Abstract
To be prepared for alternating metabolic demands occurring over the 24-hour day, the body preserves information on time in skeletal muscle, and all cells, through a circadian clock mechanism. Skeletal muscle can be considered the largest collection of peripheral clocks in the body with a major contribution to whole body energy metabolism. Comparison of circadian clock gene expression between skeletal muscle of nocturnal rodents and diurnal humans reveals very common patterns based on rest/active cycles rather than light/dark cycles. Rodent studies in which the circadian clock is disrupted in skeletal muscle demonstrate impaired glucose handling and insulin resistance. Experimental circadian misalignment in humans modifies the skeletal muscle clocks and leads to disturbed energy metabolism and insulin resistance. Preclinical studies have revealed that timing of exercise over the day can influence the beneficial effects of exercise on skeletal muscle metabolism and studies suggest similar applicability in humans. Current strategies to improve metabolic health, e.g. exercise, should be reinvestigated in their capability to modify the skeletal muscle clocks by taking timing of the intervention into account.
Keywords: Skeletal muscle, circadian clocks, metabolic health, circadian disruption, exercise
1. Introduction
Circadian rhythms are endogenously generated 24h cycles that can be observed in behavior, physiology and metabolic processes. The molecular mechanism that drives circadian rhythms is the circadian clock and this is found in virtually every cell in mammals. While the circadian clock has been studied most in the suprachiasmatic nucleus of the brain (central clock) we are now learning more about the role of the circadian clocks in peripheral tissues such as skeletal muscle. The molecular clock generates circadian rhythms through a translational-transcriptional feedback loop (TTFL) mechanism, in which CLOCK and BMAL1 proteins act as transcriptional activators of Cryptochrome (Cry1–2) and Period (Per1–3) genes, which encode proteins that repress CLOCK:BMAL1 with a periodicity of ~24h (Figure 1). In addition to the TTFL, CLOCK:BMAL1 also contribute to the expression of a large number of genes, called clock-controlled genes (CCGs). Transcriptomics has been the most used tool to identify CCGs in mammalian tissues. Early skeletal muscle transcriptomic studies relied on sampling every 4h and identified >200 rhythmic genes in mice, including components of the molecular clock and genes involved in transcription, lipid metabolism, protein degradation, ion transport, and vesicular trafficking (1, 2). Zhang et al. (2014) performed a circadian timeseries analysis of gene expression in mice every 2 hours for 48 hours in 12 different mouse organs, including skeletal muscle (gastrocnemius), using microarrays and targeted RNA-seq data. They found that skeletal muscle expresses ~1600 circadian genes and that more than 40% of all mammalian protein-coding genes are expressed rhythmically somewhere in the body, mainly in an organ-specific manner (3, 4). The components of the core circadian clock mechanism are ubiquitous across all cells; however, the CCGs are unique to each tissue. Skeletal muscle-specific CCGs include Myod1, Atrogin1 (Fbxo32), Mef2 and Glut4 highlighting the role of the clock for expression of genes important for the daily maintenance of skeletal muscle (1, 4).
Figure 1.
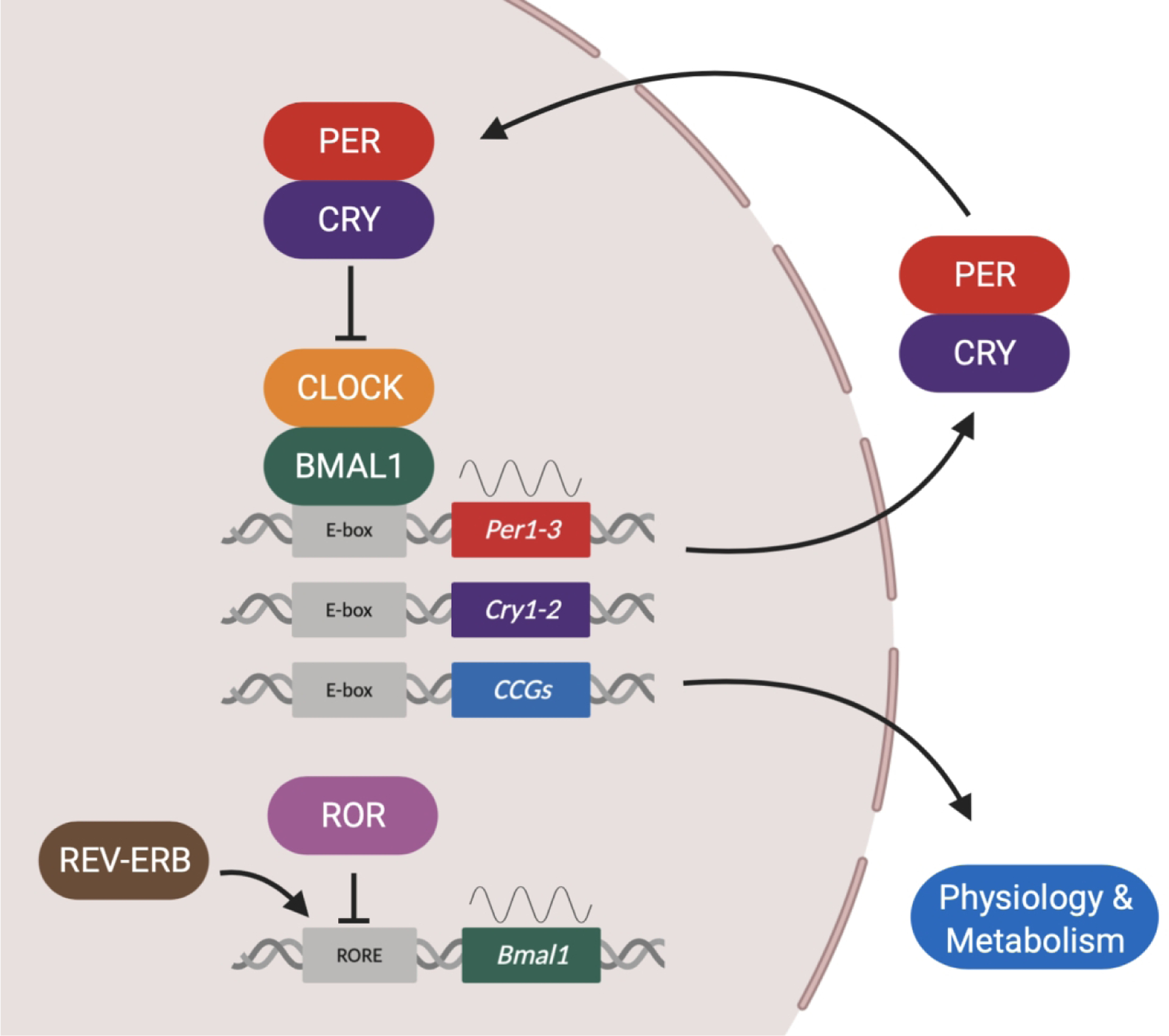
The molecular clock generates circadian rhythms through a transcriptional-translational feedback loop (TTFL) mechanism, in which the transcription of Period (Per1, Per2, and Per3) and Cryptochrome (Cry1 and Cry2) genes is activated by the heterocomplex formed by the PAS domain helix-loop-helix transcription factors CLOCK (or its paralogue NPAS2) and BMAL1 (also known as ARNTL) via E-boxes elements in their promoter regions. PER and CRY proteins dimerize, translocate to the nucleus, and repress their own CLOCK:BMAL1-mediated transcription. This creates a cycle that takes approximately 24 hours to be completed. In addition to the main loop, a secondary feedback loop comprises the nuclear receptors ROR and REV-ERB that activates or represses, respectively, the expression of Bmal1 gene by acting on ROR-binding elements (ROREs) within its promoter. CLOCK:BMAL1 activates the transcription of clock-controlled genes (CCGs) in a tissue-specific manner and these genes are critical for cell physiology and metabolism.
By constituting ~40% or more of body mass, skeletal muscles can be considered the largest collection of peripheral clocks in the human body (5). In the context of metabolic health, the relevance of studying skeletal muscle clocks in particular becomes clear considering the important role of skeletal muscle in maintaining whole body energy, substrate metabolism and glucose homeostasis; one example being that skeletal muscle accounts for ~80% of postprandial glucose uptake in humans (6, 7). Skeletal muscle is also a metabolically active tissue especially when considering the energy cost of skeletal muscle force, as well as the pumps managing intracellular calcium stores and those pumps maintaining membrane potential, thereby being the largest contributor to whole body energy requirement (8–10). Moreover, skeletal muscle is able to rapidly adapt to metabolic demand (e.g. exercise) and nutrient status by modulating substrate storage and matching substrate oxidation to glucose or fat availability, respectively (11). Whereas its flexibility to either use or store energy is mostly dependent on the change from feeding to fasting and from activity to rest, recent evidence suggests that skeletal muscle metabolism is further governed by the intrinsic skeletal muscle clocks (4).
This review summarizes the literature on how the skeletal muscle clocks of nocturnal rodents and diurnal humans are organized. The ability to perform repeated biopsies of skeletal muscle from the same individual over a 24h period provides an opportunity to directly compare the expression of circadian clock factors between humans and rodents. Based on these comparisons, we discuss the relevance of findings from clock-disruption rodent models for human metabolic health. The aim is to evaluate the physiological relevance of the skeletal muscle clocks for human metabolic health and to give an outlook on how future studies could usefully further investigate clockwork in skeletal muscle.
2. Study of circadian rhythms in nocturnal rodents and diurnal humans
The proper functioning of the circadian clock in skeletal muscle is likely to be crucial for the regulation of whole-body energy and substrate metabolism. However, scientific interest in skeletal muscle clocks in humans has only recently emerged, so that our current knowledge about skeletal muscle clocks is mostly based on studies in nocturnal rodents. Evolutionarily diverged from humans approximately 96 million years ago (12), the mouse is widely viewed as a representative model to study mammalian gene expression. However, in particular when it comes to investigating circadian rhythms, differences to humans should be considered. Among the obvious differences are the opposing times of activity and feeding versus rest. For example, in preclinical studies, mice are generally fed ad libitum but data indicates that 75–80% of intake occurs in the dark phase (13, 14). Mice also exhibit polyphasic or segmented bouts of sleep but the majority of sleep episodes are in the light phase (15). In contrast, humans are physically active and eat mostly during the light phase and exhibit consolidated sleep in the dark phase. Thus, most of the rhythms found in behavior, physiology and metabolism are antiphase when comparing nocturnal and diurnal animals against the light/dark phases of the day.
The analysis of circadian rhythms requires standardized conditions to control for environmental cues. Under normal circumstances mice are housed under defined conditions with regards to lighting, feeding and activity, and tissues can be collected at any given time of the day to investigate rhythmic patterns in skeletal muscle and other tissues. The current guidelines for discovering circadian patterns recommend that tissues are collected for at least two complete 24h cycles during which mice are under constant dark conditions with no input from light (16).
For human studies, consistent conditions (e.g., regular meals, physical activity, and bed times) are critical. However, to study the human skeletal muscle clocks a more elaborate and careful study design is required to avoid confounding factors in the face of ethical standards. For this purpose, two approaches have currently been used: constant routine or realistic life-style protocols. Constant routine protocols are designed to avoid confounding factors, known to affect skeletal muscle clocks, as much as possible. For example, Perrin et al. (2018) collected skeletal muscle biopsies every 4h over 24h in ten healthy volunteers constantly in a semi-recumbent position, under constant hourly feeding via meal-replacement solutions, under constant artificial lighting and ambient temperature (17). By contrast, van Moorsel et al. (2016) collected five skeletal muscle biopsies within 24h from twelve young healthy males under ‘normal’ living conditions (i.e. 3 meals per day, some physical activity) to mimic realistic daily lifestyle conditions as much as possible (18). Sleeping was facilitated for 8–9h with lights switched off in both protocols. The direct comparison of specific analyses, such as metabolome or transcriptome, in muscle samples derived from these two different protocols may contribute to a better understanding of the relative roles of the circadian versus behavioral components of biological rhythmicity.
3. The skeletal muscle clocks in rodents and humans
Circadian clock genes have been shown to exhibit 24h rhythmic expression in human and mouse skeletal muscle samples. In this section, we will refer to light/dark but we will also include the descriptors rest/active, as a way to compare the daily patterns of clock gene expression between rodents and humans. The core clock genes Bmal1 and Clock function as the positive arm of the transcriptional-translational feedback mechanism and in mouse skeletal muscle Bmal1 mRNA exhibits a peak expression at the transition from the dark/active to the light/rest phase. In contrast, the genes that comprise the negative arm of the clock Per1, Per2, Per3, Cry1 and Cry2 exhibit an antiphase pattern with their peak of expression prior to the transition from rest to the active phase (3, 4). Expression of core clock genes have also been measured in human skeletal muscle of lean volunteers, both when measured directly in human skeletal muscle biopsies (17–19) and by in vitro differentiation of human primary skeletal myotubes established from human donor biopsies (17, 20, 21). In vivo studies show that Bmal1 expression also peaks during the transition from active to the rest phase in human skeletal muscle, while Per1, Per2, Per3, Cry1, Cry2 are antiphase to Bmal1, and their expression peaks at the transition from rest to the activity phase. The expression of Clock mRNA is more variable with either very low amplitude (17, 18) or no circadian variation (21). Expression patterns of the clock genes in vitro are less robust. In differentiated human myotubes, Bmal1, Per1, Per2, Per3, Cry1 and Cry2 are rhythmic after synchronization with forskolin (17). A previous study found oscillations in Bmal1, Per2, Per3 and Cry1 after synchronization with dexamethasone (20). Hansen et al. reported rhythmic expression in the same genes, but Per1 and Cry2 could not be detected, after serum shock synchronization (21). In differentiated C2C12s myotubes, a mouse skeletal muscle cell line, the expression of Clock, Bmal1, Cry1, Per1, and Per2 was found to be rhythmic after dexamethasone synchronization (22). This direct juxtaposition of available data on the skeletal muscle clocks in mice and humans reveals that if expression is normalized to activity/rest cycles, the skeletal muscle clocks of humans and mice show very common patterns of gene expression as illustrated in Figure 2A and B.
Figure 2: A.
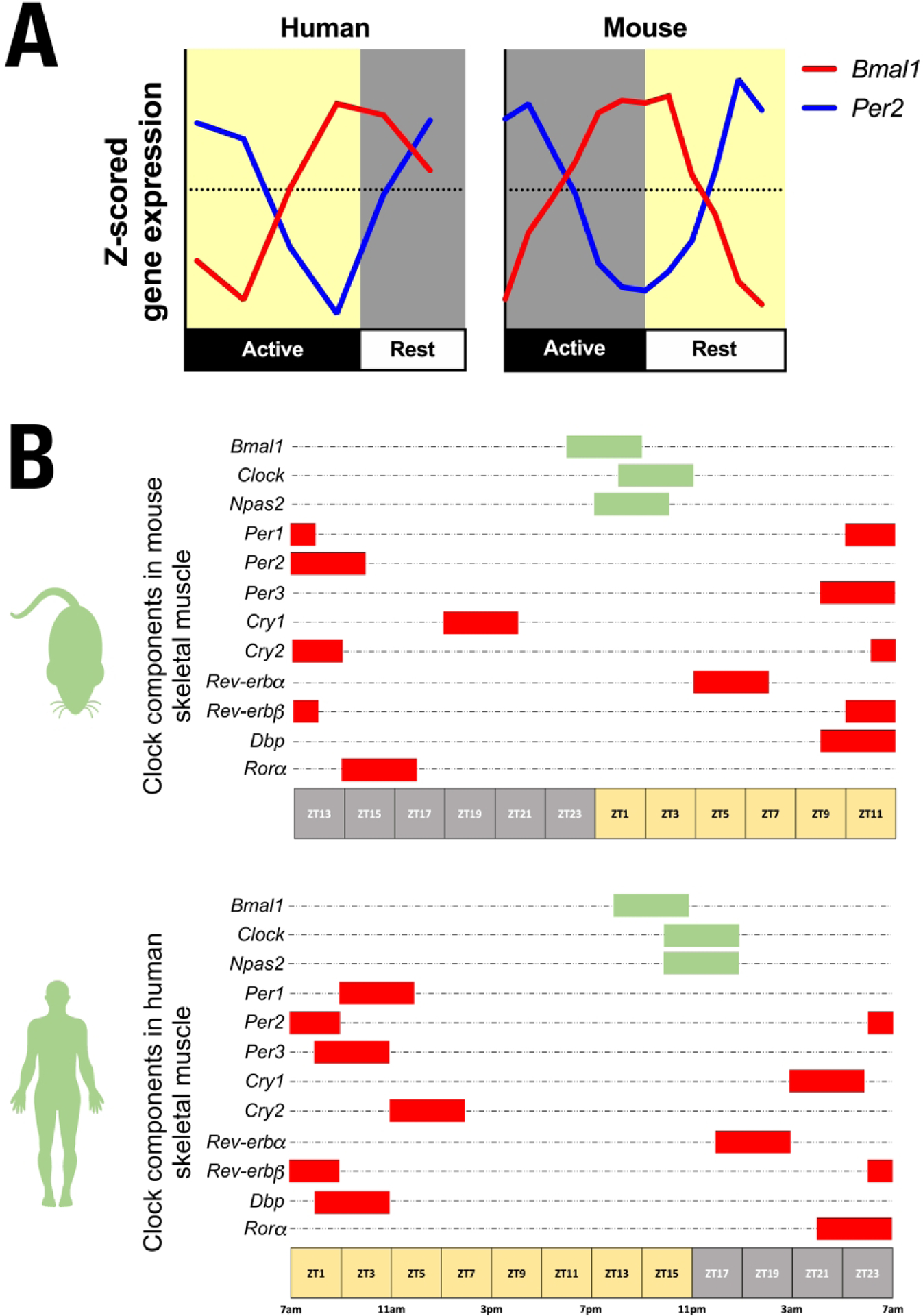
Normalized expression of core clock genes, Bmal1 and Per2, in skeletal muscle of humans and mice over time of day. The patterns of Bmal1 and Per2 expression are similar when viewed from rest/active phases of the day. Human skeletal muscle data was extracted from Perrin et al. (17) and mouse gastrocnemius data was extracted from Zhang et al. (3). Bmal1 circadian expression profile is represented by the red line and Per2 by the blue line. B. Representation of peak times of expression of a larger composite of clock genes and CCGs in mouse skeletal muscle (top panel) and human skeletal muscle (bottom panel). Genes of the positive limb are represented by green bars and genes of the negative limb by red bars. Zeitgeber time 0 (ZT0) refers to the time when lights are switched on, e.g. in human studies at 7AM. For illustration purposes, three hour time frames were used taking the mean value of peak expression of the respective gene from comparable human (17, 18) and mice (3, 4, 23) studies as the center. Note that if corrected for the rest/activity cycle, peak expression patterns across the clock components are similar in both species.
Beyond the core clock factors, the study from Perrin and colleagues (2018) performed a genome-wide transcriptome analysis by high-throughput RNA sequencing from skeletal muscle biopsies from ten healthy volunteers (17). A comparison of the oscillating genes found by Perrin et al. with the circadian gene expression dataset of mouse skeletal muscle previously published by Zhang et al. revealed 430 common circadian oscillating genes between mouse and human skeletal muscle (3, 17). Surprisingly, the phase difference in the expression of the core clock components between diurnal humans and nocturnal rodents was shorter than the expected 12h phase difference. This likely reflects the differences in entrainment as humans were entrained to a 15h:9h LD cycle while mice were kept on a 12h:12h LD prior to placing constant darkness during tissue collections. It is also important to note that the mouse dataset has a temporal resolution of 2h while the humans is 4h and this can impact phase determination (3, 17). It also needs to be taken into account that human studies are primarily done with biopsies from the vastus lateralis while most mouse studies investigate muscles of the lower leg such as gastrocnemius. So far, there are no datasets for direct skeletal muscle type comparisons between humans and mice.
To date, transcriptomic studies have been published in three different mouse skeletal muscles. Dyar et al. (2014) evaluated the expression of circadian genes in two skeletal muscles with distinct fiber type composition (23): soleus composed of a mixed Type I:Type II fiber type with a majority of oxidative fibers and tibialis anterior (TA), comprised of mostly fast-twitch glycolytic fibers (24). They identified 1359 circadian genes in the soleus, while 684 circadian genes were found in TA. Interestingly, a majority of CCGs were specific for each skeletal muscle, with 75% and 51% of circadian genes cycling only in soleus or TA, respectively (25). Circadian transcriptome studies using the mouse gastrocnemius muscle (about 75% Type II glycolytic, about 12% Type II oxidative and 13% Type I oxidative) found diverse signatures with greater than 50% of the genes differentially expressed (1–4). Thus, while all the different skeletal muscles express the core circadian clock factors, the differences in CCGs across skeletal muscles highlights the specificity of circadian regulation and may reflect differences in skeletal muscle use, metabolism and/or functional properties. Also, it is likely that there will be sex-specific differences, however, to date, there is little data on sex as a variable with the circadian clock mechanism in skeletal muscle.
Among the molecular pathways in common between human and mouse circadian datasets is the category of gene transcription. As previously demonstrated in mice, gene transcript accumulation followed a rhythmic pattern with peaks in the middle of both active and rest phases in humans (17). The active phase peak was characterized by enhanced expression of genes associated with mitochondrial activity and skeletal muscle contraction. In contrast, the homologous genes in rodents were partitioned across both the active and rest periods (1, 2, 4). In humans, the genes peaking during the middle of the rest period were enriched for the immune response and inflammation pathways. Furthermore, various transcription factors related to skeletal muscle metabolism demonstrated a rhythmic expression, such as Klf15, which plays a crucial role in regulating skeletal muscle lipid metabolism (26), Tfeb, which regulates oxidative phosphorylation and glucose homeostasis (27), and Myod1, the master myogenic regulatory factor, which peaks during the active phase. Myod1 was previously identified as having a robust circadian pattern of expression in mice with a peak expression during the active phase (2). Since this early observation, studies have demonstrated that Myod1 is a direct target of the CLOCK:BMAL1 complex (28), and as a clock co-factor regulating the expression of CCGs in skeletal muscle (29, 30). It will be interesting to determine if this role for Myod1 is conserved between mouse and human skeletal muscle. In addition to the transcription factors, genes involved in the secretion of myokines, glucose homeostasis and lipid metabolism displayed rhythmic transcription in humans (17). Taken together, these transcriptomic data strongly suggest an important role of the skeletal muscle clocks in regulating human and mouse muscle physiology.
Indeed, skeletal muscle clocks may contribute to the circadian rhythms of human resting energy expenditure, with lowest levels seen in the late biological night and highest in the afternoon/evening. Another metabolic variable, respiratory exchange ratio (RER, carbon dioxide production/oxygen consumption) exhibits a circadian pattern indicating a preference for carbohydrate oxidation in the biological morning and lipid oxidation in the evening as was reported recently (31). Furthermore, exercise performance displays diurnal variation, e.g. increased strength, power and endurance in the afternoon and evening compared with the early morning (32, 33). Also, glucose tolerance and insulin sensitivity were shown to be higher in the morning compared to later on in the day in healthy individuals (34). Further, we showed pronounced 24h rhythmicity of skeletal muscle mitochondrial function in humans with a peak during the late phase and a trough during the early phase of the day, both for basal and maximally-stimulated mitochondrial respiration (18). However, with the ‘normal’ lifestyle protocols applied in these examples the rhythms in these physiological markers cannot only be attributed to the influence of the skeletal muscle clocks per se but also on external factors such as nutrient composition of the provided meals, i.e. higher fat content of the dinner compared to breakfast and lunch, as well as timing of the meal relative to the biopsy taken (although biopsies were always taken just before each meal to avoid direct postprandial effects).
4. Disruption of skeletal muscle clocks and its effects on metabolism
Chronic disruption of circadian rhythms has been associated with metabolic disturbances in humans and mice (35). The importance of skeletal muscle clocks in physiology and behavior has been addressed using genetic loss-of-function mouse models targeting the Bmal1 gene. Bmal1 is the common target as it is the one non-redundant component of the clock mechanism. Global Bmal1 knockout (KO) mice are characterized by an arrhythmic behavior under free-running conditions, growth retardation at 16–18 weeks of age, and a very short lifespan (average of 37 weeks) with hallmarks associated with premature aging, such as skeletal muscle wasting, cataracts, hair loss, arthropathy, and age-related body and organ reduction (36, 37). Metabolically, these mice show no circadian variation in plasma glucose or triglycerides, a profound hypoglycemic response after insulin administration, and an impaired conversion of exogenous pyruvate to glucose (38). The size of skeletal muscle in the global Bmal1 KO was first described to be reduced in an age-dependent manner (37). A more detailed analysis of the skeletal muscle cell structure and function showed that the global Bmal1 KO mice have a reduction in force generation, a significant myofilament and sarcomere disorganization, and mitochondrial pathologies, highlighting the importance of the skeletal muscle clocks in the maintenance of skeletal muscle function and metabolism (28).
Skeletal muscle-specific Bmal1 KO mouse models have been generated to study the effects of circadian disruption on skeletal muscle. While there are some differences in strength outcomes, these mouse models have a common phenotype that includes disrupted regulation of insulin-stimulated glucose uptake and its metabolism (23, 39). Skeletal muscle-specific Bmal1 KOs exhibited reductions in the mRNA and protein levels of GLUT4 and TBC1D1, glucose transporter in skeletal muscle and the translocator of GLUT4-containing vesicles, respectively. In addition to the change in glucose transport, the decrease in Bmal1 in skeletal muscle causes a downregulation of the key enzymes for the use of glucose, such as hexokinase 2 (HK2) and pyruvate dehydrogenase (PDH) (4, 23). The skeletal muscle-specific Bmal1 KO mice display normal circadian behavior under free-running conditions, unlike the global Bmal1 KO, suggesting that the changes observed in the metabolism are more likely to be downstream of the skeletal muscle clock mechanism in skeletal muscle. These results indicate that the circadian clock generates the circadian rhythm of carbohydrate metabolism in skeletal muscle and that its disruption leads to metabolic dysfunction in skeletal muscle.
Complementary to the data from the Bmal1 KO mice, circadian transcriptomic studies have been performed using the Clock mutant mouse. It is important to note that this is neither a KO nor a knock down but it is a mouse that carries a non-specific skeletal muscle mutation in the Clock gene that leads to a dominant negative type version of CLOCK in the whole mouse (40, 41). These studies (1, 2) found that 78% of the rhythmic genes in wild-type skeletal muscle had an altered magnitude of expression levels in Clock mutant skeletal muscle, while 11% of the rhythmic genes from wild-type skeletal muscle were shifted out of phase in the Clock mutant mice. Analysis of these datasets revealed that CLOCK-regulated genes were associated with the cell cycle and cell proliferation, the insulin signaling pathway, protein translation, gluconeogenesis, and muscle contraction (1).
The silencing of core clock genes and their effect on metabolism have also been evaluated in vitro using human skeletal muscle cells. Perrin et al. cultured primary skeletal myotubes and disrupted their circadian clock by transfecting siRNA targeting Clock (siClock: 11, 14). Genes involved in contraction-induced and insulin-stimulated glucose uptake were downregulated upon siClock. Accordingly, basal and insulin-stimulated glucose uptake in these myotubes were markedly reduced. Taken together, these findings suggest that like Bmal1, the Clock gene/protein in skeletal muscle is important for coordinating glucose uptake in vitro (17). Moreover, Clock depletion induced changes in lipid homeostasis (17) and myokine secretion (20). As shown in the skeletal muscle-specific Bmal1 KO mouse model, Clock disruption also facilitated a global switch at the transcriptomic program towards a more oxidative program (4, 17). Various genes involved in lipid transport and storage were affected after Clock silencing, leading to alterations in total phosphatidylcholine and glycosylceramide levels. Particularly, an increase in the long chain fatty acid transporter CD36 and in FABP3, consistent with the mouse skeletal muscle clock disruption model was found upon Clock silencing (4).
Loizides-Mangold et al. performed a targeted lipidomics analysis to quantify circadian rhythmicity within different lipid classes in human skeletal muscle samples (19). In line with findings from lipidomic analysis of human plasma (42), ~20% of detected lipids displayed diurnal oscillation in vivo and in vitro. Most lipids peaked in the early morning phase prior to awakening. As lipids largely contribute to the formation of the plasma membrane, it can be assumed that skeletal muscle cell membrane properties are subjected to substantial changes over the active/rest cycles potentially dictating important metabolic processes, e.g. receptor signaling and glucose uptake. Moreover, peak levels of major glycerophospholipids and sphingolipids were found to correlate with the peak of Bmal1 gene expression in both in vivo and in vitro conditions (19), indicating a potential role for skeletal muscle clocks in the regulation of lipid metabolism. Importantly, ~40% of oscillating lipid metabolites were downregulated in myotube cultures after siClock treatment. Furthermore, the analysis of lipidomic data indicated that there were also changes in the period length and amplitude of the oscillations after siClock treatment (19). It is not clear what the impact of period length changes will be for skeletal muscle but it highlights the variety of possibilities how the circadian clock can impact metabolic outcomes. Mice skeletal muscle transcriptomics have demonstrated that genes involved in fatty-acid uptake and β-oxidation peak in the middle of the rest phase, while lipogenic genes reach peak expression at the end of the active phase, suggesting that the clock in skeletal muscle promotes storage of excess energy at the end of the active phase (4).
5. Circadian misalignment and its impact on skeletal muscle metabolism
Circadian misalignment is often used in both mice and human studies to explore the consequences of circadian disruption in an experimental setting. Circadian misalignment is defined by disruption of clock phases across the tissues/organs within the system. This occurs through disturbing normal circadian time cues relative to the behavioral cycle including altered phases of light/dark, feeding/fasting, activity/rest. In modern society, many forms of circadian misalignment already exist, such as common daily routines like artificial lighting at night, eating at night and sleep deprivation, but also given by the working conditions, e.g. with shift work and time-zone transitions resulting in jet-lag. While there are many different ways to induce circadian misalignment, it is unclear if all these forms of misalignment lead to similar effects on parameters of metabolic health. However, a recent meta-analysis of observational studies revealed that shift workers, which are exposed to a mixture of disrupted light exposure, eating at night and sleep deprivation, have an increased risk to develop type 2 diabetes mellitus (T2DM) (43) and the risk is positively correlated to the number of night shifts per month (44). To study the underlying mechanisms of this relationship, circadian misalignment protocols have been developed to simulate conditions of shift work by shifting the behavioral cycle (including the sleep/wake and fasting/feeding cycles).
Wefers and colleagues (2018) tested fourteen healthy lean volunteers in a randomized cross-over design for 3.5 days of either shifting the behavioral cycle by 12h or a control condition (45). At the end of both periods, insulin sensitivity was determined using the golden standard hyperinsulinemic-euglycemic clamp at the beginning of the active phase for both control or misaligned protocols, and skeletal muscle biopsies were taken for mRNA analysis. Wefers et al. found that the circadian rhythm of core body temperature did not adapt to the new day-night rhythm, i.e. indicating circadian misalignment, leading to a higher body temperature and energy metabolism during sleep and a reduced body temperature during the waking period in the misaligned condition. Interestingly, this circadian misalignment resulted in an increase in plasma glucose and free fatty acids levels and a rapid induction of insulin resistance, which was mainly located at the level of the skeletal muscle as indicated by a 23% reduction in insulin-stimulated non-oxidative glucose disposal. Whole genome expression profiling revealed that many of the most highly enriched gene sets among the genes changed in expression in the misaligned condition were related to fatty acid metabolism and PPAR signaling (45). In addition, the expression of clock genes at two time points, 7AM and 7PM, was determined in both conditions. In the control condition, the core clock genes Bmal1, Cry1 and Per2 displayed diurnal differences in line with the previously described studies (17, 18). Despite reversing the activity/rest and feeding/fasting cycle, the expression pattern of Bmal1 and Clock was conserved highlighting the robustness of the positive limb of the skeletal muscle clocks to feeding and activity, while the expression of Per2 and Cry1 mRNAs was altered.
Another study investigated the effects of acute sleep loss compared to a full night of sleep on the transcriptome of skeletal muscle and adipose tissue including their clock machinery (46). Strikingly, after only one night of sleep loss, pathways indicative of skeletal muscle breakdown and anabolic processes in adipose tissue were evident. At the same time, only the skeletal muscle clocks were altered with an upregulation of BMAL1 protein levels, whereas the molecular clock in adipose tissue remained unchanged. Interestingly, sleep deprivation also resulted in molecular changes in skeletal muscle indicating a shift to fatty acid metabolism, supporting the findings by Wefers et al. (45). Sleep loss further led to higher postprandial glucose levels, whereas insulin was stable, suggesting reduced glucose handling in peripheral tissues (46). Accordingly, it has been shown that persistent sleep restriction with concurrent circadian disruption alters metabolism and could increase the risk of obesity and T2DM (47). Taken together, these data indicate that circadian misalignment results in disturbed energy metabolism and insulin resistance in human skeletal muscle which both could be mediated by impaired skeletal muscle clocks.
6. The skeletal muscle clocks in obesity and under diabetogenic conditions
The circadian metabolic profile of skeletal muscle cells has further been evaluated under diabetogenic conditions in vitro. Hansen and colleagues (2016) compared clock gene expression of human primary myotubes derived from four different groups of donors: (i) young endurance trained athletes, (ii) their age-matched young, lean sedentary controls, (iii) healthy, obese subjects and (iv) body mass index- and age-matched T2DM patients (21). They did not find any differences in the expression of the core clock components between donor groups. Likewise, Perrin et al. did not find any differences in period length of the Bmal1-luc or Per2-luc reporter between myotubes established from obese and non-obese donors (20). However, T2DM patients lacked robust circadian rhythmicity of components of the core clock, Rev-erbα and Rev-erbβ (21). Downstream of the core clock, circadian rhythmicity in the expression of nicotinamide phosphoribosyltransferase (Nampt), sirtuin 1 (Sirt1) and insulin receptor substrate 1 (Irs1), genes associated with mitochondrial function and metabolic health, were disrupted in myotubes from T2DM subjects (21). Nampt has been shown to be regulated by Bmal1 and Clock in complex with Sirt1 (48) and sleep loss seems to affect rhythmicity of Nampt expression and might thereby contribute to impaired postprandial glucose metabolism (49). These results show that primary skeletal muscles cells were able to maintain the circadian metabolic characteristics in vitro dependent on the donor characteristics, resulting in an advantageous in vitro tool to study the circadian clock from skeletal muscle cells derived from patients with distinct metabolic diseases.
Recently, Sardon Puig and colleagues (2019) assessed clock gene expression in the vastus lateralis muscle before and six months after gastric bypass surgery in obese women and men and compared this data to baseline gene expression in normal weight controls (50). They found that skeletal muscle clock gene expression is affected by obesity and by the gastric bypass induced weight loss. However, these results must be interpreted with caution since only one biopsy was taken at each occasion and the time of day was not standardized for the biopsies taken at baseline. Accordingly, reported differences can rather be a result of timing differences than reflecting weight loss or obesity-induced effects. In the same study, unsynchronized myotubes from the normal weight controls were exposed to plasma free fatty acids, i.e. palmitate or oleate, to simulate diabetogenic conditions in vitro and study the plasticity of the skeletal muscle clocks under these conditions. For both fatty acids, the expression of a ubiquitous CCG, Dbp, was reduced while Rev-erbβ was upregulated. Also, serum shock synchronized myotubes were exposed to palmitate for 54h. Over time, palmitate treatment was found to alter the expression of Bmal1, Cry2, Dbp, Per1 and Per3. These findings suggest that circulating lipid metabolites can modify the phase and/or pattern of skeletal muscle clocks gene expression. It could therefore be speculated that increased lipid signaling represents a putative component of the time of feeding cue for skeletal muscle considering that free fatty acids rise during the fasted night under metabolically healthy conditions in humans (18). As free fatty acids are generally elevated in T2DM patients (51), diabetic skeletal muscle might lose its sense of time based on nutrient status and is further confronted with contradicting time cues during daytime.
7. Exercise as a tool to reset the clock and improve metabolic health?
It is well known that exercise training is an effective strategy for restoring metabolic outcomes in skeletal muscle of T2DM patients to levels of age- and BMI-matched healthy controls, e.g. mitochondrial function, RER, skeletal muscle insulin sensitivity, by improving skeletal muscle function (52). Whether exercise exerts its benefits through the molecular clock mechanism has not been studied yet. However, acute exercise is now considered a time cue for clocks in peripheral tissues and joins other factors such as glucocorticoids, feeding, neurohumoral input from the central clock and likely temperature (53, 54). Many studies using the circadian reporter Per2::Luc mice have shown that running exercise can produce a significant time-dependent phase shift of the Per2::Luc bioluminescence rhythms in several skeletal muscles. It has also been shown that exercise can improve circadian rhythms in a genetic mouse model that uncouples the temporal communication within the central clock and therefore exhibits diminished behavioral and physiological rhythms (55). Schroeder et al. found that allowing these mutant mice to run on a wheel in the later part of the active/dark phase improved their rhythmic deficits, including an improved power of locomotion activity rhythms, a shifting in acrophase of locomotion activity rhythms such that the phase was no longer different when compared with wild-type mice, and increased the amplitude of Per2::Luc rhythms in the central clock. Interestingly, the phase of Per2::Luc rhythms in peripheral tissues, e.g. adrenals and liver, was also rescued (55).
In humans, data on acute exercise and exercise training effects on the skeletal muscle clocks are more limited. Zambon et al. compared skeletal muscle clock gene expression in the exercised and non-exercised leg of four human volunteers 6h and 18h after an acute bout of resistance exercise (56). First of all, they could confirm diurnal differences in the core clock factors within the non-exercise control limb. Strikingly, they found that Bmal1, Per2 and Cry1 were upregulated 6h after exercise , whereas Per1, Cry2 and Rev-erbβ remained unchanged. This suggests that skeletal muscle contraction maybe sufficient to alter the gene expression of skeletal muscle clocks, but the effect of exercise on physiological whole-body or muscle-specific circadian outcomes was not investigated. Youngstedt et al. (2019) investigated human circadian phase response curves of urinary melatonin profiles after different times of moderate-intensity walking exercise performed for 1h per day for 3 days (57). Based on a large sample size and under highly standardized conditions, exercise induced consistent phase advances when performed either at 7 AM or between 1 and 4 PM, and consistent phase delays when exercise was performed at 10 PM. Future studies are needed to determine if such phase advances or delays also occur within the skeletal muscle clock. Previous studies investigating the effect of repeated daytime endurance exercise suggested a phase advance based on dim-light melatonin onset (58) and rectal temperature (59). In contrast, acute nocturnal endurance exercise (for 3h) induced a phase delay in melatonin secretion(60, 61).
There have been several recent studies investigating the time-dependent effects of exercise on metabolism. One recent study showed that time of acute exercise (early rest phase vs. early active phase) modifies the diurnal rhythmicity of the transcriptome in mouse skeletal muscle in a time-of-day-dependent manner (62). A combined analysis of transcriptomics and metabolomics in skeletal muscle showed that exercise at the early active/dark phase disrupts circadian rhythmicity of genes and metabolites related to carbohydrate metabolism, while exercise at the early rest/light phase stimulates the circadian expression of genes and metabolites related to carbohydrate metabolism and decreases rhythmicity of genes and metabolites related to glycerol metabolism (62). Moreover, the impact of acute exercise on systemic energy homeostasis appears to be dependent on the time of day, since oscillations in RER and energy expenditure respond differently according to the timing of exercise (62). Another study examined the difference in exercise capacity of mice between two time points within their active phase, 2h after lights-off and 2h before lights-on (named Early and Late, respectively) (63). First, they reported that running duration at 55% and 45% of the maximal aerobic capacity was longer at the Late time. Gene expression analysis of skeletal muscle samples of non-exercised mice at Early and Late time points showed daily variation in exercise-related signaling pathways, including peroxisome proliferator-activated receptor (PPAR), AMP-activated protein kinase (AMPK), and hypoxia inducible factor (HIF) (63), suggesting that circadian regulation of these pathways may explain daily variation in exercise capacity that has also been reported previously (62, 64–66).
So far, human studies investigating the effect of exercise timing on the outcomes of training are very limited. However, in line with the above mentioned findings in acute exercise studies, Savikj et al. used a randomized cross over design to compare outcomes with T2DM subjects that trained in the morning (1h after a meal) vs. afternoon training (3h after a meal). They found that two weeks of exercise training when performed in the afternoon is more efficacious than morning exercise at improving 24h blood glucose levels in T2DM patients (67). Ezagouri et al. exercised human volunteers using a submaximal constant-load exercise protocol at two times points, 8AM (Early) and 6PM (Late)(63). The results confirmed a diurnal variance in exercise capacity, where the Late group (vs Early) was characterized by a lower oxygen consumption, higher RER, lower heart rate, and lower blood glucose levels after exercise. To what extent the skeletal muscle clocks might contribute to improved glucose tolerance in response to different timing of exercise training still needs to be elucidated, as for example also the timing of meals may have influenced these results. Nevertheless, these studies provide a rationale to continue to test the application of exercise timing as a therapeutic strategy to treat metabolic diseases in which the skeletal muscle clocks might be disrupted.
8. Conclusion and future directions
Nowadays, in a mostly unnoticed manner, circadian disruption is omnipresent. In our modern society, especially food and artificial lighting are available around the clock. These environmental cues are coming at irregular times potentially providing conflicting time cues for all the clocks in our body. With physical activity levels declining the contribution of exercise as a time cue for our clocks is under-represented. Skeletal muscle is an important organ for metabolic health, and animal and human data reveal the relevance of circadian rhythmicity for skeletal muscle metabolic health. The common features of the skeletal muscle clocks in rodents and humans and the similar impact of clock disruption on substrate metabolism are important. An implication of these similarities is that preclinical research and clinical interventions can work together in the future to more rapidly advance our understanding and use of circadian principles for skeletal muscle and metabolic health. Such knowledge can help to develop timed interventions that may aid to optimize treatment and prevention options for obesity and T2DM.
Questions:
What is already known about this subject?
Skeletal muscle is important in maintaining whole body energy, and substrate metabolism and glucose homeostasis.
Disruption of the skeletal muscle clocks in mice leads to impaired glucose metabolism and increased insulin resistance.
Circadian misalignment in humans results in disturbed energy metabolism and insulin resistance in skeletal muscle.
What are the new findings in your manuscript?
This is the first review focused on comparing the findings on skeletal muscle clocks between humans and mice.
We review the latest progress on the role of the skeletal muscle clocks in metabolic health.
We discuss time-dependent exercise as a therapeutic strategy for restoring metabolic disruption.
Funding information:
Support from NIH grants RO1 AR 066082 and UO1 AG 055137 to KAE
Footnotes
Conflict of interest disclosure: All authors declared no conflicts of interest.
Clinical trial registration number: N/A.
References
- 1.Miller BH, McDearmon EL, Panda S, et al. Circadian and CLOCK-controlled regulation of the mouse transcriptome and cell proliferation. Proc Natl Acad Sci U S A 2007;104:3342–3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCarthy JJ, Andrews JL, McDearmon EL, et al. Identification of the circadian transcriptome in adult mouse skeletal muscle. Physiol Genomics 2007;31:86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang R, Lahens NF, Ballance HI, Hughes ME, Hogenesch JB. A circadian gene expression atlas in mammals: Implications for biology and medicine. Proc Natl Acad Sci 2014;111:16219–16224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hodge BA, Wen Y, Riley LA, et al. The endogenous molecular clock orchestrates the temporal separation of substrate metabolism in skeletal muscle. Skelet Muscle 2015;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Janssen I, Heymsfield SB, Wang ZM, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. J Appl Physiol Bethesda Md 1985 2000;89:81–88. [DOI] [PubMed] [Google Scholar]
- 6.Defronzo RA, Simonson D, Ferrannini E, Barrett E. Insulin resistance: a universal finding in diabetic states. Bull Schweiz Akad Med Wiss 1981:223–238. [PubMed] [Google Scholar]
- 7.Ferrannini E, Simonson DC, Katz LD, et al. The disposal of an oral glucose load in patients with non-insulin-dependent diabetes. Metabolism 1988;37:79–85. [DOI] [PubMed] [Google Scholar]
- 8.Periasamy M, Herrera JL, Reis FCG. Skeletal Muscle Thermogenesis and Its Role in Whole Body Energy Metabolism. Diabetes Metab J 2017;41:327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zurlo F, Larson K, Bogardus C, Ravussin E. Skeletal muscle metabolism is a major determinant of resting energy expenditure. J Clin Invest 1990;86:1423–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zurlo F, Nemeth PM, Choksi RM, Sesodia S, Ravussin E. Whole-body energy metabolism and skeletal muscle biochemical characteristics. Metabolism 1994;43:481–486. [DOI] [PubMed] [Google Scholar]
- 11.McCarthy JJ, Esser KA. Anabolic and catabolic pathways regulating skeletal muscle mass. Curr Opin Clin Nutr Metab Care 2010;13:230–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nei M, Xu P, Glazko G. Estimation of divergence times from multiprotein sequences for a few mammalian species and several distantly related organisms. Proc Natl Acad Sci U S A 2001;98:2497–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Acosta-Rodríguez VA, de Groot MHM, Rijo-Ferreira F, Green CB, Takahashi JS. Mice under Caloric Restriction Self-Impose a Temporal Restriction of Food Intake as Revealed by an Automated Feeder System. Cell Metab 2017;26:267–277.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kohsaka A, Laposky AD, Ramsey KM, et al. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab 2007;6:414–421. [DOI] [PubMed] [Google Scholar]
- 15.Campbell SS, Tobler I. Animal sleep: a review of sleep duration across phylogeny. Neurosci Biobehav Rev 1984;8:269–300. [DOI] [PubMed] [Google Scholar]
- 16.Hughes ME, Abruzzi KC, Allada R, et al. Guidelines for Genome-Scale Analysis of Biological Rhythms. J Biol Rhythms 2017;32:380–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perrin L, Loizides-Mangold U, Chanon S, et al. Transcriptomic analyses reveal rhythmic and CLOCK-driven pathways in human skeletal muscle. eLife 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Moorsel D, Hansen J, Havekes B, et al. Demonstration of a day-night rhythm in human skeletal muscle oxidative capacity. Mol Metab 2016;5:635–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loizides-Mangold U, Perrin L, Vandereycken B, et al. Lipidomics reveals diurnal lipid oscillations in human skeletal muscle persisting in cellular myotubes cultured in vitro. Proc Natl Acad Sci U S A 2017;114:E8565–E8574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perrin L, Loizides-Mangold U, Skarupelova S, et al. Human skeletal myotubes display a cell-autonomous circadian clock implicated in basal myokine secretion. Mol Metab 2015;4:834–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hansen J, Timmers S, Moonen-Kornips E, et al. Synchronized human skeletal myotubes of lean, obese and type 2 diabetic patients maintain circadian oscillation of clock genes. Sci Rep 2016;6:35047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barnea M, Cohen-Yogev T, Chapnik N, Madar Z, Froy O. Effect of metformin and lipid emulsion on the circadian gene expression in muscle cells. Int J Biochem Cell Biol 2014;53:151–161. [DOI] [PubMed] [Google Scholar]
- 23.Dyar KA, Ciciliot S, Wright LE, et al. Muscle insulin sensitivity and glucose metabolism are controlled by the intrinsic muscle clock. Mol Metab 2014;3:29–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schiaffino S, Reggiani C. Fiber types in mammalian skeletal muscles. Physiol Rev 2011;91:1447–1531. [DOI] [PubMed] [Google Scholar]
- 25.Dyar KA, Ciciliot S, Tagliazucchi GM, et al. The calcineurin-NFAT pathway controls activity-dependent circadian gene expression in slow skeletal muscle. Mol Metab 2015;4:823–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haldar SM, Jeyaraj D, Anand P, et al. Kruppel-like factor 15 regulates skeletal muscle lipid flux and exercise adaptation. Proc Natl Acad Sci U S A 2012;109:6739–6744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mansueto G, Armani A, Viscomi C, et al. Transcription Factor EB Controls Metabolic Flexibility during Exercise. Cell Metab 2017;25:182–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andrews JL, Zhang X, McCarthy JJ, et al. CLOCK and BMAL1 regulate MyoD and are necessary for maintenance of skeletal muscle phenotype and function. Proc Natl Acad Sci U S A 2010;107:19090–19095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang X, Patel SP, McCarthy JJ, Rabchevsky AG, Goldhamer DJ, Esser KA. A non-canonical E-box within the MyoD core enhancer is necessary for circadian expression in skeletal muscle. Nucleic Acids Res 2012;40:3419–3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hodge BA, Zhang X, Gutierrez-Monreal MA, et al. MYOD1 functions as a clock amplifier as well as a critical co-factor for downstream circadian gene expression in muscle. eLife 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zitting K-M, Vujovic N, Yuan RK, et al. Human Resting Energy Expenditure Varies with Circadian Phase. Curr Biol CB 2018;28:3685–3690.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chtourou H, Souissi N. The effect of training at a specific time of day: a review. J Strength Cond Res 2012;26:1984–2005. [DOI] [PubMed] [Google Scholar]
- 33.Facer-Childs E, Brandstaetter R. The impact of circadian phenotype and time since awakening on diurnal performance in athletes. Curr Biol CB 2015;25:518–522. [DOI] [PubMed] [Google Scholar]
- 34.Saad A, Dalla Man C, Nandy DK, et al. Diurnal pattern to insulin secretion and insulin action in healthy individuals. Diabetes 2012;61:2691–2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bae S-A, Fang MZ, Rustgi V, Zarbl H, Androulakis IP. At the Interface of Lifestyle, Behavior, and Circadian Rhythms: Metabolic Implications. Front Nutr 2019;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bunger MK, Wilsbacher LD, Moran SM, et al. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell 2000;103:1009–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kondratov RV, Kondratova AA, Gorbacheva VY, Vykhovanets OV, Antoch MP. Early aging and age-related pathologies in mice deficient in BMAL1, the core componentof the circadian clock. Genes Dev 2006;20:1868–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rudic RD, McNamara P, Curtis A-M, et al. BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biol 2004;2:e377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harfmann BD, Schroder EA, Kachman MT, Hodge BA, Zhang X, Esser KA. Muscle-specific loss of Bmal1 leads to disrupted tissue glucose metabolism and systemic glucose homeostasis. Skelet Muscle 2016;6:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vitaterna MH, King DP, Chang AM, et al. Mutagenesis and mapping of a mouse gene, Clock, essential for circadian behavior. Science 1994;264:719–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.King DP, Zhao Y, Sangoram AM, et al. Positional cloning of the mouse circadian clock gene. Cell 1997;89:641–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chua EC-P, Shui G, Lee IT-G, et al. Extensive diversity in circadian regulation of plasma lipids and evidence for different circadian metabolic phenotypes in humans. Proc Natl Acad Sci U S A 2013;110:14468–14473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gan Y, Yang C, Tong X, et al. Shift work and diabetes mellitus: a meta-analysis of observational studies. Occup Environ Med 2015;72:72–78. [DOI] [PubMed] [Google Scholar]
- 44.Vetter C, Dashti HS, Lane JM, et al. Night Shift Work, Genetic Risk, and Type 2 Diabetes in the UK Biobank. Diabetes Care 2018;41:762–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wefers J, van Moorsel D, Hansen J, et al. Circadian misalignment induces fatty acid metabolism gene profiles and compromises insulin sensitivity in human skeletal muscle. Proc Natl Acad Sci U S A 2018;115:7789–7794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cedernaes J, Schönke M, Westholm JO, et al. Acute sleep loss results in tissue-specific alterations in genome-wide DNA methylation state and metabolic fuel utilization in humans. Sci Adv 2018;4:eaar8590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Buxton OM, Cain SW, O’Connor SP, et al. Adverse metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Sci Transl Med 2012;4:129ra43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ramsey KM, Yoshino J, Brace CS, et al. Circadian clock feedback cycle through NAMPT-mediated NAD+ biosynthesis. Science 2009;324:651–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Benedict C, Shostak A, Lange T, et al. Diurnal rhythm of circulating nicotinamide phosphoribosyltransferase (Nampt/visfatin/PBEF): impact of sleep loss and relation to glucose metabolism. J Clin Endocrinol Metab 2012;97:E218–222. [DOI] [PubMed] [Google Scholar]
- 50.Sardon Puig L, Pillon NJ, Näslund E, Krook A, Zierath JR. Influence of Obesity, Weight Loss, and Free Fatty Acids on Skeletal Muscle Clock Gene Expression. Am J Physiol Endocrinol Metab 2019. [DOI] [PubMed] [Google Scholar]
- 51.Spiller S, Blüher M, Hoffmann R. Plasma levels of free fatty acids correlate with type 2 diabetes mellitus. Diabetes Obes Metab 2018;20:2661–2669. [DOI] [PubMed] [Google Scholar]
- 52.Meex RCR, Schrauwen-Hinderling VB, Moonen-Kornips E, et al. Restoration of muscle mitochondrial function and metabolic flexibility in type 2 diabetes by exercise training is paralleled by increased myocellular fat storage and improved insulin sensitivity. Diabetes 2010;59:572–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bass J Circadian topology of metabolism. Nature 2012;491:348–356. [DOI] [PubMed] [Google Scholar]
- 54.Schroder EA, Esser KA. Circadian rhythms, skeletal muscle molecular clocks, and exercise. Exerc Sport Sci Rev 2013;41:224–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schroeder AM, Truong D, Loh DH, Jordan MC, Roos KP, Colwell CS. Voluntary scheduled exercise alters diurnal rhythms of behaviour, physiology and gene expression in wild-type and vasoactive intestinal peptide-deficient mice. J Physiol 2012;590:6213–6226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zambon AC, McDearmon EL, Salomonis N, et al. Time- and exercise-dependent gene regulation in human skeletal muscle. Genome Biol 2003;4:R61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Youngstedt SD, Elliott JA, Kripke DF. Human circadian phase-response curves for exercise. J Physiol 2019;597:2253–2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Miyazaki T, Hashimoto S, Masubuchi S, Honma S, Honma KI. Phase-advance shifts of human circadian pacemaker are accelerated by daytime physical exercise. Am J Physiol Regul Integr Comp Physiol 2001;281:R197–205. [DOI] [PubMed] [Google Scholar]
- 59.Eastman CI, Hoese EK, Youngstedt SD, Liu L. Phase-shifting human circadian rhythms with exercise during the night shift. Physiol Behav 1995;58:1287–1291. [DOI] [PubMed] [Google Scholar]
- 60.Van Reeth O, Sturis J, Byrne MM, et al. Nocturnal exercise phase delays circadian rhythms of melatonin and thyrotropin secretion in normal men. Am J Physiol 1994;266:E964–974. [DOI] [PubMed] [Google Scholar]
- 61.Baehr EK, Eastman CI, Revelle W, Olson SHL, Wolfe LF, Zee PC. Circadian phase-shifting effects of nocturnal exercise in older compared with young adults. Am J Physiol Regul Integr Comp Physiol 2003;284:R1542–1550. [DOI] [PubMed] [Google Scholar]
- 62.Sato S, Basse AL, Schönke M, et al. Time of Exercise Specifies the Impact on Muscle Metabolic Pathways and Systemic Energy Homeostasis. Cell Metab 2019;30:92–110.e4. [DOI] [PubMed] [Google Scholar]
- 63.Ezagouri S, Zwighaft Z, Sobel J, et al. Physiological and Molecular Dissection of Daily Variance in Exercise Capacity. Cell Metab 2019;30:78–91.e4. [DOI] [PubMed] [Google Scholar]
- 64.Jordan SD, Kriebs A, Vaughan M, et al. CRY1/2 Selectively Repress PPARδ and Limit Exercise Capacity. Cell Metab 2017;26:243–255.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lassiter DG, Sjögren RJO, Gabriel BM, Krook A, Zierath JR. AMPK activation negatively regulates GDAP1, which influences metabolic processes and circadian gene expression in skeletal muscle. Mol Metab 2018;16:12–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Peek CB, Levine DC, Cedernaes J, et al. Circadian Clock Interaction with HIF1α Mediates Oxygenic Metabolism and Anaerobic Glycolysis in Skeletal Muscle. Cell Metab 2017;25:86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Savikj M, Gabriel BM, Alm PS, et al. Afternoon exercise is more efficacious than morning exercise at improving blood glucose levels in individuals with type 2 diabetes: a randomised crossover trial. Diabetologia 2019;62:233–237. [DOI] [PMC free article] [PubMed] [Google Scholar]


