have been raised on the reliance on self-reported symptoms like temporomandibular disorders, sleep disorders, headache and hyperacusis extrapolated from the answers of the TSCH. Nevertheless, the TSCH is a validated instrument for standardized collection of information about the characteristics of the tinnitus patient and by the time of the data collection its use still was recommended to facilitate comparability of studies in tinnitus research (Langguth et al., 2007). Third, we did not investigate presence of clinical comorbidities (hypertension, migraine, insomnia), the level of hearing loss, tinnitus pitch and loudness or the role of traits of personality among tinnitus patients as this was not the focus of the study. We focused our attention on the psychological comorbidities, and we only analyzed the presence of normal hearing/hearing loss measured with a standard pure tone audiogram (250–8,000 Hz). No absolute correlation between hearing loss and tinnitus was demonstrated since, as recently proposed, a mild hearing impairment could be present in patients with tinnitus and yet be missed by a standard pure tone audiogram (PTA) measured at octave or half octave intervals from 250 to 8,000 Hz (Xiong et al., 2019). Thus, a strong audiological evaluation would require a high definition audiogram with the study of high frequencies (Liberman et al., 2016; Lefeuvre et al., 2019), DPOAE (Xiong et al., 2019) and the threshold equalizing noise (TEN) test between 500 and 4 kHz (Kara et al., 2020) to detect hidden hearing loss and synaptopathy. Also, as proposed by Simoes et al. (2019), neuroticism and extraversion might be relevant markers of tinnitus distress over time and may be used to statistically distinguish patient groups with clinically relevant changes of tinnitus distress. Finally, we did not assess the duration of the psychiatric comorbidities, thus it is not entirely clear, whether patients suffered from pre-existing psychiatric complaints or if these symptoms unfolded associated with the tinnitus onset.
Conclusion
In conclusion, our results indicate general gender differences for psychological comorbidities in tinnitus patients, with women reporting more depression, anxiety and worries. Men, on the other hand, showed a higher need to control their thoughts. The question about the distinction between female and male tinnitus patients remains open, considering that (1) both genetic and psychosocial risk factors may contribute to this gender difference, (2) epidemiological studies reports higher prevalence of anxiety disorders and depression in female than in man [World Health Organization (WHO), 2017]. Despite the high heterogeneity of prevalence estimates, there is emerging evidence of substantial prevalence of anxiety disorders generally (3.8–25%), and particularly in women (5.2–8.7%) (Remes et al., 2016). Women have higher rates of major depression compared to men and, in average, the ratio is 2:1 (Bromet et al., 2011). Yet, there is substantial evidence that the gender difference in major depression diagnoses and depression symptoms peaks in adolescence and the gender gap then narrows and remains stable in adulthood (Salk et al., 2017). It could be hypothesized that gender differences in tinnitus and its relief arise from an interaction of genetic, anatomical, physiological, neuronal, hormonal, psychological and social factors which modulate tinnitus differently in the sexes. Our results suggest that recognition of the evidence underlying sex differences in tinnitus will guide development of treatments and provide better options for patients that are tailored to their physical and psychological comorbidities.
Data Availability Statement
The datasets for this article are not publicly available since the consent of the patients to do so was not obtained. Requests to access the datasets should be directed to the corresponding author DR.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics committee of the University of L’Aquila ID number 18/2020. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
AF: conceptualization, methodology, supervision, writing (original draft), and project administration. EN: writing (original draft) and data curation. DR: methodology, formal analysis, and writing (original draft). RM: writing (review and editing). AE: writing (review and editing) and supervision. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The handling Editor declared a past co-authorship with one of the authors AF.
References
- Aazh H., Baguley D. M., Moore B. C. J. (2019). Factors related to insomnia in adult patients with tinnitus and/or hyperacusis: an exploratory analysis. J. Am. Acad. Audiol. 30 802–809. 10.3766/jaaa.18020 [DOI] [PubMed] [Google Scholar]
- Algieri G. M. A., Leonardi A., Arangio P., Vellone V., Paolo C. D., Cascone P. (2017). Tinnitus in Temporomandibular joint disorders: is it a specific somatosensory tinnitus subtype? Int. Tinnitus J. 20 83–87. [DOI] [PubMed] [Google Scholar]
- Altissimi G., Salviati M., Turchetta R., Orlando M. P., Greco A., De Vincentiis M., et al. (2016). When alarm bells ring: emergency tinnitus. Eur. Rev. Med. Pharmacol. Sci. 20 2955–2973. [PubMed] [Google Scholar]
- Bashir A., Ammar A., Aqeel M., Tanvir A., Sammeen S. (2017). Impact of tinnitus perception on psychological distress in male and female tinnitus patients. Foundation Univ. J. Psychol. 1 56–77. [Google Scholar]
- Beck A. T., Epstein N., Brown G., Steer R. A. (1988). An inventory for measuring clinical anxiety: psychometric properties. J. Consult. Clin. Psychol. 56 893–897. 10.1037/0022-006x.56.6.893 [DOI] [PubMed] [Google Scholar]
- Beck A. T., Steer R. A., Brown G. K. (1996). Manual for the Beck Depression Inventory–II. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Bromet E., Andrade L. H., Hwang I. (2011). Cross-national epidemiology of DSM-IV major depressive episode. BMC Med. 9:90. 10.1186/1741-7015-9-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buse D. C., Loder E. W., Gorman J. A., Stewart W. F., Reed M. L., Fanning K. M., et al. (2013). Sex differences in the prevalence, symptoms, and associated features of migraine, probable migraine and other severe headache: results of the American Migraine Prevalence and Prevention (AMPP) Study. Headache 53 1278–1299. 10.1111/head.12150 [DOI] [PubMed] [Google Scholar]
- Caldirola D., Teggi R., Dacco S., Sangiorgio E., Bussi M., Perna G. (2016). Role of worry in patients with chronic tinnitus and sensorineural hearing loss: a preliminary study. Eur. Arch. Otorhinolaryngol. 273 4145–4151. 10.1007/s00405-016-4100-8 [DOI] [PubMed] [Google Scholar]
- Chen Y. C., Tsai S. J., Chen J. C., Hwang J. H. (2019). Risks of tinnitus, sensorineural hearing impairment, and sudden deafness in patients with non-migraine headache. PLoS One 14:e0222041. 10.1371/journal.pone.0222041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cima R. F. F., Mazurek B., Haider H., Kikidis D., Lapira A., Norena A., et al. (2019). A multidisciplinary European guideline for tinnitus: diagnostics, assessment, and treatment. HNO 67 10–42. 10.1007/s00106-019-0633-7 [DOI] [PubMed] [Google Scholar]
- Durai M., Searchfield G. (2016). Anxiety and depression, personality traits relevant to tinnitus: A scoping review. Int. J. Audiol. 55 605–615. 10.1080/14992027.2016.1198966 [DOI] [PubMed] [Google Scholar]
- Edvall N. K., Gunan E., Genitsaridi E., Lazar A., Mehraei G., Billing M., et al. (2019). Impact of temporomandibular joint complaints on tinnitus-related distress. Front. Neurosci. 13:879. 10.3389/fnins.2019.00879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlandsson S. I., Holgers K. M. (2001). The impact of perceived tinnitus severity on health-related quality of life with aspects of gender. Noise Health 3 39–51. [PubMed] [Google Scholar]
- Figueiredo R. R., Azevedo A. A., Penido N. O. (2016). Positive association between tinnitus and arterial hypertension. Front. Neurol. 7:171. 10.3389/fneur.2016.00171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiredo R. R., De Azevedo A. A., Penido Nde O. (2015). Tinnitus and arterial hypertension: a systematic review. Eur. Arch. Otorhinolaryngol. 272 3089–3094. 10.1007/s00405-014-3277-y [DOI] [PubMed] [Google Scholar]
- Fioretti A., Fusetti M., Eibenstein A. (2013). Association between sleep disorders, hyperacusis and tinnitus: evaluation with tinnitus questionnaires. Noise Health 15 91–95. [DOI] [PubMed] [Google Scholar]
- Gallus S., Lugo A., Garavello W., Bosetti C., Santoro E., Colombo P., et al. (2015). Prevalence and determinants of tinnitus in the italian adult population. Neuroepidemiology 45 12–19. 10.1159/000431376 [DOI] [PubMed] [Google Scholar]
- Gedikli O., Kemal O., Yildirim U., Cecen A. B., Karabulut H., Akcay M., et al. (2020). Is there an association between the parameters of arterial stiffness and tinnitus? Acta Otolaryngol. 140 128–132. 10.1080/00016489.2019.1668569 [DOI] [PubMed] [Google Scholar]
- Gomaa M. A., Elmagd M. H., Elbadry M. M., Kader R. M. (2014). Depression, Anxiety and Stress Scale in patients with tinnitus and hearing loss. Eur. Arch. Otorhinolaryngol. 271 2177–2184. 10.1007/s00405-013-2715-6 [DOI] [PubMed] [Google Scholar]
- Gun T., Ozkan S., Yavuz B. (2019). Is tinnitus an early voice of masked hypertension? High masked hypertension rate in patients with tinnitus. Clin. Exp. Hypertens. 41 231–234. 10.1080/10641963.2018.1465077 [DOI] [PubMed] [Google Scholar]
- Han T. S., Jeong J. E., Park S. N., Kim J. J. (2019). Gender differences affecting psychiatric distress and tinnitus severity. Clin. Psychopharmacol. Neurosci. 17 113–120. 10.9758/cpn.2019.17.1.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes A. F. (2013). Introduction to mediation, moderation, and conditional process analysis: a regression? Based Approach. New York, NY: The Guilford Press. [Google Scholar]
- Hwang J. H., Tsai S. J., Liu T. C., Chen Y. C., Lai J. T. (2018). Association of Tinnitus and Other Cochlear Disorders With a History of Migraines. JAMA Otolaryngol. Head. Neck. Surg. 144 712–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii C., Nishino L. K., Campos C. A. (2009). Vestibular characterization in the menstrual cycle. Braz. J. Otorhinolaryngol. 75 375–380. 10.1590/s1808-86942009000300012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kara E., Aydin K., Akbulut A. A., Karakol S. N., Durmaz S., Yener H. M., et al. (2020). Assessment of hidden hearing loss in normal hearing individuals with and without tinnitus. J. Int. Adv. Otol. 16 87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurre A., Straumann D., Van Gool C. J., Gloor-Juzi T., Bastiaenen C. H. (2012). Gender differences in patients with dizziness and unsteadiness regarding self-perceived disability, anxiety, depression, and its associations. BMC Ear Nose Throat Disord. 12:2. 10.1186/1472-6815-12-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgrebe M., Zeman F., Koller M., Eberl Y., Mohr M., Reiter J., et al. (2010). The Tinnitus Research Initiative (TRI) database: a new approach for delineation of tinnitus subtypes and generation of predictors for treatment outcome. BMC Med. Inform. Decis. Mak. 10:42. 10.1186/1472-6947-10-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langguth B., Goodey R., Azevedo A., Bjorne A., Cacace A., Crocetti A., et al. (2007). Consensus for tinnitus patient assessment and treatment outcome measurement: Tinnitus Research Initiative meeting, Regensburg, July 2006. Prog. Brain Res. 166 525–536. 10.1016/s0079-6123(07)66050-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langguth B., Hund V., Landgrebe M., Schecklmann M. (2017). Tinnitus patients with comorbid headaches: the influence of headache type and laterality on tinnitus characteristics. Front. Neurol. 8:440. 10.3389/fneur.2017.00440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefeuvre J., Chedeau J., Boulet M., Fain G., Papon J. F., Nguyen Y., et al. (2019). Hidden hearing loss and tinnitus: Utility of the high-definition audiograms in diagnosis. Clin. Otolaryngol. 44 1170–1175. 10.1111/coa.13435 [DOI] [PubMed] [Google Scholar]
- Liberman M. C., Epstein M. J., Cleveland S. S., Wang H., Maison S. F. (2016). Toward a differential diagnosis of hidden hearing loss in humans. PLoS One 11:e0162726. 10.1371/journal.pone.0162726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Escamez J. A., Amanat S. (2020). Heritability and genetics contribution to tinnitus. Otolaryngol. Clin. North Am. 10.1016/j.otc.2020.03.003 [Online ahead of print] [DOI] [PubMed] [Google Scholar]
- Lugo A., Trpchevska N., Liu X., Biswas R., Magnusson C., Gallus S., et al. (2019). Sex-specific association of tinnitus with suicide attempts. JAMA Otolaryngol. Head. Neck Surg. 145 685–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas I. L., Bruggemann P., Requena T., Bulla J., Edvall N. K., Hjelmborg J. V. B., et al. (2017). Genetic susceptibility to bilateral tinnitus in a Swedish twin cohort. Genet. Med. 19 1007–1012. 10.1038/gim.2017.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks E., Mckenna L., Vogt F. (2019). Cognitive behavioural therapy for tinnitus-related insomnia: evaluating a new treatment approach. Int. J. Audiol. 58 311–316. 10.1080/14992027.2018.1547927 [DOI] [PubMed] [Google Scholar]
- Martines F., Sireci F., Cannizzaro E., Costanzo R., Martines E., Mucia M., et al. (2015). Clinical observations and risk factors for tinnitus in a Sicilian cohort. Eur. Arch. Otorhinolaryngol. 272 2719–2729. 10.1007/s00405-014-3275-0 [DOI] [PubMed] [Google Scholar]
- McCormack A., Edmondson-Jones M., Somerset S., Hall D. (2016). A systematic review of the reporting of tinnitus prevalence and severity. Hear. Res. 337 70–79. 10.1016/j.heares.2016.05.009 [DOI] [PubMed] [Google Scholar]
- McFerran D. J., Stockdale D., Holme R., Large C. H., Baguley D. M. (2019). Why is there no cure for tinnitus? Front. Neurosci. 13:802. 10.3389/fnins.2019.00802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meric C., Gartner M., Collet L., Chery-Croze S. (1998). Psychopathological profile of tinnitus sufferers: evidence concerning the relationship between tinnitus features and impact on life. Audiol. Neurootol. 3 240–252. 10.1159/000013796 [DOI] [PubMed] [Google Scholar]
- Meyer T. J., Miller M. L., Metzger R. L., Borkovec T. D. (1990). Development and validation of the penn state worry questionnaire. Behav. Res. Ther. 28 487–495. 10.1016/0005-7967(90)90135-6 [DOI] [PubMed] [Google Scholar]
- Newman C. W., Jacobson G. P., Spitzer J. B. (1996). Development of the tinnitus handicap inventory. Arch. Otolaryngol. Head Neck Surg. 122 143–148. [DOI] [PubMed] [Google Scholar]
- Passi S., Ralli G., Capparelli E., Mammone A., Scacciatelli D., Cianfrone G. (2008). The THI questionnaire: psychometric data for reliability and validity of the Italian version. Int. Tinnitus J. 14 26–33. [PubMed] [Google Scholar]
- Pattyn T., Van Den Eede F., Vanneste S., Cassiers L., Veltman D. J., Van De Heyning P., et al. (2016). Tinnitus and anxiety disorders: a review. Hear. Res. 333 255–265. 10.1016/j.heares.2015.08.014 [DOI] [PubMed] [Google Scholar]
- Paulin J., Andersson L., Nordin S. (2016). Characteristics of hyperacusis in the general population. Noise Health 18 178–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto P. C., Sanchez T. G., Tomita S. (2010). The impact of gender, age and hearing loss on tinnitus severity. Braz. J. Otorhinolaryngol. 76 18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quattropani M. C., Lenzo V., Mucciardi M., Toffle M. E. (2014). Psychometric properties of the Italian version of the Short Form of the MCQ-30. Boll. Psicol. Appl. 269 29–41. [Google Scholar]
- Reiss M., Reiss G. (2014). Laterality of sudden sensorineural hearing loss. Ear Nose. Throat. J. 93 318–320. 10.1177/014556131409300809 [DOI] [PubMed] [Google Scholar]
- Remes O., Brayne C., van der Linde R., Lafortune L. (2016). A systematic review of reviews on the prevalence of anxiety disorders in adult populations. Brain Behav. 6:e00497. 10.1002/brb3.497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar J. W., Meisel K., Smith E. R., Quiggle A., Mccoy D. B., Amans M. R. (2019). Depression in patients with tinnitus: a systematic review. Otolaryngol. Head Neck Surg. 161 28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salk R. H., Hyde J. S., Abramson L. Y. (2017). Gender differences in depression in representative national samples: meta-analyses of diagnoses and symptoms. Psychol. Bull. 143 783–822. 10.1037/bul0000102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salviati M., Macri F., Terlizzi S., Melcore C., Provenzano A., Capparelli E., et al. (2013). The Tinnitus Handicap Inventory as a screening test for psychiatric comorbidity in patients with tinnitus. Psychosomatics 54 248–256. 10.1016/j.psym.2012.05.007 [DOI] [PubMed] [Google Scholar]
- Seydel C., Haupt H., Olze H., Szczepek A. J., Mazurek B. (2013). Gender and chronic tinnitus: differences in tinnitus-related distress depend on age and duration of tinnitus. Ear Hear 34 661–672. 10.1097/aud.0b013e31828149f2 [DOI] [PubMed] [Google Scholar]
- Shargorodsky J., Curhan G. C., Farwell W. R. (2010). Prevalence and characteristics of tinnitus among US adults. Am. J. Med. 123 711–718. 10.1016/j.amjmed.2010.02.015 [DOI] [PubMed] [Google Scholar]
- Shlamkovich N., Gavriel H., Eviatar E., Lorberboym M., Aviram E. (2016). Brain positron emission tomography-computed tomography gender differences in tinnitus patients. J. Am. Acad. Audiol. 27 714–719. 10.3766/jaaa.15067 [DOI] [PubMed] [Google Scholar]
- Simoes J., Schlee W., Schecklmann M., Langguth B., Farahmand D., Neff P. (2019). Big five personality traits are associated with tinnitus improvement over time. Sci. Rep. 9:18234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strumila R., Lengvenyte A., Vainutiene V., Lesinskas E. (2017). The role of questioning environment, personality traits, depressive and anxiety symptoms in tinnitus severity perception. Psychiatr. Q. 88 865–877. 10.1007/s11126-017-9502-2 [DOI] [PubMed] [Google Scholar]
- Tunkel D. E., Bauer C. A., Sun G. H., Rosenfeld R. M., Chandrasekhar S. S., Cunningham E. R., et al. (2014). Clinical practice guideline: tinnitus. Otolaryngol. Head Neck Surg. 151 S1–S40. [DOI] [PubMed] [Google Scholar]
- Udupi V. A., Uppunda A. K., Mohan K. M., Alex J., Mahendra M. H. (2013). The relationship of perceived severity of tinnitus with depression, anxiety, hear- ing status, age and gender in individuals with tinnitus. Int. Tinnitus J. 18 29–34. [DOI] [PubMed] [Google Scholar]
- Vielsmeier V., Strutz J., Kleinjung T., Schecklmann M., Kreuzer P. M., Landgrebe M., et al. (2012). Temporomandibular joint disorder complaints in tinnitus: further hints for a putative tinnitus subtype. PLoS One 7:e38887. 10.1371/journal.pone.0038887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells A. (2009). Metacognitive Therapy for Anxiety and Depression. New York, NY: Guilford Press. [Google Scholar]
- Wells A., Cartwright-Hatton S. (2004). A short form of the metacognitions questionnaire: properties of the MCQ-30. Behav. Res. Ther. 42 385–396. 10.1016/s0005-7967(03)00147-5 [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO) (2017). Depression and other common mental disorders: global health estimates. Geneva: World Health Organization. [Google Scholar]
- Xiong B., Liu Z., Liu Q., Peng Y., Wu H., Lin Y., et al. (2019). Missed hearing loss in tinnitus patients with normal audiograms. Hear. Res. 384:107826. 10.1016/j.heares.2019.107826 [DOI] [PubMed] [Google Scholar]
- Yang P., Ma W., Zheng Y., Yang H., Lin H. (2015). A systematic review and meta-analysis on the association between hypertension and tinnitus. Int. J. Hypertens. 2015:583493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziai K., Moshtaghi O., Mahboubi H., Djalilian H. R. (2017). Tinnitus patients suffering from anxiety and depression: a review. Int. Tinnitus J. 21 68–73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets for this article are not publicly available since the consent of the patients to do so was not obtained. Requests to access the datasets should be directed to the corresponding author DR.